Abstract
Objective
The 5-year survival rate of patients with esophageal squamous cell cancer (ESCC) is very low. However, long-term aspirin use has been suggested to have an adjuvant therapeutic effect. We therefore investigated the effect of long-term aspirin use before ESCC diagnosis on postoperative patient survival.
Methods
We carried out a retrospective cohort study of patients who underwent esophageal cancer resection in our hospital from 2008 to 2018. Patients were divided into an aspirin group (n = 79) and control group (n = 79), and were followed up until December 2019. We analyzed the clinicopathological and follow-up data of the patients during hospitalization, and the cyclooxygenase-2 (COX-2) protein expression levels by immunohistochemistry, and related these to postoperative survival.
Results
Patients who took aspirin had significantly lower survival rates than those who did not. COX-2-negative patients had better survival than patients with either low or high COX-2 expression levels. T stage was the only independent predictor of survival in patients who took aspirin.
Conclusions
Long-term regular use of aspirin before diagnosis had an adverse effect on postoperative survival in patients with ESCC. Different COX-2 protein expression levels were associated with significantly different postoperative survival rates, with COX-2-positive patients having the poorest survival.
Keywords: Aspirin, esophageal squamous cell carcinoma, cyclooxygenase-2 expression, survival analysis, independent risk factor, long-term use
Introduction
Esophageal squamous cell carcinoma (ESCC) is the most common malignant lesion of the esophagus. The prognosis is typically poor, with a 5-year overall survival (OS) rate of approximately 20%. 1 China has among the highest incidence and mortality rates of ESCC, with particularly high incidences in certain areas of the Taihang Mountains in southern Hebei Province, such as Shexian and Cixian in Handan City. 2 Even after surgical resection, the 5-year survival rate is only approximately 30%. 3
Aspirin is the oldest and most widely used antipyretic, analgesic, and anti-inflammatory drug. 4 It is commonly used for the prevention of coronary artery disease and cerebrovascular disease. 5 Aspirin can reportedly inhibit the proliferation of cancer cells, promote cancer cell apoptosis, and reduce the morbidity and mortality of malignant tumors.6–8 Its anti-tumor effects are mainly achieved via inhibition of cyclooxygenase-2 (COX-2), which can promote the synthesis of prostaglandin (PG) by arachidonic acid (AA). PGs accordingly promote the proliferation of cancer cells, enhance tumor invasion and metastasis, and inhibit the occurrence of apoptosis.9,10
In a previous prospective study, 11 we showed that using aspirin for more than 12 months after esophagectomy for cancer improved the 5-year survival rate of patients with T2N0M0 stage disease. However, we did not examine the effects of aspirin use before surgery or evaluate the extent of COX-2 expression in cancer tissues. The current study therefore aimed to investigate the associations of long-term use of aspirin before cancer resection and COX-2 tissue expression levels with postoperative survival.
Materials and methods
Research subjects
This was a single-center retrospective cohort study. Patients aged ≥18 years who underwent esophagectomy for ESCC in the Department of Thoracic Surgery, Fourth Hospital, Hebei Medical University from January 2008 to June 2018 were included in the study, irrespective of sex or their acceptance of neoadjuvant or postoperative adjuvant therapy.
All patients underwent esophagectomy for cancer. The esophagus and peri-esophageal fat tissues and lymph nodes were resected via left thoracotomy. The stomach was mobilized, and the lymph nodes along the celiac artery, common hepatic artery, splenic artery, and left gastric artery were removed through diaphragm incision. The total stomach was used as an esophageal substitute. Esophagogastric anastomosis was performed in the upper thorax if the cancer was located in the lower and middle third of the esophagus, and in the left neck if the cancer was located in the upper third of the esophagus. The reporting of this study conforms to the STROBE guidelines. 12
Patients were divided into two groups: Group A, including ESCC patients who had used aspirin 50 to 100 mg/day for more than 3 months for various non-cancer-related reasons before diagnosis, and Group P, including patients who had not taken long-term aspirin or any type of non-steroidal anti-inflammatory drug (NSAID) before diagnosis. “Long-term” aspirin use was defined as the continuous use of aspirin for more than 3 months, for at least 5 days a week. The predictive variables that required matching included sex, age, smoking and drinking history, and history of hypertension/coronary heart disease/diabetes. We carried out 1:1 propensity score matching using the statistical software SPSS 26.0 (IBM Corp., Armonk, NY, USA) to obtain the same numbers of patients in both groups.
Clinicopathological and follow-up data
All medical records of the enrolled patients were reviewed using the case retrieval system of the Fourth Hospital of Hebei Medical University, and followed up at the Follow-up Center of the Fourth Hospital of Hebei Medical University. The Esophageal Cancer Research Follow-up Questionnaire (2018 Edition) was designed with reference to previous cohort studies combined with disease characteristics, and information was collected according to the content of the questionnaire.13–15
All patients were followed-up by a mail-out questionnaire or telephone call at 6-monthly intervals until either death or censoring on the date when the patient was last known to be alive. Any patient who failed to respond to two consecutive follow-up reminders was defined as ‘lost to follow-up’, and these individuals were considered to be dead at the date of the first mail-out when calculating survival outcomes. The follow-up deadline was 31 December 2019. The study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University and conducted in accordance with the Declaration of the World Medical Association of Helsinki. All follow-ups were approved by the patient verbally or in writing, and all patients provided signed informed consent. The follow-up data were kept strictly confidential, and all patient details were de-identified.
Immunohistochemistry (IHC)
Retrieval of pathological sections and staining
Formalin-fixed paraffin-embedded tissue blocks were obtained from the Department of Pathology, the Fourth Hospital of Hebei Medical University, and cut into 4-μm-thick sections. The sections were stained using the streptavidin-peroxidase method, as described previously. 16 COX-2 mouse monoclonal antibody was obtained from Hangzhou Huaan Biotechnology Co., Ltd. (Cat#: RT1159; Hangzhou, China) and used at a dilution of 1:100.
Evaluation of staining results
The extent of staining was assessed by two pathologists using the modified German Immune Scoring System. 17 The number of positive cells and the overall intensity of the immune response were each scored separately by two pathologists. Positive staining was defined as clear yellow or brown particles that were clearly located in the cytoplasm (0 points: no staining; 1 point: weak staining; 2 points: moderate staining; and 3 points: strong staining) and the percentage of positive cells was calculated as the proportion of positive cells within 100 cells in a single field of view under the microscope (0 point: no positive cells; 1 point: 1% to 10% positive cells; 2 points: 11% to 50% positive cells; 3 points: 51% to 80% positive cells; and 4 points: 81% to 100% positive cells). The final score was calculated by multiplying the scores of the two variables: a final score of 0 was negative, 1 to 3 was a low positive, and ≥4 points was a high positive. The final result for each patient was the average of the final scores according to the two pathologists.
Statistical analysis
Patients in Group A provided the benchmark group and were propensity score matched with non-users in a 1:1 ratio. Descriptive statistical analysis of the cohort and comparison of COX-2-positivity rates between the groups were carried out by χ2 tests. OS was defined as the time from randomization to death from any cause, and disease-free survival (DFS) was defined as the time from randomization to disease recurrence or distant metastasis. Survival curves were generated using the Kaplan–Meier method and assessed with log-rank tests for univariate survival analysis. Multivariate survival analysis was then performed using Cox regression to calculate hazard ratios and 95% confidence intervals for the associations between baseline patient characteristics and survival. A P value <0.05 was considered statistically significant. All analyses were performed using SPSS 26.0 software (IBM Corp.).
Results
Patients
A total of 2310 patients underwent esophagectomy for ESCC in the Department of Thoracic Surgery, Fourth Hospital, Hebei Medical University, from January 2008 to June 2018, of whom, 301 (13.0%) were lost to follow-up and 140 (6.1%) were pathologically non-SCC. The remaining 1869 cases were included in the study. Eighty-one patients met the criteria and were included in Group A, of whom two were subsequently excluded because of an inability to perform sectioning and staining. The remaining 79 cases were included in the final analysis. Propensity matching produced 79 matched pairs for Groups A and P.
The clinicopathological data of the two groups of patients were subjected to descriptive statistical analysis (Table 1).
Table 1.
Clinicopathological information in patients with esophageal squamous cell carcinoma.
| Variable | Group | Group A | Group P | χ2 | P-value |
|---|---|---|---|---|---|
| Sex | Male | 54 | 53 | 0.03a | 0.86 |
| Female | 25 | 26 | |||
| Age, years | ≤60 | 23 | 20 | 0.29a | 0.59 |
| >60 | 56 | 59 | |||
| History of smoking | Yes | 40 | 38 | 0.10a | 0.75 |
| No | 39 | 41 | |||
| History of alcohol use | Yes | 50 | 49 | 0.03a | 0.87 |
| No | 29 | 30 | |||
| History of hypertension | Yes | 55 | 58 | 0.28a | 0.60 |
| No | 24 | 21 | |||
| History of CHD | Yes | 16 | 6 | 5.28a | 0.02 |
| No | 63 | 73 | |||
| History of diabetes | Yes | 11 | 12 | 0.05a | 0.82 |
| No | 68 | 67 | |||
| Tumor location | Upper 1/3 thoracic segment | 7 | 8 | 0.08a | 0.96 |
| Middle 1/3 | 53 | 52 | |||
| Lower 1/3 | 19 | 19 | |||
| Tumor size, cm | <5/ | 43 | 51 | 1.68a | 0.20 |
| ≥5 | 36 | 28 | |||
| Degree of differentiation | Undetermined and well differentiated | 71 | 73 | 0.31a | 0.58 |
| Moderate and poorly differentiated | 8 | 6 | |||
| T stage | Tis | 1 | 3 | 6.34a | 0.18 |
| T1 | 15 | 14 | |||
| T2 | 18 | 13 | |||
| T3 | 39 | 48 | |||
| T4 | 6 | 1 | |||
| N stage | N0 | 52 | 57 | 3.58a | 0.31 |
| N1 | 19 | 16 | |||
| N2 | 5 | 6 | |||
| N3 | 3 | 0 | |||
| TNM stage | 0 | 2 | 3 | 0.70a | 0.87 |
| I | 17 | 17 | |||
| II | 37 | 40 | |||
| III | 23 | 19 | |||
| Lymph node metastasis | Yes | 27 | 22 | 0.74a | 0.39 |
| No | 52 | 57 | |||
| LNR | 0 | 52 | 57 | 4.37a | 0.11 |
| >0 to ≤0.1 | 11 | 15 | |||
| >0.1 | 16 | 7 | |||
| Margin-positive | Yes | 5 | 3 | 0.53a | 0.47 |
| No | 74 | 76 | |||
| Vascular tumor invasion | Yes | 6 | 2 | 2.11a | 0.15 |
| No | 73 | 77 | |||
| Neural tumor invasion | Yes | 9 | 6 | 0.66a | 0.42 |
| No | 70 | 73 | |||
| Neoadjuvant radiochemotherapy | Yes | 5 | 2 | 1.35a | 0.25 |
| No | 74 | 77 | |||
| Postoperative complication | Yes | 22 | 21 | 0.03a | 0.86 |
| No | 57 | 58 | |||
| Postoperative neoadjuvant therapy | No adjuvant therapy | 42 | 40 | 11.98a | 0.01 |
| Radiotherapy alone | 2 | 12 | |||
| Chemotherapy alone | 22 | 23 | |||
| Radiochemotherapy | 13 | 4 |
aExpected count of 0 cells (0.0%) <5; minimum expected count 25.50.
CHD, coronary heart disease; LNR, lymph node ratio.
IHC staining results
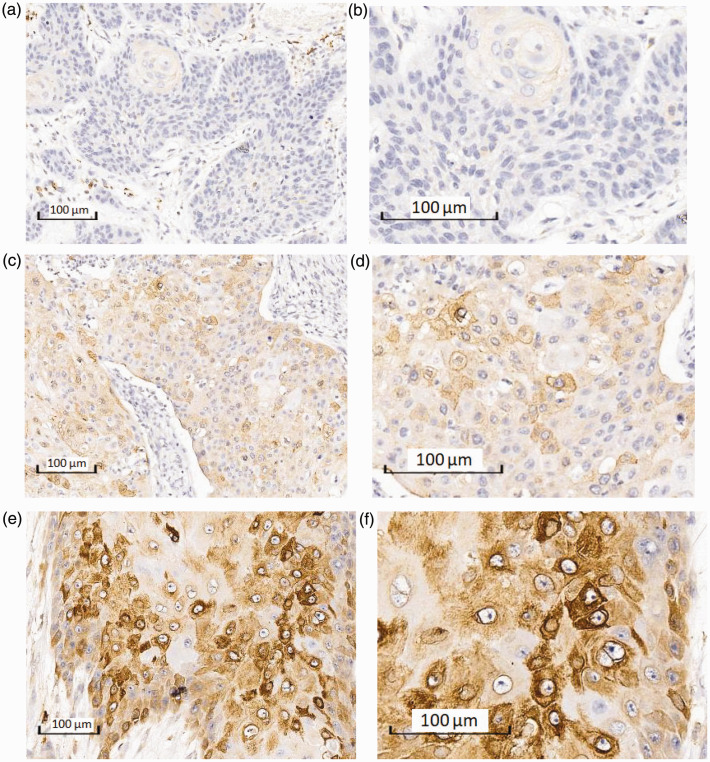
COX-2 protein expression levels in ESCC tissues in both groups are shown in Table 2. COX-2 expression was significantly lower in tissues from aspirin users compared with non-users (χ2 = 7.67, P = 0.02). Representative IHC staining images are shown in Figure 1.
Table 2.
Staining results.
| Group A | Group P | Total | |
|---|---|---|---|
| Negative (0 points) | 27 (34.2%) | 12 (15.2%) | 39 |
| Low (1–3 points) | 36 (45.6%) | 47 (59.5%) | 83 |
| High (>4 points) | 16 (20.3%) | 20 (25.3%) | 36 |
| Total | 79 | 79 | 158 |
| χ2 | 7.67a | ||
| P value | 0.02 | ||
aExpected count of 0 cells (0.0%) <5; minimum expected count 18.00.
Figure 1.
Immunohistochemical staining of cyclooxygenase-2 (COX-2) in esophageal squamous cell carcinoma tissues. Negative COX-2 staining at (a) ×10 and (b) ×20 magnification. Low COX-2 protein expression at (c) ×10 and (d) ×20 magnification. High COX-2 protein expression at (e) ×10 and (f) ×20 magnification.
Survival analysis
Comparison of survival and recurrence between the two groups
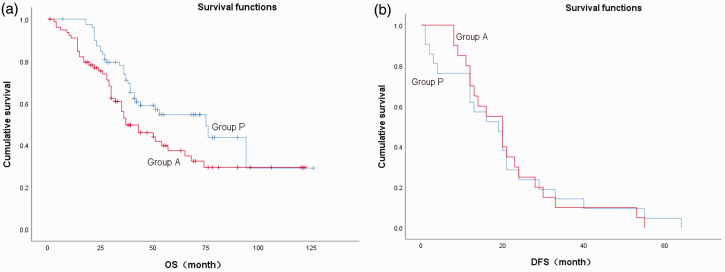
The mean ( ± standard deviation) follow-up time of all patients was 68 ± 7.16 months and the mean survival time was 51.0 ± 9.35 months. At the end of the follow-up period, there had been 45 deaths in Group A (57.0%) and 35 deaths in Group P (44.3%), and this difference was not statistically significant. However, the 1-, 3-, and 5-year survival rates and median survival time (P = 0.02) were all significantly higher in the group A compared with the group P. However, there was no significant difference in recurrence rates or recurrence time between the two groups (Table 3, Figure 2).
Table 3.
Comparison of survival and recurrence.
| Group A | Group P | P-value | |
|---|---|---|---|
| 1-year survival rate (%) | 91.0 | 97.4 | |
| 3-year survival rate (%) | 52.8 | 73.8 | |
| 5-year survival rate (%) | 37.4 | 54.6 | |
| Median survival time (months) | 37 | 75 | 0.02 |
| 1-year recurrence rate (%) | 70.0 | 61.9 | |
| 3-year recurrence rate (%) | 10.0 | 14.3 | |
| 5-year recurrence rate (%) | 0.0 | 4.8 | |
| Median recurrence time (months) | 20 | 19 | 0.95 |
Figure 2.
Survival functions in patients with esophageal squamous cell carcinoma. (a) Overall survival (OS) and (b) disease-free survival (DFS) in patients with (Group A = 1) and without aspirin (Group P = 0).
Comparison of survival rates in relation to COX-2 expression levels
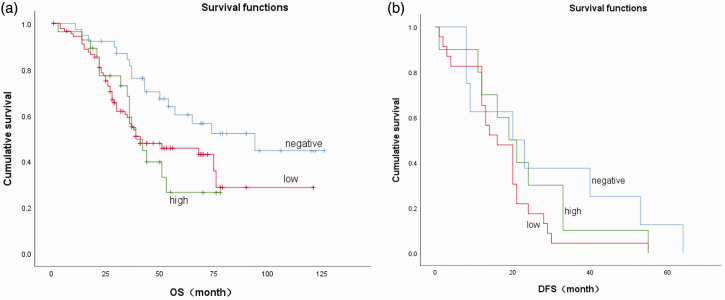
Survival analysis was performed according to COX-2 protein expression levels. COX-2 expression levels were significantly related to long-term survival rates, with higher COX-2 expression levels associated with shorter OS (P = 0.001) and DFS (P = 0.05). The specific results are shown in Table 4 and Figure 3.
Table 4.
Survival analysis of patients with esophageal squamous cell carcinoma according to cyclooxygenase-2 protein expression levels.
| Cyclooxygenase-2 protein expression |
P-value | |||
|---|---|---|---|---|
| Negative | Low | High | ||
| 1-year survival rate (%) | 97.4 | 95.1 | 94.4 | |
| 3-year survival rate (%) | 81.6 | 61.3 | 46.7 | |
| 5-year survival rate (%) | 60.3 | 50.0 | 21.0 | |
| Median survival time (months) | 94 | 68 | 36 | 0.001 |
| 1-year recurrence rate (%) | 62.5 | 72.2 | 60.0 | |
| 3-year recurrence rate (%) | 37.5 | 11.1 | 0.0 | |
| 5-year recurrence rate (%) | 12.5 | 0.0 | 0.0 | |
| Median recurrence time (months) | 20 | 20 | 16 | 0.05 |
Figure 3.
Overall survival (OS) and disease-free survival (DFS) in relation to cyclooxygenase-2 (COX-2) expression levels in patients with esophageal squamous cell carcinoma. (a) OS and (b) DFS in patients with different COX-2 protein expression levels (0 = negative, 1 = low, 2 = high).
Survival analysis of Group A ESCC patients
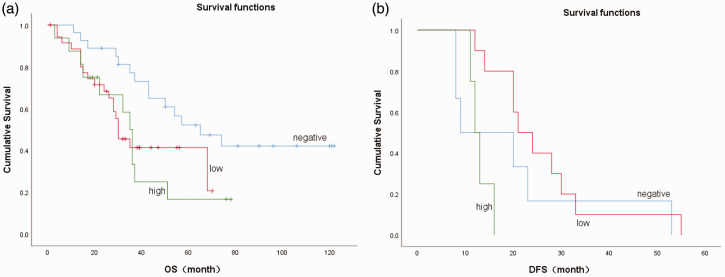
We conducted survival analysis of patients in Group A (aspirin users) according to their COX-2 protein expression levels. COX-2 expression was significantly associated with long-term OS in these patients (P = 0.02) but there was no significant difference in DFS among the different COX-2 expression groups. The 5-year DFS rates were all 0%, irrespective of the COX-2 expression level. The detailed results are shown in Table 5 and Figure 4.
Table 5.
Survival analysis of patients with esophageal squamous cell carcinoma in Group A according to cyclooxygenase-2 protein expression levels.
| Cyclooxygenase-2 protein expression |
P-value | |||
|---|---|---|---|---|
| Negative | Low | High | ||
| 1-year survival rate (%) | 96.3 | 88.6 | 87.5 | 0.02 |
| 3-year survival rate (%) | 77.1 | 41.4 | 33.3 | |
| 5-year survival rate (%) | 52.2 | 41.4 | 16.7 | |
| Median survival time (months) | 65 | 30 | 35 | |
| 1-year recurrence rate (%) | 50.0 | 90.0 | 50.0 | 0.56 |
| 3-year recurrence rate (%) | 16.7 | 10.0 | 0.0 | |
| 5-year recurrence rate (%) | 0.0 | 0.0 | -- | |
| Median recurrence time (months) | 9 | 21 | 12 | |
Figure 4.
Overall survival (OS) and disease-free survival (DFS) in relation to cyclooxygenase-2 (COX-2) expression levels in patients in Group A with esophageal squamous cell carcinoma. (a) OS and (b) DFS in patients in Group A with different COX-2 protein expression levels (0 = negative, 1 = low, 2 = high).
Analysis of independent risk factors in ESCC patients taking aspirin
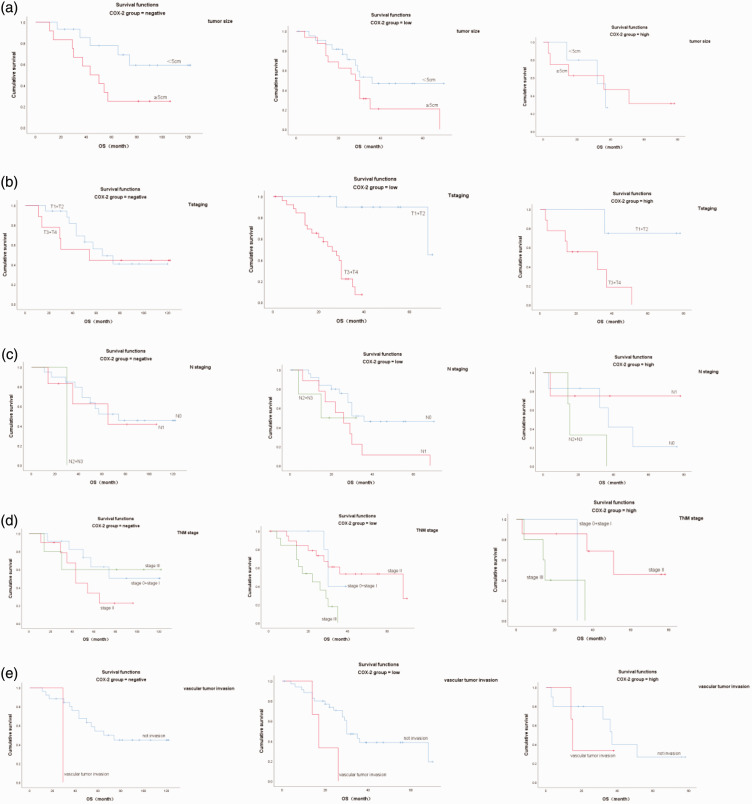
Univariate analysis suggested that tumor size (P = 0.03), T stage (P < 0.01), N stage (P = 0.02), TNM stage (P = 0.02), and vascular invasion (P = 0.02) were significantly associated with OS. However, age, sex, smoking history, alcohol consumption history, and tumor location were not associated with OS. These significant factors were then entered into Cox regression analysis, and multivariate analysis identified T stage as the only independent prognostic factor. The detailed results are shown in Tables 6 and 7 and Figures 5 and 6.
Table 6.
Univariate analysis of overall survival in cyclooxygenase-2 (COX-2) negative and positive groups among patients with esophageal squamous cell carcinoma in group A.
| Variable | Group | Median survival time (months) |
P-value | ||
|---|---|---|---|---|---|
| Negative COX-2 | Low COX-2 | High COX-2 | |||
| Sex | Male | 65 | 28 | 51 | 0.38 |
| Female | --a | --a | 35 | ||
| Age, years | ≤60 | --a | 28 | 35 | 0.92 |
| >60 | 50 | 30 | 51 | ||
| History of smoking | Yes | 57 | 30 | --a | 0.81 |
| No | 74 | 29 | 36 | ||
| History of alcohol use | Yes | 43 | 15 | 51 | 0.20 |
| No | 65 | 30 | 36 | ||
| History of hypertension | Yes | 65 | 30 | 37 | 0.63 |
| No | 57 | 15 | 36 | ||
| History of CHD | Yes | --a | 14 | 37 | 0.75 |
| No | 57 | 30 | 36 | ||
| History of diabetes | Yes | 65 | 35 | 22 | 0.83 |
| No | 57 | 28 | 37 | ||
| Tumor location | Upper 1/3 thoracic segment | 54 | 14 | 37 | 0.82 |
| Middle 1/3 | 65 | 28 | 51 | ||
| Lower 1/3 | 74 | 30 | 35 | ||
| Tumor size, cm | <5 | --a | 30 | 36 | 0.03 |
| ≥5 | 43 | 26 | 51 | ||
| Degree of differentiation | Undetermined and well differentiated | 65 | 30 | 37 | 0.13 |
| Moderate and poorly differentiated | 35 | 14 | 36 | ||
| T stage | T1 + T2 | 65 | 68 | --a | 0.00 |
| T3 + T4 | 54 | 20 | 35 | ||
| N stage | N0 | 74 | 30 | 37 | 0.02 |
| N1 | 65 | 26 | --a | ||
| N2 + N3 | 30 | 15 | 36 | ||
| TNM stage | 0 + I | --a | 30 | 32 | 0.02 |
| II | 43 | 68 | 51 | ||
| III | --a | 15 | 22 | ||
| Lymph node metastasis | Yes | 65 | 17 | 36 | 0.07 |
| No | 74 | 30 | 37 | ||
| LNR | 0 | --c | --c | --c | 0.05 |
| >0 to ≤0.1 | --c | --c | --c | ||
| >0.1 | --c | --c | --c | ||
| Margin-positive | Yes | 54 | 14 | 51 | 0.95 |
| No | 65 | 29 | 36 | ||
| Vascular tumor invasion | Yes | 29 | 15 | 14 | 0.02 |
| No | 65 | 30 | 36 | ||
| Neural tumor invasion | Yes | --c | --c | --c | 0.82 |
| No | --c | --c | --c | ||
| Neoadjuvant radiochemotherapy | Yes | -- | 22 | 3 | --b |
| No | 65 | 30 | 37 | ||
| Postoperative complication | Yes | 74 | 30 | 51 | 0.79 |
| No | 57 | 29 | 36 | ||
| Postoperative neoadjuvant therapy | No adjuvant therapy | --c | --c | --c | 0.55 |
| Chemotherapy alone | --c | --c | --c | ||
| Radiochemotherapy | --c | --c | --c | ||
aIf the estimate had been censored, it would be limited to the longest survival time; bno pooled comparison because at least one layer had no valid cases for every factor level; cno statistical analysis because all cases censored
CHD, coronary heart disease; LNR, lymph node ratio.
Table 7.
Regression analysis of multivariate Cox model for overall survival in patients with esophageal squamous cell carcinoma in group A.
| Variable | Hazard ratio | 95% Confidence interval | I value |
|---|---|---|---|
| Tumor size | 1.30 | 0.68–2.49 | 0.43 |
| T stage | 2.12 | 1.41–3.20 | <0.001 |
| N stage | 1.83 | 0.88–3.83 | 0.11 |
| TNM staging | 0.70 | 0.35–1.37 | 0.70 |
| Vascular tumor invasion | 2.00 | 0.74–5.26 | 0.18 |
Figure 5.
Significant factors affecting overall survival (OS) according to univariate analysis. (a) Tumor length (1 = <5 cm, 2 = ≥5 cm); (b) T staging (1 = T1 + T2, 3 = T3 + T4); (c) N staging (1 = N0, 2 = N1, 3 = N2 + N3); (d) TNM staging (1 = stage I, 2 = stage II, 3 = stage III); and (e) vascular tumor invasion (0 = no, 1 = yes). COX-2, cyclooxygenase-2.
Figure 6.
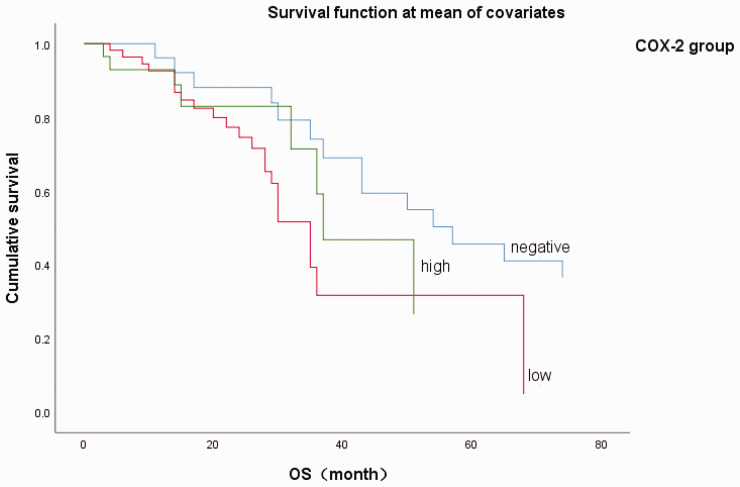
Cox regression analysis of overall survival (OS) in patients in Group A with esophageal squamous cell carcinoma. 0 = cyclooxygenase-2 (COX-2) negative, 1 = COX-2 weak positive, 2 = COX-2 strong positive.
Discussion
The development of cancer is a complex process that is affected by a variety of factors. Expression levels of COXs and the production of AA metabolites, such as PGs, have been shown to be closely related to the occurrence, development, and outcome of various cancers.18,19 COX-2 is a rate-limiting enzyme for PG synthesis. PGs are inflammatory signaling mediators that can regulate cell survival and growth, promote cancer cell proliferation, enhance tumor immune invasion, and induce the formation of cancer stem cell-like cells.20,21 We previously 22 found that higher COX-2 expression levels in tumor tissues of patients with esophageal cancer (EC) were correlated with lower 5-year survival rates. COX-2 expression is thus considered to be an indicator of a poor prognosis in patients with EC.
The current study aimed to clarify the effect of regular long-term aspirin use before diagnosis on the prognosis of ESCC patients, and the effect of different COX-2 expression levels on patient survival. We conducted a detailed survival analysis and compared survival rates between aspirin-users and non-aspirin users, as well as between patients with different COX-2 expression levels. We found that the median survival rate was significantly higher in non-aspirin users than in aspirin users, suggesting that long-term aspirin use before diagnosis had an adverse effect on the survival of patients with ESCC.
Aspirin, also known as acetylsalicylic acid, can be used as an analgesic, antipyretic, and anti-inflammatory drug. However, the results of numerous studies on the effects of long-term low-dose aspirin use before diagnosis on cancer survival have been mixed. Schneider et al. 23 concluded that regular use of aspirin had a protective effect in patients with on esophageal adenocarcinoma, especially in people with gastroesophageal reflux disease. Jonsson et al. 24 analyzed the use of low-dose aspirin before diagnosis and found that use of aspirin 1 year before diagnosis reduced the scope of the tumors and metastatic disease in patients with colorectal cancer and lung cancer, suggesting that aspirin use could be beneficial in these types of tumors.
Other researchers have proposed different views. Staalduinen et al. 25 compared the survival effects of taking aspirin and other NSAIDs before and after the diagnosis of EC, and found that only aspirin use after diagnosis was significantly related to survival. Li et al. 26 analyzed the survival benefits of aspirin before and after the diagnosis of colon cancer, and showed that the survival benefit of aspirin after diagnosis was limited to patients with positive COX-2 expression and PIKCA mutations. Their data suggested no association between pre-diagnosis use of aspirin and colon cancer mortality. Araujo et al. 27 also concluded that taking aspirin before diagnosis was not related to all-cause or cancer-specific mortality in EC patients, but was related to an increased risk of metastasis.
The current study indicated that long-term, pre-diagnosis aspirin use could lead to poor postoperative survival among ESCC patients. This result, showing that long-term use of low-dose aspirin had an adverse effect on cancer survival, appears to contradict previous results regarding the anti-tumor mechanism of aspirin.
These apparently contradictory results may be due to several factors. First, the patients included in the currents study all needed long-term aspirin use for various chronic diseases. The clinicopathological data indicated differences between the two patient groups in terms of the history of coronary heart disease, which would also affect patient survival to some certain extent. Second, aspirin can affect platelet function, 28 and its long-term use can aggravate bleeding tendencies. Aspirin has demonstrated adverse survival effects in other studies. Yokoyama et al. 29 found that low-dose aspirin failed to reduce the incidence of cancer or cancer mortality in an elderly Japanese population, while McNeill et al. 30 found that all-cause mortality was higher among elderly people who took aspirin every day compared with elderly people who received placebo, and the main cause of death was related to cancer.
Finally, we also considered changes in the tumor cells themselves. Carcinogenesis involves aberrant gene expression and changes in many signaling pathways. We observed lower COX-2 protein expression levels in the aspirin-user group, probably related to inhibition of the COX pathway by aspirin. In addition, further analysis of the subgroup of patients taking aspirin showed that negative COX-2 expression was associated with higher survival than either low or high COX-2 expression levels. This suggests that the prognosis of patients with long-term pre-diagnosis aspirin use was dependent on their COX-2 expression status. Hu et al. 31 indicated that overexpression of COX-2 in EC tissues was associated with poor OS, supporting the theory that increased COX-2 levels may be a prognostic indicator for EC.
This study had some limitations. It was a single-center retrospective cohort study. Most of the patients lived in the local and nearby areas, and the patients’ recall of previous exposure to aspirin may have been biased (information bias). In addition, the nature of the study also contributed to the small number of enrolled cases. The preventive use of aspirin may take many years to improve patient survival, and it is therefore necessary to expand the sample size and extend the follow-up time. In addition, some patients continued to take aspirin for underlying diseases after surgery; however, this was not recorded and the impact of postoperative aspirin use was not included in the analysis. Further prospective randomized controlled experimental studies are therefore needed to clarify the role of aspirin.
Conclusions
The results of this study indicated that long-term, regular use of aspirin before diagnosis may have an adverse effect on postoperative survival in patients with ESCC patients, but not on DFS. Postoperative survival times differed significantly in relation to COX-2 expression levels, and ESCC patients who did not use aspirin or with negative COX-2 protein expression had better survival rates. Depth of tumor invasion was the only independent predictor of postoperative survival in ESCC patients who took aspirin before diagnosis.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221089799 for Effect of taking aspirin before diagnosis on the prognosis of esophageal squamous cell carcinoma and analysis of prognostic factors by Jiang Jiang, Junfeng Liu, Ping Gao and Junying Liu in Journal of International Medical Research
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jiang Jiang https://orcid.org/0000-0001-7880-7232
Supplemental material
Supplemental material for this article is available online.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin 2019; 69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Totsuka Y, Shan B, et al. Esophageal cancer in high-risk areas of China: research progress and challenges. Ann Epidemiol 2017; 27: 215–221. [DOI] [PubMed] [Google Scholar]
- 3.Liu JF, Wang QZ, Hou J. Surgical treatment for cancer of the esophagus and gastric cardia in Hebei, China [J]. Br J Surg 2004; 91: 90–98. [DOI] [PubMed] [Google Scholar]
- 4.Awtry EH, Loscalzo J. Aspirin. Circulation 2000; 101: 1206–1218. [DOI] [PubMed] [Google Scholar]
- 5.Patrono C. The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J Am Coll Cardiol 2015; 66: 74–85. [DOI] [PubMed] [Google Scholar]
- 6.Yousif NG, Al-Amran FG, Hadi N, et al. Expression of IL-32 modulates NF-kappaB and p38 MAP kinase pathways in human esophageal cancer. Cytokine 2013; 61: 223–227. [DOI] [PubMed] [Google Scholar]
- 7.Qiao Y, Yang T, Gan Y, et al. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer 2018; 18: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao W, Shen Y, Feng M, et al. Aspirin acts in esophageal cancer: a brief review. J Thorac Dis 2018; 10: 2490–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y, Kemper T, Qiu J, et al. Defining the therapeutic time window for suppressing the inflammatory prostaglandin E2 signaling after status epilepticus. Expert Rev Neurother 2016; 16: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata H, Kawano S, Tsuji S, et al. Cyclooxygenase-2 over expression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol 1999; 94: 451–455. [DOI] [PubMed] [Google Scholar]
- 11.Liu JF, Jamieson GG, Wu TC, et al. A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Ann Surg Oncol 2009; 16: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 13.Pourshams A, Khademi H, Malekshah AF, et al. Cohort profile: The Golestan Cohort Study-a prospective study of oesophageal cancer in Northern Iran. Ann Surg Oncol 2010; 39: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12: e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos-Vara JA. Principles and Methods of Immunohistochemistry. Methods in Molecular Biology (Clifton, NJ) 2011; 691: 83–96. [DOI] [PubMed] [Google Scholar]
- 17.Mougiakakos D, Johansson CC, Trocme E, et al. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2–positive uveal melanoma. Cancer 2010; 116: 2224–2233. [DOI] [PubMed] [Google Scholar]
- 18.Zha S, Yegnasubramanian V, Nelson WG, et al. Cyclooxygenases in cancer: progress and perspective. Cancer Lett 2004; 215: 1–20. [DOI] [PubMed] [Google Scholar]
- 19.Greenhough A, Smartt HJ, Moore AE, et al. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009; 30: 377–386. [DOI] [PubMed] [Google Scholar]
- 20.Majumder M, Xin X, Liu L, et al. Cox-2 induces breast cancer stem cells via EP4/PI3K/AKT/NOTCH/WNT axis. Stem Cells 2016; 34: 2290–2305. [DOI] [PubMed] [Google Scholar]
- 21.Che D, Zhang S, Jing Z, et al. Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE 2/β-catenin signalling pathway. Mol Immunol 2017; 90: 197–210. [DOI] [PubMed] [Google Scholar]
- 22.Liu JF, Jamieson G, Wu TC, et al. Cyclooxygenase-2 expression in squamous cell carcinoma of the esophagus. Dis Esophagus 2006; 19: 350–354. [DOI] [PubMed] [Google Scholar]
- 23.Schneider J, Zhao W, Corley D. Aspirin and non-steroidal anti-inflammatory drug use and the risk of Barrett’s esophagus. Dig Dis Sci 2015; 60: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonsson F, Yin L, Lundholm C, et al. Low-dose aspirin use and cancer characteristics: a population-based cohort study. Br J Cancer 2013; 109: 1921–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Staalduinen J, Frouws M, Reimers M, et al. The effect of aspirin and nonsteroidal anti-inflammatory drug use after diagnosis on survival of oesophageal cancer patients [J]. Br J Cancer 2016; 114: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Wu H, Zhang H, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut 2015; 64: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 27.Araujo JL, Altorki NK, Sonett JR, et al. Prediagnosis aspirin use and outcomes in a prospective cohort of esophageal cancer patients. Therap Adv Gastroenterol 2016; 9: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest 1975; 56: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokoyama K, Ishizuka N, Uemura N, et al. Effects of daily aspirin on cancer incidence and mortality in the elderly Japanese. Res Pract Thromb Haemost 2018; 2: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 2018; 379: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Z, Yang Y, Zhao Y, et al. The prognostic value of cyclooxygenase-2 expression in patients with esophageal cancer: evidence from a meta-analysis. Onco Targets Ther 2017; 10: 2893–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221089799 for Effect of taking aspirin before diagnosis on the prognosis of esophageal squamous cell carcinoma and analysis of prognostic factors by Jiang Jiang, Junfeng Liu, Ping Gao and Junying Liu in Journal of International Medical Research