Abstract
Background
People with type 2 diabetes mellitus are at increased risk from cardiovascular disease. Dietary omega‐3 polyunsaturated fatty acids (PUFAs) are known to reduce triglyceride levels, but their impact on cholesterol levels, glycemic control and vascular outcomes are not well known.
Objectives
To determine the effects of omega‐3 PUFA supplementation on cardiovascular outcomes, cholesterol levels and glycemic control in people with type 2 diabetes mellitus.
Search methods
We carried out a comprehensive search of The Cochrane Library, MEDLINE, EMBASE, bibliographies of relevant papers and contacted experts for identifying additional trials.
Selection criteria
All randomised controlled trials were included where omega‐3 PUFA supplementation or dietary intake was randomly allocated and unconfounded in people with type 2 diabetes. Authors of large trials were contacted for missing information.
Data collection and analysis
Trials were assessed for inclusion. Authors were contacted for missing information. Data was extracted and quality assessed independently in duplicate. Fixed‐effect meta‐analysis was carried out.
Main results
Twenty three randomised controlled trials (1075 participants) were included with a mean treatment duration of 8.9 weeks. The mean dose of omega‐3 PUFA used in the trials was 3.5 g/d. No trials with vascular events or mortality endpoints were identified. Among those taking omega‐3 PUFA triglyceride levels were significantly lowered by 0.45 mmol/L (95% confidence interval (CI) ‐0.58 to ‐0.32, P < 0.00001) and VLDL cholesterol lowered by ‐0.07 mmol/L (95% CI ‐0.13 to 0.00, P = 0.04). LDL cholesterol levels were raised by 0.11 mmol/L (95% CI 0.00 to 0.22, P = 0.05). No significant change in or total or HDL cholesterol, HbA1c, fasting glucose, fasting insulin or body weight was observed. The increase in VLDL remained significant only in trials of longer duration and in hypertriglyceridemic patients. The elevation in LDL cholesterol was non‐significant in subgroup analyses. No adverse effects of the intervention were reported.
Authors' conclusions
Omega‐3 PUFA supplementation in type 2 diabetes lowers triglycerides and VLDL cholesterol, but may raise LDL cholesterol (although results were non‐significant in subgroups) and has no statistically significant effect on glycemic control or fasting insulin. Trials with vascular events or mortality defined endpoints are needed.
Plain language summary
Omega‐3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus
People with type 2 diabetes are known to be at increased risk of cardiovascular disease (such as heart attack or stroke). Type 2 diabetes mellitus is the fourth leading cause of death in developed countries with a two fold excess mortality and a two to four fold increased risk of coronary heart disease and stroke. The typical dyslipidemia (abnormality in blood lipids) associated with type 2 diabetes is a combination of hypertriglyceridemia (high levels of fats (triglycerides) in the blood), low levels of HDL (high density lipoprotein) cholesterol and abnormal LDL (low density lipoprotein) composition. Low levels of HDL cholesterol and high levels of LDL cholesterol are associated with an increased risk of cardiovascular disease, while the raised levels of triglycerides are less clearly linked to an increased risk of cardiovascular disease. Several pharmacologic approaches have been used to treat diabetic dyslipidemia and standard dietary approaches focus on restriction of saturated fat and limitation of simple carbohydrate and alcohol intake. In the late 1980s, several investigators reported on the use of dietary supplementation with fish oil as a means of treating diabetic dyslipidemia. Dietary fats and oils from different sources differ considerably in their fatty acid composition. Animal fat is rich in saturated fatty acids, vegetable and marine oils are rich in polyunsaturated fatty acids. Most fish oils are of the so‐called omega‐3 variety (omega‐3 polyunsaturated fatty acids (PUFAs)). We identified 23 randomised trials (maximum duration of eight months) including 1075 people in which omega‐3 PUFA was compared to a vegetable oil or placebo. None of the trials looked at cardiovascular endpoints in cardiovascular disease or death as an outcome measure. The review shows that although some types of fat in the blood are reduced through omega‐3 supplementation, others including LDL cholesterol (which may promote heart disease) were increased. Control of blood sugar levels was not affected by the treatment. There were no other adverse effects of the interventions noted. Clinical outcome trials of sufficient duration are required to establish conclusively the role of omega‐3 PUFA in type 2 diabetes but our results do not suggest a major harmful effect on the balance of blood fats and confirm that it has no adverse affect on blood sugar control.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups'). For an explanation of methodological terms, see the main Glossary in The Cochrane Library.
Type 2 diabetes mellitus is the fourth leading cause of death in developed countries with a two fold excess mortality and a two to four fold increased risk of coronary heart disease and stroke. The typical dyslipidemia (abnormality in blood lipids) associated with type 2 diabetes is a combination of hypertriglyceridemia (high levels of fats (triglycerides) in the blood), low levels of HDL (high density lipoprotein) cholesterol and abnormal LDL (low density lipoprotein) composition (Howard 1987). Low levels of HDL cholesterol and high levels of LDL cholesterol are associated with an increased risk of cardiovascular disease (CVD), while the raised levels of triglycerides are less clearly linked to an increased risk of CVD. Several pharmacologic approaches have been used to treat diabetic dyslipidemia (ADA 1998). These include use of 3‐hydroxy 3‐methylglutaryl coenzyme A (HMG Co‐A) reductase inhibitors (promoting the removal of LDL cholesterol from the blood) (Pyorala 1997), fibric acid derivatives (exact mechanism of action unclear, but probably includes stimulating triglyceride breakdown and LDL cholesterol removal from the blood) (Elkeles 1998) and niacin (inhibits triglyceride production in the liver and VLDL (very low density lipoprotein) secretion) (Garg 1990). Standard dietary approaches focus on restriction of saturated fat and limitation of simple carbohydrate and alcohol intake (ADA 1998). In the late 1980s, several investigators reported on the use of dietary supplementation with fish oil as a means of treating diabetic dyslipidemia (Glauber 1988; Friday 1989).
Description of the intervention
A potential role for marine‐derived omega‐3 polyunsaturated fatty acids (PUFA) in CVD risk reduction first came from observations of the native inhabitants of Greenland (Inuits) (Mouraoff 1967). Despite ingesting up to 40 percent of calories as fat (predominantly of marine origin), this population had a lower incidence of coronary heart disease compared to individuals with similar fat intake on a more conventional diet (Bang 1976). Dietary fats and oils from different sources differ considerably in their fatty acid composition. Animal fat is rich in saturated fatty acids. Vegetable and marine oils are rich in polyunsaturated fatty acids. Polyunsaturated fatty acids are characterised by the presence of more than one double bond (allowing them to stay liquid at very low temperatures). The designation using n‐3 or the Greek symbol omega‐3, or n‐6 and omega‐6, has been applied in the case of fatty acids with the first double bond three or six carbon atoms from the end of the chain. Most fish oils are of the omega‐3 variety and most vegetable oils are of the omega‐6 variety, although alpha‐linoleic acid is an omega‐3 fatty acid found in canola oil. The omega‐3 fatty acids found in fish oils are predominantly eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA).
How the intervention might work
The beneficial effect of marine‐derived omega‐3 PUFA on cardiac risk markers and on lowering cardiovascular mortality and sudden death in the general population have previously been reported in the GISSI‐Prevenzione (GISSI 1999) and DART 1 trials (Burr 1989) and in a subsequent meta‐analysis (Bucher 2002). However, the results of a later secondary prevention trial on coronary heart disease (CHD) and mortality (Burr 2003) do not support the earlier conclusions and a subsequent review has also raised doubts that omega‐3 PUFA reduce cardiovascular endpoints (Hooper 2004). The possibility of enhanced benefit from omega‐3 PUFA in people with diabetes has been shown in two previous reviews. In a previous review of the role of omega‐3 PUFA in diabetes (Friedberg 1998), benefit in reducing triglyceride levels was suggested. However, the authors included non‐randomized studies and studies including people with both type 1 and type 2 diabetes. Their review included studies up to June 1995. Concerns were also raised about the possibility of harm from omega‐3 PUFA supplementation. Early non‐randomised studies in patients with type 2 diabetes suggested that omega‐3 PUFA might be associated with a deterioration in glycemic control (Friday 1989; Glauber 1988). This concern was addressed in the first publication of this systematic review, which showed that omega‐3 PUFA supplementation has no adverse effects on glycemic control (Farmer 2001; Montori 2000).
Why it is important to do this review
The first publication of this Cochrane review was limited to randomized trials involving patients with type 2 diabetes and included searches for trials up to September 2000 (Farmer 2001). The current review includes randomised trials searched up to September 2006 and differs to our previous review in the following respects:
title was changed from 'fish oil in people with type 2 diabetes mellitus' to 'Omega‐3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus';
first author was changed to Janine Hartweg;
change in outcomes from baseline to end‐of‐trial was used to calculate the pooled effect sizes;
a further five trials up to 2006 were identified and included in the analysis;
two further outcomes are included in addition to those previously pooled.
We therefore set out to update our previous systematic review of dietary supplementation using omega‐3 PUFA among people with type 2 diabetes mellitus. Although our primary aim was to identify trials in which morbidity was studied, we also identified secondary aims of establishing the extent to which changes in serum lipids and deterioration in glucose control occurs following omega‐3 PUFA supplementation .
Objectives
To assess the effects of omega‐3 PUFA supplementation on death and vascular events in people with type 2 diabetes mellitus. We also wished to establish changes in lipids and whether deterioration in glucose control occurs.
Methods
Criteria for considering studies for this review
Types of studies
Papers of any language were considered. Trials were eligible if they were randomized placebo or vegetable oil controlled trials of omega‐3 polyunsaturated fatty acids (PUFA) (including cross‐over trials) as the only intervention in participants with type 2 diabetes. As no phase‐specific information was available for cross‐over trials, data were used only from the first intervention period to prevent measurements from the second period being affected by effects carried over from the first intervention period. Where serial measurement of an outcome was given during the intervention phase, data were obtained from the final measurement since that measurement was considered the conclusion of the study. The effect of trial design was explored in a sensitivity analysis.
Types of participants
Adults with type 2 diabetes mellitus. The diagnosis of type 2 diabetes among the participants of trials included in the review was established using the standard criteria valid at the time of the beginning of the trial.
Types of interventions
Trials in which participants were randomised to any type of dietary supplementation with omega‐3 PUFA were included. No restrictions were imposed on dose or formulation, although trials where the effect of omega‐3 PUFA could not be separated from the effect of simultaneously applied interventions, such as exercise or monounsaturated fatty acids, were not included.
No restrictions were placed on the range of compounds used as controls in the study. Some vegetable oils contain omega‐3 PUFA, or complex fatty acids that might be metabolised to form omega‐3 PUFA.
Types of outcome measures
Primary outcomes
fatal myocardial infarction or sudden cardiac death;
proven non‐fatal myocardial infarction;
coronary or peripheral revascularization procedures.
Secondary outcomes
triglycerides
total cholesterol
HDL cholesterol
LDL cholesterol
VLDL cholesterol
HbA1c
fasting glucose
fasting insulin
body weight
adverse effects
Timing of outcome measurement
Primary outcome measures will require studies of long duration to yield meaningful results. We anticipated that changes in secondary outcome measures would develop and remain stable over a short period of time and so we included studies of any duration, combining studies of short duration (three to eight weeks) and medium duration (three to six months).
Search methods for identification of studies
Electronic searches
We searched the specialised register of the former Cochrane Diabetes Group and the Cochrane Central Register of Controlled Trials, as well as an electronic literature search of MEDLINE and EMBASE (from the beginning of each database until April 2007) in two phases to identify trials involving omega‐3.
Our original search was conducted for publications from 1966 to 2000, and the second search was conducted up to 2006 using a protocol that included the Cochrane Collaboration's search strategy for randomized controlled trials (Dickersin 1994, adapted for each database), using a similar search strategy for both phases (see Appendix 1).
We searched for records in all languages.
The bibliographic sections of all publications of included or excluded trials were searched for additional trials.
Searching other resources
Dr CR Sirtori (Milan) and Dr E Ryan (Edmonton, Alberta), two trialists, were consulted in an attempt to identify any other overlooked, unpublished or ongoing studies. We did not attempt to contact other authors where the size of the trials was small.
Data collection and analysis
Selection of studies
The titles, abstracts and keywords of every record were retrieved to determine the relevant trials. Full articles were retrieved for further assessment if the information given suggested that the trial (1) included patients with type 2 diabetes mellitus, (2) compared fish oil with placebo or vegetable oil, (3) assessed one or more clinically relevant outcome measures, (4) used random allocation for the comparison groups. When there was any doubt regarding these criteria from the information given in the title and abstract, the full article was retrieved for clarification. When differences in opinion existed, these were resolved by consensus referring back to the original article.
The full articles retrieved were examined independently by the two investigators to identify relevant trials. Discrepancies were resolved by consensus.
Data extraction and management
Two reviewers extracted data from the studies independently. Disagreements were resolved by consensus. The data extraction form included the type of trial (randomised or cross‐over), type of omega‐3 polyunsaturated fatty acids (PUFA) and type of control (including dose), length of intervention, trial setting, diabetes diagnosis, baseline characteristics of intervention and control groups (including age, gender, duration of diabetes, co‐morbidity and complications, and treatment), outcomes assessed and biochemical outcome data in relation to study duration.
Assessment of risk of bias in included studies
Two investigators independently assigned quality scores to studies with discrepancies resolved by consensus. A score developed from the criteria of Jadad and Schulz (Jadad 1996; Schulz 1995) was used to assess study quality, which had a possible range from zero to five with a cutoff of two used to designate studies of high versus low quality. The criteria used were:
Was the study randomised? Was the method of randomisation appropriate?
Was the study double‐blinded? Were the methods of blinding appropriate?
Was compliance assessed?
Were there dropouts and withdrawals and were the numbers and reasons for withdrawal stated? Did more than 80 percent of those randomized complete the study?
Kappa values were calculated for inter‐rater agreement on quality.
Data synthesis
Extracted data were analyzed using the Cochrane Review Manager software. Quantitative analysis was based on changes in the means between baseline and endpoint measures. Standard deviation of the mean difference was calculated from the standard deviations of the mean at the beginning and end of each trial by assuming a degree of correlation of 0.5 (Rice 1995). Trials were included in the pooled analysis where change data of the intervention and control groups could be obtained from calculations of the mean difference and standard deviation (SD).
A fixed‐effect model was used for the pooled results. Where heterogeneity was indicated in the pooled analysis, a random‐effects model was applied. Effect sizes are presented as weighted mean differences with 95 percent confidence intervals. Heterogeneity was assessed using the chi‐squared test with the significance set at a P value of < 0.1. Where serial measurement of an outcome was given during the intervention phase, comparisons were made with the final measurement. Where a trial used two sets of doses, included comparisons of EPA and DHA, or more than one control group, a sensitivity analysis was carried out to determine which comparison gave the smallest effect size (Tramer 1997), which was then included.
Publication bias was evaluated using a funnel plot method (Egger 1997).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned a priori and undertaken for the following variables:
length of intervention (less than two months, longer than two months);
dose of omega‐3 PUFA (more than 2 g eicosapentaenoic acid (EPA), less than 2 g EPA);
type of omega‐3 PUFA (where EPA or docosahexaenoic acid (DHA) was given separately, the result which had the smallest effect size was included for statistical analysis to prevent sample
duplication (Tramer 1997);
baseline triglyceride level (studies including only hypertriglyceridemic patients, studies including not only hypertriglyceridemic patients).
Sensitivity analysis
Sensitivity analyses were carried out on:
quality (two points or less on quality scale (low quality), more than two points on quality scale (high quality));
blinding;
trial design (cross‐over versus parallel design studies);
exclusion of any particularly large study (to see how much they dominate the results).
Results
Description of studies
Results of the search
We identified 886 citations with their abstracts from electronic searches carried out in 2006, of which 197 were deemed relevant. One further trial was found from handsearching. These 197 abstracts included 38 publications that described 23 trials which are further detailed below. Six of the trials were presented in more than one publication, accounting for 17 of the published papers.
Assessment of publication bias inter‐rater agreement
The interrater reliability for the assignment of a quality score was kappa = 0. 71.
Included studies
Twenty‐three trials met inclusion criteria and were included in the review. The effect of omega‐3 PUFA on glycemic control and lipid levels was the focus of twenty of the included trials. Two trials were designed to assess the effect of omega‐3 PUFA on vascular physiology; however, these investigators also reported glycemic and lipid endpoints (McGrath 1996; Woodman 2002). Characteristics of the included trials are tabulated. Vegetable oil comparison groups included olive oil, safflower oil and corn oil.
Characteristics of included studies
The 23 trials included twelve parallel group design (Alekseeva 2000; Axelrod 1994; Hendra 1990; Jain 2002; Morgan 1995; Mostad 2006; Pelikanova 1992; Petersen 2002; Silvis 1990; Sirtori 1997; Westerveld 1993; Woodman 2002) and eleven cross‐over trials (Annuzzi 1991; Boberg 1992; Borkman 1989; Connor 1993; Goh 1997; Luo 1998; McGrath 1996; McManus 1996; Puhakainen 1995; Schectman 1988; Vessby 1990). The parallel group trials ranged in duration from three weeks to eight months. The cross‐over trials had phases that ranged in duration from 2 to 24 weeks. None of the eleven cross‐over trials reported phase‐specific data. Four trials had a washout period (3 to 8 weeks in duration) and one of these looked for but did not find a carry‐over effect (Borkman 1989). Of the seven trials that did not have a washout period, five looked for and two found a carry‐over effect (Boberg 1992; McManus 1996). Five new trials were identified since the first review was conducted (Alekseeva 2000; Jain 2002; Mostad 2006; Petersen 2002; Woodman 2002).
Interventions
The dose of omega‐3 ranged from 1.08 to 5.2 grams of eicosapentaenoic acid and 0.3 to 4.8 grams of docosahexaenoic acid. The omega‐3 was usually given in capsules except for one trial in which a liquid form was used (Pelikanova 1992). The dose of vegetable oil or placebo was matched to the dose of omega‐3. Although most trials used vegetable oils (including olive oil, safflower oil, linseed oil and corn oil) one used saline solution as a placebo (Pelikanova 1992) and two used diet (Alekseeva 2000; Jain 2002). In all of the trials omega‐3 was added to the diet rather than being a replacement for some component of the dietary fat intake, however one trial reduced the high intake of omega‐6 in the patients (Jain 2002).
Participants
A total of 1075 participants were included in the 23 trials. The individual trial sample size ranged from 8 to 418. The majority of participants were male and the ages ranged between 21 and 85 years. Most participants had type 2 diabetes of 5 to 10 years duration and were treated with diet or oral hypoglycemic agents. Few had diabetes‐related complications. In three trials, all participants were hypertriglyceridemic (Connor 1993; Morgan 1995; Vessby 1990). Two other trials included a subset of hypertriglyceridemic participants and these comprised 46% (Schectman 1988) and 10% (Luo 1998) of all participants. Individual study exclusion criteria are outlined in the tables below.
Outcomes
No trials were identified that included the primary outcome measures of fatal myocardial infarction or sudden cardiac death, myocardial infarction or coronary revascularization procedures.
Eighteen trials reported data on triglycerides, 17 trials reported data on total cholesterol, 16 trials reported data on LDL cholesterol, 16 trials reported data on HDL cholesterol, seven trials reported on VLDL cholesterol that could be pooled for analysis. Eight of the 10 cross‐over trials and eight of the 12 parallel trials reported on glycated hemoglobin and five had a phase duration of less than eight weeks (that is less than the time normally required for HbA1c to stabilize). Of the 23 trials identified in this review, only 18 reported their fasting glucose and six on fasting insulin results in a way that permitted pooling of data. Ten trials reported on changes in body weight.
Missing Data
We contacted Dr CR Sirtori in order to clarify details of the Italian Multicenter Fish Oil Study. We were able to obtain unpublished information about the inclusion of participants with type 2 diabetes, data about disease duration, the use of oral hypoglycemic agents and also clarify the issue of duplicate publication by one of the centres in the multicentre study. We did not attempt to contact other authors where the size of the trials was small.
Details of missing data from each of the included trials are described in the tables. One trial (Silvis 1990) reported total glycated hemoglobin. This measure was converted to HbA1c using the formula HbA1c=0.61 x (reported glycated Hb) + 2.1 (Nutall 1998; Fairbanks, personal communication). Four trials (Connor 1993; Jain 2002; Schectman 1988; Sirtori 1997) reported lipid measures in mg/dl, which were converted to mmol/L (Kratz 1998) and two trials (Boberg 1992; Vessby 1990) reported only the P value from which the SD was obtained to calculate the SD of change (Rice 1995).
Excluded studies
One hundred and ninety‐seven of 886 citations with their abstracts identified from the electronic and handsearches were deemed appropriate for further consideration. One further trial was found from handsearching. One hundred and sixty‐four of the 197 abstracts were excluded because they had multi‐factorial interventions from which the effect of omega‐3 polyunsaturated fatty acids (PUFA) could not be separated, or did not use omega‐3 PUFA derivatives (Adler 1994; Das 1994a; Das 1994b; Das 1995; Dunstan 1997; Holler 1996; Howard 1987; Lee 1994; Morris 1995; Okuda 1992; Okuda 1996; Prince 1997; Sirtori 1998; Tonstad 1997; Urano 1991; Zambon 1992), were non‐randomised studies (Friedberg 1998; Herrmann 1992; Kasim 1988; Malasanos 1991; Schaap 1991; Semplicini 1994; Sheehan 1997; Shunto 1992; Silva 1996; Stender 1990; Zak 1996), included patients without diabetes or patients with type 1 diabetes (Bonnema 1995; Eritsland 1994; Fasching 1991; Hamazaki 1990; Lungershausen 1997; Mackness 1994; Rossing 1996; Stacpoole 1989), did not include a placebo arm (Fasching 1991; Friday 1989; Glauber 1988; Kasim 1988; Mori 2000; Shimizu 1993; Shimizu 1995), did not include human participants (Yamada 1995), lacked data or did not report on outcomes that were relevant to this review. The 12‐month follow‐up report of the Italian Multicenter Fish Oil Study (Sirtori 1998) was excluded because it is a non‐randomised non‐placebo‐controlled addition to the original trial (Sirtori 1997). The remaining 33 publications described 23 trials that met the inclusion criteria of this review.
Risk of bias in included studies
The trials could be classified by their quality scores into eleven trials of equal or less than two points (Alekseeva 2000; Annuzzi 1991; Borkman 1989; Connor 1993; Hendra 1990; Jain 2002; Morgan 1995; Pelikanova 1992; Schectman 1988; Silvis 1990; Woodman 2002) and twelve trials of greater than two points (Axelrod 1994; Boberg 1992; Goh 1997; Hendra 1990; Luo 1998; McGrath 1996; McManus 1996; Mostad 2006; Petersen 2002; Sirtori 1997; Vessby 1990; Westerveld 1993).
Allocation
Since randomisation was an inclusion criterion, all trials started with a score of one. Most of the articles of less than two scores failed to describe the method of randomisation.
Blinding
An additional point was assigned for the presence of blinding in 19 trials (Axelrod 1994; Boberg 1992; Borkman 1989; Connor 1993; Goh 1997; Hendra 1990; Jain 2002; Luo 1998; McGrath 1996; McManus 1996; Morgan 1995; Mostad 2006; Petersen 2002; Puhakainen 1995; Schectman 1988; Sirtori 1997; Vessby 1990; Westerveld 1993; Woodman 2002). Most of the articles of low scores failed to describe the method of blinding. Some failed to mask the odour of the fish oil supplement affecting blinding.
Incomplete outcome data
Six trials reported drop‐outs or withdrawals (Axelrod 1994; Luo 1998; Mostad 2006; Petersen 2002; Silvis 1990; Woodman 2002).
Effects of interventions
Primary outcomes
No trials were identified that included the primary outcome measures of fatal myocardial infarction or sudden cardiac death, myocardial infarction or coronary revascularization procedures.
Secondary outcomes
As a guide, reference levels of triglycerides are 0.45‐1.69 mmol/L (serum), cholesterol less than 5.17 mmol/L (serum), HDL cholesterol greater than 0.91 mmol/L (serum), LDL cholesterol less than 3.36 mmol/L (serum), VLDL cholesterol 0.09 to 0.34 mmol/L (serum), insulin 35 to 145 pmol/L, HbA1c 3.8% to 6.4%, fasting plasma glucose 3.9 to 6.1 mmol/L (Kratz 1998).
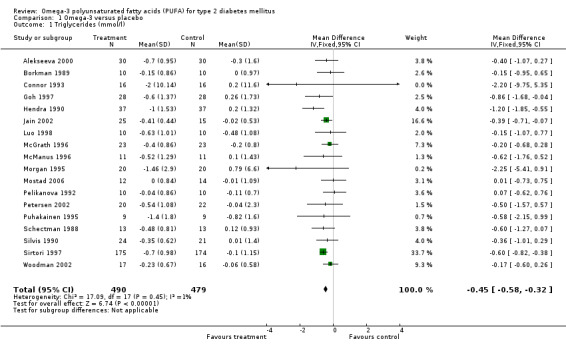
Eighteen of 23 trials reported data on triglycerides (comparison 01.01) including 969 participants. Omega‐3 supplementation was associated with a mean (pooled weighted mean difference) lowering of plasma triglyceride concentration by 0.45 mmol/L (95% confidence interval (CI) ‐0.58 to ‐0.32) compared to controls (including a placebo of vegetable oils). This reduction was statistically significant (P < 0.00001).
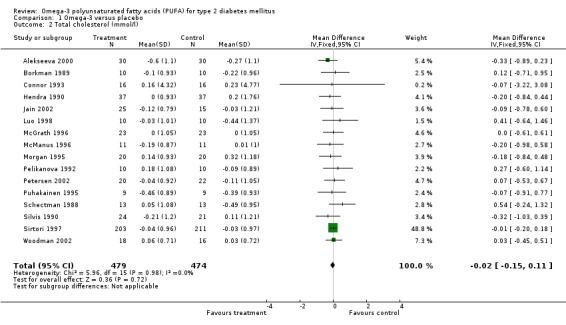
Sixteen of 23 trials reported data on total cholesterol (comparison 01.02), in which 953 participants had a statistically non‐significant pooled weighted mean difference of ‐0.02 (95% CI ‐0.15 to 0.11). Omega‐3 supplementation was not associated with a change in plasma cholesterol concentration compared to controls (P = 0.72).
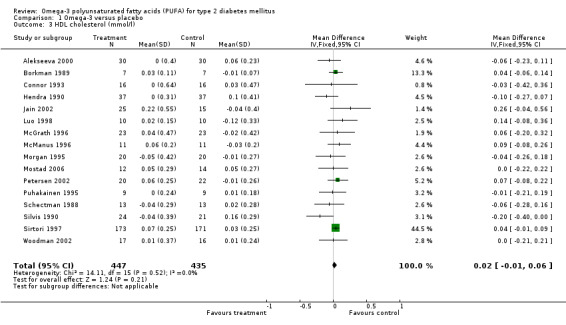
Sixteen trials reported data on HDL cholesterol (comparison 01.03) in 882 participants. Omega‐3 supplementation was associated with an increase in HDL concentration compared to controls, with a change of 0.02 mmol/L (95% CI ‐0.01 to 0.06, P = 0.21).
Of 22 trials, 16 reported data on LDL cholesterol (comparison 01.04) including 565 participants. Omega‐3 supplementation was associated with an increase in plasma LDL cholesterol concentration of 0.11 mmol/L (95% CI 0.00 to 0.22, P = 0.05).
Seven of eight trials reported data that could be pooled on VLDL cholesterol including 238 participants (comparison 01.05). Omega‐3 supplementation was associated with a decrease in VLDL concentration compared to controls, a weighted mean difference of ‐0.07 mmol/L (95% CI ‐0.13 to 0.00, P = 0.04).
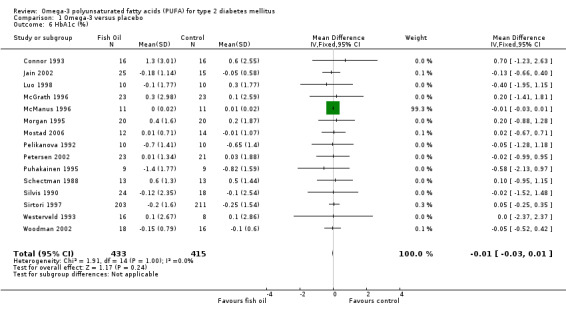
Of the 23 trials included in the review, 15 reported measurements of glycated haemoglobin (comparison 01.06). The pooled weighted mean difference for HbA1c in 848 participants was ‐0.01 % (95% CI ‐0.03 to 0.01). Omega‐3 supplementation was not associated with a statistically significant mean change in glycated haemoglobin compared with controls (P = 0.24).
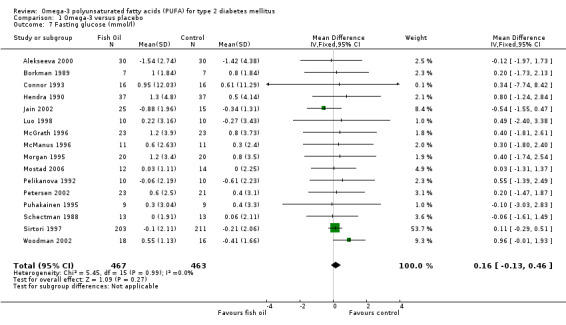
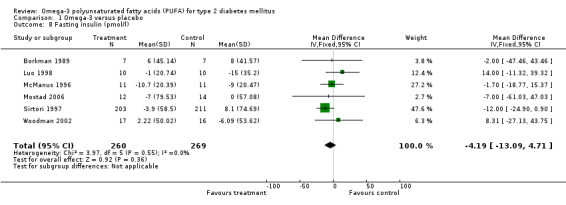
Twenty‐one of the 23 trials reported fasting glucose results, of which only sixteen with 930 participants reported their results in such a way to enable pooled analysis (comparison 01.07). The weighted mean difference was 0.16 mmol/L (95% CI ‐0.13 to 0.46, P = 0.27) showing that omega‐3 supplementation did not significantly change fasting glucose compared to controls. Fasting insulin was reported by eight trials of which only six reported data that could be pooled with 529 participants (comparison 01.08). The pooled results showed a statistically non‐significant reduction, a weighted mean difference of ‐4.19 pmol/L (95 % CI ‐13.09 to 4.71, P = 0.36). Compared to controls, omega‐3 was not associated with a significant change in fasting insulin.
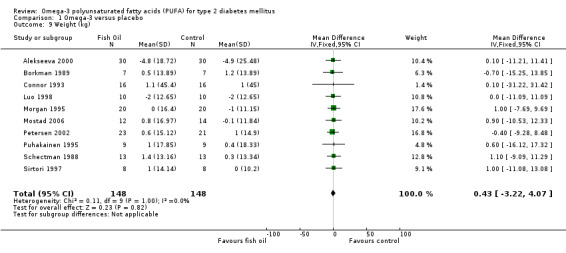
Ten trials reporting data on weight were pooled (comparison 01.09). Omega‐3 PUFA compared with controls was not associated with a significant weight change, and the weighted mean difference was 0.4 kg (95% CI ‐3.2 to 4.1, P = 0.82).
Trials did not report the incidence of adverse effects of nausea, vomiting, belching, diarrhoea, constipation, eczema, acne or arrhythmias.
Heterogeneity
The results for the test of heterogeneity for the overall results (omega‐3 versus control in all participants, comparison 01) were non‐significant (P > 0.1) for all outcomes studied, except for the subgroup analysis on low dose of omega‐3 polyunsaturated fatty acids (PUFA) for VLDL cholesterol. These did not change when different statistical models were applied.
Subgroup analyses
Subgroup analyses were carried out for outcomes that resulted in significant results in the overall analysis, that is for triglyceride, LDL and VLDL cholesterol levels. Results should be regarded as hypothesis‐generating:
Hypertriglyceridemic patients
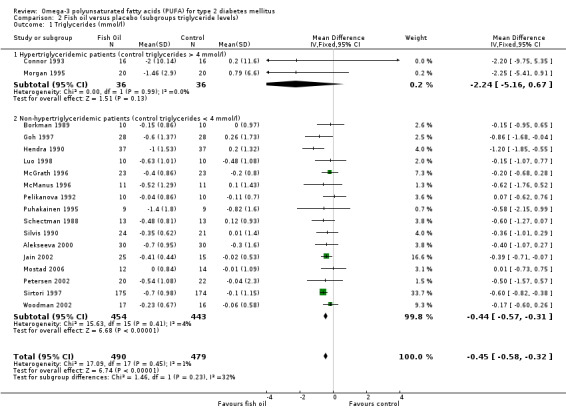
The pooled weighted mean difference for triglycerides in two trials that recruited 72 hypertriglyceridemic participants was ‐2.24 mmol/L (95% CI ‐5.16 to 0.67, P = 0.13), and ‐0.44 mmol/L (95% CI ‐0.58 to ‐0.32, P < 0.00001) for 16 trials with 897 non‐hypertriglyceridemic participants (comparison 02.01).
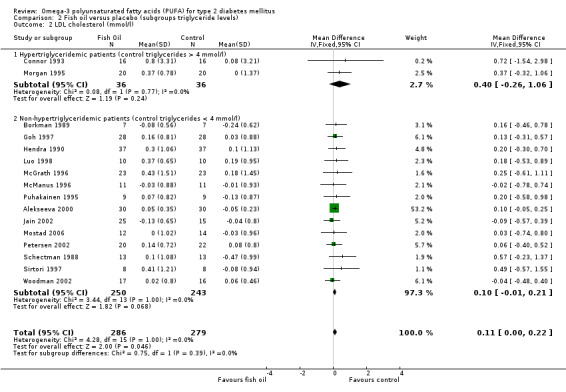
Increases in LDL cholesterol levels were statistically non‐significant in two trials with 72 hypertriglyceridemic patients, with a weighted mean difference of 0.40 mmol/L (95% CI ‐0.26 to 1.06, P = 0.24) using a fixed‐effect model. The weighted mean difference of 14 trials with 493 non‐hypertriglyceridemic participants was 0.11 mmol/L (95% CI 0.00 to 0.22, P = 0.05) (comparison 02.02).
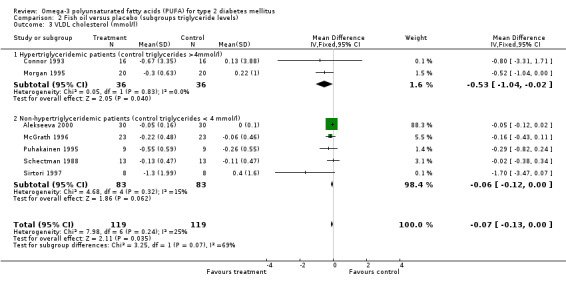
VLDL cholesterol was significantly reduced by 0.53 mmol/L (95% CI ‐1.04 to ‐0.02, P = 0.04) in two trials with 72 hypertriglyceridemic participants. The pooled weighted mean difference of VLDL cholesterol in five trials with 166 non‐hypertriglyceridemic participants was ‐0.06 mmol/L (95% CI ‐0.12 to 0.00, P = 0.06, comparison 02.03).
Dose of omega‐3 PUFA
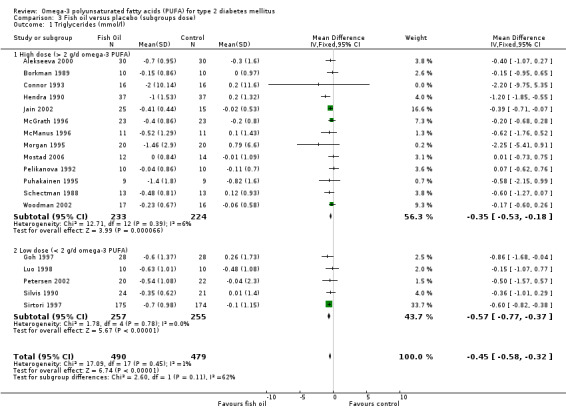
Comparison 03 shows data from trials with high doses of omega‐3 PUFA (more than 2 g eicosapentaenoic acid and docosahexaenoic acid). Pooled results for triglycerides levels showed a decrease, a weighted mean difference of ‐0.35 mmol/L (95% CI 0.53 to ‐0.18, P < 0.0001) in the pooled analysis of 13 high dose trials with 457 participants, and ‐0.57 mmol/L (95 % CI ‐0.77 to ‐0.37, P < 0.00001) in five low dose trials of 512 participants.
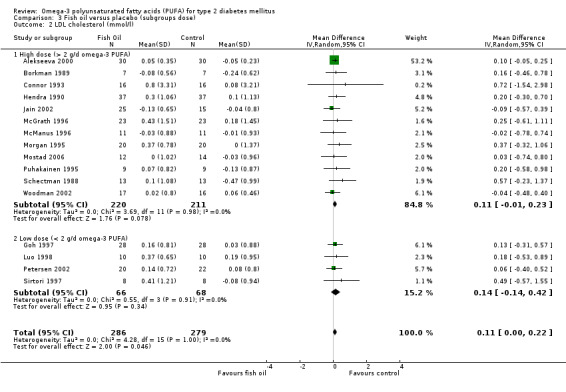
The increase in LDL cholesterol was 0.11 mmol/L (95% CI ‐0.01 to 0.23 mmol/L, P = 0.08) in 12 trials with 431 participants that administered the high doses of omega‐3 and also statistically non‐significant in four trials with 134 participants using lower doses, a weighted mean difference of 0.14 mmol/L (95% CI ‐0.14 to 0.42 mmol/L, P = 0.34) (Comparison 03.02).
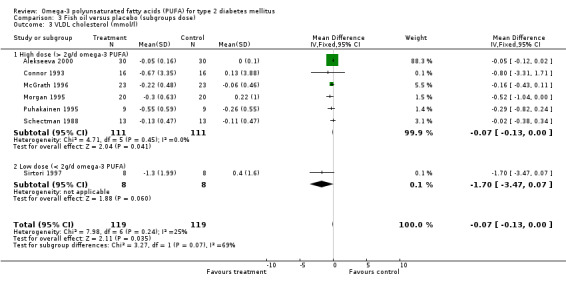
For VLDL cholesterol, the weighted mean difference was ‐0.07 mmol/L (95% CI ‐0.13 to 0.00, P = 0.04) for six high dose trials including 222 participants, with only one trial using a low dose of omega‐3.
Study duration
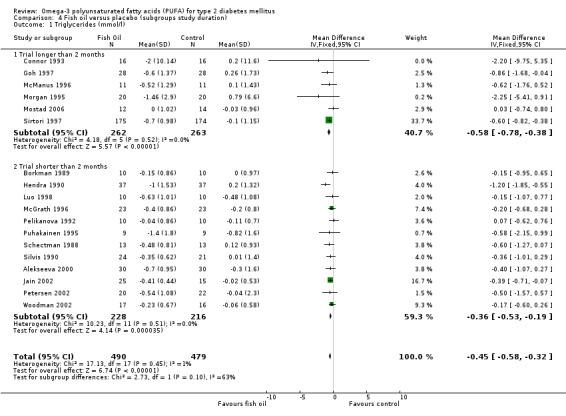
Comparison 04 shows data for trials with long (more than two months) and short (two months and less) trial duration. Triglyceride levels were reduced, a weighted mean difference of ‐0.58 mmol/L (95% CI ‐0.78 to ‐0.38, P < 0.00001) in six trials of longer duration with 525 participants and by ‐0.36 mmol/L (95% CI ‐0.53 to ‐0.19 mmol/L, P < 0.0001) in 12 shorter trials with 444 participants (comparison 04.01).
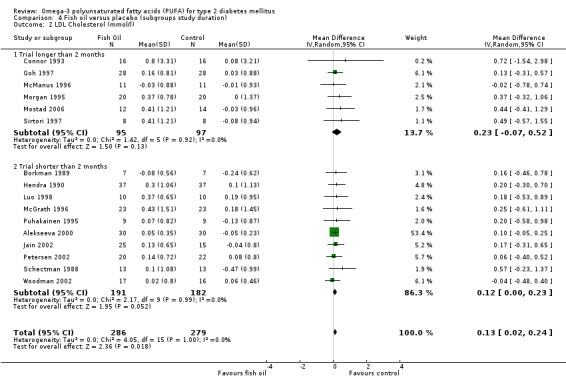
LDL cholesterol levels increased non‐significantly by 0.23 mmol/L (95% CI ‐0.07 to 0.52, P = 0.13) in six trials lasting longer than two months with 192 participants. In 10 trials less than two months duration with 373 participants, the weighted mean difference was 0.12 mmol/L (95% CI 0.00 to 0.23 mmol/L, P = 0.05) after omega‐3 supplementation compared to controls (comparison 04.02).
The weighted mean difference after omega‐3 supplementation compared to controls for VLDL cholesterol levels was ‐0.62 mmol/L (95% CI ‐1.11 to ‐0.13, P = 0.01) in three trials of longer duration with 88 patients and was ‐0.06 mmol/L (95% CI ‐0.12 to 0.01, P = 0.07) in four trials shorter than two months including 150 patients (comparison 04.03).
Sensitivity analyses
Sensitivity analyses are shown in Appendix 2. For most outcomes (total cholesterol, HDL cholesterol, triglycerides, HbA1c, fasting plasma glucose, fasting plasma insulin) the conclusions of the main analysis were unchanged when
only studies with a quality score of three or more were included, or
when only blinded studies were included, or
when only parallel design studies were included, or
when the only large study (Sirtori 1997) was excluded;
when the statistical model was adjusted.
However, conclusions regarding LDL and VLDL cholesterol levels were more sensitive to these factors, with increases in LDL becoming non‐significant when only blinded or parallel group trials were included. Pooled results for VLDL cholesterol were non‐significant when blinded, and parallel designs were included. Using a random‐effects model for VLDL cholesterol changed the pooled results to a non‐significant weighted mean difference of ‐0.13 mmol/L (95% CI ‐0.28 to 0.02, P = 0.08), but did not change the conclusions with standardised mean differences, or using weighted mean difference with a fixed‐effect model. Trials measuring VLDL cholesterol with low doses of omega‐3 PUFA showed heterogeneity (P = 0.09) using both fixed‐effect or random‐effects models ‐0.67 (95 % CI ‐2.09 to 0.75), P = 0.35). The weighted mean difference for both high and low dose trials were statistically non‐significant, using either a fixed‐effect or random‐effects model, however with standardized mean difference fixed‐effect or random‐effects models the pooled reductions were significant in trials only with high doses ‐0.36 mmol/L (95% CI ‐0.66 to ‐0.06, P = 0.02). In hypertriglyceridaemic patients, using a standardised mean difference fixed‐effect or random effects model for VLDL‐cholesterol changed the results to ‐0.43 (95% CI ‐0.90 to 0.40, P = 0.07) and ‐0.36 (95% CI ‐0.67 to ‐0.06, P = 0.02) in non‐hypertriglyceridemic patients. For fasting plasma glucose, the pooled results for trials of shorter duration were significant with weighted mean difference and a fixed‐effect or random‐effects model (0.55 mmol/L (95% CI 0.02 to 1.08, P = 0.04), but were non‐significant when using standardised mean differences (0.17 mmol/L (95% CI ‐0.04 to 0.38, P = 0.10).
Discussion
Summary of main results
This systematic review pools 23 randomized controlled trials of omega‐3 supplementation studying a total of 1075 patients with type 2 diabetes mellitus. None of the trials examined hard clinical endpoints (such as cardiovascular events or death). In the trials reviewed, omega‐3 supplementation had a statistically significant triglyceride‐ and VLDL cholesterol lowering effect. A statistically significant increase in LDL cholesterol was noted after omega‐3 supplementation. LDL was not significantly increased in subgroup analyses of hypertriglyceridemic patients, high or low omega‐3 polyunsaturated fatty acids (PUFA) doses and in trials lasting longer than two months. Omega‐3 supplementation did not result in any statistically significant increase in fasting glucose, HbA1c, or fasting insulin. No other adverse effects were reported.
Overall, the subgroup analyses are difficult to interpret as up to 50% of the trials included in the hypertriglyceridemia, high dose and long duration subgroups were identical (that is including hypertriglyceridemic patients on a high dose of fish oil in a long trial) (Connor 1993; Morgan 1995), making it therefore difficult to determine which of these factors really caused the differential response. Non‐hypertriglyceridemia, long study duration and low doses of omega‐3 PUFA may have contributed to a greater reduction in triglyceride and levels, whereas hypertriglyceridemia and trials of longer duration may have had a contribution to the larger reductions in VLDL levels. Subgroup analyses did not indicate variables that increased in LDL cholesterol levels.
Overall completeness and applicability of evidence
Our data are relevant to clinicians managing patients with type 2 diabetes. They indicate that, in hypertriglyceridemic and normotriglyceridemic patients, dietary supplementation with omega‐3 PUFA leads to a modest lowering of triglycerides without any statistically significant effect on glycemic control. The increases in LDL are not significant in hypertriglyceridemic patients. It is unlikely that omega‐3 PUFA will be prescribed in normotriglyceridemic patients, but our results do not provide evidence to discourage their use as over‐the‐counter preparations provided the formulation has been manufactured to eliminate undesirable contaminants. Omega‐3 PUFA has been suggested to have beneficial effects in other diseases including Crohn's disease, rheumatoid arthritis and breast, colon and prostate malignancies (Connor 2000), and our results show that omega‐3 PUFA represents a reasonable therapeutic strategy in hypertriglyceridemic individuals. We are not aware of any studies that have reported the combination of omega‐3 PUFA with other lipid lowering drugs, and few trials have compared omega‐3 PUFA with fibric acid derivatives (Fasching 1996).
The slight increase in LDL cholesterol seen with the use of omega‐3 PUFA can occur with other triglyceride lowering agents, in patients without diabetes (Fisher 1998; Ouguerram 2006; Theobald 2004) and is consistent with physiological studies proposing the mechanism of the LDL increase with omega‐3 PUFA (Lindsey 1992; Schectman 1996; Surette 1992). In addition, large buoyant LDL is known to be less atherogenic than small dense LDL and this may be the type of LDL produced in response to omega‐3 PUFA (Minihane 2000; Mori 2000; Suzukawa 1995). The impact of omega‐3 PUFA on LDL levels in a larger trial included in this systematic review of patients with diabetes have not yet been published (Sirtori 1997).
Although the GISSI‐Prevenzione trial has published its findings on the administration of fish oil to 11,324 survivors of myocardial infarction (GISSI 1999), the analysis for the diabetes sub‐group (15% of participants) has not yet been reported. However, the findings of reduced triglycerides and an overall beneficial effect on survival on the patients surviving myocardial infarction (relative risk reduction of 10% for the primary endpoint of death, non‐fatal myocardial infarction and stroke) are encouraging.
Quality of the evidence
Several methodological challenges were encountered in the course of this review. Eleven of 23 trials used a cross‐over design and phase‐specific data were not available for any of these. For pooling results from cross‐over and parallel group design studies, ideally, individual patient data or at least phase‐specific data should be available. In the absence of these data, three approaches are possible. The first is not to analyse data from cross‐over studies. The second is to pool parallel group design and cross‐over trials separately. The third is to treat first phase data from cross‐over studies as coming from parallel group design studies, pool these with data from parallel group design studies and look for heterogeneity in the analysis. We adopted the latter approach and our sensitivity analysis did not show any association between study design and direction or magnitude of effect. Use of the cross‐over design to study omega‐3 PUFA supplementation has other potential drawbacks. Omega‐3 PUFA is incorporated into biologic membranes and presumably would require washout periods of appropriate duration to minimize any carryover effect. In our review, only four of the 11 cross‐over studies had a washout period. Despite these limitations, the main findings of the review were similar if cross‐over studies were included or excluded from the analysis.
Another methodological problem is the use of HbA1c as an outcome measure in trials of short duration. Glycated haemoglobin or HbA1c provide an integrated measure of glycemic control over a period of approximately 12 weeks. The use of such measurements in studies of short duration will underestimate any effects on glycemic control. This may have occurred in several trials included in this review (see tables).
The random‐effects model was used where the studies were sufficiently different to assume some level of heterogeneity that could have been ignored in a fixed‐effect model, but except for VLDL cholesterol, the conclusions did not change when the results were analyzed with either model.
It is interesting to compare the current systematic review with that of Friedberg et al. (Friedberg 1998). Despite the differences in design of that review to Farmer et al (Farmer 2001) and our current review, the findings of the two reviews are similar and are in keeping with the results of the largest trial performed in this area (Sirtori 1997).
Potential biases in the review process
The subgroup and sensitivity analyses require elaboration to undertake a more comprehensive comparison between groups with different characteristics. This will be addressed in a further publication.
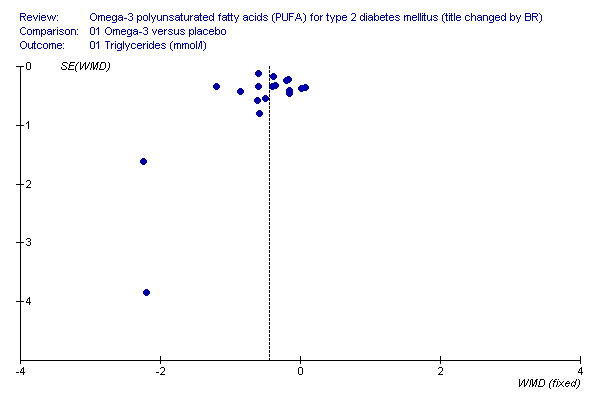
Limitations of our systematic review include the limited number of trials with emerging cardiovascular risk markers as outcomes, and small trial sizes with a median of 23 participants. Another significant limitation of our study is the shorter duration of the included trials. Some trials did not describe methods of randomisation or blinding so that the degree of rigor with which they were conducted was not clear. It was not possible to pool all the identified outcomes because of non‐standardised measurement units, and non‐reporting of changes in outcomes. The findings from the funnel plot analysis (Figure 1) may indicate bias in reporting, selection or methodology of the trials. However, we included trials reported in any language to reduce selection and language bias, and an assessment was made of the quality of the trials.
1.
Authors' conclusions
Implications for practice.
In hypertriglyceridemic patients, dietary supplementation with omega‐3 PUFA leads to a modest lowering of triglycerides without any clinically significant effect on glycemic control, and omega‐3 polyunsaturated fatty acids (PUFA) may represent a reasonable therapeutic strategy in these individuals.
Implications for research.
A recent review included patients with diabetes as part of a high risk group analysis, but also included non‐randomised control trials (Balk 2004). Three previous systematic reviews have evaluated the effect of omega‐3 PUFA on cardiovascular events, lipid and glycemic markers in type 2 diabetes (Farmer 2001; Friedberg 1998; Montori 2000). However, we considered lipid cardiovascular risk factors beyond these markers, and used changes in the mean from baseline to the end of the trial in the pooled analysis. We have also identified more recent randomised trials.
The slight increase in LDL cholesterol seen with the use of fish oil represents a cause for concern and long‐term studies assessing hard cardiovascular endpoints in patients with diabetes are needed. In conclusion, our systematic review demonstrates the difficulties of existing trial designs. Rigorously designed and conducted randomised controlled trials are required, using standardised units measuring both established and emerging cardiovascular risk markers in type 2 diabetes, to enable more conclusive pooled analyses and improve the precision of the effect size estimates. Larger trials of longer duration would conclusively establish the role and mechanisms of omega‐3 PUFA in cardiovascular disease risk reduction in type 2 diabetes. One trial sub‐group analysis awaits reporting (GISSI 1999), and four such end‐point trials are in progress (AFORRD 2004; ASCEND 2005; Galan 2003; ORIGIN 2005).
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 4 April 2007 | New search has been performed | This review is a 2007 update of the initial Farmer 2001 review. The title was changed from 'fish oil in people with type 2 diabetes mellitus' to 'omega‐3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus'. First author now is Janine Hartweg. In the current review, we further developed the search criteria, and have used change data calculated from the difference between baseline and after intervention values for the meta‐analysis, whereas in the previous review only the values after intervention were used. We have also included additional outcomes to those included in the previous review, i.e. VLDL and insulin. A total of eight new trials have been identified in a literature search up to April 2007, of which four have been included in this review from the search conducted up to December 2006. This brings the total number of randomized controlled trials to 31 considering the effects of omega‐3 fatty acids in patients with type 2 diabetes. One of these has endpoint data (myocardical infarction) not previously assessed by trials identified in the original review. |
Acknowledgements
Dr CR Sirtori kindly provided us with additional data of the Italian Multicenter Fish Oil Study.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical Subject Heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = substitute for one or no characters; ab = abstract; ti = titel; ot = original titel; pt = publication type; sh = MeSH: Medical subject heading (MEDLINE medical index term); adj = adjacency. Search strategy for meta‐analyses/systematic review 1.exp Fish Oils/ 2.fish‐oil$.tw. 3.omega‐3‐fatty acid$.tw. 4.polyunsaturated fatty acid$.tw. 5.n‐3‐fatty acid$.tw. 6.nycomed.tw. 7.eicosapen.tw. 8.(himega or lipitac or maxepa).tw. 9.n‐3 FAs.tw. 10.EPA.tw. 11.DHA.tw. 12.(pikasol or epax or superepa).tw. 13.exp alpha‐Linolenic Acid/ 14.alpha‐linolenic acid$.tw. 15.docosahexaenoic acid$.tw. 16.eicosapentaenoic acid$.tw. 17.cod liver oil$.tw. 18.exp Fatty Acids, Omega‐3/ 19.or / 1‐18 20.exp diabetes mellitus, non‐insulin‐dependent/ 21.exp insulin resistance/ 22.impaired glucose toleranc$.tw. 23.glucose intoleranc$.tw. 24.insulin$ resistanc$.tw. 25. (obes$ adj diabet$).tw. 26.(MODY or NIDDM).tw. 27.(non insulin$ depend$ or noninsulin$ depend$ or noninsulin?depend$ or non insulin?depend$).tw. 28.((typ$ 2 or typ$ II) adj diabet$).tw. 29.((keto?resist$ or non?keto$) adj diabet$).tw. 30.((adult$ or matur$ or late or slow or stabl$) adj diabet$).tw. 31.(insulin$ defic$ adj relativ$).tw. 32.pluri?metabolic$ syndrom$.tw. 33.or / 20‐32 34.exp diabetes insipidus/ 35.diabet$ insipidus.tw. 36.34 or 35 37.33 not 36 38.exp meta‐analysis/ 39.exp Review Literature/ 40.meta‐analysis.pt. 41.review.pt. 42.or/38‐41 43.letter.pt. 44.comment.pt. 45.editorial.pt. 46.historical‐article.pt. 47.or/43‐46 48.42 not 47 49.((systematic$ or quantitativ$ or methodologic$) adj (review$ or overview$)).tw. 50.meta?anal$.tw. 51.(integrativ$ research review$ or research integration$).tw. 52.quantitativ$ synthes$.tw. 53.(pooling$ or pooled analys$ or mantel$ haenszel$).tw. 54.(peto$ or der?simonian$ or fixed effect$ or random effect$).tw. 55.or / 49‐54 56.48 or 55 57.limit 56 to human 58.19 and 37 and 57 |
Appendix 2. Sensitivity analyses
| Criterion | Triglycerides | Total cholesterol | HDL cholesterol | LDL cholesterol | VLDL cholesterol | HbA1c (%) | Fasting glucose | Insulin (pmol/L) | Body weight (kg) |
| Quality (studies with score of 3 and above) | WMD ‐0.49 (‐0.65 to ‐0.34), p<0.00001; 11 trials included | WMD ‐0.01 (‐0.15 to 0.13), p=0.88; 10 trials included | WMD 0.03 (‐0.01 to 0.07), p=0.12; 10 trials included | WMD 0.18 (0.00 to 0.36), p=0.05; 11 trials included | WMD ‐0.27 (‐0.49 to 0.05), p=0.02; 4 trials included | WMD ‐0.01 (‐0.03 to 0.01), p=0.24; 10 trials included | WMD ‐0.03 (‐0.36, 0.30), p=0.88; 10 trials included | WMD ‐4.28 (‐13.35, 4.80), p=0.36; 5 trials included | WMD 0.46 (‐3.92, 4.84), p=0.84; 6 trials included |
| Blinding (blinded studies only) | WMD ‐0.49 (‐0.64 to ‐0.34), p<0.00001; 14 trials included | WMD ‐0.01 (‐0.15 to 0.12), p=0.85; 14 trials included | WMD 0.03 (0.00 to 0.07), p=0.08; 13 trials included | WMD 0.15 (‐0.02 to 0.32), p=0.08; 14 trials included | WMD ‐0.21 (‐0.39 to ‐0.02), p=0.3; 6 trials included | WMD ‐0.01 (‐0.03 to 0.01), p=0.24; 10 trials included | WMD 0.23 (‐0.09, 0.55), p=0.15; 13 trials included | WMD ‐4.19 (‐13.09, 4.17), p=0.36; 6 trials included | WMD 0.47 (‐3.38, 4.31), p=0.81; 9 trials included |
| Study design (parallel trials only) | WMD ‐0.48 (‐0.64 to ‐0.32), p<0.00001; 9 trials included | WMD ‐0.04 (‐0.18 to 0.11), p=0.61; 9 trials included | WMD 0.02 (‐0.01 to 0.06), p=0.51; 7 trials included | WMD 0.10 (‐0.02 to 0.23), p=0.10; 7 trials included | WMD (random effects) ‐0.36 (‐0.91 to 0.19), p=0.20; 3 trials included | WMD ‐0.01 (‐0.03 to 0.01), p=0.25; 9 trials included | WMD 0.26 (‐0.07, 0.59),p=0.12; 8 trials included | WMD ‐5.92 (‐16.68, 4.84); p=0.28; 4 trials included (using the DHA intervention group for Woodman et al.) | WMD 0.54 (‐4.43, 5.51), p=0.83; 4 trials included |
| Study size (large trial excluded) | WMD ‐0.37 (‐0.53 to ‐0.21), p<0.00001; 17 trials included | WMD ‐0.02 (‐0.20 to 0.15), p=0.80; 16 trials included | WMD 0.01 (‐0.04 to 0.06), p=0.73; 15 trials included | No large trial included | No large trial included | WMD ‐0.01 (‐0.03 to 0.01), p=0.23; 14 trials included | WMD 0.23 (‐0.21, 0.66), p=0.30; 15 trials included | WMD 2.91 (‐9.39, 15.21), p=0.64; 5 trials included | WMD 0.43 (‐3.22, 4.07), p=0.82; 10 trials included |
| NOTES: units (except HbA1c, insulin and body weight) ‐ mmol/l |
Data and analyses
Comparison 1. Omega‐3 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Triglycerides (mmol/l) | 18 | 969 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.58, ‐0.32] |
| 2 Total cholesterol (mmol/l) | 16 | 953 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.15, 0.11] |
| 3 HDL cholesterol (mmol/l) | 16 | 882 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.06] |
| 4 LDL cholesterol (mmol/l) | 16 | 565 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.00, 0.22] |
| 5 VLDL cholesterol (mmol/l) | 7 | 238 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 6 HbA1c (%) | 15 | 848 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 7 Fasting glucose (mmol/l) | 16 | 930 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.13, 0.46] |
| 8 Fasting insulin (pmol/l) | 6 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐4.19 [‐13.09, 4.71] |
| 9 Weight (kg) | 10 | 296 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐3.22, 4.07] |
1.1. Analysis.
Comparison 1 Omega‐3 versus placebo, Outcome 1 Triglycerides (mmol/l).
1.2. Analysis.
Comparison 1 Omega‐3 versus placebo, Outcome 2 Total cholesterol (mmol/l).
1.3. Analysis.
Comparison 1 Omega‐3 versus placebo, Outcome 3 HDL cholesterol (mmol/l).
1.4. Analysis.

Comparison 1 Omega‐3 versus placebo, Outcome 4 LDL cholesterol (mmol/l).
1.5. Analysis.

Comparison 1 Omega‐3 versus placebo, Outcome 5 VLDL cholesterol (mmol/l).
1.6. Analysis.
Comparison 1 Omega‐3 versus placebo, Outcome 6 HbA1c (%).
1.7. Analysis.
Comparison 1 Omega‐3 versus placebo, Outcome 7 Fasting glucose (mmol/l).
1.8. Analysis.
Comparison 1 Omega‐3 versus placebo, Outcome 8 Fasting insulin (pmol/l).
1.9. Analysis.
Comparison 1 Omega‐3 versus placebo, Outcome 9 Weight (kg).
Comparison 2. Fish oil versus placebo (subgroups triglyceride levels).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Triglycerides (mmol/l) | 18 | 969 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.58, ‐0.32] |
| 1.1 Hypertriglyceridemic patients (control triglycerides > 4 mmol/l) | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐5.16, 0.67] |
| 1.2 Non‐hypertriglyceridemic patients (control triglycerides < 4 mmol/l) | 16 | 897 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.57, ‐0.31] |
| 2 LDL cholesterol (mmol/l) | 16 | 565 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.00, 0.22] |
| 2.1 Hypertriglyceridemic patients (control triglycerides > 4 mmol/l) | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.26, 1.06] |
| 2.2 Non‐hypertriglyceridemic patients (control triglycerides < 4 mmol/l) | 14 | 493 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.01, 0.21] |
| 3 VLDL cholesterol (mmol/l) | 7 | 238 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 3.1 Hypertriglyceridemic patients (control triglycerides >4mmol/l) | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.04, ‐0.02] |
| 3.2 Non‐hypertriglyceridemic patients (control triglycerides < 4 mmol/l) | 5 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.12, 0.00] |
2.1. Analysis.
Comparison 2 Fish oil versus placebo (subgroups triglyceride levels), Outcome 1 Triglycerides (mmol/l).
2.2. Analysis.
Comparison 2 Fish oil versus placebo (subgroups triglyceride levels), Outcome 2 LDL cholesterol (mmol/l).
2.3. Analysis.
Comparison 2 Fish oil versus placebo (subgroups triglyceride levels), Outcome 3 VLDL cholesterol (mmol/l).
Comparison 3. Fish oil versus placebo (subgroups dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Triglycerides (mmol/l) | 18 | 969 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.58, ‐0.32] |
| 1.1 High dose (> 2 g/d omega‐3 PUFA) | 13 | 457 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.53, ‐0.18] |
| 1.2 Low dose (< 2 g/d omega‐3 PUFA) | 5 | 512 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐0.77, ‐0.37] |
| 2 LDL cholesterol (mmol/l) | 16 | 565 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.00, 0.22] |
| 2.1 High dose (> 2 g/d omega‐3 PUFA) | 12 | 431 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.01, 0.23] |
| 2.2 Low dose (< 2 g/d omega‐3 PUFA) | 4 | 134 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.14, 0.42] |
| 3 VLDL cholesterol (mmol/l) | 7 | 238 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 3.1 High dose (> 2g/d omega‐3 PUFA) | 6 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 3.2 Low dose (< 2g/d omega‐3 PUFA) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.47, 0.07] |
3.1. Analysis.
Comparison 3 Fish oil versus placebo (subgroups dose), Outcome 1 Triglycerides (mmol/l).
3.2. Analysis.
Comparison 3 Fish oil versus placebo (subgroups dose), Outcome 2 LDL cholesterol (mmol/l).
3.3. Analysis.
Comparison 3 Fish oil versus placebo (subgroups dose), Outcome 3 VLDL cholesterol (mmol/l).
Comparison 4. Fish oil versus placebo (subgroups study duration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Triglycerides (mmol/l) | 18 | 969 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.58, ‐0.32] |
| 1.1 Trial longer than 2 months | 6 | 525 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.78, ‐0.38] |
| 1.2 Trial shorter than 2 months | 12 | 444 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.53, ‐0.19] |
| 2 LDL Cholesterol (mmol/l) | 16 | 565 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.02, 0.24] |
| 2.1 Trial longer than 2 months | 6 | 192 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.07, 0.52] |
| 2.2 Trial shorter than 2 months | 10 | 373 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.00, 0.23] |
| 3 VLDL cholesterol (mmol/l) | 7 | 238 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 3.1 Trials longer than 2 months | 3 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.11, ‐0.13] |
| 3.2 Trials shorter than 2 months | 4 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.12, 0.01] |
4.1. Analysis.
Comparison 4 Fish oil versus placebo (subgroups study duration), Outcome 1 Triglycerides (mmol/l).
4.2. Analysis.
Comparison 4 Fish oil versus placebo (subgroups study duration), Outcome 2 LDL Cholesterol (mmol/l).
4.3. Analysis.
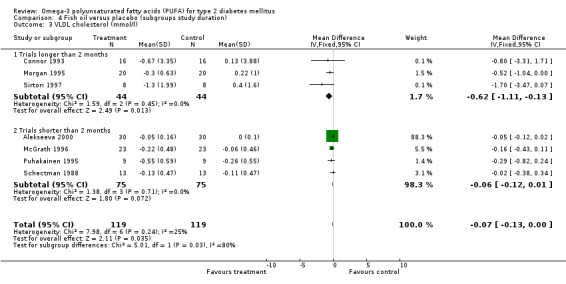
Comparison 4 Fish oil versus placebo (subgroups study duration), Outcome 3 VLDL cholesterol (mmol/l).
Comparison 5. Sensitivity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c (%) | 13 | 800 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.21, 0.20] |
5.1. Analysis.
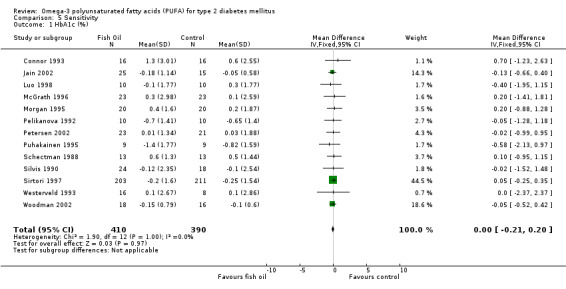
Comparison 5 Sensitivity, Outcome 1 HbA1c (%).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alekseeva 2000.
| Methods | DESIGN: Parallel DURATION: 4 weeks BLINDING: No | |
| Participants | 60 patients with type 2 diabetes (30 in each arm); 30 patients in a further treatment arm receiving linseed oil measuring lipid markers EXCLUSION CRITERIA: Diabetes confirmed diagnosis less than 1 year | |
| Interventions | 0.9 g of eicosapentaenoic acid plus 1.4 g of docosahexaenoic acid in codliver oil vs standard diet with 15g/d sunflower oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, LDL cholesterol, HDL and VLDL cholesterol MARKERS OF OXIDATION: Diene conjugates, melonaldehyde | |
| Notes | Quality score: 1 Trial divided into diet groups, with standard diet as controls, and 2 treatment groups of fish oil and linseed oil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Annuzzi 1991.
| Methods | DESIGN: Crossover DURATION: 2 weeks per phase BLINDING: no | |
| Participants | 8 male patients with type 2 diabetes EXCLUSION CRITERIA: renal/liver failure | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 1.2 g of docosahexaenoic acid VERSUS 10 g of olive oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides; only final HDL and LDL cholesterol, and final HDL, LDL and VLDL subfractions GLUCOSE PROFILE: only final fasting plasma glucose, postprandial glucose, fasting insulin, insulin sensitivity index | |
| Notes | Quality score: 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Axelrod 1994.
| Methods | DESIGN: Parallel DURATION: 6 weeks BLINDING: yes | |
| Participants | 20 ambulatory participants (10 in each arm, 9 in each arm for final analysis) with type 2 diabetes COMPLICATIONS/CO‐MORBIDITY: macro/micro vascular complications (22%) DROP‐OUTS: 2 patients ‐ colon cancer, non‐compliance EXCLUSION CRITERIA: bleeding, anemia, steroids, poorly controlled diabetes, proliferative retinopathy, medication with aspirin, NSAIDS | |
| Interventions | 1.1 g of eicosapentaenoic acid plus 1.5 g of docosahexaenoic acid VERSUS 5 g of safflower oil | |
| Outcomes | Baseline ‐
LIPID PROFILE: total cholesterol, triglycerides, HDL and LDL cholesterol
GLUCOSE PROFILE: fasting plasma glucose, HbA1c
OTHER: weight, blood pressure % of change and p‐value ‐ LIPID PROFILE: total cholesterol, triglycerides GLUCOSE PROFILE: HbA1c OTHER: systolic blood pressure, platelet aggregation, thromboxanes |
|
| Notes | Quality score: 5 Only % of change and p‐values available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Boberg 1992.
| Methods | DESIGN: Crossover DURATION: 8 weeks per phase BLINDING: yes | |
| Participants | 14 participants (86% male) with type 2 diabetes COMPLICATIONS/CO‐MORBIDITY: 43% hypertension, 7% coronary artery disease. EXCLUSION CRITERIA: renal and liver failure, hypothyroidism | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 1.2 g of docosahexaenoic acid VERSUS 10 g of olive oil | |
| Outcomes | LIPID PROFILE: triglycerides, LDL and VLDL cholesterol, apolipoproteins, LDL:HDL ratio
GLUCOSE PROFILE: HbA1c
OTHER: plasminogen activator inhibitor only % change, no p‐value: LIPID PROFILE: total cholesterol, HDL cholesterol GLUCOSE PROFILE: fasting plasma glucose, fasting insulin, insulin sensitivity index, OTHER: Factor VIIc, Fibrinogen |
|
| Notes | Quality score: 3 Only least‐square means, % of change an p‐values are provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Borkman 1989.
| Methods | DESIGN: Crossover DURATION: 3 weeks per phase with a 3 week run‐in and 3 week wash‐out periods BLINDING: yes | |
| Participants | 10 participants (70% male, 57±6.3 years old) with mild type 2 diabetes for 3.5±2.8 years of disease. (7 in the final analysis for HDL and LDL). COMPLICATIONS/CO‐MORBIDITY: 20% hypertension, 10% coronary artery disease. EXCLUSION CRITERIA: renal or liver failure, microvascular disease | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 1.2 g of docosahexaenoic acid VERSUS 10 g of safflower oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL and LDL cholesterol GLUCOSE PROFILE: fasting plasma glucose, fasting insulin, insulin sensitivity, C‐peptide OTHER: weight | |
| Notes | Quality score: 2 Blinding was invalid: 80% of participants were able to identify the capsules with fish oil. Investigators remained blind. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Connor 1993.
| Methods | DESIGN: Crossover DURATION: 24 weeks per phase with 3 month run‐in diet intervention BLINDING: yes | |
| Participants | 16 participants (81% male, 58.7±8 years‐old) with type 2 diabetes COMPLICATIONS/CO‐MORBIDITY: hypertriglyceridemia EXCLUSION CRITERIA: none | |
| Interventions | 4.1 g of eicosapentaenoic acid plus 1.9 g of docosahexaenoic acid VERSUS 15 g of olive oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL, VLDL and LDL cholesterol GLUCOSE PROFILE: fasting plasma glucose, HbA1c, C‐peptide OTHER: weight | |
| Notes | Quality score: 2 The reported SEM is most probably a SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Goh 1997.
| Methods | DESIGN: Crossover DURATION: 12 weeks of follow‐up without washout period BLINDING: yes | |
| Participants | 28 people with type 2 diabetes were divided into a low polyunsaturated/saturated fat and a high polyunsaturated/saturated fat diet group. Each group was then randomized to each crossover arm. EXCLUSION CRITERIA: heart disease, medication with lipid lowering agent | |
| Interventions | 1.4 g of eicosapentaenoic acid plus 0.88 g of docosahexaenoic acid VERSUS 35 mg/kg per day of linseed oil | |
| Outcomes | LIPID PROFILE: total cholesterol, HDL cholesterol: only baseline; complete record for triglycerides, LDL cholesterol GLUCOSE PROFILE: fasting plasma glucose, HbA1c, insulin, C‐peptide | |
| Notes | Quality score: 3 Trial divided into diet groups, with high and low doses, and two control groups. Results reported per group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hendra 1990.
| Methods | DESIGN: Parallel DURATION: 6 weeks BLINDING: yes | |
| Participants | 80 people (40 in each group, 56±1.3 years old) with type 2 diabetes COMPLICATIONS/CO‐MORBIDITY: micro/macro vascular complications (70% of the intervention group had microvascular complications compared with 42.5% in control group; 35% in control group had coronary artery disease compared with 7.5% in fish oil group). EXCLUSION CRITERIA: pregnant, oral contraceptive pills, hypercholesterolemia, recent heart attack or stroke | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 1.2 g of docosahexaenoic acid VERSUS 10 g of olive oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL and LDL cholesterol GLUCOSE PROFILE: fasting plasma glucose, HbA1 change not reported OTHER: blood pressure, factor VII, factor X, fibrinogen, thromboxane, platelet aggregation, clotting time | |
| Notes | Quality score: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jain 2002.
| Methods | DESIGN: Parallel DURATION: 6 weeks BLINDING: yes | |
| Participants | 65 people with type 2 diabetes (34 with vascular complications and 31 without) and 30 controls without type 2 diabetes. 40 of the patients with type 2 diabetes (66% male) were randomized to 25 in the treatment group and 15 in the control arm ( 52.3±8.8 years old and 5.41±4.31 years mean duration of diabetes) CO‐MORBIDITIES/COMPLICATIONS: 16% neuropathy, 18% nephropathy, 20% retinopathy, 15% ischaemic heart disease EXCLUSION CRITERIA: Anti‐oxidant medication, obese, smokers | |
| Interventions | 1.8g of eicosapentaenoic acid and 1.2 g of docosahexaenoic acid with 53.6 mg Vit E vs 4g corn oil (1g oil with 13.4mg Vit E) and both groups were prescribed dietary modifications according to WHO guidelines of fat intake | |
| Outcomes | LIPID PROFILE: total cholesterol, LDL and HDL cholesterol, triglycerides GLUCOSE PROFILE: HbA1c, fasting and postprandial glucose OTHER: blood pressure, lipid peroxides, diene conjugates, glutathione, weight (only baseline data shown) | |
| Notes | Quality score: 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Luo 1998.
| Methods | DESIGN: Crossover DURATION: 2 months run in, 2 months per period with 2 months of wash‐out BLINDING: yes | |
| Participants | 12 male participants (54±9.5 years old) with 6±3.2 years of type 2 diabetes, not on insulin COMPLICATIONS/CO‐MORBIDITY: 20% hypertension and 10% hyperlipidemia, DROP‐OUTS: 10 completed the protocol EXCLUSION CRITERIA: hepatic disease, renal failure, thyroid and gastrointestinal disorders | |
| Interventions | 1.08 g of eicosapentaenoic acid plus 0.72 g of docosahexaenoic acid VERSUS 6 g of sunflower oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL and LDL cholesterol, HDL subfractions, Lipoproteins, Apolipoproteins GLUCOSE PROFILE: fasting plasma glucose, HbA1c, insulin OTHER: weight, GLUT 4, HSL and LPL expression (end values not given) | |
| Notes | Quality score: 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McGrath 1996.
| Methods | DESIGN: Crossover DURATION: 6 weeks per phase with a 6 week wash‐out period BLINDING: yes | |
| Participants | 23 participants (87% male) with type 2 diabetes EXCLUSION CRITERIA: renal failure, cerebrovascular disease, coronary artery disease, peripheral vascular disease, hypertension, cardiovascular drugs, lipid‐lowering agents or vitamin intake. | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 1.2 g of docosahexaenoic acid VERSUS 10 g of olive oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL and LDL cholesterol, LDL, HDL and VLDL subfractions, apolipoproteins, lipid ratios GLUCOSE PROFILE: fasting plasma glucose, HBA1c OTHER: melonaldehyde, forearm blood flow, blood pressure, heart rate, cardiac output, stroke volume, platelet adhesion | |
| Notes | Quality score: 3 Data completed from McVeigh 1993 and 1994 report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McManus 1996.
| Methods | DESIGN: Crossover DURATION: 3 months BLINDING: yes | |
| Participants | 11 participants (61.8±9.6 years old, 73% male) with 7.7±6.9 years of well‐controlled type 2 diabetes COMPLICATIONS/CO‐MORBIDITY: 36% obese EXCLUSION CRITERIA: medication with insulin or lipid‐lowering agents | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 1.2 g of docosahexaenoic acid VERSUS 35 mg/kg of linseed oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL and LDL cholesterol GLUCOSE PROFILE: fasting plasma glucose, HbA1c, fasting insulin OTHER: weight | |
| Notes | Quality score: 4, but allocation was predictable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Morgan 1995.
| Methods | DESIGN: Parallel DURATION: 4 weeks of run‐in, 12 weeks of intervention, 4 weeks of post‐ingestion phase BLINDING: yes | |
| Participants | 40 participants (50% males, mean age 54 years with 7‐10 years of diabetes) with hypertriglyceridemia and well‐controlled type 2 diabetes. They were divided into 4 groups: 2 doses of fish oil and 2 doses of placebo (10 patients per group). COMPLICATIONS/CO‐MORBIDITY: hypertriglyceridemia; the intervention group had a greater weight. EXCLUSION CRITERIA: none | |
| Interventions | Low dose: 2.6 g of eicosapentaenoic acid plus 2.4 g of docosahexaenoic acid High dose: 5.2 g of eicosapentaenoic acid plus 4.8 g of docosahexaenoic acid VERSUS 9 or 18 g of corn oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL, LDL and VLDL cholesterol at 6 and 12 weeks of intervention GLUCOSE PROFILE: fasting plasma glucose, HbA1c OTHER: weight, blood pressure | |
| Notes | Quality score: 3 in final analysis all fish oil doses are reported together | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Mostad 2006.
| Methods | DESIGN: Parallel DURATION: 9 weeks | |
| Participants | 27 participants (13 in the fish oil arm, and 14 in the placeob arm, 55% male, aged 40 to 75 years) EXCLUSION CRITERIA: previous use of supplement with fish oil or marine n‐3 fatty acids less than 6 months before baseline, insulin treatment, hypertriglyceridemia >2.2 mmol/L, proliferative retinopathy, pregnancy or lactation, allergy to fish or citrus, smoking, alcoholism, congestive heart failure or other serious diseases | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 3 g decosahexaenoic acid with 60 mg/d Vitamin C and 51 mg/d Vitamin E vs 8,5 g linoleic acid with 58 mg/d Vitamin C and 52 mg/d Vitamin E | |
| Outcomes | LIPID PROFILE: Total cholesterol, LDL, HDL, triglycerides, energy metabolism, leptin and glucagon hormones GLUCOSE PROFILE: insulin, fasting plasma glucose, HbA1c, C‐peptide, insulin sensitivity, glucose utlisation, ketones | |
| Notes | Quality score: 5 1 Participant was excluded from the final analysis of the fish oil group due to early withdrawal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pelikanova 1992.
| Methods | DESIGN: Parallel DURATION: 4 weeks run‐in, 3 weeks of intervention BLINDING: no | |
| Participants | 20 (10 in each arm) male participants with type 2 diabetes EXCLUSION CRITERIA: obesity, hypertriglyceridemia, renal or liver failure | |
| Interventions | 15 cc (3 g) of fish oil VERSUS 15 cc of saline | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides GLUCOSE PROFILE: fasting plasma glucose, 2 h post‐prandial glucose, HbA1c. OTHER: plasma immunoreactive insulin after meal, C peptide given as area under the curve | |
| Notes | Quality score: 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Petersen 2002.
| Methods | DESIGN: Parallel DURATION: 8 weeks BLINDING: yes | |
| Participants | 49 participants (62% male) with type 2 diabetes (20 in the treatment and 22 in the control arm in one publication and 23 in treatment and 21 in controls in another publication) with ± 9.5 years duration of diabetes, aged 33 to 85 years. COMPLICATIONS/CO‐MORBIDITY: hypertriglyceridemia PARTICIPANTS WITHDRAWN: 7 (1 hospitalised, 1 gained weight during run‐in phase, 1 had pneumonia, 3 had raised C‐reactive protein in plasma, 1 was not fasting at blood sampling) EXCLUSION CRITERIA: Diabetes diagnosis of less than 2 years, age of onset less than 30 years, fasting plasma less than 1.5 mmol/L, use of lipid‐lowering drugs, use of dietary supplementation with fish oil/garlic, more than 5 drinks of alcohol a day, hormone replacement therapy | |
| Interventions | 2.6g eicosapentaenoic and docosahexaenoic acid with 53.6mg Vit E vs 4g corn oil (1g oil with 13.4mg Vit E) | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, LDL and HDL cholesterol, LDL and HDL subfractions, Lipid ratios GLUCOSE PROFILE: plasma glucose, HbA1c OTHER: Diene Conjugates (values not given) | |
| Notes | Quality score: 3 Glucose profile was taken from Pedersen 2003, with 23 in treatment arm and 21 in controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Puhakainen 1995.
| Methods | DESIGN: Crossover DURATION: 6 weeks per phase without washout period BLINDING: yes | |
| Participants | 9 community‐dwelling participants (44% male, 53±4 years old) with type 2 diabetes COMPLICATIONS/CO‐MORBIDITY: obesity EXCLUSION CRITERIA: macro or microvascular complications, liver or renal failure, bleeding or insulin requirement, hypothyroidism | |
| Interventions | 2.16 g of eicosapentaenoic acid plus 1.44 g of docosahexaenoic acid VERSUS 12 g of corn plus olive oil (6 g each) | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL , LDL and VLDL cholesterol, HDL, LDL and VLDL subfractions GLUCOSE PROFILE: fasting plasma glucose, HbA1c OTHER: weight | |
| Notes | Quality score: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Schectman 1988.
| Methods | DESIGN: Crossover DURATION: 4 weeks per phase (low dose) with 4 weeks of washout in between phases. At the end all participants received the high dose fish oil. BLINDING: yes | |
| Participants | 13 participants (69% males, 52±14.4 years old) with type 2 diabetes COMPLICATIONS/CO‐MORBIDITY: 46% with hypertriglyceridemia, 46% hypertension, 15% coronary artery disease EXCLUSION CRITERIA: liver failure, renal failure, hypothyroidism, poorly controlled diabetes, medication with lipid‐lowering agents | |
| Interventions | Low dose:
2.6 g of eicosapentaenoic acid plus
1.4 g of docosahexaenoic acid
VERSUS
12 g of safflower oil High dose: (non‐randomized) 5.0 g of eicosapentaenoic acid plus 2.5 g of docosahexaenoic acid |
|
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL, LDL and VLDL cholesterol, VLDL triglycerides, lipid ratios, apolipoproteins GLUCOSE PROFILE: fasting plasma glucose, 2 h post‐prandial glucose, glycated hemoglobin, C‐peptide | |
| Notes | Quality score: 2 Information is provided for both low and high dose phases. High dose phase is not randomized | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Silvis 1990.
| Methods | DESIGN: Parallel DURATION: 8 weeks for each phase with 8 weeks of washout BLINDING: no | |
| Participants | 63 participants with type 2 diabetes (not well controlled (HbA1c 9‐11), 32‐46% male, Black, age 54±10 years) randomized to placebo (21, analyzed 18 in phase II), first fish oil then fiber (24, analyzed 21 in phase II) and first fiber then fish oil (18, analyzed 17 in phase II) COMPLICATIONS/CO‐MORBIDITY: 52‐67% hypertension EXCLUSION CRITERIA: none Ranges given refer to data for the different comparison groups | |
| Interventions | 1.4 g of eicosapentaenoic acid plus 0.3 g of docosahexaenoic acid VERSUS 12 g of olive oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, HDL cholesterol reported at initial 4 weeks and initial 8 weeks, HDL:TC ratio GLUCOSE PROFILE: HbA1c OTHER: Factor VII, Fibrinogen, Bleeding and clotting time, weight | |
| Notes | Quality score: 1 Although described as crossover, this is really a parallel study between fish oil and placebo and between fiber and placebo, but the fiber and fish oil groups cross over. A subgroup received extra vitamin E. Measurements were given at weeks 4 and 8 for phases I and II. For the pooled analyses, data from the Fiber group was used as control when data for placebo group was not available. Only data from phase I taken for all analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Sirtori 1997.
| Methods | DESIGN: Parallel DURATION: 8 months BLINDING: yes | |
| Participants | 935 participants of whom 418 had type 2 diabetes divided into 211 for control and 207 for fish oil (203 finished ‐ Intention to treat used). Control group: 58.8±8.9 years old, 62% male Intervention: 58.2±9 years old, 62% male COMPLICATIONS/CO‐MORBIDITY: hyperlipidemia EXCLUSION CRITERIA: obesity, malabsortion, duodenal ulcer, noncompliance or unreliable, epilepsy, alcoholism, insulin use, unstable angina or recent heart attack, severe hypertension, severe dyslipidemia | |
| Interventions | 1.5 g of eicosapentaenoic acid plus 1.0 g of docosahexaenoic acid for 2 months, then 1.0 g of eicosapentaenoic acid plus 0.7 g of docosahexaenoic acid for 6 months VERSUS 3 g olive oil for 24 weeks | |
| Outcomes | LIPID PROFILE: total cholesterol, HDL, LDL and VLDL cholesterol, triglycerides, LDL:HDL ratio, lipoprotein and heparin lipases, lipid subfractions , composition and particle size GLUCOSE PROFILE: fasting plasma glucose, HbA1c, fasting insulin OTHER: Blood pressure | |
| Notes | Quality score: 4 Lipid subfractions, particle sizes, ratios measured in subgroup of 16 participants. Waiting for author's submission of LDL data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vessby 1990.
| Methods | DESIGN: Crossover DURATION: 8 weeks per phase, without washout period BLINDING: yes | |
| Participants | 14 participants with type 2 diabetes (78% males, ages 39‐72) COMPLICATIONS/CO‐MORBIDITY: hyperlipidemia EXCLUSION CRITERIA: medication with lipid lowering agents | |
| Interventions | 1.8 g of eicosapentaenoic acid plus 1.2 g of docosahexaenoic acid VERSUS 10 g olive oil | |
| Outcomes | LIPID PROFILE: total cholesterol, HDL and LDL cholesterol, triglycerides, VLDL, LDL and HDL‐triglycerides, Lipid ratios, Apolipoproteins GLUCOSE PROFILE: fasting plasma glucose, HbA1c OTHER: blood pressure, melonaldehyde, serum insulin after glucagon IV infusion, C‐peptide, weight (data not given) | |
| Notes | Quality score: 2 Least‐square means are presented, % change and p‐values given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Westerveld 1993.
| Methods | DESIGN: Parallel DURATION: 8 weeks BLINDING: yes | |
| Participants | 24 participants (62.5% male) with type 2 diabetes divided into three groups of 8 EXCLUSION CRITERIA: liver or renal failure, bleeding, cardiovascular disorder in last 3 months, insulin use | |
| Interventions | Low dose: 0.9 g eicosapentaenoic acid High dose: 1.8 g eicosapentaenoic acid VERSUS 1.6 g olive oil | |
| Outcomes | LIPID PROFILE: baseline total cholesterol and triglycerides, complete LDL cholesterol record for fish oil group in high dose, apolipoprotein A and B and plasma Lp (a) (data not given) GLUCOSE PROFILE: HbA1c OTHER: weight (no SD and no p‐value for placebo group), platelet aggregation, platelet adhesion, plasma viscosity, bleeding time, fibrinogen (data not shown for any of these) | |
| Notes | Quality score: 4 Least‐square means are presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Woodman 2002.
| Methods | DESIGN: Parallel DURATION: 6 weeks BLINDING: yes | |
| Participants | 59 participants with type 2 diabetes, (39 men and 12 women; age 61.2±1.2 years; duration of diabetes 5.2±4.8 years). 17 were randomized to eicosapentaenoic acid, 18 to docosahexaenoic acid, and 16 in the placebo arm DROP‐OUTS: 8 COMPLICATIONS/CO‐MORBIDITIES: All had hypertension EXCLUSION CRITERIA: Smokers, non‐hypertensive, pre‐menopausal participants, diagnosis less than 3 months, insulin use, more than 2 fish meals per week or supplementation, symptomatic heart disease, myocardial infarction, stroke, liver or renal disease, symptomatic autonomic neuropathy, regular use of non‐steroidal anti‐inflammatory drugs, recent major surgery | |
| Interventions | 4g eicosapentaenoic acid or 4g docosahexaenoic acid vs 4g olive oil | |
| Outcomes | LIPID PROFILE: total cholesterol, triglycerides, LDL and HDL cholesterol, HDL2 and HDL3 cholesterol GLUCOSE PROFILE: HbA1c, fasting glucose, fasting insulin, insulin sensitivity index, C‐peptide OTHER: F2 Isoprostanes, von Willebrand factor, blood flow, blood pressure, heart rate, interleuken‐6, tumor‐necrosis factor‐alpha, C‐reactive protein, P‐selectin, plasminogen activator inhibitor‐1, tissue plasminogen activator, thromboxane B2 and platelet aggregation (calculated as area under the curve), weight (change data not given) | |
| Notes | Quality score: 3 Trial divided into diet groups, with EPA and DHA treatment groups compared to olive oil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 1994 | Did not assess fish oil supplementation |
| Bonnema 1995 | Participants not all diabetic or had type 1 diabetes |
| Das 1994a | Not a randomized trial |
| Das 1994b | Not a randomized trial |
| Das 1995 | Not a randomized trial |
| Dunstan 1997 | Did not assess fish oil supplementation |
| Eritsland 1994 | Participants not all diabetic or had type 1 diabetes |
| Fasching 1991 | Participants not all diabetic or had type 1 diabetes No placebo arm included |
| Friedberg 1998 | A meta‐analysis |
| Hamazaki 1990 | Participants not all diabetic or had type 1 diabetes. Also a before and after comparison |
| Herrmann 1992 | Not a randomized trial |
| Holler 1996 | Did not assess fish oil supplementation |
| Howard 1993 | Did not assess fish oil supplementation and was a non‐randomized study |
| Kasim 1988 | Not a randomized trial |
| Lee 1994 | Did not assess fish oil supplementation |
| Lungershausen 1997 | Participants not all diabetic or had type 1 diabetes |
| Mackness 1994 | Participants not all diabetic or had type 1 diabetes |
| Malasanos 1991 | Not a randomized trial |
| Morris 1995 | Did not assess fish oil supplementation ‐ although included fish oil |
| Okuda 1992 | Before and after comparison |
| Okuda 1996 | Before and after comparison |
| Prince 1997 | Not a randomized trial |
| Rossing 1996 | Participants not all diabetic or had type 1 diabetes |
| Schaap 1991 | Not a randomized trial |
| Semplicini 1994 | Not a randomized trial |
| Sheehan 1997 | Not a randomized trial |
| Shimizu 1993 | No placebo arm included |
| Shimizu 1995 | No placebo arm included |
| Shunto 1992 | Not a randomized trial |
| Silva 1996 | Not a randomized trial |
| Sirtori 1998 | Non‐randomized extension of previous study |
| Stacpoole 1989 | Participants not all diabetic or had type 1 diabetes |
| Stender 1990 | Not a randomized study |
| Tonstad 1997 | Did not assess fish oil supplementation ‐ a non‐randomized trial |
| Urano 1991 | Did not assess fish oil supplementation |
| Yamada 1995 | Did not include human participants |
| Zak 1996 | Not a randomized study |
| Zambon 1992 | Did not assess fish oil supplementation |
Contributions of authors
VICTOR MONTORI: Initial review data extraction, review development.
ANDREW FARMER: Protocol development, quality assessment of trials, data extraction, data analysis, review development and editing.
SEAN DINNEEN: Initial review data extraction, review development.
JANINE HARTWEG: Protocol development, searching for trials, quality assessment of trials, data extraction, data analysis, review development and editing.
ANDREW NEIL: Data analysis, review development
RAFAEL PERERA: Statistical assessment
Sources of support
Internal sources
Division of Public Health and Primary Care, University of Oxford, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Alekseeva 2000 {published and unpublished data}
- Alekseeva RI, Sharafetdinov K, Plotnikova OA, Meshcheriakova VA, Mal'tsev GI, Kulakova SN. Effects of diet therapy including eiconol on clinical and metabolic parameters in patients with type 2 diabetes mellitus. Vopr Pitan 2000;69:36‐9. [PubMed] [Google Scholar]
- Meshcheriakova VA, Plotnikova OA, Sharafetdinov KH, Alekseeva RI, Mal'tsev GI, Kulakova SN. Comparative study of effects of diet therapy including eiconol or linseed oil on several parameters of lipid metabolism in patients with type 2 diabetes mellitus. Vopr Pitan 2001;70(28):28‐31. [PubMed] [Google Scholar]
Annuzzi 1991 {published data only}
- Annuzzi G, Rivellese A, Capaldo B, Marino L, Iovine C, Marotta G, et al. A controlled study on the effects of n‐3 fatty acids on lipid and glucose metabolism in non‐insulin‐dependent diabetic patients. Atherosclerosis 1991;87:65‐73. [DOI] [PubMed] [Google Scholar]
Axelrod 1994 {published data only}
- Axelrod L, Camuso J, Williams E, Kleinman K, Briones E, Schoenfeld D. Effects of a small quantity of omega‐3 fatty acids on cardiovascular risk factors in NIDDM. A randomized, prospective, double‐blind, controlled study. Diabetes Care 1994;17:37‐44. [DOI] [PubMed] [Google Scholar]
Boberg 1992 {published data only}
- Boberg M, Pollare T, Siegbahn A, Vessby B. Supplementation with n‐3 fatty acids reduces triglycerides but increases PAI‐1 in non‐insulin‐dependent diabetes mellitus. European Journal of Clinical Investigation 1992;22:645‐50. [DOI] [PubMed] [Google Scholar]
Borkman 1989 {published data only}
- Borkman M, Chisholm DJ, Furler SM, Storlien LH, Kraegen EW, Simons LA, et al. Effects of fish oil supplementation on glucose and lipid metabolism in NIDDM. Diabetes 1989;38:1314‐9. [DOI] [PubMed] [Google Scholar]
Connor 1993 {published data only}
- Connor WE, Prince MJ, Ullmann D, Riddle M, Hatcher L, Smith FE, et al. The hypotriglyceridemic effect of fish oil in adult‐onset diabetes without adverse glucose control. Annals of the New York Academy of Sciences 1993;683:337‐40. [DOI] [PubMed] [Google Scholar]
Goh 1997 {published data only}
- Goh YK, Jumpsen JA, Ryan EA, Clandinin MT. Effect of omega 3 fatty acid on plasma lipids, cholesterol and lipoprotein fatty acid content in NIDDM patients. Diabetologia 1997;40:45‐52. [DOI] [PubMed] [Google Scholar]
Hendra 1990 {published data only}
- Hendra TJ, Britton ME, Roper DR, Wagaine‐Twabwe D, Jeremy JY, Dandona P, et al. Effects of fish oil supplements in NIDDM subjects. Controlled study. Diabetes Care 1990;13:821‐9. [DOI] [PubMed] [Google Scholar]
Jain 2002 {published and unpublished data}
- Jain S, Gaiha M, Bhattacharjee J, Anuradha S. Effects of low‐dose omega‐3 fatty acid substitution in type‐2 diabetes mellitus with special reference to oxidative stress‐‐a prospective preliminary study. Journal of the Association of Physicians of India 2002;50:1028‐33. [PubMed] [Google Scholar]
Luo 1998 {published data only}
- Luo J, Rizkalla SW, Vidal H, Oppert JM, Colas C, Boussairi C, et al. Moderate intake of n‐3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care 1998;21:717‐24. [DOI] [PubMed] [Google Scholar]
McGrath 1996 {published data only}
- McGrath LT, Brennan GM, Donnelly JP, Johnston GD, Hayes JR, McVeigh GE. Effect of dietary fish oil supplementation on peroxidation of serum lipids in patients with non‐insulin dependent diabetes mellitus. Atherosclerosis 1996;121:275‐83. [DOI] [PubMed] [Google Scholar]
- McVeigh G, Brennan G, Hayes R, Johnston D. Primary nitrate tolerance in diabetes mellitus. Diabetologia 1994;37:115‐7. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Cohn JN, Finkelstein SM, Hayes RJ, Johnston GD. Fish oil improves arterial compliance in non‐insulin‐dependent diabetes mellitus. Arteriosclerosis and Thrombosis 1994;14:1425‐9. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, et al. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non‐insulin‐dependent) diabetes mellitus. Diabetologia 1993;36:33‐8. [DOI] [PubMed] [Google Scholar]
McManus 1996 {published data only}
- McManus RM, Jumpson J, Finegood DT, Clandinin MT, Ryan EA. A comparison of the effects of n‐3 fatty acids from linseed oil and fish oil in well‐controlled type II diabetes. Diabetes Care 1996;19:463‐7. [DOI] [PubMed] [Google Scholar]
Morgan 1995 {published data only}
- Morgan WA, Raskin P, Rosenstock J. A comparison of fish oil or corn oil supplements in hyperlipidemic subjects with NIDDM. Diabetes Care 1995;18:83‐6. [DOI] [PubMed] [Google Scholar]
Mostad 2006 {published data only}
- Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n‐3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time‐dependent alteration from carbohydrate to fat oxidation [published erratum appears in The American journal of clinical nutrition 2007;85(6):1668]. The American journal of clinical nutrition 2006;84(3):540‐50. [DOI] [PubMed] [Google Scholar]
Pelikanova 1992 {published data only}
- Pelikanova T, Kohout M, Valek J, Kazdova L, Base J. Metabolic effects of omega‐3 fatty acids in type 2 (non‐insulin‐dependent) diabetic patients. Annals of the New York Academy of Sciences 1993;683:2722‐8. [DOI] [PubMed] [Google Scholar]
- Pelikanova T, Kohout M, Valek J, Kazdova L, Karasova L, Base J, et al. The effect of fish oil on the secretion and effect of insulin in patients with type II diabetes. Casopis Lekaru Ceskych 1992;131:668‐72. [PubMed] [Google Scholar]
Petersen 2002 {published and unpublished data}
- Pedersen H, Petersen M, Major‐Pedersen A, Jensen T, Nielsen NS, Lauridsen ST, et al. Influence of fish oil supplementation on in vivo and in vitro oxidation resistance of low‐density lipoprotein in type 2 diabetes. European Journal of Clinical Nutrition 2003;57:713‐20. [DOI] [PubMed] [Google Scholar]
- Petersen M, Pedersen H, Major‐Pedersen A, Jensen T, Marckmann P. Effect of fish oil versus corn oil supplementation on LDL and HDL subclasses in type 2 diabetic patients. Diabetes Care 2002;25:1704‐8. [DOI] [PubMed] [Google Scholar]
Puhakainen 1995 {published data only}
- Puhakainen I, Ahola I, Yki‐Jarvinen H. Dietary supplementation with n‐3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non‐insulin‐dependent diabetes mellitus. American Journal of Clinical Nutrition 1995;61:121‐6. [DOI] [PubMed] [Google Scholar]
Schectman 1988 {published data only}
- Schectman G, Kaul S, Kissebah AH. Effect of fish oil concentrate on lipoprotein composition in NIDDM. Diabetes 1988;37:1567‐73. [DOI] [PubMed] [Google Scholar]
Silvis 1990 {published data only}
- Silvis N, Vorster HH, Mollentze WF, Jager JD, Huisman HW. Metabolic and Haemostatic Consequences of Dietary Fibre and N‐3 Fatty Acids in Black Type 2 (NIDDM) Diabetic Subjects: A Placebo Controlled Study. International Clinical Nutrition Review 1990;10:362‐80. [Google Scholar]
Sirtori 1997 {published data only}
- Maffettone A. Long‐term effects (six months) of omega‐3 polyunsaturated fatty acids on insulin sensitivity and lipid metabolism in patients with type 2 diabetes and hypertriglycaeridemia. Giornale Italiano di Diabetologia 1996;16:185‐93. [Google Scholar]
- Patti L, Maffetone A, Iovine C, Marino L, Annuzzi G, Raccardi G, et al. Long‐term effects of fishoil on lipoprotein subfractions and low density lipoprotein size in non‐insulin‐dependent diabetic patients with hypertriglyceridemia. Atherosclerosis 1999;146:361‐7. [DOI] [PubMed] [Google Scholar]
- Rivellese AA, Maffettone A, Iovine C, Marino L, Annunzi G, Mancini M, et al. Long‐term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care 1996;19:1207‐13. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Paoletti R, Mancini M, Crepaldi G, Manzato E, Rivellese A et al on behalf of the Italian Fish Oil Multicenter Study. N‐3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. American Journal of Clinical Nutrition 1997;65:1874‐81. [DOI] [PubMed] [Google Scholar]
Vessby 1990 {published data only}
- Vessby B, Boberg M. Dietary supplementation with n‐3 fatty acids may impair glucose homeostasis in patients with non‐insulin‐dependent diabetes mellitus. Journal of Internal Medicine 1990;228:165‐71. [DOI] [PubMed] [Google Scholar]
Westerveld 1993 {published data only}
- Westerveld HT, Graaf JC, Breugel HH, Akkerman JWN, Sixma JJ, Erkelens DW, et al. Effects of low‐dose EPA‐E on glycemic control, lipid profile, lipoprotein(a), platelet aggregation, viscosity, and platelet and vessel wall interaction in NIDDM. Diabetes Care 1993;16:683‐8. [DOI] [PubMed] [Google Scholar]
Woodman 2002 {published and unpublished data}
- Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated‐hypertensive type 2 diabetic subjects. Free Radical Biological Medicine 2003;35:772‐81. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, et al. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular functino in hypertensive type 2 diabetic patients. Atherosclerosis 2003;166:85‐93. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetes with treated hypertension. Clinical Nutrition 2002;76:1007‐15. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adler 1994 {published data only}
- Adler AI, Boyko EJ, Schraer CD, Murphy NJ. Lower prevalence of impaired glucose tolerance and diabetes associated with daily seal oil or salmon consumption among Alaska Natives. Diabetes Care 1994;17:1498‐501. [DOI] [PubMed] [Google Scholar]
Bonnema 1995 {published data only}
- Bonnema SJ, Jespersen LT, Marving J, Gregersen G. Supplementation with olive oil rather than fish oil increases small arterial compliance in diabetic patients. Diabetes, Nutrition and Metabolism Clinical and Experimental 1995;8:81‐7. [Google Scholar]
Das 1994a {published data only}
- Das UN, Kumar KV, Mohan IK. Lipid peroxides and essential fatty acids in patients with diabetes mellitus and diabetic nephropathy. Journal of Nutritional Medicine 1994;4:149‐55. [Google Scholar]
Das 1994b {published data only}
- Das UN, Kumar KV, Ramesh G. Essential fatty acid metabolism in south Indians. Prostaglandins leukotrienes and essential fatty acids 1994;50:253‐5. [DOI] [PubMed] [Google Scholar]
Das 1995 {published data only}
- Das UN. Essential fatty acid metabolism in patients with hypertension, diabetes mellitus and coronary heart disease. Prostaglandins, leukotrienes and essential fatty acids 1995;52:387‐91. [DOI] [PubMed] [Google Scholar]
Dunstan 1997 {published data only}
- Dunstan DW, Mori TA, Puddey IB, Beilin LJ, Burke V, Morton AR, et al. The independent and combined effects of aerobic exercise and dietary fish intake on serum lipids and glycemic control in NIDDM. A randomized controlled study. Diabetes Care 1997;20:913‐21. [DOI] [PubMed] [Google Scholar]
Eritsland 1994 {published data only}
- Eritsland J, Seljeflot I, Abdelnoor M, Arnesen H, Torjesen PA. Long‐term effects of n‐3 fatty acids on serum lipids and glycaemic control. Scandinavian Journal of Clinical & Laboratory Investigation 1994;54:273‐80. [DOI] [PubMed] [Google Scholar]
Fasching 1991 {published data only}
- Fasching P, Ratheiser K, Waldhausl W, Rohac M, Osterode W, et al. Metabolic effects of fish‐oil supplementation in patients with impaired glucose tolerance. Diabetes 1991;40:583‐9. [DOI] [PubMed] [Google Scholar]
Friedberg 1998 {published data only}
- Friedberg CE, Janssen M, Heine RJ, Grobbee DE. Fish oil and glycemic control in diabetes: A meta‐analysis. Diabetes Care 1998;21:494‐500. [DOI] [PubMed] [Google Scholar]
Hamazaki 1990 {published data only}
- Hamazaki T, Takazakura E, Osawa K, Urakaze M, Yano S. Reduction in microalbuminuria in diabetics by eicosapentaenoic acid ethyl ester. Lipids 1990;25:541‐5. [DOI] [PubMed] [Google Scholar]
Herrmann 1992 {published data only}
- Herrmann W, Biermann J, Ratzmann KP, Lindhofer HG. Effect of fish oil concentrate on the lipoprotein profile of patients with type II diabetes mellitus. Medizinische Klinik 1992;87:12‐5. [PubMed] [Google Scholar]
Holler 1996 {published data only}
- Holler C, Auinger M, Ulberth F, Irsigler K. Eicosanoid precursors: potential factors for atherogenesis in diabetic CAPD patients?. Peritoneal Dialysis International 1996;16:S250‐3. [PubMed] [Google Scholar]
Howard 1993 {published data only}
- Howard WJ. Is it time for a clinical trial of dietary fish oil supplementation in individuals with NIDDM?. Annals of the New York Academy of Sciences 1993;683:341‐2. [DOI] [PubMed] [Google Scholar]
Kasim 1988 {published data only}
- Kasim SE, Stern B, Khilnani S, McLin P, Bacioowski S, Jen K‐LC. Effects of omega‐3 fish oils on lipid metabolism, glycemic control and blood pressure in type II diabetic patients. Journal of Clinical Endocrinology and Metabolism 1988;67:1‐4. [DOI] [PubMed] [Google Scholar]
Lee 1994 {published data only}
- Lee R. Fish oil, essential fatty acids, and hypertension. Canadian Journal of Physiology & Pharmacology 1994;72:945‐953. [DOI] [PubMed] [Google Scholar]
Lungershausen 1997 {published data only}
- Lungershausen YK, Howe PR, Clifton PM, Hughes CR, Philips P, Graham JJ, et al. Evaluation of an omega‐3 fatty acid supplement in diabetics with microalbuminuria. Annals of the New York Academy of Sciences 1997;827:369‐81. [DOI] [PubMed] [Google Scholar]
Mackness 1994 {published data only}
- Mackness MI, Bhatnagar D, Durrington PN, Prais H, Haynes B, Morgan J, et al. Effects of a new fish oil concentrate on plasma lipids and lipoproteins in patients with hypertriglyceridaemia. European Journal of Clinical Nutrition 1994;48:859‐65. [PubMed] [Google Scholar]
Malasanos 1991 {published data only}
- Malasanos TH, Stacpoole PW. Biological effects of omega‐3 fatty acids in diabetes mellit. Diabetes Care 1991;14:1160‐79. [DOI] [PubMed] [Google Scholar]
Morris 1995 {published data only}
- Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians' health study: a prospective study. American Journal of Epidemiology 1995;142:166‐75. [DOI] [PubMed] [Google Scholar]
Okuda 1992 {published data only}
- Okuda Y, Mizutani M, Tanaka K, Isaka M, Yamashita K. Beneficial effects of eicosapentaenoic acid for diabetic patients with arteriosclerosis obliterans [1]. Diabetes Research and Clinical Practice 1992;18:139‐40. [DOI] [PubMed] [Google Scholar]
Okuda 1996 {published data only}
- Okuda Y, Mitzutani M, Ogawa M. Long term effects of eicosapentaenoic acid on diabetic peripheral neuropathy and serum lipids in patients with type II diabetes mellitus. Journal of Diabetes and its Complications 1996;10:280‐7. [DOI] [PubMed] [Google Scholar]
Prince 1997 {published data only}
- Prince MJ, Deeg MA. Do n‐3 fatty acids improve glucose tolerance and lipaemia in diabetics. Current Opinion in Lipidology 1997;8:280‐7. [DOI] [PubMed] [Google Scholar]
Rossing 1996 {published data only}
- Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G, Parving HH. Fish oil in diabetic nephropathy. Diabetes Care 1996;19:1214‐9. [DOI] [PubMed] [Google Scholar]
Schaap 1991 {published data only}
- Schaap GH, Bilo HJG, Beukhof JR, Gans ROB, Popp‐Snijders C, Donker AJM. The effects of short‐term omega‐3 polyunsaturated fatty acid supplementation in patients with chronic renal insufficiency. Current Therapeutic Research, Clinical and Experimental 1991;49:1061‐70. [Google Scholar]
Semplicini 1994 {published data only}
- Semplicini A, Valle R. Fish oils and their possible role in the treatment of cardiovascular disorders. Pharmacology and Therapeutics 1994;61:385‐97. [DOI] [PubMed] [Google Scholar]
Sheehan 1997 {published data only}
- Sheehan JP, Wei IW, Ulchaker M, Tserng KY. Effects of high fiber intake in fish‐oil treated patients with non‐insulin‐dependent diabetes mellitus. American Journal of Clinical Nutrition 1997;66:1183‐7. [DOI] [PubMed] [Google Scholar]
Shimizu 1993 {published data only}
- Shimizu H, Sato N, Tanaka Y, Kashima K, Ohtani K‐I, Mori M. Effect of eicosapentaenoic acid ethyl on urine albumin excretion in NIDDM. Diabetes Care 1993;16:1406‐7. [DOI] [PubMed] [Google Scholar]
Shimizu 1995 {published data only}
- Shimizu H, Ohtani K, Tanak Y, Sato N, Mori M, Shimomura Y. Long term effect of eicosapentaenoic acid ethyl (EPA‐E) on albuminuria of non‐insulin dependent diabetic patients. Diabetes Research and Clinical Practice 1995;28:35‐40. [DOI] [PubMed] [Google Scholar]
Shunto 1992 {published data only}
- Shunto S, Takahashi K, Negishi K, Suzuki M, Moritani S, Itabashi A, et al. Effects of eicosapentaenoic acid on glycemic control and lipid metabolism in healthy and NIDDM subjects. Seraputikku Risachi 1992;13:257‐65. [Google Scholar]
Silva 1996 {published data only}
- Silva JM, Souza I, Silva R, Tavares P, Teixeira F, Silva PS. The triglyceride lowering effect of fish oils is affected by fish consumption. International Journal of Cardiology 1996;57:75‐80. [DOI] [PubMed] [Google Scholar]
Sirtori 1998 {published data only}
- Sirtori CR, Crepaldi G, Manzato E, Mancini M, Rivellese A, Paoletti R, et al. One year treatment with ethyl‐esthers of n‐3 fatty acids in patients with hypertriglyceridemia and glucose intolerance reduced triglyceridemia, total cholessterol and increased HDL‐C without glycemic alterations. Atherosclerosis 1998;137:419‐27. [DOI] [PubMed] [Google Scholar]
Stacpoole 1989 {published data only}
- Stacpoole PW, Alig J, Ammon L, Crockett SE. Dose response effects of dietary marine oil on carbohydrate and lipid metabolism in normal subjects and patients with hypertriglyceridemia. Metabolism: Clinical and Experimental 1989;38:946‐56. [DOI] [PubMed] [Google Scholar]
Stender 1990 {published data only}
- Stender S, Jensen T, Deckert T. Experience with fish oil treatment with special emphasis on diabetic nephropathy. Journal of Diabetic Complications 1990;4:70‐1. [DOI] [PubMed] [Google Scholar]
Tonstad 1997 {published data only}
- Tonstad S. Drug therapy of hyperlipidaemia ‐ unanswered questions. Tidsskrift for Den Norske Laegeforening 1997;117:674‐7. [PubMed] [Google Scholar]
Urano 1991 {published data only}
- Urano S, Hoshi‐Hashizume M, Tochigi N, Matsuo M, Ito H. Vitamin E and the susceptibility of erythrocytes and reconstituted liposomes to oxidative stress in aged diabetics. Lipids 1991;26:58‐61. [DOI] [PubMed] [Google Scholar]
Yamada 1995 {published data only}
- Yamada Y, Fushimi H, Inoue T. Effect of eicosapentaenoic acid and docosahexaenoic acid on diabetic osteopenia. Diabetes Research and Clinical Practice 1995;30:37‐42. [DOI] [PubMed] [Google Scholar]
Zak 1996 {published data only}
- Zak A, Zeman M, Tvrzicka E, Stolba P. Effects of fish oils in patients with type 2 diabetes with associated dyslipidaemia. Casopis Lekaru Ceskych 1996;135:354‐9. [PubMed] [Google Scholar]
Zambon 1992 {published data only}
- Zambon S, Friday KE, Childs MT, Fujimoto WY, Bierman EL, Ensinck JY. Effects of glyburide and omega 3 fatty acid dietary supplements on glucose and lipid metabolism in patients with non insulin dependent diabetes mellitus. American Journal of Clinical Nutrition 1992;56:447‐54. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
AFORRD 2004 {published data only}
- The AFORRD Trial. Atorvastatin in factorial with omega‐3 risk reduction in diabetes) (Abstract) Register for Randomised Controlled Trials. http://www.controlled‐trials.com/isrctn/trial/0‐76737502.html 2004.
ASCEND 2005 {published data only}
- The ASCEND Trial (Abstract). Oxford Clinical Trials Service Unit. http://www.ctsu.ox.ac.uk/ascend/ 2005.
Galan 2003 {published data only}
- Galan P, Bree A, Mennen L, Courcy P, Preziozi P, Bertrais S, Castetbon K, Hercberg S. Background and rationale of the SU.FO.LOM3 study: double blind placebo‐controlled secondary prevention trial to test the impact with supplementation with folate, vitamin B6 and B12 and/or omega‐3 fatty acids on the prevention of recurrent ischemic events in subjects with artherosclerosis in the coronary or cerebral arteries. Journal of Nutrion, Health and Aging 2003;7(6):428‐35. [PubMed] [Google Scholar]
ORIGIN 2005 {published data only}
- The ORIGIN Trial. Outcome Reduction with Initial Glargine Intervention (Abstract) Clinical Trials.gov Identifier: NCT00069784. http://www.controlledtrials.com/mrct/trial/OMEGA%2D3%7CDIABETES/1059/61673.html 2005.
Additional references
ADA 1998
- American Diabetes Association. Management of Dyslipidemia in Adults with Diabetes. Diabetes Care. Diabetes Care 1998;21(Suppl 1):S36‐9. [DOI] [PubMed] [Google Scholar]
Balk 2004
- Balk E, Chung M, Lichtenstein A, Kupelnick B, Lawrence A, Vine D, et al. Effects of omega‐3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Prepared by Tufts New England Medical Center Evidence‐based Practice Center, Rockville, MD.. Agency for Health Care Research and Quality; Evidence Report/Technology Assessment No. 93 2004. [PMC free article] [PubMed]
Bang 1976
- Bang HO, Dyerberg J, Hjorne N. The composition of food consumed by Greenland Eskimos. Acta Medica Scandanavica 1976;200:69‐73. [DOI] [PubMed] [Google Scholar]
Bucher 2002
- Bucher, HC, Hengstler, P, Schindler C, Meier G. N‐3 polyunsaturated fatty acids in coronary heart disease: a meta‐analysis of radomized controlled trials. American Journal of Medicine 2002;112:298‐304. [DOI] [PubMed] [Google Scholar]
Burr 1989
- Burr ML, Gilbert JF, Holliday RM, Elwood PC, Fehily AM, Rogers S, et al. Effects of changes in fat, fish and fibre intakes on death and myocardial reinfarction: Diet and Reinfarction Trial (DART). Lancet 1999;354:757‐61. [DOI] [PubMed] [Google Scholar]
Burr 2003
- Burr ML, Ashfield‐Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. European Journal of Clinical Nutrition 2003;57:193‐200. [DOI] [PubMed] [Google Scholar]
Connor 2000
- Connor WE. Importance of n‐3 fatty acids in health and disease. American Journal of Clinical Nutrition 2000;71:171‐5S. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. British Medical Journal 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elkeles 1998
- Elkeles RS, Diamond JR, Poulter C, Dhanjil S, Nicolaides AN, Mahmood S, et al. Cardiovascular outcomes in type 2 diabetes: a double blind placebo‐controlled study of bezafibrate: the St. Mary's, Ealing, Northwick Park Diabetes Cardiovascular Prevention (SENDCAP) Study. Diabetes Care 1998;21:641‐8. [DOI] [PubMed] [Google Scholar]
Farmer 2001
- Farmer A, Montori V, Dinneen S, Clar C. Fish oin in people with type 2 diabetes. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI] [PubMed] [Google Scholar]
Fasching 1996
- Fasching P, Rohac M, Liener K, Schneider B, Nowotny P, Waldhausl W. Fish oil supplementation versus gemfibrozil treatment in hyperlipidemic NIDDM: a randomized crossover study. Hormone and Metabolism Research 1996;28:230‐6. [DOI] [PubMed] [Google Scholar]
Fisher 1998
- Fisher WR, Zech LA, Stacpoole PW. Apolipoprotein B metabolism in hypertriglyceridemic patients administered either a fish oil‐ or vegetable oil‐enriched diet. Journal of Lipid Research 1998;39:388‐401. [PubMed] [Google Scholar]
Friday 1989
- Friday KE, Childs MT, Tsunehara C, Fujimoto WY, Bierman EL, Ensinck JW. Elevated plasma glucose and lowered triglyceride levels from omega‐3 fatty acid supplementation in type II diabetes. Diabetes Care 1989;12:276‐81. [DOI] [PubMed] [Google Scholar]
Garg 1990
- Garg A, Grundy SM. Nicotinic acid as therapy for dyslipidaemia in non‐insulin dependent diabetes mellitus. JAMA 1990;264:2994‐6. [PubMed] [Google Scholar]
GISSI 1999
- GISSI‐Prevenzione Investigators. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Lancet 1999;354:447‐55. [PubMed] [Google Scholar]
Glauber 1988
- Glauber H, Wallace P, Griver K, Brechtel G. Adverse metabolic effect of omega‐3 fatty acids in non‐insulin dependent diabetes mellitus. Annals of Internal Medicine 1988;108:663‐8. [DOI] [PubMed] [Google Scholar]
Hooper 2004
- Hooper L, Thompson R, Harrison R, Summerbell C, Moore H, Worthington H, et al. Omega‐3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 1987
- Howard BV. Lipoprotein metabolism in diabetes mellitus. Journal of Lipid Research 1987;28:613‐28. [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds DJM, Gavaghan DJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kratz 1998
- Kratz A, Lewandrowski KB. Normal reference laboratory values. New England Journal of Medicine 1998;339(15):1063‐72. [DOI] [PubMed] [Google Scholar]
Lindsey 1992
- Lindsey S, Pronczuk A, Hayes KC. Low density lipoprotein from humans supplemented with n‐3 fatty acids depresses both LDL receptor activity and LDLr mRNA abundance in HepG2 cells. Journal of Lipid Research 1992;33:647‐58. [PubMed] [Google Scholar]
Minihane 2000
- Minihane AM, Khan S, Leigh‐Firbank EC, Talmud P, Wright JW, Murphy MC, et al. Apo E polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arteriosclerosis, Thrombosis and Vascular Biology 2000;20:1990‐7. [DOI] [PubMed] [Google Scholar]
Montori 2000
- Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes. Diabetes Care 2000;23:1407‐15. [DOI] [PubMed] [Google Scholar]
Mori 2000
- Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. American Journal of Clinical Nutrition 2000;71:1085‐94. [DOI] [PubMed] [Google Scholar]
Mouraoff 1967
- Mouratoff GJ, Carroll NV, Scott EM. Diabetes mellitus in eskimos. Journal of the American Medical Association 1967;199:107‐12. [DOI] [PubMed] [Google Scholar]
Nutall 1998
- Nutall FQ. Comparison of percent total GHb with percent HbA1c in people with and without known diabetes. Diabetes Care 1998;21:1475‐80. [DOI] [PubMed] [Google Scholar]
Ouguerram 2006
- Ouguerram K, Maugeais C, Gardette J, Margot T, Krempf M. Effect of n‐3f fatty acids on metabolism of apoB100‐containing lipoproteins in type 2 diabetic subjects. British Journal of Nutrition 2006;96(1):100‐6. [DOI] [PubMed] [Google Scholar]
Pyorala 1997
- Pyorala K, Pedersen TR, Kjiekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a sub‐group analysis of the Scandinavian Simvastatin Survival Study. Diabetes Care 1997;20:614‐20. [DOI] [PubMed] [Google Scholar]
Rice 1995
- Rice JA. Mathematical Statistics and Data Analysis. California, USA: Duxbury Press, 1995. [Google Scholar]
Schectman 1996
- Schectman G, Boerboom LE, Hannah J, Howard BV, Mueller RA, Kissebah AH. Dietary fish oil decreases low‐density‐lipoprotein clearance in non‐human primates. American Journal of Clinical Nutrition 1996;64:215‐21. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Surette 1992
- Surette, Whelan J, Lu GP, Broughton KS, Kinsella JE. Dependence on dietary cholesterol for n‐3 polyunsaturated fatty acid‐induced changes in plasma cholesterol in the Syrian hamster. Journal of Lipid Research 1992;33:263‐71. [PubMed] [Google Scholar]
Suzukawa 1995
- Suzukawa M, Abbey M, Howe PR, Nestel PJ. Effects of fish oil fatty acids on low density lipoprotein size, oxidizability, and uptake by macrophages. Journal of Lipid Research 1995;36:473‐84. [PubMed] [Google Scholar]
Theobald 2004
- Theobald HE, Chowienczyk PJ, Whittall R, Humphries SE, Sanders TA. LDL cholesterol‐raising effect of low‐dose docosahexaenoic acid in middle‐aged men and women. American Journal of Clinical Nutrition 2004;79(4):558‐63. [DOI] [PubMed] [Google Scholar]
Tramer 1997
- Tramer Mr, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta‐analysis: a case study. BMJ 1997;315:635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]


