Abstract
Background
Pancreatic metastasis (PM) from renal cell carcinoma (RCC) is relatively rare. Surgical resection of PM from RCC is considered as the first choice treatment for achieving long-term prognosis. Herein, we report a case of secondary multiple metastases from RCC to the remnant pancreas following pancreatectomy, with a review of the relevant literature.
Presentation of case
A 69-year-old man who underwent left nephrectomy for RCC (T2N0M0, stage II) 12 years ago was referred to our hospital. Multiple metastases to the pancreatic head from RCC occurred 2 years after the primary surgery, for which pancreaticoduodenectomy was performed. Nine years after metastatic resection, multiple tumors of the remnant pancreas were detected on dynamic computed tomography (CT); all tumors showed strong enhancement in the early phase, which persisted into the late phase. The tumors were round, the maximum diameter of the tumor was 20 mm, and they were hyperintense on T2-weighted magnetic resonance imaging. Positron emission tomography-CT revealed slight fluorodeoxyglucose uptake in the tumor. Multiple PMs were diagnosed, and the remnant pancreas was completely resected. Two years later, the patient was alive and showed no recurrence.
Conclusions
Surgical resection could provide long-term prognosis, even if secondary PM from RCC occurs metachronously. Long-term follow-up is recommended after primary resection, and vigilance regarding the occurrence of PM is needed.
Keywords: Pancreatic metastasis, Renal cancer, Surgical resection
Highlights
-
•
Surgical resection for pancreatic metastasis from renal cell carcinoma is considered as the first choice treatment.
-
•
We report a case of secondary multiple metastases from renal cell carcinoma to the remnant pancreas following pancreatectomy.
-
•
Surgical resection could provide long-term prognosis even if secondary pancreatic metastasis is occurred metachronously.
1. Introduction
Renal cell carcinoma (RCC) is a common malignancy of the genitourinary tract and accounts for 2–3% of all adult cancers [1]. Radical tumor resection is the gold-standard treatment for RCC. The 5-year survival rate after curative surgery is 95% [2]. Non-clear cell type and advanced TNM stage are reported to be poor prognostic factors for RCC after curative surgery. RCC most commonly metastasizes to the lungs, liver, and bones [3], [4]. Pancreatic metastasis (PM) is rare, accounting for 2% of all pancreatic malignancies [5]. PM from RCC has several distinguishing characteristics, including presentation as an isolated metastasis, slow growth with a long disease-free period after the initial surgery, and a relatively favorable prognosis [6], [7]. Owing to these characteristics, pancreatic resection is warranted in selected cases to ensure maximal benefits [8]. As repeat pancreatic resection for metachronous PM from RCC is rare, the treatment strategy remains uncertain. Herein, we report a case of metachronous recurrent metastases at remnant pancreas from RCC, nine years after pancreaticoduodenectomy for the initial PM, following remnant pancreatectomy, and review the relevant literature. This case was reported in accordance with the SCARE criteria [9].
2. Presentation of case
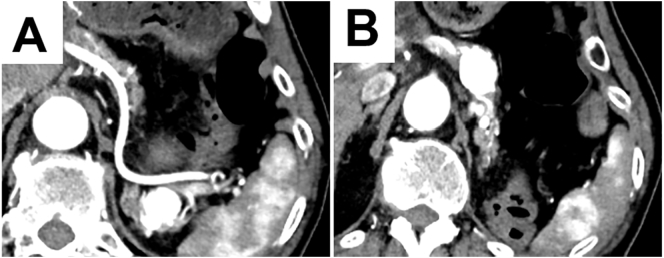
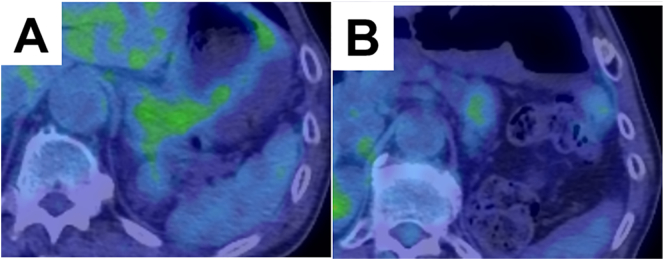
A 69-year-old man who had undergone left nephrectomy for RCC (T2N0M0, stage II) 11 years previously was referred to our hospital. Multiple pancreatic metastases from RCC occurred 2 years after the primary surgery. Dynamic computed tomography (CT) revealed tumors with clear margins and strong enhancement in the early phase, which persisted into the late phase (Fig. 1). Abdominal ultrasonography revealed enhancement of all tumors after Sonazoid injection. Tumors with clear margins and maximum diameters of 12 and 8 mm were observed on endoscopic ultra-sonography (EUS; Fig. 2). The patient was diagnosed with PM from RCC, and pancreatoduodenectomy was performed. Nine years later, dynamic CT revealed multiple tumors in the remnant pancreas. All tumors showed strong enhancement in the early phase that persisted in the late phase. The tumors were round, with the largest tumor having a maximum diameter of 20 mm (Fig. 3A, B). Slight fluorodeoxyglucose uptake was observed in the tumors on positron emission tomography-CT, with a maximum standardized uptake value of 2.0 (Fig. 4A, B). The tumors were hyperintense on T2-weighted magnetic resonance imaging. The Memorial Sloan-Kettering Cancer Center (MSKCC) risk factors for metastatic RCC (mRCC) were 0. We diagnosed the patient as having multiple PMs and completely resected the remnant pancreas. The operative time was 203 min, and intraoperative blood loss was 202 ml. The patient's postoperative course was uneventful. The pathological diagnosis was PM from RCC, and we observed sheet-like growth of tumor cells with clear cytoplasm and small rounded nuclei on histopathological examination. Two years later, the patient was alive, and there was no recurrence.
Fig. 1.
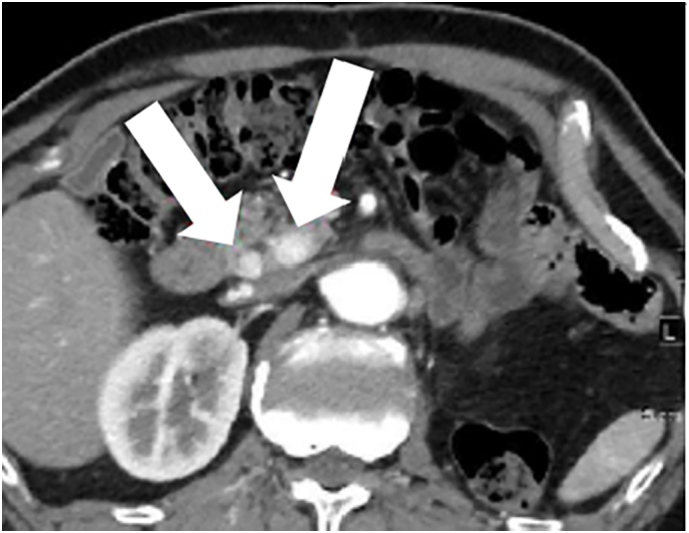
The dynamic computed tomography image shows a tumor with a clear margin and strong enhancement from the early to the late phase.
Fig. 2.
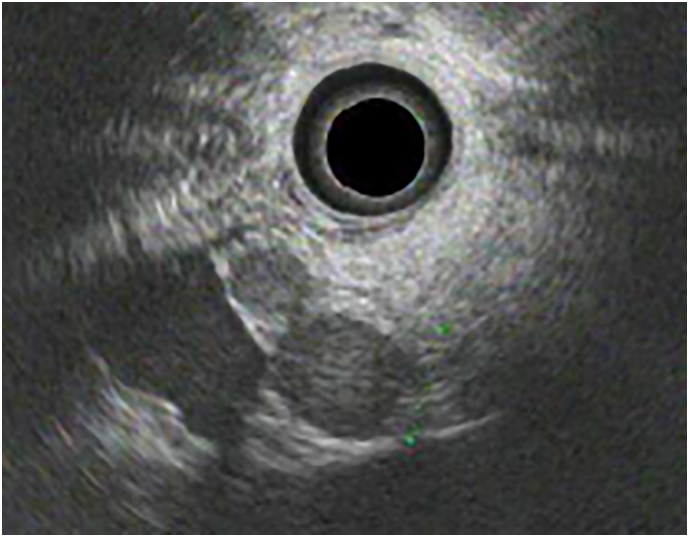
The endoscopic ultrasonography image shows tumors with clear margins. The maximum diameters of the tumors are 12 mm and 8 mm.
Fig. 3.
The dynamic computed tomography image shows strong enhancement of the tumor in the early phase.
A: Strong enhancement of the tumor in the pancreatic tail.
B: Strong enhancement of the tumor in the pancreatic body and tail.
Fig. 4.
The positron emission tomography-computed tomography image shows slight fluorodeoxyglucose uptake in the tumors.
A: Light fluorodeoxyglucose uptake by the tumor in the pancreatic body and tail.
B: Light fluorodeoxyglucose uptake of the tumor in the pancreatic tail.
3. Discussion
Surgical resection of PM without concomitant distant metastasis from RCC is considered the preferred treatment for achieving long-term prognosis. According to previous report, the overall survival rates after pancreatic resection for PM from RCC at 1, 3, and 5 years were 96%, 88%, and 83%, respectively, whereas the disease-free survival rates at 1, 3, and 5 years were 73%, 49%, and 35%, respectively [9]. Some patients experience survival benefits following pancreatic resection [6], [9], [10], [11].
Well-known risk factors for poor long-term survival in patients with PM from RCC include large diameter of the recurrent tumor, short disease-free period, non-curative resection, and no primary nephrectomy [12]. Paolo et al. reported that patients with metastatic RCC with PM survived for significantly longer than those with metastatic RCC without PM, with a median overall survival from the initiation of first-line targeted therapy of 56 months and 23 months and 5-year overall survival rates of 48.7% and 24.9% for patients with and without PM, respectively [10]. In contrast, patients with brain, pleura, liver, and bone metastases have worse prognoses than those with endocrine organ metastases (pancreas, adrenal, thyroid) [13]. Di Firanco et al. reported that aggressive surgical resection of PM from RCC is beneficial even in patients with associated extrapancreatic localization or recurrent metastasis [11]. Owing to the lack of data on repeat surgical resection for PM from RCC, it was challenging to select the optimal treatment in this case. However, in a previous report, the patient underwent complete resection of secondary recurrent PM from RCC and survived for 12 years [11]. Considering the good 2-year prognosis after remnant pancreatectomy in our case, surgical resection could provide a favorable long-term prognosis, even if secondary PM from RCC occurred metachronously.
The radiological characteristics of PM from RCC are hypervascularity, a consequent intense homogeneous contrast enhancement in the arterial phase that is greater than that of normal pancreatic parenchyma, and a tendency to pass undetected in the delayed post-contrast phases [14]. EUS is a highly sensitive diagnostic tool for the detection of pancreatic lesions, particularly small lesions. In general, metastatic pancreatic tumors appear as solid intraparenchymal space-occupying lesions with an internal structure that is either significantly more hypoechoic than, or isoechoic to, normal pancreatic tissue. These lesions are homogeneous, round, and well circumscribed [14]. In our case, the pancreatic tumor was highly suggestive of PM from RCC, and fine-needle aspiration was not performed owing to the hypervascularity of the tumor. Regardless of PM from RCC with hypervascularity, EUS-FNA is a useful intervention for precise preoperative diagnosis [15]. In this case, EUS could detect very small nodules in the pancreas during each preoperative evaluation; therefore, EUS is recommended to determine the appropriate surgical approach. The median interval from primary RCC to PM has been reported to be 83 months, and only 14.3% of patients with PM are symptomatic [8]. EUS is useful for detecting small tumors in the pancreas, and annual EUS is recommended over repeat contrast-enhanced CT to preserve the remnant renal function. Therefore, patients with RCC may require surveillance for a longer period than those with other cancers.
RCC is highly immunosensitive. The immunogenic nature of mRCC requires high-dose interleukin-2 to treat these patients, although response rates are poor and treatment-related side effects are toxic [16]. Subsequently, agents targeting the vascular endothelial growth factor and mammalian target of rapamycin (mTOR) pathways and immunotherapy with immune checkpoint inhibitors (ICIs) have become primary treatment options for patients with mRCC. Notably, ICIs and agents targeting mTOR have been found to have no impact on the prognosis of PM from RCC [16]. Patients with PM have a higher genomic instability index than those with metastases to other sites, which may explain their remarkable outcomes. Further randomized controlled studies on the use of anti-angiogenesis treatments for PM from RCC are required.
4. Conclusion
We performed remnant pancreatic resection for secondary metachronous PM from the RCC. Complete resection may improve the survival of patients with PM due to RCC.
Sources of funding
No funding body was involved in the design of the study; collection, analysis, and interpretation of data; and writing of the manuscript.
Ethical approval
All procedures used in this study were approved by the ethics committee of our institution.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Registration of research studies
This is not the ‘First in Man’ study.
Guarantor
Tomoyuki Abe
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
SI and TA conceived the presented idea, developed the theory, and performed the computations. AO, KH, MN, and TN and encouraged the investigation of a specific aspect and supervised the findings of this study. All authors have discussed the results and contributed to the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
None.
Contributor Information
Shingo Itamoto, Email: ittason.soccer2114@gmail.com.
Tomoyuki Abe, Email: t.abe.hiroshima@gmail.com.
Akihiko Oshita, Email: oshita-akihiko@umin.ac.jp.
Keiji Hanada, Email: kh-ajpbd@nifty.com.
Masahiro Nakahara, Email: masa.samurai@go7.enjoy.ne.jp.
Toshio Noriyuki, Email: nori0509@hotmail.co.jp.
References
- 1.Chow W.H., Devesa S.S., Warren J.L., Fraumeni J.F., Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281(17):1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Pantuck A.J., Zisman A., Belldegrun A.S. The changing natural history of renal cell carcinoma. J. Urol. 2001;166(5):1611–1623. [PubMed] [Google Scholar]
- 3.Alt A.L., Boorjian S.A., Lohse C.M., Costello B.A., Leibovich B.C., Blute M.L. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117(13):2873–2882. doi: 10.1002/cncr.25836. [DOI] [PubMed] [Google Scholar]
- 4.Santoni M., Conti A., Partelli S., Porta C., Sternberg C.N., Procopio G., et al. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann. Surg. Oncol. 2015;22(6):2094–2100. doi: 10.1245/s10434-014-4256-7. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S., Wolfgang C.L. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10(3):287–293. doi: 10.1016/S1470-2045(09)70065-8. [DOI] [PubMed] [Google Scholar]
- 6.Shin T.J., Song C., Jeong C.W., Kwak C., Seo S., Kang M., et al. Metastatic renal cell carcinoma to the pancreas: clinical features and treatment outcome. J. Surg. Oncol. 2021;123(1):204–213. doi: 10.1002/jso.26251. [DOI] [PubMed] [Google Scholar]
- 7.Hijioka S., Hifumi M., Mekky M.A., Takekuma Y., Kawaguchi T., Yokomizo H., et al. Total pancreatectomy for metastatic renal cell carcinoma with marked extension into the main pancreatic duct. Intern. Med. 2010;49(6):557–562. doi: 10.2169/internalmedicine.49.2943. [DOI] [PubMed] [Google Scholar]
- 8.Blanco-Fernández G., Fondevila-Campo C., Sanjuanbenito A., Fabregat-Prous J., Secanella-Medayo L., Rotellar-Sastre F., et al. Pancreatic metastases from renal cell carcinoma. Postoperative outcome after surgical treatment in a spanish multicenter study (PANMEKID) Eur. J. Surg. Oncol. 2022;48:133–141. doi: 10.1016/j.ejso.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., et al. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Grassi P., Doucet L., Giglione P., Grunwald V., Melichar B., Galli L., et al. Clinical impact of pancreatic metastases from renal cell carcinoma: a multicenter retrospective analysis. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0151662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Franco G., Gianardi D., Palmeri M., Furbetta N., Guadagni S., Bianchini M., et al. Pancreatic resections for metastases: a twenty-year experience from a tertiary care center. Eur. J. Surg. Oncol. 2020;46(5):825–831. doi: 10.1016/j.ejso.2019.11.514. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y.J., Lee J.H., Lee C.R., Han W.K., Kang C.M., Lee W.J. Laparoscopic total pancreatectomy for multiple metastasis of renal cell carcinoma of the pancreas: a case report and literature review. Ann Hepatobiliary Pancreat Surg. 2017;21(2):96–100. doi: 10.14701/ahbps.2017.21.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudani S., de Velasco G., Wells J.C., Gan C.L., Donskov F., Porta C., et al. Evaluation of clear cell, papillary, and chromophobe renal cell carcinoma metastasis sites and association with survival. JAMA Netw. Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballarin R., Spaggiari M., Cautero N., De Ruvo N., Montalti R., Longo C., et al. Pancreatic metastases from renal cell carcinoma: the state of the art. World J. Gastroenterol. 2011;17(43):4747–4756. doi: 10.3748/wjg.v17.i43.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pannala R., Hallberg-Wallace K.M., Smith A.L., Nassar A., Zhang J., Zarka M., et al. Endoscopic ultrasound-guided fine needle aspiration cytology of metastatic renal cell carcinoma to the pancreas: a multi-center experience. Cytojournal. 2016;13:24. doi: 10.4103/1742-6413.192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singla N., Xie Z., Zhang Z., Gao M., Yousuf Q., Onabolu O. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight. 2020;5(7) doi: 10.1172/jci.insight.134564. [DOI] [PMC free article] [PubMed] [Google Scholar]