Abstract
Objective
To determine the impact of lifestyle factors on life expectancy lived with and without Alzheimer’s dementia.
Design
Prospective cohort study.
Setting
The Chicago Health and Aging Project, a population based cohort study in the United States.
Participants
2449 men and women aged 65 years and older.
Main exposure
A healthy lifestyle score was developed based on five modifiable lifestyle factors: a diet for brain health (Mediterranean-DASH Diet Intervention for Neurodegenerative Delay—MIND diet score in upper 40% of cohort distribution), late life cognitive activities (composite score in upper 40%), moderate or vigorous physical activity (≥150 min/week), no smoking, and light to moderate alcohol consumption (women 1-15 g/day; men 1-30 g/day).
Main outcome
Life expectancy with and without Alzheimer’s dementia in women and men.
Results
Women aged 65 with four or five healthy factors had a life expectancy of 24.2 years (95% confidence interval 22.8 to 25.5) and lived 3.1 years longer than women aged 65 with zero or one healthy factor (life expectancy 21.1 years, 19.5 to 22.4). Of the total life expectancy at age 65, women with four or five healthy factors spent 10.8% (2.6 years, 2.0 to 3.3) of their remaining years with Alzheimer’s dementia, whereas women with zero or one healthy factor spent 19.3% (4.1 years, 3.2 to 5.1) with the disease. Life expectancy for women aged 65 without Alzheimer’s dementia and four or five healthy factors was 21.5 years (20.0 to 22.7), and for those with zero or one healthy factor it was 17.0 years (15.5 to 18.3). Men aged 65 with four or five healthy factors had a total life expectancy of 23.1 years (21.4 to 25.6), which is 5.7 years longer than men aged 65 with zero or one healthy factor (life expectancy 17.4 years, 15.8 to 20.1). Of the total life expectancy at age 65, men with four or five healthy factors spent 6.1% (1.4 years, 0.3 to 2.0) of their remaining years with Alzheimer’s dementia, and those with zero or one healthy factor spent 12.0% (2.1 years, 0.2 to 3.0) with the disease. Life expectancy for men aged 65 without Alzheimer’s dementia and four or five healthy factors was 21.7 years (19.7 to 24.9), and for those with zero or one healthy factor life expectancy was 15.3 years (13.4 to 19.1).
Conclusion
A healthy lifestyle was associated with a longer life expectancy among men and women, and they lived a larger proportion of their remaining years without Alzheimer’s dementia. The life expectancy estimates might help health professionals, policy makers, and stakeholders to plan future healthcare services, costs, and needs.
Introduction
Preventing Alzheimer’s dementia through lifestyle modifications has gained considerable attention in recent years owing to growing evidence that they help to slow cognitive decline and potentially reduce the risk of Alzheimer’s dementia.1 2 3 4 5 6 Specifically, we have shown that adherence to a healthy lifestyle is associated with a 60% lower risk of Alzheimer’s dementia.4 However, in addition to attenuating dementia risk, successful modification of these lifestyle factors is also associated with an increased life expectancy.7 8 With greater life expectancy, many more people will attain older ages, and because the risk of dementia increases exponentially with increasing age,9 10 they will be predisposed to the risk of cognitive impairment and Alzheimer’s dementia. Consequently, it might be plausible that lifestyle interventions could delay Alzheimer’s dementia to later ages, but the overall prevalence and years lived with the disease might not change or even increase. If that is the case, health professionals, policy makers, and stakeholders should plan future healthcare costs and needs adequately.
We conducted a multistate life table analysis by using data from a population cohort of older adults living on the south side of Chicago, Illinois, United States to determine the impact of lifestyle factors on life expectancy lived with and without Alzheimer’s dementia for women and men independently. This investigation will help us to understand whether an increased life expectancy through lifestyle modifications influences the overall years lived with Alzheimer’s dementia across the lifespan.
Methods
Study design, settings, and population
This study was performed within the Chicago Health and Aging Project (CHAP), a prospective population based cohort study designed to assess the risk factors of Alzheimer’s dementia in the general population. The objectives and design have been reported previously.11 12 Briefly, in 1993 all residents aged 65 years and older from a geographically defined community on the south side of Chicago received an invitation to participate, and 6157 (78.7% of all age eligible people established by community census) were enrolled. Starting in 2000, the CHAP study was extended with successive cohorts of residents (n=4645) in the study catchment area. A total of 10 802 people were enrolled from 1993 to 2012. Besides the minimum age of 65 and being a resident of geographically defined research communities, no other eligibility criteria for participation in the CHAP study were applied.
Data on a wide range of social and clinical phenotypes, including lifestyle factors, medical history, genotyping, and neurocognitive tests, were obtained during in-home assessments through structured self or interviewer administered questionnaires and biospecimen collection. Specifically, the neurocognitive tests were administered to all participants every three years up to six times throughout the study. At each of these population assessments, a stratified random sample was selected for a detailed clinical evaluation to determine the prevalence and incidence of Alzheimer’s dementia (supplementary table 1 and supplementary fig 1). Random sampling for clinical evaluation was based on stratums defined by age, sex, race, and categories of cognitive function.11 To avoid biased estimates because people could have unequal chances of being in the random sample, all our analyses were weighted for the sampling design. A total of 4021 clinical evaluations were performed on 2794 participants from 1994 to 2012. Of 4021 clinical evaluations, 1056 were used to determine the prevalence of Alzheimer’s dementia and 2965 clinical evaluations were used to assess the incidence.
The clinical evaluation for disease prevalence identified 336 participants with Alzheimer’s dementia (supplementary fig 1). The 2965 clinical evaluations for the incidence of Alzheimer’s dementia were performed among 2130 participants free of Alzheimer’s dementia at baseline. A participant without Alzheimer’s dementia at baseline was determined based on the cognitive assessment in-home interview and the clinical evaluation information.11 For example, an individual free of Alzheimer’s dementia at baseline had good cognitive function during the population interview or, if the cognitive function was intermediate or poor, was free from Alzheimer’s dementia according to the baseline clinical evaluation for prevalent dementia.11 The definition of disease free cohort has been previously described.11 Of 2130 participants in the incident sample, we excluded 20 (0.9%) because they returned the food frequency questionnaire after examination for incident Alzheimer’s dementia. The cycle in which participants returned dietary questionnaires established the baseline and the start of follow-up for our investigation. Of these 20 participants, three had incident Alzheimer’s dementia. Our overall study population included 2449 participants, 2110 free of Alzheimer’s dementia at baseline and 339 with prevalent Alzheimer’s dementia.
The Rush University Medical Center Institutional Review Board approved this study. All participants provided informed consent to participate in this study.
Assessment of lifestyle factors
Healthy lifestyle score was developed based on information about five modifiable lifestyle factors: diet,13 cognitive activities,14 physical activity,15 smoking,16 and alcohol consumption.17 In CHAP, dietary behavior was assessed by a validated food frequency questionnaire estimating how often, on average, a participant had consumed a specified amount of foods during the previous year. Dietary questionnaires were distributed to study participants for completion and mail return. The validity and reproducibility of the questionnaire were assessed in random samples selected by race from the study population.18 Quality of diet was determined using the Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) diet score, which has been significantly associated with a slower cognitive decline and lower risk of incident Alzheimer’s dementia.13 19 We defined a low risk or healthy diet as a MIND score (without alcohol) in the top 40% of the cohort distribution; that is, MIND score >7.5.6
We assessed late life cognitive activities by using a structured questionnaire that measures participation in seven cognitively stimulating activities during the past year.14 These activities include reading, visiting a museum, and playing games like cards, checkers, crosswords, or puzzles. We developed a composite cognitive activity score and defined a low risk or healthy cognitive activity score as 40% of the cohort distribution; that is, a cognitive activity score >3.43.6 Physical activity was assessed through the 1985 US Health Interview Survey where participants reported time spent in six moderate or vigorous activities, including walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding, and swimming.20 Following guidelines for physical activity in adults,21 people who were healthy or at low risk were defined as those who spent at least 150 min/week doing moderate or vigorous activity. Smoking status was self-reported, where participants were categorized as never smokers, current smokers, and former smokers.16 Former and never smokers were classified as healthy or at low risk. The frequency of alcoholic beverage intake was obtained through the food frequency questionnaires, and the average consumption was calculated.18 Following dietary guidelines for Americans 2020-25,22 those with light to moderate alcohol consumption (1-15 g/day in women and 1-30 g/day in men) were considered healthy or at low risk of Alzheimer’s dementia.
For each of these lifestyle factors, participants received a score of 1 if they met the threshold for healthy or low risk and 0 if they did not meet the criteria. The five scores were then summed to yield a final score within the range of 0-5, with higher scores indicating a healthier lifestyle.4 5 6
Race, sex, and education (years of formal schooling) were determined using the 1990 US census questions. History of heart disease, stroke, diabetes, and cancer was determined by self-report questions from the Established Populations for the Epidemiologic Study of the Elderly. Apolipoprotein E (APOE ε4) genotyping was measured on the single nucleotide polymorphisms of the rs7412 and rs429358 by Broad Institute Center for Genotyping (Cambridge, Massachusetts) using the hME Sequenom MassARRAY platform.23 We computed the body mass index for study participants by dividing their weight (kg) by their height (m2). The Center for the Epidemiological Study of Depression scale was used to quantify symptoms of depression.24
Clinical evaluation and diagnosis of Alzheimer’s dementia
Clinical evaluation and diagnosis of Alzheimer’s dementia have been described in detail previously.11 12 In short, cognitive measures included 17 tests (eg, verbal fluency test, Boston naming test, mini-mental state examination, word list memory) and were administered uniformly with examiners blinded to population interview and sampling category. A board certified neuropsychologist used data from these cognitive tests to summarize impairment in each of five cognitive domains, including memory, language, orientation, attention, and perception. Additionally, trained nurse clinicians performed a structured neurological examination and screened participants’ medical history. A board certified neurologist then reviewed all the data and re-examined each participant, emphasizing findings considered to be of clinical importance or atypical. The neurological examination was in agreement with the National Institutes of Health stroke scale.25
An impairment in two or more functions on cognitive performance tests and a loss of cognitive function determined by the neurologist were required to diagnose dementia. The diagnosis of Alzheimer’s dementia was determined by the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS and ADRDA) for probable Alzheimer’s disease.26 In the CHAP study, data on prevalent and incident Alzheimer’s dementia were collected up to February 2012.
Mortality assessment
We obtained mortality data from the Social Security Administration Death Master File, supplemented by field contacts with families and neighbors.27 Data on mortality were collected up to November 2017.
Statistical analysis
We developed a multistate life table to calculate the life expectancy and years lived with and without Alzheimer’s dementia according to adherence to a healthy lifestyle. A multistate life table is a demographic tool that allows the experience of people in different health states to be combined in the total life expectancy and the number of years that people could expect to live in the different health states. We have previously described similar calculations.7 8 28 29 30 31 32 33 In short, the current study included three health states, free of Alzheimer’s dementia, Alzheimer’s dementia, and death. In each of these health states, we investigated three transitions: free of Alzheimer’s dementia to Alzheimer’s dementia; free of Alzheimer’s dementia to death; and Alzheimer’s dementia to death. We estimated overall age and sex specific rates and sex specific hazard ratios for each transition by adherence to a healthy lifestyle using survival analysis with Gompertz distribution.34 The regression models were adjusted by age, race, marital status, education, APOE ε4 status, and comorbidities. Additionally, to account for lifestyle changes among people over time, we adjusted our models with the calendar cycle/cohort, in which the lifestyle factors were assessed. The prevalence of adherence to lifestyle score was calculated by sex and 10 year age groups separately for people with and without Alzheimer’s dementia. We used adjusted hazard ratios and prevalence of adherence to the lifestyle score to weight rates, which ultimately were used to create multistate life tables. We developed life tables for the overall population and three life tables according to the number of healthy lifestyle factors (zero or one, two or three, and four or five). In line with our previous studies,4 5 6 we used these categories because not many people had zero or five lifestyle factors. The multistate life table started at age 65 and ended at age 100. Confidence intervals of life expectancy estimates were predicted using a Monte Carlo simulation with parametric bootstrapping.
We conducted a series of sensitivity analyses to evaluate the robustness of our primary findings. Firstly, we adjusted our models for depressive symptoms and body mass index, in addition to the primary adjustment, and evaluated the association of lifestyle score with Alzheimer’s dementia and mortality.6 Depression and weight have a complex relation with Alzheimer’s dementia. Although both might qualify as risk factors for cognitive impairment, they could also qualify as outcomes of dementia (eg, depressive symptoms and weight loss). Therefore, we conducted this examination as a sensitivity analysis. Secondly, based on dietary guideline recommendations22 and data focused on alcohol and dementia,17 we included light to moderate alcohol use in the low risk or healthy score to limit alcohol intake in those who drink; however, people who do not drink should not be encouraged to start alcohol consumption. Therefore, we created a new lifestyle score without alcohol consumption and examined the association of lifestyle score (without alcohol) and risk of Alzheimer’s dementia and mortality. We also developed life tables for the lifestyle score without alcohol.
Thirdly, study participants were enrolled in our cohort on different calendar times from 1993 to 2009 (supplementary table 1), and therefore, we anticipate differences between people in adherence to lifestyle factors across calendar times, for example, being more healthy in recent years. To address this concern, we additionally adjusted all our models by the calendar cycle/cohort, in which participants provided lifestyle factors, and as sensitivity analysis, we limited the study population to participants who were enrolled in the study before 2000 only. We also explored the trajectory of lifestyle scores across calendar time, from 1993 to 2009. Fourthly, in the primary analyses, we censored participants who fulfilled the criteria for diagnosis of dementia by NINCDS and ADRDA but had another condition that impaired cognition. We conducted a sensitivity analysis by excluding these participants and re-evaluating the association between lifestyle, Alzheimer’s dementia, and mortality. Fifthly, we analyzed the role of lifestyle factors in relation to Alzheimer’s dementia and mortality separately in black or African American people and white people, and also examined the lifestyle score as a continuous variable in relation to the risk of Alzheimer’s dementia and mortality per one point increase (eg, one additional healthy factor) in the lifestyle score.
About 18% of participants with clinical evaluation for Alzheimer’s dementia did not respond to dietary or genetic study questions, introducing missing data to our investigation for the MIND score and APOE ε4 status. We imputed these missing values using multivariate imputations by chained equations35 with the mice package.36 We used a single imputation method and maximized the accuracy with a wide range of predictors related to diet and APOE ε4, such as physical activity, cognitive activity, body mass index, depressive symptoms, the incidence of Alzheimer’s dementia, and mortality. However, given statistical uncertainty in the imputation method, we conducted sensitivity analyses in the sample without missing or imputed data. In these sensitivity analyses, we repeated our primary analyses and computed the hazard ratios for three transitions (free of dementia, dementia, and mortality) and life expectancy with and without Alzheimer’s dementia in participants with no missing data. Analyses were performed using R statistical computing, version 4.0 (R Foundation for Statistical Computing, Vienna, Austria).37
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study.
Results
Table 1 shows the baseline characteristics in women (n=1540) and men (n=909) for the overall sample and by lifestyle score. The average age of women and men in the study was 76 years, and they had about 12.5 years of schooling; 57% of women and 56% of men were black or African American. Women and men with four or five low risk (or healthy) lifestyle factors were younger, mainly white, and had more years of education than those with zero or one lifestyle factor.
Table 1.
Baseline characteristics of women and men in Chicago Health and Aging Population (n=2449). Data are numbers (%) unless stated otherwise
| Characteristics | Women: lifestyle score | Men: lifestyle score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (0-5) | 0-1 healthy factor | 2-3 healthy factors | 4-5 healthy factors | Overall (0-5) | 0-1 healthy factor | 2-3 healthy factors | 4-5 healthy factors | ||
| No of participants (%) | 1540 (100) | 411 (26.7) | 878 (57.0) | 251 (16.3) | 909 (100) | 215 (23.7) | 536 (59.0) | 158 (17.3) | |
| Demographics | |||||||||
| Age (years), mean (SD) | 76.2 (6.8) | 77.7 (7.3) | 76.1 (6.8) | 73.8 (5.4) | 75.8 (6.7) | 76.6 (7.2) | 76.0 (6.8) | 74.0 (5.3) | |
| Black or African American | 884 (57.4) | 283 (68.9) | 519 (59.1) | 82 (32.7) | 505 (55.6) | 160 (74.4) | 297 (55.4) | 48 (30.4) | |
| Education (years), mean (SD) | 12.5 (3.3) | 11.4 (3.3) | 12.5 (3.2) | 14.2 (2.9) | 12.6 (4.1) | 10.7 (3.6) | 12.7 (4.1) | 15.0 (3.2) | |
| Marital status | |||||||||
| Single | 85 (5.5) | 25 (6.1) | 44 (5.0) | 16 (6.4) | 42 (4.6) | 9 (4.2) | 26 (4.9) | 7 (4.4) | |
| Married | 520 (33.8) | 126 (30.7) | 280 (31.9) | 114 (45.4) | 636 (70.0) | 147 (68.4) | 362 (67.5) | 127 (80.4) | |
| Divorced or separated | 156 (10.1) | 46 (11.2) | 89 (10.1) | 21 (8.4) | 61 (6.7) | 19 (8.8) | 34 (6.3) | 8 (5.1) | |
| Widowed | 778 (50.6) | 214 (52.1) | 464 (52.9) | 100 (39.8) | 170 (18.7) | 40 (18.6) | 114 (21.3) | 16 (10.1) | |
| Genetics | |||||||||
| APOE ε4 carrier | 502 (32.6) | 143 (34.8) | 280 (31.9) | 79 (31.5) | 307 (33.8) | 80 (37.2) | 175 (32.6) | 52 (32.9) | |
| Lifestyle factors | |||||||||
| MIND diet, mean score (SD) | 6.9 (1.6) | 5.9 (1.1) | 7.0 (1.5) | 8.1 (1.4) | 6.6 (1.6) | 5.7 (1.1) | 6.6 (1.6) | 7.8 (1.5) | |
| Late life cognitive activity, mean score (SD) | 3.2 (0.7) | 2.7 (0.6) | 3.2 (0.7) | 3.8 (0.4) | 3.0 (0.7) | 2.6 (0.6) | 3.0 (0.6) | 3.6 (0.5) | |
| Physical activity (min/week), median (IQR) | 50.0 (0.0-210.0) | 0.0 (0.0-47.5) | 55.5 (0.0-201.9) | 270.0 (176.2-420.0) | 120.0 (0.0-330.0) | 0.0 (0.0-63.8) | 145.0 (10.0-360.0) | 312.5 (210.0-555.6) | |
| Current smoker | 153 (9.9) | 85 (20.7) | 61 (6.9) | 7 (2.8) | 108 (11.9) | 58 (27.0) | 49 (9.1) | 1 (0.6) | |
| Alcohol consumption (g/day), mean (SD) | 3.0 (6.5) | 1.7 (6.1) | 3.1 (6.5) | 4.9 (6.9) | 6.8 (12.8) | 4.2 (13.7) | 7.5 (13.2) | 7.7 (8.9) | |
| Lifestyle score 0-5, mean (SD) | 2.3 (1.1) | — | — | — | 2.4 (1.2) | — | — | — | |
| Comorbidities | |||||||||
| 0 | 822 (53.4) | 184 (44.8) | 485 (55.2) | 153 (61.0) | 455 (50.1) | 109 (50.7) | 267 (49.8) | 79 (50.0) | |
| 1 | 508 (33.0) | 149 (36.3) | 275 (31.3) | 84 (33.5) | 341 (37.5) | 72 (33.5) | 213 (39.7) | 56 (35.4) | |
| 2 | 182 (11.8) | 66 (16.1) | 104 (11.8) | 12 (4.8) | 101 (11.1) | 27 (12.6) | 54 (10.1) | 20 (12.7) | |
| 3-4 | 28 (1.8) | 12 (2.9) | 14 (1.6) | 2 (0.8) | 12 (1.3) | 7 (3.3) | 2 (0.4) | 3 (1.9) | |
| Others risk factors | |||||||||
| Body mass index, mean (SD) | 27.5 (6.1) | 27.5 (6.8) | 27.7 (6.0) | 26.8 (4.8) | 26.5 (4.7) | 25.5 (5.2) | 26.7 (4.5) | 27.2 (4.4) | |
| Depressive symptoms, mean CESD scale score (SD) | 1.7 (2.1) | 2.4 (2.4) | 1.6 (1.9) | 1.1 (1.7) | 1.4 (1.9) | 1.9 (2.1) | 1.3 (1.8) | 0.9 (1.5) | |
Comorbidities include heart disease, stroke, diabetes, and cancers. MIND diet score does not include the point from alcohol (wine) intake; that is, MIND diet without alcohol.
APOE=apolipoprotein E; CESD=Center for Epidemiologic Studies Depression; MIND=Mediterranean-DASH Diet Intervention for Neurodegenerative Delay.
Incident Alzheimer’s dementia and mortality
Table 2 presents the association between lifestyle score and risk of Alzheimer’s dementia and mortality in women and men. During 13 047 person years of follow-up for incident Alzheimer’s dementia, 439 (20.8%) participants developed the disease. Higher adherence to a healthy lifestyle, defined as people with four or five healthy factors, was associated with a lower risk of Alzheimer’s dementia in women (hazard ratio 0.44, 95% confidence interval 0.32 to 0.59) and men (0.30, 0.19 to 0.47) compared with participants with zero or one healthy factor. Similarly, women and men with four or five healthy factors had a lower mortality risk than those with zero or one healthy factor. The corresponding hazard ratios were 0.56 (0.49 to 0.65) for women and 0.47 (0.39 to 0.57) for men. Among people living with Alzheimer’s dementia, women with four or five healthy factors had a higher risk of mortality after the development of dementia (1.31, 1.03 to 1.67) compared with women with zero or one health behavior. No significant associations were noted in men (1.00, 0.73 to 1.39).
Table 2.
Associations between healthy lifestyle score and risk of Alzheimer’s dementia and mortality in women and men
| Transition | Women | Men | |||||
|---|---|---|---|---|---|---|---|
| Number*; person years | Lifestyle score (healthy factors) | Hazard ratio (95% CI) | Number*; person years | Lifestyle score (healthy factors) | Hazard ratio (95% CI) | ||
| Incident Alzheimer’s dementia | 275; 8332 | 0-1 | 1 (reference) | 164; 4715 | 0-1 | 1 (reference) | |
| — | 2-3 | 0.71 (0.59 to 0.87) | — | 2-3 | 0.70 (0.53 to 0.93) | ||
| — | 4-5 | 0.44 (0.32 to 0.59) | — | 4-5 | 0.30 (0.19 to 0.47) | ||
| Mortality in participants without Alzheimer’s dementia | 787; 17 753 | 0-1 | 1 (reference) | 527; 9626 | 0-1 | 1 (reference) | |
| — | 2-3 | 0.69 (0.62 to 0.77) | — | 2-3 | 0.57 (0.50 to 0.67) | ||
| — | 4-5 | 0.56 (0.49 to 0.65) | — | 4-5 | 0.47 (0.39 to 0.57) | ||
| Mortality in participants with Alzheimer’s dementia | 424; 2521 | 0-1 | 1 (reference) | 260; 1321 | 0-1 | 1 (reference) | |
| — | 2-3 | 1.11 (0.99 to 1.24) | — | 2-3 | 0.83 (0.69 to 1.00) | ||
| — | 4-5 | 1.31 (1.03 to 1.67) | — | 4-5 | 1.00 (0.73 to 1.39) | ||
Models adjusted by age, race, marital status, education, apolipoprotein E ε4 and comorbidities. A behavior was classified as low risk (or healthy) if it met several criteria: Mediterranean-DASH Diet Intervention for Neurodegenerative Delay—MIND score (without alcohol) >7.5, corresponding to upper 40% of cohort distribution; cognitive activity score >3.43, corresponding to upper 40% of cohort distribution; not being a current smoker; moderate or vigorous exercise for ≥150 min/week; and light to moderate alcohol consumption (1-15 g/day for women and 1-30 g/day for men). Participants with Alzheimer’s dementia (transition 3: mortality in participants with Alzheimer’s dementia) include prevalent dementia at baseline (n=339) and incident dementia (n=439) during follow-up.
Number refers to the number of events (Alzheimer’s dementia or death) during the follow-up for each transition.
Life expectancy and years lived with and without Alzheimer’s dementia
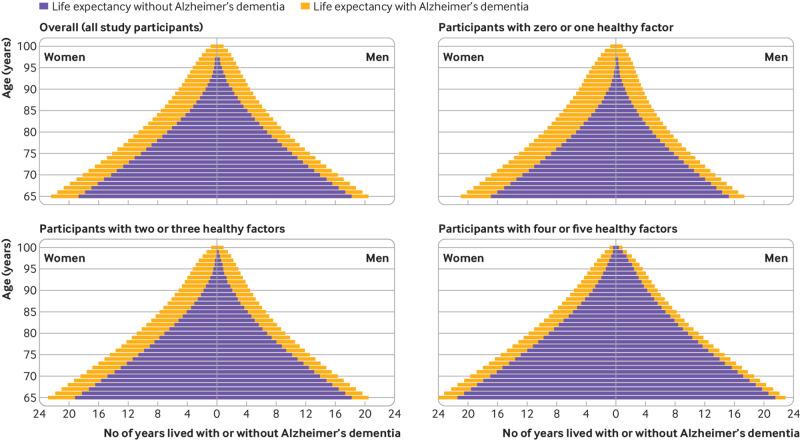
Figure 1 shows the total life expectancy and years lived with and without Alzheimer’s dementia in women and men, overall and by lifestyle score. Figure 2 illustrates the proportion of life expectancy lived with Alzheimer’s dementia. At age 65, the life expectancy in women was 22.5 years (95% confidence interval 21.0 to 23.8), of which 3.7 years (2.9 to 4.6), corresponding to 16.3% of life expectancy, were lived with Alzheimer’s dementia. For men, the total life expectancy at age 65 was 20.6 years (18.9 to 23.4) and 2.3 years (0.4 to 3.0), or 11.0% of life expectancy, were lived with Alzheimer’s dementia. The life expectancy at age 85 was 7.6 years (6.8 to 8.5) in women and 6.6 years (5.4 to 8.4) in men; women lived 49.9% (3.8 years, 3.0 to 4.8) and men 37.2% (2.5 years, 0.5 to 3.3) of their life expectancy with Alzheimer’s dementia.
Fig 1.
Overall life expectancy and life expectancy with and without Alzheimer’s dementia according to categories of lifestyle score in women and men. A behavior was classified as low risk or healthy if it met several criteria: Mediterranean-DASH Diet Intervention for Neurodegenerative Delay—MIND score (without alcohol) >7.5, corresponding to upper 40% of cohort distribution; cognitive activity score >3.43, corresponding to upper 40% of cohort distribution; not being a current smoker; moderate or vigorous exercise for ≥150 min/week; and light to moderate alcohol consumption (1-15 g/day for women and 1-30 g/day for men)
Fig 2.
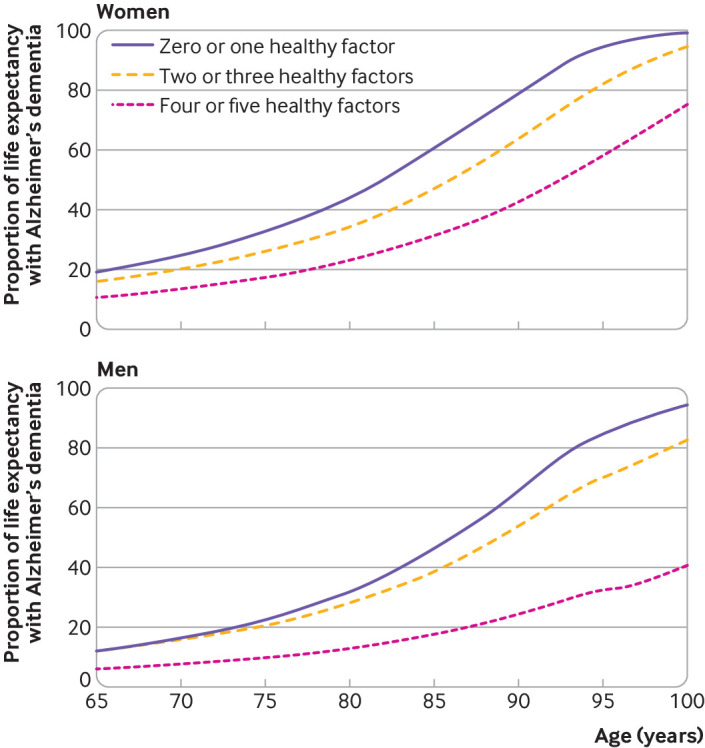
Proportion of life expectancy spent with Alzheimer’s dementia according to categories of lifestyle score in women and men. Proportion is computed by dividing life expectancy lived with Alzheimer’s dementia by total life expectancy at a given age (supplementary tables 2 and 3). A behavior was classified as low risk or healthy if it met several criteria: Mediterranean-DASH Diet Intervention for Neurodegenerative Delay—MIND score (without alcohol) >7.5, corresponding to upper 40% of cohort distribution; cognitive activity score >3.43, corresponding to upper 40% of cohort distribution; not being a current smoker; moderate or vigorous exercise for ≥150 min/week; and light to moderate alcohol consumption (1-15 g/day for women and 1-30 g/day for men)
Total life expectancy differed by lifestyle score in women and men (fig 1 and fig 2). Women aged 65 with four or five healthy factors had a life expectancy of 24.2 years (95% confidence interval 22.8 to 25.5) and lived 3.1 years more than women aged 65 with zero or one healthy factor (life expectancy 21.1 years, 19.5 to 22.4). These differences were also present in years lived with and without Alzheimer’s dementia. Of the total life expectancy at age 65, women with four or five healthy factors spent 10.8% (2.6 years, 2.0 to 3.3) of their life expectancy with Alzheimer’s dementia, whereas women with zero or one healthy factor spent 19.3% (4.1 years, 3.2 to 5.1) with the disease. Life expectancy in women aged 65 without Alzheimer’s dementia with four or five healthy factors was 21.5 years (20.0 to 22.7) and in those with zero or one healthy factor it was 17.0 years (15.5 to 18.3). For women aged 85, the differences in years lived with and without Alzheimer’s dementia were more notable. Those with four or five healthy factors had a life expectancy of 8.5 years (7.7 to 9.4), of which 30.9% (2.6 years, 2.0 to 3.4) were lived with Alzheimer’s dementia, whereas women with zero or one healthy factor had a total life expectancy of 7.2 years (6.3 to 8.1) and 60.3% (4.4 years, 3.4 to 5.4) were lived with Alzheimer’s dementia. Life expectancy in women aged 85 without Alzheimer’s dementia with four or five healthy factors was 5.9 years (4.8 to 6.8) and in those with zero or one healthy factor it was 2.9 years (2.0 to 3.6).
Men aged 65 with four or five healthy factors had a total life expectancy of 23.1 years (21.4 to 25.6), which is 5.7 years more than men aged 65 with zero or one healthy behavior (life expectancy 17.4 years, 15.8 to 20.1). Of the total life expectancy at age 65, men with four or five healthy factors spent 6.1% (1.4 years, 0.3 to 2.0) of their life expectancy with Alzheimer’s dementia, and those with zero or one healthy behavior spent 12.0% (2.1 years, 0.2 to 3.0) with the disease. Life expectancy for men aged 65 without Alzheimer’s dementia with four or five healthy factors was 21.7 years (19.7 to 24.9), and for those with zero or one healthy factor it was 15.3 years (13.4 to 19.1). At age 85, the proportion of life expectancy spent with Alzheimer’s dementia was 17.7% (1.5 years, 0.5 to 2.1) in men with four or five healthy factors and 46.0% (2.4 years, 0.3 to 3.3) in those with zero or one healthy factor. Life expectancy for men aged 85 without Alzheimer’s dementia with four or five healthy factors was 6.8 years (5.3 to 9.3) and for those with zero or one healthy factor it was 2.8 years (1.6 to 6.1). Supplementary tables 2 and 3 show life expectancy estimates by age in women and men with four or five and zero or one healthy factors.
Sensitivity analyses
The results of sensitivity analyses are presented in the supplementary material. Additional adjustment for body mass index and depressive symptoms did not change the strength or significance of the association between lifestyle score, Alzheimer’s dementia, and mortality in women and men. For example, for the risk of Alzheimer’s dementia the hazard ratio was 0.46 (95% confidence interval 0.34 to 0.62) in women and 0.31 (0.20 to 0.48) in men with four or five healthy factors compared with those with zero or one healthy factor (supplementary table 4). The lifestyle score without alcohol scoring was associated with a lower risk of Alzheimer’s dementia and mortality. The results were similar to the primary analysis, although the strength of the relation with Alzheimer’s dementia was slightly weakened in women (0.49 vs 0.44) and men (0.34 vs 0.30; supplementary table 5 and supplementary fig 2). We did not observe significant differences in the average lifestyle score from 1993 to 2009 (supplementary fig 3), and restricting our study population to participants enrolled in the cohort before 2000 showed similar results as the primary analysis; that is, a healthy lifestyle is associated with a lower risk of Alzheimer’s dementia and mortality (supplementary table 6).
When we excluded participants who fulfilled the criteria for diagnosis of Alzheimer’s dementia by NINCDS and ADRDA but had another condition that impaired cognition (eg, atypical dementia), the strength or the significance of the associations did not change (supplementary table 7). Overall, associations between lifestyle, Alzheimer’s dementia, and mortality were similar for white people and black or African American people; however, we noted a stronger association of lifestyle factors and risk of Alzheimer’s dementia in white people than in black or African American people (hazard ratio 0.26 vs 0.37; supplementary table 8). The lifestyle score as a continuous variable was associated with a lower risk of Alzheimer’s dementia and mortality in women and men (supplementary table 9). When we excluded participants with missing or imputed data, the results were similar to the original analysis for associations of lifestyle score with risk of Alzheimer’s dementia and mortality (supplementary table 10) or life expectancy with and without Alzheimer’s dementia in women and men (supplementary fig 4).
Discussion
This study provides quantitative data about the influence of lifestyle factors on life expectancy with and without Alzheimer’s dementia. Using data from a population based cohort study in the US, adherence to a healthy lifestyle was associated with a longer life expectancy and fewer years lived with Alzheimer’s dementia across the lifespan. On average, the total life expectancy at age 65 in women and men with four or five healthy factors was 24.2 and 23.1 years, of which 10.8% and 6.1% were spent with Alzheimer’s dementia. For women and men with zero or one healthy factor, life expectancy was shorter—21.1 and 17.4 years—and more of their remaining life expectancy was spent with Alzheimer’s dementia (19.3% and 12.0%, respectively). At age 85, these differences were even more notable. This investigation suggests that a prolonged life expectancy owing to a healthy lifestyle is not accompanied by an increased number of years living with Alzheimer’s dementia.
Comparison with other studies
Adherence to a healthy lifestyle has been associated with a slower cognitive decline and a lower risk of Alzheimer’s dementia.2 3 4 5 6 Lifestyle factors are also predictors of longevity, and so adopting a healthy lifestyle could prolong life expectancy.7 8 With increasing life expectancy, more people will reach older ages when cognitive impairment and Alzheimer’s dementia become increasingly common,9 10 questioning the long term consequence of lifestyle interventions on Alzheimer’s dementia. Acknowledging the influence of lifestyle on Alzheimer’s dementia (morbidity) and longevity (mortality), we used a multistate life expectancy analysis that considers morbidity (eg, Alzheimer’s dementia) and mortality, and provides estimates on years lived with and without the disease.7 8 28 29 30 31 32 33 We reported that a healthy lifestyle is associated with a longer life expectancy and most of these years were lived free of Alzheimer’s dementia. Years lived free from Alzheimer’s dementia are estimated based on the risk of incident dementia and mortality in those without dementia. We observed that a healthy lifestyle is associated with a lower risk of Alzheimer’s dementia and mortality, suggesting that the event of dementia could be postponed in older ages and people will live longer, which ultimately will contribute to increased years lived free from Alzheimer’s dementia. The number of years lived with Alzheimer’s dementia is a result of mortality risk among participants living with dementia. We did observe an increased risk of mortality in women with dementia, and no significant association in men, suggesting that after the event of dementia, fewer years are expected to be lived with Alzheimer’s dementia in people with four or five healthy behaviors. Life expectancy estimates are also helpful for health professionals to estimate future healthcare costs and to plan for healthcare needs.
Studies that estimate life expectancy with and without Alzheimer’s dementia according to adherence to lifestyle factors seem to be lacking. Most of the studies in the literature have focused on calculating the overall life expectancy lived with dementia,38 39 40 and only a few studies have examined the role of risk factors, such as educational attainment or genetic risk (defined by the presence of the APOE ε4 allele).33 41 Specifically, a study in the US (Seattle, Washington) using a Markov model to estimate life expectancy with and without dementia showed that people with higher educational attainment have an increased life expectancy without dementia and a greater percentage of life expectancy lived without dementia.41 A similar investigation from Europe (Rotterdam, Netherlands) confirmed that people with primary education spent more time of their remaining life expectancy with dementia than those with higher education.33 Additionally, this study showed that the presence of the APOE ε4 allele was associated with an increased number of years living with dementia in women and men.33 In this study, we controlled regression models for education and the APOE ε4 allele and focused our investigation on modifiable risk factors, such as lifestyle factors.
Consistent with our sex difference findings, a previous study found that women have a higher life expectancy than men and a higher percentage of life expectancy lived with dementia.41 Sex differences in the prevalence and incidence of Alzheimer’s dementia are recognized by the research community.42 43 In particular, a recently published study focusing on sex differences in dementia risk showed that while vascular, lifestyle, and psychosocial risk factors might have a similar effect on women and men, women had a higher risk of dementia after reaching age 80.44 Therefore, our study supports the hypothesis that a higher life expectancy in women could be a potential explanation for seeing more women than men living with dementia. However, further research is required to better understand sex differences in Alzheimer’s dementia risk.
Strengths and limitations of study
The strengths of our study are a population based design, long term follow-up, and accurate diagnosis of Alzheimer’s dementia through structured clinical neurological evaluations with neuropsychological testing. Our study has several limitations. Firstly, adherence to lifestyle factors was determined at baseline and not updated during the follow-up because we think that lifestyle changes could be attributed to cognitive impairment (eg, reverse causality) as the population ages. Secondly, the proportion of people with an unhealthy lifestyle could be underestimated in our study because people with poor health are less likely to participate in research studies (healthy volunteers effect), or due to their overall health, they might have died before having the opportunity to participate in our study (survival bias). Thirdly, assessments of lifestyle factors were based on self-report and could be prone to measurement error, although these questionnaires were validated.18 45 Fourthly, the life expectancy estimates provided in this study should not be generalized to other populations without additional research and validation.
Fifthly, the study population included in this analysis consisted of people recruited from 1993 to 2009. We adjusted for the calendar year when lifestyles were assessed and repeated analysis in those recruited before 2000; however, cohort effects might still impact our estimates. The cohort effect could be significant in dementia research, recognizing that dementia incidence and prevalence change over time, like any other non-communicable chronic disease. Finally, our estimates are based on observation data, and do not imply causal inferences.
Conclusion and public health implications
A healthy lifestyle was associated with a longer life expectancy among men and women, and with a larger proportion of remaining years lived without Alzheimer’s dementia. The life expectancy estimates presented here could help health professionals, policy makers, and stakeholders to plan future healthcare services, costs, and needs.
What is already known on this topic
Adherence to a healthy lifestyle is associated with a lower risk of Alzheimer’s dementia and longer life expectancy
With increasing life expectancy, more people will reach older ages when cognitive impairment and Alzheimer’s dementia become increasingly common
The impact of lifestyle on life expectancy with and without Alzheimer’s dementia needs to be investigated
What this study adds
The study findings suggest a healthy lifestyle could increase life expectancy among men and women
A healthy lifestyle might also increase the proportion of remaining years lived without Alzheimer’s dementia
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: KD designed and conceptualized the study, conducted data analysis, interpreted the findings, and drafted and revised the manuscript for intellectual content. OHF interpreted the findings, revised the manuscript, and provided intellectual content. CNF, PD, KRK, TMH, AD, XL, and NTA reviewed and revised the manuscript and provided intellectual content. KD and EMR did the statistical analysis. DAE oversaw data collection for the study and provided intellectual content. KBR interpreted the findings, reviewed data analysis, revised the manuscript, provided intellectual content, and supervised the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. KD and KBR are the guarantors. CNF is deceased.
Funding: This study was supported by the National Institutes On Aging of the National Institute of Health under award No R21AG070287, R01AG051635, RF1AG057532, R01AG058679, and R01AG073627. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Competing interests: Authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institutes On Aging of the National Institute of Health for the submitted work; support from National Institutes On Aging of the National Institute of Health outside the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Dissemination to participants and related patient and public communities: We plan to disseminate these findings to participants in our annual newsletter and to the general public in a press release.
The lead author (KD) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The Rush University Medical Center Institutional Review Board approved this study. All participants provided informed consent to be eligible to participate in this study.
Data availability statement
Deidentified data are available on request for qualified investigators from www.riha.rush.edu/dataportal.html
References
- 1. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255-63. 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 2. Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA 2019;322:430-7. 10.1001/jama.2019.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Licher S, Ahmad S, Karamujić-Čomić H, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med 2019;25:1364-9. 10.1038/s41591-019-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology 2020;95:e374-83. 10.1212/WNL.0000000000009816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhana K, Aggarwal NT, Rajan KB, Barnes LL, Evans DA, Morris MC. Impact of the apolipoprotein E4 allele on the relationship between healthy lifestyle and cognitive decline: a population-based study. Am J Epidemiol 2021;190:1225-33. 10.1093/aje/kwab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhana K, Barnes LL, Liu X, et al. Genetic risk, adherence to a healthy lifestyle, and cognitive decline in African Americans and European Americans. Alzheimers Dement 2021. 10.1002/alz.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Schoufour J, Wang DD, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ 2020;368:l6669. 10.1136/bmj.l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 2018;138:345-55. 10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ott A, Breteler MM, van Harskamp F, et al. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. BMJ 1995;310:970-3. 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement 2021;17:1966-75. 10.1002/alz.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago health and aging project (CHAP). J Alzheimers Dis 2003;5:349-55. 10.3233/JAD-2003-5501. [DOI] [PubMed] [Google Scholar]
- 12. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol 2003;60:185-9. 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 13. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015;11:1007-14. 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology 2007;69:1911-20. 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 15. Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014;14:510. 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aggarwal NT, Bienias JL, Bennett DA, et al. The relation of cigarette smoking to incident Alzheimer’s disease in a biracial urban community population. Neuroepidemiology 2006;26:140-6. 10.1159/000091654. [DOI] [PubMed] [Google Scholar]
- 17. Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 2018;362:k2927. 10.1136/bmj.k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 2003;158:1213-7. 10.1093/aje/kwg290 [DOI] [PubMed] [Google Scholar]
- 19. Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015;11:1015-22. 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65-72. 10.1016/S0749-3797(18)31107-3 [DOI] [PubMed] [Google Scholar]
- 21. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020-8. 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th edition. December 2020. URL: https://www.dietaryguidelines.gov/ (accessed 29 March 2021).
- 23. Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet 1991;337:1158-9. 10.1016/0140-6736(91)92823-K. [DOI] [PubMed] [Google Scholar]
- 24. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385-401. 10.1177/014662167700100306. [DOI] [Google Scholar]
- 25. Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol 1989;46:660-2. 10.1001/archneur.1989.00520420080026 [DOI] [PubMed] [Google Scholar]
- 26. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 1984;34:939-44. 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27. Rajan KB, Aggarwal NT, Wilson RS, Everson-Rose SA, Evans DA. Association of cognitive functioning, incident stroke, and mortality in older adults. Stroke 2014;45:2563-7. 10.1161/STROKEAHA.114.005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med 2005;165:2355-60. 10.1001/archinte.165.20.2355. [DOI] [PubMed] [Google Scholar]
- 29. Dhana K, Nano J, Ligthart S, et al. Obesity and life expectancy with and without diabetes in adults aged 55 years and older in the Netherlands: a prospective cohort study. PLoS Med 2016;13:e1002086. 10.1371/journal.pmed.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhana K, Berghout MA, Peeters A, et al. Obesity in older adults and life expectancy with and without cardiovascular disease. Int J Obes (Lond) 2016;40:1535-40. 10.1038/ijo.2016.94. [DOI] [PubMed] [Google Scholar]
- 31. Dhana K, Koolhaas CM, Berghout MA, et al. Physical activity types and life expectancy with and without cardiovascular disease: the Rotterdam Study. J Public Health (Oxf) 2017;39:e209-18. 10.1093/pubmed/fdw110. [DOI] [PubMed] [Google Scholar]
- 32. Bano A, Dhana K, Chaker L, et al. Association of thyroid function with life expectancy with and without cardiovascular disease: the Rotterdam study. JAMA Intern Med 2017;177:1650-7. 10.1001/jamainternmed.2017.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolters FJ, Tinga LM, Dhana K, et al. Life expectancy with and without dementia: a population-based study of dementia burden and preventive potential. Am J Epidemiol 2019;188:372-81. 10.1093/aje/kwy234. [DOI] [PubMed] [Google Scholar]
- 34. Olshansky SJ, Carnes BA. Ever since Gompertz. Demography 1997;34:1-15. 10.2307/2061656 [DOI] [PubMed] [Google Scholar]
- 35. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40-9. 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Buuren S, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1-67 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 37. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2021. [Google Scholar]
- 38. Ritchie K, Robine JM, Letenneur L, Dartigues JF. Dementia-free life expectancy in France. Am J Public Health 1994;84:232-6. 10.2105/AJPH.84.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ritchie K, Mathers C, Jorm A. Dementia-free life expectancy in Australia. Aust J Public Health 1994;18:149-52. 10.1111/j.1753-6405.1994.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 40. Sauvaget C, Tsuji I, Minami Y, et al. Dementia-free life expectancy among elderly Japanese. Gerontology 1997;43:168-75. 10.1159/000213846. [DOI] [PubMed] [Google Scholar]
- 41. Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer’s disease in an older population: updated incidence and life expectancy with and without dementia. Am J Public Health 2015;105:408-13. 10.2105/AJPH.2014.301935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63-75.e2. 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 43. Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015;11:310-20. 10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sindi S, Kåreholt I, Ngandu T, et al. Sex differences in dementia and response to a lifestyle intervention: evidence from Nordic population-based studies and a prevention trial. Alzheimers Dement 2021;17:1166-78. 10.1002/alz.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65-72. 10.1016/S0749-3797(18)31107-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
Deidentified data are available on request for qualified investigators from www.riha.rush.edu/dataportal.html