Key Points
Bendamustine with rituximab is a highly effective frontline therapy in extranodal marginal zone lymphoma across all extranodal sites.
Bendamustine with rituximab overcomes known poor prognosis features in extranodal marginal zone lymphoma.
Visual Abstract
Abstract
Extranodal marginal zone lymphoma (EMZL) is a heterogeneous non-Hodgkin lymphoma. No consensus exists regarding the standard-of-care in patients with advanced-stage disease. Current recommendations are largely adapted from follicular lymphoma, for which bendamustine with rituximab (BR) is an established approach. We analyzed the safety and efficacy of frontline BR in EMZL using a large international consortium. We included 237 patients with a median age of 63 years (range, 21-85). Most patients presented with Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1 (n = 228; 96.2%), stage III/IV (n = 179; 75.5%), and intermediate (49.8%) or high (33.3%) Mucosa Associated Lymphoid Tissue International Prognosis Index (MALT-IPI). Patients received a median of 6 (range, 1-8) cycles of BR, and 20.3% (n = 48) received rituximab maintenance. Thirteen percent experienced infectious complications during BR therapy; herpes zoster (4%) was the most common. Overall response rate was 93.2% with 81% complete responses. Estimated 5-year progression-free survival (PFS) and overall survival (OS) were 80.5% (95% CI, 73.1% to 86%) and 89.6% (95% CI, 83.1% to 93.6%), respectively. MALT-IPI failed to predict outcomes. In the multivariable model, the presence of B symptoms was associated with shorter PFS. Rituximab maintenance was associated with longer PFS (hazard ratio = 0.16; 95% CI, 0.04-0.71; P = .016) but did not impact OS. BR is a highly effective upfront regimen in EMZL, providing durable remissions and overcoming known adverse prognosis factors. This regimen is associated with occurrence of herpes zoster; thus, prophylactic treatment may be considered.
Introduction
Extranodal marginal zone lymphoma (EMZL) of mucosa-associated lymphoid tissue is a rare disease representing 5% to 8% of all B-cell lymphomas.1,2 EMZL may arise at any mucosal site but most commonly involves the stomach (30%), followed by ocular adnexa (12%), skin (10%), lung (9%), and salivary gland (7%).3 EMZL is generally diagnosed at an early stage, remaining confined to the site of origin for a prolonged time.4,5 In patients with stage I EMZL of any location, including those with gastric involvement after failure of Helicobacter pylori eradication therapy, the preferred approach is radiation therapy.6-11 However, 20% to 40% of patients with EMZL present with advanced-stage disease that is associated with inferior outcomes.5,12 The treatment approach in patients with advanced-stage disease remains controversial, and there is lack of consensus for the choice of first-line therapy and number of treatment cycles in the absence of randomized clinical trial data.
Several agents have been tested in patients with untreated EMZL, including single-agent rituximab,13,14 lenalidomide with rituximab,15,16 R-CVP (rituximab, cyclophosphamide, vincristine, and prednisolone),17,18 R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone),19,20 and others. The International Extranodal Lymphoma Study Group (IELSG) 19 remains the only phase 3 study focusing on the frontline treatment of EMZL. This study randomized 454 patients to the combination of rituximab with chlorambucil, single-agent chlorambucil, or single-agent rituximab. The combination arm led to significantly longer 5-year event-free survival (EFS) (68%) compared with single-agent chlorambucil (51%) or rituximab (50%; P = .0009), without significant toxicity noted in any treatment arm.21 Although chlorambucil with rituximab had shown superior efficacy in a randomized study, this regimen has not gained wide acceptance in the United States, and novel therapies were subsequently developed.22
The National Comprehensive Cancer Network guidelines list bendamustine combined with rituximab (BR) as 1 of 3 preferred regimens in EMZL.22 However, data supporting this regimen in EMZL are limited. Two randomized clinical trials tested BR vs R-CHOP and/or R-CVP but largely included patients with follicular lymphoma.18,23 A relatively small number of patients with MZL was enrolled in these studies, and both clinical trials analyzed all MZL subtypes as a single entity. The MALT2008-01 is the largest study testing BR in patients with untreated EMZL. Following a response-adapted approach, 57 evaluable patients received BR with a complete response (CR) and unconfirmed CR (CRu) rate of 98%, associated with a 7-year EFS rate of 88%. No differences in response rate or survival by disease location or presence of t(11;18) were observed.24,25
Considering the small number of patients with MZL enrolled in clinical trials, larger observational studies are needed to provide more accurate data on safety and efficacy of BR in untreated EMZL. Therefore, we explored BR activity in EMZL as part of an international consortium created to address this question.
Methods
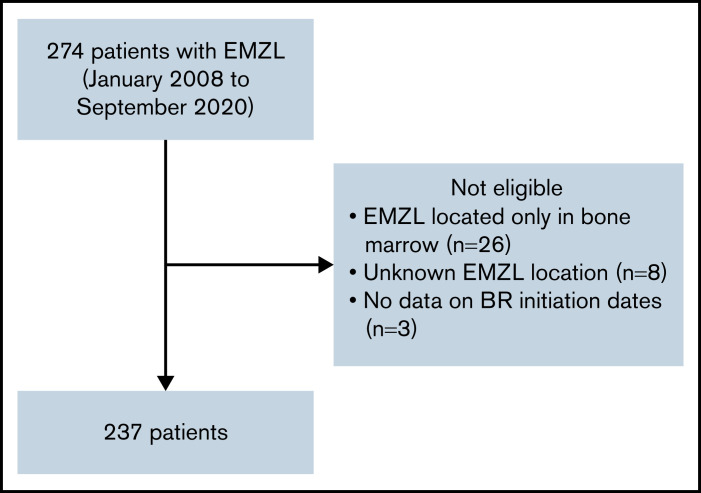
The consortium, comprised of 20 institutions from Italy and the United States, identified 274 adult patients with EMZL treated with frontline BR from January 2008 to September 2020. Patients with prior radiation therapy, surgery, or H. pylori eradication therapy were allowed. Prior rituximab or other systemic therapies were excluded. Patients with the following factors were excluded from the study: EMZL located only in bone marrow to avoid potential inclusion of cases with lymphoplasmacytic lymphoma, unknown EMZL location, and absence of data on BR initiation dates (Figure 1). A total of 237 patients were included in this analysis, including 182 patients with EMZL in whom pertinent data were collected retrospectively and 55 patients prospectively enrolled in the NF10 project from the Fondazione Italiana Linfomi treated with frontline BR from January 2008 to December 2019.26 The study was approved by the institutional review boards of all participating institutions and performed in accordance with the Declaration of Helsinki.
Figure 1.
CONSORT diagram.
Expert pathology review was performed at each participating institution using the World Health Organization classification without central pathology review.1 Staging evaluation and therapy for patients were completed at the discretion of treating physicians and following local institutional standards. Opportunistic infection prophylaxis was given according to institutional guidelines.
Investigators collected detailed demographic, clinicopathologic, laboratory, treatment, and outcome data using a standardized protocol. Demographic and clinical characteristics were summarized using descriptive statistics. Performance status (PS) was assigned according to the Eastern Cooperative Oncology Group (ECOG) scale. Serum lactate dehydrogenase (LDH) was considered elevated based on the institutional upper limit of normal. The mucosa-associated lymphoid tissue International Prognosis Index (MALT-IPI) was calculated as previously described.27 Patients with EMZL located in the skin, adipose tissue, fascia, and skeletal muscle were included in soft tissue extranodal site. Progression-free survival (PFS) was defined as the time from diagnosis to progression, relapse, death, or last follow-up, whichever occurred first. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Event-free patients were censored at the date of last follow-up. PFS and OS were estimated by Kaplan-Meier method and associations with prognostic factors assessed by log-rank test, univariable and multivariable Cox proportional-hazard regression models. Multivariable Cox model was constructed by selecting variables significant in the univariable analysis with a P value of <.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
In total, 237 patients with EMZL treated with frontline BR were identified. Median age at diagnosis was 63 years (range, 21-85) with a female to male ratio of 1.2:1. Prior history of autoimmune disease was present in 29 patients (12%), with Hashimoto’s thyroiditis and rheumatoid arthritis (each in 4 patients) constituting the most common diseases. Most patients had ECOG PS 0-1 (65% PS 0, 31.2% PS 1). Staging studies at baseline included computed tomography (CT) scan in 68 (28.7%) and positron emission tomography (PET)/CT in 169 patients (71.3%). One hundred seventy-nine (75.5%) patients had advanced-stage (III-IV) disease; however, elevated LDH (19%; n = 45) and B symptoms (17.7%; n = 42) were uncommon. Most patients had no bone marrow involvement (60.8%; n = 144) and had fewer than 4 nodal sites (79.7%; n = 189). Most patients had 1 extranodal site of disease (54.9%; n = 130), followed by 2 sites in 27.4% (n = 65) and 3 in 12.7% (n = 30). Common extranodal sites included lung (25.3%; n = 60) followed by stomach (17.3%; n = 41) and soft tissue (10.1%; n = 24). Only 2 patients had H. pylori-associated gastric EMZL (4.4%), and both had experienced failure of antibiotic therapy before receiving BR. The MALT-IPI risk was intermediate (1 risk factor) in 49.8% and high (≥2 risk factors) in 33.3% of patients (Table 1). Presence of paraprotein was examined in 160 (67.5%) patients and was present in 63 (39.4%). IgM was the most common paraprotein (40 patients: IgM κ: 24, IgM λ: 8, and unspecified: 8), followed by IgG (IgG λ and κ 5 cases each), free light chain (4 cases), 2 paraproteins (2 patients), and unknown (7 patients).
Table 1.
Baseline patient’s characteristics
| Variable | N | % |
|---|---|---|
| Age | ||
| <70 y | 176 | 74.3 |
| ≥70 y | 61 | 25.7 |
| Sex | ||
| Female | 128 | 54.0 |
| Male | 109 | 46.0 |
| Race/ethnicity | ||
| Non-Hispanic white | 192 | 81.0 |
| Hispanic white | 14 | 5.9 |
| African American | 18 | 7.6 |
| Other | 13 | 5.5 |
| Tumor stage | ||
| Stage I-II (early stage) | 58 | 24.5 |
| Stage III-IV (advanced stage) | 179 | 75.5 |
| ECOG performance status * | ||
| 0 | 154 | 65.0 |
| 1 | 74 | 31.2 |
| 2 | 9 | 3.8 |
| B symptoms * | ||
| Non-B symptoms | 195 | 82.3 |
| B symptoms | 42 | 17.7 |
| LDH * | ||
| Normal LDH | 192 | 81.0 |
| Elevated LDH | 45 | 19.0 |
| Number of extranodal site | ||
| 1 | 130 | 54.9 |
| ≥2 | 107 | 45.1 |
| Unknown | 1 | 0.4 |
| Bone marrow involvement | ||
| Negative | 144 | 60.8 |
| Positive | 64 | 27.0 |
| Unknown | 29 | 12.2 |
| MALT-IPI score | ||
| 0 | 40 | 16.9 |
| 1 | 118 | 49.8 |
| 2-3 | 79 | 33.3 |
Adjust study site as random effect. NE, not estimable.
ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; MALT-IPI, Mucosa associated lymphoid tissue International Prognostic Index.
Missing values included in normal/no symptoms categories.
Treatment characteristics
The median time from diagnosis to BR therapy was 56 (interquartile range 31-98) (95% CI, 49-64) days with a median of 6 cycles (range, 1-8) in persons with available data (n = 181). Rituximab maintenance was administered in 48 patients (20.3%) with a median duration of 16 months. Nine (3.8%) patients received consolidation with involved site radiation therapy. Treatment response was evaluated with PET/CT in 140 patients (59%) and with CT scans in the rest. The overall response rate (ORR) to frontline BR was 93.2% with a CR rate of 81% and partial response (PR) rate of 12.2%. In patients assessed for response by PET/CT, the ORR was 96.5% with CR of 82.9%. In patients assessed for response by CT, the ORR was 92.5% with CR of 81.7%. Patients with localized disease (stage I-II) experienced higher rates of CR compared with those with advanced stage (III-IV) (93% vs 79%; P = .016, respectively).
The incidence of infectious complications during BR therapy was 13% among 180 patients with data. Among those patients, we observed 7 cases of herpes zoster reactivation (4%), 5 cases of pneumonia (3%), and influenza (3%). In patients treated with rituximab maintenance, 3 patients experienced neutropenia (6.2%) and we observed 1 case of herpes zoster reactivation (2%) and facial cellulitis (2%). Eight patients (3.4%) developed secondary malignancies after BR treatment, including one case each of acute myeloid leukemia and squamous cell carcinoma of the skin.
Survival analysis
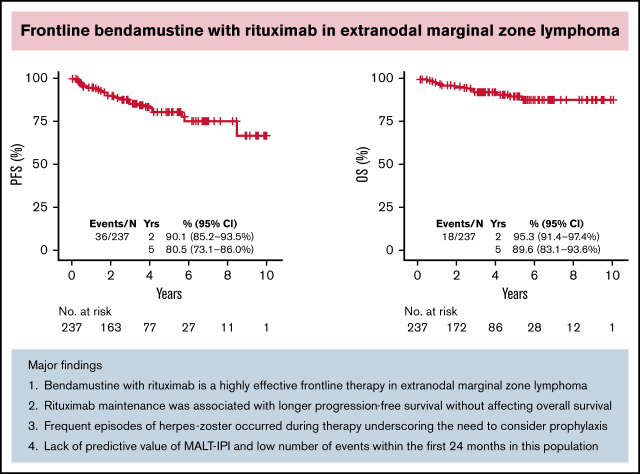
Thirty-six patients had progression of disease and 18 died. With a median follow-up of 3.21 years (range, 0-10.75), the estimated 5-year PFS and OS were 80.5% (95% CI, 73.1% to 86.0%) and 89.6% (95% CI, 83.1% to 93.6%), respectively (Figure 2A-B). The 5-year cumulative incidence of lymphoma-specific death was 3.8% (95% CI, 1.6% to 7.7%) with only 8 deaths attributable to lymphoma. The causes of death in the other 10 patients were head trauma, lung cancer, liver cirrhosis/hepatocellular carcinoma, respiratory failure, bladder cancer, acute myeloid leukemia, heart arrhythmia, and amyotrophic lateral sclerosis, each in 1 patient and unknown in 2. No difference in PFS was observed among patients achieving CR or PR after BR (hazard ration [HR]: 0.47; 95% CI, 0.18-1.27; P = .12). We did not observe better PFS by number of BR cycles (P = .222). However, we observed shorter OS in patients treated with 1 to 3 cycles (P = .009; data not shown). Patients treated with rituximab maintenance after achieving CR or PR following BR treatment exhibited longer PFS (5-year rate 94.4% vs 81.1%; P = .0121) with no difference in OS (P = .168) (Figure 2C-D). We did not observe differences in PFS or OS comparing gastric vs other EMZL locations (PFS P = .885 and OS P = .486) (Figure 2E-F). Interestingly, MALT-IPI score did not predict PFS and OS (Figure 2G-H). We were not able to estimate the effect of progression of disease within 24 months (POD24) on outcomes in this cohort due to few early events (n = 21).
Figure 2.
Survival of EMZL patient treated with BR. PFS (A) and OS (B) in patients with extranodal marginal zone lymphoma treated with bendamustine and rituximab; by rituximab maintenance (C-D); by disease location (E-F); and by MALT-IPI risk group (G-H).
Variables associated with shorter PFS in univariable Cox regression analysis were age ≥70 years (HR = 2.07; 95% CI, 1.03-4.14; P = .040), B symptoms (HR = 2.56; 95% CI, 1.25-5.24; P = .01), and not attaining a CR (HR = 5.31; 95% CI, 2.63-10.73; P < .001), while rituximab maintenance (HR = 0.18; 95% CI, 0.04-0.79; P = .023) was associated with longer PFS. The variables associated with shorter OS were not attaining CR (HR = 4.67; 95% CI, 1.71-12.79; P = .003) and treatment with 1 to 3 cycles of BR (HR = 5.19; 95% CI, 1.60-16.90; P = .006) (Table 2). In a multivariable Cox model, presence of B symptoms and absence of rituximab maintenance were significantly associated with shorter PFS; no variables were associated with OS, likely due to a small number of events (Table 3).
Table 2.
UVA Cox models for PFS and OS
| Variable | Category | PFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | <70 y | Reference | Reference | ||
| ≥70 y | 2.07 (1.03, 4.14) | .040 | 1.74 (0.64, 4.71) | .279 | |
| Sex | Female | Reference | Reference | ||
| Male | 0.67 (0.34, 1.33) | .258 | 0.83 (0.32, 2.18) | .712 | |
| Race/ethnicity | Non-Hispanic white | Reference | Reference | ||
| Hispanic white | 1.22 (0.36, 4.13) | .746 | 1.46 (0.29, 7.49) | .648 | |
| African American | 0.32 (0.04, 2.35) | .261 | NE | ||
| Other | 1.19 (0.28, 5.01) | .814 | 1.15 (0.15, 8.91) | .893 | |
| Tumor stage | I-II | Reference | Reference | ||
| III-IV | 1.31 (0.59, 2.88) | .503 | 0.98 (0.35, 2.78) | .970 | |
| B symptoms | No B symptoms | Reference | Reference | ||
| B symptoms | 2.56 (1.25, 5.24) | .010 | 2.35 (0.82, 6.78) | .113 | |
| LDH | Normal LDH | Reference | Reference | ||
| Elevated LDH | 1.10 (0.48, 2.52) | .821 | 1.28 (0.41, 3.98) | .665 | |
| Number of nodal sites | <4 | Reference | Reference | ||
| ≥4 | 1.03 (0.44, 2.38) | .948 | 2.05 (0.69, 6.10) | .197 | |
| Bone marrow involvement | Negative | Reference | Reference | ||
| Positive | 0.57 (0.23, 1.41) | .227 | 0.40 (0.09, 1.80) | .232 | |
| MALT-IPI | 0 | Reference | Reference | ||
| 1 | 1.83 (0.62, 5.43) | .274 | 5.01 (0.65, 38.81) | .123 | |
| 2-3 | 2.36 (0.77, 7.21) | .132 | 3.28 (0.38, 28.34) | .281 | |
| Rituximab maintenance | No | Reference | Reference | ||
| Yes | 0.18 (0.04, 0.79) | .023 | 0.24 (0.03, 1.95) | .184 | |
| Clinical response | Complete response | Reference | Reference | ||
| Noncomplete response | 5.31 (2.63, 10.73) | <.001 | 4.67 (1.71, 12.79) | .003 | |
| Cycles of bendamustine and rituximab | ≥6 | Reference | Reference | ||
| 4-5 | 1.63 (0.69, 3.84) | .268 | 2.24 (0.69, 7.29) | .180 | |
| 1-3 | 2.25 (0.77, 6.56) | .139 | 5.19 (1.60, 16.90) | .006 | |
Table 3.
Multivariable Cox models for PFS and OS
| Variable | Category | PFS (36 events) | OS (18 events) | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| B symptoms | No B symptoms | Reference | Reference | ||
| B symptoms | 2.56 (1.18, 5.52) | .017 | 2.15 (0.69, 6.67) | .184 | |
| MALT-IPI scores | 0 | Reference | Reference | ||
| 1 | 1.68 (0.54, 5.20) | .371 | 3.91 (0.48, 31.99) | .203 | |
| 2-3 | 1.79 (0.55, 5.81) | .333 | 1.80 (0.19, 16.76) | .607 | |
| Rituximab maintenance | No | Reference | Reference | ||
| Yes | 0.15 (0.03, 0.65) | .012 | 0.17 (0.02, 1.33) | .091 | |
| Clinical response | Complete response | Reference | Reference | ||
| Noncomplete response | 2.00 (0.73, 5.52) | .180 | 0.52 (0.05, 5.01) | .569 | |
| Cycles of bendamustine and rituximab | ≥6 | Not included | Reference | ||
| 4-5 | 2.56 (0.75, 8.79) | .134 | |||
| 1-3 | 2.84 (0.60, 13.39) | .186 | |||
Higher grade transformation.
During the follow-up period, 11 patients (4.6%) developed biopsy-confirmed higher-grade transformation (HGT) to diffuse large B-cell lymphoma, with most demonstrating a nongerminal center phenotype (55.6%). The cumulative incidence of HGT at 5 and 10 years with death as competing risk was 6% (95% CI, 2.9% to 10.8%) and 9.1% (95% CI, 3.6% to 17.8%), respectively (Figure 3). The median time to HGT was 0.74 (range, 0.37-5.79) years. Patients experiencing HGT had higher median standardized uptake values on diagnostic PET/CT than patients who did not experience transformation (18 vs 6.7; P = .016, respectively). The median number of BR cycles in those patients with subsequent HGT was 6 (range, 3-6). Only 1 (9%) patient treated with rituximab maintenance experienced HGT. Treatments for HGT included an anthracycline-based regimen with R-CHOP in 7 patients and dose-adjusted infusional etoposide, doxorubicin, vincristine, prednisone, cyclophosphamide, and rituximab (DA-EPOCH-R) regimen in 3 patients; 1 patient opted for hospice. Three patients underwent consolidation with autologous stem cell transplant, with 2 patients (66.7%) alive at last follow-up. In 7 patients not treated with autologous stem cell transplant, 4 (57%) remain alive with a median follow-up of 2.76 years (95% CI, 0.91-5.95) by reverse Kaplan-Meier method.
Figure 3.
Kaplan-Meier curve for cumulative incidence of HGT with dead as competing risk.
Discussion
To our knowledge, this study represents the largest efficacy analysis of BR in untreated EMZL. The main findings of our study are as follows: (1) BR is a highly effective frontline therapy in EMZL, with an ORR of 93.2% and CR rate of 81%, taking into account disease across all extranodal sites; (2) rituximab maintenance was associated with longer PFS without affecting OS; (3) frequent infectious complications, including herpes zoster reactivation, pneumonia, and influenza, were noted with BR, which have not previously been reported in MZL clinical trials; (4) MALT-IPI lacked predictive value; and (5) few progression and death events occurred within the first 24 months after BR treatment.
Two randomized clinical trials have previously tested BR in MZL. The Study Group for Indolent Lymphomas (StiL) demonstrated significantly longer PFS in a phase 3 noninferiority study in which BR was compared with R-CHOP in indolent and mantle cell lymphomas. However, median PFS was not significantly different among 37 patients with MZL treated with BR compared with 30 patients treated with R-CHOP (57.2 vs 47.2 months; P = .32, respectively).23 The BRIGHT study randomized patients to BR vs R-CHOP or R-CVP. In this study, 46 patients with MZL (BR arm: 28 and R-CHOP/R-CVP arm: 18) were included, achieving an ORR of 92% with a CR rate of 20%; however, the CR rate ratio in indolent non-Hodgkin lymphoma group was not significantly better for BR compared with R-CHOP/R-CVP (rate ratio = 1.16; P = .129).18
Our results are consistent with the excellent responses observed by Salar et al in the MALT2008-01 trial, although we observed lower CR rates (81% compared with 98%).24 Contrary to our study, the MALT2008-01 cohort included 66% of treated patients with limited-stage disease while 75.5% of our patients had advanced-stage disease. In MALT2008-01, patients received BR in a response-adapted approach for which response was evaluated after 3 cycles and those achieving CR received a total of 4 cycles, whereas patients achieving PR received a total of 6 cycles. After 3 cycles, 75% (n = 43) of the patients achieved CR/CRu, with only 14 patients requiring completion of 6 cycles. Overall, the CR/CRu rate was 98%, with median duration of response not reached.24 Whether this approach can be applied with similar efficacy to patients with advanced-stage disease needs further evaluation, because in our retrospective analysis, CR rate was lower and most patients received 6 cycles of BR. In MALT2008-01,24 patients achieving CR after the initial 3 cycles were mainly those with gastric and multifocal site involvement compared with those with nongastric locations (P = .0226). In contrast, we observed similar responses and survival in patients with gastric and nongastric EMZL. However, in the IELSG-19 study, patients with gastric EMZL demonstrated better 5-year EFS (62% vs 53%; P = .024), 5-year PFS (71% vs 57%; P = .001) and CR rates (72% vs 61%; P = .015) compared with nongastric locations.21 Therefore, it is possible that BR may overcome poorer results obtained in patients with nongastric EMZL.
The timing of treatment initiation for advanced-stage EMZL remains controversial, with current guidelines recommending therapy initiation based on Groupe d’Etude des Lymphomes Folliculaire (GELF) criteria.22 However, these criteria were designed for follicular lymphoma and extrapolated, without confirmation, to patients with EMZL. Whether GELF criteria are appropriate to determine therapy initiation in EMZL remains unclear and unanswerable with this dataset. Single-agent rituximab demonstrated clinical activity in a study including 34 patients with untreated and relapsed EMZL. In chemotherapy-naïve patients (n = 23), rituximab achieved an ORR of 87% with CR rate of 48% and median time to treatment failure of 22 months.14 Nevertheless, subsequent studies demonstrated moderate activity of single-agent rituximab with lower rates of responses and early relapses in patients not achieving CR.13,28,29 Our results support the selection of BR in patients with advanced-stage EMZL requiring therapy initiation, leading to excellent results with prolonged remissions.
Evidence supporting rituximab maintenance in EMZL is limited without prior data after frontline BR. The ECOG E4402 study tested maintenance rituximab vs retreatment in asymptomatic and low tumor burden patients with indolent lymphoma who had responded to rituximab.30 This study included 71 patients with MZL (29 with EMZL), most of whom achieved stable disease after rituximab induction (ORR: 39.5% and CR rate: 15.8%). The maintenance arm achieved longer time to rituximab failure compared with retreatment arm (5-year treatment failure-free 45% vs 20%; P = .012) in patients with small lymphocytic lymphoma and MZL without specific data on EMZL. The only study testing rituximab maintenance after BR induction is the StiL NHL7-2008 MAINTAIN trial presented so far in an abstract form.31 In this study, 119 patients with nodal and splenic MZL were randomized to rituximab maintenance vs observation after response to BR with a significant PFS benefit in the maintenance arm (median PFS not reached vs 92.2 months, HR = 0.35; 95% CI, 0.17-0.76; P = .008) although patients with EMZL were excluded from this study.31 Similarly, a preliminary report from the IELSG group examined the role of subcutaneous rituximab maintenance following frontline therapy with rituximab and chlorambucil in patients with EMZL achieving CR, PR, or stable disease.32 The CR rate at the end of induction in the intent-to-treat population (n = 112) was 53% (gastric 69% and nongastric 45%). After 1 year of maintenance, 70 evaluable patients demonstrated a CR rate of 69%, PR: 26%, stable disease 2.5%, and progressive disease 2.5%. Interestingly, among the 30 patients who achieved PR at the end of induction, 30% (n = 9) had an improved response of CR after 1 year of maintenance rituximab. As with follicular lymphoma,33,34 our data demonstrated longer PFS in EMZL with the use of rituximab after frontline BR without impacting the OS. However, several caveats need to be considered when interpreting these data: a short median follow-up (3 years) mainly censoring early events, underscoring the need for longer follow-up to capture late events; small number of patients (n = 48) selected for rituximab maintenance; and, more importantly, the absence of randomization regarding which patients received rituximab maintenance. Furthermore, the concerning number of fatal events observed in the GALLIUM study with anti-CD20 maintenance after a bendamustine-containing regimen suggests the importance of testing the safety and efficacy of rituximab maintenance in a randomized clinical trial.35
Immunosuppression characterized by CD4+ lymphopenia is common after bendamustine administration, usually lasting for 7 to 9 months upon completion of therapy.20,36 CD4+ lymphopenia is associated with cumulative bendamustine dose and is independent of absolute lymphocyte recovery.37 A systematic review and meta-analysis of randomized controlled trials (n = 2620 patients) demonstrated no effect of bendamustine on the rate of infections (relative risk: 1.09) compared with alkylating agents or fludarabine.38 Similarly, evaluating Medicare beneficiaries treated with BR for B-cell lymphomas, Olszewski et al observed only a slight absolute increase in infections and pneumonia risks (0.9 per 100 person-months and 0.6, respectively) during the second year after BR compared with R-CHOP and R-CVP.39 A retrospective analysis in 82 patients with chronic lymphocytic leukemia treated with frontline BR suggested the need for herpes zoster prophylaxis beyond 8 months.40 We observed infections associated with herpes zoster during therapy with BR in 4% of patients, an observation previously not described in clinical trials.18,23-25 Thus, our data suggest integrating herpes zoster vaccination prior to starting therapy or prophylaxis during, and probably beyond, BR treatment. Furthermore, because myelosuppression correlates with cumulative doses of bendamustine, a response-adapted approach proposed by Salar et al25 may decrease the incidence of infections warranting evaluation in randomized clinical studies. Similar to prior reports evaluating extended adverse events in patients treated with bendamustine,41,42 we observed no excess of secondary malignancies associated with BR.31,43 However, higher incidence of nonmelanoma skin cancer was reported in the BR arm in the 5-year follow-up of BRIGHT study, highlighting the need for regular skin cancer screening upon therapy completion.43
The MALT-IPI based on age ≥70 years, elevated LDH, and Ann Arbor stage III/IV is a valuable stratification tool to identify patients at risk of shorter survival.27 Further, 3 independent cohorts demonstrated shorter survival in patients with EMZL experiencing POD24 from diagnosis.5,44,45 Prior studies established the failure to achieve CR after frontline therapy as an important prognostic factor in EMZL.5,46 In the cohort reported herein, we observed a high CR rate; consequently, only a few events occurred in the first 24 months after BR therapy, likely contributing to the findings that MALT-IPI did not predict survival. The high efficacy of BR might overcome known poor prognosis features and will need to be verified in future studies.
Inherent to all retrospective analyses, our study is limited by the lack of data on indications for treatment selection, initiation, bendamustine dosage, and treatment length. Advanced stage disease and good ECOG PS were frequently observed in our cohort, and these features may have influenced BR selection over other regimens. We did not have enough data to analyze the effect of t(11;18) in this population, but it does not seem to affect response to BR based on previously published reports.24
In summary, BR is a highly effective regimen in upfront treatment of EMZL, capable of inducing durable remissions and possibly overcoming known adverse prognosis factors. We identified presence of B symptoms as the only factor associated with worse outcome in patients treated with BR. In contrast, rituximab maintenance was associated with longer PFS but no impact on OS, underscoring the need to confirm these results in a randomized study before integrating this approach into clinical practice. Importantly, we observed frequent infections attributed to herpes zoster, highlighting the need to consider prophylaxis during BR treatment. Overall, BR is an excellent regimen for upfront treatment of patients with EMZL with advanced-stage disease requiring treatment.
Authorship
Contribution: J.P.A. and I.S.L. designed and performed the research, analyzed data, and wrote the paper; and L.A., M.P.W., A.W.B., G.S., N.E., M.S., A.S., J.S.-S., P.T., A.B.A., A.J.O., S.-H.K., B.H., S.G., S.A., J.J.C., L.A., T.J.V., R.S., S.M.C., F.V., I.M.R., D.K., J.S.A., W.Z., D.E., P.M., E.C., M.K., B.L., C.N.L., A.F.H., J.W.F., B.S.K., S.L., and P.L.Z. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.P.A. received honoraria from OncLive and Oncinfo and served on the advisory board and received research support of ADC Therapeutics. An immediate family member has served on the advisory boards of Puma Biotechnology, Inovio Pharmaceuticals, Agios Pharmaceuticals, Forma Therapeutics, and Foundation Medicine. L.A. reported research funding from Gilead Sciences, served as consultant for Bayer, Celgene, Roche, Gilead Sciences, Roche, Sandoz, Janssen-Cilag, Verastem, and ADC Therapeutics, was on the speaker bureau for Novartis, and received travel expenses from Roche, Celgene, Janssen-Cilag, and EUSA Pharma. G.S. received honoraria from Beigene and Kite Pharma. N.E. received honoraria from Genzyme and served on the advisory board for Verastem, Beigene, and Karyopharm. A.S. received honoraria from OncLive and Curio Science and served on the advisory board for Kite Pharma. J.S.-S. served on advisory boards for SeaGen, Janssen, MassiveBio, TG Therapeutics, Incyte, and BMS. P.T. served on the advisory board for TG Therapeutics. A.J.O. received research funding from Celldex Therapeutics, PrecisionBio, TG Therapeutics, Acrotech Pharma, Genentech, and Genmab. B.H. received honoraria from BMS and ADC Therapeutics. S.G. served as a consultant for Beigene, TG Therapeutics, Epizyme, and ADC Therapeutics, has received research funding from Adaptive Biotechnologies, and was on the speaker bureau for ADC Therapeutics and TG Therapeutics. S.A. served as a consultant for Fate Therapeutics and served on the advisory board for AstraZeneca, Beigene, TG Therapeutics, and Seagen. J.J.C. received honoraria from AbbVie, Beigene, Pharmacyclics, Janssen, Roche, and TG Therapeutics. T.J.V. received research funding from AstraZeneca. P.M. received honoraria from ADC Therapeutics. M.K. served as consultant for Celgene, Adaptive, Biotechnologies, ADC Therapeutics, AstraZeneca, Genentech, Kite, KaryoPharm, AbbVie, Genetech, TG Therapeutics, and SeaGen. B.L. served as consultant for Genentech/Roche, MEI, Novartis and received research funding from Novartis and Jannsen. A.F.H. received research funding from Kite, a Gilead Company, ADC Therapeutics, Tubulis, Bristol, Genentech, and AstraZeneca and was a consultant for Seagen, Bristol Myers Squibb, Merck, and Karyopharm. J.W.F. served on advisory boards for Acerta, Bayer, and Novartis. B.S.K. served as consultant for AbbVie, Adaptive, ADC Therapeutics, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Genentech, Incyte, Janssen, Karyopharm, Kite, MEI, Pharmacyclics, Roche, TG Therapeutics, and Teva and has received research funding from AbbVie, Acerta, ADC Therapeutics, AstraZeneca, Beigene and Genetech. S.L. served on advisory boards for Celgene, Janssen, Gilead, Regeneron, Genelab SRL, and Roche. P.L.Z. served on advisory boards for Secura Bio, Sandoz, and ADC Therapeutics, served on advisory boards and speakers bureau for Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG Therapeutics, Takeda, Roche, EUSA Pharma, Kyowa Kirin, Novartis, Incyte, and Beigene, and was a consultant for EUSA Pharma, Novartis, and MSD. I.S.L. served on the advisory boards of Seattle Genetics, Janssen Scientific, and Verastem. The remaining authors declare no competing financial interests.
The current affiliation for D.E. is Division of Hematology and Medical Oncology, University of Rochester Medical Center, Rochester, NY.
Correspondence: Izidore S. Lossos, Division of Hematology, Department of Medicine, University of Miami Miller School of Medicine, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: ilossos@med.miami.edu.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed., revised. Lyon, France: IARC Press; 2017. [Google Scholar]
- 2.Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119(3):629-638. [DOI] [PubMed] [Google Scholar]
- 3.Cerhan JR, Habermann TM. Epidemiology of marginal zone lymphoma. Ann Lymphoma. 2021;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starr AG, Caimi PF, Fu P, et al. Dual institution experience of extranodal marginal zone lymphoma reveals excellent long-term outcomes. Br J Haematol. 2016;173(3):404-412. [DOI] [PubMed] [Google Scholar]
- 5.Alderuccio JP, Zhao W, Desai A, et al. Short survival and frequent transformation in extranodal marginal zone lymphoma with multiple mucosal sites presentation. Am J Hematol. 2019;94(5):585-596. [DOI] [PubMed] [Google Scholar]
- 6.Desai A, Joag MG, Lekakis L, et al. Long-term course of patients with primary ocular adnexal MALT lymphoma: a large single-institution cohort study. Blood. 2017;129(3):324-332. [DOI] [PubMed] [Google Scholar]
- 7.Olszewski AJ, Desai A. Radiation therapy administration and survival in stage I/II extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Int J Radiat Oncol Biol Phys. 2014;88(3):642-649. [DOI] [PubMed] [Google Scholar]
- 8.Teckie S, Qi S, Chelius M, et al. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann Oncol. 2017;28(5):1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderuccio JP, Florindez JA, Reis IM, Zhao W, Lossos IS. Treatments and outcomes in stage I extranodal marginal zone lymphoma in the United States. Cancers (Basel). 2021;13(8):1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling DC, Vargo JA, Balasubramani GK, Beriwal S. Underutilization of radiation therapy in early-stage marginal zone lymphoma negatively impacts overall survival. Pract Radiat Oncol. 2016;6(4):e97-e105. [DOI] [PubMed] [Google Scholar]
- 11.Yahalom J, Xu AJ, Noy A, et al. Involved-site radiotherapy for Helicobacter pylori-independent gastric MALT lymphoma: 26 years of experience with 178 patients. Blood Adv. 2021;5(7):1830-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer JP, Hiddink RF, Raderer M, et al. Dissemination patterns in non-gastric MALT lymphoma. Haematologica. 2008;93(2):201-206. [DOI] [PubMed] [Google Scholar]
- 13.Lossos IS, Morgensztern D, Blaya M, Alencar A, Pereira D, Rosenblatt J. Rituximab for treatment of chemoimmunotherapy naive marginal zone lymphoma. Leuk Lymphoma. 2009;48(8):1630-1632. [DOI] [PubMed] [Google Scholar]
- 14.Conconi A, Martinelli G, Thiéblemont C, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003;102(8):2741-2745. [DOI] [PubMed] [Google Scholar]
- 15.Becnel MR, Nastoupil LJ, Samaniego F, et al. Lenalidomide plus rituximab (R2 ) in previously untreated marginal zone lymphoma: subgroup analysis and long-term follow-up of an open-label phase 2 trial. Br J Haematol. 2019;185(5):874-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiesewetter B, Willenbacher E, Willenbacher W, et al. ; AGMT Investigators . A phase 2 study of rituximab plus lenalidomide for mucosa-associated lymphoid tissue lymphoma. Blood. 2017;129(3):383-385. [DOI] [PubMed] [Google Scholar]
- 17.Oh SY, Kim WS, Kim JS, et al. Phase II study of R-CVP followed by rituximab maintenance therapy for patients with advanced marginal zone lymphoma: consortium for improving survival of lymphoma (CISL) study. Cancer Commun (Lond). 2019;39(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walewski J, Paszkiewicz-Kozik E, Michalski W, et al. First-line R-CVP versus R-CHOP induction immunochemotherapy for indolent lymphoma with rituximab maintenance: a multicentre, phase III randomized study by the Polish Lymphoma Research Group PLRG4. Br J Haematol. 2020;188(6):898-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rummel MJ, Al-Batran SE, Kim S-Z, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(15):3383-3389. [DOI] [PubMed] [Google Scholar]
- 21.Zucca E, Conconi A, Martinelli G, et al. Final results of the IELSG-19 randomized trial of mucosa-associated lymphoid tissue lymphoma: improved event-free and progression-free survival with rituximab plus chlorambucil versus either chlorambucil or rituximab monotherapy. J Clin Oncol. 2017;35(17):1905-1912. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. B-cell lymphomas (version 5.2021). https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed 1 October 2021.
- 23.Rummel MJ, Niederle N, Maschmeyer G, et al. ; Study group indolent Lymphomas (StiL) . Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210. [DOI] [PubMed] [Google Scholar]
- 24.Salar A, Domingo-Domenech E, Panizo C, et al. ; Grupo Español de Linfomas/Trasplante de Médula Ósea (GELTAMO) . First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma (MALT2008-01): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2014;1(3):e104-e111. [DOI] [PubMed] [Google Scholar]
- 25.Salar A, Domingo-Domenech E, Panizo C, et al. Long-term results of a phase 2 study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma. Blood. 2017;130(15):1772-1774. [DOI] [PubMed] [Google Scholar]
- 26.Luminari S, Goldaniga MC, Cesaretti M, et al. Pattern of care in indolent non follicular lymphoma: a report from NF10 Project, an international, prospective, observational study coordinated by the Fondazione Italiana Linfomi. Blood. 2015;126(23):2686.26472751 [Google Scholar]
- 27.Thieblemont C, Cascione L, Conconi A, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409-1417. [DOI] [PubMed] [Google Scholar]
- 28.Raderer M, Jäger G, Brugger S, et al. Rituximab for treatment of advanced extranodal marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue lymphoma. Oncology. 2003;65(4):306-310. [DOI] [PubMed] [Google Scholar]
- 29.Ferreri AJ, Ponzoni M, Martinelli G, et al. Rituximab in patients with mucosal-associated lymphoid tissue-type lymphoma of the ocular adnexa. Haematologica. 2005;90(11):1578-1579. [PubMed] [Google Scholar]
- 30.Williams ME, Hong F, Gascoyne RD, et al. Rituximab extended schedule or retreatment trial for low tumour burden non-follicular indolent B-cell non-Hodgkin lymphomas: Eastern Cooperative Oncology Group Protocol E4402. Br J Haematol. 2016;173(6):867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rummel MJ, Koenigsmann M, Chow KU, et al. Two years rituximab maintenance vs. observation after first line treatment with bendamustine plus rituximab (B-R) in patients with marginal zone lymphoma (MZL): results of a prospective, randomized, multicenter phase 2 study (the StiL NHL7-2008 MAINTAIN trial). J Clin Oncol. 2018;36(suppl 15):7515. [Google Scholar]
- 32.Stathis A, Gregorini A, Gressin R, et al. IELSG-38: a phase II study of chlorambucil in combination with rituximab followed by maintenance therapy with subcutaneous rituximab in patients with extranodal marginal zone b-cell lymphoma of mucosa associated lymphoid tissue (MALT). Blood. 2017;130(suppl 1):1506. [Google Scholar]
- 33.Hill BT, Nastoupil L, Winter AM, et al. Maintenance rituximab or observation after frontline treatment with bendamustine-rituximab for follicular lymphoma. Br J Haematol. 2019;184(4):524-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahl BS, Burke JM, van der Jagt R, et al. Assessment of maintenance rituximab after first-line bendamustine-rituximab in patients with follicular lymphoma: an analysis from the BRIGHT Trial. Blood. 2017;130(suppl 1):484. [Google Scholar]
- 35.Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM Study: influence of chemotherapy on efficacy and safety. J Clin Oncol. 2018;36(23):2395-2404. [DOI] [PubMed] [Google Scholar]
- 36.Saito H, Maruyama D, Maeshima AM, et al. Prolonged lymphocytopenia after bendamustine therapy in patients with relapsed or refractory indolent B-cell and mantle cell lymphoma. Blood Cancer J. 2015;5(10):e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez-Calle N, Hartley S, Ahearne M, et al. Kinetics of T-cell subset reconstitution following treatment with bendamustine and rituximab for low-grade lymphoproliferative disease: a population-based analysis. Br J Haematol. 2019;184(6):957-968. [DOI] [PubMed] [Google Scholar]
- 38.Gafter-Gvili A, Gurion R, Raanani P, Shpilberg O, Vidal L. Bendamustine-associated infections: systematic review and meta-analysis of randomized controlled trials. Hematol Oncol. 2017;35(4):424-431. [DOI] [PubMed] [Google Scholar]
- 39.Olszewski AJ, Reagan JL, Castillo JJ. Late infections and secondary malignancies after bendamustine/rituximab or RCHOP/RCVP chemotherapy for B-cell lymphomas. Am J Hematol. 2018;93(1):E1-E3. [DOI] [PubMed] [Google Scholar]
- 40.Allen JM, Rybicki LA, Caimi PF, et al. Standard duration of varicella zoster virus prophylaxis may be inadequate to prevent viral reactivation in patients with chronic lymphocytic leukemia treated with front-line bendamustine plus rituximab. Blood. 2015;126(23):4167. [Google Scholar]
- 41.Olszewski AJ, Butera JN, Reagan JL, Castillo JJ. Outcomes of bendamustine- or cyclophosphamide-based first-line chemotherapy in older patients with indolent B-cell lymphoma. Am J Hematol. 2020;95(4):354-361. [DOI] [PubMed] [Google Scholar]
- 42.Penne M, Sarraf Yazdy M, Nair KS, Cheson BD. Extended follow-up of patients treated with bendamustine for lymphoid malignancies. Clin Lymphoma Myeloma Leuk. 2017;17(10):637-644. [DOI] [PubMed] [Google Scholar]
- 43.Flinn IW, van der Jagt R, Kahl B, et al. First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol. 2019;37(12):984-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conconi A, Thieblemont C, Cascione L, et al. Early progression of disease predicts shorter survival in MALT lymphoma patients receiving systemic treatment. Haematologica. 2020;105(11):2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luminari S, Merli M, Rattotti S, et al. Early progression as a predictor of survival in marginal zone lymphomas: an analysis from the FIL-NF10 study. Blood. 2019;134(10):798-801. [DOI] [PubMed] [Google Scholar]
- 46.Alderuccio JP, Zhao W, Desai A, et al. Risk factors for transformation to higher-grade lymphoma and its impact on survival in a large cohort of patients with marginal zone lymphoma from a single institution. J Clin Oncol. 2018;36(34):JCO1800138. [DOI] [PubMed] [Google Scholar]