Abstract
Background
Acute otitis media (AOM) is a spontaneously remitting disease of which pain is the most distressing symptom. Antibiotics are now known to have less benefit than previously assumed. Topical pain relief may be a satisfactory intervention for AOM sufferers and encourage clinicians to prescribe fewer antibiotics.
Objectives
To assess the effectiveness of topical analgesia for AOM in adults and children.
Search methods
For this second update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1), Ovid MEDLINE (2008 to February Week 1 2011), Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 10 February 2011), Ovid EMBASE (2008 to 2011 Week 05), EBSCO CINAHL (2008 to 4 February 2011) and Ovid AMED (2008 to April 2011).
Selection criteria
Double‐blind randomised controlled trials (RCTs) or quasi‐RCTs comparing an otic preparation with an analgesic effect (excluding antibiotics) versus placebo or an otic preparation with an analgesic effect (excluding antibiotics) versus any other otic preparation with an analgesic effect, in adults or children presenting at primary care settings with AOM without perforation.
Data collection and analysis
Three review authors independently screened studies, assessed trial quality and extracted data. Attempts to obtain additional information from the trial authors of the included trials were unsuccessful.
Main results
Five trials including 391 children aged three to 18 years met our criteria. Two studies (117 children) compared anaesthetic ear drops versus placebo immediately at diagnosis. All children received some form of oral pain relief. In all five studies it was clear that ear pain diminishes rapidly for most sufferers. Nevertheless there was a statistically significant difference in the proportion of children achieving a 50% reduction in pain in favour of anaesthetic drops 10 minutes after instillation (risk ratio (RR) 2.13, 95% confidence interval (CI) 1.19 to 3.80) and 30 minutes after instillation (RR 1.43, 95% CI 1.12 to 1.81) on the day AOM was diagnosed but not at 20 minutes (RR 1.24, 95% CI 0.88 to 1.74). Three trials (274 children) compared anaesthetic ear drops with naturopathic herbal ear drops. Naturopathic drops were favoured 15 and 30 minutes after instillation, one to three days after diagnosis, but the differences were not statistically significant. Only one trial looked at adverse reactions and found none. Overall the findings of this review are based on trial evidence that is at low or unclear risk of bias.
Authors' conclusions
Evidence from five RCTs, only two of which addressed the most relevant question of primary effectiveness, provides limited evidence that ear drops are effective 30 minutes after administration in older children with AOM. Uncertainty exists as to the magnitude of this effect and more high‐quality studies are needed.
Plain language summary
Topical analgesics for acute otitis media
Antibiotics make little difference to children with an uncomplicated ear infection and ear pain. Some advocate ear drops with local anaesthetic such as amethocaine, benzocaine or lidocaine. Five trials (391 participants) were identified; two compared anaesthetic drops to placebo (inactive) drops; and three compared anaesthetic drops to herbal ear drops. There was no strong evidence that herbal ear drops were effective, but anaesthetic drops did provide better pain relief than the inactive drops. Only one trial looked at adverse reactions and reported no cases of ringing in the ears or unsteadiness when walking and three cases of very mild dizziness.
Children in all the trials experienced a rapid, short‐term reduction in pain after using ear drops. It is hard to know if this was the result of the natural course of the illness; the placebo effect of receiving treatment; the soothing effect of any liquid in the ear or the pharmacological effects of the ear drops themselves. Nevertheless, there is some evidence that when combined with oral pain medication, anaesthetic ear drops may help to relieve pain more rapidly in children aged three to 18 years. More good‐quality trials are needed.
Background
Description of the condition
Acute otitis media (AOM) is a very common disease of childhood and a leading cause of visits to the family doctor and antibiotic prescribing for children in high‐income countries (Charles 2004; Froom 1997; Pirozzo 2004; Sanders 2009). Although it is difficult to establish a global estimate, childhood incidence ranges between 17% and 32% per year (Pirozzo 2004). In a national sampling of emergency department visits in the USA for 1996 to 2005, there were 2.6 million and 2.1 million visits for AOM during the first and last years of the study (Fischer 2007).
AOM is characterised by inflammation and effusion of the middle ear accompanied by varying degrees of local pain, fever, irritability and possible erythema and deafness. The onset of symptoms and signs is rapid and the acute infection usually resolves within days. The illness can affect people at any age but occurs mainly in children, where incidence peaks between 6 and 15 months (Klein 1989). Although the morbidity rate is high, the mortality rate for healthy children in high‐income countries is low. Suppurative complication rates are also low (Marcy 2001) and severe illness requiring antibiotic therapy only occurs in about 2.7% of children (Van Buchem 1985). This may not be true of low‐income countries where the burden of AOM is heavier because access to medical care is limited and the risk of complications is higher (Berman 1995; Klein 2001). The impact of AOM is also greater among some indigenous populations living in high‐income countries such as Australia and Canada (WHO/CIBA 1996). Potential hearing loss is of particular concern in countries where illiteracy is high and the comprehension of normal speech is vital (Klein 2001).
Description of the intervention
Antibiotics have been a mainstay of treatment based on a pathophysiological model (Pirozzo 2004). Two Cochrane Reviews challenge this approach to treatment by demonstrating that the benefits from antibiotics are modest and may not outweigh their risks (Kozyrskyj 2010; Sanders 2009). Approximately 17 children needed to be treated to prevent one child experiencing pain after two to seven days (Sanders 2009). Another systematic review showed that 60% of children will improve spontaneously in 24 hours without any antibiotic treatment and 80% of cases will resolve within three days (Rosenfeld 2003). Antibiotics also threaten adverse effects in the individual, such as diarrhoea, stomach pain, rash and vomiting. Antibiotic use also inevitably promotes resistance by natural selection, thus limiting their usefulness for future generations (Nasrin 2002). In recent years there has been a trend away from clinicians prescribing antibiotics for all AOM. Generally, children over the age of two years can be managed with analgesia and watchful waiting (Sanders 2009). In an effort to minimise adverse effects and help guard against the selection of resistant strains some current guidelines advise against routine antibiotic treatment for uncomplicated AOM (DoH 2000; SIGN 2003; Spicer 2003). The AAP guidelines (AAP 2004) offer the option, not recommendation, of initially observing selected children, primarily those aged two years or older with non‐severe illness. A small number of studies have examined the value of using topical pain‐relieving ear drops in cases of uncomplicated AOM (Bolt 2008; Hoberman 1997; Sarrell 2001; Sarrell 2003a). The composition of ear drops varies slightly, but generally they are comprised of an anaesthetic such as amethocaine, benzocaine or lidocaine and an analgesic such as phenazone (antipyrine), in a glycerin base.
How the intervention might work
Pain is a common aspect of AOM because the tympanic membrane is well‐innervated with pain sensors and pressure from pus in the middle ear stretches these. Topical analgesia may provide pain relief through the local anaesthetic effect of directly inactivating the sensors (Schecter 2003). In addition, topical analgesic drops may reduce middle ear pressure by the hygroscopic activity of glycerin (Hoberman 1997; Sarrell 2001). Topical treatments may be prescribed or purchased over the counter.
Why it is important to do this review
Even though AOM is not usually life‐threatening, the symptoms may be very distressing, especially for children and their parents/carers. Pain is central to the patient's experience of the illness (Schecter 2003) and the most common reason to seek treatment. A survey of US primary care physicians found that many remain reticent about the 'watchful waiting' approach to managing AOM because they believe this approach will not be acceptable to parents/carers (Vernacchio 2007). Being able to offer an effective treatment for the principle symptom of pain may help doctors to reduce antibiotic prescribing for uncomplicated AOM.
Objectives
The aim of this review was to:
assess the effectiveness of topical analgesia in adults and children suffering from AOM without perforation; and
assess whether different topical analgesic preparations differ in effect.
The primary outcome measures were severity and duration of pain. Secondary measures were parental satisfaction, days missed from school or work (for both children and parents/carers) and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
Double‐blind randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Adults and children presenting at primary care settings, suffering from AOM without perforation.
Types of interventions
Any otic preparation with an analgesic effect (excluding antibiotics) versus placebo.
Any otic preparation with an analgesic effect (excluding antibiotics) versus any other otic preparation with an analgesic effect.
Types of outcome measures
Data extraction focused on patient‐relevant outcomes.
Primary outcomes
Severity and duration of pain.
Secondary outcomes
At least one of the following:
parental satisfaction;
days missed from school or work; or
adverse events.
Search methods for identification of studies
Electronic searches
See Appendix 1 for details of the search methods used in the first published version of this review.
For the first review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, Issue 4) which contains the Acute Respiratory Infections (ARI) Group's Specialised Register, Ovid MEDLINE (2006 to January Week 2 2009), Ovid EMBASE (2006 to 2009 Week 03), Ovid CINAHL (2006 to January Week 2 2009) and Ovid AMED (1985 to January 2009).
For this second review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 1, part of The Cochrane Library,www.thecochranelibrary.com (accessed 11 February 2011), Ovid MEDLINE (2008 to February Week 1 2011), Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 10 February 2011), Ovid EMBASE (2008 to 2011 Week 05), EBSCO CINAHL (2008 to 4 February 2011) and Ovid AMED (2008 to April 2011).
We used the following search strategy in Ovid MEDLINE:
MEDLINE (Ovid)
1 exp Otitis Media/ 2 (otitis media or AOM or OM).mp. 3 or/1‐2 4 exp Benzocaine/ 5 benzocaine.mp. 6 exp Tetracaine/ 7 amethocaine.mp. 8 exp Lidocaine/ 9 lidocaine.mp. 10 (anesthetic or anaesthetic).mp. 11 topical analgesi$.mp. 12 exp Antipyrine/ 13 (antipyrine or phenazone).mp. 14 (americaine otic or aurafair or auralgan or auralgesic or auraphene or aurisan or auroto or dolotic or lanaurine otocain or omedia or oticaine or otigesic or otocalm or Rx‐Otic or sedaural or tympagesic).mp. 15 or/4‐14 16 exp Anti‐Inflammatory Agents/ 17 (antiinflammator$ or anti inflammator$).mp. 18 or/16‐17 19 exp Administration, Topical/ 20 (topical or otic).mp. 21 or/19‐20 22 18 and 21 23 exp Histamine H1 Antagonists/ 24 (antihistamine$ or anti‐histamine$).mp. 25 or/23‐24 26 25 and 21 27 exp Steroids/ 28 steroid$.mp. 29 or/27‐28 30 29 and 21 31 15 or 22 or 26 or 30 32 3 and 31
Search strategies for EMBASE, CINAHL, AMED and CENTRAL are shown in the appendices (Appendix 2; Appendix 3; Appendix 4; Appendix 5). We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE and CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no publication or language restrictions.
Searching other resources
We scanned the references of all included trials to identify other potentially relevant studies. We contacted the manufacturers of topical analgesic preparations and authors of published trials to enquire if they were aware of any unpublished trials. Only one reply was received, stating that the company had no additional unpublished data about their product. We searched the reference lists of all eligible studies for further references not identified through the electronic searches.
Data collection and analysis
Three review authors (JD, ACJ, JW) independently assessed the trials. We resolved differences of opinion by discussion. Five trials fulfilled the criteria (Bolt 2008; Hoberman 1997; Sarrell 2001; Sarrell 2003a; Sarrell 2003b); two trials were reported in one paper (Sarrell 2003a; Sarrell 2003b). We identified one unpublished trial (Matz 2001a) through personal communications but then excluded it. Reasons for exclusion for all papers, whether appraised or not, are detailed in the Characteristics of excluded studies table. We scanned the references of the included trials to identify other potentially relevant studies. We sent letters of enquiry to 17 companies that were listed in MicroMedex as manufacturers of otic pain relief preparations, in order to locate unpublished trials or data. Only one reply was received stating that the company had no additional unpublished data about their product.
Selection of studies
Three review authors (ACJ, JW, JD) independently reviewed titles and abstracts to exclude trials which clearly did not meet the inclusion criteria of the review. We obtained the full paper for further examination if any review author felt that the trial might possibly meet the inclusion criteria.
Data extraction and management
Two review authors (ACJ, JW) independently extracted data from the studies using data extraction forms designed and validated by the review authors. Differences were resolved by discussion. Attempts to obtain missing data from trial authors were unsuccessful.
Assessment of risk of bias in included studies
In the original version of this review we assessed the risk of bias using a modification of a published method by Chalmers et al (Chalmers 1990). In subsequent updates including this one, we independently reassessed each included study using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding of either participants, personnel or assessors, or any combination of the three, incomplete outcome data, selective outcome reporting and other issues (for example, extreme baseline imbalance) (see Appendix 6 for details of criteria on which the judgements were based).
Measures of treatment effect
Pain was measured as a dichotomous outcome in two trials (Bolt 2008 and Hoberman 1997) and as a continuous outcome in the other three trials (Sarrell 2001, Sarrell 2003a and Sarrell 2003b). Forest plots for the trials with dichotomous outcomes were drawn using risk ratios (RR) and 95% confidence interval (CI). We combined and analysed trials measuring continuous outcomes using mean differences (MD) and 95% CI. Our attempts to obtain individual patient data in order to reconstruct an intention‐to‐treat (ITT) analysis in three of the trials were unsuccessful.
Assessment of heterogeneity
We examined heterogeneity using both fixed‐ and random‐effects models in Review Manager software (RevMan 2011). In trials measuring dichotomous outcomes (anaesthetic versus placebo) there was very little heterogeneity so we used a fixed‐effect model. In the trials using continuous data (naturopathic drop trials) we used a random‐effects model as heterogeneity was identified.
Subgroup analysis and investigation of heterogeneity
The planned subgroup analyses of the primary outcomes were:
age groups; a) children aged less than 24 months at time of randomisation; b) children aged 24 months up to 18 years at time of randomisation; c) adults aged 18 years and over at time of randomisation;
different types of otic preparations with an analgesic effect ‐ local anaesthetics, antihistamines, nonsteroidal anti‐inflammatories, steroids or complementary medicines; and
concurrent use of antibiotics.
We were unable to carry out any subgroup analyses because there were too few trials and insufficient data in the categories outlined in the protocol.
Results
Description of studies
Results of the search
In the original review combined searches of MEDLINE, EMBASE, CENTRAL and LILACS retrieved 356 citations. We reviewed full copies of 29 studies; eight trials were identified as possibly meeting the review inclusion criteria and four trials were included in reports published in three papers. The 2003 paper by Sarrell was considered as two trials for the purposes of analysis (Sarrell 2003a; Sarrell 2003b). In the first update combined searches of MEDLINE, EMBASE, CENTRAL retrieved 10 unique citations, two reports were identified as possibly meeting the review inclusion criteria and one new trial was included (Bolt 2008). For this second update combined searches of MEDLINE, EMBASE, CENTRAL, CINAHL and AMED retrieved 109 citations, none of which were eligible for inclusion.
Included studies
Five trials fulfilled the review inclusion criteria; of these, two were found in the same paper (Sarrell 2003a; Sarrell 2003b). Two of the trials (Bolt 2008; Hoberman 1997) evaluated the efficacy of anaesthetic ear drops for treating ear pain in children with acute otitis media. Adults were not included in any of the studies, nor were children under the age of three.
In Hoberman 1997 54 children aged 5 to 19 years, with ear pain and a clinical diagnosis of AOM, who presented to the primary care settings or the emergency department of the Children's Hospital of Pittsburgh, Pennsylvania, USA were eligible for enrolment in the study. Children were excluded if they had received any analgesic medication or ear drops within the preceding five hours. The anaesthetic drops consisted of antipyrine, benzocaine and glycerine. Eligible children were randomly assigned to either five drops of the anaesthetic preparation or olive oil placebo. All children were also treated with 15 mg/kg of acetaminophen as a single dose. Ear pain was assessed upon entry to the study, then 10, 20 and 30 minutes after instillation and an average ear pain score was determined. Two visual analogue scales were used; a 10 cm horizontal line and a 10 cm colour scale ranging from white (indicating no pain) through gradations of red to dark red (indicating severe pain). A 1 cm span in each scale was equivalent to an ear pain point. A pain score of at least three out of 10 at the onset of treatment was required for study participation.
In the study by Bolt 2008 children aged between 3 and 17 years who presented to the emergency department of an Australian hospital with ear pain of less than three days’ duration and evidence of AOM were eligible for enrolment in the study. Preceding oral analgesic was not a criterion for exclusion. However, details of medication use were collected. Eligible children were randomly assigned to receive three drops of topical aqueous 2% lignocaine or normal saline (placebo). Drops were instilled in the painful ear; if the pain was bilateral, the most painful ear was treated first. If the patient had not received analgesia in the preceding four hours, they were offered 15 mg/kg paracetamol. Further oral analgesia was offered between 10 (T10) and 30 minutes (T30) of the study period at the discretion of the treating doctor. Any analgesia given in hospital was recorded. Ear pain was assessed at study entry and 10, 20 and 30 minutes later (T0, T10, T20 and T30, respectively) by the patient and at T0 and T30 by the treating physician. Ear pain was measured using a faces pain scale for patients up to six years of age (Bieri Faces Pain Scale‐Revised) and a visual analogue scale (VAS; score 0 to 10) for use by staff and patients seven years and older. Three children were lost to phone follow up the next day; two in the anaesthetic group and one in the control group.
Three trials investigated the efficacy of naturopathic herbal extracts in the management of ear pain associated with AOM. One of these (Sarrell 2001) included 110 children aged between 6 to 18 years with ear pain and for whom a clinical diagnosis of otitis media was made, enhanced by tympanometry. Children were excluded if they had used any ear drops or analgesics within the preceding four hours.
Participants in this trial (Sarrell 2001) were randomised to receive either anaesthetic ear drops (amethocaine, phenazone and glycerine) or naturopathic herbal extract ear drops (Allium sativum, Verbascum thapsus, calendula flores and Hypericum perforatum in olive oil). Five drops of either solution were instilled into the ear three times daily for three days. All children were treated with acetaminophen (15 mg/kg given as a single dose). Ear pain reduction was assessed using two visual analogue scales, graded 1 to 10, with 1 signifying no pain and 10 signifying excruciating pain. Measurements of both scales were recorded separately at each time point, and then averaged to determine an overall ear pain score for each treatment group. Pain was measured upon diagnosis of AOM and then daily for three days; before instillation and then 15 and 30 minutes after the first instillation each day. A pain score of at least three at the onset of treatment was required for study participation. The participants were educated in the use of the pain scale and telephone interviews were conducted with parents 24 and 48 hours after the treatment period. Seven children were not included in the final analysis (five due to non‐compliance and two because they were overcome by the smell of the ear drops).
In the second trial by Sarrell (Sarrell 2003a), 90 children aged 5 to 18 years with ear pain were enrolled. The children were assigned by computer‐numbered randomisation to receive either anaesthetic ear drops (amethocaine, phenazone and glycerine) or naturopathic herbal extract ear drops (Allium sativum, Verbascum thapsus, calendula flores, Hypericum perfoliatum, Lavandula officinalis and vitamin E in olive oil). The dosing schedule was five drops three times daily for three days. Ear pain was assessed by using a linear numbered scale, from 1 (no pain) to 10 (worst possible pain), a scale of five facial expressions, and a colour scale. Four children were excluded from the final analysis because of non‐compliance (they forgot to take the medicine or could not be reached for the follow‐up interview).
In the third trial by Sarrell (Sarrell 2003b), 90 children aged 5 to 18 years with ear pain were enrolled. Computer‐numbered randomisation was used to assign children to treatment. Anaesthetic ear drops (five drops three times daily for three days) plus oral amoxicillin (80 mg/kg/day divided into three doses) were compared with naturopathic herbal extract ear drops (five drops three times daily for three days) plus oral amoxicillin (80 mg/kg/day divided into three doses). Ear pain assessment and outcome measures were conducted as per Sarrell 2003a. Five children were excluded from the final analysis because of non‐compliance (they forgot to take the medicine or could not be reached for the follow‐up interview).
Excluded studies
We excluded two trials (Laxdal 1970; Matz 2001a) because they compared a topical otic preparation to oral antibiotics. One trial (Matz 2001a) assessed pain and parental satisfaction in children with AOM treated with either anaesthetic ear drops or amoxicillin and the trial was not double‐blinded. Another trial (Laxdal 1970), which compared anaesthetic ear drops to penicillin, did not assess pain and was not double‐blinded. A third trial (Weippl 1985) compared the analgesic effect of suprofen syrup to anaesthetic ear drops. This trial was neither randomised nor double‐blinded and as the syrup was administered orally it was excluded. The fourth trial (Abramson 1969) examined topical anaesthesia for the tympanic membrane prior to surgery.
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
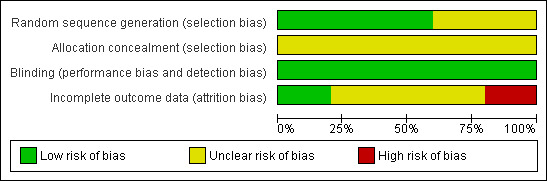
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
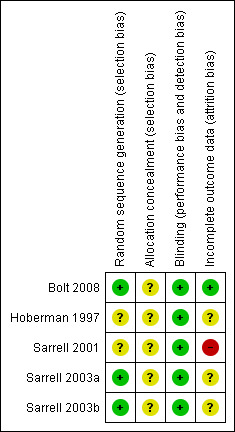
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sarrell 2003a and Sarrell 2003b state that computer‐generated randomisation was used and the Bolt 2008 study report states that the drops were randomised in blocks of 10 but the method of randomisation is not discussed in the other two studies (Hoberman 1997; Sarrell 2001). The method of allocation concealment is unclear in all five trials. In the anaesthetic versus placebo trials (Bolt 2008; Hoberman 1997) the children were comparable in age, sex, race, laterality of AOM and baseline ear pain score. The same was true for the naturopathic drop trials (Sarrell 2001; Sarrell 2003a; Sarrell 2003b). This goes some way to indicating that allocation procedures were satisfactory. However, this cannot be known for certain due to the lack of information in all trial reports.
Blinding
Blinding for a subjective outcome such as pain measurement is important and this aspect of trial design was adequate in all five studies. Double‐blinding is stated in all three naturopathic drop trials but no details were provided for the earlier trial (Sarrell 2001). In Sarrell 2003a and Sarrell 2003b all ear drops were placed in identical bottles, the contents of which were unknown to both the participants and the study nurse. Blinding in both anaesthetic versus placebo trials (Hoberman 1997) was adequate. In Hoberman 1997 children received either the anaesthetic drops or olive oil placebo and investigators were also unaware of treatment assignment. Bolt 2008 reports that patients and parents, those administering ear drops and those assessing ear pain were all blinded to group assignment.
Incomplete outcome data
In Hoberman 1997 no children were reported to have dropped out, but data were missing for one child at the 20 minutes evaluation time point. This child, representing 3.7%, was not accounted for in the final analysis of that time point. In Bolt 2008 two children in the intervention group were reported to have dropped out (6.5%). Data were missing for one of the children at the 10 minutes evaluation time point and the child was thus assumed to have been a failure at T10. One child in the placebo group dropped out (3.2%).
Seven children (6.4%) were excluded after randomisation in Sarrell 2001 but no information about which groups they came from was provided. We contacted the authors on this matter but no further information was forthcoming. Four out of 90 children enrolled in Sarrell 2003a were excluded due to non‐compliance (one (2.2%) in the naturopathic group and three (6.7%) in the anaesthetic group). Five out of 90 children enrolled in the Sarrell 2003b trial were excluded due to non‐compliance (three (6.7%) in the naturopathic ear drops plus antibiotics group and two (4.4%) in the anaesthetic ear drops plus antibiotics group). The authors stated that five children in Sarrell 2003b forgot to take their medication which may indicated that pain symptoms were improving. However, it is not stated which group(s) these children were assigned to. Only two of the four arms were included in our analyses and in both of those groups losses were under 20%. However, attrition bias cannot be ruled out.
Overall losses in Hoberman 1997, Sarrell 2001, Sarrell 2003a and Sarrell 2003b were well under 20% but none of the naturopathic herbal extract trials (Sarrell 2001;Sarrell 2003a;Sarrell 2003b) carried out an intention‐to‐treat analysis.
Other potential sources of bias
In Hoberman 1997 the authors calculated that 27 subjects per study arm were required to detect a clinically significant reduction after 10, 20 and 30 minutes of at least 50% from the baseline score. In Bolt 2008 an indicative power calculation was based on comparison of groups at 30 minutes. They also considered a 50% reduction in pain at each time point from the baseline pain score to be clinically significant. A sample size of 28 per group was calculated to give 80% power. Neither of the naturopathic drop trials reported a power calculation.
Effects of interventions
The primary outcome measures specified in our protocol (severity and duration of pain) matched those in the included trials. However, the secondary outcomes of parental satisfaction and days missed from school or work were not addressed in any of the trials. The trial by Bolt 2008 was the only one to report on adverse events. No episodes of tinnitus or dizziness occurred in the emergency department. Three patients reported mild dizziness the following day but did not require any medical treatment (Bolt 2008). No incidents of tinnitus, dizziness or unsteady gait were reported during the remainder of the follow‐up period (Bolt 2008). Other adverse events such as including stinging, pain, dermatitis and sensitisation (Rosenfeld 2005) were not reported.
Anaesthetic drops versus placebo
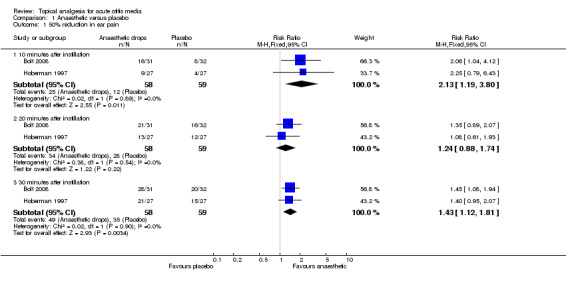
In the two trials (Bolt 2008; Hoberman 1997) that compared anaesthetic ear drops with placebo, the anaesthetic ear drops were favoured at each time point, irrespective of which outcome measure was used ‐ 50% pain reduction or 25% pain reduction. Additional measurements included a one or more point reduction in pain score (Hoberman 1997); a two‐point reduction (Bolt 2008) and mean score over time (Hoberman 1997). Data on these outcomes could not be pooled. However, the anaesthetic ear drops were favoured for all three measures.
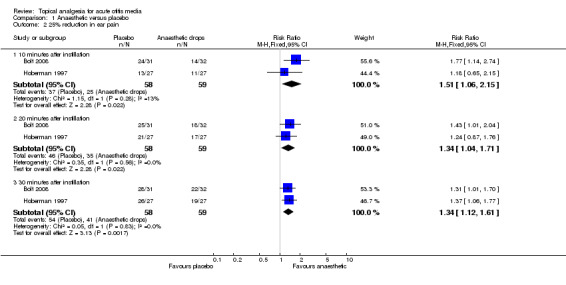
There was a statistically significant difference in the proportion of children achieving a 50% reduction in pain in favour of anaesthetic drops at T10 (10 minutes) (risk ratio (RR) 2.1, 95% confidence interval (CI) 1.2 to 3.8) (Analysis 1.1.1) and T30 (30 minutes) (RR 1.4, 95% CI 1.1 to 1.8) (Analysis 1.1.2) but not at T20 (20 minutes) (RR 1.2, 95% CI 0.9 to 1.7) (Analysis 1.1.2) (Figure 3). The proportion of children achieving a 25% reduction in pain was significantly higher in the anaesthetic group at all time points (T10, RR 1.5, 95% CI 1.1 to 2.2; T20, RR 1.3, 95% CI 1 to 1.7 and T30, RR 1.3, 95% CI 1.1 to 1.6) (Figure 4). The number of children the clinician would need to treat in order for one child to achieve a 50% reduction in pain at T10 is four (95% CI 3 to 16) and at T30 it is also four (95% CI 3 to 11). The number needed to treat for a 25% reduction in pain at T10 is five (95% CI 3 to 27); at T20 it is also five (95% CI 3 to 27) and at T30 it is four (95% CI 3 to 10).
3.
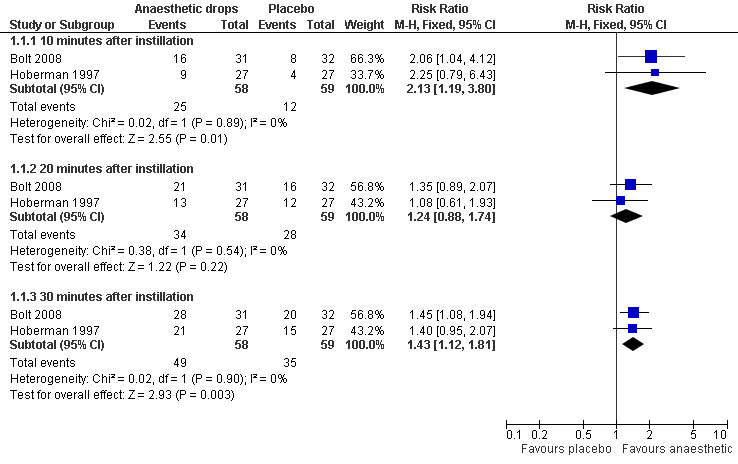
Forest plot of comparison: 1 Anaesthetic versus placebo, outcome: 1.1 50% reduction in ear pain.
4.
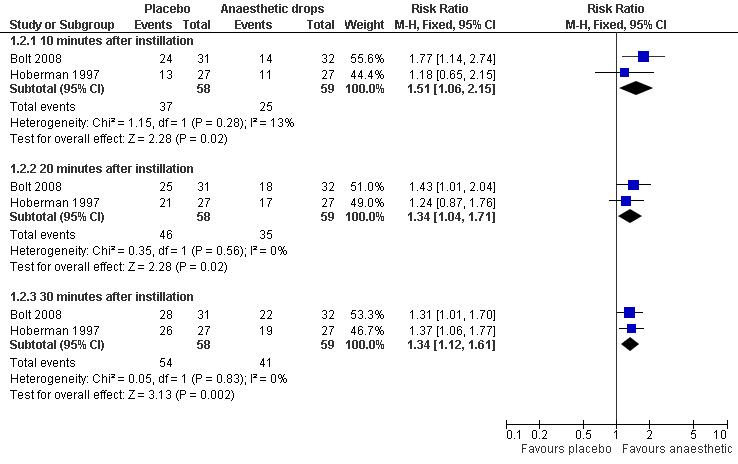
Forest plot of comparison: 1 Anaesthetic versus placebo, outcome: 1.2 25% reduction in ear pain.
Anaesthetic drops versus naturopathic drops
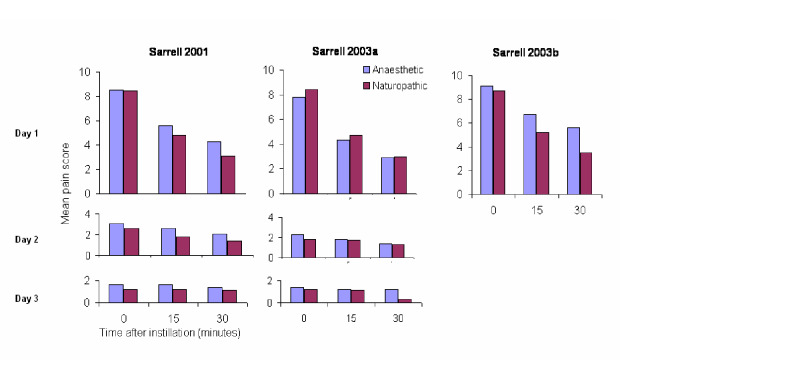
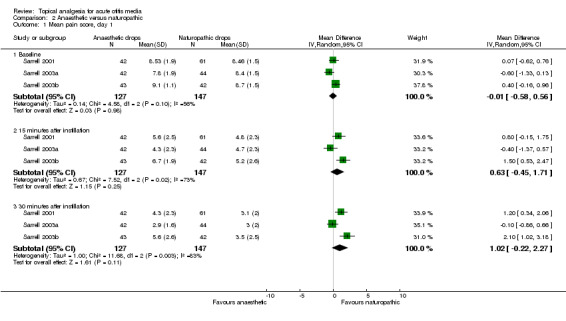
For the anaesthetic versus naturopathic drops (Sarrell 2001; Sarrell 2003a; Sarrell 2003b), a reduction in pain was seen over time in both treatment groups across all trials. In the first trial (Sarrell 2001), the anaesthetic group showed a mean pain score of 8.53 at baseline. It had declined to 5.6 15 minutes after instillation, and to 4.3 30 minutes after instillation on day one. The naturopathic group showed a mean pain score of 8.46 at baseline, 4.8 15 minutes after instillation, and 3.1 30 minutes after instillation.
In the second trial (Sarrell 2003a), the anaesthetic group had a mean pain score of 7.8 at baseline, 4.3 15 minutes after instillation, and 2.9 30 minutes after instillation on day one. The naturopathic group had a mean pain score of 8.4 at baseline, 4.7 15 minutes after instillation, and 3.0 30 minutes after instillation.
In the third trial (Sarrell 2003b), the group that was given anaesthetic drops plus oral amoxicillin showed a mean pain score of 9.1 at baseline, 6.7 15 minutes after instillation, and 5.6 30 minutes after instillation on day one. The group that was given naturopathic drops plus oral amoxicillin showed a mean pain score of 8.7 at baseline, 5.2 15 minutes after instillation, and 3.5 30 minutes after instillation. According to the above results, there is a clear reduction in pain in all groups on day one. The following two days also show a reduction in pain, but the drop is not as pronounced. Initially we believed we should not pool the data because the two arms of Sarrell 2003b used antibiotics. However, there is strong evidence to suggest that antibiotics make no difference to the level of pain within the first 24 hours (Sanders 2009), therefore we performed a meta‐analysis using all the Sarrell trials for day one only.
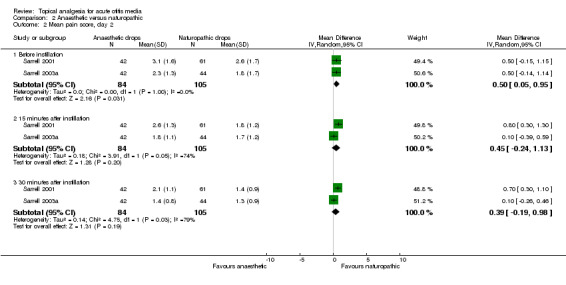
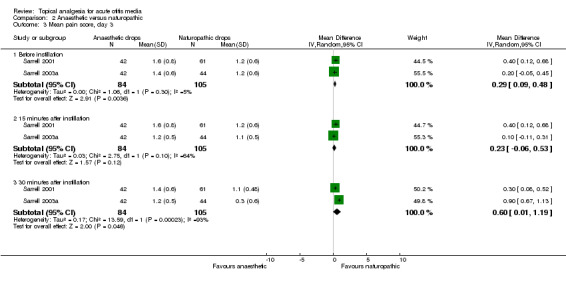
The first trial (Sarrell 2001) achieved statistical significance between the groups at 30 minutes on day one (P value less than 0.01), favouring the naturopathic ear drops. In the second trial (Sarrell 2003a) there was a significant difference in pain on day three (P value less than 0.001), 30 minutes after instilling the drops, also favouring naturopathic ear drops. Antibiotics were given to both groups in the third trial (Sarrell 2003b), in which the naturopathic ear drops were favoured again at each time point, and the differences reached statistical significance at 15 and 30 minutes on day one (P value less than 0.01); before instillation on day two (P value less than 0.001); before (P value less than 0.05) and 30 minutes after instillation on day three (P value less than 0.01) (Figure 5).
5.
We performed a meta‐analysis on two of the naturopathic drop trials (Sarrell 2001; Sarrell 2003a) (Figure 6). The forest plot indicates significant heterogeneity and provides no clear evidence of the effectiveness of naturopathic drops over anaesthetic drops.
6.
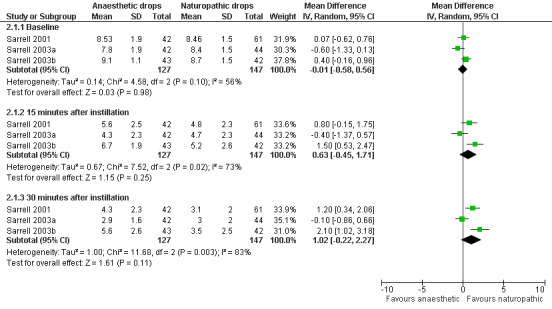
Forest plot of comparison: 2 Anaesthetic versus naturopathic, outcome: 2.1 Mean pain score, day 1.
Discussion
Adequate pain relief early in the course of acute otitis media (AOM) is important. The delay in effect of systemic analgesia may establish pain response pathways and therefore a more a painful illness overall. Antibiotics do not reduce pain within the first day, only slightly reduce it in the few days following, and have a modest overall impact in most children (Sanders 2009).
The risk of selection bias is unclear in all five trials. Blinding, which is especially important in trials with subjective outcomes such as pain measurement, was adequate but the impact of incomplete outcome assessment is unclear. Overall the findings of this review are based on trial evidence that is at low or unclear risk of bias.
The potential impact of bias was most unclear (or high) in the naturopathic drop trials. Only marginal and insignificant differences were shown between the groups in these three trials. The two anaesthetic versus placebo trials showed topical analgesia significantly reduced AOM ear pain. Whilst the risk of bias in the two studies was unclear in some regards, it was lower overall than for the naturopathic drop trials.
These findings are limited to children aged three to 18 years. The study by Bolt 2008 included children as young as three, but in the studies by Hoberman 1997, Sarrell 2001 and Sarrell 2003a, the lower limits were five, six and five years, respectively. This is a weakness given that incidence peaks between six and 15 months (Klein 1989). Although we aimed to study adults as well as children, no patients over the age of 19 years were eligible for inclusion in any study. We can only generalise this effect to younger children (infants) or adults if we assume an identical biological mechanism.
Participant numbers were modest in all five trials. Even though anaesthetic drops were favoured over placebo in regard to both the 50% and 25% pain reduction outcomes, the lower limit of the confidence intervals is close to unity at all the time points where a statistically significant difference was observed. This proximity to unity indicates that we cannot rule out the possibility of a chance effect.
Only one of the trials (Bolt 2008) reported on a limited range of adverse events (tinnitus, dizziness or unsteady gait). All participants had to have intact tympanic membranes to participate in this study. Therefore, we know nothing about whether topical pain drops could cause ototoxicity through perforated tympanic membranes. Overall individual trial numbers were too small to detect anything other than very common events.
There was a rapid reduction in pain after instilling ear drops in both intervention and control groups in all five trials. It is hard to know if this was the result of the natural course of the illness; the placebo effect of being in a clinical setting; the ear drops; or the soothing effect of (any) liquid on the inflamed tympanic membrane. It is also possible that the pain reduction resulted from the concomitant administration of oral acetaminophen (paracetamol). The Bolt trial (Bolt 2008) identified oral analgesics as a likely contributor to the relief of ear pain. However, the reduction seen at 10 minutes is not likely to be an effect of acetaminophen, for which the complete absorption rate ranges from 23 to 60 minutes (Watson 1989). A no ear drop control group as well as a placebo control group might help to disentangle these factors. However, the statistically significant difference in the proportion of children achieving a 50% reduction in pain, in favour of anaesthetic drops, would indicate that this intervention is a useful strategy for dealing with a common and distressing childhood illness.
Reporting was poor in four out of the five included trials for some quality elements. Allocation concealment was not mentioned in any of the trials and is a potential source of bias in all studies. Intention‐to‐treat (ITT) was not performed in three of the trials (Sarrell 2001; Sarrell 2003a; Sarrell 2003b) but the number of drop‐outs was given in each trial. One of the trials (Sarrell 2001) did not specify which arm the patients dropped out of, but even in the worst‐case scenario, the drop‐out rate in that trial would have been less than 15%. Although these losses are within conventional limits it would have been reassuring to know that they did not all occur in one arm. The drop‐out rate in the other two trials did not exceed 7% (Sarrell 2003a; Sarrell 2003b). To be able to reconstruct an ITT analysis (Sarrell 2001; Sarrell 2003a; Sarrell 2003b), individual patient data were required. Attempts to contact the trial authors for further information were unsuccessful.
The meta‐analysis of the two anaesthetic versus placebo trials (Bolt 2008; Hoberman 1997) indicated no heterogeneity. However, meta‐analysis of two of the naturopathic drop trials (Sarrell 2001; Sarrell 2003a) revealed significant heterogeneity. The remaining naturopathic drop trial (Sarrell 2003b) used antibiotics in both groups, excluding the possibility of combining their data with the two other trials (Sarrell 2001; Sarrell 2003a).
The five included trials (Bolt 2008; Hoberman 1997; Sarrell 2001; Sarrell 2003a; Sarrell 2003b) all used two visual analogue scales to assess pain. Pain is a subjective outcome and related to many variables, therefore self‐reporting is considered as the most trustworthy way to measure pain (Mathews 1993). In four of the five trials (Hoberman 1997; Sarrell 2001; Sarrell 2003a; Sarrell 2003b) only children older than five years of age were selected to participate in the trials. Only the study by Bolt 2008 included children as young as three. The likely reason for enrolling older children is that younger children have a limited ability to describe their pain experience (Mathews 1993). However, some clinicians might be concerned about generalising the results of this review to the population most at risk.
Finally, one must ask whether naturopathic preparations have known analgesic properties. We contacted a naturopathy practitioner (Morgan 2006) who stated that at least some of the compounds have a reputation for analgesic properties when used topically (for example, calendula flower, mullein flower and lavender oil). Other explanations of the intervention effect of naturopathic preparations other than a real effect require impugning the quality or fairness of the trial.
Authors' conclusions
Implications for practice.
The data from these five RCTs, two of which addressed the most relevant question of primary effectiveness, indicate that analgesic ear drops may be helpful in treating the pain associated with AOM in children. Ear pain diminishes rapidly for most sufferers and it is likely that the concomitant administration of oral analgesics aids this process. Nevertheless, this review indicates that topical analgesia offers an effective and accessible treatment option for children with AOM.
Implications for research.
Only two trials addressed the question of primary effectiveness. Further high‐quality, randomised, placebo‐controlled trials would help to establish more clearly the safety and efficacy of analgesic drops for AOM.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2011 | New search has been performed | Searches conducted. No new trials were identified and our conclusions remain unchanged. |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 22 January 2009 | New search has been performed | Searches conducted. One new trial was included. |
| 4 March 2008 | Amended | Converted to new review format. |
| 20 May 2006 | New search has been performed | Searches conducted. |
Acknowledgements
We would like to thank Juliet Dawkins for her help in assessing risk of bias in the original version of this review. ACJ and JW would like to thank their supervisor Prof. Gunnar Tobin for his invaluable assistance with organising the work placement at Bond University. They also wish to acknowledge the support of The Sahlgrenska Academy at Göteborg University; Farmaciforbundet and the Swedish Pharmaceutical Association. The review authors also wish to thank the following people for commenting on the first draft protocol: Amy Zelmer, Betsy Blazek‐O'Neill, Richard Rosenfeld, Nelcy Rodriguez and Paul Glasziou. We would also like to thank the following people for commenting on the updated draft: Anne Lyddiatt, Richard Rosenfeld, Sree Nair and Paul Glasziou. In writing the original review, the review authors wish to thank Katja Ullrich for translating German language articles and assistance writing the background of the protocol. Also, Kumar Kuldeep for commenting on the statistical components of the draft review. Finally, we wish to thank Michelle Morgan for assisting us with information on the active ingredients present in naturopathic herbal extract ear drops.
Appendices
Appendix 1. Original search strategy 2006
For the first published version of this review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2006, Issue 2), MEDLINE (1966 to May Week 3 2006), EMBASE (1990 to December 2005) and LILACS (1982 to September 2005) without date or language restrictions.
The following search strategy was used in Ovid MEDLINE and adapted as appropriate for other databases 1 exp Otitis Media/ 2 (otitis media or AOM or OM).mp. 3 or/1‐2 4 exp Benzocaine/ 5 benzocaine.mp. 6 exp Tetracaine/ 7 amethocaine.mp. 8 exp Lidocaine/ 9 lidocaine.mp. 10 (anesthetic or anaesthetic).mp. 11 topical analgesi$.mp. 12 exp Antipyrine/ 13 (antipyrine or phenazone).mp. 14 (americaine otic or aurafair or auralgan or auralgesic or auraphene or aurisan or auroto or dolotic or lanaurine otocain or omedia or oticaine or otigesic or otocalm or Rx‐Otic or sedaural or tympagesic).mp. 15 or/4‐14 16 exp Anti‐Inflammatory Agents/ 17 (antiinflammator$ or anti inflammator$).mp. 18 or/16‐17 19 exp Administration, Topical/ 20 (topical or otic).mp. 21 or/19‐20 22 18 and 21 23 exp Histamine H1 Antagonists/ 24 (antihistamine$ or anti‐histamine$).mp. 25 or/23‐24 26 25 and 21 27 exp Steroids/ 28 steroid$.mp. 29 or/27‐28 30 29 and 21 31 15 or 22 or 26 or 30 32 3 and 31
Appendix 2. Ovid EMBASE search strategy
1 exp Otitis Media/ 2 (otitis media or AOM or OM).mp. 3 or/1‐2 4 exp Benzocaine/ 5 benzocaine.mp. 6 exp Tetracaine/ 7 amethocaine.mp. 8 exp Lidocaine/ 9 lidocaine.mp. (18813) 10 (anesthetic or anaesthetic).mp. 11 topical analgesi$.mp. 12 exp Antipyrine/ 13 (antipyrine or phenazone).mp. 14 (americaine otic or aurafair or auralgan or auralgesic or auraphene or aurisan or auroto or dolotic or lanaurine otocain or omedia or oticaine or otigesic or otocalm or Rx‐Otic or sedaural or tympagesic).mp. 15 or/4‐14 16 exp Anti‐Inflammatory Agents/ 17 (antiinflammator$ or anti inflammator$).mp. 18 or/16‐17 19 exp Administration, Topical/ 20 (topical or otic).mp. 21 or/19‐20 22 18 and 21 23 exp Histamine H1 Antagonists/ 24 (antihistamine$ or anti‐histamine$).mp. 25 or/23‐24 26 21 and 25 27 exp Steroids/ 28 steroid$.mp. 29 or/27‐28 30 21 and 29 31 15 or 22 or 26 or 30 32 3 and 31 33 Clinical trial/ 34 Randomized controlled trials/ 35 Random Allocation/ 36 Single‐Blind Method/ 37 Double‐Blind Method/ 38 Cross‐Over Studies/ 39 Placebos/ 40 Randomi?ed controlled trial$.tw. 41 RCT.tw. 42 Random allocation.tw. 43 Randomly allocated.tw. 44 Allocated randomly.tw. 45 (allocated adj2 random).tw. 46 Single blind$.tw. 47 Double blind$.tw. 48 ((treble or triple) adj blind$).tw. 49 Placebo$.tw. 50 Prospective Studies/ 51 or/33‐50 52 Case study/ 53 Case report.tw. 54 Abstract report/ or letter/ 55 or/52‐54 56 51 not 55 57 animal/ 58 human/ 59 57 not 58 60 56 not 59 61 32 and 60
Appendix 3. Ovid CINAHL search strategy
1 exp Otitis Media/ 2 (otitis media or AOM or OM).mp. 3 or/1‐2 4 exp Benzocaine/ 5 benzocaine.mp. 6 exp Tetracaine/ 7 amethocaine.mp. 8 exp Lidocaine/ 9 lidocaine.mp. 10 (anesthetic or anaesthetic).mp. 11 topical analgesi$.mp. 12 (antipyrine or phenazone).mp. 13 (americaine otic or aurafair or auralgan or auralgesic or auraphene or aurisan or auroto or dolotic or lanaurine otocain or omedia or oticaine or otigesic or otocalm or Rx‐Otic or sedaural or tympagesic).mp. 14 or/4‐13 15 exp Anti‐Inflammatory Agents/ 16 (antiinflammator$ or anti inflammator$).mp. 17 or/15‐16 18 exp Administration, Topical/ 19 (topical or otic).mp. 20 or/18‐19 21 17 and 20 22 exp Histamine H1 Antagonists/ 23 (antihistamine$ or anti‐histamine$).mp. 24 or/22‐23 25 20 and 24 26 exp Steroids/ 27 steroid$.mp. 28 or/26‐27 29 20 and 28 30 15 or 21 or 25 or 29 31 3 and 30
Appendix 4. Ovid AMED search strategy
1 exp Otitis Media/ 2 (otitis media or AOM or OM).mp. 3 or/1‐2 4 exp Lidocaine/ 5 (benzocaine or tetracaine or amethocaine or lidocaine).mp. 6 (anesthetic or anaesthetic).mp. 7 topical analgesi$.mp. 8 (antipyrine or phenazone).mp. 9 (americaine otic or aurafair or auralgan or auralgesic or auraphene or aurisan or auroto or dolotic or lanaurine otocain or omedia or oticaine or otigesic or otocalm or Rx‐Otic or sedaural or tympagesic).mp. 10 (topical or otic).mp. 11 or/4‐10 12 3 and 11
Appendix 5. CENTRAL search strategy
#1 MeSH descriptor Otitis Media explode all trees #2 ("otitis media" or AOM or OM):it,ab,kw #3 (#1 OR #2) #4 MeSH descriptor Benzocaine explode all trees #5 MeSH descriptor Tetracaine explode all trees #6 MeSH descriptor Lidocaine explode all trees #7 (benzocaine or tetracaine or amethocaine or lidocaine):ti,ab,kw #8 (anesthetic or anaesthetic):ti,ab,kw #9 topical NEXT analgesi*:ti,ab,kw #10 MeSH descriptor Antipyrine explode all trees #11 (antipyrine or phenazone):ti,ab,kw #12 (americaine otic or aurafair or auralgan or auralgesic or auraphene or aurisan or auroto or dolotic or lanaurine otocain or omedia or oticaine or otigesic or otocalm or Rx‐Otic or sedaural or tympagesic):ti,ab,kw #13 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 MeSH descriptor Anti‐Inflammatory Agents explode all trees #15 (antiinflammator* or anti‐inflammator*):ti,ab,kw #16 (#14 OR #15) #17 MeSH descriptor Administration, Topical explode all trees #18 (topical or otic):ti,ab,kw #19 (#17 OR #18) #20 (#16 AND #19) #21 MeSH descriptor Histamine H1 Antagonists explode all trees #22 (antihistamine* or anti‐histamine*):ti,ab,kw #23 (#21 OR #22) #24 (#16 AND #23) #25 MeSH descriptor Steroids explode all trees #26 steroid*:ti,ab,kw #27 (#25 OR #26) #28 (#16 AND #27) #29 (#13 OR #20 OR #24 OR #28) #30 (#3 AND #29)
Appendix 6. Criteria for judging risk of bias in the ‘Risk of bias’ assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for a judgement of ‘Low risk’ of bias | The investigators describe a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random |
| Criteria for the judgement of ‘High risk’ of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example:
|
| Criteria for the judgement of ‘Unclear risk’ of bias | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment | |
| Criteria for a judgement of ‘Low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of ‘High risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of ‘Unclear risk’ of bias | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of ‘Low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias | Any one of the following:
|
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for a judgement of ‘Low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias | Any one of the following:
|
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature or handling of incomplete outcome data | |
| Criteria for a judgement of ‘Low risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias | Any one of the following:
|
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for a judgement of ‘Low risk’ of bias | Any of the following:
|
| Criteria for the judgement of ‘High risk’ of bias | Any one of the following:
|
| Criteria for the judgement of ‘Unclear risk’ of bias | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that the majority of studies will fall into this category |
Data and analyses
Comparison 1. Anaesthetic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50% reduction in ear pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 10 minutes after instillation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.19, 3.80] |
| 1.2 20 minutes after instillation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.88, 1.74] |
| 1.3 30 minutes after instillation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.12, 1.81] |
| 2 25% reduction in ear pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 10 minutes after instillation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.06, 2.15] |
| 2.2 20 minutes after instillation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.04, 1.71] |
| 2.3 30 minutes after instillation | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.12, 1.61] |
1.1. Analysis.
Comparison 1 Anaesthetic versus placebo, Outcome 1 50% reduction in ear pain.
1.2. Analysis.
Comparison 1 Anaesthetic versus placebo, Outcome 2 25% reduction in ear pain.
Comparison 2. Anaesthetic versus naturopathic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score, day 1 | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Baseline | 3 | 274 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.58, 0.56] |
| 1.2 15 minutes after instillation | 3 | 274 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.45, 1.71] |
| 1.3 30 minutes after instillation | 3 | 274 | Mean Difference (IV, Random, 95% CI) | 1.02 [‐0.22, 2.27] |
| 2 Mean pain score, day 2 | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Before instillation | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.05, 0.95] |
| 2.2 15 minutes after instillation | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.24, 1.13] |
| 2.3 30 minutes after instillation | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.19, 0.98] |
| 3 Mean pain score, day 3 | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Before instillation | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.29 [0.09, 0.48] |
| 3.2 15 minutes after instillation | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.06, 0.53] |
| 3.3 30 minutes after instillation | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.01, 1.19] |
2.1. Analysis.
Comparison 2 Anaesthetic versus naturopathic, Outcome 1 Mean pain score, day 1.
2.2. Analysis.
Comparison 2 Anaesthetic versus naturopathic, Outcome 2 Mean pain score, day 2.
2.3. Analysis.
Comparison 2 Anaesthetic versus naturopathic, Outcome 3 Mean pain score, day 3.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bolt 2008.
| Methods | Block randomisation Baseline comparability documented. Patients, treating clinicians and outcome assessors were all unaware of treatment assignment ITT analysis | |
| Participants | Australia Children aged between 3 and 17 years who presented to an emergency department with ear pain of less than 3 days duration and evidence of AOM | |
| Interventions | Treatment: anaesthetic ear drops (2% aqueous lignocaine drops) Control: normal saline drops (aqueous solution) Duration: 30 minutes If the patient had not received analgesia in the preceding 4 hours, they were offered 15 mg/kg paracetamol. Further oral analgesia was offered between 10 (T10) and 30 minutes (T30) of the study period at the discretion of the treating doctor | |
| Outcomes | Ear pain was assessed by means of 2 visual analogue scales at baseline, 10, 20, 30 minutes after instillation, and an average ear pain score was determined Four measures were used: 1) proportion of subjects who showed 50% reduction; 2) proportion of subjects who showed 25% reduction; and 3) proportion of participants showing a 2‐point reduction in pain score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The drops were randomised in blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | No mention of sequentially numbered, opaque, sealed envelopes or of central randomisation by a third party |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patients and parents, those administering ear drops and those assessing ear pain were blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "On the basis of an intention to treat analysis, two patients were retained in the study and analysed despite pain resolution subsequent to enrolment with a pain score of zero at T0". Numbers in Table 2 indicate all participants were included in the analyses |
Hoberman 1997.
| Methods | Randomisation claimed, but method not described Baseline comparability documented. Investigators were unaware of treatment assignment ITT analysis |
|
| Participants | USA 54 children in primary care or emergency department aged 5 to 19 years with ear pain and eardrum findings indicative of AOM | |
| Interventions | Treatment: anaesthetic ear drops (antipyrine, benzocaine, glycerine) Control: olive oil drops Duration: 30 minutes All children were also given acetaminophen (15 mg/kg in a single dose) | |
| Outcomes | Ear pain was assessed by means of 2 visual analogue scales at baseline, 10, 20, 30 minutes after instillation, and an average ear pain score was determined Four measures were used: 1) proportion of subjects who showed 50% reduction; 2) proportion of subjects who showed 25% reduction; 3) proportion of subjects who showed a 1 or more point reduction; 4) mean score over time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were "randomly assigned". No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No mention of sequentially numbered, opaque, sealed envelopes or of central randomisation by a third party |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Investigators were unaware of the study drug assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "One patient in the Auralgan group did not receive a T20 evaluation". However the difference between the 2 groups was not statistically significant at this time point and even if treatment failure was assumed the missing value is not likely to have a significant impact |
Sarrell 2001.
| Methods | Randomisation claimed, but method not described Baseline comparability stated Double‐blind Not ITT (7 of 110 patients excluded) | |
| Participants | Israel 103 children aged 6 to 18 years who were diagnosed with otalgia associated with AOM | |
| Interventions | Treatment: anaesthetic ear drops (amethocaine, phenazone, glycerine)
Control: naturopathic ear drops (Allium sativum, Verbascum thapsus, calendula flores, Hypericum perforatum in olive oil)
Drops were instilled 3 times daily for 3 days
All children were also given acetaminophen (15 mg/kg in a single dose) Duration: 3 days |
|
| Outcomes | Ear pain was assessed using 2 visual analogue scales and an overall ear pain score was determined. The first data point was assessed at the diagnosis of AOM and then pain was assessed during 3 days; before the drops were instilled, and at 15 and 30 minutes after instillation Outcome: mean pain score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...the children were randomly assigned to 1 of 2 treatment groups". No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No mention of sequentially numbered, opaque, sealed envelopes or of central randomisation by a third party |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study was conducted in a double‐blind, randomized manner" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "A total of 110 children were enrolled in the study. Seven children were excluded: 5 due to noncompliance (those who could not be reached by telephone for the interview or those who forgot to take the medication), and 2 additional children were overcome by the smell of the ear drops." Only 103 children were included in the analyses and no information about which groups the drop‐out occurred in was provided |
Sarrell 2003a.
| Methods | Computer‐numbered randomisation. Baseline comparability documented Identical bottles Double‐blind Not ITT (4 of 90 patients were excluded) | |
| Participants | Israel 86 children in an ambulatory clinic aged 5 to 18 years with ear pain caused by AOM | |
| Interventions | Treatment: anaesthetic ear drops (amethocaine, phenazone, glycerine) Control: naturopathic ear drops (Allium sativum, Verbascum thapsus, calendula flores, Hypericum perforatum, Lavandula officinalis and vitamin E in olive oil) Drops were instilled 3 times daily for 3 days Duration: 3 days | |
| Outcomes | Ear pain was assessed using 2 visual analogue scales and an overall ear pain score was determined. The first data point was assessed at the diagnosis of AOM and then pain was assessed during 3 days; before the drops were installed, and at 15 and 30 minutes after instillation Outcome: mean pain score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...the children were assigned by computer‐numbered randomization..." |
| Allocation concealment (selection bias) | Unclear risk | No mention of sequentially numbered, opaque, sealed envelopes or of central randomisation by a third party |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind design was used, and all ear drops were placed in identical bottles" "The contents of the bottles were unknown by both the subjects and the nurse" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Nine of the 180 children enrolled in the study (1 in group A, 3 in group B, 3 in group C, and 2 in group D) were excluded from the final analysis because of noncompliance: 5 forgot to take the medicine, and 4 could not be reached for the follow‐up interview." |
Sarrell 2003b.
| Methods | Computer‐numbered randomisation Baseline comparability documented Ear drops in identical bottles Double‐blind Not ITT (5 of 90 patients were excluded) | |
| Participants | Israel 85 children in an ambulatory clinic aged 5 to 18 years with ear pain caused by AOM | |
| Interventions | Treatment: anaesthetic ear drops (amethocaine, phenazone, glycerine). Control: naturopathic ear drops (Allium sativum, Verbascum thapsus, calendula flores, Hypericum perforatum, Lavandula officinalis and vitamin E in olive oil) Drops were instilled 3 times daily for 3 days All children were also given oral amoxicillin (80 mg/kg/day divided into 3 doses) Duration: 3 days | |
| Outcomes | Ear pain was assessed using 2 visual analogue scales and an overall ear pain score was determined. The first data point was assessed at the diagnosis of AOM and then pain was assessed during 3 days; before the drops were installed, and at 15 and 30 minutes after instillation Outcome: mean pain score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...the children were assigned by computer‐numbered randomization..." |
| Allocation concealment (selection bias) | Unclear risk | No mention of sequentially numbered, opaque, sealed envelopes or of central randomisation by a third party |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind design was used, and all ear drops were placed in identical bottles" "The contents of the bottles were unknown by both the subjects and the nurse" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Nine of the 180 children enrolled in the study (1 in group A, 3 in group B, 3 in group C, and 2 in group D) were excluded from the final analysis because of noncompliance: 5 forgot to take the medicine, and 4 could not be reached for the follow‐up interview." |
AOM: acute otitis media ITT: intention‐to‐treat
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramson 1969 | For surgery, not for AOM |
| Brunet 1970 | No control group |
| Busmann 1967 | No original data, review only |
| Comeau 1978 | Iontophoresis For myringotomy or ventilation tube placement, not AOM |
| Fay 2003 | No original data, comments on a previous trial (Sarrell 2003a; Sarrell 2003b) |
| Fort 2000 | Oral not topical administration of pain relief |
| Francois 1993 | Treatment of congestive myringitis, not AOM |
| Francois 1995 | No original data, an overview |
| Koeppel 1970 | No original data, review only |
| Lacher 1969 | No control group |
| Laszlo 1981 | Anaesthesia of tympanic membrane, not for AOM |
| Laxdal 1970 | Not double‐blinded; intervention not appropriate |
| MacPhail 1996 | Descriptive article |
| Matz 2001a | Not double‐blinded; intervention not appropriate |
| Matz 2001b | No original data, referring to data in an unpublished study (Matz) |
| McConaghy 2001 | No original data; review only |
| Menshikov 1968 | No control group |
| Millard 1969 | No control group |
| Milvio 1984 | Oral non‐steroidal anti‐inflammatory drug |
| Polyakova 1991 | Unclear if randomised and blinded, unable to contact authors |
| Reiss 2002 | No original data; an overview |
| Sano 1995 | No original data; an overview |
| Shikowitz 1989 | No original data; review only |
| Silverstein 1969 | No control group; for insertion of tympanic membrane tubes, not for AOM |
| Weippl 1985 | Neither randomised nor double‐blinded; oral treatment |
| Willenberg 1975 | No control group |
| Woldman 1998 | No original data; comments on a previous trial (Hoberman 1997) |
AOM: acute otitis media
Contributions of authors
Designing the review: Ruth Foxlee (RF), Ann‐Charlott Johansson (ACJ), Jessika Wejfalk (JW), Chris Del Mar (CDM) and Liz Dooley (LD). Coordinating the review: RF. Literature searches: RF, ACJ and JW. Quality assessment: ACJ, JW, RF and Juliet Dawkins (JD). Data extraction: RF, ACJ and JW. Data analysis: RF, ACJ, JW and CDM. Writing the review: RF, ACJ, JW, CDM, JD and LD.
Sources of support
Internal sources
Sahlgrenska Academy, Sweden.
Farmaciforbundet, Sweden.
The Swedish Pharmaceutical Association, Sweden.
External sources
No sources of support supplied
Declarations of interest
One of the co‐authors, Prof Chris Del Mar, acted briefly as a consultant for Key Pharmaceuticals. This Australian‐based company is the manufacturer of the otic solution Ear Clear for Ear Ache Relief. He evaluated the results of one trial and presented evidence to the Australian Therapeutic Goods Association.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bolt 2008 {published data only}
- Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double‐blind placebo‐controlled randomised trial. Archives of Disease in Childhood 2008;93(1):40‐4. [DOI] [PubMed] [Google Scholar]
Hoberman 1997 {published data only}
- Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Archives of Pediatrics & Adolescent Medicine 1997;151(7):675‐8. [DOI] [PubMed] [Google Scholar]
Sarrell 2001 {published data only}
- Sarrell EM, Mandelberg A, Cohen HA. Efficacy of naturopathic extracts in the management of ear pain associated with acute otitis media. Archives of Pediatrics and Adolescent Medicine 2001;155(7):796‐9. [DOI] [PubMed] [Google Scholar]
Sarrell 2003a {published data only}
- Sarrell EM, Cohen HA, Kahan E. Naturopathic treatment for ear pain in children. Pediatrics 2003a;111(5 Pt 1):574‐9. [DOI] [PubMed] [Google Scholar]
Sarrell 2003b {published data only}
- Sarrell EM, Cohen HA, Kahan E. Naturopathic treatment for ear pain in children. Pediatrics 2003b;111(5 Pt 1):e574‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abramson 1969 {published data only}
- Abramson M. Topical anesthesia of the tympanic membrane. Archives of Otolaryngology 1969;90(2):147‐9. [DOI] [PubMed] [Google Scholar]
Brunet 1970 {published data only}
- Brunet J, Cozette P. Clinical study of Otipax in infectious and barotraumatic pathology of the middle ear. Therapeutique 1970;46(4):399‐401. [PubMed] [Google Scholar]
Busmann 1967 {published data only}
- Busmann GJ. Local therapy of diseases of the middle ear. Therapie der Gegenwart 1967;106(10):1326‐9. [PubMed] [Google Scholar]
Comeau 1978 {published data only}
- Comeau M, Brummett R. Anesthesia of the human tympanic membrane by iontophoresis of a local anesthetic. Laryngoscope 1978;88(2 Pt 1):277‐85. [DOI] [PubMed] [Google Scholar]
Fay 2003 {published data only}
- Fay DL, Schellhase KG, Wujek D. Naturopathic ear drops minimally effective for acute otitis media. Journal of Family Practice 2003;52(9):673, 676. [PubMed] [Google Scholar]
Fort 2000 {published data only}
- Fort G. Analgesia in otorhinolaryngological diseases: lysine clonixinate versus ibuprofen [Analgesia en afecciones otorrinolaringologicas Clonixinato de Lisina vs. Ibuprofeno]. Prensa Medica Argentina 2000;87(4):409‐18. [Google Scholar]
Francois 1993 {published data only}
- Francois M. Efficacy and tolerance of a local application of phenazone and chlorhydrate lidocaine (Otipax) in infants and children with congestive otitis. Annales de Pediatrie 1993;40(7):481‐4. [PubMed] [Google Scholar]
Francois 1995 {published data only}
- Francois M. Treatment of acute otitis media. Archives de Pédiatrie 1995;2(1):86‐8. [DOI] [PubMed] [Google Scholar]
Koeppel 1970 {published data only}
- Koeppel FW. Treatment of diseases of the ear canal and middle ear with Otobacid. Munchener Medizinische Wochenschrift 1970;112(17):806‐9. [PubMed] [Google Scholar]
Lacher 1969 {published data only}
- Lacher G. Clinical trial of Otipax: auricular pulverizations. Revue de Laryngologie, D' Otologie et de Rhinologie 1969;90(11):719‐22. [PubMed] [Google Scholar]
Laszlo 1981 {published data only}
- Laszlo I, Pupp L. Anaesthesia of tympanic membrane with lidocaine. Therapia Hungarica 1981;29(4):176‐9. [PubMed] [Google Scholar]
Laxdal 1970 {published data only}
- Laxdal OE. Treatment of acute otitis media: a controlled study of 142 children. Canadian Medical Association Journal 1970;102(3):263‐8. [PMC free article] [PubMed] [Google Scholar]
MacPhail 1996 {published data only}
- MacPhail E. Acute otitis media. Canadian Pharmaceutical Journal 1996;129(1):29‐31. [Google Scholar]
Matz 2001a {unpublished data only}
- Matz PS, Webster RA, Vivier P, Alario A. Use of a topical otic analgesic as a treatment strategy for acute otitis media. Unpublished manuscript presented at the Pediatric Academic Society Meeting; Baltimore. 2001.
Matz 2001b {published data only}
- Matz PS, Batista D, Vivier P, Alario AJ. Can a topical otic anesthetic reduce the need for antibiotics in acute otitis media?. Pediatric Research 2001;49:131A. [Google Scholar]
McConaghy 2001 {published data only}
- McConaghy JR. The evaluation and treatment of children with acute otitis media. Journal of Family Practice 2001;50(5):457‐9, 463‐5. [PubMed] [Google Scholar]
Menshikov 1968 {published data only}
- Menshikov NM. The use of lyase and desoxyribonuclease in acute otitis in children. Zhurnal Ushnykh, Nosovykh i Gorlovykh Boleznei 1968;28(2):77‐9. [PubMed] [Google Scholar]
Millard 1969 {published data only}
- Millard J. On the local treatment of infections of the ear canal and middle ear. Deutsches Medizinisches Journal 1969;20(6):181‐4. [PubMed] [Google Scholar]
Milvio 1984 {published data only}
- Milvio C. Nimesulide for the treatment of painful inflammatory process in the ear, nose and throat areas: a double‐blind controlled study with benzydamine. Journal of International Medical Research 1984;12(6):327‐32. [DOI] [PubMed] [Google Scholar]
Polyakova 1991 {published data only}
- Polyakova TS, Voznesensky NL, Mironov AA, Al Sakir M. Otinum in the therapy of middle ear diseases. Vestnik‐Oto‐Rino‐Laringologii 1991;53(2):56‐8. [PubMed] [Google Scholar]
Reiss 2002 {published data only}
- Reiss G, Reiss M. Otorhinolaryngology ‐ 4: Pro and contra of ear drops. Pflege Zeitschrift 2002;55(2):86‐8. [PubMed] [Google Scholar]
Sano 1995 {published data only}
- Sano F, Sole D, Naspitz CK, Takara CK. Otitis media in children. Brasileira‐de‐Medicina 1995;52:113‐22. [Google Scholar]
Shikowitz 1989 {published data only}
- Shikowitz MJ. Otitis media. Children's Hospital Quarterly 1989;1(4):289‐99. [Google Scholar]
Silverstein 1969 {published data only}
- Silverstein H, Call DL. Tetracaine base. An effective surface anesthetic for the tympanic membrane. Archives of Otolaryngology 1969;90(2):150‐1. [DOI] [PubMed] [Google Scholar]
Weippl 1985 {published data only}
- Weippl G, Michos N, Stocker H. Clinical experience and results of treatment with suprofen in pediatrics. 4th communication: assessment of pain in babies and infants/analgesic effect of suprofen syrup in otitis media. Arzneimittelforschung 1985;35(11):1732‐4. [PubMed] [Google Scholar]
Willenberg 1975 {published data only}
- Willenberg W. New preparation for the treatment of inflammatory ear diseases. Zeitschrift fur Allgemeinmedizin 1975;51(16):759‐62. [PubMed] [Google Scholar]
Woldman 1998 {published data only}
- Woldman S. Treating ear pain in children with acute otitis media. Archives of Pediatrics and Adolescent Medicine 1998;152(1):102. [PubMed] [Google Scholar]
Additional references
AAP 2004
- American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451‐65. [DOI] [PubMed] [Google Scholar]
Berman 1995
- Berman S. Otitis media in developing countries. Pediatrics 1996;96:126‐31. [PubMed] [Google Scholar]
Chalmers 1990
- Chalmers I, Adams M, Dickersin K, Hetherington J, Tarnow‐Mordi W, Meinert C, et al. A cohort study of summary reports of controlled trials. JAMA 1990;263(10):1401‐5. [PubMed] [Google Scholar]
Charles 2004
- Charles J, Pan Y, Britt H. Trends in childhood illness and treatment in Australian general practice, 1971‐2001. Medical Journal of Australia 2004;180:216‐9. [DOI] [PubMed] [Google Scholar]
DoH 2000
- Department of Health Standing Medical Advisory Committee Subgroup on Antimicrobial Resistance. The Path of Least Resistance ‐ main report. London: DoH 2000. www.advisorybodies.doh.gov.uk/smac1.htm 2000 (accessed 16 August 2005).
Fischer 2007
- Fischer T, Singer AJ, Lee C, Thode HC, Jr. National trends in emergency department antibiotic prescribing for children with acute otitis media, 1996‐2005. Academic Emergency Medicine 2007;14(12):1172‐5. [DOI] [PubMed] [Google Scholar]
Froom 1997
- Froom J, Culpepper L, Jacobs M. Antimicrobials for acute otitis media? A review from the International Primary Care Network. BMJ 1997;315(7100):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Klein 1989
- Klein JO. Epidemiology of otitis media. Pediatric Infectious Disease Journal 1989;8(Suppl 1):89. [DOI] [PubMed] [Google Scholar]
Klein 2001
- Klein JO. The burden of otitis media. Vaccine 2001;19(Suppl):S2‐S8. [DOI] [PubMed] [Google Scholar]
Kozyrskyj 2010
- Kozyrskyj AL, Klassen TP, Moffatt M, Harvey K. Short‐course antibiotics for acute otitis media. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001095.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Marcy 2001
- Marcy M, Takata G, Shekelle P, Chan LS, Mason W, Wachsman L, Ernst R, et al. Management of Acute Otitis Media. Evidence Report/Technology Assessment No. 15. (Prepared by the Southern California Evidence‐based Practice Center under Contract No. 290‐97‐0001). 2001 AHRQ Publication No. 01‐E010. Rockville, MD: Agency for Healthcare Research and Quality.
Mathews 1993
- Mathews JR, McGrath PJ, Pigeon H. Assessment and measurement of pain in children. Pain in Infants, Children, and Adolescents. Lippincott, Williams and Wilkins, 1993. [Google Scholar]
Morgan 2006
- Morgan M. Personal communication 2006.
Nasrin 2002
- Nasrin D, Collignon PJ, Roberts L, Wilson EJ, Pilotto LS, Douglas RM. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ 2002;324(7328):324‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pirozzo 2004
- Pirozzo S, Mar C. Otitis media. In: Evidence‐based Pediatrics and Child Health. 2nd Edition. BMJ Books, 2004. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2011.
Rosenfeld 2003
- Rosenfeld RM. Natural history of untreated otitis media. Laryngoscope 2003;113(10):1645–57. [DOI] [PubMed] [Google Scholar]
Rosenfeld 2005
- Rosenfeld R. Personal communication 2005.
Sanders 2009
- Sanders S, Glasziou PP, Mar C, Rovers M. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000219.pub2] [DOI] [PubMed] [Google Scholar]
Schecter 2003
- Schecter NL. Management of common pain problems in the primary care pediatric setting. Pain in Infants, Children and Adolescents. 2nd Edition. Lippincott Williams & Wilkins, 2003. [Google Scholar]
SIGN 2003
- Scottish Intercollegiate Guidelines Network. Guideline 66: Diagnosis and management of childhood otitis media in primary care: a national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/66/index.html 2003.
SIGN 2011
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 16 February 2011).
Spicer 2003
- Spicer WJ, Christiansen K, Currie BJ, Dartnell JGA, Ferguson JK, Garland SM. Therapeutic Guidelines: Antibiotic. Version 12. North Melbourne: Therapeutic Guidelines Limited, 2003. [Google Scholar]
Van Buchem 1985
- Buchem FL, Peeters MF, van'T Hof MA. Acute otitis media: a new treatment strategy. British Medical Journal (Clinical Research Edition) 1985;290(6474):1033‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernacchio 2007
- Vernacchio L, Vezina RM, Mitchell AA. Management of acute otitis media by primary care physicians: trends since the release of the 2004 American Academy of Pediatrics/American Academy of Family Physicians clinical practice guideline. Pediatrics 2007;120(2):281‐7. [PUBMED: 17671053] [DOI] [PubMed] [Google Scholar]
Watson 1989
- Watson PD, Mortensen ME. Pharmacokinetics of common analgesics, antiinflammatories and antipyretics in children. Clinical Pharmacokinetics 1989;17:116‐37. [DOI] [PubMed] [Google Scholar]
WHO/CIBA 1996
- WHO/CIBA Foundation Workshop. Prevention of hearing impairment from chronic otitis media. WHO/PDH/98.4 1996.
References to other published versions of this review
Foxlee 2006
- Foxlee R, Johansson A, Wejfalk J, Dawkins J, Dooley L, Del Mar C. Topical analgesia for acute otitis media. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005657.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foxlee 2009
- Foxlee R, Johansson A, Wejfalk J, Dawkins J, Dooley L, Del Mar C. Topical analgesia for acute otitis media. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005657.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]