Key Points
Forty percent bacterial, 53% viral, and 6% fungal infections in 29 of 55 patients with multiple myeloma were observed after BCMA CAR-T.
Most infections after BCMA CAR-T were mild-moderate and involved upper or lower respiratory system.
Visual Abstract
Abstract
B-cell maturation antigen-targeted chimeric antigen receptor T-cell therapy (BCMA CAR-T) is an effective treatment of relapsed refractory multiple myeloma (MM). However, the pattern of infectious complications is not well elucidated. We performed a single-center retrospective analysis of infection outcomes up to 1 year after BCMA CAR-T for MM from 2018 to 2020. Fifty-five patients with MM were treated with BCMA CAR-T. Before lymphodepletion (LD), 35% of patients had severe hypogammaglobulinemia and 18% had severe lymphopenia. Most patients (68%) received bridging chemotherapy (BC) before LD. In the first month after CAR-T, 98% patients had grade 3 to 4 neutropenia. At 1 year after infusion, 76% patients had hypogammaglobulinemia. With a median follow-up of 6.0 months (95% confidence interval, 4.7-7.4), there were a total of 47 infection events in 29 (53%) patients: 40% bacterial, 53% viral, and 6% fungal. Most (92%) were mild-moderate and of the lower/upper respiratory tract system (68%). Half of the infections (53%) occurred in the first 100 days after CAR-T infusion. Although no statistically significant risk factors for infection were identified, prior lines of therapy, use of BC, recent infections, and post–CAR-T lymphopenia were identified as possible risk factors that need to be further explored. This is the largest study to date to assess infectious complications after BCMA CAR-T. Despite multiple risk factors for severe immunosuppression in this cohort, relatively few life-threatening or severe infections occurred. Further larger studies are needed to better characterize the risk factors for and occurrence of infections after BCMA CAR-T.
Introduction
Relapsed refractory multiple myeloma (MM) remains incurable despite a wide variety of approved therapeutic agents. Using chimeric antigen receptor T-cell therapy (CAR-T) against CD19 in lymphoma has been quite successful, and recently, idecabtagene vicleucel B-cell maturation antigen-directed CAR-T therapy (BCMA CAR-T) gained US Food and Drug Administration approval for relapsed refractory MM.1 Toxicities associated with CAR-T include cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS),2 cytopenias,3,4 tumor lysis syndrome, hypogammaglobulinemia, and infections, among others.5,6
Although infections have been observed after CAR-T, there is currently limited insight on the rate, type, and severity of infections and the risk factors that predispose patients to infectious complications. Infection rates after CAR-T range from 23% to 63%,7-9 with some possible risk factors identified including steroids, impaired performance status, history of infections, treatment history, and CRS.7,8 A single-center retrospective study of 144 patients treated with various CAR-T products included 26 patients treated with anti–BCMA CAR-T.10 Fifty-eight (40.3%) subjects experienced a total of 103 infections from initiation of lymphodepletion (LD) through day 30 after CAR-T. Increased risk of infection was associated with age > 18 years, prior history of infection, and prior lines of therapies.10 This study was limited by the heterogenous patient population. Indeed, the most common cause of morbidity and mortality in MM is the underlying disease itself, which can be associated with deficiencies in both humoral and cellular immunity. Hence, studying a homogenous patient population to assess infectious outcomes and risk factors for infections after CAR-T is very important.
Although several studies have described infectious complications after CAR-T,7-10 long-term infection studies in patients with MM treated with BCMA CAR-T are still lacking. We report infection rates of incidence, risk factors, and outcomes for up to 1 year after BCMA CAR-T in patients with MM.
Methods
Patients and characteristics
This is a single-center retrospective study analyzing the infectious complications of all patients with MM who underwent BCMA CAR-T between 2018 and 2020. All clinical information was extracted from electronic health records. The data cutoff for statistical analysis was 1 May 2020. The University of California, San Francisco, institutional review board approved this study through the Multiple Myeloma Translational Initiative protocol, which allows us to access patient records for patients who have consented to the protocol, and all the patients included in this study consented to this protocol.
Antimicrobial prophylaxis and toxicity grading
There was no institutional mandate for antimicrobial prophylaxis or infection surveillance; thus, prophylaxis used was based on institutional practice or primary physician’s preference. General institutional guidelines of infection prophylaxis were as follows: granulocyte colony-stimulating factor (G-CSF) was administered when the absolute neutrophil count (ANC) was <500 cells/mm3, although not before 14 days after CAR-T. All patients received standard varicella zoster virus (VZV) prophylaxis. Antibacterial and antifungal prophylaxis were given while ANC was <500 cells/mm3, and Pneumocytis jiroveci pneumonia (PCP) prophylaxis was given when CD4 count was <200 cells/mm3. Cytomegalovirus monitoring was not routinely done after CAR-T. However, if patients had any clinical indication for infection, they underwent testing. The serum immunoglobulin G (IgG) concentration was evaluated before and approximately monthly after CAR-T, and immunoglobulin (400 mg/kg, IV) was given if the serum IgG concentration was <400 mg/dL.
The most severe grade of neutropenia, lymphopenia, and hypogammaglobulinemia was recorded for each patient in blocks of 3 months after CAR-T. Neutropenia and lymphopenia were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Hypogammaglobulinemia was graded as normal if IgG > 600 mg/dL, mild-moderate if IgG was 300 to 600 mg/dL, significant if IgG was 100 to 299 mg/dL, and profoundly reduced if IgG < 100 mg/dL.11 The most severe values of counts and IgG were used in each time block to classify severity, irrespective of the supportive care given. Functional hypogammaglobulinemia was assessed by subtracting out the monoclonal IgGs in the patients with IgG isotype MM.
The severity of CRS was graded by the American Society for Transplantation and Cellular Therapy consensus criteria.12 Neurotoxicity was graded using the standard ICANS tool and did not contribute to organ toxicity grading in CRS.12
Infection categorization
All infections were documented from the day of CAR-T through 1 year after CAR-T, last follow-up, or relapse/progression, whichever came first. Infections were recorded if there was a microbiologic or histopathologic diagnosis or if treatment was received for the infection, and infection-onset day was defined as the day on which the diagnostic test was performed. Infection events included both confirmed infections in which causative pathogens were identified and probable infections diagnosed by the presence of localized physical examination, symptoms, and/or radiologic findings that warranted treatment with antimicrobials. Any episode of culture-negative neutropenic fever in the absence of localized infection within the first 30 days after CAR-T was excluded from the analysis because of the high probability of overlap with CRS. Bacterial infections were categorized as bacteremia or site-specific infections. Viral infections were classified as respiratory virus or other. Fungal infections were characterized as mold or nonmold infections. Infections were also classified by the organ system they affected: lower respiratory, upper respiratory, bloodstream, central nervous system, skin, and other.
Classification of infection severity
Infection severity was classified as mild, moderate, severe, life-threatening, or fatal as previously established.13,14 Mild infections required no treatment. Moderate infections required oral treatment only. Severe infections required IV antimicrobial therapy or were associated with other clinical circumstances that were considered severe, with the exception of bacteremia because of possible skin contaminants and fever without systemic symptoms (categorized as moderate). Life-threatening infections were complicated by symptoms considered life-threatening. Fatal infections contributed significantly to death. Patients with severe, life-threatening, or fatal infections were classified as high-severity infections, whereas patients with mild or moderate infections were classified as low severity. Patients were censored at time of progression, time of starting next therapy, or death, whichever occured first.
Statistical analysis
Overall survival (OS) was defined as the time from starting anti–BCMA CAR-T to death from any causes. For those who were alive, OS was censored at the date of last known contact. Progression-free survival (PFS) was defined as the time from starting CAR-T to evidence of disease progression, starting next line of therapy, or death, whichever occurred first. PFS was censored as date of last encounter within 1 year after CAR-T. Descriptive statistics were used to summarize baseline characteristics, supportive care, and incidence of infections. We reported median and range for continuous variables. Categorical data were summarized using proportions. OS and PFS were analyzed using Kaplan-Meier method and Cox regressions, with infection being treated as a binary time-dependent covariate with value jumped from 0 to 1 at the time of first infection. Recurrent infections were summarized using the Nelson-Aalen estimator15 and analyzed using the proportional rate model,16 an extension of the Cox model to the recurrent events setting, where the robust sandwich-type variance estimates were used in deriving P values and confidence intervals. Post–CAR-T neutropenia and hypogammaglobulinemia were treated as binary time-dependent covariates in the recurrent event analysis. All statistical analyses were performed by R program version 3.6.0.
Results
Patient and treatment characteristics
Fifty-five adult patients with MM who were treated with BCMA CAR-T were included in the study. Patient and treatment characteristics are detailed in Table 1. The median age was 62 years (range, 33-77 years). Most patients (53%) were IgGĸ MM followed by IgAĸ (18%) and IgGλ (15%). Median time from diagnosis of MM to CAR-T was 6.8 years (range, 0.6-14.3 years). Before receiving LD and CAR-T, patients had received a median of 6 treatment regimens (range, 1-13). Eighty-nine percent of patients had undergone prior transplantation with median time from transplant being 60.9 months (range, 4.6-146.9 months). Eighty-nine percent of patients had received prior monoclonal antibody (McAb) treatment, with median time from most recent McAb therapy before CAR-T being 7.0 months (range, 0.9-54.3 months). Eighty-nine percent of patients were triple class refractory. In the 30 days before CAR-T infusion, 24% of patients had an infection. Before LD, 42% of patients had serum IgG < 300 mg/dL, 18% had an ALC < 200 cells/mm3, and 2% had an absolute neutrophil count (ANC) < 500 cells/mm3. The median CD19 count before CAR-T for the 40 patients with available data was 17.5 × 106 cells/L (range, 1-537 × 106 cells/L). Most patients (64%) received bridging chemotherapy (BC), commonly cyclophosphamide (Cy)-based therapy, before LD. LD consisted of fludarabine (30 mg/m2 of body surface area per day) and Cy (300 mg/m2 per day) on days −5, −4, and −3. Patients were treated with 1 of 4 different BCMA CAR-T products, JCARH125, BB2121, BB21217, or JNJ4528, and CAR-T doses ranged from <100 × 106 cells/kg to 600 × 106 cells/kg. The median time to neutrophil recovery was 8.5 days (range, 0-63.0 days) after CAR-T. Most patients (87%) had grade 1 to 2 CRS, and 11% of patients had grade 1 to 2 neurotoxicity. Admission to the intensive care unit was needed in 5% of patients within 28 days of CAR-T.
Table 1.
Baseline characteristics
| BCMA CAR-T (n = 55) | |
|---|---|
| Demographics | |
| Age (y), median (range) | 62 (33-77) |
| Sex | |
| Female, n (%) | 28 (51) |
| Race | |
| White, n (%) | 36 (65) |
| Asian, n (%) | 5 (9) |
| African American, n (%) | 5 (9) |
| Other, n (%) | 9 (16) |
| Ethnicity | |
| Hispanic, n (%) | 8 (15) |
| Non-Hispanic, n (%) | 47 (85) |
| BMI, median (range) | 26.7 (20.5-56.2) |
| Charlson comorbidity index, median (range) | 2 (0-7) |
| MM diagnosis and type | |
| Time from diagnosis of MM (mo), median (range) | 6.8 (0.6-14.3) |
| Type of MM | |
| IgAĸ/IgAλ | 10 (18)/2 (4) |
| IgGĸ/IgGλ | 29 (53)/8 (15) |
| ĸ light chains | 5 (9) |
| IgDĸ | 1 (2) |
| Prior treatment | |
| Prior lines of therapy, median (range) | 6 (1-13) |
| Prior auto-transplant, n (%) | 48 (87) |
| Prior Allo-transplant, n (%) | 1 (2) |
| Time from transplant (mo), median (range) | 60.9 (4.6-146.9) |
| Prior McAb, n (%) | 49 (89) |
| Time from McAb (mo), median (range) | 7.0 (0.9-54.3) |
| Triple class refractory, n (%) | 49 (89) |
| Prior infections | |
| Hx of infection in 30 days before CAR-T, n (%) | 13 (24) |
| Baseline laboratory values | |
| Before LD | |
| IgG < 300 (mg/dL), n (%) | 23 (42) |
| ALC < 200 (cells/mm3), n (%) | 10 (18) |
| ANC < 500 (cells/mm3), n (%) | 1 (2) |
| Ferritin (μg/L), median (range) | 247 (29-5894) |
| Prior to CAR-T | |
| CD19 B cells absolute (×106cells/L), median (range) | 17.5 (1-537) |
| Treatment | |
| BC received, n (%) | 35 (64) |
| Type of BC, n (%) | |
| Cy-based | 24 (44) |
| Dexamethasone | 5 (40) |
| Carfilzomib or carfilzomib/Dex | 3 (5) |
| Dara/Pom/Dex | 1 (2) |
| Benda/Dex | 1 (2) |
| XRT | 1 (2) |
| BCMA CAR-T product, n (%) | |
| JCARH125 | 17 (31) |
| BB2121 | 23 (42) |
| BB21217 | 7 (13) |
| JNJ-4528 | 8 (15) |
| CAR-T dose (cells/kg), n (%) | |
| 600 × 106 | 6 (11) |
| 450 × 106 | 16 (29) |
| 300 × 106 | 22 (40) |
| 150 × 106 | 3 (5) |
| <100 × 106 | 8 (15) |
| Post–CAR-T characteristics | |
| Time to neutrophil recovery (days), median (range) | 8.5 (0-63.0) |
| Cytokine release syndrome (grade 1-2), n (%) | 48 (87) |
| Cytokine release syndrome (grade ≥ 3), n (%) | 0 (0) |
| Neurotoxicity (grade 1-2), n (%) | 6 (11) |
| Neurotoxicity (grade ≥ 3), n (%) | 2 (4) |
| Corticosteroids received, n (%) | 22 (40) |
| Tocilizumab received, n (%) | 42 (76) |
| Maximum ferritin level, median (range) | 1081 (32-40 000) |
| ICU admission by day 28, n (%) | 3 (5) |
Allo, allogeneic; Auto, autologous; Hx, history.
Antimicrobial prophylaxis and supportive care
Most patients (98%) received antibacterial prophylaxis/treatment after CAR-T, with 22% of patients starting this before LD. Median duration on antibacterial prophylaxis after CAR-T was 22.5 days (range, 7.0-198.0 days). All patients were on VZV prophylaxis, and 95% of patients started this before LD. Median duration on antiviral prophylaxis after CAR-T was 182.0 days (range, 16.0-371.0 days). Most patients (95%) received antifungal prophylaxis after CAR-T, with 10% starting this before LD. Median duration of antifungal therapy was 16.5 days (range, 5.0-3665.0 days) after CAR-T. Most patients (98%) received prophylaxis for PCP, with 51% of patients starting before LD. The median duration of PCP prophylaxis after CAR-T was 141.5 days (range, 14.0-463.0 days; supplemental Table 1).
There were 58% (32 of 55) patients who received at least 1 dose of intravenous immunoglobulin (IVIG) within 12 months after CAR-T. The median number of total IVIG doses received after CAR-T was 1.0 (range, 0-6.0). The time block after CAR-T in which the largest proportion of patients received ≥1 dose of IVIG was 3 to 6 months after CAR-T at 38% (17 of 45) of patients. At 9 to 12 months after CAR-T, there were 24% (4 of 17) patients who received ≥1 dose of IVIG. Ninety-three percent (51 of 55) of patients received ≥1 dose of G-CSF within 12 months after CAR-T. The median number of total G-CSF doses received after CAR-T was 4.0 (range, 0-23.0). Ninety-one percent (50 of 55) of patients required ≥1 G-CSF dose within 1 month after CAR-T. The G-CSF requirement gradually decreased over time, with 6% (1 of 17) patients receiving ≥1 dose of G-CSF 9 to 12 months after CAR-T (supplemental Figure 1A-B).
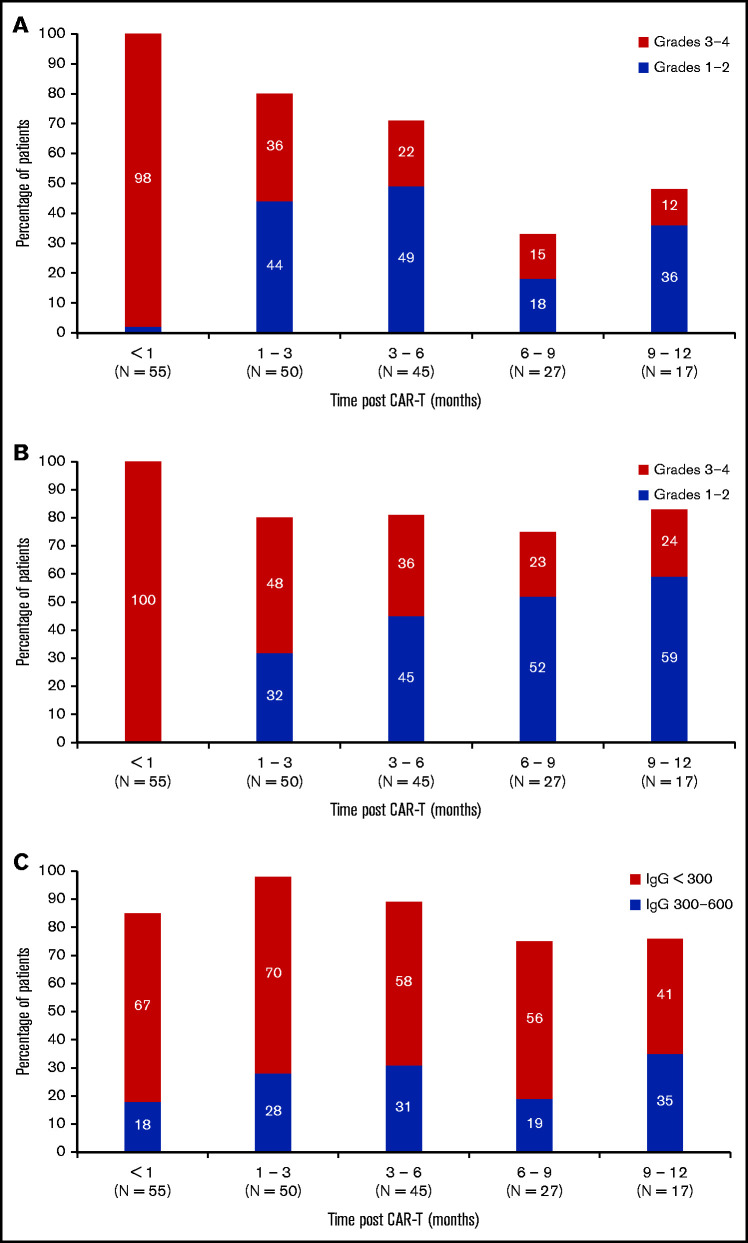
Recovery of neutrophil counts, lymphocyte counts, and immunoglobulin levels
Rates of grade 3 to 4 neutropenia and lymphopenia were most severe in the first month after CAR-T and gradually improved over time. Ninety-eight percent (54 of 55) of patients had grade 3 to 4 neutropenia in the first month after CAR-T, whereas only 12% (2 of 17) patients had grade 3 to 4 neutropenia 9 to 12 months after CAR-T (Figure 1A). A similar trend was seen with lymphopenia after CAR-T (Figure 1B). All patients experienced grade 3 to 4 lymphopenia within the first month after CAR-T, whereas only 24% (4 of 17) of patients had grade 3 to 4 lymphopenia 9 to 12 months after CAR-T. With respect to hypogammaglobulinemia, 18% (10 of 55) of patients had mild-moderate hypogammaglobulinemia and 67% (37 of 55) of patients had significant or profoundly reduced hypogammaglobulinemia in the first month after CAR-T. By 9 to 12 months after CAR-T, 35% (6 of 17) of patients had mild-moderate hypogammaglobulinemia and 41% (7 of 17) of patients had significant or profoundly reduced hypogammaglobulinemia (Figure 1C). There was no association between the level of CD19 count before CAR-T and the development of post–CAR-T significant or profoundly reduced hypogammaglobulinemia (odds ratio, 1.0; P = .2). There was also no association between significant or profoundly reduced pre–CAR-T hypogammaglobulinemia and development of post–CAR-T hypogammaglobulinemia of the same severity (odds ratio, 1.7; P = .3).
Figure 1.
Neutropenia, lymphopenia, and hypogammaglobulinemia after BCMA CAR-T. (A) Incidence and severity of neutropenia up to 1 year after BCMA CAR-T. (B) Incidence and severity of lymphopenia up to 1 year after BCMA CAR-T. (C) Incidence and severity of hypogammaglobulinemia up to 1 year after BCAM CAR-T. Number of patients evaluable in each 3-month time block is shown on the x axis.
CRS and ICANS.
CRS was observed in 48 patients (87%; grade 1 in 23 [42%] patients and grade 2 in 25 [45%] patients) at a median onset of 1.0 day after CAR-T (range, 0-12.0 days) and median duration of 2.0 days (range, 0-8.0 days). ICANS was observed in 8 patients (14%; grade 1 in 3 patients, grade 2 in 3 patients, and grade 3 in 2 patients). The median onset of ICANS was 3.5 days after CAR-T (range, 1.0-10.0 days), and median duration of ICANS was 1 day (range, 0-21.0 days). Forty-two patients (76%) received at least 1 dose of tocilizumab. Twenty-nine patients (53%) required 1 dose of tocilizumab, 12 patients (22%) received 2 doses of tocilizumab, and 1 patient (2%) received 3 doses. The median time to first tocilizumab after CAR-T was 1 day (range, 0-10.0 days). Twenty-two (40%) patients received steroids after CAR-T, with median time to first steroid dose being 2.0 days (range, 0-8.0 days) and median cumulative dose of dexamethasone being 70 mg (range, 30-150 mg) over the 14-day hospitalization period. There were 12 patients who received at least 1 dose of anakinra, with the number of median doses received being 3.5 (range, 2.0-11.0). Median time to first anakinra dose after CAR-T was 6.5 days (range, 4.0-113.0 days; supplemental Table 2).
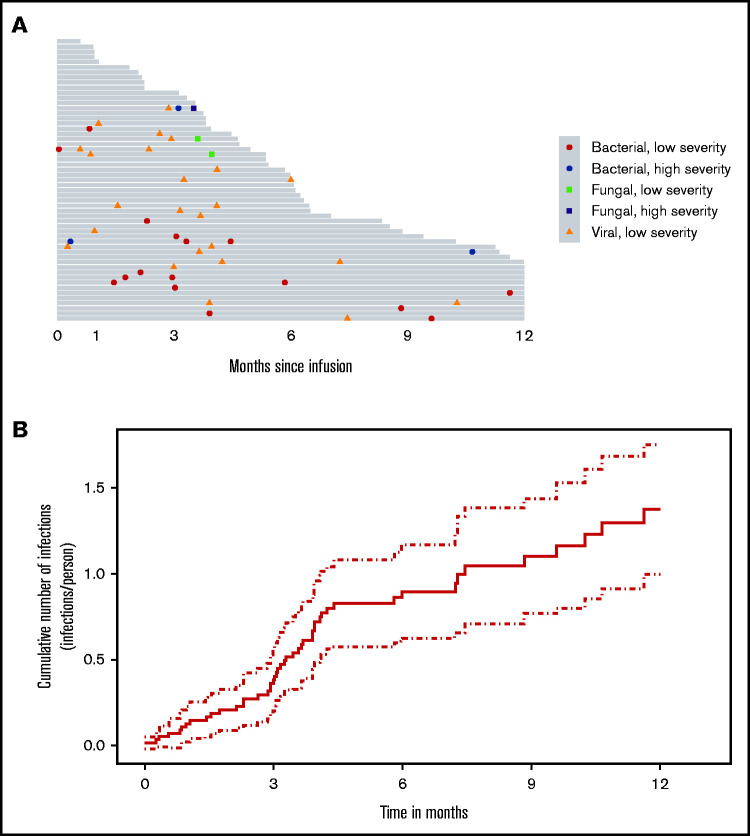
Incidence, characteristics, and patterns of infection after CAR-T
With a median follow-up of 6.0 months (95% confidence interval [CI]: 4.7-7.4), there were a total of 47 infection events after CAR-T and before progression or death (Figure 2A). At 12 months after CAR-T, the expected cumulative number of infections per person was 1.4 (95% CI: 1.0-1.8) had the patient remained free of progression/death (Figure 2B).
Figure 2.
Infection incidence after BCMA CAR-T. (A) Swimmer’s plot of infections up to disease progression within 1 year after BCMA CAR-T (gray bars on this plot indicate the duration from CAR-T infusion to either progression or death). (B) Cumulative number of infections/person over time after BCMA CAR-T (solid line indicates rate of infections over time, dotted lines indicate the corresponding pointwise 95% interval).
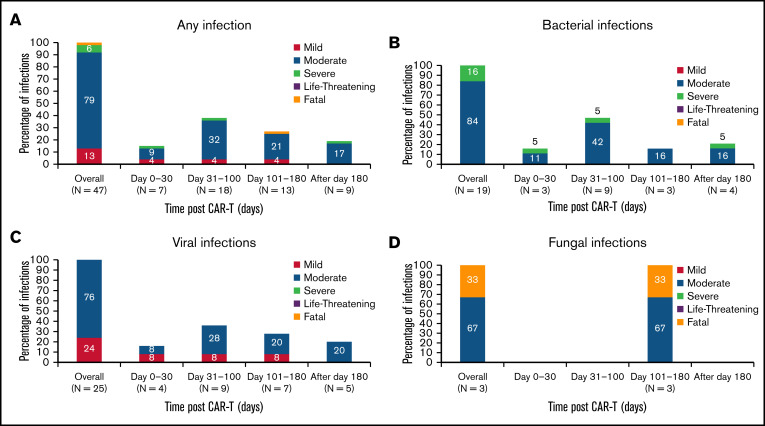
Infectious complications were predominantly viral and of low severity (Figure 2A). There were 19 (40%) bacterial, 25 (53%) viral, and 3 (6%) fungal infections in 29 patients (53%) during the entire study period. Of the bacterial infections, there were 14 (30%) bacterial site infections, 2 (4%) gram-positive bacteremia, and 3 (6%) gram-negative bacteremia. All the viral infections were respiratory viruses, and no cases of cytomegalovirus infections were identified. Of the fungal infections, 2 (4%) were mold (both had Aspergillus fumigatus) and 1 (2%) was nonmold. One of the patients with Aspergillus pneumonia was persistently neutropenic including at the time of infection despite recurrent doses of G-CSF. Fatal Aspergillus pneumonia developed in the other patient after severe influenza pneumonia while non-neutropenic. Of the 47 infection events, 6 (13%) were classified as mild, 37 (79%) were moderate, 3 (6%) were severe, and 1 (2%) was classified as fatal (Table 2; Figure 3A). The infections were predominantly infections of the lower or upper respiratory system (34% each). Bloodstream infections were present at a prevalence of 2%. There were no gastrointestinal or central nervous system infections. All other infections such as skin and urinary tract infections were seen in 30% of patients (Figure 3B).
Table 2.
Infections organized by type and subtype and organ system
| Severity of infection | ||||
|---|---|---|---|---|
| Number of infection events (n = 47) | Number of patients (n = 55) | |||
| Any severity* | High severity† | Any severity* | High severity† | |
| Any infection, n (%) | 47 (100) | 4 (9) | 29 (53) | 3 (6) |
| Bacterial infections, n (%) | 19 (40) | 3 (6) | 15 (27) | 3 (6) |
| Bacterial site,‡ n (%) | 14 (30) | 2 | 12 (22) | 2 (4) |
| Gram-positive bacteremia, n (%) | 2 (4) | 0 (0) | 2 (4) | 0 (0) |
| Gram-negative bacteremia, n (%) | 3 (6) | 1 (2) | 3 (6) | 2 (4) |
| Viral infections, n (%) | 25 (53) | 0 (0) | 18 (33) | 2 (4) |
| Respiratory virus, n (%) | 25 (53) | 0 (0) | 18 (33) | 2 (4) |
| Other, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fungal infection, n (%) | 3 (6) | 1 (2) | 3 (6) | 1 (2) |
| Mold fungal, n (%) | 2 (4) | 1 (2) | 2 (4) | 1 (2) |
| Nonmold fungal, n (%) | 1 (2) | 0 (0) | 1 (2) | 0 (0) |
| Organ system | ||||
| Lower respiratory, n (%) | 16 (34) | 0 (0) | 14 (30) | 2 (4) |
| Upper respiratory, n (%) | 16 (34) | 0 (0) | 14 (30) | 2 (4) |
| Bloodstream, n (%) | 1 (2) | 1 (2) | 1 (2) | 1 (2) |
| GI, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CNS, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other,§ n (%) | 14 (30) | 3 (6) | 9 (16) | 2 (4) |
CNS, central nervous system; GI, gastrointestinal.
Any severity: infections that are mild, moderate, severe, life-threatening, or fatal.
High severity: infections that are severe, life-threatening, or fatal.
Site-specific bacterial infections were defined as evidence of bacterial infection by culture of a normally sterile site or by culture and evidence of tissue invasion of a nonsterile site.
Other includes skin and urinary tract, among other infections.
Figure 3.
Classification of infections after BCMA CAR-T. (A) Infections by type and subtype. (B) Infections by organ system.
Seven (15%) infections occurred in the first 30 days after CAR-T, with 4 (2%) being severe. Most infections (25 events, 53%) occurred in the first 100 days after CAR-T, predominantly being moderate in severity (16 events, 34%; Figure 4). The distribution of any infection, viral infections, bacterial infections, and fungal infections over time after CAR-T organized by severity is shown in Figure 4. Among the 29 patients with first infections, the median time to onset of first infection was 3.9 months (95% CI: 3.2-not reached [NR]).
Figure 4.
Characterization of infections after BCMA CAR-T over time. (A) Infections of any type after BCMA CAR-T. (B) Bacterial infections after BCMA CAR-T. (C) Viral infections after BCMA CAR-T. (D) Fungal infections after BCMA CAR-T. The total number of infections and number of infection events observed in each time period is shown on the x axis.
Risk factors associated with infection
Recurrent event analysis was performed to identify risk factors for development of infection after CAR-T up to 1 year with censoring at the time of progression or death. The risk factors assessed were prior lines of therapy (>3 lines of therapy), use of BC, baseline lymphopenia (ALC < 200 cells/mm3), baseline hypogammaglobulinemia (IgG < 300 mg/dL), triple class refractoriness, prior McAb therapy, CAR-T dose (<300 × 106 cells/kg vs ≥300 × 106 cells/kg), infection 30 days before CAR-T, post–CAR-T neutropenia (ANC < 500 cells/mm3) as a time-dependent variable, post–CAR-T lymphopenia (ALC < 200 cells/mm3) as a time-dependent variable, post–CAR-T hypogammaglobulinemia (IgG < 300 mg/dL) as a time-dependent variable, depth of best clinical response achieved after CAR-T, tocilizumab use, steroid use, and maximum CRS grade 1 to 2 vs none. Baseline neutropenia (ANC < 500 cells/mm3) was not assessed as a risk factor given only 1 patient met the criteria. None of these risk factors were found to have an association that reached statistical significance, although prior lines of therapy > 3, use of BC, infections 30 days before CAR-T, and post–CAR-T lymphopenia had a trend toward increasing infection risk (Table 3). Although post–CAR-T hypogammaglobulinemia was not associated with an increased risk for infection in a statistically significant manner, 60% of post–CAR-T infections (30% viral, 26% bacterial, and 4% fungal) occurred when patients had either significant or profoundly reduced hypogammaglobulinemia at the time of infection (supplemental Table 3). The cumulative number of infections over time was not significantly associated with whether patients received IVIG support for IgG < 300 mg/dL at any time point before or after CAR-T. Tocilizumab use, tocilizumab total dose, dexamethasone use, dexamethasone total dose, and the maximum grade of CRS or neurotoxicity were not significantly associated with developing high severity infections. Although depth of best clinical response achieved after CAR-T had no correlation with infection risk, 85% of infections after CAR-T occurred when patients had achieved at least a partial response to CAR-T at the time of developing the infections (supplemental Table 4).
Table 3.
Risk factors for infection
| Rate ratio | CI (lower) | CI (upper) | P | |
|---|---|---|---|---|
| Prior lines of therapy (>3 vs ≤3) | 1.20 | 0.48 | 3.02 | .70 |
| Prior monoclonal antibody therapy (yes or no) | 1.0 | 0.28 | 3.52 | 1.0 |
| Triple class refractory (yes or no) | 1.0 | 0.28 | 3.52 | 1.0 |
| BC (yes or no) | 1.22 | 0.70 | 2.13 | .48 |
| Dose of CAR-T (<300 × 106 cells/kg vs ≥300 × 106 cells/kg) | 0.94 | 0.44 | 1.99 | .87 |
| Infections 30 days before CAR-T (yes or no) | 1.46 | 0.86 | 2.50 | .16 |
| Baseline lymphopenia (ALC <200 cells/mm3 vs ≥200 cells/mm3) | 1.0 | 0.50 | 2.05 | 1.0 |
| Baseline hypogammaglobulinemia (IgG <300 mg/dL vs ≥300 mg/dL) | 0.97 | 0.57 | 1.65 | .91 |
| Post–CAR-T neutropenia (grade ≥3 vs grade <3) | 1.14 | 0.56 | 2.30 | .72 |
| Post–CAR-T lymphopenia (grade ≥3 vs grade <3) | 1.34 | 0.70 | 2.55 | .37 |
| Post–CAR-T hypogammaglobulinemia (IgG <300 mg/dL vs ≥300 mg/dL) | 0.83 | 0.45 | 1.55 | .56 |
| Post–CAR-T best response (≥ partial response (PR) vs < PR) | 0.50 | 0.07 | 3.74 | .50 |
| Tocilizumab (yes or no) | 1.12 | 0.60 | 2.12 | .72 |
| Steroids (yes or no) | 1.12 | 0.66 | 1.89 | .68 |
| Max CRS grade (1-2 vs 0) | 1.06 | 0.42 | 2.64 | .91 |
Impact of infection on patients’ survival outcomes
The median OS of the cohort was 24.8 months (95% CI: 18.59-NR), and the median PFS was 12.0 months (95% CI: 9.4-NR). Of all infectious complications, 1 resulted in death attributed to an invasive fungal infection with Aspergillus fumigatus. There was no statistically significant association between development of infection and mortality risk in CAR-T–treated patients when analyzed by univariate Cox regression (hazard ratio, 0.88; 95% CI: 0.2-3.7; P = .9).
Discussion
This is the largest study to date to assess the infectious complications after BCMA CAR-T for MM. In this cohort of 55 patients, we found 47 total infection events up to 1 year after CAR-T. Despite multiple risk factors that indicate significant immunosuppression in this patient cohort before commencing LD and after CAR-T infusion, few of the infections occurring after CAR-T were life-threatening or severe. Most of these infections were mild to moderate, with only 9% of infections being classified as high severity. Viral infections were the most frequent at 53%, followed by bacterial infections at 40%. Fungal infections were very rare, although fatal in 1 patient. The infectious complications predominantly affected the upper or lower respiratory system. Most infections occurred in the first 100 days after CAR-T. A variety of risk factors were assessed, none of which increased risk for infections in a statistically significant manner. Although high-grade neutropenia was present in most patients in the first month after CAR-T, the severity and incidence of neutropenia resolved by 9 to 12 months after CAR-T. Most patients, however, had persistent hypogammaglobulinemia 9 to 12 months after CAR-T.
There are both similarities and differences in the infectious complications seen in our cohort of patients with MM after BCMA CAR-T and prior studies of CD19 CAR-T. The prevalence of both viral and bacterial infections seen in patients with MM after BCMA CAR-T is similar to the study assessing infections in diffuse large B-cell lymphoma (DLBCL) patients up to 1 year after CD19 CAR-T.8 However in the DLBCL CD19 cohort, bacterial pathogens were the most predominant, unlike our study, where viral pathogens were most frequent.8 Both in our study and that study, infections were mostly mild to moderate in severity, and the upper or lower respiratory system was the organ system most frequently affected.8,17 Prior studies assessing infectious complications after CD19 CAR-T also found, similar to our study, relatively low incidence rates of late infections beyond 90 days after CAR-T.18 Prior risks factors identified to increase risk of infections after CD19 CART, such as use of steroids, increased prior lines of therapy, impaired performance status, and CRS, were not observed in our study.7,8 The median dose of steroids used, rates of grade 1 to 3 CRS, and median lines of prior therapy were higher in our study than that reported in some of these prior CD19 CAR-T infection studies,7,8 suggesting that the lack of these risk factors having a signal in our study is either because of the difference in patient population and type of CAR-T used or the fact that our study sample size was too small to detect these risk factors. Although poor performance status was a risk factor for increased infections in a prior CD19 CAR-T study,8 this could not be assessed as a risk factor in our study because all our patients received BCMA CAR-T in clinical trials, thus requiring good performance status to meet eligibility criteria.
The only other prior study focusing on infectious complications of CAR-T that included patients who had BCMA CAR-T was a heterogenous study with short follow-up of 30 days after CAR-T.10 This study also showed predominance of bacterial and viral infections over fungal infections in the BCMA cohort, although the largest predominance of viral infections observed in our study was not seen in this small cohort.10 The phase 1 BB2121 study19 demonstrated 15% any grade upper respiratory tract infection, which is lower than that seen in our study, but may be explained by the differences in characterizing and including infections.
The limitations of this study include the small sample size and its retrospective nature. Infections were included both if there was an actual causative pathogen identified and if there was a suspected infection that required antimicrobials, which may have led to overcharacterization of infections. However, this is in line with prior studies of infectious complications in CAR-T8 and stem cell transplant.20 The absence of finding any risk factors that increased the risk of infections in a statistically significant manner may have been because of our small sample size and our study not being powered enough to identify these risk factors. The absence of finding an association between receiving IVIG for significant or profoundly reduced hypogammaglobulinemia may be secondary to not only small sample size but also missing data, given many patients were given supportive care locally. This study also examines infectious complications in patients who received BCMA CAR-T on a clinical trial, raising the issue of selection bias. Real-world studies are needed in the future to assess infectious complications of this treatment.
Although the rates of severe infections were low, our study highlights that infectious complications after BCMA CAR-T can occur despite a very high rate of antimicrobial prophylaxis. The highest prevalence of bacterial and viral infections was in months 1 to 3 and fungal infections was in months 3 to 4. Although 2% of severe fungal infections in this population is comparable to other novel myeloma therapies,21 our patients had high rates of neutropenia within the first 3 months after CAR-T. Based on these findings, we recommend antibacterial prophylaxis not only during early neutropenia but also with late or recurrent neutropenia and likely for at least 1 month after CAR-T. We recommend VZV prophylaxis for 6 months and PCP prophylaxis for 3 months. Fluconazole prophylaxis should be tailored to patient’s risk for prolonged neutropenia. G-CSF support to keep ANC > 1000 cells/mm3 may be considered in the first 3 months after CAR-T. However, because of the theoretical concern of increased risk of CRS with G-CSF, the risks and benefits should be considered before initiation of G-CSF in the early period after CAR-T. We also recommend early use of pegfilgrastim and consideration of mold prophylaxis for patients with recurrent neutropenia. The overall low infection rate in our study despite persistent hypogammaglobulinemia is possibly secondary to IVIG, but no clear association was identified. Although we did not identify any statistically significant risk factors for infection because of the study’s small sample size, risk factors such as prior lines of therapy, use of BC, recent infections, and post–CAR-T lymphopenia were identified as possible risk factors that need to be further explored. Although this study sheds light on the infectious complications of BCMA CAR-T in patients with MM, which is poorly understood at this time, we need larger studies to evaluate this question further, standardize criteria for defining infections, characterize the underlying risk factors, and establish appropriate prophylactic approaches in patients undergoing BCMA CAR-T.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgment
The authors thank the patients included in this study.
Authorship
Contribution: S.K. and S.W.W. wrote the manuscript; S.K., S.B., M.L., V.K., and K.N. acquired the data; S.K., Y.S., C.-Y.H., and S.W.W. interpreted the data and performed the statistical analyses; S.K., Y.S., C.-Y.H., S.B., M.L., V.K., K.N., T.M., J.W., N.S., and S.W.W. helped revise the manuscript; and all authors discussed the data and the analytic methods and contributed to the manuscript.
Conflict-of-interest disclosure: T.M. discloses conflicts of interest with Amgen (research), GSK (consulting), Janssen (research), Juno (consulting), Roche (consulting), Sanofi (research), and Seattle Genetics (research). J.W. discloses conflicts of interest with Amgen (consulting), Celgene (consulting), Janssen (consulting), Novartis (consulting), and Takeda (consulting). N.S. discloses conflicts of interest with Amgen (consulting), Bluebird (research), BMS (consulting), Celgene (consulting), Genentech (research), GSK (consulting), Indapta (equity), Janssen (research), Karyopharm (consulting), Kite (consulting), Nektar (consulting), Nkarta (consulting), Oncopeptides (research), Poseida (research), Precision Biosciences (consulting), Seattle Genetics (research), Surface Oncology (research), Sutro (research), and Teneobio (consulting). S.W.W. discloses conflicts of interest with Celgene (research), Fortis (research), Genentech (research), Janssen (research), Juno (research), Sanofi (consulting), and Amgen (consulting). All remaining authors declare no competing financial interests.
Correspondence: Swetha Kambhampati, UCSF Medical Center, 505 Parnassus Ave, PO Box 1270, San Francisco, CA 94143; e-mail: swetha.kambhampati@ucsf.edu.
References
- 1.Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716. [DOI] [PubMed] [Google Scholar]
- 2.Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39(39):433-444. [DOI] [PubMed] [Google Scholar]
- 3.Nahas GR, Komanduri KV, Pereira D, et al. Incidence and risk factors associated with a syndrome of persistent cytopenias after CAR-T cell therapy (PCTT). Leuk Lymphoma. 2020;61(4):940-943. [DOI] [PubMed] [Google Scholar]
- 4.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yáñez L, Sánchez-Escamilla M, Perales M-A. CAR T cell toxicity: current management and future directions. HemaSphere. 2019;3(2):e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10(8):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkilineni L, Shahani S, Yates B, et al. Infectious complications associated with CAR T-cell therapy. Blood. 2019;134(suppl 1):4449-4449. [Google Scholar]
- 11.Agarwal S, Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol. 2007;99(3):281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PubMed] [Google Scholar]
- 13.van Burik JA, Carter SL, Freifeld AG, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(12):1487-1498. [DOI] [PubMed] [Google Scholar]
- 14.Young JH, Logan BR, Wu J, et al. ; Blood and Marrow Transplant Clinical Trials Network Trial 0201 . Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22(2):359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aalen OO, Husebye E. Statistical analysis of repeated events forming renewal processes. Stat Med. 1991;10(8):1227-1240. [DOI] [PubMed] [Google Scholar]
- 16.Lin DY, Wei LJ, Yang I, et al. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B Stat Methodol. 2000;62(4):711-730. [Google Scholar]
- 17.Kambhampati S, Fakhri B, Sheng Y, et al. Infectious complications of BCMA-targeted and CD19-targeted chimeric antigen receptor T-cell immunotherapy. Blood. 2020;136(suppl 1):4-5.32614961 [Google Scholar]
- 18.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26(1):26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman S, Rybicki L, Ky Hamilton B, et al. Early infectious complications after autologous hematopoietic cell transplantation for multiple myeloma. Transpl Infect Dis. 2019;21(4):e13114. [DOI] [PubMed] [Google Scholar]
- 21.Teh BW, Teng JC, Urbancic K, et al. Invasive fungal infections in patients with multiple myeloma: a multi-center study in the era of novel myeloma therapies. Haematologica. 2015;100(1):e28-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.