Key Points
Clonal hematopoiesis is present in at least 14% of patients with WM.
Patients with CH are more likely to progress from IgM MGUS or smoldering WM to symptomatic WM.
Abstract
Clonal hematopoiesis (CH) is associated with adverse outcomes in patients with non-Hodgkin lymphoma (NHL) and multiple myeloma undergoing autologous stem cell transplantation. Still, its implications for patients with indolent NHL have not been well studied. We report the prevalence of CH in patients with Waldenström macroglobulinemia (WM) and its association with clinical outcomes. To unambiguously differentiate CH mutations from those in the WM clone, CH was defined by the presence of somatic mutations in DNMT3A, TET2, or ASXL1 (DTA) and was detected in 14% of 587 patients with IgM monoclonal gammopathy of undetermined significance (MGUS), smoldering WM (SWM) or WM. The presence and size of DTA clones were associated with older age. Patients with CH had an increased risk of progression from MGUS or SWM to WM, but not worse overall survival in this cohort. These findings further illuminate the clinical effects of CH in patients with indolent NHL such as WM.
Introduction
Clonal hematopoiesis (CH), a phenomenon in which somatic mutations in hematopoietic stem cells leads to clonal expansion, has been associated with a variety of adverse outcomes, including increased risk of developing hematologic neoplasms, such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), and cardiovascular disease.1,2 CH has also been associated with decreased overall survival (OS) in patients with non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) who undergo autologous stem cell transplantation.3,4 Whether CH is associated with similar adverse outcomes in patients with indolent NHL and outside the context of stem cell transplantation is unknown.
Waldenström macroglobulinemia (WM) is an indolent NHL characterized by immunoglobulin M (IgM)–secreting lymphoplasmacytic cells and hallmark mutations in genes involved in B-cell signaling, including MYD88, CXCR4, ARID1A, and CD79B.5,6 Patients often present with precursor states, such as IgM monoclonal gammopathy of undetermined significance (MGUS) and smoldering WM (SWM) and are observed for years without therapeutic intervention. When patients become symptomatic, standard treatments include cytotoxic chemotherapy (eg, nucleoside analogues and alkylating agents), proteasome inhibitors, Bruton tyrosine kinase inhibitors, and anti-CD20 antibodies.7 We investigated the prevalence and clinical implications of CH in patients with WM.
Methods
We retrospectively reviewed clinical data of 602 patients with IgM MGUS, SWM, and WM who had clinical next-generation sequencing (NGS) performed on bone marrow aspirates or peripheral blood obtained from October 2014 through February 2020 at the Dana-Farber Cancer Institute (DFCI). The study was approved by the DFCI institutional review board (DF/HCC-18-194) and was performed in accordance with the Declaration of Helsinki. Supplemental Figure 1 summarizes the study workflow.
An Illumina Truseq amplicon-based NGS assay of 95 genes recurrently mutated in myeloid and lymphoid neoplasms was used (supplemental Table 1).8 Each specimen yielded ∼2 million reads and ∼1500× average coverage with 90% of amplicons having >200× coverage. Pathogenic driver variants were identified based on mutation type, position, and frequency in published reports1,3,9 and public databases.10 Supplemental Table 2 lists the queried variants, and supplemental Figure 2 summarizes the mutational profile.
Detailed statistical analysis methods are provided in the supplemental Methods.
Results and discussion
The cohort included 453 patients with symptomatic WM with a median age of 61 years (range, 22-90) at diagnosis and 68 years (range, 33-93) at time of first NGS assay. Thirteen patients (3%) had a coincident diagnosis of MDS or AML and 1 had acute lymphoblastic leukemia at the time of NGS; those 14 patients were excluded from further analysis. Before NGS testing, 304 patients had received therapy, 145 of whom had received cytotoxic agents (supplemental Table 3).
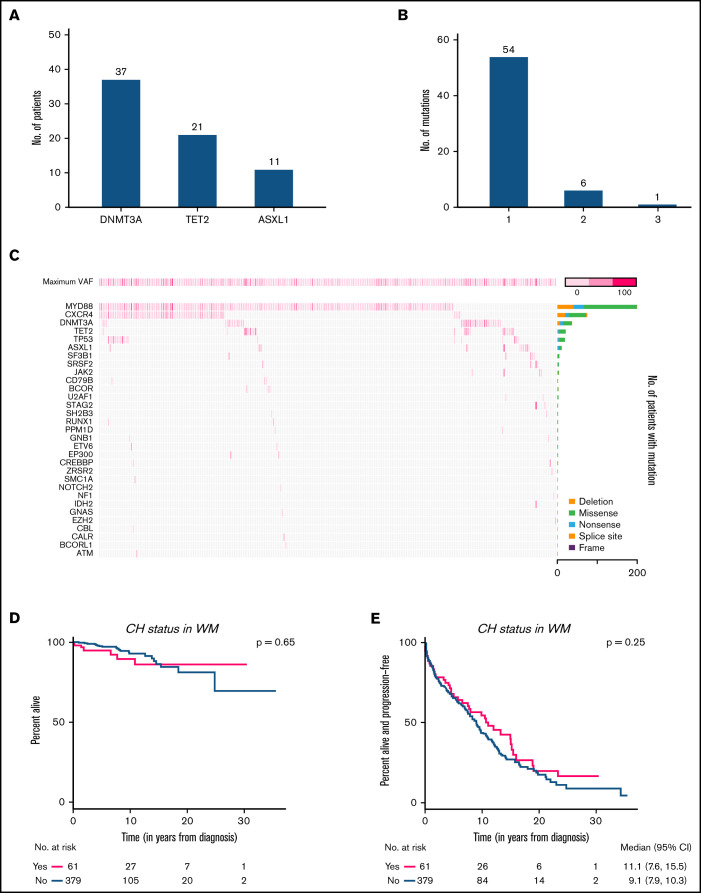
We identified 413 somatic mutations in 258 of 440 individuals (59%), with the most common being MYD88 p.L265P (46%) and CXCR4 mutations (17.0%) (supplemental Figure 3). Because some genes can be mutated in both CH and WM cells (ie, TP53) we restricted our analysis of CH to cases with DNMT3A, TET2, or ASXL1 (CH-DTA) mutations with a variant allele frequency (VAF) of ≥2%, where we could unambiguously assign the mutation to the CH clone. DTA mutations represent the 3 most commonly identified mutations in CH and have been reported primarily in myeloid malignancies. The prevalence of CH-DTA in WM was 14%, encompassing 69 mutations in 61 patients with a median VAF of 10.4% (Figure 1A-C). CH-DTA detection was not affected by BM infiltration by lymphoplasmacytic cells (supplemental Figure 4A-B) or MYD88 status (supplemental Figure 4C) in our cohort. CH-DTA was positively associated with older age (P < .001) at the time of NGS, with a median age of 72 vs 67 years for patients with vs those without CH-DTA, respectively.1,4,11 Cytotoxic chemotherapy had been administered before NGS testing in 43% of patients with CH-DTA vs 30% of patients without (P = .08).
Figure 1.
Mutational spectrum in patients with WM. (A) The total number of symptomatic patients with WM harboring ≥1 mutations in each gene. (B) The number of symptomatic patients with WM harboring mutations in 1, 2, and 3 different genes. (C) Co-mutation plot showing mutations present in all 258 patients: each column represents a single patient. The top row denotes the maximum VAF in each patient, with darker shades of pink indicating higher VAF. The bar graph on the right designates the proportion of the different mutation subtypes for each gene. OS (D) and PFS (E) among patients with WM with CH vs those without CH.
CH-DTA prevalence in this study was higher than that reported in other large healthy cohort studies (5% to 10% among subjects ≥60 years old), particularly when considering the more inclusive CH definition of those studies compared with ours.1,2,11 However, those studies used whole-exome or whole-genome sequencing and thus had lower sensitivity for mutation detection. In smaller cohorts of patients with cancer, NGS showed the prevalence of DTA mutations at a VAF ≥2% has been similar: 9% in patients with MM,4 17% in those with NHL,3 and 21% in those with metastatic melanoma.12 These differences are likely caused by several factors including the cohorts’ age distribution, prior cytotoxic therapy exposure, and differences in sequencing platforms.
CH-DTA was not associated with inferior OS (Figure 1D) with a relatively short median follow-up from diagnosis and NGS assay of 6.7 (95% CI, 6.1-7.6) and 2.5 (95% CI, 2.2-2.8) years, respectively. The most common cause of death was disease progression with no significant difference between those with or without CH-DTA. Patients with CH-DTA had an increased risk of cardiovascular disease (30% vs 18%; P = .036).1,13 Whether survival differences will become apparent with longer follow-up remains unknown.
Patients with CH-DTA had elevated β2-microglobulin at the time of NGS (4.0 mg/dL in patients with CH-DTA vs 3.2 mg/dL in those without CH-DTA; P = .004), consistent with our prior observation in MM (supplemental Table 4).4 Patients with CH-DTA also had a higher frequency of amyloidosis (11% vs 3%, P = .009). DTA mutations have been associated with a hyperinflammatory phenotype related to increased interleukin-6 (IL-6) and -1β, mediated by mutant myeloid cells.14 Whether these clinical findings are associated with an inflammatory microenvironment and how this may affect the development and evolution of disease warrants additional study.
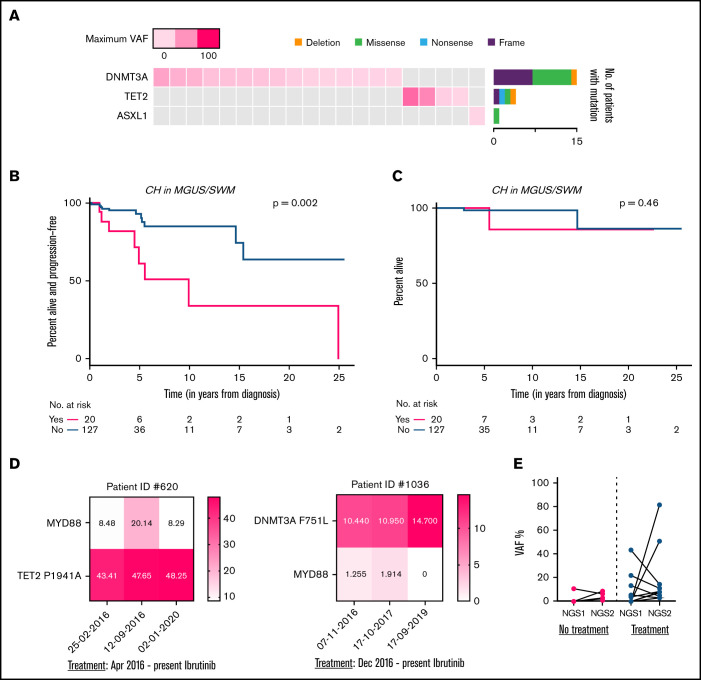
Next, we looked at the 31 patients with IgM MGUS and the 116 patients with SWM who had NGS performed. The prevalence of CH-DTA in MGUS and SWM did not differ significantly from that in symptomatic WM (13%, 14%, and 14%, respectively; Figure 2A; supplemental Figure 5). Among precursor patients, there was an increased risk of progression to symptomatic WM in those with CH-DTA (7 of 20 patients with vs 11 of 116 without CH-DTA progressing over a median of 54 months; P = .002; Figure 2B-C). Although patients with MGUS and asymptomatic WM progressed at different rates to WM, we still found that CH-DTA was significantly associated with risk of progression to symptomatic WM when studied separately. Whether this increased risk is related to changes in the bone marrow niche that promote disease, lymphoplasmacytic cells supporting CH expansion, or increased risk of defective hematopoiesis and subsequent cytopenias in patients with CH remains unclear.
Figure 2.
CH in patients with IgM MGUS and SWM. (A) Co-mutation plot showing mutations present in all 20 patients with IgM MGUS and patients with SWM: each column represents a single patient. The top row denotes the maximum VAF in each patient, with darker shades of pink indicating higher VAF. The bar graph on the right designates the proportion of the different mutation subtypes for each gene. PFS (B) and OS (C) among patients with IgM MGUS and those with SWM with CH vs those without CH. (D) Representative heat maps for the clonal dynamics of WM-related mutations and DTA mutations in WM. Values depicted in each square represent VAF. (E) Average change in VAF of DTA mutations assessed between consecutive time points, with or without intervening therapy.
One-hundred four patients had samples sequenced at more than 1 time point, with 23 having CH-DTA mutations (supplemental Figure 6). After WM-directed therapy, most patients exhibited a VAF decrease in their WM-related MYD88 and/or CXCR4 mutations (Figure 2D). On average, DTA clones expanded 6.2-fold after therapy, compared with the increase in those without treatment (Figure 2E). These data show that DTA mutations originate from a distinct myeloid clone and do not respond to WM-directed therapy. It is unclear whether CH-DTA clones truly expand in response to therapy, or this represents pseudoexpansion in the setting of a shrinking WM clone. Further analysis of the expansion kinetics and outcomes of CH clones is important in understanding clonal dynamics during therapy. These changes are important to take into account as use of NGS becomes more frequent as a method to track response to therapy.
Increased risk of MDS and AML is well documented in WM,15-18 and CH is associated with an increased risk of myeloid malignancy.3 We performed an exploratory analysis of patients with both WM and a myeloid malignancy. Fourteen patients had a concurrent myeloid malignancy at the time of NGS, developing at a median of 7.9 years from diagnosis of WM, 13 of which were MDS (87%) (supplemental Figure 7A). During follow-up, 1 additional patient with SF3B1 and SRSF2 mutations developed MDS after receiving bendamustine and rituximab (supplemental Figure 7B). All but 3 patients had received cytotoxic therapy before developing MDS or AML (supplemental Figure 7C-D). TP53-mutant CH is among the highest risk lesions for development of a secondary MDS or AML.19,20 In this cohort, TP53 was the fifth most commonly mutated gene (n = 19; supplemental Table 5; supplemental Figure 3B) and was further enriched in patients with concurrent WM and MDS/AML (4 of 14; supplemental Figure 7D). In contrast to a previous report,17 patients with TP53-mutations did not have an inferior OS or PFS (supplemental Figure 8A-B), which could be related to the fact that TP53 mutations may reside in the CH clone rather than the WM clone. Longer follow-up and prospective studies are needed to determine which patients with WM and CH have the highest risk of developing a secondary myeloid malignancy.
In summary, we demonstrated that CH is common in patients with WM and is associated with increased risk of progression from precursor states but not with inferior survival. Further work is needed to determine how the presence of CH may promote progression to WM and whether WM-related microenvironmental changes within the BM niche, leading to immunosuppression, could play a role in CH expansion. Incorporation into risk stratification models will also require further investigation. Importantly, our data do not support changes in clinical management or alterations in therapy for patients with WM and coexistent CH and reinforce the need to interpret NGS results within their specific clinical context.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all physicians and clinical research assistants for their participation and work.
This work was supported by grants from the Kirsch Foundation, Adelson Medical Research Foundation (AMRF), and Stand Up to Cancer (SU2C) (I.M.G) and National Institutes of Health, National Cancer Institute grant K08CA252174.
Authorship
Contribution: S.T., T.H.M., A.S.S., and I.M.G. conceived the study; R.R. and L.T. developed the methodology; S.T., T.H.M., R.R., L.L., K.I.N., H.E.-K., N.K.S., A.H.N., E.A., G.B. and S.A.A conducted the investigations; S.T. wrote the original draft; S.T., T.H.M., H.E.-K., A.H.N., E.A., D.P.S., J.J.C., S.P.T., A.S.S. and I.M.G wrote, reviewed, and edited the final manuscript; I.M.G. acquired funding; and A.S.S. and I.M.G. supervised the study.
Conflict-of-interest disclosure: D.P.S is currently employed by Novartis. J.J.C. has acted as a consultant for Pharmacyclis, Janssen Pharmaceuticals, Roche, and BeiGene and has received institutional research funding from Pharmacyclics, AbbVie, Janssen Pharmaceuticals, BeiGene, and TG Therapeutics. S.P.T. has acted as a consultant for Janssen Pharmaceuticals, Pharmacyclics, and BeiGene and has received research funding from Janssen Pharmaceuticals and Bristol Myers Squibb. I.M.G. has acted as a consultant for Bristol Myers Squibb, Novartis, Amgen, Takeda, Noxxon Pharma, Celgene, Sanofi, Genentech, GlaxoSmithKline, GNS Healthcare, Karyopharm Therapeutics, Adaptive Biotechnologies, Janssen, Medscape, and AbbVie and has received honoraria from Celgene, Bristol Myers Squibb, Takeda, Amgen, Janssen, Karyopharm Therapeutics, Cellectar, Adaptive Biotechnologies, Sanofi, and Medscape. The remaining authors declare no competing financial interests.
Correspondence: Adam S. Sperling, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: adam_sperling@dfci.harvard.edu; and Irene M. Ghobrial, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: irene_ghobrial@dfci.harvard.edu.
References
- 1.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35(14):1598-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouhieddine TH, Sperling AS, Redd R, et al. Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Nat Commun. 2020;11(1):2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polyatskin IL, Artemyeva AS, Krivolapov YA. [Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition): lymphoid tumors] [in Russian. Arkh Patol. 2019;81(3):59-65. [DOI] [PubMed] [Google Scholar]
- 6.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30(2):110-115. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Kastritis E. How I treat Waldenström macroglobulinemia. Blood. 2019;134(23):2022-2035. [DOI] [PubMed] [Google Scholar]
- 8.Kluk MJ, Lindsley RC, Aster JC, et al. Validation and implementation of a custom next-generation sequencing clinical assay for hematologic malignancies. J Mol Diagn. 2016;18(4):507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller PG, Gibson CJ, Mehta A, et al. Fitness landscape of clonal hematopoiesis under selective pressure of immune checkpoint blockade. JCO Precis Oncol. 2020;4(4):00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo JJ, Olszewski AJ, Hunter ZR, Kanan S, Meid K, Treon SP. Incidence of secondary malignancies among patients with Waldenström macroglobulinemia: an analysis of the SEER database. Cancer. 2015;121(13):2230-2236. [DOI] [PubMed] [Google Scholar]
- 16.Castillo JJ, Gertz MA. Secondary malignancies in patients with multiple myeloma, Waldenström macroglobulinemia and monoclonal gammopathy of undetermined significance. Leuk Lymphoma. 2017;58(4):773-780. [DOI] [PubMed] [Google Scholar]
- 17.Leleu X, Soumerai J, Roccaro A, et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenström macroglobulinemia treated with nucleoside analogs. J Clin Oncol. 2009;27(2):250-255. [DOI] [PubMed] [Google Scholar]
- 18.Hanzis C, Ojha RP, Hunter Z, et al. Associated malignancies in patients with Waldenström’s macroglobulinemia and their kin. Clin Lymphoma Myeloma Leuk. 2011;11(1):88-92. [DOI] [PubMed] [Google Scholar]
- 19.Abelson S, Collord G, Ng SWK, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559(7714):400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai P, Mencia-Trinchant N, Savenkov O, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med. 2018;24(7):1015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.