Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in >5 million deaths worldwide,1 with the Delta (B.1.617.2) and, more recently, the Omicron (B.1.1.529) variants accounting for most cases.2 Kidney transplant recipients (KTRs) are at increased risk of mortality following SARS-CoV-2 infection,3 and develop blunted antiviral responses following SARS-CoV-2 vaccination compared with nontransplant patients.4, 5, 6 Furthermore, the Delta and Omicron variants have been shown to be less sensitive to neutralizing antibodies from sera of vaccinated immunocompetent individuals,7 , 8 and similar data from vaccinated KTRs are limited.9 The aim of this study was to evaluate antiviral humoral responses against SARS-CoV-2 variants, including the Delta and Omicron variants, following the third dose of SARS-CoV-2 mRNA vaccination in KTRs.
Results
Patient characteristics
Fifty-one vaccinated KTRs were enrolled in the study. Baseline characteristics are shown in Supplementary Table S1. Median age was 63 years, most had received a living donor transplant (65%), and the median time between transplantation and vaccination was 43 months. Baseline samples were collected at a median of 5 days (interquartile range [IQR], 0–18 days) before the third vaccine dose, and postvaccination samples were obtained at a median of 29 days (IQR, 26–33 days) after vaccination. Methods of subject recruitment and antibody assays are described in the Supplementary Methods.
Anti-spike antibody measurement
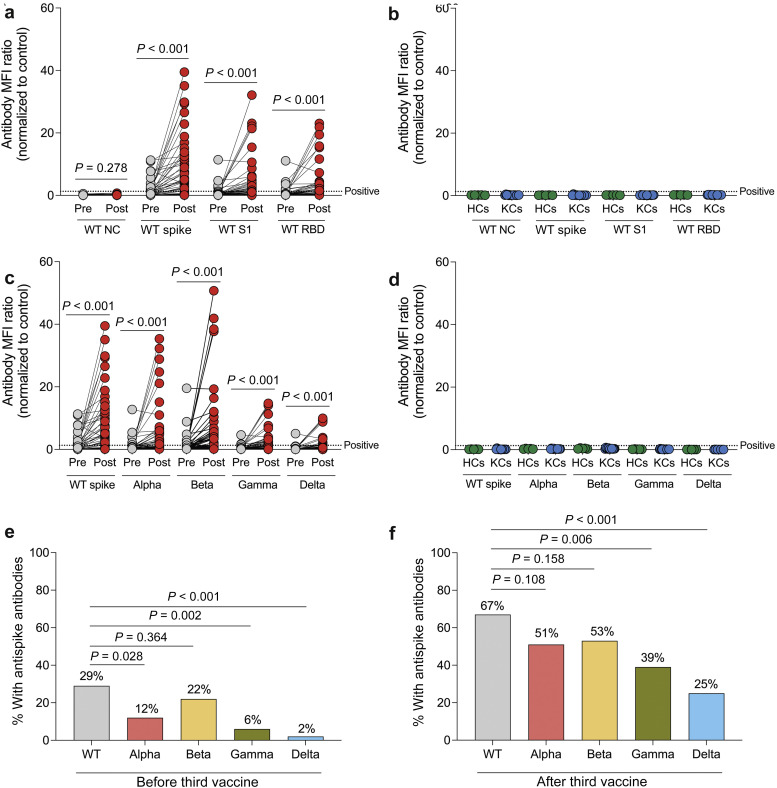
To evaluate antibody-mediated antiviral responses, we measured total antibodies (IgM, IgG, and IgA) directed against the nucleocapsid, spike trimer, S1, and receptor-binding domain (RBD) regions of wild-type (WT) SARS-CoV-2 before and following the third vaccine dose in KTRs using a Luminex-based multiplex assay. After the third vaccine dose, there was a significant increase in the mean fluorescence intensity ratios for antibodies directed against the WT spike trimer, S1, and RBD regions (P < 0.001 for all; Figure 1 a), but no significant change in the mean fluorescence intensity ratios of anti-nucleocapsid antibodies (P = 0.278). Using the recommended assay positivity threshold, the percentage of KTRs with anti–WT-spike antibodies increased from 29% to 67%, anti–WT-S1 antibodies increased from 10% to 47%, and anti–WT-RBD antibodies increased from 12% to 45% after the third vaccine dose (P < 0.001 for all). In comparison, all prepandemic healthy controls and prepandemic kidney transplant patient controls had negative results for antibodies against all 4 WT SARS-CoV-2 antigens (Figure 1b). After the third vaccine dose, there was a significant increase in the mean fluorescence intensity ratios of anti-spike antibodies directed against the Alpha, Beta, Gamma, and Delta variants (P < 0.001 for all; Figure 1c), whereas all prepandemic healthy controls and prepandemic kidney transplant patient controls had undetectable antibodies against those variants (Figure 1d). When evaluating differences in anti-spike antibody responses to SARS-CoV-2 variants, we found that before the third vaccine dose, a higher percentage of KTRs (29%) had anti-spike antibodies against WT SARS-CoV-2 compared with the Alpha (12%; P = 0.028), Gamma (6%; P = 0.002), and Delta (2%; P < 0.001) variants but not the Beta variant (22%; P = 0.364; Figure 1e). After the third vaccine dose, a higher percentage of KTRs (67%) had anti-spike antibodies against WT SARS-CoV-2 compared with the Gamma (39%; P = 0.006) and Delta (25%; P < 0.001) variants but not the Alpha (51%; P = 0.108) or Beta variants (53%; P = 0.158; Figure 1f).
Figure 1.
Increase in anti-spike antibody levels after the third dose of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine in kidney transplant recipients (KTRs). (a,b) Total antibody (IgM, IgG, and IgA) mean fluorescence intensity (MFI) ratios to wild-type (WT) SARS-CoV-2 antigens before and after the third vaccine dose in KTRs (n = 51; a) and in prepandemic healthy controls (HCs; n = 5) and prepandemic kidney transplant control patients (KCs; n = 15), measured by Luminex-based multiplex assay (b). (c,d) Antibody MFI ratios for anti-spike antibodies of WT and variants of SARS-CoV-2 before and after the third vaccine dose in KTRs (c) and prepandemic HCs and KCs (d). Horizontal lines indicate positivity threshold for assay. (e,f) Proportion of KTRs with anti-spike antibodies against WT and variants of SARS-CoV-2 before (e) and after (f) the third vaccine dose. (a,c) Statistic by Wilcoxon matched-pairs signed rank test. (e,f) Statistic by χ2 test. NC, nucleocapsid; RBD, receptor-binding domain.
Anti-RBD antibody measurement against Omicron variant and its neutralization capacity
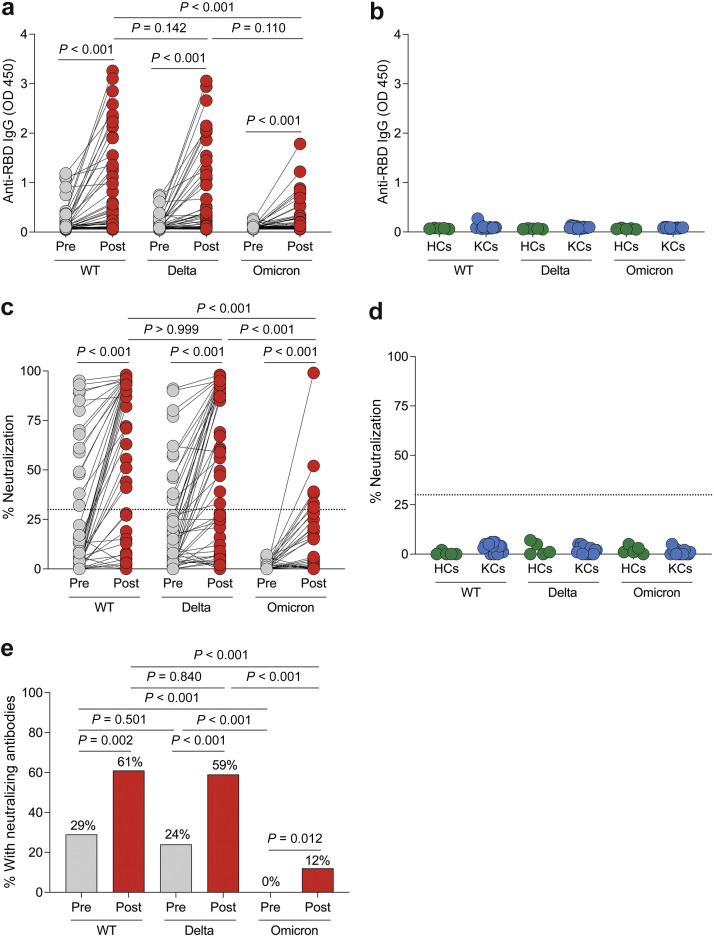
Because the multiplex assay lacked assessment of antibodies directed against the new Omicron variant, we evaluated anti-RBD IgG antibodies against the WT, Delta, and Omicron variants using enzyme-linked immunosorbent assay. After the third vaccine dose, there was a significant increase in anti-RBD IgG antibody levels for the WT, Delta, and Omicron variants (P < 0.001 for all; Figure 2 a). In comparison, prepandemic healthy controls and prepandemic kidney transplant patient controls had no detectable IgG antibodies against the RBD of the WT, Delta, and Omicron variants (Figure 2b). After the third vaccine dose in KTRs, anti-RBD IgG antibody levels were higher for WT SARS-CoV-2 compared with the Omicron variant (P < 0.001) and similar between WT SARS-CoV-2 and the Delta variant (P = 0.142).
Figure 2.
Poor anti–receptor binding domain (RBD) antibody and neutralization responses to the Omicron variant before and after the third dose of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine in kidney transplant recipients (KTRs). (a,b) Anti–SARS-CoV-2 RBD IgG antibody OD 450 values to wild-type (WT), Delta (B.1.617.2), and Omicron (B.1.1.529) variants of SARS-CoV-2 before and after the third vaccine dose in KTRs (n = 51; a) and in prepandemic healthy controls (HCs; n = 5) and prepandemic kidney transplant control patients (KCs; n = 15), measured by enzyme-linked immunosorbent assay (b). (c,d) Percentage of neutralization to WT, Delta, and Omicron variants of SARS-CoV-2 before and after the third vaccine dose in KTRs (n = 51; c) and in prepandemic HCs (n = 5) and KCs (n = 15), measured by surrogate virus neutralization test (d). Horizontal lines indicate positivity threshold for assay. (e) Proportion of KTRs with a positive neutralization response before and after the third vaccine dose in KTRs. (a,c) Statistic by Friedman test with Dunn correction for multiple comparisons. (e) Statistic by χ2 test.
We then evaluated neutralizing antibody responses using a surrogate virus neutralization test.S1 We found a significant increase in the percentage of neutralization against the WT, Delta, and Omicron variants after the third vaccine dose (P < 0.001 for all; Figure 2c). However, the percentage of neutralization was significantly lower for the Omicron variant compared with the WT and Delta variants (P < 0.001). In comparison, there was no significant neutralizing response against the WT, Delta, and Omicron variants in prepandemic healthy controls and prepandemic kidney transplant patient controls (Figure 2d).
Using the assay’s ≥30% neutralization positivity threshold,S1 we found that the percentage of KTRs who had neutralizing responses increased from 29% to 61% for WT SARS-CoV-2 (P = 0.002), from 24% to 59% for the Delta variant (P < 0.001), and from 0% to 12% for the Omicron variant following the third vaccine dose (P = 0.012; Figure 2e). Before and following the third vaccine dose, fewer KTRs had a neutralizing response against the Omicron variant, compared with the WT (P < 0.001) and Delta (P < 0.001) variants, but there was no difference between the proportion of KTRs with neutralizing responses to the WT versus Delta variants. Moreover, all KTRs with neutralizing responses to the Delta variant but without anti-Delta spike antibodies by the multiplex assay had anti-WT spike antibodies. We evaluated characteristics associated with developing neutralizing responses (Supplementary Table S2) and found that older age and steroid use were associated with lower odds of developing neutralizing responses to WT and the Delta variant, whereas belatacept use was associated with lower odds of developing neutralizing responses to the Delta variant.
Breakthrough infection after vaccination
At a median follow-up of 89 days (IQR, 78–100 days) after the third vaccine dose, 3 patients (6%) developed symptomatic SARS-CoV-2 infection (Supplementary Table S3). None had neutralizing responses to the Omicron variant. All had mild symptoms, were treated with monoclonal antibodies in the outpatient setting, and did not require hospitalization.
Noninvasive rejection monitoring, allograft rejection, and adverse events
No patients experienced severe adverse events after vaccination. At a median of 29 days (IQR, 26–32 days) after the third vaccine dose, no KTRs developed de novo donor-specific antibodies and there was no significant change in serum creatinine, urine protein-to-creatinine ratio, or donor-derived cell-free DNA levels compared with prevaccination (Supplementary Table S4). At a median follow-up of 89 days (IQR, 78–100 days) after the third vaccine dose, no patients developed allograft rejection.
Discussion
This study is one of the first to characterize antiviral humoral responses against SARS-CoV-2 variants, including the Omicron variant, after a third dose of SARS-CoV-2 mRNA vaccines in KTRs. Using a Luminex-based multiplex assay, we found that two-thirds of KTRs had anti-WT spike antibodies after the third vaccine dose, similar to previous reports,S2–S7 but fewer KTRs had anti-spike antibodies against the Gamma and Delta variants. Using an enzyme-linked immunosorbent assay we developed in our laboratory, we found an increase in anti-RBD antibody levels against the WT, Delta, and Omicron variants after the third vaccine dose, although anti-RBD antibody levels were significantly lower for the Omicron variant compared with WT virus.
To assess neutralization capacity of antibody responses, we used a surrogate virus neutralization test, which has been shown to be highly correlated with live virus and pseudovirus neutralization assays.S1 , S8 , S9 We found an increase in the percentage of neutralization against the WT, Delta, and Omicron variants after the third vaccine dose, as has been reported in immunocompetent individuals.S10 , S11 About half of KTRs had neutralizing responses to the WT and Delta variants after the third vaccine dose, consistent with previous reports in KTRs.S9 The presence of Delta variant neutralization in KTRs with anti-WT but not anti-Delta spike antibodies suggests cross-reactivity of anti-WT spike antibodies with the Delta variant. Concerningly, no KTRs had neutralizing responses to the Omicron variant before the third vaccine dose, and only 12% had neutralizing responses to the Omicron variant after the third vaccine dose. The Omicron variant’s ability to escape neutralization compared with WT SARS-CoV-2 in our study is consistent with data from vaccinated immunocompetent individuals8 , S10 , S12 , S13 and is likely due to its highly mutated RBD region.S14–S16 A limitation of the surrogate virus neutralization test is that it is not able to measure neutralizing antibodies directed against non-RBD regions of the spike protein as it only measures RBD–angiotensin-converting enzyme 2 interactions. We found that older age and steroid use were associated with lower odds of developing neutralizing antibody responses against WT SARS-CoV-2, as described previously,S7 and the Delta variant. Belatacept use was associated with lower odds of developing neutralizing responses against the Delta variant, and although the odds ratio was similar for WT and the Omicron variant, it did not reach statistical significance.
We were thus able to provide a detailed characterization of antibody responses to SARS-CoV-2 variants in KTRs, including the Omicron variant, and to evaluate the alloimmune safety of a third vaccine dose. Our study has limitations, including its small sample size, observational design, lack of a control group of KTRs who did not receive a third vaccine dose, and lack of assessment of cellular responses to vaccination. Further studies evaluating the cellular response to the Omicron variant and the implications of the reduced neutralization ability with regard to risk and severity of infections in KTRs are needed.
In summary, we found that a third dose of SARS-CoV-2 mRNA vaccination in KTRs was associated with an increased antiviral antibody response against WT and variants of SARS-CoV-2. Although the neutralizing responses to the Omicron variant increased in some, overall they remained markedly diminished. Strategies designed to improve antiviral immune responses to the Omicron and future variants are needed in this vulnerable high-risk group, including monoclonal antibodies for preexposure prophylaxis and additional homologous or heterologous vaccine doses.
Disclosure
VP has a financial interest in SeQure, Dx, Inc., a company developing technologies for gene editing target profiling. VP’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. All the other authors declared no competing interests.
Data Statement
Data to support the findings in the study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank the staff of the transplant clinic for their assistance in conducting this study. The study was funded by CareDx, Inc (Brisbane, CA), grant number 2021A008053 to LVR and JRA. The study was also supported in part by the Harold and Ellen Danser Endowed/Distinguished Chair in Transplantation at Massachusetts General Hospital (Boston, MA). This was an investigator-initiated research project where the design and conduct of the study was determined by the investigator without influence from the funders.
Footnotes
Supplementary Methods.
Table S1. Baseline characteristics of kidney transplant recipients.
Table S2. Characteristics associated with developing neutralizing antibodies responses against wild-type, Delta, and Omicron variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after third dose of mRNA vaccination in kidney transplant recipients.
Table S3. Breakthrough severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after third dose of vaccination.
Table S4. Allograft status before and 4 weeks after third severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine dose.
Supplementary References.
Supplementary Material
References
- 1.World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.CDC: variant proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 3.Caillard S., Chavarot N., Francois H., et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21:1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benotmane I., Gautier-Vargas G., Cognard N., et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon-Arevalo H., Choi M., Stefanski A., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6:1–15. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 7.Mlcochova P., Kemp S.A., Dhar M.S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejnirattisai W., Shaw R.H., Supasa P., et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399:234–236. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaba A.H., Zhu X., Liang T., et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22:1253–1260. doi: 10.1111/ajt.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.