Abstract
Background
** This review is awaiting update with a new protocol. The methods used for the review were acceptable when the review was published but do not meet contemporary standards, and the review is also considerably out of date. Therefore, readers should note that the review may not represent a reliable basis for decision making. **
Since the introduction of the first cholinesterase inhibitor (ChEI) in 1997, most clinicians and probably most patients would consider the cholinergic drugs, donepezil, galantamine and rivastigmine, to be the first line pharmacotherapy for mild to moderate Alzheimer's disease.
The drugs have slightly different pharmacological properties, but they all work by inhibiting the breakdown of acetylcholine, an important neurotransmitter associated with memory, by blocking the enzyme acetylcholinesterase. The most that these drugs could achieve is to modify the clinical manifestations of Alzheimer's disease. Cochrane reviews of each ChEI for Alzheimer's disease have been completed.
Objectives
To assess the effects of donepezil, galantamine and rivastigmine in people with mild, moderate or severe dementia due to Alzheimer's disease based on evidence summarised in three existing Cochrane Reviews
Search methods
The Cochrane Dementia and Cognitive Improvement Group's Specialized Register was searched using the terms 'donepezil', 'E2020' , 'Aricept' , galanthamin* galantamin* reminyl, rivastigmine, exelon, "ENA 713" and ENA‐713 on 12 June 2005. This Register contains up‐to‐date records of all major health care databases and many ongoing trial databases.
Selection criteria
All unconfounded, blinded, randomized trials of at least six months in which treatment with a ChEI at the usual recommended dose was compared with placebo or another ChEI for patients with mild, moderate or severe dementia due to Alzheimer's disease.
Data collection and analysis
Data were extracted by one reviewer (JSB), pooled where appropriate and possible, and the pooled treatment effects, or the risks and benefits of treatment, estimated.
Main results
The results of 10 randomized, double blind, placebo controlled trials demonstrate that treatment for 6 months, with donepezil, galantamine or rivastigmine at the recommended dose for people with mild, moderate or severe dementia due to Alzheimer's disease produced improvements in cognitive function, on average ‐2.37 points (95%CI ‐2.73 to ‐2.02, p<0.00001), in the midrange of the 70 point ADAS‐Cog Scale. Study clinicians rated global clinical state more positively in treated patients. Benefits of treatment were also seen on measures of activities of daily living and behaviour. None of these treatment effects are large.
The effects are similar for patients with severe dementia, although there is very little evidence, from only two trials.
More patients leave ChEI treatment groups, (29%), than leave the placebo groups (18%).
There is evidence of more adverse events in total in the patients treated with a ChEI than with placebo. Although many types of adverse event were reported, nausea, vomiting, diarrhoea, were significantly more frequent in the ChEI groups than in placebo.
There is only one randomized, double blind study in which two ChEIs are compared, donepezil compared with rivastigmine. There is no evidence of a difference between donepezil and rivastigmine for cognitive function, activities of daily living and behavioural disturbance at two years. Fewer patients suffer adverse events on donepezil than rivastigmine.
Authors' conclusions
The three cholinesterase inhibitors are efficacious for mild to moderate Alzheimer's disease. Despite the slight variations in the mode of action of the three cholinesterase inhibitors there is no evidence of any differences between them with respect to efficacy. The evidence from one large trial shows fewer adverse events associated with donepezil compared with rivastigmine.
Keywords: Humans, Alzheimer Disease, Alzheimer Disease/drug therapy, Cholinesterase Inhibitors, Cholinesterase Inhibitors/therapeutic use, Donepezil, Galantamine, Galantamine/therapeutic use, Indans, Indans/therapeutic use, Nootropic Agents, Nootropic Agents/therapeutic use, Phenylcarbamates, Phenylcarbamates/therapeutic use, Piperidines, Piperidines/therapeutic use, Randomized Controlled Trials as Topic, Rivastigmine
Plain language summary
Cholinesterase inhibitors (ChEIs), donepezil, galantamine and rivastigmine are efficacious for mild to moderate Alzheimer's disease
Alzheimer's disease is the commonest cause of dementia affecting older people, and is associated with loss of cholinergic neurons in parts of the brain. Cholinesterase inhibitors (ChEIs), donepezil, galantamine and rivastigmine, delay the breakdown of acetylcholine released into synaptic clefts and so enhance cholinergic neurotransmission. The three cholinesterase inhibitors are efficacious for mild to moderate Alzheimer's disease. Despite the slight variations in the mode of action of the three cholinesterase inhibitors there is no evidence of any differences between them with respect to efficacy. The evidence from one large trial shows fewer adverse events associated with donepezil compared with rivastigmine.
Background
Since the introduction of the first cholinesterase inhibitor (ChEI) in 1997, most clinicians and probably most patients would consider the cholinergic drugs, donepezil, galantamine and rivastigmine, to be the first line pharmacotherapy for Alzheimer's disease. They are all licensed for mild to moderate Alzheimer's disease.
The drugs have slightly different pharmacological properties, but they all work by inhibiting the breakdown of acetylcholine, an important neurotransmitter associated with memory, by blocking the enzyme acetylcholinesterase. The most that these drugs could achieve is to modify the manifestations of Alzheimer's disease. Cochrane reviews of each ChEI for Alzheimer's disease have been completed (Birks 2005, Birks 2005b and Loy 2005). All the available evidence, published and unpublished, relating to the studies of the ChEIs has been identified, evaluated and described. The methodological quality of each study has been assessed, and those meeting the requirements of the protocol, the randomized, placebo controlled studies which test any dose of a ChEI in patients with AD, are included. Where the studies are considered sufficiently similar, in terms of the patient population, the dose, the duration of treatment, for the results to be interpretable in clinical terms, the results for each outcome may be combined in a meta‐analysis.
There are 23 included studies of donepezil (5272 patients randomized), 9 of rivastigmine (3449 patients randomized) and 9 of galantamine (5194 patients randomized) in the Cochrane reviews. The objective of the majority of these studies is to evaluate the efficacy and tolerability of a ChEI, by detecting differences between the rate of deterioration of cognitive function between treatment and placebo groups over either 3 or 6 months. Cognitive function is usually assessed by measuring the ADAS‐Cog (the cognitive scale of the Alzheimer's Disease and Associated Disorders Scale (Rosen 1984)) or MMSE (the Mini Mental State Examination) (Folstein 1975) score of a patient. In addition the clinician's overall impression of change and measures of behaviour and the ability to carry out activities of daily living have been assessed in some of the studies. The average age of patients in a study is between 72 and 78 years, with one exception, a study of older patients of mean age 86 years. The diagnosis is Alzheimer's disease, according to standardized criteria of National Institute of Neurological, Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA (McKhann 1987) and Diagnostic and Statistical Manual, DSM‐III‐R (APA 1987)) which is mild to moderate usually defined as a MMSE between 10 or 11 and 24 or 26. There are two studies of patients with more severe disease (MMSE 5 to 17) and one of mild disease. Most of these studies are funded by the pharmaceutical company that manufactures or markets the drug.
The three reviews reach similar conclusions, that, at certain doses greater than the lowest doses tested, the ChEIs show efficacy in cognitive function, activities of daily living, behaviour and global clinical state compared with placebo for treatment of up to 12 months duration, but the effects are small. For the same doses there were significantly more patients leaving the study before the end of treatment from the ChEI treatment groups than from placebo and there were more adverse effects such as nausea, vomiting, diarrhoea, anorexia, headache and abdominal pain, associated with the ChEI than with placebo. A titration period of about 3 months is needed to develop tolerance and to minimize the side effects.
Despite the evidence from the clinical studies and the intervening clinical experience the debate on whether ChEIs are effective continues.
Recently four randomized trials, three single blind, and one double blind, each comparing two ChEIs, have been completed and the results published. It would be informative to line up the evidence from the three drugs side by side, and pool the results if homogeneous, and to evaluate the direct comparisons between the drugs. It is not necessary to repeat the vast amount of evidence found in the three existing reviews. This review is limited to considering the evidence relating to the dosages recommended by the manufacturers for clinical use. The doses included are 10 mg/day once a day for donepezil, 24 mg/day divided into 2 doses for galantamine, and 6‐12 mg/day divided into 2 doses for rivastigmine. These doses may not be strictly equivalent, but the manufacturers have chosen them to balance beneficial and adverse effects and it is appropriate that we examine the evidence for the doses that are more likely to be in use.
There is other evidence available from studies which are not randomized and double blind, the open label extension studies. These studies recruit patients who have been participating in a phase 3 randomized, double blind placebo controlled study to continue on open label treatment. There are reports of further analyses of the data from the randomized double blind studies for subgroup of patients defined by severity of disease or by the presence of vascular risk factors.
Providers of health care, such as the NHS in the UK, question the cost effectiveness of the ChEIs. The question usually asked is whether the cost of health resource use associated with caring for somebody with Alzheimer's disease, and this is usually defined as cost to the public purse, is reduced sufficiently when they are prescribed a ChEI to cover the cost of the drug. In addition doctors and patients are not unaware of the cost of the ChEIs, but when evaluating the cost effectiveness they would include the costs of informal and formal care provided by the family of the patient. There have been several economic evaluations of the ChEIs. Because there are very little data from randomized controlled trials describing the progression of patients with and without ChEI treatment of longer than one year, the estimates of costs depend on extrapolation of the results from the clinical trials to predict the time until full time care in an institution is needed. Inevitably these models depend on many assumptions and interpretation of the economic evaluations is not straightforward.
This review aims to present an overview of the evidence for the efficacy, the effectiveness and the cost effectiveness of ChEIs as a group. It draws on data extracted and analysed in the three Cochrane reviews on donepezil (Birks 2005), rivastigmine (Birks 2005b) and galantamine (Loy 2005).
Objectives
To assess the effects of donepezil, galantamine and rivastigmine in people with mild, moderate or severe dementia due to Alzheimer's disease based on evidence summarised in three existing Cochrane Reviews.
Methods
Criteria for considering studies for this review
Types of studies
All unconfounded, randomized, double blind trials designed to evaluate the efficacy and tolerability of a ChEI, of patients with dementia due to Alzheimer's disease in which treatment with a ChEI was administered for approximately 6 months or longer and compared with a placebo group or another ChEI were studied. Trials in which the allocation to treatment or control was not randomized, were excluded. Trials that did not report results, or did not report them with sufficient detail for inclusion in the meta‐analyses were excluded.
Types of participants
Patients were diagnosed as having probable Alzheimer's disease using accepted criteria such as ICD‐10, DSM (APA 1987) and NINCDS‐ADRDA (McKhann 1987).
Types of interventions
A ChEI given at the dose recommended as optimal by the manufacturing pharmaceutical company (donepezil 10mg/day in one dose, galantamine 24mg/day in two doses, and rivastigmine 6‐12mg/day in 2 doses).
Types of outcome measures
The primary outcomes of interest are: 1. cognitive function (as measured by psychometric tests) 2. clinical global impression 3. changes in global disease severity 4. performance of activities of daily living 5. behavioural disturbance 6. quality of life 7. effect on carer 8. dependency (such as institutionalization) 9. death 10. acceptability of treatment as measured by withdrawal from trial 11. safety as measured by the incidence of adverse events (including side‐effects) leading to withdrawal 12. use of health care resources 13. costs.
Physiological outcomes such as plasma levels, changes on functional imaging or EEG changes are noted but not assessed as they are not primarily measures of efficacy.
Search methods for identification of studies
The Cochrane Dementia and Cognitive Improvement Group's Specialized Register was searched using the terms 'donepezil', 'E2020' , 'Aricept' , galanthamin* galantamin* reminyl, rivastigmine, exelon, "ENA 713" and ENA‐713 on 12 June 2005. This Register contains up‐to‐date records of all major health care databases and many ongoing trial databases.
The Specialized Register at that time contained records from the following databases: CENTRAL: January 2005 (issue 1); MEDLINE: 1966 to 2005/02; EMBASE: 1980 to 2005/01; PsycINFO: 1887 to 2005/01; CINAHL: 1982 to 2004/12; SIGLE (Grey Literature in Europe): 1980 to 2004/06; ISTP (Index to Scientific and Technical Proceedings): to May 2000; INSIDE (BL database of Conference Proceedings and Journals): to June 2000; Aslib Index to Theses (UK and Ireland theses): 1970 to March 2003; Dissertation Abstract (USA): 1861 to March 2003; ADEAR (Alzheimer's Disease Clinical Trials Database): to 25 March 2005; National Research Register: issue 1/2005; Current Controlled trials (last searched April 2005) which includes: Alzheimer Society GlaxoSmithKline HongKong Health Services Research Fund Medical Research Council (MRC) NHS R&D Health Technology Assessment Programme Schering Health Care Ltd South Australian Network for Research on Ageing US Dept of Veterans Affairs Cooperative Studies National Institutes of Health (NIH) ClinicalTrials.gov: last searched March 2005; LILACS (Latin American and Caribbean Health Science Literature): last searched April 2003
Pharmaceutical companies: Members of the Donepezil Study Group and Eisai Inc were contacted; Eisai Inc has made extensive information available including results from 134, 161, 201, 301, 302 and 304.
An additional Internet search using Copernic 2000 was performed on 21 and 22 June 2005 using trial names and numbers. No new trials were found other than the ones that had already been found in the update search of the CDCIG Register on 12 June 2005; we did find additional references to existing trials. Eisai/Pfizer, FDA, EMEA and NICE websites were searched. Additional information was collected from unpublished "clinical research reports" obtained from Janssen. Novartis, the developer of rivastigmine, was contacted for information about any unpublished and published trials.
Data collection and analysis
Irrelevant publications were discarded, based on the title of the publication and its abstract. In the presence of any suggestion that an article could be relevant, it was retrieved for further assessment.
The trials were reviewed for inclusion from the culled citation list.
QUALITY ASSESSMENT The methodological quality of each selected trial was assessed using the Cochrane Collaboration guidelines (Alderson 2004): Category A (adequate) is where the report describes allocation of treatment by: Some form of centralized randomized scheme Some form of randomization scheme controlled by a pharmacy Numbered or coded containers An on‐site or coded computer system Using envelopes for assignment
Category B (intermediate) is where the report describes the treatment allocation by: Use of a list of table to allocate assignments Use of envelopes or sealed envelopes Use of randomization design without further detail
Category C (Inadequate) is where the report describes allocation of treatment by: Alternation Reference to case record numbers, date of birth or any other such approach Any allocation procedure that is entirely transparent before assignment, such as an open list of random numbers or assignments
Only trials in categories A or B were included in the review.
DATA EXTRACTION Data were extracted from the published reports. The summary statistics required for each trial and each outcome for continuous data are the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and the number of people in each treatment group at each time point were extracted if available.
For binary data the number in each treatment group and the numbers experiencing the outcome of interest were sought.
The baseline assessment is defined as the latest available assessment prior to randomization, but no longer than two months before.
For each outcome measure, data were sought on every person assessed. To allow an intention‐to‐treat (ITT) analysis, the data were sought irrespective of compliance, whether or not the person was subsequently deemed ineligible, or otherwise excluded from treatment or follow‐up. If intention‐to‐treat data were not available in the publications, "on‐treatment" or the data of those who complete the trial (OC) were sought and indicated as such.
Data from 'open‐label' follow‐on phases after the randomized study were not used to assess safety or efficacy because patients were usually not randomized, nor were treatments concealed.
DATA ANALYSIS The outcomes measured in trials of dementia and cognitive impairment often arise from ordinal rating scales. Where the rating scales used in the trials have a reasonably large number of categories (more than 10) the data were treated as continuous outcomes arising from a normal distribution.
Summary statistics (n, mean and standard deviation) are required for each rating scale at each assessment time for each treatment group in each trial for change from baseline. For cross‐over trials only the data from the first treatment period are used.
Meta‐analysis requires the combination of data from trials that may not use the same rating scale to assess an outcome. The measure of the treatment difference for any outcome is the weighted mean difference when the pooled trials use the same rating scale or test, and the standardized mean difference, which is the absolute mean difference divided by the pooled standard deviation when they used different rating scales or tests.
For binary outcomes, such as improvement or no improvement, the odds ratio is used to measure treatment effect. A weighted estimate of the typical treatment effect across trials is calculated.
Overall estimates of the treatment difference are presented. In all cases the overall estimate from a fixed‐effects model is presented and a test for heterogeneity using a standard chi‐square statistic is performed. If, however, there is evidence of heterogeneity of the treatment effect between trials then either only homogeneous results are pooled, or a random‐effects model used (in which case the confidence intervals would be broader than those of a fixed‐effects model).
Results
Description of studies
Placebo controlled studies
The 13 trials which met the inclusion criteria (Table 1) were designed to evaluate the efficacy and safety of a ChEI in patients with dementia due to AD. They were all multicentre, randomized, double‐blind parallel group trials. There were 7298 patients randomized (2228 in the donepezil studies, 2267 galantamine, and 2803 rivastigmine). The mean age at baseline within a trial was between 72 and 75 years, except for DON‐311, with a mean age of 86 years, which was designed to examine the efficacy, safety and tolerability in the management of very elderly residents in nursing homes. The dementia was described as mild to moderate in 10 trials, (mean MMSE 17‐20), mild in one, DON‐402, (mean MMSE 24) and severe in two trials, DON‐311 and DON‐Feldman (mean MMSE 12 and 14). Seven trials were based in the USA, three in Europe, and one, DON‐Feldman, in Canada, Australia and France, one RIV‐B303 in Europe and North America, and one RIV‐B304 in the UK, Ireland, Australia, Canada, RSA and Italy. Twelve trials were supported by the marketing or manufacturing company of the drug, and GAL‐INT‐1 Wilcock was supported by the UK National Health Service Research and Development Health Technology Assessment programme. Over the course of the studies most of the patients, between 75% and 97%, were receiving at least one concomitant medication. The most common medications were analgesics, systemic antibacterials, psycholeptics, and anti‐inflammatory products. Antidementia drugs, anticonvulsants, antidepressants and antipsychotics were generally not allowed.
1. Description of included studies at baseline.
| Study | duration weeks | number of patients | mean age | % female | mean MMSE | dose mg/day donep. | phase | country | funded by |
| DON vs RIV/Bullock | 104 | 988 | 75.9 | 69 | 15.1 | 10 donepezil, maximum 12 (in 2 doses) rivastigmine | ‐ | Australia, Canada, France, Germany, Italy, Spain, UK | Novartis |
| Donepezil‐302 | 24 | 473 | 73.4 | 62 | 19.0 | 5, 10 | III | USA | Eisai |
| Donepezil‐304 | 24 | 818 | 71.7 | 57 | 20.0 | 5, 10 | III | EUROPE | Eisai |
| Donepezil‐311 | 24 | 208 | 85.7 | 82 | 14.4 | 10 | III | USA | Eisai |
| Donepezil‐402 | 24 | 153 | 74.0 | 53.6 | 24.1 | 10 | USA | Eisai/Pfizer | |
| Donepezil‐Feldman | 24 | 290 | 73.6 | 61 | 11.8 | 10 | CANADA, AUSTRALIA, FRANCE | Eisai/Pfizer | |
| DON‐Nordic | 52 | 286 | 72.5 | 64 | 19.3 | 10 | EUROPE | Pfizer | |
| GAL‐INT‐1 | 26 | 653 | 72.2 | 63 | 19.3 | 24, 32 | EUROPE | UK NHS R&D health technology assessment programme | |
| GAL‐USA‐1 | 26 | 636 | 70.7 | 62 | 19.3 | 24, 32 | USA | Janssen | |
| GAL‐USA‐10 | 22 | 978 | 76.9 | 64 | 17.8 | 8, 16, 24 | USA | Janssen | |
| Rivastigmine‐B303 | 26 | 725 | 72.0 | 59 | 20.0 | 6‐12 | III | EUROPE, CANADA, USA | Novartis |
| Rivastigmine‐B304 | 26 | 677 | 71.4 | 59 | 18.5 | 2‐12 | III | UK, IRELAND, AUSTRALIA, CANADA, RSA, ITALY | Novartis |
| Rivastigmine‐B351 | 26 | 702 | 74.1 | 56 | 20.0 | 6,9 | III | USA | Novartis |
| Rivastigmine‐B352 | 26 | 699 | 74.5 | 61 | 19.7 | 6‐12 | III | USA | Novartis |
The doses of donepezil used in the trials were within the range shown to be clinically useful and reasonably well tolerated in the earlier studies. Treatment was once daily. The initial dose was 5 mg/day for one week followed by the full dose for DON‐302 and DON‐304. For the two later trials, DON‐311 and DON‐Nordic, the time on 5 mg/day was prolonged to 4 weeks, before increasing to 10 mg/day. The forced titration schemes were blinded.
GAL‐INT‐1 Wilcock and GAL‐USA‐1 Raskind followed a rapid dose titration, 8 mg/day for one week, 16 mg/day for week 2, and 24 mg/day thereafter (for the 24 mg/day arm), GAL‐USA‐10 Tariot a more gradual titration scheme, 8 mg/day for 4 weeks, 16 mg/day for the next 4 weeks, and thereafter 24 mg/day, all divided into two doses per day.
RIV‐B351 tested a fixed dose of rivastigmine (mean dose at 26 weeks was 8.5 mg/day), but the other rivastigmine trials aimed at a maximum tolerated dose within a prescribed range (mean dose at 26 weeks was 9.3 ‐ 10.4 mg/day), all divided into two equal doses per day. The titration period was between 3 and 12 weeks.
Most of the trials had more than two arms, testing dosages of the ChEI that are not reviewed here.
The list of exclusions was quite extensive and fairly consistent across the studies.
Donepezil Patients were excluded from the donepezil studies if they had insulin‐dependent diabetes mellitus or other endocrine disorder, asthma, obstructive pulmonary disease or clinically significant uncontrolled gastrointestinal hepatic or cardiovascular diseases. Patients known to be hypersensitive to cholinesterase inhibitors or who had taken tacrine or other investigational medicines within one month of baseline were excluded. Concomitant medications such as anticholinergics, anticonvulsants, antidepressants and antipsychotics were not allowed. Drugs with central nervous system (CNS) activity were prohibited or partially restricted. The patients included in DON‐311 were on average older than in the other studies, and were more likely to have comorbid illness. They were required to have reported at a frequency of several times a week at least one symptom from the Neuropsychiatric Inventory Nursing Home version (NPI‐NH).
Galantamine Some stable and well‐controlled concomitant medical disorders were not reason for exclusion (hypertension, heart failure, diabetes and hypothyroidism). The list of reasons for exclusion was quite extensive and consistent across studies, other neurodegenerative illness, cardiovascular disease, or active cerebrovascular disease, clinically significant infarct dementia, psychiatric, hepatic, renal, pulmonary, metabolic, endocrine, active peptic ulcer, history of epilepsy, drug or alcohol abuse.
Rivastigmine The list of exclusions was not extensive. Patients with severe and unstable illnesses (cardiovascular or pulmonary disease, unstable diabetes mellitus, peptic ulceration within the preceding five years, evidence of alcohol or substance abuse) were excluded, as were subjects taking medications such as anticholinergic drugs, acetylcholine precursor health food supplements, memory enhancers, insulin and psychotropic drugs.
The trials designed to compare two ChEIs.
There are four randomized studies designed to compare two ChEIs, but only one, DON vs RIV/Bullock, met the inclusion criteria. The other three, DON vs GAL/Jones, DON vs GAL/Wilcock, and DON vs RIV/Wilkinson, were open label, the participants knew which drug they were taking, and in addition two were of 12 weeks duration only.
DON vs RIV/Bullock This is a 104 week, randomized, double blind multicentre international study of donepezil compared with rivastigmine. The trial was funded by Novartis, the manufacturer of rivastigmine. The number randomized was 994. The titration period was 16 weeks. The rivastigmine group started at 3 mg/day, and the dose was increased by 3 mg/day at 4 week intervals to a maximum of 12 mg/day. The donepezil group received 5 mg/day in weeks 1‐8 and 10 mg/day thereafter. Following the 16 weeks titration patients were maintained at the highest tolerated dose level. Patients were diagnosed with probable AD according to NINCDS‐ADRDA criteria. Those with AD and also symptoms of Lewy body disease were allowed in the study. Patients with other serious illness or disease were excluded. At baseline the mean age was 75.9 years, and mean MMSE was 15.1.
Outcome scales and tests
Global Assessment 1. A Clinician's Interview‐Based Impression of Change scale (CIBIC‐Plus) (Schneider 1997) was used in eight studies. It provides a global rating of patient function in four areas, general, cognitive, behaviour and activities of daily living. All patients are scored on global severity at baseline and subsequent assessments on a scale of 1‐7 are relative to baseline, with 1 showing marked improvement, 7 marked worsening with 4 representing no change. Information is obtained from the caregiver and patient and the clinician is blind to all other measures. Results are often presented in dichotomised mixed form, for example, improved compared with unchanged or worse. 2. The Gottfries, Brane and Steen scale (GBS) (Gottfries 1982) was used in the DON‐Nordic trial for the global assessment. The GBS is a comprehensive scale for rating dementia syndromes, based on a semi‐structured interview with the caregiver. A seven‐point scoring system, from 0 (normal function) to 6 (maximum disturbance or presence of symptoms) measures orientation, memory and concentration (12 items), activities of daily living (6 items), emotional function (3 items) and pathological aspects of behaviour (6 items). 3. The Global Deterioration Scale (GDS) (Reisberg 1982) was developed for the assessment of primary degenerative dementia and the delineation of the stages of disease. The stages are scored from 1 (no cognitive decline) to 7 (severe cognitive decline).
Cognitive Function 1. The primary cognitive test in ten studies was the cognitive part of the Alzheimer's Disease Assessment Scale (ADAS‐Cog) (Rosen 1984). ADAS‐Cog comprises 11 individual tests, spoken language ability (0‐5), comprehension of spoken language (0‐5), recall of test instructions (0‐5), word finding difficulty (0‐5), following commands (0‐5), naming object (0‐5), construction drawing (0‐5), ideational praxis (0‐5), orientation (0‐8), word recall (0‐10) and word recognition (0‐12). The total score ranges from 0‐70, the high score indicating greater impairment. 2. Mini Mental State Examination (MMSE) (Folstein 1975) evaluates cognition in five areas: orientation, immediate recall, attention and calculation, delayed recall, and language. The test takes only 15 minutes to administer and the score ranges from 0 (severe impairment) to 30 (normal). It was used in nine studies. 3. The Severe Impairment Battery (SIB) (Panisset 1994) is used to assess a range of cognitive functions in severely demented patients. It is composed of 6 subscales, attention, orientation, language, memory, visuoperception and construction, and includes brief assessments of social skills, praxis, and responding to name. The score ranges from 0 to 100, the lower scores indicating greater impairment.
Activities of daily living: 1. The Progressive Deterioration Scale (PDS) (DeJong 1989), which was used in the DON‐Nordic trial and the rivastigmine trials, is a disease specific measure of changes in 29 items of the activities of daily living. Each item is scored on a visual analogue scale of 0‐100 (a higher score is better), and the final score is the mean score of the items. The interview is conducted with the caregiver. 2. The Disability Assessment for Dementia (DAD) (Gélinas 1999), which was used in the DON‐Feldman and GAL‐INT‐1 Wilcock trials, is a 10 domain, 40 item instrument that measures instrumental and basic activities of daily living. A higher score indicates improvement. 3. The Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living (ADCS) (Galasko 1997)
Behavioural Disturbance 1. The Neuropsychiatric Instrument (NPI) (Cummings 1994), a 12 item, carer rated instrument, was used by DON‐311, DON‐Feldman and GAL‐USA‐10 Tariot to evaluate behavioural and neuropsychiatric symptoms, including delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy, disinhibition, irritability, aberrant motor behaviour, night‐time behaviour, and appetite/eating disorder. Frequency is rated from 1 (occasional, less than once a week) to 4 (very frequent) and severity from 1 (mild) to 3 (severe). The product of frequency and severity ranges from 1 to 12, with a total score ranging from 12 to 120 for the 10 domains summed. A lower score indicates improvement.
In all studies assessments were carried out at more than one time point between the base line assessment and the reported end‐point.
Details of adverse events were ascertained by the questioning of each patient at each assessment. Serious adverse events were reported immediately.
The reported analyses were performed on an intention‐to‐treat (ITT) basis, which included all patients who were randomized to treatment, assessed at baseline, received at least one dose of the study drug, and had at least one post baseline assessment. The ITT population consisted of those who provided complete data at endpoint regardless of compliance (the observed cases OC) plus the LOCF population, (the last observation on double‐blind treatment carried forward), for whom the last observation on double‐blind treatment was carried forward to endpoint. These data were analysed by the investigators in the endpoint analyses, which were the primary analyses and are described as ITT‐LOCF.
The change from baseline of continuous outcome measures was analysed by analysis of variance which included the centre, the treatment, centre by treatment interaction, and baseline score in the model. The ordinal data were analysed either as continuous data using analysis of variance, or as ordinal data using the Cochran‐Mantel‐Haenszel test and with adjustments for centre if necessary.
Risk of bias in included studies
The studies were all described as randomized, but usually no further details of the exact method of randomization were given. The percentage of patients leaving before the end of the study was approximately 30% from the treatment group, 18% from the placebo groups. The main cause of drop‐outs was an adverse event. The results of analyses of the ITT‐LOCF and OC data sets are usually reported and these can be compared to assess the effect of drop‐outs.
All the included studies were described as double‐blind.
Effects of interventions
ChEI vs placebo 13 trials met the criteria for inclusion. All studies examined the cognitive, functional and global effects of a ChEI.
Meta‐analyses on the ITT population, where LOCF assessments were incorporated when assessments were missing, are reported where data are available. Models were fitted using fixed effects. There is evidence of heterogeneity between the studies for a few meta‐analyses, but not a high level of heterogeneity as measured by I‐squared (Higgins 2003).
The rating scales and cognitive tests differ in the direction representing improvement. A decrease in score indicates improvement with the ADAS‐Cog, CIBIC‐Plus, GBS, and ADL, whereas increase shows improvement for the MMSE.
Global assessment The 7‐point CIBIC‐Plus scale, measuring global clinical state, was dichotomized, counting those showing no change or decline, against those showing improvement, and analysed using the odds ratio. There are benefits associated with ChEI compared with placebo after approximately 6 months of treatment as shown by the ITT‐LOCF analyses (numbers improved 428/1755 (24%) vs 277/1647 (17%), OR 1.56, 95% CI 1.32 to 1.85, p<0.00001, 8 studies). The results are fairly homogeneous across the three ChEIs. The 7‐point CIBIC‐Plus scale, measuring global clinical state, was dichotomized, counting those showing decline, against those showing improvement or no change, and analysed using the odds ratio. There are benefits associated with a ChEI compared with placebo after approximately 6 months of treatment as shown by the ITT‐LOCF analyses (numbers improved or unchanged 425/645 vs 340/661, OR 1.84, 95% CI 1.47 to 2.30, p<0.00001, 2 studies). The GBS is a global assessment scale. Only one trial used this scale, the DON‐Nordic and there is no evidence of benefit or risk associated with a ChEI after one year of treatment.
Cognitive function The meta‐analysis reveals benefits associated with a ChEI compared with placebo on cognitive function as shown by improvement in the ADAS‐Cog and MMSE test scores after treatment of approximately 6 months. Ten studies contribute data to the ADAS‐Cog meta analysis, three of donepezil, 3 of galantamine and four of rivastigmine, nine studies to the MMSE meta analysis, five of donepezil and four of rivastigmine. ADAS‐cog (MD ‐2.37, 95%CI ‐2.73 to ‐2.02, P=<0.00001, 10 studies) MMSE (MD 1.37, 95%CI 1.13 to 1.61, P=<0.00001, 9 studies) For ADAS‐Cog, the treatment effect for individual trials is between ‐1.4 and ‐3.9 points. The rivastigmine trials show the most variation, with low and high treatment effects within this range. They also show decline for treatment and placebo groups, whereas the donepezil and galantamine trials show improvement on treatment, and decline on placebo. There is heterogeneity between trials for MMSE which is due to RIV‐B352. Whereas the treatment effect is between 0.65 and 1.80 points for eight trials, that of RIV‐B352 is 2.9 points.
Activities of daily living The DON‐Nordic and the 4 rivastigmine trials assessed activities of daily living using the PDS scale. ChEI showed benefit compared with placebo after 6 months or more of treatment (MD 2.40, 95% CI 1.55 to 3.37, p<0.00001, ITT‐LOCF analysis) DON‐Feldman and GAL‐INT‐1 Wilcock used the DAD scale. ChEI showed benefit compared with placebo after 6 months or more of treatment (MD 4.39, 95% CI 1.96 to 6.81, p=0.0004, ITT‐LOCF analysis)
Behavioural disturbance DON‐311, DON‐Feldman and GAL‐USA‐10 Tariot assessed behavioural disturbance (NPI‐TOTAL), and ChEI showed benefit compared with placebo at 6 months (ITT‐LOCF). (MD ‐2.44, 95%CI ‐4.12 to ‐0.76, P=0.004).
Side effects The ChEIs were judged to be fairly well tolerated. The meta‐analyses of withdrawals before the end of treatment, using the odds ratio, showed significant differences in withdrawals between the ChEI group and the placebo group in favour of placebo after 6 months or more of treatment (778/2672 29% vs 453/2471 18%, OR 1.76, 95% CI 1.54 to 2.02, p<0.00001, 14 studies). The percentage of withdrawals from the treatment group varies from 16% to 43%, and from the placebo group from 0% to 33%, and variation over this range is seen within the results for each ChEI.
Various adverse events were recorded. The meta‐analyses of withdrawals before the end of treatment due to an adverse event, using the odds ratio, show that there were significant differences between withdrawals from the ChEI group compared with the placebo group in favour of placebo (488/2672 18% ChEI, 209/2471 8% placebo) (OR 2.32 95%CI 1.95 to 2.76, p<0.00001, 13 studies). The percentage of withdrawals from the treatment group varies from 7% to 29%, and from the placebo group from 7% to 18%, and variation over this range is seen within the results for each ChEI.
The meta‐analyses of the total number of patients who suffered at least one adverse event before the end of treatment, using the odds ratio, show that there were significant differences between the ChEI group compared with the placebo group in favour of placebo (1802/2515 72% ChEI, 1326/2309 57% placebo) (OR 2.51 95%CI 2.14 to 2.95, p<0.00001, 12 studies). There were far fewer adverse events in both groups of the galantamine trials, although overall the odds of an adverse event was highest in the galantamine trials and lowest in the donepezil trials.
Forty seven different types of adverse event were reported in the trials. There were significant differences, in favour of placebo, compared with ChEI for several types of adverse events during treatment of 6 months or more. Abdominal pain (159/1441 compared with 74/1263) (OR 1.95, 95% CI 1.46 to 2.61, p<0.00001, 7 studies) Abnormal dreams (9/96 compared with 0/105) (Peto OR 5.38, 95% CI 1.34 to 21.55, p=0.02, 1 study) Anorexia (281/2296 compared with 76/2123) (OR 3.75 95%CI 2.89 to 4.87, p<0.00001, 10 studies) Asthenia (47/485 compared with 22/452) (OR 2.47 95%CI 1.27 to 4.81, p=0.008, 3 studies) Diarrhoea (386/2686 compared with 197/2487) (OR 1.91 95%CI 1.59 to 2.30, p=<0.00001, 13 studies) Dizziness (355/2399 compared with 171/2184) (OR 1.99 95%CI 1.64 to 2.42, p<0.00001, 12 studies) Fatigue (12/157 compared with 3/162) (OR 4.39 95%CI 1.21 to 15.85, p=0.02, 1 study) Headache (280/1934 compared with 170/1752) (OR 1.56 95%CI 1.27 to 1.91, p<0.0001, 9 studies) Insomnia (133/1564 compared with 79/1342) (OR 1.49 95%CI 1.12 to 2.00, p=0.007, 7 studies) Muscle cramp (12/157 compared with 1/162) (OR 13.32 95%CI 1.71 to 103.74, p=0.01, 1 study) Nausea (833/2648 compared with 222/2441) (OR 4.87 95%CI 4.13 to 5.74, p<0.00001, 13 studies) Peripheral oedema (25/103 compared with 14/105) (OR 2.08 95%CI 1.01 to 4.28, p=0.05, 1 study) Syncope (41/1194 compared with 19/1012) (OR 1.90 95%CI 1.09 to 3.33, p=0.02, 5 studies) Tremor (19/315 compared with 3/318) (OR 6.82 95%CI 1.99 to 23.37, p=0.002, 2 studies) Vertigo (11/142 compared with 3/144) (OR 3.95 95%CI 1.08 to 14.46, p=0.04, 1 study) Vomiting (521/2434 compared with 122/2269) (OR 4.82 95%CI 3.91 to 5.94, p<0.00001, 11 studies) Weight loss (73/679 compared with 27/679) (OR 2.99 95%CI 1.89 to 4.75, p<0.00001, 4 studies)
Several donepezil trials reported only adverse events suffered by more than 5% of patients.
Direct comparisons between the cholinesterase inhibitors
There was one included trial, DON vs RIV/Bullock, which compares donepezil with rivastigmine. Donepezil vs rivastigmine
There was no significant difference between donepezil and rivastigmine for cognitive function, activities of daily living and behavioural disturbance and global assessment as measured by the Global Deterioration Scale (GDS).
The analysis of withdrawals before the end of treatment, using the odds ratio, showed significant differences in withdrawals between donepezil and rivastigmine in favour of donepezil after 2 years of treatment (182/499 vs 234/495, OR 0.64 95% CI 0.50 to 0.83, p=0.0006).
Various adverse events were recorded. The meta‐analyses of withdrawals before the end of treatment due to an adverse event, using the odds ratio, show that there were significant differences between withdrawals from the donepezil group compared with the rivastigmine group in favour of donepezil (47/499 donepezil, 90/495 rivastigmine) (OR 0.47 95%CI 0.32 to 0.68, p<0.0001).
There were significant differences, in favour of donepezil, compared with rivastigmine for several types of adverse events during treatment of 12 ‐16 weeks Nausea (76/499 donepezil 163/495 rivastigmine) (OR 0.37, 95% CI 0.27 to 0.5, p<0.00001) Vomiting (29/499 donepezil 138/495 rivastigmine) (OR 0.16, 95% CI 0.10 to 0.24, p<0.00001) Falls (10/499 donepezil 25/495 rivastigmine) (OR 0.38, 95% CI 0.18 to 0.81, p=0.01) Hypertension(7/499 donepezil 20/495 rivastigmine) (OR 0.34, 95% CI 0.14 to 0.81, p=0.01) Anorexia (20/499 donepezil 45/495 rivastigmine) (OR 0.42, 95% CI 0.23 to 0.66, p=0.0005) Weight loss (9/499 donepezil 30/495 rivastigmine) (OR 0.28, 95% CI 0.13 to 0.61, p=0.001)
and between 16 weeks and 2 years of treatment Nausea (24/453 donepezil 52/404 rivastigmine) (OR 0.38, 95% CI 0.23 to 0.63, p=0.0002) Vomiting (20/453 donepezil 62/404 rivastigmine) (OR 0.25, 95% CI 0.15 to 0.43, p<0.00001) Anorexia (14/453 donepezil 26/404 rivastigmine) (OR 0.46, 95% CI 0.24 to 0.90, p=0.02)
The analysis of serious adverse events, using the odds ratio, show that there was no significant difference between the donepezil group compared with the rivastigmine group.
CONCLUSIONS The results of 13 trials demonstrate beneficial effect of ChEI compared with placebo on cognitive function and measures of global clinical state at 6 months or more. There is nothing to suggest the effects are less for those with severe dementia or mild dementia, although there is very little evidence for other than mild to moderate dementia.
There are fewer data on measures of behavioural disturbance and activities of daily living, but there is evidence of benefit of ChEI in these domains.
The percentage of patients leaving the trials from the ChEI group is about 29% compared with 18% from the placebo group. There is evidence that more participants leave ChEI treatment groups on account of adverse events than leave the placebo groups.
There is evidence of more adverse events in total in the ChEI groups than in placebo. Many types of adverse event were reported, and seventeen of these were significantly more frequent in the ChEI groups than in placebo. Sometimes the evidence arose from one trial only. There was pooled evidence from six or more studies that adverse events of abdominal pain, anorexia, dizziness, nausea, vomiting, diarrhoea, headache, and insomnia were significantly more frequent in the ChEI groups than in placebo.
There are data on health resource use and costs from two donepezil trials, but none from trials of galantamine or rivastigmine. Therefore there is no meta‐analysis of health costs included in this review.
There are four trials which compared a ChEI with another, only one trial, which compares donepezil with rivastigmine, is double blind. Two of these trials are of 12 weeks duration which is not long enough because patients need time to adjust and tolerate treatment .
There is no evidence of a difference between donepezil and rivastigmine for cognitive function, activities of daily living and behavioural disturbance. Fewer patients suffer adverse events on donepezil than rivastigmine.
In summary, we conclude that from the evidence from 13 trials the three drugs show similar benefit for cognitive function and global assessment. From the evidence of one trial patients suffer less from adverse events on donepezil compared with rivastigmine.
Discussion
This review has brought together the evidence from 13 randomized, placebo controlled, double blind trials of donepezil, galantamine or rivastigmine used at the recommended doses, for periods of 6 months or more, and from a single trial which compared donepezil with rivastigmine. There is additional evidence for the cholinesterase inhibitors from the Cochrane reviews of each ChEI, and from other sources which we now discuss.
Excluded trial ‐ AD2000 Almost all of the trials included are not independent of the drug manufacturing or marketing companies. Results have been published from AD2000, which is a large, randomized, placebo controlled trial of donepezil independently funded in the UK, and whose participants were typical patients referred to memory clinics by general practitioners. AD2000 has not been included in this review. Interpretation of the published results is not straight forward due to the complex design of the study and the form in which results were reported. Patients were randomized twice, at baseline and at 12 weeks making it difficult to define a patient's treatment status which adds to the complexities of the analyses. There has never been an adequate explanation for this double randomization. It does not, as stated by the AD2000 investigators, provide an additional estimation on the 13 week treatment effect, the second estimate is not independent of the first, and cannot be described as a 13 week effect when patients have already been treated for the previous 13 weeks. There are many other estimates of the 13 week treatment effect, this is not important enough to compromise the design of a trial that promised to answer questions that had not been addressed previously. There are problems not just with the design of the trial, but with the execution. The actual recruitment of 566 was far short of intended recruitment of 3000, and was underpowered for achieving the original objectives. In addition, there were many leaving the trial. Reasons for the loss of patients were death, simple withdrawal and withdrawal to open‐label donepezil. Patients were also withdrawn if they entered institutional care. In the first year of this trial 40% of patients were lost, and some of this loss was related to treatment. The losses continued over the several phases of this long trial. The patients continued with treatment only if the patient, the doctor and the carer judged it appropriate. It is not possible to analyse the trials data using simple methods. The trialists have analysed the data from the trial using complex multilevel models which take account of the repeated measurements on patients, the change in their treatment status and the time of leaving the trial. Although patients taking donepezil were randomized to 10 mg or 5 mg per day the results for each dose are not reported separately. These analyses and results do not translate easily into the form required for the meta‐analyses, and in addition interpretation is hindered by the differential loss of patients and the complexity of the design and analysis. AD2000 failed to meet its objectives. The intention had been to provide independent estimates of efficacy and cost effectiveness for donepezil for a population of patients not selected for being in better health than average, treated for much longer than six months. Although the investigators have published results they are dependent on correction for bias due to differential dropouts from the donepezil and placebo groups. It would be unwise to base important decisions on the provision and use of donepezil on the results of this trial. Subgroup analyses
Subgroup analyses are further analyses of data from the randomized trials which have been undertaken in an attempt to identify those who may preferentially benefit from treatment with a ChEI, sometimes described as the responders. The data set is limited to patients who belong to a particular subgroup. In published reports such subgroups have been defined as those with severe disease, those with mild disease, with hypertension or those with vascular risk factors (VRF). Analysing the complete data set there are two interesting questions which could be asked. The first is whether there is a treatment effect for those in the subgroup, and those not, and secondly whether the treatment effect is the same for those within the subgroup compared with those not, that is, is there an interaction between treatment and subgroup? Such analyses have been reported for rivastigmine and discussed by Birks 2005b. Erkinjuntti 2002, funded by Novartis Pharmaceuticals, investigated the response to rivastigmine of those without hypertension compared to those with, using the data from RIV‐B303. Although it is reported that particular benefits may be observed in those with vascular risk factors, on examination of the results of the analyses there is no evidence for this assertion. Kumar 2000 carried out a similar sub group analysis, the groups defined as those with or without vascular risk factors, using the data from RIV‐B352. Kumar asserted that those with VRF experience greater clinical benefit than those without VRF, but the evidence does not support this claim.
At most these subgroup analyses may generate hypotheses that can be tested in further trials; they do not identify those who may respond better to treatment. There will not be sufficient power to examine treatment effects for a smaller group of patients, and usually these analyses are carried out retrospectively; they were not part of the original protocol.
Open label extension studies As Alzheimer's disease generally progresses slowly and a clinical course of 5 or 10 years is not unusual, clinical trials of 6 or 12 month duration of treatment are of limited use. Unfortunately, randomized trial evidence of longer term effects is not currently available and given the widely differing rates of progression of Alzheimer's disease in different individuals and groups selected in different ways, extrapolation could be misleading. There are reports of open‐label extensions to some of the included studies. Generally the patients who complete the randomized phase were eligible for entry to the open‐label phase. All patients began on a low dose for several weeks and progressed to a higher dose if this dose was tolerated. It has been reported that patients maintained their level of performance at better than baseline for more than 40 weeks, and that there was no reduction in treatment effect for up to two years (open label extension studies to the trials of donepezil, DON‐302). It is of great interest to see that patients are still doing well after more than two years on a ChEI, although the results are likely to be biased. There are several reasons for possible bias; not all patients enter the extensions to the trials, only a self selected group, the comparisons are made with historical controls, or with a hypothetical placebo decline obtained by extrapolation from the randomized phase, and patients drop out at a much faster rate than from the original randomized trial possibly leaving only the healthiest and least impaired. The results of open‐label extension trials must be interpreted with caution.
Health resource utilization and costs
Systematic reviews of interventions provide us with evidence on efficacy and effectiveness. If the intervention is not cheap those who make decisions about the use of resources within a health care system need cost benefit information as well. This type of information is not usually available in Cochrane reviews, unless data on resource utilization have been collected as an outcome for a randomized controlled trial.
Only two studies assessed outcomes relating to health care resource use and the associated costs, the DON‐Feldman and DON‐Nordic studies. These are discussed in Birks 2005. The two studies reported on costs accruing during the randomized treatment for patients, the first over six months in 1998 in Australia, France and Canada, (in the ratio 25:10:65) and the other over one year in 1999 in Northern Europe, mostly Finland and Sweden, with smaller groups in Denmark, the Netherlands and Norway. The costs covered the utilization of healthcare resources by the patient and carer and the cost of unpaid carer time. The results are specific to the countries in which the studies were undertaken because of differences between the public health systems. The detailed reports show very different apportionment of costs between different items in different countries.
The DON‐Feldman study reports that there was no benefit or disadvantage of donepezil, for any individual item of health care resource, for the cost of any item, for the total cost of health care resources for the patient including the cost of donepezil, and total costs for the carer. The unpaid carer time was also estimated and costed. The DON‐Nordic study reports that there was no benefit or disadvantage of donepezil for total cost of health care resource use for the patient, or for the carer, or for the total costs of patient and carer. The results were not pooled as the outcomes were not considered comparable across trials. Many items were assessed and reported separately, and total costs were reported.
There is little evidence of a difference in the cost of health resource utilization between the donepezil and placebo groups. There are no data on these outcomes for galantamine or rivastigmine.
One of the main costs of Alzheimer's disease is the cost of care, especially the cost of care in an institution. If the costs could be shown to be significantly less with treatment with a ChEI then the cost effectiveness would be proven. But there are no randomized double blind placebo controlled trials of a ChEI that continue long enough to provide this evidence. The estimates of costs rely on statistical models based on the prediction of the progression of disease by extrapolation of the results from the short term trials, together with epidemiological data, mortality data and data on resource use applicable to a particular situation or health care system. As a recent example see the economic analyses that NICE has commissioned for the review of the ChEIs The weakness of these evaluations is the assumptions on which they are based. Every health economist has their own model and predictions. These models are very imprecise; it is impossible to judge where the truth might lie without long term data. The only sensible conclusion must be that it would be inappropriate for any provider of health care to make a decision regarding the availability of ChEIs for patients based on these economic models.
Authors' conclusions
Implications for practice.
In people with mild, moderate or severe dementia due to Alzheimer's disease treated for periods of 6 months and one year, treatment with a donepezil, galantamine or rivastigmine at the recommended dosage produced improvements in cognitive function, on average ‐2.7 points (95%CI ‐3.0 to ‐2.3), in the midrange of the 70 point ADAS‐Cog Scale. Study clinicians blind to other measures rated global clinical state more positively in treated patients. Benefits of treatment were also seen on measures of activities of daily living and behaviour. None of these treatment effects are large. Despite the slight variations in the mode of action of the three cholinesterase inhibitors there is no evidence of any differences between them with respect to efficacy. From the evidence provided by one trial there appears to be less adverse events associated with donepezil compared with rivastigmine. It may be that galantamine and rivastigmine match donepezil in tolerability if a careful and gradual titration routine over more than three months is used.
Implications for research.
Ideally there is still a need for randomized, placebo controlled trials of longer than one year that examine not only cognitive function, behaviour, activities of daily living and global clinical state, but also the use of health care resources. Randomized trials of treatment involving the use of placebos over many years are unlikely to be either a practical or ethical option. Other robust randomized trial designs will be needed to help establish the maximum duration of treatment, and the indicators that could show when treatment is no longer beneficial.
Feedback
Interpretation of results of Bullock 2005 study
Summary
I should like to draw your attention to a possible error in Dr Birks' discussion of our recent paper (DON vs RIV/Bullock) which describes a 104‐week study of donepezil compared with rivastigmine:
1. Dr Birks stated in her review that there were no significant differences between treatment groups on any outcome measures used in this study (pages 1 and 9). However, statistically significant differences between donepezil and rivastigmine (in favour of rivastigmine) were observed at week 104 on the Global Deterioration Scale (according to ANCOVA and Wilcoxon analyses) and the Alzheimer's Disease Cooperative Society Activities of Daily Living scale (according to Wilcoxon analyses).
2. I am not sure that the odds ratio calculations of adverse events (page 9) are correct because, for example, Dr Birks reported an odds ratio in favour of donepezil for weight loss during the maintenance phase of treatment, whereas percentages of patients experiencing weight loss (as an adverse event) during that phase of the study were actually higher in the donepezil group than the rivastigmine group.
3. I am not sure how the statement that 'the analysis of serious adverse events, using the odds ratios, show that there was a significant difference…' can be correct. I believe the odds ratio for serious adverse events in this study would be 1.03 with a confidence interval of 0.79 to 1.35, which is not only far from significant, but also suggests a numerically greater incidence of these events with donepezil than with rivastigmine.
I appreciate that this is a complex topic and that Dr Birks has done well to assimilate a lot of information so succinctly, but I would appreciate it if at least the three issues notes above in relation to my own publication could be investigated further, and corrected if necessary and possible.
Thank you for your consideration.
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
1. Dr Bullock correctly reports the p‐values reported in his paper from the comparison between donepezil and rivastigmine. When I enter the data as reported in Table 3 of his paper I get a non‐significant result. If the published means and standard deviations are from an analysis of variance then I should get the same result with a t‐test which is equivalent to an F test on one degree of freedom. We need to discover what is causing the discrepancy by studying the results, by which I mean the tables of sum of squares. I would appreciate being allowed access to the more detailed results. Many people did not complete this trial, 48% in the rivastigmine arm. Therefore it would be very helpful if we could also report the analyses of the completers, to compare with the ITT‐LOCF population to assess whether there is bias caused by non‐completion and the imputation of results using LOCF.
2. Dr Bullock is correct. I have reported a significant difference between donepezil and rivastigmine for the number of adverse events of weight loss in favour of donepezil for the maintenance phase when there is no significant difference between donepezil and rivastigmine. There is a significant difference in favour of donepezil for the titration phase and this had been reported in error. I apologise for the error which will be corrected in the next version.
3. Dr Bullock has misread the report. I actually write 'The analysis of serious adverse events, using the odds ratio, show that there was no significant difference between the donepezil group compared with the rivastigmine group'.
Contributors
Dr Roger Bullock, Chief Investigator of DON vs RIV/Bullock Mrs Jacqueline Birks, contact author Roy Jones, CDCIG Comments and Criticisms Editor Kate Hicks, CDCIG Review Coordinator
Tam et al: Methodology
Summary
Comment: Written by Tina SC Tam, Brian KC Wong, Aaron M Tejani
Dear Dr. Birks,
We read with great interest your systematic review of cholinesterase inhibitors for Alzheimer’s disease. Although this systematic review has taken on the arduous task of ascertaining the highest level of available evidence, we suggest the review be withdrawn as several of the MECIR standards for conducting and reporting of new Cochrane Intervention Reviews that are considered ‘mandatory’ or ‘highly desirable’ have not been met. (1)
A critical problem with this review is the lack of a secondary author. According to the MECIR standards C39, C45, C46, and C53, it is mandatory to use at least two people working independently to determine included studies, to extract study characteristics and outcome data, and to assess risk of bias. (1) Another issue we’ve identified in the review is a lack of discussion of the clinical importance of a ‐2.7 point change in ADAS‐Cog score. (2) It would be helpful to readers to discuss the minimal clinically important difference (MCID) related to scales such as ADAS‐Cog, as a ‐2.7 point change, although statistically significant, may be not be clinically meaningful to patients. (3) We are also concerned with the lack of exploration of the sources of statistical heterogeneity in the analyses across multiple outcomes. The Cochrane handbook lists a number of options available to address statistical heterogeneity and MECIR standard C70 requires any statistical heterogeneity to be taken into account when interpreting the results. (1,2) You do not discuss the statistical heterogeneity present across the included studies when drawing conclusions about the positive effect of cholinesterase inhibitors on cognitive function as measured by changes in the ADAS‐Cog score (P = 0.004; I2 = 62%).
In addition, we would like to focus specifically on two main issues in this letter: the use of the last observation carried forward (LOCF) method in the included trials and the lack of a risk of bias assessment for any of the included trials. Intention to treat (ITT) analyses were employed in the trials, with any missing information being accounted for by the LOCF method. We argue that LOCF is an inappropriate method to estimate the missing data due to the progressive nature of Alzheimer’s Disease. It has been well studied that disease progression is an established feature of Alzheimer’s Disease related to dementia. (4) By using participants’ responses at the dropout point of the trial, we are ignoring the deterioration of the disease status from the point of last observation to the end of the trial. (5) As the author has pointed out, the dropout rate is 29% for the treatment group and 18% for the placebo group, with the discrepancy being attributed to adverse events. Thus, a larger proportion of study participants from the treatment group would have results reported from an earlier stage of their disease, thereby skewing the treatment effect in favour of the intervention arm or even contributing to false‐positive results. (5) In other words, patients in the cholinesterase inhibitor arm who dropped out while having good control of their condition will not have their disease progression captured, unlike participants randomized to the placebo group where a larger proportion remained to the end of the study. (5) We believe it is paramount for readers to understand this principle and that a discussion to caution readers in interpreting the results is warranted. We would state in the discussion that notwithstanding the findings of improvement in cognitive function, the treatment effect could be exaggerated given the ITT‐LOCF bias and given the small magnitude of the observed effect, it is possible that cholinesterase inhibitors may not improve, or may even worsen, cognitive function.
We hereby suggest a few ways to improve the quality of the review and interpretation of the results. Firstly, the handbook suggests seeking statistical advice for dealing with data not missing at random. (2) Second, the discussion should state clearly that the cognitive function analyses suffers from LOCF bias as mentioned earlier and the results should be viewed with caution. Third, when the review is updated, the section on incomplete outcome data for trials that use LOCF should be properly assessed and will be rated with a high risk of bias.
Our second major concern with the review is the lack of a risk of bias assessment for any of the included trials. In the Cochrane Handbook for Systematic Reviews of Interventions, it states that “the extent to which a Cochrane review can draw conclusions about the effects of an intervention depends on whether the data and results from the included studies are valid.” (2) A meta‐analysis of invalid studies may produce misleading results. Thus, as suggested by the handbook, “the evaluation of the validity of the included studies is therefore an essential component of a Cochrane review, and should influence the analysis, interpretation and conclusions of the review.” You fail to include an adequate assessment of the risk of bias in the included studies and does not reflect this lack of assessment in the abstract, conclusions, or discussion of the review. Given the significance of this review and its potential to impact care and policy makers (cited by Google Scholar over 1200 times), it is important a thorough risk of bias assessment is conducted to examine the internal validity of the review. We would emphasize that the domain of incomplete outcome data contributing to attrition bias be looked at with special attention based on our discussion of LOCF on cognitive function assessments above.
The Cochrane Collaboration recommends a domain‐based evaluation to assess risk of bias in each included study, where a judgement is made for each entry in a ‘Risk of bias’ table. Each entry is assessed as ‘low risk’, ‘high risk’, or ‘unclear risk’. We conducted a risk of bias assessment on GAL‐USA‐10 Tariot and found an unclear risk in selection, performance, and reporting bias. No information was provided regarding allocation concealment. The blinding of participants was not described in detail and a protocol was not registered to determine possibilities of selective outcome reporting. We also found an unclear risk of detection and attrition bias due to the unclear double‐blinding of outcome assessors that was reported and unaccounted patients in their ITT analysis. Lack of or unclear double‐blinding can be associated with an average of 13% exaggeration of intervention effects. (6) Due to the difference in dropout rates in the placebo group and galantamine 24 mg/day treatment arm (16% vs 22%), outcome assessors may have been able to identify specific patients in each treatment arm. (7) Additionally, there was no mention of the method used to blind outcome assessors. Because the ADAS‐cog assessment contains components of subjectivity, unblinding of outcome assessors can increase or decrease the effect of galantamine. In GAL‐USA‐10, 20 participants were not accounted for in the ITT analysis in the 24 mg/day treatment group (7), leading to an unclear risk of incomplete outcome data reporting and possibly contributing to attrition bias. We did not have the chance to contact the authors of the GAL‐USA‐10 trial regarding the missing information and more work will be needed to conduct a proper bias assessment. However, given that our immediate categorization for one of the trials is unclear for most risk of bias domains, it makes us extremely concerned about the internal validity of the other included studies in the review.
You have presented 17 adverse events where the rates differ significantly between groups receiving treatment and placebo. However, even though death is listed under “types of outcome measures” as one of the primary outcomes of interest, no mortality figures were presented in the result section of the review. This leads us to wonder if the exclusion of mortality data can contribute to bias in selective outcome reporting. Furthermore, it has been reported that there is a trend towards increase in serious adverse events (RR of 1.22) with donepezil in a meta‐analysis examining data from 9 randomized controlled trials. (8) Because serious adverse events are not included as outcomes in this review and information related to death is not reported, the safety of cholinesterase inhibitors remains unclear at this point and the conclusion should be drawn as such.
You concluded that “treatment with donepezil, galantamine, or rivastigmine at recommended dosage produced improvements in cognitive function, on average ‐2.7 points (95% CI ‐3.0 to ‐2.3), in the midrange of the 70 point ADAS‐Cog Scale.” Based on the issues identified above, we are uncertain if these are valid conclusions. Until a comprehensive risk of bias assessment and a discussion on the implications of LOCF analysis on ADAS‐Cog scores are addressed, it is our opinion that the review be withdrawn and only republished if all the above issues are properly addressed.
References:
1. Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Standards for the conduct of new Cochrane Intervention Reviews 2012 V2.3. Methodological Expectations of Cochrane Intervention Reviews: Standards for the conduct and reporting of new Cochrane Intervention Reviews 2012. Version 2. 2013: Cochrane, London.
2. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [cited 2015 Aug 19] Available from www.cochrane‐handbook.org.
3. McGlothlin AE, Lewis RJ. Minimal clinically important difference, defining what really matters to patients. JAMA‐J Am Med Assoc. 2014 Oct;312(13):1342‐43.
4. Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, Weiner MF, Farlow MR, Sano M, Grundman M, Thal LJ. A Randomized Controlled Trial of Prednisone in Alzheimer’s Disease. Neurology. 2000 Feb;54(3):588‐593.
5. Molnar FJ, Hutton B, Fergusson D. Does Analysis Using “Last Observation Carried Forward” Introduce Bias in Dementia Research? Can Med Assoc J. 2008 Oct;179(8):751‐753.
6. Savovic J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med. 2012 Sept;157:429‐38.
7. Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C, the Galantamine USA Study Group. A 5‐month, randomized, placebo‐controlled trial of galantamine in AD. Neurology. 2000 Jun;54(12):2269‐76.
8. Therapeutics Initiative. Drugs for Alzheimer’s Disease. Therapeutics Letter [Internet]. 2005 Apr [cited 2015 Aug 19];56. Available from: http://www.ti.ubc.ca/newsletter/drugs‐alzheimers‐disease
Reply
We thank Tina SC Tam, Brian KC Wong, Aaron M Tejani for their comment and interest on our review.
This review was published in 2005 and complies with the standards set out by Cochrane at that time. The current requirements demanded of a Cochrane review are not the same. Currently there is a requirement for a risk of bias assessment, but this was not required in 2005. We accept that the review should be updated. An updated review would comply with current standards.
This is a summary review of the reviews of the cholinesterase inhibitors. The review states that the purpose is to provide a summary of the evidence, and not to repeat the vast amounts of information in the individual reviews. Cochrane now provides a template and instructions for carrying out a summary review, this is a recognised format, but this was not the case in 2005.
Each of the three reviews of the individual cholinesterase inhibitors was carried out by a team of authors.
We agree that missing data in the trials of the cholinesterase inhibitors is a cause for concern. The use of the last observation carried forward (LOCF) as a method of imputation for missing data at endpoint is discussed in great detail in the Cochrane review of rivastigmine. The review also includes analyses of several populations of patients including intention to treat with LOCF, the population of patients who complete the trial, and the population that included retrieving patients who had dropped out but were still assessed at endpoint. The investigation of the different populations was extensive in order to estimate any bias that may result from the different methods of dealing with missing data. It was not necessary to repeat this research in the summary review.
Regarding deaths and serious adverse events, the results appear in the individual reviews. There were no statistically significant differences between a cholinesterase inhibitor and placebo for these outcomes. These outcomes can be included in the updated summary review.
Contributors
Tina SC Tam
Brian KC Wong
Aaron M Tejani
Jacqueline Birks, Contact Author
Mario Fioravanti, Feedback Editor
Gøtzsche, P: Miscellaneous
Summary
Comment: written by Peter Gøtzsche
This review concludes that, “The three cholinesterase inhibitors are efficacious for mild to moderate Alzheimer’s disease.” I believe these drugs don’t work. The improvement in cognitive function was 2.7 points, in the midrange of a 70‐point scale. This is less than the 4 points the FDA considers the minimally relevant clinical change (1). We can also compare with the smallest effect that can be perceived on the Hamilton scale for depression, which is 5‐6 (2), although the maximum on this scale is only 52.
The placebo controlled trials have not been effectively blinded, as cholinesterase inhibitors have conspicuous side effect. This lack of blinding can in itself easily have caused the very minor effect that was noted in the trials (3).
A long‐term trial of 565 patients with mild to moderate Alzheimer’s disease that compared donepezil with placebo found no meaningful effects whatsoever, and the authors concluded that donepezil isn’t cost‐effective, with benefits below minimally relevant thresholds (4). In contrast to other trials, it was publicly funded. This trial was excluded from the Cochrane review, for no good reason, as far as I can see. The outcomes after three years were similar on drug and placebo with respect to institutionalisation, progression of disability, and behavioural and psychological symptoms.
The author of the Cochrane review wrote that “donepezil appears to have no serious or common side effects.” This sentence is highly misleading. The harms are both common and serious, and she documents in her review that 29% of the patients left the drug group on account of adverse events, as compared to only 18% in the placebo groups. The most common side effects of donepezil are nausea, diarrhoea, not sleeping well, vomiting, muscle cramps, feeling tired, and not wanting to eat. I believe this is not what we would want for an old person who might already have problems with not sleeping well, feeling tired, and not wanting to eat.
The list of frequent side effects in Pfizer’s product information for Aricept (donepezil) is very long (5). Hypotension and syncope occurs in more than 1% and when old people fall, there is a considerable risk that they break their hip and die. A large Canadian cohort study showed that people who took anti‐dementia drugs had almost a doubled risk of hospitalisation for syncope compared to demented people who didn’t take these drugs, and they had more pacemakers inserted and more hip fractures (6). Most astonishingly, more than half the patients who were admitted to hospital for bradycardia were retreated with the drug.
There are many good reasons not to use anti‐dementia drugs.
References:
1. Molnar FJ, Man‐Son‐Hing M, Fergusson D. Systematic review of measures of clinical significance employed in randomized controlled trials of drugs for de¬mentia. J Am Geriatr Soc 2009;57:536‐46.
2. Leucht S, Fennema H, Engel R, et al. What does the HAMD mean? J Affect Disord 2013;148:243‐8.
3. Gøtzsche PC. Deadly psychiatry and organised denial. Copenhagen: People’s Press; 2015.
4. Courtney C, Farrell D, Gray R, et al. Long‐term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double‐blind trial. Lancet 2004;363:2105‐15.
ARICEPT ® (donepezil hydrochloride) tablets. http://labeling.pfizer.com/ShowLabeling.aspx?id=510.
5. Syncope with cholinesterase inhibitors. Rev Prescrire 2011;31:434.
Reply
We thank you for your comment and your interest in our review.
You believe that the cholinesterase inhibitors do not work. This depends of course on the definition of ‘work’. We actually qualify the statement of the effect derived from the meta analysis, we describe the treatment effect as small.
The cholinesterase inhibitors do have side effects, most noticeably of nausea (31% on ChEI versus 9% on placebo). It is not possible to quantify any impact that unblinding due to side effects may have had on the results. We agree that not assessing how effectively the trials were blinded is a methodological limitation of the trials.
We have discussed the excluded trial to which you refer, AD2000, and the reason for the exclusion from this review in depth in the review. Although you state that this trial was excluded for no good reason it is difficult to answer your criticism without further detail as to why our reasons are not good.
We agree that the sentence you quote about common and serious adverse effects of donepezil (in a paragraph about titration schedules) is misleading and we are deleting it from the review. We agree that our data show that there are significantly more adverse events among patients taking cholinesterase inhibitors than among patients taking placebo and this was clearly stated at key points in the conclusions and abstract of the review. While preparing this response, we have noted an error in the abstract: the total numbers leaving both groups (29% in drug group, 18% in placebo group) were erroneously labelled as the numbers leaving due to adverse events. This has also now been corrected. We found no evidence of a difference in the number of fractures between the two groups, there were 2 % in both group, nor in the numbers suffering an event of syncope, 3% in the drug group and 2% in the placebo group. There is no evidence of a difference in serious adverse events between the treatment and placebo arms in the trials, but we accept that RCTs alone may not be adequate to identify less common adverse effects.
We believe that our conclusions of small effects in favour of the treatment group and a higher rate of adverse effects in the treatment group are supported by the evidence in the review.
An update of the review, conducted according to current Cochrane methodological guidance, is in preparation.
Contributors
Peter Gøtzsche
Jacqueline Birks, Contact Author
Jenny McCleery, Co‐ordinating Editor
Rupert McShane, Co‐ordinating Editor
Mario Fioravanti, Feedback Editor
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2016 | Amended | This review is awaiting update with a new protocol |
History
Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 23 November 2015 | Feedback has been incorporated | Two sets of feedback incorporated |
| 12 April 2012 | Amended | Additional table linked to text. |
| 23 October 2008 | Amended | Converted to new review format. |
| 22 August 2006 | Feedback has been incorporated | Reply to feedback added |
| 3 July 2006 | Feedback has been incorporated | Feedback added |
| 14 September 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
I gratefully acknowledge the contribution of Dymphna Hermans for invaluable assistance in performing the search of electronic databases. Novartis (Pharma UK) has provided access to all the company results from the four phase III studies. Novartis (Pharma UK) and Novartis (Hellas) have provided abstracts of conference presentations and lists and descriptions of trials.
Data and analyses
Comparison 1. Cholinesterase inhibitor (optimum dose) vs placebo.
1.1. Analysis.
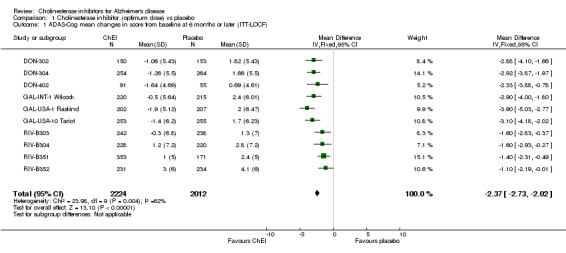
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 1 ADAS‐Cog mean changes in score from baseline at 6 months or later (ITT‐LOCF).
1.2. Analysis.
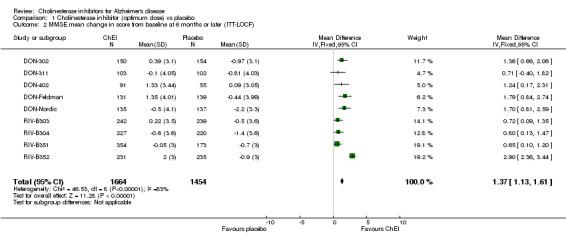
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 2 MMSE mean change in score from baseline at 6 months or later (ITT‐LOCF).
1.3. Analysis.
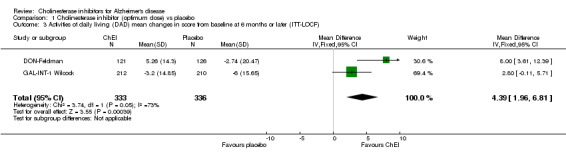
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 3 Activities of daily living (DAD) mean changes in score from baseline at 6 months or later (ITT‐LOCF).
1.4. Analysis.
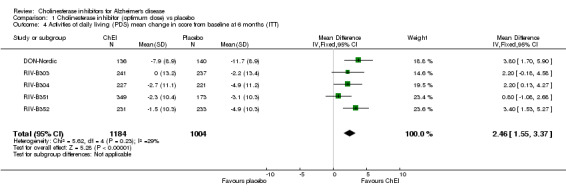
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 4 Activities of daily living (PDS) mean change in score from baseline at 6 months (ITT).
1.5. Analysis.
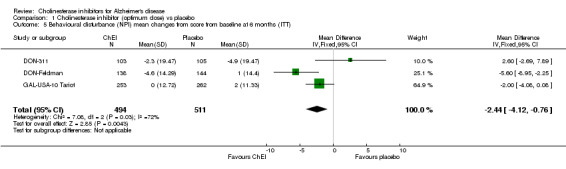
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 5 Behavioural disturbance (NPI) mean changes from score from baseline at 6 months (ITT).
1.6. Analysis.
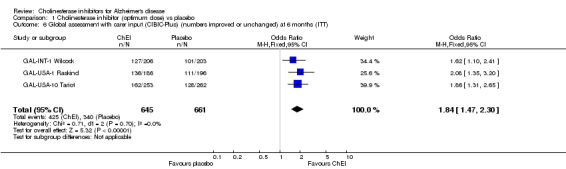
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 6 Global assessment with carer input (CIBIC‐Plus) (numbers improved or unchanged) at 6 months (ITT).
1.7. Analysis.
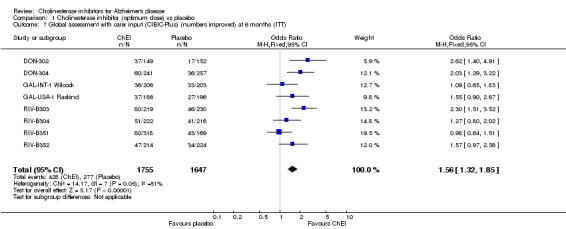
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 7 Global assessment with carer input (CIBIC‐Plus) (numbers improved) at 6 months (ITT).
1.8. Analysis.
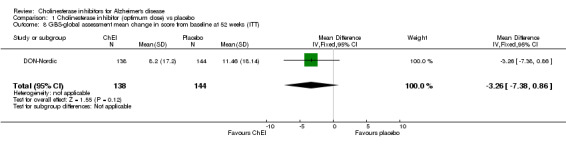
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 8 GBS‐global assessment mean change in score from baseline at 52 weeks (ITT).
1.9. Analysis.
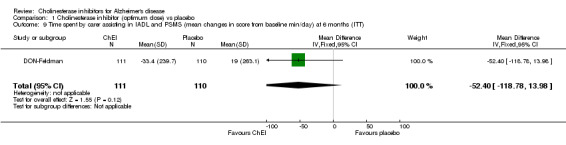
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 9 Time spent by carer assisting in IADL and PSMS (mean changes in score from baseline min/day) at 6 months (ITT).
1.10. Analysis.
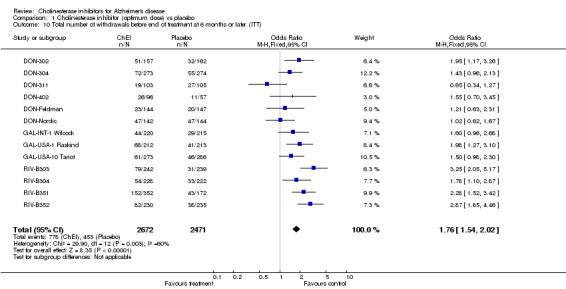
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 10 Total number of withdrawals before end of treatment at 6 months or later (ITT).
1.11. Analysis.
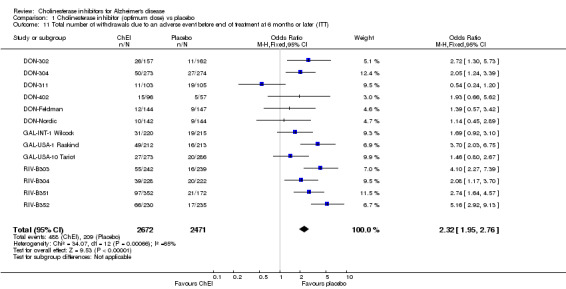
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 11 Total number of withdrawals due to an adverse event before end of treatment at 6 months or later (ITT).
1.12. Analysis.
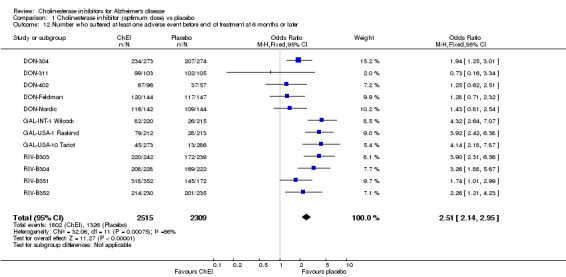
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 12 Number who suffered at least one adverse event before end of treatment at 6 months or later.
1.13. Analysis.
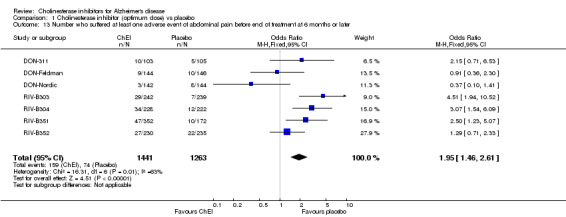
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 13 Number who suffered at least one adverse event of abdominal pain before end of treatment at 6 months or later.
1.14. Analysis.
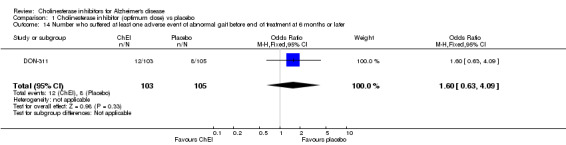
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 14 Number who suffered at least one adverse event of abnormal gait before end of treatment at 6 months or later.
1.15. Analysis.
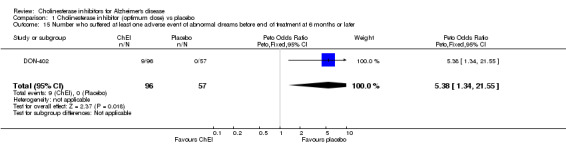
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 15 Number who suffered at least one adverse event of abnormal dreams before end of treatment at 6 months or later.
1.16. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 16 Number who suffered at least one adverse event of accidental injury before end of treatment at 6 monthsorlater.
1.17. Analysis.
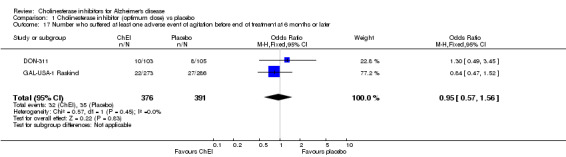
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 17 Number who suffered at least one adverse event of agitation before end of treatment at 6 months or later.
1.18. Analysis.
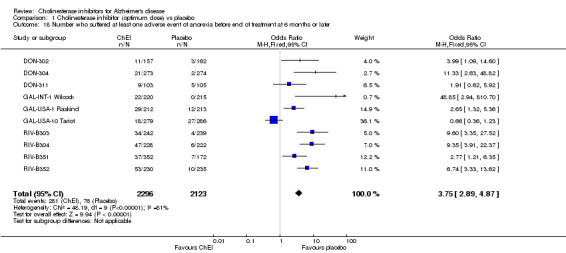
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 18 Number who suffered at least one adverse event of anorexia before end of treatment at 6 months or later.
1.19. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 19 Number who suffered at least one adverse event of anxiety before end of treatment at 6 months or later.
1.20. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 20 Number who suffered at least one adverse event of arthralgia before end of treatment at 6 months or later.
1.21. Analysis.
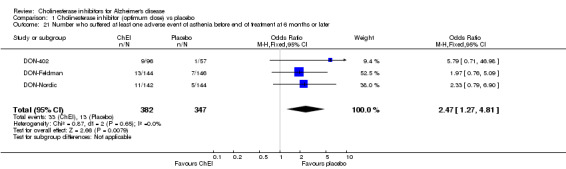
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 21 Number who suffered at least one adverse event of asthenia before end of treatment at 6 months or later.
1.22. Analysis.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 22 Number who suffered at least one adverse event of back pain before end of treatment at 6 months or later.
1.23. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 23 Number who suffered at least one adverse event of confusion before end of treatment at 6 months or later.
1.24. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 24 Number who suffered at least one adverse event of conjunctivitis before end of treatment at 6 months or later.
1.25. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 25 Number who suffered at least one adverse event of constipation before end of treatment at 6 months or later.
1.26. Analysis.
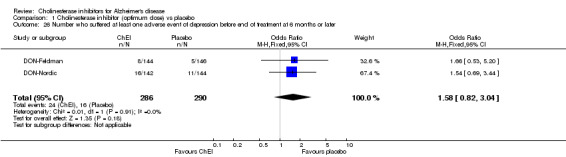
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 26 Number who suffered at least one adverse event of depression before end of treatment at 6 months or later.
1.27. Analysis.
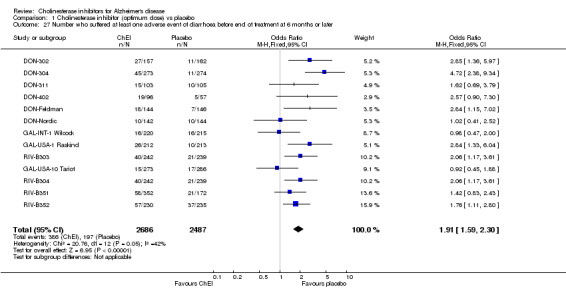
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 27 Number who suffered at least one adverse event of diarrhoea before end of treatment at 6 months or later.
1.28. Analysis.
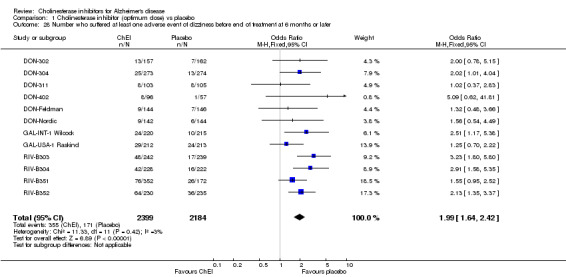
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 28 Number who suffered at least one adverse event of dizziness before end of treatment at 6 months or later.
1.29. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 29 Number who suffered at least one adverse event of ecchymosis before end of treatment at 6 months or later.
1.30. Analysis.
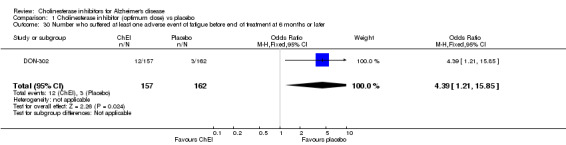
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 30 Number who suffered at least one adverse event of fatigue before end of treatment at 6 months or later.
1.31. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 31 Number who suffered at least one adverse event of fever before end of treatment at 6 months or later.
1.32. Analysis.
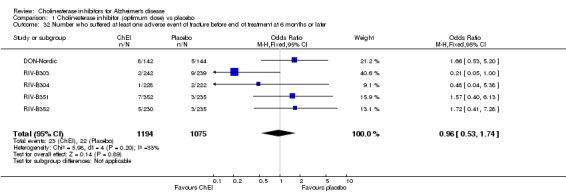
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 32 Number who suffered at least one adverse event of fracture before end of treatment at 6 months or later.
1.33. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 33 Number who suffered at least one adverse event of haemorrhage before end of treatment at 6 months or later.
1.34. Analysis.
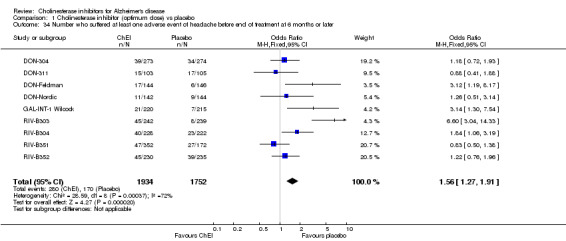
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 34 Number who suffered at least one adverse event of headache before end of treatment at 6 months or later.
1.35. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 35 Number who suffered at least one adverse event of hostility before end of treatment at 6 months or later.
1.36. Analysis.

Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 36 Number who suffered at least one adverse event of increased cough before end of treatment at 6 months or later.
1.37. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 37 Number who suffered at least one adverse event of infection before end of treatment at 6 months or later.
1.38. Analysis.
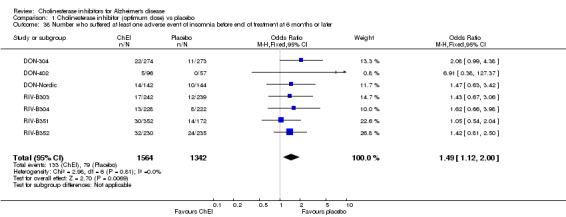
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 38 Number who suffered at least one adverse event of insomnia before end of treatment at 6 months or later.
1.39. Analysis.
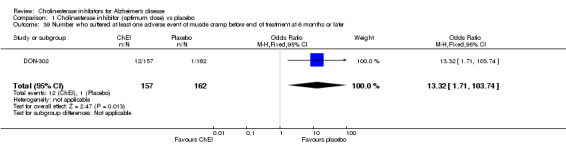
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 39 Number who suffered at least one adverse event of muscle cramp before end of treatment at 6 months or later.
1.40. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 40 Number who suffered at least one adverse event of myasthenia before end of treatment at 6 months or later.
1.41. Analysis.
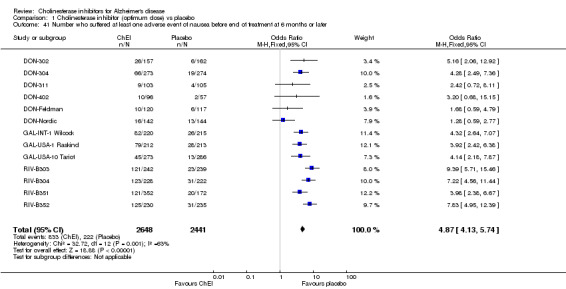
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 41 Number who suffered at least one adverse event of nausea before end of treatment at 6 months or later.
1.42. Analysis.
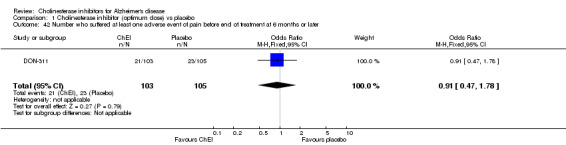
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 42 Number who suffered at least one adverse event of pain before end of treatment at 6 months or later.
1.43. Analysis.
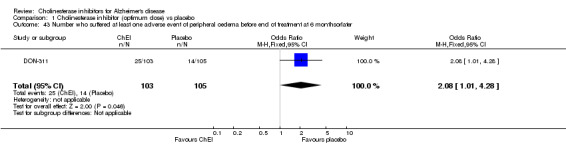
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 43 Number who suffered at least one adverse event of peripheral oedema before end of treatment at 6 monthsorlater.
1.44. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 44 Number who suffered at least one adverse event of a rash before end of treatment at 6 months or later.
1.45. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 45 Number who suffered at least one adverse event of a respiratory tract infection before end of treatment at 6 m.
1.46. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 46 Number who suffered at least one adverse event of rhinitis before end of treatment at 6 months or later.
1.47. Analysis.
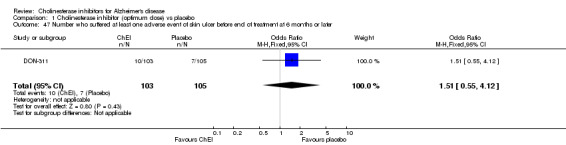
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 47 Number who suffered at least one adverse event of skin ulcer before end of treatment at 6 months or later.
1.48. Analysis.
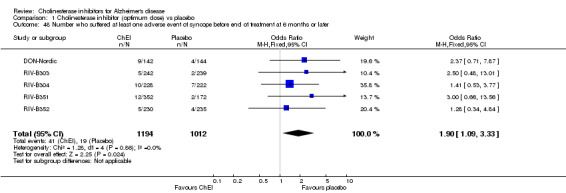
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 48 Number who suffered at least one adverse event of syncope before end of treatment at 6 months or later.
1.49. Analysis.
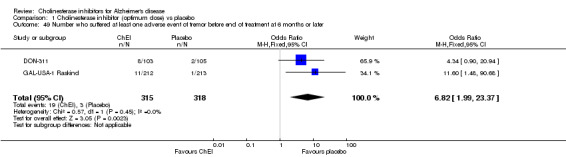
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 49 Number who suffered at least one adverse event of tremor before end of treatment at 6 months or later.
1.50. Analysis.
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 50 Number who suffered at least one adverse event of urinary tract infection before end of treatment at 6 month.
1.51. Analysis.
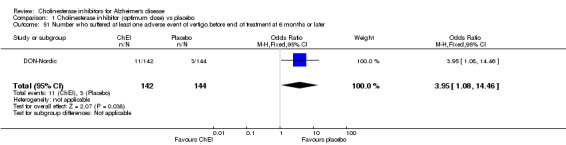
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 51 Number who suffered at least one adverse event of vertigo before end of treatment at 6 months or later.
1.52. Analysis.
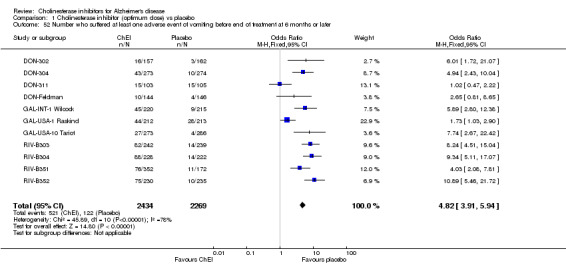
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 52 Number who suffered at least one adverse event of vomiting before end of treatment at 6 months or later.
1.53. Analysis.
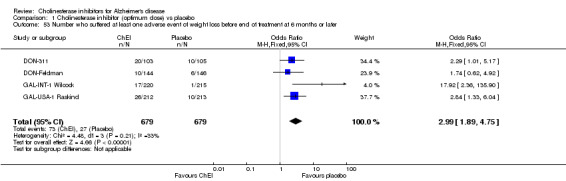
Comparison 1 Cholinesterase inhibitor (optimum dose) vs placebo, Outcome 53 Number who suffered at least one adverse event of weight loss before end of treatment at 6 months or later.
Comparison 2. Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE mean change from baseline (ITT‐LOCF) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 24 months | 1 | 955 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.33, 0.33] |
| 2 Activities of daily living (ADCS‐ADL) (ITT‐LOCF) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 24 months | 1 | 929 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐4.58, 0.42] |
| 3 Behavioural disturbance (NPI‐10) (ITT‐LOCF) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 24 months | 1 | 955 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐1.68, 2.76] |
| 4 Cognitive function (SIB) (ITT‐LOCF) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 24 months | 1 | 954 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐3.66, 2.44] |
| 5 Global Deterioration Scale (GDS) (ITT‐LOCF) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 24 months | 1 | 954 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.00, 0.22] |
| 6 Total number of patients who withdrew before end of treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 104 weeks | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.83] |
| 7 Total number of patients who withdrew before end of treatment due to an adverse event | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 104 weeks | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 8 Total number of patients who suffered an adverse event of nausea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.50] |
| 8.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.23, 0.63] |
| 9 Total number of patients who suffered an adverse event of vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.10, 0.24] |
| 9.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.15, 0.43] |
| 10 Total number of patients who suffered an adverse event of agitation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.93, 2.30] |
| 10.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.79, 2.00] |
| 11 Total number of patients who suffered an adverse event of anorexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.72] |
| 11.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 12 Total number of patients who suffered an adverse event of diarrhoea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.30] |
| 12.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.60, 1.77] |
| 13 Total number of patients who suffered an adverse event of weight loss | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.61] |
| 13.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.71] |
| 14 Total number of patients who suffered an adverse event of headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.48] |
| 14.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.81] |
| 15 Total number of patients who suffered an adverse event of a fall | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 15.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.75, 1.94] |
| 16 Total number of patients who suffered an adverse event of hypertension | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.81] |
| 16.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.44] |
| 17 Total number of patients who suffered an adverse event of depression | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.11] |
| 17.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.30] |
| 18 Total number of patients who suffered an adverse event of a urinary tract infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.67, 3.96] |
| 18.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.70, 2.42] |
| 19 Total number of patients who suffered an adverse event of aggression | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 By 16 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.60, 4.09] |
| 19.2 Between 16 and 104 weeks of treatment | 1 | 857 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.64, 2.18] |
| 20 Total number of patients who suffered a serious adverse event | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 At 104 weeks of treatment | 1 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 21 Total number of patients who died before end of treatment | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 21.1 At 104 weeks | 1 | 994 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.78, 2.22] |
2.1. Analysis.
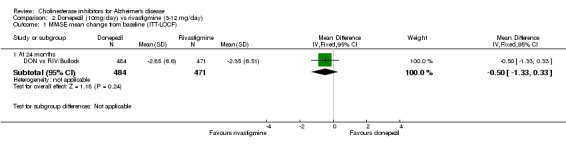
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 1 MMSE mean change from baseline (ITT‐LOCF).
2.2. Analysis.
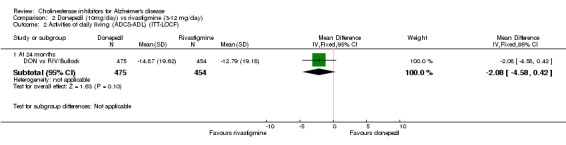
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 2 Activities of daily living (ADCS‐ADL) (ITT‐LOCF).
2.3. Analysis.
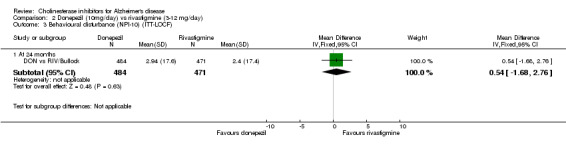
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 3 Behavioural disturbance (NPI‐10) (ITT‐LOCF).
2.4. Analysis.
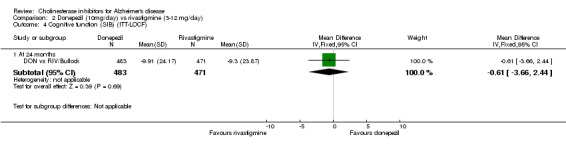
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 4 Cognitive function (SIB) (ITT‐LOCF).
2.5. Analysis.
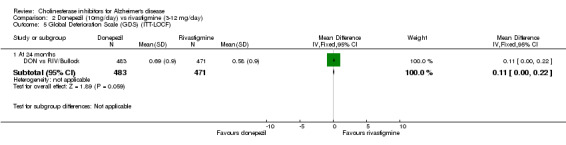
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 5 Global Deterioration Scale (GDS) (ITT‐LOCF).
2.6. Analysis.
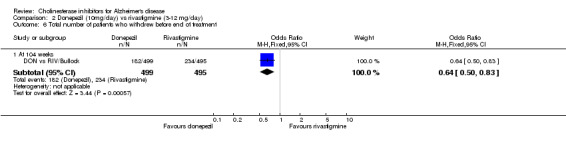
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 6 Total number of patients who withdrew before end of treatment.
2.7. Analysis.
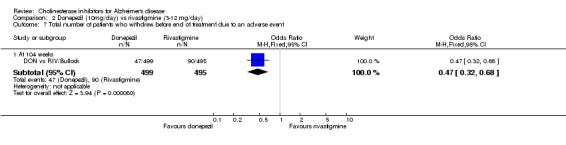
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 7 Total number of patients who withdrew before end of treatment due to an adverse event.
2.8. Analysis.
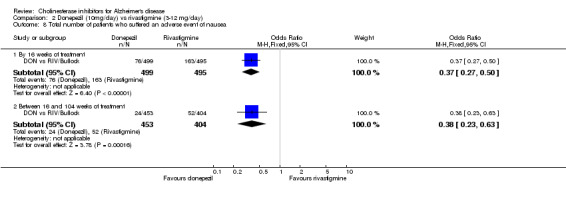
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 8 Total number of patients who suffered an adverse event of nausea.
2.9. Analysis.
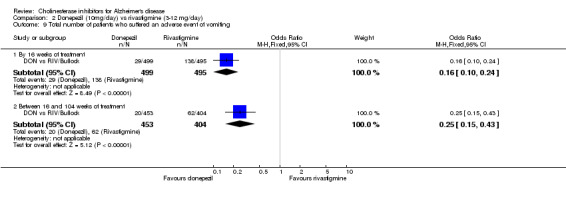
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 9 Total number of patients who suffered an adverse event of vomiting.
2.10. Analysis.
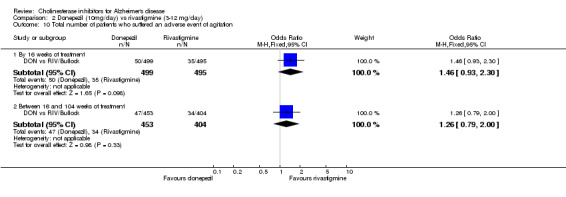
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 10 Total number of patients who suffered an adverse event of agitation.
2.11. Analysis.
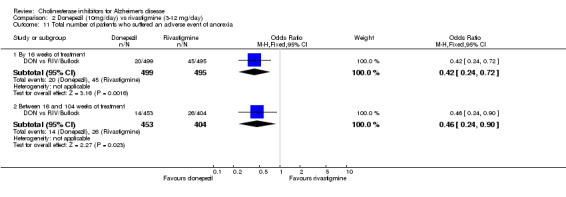
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 11 Total number of patients who suffered an adverse event of anorexia.
2.12. Analysis.
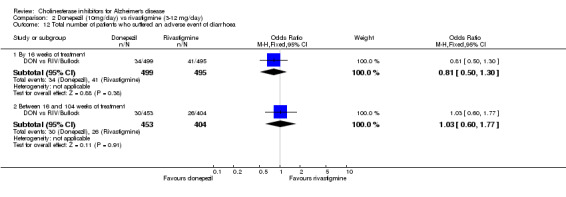
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 12 Total number of patients who suffered an adverse event of diarrhoea.
2.13. Analysis.
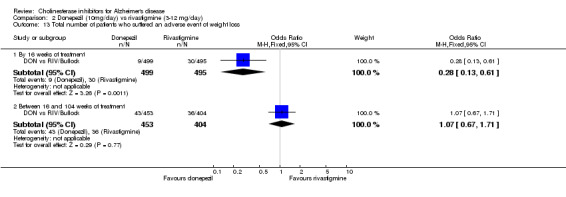
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 13 Total number of patients who suffered an adverse event of weight loss.
2.14. Analysis.
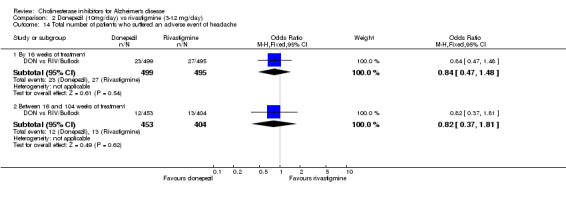
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 14 Total number of patients who suffered an adverse event of headache.
2.15. Analysis.
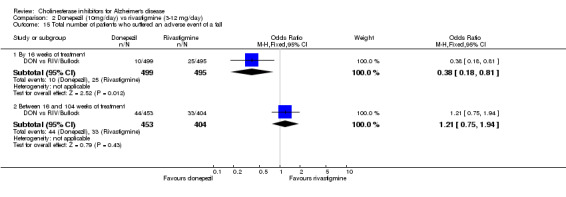
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 15 Total number of patients who suffered an adverse event of a fall.
2.16. Analysis.
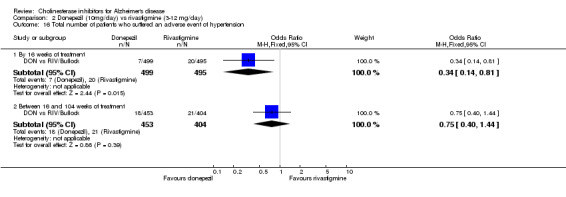
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 16 Total number of patients who suffered an adverse event of hypertension.
2.17. Analysis.
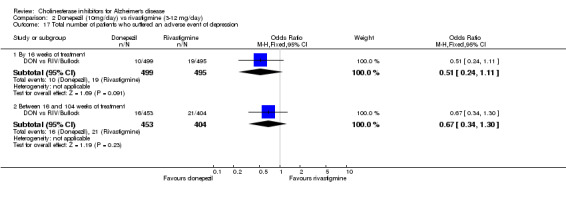
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 17 Total number of patients who suffered an adverse event of depression.
2.18. Analysis.
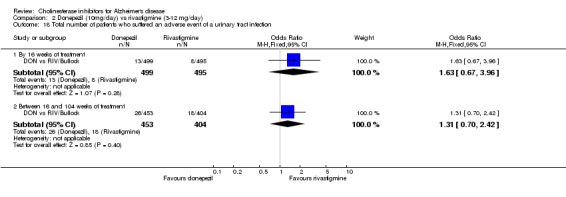
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 18 Total number of patients who suffered an adverse event of a urinary tract infection.
2.19. Analysis.
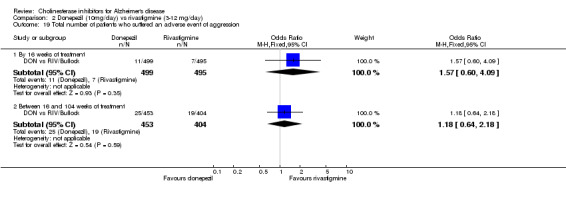
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 19 Total number of patients who suffered an adverse event of aggression.
2.20. Analysis.
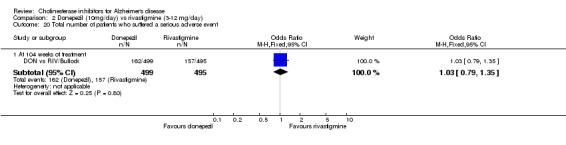
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 20 Total number of patients who suffered a serious adverse event.
2.21. Analysis.
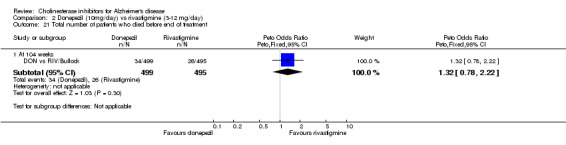
Comparison 2 Donepezil (10mg/day) vs rivastigmine (3‐12 mg/day), Outcome 21 Total number of patients who died before end of treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DON vs RIV/Bullock.
| Methods | 104 week, randomized, double‐blind, parallel group | |
| Participants | Country: Australia, Canada, France, Germany, Italy, Spain, UK 94 centres, 998 participants with mild to moderate probable Alzheimer's disease (DSM‐IV and NINCDS‐ADRDA criteria), mean MMSE 15.1(3.0), mean age 75.9 (6.7) Inclusion criteria: MMSE 10‐20 Exclusion criteria: other neurodegenerative disease, any advanced, unstable, severe disease, major depressive episode, seizure disorder, peptic ulceration, acute or severe asthma or cardiovascular disease, cerebrovascular disease, certain other medication | |
| Interventions | 1. donepezil (10mg/day) 2. rivastigmine (maximum 12mg/day in two doses) | |
| Outcomes | SIB GDS ADCS‐ADL MMSE NPI | |
| Notes | ||
DON‐302.
| Methods | 24‐week, randomized, double‐blind, parallel‐group, placebo‐controlled study ‐ a computer randomization schedule was used | |
| Participants | Country: USA Multi‐centre (20 sites) 473 participants, aged 51‐94 years, 180 men and 293 women. Selection criteria: Eligible patients had a diagnosis of uncomplicated AD, according to the NINCDS‐ADRDA criteria and DSM‐III‐R categories 290.00 and 290.10, with no clinical or laboratory evidence of a cause other than AD for their dementia. MMSE between 10 and 26, and CDR=1 (mild dementia) or 2 (moderate dementia). All participants had a reliable caregiver. Exclusion criteria: evidence of insulin dependent diabetes mellitus or other endocrine disorder. Asthma, obstructive pulmonary disease or clinically significant uncontrolled gastrointestinal hepatic or cardiovascular diseases. Patients known to be hypersensitive to ChE inhibitors or who had taken tacrine or other investigational medicines within 1 month of baseline were excluded. Concomitant medications such as anticholinergics, anticonvulsants, antidepressants and antipsychotics were not allowed. Drugs with CNS activity were prohibited or partially restricted. | |
| Interventions | 1. placebo 2. donepezil 5mg/day 3. donepezil 10mg/day | |
| Outcomes | Primary: ADAS‐Cog CIBIC plus (including caregiver information) secondary: MMSE QoL (patient rated) CDR‐SB (Clinical dementia scale, sum of boxes) | |
| Notes | The group on 10mg/d of donepezil was on a blinded forced titration scheme of 5mg/d for week 1, and 10mg/d for the remainder of the study. Measures of clinical outcome were assessed at baseline and at 6‐week intervals | |
DON‐304.
| Methods | 24‐week double‐blind, parallel‐group, placebo‐controlled, randomized study ‐ the randomization schedule was computer‐generated | |
| Participants | Country: Europe Multi‐centre 818 participants, 348 men and 470 women, with mild to moderately severe AD, mean age 71.7 (8.3), mean MMSE 20.2 (5.0) Country: Europe, South Africa, New Zealand, Australia and Canada Multiple centre (82 sites) Selection criteria: Eligible patients had a diagnosis of probable AD, according to the NINCDS‐ADRDA criteria and DSM‐III‐R categories 290.00 and 290.10, with no clinical or laboratory evidence of a cause other than AD for their dementia. MMSE between 10 and 26, and CDR=1 (mild dementia) or 2 (moderate dementia). All participants had a reliable caregiver. CT or MRI within 6 months of entry. Exclusion criteria: evidence of insulin dependent diabetes mellitus or other endocrine disorder. Asthma, obstructive pulmonary disease or clinically significant uncontrolled gastrointestinal hepatic or cardiovascular diseases. Patients known to be hypersensitive to ChE inhibitors or who had taken tacrine or other investigational medicines within 1 month of baseline were excluded. Concomitant medications such as anticholinergics, anticonvulsants, antidepressants and antipsychotics were not allowed. Drugs with CNS activity were prohibited or partially restricted. | |
| Interventions | 1. placebo 2. donepezil 5 mg/day 3. donepezil 10 mg/day | |
| Outcomes | ADAS‐Cog CIBIC‐Plus CDR‐SB (CDR sum of boxes) QoL IDDD (functional evaluations) | |
| Notes | Patients in the 10mg/day group received 5mg/day for the first week of treatment. 6‐week placebo washout phase followed the double‐blind phase. The group on 10mg/d of donepezil was on a blinded forced titration scheme of 5mg/d for week 1, and 10mg/d for the remainder of the study. Measures of clinical outcome were assessed at baseline and at 6‐week intervals | |
DON‐311.
| Methods | 24‐week double‐blind, parallel group, placebo controlled, randomized study | |
| Participants | Country: USA Multi‐centre (27 sites) study with 208 participants, 37 men and 171 women, with possible or probable AD, or AD with cerebrovascular disease (but not vascular dementia) Inclusion criteria: MMSE between 5 and 26 inclusive, residence in nursing home, at least one NPI symptom reported at a frequency of at least several times per week. Exclusion: most concomitant medications were allowed except those with significant cholinergic or anticholinergic effects | |
| Interventions | 1. placebo 2. donepezil 10 mg/day | |
| Outcomes | NPI‐NH MMSE CDR‐SB | |
| Notes | The group on donepezil took 5 mg/d for the first 4 weeks, followed by 10 mg/d for 20 weeks. | |
DON‐402.
| Methods | 24‐week double‐blind, parallel‐group, placebo‐controlled, randomized study | |
| Participants | Country: USA Multi‐centre (17 sites) 153 participants, 71 men and 82 women, with probable AD diagnosed within the last year (DSM‐IV and NINCDS‐ADRDA), mean age 74.0 years, mean MMSE=24.1. Inclusion criteria: modified Hachinski <=4, CDR 0.5 or 1.0, MMSE 21‐26, only mild impairment of ADL Exclusion criteria: if memory impairment was due to stroke or Parkinson's disease, previous treatment with cholinesterase inhibitor | |
| Interventions | 1. placebo 2. donepezil 5mg/day for 6 weeks followed by forced escation to 10mg/day thereafter | |
| Outcomes | mADAS‐Cog MMSE CDR‐sum of boxes CMBT Apathy scale patient rated global assessment | |
| Notes | patients unable to tolerate 10mg/day were dropped from the study | |
DON‐Feldman.
| Methods | 24‐week double‐blind, parallel‐group, placebo‐controlled, randomized study ‐ the randomization schedule was computer‐generated | |
| Participants | Country: Canada, Australia, France Multi‐centre (32 sites) 292 participants, aged 51‐94 years, 115 men and 177 women. Selection criteria: Eligible patients had a diagnosis of probable or possible AD, of moderate or severe severity, according to the NINCDS‐ADRDA criteria with no clinical or laboratory evidence of a cause other than AD for their dementia. MMSE between 5 and 17. All participants had a reliable caregiver. Exclusion criteria: delirium, depression or other illness that may interfere with the study. Other neurologic or psychiatric diagnosis. History of drug or alcohol misuse. Hypersensitivity to AChE inhibitors. Clinically obstructive airway disease, asthma, haematologic or oncologic disorder within last 2 years. B12 or folate deficiency, active gastrointestinal, renal, hepatic, endocrine or cardiovascular system disease. Most concomitant medications were allowed except those with notable cholinomimetic or anticholinergic effects. | |
| Interventions | 1. placebo 2. donepezil 10 mg/day | |
| Outcomes | CIBIC plus MMSE SIB DAD IADL PSMS NPI FRS CSS SF‐36 CAUST | |
| Notes | The group on donepezil took 5 mg/d for the first 4 weeks, followed by 10 mg/d for 20 weeks. The dose could be reduced to 5mg/day at any point if necessary | |
DON‐Nordic.
| Methods | 52‐week, double‐blind, parallel‐group, placebo‐controlled, randomized study | |
| Participants | Country: Northern Europe multi‐centre (28 sites), 286 participants, 102 men and 184 women, age range 49‐88 years, with mild to moderate possible or probable AD. Selection criteria: Diagnosis of AD with DSM‐IV and NINCDS‐ADRDA criteria, with 9< MMSE <27 | |
| Interventions | 1. placebo 2. donepezil 10 mg/day | |
| Outcomes | GBS MMSE PDS GDS IADL PSMS RUD | |
| Notes | The group on donepezil received 5mg/d for 28 days initially, and then 10mg/d according to the clinician's judgement for 1 year. | |
GAL‐INT‐1 Wilcock.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled, with 4‐week placebo run‐in Duration: 26 weeks | |
| Participants | Country: 8 European No. of Centers: 86 Diagnosis: At least 6 month history of progressive cognitive decline, Senile Dementia Alzheimer's Type defined by: NINCDS‐ADRDA. Inclusion: MMSE score of 11 to 24, ADAS‐cog score > 11; CT or MRI < 12 months previously with no evidence of multi‐infarct dementia or active cerebrovascular disease; responsible caregiver; discontinued from antidementia medications; discontinued where possible form anticholinergic or cholinomimetic agents. Exclusion: Past cholinesterase inhibitor use; uncontrolled hypertension, heart failure, type II diabetes mellitus, hypothyroidism; other neurodegenerative disorders; cardiovascular disease that would affect completion of the trial; clinically significant psychiatric, hepatic, renal, pulmonary, metabolic, endocrine conditions; urinary outflow obstruction; active peptic ulcer; history of epilepsy, significant substance abuse. Total No. of patients: 653 Sex: Not stated. Age: [placebo 72.7 (7.6)] [galantamine 24mg 71.9 (8.3)] [galantamine 32mg 72,1 (8.6)] | |
| Interventions | Route: oral Treatment: galantamine 12mg b.i.d. galantamine 16mg b.i.d. Treatment commenced at 4mg b.i.d. and was progressively increased weekly by 8mg/d to assigned maximum dose. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog ADCS‐CGIC Expanded ADAS‐cog DAD (Disability Assessment for Dementia) | |
| Notes | No. excluded after randomization: 128 No. not included in analysis: 128 | |
GAL‐USA‐1 Raskind.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled, with 4‐week placebo run‐in Duration: 26 weeks/ 6 months | |
| Participants | Country: USA No. of Centers: 33 Diagnosis: Senile Dementia Alzheimer's Type defined by: NINCDS‐ADRDA. Inclusion: MMSE score of 11 to 24 inclusive, ADAS‐cog score > 11; responsible caregiver; free for 30 days of medications indicated for dementia (3 months for cholinesterase inhibitors); written informed consent by patient or appropriate representative. Exclusion: Uncontrolled hypertension, heart failure, type II diabetes mellitus, hypothyroidism; other neurodegenerative disorders; cardiovascular disease that would affect completion of the trial; clinically significant psychiatric, hepatic, renal, pulmonary, metabolic, endocrine conditions; urinary outflow obstruction; active peptic ulcer; history of epilepsy, significant substance abuse. Total No. of patients: 636 Sex: 242 males. Age: 70.3 +/‐ 1.6 to 71.1 +/‐ 1.5 (broken down by treatment group) | |
| Interventions | Route: oral Treatment: galantamine 12mg b.i.d. galantamine 16mg b.i.d. Treatment commenced at 4mg b.i.d. and was increased weekly by 8mg/d to assigned maximum dose. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog ADCS‐CGIC DAD (Disability Assessment for Dementia) | |
| Notes | No. excluded after randomization: 198 No. not included in observed case analysis: 198 | |
GAL‐USA‐10 Tariot.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled, with 4‐week placebo run in Duration: 5 months | |
| Participants | Country: United States No. of Centers: Unstated Diagnosis: At least 6 month history of progressive cognitive decline, Senile Dementia Alzheimer's Type defined by: NINCDS‐ADRDA. Inclusion: MMSE score of 10 to 22, ADAS‐cog score > 17; CT or MRI < 12 months previously with no evidence of multi‐infarct dementia or active cerebrovascular disease; responsible caregiver; free for 30 days of medications indicated for dementia; free for 60 days for cholinomimetic agents. Exclusion: Uncontrolled hypertension, heart failure, type II diabetes mellitus, hypothyroidism; other neurodegenerative disorders; cardiovascular disease that would affect completion of the trial; clinically significant psychiatric, hepatic, renal, pulmonary, metabolic, endocrine conditions; urinary outflow obstruction; active peptic ulcer; history of epilepsy, significant substance abuse. Total No. of patients: 978 Sex: 353 males Age: 76.0 +/‐ 0.6 to 77.7 +/‐ 0.4 | |
| Interventions | Route: oral Treatment: galantamine 4mg b.i.d. galantamine 8mg b.i.d. galantamine 12mg b.i.d. Treatment commenced at 8mg/d and was increased 8mg/d every 4 weeks until the target dose had been reached. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog ADCS‐CGIC ADCS‐ADL (Alzheimer's Disease Cooperative Study Activities of Daily Living), NPI (Neuropsychiatric Inventory) | |
| Notes | No. excluded after randomization: 199 No. not included in observed cases analysis: 199 | |
RIV‐B303.
| Methods | 26 week double‐blind randomized: method described placebo‐controlled parallel‐group | |
| Participants | Country: Europe and North America 45 centres 725 participants (428 female, 297 male) age range 45‐95 years mean age=72 years Inclusion: DSM‐IV, NINCDS‐ADRDA criteria for probable AD. MMSE 10‐26 inclusive 50‐85 years old (outside this range with approval of medical expert), most concomitant disease, most medications Exclusion: severe and unstable cardiac disease, severe obstructive pulmonary disease, other life threateneing conditions (eg rapidly progressing malignancies), , anticholinergic drugs, acetylcholine precursor health food supplements, memory enhancers, insulin, psychotropic drugs (apart from occasional use of chloral hydrate for agitation or insomnia) | |
| Interventions | 1.rivastigmine 1‐4mg/day divided into 2 doses 2.rivastigmine 6‐12mg/day divided into 2 doses 3.placebo doses increased weekly insteps of 1.5mg/day during weeks 1‐12, but had to be within target range by week 7 | |
| Outcomes | ADAS‐Cog CIBIC‐plus PDS GDS CAS MMSE | |
| Notes | Main hypothesis: to assess the effects of rivastigmine on the core domains of AD Assessments: baseline, 12,18,26 weeks | |
RIV‐B304.
| Methods | 26 week double‐blind randomized placebo‐controlled parallel‐group | |
| Participants | Country: Australia, Canada, Italy, South Africa, UK 38 centres 678 participants Inclusion: DSM‐IV, NINCDS‐ADRDA criteria for probable AD. MMSE range 10‐26 inclusive Exclusion: significant illness, severe chronic pulmonary disease, psychiatric or neurological disorder, severe cardiovascular problems, clinically significant lab tests, including those indicative of impaired renal or liver function | |
| Interventions | 1.rivastigmine 2‐12 mg/day divided into 2 doses 2.rivastigmine 2‐12 mg/day divided into 3 doses 3.placebo titration to highest tolerated dose during weeks 1 and 2. During weeks 3‐26 dose variation allowed | |
| Outcomes | ADAS‐Cog CIBIC‐plus PDS GDS CAS MMSE | |
| Notes | Main hypothesis: to evaluate the efficacy and safety of individual highest well tolerated doses (range 2‐12 mg/d) of rivastigmine bid or tid for 26 weeks compared to placebo, in the therapy of patients with probable AD | |
RIV‐B351.
| Methods | 26 week double‐blind randomized placebo‐controlled parallel‐group | |
| Participants | Country: USA 14 centres 702 participants (393 female, 309 male) age range 45‐89 years, mean =74.5 years Inclusion: DSM‐IV, NINCDS‐ADRDA criteria for probable AD. MMSE range 10‐26 inclusive head computed tomography or magnetic resonance imaging scan consistent with AD within 12 months , most concomitant disease, most medications Exclusion: severe and unstable medical illnesses, anticholinergic drugs, acetylcholine precursor health food supplements, memory enhancers, insulin, psychotropic drugs (apart from occasional use of chloral hydrate for agitation or insomnia) | |
| Interventions | 1.rivastigmine:3 mg/day divided into 2 doses 2.rivastigmine:6 mg/day divided into 2 doses 3.rivastigmine:9 mg/day divided into 2 doses 4.placebo titration during weeks 1‐12 to the fixed dose, no dose reductions allowed | |
| Outcomes | ADAS‐Cog CIBIC‐plus PDS GDS CAS MMSE CAS | |
| Notes | Main hypothesis: to evaluate the efficacy and safety of 3 fixed doses of rivastigmine (3,6,9 mg/d) and placebo for 26 weeks of treatment, and dose/efficacy and dose/safety relationships in patients with probable mild to moderate AD Assessments: baseline, 12,18,26 weeks | |
RIV‐B352.
| Methods | 26 week double‐blind randomized:method described placebo‐controlled parallel‐group | |
| Participants | Country: USA 22 centres 699 participants (426 female, 273 male) age range 45‐89 years, mean =74.5 years inclusion: DSM‐IV, NINCDS‐ADRDA criteria for probable AD. MMSE range 10‐26 inclusive head computed tomography or magnetic resonance imaging scan consistent with AD within 12 months, most concomitant disease, most medications exclusion: severe and unstable medical illnesses, anticholinergic drugs, acetylcholine precursor health food supplements, memory enhancers, insulin, psychotropic drugs (apart from occasional use of chloral hydrate for agitation or insomnia) | |
| Interventions | 1.rivastigmine 1‐4mg/day divided into 2 doses 2.rivastigmine 6‐12mg/day divided into 2 doses 3.placebo titration phase week 0‐7, flexible phase weeks 8‐26, dose bid with food | |
| Outcomes | ADAS‐Cog CIBIC‐plus PDS GDS CAS MMSE | |
| Notes | primary hypothesis:to evaluate efficacy and safety of rivastigmine assessments: baseline, 12,18,26 weeks open label extension | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AD2000 | Randomized, placebo controlled, double blind trial of donepezil. Results for the 5 and 10 mg/day groups were not reported separately. Complex design and high numbers of dropouts made analysis and interpretation difficult. |
| DON vs GAL/Jones | Single‐blinded study of 12 weeks only. |
| DON vs GAL/Wilcock | Single blinded study. |
| DON vs RIV/Wilkinson | Single‐blinded study of 12 weeks only. |
| Donepezil‐203 | Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate effect on brain glucose metabolism and no data to contribute to this review. |
| Donepezil‐204 | Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate effect on brain glucose metabolism and no data to contribute to this review. |
| Fuschillo 2001 | Randomized study of donepezil 5 mg/day compared with rivastigmine 6‐9 mg/day for AD. Not blinded. |
| GAL‐INT‐10 | Randomized, double‐blind, placebo controlled study of galantamine. Dose available could be as high as 24 mg/day, but the mean dose was 17mg/day, which was too low for this review. |
| GAL‐INT‐6Erkinjuntti | Diagnosis of the included patients was not for simply AD. Randomized, placebo controlled, double blind trial of donepezil, for AD with cerebrovascular disease |
| Krishnan 2003 | Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate effect on N‐acetylasparate concentration and hippocampal volume and no data to contribute to this review. |
| Mega 2002 | Non‐randomized study of donepezil, metrifonate or galantamine. Outcome is response to cerebral metabolic activation. |
| Rozzini 2002 | Donepezil compared with rivastigmine in a non‐randomzied study. |
| Shua‐Haim 2002b | Donepezil compared with rivastigmine compared with galantamine. No mention of randomization |
| Study 312/314 | Randomized, placebo controlled, double blind trial of donepezil. Designed to evaluate preservation of function. Patients left the trial when function declined to a specified level and no data to contribute to this review. |
| Tsolaki 2002 | Donepezil compared with rivastigmine in a non‐randomized study. |
| Wang 2001 | Open label, randomized study, comparing rivastigmine with donepezil. |
| Werber 2002 | Non‐randomized study of tacrine, donepezil or rivastigmine. |
Contributions of authors
JB: All correspondence; drafting of review versions, selection of trials, extraction of data; entry of data and interpretation of data analysis
Contact editor: Rupert McShane Consumer editor: Mervyn Richardson
This review has been peer reviewed anonymously in November 2005.
Sources of support
Internal sources
No sources of support supplied
External sources
Alzheimer's Society, UK.
New Source of support, Other.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
DON‐302 {published data only}
- Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD, Donepezil Study Group. Open‐label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Archives of Neurology 2001;58(3):427‐33. [DOI] [PubMed] [Google Scholar]
- Feldman H. Gauthier S. Hecker J. Vellas B. Subbiah P. Whalen E. Donepezil MSAD Study Investigators Group. Erratum: a 24‐week, randomized, double‐blind study of donepezil in moderate to severe alzheimer's disease. Neurology 2001; Vol. 57, issue 11:2153. [DOI] [PubMed]
- Friedhoff LT, Rogers L. Donepezil lengthens time to loss of activities of daily living in patients with mild to moderate Alzheimer's disease ‐ results of a preliminary evaluation. Presented at the annual meeting of the American Academy of Neurology. Boston. Neurology 1997;48(3):A100. [Google Scholar]
- Friedhoff LT, Rogers SL. Donepezil lengthens time to loss of activities of daily living and cognition in patients with mild to moderate Alzheimer's disease. Proceedings of the 10th European College of Neuropsychopharmacology Congress; 1997 Sep 13‐17, Vienna, Austria 1997. [MEDLINE: ]
- Friedhoff LT, Rogers SL. Donepezil maintains activities of daily living in patients with mild to moderately severe Alzheimer's disease: results of a retrospective analysis. Eur J Neurology 1997;4, Suppl 1:S9. [Google Scholar]
- Rogers SL. Donepezil new clinical trials support long term use. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm. 2000:133.
- Rogers SL, Doody R, Mohs R, Friedhoff LT. E2020 produces both clinical, global and cognitive test improvement in patients with mild to moderately severe Alzheimer's disease: Results of a 30‐week phase III trial. Neurology 1996;46:A217 S14.001 ARI‐8. [Google Scholar]
- Rogers SL, Doody R, Mohs R, Friedhoff T, Donepezil Study Group. E2020 (Aricept TM) improves global and cognitive function in patients with Alzheimer's Disease. Results of a 30‐week trial. unpublished paper 1996b.
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT, and the Donepezil Study Group. A 24‐week, double‐blind, placebo‐controlled trial of donepezil in patients with Alzheimer's disease. Neurology 1998;50(1):136‐145. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Friedhoff LT, Farlow MR, Doody RS, Mohs R. Efficacy of donepezil in Alzheimer's disease: Fact or artifact? [Reply]. Neurology 1999; Vol. 52, issue 1:218‐9. [DOI] [PubMed]
- Rogers SL, Mohs RC, Friedhoff LT. Donepezil (E2020) improves cognition and function in patients with mild to moderately severe Alzheimer's disease. Results from Phase III trials. American Psychiatric Association 150th Annual Meeting, San Diego 1997.
DON‐304 {published and unpublished data}
- Bayer AJ, Rossor M, Hecker J, Gauthier S, Burns A, Petite H, Moller HJ, Rogers SL, Friedhoff LT, International Donepezil Study Group. Donepezil improves functional activity in patients with Alzheimer's disease. Proceedings of the 21st Collegium Internationale Neuro psychopharmacologicum; 1998 Jul 12‐16, Glasgow, Scotland 1998. [MEDLINE: ]
- Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Möller H‐J, Rogers SL, Friedhoff LT and the International Donepezil Study Group. The effects of donepezil in Alzheimer's disease ‐ results from a multinational trial. Dement Geriatr Cogn Disord 1999;10(3):237‐244. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Rosser M, Hecker J, Petite H, Rogers S, Mohr E, Burns A, Friedhoff LT, Rogers S. Donepezil Produces Both Clinical Global and Cognitive Test Improvement in Patients with Alzheimer's Disease. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐Jun 4, Toronto, Canada 1998b. [MEDLINE: ]
- Gauthier S, Rossor M, Hecker J, et al. Results from a multinational Phase III clinical trial of donepezil in Alzheimer's disease. [abstract] [poster presentation at 5th International Geneva/Springfield Symposium on Advances in Alzheimer therapy] April 15‐18 1998.
- Pratt RD, Gauthier S, Burns A, Perdomo CA. Donepezil provides long‐term clinical benefits for patients with Alzheimers Disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington 2000a.
DON‐311 {published data only}
- Finucane TE, Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, Whalen E. Getting donepezil into the nursing home. A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. Journal of the American Geriatrics Society 2003; Vol. 51, issue 1:133‐134. [PubMed]
- Steinman MA, Covinsky KE, Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, Whalen E. Donepezil for nursing home patients with dementia: a reinterpretation of the evidence. A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting J Am Geriatr Soc 2001;49:1590‐9.. Journal of the American Geriatrics Society 2003;51(1):132‐133. [PubMed] [Google Scholar]
- Tariot P, Cummings JL, Katz IR, Perdomo CA, Whalen E, Sovel MA, Schwam EM. Donepezil was well‐tolerated and enhanced cognition in nursing home patients with alzheimer's disease. Journal of the American Geriatrics Society 1999; Vol. 47:S3. [PubMed]
- Tariot P, Perdomo CA, Whalen E, Sovel MA, Scham EM. Age is not a barrier to donepezil treatment of Alzheimer's disease in the long‐term care setting. International Psychogeriatrics. 1999; Vol. 11, issue Supplement 1:134.
- Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, Whalen E. A randomised, double‐blind, placebo‐controlled study of the efficacy and safety of Donepezil in patients with Alzheimer's disease in the nursing home setting. Journal of the American Geriatrics Society 2001; Vol. 49, issue 12:1590‐9. [PubMed]
DON‐402 {published data only}
- Seltzer B, Zolnouni P, Nunez M, Goldman R, Kumar D, Ieni J, Richardson S, Donepezil 402 Study Group. Efficacy of donepezil in early‐stage Alzheimer disease. Archives of Neurolology 2004;61:1852‐6. [DOI] [PubMed] [Google Scholar]
DON‐Feldman {published data only}
- Feldman H. Therapeutic benefits of acetylcholinesterase inhibitor therapy in the moderate to severe stage of alzheimer's disease. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm 2000:59.
- Feldman H, Gauthier S, Hecker J, Vellas B, Hux M, Emir B, Subbiah P, Mastery V. Improved health outcomes with donepezil in moderate to severe Alzheimer's disease are associated with economic benefits. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:285.
- Feldman H, Gauthier S, Hecker J, Vellas B, Emir B, Mastey V, Subbiah P, and the Donepezil Study Group. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer's disease and the effect on caregiver burden. Journal of the American Geriatrics Society 2003;51:737‐744. [DOI] [PubMed] [Google Scholar]
- Feldman H, Gauthier S, Hecker J, Vellas B, Hux M, Xu Y, Schwam EM, Shah S, Mastey V, Donepezil MSAD Study Investigators Group. Economic evaluation of donepezil in moderate to severe Alzheimer disease. Neurology 2004;63(4):644‐650. [DOI] [PubMed] [Google Scholar]
- Feldman H, Gauthier S, Hecker J, Vellas B, Ieni J, Xu Y, Schwam E. Treatment Benefits of Donepezil in Patients with Severe Alzheimer's Disease and Their Caregivers. 57th Annual Meeting of the American Academy of Neurology, Miami Beach, April 2005 2005b:P02.097. 2005.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. Donepezil provides benefits in global function in moderate to severe Alzheimer's Disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. Donepezil's benefits on cognition, global function, activities of daily living and behavior in patients with moderate to severe alzheimer's disease. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm 2000:174.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E, Donepezil MSAD Study Group. Benefits of Donepezil on global function, behavior, cognition and ADLs in patients with moderate to severe Alzheimer's disease. Neurology 2000; Vol. 54, issue Suppl 3:A469.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E, Donepezil MSAD Study Investigators Group. A 24‐week, randomized, double‐blind study of donepezil in moderate to severe Alzheimer's disease. Neurology 2001a; Vol. 57, issue 4:613‐20. [DOI] [PubMed]
- Gauthier S, Feldman H, Hecker J, Vellas B, Emir B, McGill PS. Exploratory analysis of the effects af donepezil in moderate and severe Alzheimer's disease patients. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:277.
- Gauthier S, Feldman H, Hecker J, Vellas B, Ames D, Subbiah P, Whalen E, Emir B, Donepezil MSAD Study Investigators Group. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer's disease. International Psychogeriatrics 2002;14(4):389‐404. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Feldman H, Hecker J, Vellas B, Emir B, Subbiah P, The Donepezil MSAD Study Investigators' Group. . 2002a, 18(6):347‐54. Functional, cognitive and behavioral effects of donepezil in patients with moderate Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2002;18(6):347‐354. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Feldman H, Hecker J, Vellas B, Subbiah P, Whalen E. Benefits of Donepezil on performance of basic and instrumental activities of daily living in moderate to sever Alzheimer's Disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000a.
- Gauthier S, Feldman H, Hecker J, Vellas B, Subbiah P, Whalen E. Effects of donepezil on behaviour and other domains in moderate to severe alzheimer's disease. Journal of the European College of Neuropsychopharmacology. 2000b; Vol. 10, issue Suppl 3:S359. [MEDLINE: ]
- Gauthier S, Feldman H, Vellas B, Subbiah P. Efficacy of donepezil on functional, behavioural and cognitive symptoms in patients with moderate to severe alzheimer's disease. Journal of the American Geriatrics Society 2000; Vol. 48, issue 8:S2.
- Hecker J, Foti D, Gauthier S, Vellas B, Subbiah P, Whalen E. Benefits of Donepezil in the treatment of behavioural problems in moderate to severe Alzheimer's Disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000.
- Luckmann R. Donepezil improved the clinical state and quality of life in moderate‐to‐severe Alzheimer disease. ACP‐Journal‐Club 2002; Vol. 136, issue 2:59. [PubMed]
- Panisset M, Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. Use of the severe impairment battery in a clinical trial of Donepezil in moderate to severe Alzheimers Disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington 2000.
- Shah SN, Feldman H, Gauthier S, Hecker J, Vellas B, Hux M, Xu Y, Schwam E, Leaderer M, Pfizer Inc, New York, NY, USA. Pharmacoeconomic Benefits of Donepezil Treatment in Severe Alzheimer's Disease. NeuroBiology of Aging 2004;25(S2):208. [Google Scholar]
- Vellas B, Feldman H, Gauthier S, Hecker J, Subbiah P, Whalen E, Mastey V. Donepezil treatment in patients with moderate to severe Alzheimer's Disease reduces caregiver stress. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington 2000.
DON‐Nordic {published data only}
- Engedal K, Soininen H, Verhey F, Waldemar G, Winblad B, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P. Donepezil improved or stabilized cognition over one year in patients with mild and moderate alzheimer's disease. Journal of the European College of Neuropsychopharmacology 2000;10(Suppl 3):S368. [Google Scholar]
- Mastey V, Wimo A, Winblad B, Haglund A, Jacobson L, Miceli R, Zhang R, Subbiah P. An economic evaluation of donepezil in mild to moderate alzheimer's disease: results of a one year, double‐blind, randomized trial. Journal of the American Geriatrics Society 2001;49(4):S131. [Google Scholar]
- Mastey V, Wimo A, Winblad B, Haglund A, Jacobson L, Miceli R, Zhang R, Subbiah P. Donepezil reduces the time caregivers spend providing care: results of a one‐year, double‐blind, randomized trial in patients with mild to moderate Alzheimer's disease. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco 2001.
- Soininen H, Winblad B, Engedal K, Verhey F, Waldemar G, Wimo A, Wetterholme AL, Zhang R, Hugland A, Subbiah P. Long term benefits of donepezil on ADLs in AD patients. Proceedings of the Annual Scientific Meeting of the American Geriatric Society and the American Federation for Aging Research; 2000 May 17‐21, Nashville. 2000:171.
- Soininen H, Winblad B, Engedal K, Verhey F, Waldemar G, Wimo A, Zhang R, Subbiah P, Donepezil Nordic Study Group. Response to Donepezil is not predicted by apolipoprotein E genotype and/or gender. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington 2000.
- Waldemar G, Winblad B, Engedal K, Soininen H, Donepezil Nordic Study Group et al. Benefits of Donepezil on cognition, function and/or neuropsychiatric symtoms in patients with Alzheimers Disease over one year. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000.
- Waldemar G, Winblad B, Engedal K, Soininen HS, Verhey FR, Wimo A, Wetterholm AL, Zhang R, Haglund AA, Subbiag P, Donepezil Nordic Study Group. Donepezil benefits patients with either mild of moderate Alzheimer's disease over one year. Neurology 2000; Vol. 54, issue Suppl 3:A470. [DOI] [PubMed]
- Wimo A. Erratum: an economic evaluation of donepezil in mild to moderate alzheimer's disease: results of a 1‐year, double‐blind, randomized trial (dementia and geriatric cognitive disorders (2003) 15 (44‐54)). Dementia and Geriatric Cognitive Disorders 2003;16(2):102. [DOI] [PubMed] [Google Scholar]
- Wimo A, Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wetterholm AL, Mastey V, Haglund A, Zhang R, Miceli R, Chin W, Subbiah P, Donepezil Nordic Study Group. An economic evaluation of donepezil in mild to moderate Alzheimer's disease: results of a 1‐year, double‐blind, randomized trial. Dementia and geriatric cognitive disorders 2003;15(1):44‐54. [DOI] [PubMed] [Google Scholar]
- Wimo A, Winblad B, Mastey V, et al. An economic evaluation of Donepezil in mild to moderate Alzheimers Disease: Results of a one‐year, double‐blind, randomized trial. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000.
- Wimo A, Winblad B, Mastey V, Haglund A, Hertzman P, Miceli R, Jacobson L, Subbiah P. An economic evaluation of donepezil in mild to moderate alzheimer's disease patients: results of a one‐year, double‐blind, randomized trial. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18, Chicago, Illionois 2000. [MEDLINE: ]
- Winblad B. Long term therapeutic benefits of acetylcholinesterase inhibitor therapy in patients with alzheimer's disease. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm 2000:164.
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm A, Zhang R, Haglund A, Subbiah P. A 1‐year, randomized, placebo‐controlled study of donepezil in patients with mild to moderate AD. Neurology 2001; Vol. 57, issue 3:489‐95. [DOI] [PubMed]
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P. Donepezil enhances global function and acitivities of daily living compared with placebo in a one‐year, double‐blind trial in patients with mild to moderate alzheimer's disease. Proceedings of the Quality Research in Dementia Conference; 2000 Nov 19‐22, London 2000.
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P. Donepezil enhances global function, cognition and activities of daily living compared with placebo in a one‐year, double‐blind trial in patients with mild to moderate Alzheimer's disease. International Psychogeriatrics 1999;11(Supplement 1):138. [Google Scholar]
DON vs RIV/Bullock {published data only}
- Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, Nagel J, Lane R. Rivastigmine and donepezil treatment in moderate to moderately severe Alzheimer's disease over a 2‐year period. Current Medical Research and Opinions 2005;21(8):1317‐1327. [DOI] [PubMed] [Google Scholar]
GAL‐INT‐1 Wilcock {published data only}
- Wilcock GK. Erratum: Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: Multicentre randomised controlled trial (British Medical Journal (December 9)(1445‐1449)). BR‐MED‐J: British‐Medical‐Journal 2001;322(7278):90. [Google Scholar]
- Wilcock GK. GALANTAMINE ALLEVIATES CAREGIVER BURDEN IN ALZHEIMER'S DISEASE: A 6‐MONTH PLACEBO‐CONTROLLED STUDY. Conference Proceedings World Alzheimer Congress; 9‐13 July, 2000, Washington. 2000.
- Wilcock GK, Lilienfeld S, Gaens E on behalf of the Galantamine International‐1 Study Group. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: a multicentre randomised controlled trial. British Medical Journal 2000;321:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
GAL‐USA‐10 Tariot {published data only}
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C, Galantamine USA‐10 Study Group. A 5‐month, randomized, placebo‐controlled trial of galamtine in AD. Neurology 2000;54(12):2269‐2276. [DOI] [PubMed] [Google Scholar]
GAL‐USA‐1 Raskind {published data only}
- Raskind MA, Peskind ER, Wessel T, Yuan W, Galantamine USA‐1 Study Group. Galantamine in AD: A 6‐month randomized placebo‐controlled trial with a 6‐month extension. Neurology 2000;54(12):2261‐2268. [DOI] [PubMed] [Google Scholar]
RIV‐B303 {published data only}
- Anand R, Hartman R, Graham S. Effects dof Alzheimer's disease severity on activities of daily living with long‐term rivastigmine treatment. Journal of the American Geriatrics Society 2001;49(4):S151. [Google Scholar]
- Anand R, Messina J, Veach J, Hartman R. Effects of Rivastigmine in patients with moderately severe Alzheimer's disease. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000:199.
- Farlow M, Anand R, Messina J, Hartman R. Increased cognitive efficacy of rivastigmine in patients with moderate to severe Alzheimer's disease with co‐existing vascular risk. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000:206.
- Farlow M, Messina J, Anand R, Hartman R, Veach J. Dose dependent effect of rivastigmine on progression of cognitive deterioration in Alzheimer's disease. In: Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000. 2000:172.
- Hebert M. [Alzheimer disease: efficacy and tolerance of rivastigmine] [Maladie d'Alzheimer: efficacite et tolerance de la rivastigmine]. Presse Medicale 1999;28(32):1757‐8. [PubMed] [Google Scholar]
- Kumar V, Messina J, Hartman R, Anand R. Long‐term cognitive benefits of rivastigmine in Alzheimer's disease patients with vascular risk. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000:215.
- Lindesay J. An open label one‐year extension of SDZ‐ENA‐713 studies B303, B304 and B305 to prospectively evaluate long‐term safety, tolerability and efficacy of SDZ‐ENA‐713 in out‐patients with probable Alzheimer's disease. National Research Register 2000.
- Roesler M, Retz W, Retz Junginger P, Dennler HJ. Effect of two‐year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer's disease. Behavioural‐Neurology 1998;11(4):211‐6. [DOI] [PubMed] [Google Scholar]
- Rösler M. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: International randomised controlled trial [Erratum]. British Medical Journal 2001; Vol. 322, issue 7300:1456. [DOI] [PMC free article] [PubMed]
- Rösler M, Anand R, Cicin‐Sain A, Gauthier S, Agid Y, Dal‐Bianco P, Stähelin HB, Hartman R, Gharabawi M on behalf of the B303 Exelon Study Group. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ 1999;318(7184):633‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler M, Dennler H, Retz W, Gastpar W. A double‐blind placebo controlled study of ENA 713 in Alzheimer's disease (DAT). Pharmacopsychiatry 1997;30:212. [MEDLINE: ] [Google Scholar]
- Rösler M, Retz W, Retz Junginger P, Dennler HJ. Effects of two‐year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer's disease. Behavioural‐Neurology 1998;11(4):211‐6. [DOI] [PubMed] [Google Scholar]
- Vincent S, Andrews C, Lane R. Rivastigmine shows particular efficacy in Alzheimer patients with concomitant hypertension. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, 2002 Apr 3‐6, Geneva. 2002:253.
- Wilkinson DG. Rivastigmine was effective and safe in Alzheimer disease. ACP Journal Club 1999;131(2):34. [MEDLINE: ] [Google Scholar]
RIV‐B304 {published data only}
- Lindesay J. An open label one‐year extension of SDZ‐ENA‐713 studies B303, B304 and B305 to prospectively evaluate long‐term safety, tolerability and efficacy of SDZ‐ENA‐713 in out‐patients with probable Alzheimer's disease. National Research Register 2000.
- Novartis. No title. Unpublished. Data provided by Novartis No year.
- Novartis Pharmaceuticals. ADENA Programme. Unpublished Data 1998.
RIV‐B351 {published data only}
- Novartis. No title. Unpublished. Data provided by Novartis No year.
- Novartis Pharmaceuticals. ADENA Programme. Unpublished Data 1998.
RIV‐B352 {published data only}
- Corey‐Bloom J, Anand R and Veach J for ENA 713 B352 Study Group. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Ger Psychopharmacology 1998;1:55‐65. [Google Scholar]
- Doraiswamy M. The effects of rivastigmine on the alzheimer's disease assessment scale‐cognitive subscale items scores of patients with alzheimer's disease. Health in aging the challenge and promise of new decade. Proceedings of the annual scientific meeting of the American geriatrics society and the American federation for aging research; 2000 May 17‐21, Nashville. 2000:173.
- Doraiswamy PM, Anand R, Hartman R. Cognitive effects of rivastigmine in patients with mild to moderate alzheimer's disease compared to those with moderately‐severe to severe AD. Clinical Neuropschological Assessment 2000;1(6):12. [Google Scholar]
- Doraiswamy PM, Anand R, Hartman R. Long term cognitive effects in Alzheimer's disease patients stratified by vascular risk score and treated for 1 year with rivastigmine. Clinical Neuropschological Assessment 2000;1(6):14. [Google Scholar]
- Farlow M, Hake A, Messina J, Veach J, Anand R. The response of patients with Alzheimer's disease to rivastigmine treatment is predicted by the rate of disease progression. Neurology 2000; Vol. 54, issue Suppl 3:A469. [DOI] [PubMed]
- Farlow M, Messina J, Anand R. Long term cognitive benefits associated with the use of rivastigmine in the treatment of Alzheimer's disease results following two years of treatment. Proceedings of the Annual Scientific Meeting of the American Geriatric Society and the American Federation for Aging Research; 2000 May 17‐21, Nashville. 2000:172.
- Farlow MR, Hake A, Messina J, Hartman R, Veach J, Anand R. Response of patients with Alzheimer disease to rivastigmine treatment is predicted by the rate of disease progression. Archives of Neurology 2001;58(3):417‐22. [DOI] [PubMed] [Google Scholar]
- Ferris S. Improving day to day functioning in patients with AD. Proceedings of the Ninth Congress of the International Psychogeriatric Association; 1999 Aug 15‐20, Vancouver. 1999:77.
- Krishnan KR, Dorasiswamy PM, Messina J, Veach J, ENA 713 B352 Study Group. Rivastigmine slows stage specific global deterioration in Alzheimer's disease. Journal of the American Geriatrics Society 1999;47:S3. [Google Scholar]
- Kumar V, Anand R, Messina J, Hartman R, Veach J. An efficacy and safety analysis of Exelon in Alzheimer's disease patients with concurrent vascular risk factors. European Journal of Neurology 2000;7(2):159‐69. [DOI] [PubMed] [Google Scholar]
- Veach KR, Doraiswamy PM. Rivastigmine slows stage‐specific global deterioration in Alzheimer's disease. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20, Washington DC. 1999. [MEDLINE: ]
References to studies excluded from this review
AD2000 {published data only}
- AD2000 Collaborative Group. Long‐term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double‐blind trial. Lancet 2004;363:2105‐15. [DOI] [PubMed] [Google Scholar]
- Bentham P, Gray R, Hill R, Sellwood E, Courtney C. Twelve week respone to cholinesterase inhibitors dose not predict future benefit the AD2000 trial experiance. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:337.
- Lendon CL. Determination of respones to anticholinesterase therapy in the treatment of Alzheimer's disaese. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:1287.
- Lendon CL. Determination of responses to anticholinesterase therapy in the treatment of Alzheimer's disease. Proceedings of the 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20‐25, Stockholm, Sweden: Abstract No 1287. 2002.
- Roberts N. A reliable assessment of the efficacy and safety of donepezil and aspirin in Alzheimer's disease (AD2000). National Research Register 2001.
- Schneider LS. AD2000: donepezil in Alzheimer's disease. Lancet 2004; Vol. 363, issue 9427:2100‐1. [DOI] [PubMed]
- Waghray S. AD2000 A reliable assessment of the efficacy and safety of donepezil and aspirin in Alzheimer's disease. National Research Register 2000. [MEDLINE: ]
Donepezil‐203 {published data only}
- Tune L, Tiseo P, Hoffman J, Perdomo C, Votaw J, Rogers S, Friedhoff L. PET in AD: Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer's disease: results of a 24‐week study. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26 San Francisco. 2001.
- Tune L, Tiseo PJ, Ieni J, Perdomo C, Pratt RD, Votaw JR, Jewart RD, Hoffman JM. Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease: results of a 24‐week, double‐blind, placebo‐controlled study. American Journal of Geriatric Psychiatry 2003;11(2):169‐177. [PubMed] [Google Scholar]
- Tune LE, Tiseo PJ, Hoffman JM, Perdomo CA, Votow JR, Rogers SL, Friedhoff LT. Functional Brain Activity in Alzheimer's Disease. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐ Jun 4, Toronto. 1998:NR345. [MEDLINE: ]
Donepezil‐204 {published data only}
- Anon. No title. Eisai Inc..
DON vs GAL/Jones {published data only}
- Jones RW, Soininen H, Hager K, Aarsland D, Passmore P, The DONGAL Study Group, Murthy A, Zhang R, Bahra R. A multinational, randomised, 12‐week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19:58‐67. [DOI] [PubMed] [Google Scholar]
DON vs GAL/Wilcock {published data only}
- Wilcock G, Howe I, Coles H, Lilienfeld S, Truyen L, Young Z, Bullock R, and members of the GAL‐GBR‐2 Study Group. A long‐term comparison of galantamine and donepezil in the treatment of Alzheimer's disease. Drugs Aging 2003;20(10):777‐789. [DOI] [PubMed] [Google Scholar]
DON vs RIV/Wilkinson {published data only}
- Bullock R, Passmore F, Potocnik F, Hock C. The tolerability, ease of use and efficacy of donepezil and rivastigmine in alzeimer's disease patients: a 12‐week, multinational, comparative study. Journal of the American Geriatrics Society 2001; Vol. 49, issue 4:S19.
- Bullock R, Wilkinson DG, Passmore P, Hopker SW, Smith R, Potocnik FC, Maud CM, Hock C. Caregiver and physician determination of satisfaction with and ease of use of donepezil and rivastigamine treatment in alzheimer's disease patients. Proceedings of the 17th Alzheimer's Disease International Conference; 2001 Oct 25‐27, Christchurch, New Zealand 2001:39.
- Böttcher‐Buhler E. [Well tolerated, effective and inexpensive therapy of Alzheimer's dementia with donepezil] [Therapie der Alzheimer‐Demenz mit Donepezil: gut vertraglich, wirksam und kostengunstig]. Neurologie und Rehabilitation 2000; Vol. 6, issue 6:332‐3.
- Potocnik FC, Smith R, Passmore P, Hock C, Wilkinson D, Maud CM, Hopker S. Tolerability, ease of use, and efficacy of donepezil and rivastigmine in alzheimer's disease patients. Proceedings of the Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans 2001.
- Wilkinson D, Passmore P, Potocnik F, Maud C, Hock C. Donepezil compared to rivastigmine in Alzheimer's disease: similar efficacy but better tolerability and physician and caregiver satisfaction in a multinational randomized trial. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco 2001.
- Wilkinson DG, Passmore AP, Bullock R, Hopker SW, Smith R, Potocnik FCV, Maud CM, Engelbrecht I, Hock C, Ieni JR, Bahra RS. A multinational, randomised, 12‐week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer's disease. International Journal of Clinical Practice 2002;56(6):441‐446. [PubMed] [Google Scholar]
Fuschillo 2001 {published data only}
- Fuschillo C, Pia S, Campana F, Pinto A, Simone L. Cognitive deficits in alzheimer's disease: treatment with acetylcholinesterase inhibitor agents. Archives of Gerontology and Geriatrics 2001;33(Suppl 1):151‐8. [DOI] [PubMed] [Google Scholar]
GAL‐INT‐10 {published data only}
- Brodaty H, Corey‐Bloom J, Potocnik FCV, Truyen L, Gold M, Damaraju CRV. Galantamine Prolonged‐Release Formulation in the Treatment of Mild to Moderate Alzheimer's Disease. Dement Geriatr Cogn Disord 2005;20(2‐3):120‐132. [DOI] [PubMed] [Google Scholar]
GAL‐INT‐6Erkinjuntti {published data only}
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359:1283‐1290.. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. The safety and efficacy of Galantamine in the treatment of vascular and mixed dementia (Double‐blind part only). (GAL‐INT‐6). Johnson and Johnson Pharmaceutical Research & Development. January 2004.
Krishnan 2003 {published data only}
- Krishnan KR, Charles HC, Doraiswamy PM, Mintzer J, Weisler R, Yu X, Perdomo C, Ieni JR, Rogers S. Randomized, placebo‐controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer's disease. American journal of psychiatry, The 2003;160(11):2003‐11. [DOI] [PubMed] [Google Scholar]
Mega 2002 {published data only}
- Mega M, Dinov I, Manese M, Felix J, O'Connor S, Toga A, Cummings J. Cerebral metabolic activation with cholinesterase inhibitor therapy in Alzheimer's disease. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:430.
Rozzini 2002 {published data only}
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzani S, Leonardi R, Ranzenigo A, Rozzini R, Saviotti FM, Turla M, Trabucchi m, Ghianda D, Borroni B, Chilovi BV, Padovani A. Acetylcholinesterase inhibitors are effective in real world patients with mild to moderate Alzheimer disease evidence from a large population treated with rivastigmine or donepezil. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:329.
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzoni S, Leonardi R, Ranzenigo A, Rozzini R, Saviotti FM, Turla M, Trabucchi M, Vicini B, Borroni B, Padovani A. Comparison of efficacy and safety of rivastigmine and donepezil in patients with mild to moderate alzheimer disease: results from a multicentre randomised trial.. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:240.
Shua‐Haim 2002b {published data only}
- Shua‐Haim J, Smith J, Potel S. A head to study of donepezitl aricept rivastigmine exlon and galantamine reminyl for the treatment of Alzheimer's disease safety tolerability clinical and caregiver impression after 4‐5 months of trratment a prospective study. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:286.
Study 312/314 {published data only}
- Ebell M. Does donepezil help patients with moderate Alzheimer's dementia preserve their ability to function independently?. Evidence Based Practice 2002.
- Mohs R, Doody R, Morris J, Ieni J, Perdomo C, Pratt R, Rogers S. Donepezil preserves activities of daily living in alzheimer's disease patients: results from a one‐year placebo‐controlled functional survival study. Neurology 2000; Vol. 54, issue Suppl 3:A415.
- Mohs R, Doody R, Morris J, Ieni J, Rogers S, Perdomo C, Pratt R. Donepezil preserves functional status and improves cognition in alzheimer's disease patients: results from a 1‐year propective placebo‐controlled study. Journal of the American Geriatrics Society 2000; Vol. 48, issue 8:S46.
- Mohs R, Doody R, Morris J, Ieni JR, Rogers SL, Perdomo CA, Pratt RD. Donepezil preserves functional status in Alzheimer's disease patients: results from a 1‐year prospective placebo‐controlled attrition study. Journal of the European College of Neuropsychopharmacology 1999;9(Suppl 5):S328. [Google Scholar]
- Mohs R, Doody R, Morris J, Ieni JR, Rogers SL, Perdomo CA, Pratt RD. Donepezil preserves functional status in Alzheimer's disease patients: results from a 1‐year prospective placebo‐controlled study. Proceedings of the Quality Research in Dementia Conference; 2000 Nov 19‐22, London. 2000.
- Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo C, Pratt RD, 312 Study Group. A 1‐year, placebo‐controlled preservation of function survival study of donepezil in AD patients [Erratum]. Neurology 2001; Vol. 57, issue 10:1942. [MEDLINE: ] [DOI] [PubMed]
- Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, Pratt RD. A 1‐year, placebo‐controlled preservation of function survival study of donepezil in AD patients. Neurology 2001; Vol. 57, issue 3:481‐8. [DOI] [PubMed]
- Mohs RC, Doody RS, Morris JC, Leni JR, Rogers SL, Perdomo CA, Pratt RD. Donepezil preserves functional status in Alzheimer's disease. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18, Chicago, Illionois. 2000. [MEDLINE: ]
- Pratt R, Mohs R, Doody R, Morris J, Rogers S, Ieni J, Perdomo C. Donepezil preserves functional status in Alzheimer's disease patients results from a 1‐year prospective placebo controlled functional study. Proceedings of the 6th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden 2000b:178.
Tsolaki 2002 {published data only}
- Tsolaki M, Gerothanassis D, Aristotle CP. Efficacy and safety of cholinesterase inhibitors a longitudinal comparative study between donepezil and rivastigmine. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:2038.
Wang 2001 {published data only}
- Wang Y, Chen Q, Zhang Z, et al. The treatment by using rivastigmine for patients with alzheimer disease: results of a multicenter, randomized, open‐labeled, controlled clinical trial. Chinese Journal of Neurology 2001;34(4):210‐3. [Google Scholar]
Werber 2002 {published data only}
- Werber EA, Klein C, Rabey MJ. Evaluation of cholinergic treatment in demented by p300 evoked related potentials. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:442.
Additional references
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington DC: APA, 1987:American Psychiatric Association. [Google Scholar]
Birks 2005
- Birks JS, Harvey R. Donepezil for dementia due to Alzheimer's disease (Cochrane Review). The Cochrane Library 2005, Issue 3. [DOI] [PubMed] [Google Scholar]
Birks 2005b
- Birks J, Grimley Evans J, iakovidou V, Tsolaki M. Rivastigmine for Alzheimer's disease (Cochrane Review). The Cochrane Library 2005, Issue 3. [DOI] [PubMed] [Google Scholar]
Bucks 1996
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol activities of daily living scale. Age Ageing 1996;25:113‐120. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenburg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐2313. [DOI] [PubMed] [Google Scholar]
DeJong 1989
- DeJong R, Osterlund OW, Roy GW. Measurement of quality‐of‐life changes in patients with Alzheimer's disease. Clin Ther 1989;11(4):545‐554. [PubMed] [Google Scholar]
Erkinjuntti 2002
- Erkinjuntti T, Skoog I, Lane R, Andrews C. Rivastigmine in patients with Alzheimer's disease and concurrent hypertension. International Journal of Clinical Practice 2002;56(10):791‐796. [PubMed] [Google Scholar]
Folstein 1975
- Folstein NF, Folstein SE, McHugh PR. Mini‐Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(Suppl 2):533‐539. [PubMed] [Google Scholar]
Gottfries 1982
- Gottfries CG, Brane G, Gullberg B, Steen G. A new rating scale for dementia syndromes. Arch Gerontol Geriatr 1982;1:311‐330. [DOI] [PubMed] [Google Scholar]
Gélinas 1999
- Gélinas I, Gauthier L, McIntyre M, et al. Development of a functional measure for persons with Alzheimer's disease: the Disability Assessment for Dementia. American Journal of Occupational Therapy 1999;53:471‐481. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2000
- Kumar V, Anand R, Messina J, Hartman R, Veach J. An efficacy and safety analysis of Exelon in Alzheimer's disease patients with concurrent vascular risk factors. European Journal of Neurology 2000;7(2):159‐169. [DOI] [PubMed] [Google Scholar]
Loy 2005
- Loy C, Schneider L. Galantamine for Alzheimer's disease (Cochrane Review). The Cochrane Library 2005, Issue 3. [DOI] [PubMed] [Google Scholar]
McKhann 1987
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;4:939‐944. [DOI] [PubMed] [Google Scholar]
Olin 2005
- Olin J, Schneider L. Galantamine for Alzheimer's disease (Cochrane Library). The Cochrane Library 2005, Issue 3. [Google Scholar]
Panisset 1994
- Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neurological test for severely demented patients. Archives of Neurology 1994;51:41‐45. [DOI] [PubMed] [Google Scholar]
Reisberg 1982
- Reisberg B, Ferris SH, Leon MJ, Crook T. the global deterioration sclae for assessment of primary degenerative dementia. Am J Psychiatry 1982;139:1136‐1139. [DOI] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis K. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356‐1364. [DOI] [PubMed] [Google Scholar]
Schneider 1997
- Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study ‐ Clinical Global Impression of Change. AD Assoc Dis 1997;11(suppl 2):S22‐32. [DOI] [PubMed] [Google Scholar]
Wimo 1998
- Wimo A, Wetterholm AL, Mastey V, Winblad B. Evaluation of the healthcare resource utilization and caregiver time in anti‐dementia drug trials. In: Wimo A, Jönsson B, Karlsson G, Winblad B editor(s). Health Economics in Dementia. Chichester: Wiley, 1998:465‐477. [Google Scholar]


