Abstract
The in vivo efficacy of the echinocandin antifungal caspofungin acetate (caspofungin; MK-0991) was evaluated in models of disseminated aspergillosis and candidiasis in mice with cyclophosphamide (CY)-induced immunosuppression. Caspofungin is a 1,3-β-d-glucan synthesis inhibitor efficacious against a number of clinically relevant fungi including Aspergillus and Candida species. Models of CY-induced transient or chronic leukopenia were used with once daily administration of therapy initiated 24 h after microbial challenge. Caspofungin was effective in treating disseminated aspergillosis in mice that were transiently leukopenic (significant prolongation of survival at doses of ≥0.125 mg/kg of body weight and a 50% protective dose [PD50] of 0.245 mg/kg/day at 28 days after challenge) or chronically leukopenic (50 to 100% survival at doses of ≥0.5 mg/kg and PD50s ranging from 0.173 to 0.400 mg/kg/day). Caspofungin was effective in the treatment and sterilization of Candida infections in mice with transient leukopenia with a 99% effective dose based on reduction in log10 CFU of Candida albicans/gram of kidneys of 0.119 mg/kg and 80 to 100% of the caspofungin-treated mice having sterile kidneys at caspofungin doses from 0.25 to 2.0 mg/kg. In Candida-infected mice with chronic leukopenia, caspofungin was effective at all dose levels tested (0.25 to 1.0 mg/kg), with the log10 CFU of C. albicans/gram of kidneys of caspofungin-treated mice being significantly lower (>99% reduction) than that of sham-treated mice from day 4 to day 28 after challenge. Also, 70 to 100% of the caspofungin-treated, chronic leukopenic mice had sterile kidneys at caspofungin doses of 0.5 to 1.0 mg/kg from day 8 to 28 after challenge. Sterilization of Candida infections by caspofungin in the absence of host leukocytes provides compelling in vivo evidence for fungicidal activity against C. albicans. Further human clinical trials with caspofungin against serious fungal infections are in progress.
Caspofungin acetate (caspofungin), formerly reported as MK-0991 and L-743872, is a potent, parenteral agent currently undergoing clinical development by Merck & Co., Rahway, N.J., with efficacy against a number of clinically important fungi (Aspergillus and Candida species), including many species and strains resistant to other antifungal agents (1, 4, 6, 8–10, 17, 18, 22; E. M. Bernard et al., Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F39, 1996; P. Connolly et al., Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F81, 1997; A. M. Flattery et al., Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F40, 1996; A. M. Flattery et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J61, 1998; L. K. Najvar et al., Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F43, 1996; C. A. Sable et al., Program Addendum 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LB-33, 1997). Caspofungin is a member of the echinocandin class of antibiotics and is a water-soluble, semisynthetic derivative of the pneumocandin Bo, which in turn is a fermentation product derived from the fungus Glarea lozoyensis (5). The mechanism of action of caspofungin is inhibition of 1,3-β-d-glucan synthesis, which is critical in the formation of structural cell wall components in certain pathogenic fungi and Pneumocystis carinii cysts (3, 4, 12, 13, 19; F. A. Bouffard, J. F. Dropinski, J. M. Balkovec, R. M. Black, M. L. Hammond, K. H. Nollstadt, and S. Dreikorn, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F27, 1996).
In recent years, the increased number of immunosuppressed patients has increased the incidence of serious, life-threatening fungal infections (2, 23, 24). Despite the introduction of more-effective, less-toxic triazole agents and new formulations of amphotericin B (AmB), the incidence of fungal infections resistant to many currently available antifungal agents is still a serious concern, and the need for new antimycotics with novel modes of action continues (13, 21). This report describes the in vivo efficacy of caspofungin in models of disseminated aspergillosis and candidiasis in mice with cyclophosphamide (CY)-induced immunosuppression.
MATERIALS AND METHODS
Drugs.
Caspofungin was synthesized by the Department of Medicinal Chemistry at Merck Research Laboratories, Rahway, N.J.; formulated; and serially diluted in sterile distilled water. AmB, purchased as Fungizone (Bristol-Myers Squibb, Princeton, N.J.), was reconstituted according to the manufacturer's instructions and further diluted in sterile distilled water. Fluconazole (FCZ) (Diflucan for Injection; Pfizer, Groton, Conn.) was used as supplied (2 mg/ml) for the high dose and serially diluted in sterile distilled water for the lower doses.
Animals.
Outbred, conventionally reared, female ICR mice (average weight 23 to 25 g; Harlan, Indianapolis, Ind.), were used. Mice were housed in sterile microisolator cages with sterile bedding, feed, and water.
All procedures were performed in accordance with the highest standards for the humane handling, care, and treatment of research animals and were approved by the Merck Institutional Animal Care and Use Committee. Procedures for the care and use of research animals at Merck meet or exceed all applicable local, national, and international laws and regulations.
Immunosuppression.
ICR mice were immunosuppressed with a 6-mg/mouse dose of CY (Cytoxan; Mead Johnson, Princeton, N.J.) administered by intraperitoneal (i.p.) injection for transient suppression or orally by gavage for chronic suppression, 3 days prior to infection (day −3). For the transient-suppression aspergillosis study, immunosuppression was maintained by four additional doses of CY (2 mg/mouse, i.p.) on days 1, 4, 7, and 10 after infection. For the transient-suppression candidiasis studies, immunosuppression was maintained by two additional doses of CY (2 mg/mouse, i.p.) on days 1 and 4 after infection. For all of the chronic suppression studies, immunosuppression was maintained by nine additional doses of CY (2 mg/mouse, orally) on days 1, 4, 7, 10, 14, 16, 19, 22, and 25 after infection. Immunosuppression was monitored in representative ICR mice by differential white blood cell counts at time points following treatment with CY. Two control groups received CY treatments as described above to determine possible mortality due to immunosuppression alone. One group was noninfected and nontreated. The second group was sham-infected with sterile physiological saline and sham-treated with sterile distilled water on the same therapy schedule as the test groups.
Organisms and culture conditions.
Aspergillus fumigatus MF5668 (ATCC 13073), originally isolated from a human pulmonary lesion, was cultured on Sabouraud dextrose agar (SDA) (BBL, Cockeysville, Md.) slants at 35°C for 3 to 5 days. Conidia were washed from the surface of several (two to three) agar slants into sterile saline with 0.01% Tween 20 (Fisher Scientific, Fair Lawn, N.J.), and the conidial concentration was determined by counting with a hemacytometer. The viable count was confirmed by serially diluting the conidial suspension 10-fold and plating the inoculum on SDA plates.
Candida albicans MY1055 (Merck Culture Collection) was cultured on SDA plates at 35°C for 24 h. Yeast cells were washed from the surfaces of one to two agar plates into sterile saline, and the cell concentrations were determined by counting with a hemacytometer. The viable count was confirmed by serially diluting the yeast suspension 10-fold and plating each inoculum on SDA plates.
In vitro susceptibility.
A. fumigatus MF5668 was tested for susceptibility to caspofungin and AmB by the broth microdilution method as described in NCCLS document M38-P (15) utilizing the recommended buffered RPMI-1640 medium, an inoculum of 1.0 × 104 to 5.0 × 104 conidia/ml, and an incubation temperature of 35°C. The MIC of caspofungin was defined as the lowest concentration of the antifungal agent inhibiting 80% visible growth at 24 h, while the MIC of AmB was defined as the lowest concentration of the drug inhibiting 100% of the visible growth at 48 h.
C. albicans MY1055 was tested for susceptibility to caspofungin, AmB, and FCZ by the broth microdilution method as described in NCCLS document M27-A (16) utilizing the recommended buffered RPMI-1640 medium, an inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml, and an incubation temperature of 35°C for 48 h. The MIC of caspofungin and AmB was defined as the lowest concentration of the antifungal agent inhibiting 100% visible growth, while the MIC of FCZ was defined as the lowest concentration of the drug inhibiting 80% of the visible growth.
Aspergillosis survival studies.
For both the transient and chronic models, a disseminated Aspergillus infection was induced in immunosuppressed ICR mice by the intravenous (i.v.) inoculation of 0.2 ml of an A. fumigatus MF5668 spore suspension (1.4 × 104 to 2.4 × 104 conidia/mouse) into their lateral tail vein. Therapy was delayed until 24 h after challenge.
In the transient-suppression model, caspofungin and AmB were tested at twofold-increasing doses from 0.03 to 1.0 mg/kg of body weight administered i.p., once daily (q.d.), for a total of 14 days. There were 10 mice per therapy group.
In the chronic-suppression model, caspofungin and AmB were tested at doses of 0.25, 0.5, and 1.0 mg/kg i.p., q.d., for 7 days. In the first two chronic-suppression studies there were 10 mice per group, and in the third study there were 50 mice per group.
In both models, the infected, sham-treated control mice received sterile distilled water and morbidity and mortality were recorded daily for 28 days.
Candidiasis studies.
In all the Candida studies, a disseminated infection was induced in immunosuppressed ICR mice by the i.v. inoculation of 0.2 ml of a yeast cell suspension (2.0 × 104 to 1.22 × 105 cells/mouse) of C. albicans MY1055 into the lateral tail vein. These infectious doses for C. albicans MY1055 were used in order to attain maximum tissue colonization with minimum mortality for the course of the therapy period (7 days). Efficacy was based on reduction of CFU of C. albicans per gram of kidneys at day 8 after challenge for the transient-suppression model and at selected time points after challenge in the chronic-suppression model (target organ kidney assays). For both suppression models, efficacy was also determined based on survival at day 21 after infection.
In both transient- and chronic-suppression studies, paired kidneys from five mice were collected (as described below) at 24 h after infection and prior to therapy to determine CFU of Candida per gram of kidneys at the time therapy was initiated. Antifungal therapy was not initiated until 24 h after challenge, and mice were treated i.p., q.d., for a total of 7 days. The infected, sham-treated control animals received sterile distilled water administered i.p., q.d., for a total of 7 days.
In the transient-suppression study, mice were treated with caspofungin at twofold-increasing doses from 0.06 to 2.0 mg/kg. AmB was tested at twofold-increasing doses from 0.06 to 1.0 mg/kg. At 8 days after infection (24 h after the last dose), paired kidneys from euthanatized mice (five per group) were removed using aseptic techniques, weighed, and placed in sterile Whirl-Pak bags (Fisher Scientific, Springfield, N.J.) containing 5 ml of sterile saline. Kidneys were homogenized in the bags and serially diluted in saline, and aliquots were plated on SDA. Plates were incubated at 35°C, and CFU of C. albicans were enumerated after 30 to 48 h. Means of the CFU per gram of tissue from drug-treated groups were compared to the means from sham-treated controls. Percent sterilization was indicated by the number of mice with no detectable yeast, with the limit of detection, because of the dilution scheme, being 50 yeast cells per pair of kidneys. For data from individual mice where no detectable yeast cells were recovered from the tissues, 49 CFU per pair of kidneys was used so that the counts would be one less than the limit of detection. Mice assigned to the survival study (10 mice per group) were monitored daily, and mortality was recorded for 21 days after infection. At day 21 after challenge, the 50% protective dose (PD50) and PD90 were determined (as described below).
In the chronic-suppression studies, mice were treated with either caspofungin, AmB, or FCZ. Caspofungin and AmB were tested at titrated doses of 0.25, 0.50, or 1.0 mg/kg. FCZ was tested at titrated doses of 20.0, 40.0 or 80.0 mg/kg. At 4, 8, 14, 21, and 28 days after infection, the CFU of C. albicans per gram of paired kidneys was enumerated (five mice per group per experiment) as described above. Mice assigned to the survival study (10 mice per group per study) were monitored daily, and mortality was recorded for 28 days after infection. PD50s and PD90s were determined (as described below) at day 28 after challenge.
Statistical analyses.
In the disseminated aspergillosis models, the PD50s and PD90s based on survival were estimated by a robust probit method (14, 20) from survival rates calculated by the Kaplan-Meier technique (11) at day 28 after challenge.
In the disseminated candidiasis models, means of log10 CFU of yeast per gram of kidneys from the treated groups were compared to those of the sham-treated control using Student's t test (two tailed, unpaired) on Microsoft Excel. Comparisons were deemed significant at the α = 0.05 level. Means of percent reduction in CFU of Candida per gram of kidney for treated groups at the selected time point following challenge relative to control were computed. A linear trend was typically evident when dose and CFU were both expressed on a log10 scale. Inverse regression (7) was subsequently used to estimate 90% effective doses (ED90s) and ED99s, defined as the doses (milligrams per kilogram) that reduced the number of CFU per organ by 90 and 99%, respectively. The PD50s and PD90s based on survival were estimated by a robust probit method (14, 20) from survival rates calculated by the Kaplan-Meier technique (11).
RESULTS
In vitro susceptibility.
The MICs of caspofungin and AmB for A. fumigatus MF5668 were 0.125 and 0.5 μg/ml, respectively. The MICs of caspofungin, AmB, and FCZ for C. albicans MY1055 were 0.5, 0.5, and 1.0 μg/ml, respectively.
Efficacy in the transient-immunosuppression model of disseminated aspergillosis.
The efficacy of delayed therapy (24 h after infection) with caspofungin or AmB (i.p., q.d., for 14 days) was determined for a disseminated A. fumigatus MF5668 infection (i.v. challenge with 1.6 × 104 CFU/mouse) in mice with CY-induced immunosuppression maintained for the entire therapy period. The mean total leukocyte counts of CY-treated mice remained below 2,400 cells/μl from the time of infection until day 10 after infection and then began to rise, reaching 4,300 and 10,700 cells/μl by days 14 and 17 (7 days after the last CY dose), respectively.
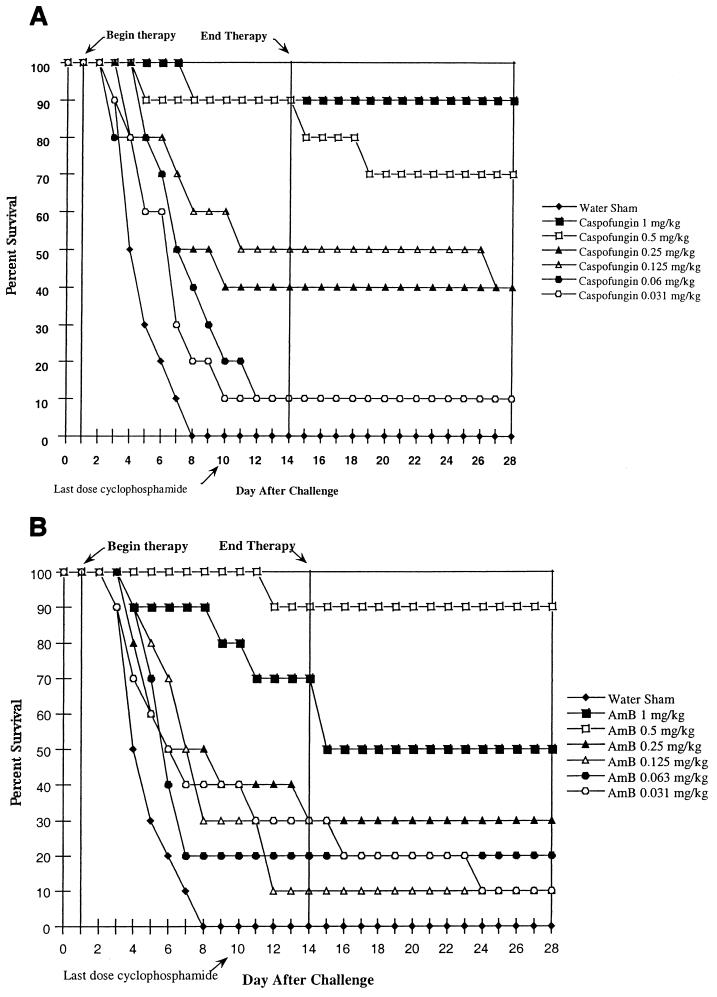
The percent survival over time for mice treated with caspofungin and AmB is shown in Fig. 1A and B, respectively. Caspofungin at concentrations of ≥0.125 mg/kg/dose significantly prolonged the survival of infected mice compared to that of infected sham-treated animals. Treatment with caspofungin at 0.5 and 1.0 mg/kg/dose resulted in 70 and 90% survival, respectively. AmB at concentrations of ≥0.25 and 0.63 mg/kg/dose significantly prolonged survival. However, the group receiving 1.0-mg/kg dose of AmB showed a sharp drop in survival compared to the group receiving the 0.5-mg/kg dose. Treatment with AmB at 0.5 and 1.0 mg/kg/dose resulted in 90 and 50% survival, respectively.
FIG. 1.
Efficacy in the transient-suppression model of disseminated aspergillosis. ICR mice were immunosuppressed with a 6-mg/mouse dose of CY administered i.p. 3 days prior to infection with 1.6 × 104 CFU of A. fumigatus MF5668 (i.v.) per mouse. Immunosuppression was maintained by additional doses of CY (2 mg/mouse, i.p.) on days 1, 4, 7, and 10 after infection. Therapy was initiated 24 h after infection, and mice (10/group) were treated i.p., q.d., for 14 days. (A) Caspofungin; (B) AmB.
The PD50s (the 95% confidence interval is given parenthetically) based on survival at day 28 (14 days after the last dose) of caspofungin and AmB were 0.245 (0.157, 0.412) and 0.264 (0.167, ∞) mg/kg, respectively.
Efficacy in the chronic-immunosuppression model of disseminated aspergillosis.
The efficacy of delayed therapy (24 h after infection) with caspofungin or AmB (i.p., q.d., for 7 days) was determined in three separate studies of disseminated A. fumigatus MF5668 infection in mice with CY-induced immunosuppression maintained for the entire experimental period (28 days after challenge). Mice were challenged i.v. with A. fumigatus MF5668 at 1.0 × 104 CFU/mouse (study 1), at 2.4 × 104 CFU/mouse (study 2), and at 1.88 × 104 CFU/mouse (study 3). The mean total leukocyte counts for normal mice (nonimmunosuppressed) ranged between 5,470 to 13,900 cells/μl for all sample times. The mean total leukocyte counts of CY-treated mice remained below 3,000 cells/μl from the time of infection until day 21 after infection and then began to rise, reaching 5,200 and 5,600 cells/μl by day 25 and 29, respectively.
The percent survival at day 28 after challenge (21 days after the last therapy) of mice treated with caspofungin at doses of ≥0.5 mg/kg ranged from 50 to 100% in the three studies. The percent survival at day 28 after challenge of mice treated with AmB at doses of ≥0.5 mg/kg ranged from 40 to 90% (Table 1). It should be noted that there was considerable variation between survival rates in the three studies. The PD50s of caspofungin at day 28 after challenge ranged from 0.173 to 0.400 mg/kg, and the PD50s of AmB ranged from 0.235 to 0.600 mg/kg (Table 1).
TABLE 1.
Percent survival and PDs (day 28) for mice in the chronic suppression model of disseminated aspergillosisa
| Treatment group | % Survival
|
PD50 (95% confidence interval)
|
PD90 (95% confidence interval)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Study 1 | Study 2 | Study 3 | Study 1 | Study 2 | Study 3 | Study 1 | Study 2 | Study 3 | |
| Caspofungin | |||||||||
| 1.0 mg/kg | 80.0 | 50.0 | 92.0 | 0.328 (0.199, 0.522) | 0.400 (0.194, ∞) | 0.173 (0.136, 0.207) | >1.0 (NC) | >1.0 (NC) | 0.486 (0.389, 0.684) |
| 0.5 mg/kg | 80.0 | 100.0 | 90.0 | ||||||
| 0.25 mg/kg | 40.0 | 30.0 | 86.0 | ||||||
| AmB | |||||||||
| 1.0 mg/kg | 80.0 | 50.0 | 90.0 | 0.500 (0.314, ∞) | 0.600 (0.329, ∞) | 0.235 (0.189, 0.282) | >1.0 (NC) | >1.0 (NC) | 0.753 (0.582, ∞) |
| 0.5 mg/kg | 40.0 | 70.0 | 80.0 | ||||||
| 0.25 mg/kg | 30.0 | 30.0 | 56.0 | ||||||
| Infected, sham treated | 10.0 | 10.0 | 22.0 | ||||||
| CY controls | |||||||||
| Sham infected, sham treated | 100.0 | 100.0 | 95.0 | ||||||
| Noninfected, nontreated | 90.0 | 100.0 | 100.0 | ||||||
Mice were challenged i.v. with A. fumigatus MF5668 at 1.0 × 104 CFU/mouse (study 1), at 2.4 × 104 CFU/mouse (study 2), and 1.88 × 104 CFU/mouse (study 3). Mice received the first treatment 24 h after challenge (delayed therapy) and were treated i.p., q.d., for 7 days. Mice were immunosuppressed throughout the experimental period (28 days). There were 10 mice per group in studies 1 and 2 and 50 mice per group in study 3, except for the CY control groups, which had 20 mice per group. PD50s and PD90s were calculated based on survival. NC, not calculated; ∞, infinitely large.
Efficacy in the transient-immunosuppression model of disseminated candidiasis.
The efficacy of delayed therapy (24 h after infection) with caspofungin or AmB (i.p., q.d., for 7 days) was determined against a disseminated C. albicans MY1055 infection (i.v. challenge with 2.0 × 104 CFU/mouse) in mice with CY-induced immunosuppression maintained for the entire therapy period (7 days after challenge). At 24 h after challenge and just prior to the initiation of therapy, the mean C. albicans count (five mice) was 3.2 × 104 CFU/g of kidney. Efficacy based on CFU of C. albicans per gram of kidneys was determined 8 days after challenge (1 day after discontinuation of therapy). Efficacy was also based on survival for 21 days after challenge (14 days after discontinuation of therapy).
Caspofungin was effective at doses from 0.125 to 2.0 mg/kg, since the log10 CFU of C. albicans per gram of kidneys of caspofungin-dosed mice were significantly lower than those of vehicle-treated mice. The percent of mice with sterile kidneys ranged between 80 and 100% at caspofungin doses from 0.25 to 2.0 mg/kg. Although AmB gave significant reductions in CFU per gram of kidneys at all doses tested (0.06 to 1.0 mg/kg), there was only 20% renal sterilization at the 1.0-mg/kg dose and no sterilization at lower doses (Table 2). The ED90s and ED99s, based on reduction in CFU of C. albicans per gram kidneys, for caspofungin were 0.049 and 0.119 mg/kg, respectively. The ED90s and ED99s for AmB were 0.071 and 0.198 mg/kg, respectively (Table 2).
TABLE 2.
Efficacy of delayed therapy against a disseminated C. albicans MY1055 infection in the CY-induced, transient-suppression model in ICR micea
| Dose (mg/kg) | Mean log10 CFU/g of kidneys (% sterilization)b
|
|
|---|---|---|
| Caspofunginc | AmBd | |
| 2.0 | 2.10*f ± 0.01 (100) | NTe |
| 1.0 | 2.14* ± 0.04 (100) | 2.89* ± 0.63 (20) |
| 0.5 | 2.13* ± 0.05 (100) | 3.48* ± 0.62 (0) |
| 0.25 | 2.38* ± 0.58 (80) | 4.46* ± 0.99 (0) |
| 0.125 | 4.62* ± 0.80 (0) | 4.78* ± 0.92 (0) |
| 0.063 | 6.06 ± 0.57 (0) | 5.61* ± 0.69 (0) |
| 0 | 6.47 ± 0.12 (0) | 6.47 ± 0.12 (0) |
Mice were challenged i.v. with C. albicans MY1055 at 2.0 × 104 CFU/mouse. Mice received first treatment 24 h after challenge (delayed therapy) and were treated i.p., q.d., for 7 days. Kidneys were aseptically collected at day 8 after challenge.
Mean log10 CFU/gram ± standard deviation at 8 days after challenge for paired kidneys. There were five mice per group except for the groups receiving no drug (three mice per group). Percent sterilization indicates the number of mice with no detectable yeast, where the limit of detection was 50 yeast cells per pair of kidneys.
ED90s and ED99s (95% confidence intervals) were calculated based on reduction in CFU/gram of kidneys of treated groups compared to sham-treated control animals and were 0.049 (0.014, 0.180) and 0.119 (0.038, 0.374), respectively.
See footnote c. Corresponding values were 0.071 (0.020, 0.254) and 0.198 (0.069, 0.571), respectively.
NT, not tested.
*, significantly different from result for sham-treated control (P < 0.05; Excel t test).
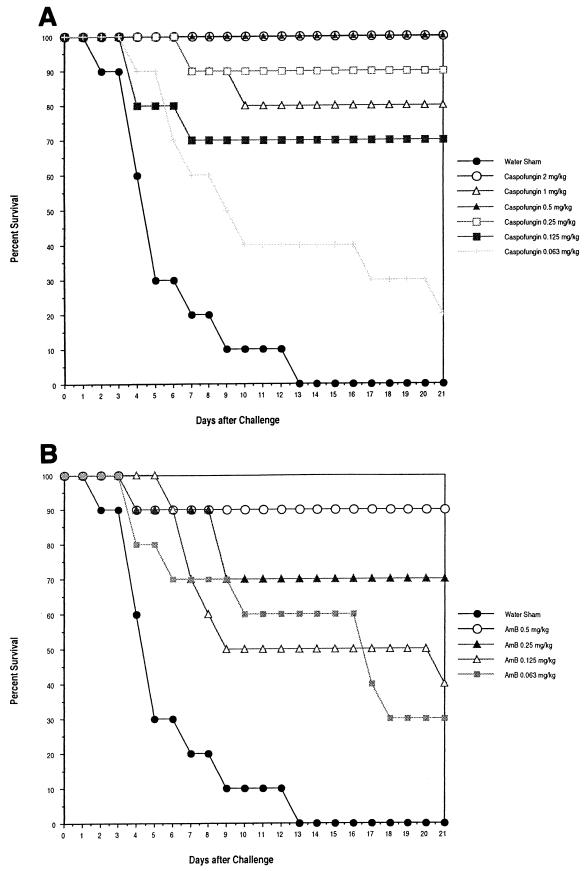
The percent survival over time for mice treated with caspofungin and AmB is shown in Fig. 2A and B, respectively. The percent survival at day 21 after challenge of mice treated with caspofungin at doses of ≥0.25 mg/kg ranged from 80 to 100% (Fig. 2A). The percent survival at day 21 after challenge of mice treated with AmB at doses of ≥0.25 mg/kg ranged from 70 to 90% (Fig. 2B). The PD50 (the 95% confidence interval is shown parenthetically) value based on survival at 21 days after challenge was 0.113 (0.075, 0.164) mg/kg for caspofungin and 0.222 (0.109, 0.513) mg/kg for AmB.
FIG. 2.
Efficacy of delayed therapy against disseminated C. albicans MY1055 infection in CY-treated, transient-suppression model in ICR mice. Mice were challenged i.v. with C. albicans MY1055 at 2.0 × 104 CFU/mouse. Mice (10/group) received first treatment 24 h after challenge (delayed therapy) and were treated i.p., q.d., for 7 days. Mice were immunosuppressed with a 6-mg/mouse dose of CY on day −3. Immunosuppression was maintained by additional doses of CY on days 1 and 4 after challenge. (A) Caspofungin; (B) AmB.
Efficacy in the chronic-immunosuppression model of disseminated candidiasis.
The efficacy of delayed therapy (24 h after infection) with caspofungin, AmB, and FCZ (i.p., q.d., for 7 days) was determined in separate studies of disseminated C. albicans MY1055 infection with CY-induced immunosuppression maintained for the entire experimental period (28 days after challenge). Mice were challenged i.v. with C. albicans MY1055 at 5.6 × 104 CFU/mouse (study 1) and 1.22 × 105 CFU/mouse (study 2). At 24 h after challenge and just prior to the initiation of therapy, the mean C. albicans count (10 mice) was 7.6 × 104 CFU/g of kidney. Efficacy was also based on survival for 28 days after challenge (21 days after discontinuation of therapy).
Caspofungin was effective at all doses tested (0.25 to 1.0 mg/kg), with the log10 CFU of C. albicans per gram of kidneys of caspofungin-treated mice being significantly lower (>99% reduction) than that of sham-treated mice from day 4 to day 28 after challenge. At caspofungin doses of 0.5 and 1.0 mg/kg, the percent of mice with sterile kidneys ranged between 70 and 100% from day 8 to 28 after challenge. AmB gave significant reductions in CFU per gram kidney at all doses tested (0.25 to 1.0 mg/kg) from day 8 to 28, except on day 21 at 0.25 mg/kg (Table 3). At AmB doses of 0.5 and 1.0 mg/kg, the renal sterilization ranged from 20 to 80% of the mice sampled after day 8 (Table 3). FCZ significantly reduced the CFU of C. albicans per gram of kidneys compared to those for the sham-treated mice at days 4 and 8 (1 day posttherapy) after challenge. However, by day 14 after challenge, the recovery of C. albicans from kidneys began to rise and reached a no-effect level by 21 days after challenge, except for the 20-mg/kg dose on day 21 (Table 3).
TABLE 3.
Efficacy of delayed therapy against disseminated C. albicans MY1055 infection in ICR mice with CY-induced, chronic immunosuppressiona
| Compound | Dose (mg/kg) | Log10 CFU/g kidneys (% sterilizationb [% reduction from control]c) at time point after challenge
|
||||
|---|---|---|---|---|---|---|
| Day 4 | Day 8 | Day 14 | Day 21 | Day 28 | ||
| Caspofungin | 1.00 | 3.64* ± 0.18 (0 [99.85]) | 2.30* ± 0.83 (90 [99.98]) | 2.07* ± 0.07 (70 [99.99]) | 2.07* ± 0.07 (100 [99.99]) | 2.09* ± 0.08 (100 [99.99]) |
| 0.50 | 3.94* ± 0.28 (0 [99.70]) | 2.10* ± 0.04 (100 [99.99]) | 2.06* ± 0.06 (100 [99.99]) | 2.08* ± 0.08 (100 [99.99]) | 2.10* ± 0.05 (90 [99.99]) | |
| 0.25 | 4.34* ± 0.34 (0 [99.26]) | 2.10* ± 0.15 (90 [99.99]) | 2.78* ± 0.96 (60 [99.97]) | 3.44* ± 1.66 (50 [99.68]) | 3.80* ± 1.74 (40 [99.93]) | |
| AmB | 1.00 | 5.09* ± 0.80 (0 [95.82]) | 2.07* ± 0.07 (80 [99.99]) | 2.46* ± 0.79 (60 [99.98]) | 2.37* ± 0.82 (80 [99.97]) | 3.41* ± 2.11 (80 [99.97]) |
| 0.50 | 5.63* ± 0.92 (0 [85.49]) | 2.91* ± 0.49 (60 [99.93]) | 2.93* ± 1.00 (50 [99.95]) | 3.47* ± 1.40 (20 [99.66]) | 4.14* ± 2.11 (40 [99.84]) | |
| 0.25 | 6.20 ± 0.39 (0 [46.79]) | 3.58* ± 0.51 (10 [99.67]) | 4.08* ± 0.93 (10 [99.33]) | 4.56 ± 1.30 (10 [95.78]) | 4.44* ± 2.57 (50 [99.68]) | |
| FCZ | 80.00 | 5.57* ± 0.51 (0 [90.53]) | 3.51* ± 1.35 (20 [99.87]) | 4.37* ± 0.38 (0 [99.69]) | 6.75 ± 0.91 (0 [NC]d) | 6.01 ± 2.26 (20 [NC]) |
| 40.00 | 5.91* ± 0.18 (0 [79.26]) | 3.45* ± 0.30 (0 [99.89]) | 5.35 ± 0.48 (0 [97.11]) | 6.97 ± 0.41 (0 [NC]) | 6.17 ± 2.36 (20 [NC]) | |
| 20.00 | 5.70* ± 0.36 (0 [87.12]) | 2.87 ± 0.59 (20 [99.97]) | 5.22 ± 1.01 (0 [97.85]) | 4.15 ± 2.01 (40 [NC]) | 7.68 (0 [NC]) | |
| Sham treated | 6.47 ± 0.30 (0) | 6.06 ± 0.30 (0) | 6.26 ± 1.28 (0) | 5.94 ± 2.24 (0) | 6.93 ± 0.41 (0) | |
Mice were challenged i.v. with C. albicans MY1055 at 5.6 × 104 CFU/mouse (study 1) and 1.22 × 105 CFU/mouse (study 2). Mice received the first treatment 24 h after challenge (delayed therapy) and were treated i.p., q.d., for 7 days. Mice were immunosuppressed throughout the experimental period (28 days). Kidneys were aseptically collected at days 4, 8, 14, 21, and 28 after challenge.
Mean log10 CFU/gram ± standard deviation at time points after challenge for paired kidneys. There were 10 mice per group except as follows. For FCZ-treated mice, there were five mice per group, except for the 20-mg/kg dose at day 28 (one mouse). Groups of sham-treated mice contained eight, five, and two mice on days 14, 21, and 28, respectively. Percent sterilization indicates the number of mice with no detectable yeast, where the limit of detection was 50 yeast cells per pair of kidneys. Data for caspofungin are pooled from studies 1 and 2.
Percent reduction calculated based on reduction in CFU/g of kidneys of treated groups compared to sham-treated control animals.
NC, not calculated.
*, significantly different from result for sham-treated control (P < 0.05; Excel t test).
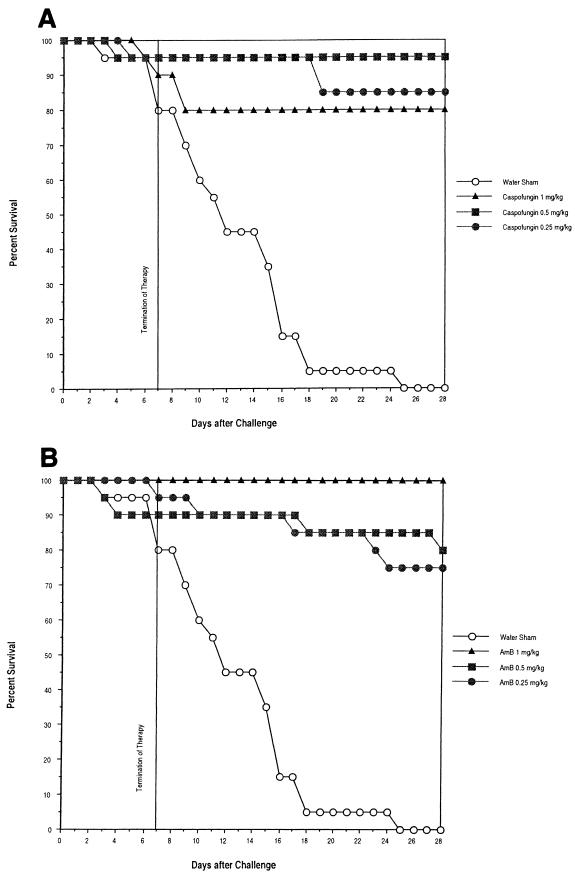
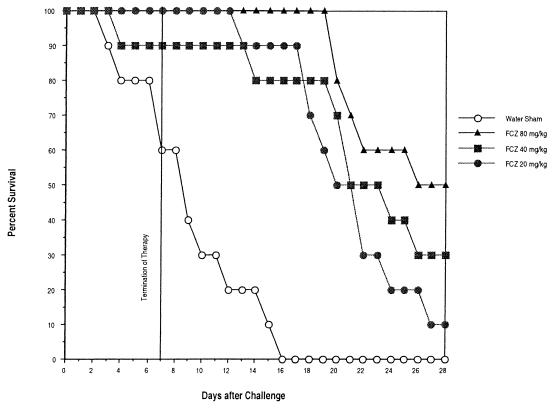
Percent survival over time for caspofungin, AmB, and FCZ is shown in Fig. 3A and B and 4, respectively. When day 28 survival data were compared, caspofungin's efficacy was comparable to that of AmB, and both were superior to FCZ. Percents survival at day 28 after challenge of mice treated with caspofungin at doses of 0.25, 0.5, and 1.0 mg/kg were 85, 95, and 80%, respectively. Percents survival at day 28 after challenge of mice treated with AmB at doses of 0.25, 0.50, and 1.0 mg/kg were 75, 80, and 100%, respectively. Percents survival at day 28 with FCZ at doses of 20.0, 40.0, and 80.0 mg/kg were 10, 30, and 50%, respectively.
FIG. 3.
Efficacy of delayed therapy against disseminated C. albicans MY1055 infection in CY-treated, chronically immunosuppressed ICR mice. Mice were challenged i.v. with C. albicans MY1055 at 5.6 × 104 CFU/mouse (study 1) and 1.22 × 105 CFU/mouse (Study 2). Mice received first treatment 24 h after challenge (delayed therapy) and were treated i.p., q.d., for 7 days. Survival data were pooled from both studies (20 mice total). Mice were immunosuppressed throughout the experimental period (28 days). (A) Caspofungin; (B) AmB.
DISCUSSION
Caspofungin, a new echinocandin in clinical development at Merck & Co. has been shown to have highly potent and reproducible in vitro activity on a wide variety of Candida species, including strains that have intrinsic or acquired resistance to other currently available antifungal agents (4, 17, 18, 22). Caspofungin has clear in vitro activity against Aspergillus species and against other filamentous and dimorphic fungi, although there are considerable species and strain variations (4, 6, 8; Connolly et al., 37th ICAAC). Preclinical evaluation in animal model infections has shown caspofungin to have efficacy against Candida species in both immunocompetent and immunocompromised animals (1, 10; Flattery et al., 36th and 38th ICAAC; J. G. Smith, G. K. Abruzzo, C. J. Gill, A. M. Flattery, L. Kong, H. Rosen, H. Kropp, and K. Bartizal, Abstr. 36th Int. Conf. Antimicrob. Agents Chemother., abstr. F41, 1996). In a multicenter, double-blind study, parenterally administered caspofungin at doses of 50 and 70 mg/day was efficacious and well tolerated in patients (78% human immunodeficiency virus positive) with endoscopically confirmed Candida esophagitis. Favorable clinical responses (confirmed by endoscopy) were seen in the majority of patients (∼85%) in the combined caspofungin groups, which was comparable to the clinical response (∼67%) seen in patients on AmB at 0.5 mg/kg/day (Sable et al., 37th ICAAC). Caspofungin has been reported to be highly efficacious in animal models of disseminated aspergillosis in complement component 5-deficient mice (1), neutropenic mice (Smith et al., 36th ICAAC), and mice with CY-induced leukopenia (Flattery et al., 38th ICAAC), as well as in a pulmonary aspergillosis model in immunocompromised rats (Bernard et al., 36th ICAAC). Although caspofungin has measurable in vitro activity against Cryptococcus neoformans (MICs ranging from 16 to 32 μg/ml [4]), previous studies have shown that it is not effective in mouse models of disseminated cryptococcosis (1).
This report describes the efficacy of caspofungin against disseminated C. albicans and A. fumigatus infections in mice with either CY-induced transient or prolonged leukopenia. In the transient-suppression models, mice were treated with CY to achieve leukopenia at the time of infection and to maintain immunosuppression throughout the therapy period. Generally, total leukocyte counts returned to normal values by 5 to 7 days after the last dose of CY. In the chronic suppression models, mice were treated with CY to maintain leukopenia for the entire experimental period.
The efficacy of caspofungin under all of these conditions, including those of prolonged CY-induced leukopenia, was equivalent to that of AmB for both Aspergillus and Candida infections. The degree of tissue sterilization achieved against C. albicans reflects the intrinsic activity and fungicidal capacity of caspofungin even when the host's cellular immune response is severely reduced. These preclinical evaluations of caspofungin support its usage for the treatment of fungal infections in patients who are refractory to or intolerant of other therapies. It is hoped that caspofungin may help meet a significant medical need in the treatment of disseminated fungal infections in the immunocompromised patient population, with advantages of both enhanced antifungal efficacy and tolerability.
FIG. 4.
Efficacy of delayed therapy with FCZ against disseminated C. albicans MY1055 infection in CY-treated, chronically immunosuppressed ICR mice. Mice were challenged i.v. with C. albicans MY1055 at 5.6 × 104 CFU/mouse (study 1) and 1.22 × 105 CFU/mouse (study 2). Mice (10/group) received first treatment 24 h after challenge (delayed therapy) and were treated i.p., q.d., for 7 days. Mice were immunosuppressed throughout the experimental period (28 days).
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992;14:S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 3.Balkovec J M, Black R M, Abruzzo G K, Bartizal K, Dreikorn S, Nollstadt K. Pneumocandin antifungal lipopeptides. The phenolic hydroxyl is required for 1,3-β-d-glucan synthesis inhibition. Bioorganic Med Chem Lett. 1993;3:2039–2042. [Google Scholar]
- 4.Bartizal K, Gill C J, Abruzzo G K, Flattery A M, Kong L, Scott P M, Smith J G, Leighton C E, Bouffard A, Dropinski J F, Balkovec J. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872) Antimicrob Agents Chemother. 1997;41:2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bills G F, Platas G, Peláez F, Mazurekar P. Reclassification of a pneumocandin-producing anamorph, Glarea lozoyensis, gen et sp. nov., previously identified as Zalerion arboricola. Mycol Res. 1998;103:179–192. [Google Scholar]
- 6.Del Poeta M, Schell W A, Perfect J. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother. 1997;41:1835–1836. doi: 10.1128/aac.41.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draper N R, Smith H. Applied regression analysis. New York, N.Y: John Wiley & Sons, Inc.; 1981. [Google Scholar]
- 8.Espinel-Ingroff A. A comparison of the in vitro activities of the new triazole SCH556592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graybill J R, Najvar L K, Luther M F, Fothergill A W. Treatment of murine disseminated candidiasis with L-743,872. Antimicrob Agents Chemother. 1997;41:1775–1777. doi: 10.1128/aac.41.8.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graybill J R, Bocanegra R, Luther M F, Fothergill A W, Rinaldi M J. Treatment of murine disseminated Candida krusei or Candida glabrata infection with L-743,872. Antimicrob Agents Chemother. 1997;41:1937–1939. doi: 10.1128/aac.41.9.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan E L, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 12.Kurtz M B, Heath I B, Marrinan J, Dreikorn S, Onishi J, Douglas C. Morphological effects of lipopeptides against Aspergillus fumigatus correlates with activity against (1,3)-β-d-glucan synthase. Antimicrob Agents Chemother. 1994;38:1480–1489. doi: 10.1128/aac.38.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz M B, Douglas C M. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 14.Morgan B J T. The analysis of quantal response data. London, United Kingdom: Chapman and Hall; 1992. pp. 59–65. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Nelson P W, Lozano-Chiu M, Rex J H. In vitro growth-inhibitory activity of pneumocandins, L-733,560 and L-743,872 against putatively amphotericin B- and fluconazole-resistant Candida isolates: influence of assay conditions. J Med Vet Mycol. 1997;35:285–287. [PubMed] [Google Scholar]
- 18.Pfaller M, Messer S A, Gee S, Joly S, Pujol C, Sullivan D J, Coleman D C, Soll D R. In vitro susceptibility of Candida dubliniensis isolates tested against the new triazole and echinocandin antifungal agents. J Clin Microbiol. 1999;37:870–872. doi: 10.1128/jcm.37.3.870-872.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powles M A, Liberator P, Anderson J, Karkhanis Y, Dropinski J F, Bouffard F A, Balkovec J M, Fujioka H, Aikawa M, McFadden D, Schmatz D. Efficacy of MK-0991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:1985–1989. doi: 10.1128/aac.42.8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pregibon D. Resistant fits for some commonly used logistic models with medical applications. Biometrics. 1982;38:485–498. [PubMed] [Google Scholar]
- 21.Rex J H, Bennett J E, Sugar A M. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994;331:1325–1330. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 22.Vasquez J A, Lynch M, Boikov D, Sobel J D. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob Agents Chemother. 1997;38:1480–1489. doi: 10.1128/aac.41.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh T J, Gonzales C, Roilides E, Mueller B U, Ali N, Lewis L L, Whitcomb T O, Marshall D J, Pizzo P A. Fungemia in children infected with the human immunodeficiency virus: new epidemiologic patterns, emerging pathogens, and improved outcome with antifungal therapy. Clin Infect Dis. 1995;20:900–906. doi: 10.1093/clinids/20.4.900. [DOI] [PubMed] [Google Scholar]
- 24.Wheat L J. Fungal infections in the immunocompromised host. In: Rubin R H, Young L S, editors. Clinical approach to infection in the immunocompromised host. Vol. 3. New York, N.Y: Plenum Publishing Corporation; 1994. pp. 211–237. [Google Scholar]