Abstract
Cardiorespiratory fitness is impaired in type 2 diabetes (T2D), conferring significant cardiovascular risk in this population; interventions are needed. Previously, we reported that a T2D-associated decrement in skeletal muscle oxidative flux is ameliorated with acute use of supplemental oxygen, suggesting that skeletal muscle oxygenation is rate-limiting to in vivo mitochondrial oxidative flux during exercise in T2D. We hypothesized that single-leg exercise training (SLET) would improve the T2D-specific impairment in in vivo mitochondrial oxidative flux during exercise. Adults with (n = 19) and without T2D (n = 22) with similar body mass indexes and levels of physical activity participated in two weeks of SLET. Following SLET, in vivo oxidative flux measured by 31P-MRS increased in participants with T2D, but not people without T2D, measured by the increase in initial phosphocreatine synthesis (P = 0.0455 for the group × exercise interaction) and maximum rate of oxidative ATP synthesis (P = 0.0286 for the interaction). Additionally, oxidative phosphorylation increased in all participants with SLET (P = 0.0209). After SLET, there was no effect of supplemental oxygen on any of the in vivo oxidative flux measurements in either group (P > 0.02), consistent with resolution of the T2D-associated oxygen limitation previously observed at baseline in subjects with T2D. State 4 mitochondrial respiration also improved in muscle fibres ex vivo. Skeletal muscle vasculature content and calf blood flow increased in all participants with SLET (P < 0.0040); oxygen extraction in the calf increased only in T2D (P = 0.0461). SLET resolves the T2D-associated impairment of skeletal muscle in vivo mitochondrial oxidative flux potentially through improved effective blood flow/oxygen delivery.
Keywords: blood flow, diabetes, exercise, skeletal muscle
Introduction
The 463 million adults worldwide living with type 2 diabetes (T2D) (Saeedi et al. 2019) have three times the risk of premature cardiovascular disease (CVD) mortality compared with individuals without T2D (Baena-Diez et al. 2016). All-cause and CVD mortality are inversely associated with cardiorespiratory fitness, and adults with T2D have 20% lower fitness compared with their age-, activity- and adiposity-matched peers (Regensteiner et al. 2015). Peak oxygen consumption (VO2peak), the gold standard measure of fitness, represents the product of cardiovascular blood flow and peripheral oxygen uptake. During aerobic exercise, the primary site of oxygen uptake is skeletal muscle mitochondria for oxidative phosphorylation of ATP. Oxidative flux is impaired in people with T2D (Cree-Green et al. 2017, 2018) and contributes to lower cardiorespiratory fitness in this population (Coen et al. 2013).
Oxidative flux through mitochondria in response to ATP demand is dependent on the intrinsic properties of the mitochondria, insulin action and blood flow for the delivery of oxygen and substrate necessary for oxidative flux. Intrinsic properties of skeletal muscle mitochondria include their respiratory capacity and coupling control ratios of different states of mitochondrial respiration. The impact of T2D on these intrinsic properties of mitochondria is unclear. Some reports detail lower mitochondrial content and function in people with T2D (Mogensen et al. 2007; Phielix et al. 2008; van Tienen et al. 2012; Antoun et al. 2015), while others report no difference in mitochondrial parameters in people with and without T2D (Asmann et al. 2006; Boushel et al. 2007; Abdul-Ghani et al. 2009). Impaired intrinsic properties of skeletal muscle mitochondria could be a source of lower cardiorespiratory fitness in people with T2D (Larson-Meyer et al. 2000). Of note, whole-body aerobic exercise training results in a large increase in skeletal muscle mitochondrial content with a relatively smaller improvement in VO2peak (Gollnick et al. 1973; Davies et al. 1981). These data suggest a nonlinear relationship between muscle mitochondrial content and fitness and highlight the importance of appreciating the local factors in skeletal muscle that contribute to oxidative flux, such as blood flow and tissue oxygenation.
Data from our group and others demonstrate the importance of skeletal muscle blood flow-related factors to oxidative flux and cardiorespiratory fitness, especially in people with T2D (Nielsen et al. 1990; Sjoberg et al. 2017). To meet the energetic demands of exercise, skeletal muscle blood flow increases significantly from rest (Richardson et al. 1993) and skeletal muscle capillarization is a determinant of aerobic exercise capacity (Olfert et al. 2009). Specific to people with T2D, in vivo skeletal muscle oxidative flux was lower compared with body mass index (BMI)-similar peers and was improved by acutely augmenting tissue oxygenation with supplemental oxygen (Cree-Green et al. 2018). In contrast, supplemental oxygen did not alter oxidative flux in participants with similar BMI and physically inactive lifestyle who did not have T2D; there were minor differences in the intrinsic mitochondrial properties between groups (Cree-Green et al. 2018). Further, skeletal muscle oxygenation correlates with VO2peak in people without T2D; however, this relationship is lost in people with T2D (Mason McClatchey et al. 2017a). These data suggest that in T2D there is discoordination of the delivery of oxygen to skeletal muscle mitochondria and mitochondrial function that is associated with impaired VO2peak.
The purpose of this investigation was to determine whether single, lower-leg exercise training (SLET) would improve skeletal muscle oxidative flux in adults with T2D. We were particularly interested in understanding the changes in the local environment associated with both blood flow and mitochondria to pinpoint any exercise training-mediated changes, given the previously described impairment in oxidative flux (Cree-Green et al. 2018). Therefore, we employed a single-leg model to address the hypothesis that SLET would improve the T2D-specific impairment in in vivo mitochondrial oxidative flux during exercise.
Methods
Ethical approval
This study was approved by the University of Colorado Anschutz Medical Campus Institutional Review Board (IRB# 06–0062) and written informed consent was obtained from all participants. The study conformed to the standards set by the Declaration of Helsinki and was registered on Clinicaltrials.gov (identifier: NCT01793909).
Participants
Forty-one adults with (n = 19) and without (n = 22) T2D between the ages of 30 and 70 years and with overweight or obesity (BMI 25–40 kg/m2) were recruited. Presence of T2D was confirmed by medical chart review. All were physically inactive, defined as not participating in a regular exercise programme (<one bout of exercise/week), which was confirmed by use of a questionnaire (Low level Physical Activity Recall, LOPAR) (Sallis et al. 1985). Participants were excluded if they had: (1) HbA1c >9% (75 mmol/mol); (2) insulin, thiazolidinedione, glucagon-like peptide-1 agonists/dipeptidyl peptidase 4 inhibitors, sodium-glucose transport protein 2 inhibitors, or oral steroid use; (3) documented coronary or peripheral arterial disease, electrocardiography findings of cardiac ischaemia or conduction system abnormalities with a stress exercise test, or use of beta blockers or other symptoms potentially limiting exercise function; (4) uncontrolled hypertension (systolic blood pressure >150 mmHg and/or diastolic blood pressure >110 mmHg); (5) obstructive pulmonary disease or asthma; (6) peripheral neuropathy; (7) current or past smoking within the last 2 years; (8) anaemia (Hb < 10 mg dl−1); (9) autonomic dysfunction (e.g. fall in BP >20 mmHg on standing without change in HR); or (10) implanted metal (due to use of MRI). Participants without T2D but with overweight/obesity (overweight/obese controls (OWC)) had no health conditions other than excess weight and could not have more than one first-degree relative with diabetes.
Participants underwent a screening visit to confirm eligibility followed by four additional study visits prior to the start of SLET. Visit one included consent and a fasting blood sample for a lipid panel, glucose, insulin and HbA1c. Visit two included VO2peak testing using a bicycle ergometer to establish peak aerobic power. During visit three a biopsy of the dominant gastrocnemius was performed. Visit four included out-of-magnet leg exercise testing performed in combination with near infrared spectroscopy (NIRS), then plethysmography to measure leg blood flow. Visit five included MRI imaging of the leg for maximal cross-sectional area and in-MRI single-leg exercise testing with31P magnetic resonance spectroscopy (MRS) to assess mitochondrial function. Visits two to five were each separated by 1 to 3 days. Following SLET, the skeletal muscle biopsy, NIRS data collection and MRI imaging were repeated with the same rest interval between visits.
Graded exercise test
VO2peak was determined via graded exercise to exhaustion as previously described (Regensteiner et al. 1995, 1998; Brandenburg et al. 1999) using a stationary cycle ergometer (Lode Bike, Groningen, The Netherlands) and a metabolic cart (Medgraphics Ultima CPX, Medical Graphics Corp., St. Paul, MN, USA). After the start of exercise, the work rate was increased in 10–20 watt/min increments (depending on age and sex) in order to allow each participant to reach peak aerobic power within 8–12 min. Peak VO2 was confirmed by a respiratory exchange ratio greater than 1.1. During incremental exercise testing, the highest VO2 and heart rate averaged over 15 s were defined as the peak values.
Magnetic resonance imaging and spectroscopy
Imaging and spectroscopy acquisition.
All MR imaging and 31P MRS equipment and procedures have been previously described (Cree-Green et al. 2014a, 2014b, 2017). In summary, imaging and MRS were performed on a General Electric (GE) 3T magnet with HDx MRI running Version 15M4 software and a Siemens 3T magnet with a Skyra platform. Our scanners were also equipped with multi-nuclear spectroscopy hardware and research software upgrades and utilized a custom-built 1H/31P leg coil (Clinical MR Solutions, Brookfield, WI).
MRS exercise protocol.
Strength testing of the dominant leg via single-leg exercise was done on a custom-built MR-compatible plantar flexion device with force measurement capability as previously described (Bamman & Caruso, 2000; Larson-Meyer et al. 2000; Cree-Green et al. 2014a, 2017).
The 31P MRS exercise protocol consisted of measurements during rest for 60 s, isometric plantar flexion for 90 s at 70% maximal volitional contraction, and recovery for 8 min post-exercise. We selected a 90 s isometric exercise bout as this perturbation has been extensively modelled and utilized for assessing both aerobic and anaerobic processes (Newcomer & Boska, 1997; Larson-Meyer et al. 2000; Sirikul et al. 2007; Cree-Green et al. 2014b, 2017). Force was monitored and recorded in kg continuously throughout the exercise, with verbal and visual feedback to keep the force within the target goal. Following this room-air exercise bout, participants received 6–81 of O2 by face mask. After 5 min of O2 saturation of 99%–100%, a second identical exercise bout was performed.
Spectroscopy analysis.
Analysis of the spectroscopy was done as previously reported (Newcomer & Boska, 1997; Larson-Meyer et al. 2000; Cree-Green et al. 2014b, 2017). Briefly, peak positions and areas of interest (phosphocreatine (PCr), inorganic free phosphate (Pi), β-adenosine triphosphate (ATP) (3 peaks), α-ATP (2 peaks), γ-ATP (2 peaks) and phosphomonoester) were determined by time domain fitting using jMRUi (van den Boogaart A, 1997) utilizing AMARES (A Method of Accurate, Robust and Efficient Spectral fitting), a nonlinear least-square-fitting algorithm with previously built prior knowledge files (Rico-Sanz et al. 1999) as previously described (Newcomer & Boska, 1997; Cree-Green et al. 2014b). ADP concentrations were calculated using a Michaelis–Menten model of the creatine kinase reaction, as previously reported (Newcomer & Boska, 1997). All exercise spectra were corrected for saturation using the fully relaxed spectra for that day. Calculations utilized data from the end of exercise and during the immediate recovery period, and included the rates of oxidative phosphorylation (OxPhos) calculated as ΔPCr/time from the first 10 s following cessation of exercise, initial PCr synthesis (VPcr) and apparent maximum rate of oxidative ATP synthesis (Qmax), which is calculated with the initial rate of PCr resynthesis relative to end-exercise ADP concentration using an assumed Km = 30 μM. Time constants (TC) for ADP and PCr were calculated via regression analyses with Sigmaplot (Systat Software, Inc, San Jose, CA, USA). Reported values (VPCr, QMAX, OxPhos and ADP TC; Fig. 1) characterize several aspects of oxidative phosphorylation. VPCr in the synthesis rate of creatine to phosphocreatine, the high-energy phosphate used for skeletal muscle contraction. QMAX is the calculated maximal rate of oxidative ATP production. OxPhos is the rate of oxidative phosphorylation. The ADP TC is the time required to phosphorylate ADP, generating ATP. Together, QMAX, OxPhos and ADP TC reflect the muscle’s ability to generate ATP through oxidative phosphorylation.
Figure 1. Mitochondrial oxidative phosphorylation flux measured by 31P-MRS.
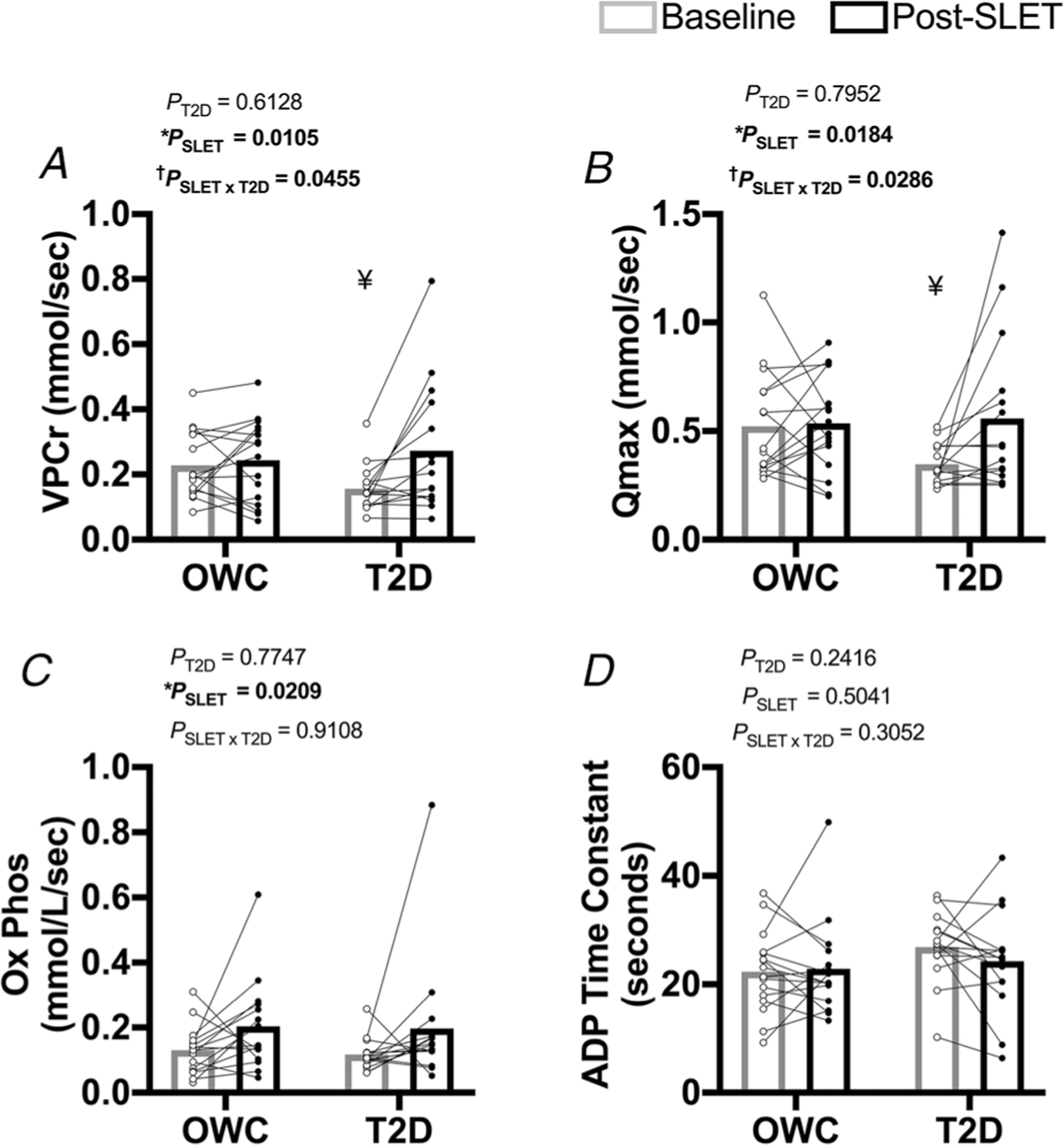
A, rate of PCr synthesis (VPCr; †P = 0.0455 for the interaction of single-leg exercise training (SLET) and T2D) ¥P = 0.0333 for the difference in groups at baseline. B, maximal rate of ATP synthesis (QMAX; †P = 0.0286 for the SLET × T2D interaction). ¥P =0.0143 for the difference in groups at baseline. C, rate of oxidative phosphorylation (OXPhos; *Effect of SLET P = 0.0209). D, ADP Time Constant. Participants with overweight/obesity, without T2D (OWC; n =16). Participants with T2D (T2D; n =15).
NIRS data acquisition and analysis
Tissue total haemoglobin + myoglobin ([tHb]), deoxy [haemoglobin + myoglobin] ([HHb]) and oxy [haemoglobin + myoglobin] ([OHb]) were assessed by a frequency domain multi-distance NIRS monitor (Optiplex TS, ISS, Champaign, IL, USA) which is placed at the maximal circumference of the calf during the exercise bouts. The NIRS monitor emits two wavelengths (690 and 830 nm) and measures absorbance at distances of 2.0, 2.5, 3.0 and 3.5 cm. The NIRS data were sampled continuously and recorded at 50 Hz. Upon export, data were down-sampled to 1 Hz using a running average of the higher-resolution 50 Hz data. The NIRS monitor was calibrated prior to each visit using a calibration phantom of known scattering and optical properties. Resting values of tissue [tHb], [HHb] and [OHb] were obtained by averaging the 30 s.
Plethysmography measurements
Calf blood flow at rest and post-limb occlusion was determined in semi-seated participants by venous occlusion strain gauge plethysmography (D.E. Hokanson Inc. Issaquah, WA, USA), using calibrated mercury-in-silastic strain gauges and expressed as ml/100 ml min−1 as previously reported by our laboratory and others (Hiatt et al. 1989; Regensteiner et al. 2003). Reactive hyperaemia was calculated by subtracting baseline blood flow from peak blood flow measured following cuff occlusion.
Skeletal muscle biopsy and sample analyses
Skeletal muscle samples were taken from the medial aspect of the gastrocnemius muscle from the same leg (dominant leg) that underwent MRI. Biopsies were performed at least 24 h after the last known exercise bout (either MRS or NIRS exercise) to ensure that the skeletal muscle was in a resting metabolic state. A modified Bergstrom needle technique was utilized to obtain a skeletal muscle sample following local anaesthesia with 2% lidocaine and a 1 cm skin incision (Bergstrom, 1975). Samples were processed for skeletal muscle mitochondrial respiration, histology and protein content by Western blot.
Mitochondrial respiration.
Mitochondrial respiration was measured using Oroboros Oxygraph-2k (O2k, Oroboros Instruments Corp., Innsbruck, Austria) according to modifications from previously described protocols (Kuznetsov et al. 2008; Pesta & Gnaiger, 2012; Keller et al. 2015). Immediately after biopsy, ~ 10–20 mg of skeletal muscle tissue was placed in ice-cold mitochondrial preservation buffer (BIOPS (10 mM Ca-EGTA, 0.1 mM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-MES, 0.5 mM DTT, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, pH 7.1)). Muscle fibres were separated mechanically (in BIOPS and on ice), partially teased apart by fine forceps, then permeabilized by incubation with saponin (30 μg ml−1) in BIOPS on ice (30 min), then washed in mitochondrial respiration buffer (MiR06 (0.5 mM EGTA, 3 mM magnesium chloride, 60 mM K-lactobionate, 20 mM taurine, 10 mM potassium phosphate, 20 mM Hepes, 110 mM sucrose, 1 g l−1 bovine serum albumin, 280 U ml−1 catalase, pH 7.1)). The 2–3 mg of fibres were added to prewarmed MiR06 + 25μM blebbistatin in the O2k. Oxygen in the MiR06 was started at 400 μM and maintained >250 μM
Two sets of substrates and inhibitors were added to assess respiration rates at several states. Rates for Run 1 were measured following the addition of pyruvate (P, 5 mM) and malate (M, 2 mM) (PM leak (L)); PM with ADP (2 mM) (PM OXPHOS (P)); PM, ADP and glutamate (G, 10 mM) and succinate (S, 6 mM) (PMGS P); PMGS, ADP and oligomycin (10 μg ml−1, PMGS L). Rates for Run 2 were measured following the addition of octanoylcarnitine (OC, 200 μM) and malate (M, 1 mM) (OCM L); OCM with diphosphate (ADP, 2 mM) (OCM P); OCM, ADP and glutamate (G, 10 mM) and succinate (S, 6 mM) (OCMGS P); OCMGS, ADP and oligomycin (10 μg ml−1, OCMGS L). Both runs ended with 0.5 μM stepwise titration of 4-(trifluoromethoxy) phenylhydrazone (FCCP) until maximal uncoupling was achieved (electron transfer system capacity (E)). Cytochrome c (10 μM) was also added during both runs to determine that mitochondrial membrane damage was minimal and the same between runs. Respiration ratios were calculated as a ratio of L/P PM, L/P PMGS, L/E PMGS, P/E PMGS, L/P OCM, L/P OCMGS, L/E OCMGS and P/E OCMGS.
Biochemistry.
Flash-frozen skeletal muscle was homogenized in a lysis buffer (Scalzo et al. 2018b) then centrifuged (18,000 g for 10 min at 4°C). The supernatant was analysed for protein concentration by Bradford assay. Citrate synthase enzyme activity was determined by previously described methods (Keller et al. 2015). Protein in Laemmli sample buffer was run on 4%–20% Tris-HCl gels. Proteins were electrophoretically transferred to PVDF membranes, and equivalence of protein loading was assessed by staining of membrane-bound proteins by Ponceau S stain and the protein vinculin. Blots were probed using antibodies against mitochondrial OXPhos complexes (Abcam #ab110412), CD31 (Cell Signalling #3528S), vascular endothelial growth factor (VEGF; Abcam #ab46154), and vinculin (Cell Signalling # 4650s); all antibodies were used at a concentration of 1:1000 overnight at 4°C and followed by fluorescent secondary. Proteins were detected by Li-COR (Odyssey CLX) Western blot scanner, and densitometric analysis was performed using Image Studio v4.1. In addition to the above-mentioned visual confirmation of equal protein loading and transfer across membranes with ponceau staining and the protein-loading control vinculin, a sample-loading control (human skeletal muscle) was used across all blots and all data were normalized to that lane.
Determination of myosin heavy chain (MyHC) fibre-type distribution
A section of muscle from the biopsy was frozen in Tissue-Tek OCT (Sakura Finetek, Torrance, CA, USA). Frozen muscle was equilibrated to the cryostat thermostat (−18–20°C) prior to cutting 8 μm thick sections from the mounted tissue onto charged slides. Sections were dried at room temperature for 20 min, then incubated in PBS for 5 min. The sections were then incubated for 90 min at room temperature in primary antibodies: MyHC Type 1 fibres: BA.D5 IgG2b supernatant (5 μg ml−1 in PBS; Developmental Studies Hybridoma Bank (DHSB), Iowa City, IA, USA); MyHC Type 2× fibres: SC.71 IgG1 supernatant (5 μg ml−1 in PBS; DHSB); MyHC Type 2a fibres: 6H1 IgM supernatant (3.75 μg ml−1 in 5% BSA; DHSB). After the incubation, slides were washed three times in PBS for 5 min each. Sections were incubated in corresponding secondary antibodies for 60 min at room temperature (AF555 goat anti-IgG2b (1:400, Invitrogen, Rockford, IL, USA), AF488 goat anti-IgG1 (1:500, Invitrogen); APC/Cy7 goat anti-IgM (1:50, Biolegend, San Diego, CA, USA)). Slides were washed three times in PBS and covered with ProLong Gold Antifade mounting media with DAPI (Invitrogen). Positive fibre identification was completed by an individual blinded to participant group using a BZ-X800 fluorescent slide reader (Keyence, Itasca IL, USA). Results are presented as Type 1 versus all Type 2 fibres.
Single, lower-leg exercise training
Participants completed 10 sessions of SLET with their dominant leg (5 days per week for 2 weeks). Sessions included 30–45 min per day of six body-weight and resistance exercises: (1) Single-legged leg press at full knee extension – on a seated leg press (Cybex, Life Fitness, Rosemont, IL, USA), participants extend the leg press plate to reach full knee extension, then slide the dominant foot to the lower edge of plate so that the heel was off and removed the dominant leg from the plate (3 ×30 repetitions at 40%–50% MVC). (2) Weighted heel raise – participants used a smith machine (Cybex, Life Fitness, Rosemont, IL, USA) with the bar on their shoulder in the proper position, lifted the non-dominant leg so they were standing only on the dominant leg, and performed 3 ×15–20 repetitions with the weight with which they felt they could comfortably complete the sets. (3) Body-weight heel raises timed 1 min – participants performed single-leg heel raises with their heel extended beyond the edge of an exercise step for 1 min (three sets). (4) Body-weight heel raises with pauses – participants performed heel raises using the set-up described above (item 3), and completed 12 repetitions. In each repetition, when maximum dorsiflexion was achieved, the subject held that position for 5 s and then returned to neutral. (5) Body-weight heel raises timed 30 s – participants performed heel raises using the set-up described above (item 3) for 30 s. (6) Seated plate dorsiflexion – participants sat on a standard chair with knees bent at approximately 90 degrees. A weight plate (such as used in the smith machine) was rested on their dominant thigh, and participants performed dorsiflexion of the dominant leg for 2 min. Each set of exercises included 1 min of rest between repetitions, each completed exercise set was followed by 3 min of rest, and each session was preceded by a 5 min warm up and followed by a 10 min cool down. We chose a high-repetition, low-resistance protocol to specifically engage oxidative metabolism in the lower leg muscles while avoiding a central cardiovascular response to training that would make conclusions about the training stimulus ambiguous for the local skeletal muscle and vascular environment. Progression of exercises using external weight/resistance was made when participants could comfortably complete all sets and repetitions.
Statistical analysis
Adequate sample size was predicted to be 10 participants in each group to demonstrate a difference in the a priori determined primary outcome of in vivo mitochondrial outcomes, based on the variability seen in similar youth (Cree-Green et al. 2014b, 2017). A total of 2 ×2 analysis of variance (ANOVA) with repeated measures were used to determine the influence of SLET (repeated measure) and group (OWC vs. T2D) using Graphpad Prism version 8 (San Diego, CA, USA). Pairwise comparisons were performed using the Tukey test when there was a significant effect of treatment or a time by treatment interaction. Differences in participant characteristics were determined with unpaired t tests. The level of statistical significance was set at P < 0.05. Data are expressed as means ± standard deviation.
Results
Participant characteristics
Baseline physical characteristics and circulating metabolic factors obtained from research participants are presented in Table 1. There were no differences in age (P = 0.1757) or BMI (P = 0.4796) between the groups. As expected, the participants with T2D had greater HbA1c and fasting glucose then the OWC group (P < 0.0001). LDL was greater in the control group compared with people with T2D (P = 0.0238); this finding is potentially due to the greater use of statins in the people with T2D compared with OWC (47% vs. 18%, respectively; P = 0.0169). Peak VO2 was lower in people with T2D compared with the OWC group (P = 0.0050 for relative VO2peak and P = 0.0243 for absolute VO2peak) as previously observed (Regensteiner et al. 2015; Scalzo et al. 2018a). There were no group differences in peak heart rate (151 ± 20 vs. 145 ± 17; P = 0.1987) or respiratory exchange ratio (1.22 ± 0.02 vs. 1.19 ± 0.03; P = 0.3508) measured with VO2peak. The compliance rate for completing SLET was 93% ± 12% in the OWC group and 87% ± 13% in participants with T2D. There was no difference in compliance between groups (P = 0.1395).
Table 1.
Participant characteristics
| OWC | T2D | |
|---|---|---|
|
| ||
| N | 22 | 19 |
| Sex (m/f) | 10/12 | 10/9 |
| Age (years) | 56 ± 13 | 59 ± 9 |
| Body mass (kg) | 86.5 ± 16.1 | 86.5 ± 12.8 |
| Body mass index (kg/m2) | 29.8 ± 4.6 | 30.8 ± 3.8 |
| Body fat (%) | 35.4 ± 8.3 | 36.3 ± 7.6 |
| HbA-1c (%) | 5.4 ± 0.2 | 6.4 ± 0.7† |
| Insulin (μU ml−1) | 15.6 ± 6.2 | 18.4 ± 9.4 |
| Glucose (mg dl−1) | 88.8 ± 8.2 | 113.8 ± 25.1† |
| LDL cholesterol (mg dl−1) | 111.3 ± 30.3 | 89.2 ± 29.6† |
| HDL cholesterol (mg dl−1) | 51.0 ± 10.9 | 46.2 ± 12.7 |
| Triglycerides (mg dl−1) | 100.3 ± 37.3 | 139.4 ± 105.7 |
| VO2peak (ml kg−1 min−1) | 24.0 ± 7.5 | 18.9 ± 5.0† |
| VO2peak (ml min−1) | 2086 ± 762 | 1676 ± 504† |
Data are means ± SD.
Main effect of T2D P < 0.0238.
In vivo mitochondrial measures
In vivo 31P MRS muscle mitochondrial endpoints are shown in Fig. 1. The groups had similar: (1) blood O2 saturation in response to room air and supplemental O2; (2) work relative to their calf muscle size; and (3) ADP generation during each calf exercise bout. SLET increased VPCr and QMax in the participants with T2D (Fig. 1A and B; P = 0.0455 and P = 0.0286, respectively, for the interaction of training and disease for both variables). Post hoc analyses reveal that VPCr and QMax were both lower in participants with T2D at baseline (P = 0.0333 and P = 0.0143, respectively). However, these differences were not present post-SLET (VPCr P = 0.6270, QMax P = 0.8266). OxPhos increased following exercise training in all participants (Fig. 1C; P = 0.0209). The ADP time constant was not affected by SLET in either group (P = 0.5041).
Our previous report demonstrated that supplemental oxygen improved oxidative flux in participants with T2D (Cree-Green et al. 2018). We repeated the assessment of oxidative flux with and without supplemental oxygen after SLET to determine whether skeletal muscle oxygenation persisted as a limitation to oxidative flux in people with T2D. Comparison of the post-training values with and without oxygen administration reveals that none of the variables representing oxidative flux were affected by supplemental oxygen in participants with T2D or OWC (P > 0.2; data not shown), consistent with resolution of the skeletal muscle oxygenation limitation in T2D by SLET.
Skeletal muscle near infrared spectroscopy
Skeletal muscle NIRS was used to assess both blood flow and oxygenation of the gastrocnemius during constant work rate exercise. There was an interaction of diabetes and exercise training for change in oxygen saturation with acute exercise (Fig. 2A; P = 0.0461). The decrease in oxygen saturation with acute exercise was greater following SLET in the participants with T2D (P = 0.0241); this variable did not change with training in the OWC participants (P = 0.8608). For this outcome measure, there appears to be an outlier in the OWC group. With the pre-/post-SLET data pair removed, the statistical interaction of T2D and SLET is no longer significant (P = 0.0765). In this analysis, there is a significant effect of SLET (P = 0.0021 vs. P = 0.0343 in the primary analysis) and no main effect of T2D (P = 0.0623). Accumulation of deoxyhaemoglobin was greater following SLET in both groups (Fig. 2D; P = 0.0486). Together, the oxygen saturation and oxyhaemoglobin data suggest greater oxygen extraction/use in the participants with T2D following SLET. The changes in total haemoglobin (reflective of local increase in microvascular blood volume) and oxygenated haemoglobin were not different with exercise training or presence of diabetes (Fig. 2B and D; P > 0.1478).
Figure 2. Skeletal muscle oxygenation and blood flow.
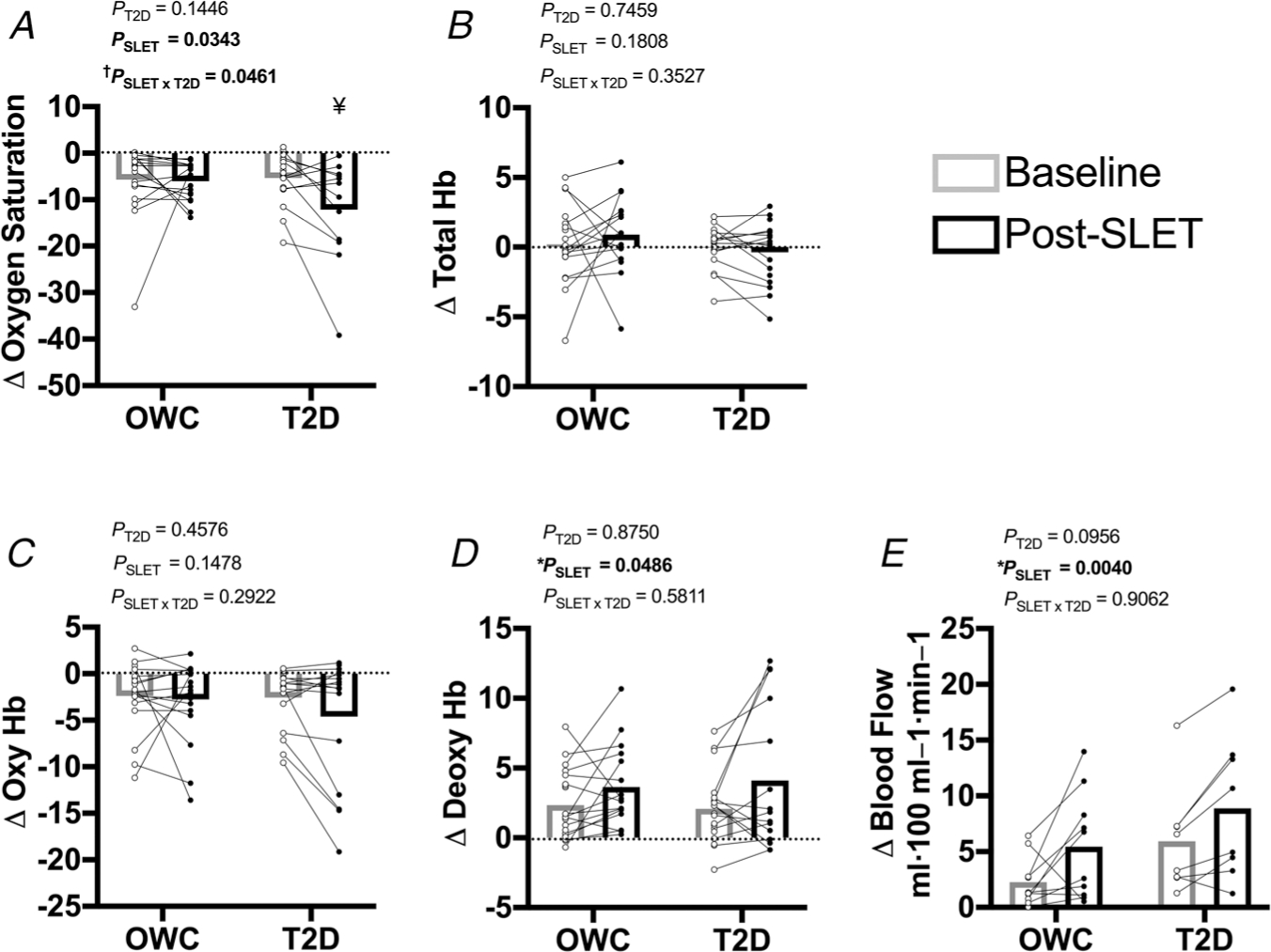
Change in skeletal muscle oxygenation with acute exercise measures with NIRS and plethysmography: A, skeletal muscle oxygen saturation. †P = 0.0461 for the interaction of single-leg exercise training (SLET) and T2D. ¥P = 0.0241 for the change with SLET in participants with T2D. B, total tissue haemoglobin (Hb) C, oxygenated Hb. D, deoxygenated Hb. *Effect of SLET P = 0.049. E, reactive hyperaemia. *Effect of SLET P = 0.0040. Participants with overweight/obesity, without T2D (OWC; NIRS n = 20, plethysmography n =10). Participants with T2D (T2D; NIRS n =18, plethysmography n = 8).
Reactive hyperaemia
Reactive hyperaemia, an assessment of skeletal muscle blood flow, was measured at the calf and expressed as the change in blood flow at rest versus post-occlusion. There was no difference in resting blood flow between groups or after SLET (OWC Baseline: 2.742 ± 2.621 vs. Post-SLET: 1.373 ± 0.532; T2D Baseline: 1.639 ± 0.780 vs. Post-SLET: 1.679 ± 1.185; P > 0.2102). Reactive hyperaemia increased with SLET in all participants (Fig. 2E; P = 0.0040).
Skeletal muscle mitochondrial respiration
Mitochondrial respiration results from gastrocnemius muscle biopsy samples are presented in Fig. 3 and Tables 2 and 3. Representative tracings are presented in Fig. 4. There was no effect of group or SLET on the L/P ratio with PM or OCM (Fig. 3A and E; all P > 0.1333). The L/P ratio was increased with SLET for both the PMGS and OCMGS SUITs (Fig. 3B and F; P = 0.0221 and P = 0.0157, respectively). The increase in the PMGS L/P ratio was the result of greater state 4 respiration (leak) following SLET (Table 2; P = 0.0014); similarly, there was a suggestion that OCMGS state 4 respiration was greater following single-leg training (Table 3; P = 0.1120). The L/E ratio with PMGS (Fig. 3C) was not affected by group (P = 0.5650) or SLET (P = 0.0635); with OCMGS the L/E ratio increased with SLET (Fig. 3G; P = 0.0185) but was unaffected by group (P = 0.3940). There was an interaction of SLET and T2D for the P/E ratio with the PMGS SUIT (Fig. 3D; P = 0.0084). This ratio increased in OWC participants, but not in participants with T2D. OXPHOS with OCMGS was lower in people with T2D than controls (Table 3; P = 0.0432). All other measures were unaffected by SLET or disease status (P > 0.1). To summarize, SLET increased state 4 respiration leading to greater L/P ratios with each substrate suit and greater L/E with OCMGS following training in all participants.
Figure 3. Ex vivo mitochondrial respiration measured by Oroboros O2k.
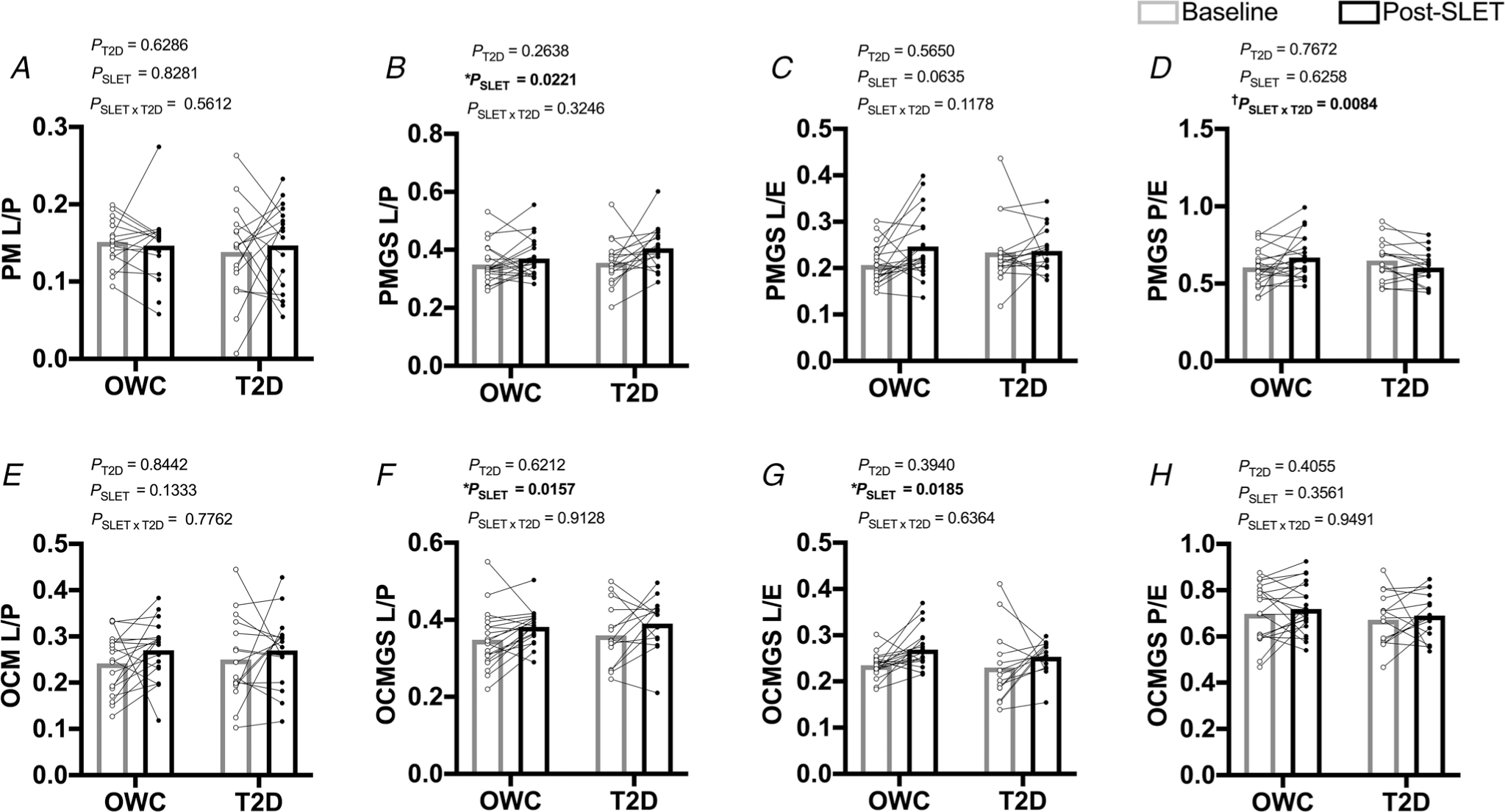
A and E, ratio of O2 consumption from state 2 (L) and state 3 (P) respiration (inverses respiratory control ratio (RCR)) (P: Pyruvate, M: Malate (A); OCM: Octanoylcarnitine and Malate (E)). B and F, ratio of O2 consumption from state 4 (L) and state 3 (P) respiration (inverses respiratory control ratio (RCR)) (PMGS: Pyruvate, Malate, Glutamate, Succinate (B); OCMGS: Octanoylcarnitine, Malate, Glutamate, Succinate (F)). C and G, ratio of O2 consumption from state 4 (L) and max-uncoupled (E) respiration from 2 SUIT protocols (PMGS (C); OCMGS (G)). D and H, ratio of O2 consumption from state 3 (P) and max-uncoupled (E) respiration (OxPhos Ratio) from 2 SUIT protocols (PMGS (D); OCMGS (H)). Tables 2 and 3: corresponding raw respiration values. *Effect of single-leg exercise training (SLET). †P = 0.0084 for the interaction of SLET and T2D. Participants with overweight/obesity, without T2D (OWC; n = 20). Participants with T2D (T2D; n = 18).
Table 2.
Gastrocnemius mitochondrial respiration: carbohydrate-linked substrate SUIT
| OWC (n = 20) |
T2D (n = 18) |
|||
|---|---|---|---|---|
| Baseline | Trained | Baseline | Trained | |
|
| ||||
| State 2: PM | 5.55 ± 1.47 | 5.88 ± 1.71 | 4.73 ± 2.51 | 4.79 ± 2.03 |
| OXPHOS (State 3): PM | 36.5 ± 8.8 | 40.2 ± 8.9 | 34.2 ± 11.5 | 33.1 ± 8.1 |
| OXPHOS (State 3): PMGS | 53.3 ± 14.5 | 58.9 ± 14.7 | 49.7 ± 16.2 | 49.4 ± 13.2 |
| State 4: PMGS | 18.0 ± 4.3 | 21.3 ± 5.1* | 17.6 ± 7.0 | 19.5 ± 4.1* |
| Uncoupled PMGS | 89.1 ± 31.7 | 91.1 ± 26.3 | 78.7 ± 27.7 | 84.5 ± 24.0 |
Data are means ± SD.
Main effect of training P = 0.0014.
P: pyruvate; M: malate; G: glutamate; S: succinate.
Table 3.
Gastrocnemius mitochondrial respiration: lipid-linked substrate SUIT
| OWC (n = 20) |
T2D (n = 18) |
|||
|---|---|---|---|---|
| Baseline | Trained | Baseline | Trained | |
|
| ||||
| State 2: OCM | 5.70 ± 1.92 | 6.10 ± 1.56 | 4.90 ± 2.36 | 5.11 ± 2.33 |
| OXPHOS (State 3): OCM | 23.3 ± 7.5 | 22.5 ± 5.8 | 19.2 ± 6.5 | 19.0 ± 6.1 |
| OXPHOS (State 3): OCMGS | 57.9 ± 16.5 | 58.0 ± 13.8 | 49.8 ± 15.5† | 47.8 ± 10.5† |
| State 4: OCMGS | 19.6 ± 5.5 | 21.8 ± 5.5 | 17.6 ± 7.8 | 18.2 ± 4.7 |
| Uncoupled OCMGS | 83.3 ± 20.1 | 81.1 ± 18.0 | 74.5 ± 19.9 | 71.0 ± 21.5 |
Data are mean ± SD.
Main effect of T2D P = 0.0432.
OC: octanolycarnitine; M: malate; G: glutamate; S: succinate.
Figure 4. Representative tracings from ex vivo mitochondrial respiration measures.
Top: Data collected with the Pyruvate (P), Malate (M), Glutamate (G) and Succinate (S) SUIT measured at Baseline and following single-legged exercise training (SLET) in participants with (T2D) and without (OWC) type 2 diabetes. Bottom: Data collected with the Octanoylcarnitine (OC), Malate (M), Glutamate (G) and Succinate (S) SUIT measured at Baseline and following SLET in participants with (T2D) and without (OWC) type 2 diabetes. [Colour figure can be viewed at wileyonlinelibrary.com]
Skeletal muscle mitochondrial content
Gastrocnemius muscle lysates were analysed for citrate synthase activity and mitochondrial complex content (Fig. 5A–E). Citrate synthase activity increased with exercise; however, this did not achieve statistical significance (P = 0.0843). There was no effect of T2D on citrate synthase activity (P = 0.4394). There was no difference in the mitochondrial proteins by SLET or T2D (P > 0.3).
Figure 5. Skeletal muscle mitochondrial content.
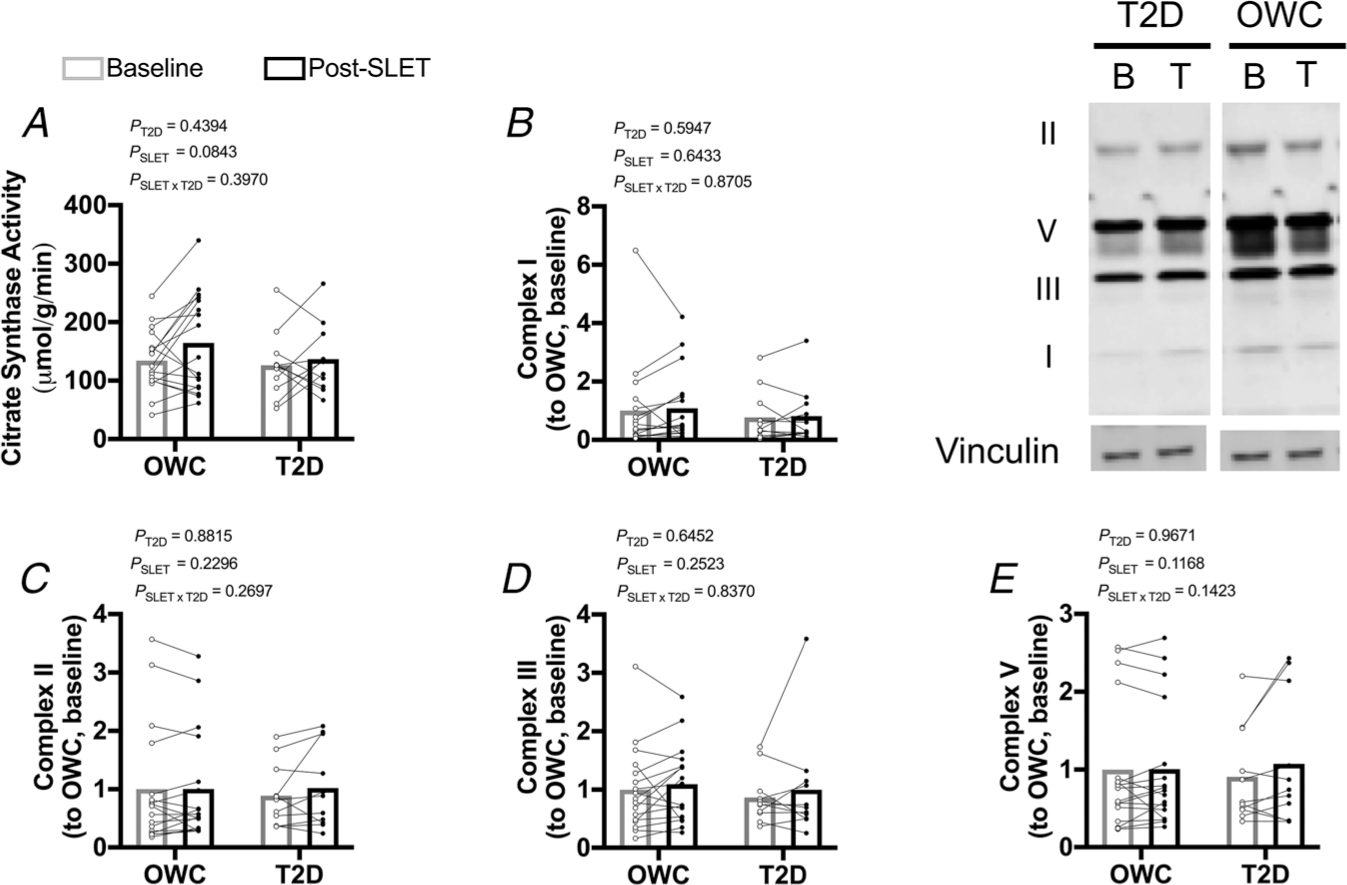
A, citrate synthase activity. B–E, protein content of mitochondrial complexes. Participants with overweight/obesity, without type 2 diabetes (T2D) (OWC; n =17). Participants with T2D (T2D; n =11). Single-leg exercise training (SLET). Baseline sample (B). Trained sample (T).
Skeletal muscle angiogenesis
To determine the impact of SLET on skeletal muscle vascular content, CD31 (a vascular endothelial marker) and VEGF (a primary signaller for angiogenesis), were measured in gastrocnemius lysates. CD31 increased with exercise in both groups (Fig. 6A; P = 0.0003), suggesting greater skeletal muscle vascular content. There was no effect of T2D on CD31 content (P = 0.6877). VEGF was unaffected by disease or exercise training after 10 days of SLET (Fig. 6B; P > 0.2150).
Figure 6. Skeletal muscle vascular content.
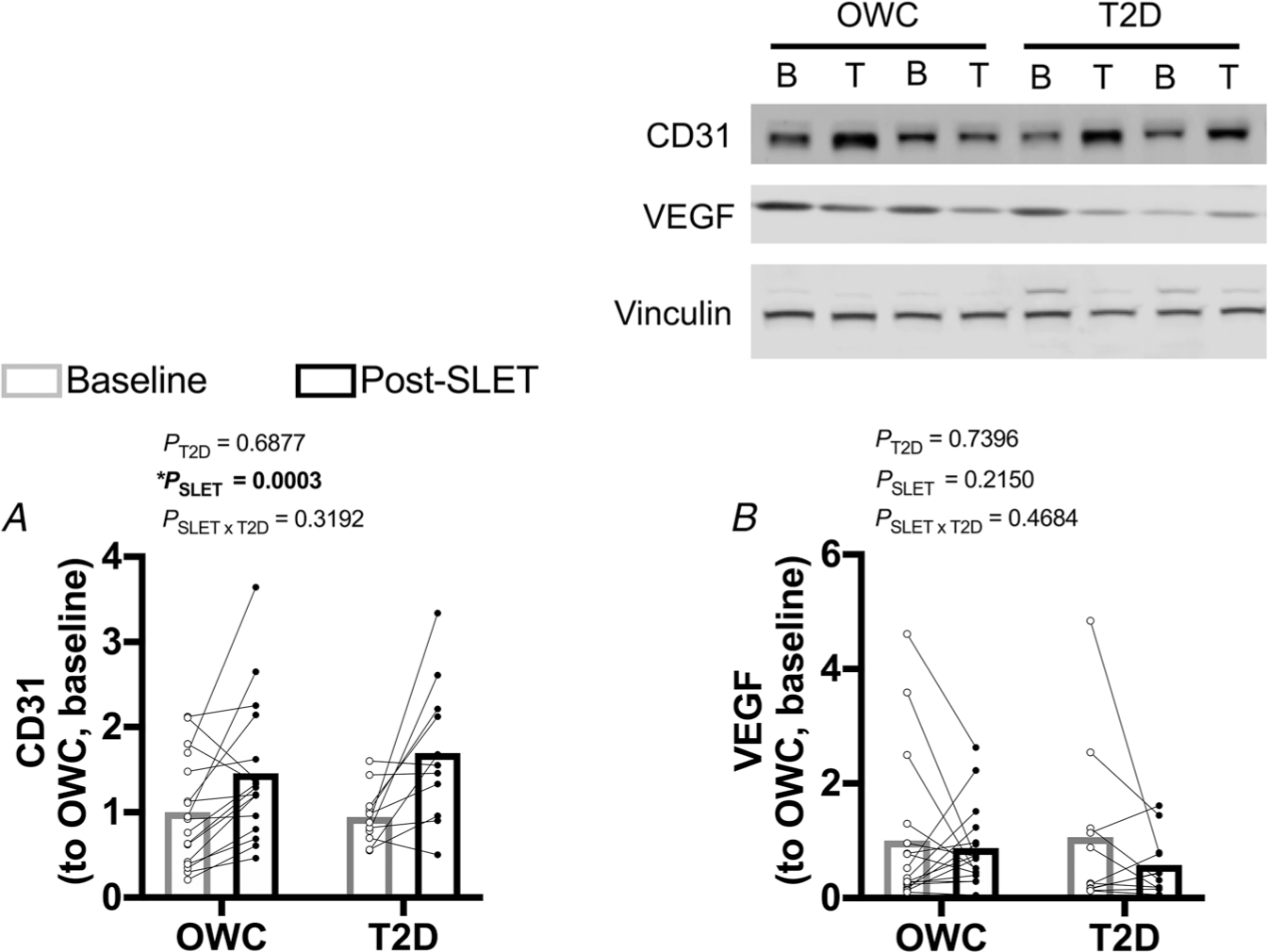
A, cluster of differentiation 31 (CD31; endothelial marker/indicator of vascular content) protein content. * Effect of single-leg exercise training (SLET) P = 0.0003. B, vascular endothelial growth factor (VEGF) protein content. Participants with overweight/obesity, without type 2 diabetes (T2D) (OWC; n =17). Participants with T2D (T2D; n = 11). Baseline sample (B). Trained sample (T).
Myosin heavy chain (MyHC) fibre-type distribution
Neither T2D nor SLET altered muscle fibre-type distribution (P > 0.3417). For OWC participants, Type I fibres were 61.9% ± 5.4% of total at baseline and 70.6% ± 4.4% after training; type IIa/xwere 38.1% ± 5.4% of total at baseline and 29.4% ± 4.4% after training. For participants with T2D, Type I fibres were 63.1% ± 3.9% of total at baseline and 59.1% ± 5.6% after training; type IIa/x were 36.9% ± 3.9% of total at baseline and 40.9% ± 5.6% after training.
Discussion
The primary finding of this study is that in vivo skeletal muscle oxidative flux is increased with 10 days of SLET. In addition, the previously identified skeletal muscle oxygenation limitation associated with T2D is corrected by SLET. Further analyses suggest that the improvement of in vivo oxidative flux with SLET was possibly mediated by improved leg blood flow and oxygen extraction (measured by NIRS and plethysmography). Additionally, state 4 mitochondrial respiration measured ex vivo increased with SLET. Together, these data suggest that the coordinated responses of skeletal muscle blood flow and mitochondria occur in response to SLET in people with T2D.
In our previous study, we found that oxygen availability in skeletal muscle was limiting for in vivo oxidative flux in people with T2D (Cree-Green et al. 2018). In this former study, oxidative flux only responded to greater skeletal muscle oxygen saturation in people with T2D. This finding is similar to the current study where people with T2D had a more robust response to SLET for measures of PCr synthesis and maximal ATP synthesis rates. Considering that blood flow measures improved with SLET in participants with and without T2D, the absence of change in PCr and ATP synthesis in the OWC group suggests that these measures are not blood flow-limited in the OWC population. We have also reported that in individuals with overweight/obesity but without T2D, there is a positive correlation between skeletal muscle blood flow and VO2peak. However, this relationship is not present in people with T2D (Mason McClatchey et al. 2017a). Data from animal models of diabetes demonstrate that capillary perfusion in skeletal muscle is heterogeneous and ineffective for appropriately matching oxygen delivery to mitochondrial demand (Frisbee et al. 2011; Mason McClatchey et al. 2017a, 2017b; Frisbee et al. 2019; MacDonald et al. 2020). Considering the importance of skeletal muscle blood flow to exercise and adaptation to exercise (Nielsen et al. 1990; Richardson et al. 1993; Olfert et al. 2009; Sjoberg et al. 2017), these data suggest that ineffective oxygen delivery to skeletal muscle mitochondria in the context of T2D limits skeletal muscle oxidative flux.
The purpose of this investigation was to determine whether the T2D-associated oxygen limitation on skeletal muscle oxidative flux could be remediated by SLET. The study design was intended to measure both skeletal muscle blood flow and mitochondrial function to pinpoint training-mediated changes related to this previously described impairment of in vivo oxidative flux. Single-leg exercise training increased skeletal muscle vascularization measured by CD31 content, calf blood flow (reactive hyperaemia), and skeletal muscle oxygen extraction assessed with NIRS. Of note, the observed increase in CD31 content was not accompanied by a concomitant increase in VEGF protein expression. VEGF is a component of the signalling cascade associated with angiogenesis (Leung et al. 1989). Previous data support that VEGF signalling events occur early in physiological adaptation to whole-body exercise training (Richardson et al. 2000) and may have been missed with our two time-point approach or our single-leg exercise model. Further, data from whole-body exercise training demonstrate that augmentation of early response of skeletal muscle blood flow during acute exercise occurs within 10 days of exercise training (Shoemaker et al. 1996), which suggests that any signalling event for angiogenesis happens quickly after exercise training commences. The changes in variables associated with skeletal muscle vascularization and blood flow, combined with the loss of an effect of supplemental oxygen on in vivo oxidative flux measurements, suggest that the oxygenation limitation in skeletal muscle associated with T2D was corrected by SLET.
The observed changes to skeletal muscle blood flow were not accompanied by major improvement to intrinsic mitochondrial function. The pathophysiology of T2D includes reports of impaired mitochondrial function across several tissues (Pinti et al. 2019). However, it is unclear whether defects in skeletal muscle mitochondria are present in people with T2D as previous reports both support impaired mitochondrial function (Mogensen et al. 2007; Phielix et al. 2008; van Tienen et al. 2012; Antoun et al. 2015), or fail to support differences in mitochondrial parameters in people with and without T2D (Asmann et al. 2006; Boushel et al. 2007; Abdul-Ghani et al. 2009). The data from the current study reflect no baseline difference in mitochondrial content and only state 3 respiration with OCMGS was lower in people with T2D. The finding of similar mitochondrial function between groups in this study may be due to the relative health (no co-morbidities) of the participants with T2D and the similar physical activity patterns of each group. Further work is necessary to understand the impact of T2D on mitochondrial function, and its implication for T2D therapies. With SLET, there was an increase in state 4 respiration and the associated increases in L/P and L/E ratios in all participants. The change in state 4 respiration may reflect an adaptation to SLET to maintain mitochondrial membrane potential (Divakaruni & Brand, 2011). However, without concomitant changes to state 3 respiration it is difficult to determine the importance of this change. The P/E ratio with PMGS did increase in OWC participants with SLET but not in T2D, but the absence of significant changes in state 3 or max-uncoupled respiration in either group make these data difficult to interpret as a change to mitochondrial function. Previous studies have reported improved skeletal muscle mitochondrial function with whole-body aerobic exercise training in adults with T2D (Meex et al. 2010; Phielix et al. 2010; Pino et al. 2019). Further, studies using a single-leg exercise model in participants without T2D have demonstrated improved mitochondrial variables with training. Alternating single-leg knee extension augmented both VO2peak and citrate synthase activity in the vastus lateralis in young, sedentary participants (Wolff et al. 2019). In young, active men, citrate synthase activity in the vastus lateralis increased, as well as mitochondrial content, in response to single-leg cycling where both legs were used separately (MacInnis et al. 2017). The absence of an effect of SLET across mitochondrial parameters in people with and without T2D could be the result of the shorter duration of the current study compared with these other examples or that our SLET intervention was not a robust enough aerobic stimulus to facilitate changes to intrinsic mitochondrial properties or required a longer duration.
We focused on skeletal muscle oxidative flux because it is a powerful contributor to cardiorespiratory fitness. Cardiorespiratory fitness, measured as VO2peak, is determined by the coordination of blood flow and oxygen extraction from arterial blood to use for oxidative ATP production, particularly in skeletal muscle. In people with T2D, cardiorespiratory fitness is lower than in their peers without T2D, but with similar age, BMI and physical activity (Regensteiner et al. 2015; Bjornstad et al. 2016; Scalzo et al. 2018a). This finding is consistent with the data from the current study. Comparing the study population from the current investigation with FRIEND Registry values (Myers et al. 2017), the participants with T2D had less than 50% of the cardiorespiratory fitness of their peers without T2D even when considering the difference in testing modalities and altitude between the current study and the FRIEND registry (~10% difference for treadmill versus cycle (Shephard, 1984); ~ 5% for the study location (1600 m) (Wehrlin & Hallen, 2006)). This dramatic impairment in cardiorespiratory fitness associated with T2D is an important clinical finding because of the inverse relationship between fitness and premature mortality (Wei et al. 1999, 2000; Harber et al. 2017). The excess premature mortality observed in people with T2D (Baena-Diez et al. 2016) highlights the need to focus on improving cardiorespiratory fitness in this population.
Whole-body aerobic exercise training improves cardiorespiratory fitness in people with T2D (Regensteiner et al. 2015) by targeting several contributing variables in both central and peripheral systems. The focus of this study was on the local environment in the skeletal muscle, so a SLET model was employed. The findings from this study suggest that there could be a coordinated response between skeletal muscle blood flow and mitochondria in response to SLET and that each of these variables should be considered when targeting cardiorespiratory fitness with whole-body exercise training studies.
The study of whole-body and single-leg exercise in people with T2D is fraught with potential limitations and considerations. Two important points relative to the current study include assessing the impact of sex on the response to exercise in people with T2D and understanding the impact of medications common in the treatment of T2D (specifically metformin and statins) on skeletal muscle mitochondria.
The current study was powered to examine the interaction of T2D and SLET on in vivo oxidative flux; sex was not included as a factor for the primary statistical analyses. However, clear indications exist that exercise performance and cardiorespiratory fitness are affected differently by T2D in men and women (Regensteiner et al. 2015; Huebschmann et al. 2019). For example, there is a greater T2D-associated decrement in cardiorespiratory fitness when women with T2D are compared with women without T2D versus the comparison between men with and without T2D (Regensteiner et al. 2015). Given that impaired cardiorespiratory fitness is associated with a greater relative risk of mortality, this sex-specific decrement associated with T2D in women is significant in terms of the implications for health (Huebschmann et al. 2019). We conducted exploratory analyses to determine whether there was a signal for an interaction between sex and diabetes on the response to exercise. In line with previous studies, women in this study with T2D appeared to have less mitochondrial content (55% less complex I and 47% less complex III) and citrate synthase activity (25% less) compared with the women without T2D. Further, in response to training, citrate synthase activity and complex I and III content were appreciably increased (respectively, 29%, 50% and 34% increases) in the OWC women while these responses were absent in the women with T2D (citrate synthase activity 2% decrease, complex I 5% increase, and complex III 10% decrease). These differences between women with and without T2D were not found in OWC men and their T2D counterparts. Using these descriptive data, future studies will be powered to include sex as a variable in the primary analyses so that conclusions regarding the interaction of sex and T2D on whole-body as well as SLET can be made.
The use of medications with implications for mitochondrial health in the participants also warrants further discussion. In the current study, 47% of the participants with T2D were prescribed a statin versus 18% of OWC participants. Statins are widely used to treat hypercholesterolaemia and lower the risk for cardiovascular events (Salami et al. 2017). The use of statins is considered the standard of care in this age group with T2D considering the increased risk for cardiovascular mortality with high cholesterol (Andary et al. 2019). There is a documented association between statins and impaired skeletal muscle mitochondrial function (Larsen et al. 2013; Dohlmann et al. 2019) and the response of mitochondria to whole-body exercise training (Mikus et al. 2013). Similar to statins, metformin administration in older individuals without T2D has been observed to blunt mitochondrial respiration adaptations to whole-body exercise training (Konopka et al. 2019). Specific to this study, the percentage of participants with T2D-prescribed statins (47%) and metformin (77%) was greater than those prescribed these medications in the OWC group (18% and 0%, respectively; P = 0.0169 for statin prescriptions and P < 0.0001 for metformin prescriptions). However, the participants with T2D had a greater in vivo oxidative flux response to exercise training than the OWC group and similar responses to training with ex vivo assessments of mitochondrial function. In people with T2D, these medications have documented benefits for diabetes complications and glycaemic control, respectively (Mills et al. 2008; An & He, 2016). The data from this study suggest that the use of the medications, in the context of T2D, does not interfere with adaptations to exercise training.
In conclusion, SLET resolved the T2D-associated impairment of in vivo mitochondrial oxidative flux and improved effective blood flow and oxygen delivery in all participants. The potential for understanding the mechanism of increased in vivo oxidative flux has key implications for cardiorespiratory fitness, a critically important clinical variable to consider in people with T2D. In vivo oxidative flux is (1) lower in people with T2D than in individuals without T2D and (2) could be dependent on blood flow and oxygen delivery to skeletal muscle. Single-leg exercise training resolves the T2D-associated impairment of skeletal muscle in vivo mitochondrial oxidative flux potentially through the improvement of effective blood flow/oxygen delivery. Future studies will examine the time course of changes to blood flow versus mitochondrial function to better target therapies to improve cardiorespiratory fitness in women and men with T2D.
Supplementary Material
Key points.
People with type 2 diabetes (T2D) have impaired skeletal muscle oxidative flux due to limited oxygen delivery.
In the current study, this impairment in oxidative flux in people with T2D was abrogated with a single-leg exercise training protocol.
Additionally, single-leg exercise training increased skeletal muscle CD31 content, calf blood flow and state 4 mitochondrial respiration in all participants.
Funding
This study was funded by the following: the Eugene Armstrong Family Foundation, an American Diabetes Association Clinical Research Grant (1-12-CT-64 to J.G.R. and J.E.B.R.), a Veterans Administration Career Development Award (BX004533 to R.L.S.), the National Institutes of Health (NIH)/National Center for Research Resources (T32-DK-063687 and K23-DK-107871 to MC-G), NIH Building Interdisciplinary Research Careers in Women’s Health (2K12-HD-057022 IES and MC-G), the Doris Duke Foundation (2015212 to MC-G), the Denver Research Institute pilot (IES), the Eastern Colorado Geriatric Research, Education, and Clinical Center (IES), a Veterans Administration Merit Award (CVP BX002046 to JEBR), the NIH/National Center for Advancing Translational Sciences (Colorado CTSA UL1-TR-001082) and Magnet NIH (1S10-OD-018435).
Biography

Rebecca L. Scalzo is an Assistant Professor of Medicine at the University of Colorado Anschutz Medical Campus and a Research Scientist at the Rocky Mountain Regional VA Medical Center. Her research is focused on understanding the impact of type 2 diabetes on cardiorespiratory fitness and skeletal muscle mitochondrial function using translational models. She is particularly interested in the sexual-dimorphic impact of type 2 diabetes on these outcomes. Dr Scalzo received her undergraduate degree from the University of Kansas, her master’s degree from Ball State University and her doctoral degree from Colorado State University.
Footnotes
Competing interests
The authors declare that there are no competing interests.
Additional information
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abdul-Ghani MA, Jani R, Chavez A, Molina-Carrion M, Tripathy D & Defronzo RA (2009). Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia 52, 574–582. [DOI] [PubMed] [Google Scholar]
- An H & He L (2016). Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol 228, R97–R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andary R, Fan W & Wong ND (2019). Control of cardiovascular risk factors among US adults with type 2 diabetes with and without cardiovascular disease. Am J Cardiol 124, 522–527. [DOI] [PubMed] [Google Scholar]
- Antoun G, McMurray F, Thrush AB, Patten DA, Peixoto AC, Slack RS, McPherson R, Dent R & Harper ME (2015). Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 58, 2861–2866. [DOI] [PubMed] [Google Scholar]
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML & Nair KS (2006). Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55, 3309–3319. [DOI] [PubMed] [Google Scholar]
- Baena-Diez JM, Penafiel J, Subirana I, Ramos R, Elosua R, Marin-Ibanez A, Guembe MJ, Rigo F, Tormo-Diaz MJ, Moreno-Iribas C, Cabre JJ, Segura A, Garcia-Lareo M, Gomez de la Camara A, Lapetra J, Quesada M, Marrugat J, Medrano MJ, Berjon J, Frontera G, Gavrila D, Barricarte A, Basora J, Garcia JM, Pavone NC, Lora-Pablos D, Mayoral E, Franch J, Mata M, Castell C, Frances A, Grau M & Investigators F (2016). Risk of cause-specific death in individuals with diabetes: A competing risks analysis. Diabetes Care 39, 1987–1995. [DOI] [PubMed] [Google Scholar]
- Bamman MM & Caruso JF (2000). Resistance exercise countermeasures for space flight: implications of training specificity. J Strength Cond Res 14, 45–49. [PubMed] [Google Scholar]
- Bergstrom J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Bjornstad P, Truong U, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Pyle L, Regensteiner JG, Reusch JE & Nadeau KJ (2016). Cardiopulmonary dysfunction and adiponectin in adolescents with type 2 diabetes. J Am Heart Assoc 5, e002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R & Dela F (2007). Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50, 790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR & Regensteiner JG (1999). Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care 22, 1640–1646. [DOI] [PubMed] [Google Scholar]
- Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FG, Shankland E, Conley KE & Goodpaster BH (2013). Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci 68, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Gupta A, Coe GV, Baumgartner AD, Pyle L, Reusch JE, Brown MS, Newcomer BR & Nadeau KJ (2017). Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J Diabetes Complications 31,141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Newcomer BR, Brown M, Hull A, West AD, Singel D, Reusch JE, McFann K, Regensteiner JG & Nadeau KJ (2014a). Method for controlled mitochondrial perturbation during phosphorus MRS in children. Med Sci Sports Exerc 46, 2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, Regensteiner JG, Pyle L, Reusch JE & Nadeau KJ (2014b). Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree-Green M, Scalzo RL, Harrall K, Newcomer BR, Schauer IE, Huebschmann AG, McMillin S, Brown MS, Orlicky D, Knaub L, Nadeau KJ, McClatchey PM, Bauer TA, Regensteiner JG & Reusch JEB (2018). Supplemental oxygen improves in vivo mitochondrial oxidative phosphorylation flux in sedentary obese adults with type 2 diabetes. Diabetes 67,1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ, Packer L & Brooks GA (1981). Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys 209, 539–554. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS & Brand MD (2011). The regulation and physiology of mitochondrial proton leak. Physiology 26, 192–205. [DOI] [PubMed] [Google Scholar]
- Dohlmann TL, Morville T, Kuhlman AB, Chrois KM, Helge JW, Dela F & Larsen S (2019). Statin treatment decreases mitochondrial respiration but muscle coenzyme Q10 levels are unaltered: the LIFESTAT study. J Clin Endocrinol Metab 104, 2501–2508. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Lewis MT, Kasper JD, Chantler PD & Wiseman RW (2019). Type 2 diabetes mellitus in the Goto-Kakizaki rat impairs microvascular function and contributes to premature skeletal muscle fatigue. J Appl Physiol 126, 626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC, Wu F, Goodwill AG, Butcher JT & Beard DA (2011). Spatial heterogeneity in skeletal muscle microvascular blood flow distribution is increased in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 301, R975–R986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saltin B, Saubert CWt, Sembrowich WL & Shepherd RE (1973). Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34, 107–111. [DOI] [PubMed] [Google Scholar]
- Harber MP, Kaminsky LA, Arena R, Blair SN, Franklin BA, Myers J & Ross R (2017). Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog Cardiovasc Dis 60, 11–20. [DOI] [PubMed] [Google Scholar]
- Hiatt WR, Huang SY, Regensteiner JG, Micco AJ, Ishimoto G, Manco-Johnson M, Drose J & Reeves JT (1989). Venous occlusion plethysmography reduces arterial diameter and flow velocity. J Appl Physiol 66, 2239–2244. [DOI] [PubMed] [Google Scholar]
- Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG & Reusch JEB (2019). Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 62, 176–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ & Reusch JE (2015). Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. J Cardiovasc Pharmacol 65,137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA, Biela LM, Bailey SM, Hamilton KL & Miller BF (2019). Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 18, e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R & Kunz WS (2008). Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3, 965–976. [DOI] [PubMed] [Google Scholar]
- Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Bang LE, Bundgaard H, Nielsen LB, Helge JW & Dela F (2013). Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol 61,44–53. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP & Weinsier RL (2000). 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed 13, 14–27. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV & Ferrara N (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309. [DOI] [PubMed] [Google Scholar]
- MacDonald TL, Pattamaprapanont P, Pathak P, Fernandez N, Freitas EC, Hafida S, Mitri J, Britton SL, Koch LG & Lessard SJ (2020). Hyperglycaemia is associated with impaired muscle signalling and aerobic adaptation to exercise. Nat Metab. 2, 902–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis MJ, Zacharewicz E, Martin BJ, Haikalis ME, Skelly LE, Tarnopolsky MA, Murphy RM & Gibala MJ (2017). Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work. J Physiol 595, 2955–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason McClatchey P, Bauer TA, Regensteiner JG, Schauer IE, Huebschmann AG & Reusch JEB (2017a). Dissociation of local and global skeletal muscle oxygen transport metrics in type 2 diabetes. J Diabetes Complications 31, 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason McClatchey P, Wu F, Olfert IM, Ellis CG, Goldman D, Reusch JEB & Frisbee JC (2017b). Impaired tissue oxygenation in metabolic syndrome requires increased microvascular perfusion heterogeneity. J Cardiovasc Transi Res 10, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P & Hesselink MK (2010). Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59, 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ & Thyfault JP (2013). Simvastatin impairs exercise training adaptations. J Am Coll Cardiol 62, 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P & Perri D (2008). Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 52, 1769–1781. [DOI] [PubMed] [Google Scholar]
- Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H & Hojlund K (2007). Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56, 1592–1599. [DOI] [PubMed] [Google Scholar]
- Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E & Arena R (2017). A reference equation for normal standards for VO2 max: analysis from the fitness registry and the importance of exercise national database (FRIEND registry). Prog Cardiovasc Dis 60, 21–29. [DOI] [PubMed] [Google Scholar]
- Newcomer BR & Boska MD (1997). Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 20, 336–346. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Savard G, Richter EA, Hargreaves M & Saltin B (1990). Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol 69, 1040–1046. [DOI] [PubMed] [Google Scholar]
- Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD & Breen EC (2009). Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587, 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesta D & Gnaiger E (2012). High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810, 25–58. [DOI] [PubMed] [Google Scholar]
- Phielix E, Meex R, Moonen-Kornips E, Hesselink MK & Schrauwen P (2010). Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia 53, 1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK & Schrauwen P (2008). Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 57, 2943–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino MF, Stephens NA, Eroshkin AM, Yi F, Hodges A, Cornnell HH, Pratley RE, Smith SR, Wang M, Han X, Coen PM, Goodpaster BH & Sparks LM (2019). Endurance training remodels skeletal muscle phospholipid composition and increases intrinsic mitochondrial respiration in men with Type 2 diabetes. Physiol Genomics 51, 586–595. [DOI] [PubMed] [Google Scholar]
- Pinti MV, Fink GK, Hathaway QA, Durr AJ, Kunovac A & Hollander JM (2019). Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab 316, E268–E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Huebschmann AG, Herlache L, Weinberger HD, Wolfel EE & Reusch JE (2015). Sex differences in the effects of type 2 diabetes on exercise performance. Med Sci Sports Exerc 47, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH & Hiatt WR (1998). Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol 85, 310–317. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S, Oliver-Pickett CK, Reusch JE & Weil JV (2003). Oral L-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med 8, 169–175. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Sippel J, McFarling ET, Wolfel EE & Hiatt WR (1995). Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc 27, 661–667. [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? JAppl Physiol 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R & Wagner PD (2000). Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol 279, H772–H778. [DOI] [PubMed] [Google Scholar]
- Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R & Bell JD (1999). Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1H-MRS. J Appl Physiol 87, 2068–2072. [DOI] [PubMed] [Google Scholar]
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D & Williams R & Committee IDFDA (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157, 107843. [DOI] [PubMed] [Google Scholar]
- Salami JA, Warraich H, Valero-Elizondo J, Spatz ES, Desai NR, Rana JS, Virani SS, Blankstein R, Khera A, Blaha MJ, Blumenthal RS, Lloyd-Jones D & Nasir K (2017). National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the medical expenditure panel survey. JAMA Cardiol 2, 56–65. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN & Paffenbarger RS Jr (1985). Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121, 91–106. [DOI] [PubMed] [Google Scholar]
- Scalzo RL, Bauer TA, Harrall K, Moreau K, Ozemek C, Herlache L, McMillin S, Huebschmann AG, Dorosz J, Reusch JEB & Regensteiner JG (2018a). Acute vitamin C improves cardiac function, not exercise capacity, in adults with type 2 diabetes. Diabetol Metab Syndr 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo RL, Knaub LA, Hull SE, Keller AC, Hunter K, Walker LA & Reusch JEB (2018b). Glucagon-like peptide-1 receptor antagonism impairs basal exercise capacity and vascular adaptation to aerobic exercise training in rats. Physiol Rep 6, e13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RJ (1984). Tests of maximum oxygen intake. A critical review. Sports Med 1, 99–124. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Phillips SM, Green HJ & Hughson RL (1996). Faster femoral artery blood velocity kinetics at the onset of exercise following short-term training. Cardiovasc Res 31, 278–286. [PubMed] [Google Scholar]
- Sirikul B, Hunter GR, Larson-Meyer DE, Desmond R & Newcomer BR (2007). Relationship between metabolic function and skeletal muscle fatigue during a 90 s maximal isometric contraction. Appl Physiol Nutr Metab 32, 394–399. [DOI] [PubMed] [Google Scholar]
- Sjoberg KA, Frosig C, Kjobsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JFP, Rattigan S, Richter EA & McConell GK (2017). Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66, 1501–1510. [DOI] [PubMed] [Google Scholar]
- van den Boogaart A (1997). MRUI MANUAL V. 96.3. A user’s guide to the Magnetic Resonance User Interface Software Package. Delft Technical University Press, Delft. [Google Scholar]
- van Tienen FH, Praet SF, de Feyter HM, van den Broek NM, Lindsey PJ, Schoonderwoerd KG, de Coo IF, Nicolay K, Prompers JJ, Smeets HJ & van Loon LJ (2012). Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J Clin Endocrinol Metab 97, 3261–3269. [DOI] [PubMed] [Google Scholar]
- Wehrlin JP & Hallen J (2006). Linear decrease in. VO2max and performance with increasing altitude in endurance athletes. Eur J Appl Physiol 96, 404–412. [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ & Blair SN (2000). Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 132, 605–611. [DOI] [PubMed] [Google Scholar]
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr. & Blair SN (1999). Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282, 1547–1553. [DOI] [PubMed] [Google Scholar]
- Wolff CA, Konopka AR, Suer MK, Trappe TA, Kaminsky LA & Harber MP (2019). Increased cardiorespiratory fitness and skeletal muscle size following single-leg knee extension exercise training. J Sports Med Phys Fitness 59, 934–940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


