Abstract
Background:
Over 400 million people are diabetic. Type 1 and type 2 diabetes are characterized by decreased functional β-cell mass and, consequently, decreased glucose-stimulated insulin secretion. A potential intervention is transplantation of β-cell containing islets from cadaveric donors. A major impediment to greater application of this treatment is the scarcity of transplant-ready β-cells. Therefore, inducing β-cell proliferation ex vivo could be used to expand functional β-cell mass prior to transplantation. Various molecular pathways are sufficient to induce proliferation of young β-cells; however, aged β-cells are refractory to these proliferative signals. Given that the majority of cadaveric donors fit an aged demographic, defining the mechanisms that impede aged β-cell proliferation is imperative.
Results:
We demonstrate that aged rat (5-month-old) β-cells are refractory to mitogenic stimuli that otherwise induce young rat (5-week-old) β-cell proliferation. We hypothesized that this change in proliferative capacity could be due to differences in cyclin-dependent kinase inhibitor expression. We measured levels of p16INK4a, p15INK4b, p18INK4c, p19INK4d, p21CIP1, p27KIP1 and p57KIP2 by immunofluorescence analysis. Our data demonstrates an age-dependent increase of p27KIP1 in rat β-cells by immunofluorescence and was validated by increased p27KIP1 protein levels by western blot analysis. Interestingly, HDAC1, which modulates the p27KIP1 promoter acetylation state, is downregulated in aged rat islets. These data demonstrate increased p27KIP1 protein levels at 5 months of age, which may be due to decreased HDAC1 mediated repression of p27KIP1 expression.
Significance:
As the majority of transplant-ready β-cells come from aged donors, it is imperative that we understand why aged β-cells are refractory to mitogenic stimuli. Our findings demonstrate that increased p27KIP1 expression occurs early in β-cell aging, which corresponds with impaired β-cell proliferation. Furthermore, the correlation between HDAC1 and p27 levels suggests that pathways that activate HDAC1 in aged β-cells could be leveraged to decrease p27KIP1 levels and enhance β-cell proliferation.
Keywords: aging, β-cell, diabetes, HDAC1, p27KIP1
INTRODUCTION
Insulin-secreting β-cells are found in the pancreatic Islets of Langerhans. Their primary role is to maintain glucose homeostasis by sensing elevated blood glucose levels and secreting insulin in response. Decreased functional β-cell mass is a hallmark of type 1 and type 2 diabetes. Islet transplantation from cadaveric donors can successfully replace lost β-cell mass and normalize glucose homeostasis. This can reduce or eliminate the occurrence of severe hypoglycemic events. This is evidenced by patients that undergo islet replacement therapy have reported insulin independence and successful control of hypoglycemic episodes 5 years posttransplantation (Hering et al., 2016). Unfortunately, a lack of transplant-ready β-cells prevents greater utilization of this treatment modality (Farney, Sutherland, & Opara, 2016). Recent islet transplantation efforts indicate that two or more pancreata are required to obtain sufficient β-cell mass for successful blood glucose maintenance (Farney et al., 2016; Shapiro, Pokrywczynska, & Ricordi, 2017). This is problematic due to the difficulty of acquiring several donors for each diabetic patient seeking treatment. Therefore, inducing β-cell proliferation ex vivo prior to transplantation could be used to increase the transplant-ready β-cell pool. This would allow for greater use of islet transplantation, as a single pancreas could provide sufficient functional β-cell mass for one, or multiple, diabetic individuals.
In humans and rodents, β-cell proliferation significantly decreases in early life, with the majority of human β-cell replication completed prior to adolescence (Butler, Meier, Butler, & Bhushan, 2007; Gregg et al., 2012; Perl et al., 2010). Rodent and human studies, however, have demonstrated that under conditions such as obesity or pregnancy, β-cell proliferation can be induced (Butler et al., 2010; Butler et al., 2007; Cox et al., 2016). This demonstrates that while the pathways that regulate β-cell proliferation are tightly controlled, the pathways are generally intact and, given the proper signals, can be initiated. Numerous studies have begun to define pathways that are sufficient to induce β-cell proliferation in rodent and human islets (Draney et al., 2018; Draney, Hobson, Grover, Jack, & Tessem, 2016; Hayes et al., 2013; Hobson et al., 2015; Salpeter et al., 2013; Tessem et al., 2014). β-cells have been shown to enter a refractory period post-replication, and the duration of this refractory period increases with age, amounting to several months in old rodents (Salpeter, Klein, Huangfu, Grimsby, & Dor, 2010). These data suggest that aging induces changes that inhibit β-cell proliferation. Given that the majority of islet donors are aged, understanding the molecular restraints that impede aged β-cell proliferation is essential if islet transplantation as a treatment method for diabetes is to become more widely available.
β-cells express most of the classical cell cycle components necessary for cellular replication (Fiaschi-Taesch et al., 2013a, 2013b). Mitogenic stimulation increases the cellular levels of D Cyclins (Dijkers et al., 2000; Fiaschi-Taesch et al., 2009; Ingham & Schwartz, 2017). In the early G1 phase of the cell cycle, D cyclins bind to and activate CDK4 and CDK6. β-cells, however, have differential expression of these factors as human β-cell lack expression of Cyclin D2 and rodent β-cells have low expression of CDK6 (Fiaschi-Taesch, Kleinberger, Salim, Troxell, Wills, Tanwir, Casinelli, Cox, Takane, Scott, et al., 2013). CDK4 knock out mice present with decreased β-cell mass and diabetes, and Cyclin D1/D2 double knock out mice are diabetic as early as 3 months of age (Kushner, 2006; Rane, Cosenza, Mettus, & Reddy, 2002). Cyclin D/CDK4 or CDK6 activity results in retinoblastoma protein phosphorylation, which in turn releases the various E2Fs. β-cells express all E2F’s with the exception of E2F8, with E2F1 and E2F4 being the predominant family members (Cozar-Castellano, Haught, & Stewart, 2006; Cozar-Castellano, Weinstock, et al., 2006; Fiaschi-Taesch et al., 2013a). Upregulation of E2F transcriptional targets, such as the Cyclin E’s, Cyclin A’s and Cyclin B’s, allow for further cell cycle progression, DNA replication and, ultimately, mitotic cell division (Li et al., 2003; Salpeter et al., 2010; Tessem et al., 2007). The majority of most cell cycle components in the β-cell are found in the cytosol, rather than the nucleus. As β-cells move from a quiescent to a proliferative state, migration of various components to the nucleus is observed (Fiaschi-Taesch et al., 2013b).
The cell cycle is negatively regulated by two families of cyclin-dependent kinase inhibitors (CDKIs). The Ink4 CDKI family (p16INK4a, p15INK4b, p18INK4c, p19INK4d) bind to the Cyclin D-CDK4/6 complex, inhibiting the catalytic activity of CDK4/6 and thereby leaving the cell in a quiescent state (Chen et al., 2009; Fiaschi-Taesch et al., 2009; Ingham & Schwartz, 2017; Stein, Milewski, & Dey, 2013). The Cip/Kip CDKI family (p21CIP1, p27KIP1, p57KIP2) is primarily responsible for inhibiting the Cyclin E-CDK2 complex (Kulkarni, Mizrachi, Ocana, & Stewart, 2012; Rankin & Kushner, 2009). Each of these CDKI’s are expressed in adult rodent and human β-cells (Cozar-Castellano, Haught, et al., 2006; Cozar-Castellano, Weinstock, et al., 2006). The presence of these CDKI’s in the β-cell nucleus can impair cell cycle progression. Interestingly, while CDKI’s can be observed in the nuclei of proliferative and non-proliferative β-cells, p27KIP1 is never observed in the nucleus of proliferating β-cells (Fiaschi-Taesch et al., 2013b).
The histone acetylation state plays a critical role in gene transcription, where acetylated histones are generally associated with highly transcribed genes, and deacetylated histones are associated with repressed gene transcription (Moser, Hagelkruys, & Seiser, 2014). Histone acetyltransferases (HATs) function by adding acetyl groups to histone lysine residues. Histone deacetylases (HDACs), in contrast, remove acetyl groups. HDAC activity can positively or negatively regulate various components of the cell cycle (Brazelle et al., 2010; Goder et al., 2018). In particular, HDAC1 regulates expression of various cell cycle inhibitors, including p16INK4a,p15INK4b, p19INK4d, p21CIP1, p27KIP1, and p57KIP2 (Hitomi et al., 2007; Jin & Datta, 2014; Martin et al., 2013; Zhou et al., 2018). While HDAC1 can regulate expression of many of the cell cycle inhibitors, it has an especially strong effect on p27KIP1 expression. Cells deficient in HDAC1 have increased p27KIP1 expression (Lagger et al., 2002; Zupkovitz et al., 2010), and HDAC1 overexpression results in decreased p27KIP1 expression (Huang et al., 2014; Pang et al., 2011). Similarly, HDAC1 overexpression has been shown to preferentially decrease the acetylation state of the p27KIP1 promoter (Huang et al., 2017; Trivedi, Lu, Wang, & Epstein, 2008). Finally, we have previously shown that HDAC1 overexpression in primary rat and human islets are sufficient to induce proliferation, and that this corresponds with decreased p27KIP1 expression (Draney et al., 2018).
Here, we present data demonstrating that 5-month-old Wistar rat β-cells are refractory to the same mitogenic signals that induce proliferation of β-cells from 5-week-old Wistar rats. We demonstrate that 5-month aged rat β-cells have increased p27KIP1 levels, with a significant increase in nuclear localization. Furthermore, aged β-cells have decreased HDAC1 mRNA and protein levels. These data suggest that decreased expression of HDAC1 in aged β-cells results in increased p27KIP1 expression, which impairs the ability of aged β-cells to respond to exogenous mitogenic signals.
RESULTS
Aged β-cells are refractory to known proliferative mechanisms
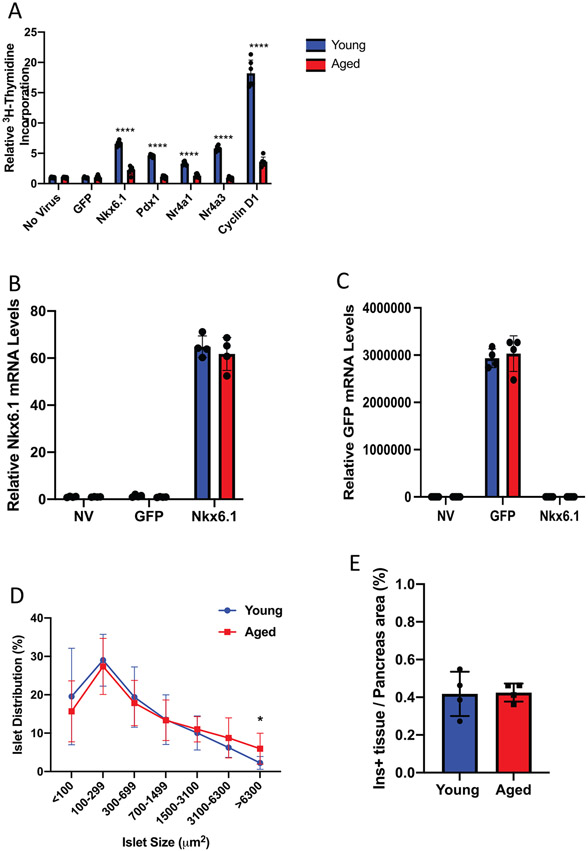
Overexpression of various transcription factors and cell cycle components can successfully stimulate rodent and human β-cell replication. We have shown that β-cell replication can be mediated by the orphan nuclear receptors Nr4a1 and Nr4a3 (Tessem et al., 2014). The β-cell transcription factors Pdx1 and Nkx6.1 are also sufficient to induce β-cell proliferation through upregulation of Cyclin D and E (Hayes et al., 2013; Schisler et al., 2008b). To understand how the β-cell responds to proliferative signals as a function of age, islets from 5-week-old or 5-month-old Wistar rats were transduced with adenovirus expressing GFP, Nkx6.1, Pdx1, Nr4a1, Nr4a3, and Cyclin D1. Proliferation was measured using 3H-thymidine incorporation. As has been previously shown through 3H-thymidine and EdU incorporation, each of these factors were sufficient to induce proliferation in young islets (5 weeks). Islets from aged (5 month) animals, however, were refractory to factor-induced proliferation (Figure 1A).
FIGURE 1.
Aged rat islets are refractory to mitogenic signals that induce proliferation in young rat islets. (A) Young (5 week) and aged (5 month) islets (n = 6 each) were left untransduced or transduced with AdCMV-GFP, AdCMV-Nkx6.1, AdCMV-Pdx1, AdCMV-Nr4a1, AdCMV-Nr4a3, or AdCMV-Cyclin D1. Young islets demonstrated increased proliferation as measured by 3H-thymidine incorporation, while aged islets had impaired proliferation (n = 6 mice per age). Young and aged islets (n = 4 mice per age) express (B) Nkx6.1 and (C) GFP mRNA levels at similar levels after adenoviral transduction. (D) Young and aged rats have equal sized islets, apart from islets with a size of 6300 μM2 being more prevalent in aged pancreata (n = 5 mice per age). (E) No difference in percent Insulin positive area between young and aged pancreata (n = 4 mice per age). (F) Entry points of tested mitogenic signals to the cell cycle, and general locations of influence of cyclin dependent kinase inhibitors. ****p < 0.0001
We hypothesized that the inability of aged islets to proliferate in response to these mitogenic signals could be due to differences in islet transfection efficiency between the young and aged islets. However, measurements of either Nkx6.1 or GFP overexpression post transduction with AdCMV-Nkx6.1 or AdCMV-GFP demonstrated no significant difference in the respective mRNA levels between young and aged islets (Figure 1B,C). This suggested that transduction efficiency between the young and aged islets is not responsible for the changes in proliferation capacity. Similarly, we hypothesized that changes in islet architecture could negatively affect proliferation in the aged islets. We measured islet size and total β-cell area of young and aged rats. Neither islet size nor total β-cell area was significantly different between 5 weeks and 5 months of age (Figure 1D,E). These results suggest that while various factors can induce proliferation in young islets, their ability to induce proliferation in aged islets is inhibited and that this inhibition is not due to changes in islet size, the ability of the islets to be transduced, or in their ability to express the defined mitogenic gene of interest.
CDKI histological analysis demonstrates increased p27KIP1 islet expression as a function of age
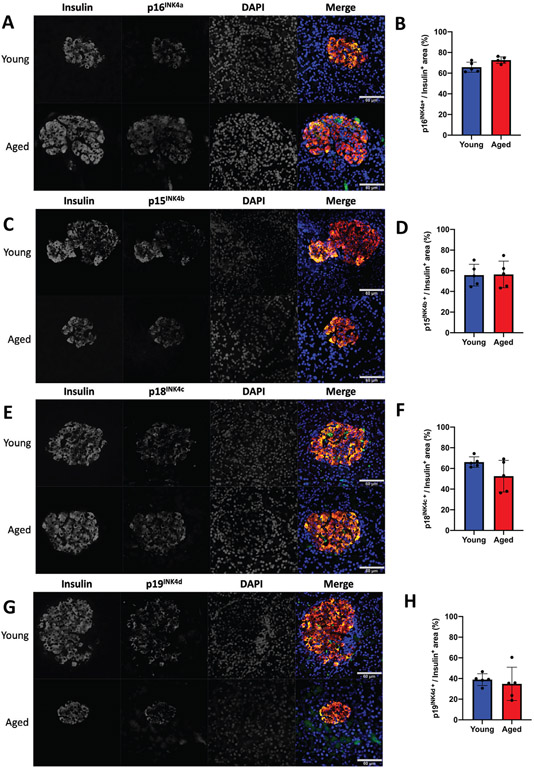
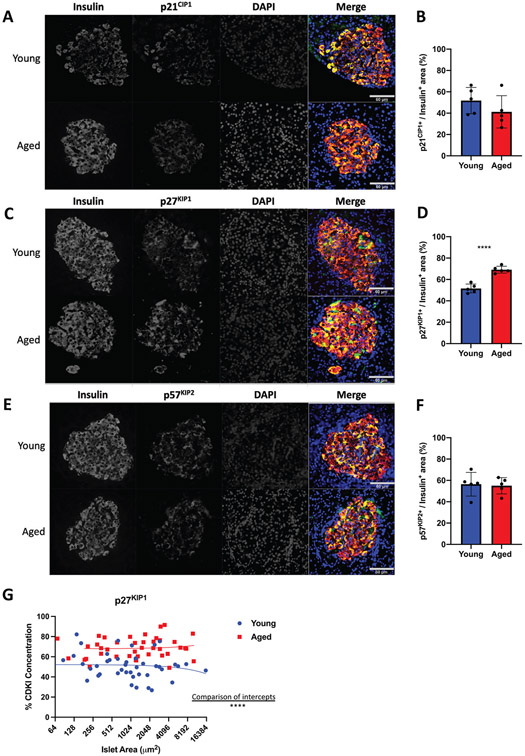
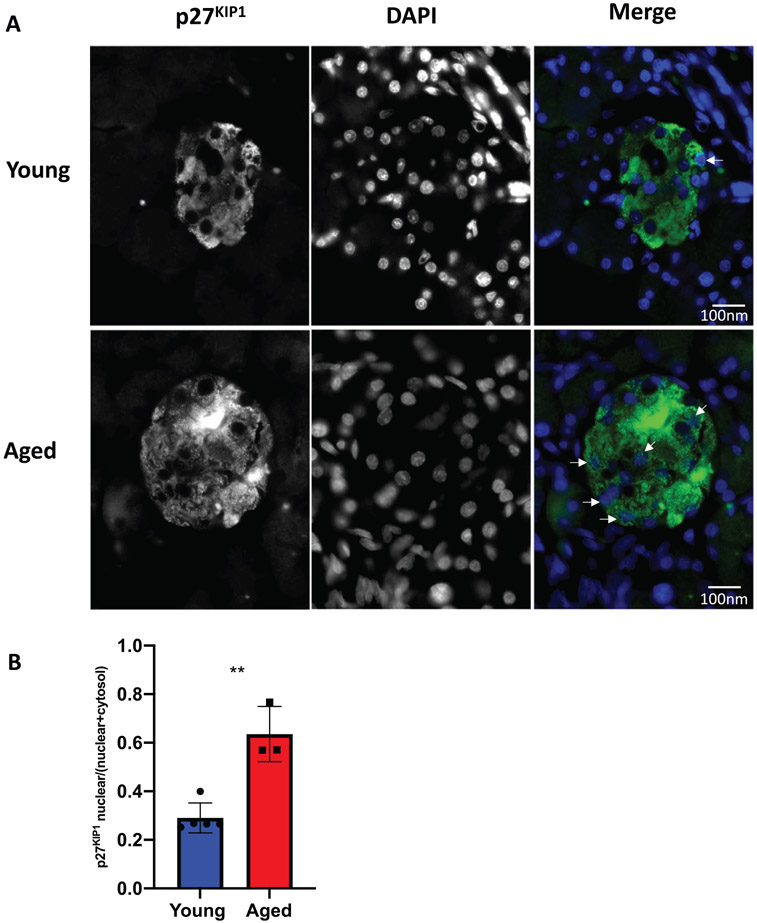
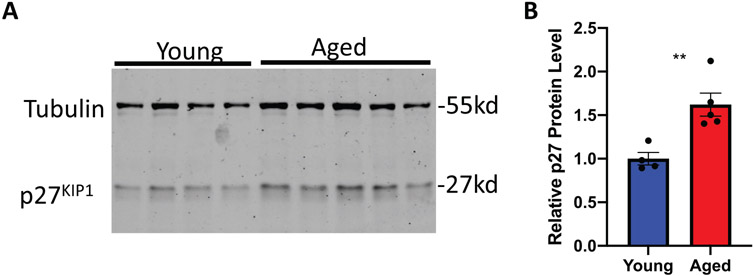
Our data demonstrates that islets from 5-month-old rats are refractory to various proliferative signals. The global inhibition of mitogenic signals suggests changes that directly affect cell cycle function. This could be due to increased protein levels of cyclin-dependent kinase inhibitors (CDKI). Given that Pdx1 induced proliferation by activating the Cyclin D-CDK4/6 complex through upregulating Cyclin D’s, and Nkx6.1, Nr4a1, and Nr4a3 induce proliferation by inducing E2F1 and Cyclin E’s, we hypothesized that cell cycle inhibitors may play a critical role in inhibiting aged β-cell proliferation (Hayes et al., 2013; Schisler et al., 2008a; Tessem et al., 2014). We measured expression of all CDKI’s in situ by immunofluorescent analysis. Measuring CDKI expression per insulin positive area demonstrated no significant changes for the majority of the CDKI’s. No significant differences were observed for p16INK4a, p15INK4b, p18INK4c, or p19INK4d (Figure 2). Similarly, no changes were observed for p21CIP1 or p57KIP2 (Figure 3A,B,E,F). Interestingly, and unlike the other CDKI’s, p27KIP1 levels were significantly upregulated in insulin positive cells of aged animals (Figure 3C,D). Furthermore, comparison of aged islets to young islets based on islet area demonstrated that aged islets have a significantly greater level of p27KIP1 expression (Figure 3G). p27KIP1 inhibits cell cycle progression by migrating to the nucleus and inhibiting cyclin-dependent kinase (CDK) activity through binding and blocking CDK-Cyclin complex activity (Rosner et al., 2007). Given this function of p27KIP1, we measured p27KIP1 nuclear localization in young and aged islets. Analysis of p27KIP1 localization revealed a significant increase in the p27KIP1 nuclear localization in aged β-cells when compared to young β-cells (Figure 4A,B). Finally, to validate the immunofluorescent observation of increased β-cell p27KIP1 levels, we measured p27KIP1 protein levels from young and aged rat islets. p27KIP1 protein expression was significantly elevated in aged rats as compared to their young counterparts (Figure 5A,B). These data demonstrate that p27KIP1 expression is modified in an age-dependent fashion, and that this change, when considered within the timeframe of our study, is unique to only this member of the INK4, CIP, or KIP families.
FIGURE 2.
Aged islets have no significant change in Ink4 protein levels as compared to young islets. No significant difference was observed between young (5 week) and aged (5 month) islet protein levels of p16INK4a (A-B), p15INK4b (C-D), p18INK4c (E-F) or p19INK4d (G-H) as measured by immunofluorescence. n = 5 mice per age, minimum of three sections per rat. All images have a set scale bar of 60 μm
FIGURE 3.
Aged islets have increased in p27KIP1 protein levels as compared to young islets by immunofluorescence assay. No significant difference was observed between young (5 week) and aged (5 month) islet protein levels of p21CIP1 (A-B) or p57KIP2 (E-F). However, p27KIP1 (C-D) protein levels were significantly increased in aged islets as measured by immunofluorescence. (G) Percent p27KIP1 is significantly greater in aged islets, regardless of islet size, with comparison of intercepts. n = 5 mice per age, minimum of three sections per rat. ****p < 0.0001. All images have a set scale bar of 60 μm
FIGURE 4.
Aged islets have increased nuclear p27KIP1 protein levels as compared to young islets by immunofluorescence assay. (A-B) p27KIP1 nuclear protein levels are significantly increased in aged islets as measured by immunofluorescence. n = 3–5 mice per age, minimum of three sections per rat. **p < 0.01. All images have a set scale bar of 100 nm
FIGURE 5.
Aged islets have increased p27KIP1 protein levels as compared to young islets. (A-B) p27KIP1 protein levels are significantly increased in aged islets as measured by western blotting. n = 4–5 mice per age. **p < 0.01
Aged islets have impaired HDAC1 expression
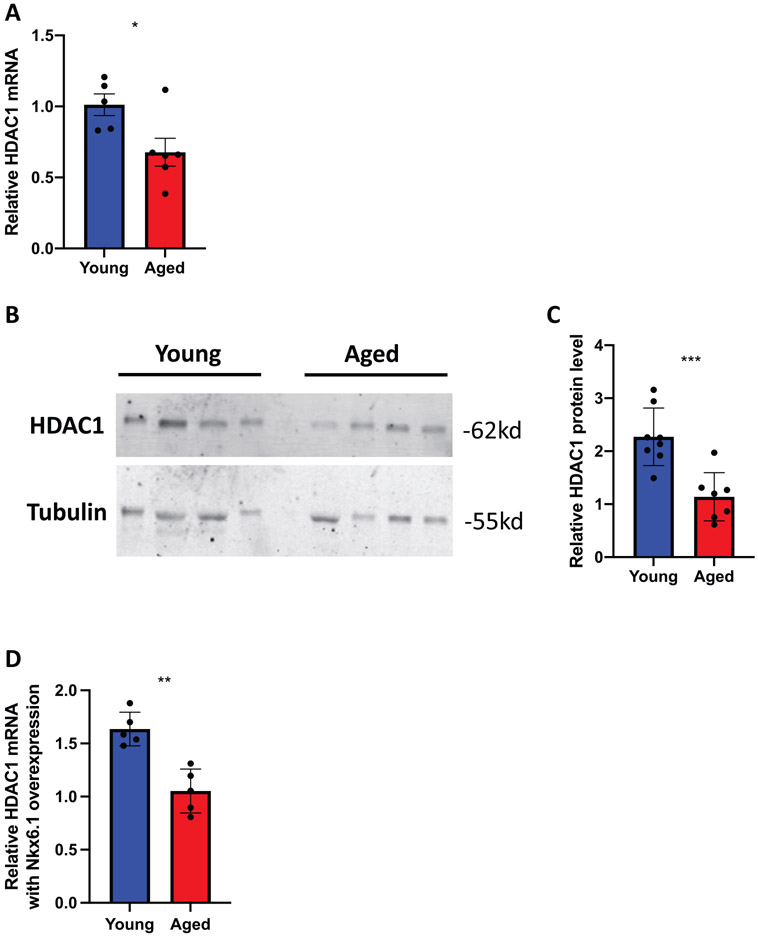
We have previously shown that HDAC1 can induce β-cell proliferation by decreasing p27KIP1 expression (Draney et al., 2018). Given the observed increased p27KIP1 expression, we hypothesized that HDAC1 mRNA and protein levels might be decreased in the aged islets. HDAC1 mRNA was in fact decreased in aged islets as compared to young islets (Figure 6A). HDAC1 protein levels were also decreased in aged islets (Figure 6B,C). Finally, we measured HDAC1 mRNA levels in young and aged β-cells after AdCMV-Nkx6.1 transduction and demonstrated that HDAC1 levels did not increase in aged β-cells in response to Nkx6.1 overexpression, as they did in young β-cells (Figure 6D). These data suggest that the increase in p27KIP1 protein levels may be due to age-dependent decreases in HDAC1 levels. As HDAC1 has been shown to preferentially deacetylate the p27KIP1 promoter, decreased HDAC1 protein levels could result in increased p27KIP1 levels and impaired cell cycle progression. Furthermore, the fact that Nkx6.1 fails to induce proliferation in the aged β-cells, and that it also fails to induce HDAC1 expression suggests impairment in Nkx6.1 mediated gene transcription in aged β-cells.
FIGURE 6.
Aged islets have impaired HDAC1 mRNA and protein expression as compared to young islets. (A) HDAC1 mRNA is decreased in aged islets (n = 5 mice per age). (B-C) HDAC1 protein levels are decreased in aged islets (n = 7–8 mice per age). (D) AdCMV-Nkx6.1 mediated HDAC1 induction is impaired in aged islets, as compared to AdCMV-GFP treated islets (n = 4–5 mice per age). *p < 0.05, **p < 0.01, ***p < 0.001
DISCUSSION
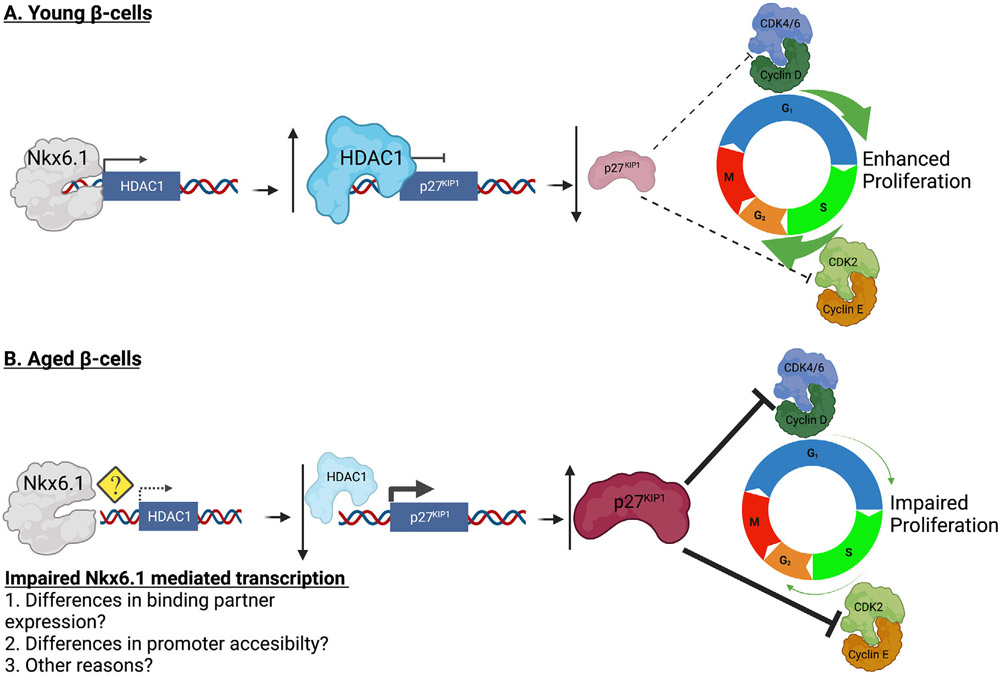
Although great progress has been made in improving the efficiency and efficacy of islet transplantation as a treatment, a major remaining barrier to the application of this treatment method is the availability of transplant-ready β-cells. Defining the molecular pathways that induce β-cell proliferation could allow us to expand the transplant ready β-cell population. As the majority of β-cell donors are from aged populations, these proliferation pathways must function in “aged” β-cells to be of use in islet transplantation. Here, we present data demonstrating that p27KIP1 protein levels are elevated in β-cells from 5-month-old Wistar rats and not in 5-week-old rats. This change in p27KIP1 levels is of particular interest, given that islets from 5-month-old rats are refractory to β-cell proliferation mediated by overexpression of Nkx6.1, Pdx1, Nr4a1, Nr4a3 or Cyclin D. Furthermore, we have shown that in addition to increased β-cell p27KIP1 levels, a decrease in HDAC1 mRNA and protein levels are observed. This is particularly important given that we and others have previously shown that HDAC1 is sufficient to induce β-cell proliferation by decreasing p27KIP1 promoter acetylation and thus decreasing p27KIP1 expression (Draney et al., 2018; Huang et al., 2014; Huang et al., 2017). Together, our data suggest that young islets, with increased HDAC1 expression, have tighter control over p27KIP1 expression that allows the β-cell to respond to mitogenic signals, resulting in cell cycle entry and proliferation (Figure 7A). Conversely, aged islets, from rats as young as 5 months of age, have decreased HDAC1 mRNA and protein levels. Conversely, decreased HDAC1 expression results in increased p27KIP1 expression and impaired β-cell responsiveness to mitogenic signals (Figure 7B).
FIGURE 7.
Decreased HDAC1 expression in aged islets results in increased p27KIP1 levels and impaired ability to respond to mitogenic signals. (A) Young β-cells have elevated HDAC1 levels. This increases deacetylation of the p27KIP1 promoter and decreases p27KIP1 expression. This allows the young β-cells to be responsive to mitogenic signals. (B) Aged β-cells have decreased HDAC1 levels. This increases acetylation of the p27KIP1 promoter and increases p27KIP1 expression. Aged β-cells become unresponsive to mitogenic signals. Figure designed using BioRender
Our data demonstrate that at 5 months of age p27KIP1 is the only CDKI that shows significant changes in expression. Various studies have demonstrated that p27KIP1 overexpression decreases β-cell proliferation and islet mass, and that p27KIP1 deletion results in greater β-cell proliferation and islet mass (Georgia & Bhushan, 2006; Rachdi et al., 2006; Stein et al., 2013; Uchida et al., 2005). If p27KIP1 levels rise, inhibition of the activity of both Cyclin D-CDK4/6 and Cyclin E-CDK2 complexes is observed (Ingham & Schwartz, 2017; Stein et al., 2013). Reduced concentrations of p27KIP1 fails to bind all the Cyclin D present in the cytosol, resulting in activated cyclin-cdk complexes, pRb phosphorylation, and activation of E2F-mediated transcription of targets necessary for cell cycle progression. Thus, elevated p27KIP1 levels prevent cell cycle progression at the G1 checkpoint. Our data suggest that elevated p27KIP1 levels may be sufficient to impede mitogenic induced β-cell proliferation in rats as early as 5 months of age.
Various studies have shown that the other CDKIs also play a critical role in β-cell proliferation. Expression of p16INK4a increases with age and is an important regulator of β-cell proliferation, and p16INK4a overexpression in β-cells impairs proliferation and induces a senescence pathway (Helman et al., 2016; Kehm et al., 2018; Salpeter et al., 2010). Upregulation of p16INK4a in β-cells has been shown to correlate with age-related downregulation of the polycomb proteins Ezh2 and Bmi1 (Chen et al., 2009; Dhawan, Tschen, & Bhushan, 2009). Interestingly, the fatty acid palmitate impairs β-cell proliferation by upregulating expression of p16INK4a and p18INK4c, both of which are necessary for the antiproliferative phenotype observed in this model (Pascoe et al., 2012). Human islets also have elevated p18INK4c levels (Stein et al., 2013). Finally, rats exposed to gestational diabetes during development have increased islet p16INK4a and p15INK4b mRNA and protein levels, and impaired beta cell function (Nazari, Shahryari, Ghafari, Nabiuni, & Golalipour, 2020). These data show that members of the Ink4 family play important roles in regulating β-cell proliferation as a function of aging.
Other members of the CIP/KIP CDKI family negatively regulate β-cell proliferation. While p21CIP1 knock out mice show no difference in β-cell proliferation or islet mass (Cozar-Castellano, Haught, et al., 2006), p21CIP1 overexpression decreases β-cell proliferation and islet mass (Yang et al., 2009). In addition, p57KIP2 levels are highly expressed in mature β-cells, and recent studies show that epigenetic modifications of the p57KIP2 promoter are sufficient to decrease p57KIP2 protein levels and enhance β-cell proliferation. These data demonstrate a connection between CDKI’s and β-cell proliferation (Ou et al., 2019). While we observed the presence of all INK4 and CIP/KIP family members in the β-cell, we only observed statistically significant changes in p27KIP1 protein levels as a function of aging. This suggests that increased p27KIP1 protein expression may predate shifts in expression of the other CDKIs, and positions p27KIP1 as an early braking mechanism to inhibit β-cell proliferation. The timing in terms of possibly changes in the protein levels of the other CDKIs still remains to be explored and will need to be determined through further studies.
Supporting our observation that increased p27KIP1 protein levels may be an early age-related change that halts β-cell proliferation, we observed no difference in the percent β-cell area and only a modest change in islet size distribution at islet areas greater than 6300 μm2 when comparing young and aged animals. Previous studies have demonstrated islet hyperplasia and hypertrophy as a function of aging (Montanya, Nacher, Biarnes, & Soler, 2000). The observed changes in β-cell p27KIP1 protein levels and nuclear localization may be important for the eventual shift in islet size observed at older time points. Our future studies will begin to address the role of p27KIP1 in permitting these changes.
Our previous data demonstrate an intimate connection between HDAC1 and p27KIP1 in the β-cell. We have shown that HDAC1 overexpression induces β-cell proliferation in part by decreasing p27KIP1 levels, and that p27KIP1 overexpression is sufficient to impede HDAC1 mediated β-cell proliferation (Draney et al., 2018). Additionally, alterations in HDAC1 expression are sufficient to modulate p27KIP1 levels in various other cell types. In T-cells, HDAC1 upregulation decreases p27KIP1 levels, while HDAC1 inhibition induces p27KIP1 levels (Jazi et al., 2016; Song et al., 2017). Changes in HDAC1 levels and HDAC1 activity has been shown to preferentially modulate the p27KIP1 promoter acetylation state, where HDAC1 overexpression results in deacetylation (Huang et al., 2014; Huang et al., 2017; Pang et al., 2011; Trivedi et al., 2008). Similarly, chemical HDAC inhibitors have been shown to enhance p27KIP1 levels in acute promyelocytic leukemia and human ovarian cancer cells (Karaca et al., 2013; Valiuliene et al., 2015). Thus, while the mechanism by which HDAC1 targets the p27KIP1 promoter and impairs its expression has not been defined, a clear link exists between HDAC1 activity and p27KIP1 levels.
While β-cell proliferation primarily occurs prior to adolescence and decreases as a function of aging, pregnancy and obesity are two biological states that are associated with increased β-cell proliferation. Pregnancy-induced β-cell expansion is coupled with decreased expression of the histone-associated scaffolding protein Menin1, which has been shown to be sufficient to inhibit p27KIP1 expression (Rieck & Kaestner, 2010). Furthermore, small molecule mediated Menin1 inhibition has been shown to induce β-cell proliferation by downregulating p27KIP1 expression (Pahlavanneshan et al., 2020). Menin1 has also been shown to bind HDAC1 (Kim, Lee, Cho, Liu, & Youn, 2003). This may suggest that in the proliferation-inhibited state, Menin1 mediates HDAC1 sequestration and prevents p27KIP1 promoter deacetylation. In the absence of Menin1, however, HDAC1 could be free to downregulate p27KIP1 expression. Various authors show that obesity-induced β-cell expansion is also associated with decreased p27KIP1 expression within the β-cell (Linnemann, Baan, & Davis, 2014; Stein, Milewski, Hara, Steiner, & Dey, 2011). These obesity-related studies demonstrate that decreases in GSK3-β, which interacts with HDAC1, correspond with increased p27KIP1 levels in the β-cell, and lead to increased β-cell proliferation (Bardai, Price, Zaayman, Wang, & D’Mello, 2012; Stein et al., 2011). Studies into these connections are currently ongoing.
In summary, our data demonstrate that increased expression of the CDKI p27KIP1 is a critical early change in β-cell aging that correlates with decreased β-cell proliferative capacity. We have demonstrated a correlation between aged β-cells and decreased HDAC1 protein levels, which has previously been shown to regulate p27KIP1 expression. Our data suggest that upregulation of HDAC1 or downregulation of p27KIP1 may be sufficient to induce proliferation of aged β-cells. Given our observations, future use of class I HDAC activators such as N-acetylthioureas or ITSA-1 may be useful in enhancing aged β-cell proliferation and should be explored (Behera et al., 2018; Du et al., 2020; Singh et al., 2011).
MATERIALS AND METHODS
Animal husbandry
Wistar rat breeding pairs were purchased from Harlan and maintained on a standard chow diet (Teklad 7001; Harlan). Pups were weaned at 21 days. Rats were allowed to feed ad libitum and were maintained on a 12-h light-dark cycle. Animals were harvested at either 5-weeks or 5-months of age for subsequent studies. All animal studies were approved and performed in accordance with Brigham Young University’s IACUC guidelines (Protocol #19-1002).
Islet isolation
Pancreatic islets were isolated from 5-week-old or 5-month-oldWistar rats as previously described (Draney et al., 2018). Islets were either immediately harvested for protein and mRNA or were cultured for adenoviral transduction. Primary rat islets were cultured in RPMI 1640 and supplemented with 10% FBS, 1% Fungizone antimycotic (Life Technologies) and 1% HEPES. Islet medium was changed every 24 h.
Islet adenovirus transduction
Adenoviruses expressing GFP, Nkx6.1, Pdx1, Nr4a1, Nr4a3, and Cyclin D1 have been described elsewhere (Draney et al., 2016; Hayes et al., 2013; Schisler et al., 2008b; Tessem et al., 2008). All recombinant viruses were shown to be replication incompetent by being E1a deficient, using an RT-PCR screen, as previously described (Tessem et al., 2014). For studies involving adenovirus-mediated gene manipulation, pools of 200 islets were transduced with ~2 × 107 IFU/ml adenovirus (moi~100–200) for 18 h, as previously described (Hobson et al., 2015). Media was changed after 18 h, and islets were cultured for 96 h. Assays were completed at 96 h after harvest.
[3H]-thymidine incorporation assay
DNA synthesis rates in 5-week and 5-month-old primary rat islets were measured by [methyl-3H]-thymidine incorporation and were completed as previously described (Draney et al., 2018; Draney et al., 2016; Hobson et al., 2015; Tessem et al., 2014). Briefly, primary rat islets were labeled for 24 h, beginning at 72 h of culture, after which islets were harvested at 96 h of culture and processed for DNA 3H-thymidine measurements.
Tissue preparation and immunohistochemistry
Pancreata were harvested from 5-week or 5-month-old rats. Fixation, embedding, cutting and mounting of pancreata for immunofluorescence was completed as previously described (Schisler et al., 2008b; Tessem et al., 2008). Antigen retrieval was performed using 10 mM citric acid buffer (pH 6.0), followed by overnight incubation with primary antibody (guinea pig anti-insulin [1:500; DAKO, Denmark], mouse anti-p16INK4a [1:100, Genetex, CA, USA], rabbit anti-p15INK4b [1:50, Genetex, CA, USA], mouse anti-p18INK4c [1:100, Santa Cruz Biotech., CA, USA], mouse anti-p19INK4d [1:50, Santa Cruz Biotech., CA, USA], rabbit anti-p21CIP1 [1:100, Santa Cruz Biotech., CA, USA], rabbit anti-p27KIP1 [1:25, Genetex, CA, USA], mouse anti-p57KIP2 [1:100, Santa Cruz Biotech., CA, USA]). Slides were incubated for 8 minutes in Sudan Black B (0.05% solution in 70% ethanol) to quench autofluorescence, followed by an hour incubation with secondary antibodies (anti-mouse [Alexa Fluor 555, 1:500, Life Technologies, OR, USA], anti-rabbit [Alexa Fluor 555, 1:500, Life Technologies, OR, USA], anti guinea pig [488 FITC, 1:250, Life Technologies, OR, USA]). A fluorophore nuclear stain (DAPI) was applied, followed by a coverslip.
Image analysis and quantification
Images were visualized using an Olympus BX43 upright microscope and acquired with an Olympus DP26 digital camera using cellSens Entry acquisition software. Images were taken at 40x magnification (islets) [UpPlan FL N, 40x/0.75], 100x. magnification (nuclear p27KIP1) [UpPlan FL N, 100x/1.30 Oil] or 4x magnification (pancreas tissue) [UPlan FL N, 4x/]. Background correction was conducted using Adobe Photoshop in accordance with Biology of the Cell’s publication parameters. Areas were quantified using ImageJ software and analyzed for significance in GraphPad Prism. ImageJ/Fiji’s WEKA segmentation program was used to automate the process of analyzing insulin positive tissue area.
Protein isolation and analysis
Protein was harvested from pools of 200 rat islets. Total protein concentration was quantified using the Pierce BCA protein assay kit. Protein samples were resolved on 10% PAGE gel. Proteins were transferred to PVDF and blocked for 1 h in LiCor blocking buffer in PBS. Proteins were incubated overnight in the appropriate primary antibody (GeneTex rabbit anti-p27KIP1 antibody GTX100446 1:500; Cell Signaling rabbit anti-HDAC1 antibody 2602 1:1000; Proteintech mouse anti-β-tubulin antibody 66240-1-Ig 1:20,000). Secondary antibodies were incubated for 1 h at room temperature (LiCor IRDye 680RD Goat anti-rabbit 1:10,000; Abcam IRDye 800CW goat anti-mouse 1:10,000). Gels were imaged using the LiCor CLx-1893 and quantified using ImageJ (National Institutes of Health, Bethesda, MD).
mRNA isolation, amplification and analysis
RNA was harvested from 50–100 primary rat islets as previously described (Draney et al., 2018). Real-time PCR was performed using the Life Technologies Quant Studio 6 Detection System and Software (Life Technologies) using sybr green primers and PCR master mix reagents (Bio-Rad) as previously described for HDAC1, p27KIP1 and PPIA. Relative mRNA levels for HDAC1 and p27KIP1 were calculated using the Delta Ct method, with PPIA used as the housekeeping gene. Sequences for all primers are available upon request.
Statistical analysis
All results are expressed as mean±Standard Deviation. Data were analyzed using two-tailed Student t-test or two-way ANOVA where appropriate (GraphPad Prism Software). Statistical significance was defined as p < 0.05.
ACKNOWLEDGEMENTS
The authors thank the members of the Tessem Lab for critical conversations and suggestions. This work was funded in part by grants from the American Diabetes Association [1-17-IBS-101], Beatson Foundation [2019-003] and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [R15DK12483501A1] to J.S.T and a Brigham Young University College Undergraduate Research Award to T.J.A. and D.M.J.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author, JST.
REFERENCES
- Bardai FH, Price V, Zaayman M, Wang L & D’Mello SR (2012) Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death. Journal of Biological Chemistry, 287(42), 35444–35453. 10.1074/jbc.M112.394544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera J, Kelly KE, Voor MJ, Metreveli N, Tyagi SC & Tyagi N (2018) Hydrogen sulfide promotes bone homeostasis by balancing inflammatory cytokine signaling in CBS-deficient mice through an epigenetic mechanism. Scientific Reports, 8(1), 15226. 10.1038/s41598-018-33149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelle W, Kreahling JM, Gemmer J, Ma Y, Cress WD, Haura E & Altiok S (2010) Histone deacetylase inhibitors downregulate checkpoint kinase 1 expression to induce cell death in non-small cell lung cancer cells. Plos One, 5(12), e14335. 10.1371/journal.pone.0014335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C & Butler PC (2010) Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia, 53(10), 2167–2176. 10.1007/s00125-010-1809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PC, Meier JJ, Butler AE & Bhushan A (2007) The replication of beta cells in normal physiology, in disease and for therapy. Nature Clinical Practice Endocrinology & Metabolism, 3(11), 758–768. 10.1038/ncpendmet0647 [DOI] [PubMed] [Google Scholar]
- Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A & Kim SK (2009) Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes & Development, 23(8), 975–985. 10.1101/gad.1742509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AR, Lam CJ, Rankin MM, King KA, Chen P, Martinez R, Li C & Kushner JA (2016) Extreme obesity induces massive beta cell expansion in mice through self-renewal and does not alter the beta cell lineage. Diabetologia, 59(6), 1231–1241. 10.1007/s00125-016-3922-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozar-Castellano I, Haught M & Stewart AF (2006) The cell cycle inhibitory protein p21cip is not essential for maintaining beta-cell cycle arrest or beta-cell function in vivo. Diabetes, 55(12), 3271–3278. 10.2337/db06-0627 [DOI] [PubMed] [Google Scholar]
- Cozar-Castellano I, Weinstock M, Haught M, Velazquez-Garcia S, Sipula D & Stewart AF (2006) Evaluation of beta-cell replication in mice transgenic for hepatocyte growth factor and placental lactogen: Comprehensive characterization of the G1/S regulatory proteins reveals unique involvement of p21cip. Diabetes, 55(1), 70–77. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16380478 [PubMed] [Google Scholar]
- Dhawan S, Tschen SI & Bhushan A (2009) Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes & Development, 23(8), 906–911. 10.1101/gad.1742609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L & Coffer PJ (2000) Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Molecular and Cellular Biology, 20(24), 9138–9148. 10.1128/mcb.20.24.9138-9148.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draney C, Austin MC, Leifer AH, Smith CJ, Kener KB, Aitken TJ, Hess KH, Haines AC, Lett LA, Hernandez-Carretero A, Fueger PT, Arlotto M & Tessem JS (2018) HDAC1 overexpression enhances beta-cell proliferation by downregulating Cdkn1b/p27. Biochemical Journal, 475(24), 3997–4010. 10.1042/BCJ20180465 [DOI] [PubMed] [Google Scholar]
- Draney C, Hobson AE, Grover SG, Jack BO & Tessem JS (2016) Cdk5r1 overexpression induces primary beta-cell proliferation. Journal of Diabetes Research, 2016, 6375804. 10.1155/2016/6375804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Li W, Liu B, Zhang Y, Yu J, Hou X & Fang H (2020) An in silico mechanistic insight into HDAC8 activation facilitates the discovery of new small-molecule activators. Bioorganic & Medicinal Chemistry, 28(16), 115607. 10.1016/j.bmc.2020.115607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farney AC, Sutherland DE & Opara EC (2016) Evolution of islet transplantation for the last 30 years. Pancreas, 45(1), 8–20. 10.1097/MPA.0000000000000391 [DOI] [PubMed] [Google Scholar]
- Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I & Stewart AF (2009) Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes, 58(4), 882–893. 10.2337/db08-0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Scott DK & Stewart AF (2013a) Human pancreatic beta-cell G1/S molecule cell cycle atlas. Diabetes, 62(7), 2450–2459. 10.2337/db12-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Srinivas H, Scott DK & Stewart AF (2013b) Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human beta-cell replication: A revised model of human beta-cell G1/S control. Diabetes, 62(7), 2460–2470. 10.2337/db12-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia S & Bhushan A (2006) p27 Regulates the transition of beta-cells from quiescence to proliferation. Diabetes, 55(11), 2950–2956. 10.2337/db06-0249 [DOI] [PubMed] [Google Scholar]
- Goder A, Emmerich C, Nikolova T, Kiweler N, Schreiber M, Kuhl T, Imhof D, Christmann M, Heinzel T, Schneider G & Kramer OH (2018) HDAC1 and HDAC2 integrate checkpoint kinase phosphorylation and cell fate through the phosphatase-2A subunit PR130. Nature communications, 9(1), 764. 10.1038/s41467-018-03096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA & Rhodes CJ (2012) Formation of a human beta-cell population within pancreatic islets is set early in life. Journal of Clinical Endocrinology and Metabolism, 97(9), 3197–3206. 10.1210/jc.2012-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HL, Moss LG, Schisler JC, Haldeman JM, Zhang Z, Rosenberg PB, Newgard CB & Hohmeier HE (2013) Pdx-1 activates islet alpha- and beta-cell proliferation via a mechanism regulated by transient receptor potential cation channels 3 and 6 and extracellular signal-regulated kinases 1 and 2. Molecular and Cellular Biology, 33(20), 4017–4029. 10.1128/MCB.00469-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman A, Klochendler A, Azazmeh N, Gabai Y, Horwitz E, Anzi S, Swisa A, Condiotti R, Granit RZ, Nevo Y, Fixler Y, Shreibman D, Zamir A, Tornovsky-Babeay S, Dai C, Glaser B, Powers AC, Shapiro AMJ, Magnuson MA, Dor Y & Ben- Porath I (2016) p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nature Medicine, 22(4), 412–420. 10.1038/nm.4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AMJ, Stock PG, Turgeon NA & Clinical Islet Transplantation, C. (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care, 39(7), 1230–1240. 10.2337/dc15-1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi T, Matsuzaki Y, Yasuda S, Kawanaka M, Yogosawa S, Koyama M, Tantin D & Sakai T (2007) Oct-1 is involved in the transcriptional repression of the p15(INK4b) gene. Febs Letters, 581(6), 1087–1092. 10.1016/j.febslet.2007.01.092 [DOI] [PubMed] [Google Scholar]
- Hobson A, Draney C, Stratford A, Becker TC, Lu DH, Arlotto M & Tessem JS (2015) Aurora Kinase A is critical for the Nkx6.1 mediated beta-cell proliferation pathway. Islets, 7(1), ARTN e1027854. doi: 10.1080/19382014.2015.1027854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Chen J, Lu C, Han J, Wang G, Song C, Li G, Kang J & Wang J (2014) HDAC1 and Klf4 interplay critically regulates human myeloid leukemia cell proliferation. Cell death & disease, 5, e1491. 10.1038/cddis.2014.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wu R, Su ZY, Guo Y, Zheng X, Yang CS & Kong AN (2017) A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin-dependent kinase inhibitors p21 and p27. Journal of Nutritional Biochemistry, 40, 155–163. 10.1016/j.jnutbio.2016.10.019 [DOI] [PubMed] [Google Scholar]
- Ingham M & Schwartz GK (2017) Cell-cycle therapeutics come of age. Journal of Clinical Oncology, 35(25), 2949–2959. 10.1200/JCO.2016.69.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazi MS, Mohammadi S, Yazdani Y, Sedighi S, Memarian A & Aghaei M (2016) Effects of valproic acid and pioglitazone on cell cycle progression and proliferation of T-cell acute lymphoblastic leukemia Jurkat cells. Iranian Journal of Basic Medical Sciences, 19(7), 779–786. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27635203 [PMC free article] [PubMed] [Google Scholar]
- Jin L & Datta PK (2014) Oncogenic STRAP functions as a novel negative regulator of E-cadherin and p21(Cip1) by modulating the transcription factor Sp1. Cell Cycle, 13(24), 3909–3920. 10.4161/15384101.2014.973310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca B, Atmaca H, Bozkurt E, Kisim A, Uzunoglu S, Karabulut B, Sezgin C, Sanli UA & Uslu R (2013) Combination of AT-101/cisplatin overcomes chemoresistance by inducing apoptosis and modulating epigenetics in human ovarian cancer cells. Molecular Biology Reports, 40(6), 3925–3933. 10.1007/s11033-012-2469-z [DOI] [PubMed] [Google Scholar]
- Kehm R, König J, Nowotny K, Jung T, Deubel S, Gohlke S, Schulz TJ & Höhn A (2018) Age-related oxidative changes in pancreatic islets are predominantly located in the vascular system. Redox Biology, 15, 387–393. 10.1016/j.redox.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee JE, Cho EJ, Liu JO & Youn HD (2003) Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Research, 63(19), 6135–6139. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14559791 [PubMed] [Google Scholar]
- Kulkarni RN, Mizrachi EB, Ocana AG & Stewart AF (2012) Human beta-cell proliferation and intracellular signaling: Driving in the dark without a road map. Diabetes, 61(9), 2205–2213. 10.2337/db12-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner JA (2006) Beta-cell growth: An unusual paradigm of organogenesis that is cyclin D2/Cdk4 dependent. Cell Cycle, 5(3), 234–237. 10.4161/cc.5.3.2399 [DOI] [PubMed] [Google Scholar]
- Lagger G, O’Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T & Seiser C (2002) Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. Embo Journal, 21(11), 2672–2681. 10.1093/emboj/21.11.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FX, Zhu JW, Tessem JS, Beilke J, Varella-Garcia M, Jensen J, Hogan CJ & Degregori J (2003) The development of diabetes in E2f1/E2f2 mutant mice reveals important roles for bone marrow-derived cells in preventing islet cell loss. PNAS, 100(22), 12935–12940. 10.1073/pnas.2231861100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann AK, Baan M & Davis DB (2014) Pancreatic beta-cell proliferation in obesity. Advances in Nutrition, 5(3), 278–288. 10.3945/an.113.005488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Popov N, Aguilo F, O’Loghlen A, Raguz S, Snijders AP, Dharmalingam G, Li S, Thymiakou E, Carroll T, Zeisig BB, So CW, Peters G, Episkopou V, Walsh MJ & Gil J (2013) Interplay between Homeobox proteins and Polycomb repressive complexes in p16(INK4a) regulation. EMBO Journal, 32(7), 982–995. 10.1038/emboj.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanya E, Nacher V, Biarnes M & Soler J (2000) Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: Role of beta-cell hyperplasia and hypertrophy. Diabetes, 49(8), 1341–1346. 10.2337/diabetes.49.8.1341 [DOI] [PubMed] [Google Scholar]
- Moser MA, Hagelkruys A & Seiser C (2014) Transcription and beyond: The role of mammalian class I lysine deacetylases. Chromosoma, 123(1-2), 67–78. 10.1007/s00412-013-0441-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari Z, Shahryari A, Ghafari S, Nabiuni M & Golalipour MJ (2020) In utero exposure to gestational diabetes alters DNA methylation and gene expression of CDKN2A/B in Langerhans islets of rat offspring. Cell J, 22(2), 203–211. 10.22074/cellj.2020.6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou K, Yu M, Moss NG, Wang YJ, Wang AW, Nguyen SC, Jiang C, Feleke E, Kameswaran V, Joyce EF, Naji A, Glaser B, Avrahami D & Kaestner KH (2019) Targeted demethylation at the CDKN1C/p57 locus induces human beta cell replication. Journal of Clinical Investigation, 129(1), 209–214. 10.1172/JCI99170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavanneshan S, Behmanesh M, Oropeza D, Furuyama K, Tahamtani Y, Basiri M, Herrera PL & Baharvand H (2020) Combined inhibition of menin-MLL interaction and TGF-beta signaling induces replication of human pancreatic beta cells. European Journal of Cell Biology, 99(5), 151094. 10.1016/j.ejcb.2020.151094 [DOI] [PubMed] [Google Scholar]
- Pang M, Ma L, Liu N, Ponnusamy M, Zhao TC, Yan H & Zhuang S (2011) Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. Journal of Cellular Biochemistry, 112(8), 2138–2148. 10.1002/jcb.23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe J, Hollern D, Stamateris R, Abbasi M, Romano LC, Zou B, O’Donnell CP, Garcia-Ocana A & Alonso LC (2012) Free fatty acids block glucose-induced beta-cell proliferation in mice by inducing cell cycle inhibitors p16 and p18. Diabetes, 61(3), 632–641. 10.2337/db11-0991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM & Tisdale JF (2010) Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. Journal of Clinical Endocrinology and Metabolism, 95(10), E234–239. 10.1210/jc.2010-0932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachdi L, Balcazar N, Elghazi L, Barker DJ, Krits I, Kiyokawa H & Bernal-Mizrachi E (2006) Differential effects of p27 in regulation of beta-cell mass during development, neonatal period, and adult life. Diabetes, 55(12), 3520–3528. 10.2337/db06-0861 [DOI] [PubMed] [Google Scholar]
- Rane SG, Cosenza SC, Mettus RV & Reddy EP (2002) Germ line transmission of the Cdk4(R24C) mutation facilitates tumorigenesis and escape from cellular senescence. Molecular and Cellular Biology, 22(2), 644–656. 10.1128/MCB.22.2.644-656.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin MM & Kushner JA (2009) Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes, 58(6), 1365–1372. 10.2337/db08-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck S & Kaestner KH (2010) Expansion of beta-cell mass in response to pregnancy. Trends in Endocrinology and Metabolism, 21(3), 151–158. 10.1016/j.tem.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M, Freilinger A, Hanneder M, Fujita N, Lubec G, Tsuruo T & Hengstschlager M (2007) p27Kip1 localization depends on the tumor suppressor protein tuberin. Human Molecular Genetics, 16(13), 1541–1556. 10.1093/hmg/ddm103 [DOI] [PubMed] [Google Scholar]
- Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B & Dor Y (2013) Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes, 62(8), 2843–2848. 10.2337/db13-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SJ, Klein AM, Huangfu D, Grimsby J & Dor Y (2010) Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development (Cambridge, England), 137(19), 3205–3213. 10.1242/dev.054304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG & Newgard CB (2008a) Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Molecular and Cellular Biology, 28(10), 3465–3476. 10.1128/MCB.01791-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG & Newgard CB (2008b) Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Molecular and Cellular Biology, 28(10), 3465–3476. 10.1128/Mcb.01791-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Pokrywczynska M & Ricordi C (2017) Clinical pancreatic islet transplantation. Nature Reviews Endrocrinology, 13(5), 268–277. 10.1038/nrendo.2016.178 [DOI] [PubMed] [Google Scholar]
- Singh RK, Mandal T, Balsubramanian N, Viaene T, Leedahl T, Sule N, Cook G & Srivastava DK (2011) Histone deacetylase activators: N-acetylthioureas serve as highly potent and isozyme selective activators for human histone deacetylase-8 on a fluorescent substrate. Bioorganic & Medicinal Chemistry Letters, 21(19), 5920–5923. 10.1016/j.bmcl.2011.07.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K-H, Choi CH, Lee H-J, Oh SJ, Woo SR, Hong SO, Noh KH, Cho H, Chung EJ, Kim J-H, Chung J-Y, Hewitt SM, Baek S, Lee K-M, Yee C, Son M, Mao C-P, Wu TC & Kim TW (2017) HDAC1 upregulation by NANOG promotes multidrug resistance and a stem-like phenotype in immune edited tumor cells. Cancer Research, 77(18), 5039–5053. 10.1158/0008-5472.CAN-17-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Milewski WM & Dey A (2013) The negative cell cycle regulators, p27(Kip1), p18(Ink4c), and GSK-3, play critical role in maintaining quiescence of adult human pancreatic beta-cells and restrict their ability to proliferate. Islets, 5(4), 156–169. 10.4161/isl.25605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Milewski WM, Hara M, Steiner DF & Dey A (2011) GSK-3 inactivation or depletion promotes beta-cell replication via down regulation of the CDK inhibitor, p27 (Kip1). Islets, 3(1), 21–34. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21278490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessem JS, Jensen JN, Pelli H, Dai XM, Zong XH, Stanley ER, Jensen J, DeGregori J (2007) Critical roles for macrophages in islet angiogenesis and maintenance during pancreatic degeneration. Diabetes, 57(6), 1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessem JS, Jensen JN, Pelli H, Dai XM, Zong XH, Stanley ER, Jensen J & DeGregori J (2008) Critical roles for macrophages in islet angiogenesis and maintenance during pancreatic degeneration. Diabetes, 57(6), 1605–1617. 10.2337/db07-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessem JS, Moss LG, Chao LC, Arlotto M, Lu D, Jensen MV, Stephens SB, Tontonoz P, Hohmeier HE & Newgard CB (2014) Nkx6.1 regulates islet beta-cell proliferation via Nr4a1 and Nr4a3 nuclear receptors. PNAS, 111(14), 5242–5247. 10.1073/pnas.1320953111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi CM, Lu MM, Wang Q & Epstein JA (2008) Transgenic overexpression of Hdac3 in the heart produces increased postnatal cardiac myocyte proliferation but does not induce hypertrophy. Journal of Biological Chemistry, 283(39), 26484–26489. 10.1074/jbc.M803686200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF & Kasuga M (2005) Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nature Medicine, 11(2), 175–182. 10.1038/nm1187 [DOI] [PubMed] [Google Scholar]
- Valiuliene G, Stirblyte I, Cicenaite D, Kaupinis A, Valius M & Navakauskiene R (2015) Belinostat, a potent HDACi, exerts antileukaemic effect in human acute promyelocytic leukaemia cells via chromatin remodelling. Journal of Cellular and Molecular Medicine, 19(7), 1742–1755. 10.1111/jcmm.12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang W, Jiang W, Sun X, Han Y, Ding M, Shi Y & Deng H (2009) P21cip-overexpression in the mouse beta cells leads to the improved recovery from streptozotocin-induced diabetes. Plos One, 4(12), e8344. 10.1371/journal.pone.0008344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Cai Y, Liu D, Li M, Sha Y, Zhang W, Wang K, Gong J, Tang N, Huang A & Xia J (2018) Pharmacological or transcriptional inhibition of both HDAC1 and 2 leads to cell cycle blockage and apoptosis via p21(Waf1/Cip1) and p19(INK4d) upregulation in hepatocellular carcinoma. Cell Proliferation, 51(3), e12447. 10.1111/cpr.12447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, Jurkin J, Rembold M, Meunier D, Egger G, Lagger S, Chiocca S, Propst F, Weitzer G & Seiser C (2010) The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Molecular and Cellular Biology, 30(5), 1171–1181. 10.1128/MCB.01500-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author, JST.