Abstract
Purpose
Ample evidence has revealed that the lymphocyte-to-monocyte ratio (LMR), albumin-to-globulin ratio (AGR), and mean platelet volume (MPV) are cancer-related inflammatory markers. The present study aimed to combine these indicators to better assess the progression of colon cancer.
Methods
This retrospective study enrolled 251 patients with colon cancer, 171 patients with benign colon diseases, and 187 healthy control subjects. The receiver operating characteristic curve and area under the curve (AUC) were used to determine the diagnostic values of the selected inflammatory index.
Results
The levels of LMR, AGR, and MPV were decreased in the colon cancer group compared with the healthy control and benign colon disease groups. The LMR, AGR, and MPV were all correlated with tumor size. Moreover, LMR and AGR was associated with lymph node metastasis and clinical stage, AGR was related to distant metastasis. Both the LMR (P = .030) and AGR (P = .005) were negatively correlated with the concentration of carcinoembryonic antigen (CEA). The AUC value of MPV combined with CEA had a good diagnostic ability for distinguishing colon cancer cases (AUC = .950) and patients with benign colon diseases (AUC = .886) from controls. Meanwhile, the combination of LMR or AGR with CEA could enhance larger AUC (.746 for LMR + CEA, .737 for AGR + CEA) than CEA, LMR, or AGR alone in detecting colon cancer from benign colon diseases.
Conclusions
CEA combined with the LMR, AGR, or MPV may be used as better blood-based biomarkers in the progression of colon cancer patients.
Keywords: colon cancer, lymphocyte-to-monocyte ratio, albumin-to-globulin ratio, mean platelet volume, diagnosis
Introduction
Colon cancer comprised the highest incidence in tumors of the digestive system and represented a commonly diagnosed malignant tumor worldwide in 2020 1 ; it was the fourth most frequent cause of cancer morbidity and fifth leading cause of cancer mortality in China in 2015. 2 Due to the lack of obvious manifestations, most patients with colon cancer have silent symptoms for years. More than 50% of colon cancer cases are clinically diagnosed at the advanced cancer stage. 3 Therefore, effective screening protocols are essential for colon cancer detection. As well known, fecal occult blood test (FOBT) is a cheap and convenient screening method for colon cancer. 4 Nevertheless, the result is frequently affected by many dietary factors, multiple drugs, and upper or lower gastrointestinal bleeding site, which may lead to false positives and subsequent unnecessary tests and panic. 5 Other methods that colonoscopy and biopsy have been used as ideal methods for the diagnosis of early colon cancer, 6 but these inspections greatly increase the physical and financial burden on patients harboring colonic diseases, resulting in poor compliance. Hence, low-cost, non-invasive and easily obtainable markers have important significance for the diagnosis and prevention of colon cancer.
Several serum tumor markers have been commonly used in colon diseases, such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and carbohydrate antigen 242 (CA242).7,8 CEA is considered the primary marker, with almost all researches and clinical practices for the detection and monitoring of colon cancer used this indicator. 9 However, several studies discovered that CEA possessed low sensitivity and could not appropriately present the complete potency for clinical diagnosis and treatment in colorectal cancer. 10 Thus, more reliable and powerful biomarkers of identifying the colon cancer are expected to obtain.
In recent years, many inflammatory parameters from peripheral blood and serum have been evaluated in the diagnosis and prognosis of multiple malignancies, including colorectal cancer,11,12 such as circulating neutrophils, 13 lymphocytes, 14 monocytes, 15 platelets, 16 mean platelet volume (MPV), 17 and albumin. 18 Due to the release of chemokines and cytokines, MPV is, as an early indicator of platelet activation, an inflammatory marker. And it is distorted and has been shown to have diagnostic/predictive value in some non-malignant inflammatory conditions. 19 Moreover, several reports discovered that it was closely related to the occurrence, features, and outcomes of many neoplasms.20-22 As crucial components of host immunity, lymphocytes can infiltrate into the tumor microenvironment, and they play a vital role in cell-mediated immunity, preventing the proliferation and metastatic activity of colorectal cancer. 23 Systemic inflammation can induce changes in the hematological system, leading to a significant decrease of lymphocytes.24,25 Lymphocytopenia is considered an insufficient immune response against tumor, resulting in hyperproliferation and tumorigenesis. Conversely, the excess circulating monocytes gather and settle in solid tumor tissues after being mediated by chemokines of inflammatory cytokines; they are then differentiated into tumor-associated macrophages with specific phenotypic characteristics. 26 Increasing evidence has demonstrated that the accumulation of tumor-associated macrophages in the tumor sites contributes to the angiogenesis, tumorigenesis, and pathogenesis of colon cancer. 27 As a result, the relatively lower number of lymphocytes is an indicator marker of weak immune response, and the elevated monocyte count is a microenvironment monitor of high tumor burden. Therefore, lymphocyte-to-monocyte ratio (LMR), as a reflection of systemic inflammation and immunological statuses, may have a crucial role in the progression of colon cancer. The albumin-to-globulin ratio (AGR) which combines serum albumin and globulin, is a routinely available and cost-effective marker and associated with the process of inflammatory and nourishment state. Accumulated evidence displayed that AGR was an independent and useful predictor in the prognosis of colon cancer by regulating cells and/or releasing several mediators 28 ; moreover, elevated AGR was a favorable factor for better clinical outcomes. 12 Hence, we hypothesized that AGR may have a significant diagnostic value in colon cancer.
Up to now, to our knowledge, studies have rarely investigated the diagnostic role of these three inflammatory parameters (LMR, AGR, and MPV) in the progression of colon cancer, especially for persons with benign colon diseases. Therefore, this study investigated the value of LMR, AGR, and MPV combined or not with CEA in the progression of colon cancer.
Material and Methods
Patients
251 patients with colon cancer, 171 benign colon diseases cases, and 187 healthy controls with complete clinical data were consecutively included in this retrospective study from January 2012 to September 2020. In colon cancer participants with new diagnoses, the disease was confirmed by histology and treated with surgical resection. Clinical staging of colon cancer was conducted accorded to the seventh edition of the American Joint Committee on Cancer/TNM tumor staging criteria. Patients with other cancers, cardiovascular disease, diabetes mellitus, hematological disease, autoimmune disease, recent blood transfusion, liver cirrhosis, nonalcoholic fatty liver disease, nephropathy, or treatment with other therapies, such as radiotherapy, chemotherapy, and hormonotherapy, were excluded. Colon polyps, colon adenomas, and colonitis were included as benign colon disease patients who were diagnosed by colonoscopy and histopathology. The healthy controls were healthy subjects without the above-mentioned diseases and clinical gastrointestinal symptoms during the same period of physical examination. Ever smokers were defined as participants who had smoked at least 100 cigarettes in their lifetime, and ever drinkers were defined as participants who had drunk alcoholic beverages once a week for at least a year. 29 All patient details have been de-identified in this study.
Data Collection
All data were collected from the hospital’s electronic medical records, including gender, age, height, weight, smoking status, drinking status, white blood cells (WBC), platelets, hemoglobin, lymphocyte, monocyte, albumin, globulin, MPV, CA19-9, CA242, and CEA. Whole blood-cell parameters were detected with a Beckmann 780 device (Beckman Coulter, Brea, CA). The levels of albumin and total protein were analyzed by a Hitachi 7600 automatic biochemical analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan). The concentration of serum CEA was tested by using a Roche E6000 analyzer (Roche Diagnostics, Basel, Switzerland). Architect i2000 and its reagents (Abbott GmBH Diagnostika, Wiesbaden, Germany) were used to evaluated the serum CA19-9 values. The level of serum CA242 was determined using Enzyme Linked Immunosorbent Assay (CanAg Company, Sweden). The ratios of interest were calculated as follows: body mass index (BMI) = weight/height, 2 LMR = lymphocyte count/monocyte count, and AGR = albumin/(total protein – albumin).
Determination of Sample Size
To ensure the credibility of the results, the sample size for this study was estimated using the PASS 15 program based on a probability of α = .05 and β = .10. The alternative hypothesis was one-sided test. The assumed of the indexes (LMR, AGR, and MPV) AUC was .50–.60. The case-control design used an approximately 1:1 ratio. According to the above parameters, the sample size of each group was 136 cases. A total of 251 patients with colon cancer were included in this retrospective data collection. If the sample size model was “enter N+, solve for N−, N+ was 251,” the power analysis showed that the N− sample size of 92.
Statistical Analysis
The Kolmogorov–Smirnov test was used to detect the distribution of the continuous variables. The mean and standard deviation were applied for normally distributed data, and non-normally distributed data were expressed as the median and quartile. Differences between groups in laboratory parameters and clinical characteristics were calculated using the Student’s T test (normally distributed data), Mann–Whitney nonparametric U test (non-normally distributed data), or χ2 test (categorical variable). The Spearman correlation coefficient was conducted to detect correlations between inflammatory index (LMR, AGR, or MPV) and CEA in the colon cancer group. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were calculated by MedCalc statistical software (version 18.1.1). Data processing and analysis were determined by SPSS 16.0 statistical software package, using a significance level of .05.
Results
Patient Characteristics
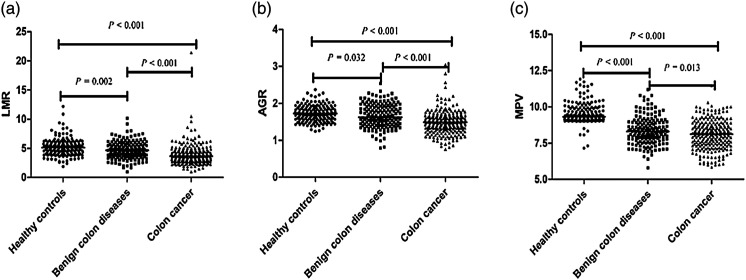
Basic information and laboratory parameters are summarized in Table 1. The median age of the colon cancer cases, benign colon diseases patients, and control individuals were 56.00, 48.00, and 53.00 years, respectively. No intergroup difference was observed in gender, BMI, smoking status and drinking status among the three groups. Patients with colon cancer had higher levels of WBCs, platelets, monocytes, and CEA compared with healthy individuals and benign colon disease patients. Conversely, the levels of hemoglobin, MPV, albumin, LMR, and AGR were significantly lower in the colon cancer group than they were in the control and benign colon disease groups, and there were statistical differences in LMR (Figure 1A), AGR (Figure 1B) and MPV (Figure 1C) among the three groups. The values of CA19-9 were evident discrepancies between benign colon diseases and healthy controls, as well as in colon cancer and benign colon diseases groups. The concentrations of CA242 were not available in the benign colon disease group, and there was difference between the healthy control group and colon cancer group (P < .001).
Table 1.
Basic information and laboratory parameters among colon cancer, benign colon diseases, and healthy control groups.
| Characteristics | Healthy controls (N = 187) | Benign colon diseases (N = 171) | Colon cancer (N = 251) | P a | P b | P c |
|---|---|---|---|---|---|---|
| Gender (Male/female) | 99/88 | 90/81 | 140/111 | .555 | .524 | .953 |
| Age (years) | 53.00 (49.00–60.00) | 48.00 (41.00–56.00) | 56.00 (46.00–64.00) | .092 | <.001 | <.001 |
| BMI * (kg/m2) | 22.20±2.67 | 22.00±2.87 | 21.94±3.56 | .382 | .867 | .482 |
| Smoking status(ever/never) | 32/155 | 30/141 | 35/216 | .362 | .315 | .914 |
| Drinking status(ever/never) | 18/169 | 15/156 | 21/230 | .647 | .884 | .780 |
| WBC (×109/L) | 6.10 (5.17–6.71) | 6.30 (5.20–7.46) | 6.41 (5.33–7.70) | .001 | .453 | .028 |
| Hemoglobin (g/L) | 143.00 (135.10–150.60) | 131.00 (120.00–142.70) | 117.00 (99.00–130.80) | <.001 | <.001 | <.001 |
| Platelet (×109/L) | 202.20 (179.50–227.80) | 230.20 (193.60–276.50) | 275.00 (228.50–345.00) | <.001 | <.001 | <.001 |
| MPV (fL) | 9.32 (9.14–9.82) | 8.30 (7.80–8.93) | 8.11 (7.42–8.70) | <.001 | .013 | <.001 |
| Lymphocyte* (×109/L) | 2.06±.46 | 2.14±.68 | 1.89±.52 | <.001 | <.001 | .200 |
| Monocyte (×109/L) | .41 (.33–.50) | .48 (.37–.58) | .50 (.41–.62) | <.001 | .012 | <.001 |
| Albumin (g/L) | 46.70 (45.0–48.20) | 42.60 (39.40–45.20) | 38.50 (36.10–41.10) | <.001 | <.001 | <.001 |
| Globulin (g/L) | 27.10 (25.30–29.20) | 26.00 (23.20–28.70) | 26.10 (23.70–28.80) | .001 | .381 | <.001 |
| CEA (ng/mL) | .71 (.39–1.30) | 1.74 (1.08–2.61) | 2.71 (1.56–7.28) | <.001 | <.001 | <.001 |
| CA242(U/mL) | 4.09 (.00–7.45) | — | 5.15 (.01–18.90) | <.001 | — | — |
| CA19-9(U/mL) | 12.31 (7.47–21.84) | 8.90 (4.44–15.95) | 10.46 (5.41–24.13) | .239 | .013 | <.001 |
| LMR | 5.13 (4.24–5.87) | 4.67 (3.67–5.60) | 3.63 (2.86–4.51) | <.001 | <.001 | .002 |
| AGR* | 1.72±.21 | 1.66±.31 | 1.50±.31 | <.001 | <.001 | .032 |
BMI, body mass index; WBC, white blood cell; MPV, mean platelet volume; CEA, carcinoembryonic antigen; CA242, carbohydrate antigen 242; CA19-9, carbohydrate antigen 19-9; LMR, lymphocyte-to-monocyte ratio; AGR, albumin-to-globulin ratio.
*Normally distributed data.
acolon cancer vs healthy controls.
bcolon cancer vs benign colon diseases.
cbenign colon diseases vs healthy controls.
Figure 1.
Comparison of LMR and AGR among three groups. (A): LMR (B): AGR. (C): MPV. Note. LMR: lymphocyte-to-monocyte ratio, AGR: albumin to globulin ratio, MPV: mean platelet volume.
Correlations Between LMR, AGR, MPV, and the Clinicopathological Characteristics in Patients With Colon Cancer
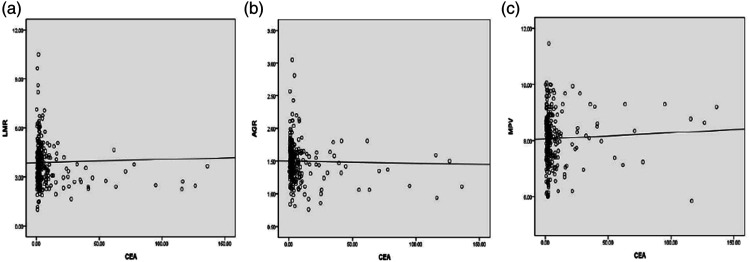
There was a negative correlation presented between CEA and the LMR (r = −.137, P = .030) (Figure 2A) and AGR (r = −.178, P = .005) (Figure 2B) in the colon cancer group, respectively. Nevertheless, no correlation was observed between AGR and MPV (r = .012, P = .846) (Figure 2C). According to the seventh edition of the American Joint Committee on Cancer/TNM tumor stage, the clinicopathological characteristics of the 251 patients carrying colon cancer are shown in Table 2. The levels of LMR, AGR, and MPV in the colon cancer group were all closely related to the tumor size, but not associated with tumor invasion. Moreover, the AGR and LMR were correlated with lymph node metastasis and clinical stage. The colon cancer subjects with stage M0 had significantly higher levels of AGR compared to the cases with stage M1 (P = .013).
Figure 2.
Correlation analysis of LMR, AGR, MPV, and CEA in patients with colon cancer. (A). LMR and CEA with colon cancer, (B). AGR and CEA with colon cancer, (C). MPV and CEA with colon cancer. Note. LMR: lymphocyte-to-monocyte ratio, AGR: albumin to globulin ratio, MPV: mean platelet volume, CEA: carcinoembryonic antigen.
Table 2.
Correlation between LMR, AGR, and MPV and clinicopathological features in colon cancer.
| N | LMR | P | AGR | P | MPV | P | |
|---|---|---|---|---|---|---|---|
| Tumor invasion (T stage) | |||||||
| T1 + T2 | 86 | 3.63 (2.83–4.87) | .513 | 1.46 (1.33–1.62) | .602 | 8.10 (7.53–8.73) | .973 |
| T3 + T4 | 165 | 3.63 (2.89–4.36) | 1.50 (1.31–1.66) | 8.14 (7.40–8.69) | |||
| Lymph node metastasis (N stage) | |||||||
| N0 | 177 | 3.75 (2.85–4.87) | .033 | 1.52 (1.33–1.68) | .025 | 8.10 (7.34–8.69) | .300 |
| N1–N3 | 74 | 3.44 (2.90–4.12) | 1.43 (1.31–1.58) | 8.17 (7.60–8.71) | |||
| Distant metastasis (M stage) | |||||||
| M0 | 242 | 3.63 (2.94–4.52) | .366 | 1.50 (1.32–1.65) | .013 | 8.11 (7.44–8.70) | .900 |
| M1 | 9 | 3.09 (2.48–4.28) | 1.19 (1.04–1.44) | 8.16 (6.90–8.97) | |||
| Tumor size (cm) | |||||||
| <5 | 159 | 3.85 (3.00–4.65) | .004 | 1.52 (1.34–1.67) | .002 | 8.20 (7.60–8.80) | .026 |
| ≥5 | 92 | 3.26 (2.70–4.17) | 1.43 (1.15–1.60) | 7.90 (7.14–8.60) | |||
| Clinical stage | |||||||
| I + II | 179 | 3.77 (2.95–4.86) | .013 | 1.52 (1.33–1.69) | .002 | 8.10 (7.40–8.70) | .833 |
| III + IV | 72 | 3.43 (2.80–4.03) | 1.44 (1.23–1.57) | 8.17 (7.49–8.69) | |||
LMR, lymphocyte-to-monocyte ratio; AGR, albumin-to-globulin ratio; MPV, mean platelet volume.
Logistic Regression Used to Distinguish Colon Cancer From Controls
The correlation between several potential risk factors and colorectal cancer was analyzed by binary logistic regression (Table 3), including gender (odd ratio [OR] = .892, 95% confidence interval [CI] = .610–1.304, P = .556), age (OR = 1.013, 95% CI = .994–1.031, P = .174), BMI (OR = .975, 95% CI = .918–1.035, P = .401), smoking status (OR = .785, 95% CI = .466–1.323, P = .363), drinking status (OR = .857, 95% CI = .443–1.659, P = .647), MPV (OR = .089, 95% CI = .055–.143, P < .001), CA242 (OR = 1.061, 95% CI = 1.033–1.089, P < .001), CA19-9 (OR = 1.012, 95% CI = 1.002–1.022, P = .019), CEA (OR = 2.855, 95% CI = 2.223–3.666, P < .001), LMR (OR = .547, 95% CI = .466–.644, P < .001), and AGR (OR = .036, 95% CI = .015–.088, P < .001). The above important indexes (P < .05) were selected as potential independent predictors for further multivariate analysis. After multivariate analysis, MPV (β = −2.352, P < .001), LMR (β = −.306, P = .001), AGR (β = −4.091, P < .001), and CEA (β = .967, P < .001) were also recognized as crucial markers in the occurrence of colon cancer. The optimal model (logit P = .967 × CEA −.306 ×LMR −4.091 × AGR −2.352 × MPV +27.383) was set up for differencing colon cancer cases from controls. The AUC, sensitivity, and specificity were reach up to .964, 90.84%, and 92.51%, respectively.
Table 3.
Screening for significant predictors that distinguished colon cancer from healthy controls by using univariate and multivariate analyses.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Gender | .892 | .610−1.304 | .556 | |||
| Age(years) | 1.013 | .994−1.031 | .174 | |||
| BMI | .975 | .918−1.035 | .401 | |||
| Smoking status | .785 | .466−1.323 | .363 | |||
| Drinking status | .857 | .443−1.659 | .647 | |||
| MPV | .089 | .055−.143 | <.001 | .095 | .050−.180 | <.001 |
| CA242 | 1.061 | 1.033−1.089 | <.001 | 1.013 | .969−1.058 | .567 |
| CA19-9 | 1.012 | 1.002−1.022 | .019 | .997 | .988−1.006 | .465 |
| CEA | 2.855 | 2.223−3.666 | <.001 | 2.630 | 1.928−3.588 | <.001 |
| LMR | .547 | .466−.644 | <.001 | .736 | .612−.886 | .001 |
| AGR | .036 | .015−.088 | <.001 | .017 | .003−.080 | <.001 |
BMI, body mass index; MPV, mean platelet volume; CA242, carbohydrate antigen 242; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; LMR, lymphocyte-to-monocyte ratio; AGR, albumin-to-globulin ratio. CI, confidence interval; OR, odd ratio.
Diagnostic efficacy of LMR, AGR, CEA, and MPV alone or in combination to differentiate patients with colon cancer from other subjects
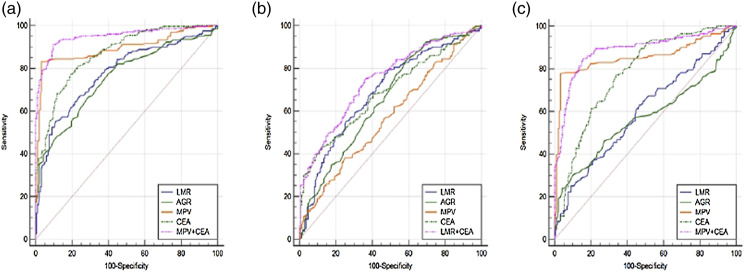
The results of the ROC curve analysis are presented in Table 4 and Figure 3. The AUC value of the combination of MPV and CEA was .950 (95% CI = .925–.968, positive likelihood ratio [PLR] = 9.48, negative likelihood ratio [NLR] = .097, positive predictive value [PPV] = 92.7%, negative predictive value [NPV] = 88.5%), which possessed a good diagnostic ability for distinguishing colon cancer cases from healthy controls. Meanwhile, the sensitivity and specificity of the combination of MPV and CEA were increased to 91.24% and 90.37%, respectively. Compared to the subjects with benign colon disease, LMR combined with CEA produced larger AUC (.746) than other combined indicators in subjects with colon cancer. However, the sensitivity (75.30%) of combination for LMR and CEA was lower than LMR (77.29%) and AGR (83.27%) alone. To predict colon cancer, the optimal cut-offs of LMR, AGR, MPV, and CEA in the benign colon disease subjects were 4.58, 1.71, 7.80, and 3.44, respectively. For the diagnosis of benign colon disease, the combined use of MPV and CEA resulted in better AUC (.886) and sensitivity (84.80%) than any single index or combination of other indicators. However, the specificity (84.49%) of the combination of MPV and CEA was inferior to MPV (96.79%) or AGR (89.84%) alone.
Table 4.
Diagnostic efficacy of LMR, AGR, and CEA used alone or in combination to differentiate colon cancer from benign colon diseases.
| Cut off | Sensitivity (%) | Specificity (%) | PLR | NLR | PPV (%) | NPV (%) | AUC (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|---|
| LMR a | 3.78 | 55.38 | 88.77 | 4.93 | .50 | 86.9 | 59.7 | .778(.736−.816) | <.001 |
| AGR a | 1.59 | 66.53 | 71.12 | 2.30 | .47 | 75.6 | 61.3 | .756(.713−.796) | <.001 |
| MPV a | 8.93 | 83.27 | 96.79 | 25.95 | .17 | 97.2 | 81.2 | .894(.862−.922) | <.001 |
| CEA a | 1.35 | 80.08 | 78.07 | 3.65 | .26 | 83.1 | 74.5 | .870(.835−.900) | <.001 |
| LMR a +AGR a | .64 | 65.74 | 88.24 | 5.59 | .39 | 88.2 | 65.7 | .819(.780 − .854) | <.001 |
| LMR a +MPV a | .60 | 82.47 | 94.65 | 15.42 | .19 | 95.4 | 80.1 | .916(.886−.940) | <.001 |
| AGR a +MPV a | .65 | 78.49 | 96.26 | 20.97 | .22 | 96.6 | 76.9 | .923(.894−.946) | <.001 |
| LMR a +CEA a | .47 | 86.45 | 81.82 | 4.75 | .17 | 86.5 | 81.8 | .895(.863−.922) | <.001 |
| AGR a +CEA a | .46 | 84.86 | 80.75 | 4.41 | .19 | 85.5 | 79.9 | .889(.856−.917) | <.001 |
| MPV a +CEA a | .45 | 91.24 | 90.37 | 9.48 | .097 | 92.7 | 88.5 | .950(.925−.968) | <.001 |
| LMR b | 4.58 | 77.29 | 53.22 | 1.65 | .43 | 70.8 | 61.5 | .688(.642−.732) | <.001 |
| AGR b | 1.71 | 83.27 | 42.11 | 1.44 | .40 | 67.9 | 63.2 | .655(.607−.700) | <.001 |
| MPV b | 7.80 | 38.25 | 74.85 | 1.52 | .82 | 69.1 | 45.2 | .571(.523−.619) | .011 |
| CEA b | 3.44 | 43.43 | 87.72 | 3.54 | .64 | 83.8 | 51.4 | .686(.639−.730) | <.001 |
| LMR b +AGR b | .66 | 53.39 | 78.95 | 2.54 | .59 | 78.8 | 53.6 | .717(.671−.759) | <.001 |
| LMR b +MPV b | .65 | 51.39 | 80.70 | 2.66 | .60 | 79.6 | 53.1 | .698(.652−.742) | <.001 |
| AGR b +MPV b | .61 | 62.15 | 69.01 | 2.01 | .55 | 74.6 | 55.4 | .680(.633−.724) | <.001 |
| LMRb+CEA b | .54 | 75.30 | 63.74 | 2.08 | .39 | 75.3 | 63.7 | .746(.702−.787) | <.001 |
| AGR b +CEAb | .58 | 58.57 | 76.02 | 2.44 | .55 | 78.2 | 55.6 | .737(.693−.779) | <.001 |
| MPV b +CEA b | .59 | 51.39 | 83.63 | 3.14 | .58 | 82.2 | 54.0 | .715(.669−.758) | <.001 |
| LMR c | 5.02 | 63.16 | 53.48 | 1.36 | .69 | 55.4 | 61.3 | .595(.542−.646) | .002 |
| AGR c | 1.46 | 30.41 | 89.84 | 2.99 | .77 | 73.2 | 58.5 | .567(.514−.619) | .032 |
| MPV c | 8.98 | 77.78 | 96.79 | 24.24 | .23 | 95.7 | 82.6 | .856(.815−.891) | <.001 |
| CEA c | 1.05 | 77.19 | 66.31 | 2.29 | .34 | 67.7 | 76.1 | .776(.729−.818) | <.001 |
| LMR c +AGR c | .54 | 40.35 | 83.42 | 2.43 | .72 | 69.0 | 60.5 | .610(.558−.661) | .000 |
| LMR c +MPV c | .55 | 74.85 | 95.19 | 15.55 | .26 | 93.4 | 80.5 | .864(.824 − .898) | <.001 |
| AGR c +MPV c | .55 | 71.35 | 96.79 | 22.24 | .30 | 95.3 | 78.7 | .851(.810–.887) | <.001 |
| LMR c +CEA c | .42 | 73.68 | 74.33 | 2.87 | .35 | 72.4 | 75.5 | .779(.733−.821) | <.001 |
| AGR c +CEA c | .43 | 70.18 | 72.19 | 2.52 | .41 | 69.8 | 72.6 | .773(.726−.816) | <.001 |
| MPV c +CEA c | .38 | 84.80 | 84.49 | 5.47 | .18 | 83.3 | 85.9 | .886(.848−.917) | <.001 |
Note. CEA, carcinoembryonic antigen; LMR, lymphocyte-to-monocyte ratio; AGR, albumin-to-globulin ratio; MPV, mean platelet volume; PLR, positive likelihood ratio; NLR, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; AUC, area under curve.
acolon cancer vs healthy controls.
bcolon cancer vs benign colon diseases.
cbenign colon diseases vs healthy controls.
Figure 3.
The diagonstic value of LMR, AGR, and MPV used alone or in combination with CEA in the progression of colon cancer. (A). colon cancer vs healthy controls, (B). colon cancer vs benign colon diseases, (C). benign colon diseases vs healthy controls. Note. LMR: lymphocyte-to-monocyte ratio, AGR: albumin to globulin ratio, MPV: mean platelet volume, CEA: carcinoembryonic antigen
Discussion
Colon cancer is closely associated with inflammation which has been unraveled as a crucial hallmark in all the steps of colon tumorigenesis, including initiation, invasion, progression, and metastasis. 30 Recent work has elucidated that cancer-associated inflammatory markers are increasingly used in the early diagnosis and prognosis of malignant tumors.31,32 However, rarely studies have investigated the diagnostic role of these inflammatory parameters (LMR, AGR, and MPV) in distinguish colon cancer from benign colon diseases. Therefore, this study assessed the diagnostic efficacy of the common inflammatory indexes and detected whether they could be used as surrogate markers in the progression of colon cancer.
In this retrospective analysis, we discovered that the healthy controls had significantly higher MPV compared to the benign colon disease patients and colon cancer cases, which was consistent with the result of Stojkovic et al., 33 but contrary to the findings of Li et al. 34 and Kilincalp et al. 35 We found that the MPV values of the control group in the first two articles were 9.06 and 10.7, which were similar to the results of this study, but the wide gulf was obtained from the Kilincalp et al.’s article in the control group. The differences may be due to health groups included criteria, different sample size, and ethnic variation in different study. As stated in the previous research that MPV was connected with obesity, smoking, and so on, 36 which may also differ among the studies population, resulting in the inconsistent outcomes. Although no association was demonstrated between clinical stage and MPV in our current finding, the results revealed that the MPV levels in patients with stage III and IV were slightly higher than that in cases with stage with I and II, which was similar to Li et al.’s study. 34 And the present study was the first research revealing the correlation between MPV and the tumor size.
In concordance with previous results, the patients with colon cancer had a lower LMR level than the benign colon disease patients and healthy controls did. For example, Aksoy et al. 37 observed that the level of monocyte-to-lymphocyte ratio (MLR) was higher in the gastric cancer group than it was in the intestinal metaplasia and healthy control groups. A study by Luo et al. 38 found that MLR was significantly elevated in patients carrying urothelial carcinoma of the bladder relative to healthy controls. Moreover, our study disclosed that the level of LMR was significantly correlated with the features of colon cancer, such as lymph node metastasis, tumor size, and clinical stage. Indeed, Ozawa et al. 39 demonstrated that cancer-specific survival was significantly worse in patients with low LMR levels than in high-LMR patients, and LMR may be an independent prognostic marker for stage III and IV colon cancer cases. 40 Peng et al. 41 assessed the prognosis of patients harboring colorectal liver-only metastases and elucidated that elevated LMR predicted a favorable outcome in both 5-year recurrence-free survival and overall survival of patients with lymph node metastases and liver tumor up to a diameter of less than 5 cm. Furthermore, several meta-analyses have demonstrated that malignant patients with high preoperative LMR have better predicted clinical outcomes compared with patients with low LMR in populations comprising Asians, digestive system carcinomas, non-metastatic diseases, and early disease stages,42,43 which confirmed our findings.
Emerging evidence suggests that AGR is mainly used as a clinical indicator for several kinds of cancers. Growing tumors induce hypoalbuminemia via secreting inflammatory cytokines, which may inhibit albumin synthesis and promote albumin loss, resulting in weak systemic response. Rasouli et al. 44 reported that patients with malignant tumors had a decreased concentration of albumin, which were measured by colorimetric methods, compared with healthy controls, which was accordant with the present study results. Globulin, as a reflector for most proinflammation protein, was increased by the accumulation of acute-phase proteins and immunoglobulins. 45 The AGR, which is compatible with hypoalbuminemia and hyperglobulinemia, may be able to more accurately reflect the nutritional and inflammatory state, and thus is associated with the progression of neoplasia. The electrophoretic data of serum proteins showed that the AGR was significantly decreased in 85 patients harboring cancer relative to controls. 44 Cheng et al. 46 confirmed that the globulin-to-albumin ratio (GAR), was significantly higher in the subjects with liver disease compared with individuals with no evidence of liver disease. Quite a few studies revealed that patients with lower pretreatment AGR were related to worse survival than higher AGR subjects in colorectal cancer, 47 gastric cancer, 48 pancreatic cancer, 49 nasopharyngeal carcinoma, 50 and esophageal cancer. 51 Moreover, a significant correlation based on the above-mentioned researches was observed between clinical characteristics and the level of AGR, such as lymph node metastasis, tumor size, distant metastasis, and tumor stage. In agreement with previous studies, this study found that the value of AGR in the colon cancer subjects was lower than that in the benign colon diseases and healthy individuals; furthermore, it showed that the AGR was associated with lymph node metastasis, distant metastasis, tumor size, and clinical stage.
CEA is a serum glycoprotein that is mainly secreted by cells of the large intestine, and it has been widely applied as a tumor marker for the malignant characteristics of colorectal cancer. Unfortunately, high levels of CEA are not present in about 15% of large intestine cancers. 52 Although CEA has a high specificity in colorectal cancer,53,54 elevated CEA is also revealed in other malignant tumors. Moreover, increased circulating levels of CEA are observed not only in cancer patients but also in some benign intestinal lesions, and the role was poor in differentiating colorectal cancer patients from those with benign colorectal diseases. 55 Therefore, the sensitivity and effectiveness of CEA are not sufficient for clinical diagnosis and treatment. Consistent with previous studies, the sensitivity (43.43%) and diagnostic value (.686) of CEA were not noticeable in identifying colon cancer from benign colon diseases in this study, while the specificity was up to 87.72%. Whereas we found that CEA combined with LMR or AGR generated a significantly better sensitivity and AUC than CEA used alone in discriminating patients with colon cancer and benign colon diseases cases. Similar to this pilot study, a previous report displayed that LMR possessed a moderate ability (AUC = .71) and could contribute to distinguishing patients carrying gastric cancer from those with intestinal metaplasia, 37 the diagnostic efficiency was similar to that of our study in colon cancer, and our research have improved diagnostic efficiency through the combination of indicators. Meanwhile, the AUC value of MPV combined with CEA had a good diagnostic ability and sensitivity for distinguishing colon cancer cases (AUC = .950 and 91.24%) and patients with benign colon diseases (AUC = .886 and 84.80%) from controls, was superior compared with individual indexes or combination of other indicators. Similarly, Stojkovic et al. 33 revealed that ROC curve analysis showed high diagnostic efficacy of NLR, PLR and MPV in CRC patients compared with individual markers (AUC = .904). In many malignancies, AGR exhibited good diagnostic efficacy (AUC = .81) in differentiating cancer patients from healthy controls, 44 which squared with our results. All these findings suggest that CEA combined with the selected inflammatory index could not only be used as a colon cancer diagnostic biomarker but may also improve the diagnostic efficiency of detecting patients harboring colon cancer from benign colon diseases cases.
There are certain potential limitations in the current research. On the one hand, this is a retrospective analysis of a relatively small sample size from a single center, so selection bias and statistical validity should be noted, which may affect the final results about the associations between the LMR or AGR and colon cancer. We failed to stratify benign colon diseases due to the relatively small sample size. On the other hand, confounding factors cannot be completely ruled out, including age, race, dietary habits and family histories, which may prevent us from drawing any firm conclusions. For example, median age of included patients was quite low in the three groups. Moreover, different populations may show different levels of "inflammatory conditions." Probably, results would be different including elderly patients and patients with chronic/inflammatory conditions or Western populations. Therefore, a large-scale, prospective study with multiple centers is still needed to validate these results.
Conclusion
This study first described that the LMR and AGR were correlated with CEA and colon cancer, as well as lymph node metastasis, tumor size, distant metastasis, and clinical stage. Moreover, the combination of the LMR, AGR, or MPV with CEA may enhance sensitivity and diagnostic efficacy and may be a helpful diagnostic marker for differentiating colon cancer from benign colon diseases and healthy controls.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was supported by the National Natural Science Foundation of China (82060036), the Natural Science Foundation of Guangxi(2021GXNSFAA196029) and the Science Study of Guangxi Health Commission (Z20190486).
Ethics Approval: This research was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No.2022-KY-E-024), and all participants verbally agreed that their data could be used for scientific research under the protection of privacy.
ORCID iD
Zhuning Mo https://orcid.org/0000-0001-9627-5241
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Xia C, Zheng R, et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: A comparative risk assessment. Lancet Glob Health. 2019;7(2):e257-e269 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics. CA Cancer J Clin. 2017;67(3):177-193. [DOI] [PubMed] [Google Scholar]
- 4.Yuan SY, Wu W, Fu J, et al. Quantitative immunochemical fecal occult blood test for neoplasia in colon cancer screening. J Dig Dis. 2019;20(2):78-82. [DOI] [PubMed] [Google Scholar]
- 5.Song LL, Li YM. Current noninvasive tests for colorectal cancer screening: An overview of colorectal cancer screening tests. World J Gastrointest Oncol. 2016;8(11):793-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappell MS. From colonic polyps to colon cancer: pathophysiology, clinical presentation, screening and colonoscopic therapy. Minerva Gastroenterol Dietol. 2007;53(4):351-373 [PubMed] [Google Scholar]
- 7.Feng Z, Shi X, Zhang Q, et al. Analysis of clinicopathological features and prognosis of 1315 cases in colorectal cancer located at different anatomical subsites. Pathol Res Pract. 2019;215(10):152560. [DOI] [PubMed] [Google Scholar]
- 8.Peng Y, Zhai Z, Li Z, Wang L, Gu J. Role of blood tumor markers in predicting metastasis and local recurrence after curative resection of colon cancer. Int J Clin Exp Med. 2015;8(1):982-990 [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi T, Shimada Y, Hsu M, et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018;4(3):309-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita K, Watanabe M. Clinical significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009;100(2):195-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018;55:128-138 [DOI] [PubMed] [Google Scholar]
- 12.Fujikawa H, Toiyama Y, Inoue Y, et al. Prognostic impact of preoperative albumin-to-globulin ratio in patients with colon cancer undergoing surgery with curative intent. Anticancer Res. 2017;37(3):1335-1342 [DOI] [PubMed] [Google Scholar]
- 13.Shaul ME, Fridlender ZG. Cancer-related circulating and tumor-associated neutrophils - subtypes, sources and function. FEBS J. 2018;285(23):4316-4342 [DOI] [PubMed] [Google Scholar]
- 14.Iseki Y, Shibutani M, Maeda K, et al. The impact of the preoperative peripheral lymphocyte count and lymphocyte percentage in patients with colorectal cancer. Surg Today. 2017;47(6):743-754 [DOI] [PubMed] [Google Scholar]
- 15.Valero C, Zanoni DK, McGill MR, et al. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer. 2020;126(5):994-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): New perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwada T, Saito Y, Terasaki Y, et al. Interstitial lung disease associated with nanoparticle albumin-bound paclitaxel treatment in patients with lung cancer. Jpn J Clin Oncol. 2019;49(2):165-173 [DOI] [PubMed] [Google Scholar]
- 19.Milovanovic Alempijevic T, Stojkovic Lalosevic M, Dumic I, et al. Diagnostic accuracy of platelet count and platelet indices in noninvasive assessment of Fibrosis in nonalcoholic fatty liver disease patients. Can J Gastroenterol Hepatol. 2017;2017:6070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo F, Zhu X, Qin X. Platelet distribution width in hepatocellular carcinoma. Med Sci Monit. 2018;24:2518-2523. [PubMed] [Google Scholar]
- 21.Karaman K, Bostanci EB, Aksoy E, et al. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. Eur J Intern Med. 2011;22(6):e95-98 [DOI] [PubMed] [Google Scholar]
- 22.Manjunath K V, Jonnada P, Sai Kiran N, Anwar A. Role of mean platelet volume in the prognosis of locally advanced gastric cancer: A tertiary cancer center experience. Cureus. 2020;12(7):e9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782-795. [DOI] [PubMed] [Google Scholar]
- 24.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Crit Care Med. 1999;27(4):733-740 [DOI] [PubMed] [Google Scholar]
- 26.De la Fuente Lopez M, Landskron G, Parada D, et al. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour Biol. 2018;40(11):1010428318810059. [DOI] [PubMed] [Google Scholar]
- 27.Kvorjak M, Ahmed Y, Miller ML, et al. Cross-talk between colon cells and macrophages increases ST6GALNAC1 and MUC1-sTn expression in Ulcerative colitis and colitis-associated colon cancer. Cancer Immunol Res. 2020;8(2):167-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xuan Q, Yang Y, Ji H, et al. Combination of the preoperative albumin to globulin ratio and neutrophil to lymphocyte ratio as a novel prognostic factor in patients with triple negative breast cancer. Cancer Manag Res. 2019;11:5125-5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han CH, Lu J, Wei Q, et al. The functional promoter polymorphism (-842G>C) in the PIN1 gene is associated with decreased risk of breast cancer in non-Hispanic white women 55 years and younger. Breast Cancer Res Treat. 2010;122(1):243-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101-2114. e2105 [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Xu Z, Huang Y, et al. Prognostic values of preoperative platelet-to-lymphocyte ratio, albumin and hemoglobin in patients with non-metastatic colon cancer. Cancer Manag Res. 2019;11:3265-3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Zhang B, Zhu K, et al. Preoperative albumin-to-globulin ratio predicts survival in patients with non-small-cell lung cancer after surgery. J Cell Physiol. 2019;234(3):2471-2479 [DOI] [PubMed] [Google Scholar]
- 33.Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, et al. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers. 2019;2019:6036979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li JY, Li Y, Jiang Z, Wang RT, Wang XS. Elevated mean platelet volume is associated with presence of colon cancer. Asian Pac J Cancer Prev. 2014;15(23):10501-10504 [DOI] [PubMed] [Google Scholar]
- 35.Kilincalp S, Coban S, Akinci H, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2015;24(4):328-333 [DOI] [PubMed] [Google Scholar]
- 36.Gasparyan AY, Sandoo A, Stavropoulos-Kalinoglou A, Kitas GD. Mean platelet volume in patients with rheumatoid arthritis: the effect of anti-TNF-alpha therapy. Rheumatol Int. 2010;30(8):1125-1129 [DOI] [PubMed] [Google Scholar]
- 37.Aksoy EK, Kantarci S, Torgutalp M, et al. The importance of complete blood count parameters in the screening of gastric cancer. Prz Gastroenterol. 2019;14(3):183-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo Y, Shi X, Li W, et al. Evaluation of the clinical value of hematological parameters in patients with urothelial carcinoma of the bladder. Medicine (Baltimore). 2018;97(14):e0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozawa T, Ishihara S, Kawai K, et al. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. J Surg Res. 2015;199(2):386-392 [DOI] [PubMed] [Google Scholar]
- 40.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J, Li H, Ou Q, et al. Preoperative lymphocyte-to-monocyte ratio represents a superior predictor compared with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for colorectal liver-only metastases survival. Onco Targets Ther. 2017;10:3789-3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971-978 [DOI] [PubMed] [Google Scholar]
- 43.Teng JJ, Zhang J, Zhang TY, Zhang S, Li BS. Prognostic value of peripheral blood lymphocyte-to-monocyte ratio in patients with solid tumors: A meta-analysis. Onco Targets Ther. 2016;9:37-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasouli M, Okhovatian A, Enderami A. Serum proteins profile as an indicator of malignancy: Multivariate logistic regression and ROC analyses. Clin Chem Lab Med. 2005;43(9):913-918 [DOI] [PubMed] [Google Scholar]
- 45.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448-454 [DOI] [PubMed] [Google Scholar]
- 46.Cheng KC, Lin WY, Liu CS, et al. Association of different types of liver disease with demographic and clinical factors. Biomedicine (Taipei). 2016;6(3):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hachiya H, Ishizuka M, Takagi K, et al. Clinical significance of the globulin-to-albumin ratio for prediction of postoperative survival in patients with colorectal cancer. Ann Gastroenterol Surg. 2018;2(6):434-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao S, Feng F, Liu N, et al. Preoperative albumin level is superior to albumin-globulin ratio as a predicting indicator in gastric cancer patients who underwent curative resection. Cancer Manag Res. 2019;11:9931-9938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng L, Gu S, Wang P, et al. Pretreatment values of bilirubin and albumin are not prognostic predictors in patients with advanced pancreatic cancer. Cancer Med. 2018;7(12):5943-5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li AC, Xiao WW, Wang L, et al. Risk factors and prediction-score model for distant metastasis in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Tumour Biol. 2015;36(11):8349-8357 [DOI] [PubMed] [Google Scholar]
- 51.Oki S, Toiyama Y, Okugawa Y, et al. Clinical burden of preoperative albumin-globulin ratio in esophageal cancer patients. Am J Surg. 2017;214(5):891-898 [DOI] [PubMed] [Google Scholar]
- 52.Lawicki S, Mroczko B, Szmitkowski M. [Neoplasm markers useful for diagnosis and monitoring of colonic neoplasms]. Postepy Hig Med Dosw. 2002;56(5):617-634 [PubMed] [Google Scholar]
- 53.Theron EJ, Albrecht CF, Kruger PB, Jenkins K, van der Merwe MJ. beta-Glucosidase activity in fetal bovine serum renders the plant glucoside, hypoxoside, cytotoxic toward B16-F10-BL-6 mouse melanoma cells. Vitro Cell Dev Biol Anim. 1994;30A(2):115-119 [DOI] [PubMed] [Google Scholar]
- 54.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313-5327 [DOI] [PubMed] [Google Scholar]
- 55.Holdenrieder S, Stieber P, Liska V, et al. Cytokeratin serum biomarkers in patients with colorectal cancer. Anticancer Res. 2012;32(5):1971-1976 [PubMed] [Google Scholar]