Highlights
-
•
Acknowledging past medical harm led to more positive COVID-19 vaccine attitudes.
-
•
Presenting general information about the vaccine did not improve vaccine attitudes.
-
•
Tailored messaging strategies showed no backfire effects in other racial groups.
Keywords: COVID-19, Vaccine acceptance, Vaccine hesitancy, Black Americans
Abstract
Black Americans have been disproportionately affected by COVID-19 but have comparatively low vaccination rates, creating a need for vaccine messaging strategies that are tailored to this population. We conducted an experimental study to examine the effects of three messaging strategies on Black Americans’ reported willingness to receive the vaccine and vaccine hesitancy. We also recruited White and Hispanic Americans to assess any potential backfire effects of the tailored strategies for non-Black participants. A total of 739 participants completed the study. Results from 4x2 ANCOVAs indicate that, among Black participants, messaging that acknowledged past unethical treatment of Black Americans in medical research and emphasized current safeguards to prevent medical mistreatment was associated with significantly less vaccine hesitancy than the control condition. The same effects were not observed for messaging strategies that provided general safety information about the vaccine or that emphasized the role of the vaccine in reducing racial inequities. There were no significant differences across conditions for participants of other races. Results demonstrate that public health messages tailored to address specific vaccine concerns may aid future vaccination campaigns.
1. Introduction
The novel coronavirus emerged in 2019, causing an international pandemic associated with widespread acute illness, excess mortality, and economic strain. In the United States, which has had the largest share of COVID-19 infections and deaths of any country (Johns Hopkins University and Johns Hopkins Coronavirus Resource Center, 2020), hospitalizations and deaths have been concentrated among Black Americans (CDC, 2020). Primary prevention strategies such as vaccination are critical for reducing COVID-19 transmission and narrowing racial/ethnic health disparities in COVID-19 outcomes. However, evidence suggests that vaccination rates have varied across racial/ethnic groups in the United States and are lower among Black Americans (Ndugga et al., 2021). To help reduce disparities in COVID-19 outcomes, research is needed to identify effective vaccine messaging tailored toward specific groups.
That COVID-19 outcomes are stratified by race/ethnicity was not unforeseen given widespread disparities in many acute and chronic disease outcomes in the United States (Williams, 2012). Many of the risk factors for severe COVID-19, such as hypertension, diabetes, asthma, and kidney disease, are also disproportionately concentrated among Black Americans (Cunningham et al., 2017). However, scholars provide compelling evidence that persistent disparities in health outcomes result from different manifestations of racism, rather than genetic or physiological differences between racial or ethnic groups. Like other health disparities, disparities in COVID-19 outcomes likely result from systemic inequality and structural factors such as housing, educational attainment, and socioeconomic status, among others, which are critical social determinants of health (Laster Pirtle, 2020, Karaye and Horney, 2020, Lopez et al., 2021, Khazanchi et al., 2020). Black Americans also face discrimination within medical settings, receiving fewer treatment referrals, analgesic medications, and time with health care professionals (Hall et al., 2015), which likely contribute to ongoing inequities in COVID-19 outcomes (Milner et al., 2020). Although Black Americans are not any more likely to be infected with COVID-19, hospitalizations are 2.8 times, and deaths are 1.9 times, that of White Americans (CDC, 2021). These figures provide a rationale for shifting resources and public health campaigns toward reducing the burden of COVID-19 among Black Americans in particular.
Importantly, despite being disproportionately affected by COVID-19, the current vaccination rate is approximately 1.5 times higher among White Americans than Black Americans (Ndugga et al., 2021). Public health experts have put forward two primary hypotheses to explain this disparity. The first is that Black Americans are disproportionately hesitant to accept the COVID-19 vaccine as a result of medical mistrust, which is supported by the fact that hesitancy is also higher in this group for other vaccines, such as the influenza (CDC, 2020, Hall et al., 2020), H1N1 (Burger et al., 2021), HPV (Kessels et al., 2012), and pneumonia vaccines (Winston et al., 2003), Recent work also shows that Black Americans are similarly reporting greater COVID-19 vaccine hesitancy than White Americans (Callaghan et al., 2021, Fisher et al., 2020, Largent et al., 2020), although this has narrowed since the vaccines were first approved. The second hypothesis is that low vaccination rates among Black Americans at least partially reflect key barriers to getting vaccinated, including the location of and hours of mass vaccination sites (Boyd, 2021).
To address vaccination disparities, public health interventions must target the causes of hesitancy in addition to ensuring that vaccinations are equitably distributed to all Americans. Much of the discussion of hesitancy to date has been on historic medical mistreatment of Black Americans (Yuko, 2021). Although the infamous Tuskegee Syphilis Study has received the most attention (Freimuth et al., 2001), Black Americans have been exploited in medical research consistently throughout American history, beginning soon after the Atlantic slave trade was established (Gamble, 1997, Scharf et al., 2010). In response, new safety guidelines were introduced as part of the Belmont Report in 1974 which established ethical guardrails for social, behavioral, and biomedical research (The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 1979). Yet, continued discrimination in medical settings may contribute to vaccine hesitancy and general iatrophobia (Best et al., 2021). Indeed, Black patients report a direct impact of prior unethical medical experimentation on their current medical attitudes and behaviors (Freimuth et al., 2001, Scharf et al., 2010), which could spill over into vaccine attitudes. Public health messages that emphasize the history of violence in medical research and continued reality of medical discrimination may be important for increasing trust in medicine and public health and improving vaccine acceptance.
Another promising vaccination messaging option is to emphasize disparities in the vaccine roll-out and encourage vaccination as a measure of social responsibility to ensure that Black Americans are not more vulnerable to serious disease and death from COVID-19. Systematic research is necessary, however, to understand the effectiveness of either messaging strategy. Important to note is that the focus on vaccination attitudes alone is not sufficient to ensure vaccine equity in the U.S. In addition to reducing vaccine hesitancy and developing messages that address concerns among different groups, public health campaigns must address other barriers to vaccination, including access barriers.
The aim of this study is to assess whether different public health messages, specifically focused on the history of medical mistreatment of Black Americans and the current racial inequities in COVID-19 outcomes, are related to COVID-19 vaccine acceptance and hesitancy among Black Americans. We use an experimental approach to test two primary endpoints: whether different messages, tailored specifically to Black Americans, produce differences in (1) vaccine acceptance and (2) vaccine hesitancy as compared to standard messaging used by the Centers for Disease Control and Prevention (CDC) or no messaging at all. Our first messaging strategy emphasizes social responsibility and the need to protect health in marginalized communities which have been disproportionately affected by COVID-19. The second framing focuses on the historical mistreatment of Black Americans in medical research and reinforces the protections currently in place to prevent further unethical treatment. We also assessed these messaging strategies within a sample of White and Hispanic Americans to examine whether there were unintended consequences among demographic groups that were not targeted by these particular messaging strategies. This study provides insight into which tailored messaging strategies may be effective among Black Americans who have had a greater burden due to COVID-19.
2. Method
2.1. Sample
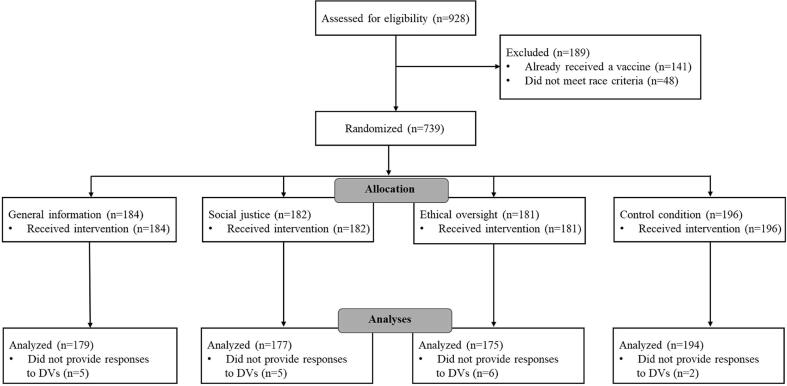
Data were collected online from 743 participants who resided in the United States and identified their race as Black (N = 244), Hispanic (N = 170), or White (N = 329). A sensitivity analysis conducted using G*Power based on the type of analyses conducted (i.e., ANCOVA), an estimate of 80% power, the obtained sample size (i.e., 743), an alpha value of 0.05, the number of groups included in the model (i.e., 8), and the number of covariates (i.e., 6) suggested that our sample was sufficient to detect an effect size of 0.14, or a small to medium effect. Participants were only eligible to participate in the study if they had not yet received any doses of the COVID-19 vaccine given that we were interested in the effectiveness of the messaging strategies among unvaccinated people. Please see Fig. 1 for a CONSORT chart displaying the recruitment process. This study was conducted in April 2021 which corresponded to when COVID-19 vaccination became open to all adults in the United States. Participants were recruited to the study via Prolific Academic, which is a web-based platform with registered participants who are matched to surveys based on their eligibility criteria. Participants were geographically diverse and represented every state in the U.S. Additional demographic information for participants by condition is available in Table 1. The study was approved by the Institutional Review Board at Ohio University and the study was completed in compliance with ethical guidelines, including obtaining consent from participants.
Fig. 1.
CONSORT flow diagram of participant enrollment.
Table 1.
Participant demographic characteristics and descriptive statistics by Condition.
| General information | Social justice | Ethical oversight | Control | |
|---|---|---|---|---|
| Total | 184 | 182 | 181 | 196 |
| Age | 32.57 (11.96) | 31.80 (11.00) | 32.30 (10.82) | 31.91 (10. 69) |
| Race | ||||
| Black | 62 (33.7%) | 59 (32.4%) | 61 (33.7%) | 62 (31.6%) |
| Hispanic | 42 (22.8%) | 42 (23.1%) | 40 (22.1%) | 46 (23.5%) |
| White | 80 (43.5%) | 81 (44.5%) | 80 (44.2%) | 88 (44.9%) |
| Sex | ||||
| Male | 99 (54.7%) | 86 (48.6%) | 92 (52.0%) | 94 (48.5%) |
| Female | 83 (45.4%) | 91 (51.4%) | 85 (48.0%) | 100 (51.5%) |
| Political affiliation | ||||
| Republican | 29 (15.7%) | 22 (12.1%) | 22 (12.2%) | 20 (10.3%) |
| Independent | 69 (37.5%) | 55 (30.2%) | 66 (36.7%) | 59 (30.3%) |
| Democrat | 86 (46.7%) | 105 (57.7%) | 92 (51.1%) | 116 (59.5%) |
| Religion | ||||
| Religious | 84 (45.7%) | 88 (48.4%) | 86 (47.5%) | 97 (49.5%) |
| Not religious | 100 (54.3%) | 94 (51.6%) | 95 (52.5%) | 99 (50.5%) |
| Education | ||||
| High school diploma | 33 (17.9%) | 41 (22.5%) | 34 (18.8%) | 23 (11.7%) |
| Some college | 55 (29.9%) | 49 (26.9%) | 52 (29.3%) | 66 (33.7%) |
| Associate’s | 20 (10.9%) | 22 (12.1%) | 19 (10.5%) | 17 (8.7%) |
| Bachelor’s | 62 (33.7%) | 56 (30.8%) | 55 (30.4%) | 66 (33.7%) |
| Master’s | 13 (7.1%) | 11 (6.0%) | 17 (9.4%) | 21 (10.7%) |
| Doctoral/professional | 1 (0.5%) | 3 (1.6%) | 3 (1.7%) | 3 (1.5%) |
Note: Means and standard deviations are reported for continuous variables and raw numbers and percentages are reported for categorical variables.
2.2. Procedure
Participants were stratified by race and then randomly assigned to receive one of four messages about the COVID-19 vaccine. A chi square test indicated that each racial group was equally represented among the four conditions (χ2(6, N = 743) = 0.31, p = 0.999). The first condition (i.e., the general information condition) received information used by the CDC to encourage people to get vaccinated and the passage emphasized the importance of vaccination for ending the pandemic as well as the efficacy rates and safety profile of the vaccines (CDC NCIRD, 2021). The second condition (i.e., the social justice condition) included the same information as the first condition but added messaging which emphasized the racial disparities in COVID-19 health outcomes and the role vaccination can play in reducing racial inequality among Black communities. The goal of the second condition was to assess whether social justice and protecting vulnerable community members might motivate vaccination among members of a racial/ethnic group that has been disproportionately affected by COVID-19.
The third condition (i.e., the ethical oversight condition) also included the information presented in the first condition and added a passage which acknowledged that the U.S. has a history of unethical treatment of Black Americans in medical research. The passage then described that new measures have been implemented to ensure that racial/ethnic minorities are not mistreated or unethically used in the development and testing of new vaccines. This condition was included to mitigate mistrust and hesitancy that might arise from the historical exploitation of Black Americans in medical research, which has been connected to Black American’s reported medical mistrust (Freimuth et al., 2001, Scharf et al., 2010). The final condition was a control condition wherein participants received no passage about the vaccine (see Appendix A for the manipulations).
3. Measures
3.1. Primary endpoints
Our primary endpoints were willingness to accept a COVID-19 vaccine and hesitancy about the COVID-19 vaccine after reading a brief passage. We included measures of acceptance and hesitancy to assess both participants’ behavioral intentions as well as their beliefs about the COVID-19 vaccine. Willingness to accept a vaccine was measured using a single item which read, “All things considered, how likely are you to get a coronavirus vaccine when one becomes available to you.” This item was taken from a recent study on COVID-19 (Ruiz & Bell, 2021) and single item measures are commonly used to assess vaccine acceptance. The response scale ranged from 1 (extremely unlikely) to 5 (extremely likely) and higher scores indicate more vaccine acceptance.
Participants then responded to a 9-item measure of their hesitancy about the COVID-19 vaccine using the Vaccine Hesitancy Scale (Larson et al., 2015). The scale was initially developed by the World Health Organization’s SAGE Working Group on Vaccine Hesitancy and has been used to assess attitudes toward a number of vaccines. The scale has also been validated, with evidence suggesting that scores are correlated with relevant vaccine attitudes and predict vaccine refusal (Shapiro et al., 2018). We modified the scale to be relevant to the COVID-19 vaccine and, in doing so, removed one item from the scale which did not apply (i.e., “My child does not need vaccines for diseases that are not common anymore”). The response scale ranged from 1 (strongly disagree) to 5 (strongly agree) and higher scores indicate less vaccine hesitancy. The adapted scale demonstrated good internal consistency reliability (α = 0.94).
3.2. Secondary endpoint
We finally asked participants a single follow up question which read, “If you were to accept a COVID-19 vaccine, where would you feel most comfortable receiving it?”. Response options included a hospital, community health center, primary care office, health department, pharmacy, government site, or another location not listed. We included this item given the ongoing concerns that a lack of access to the vaccine is contributing to the racial gap in vaccination rates.
3.3. Analyses
Data were analyzed using two 4x2 ANCOVA models in which the first factor was the four conditions, the second factor corresponded to race (coded as 1 = Black, 2 = White/Hispanic), and the dependent variable was either vaccine acceptance or vaccine hesitancy. ANCOVA was used because, despite the dependent variables being measured with a Likert scale, ANCOVA models have been shown to perform well with such data and be robust to violations of nonnormality that may be produced by ordinal scales (Olejnik and Algina, 2016, Stiger et al., 2011, Wu and Leung, 2017). We combined White and Hispanic participants given that our primary research questions were whether the vaccine messaging strategies were effective for Black Americans and whether they backfired in other racial/ethnic groups.1 The 4x2 model allowed us to assess for the interaction between race and condition, which would indicate that Black and non-Black participants responded differently to the messaging conditions. We probed any significant effects by examining the pairwise comparisons to determine what significant differences emerged between conditions. Bonferroni corrections were applied to correct for conducting multiple comparisons. We also controlled for relevant demographic variables in the ANCOVA models to ensure that differences between or among groups were not due to extraneous factors. More specifically, we controlled for participant sex (coded 1 = male, 2 = female), age, religious status (coded as religious or nonreligious), political affiliation (coded into two variables which represent identifying as republican and identifying as an independent), and education. We selected these variables given that prior work on COVID-19 vaccine acceptance and hesitancy has found differences across these demographic characteristics (Fisher et al., 2020, Largent et al., 2020, Unroe et al., 2021, Olagoke et al., 2021).
We finally assessed potential racial differences in the locations at which participants preferred to receive the COVID-19 vaccine using a chi-square test, which is a nonparametric test that assesses the degree of independence between two variables. This allowed us to examine whether location preference is independent of one’s race/ethnicity All analyses were conducted using IBM SPSS Version 27 (IBM Corp, 2020).
4. Results
4.1. Vaccine acceptance and hesitancy
Results for the ANCOVA models are shown in Table 2 and the means for each condition are shown in Table 3. Beginning with the model assessing vaccine acceptance, results indicated that each of the control variables significantly predicted vaccine acceptance. Further, there was no significant main effect of condition (F(3,711) = 1.39, p = 0.244, η2 = 0.006) but there were significant effects for race (F(1,711) = 40.24, p < 0.001, η2 = 0.054) and for the interaction between race and condition (F(3,711) = 3.64, p = 0.013, η2 = 0.015). Given that we were most interested in the differences between conditions within racial groups, we focused our interpretation on the pairwise comparisons for the interaction. Additionally, racial differences in vaccine attitudes are known and are not the subject of our research questions. As such, we only interpreted differences that emerged between conditions within racial groups. Results for the pairwise comparisons for Black participants indicated that participants who were exposed to the ethical oversight condition were significantly more likely to accept the COVID-19 vaccine (M = 3.69, SE = 0.17, 95% CI [3.36, 4.03]) in comparison to participants who were exposed to the social justice condition (M = 3.02, SE = 0.18, 95% CI [2.67, 3.36], p = 0.034). None of the remaining pairwise comparisons were significant for the Black sample. There were no significant pairwise comparisons for the non-Black sample.
Table 2.
ANCOVA Results for Messaging Condition on Vaccine Acceptance and Hesitancy.
| Acceptance |
Hesitancy |
|||||
|---|---|---|---|---|---|---|
| F (df) | p | η2 | F (df) | p | η2 | |
| Age | 6.68 (1,711) | 0.010 | 0.009 | 10.61 (1,711) | 0.001 | 0.015 |
| Gender | 6.49 (1,711) | 0.011 | 0.009 | 14.91 (1,711) | <0.001 | 0.021 |
| Education | 9.83 (1,711) | 0.002 | 0.014 | 8.65 (1,711) | 0.003 | 0.012 |
| Republican | 73.23 (1,711) | <0.001 | 0.093 | 78.47 (1,711) | <0.001 | 0.099 |
| Independent | 56.25 (1,711) | <0.001 | 0.073 | 65.69 (1,711) | <0.001 | 0.085 |
| Religion | 8.13 (1,711) | 0.004 | 0.011 | 13.37 (1,711) | <0.001 | 0.018 |
| Condition | 1.39 (3,711) | 0.244 | 0.006 | 2.03 (3,711) | 0.109 | 0.008 |
| Race | 40.24 (1,711) | <0.001 | 0.054 | 43.41 (1,711) | <0.001 | 0.058 |
| Race * Condition | 3.64 (3,711) | 0.013 | 0.015 | 2.76 (3,711) | 0.041 | 0.012 |
Note: Gender is coded as 1 = male, 2 = female; religion is coded as 1 = religious, 2 = nonreligious; race is coded as 1 = Black, 2 = White/Hispanic.
Table 3.
Means and Standard Errors for Vaccine Acceptance and Hesitancy Scores by Condition and Race.
| Acceptance |
Hesitancy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black |
Non-Black |
Black |
Non-Black |
|||||||||
| N | M (SE) | 95% CI | N | M (SE) | 95% CI | N | M (SE) | 95% CI | N | M (SE) | 95% CI | |
| General information | 60 | 3.47 (0.17) | 3.13, 3.80 | 119 | 4.10 (0.12) | 3.86, 4.33 | 60 | 3.44 (0.12) | 3.21, 3.68 | 119 | 3.94 (0.09) | 3.77, 4.11 |
| Social justice | 57 | 3.01 (0.18) | 2.67, 3.36 | 120 | 4.13 (0.12) | 3.90, 4.37 | 57 | 3.25 (0.13) | 3.00, 3.49 | 120 | 3.96 (0.09) | 3.79, 4.13 |
| Ethical oversight | 60 | 3.69 (0.17) | 3.36, 4.03 | 115 | 3.86 (0.12) | 3.61, 4.10 | 60 | 3.65 (0.12) | 3.41, 3.89 | 115 | 3.81 (0.09) | 3.64, 3.98 |
| Control | 62 | 3.13 (0.17) | 2.80, 3.47 | 132 | 3.98 (0.12) | 3.75, 4.20 | 62 | 3.17 (0.12) | 2.93, 3.41 | 132 | 3.82 (0.08) | 3.66, 3.98 |
Note: Significant differences were found for the social justice and ethical oversight conditions on vaccine acceptance among Black participants (p =.034) and for the ethical oversight and control conditions for vaccine hesitancy among Black participants (p =.028).
Results for the ANCOVA model predicting vaccine hesitancy similarly demonstrated that each of the covariates was significantly related to vaccine hesitancy. There was no significant main effect for condition (F(3,711) = 2.03, p = 0.109, η2 = 0.008) but there were significant effects for race (F(1,711) = 43.11, p < 0.001, η2 = 0.058) and for the interaction between race and condition (F(3,711) = 2.76, p = 0.041, η2 = 0.012). The pairwise comparisons for the interaction between race and condition showed that, among the Black participants, participants in the ethical oversight condition reported significantly less vaccine hesitancy (M = 3.65, SE = 0.12, 95% CI [3.41, 3.89]) than participants in the control condition (M = 3.17, SE = 0.12, 95% CI [2.93, 3.41], p = 0.028). No other pairwise comparisons were significant for the Black participants and no significant differences emerged for the non-Black participants.
4.2. Vaccination location
Results for the chi-square test assessing the relationship between race/ethnicity and preferred location for receiving the COVID-19 vaccine are shown in Table 4. The chi-square test was significant (χ2(12, N = 787) = 29.78, p = 0.003), which indicates there is a relationship between race/ethnicity and preferred location. An examination of the location responses by race/ethnicity indicated that the most commonly preferred location among Black participants was a hospital whereas the most commonly preferred location for White and Hispanic respondents was a primary care office. Further, a greater proportion of White and Hispanic participants reported preferring to receive the vaccine at a pharmacy in comparison to Black participants.
Table 4.
Chi-Square Results for Preferred Vaccination Location by Race/Ethnicity.
| Black Sample | Hispanic Sample | White Sample | % of total | |
|---|---|---|---|---|
| Hospital | 24.5% | |||
| Observed count | 84 (36.8%) | 36 (22.0%) | 62 (20.6%) | |
| Expected count | 59.8 | 41.6 | 80.6 | |
| Community health center | 9.8% | |||
| Observed count | 22 (9.6%) | 18 (11.0%) | 33 (11.0%) | |
| Expected count | 24.0 | 16.7 | 32.3 | |
| Primary care office | 31.4% | |||
| Observed count | 72 (31.6%) | 50 (30.5%) | 111 (36.9%) | |
| Expected count | 76.5 | 53.3 | 103.2 | |
| Health department | 4.4% | |||
| Observed count | 8 (3.5%) | 9 (5.5%) | 16 (5.3%) | |
| Expected count | 10.8 | 7.6 | 14.6 | |
| Pharmacy | 20.2% | |||
| Observed count | 35 (15.4%) | 44 (26.8%) | 71 (23.6%) | |
| Expected count | 49.3 | 34.3 | 66.4 | |
| Government site | 3.0% | |||
| Observed count | 7 (3.1%) | 7 (4.3%) | 8 (2.7%) | |
| Expected count | 7.2 | 5.0 | 9.7 | |
Note: observed count = the number of participants in each subsample who selected each location; expected count = the number of participants in each subsample who would be expected to have chosen each location if race/ethnicity and location were independent; % of total = the percentage of all participants who selected each location category.
5. Discussion
The aim of this study was to assess the effect of vaccine messaging on COVID-19 vaccine acceptance and hesitancy among unvaccinated Americans. Our findings support the need for vaccine messages that are tailored to demographic groups in the United States, including racial/ethnic minorities. Although public health officials have long dismissed a “one size fits all” approach (Siddiqui and Armour, 2021), our data provide empirical evidence that using messaging to address specific concerns about COVID-19 vaccines may be an important strategy to explore. We found limited support for the role of targeted messaging on vaccine acceptance, or the intention to get a COVID-19 vaccine soon. The impact of messaging was more pronounced, however, in the decrease of COVID-19 vaccine hesitancy. Although the effect size was modest, receiving messaging about ethical safeguards resulted in decreased vaccine hesitancy among Black Americans as compared to receiving no messaging. By contrast, nontailored vaccine messaging that provided information about the safety of COVID-19 vaccines did not reduce vaccine hesitancy. This finding provides support for the hypothesis that medical mistrust is a critical facet of COVID-19 hesitancy and, as such, that acknowledging the history of medical mistreatment may be an important facet of COVID-19 vaccine messaging. Most important, these findings build on evidence that disclosing past and present injustices and acknowledging their continued harm can be instrumental in rebuilding trust in medicine and public health (Best et al., 2021).
It is important to note that the standard language adopted by the CDC, that of emphasizing the safety and effectiveness of COVID-19 vaccines, did not have an effect on vaccine acceptance or hesitancy. This provides further evidence that new messaging approaches are needed, particularly messages that address the diverse concerns that exist among those who remain vaccine hesitant. Indeed, we found that effective messages may work differently among Black, Hispanic, and White Americans and that tailoring messages for subgroups did not seem to introduce any negative effects for other groups who may also be exposed to that messaging. Because it is challenging to restrict public health messages solely to specific groups, knowing that tailored messages do not harm vaccine confidence in other groups should further bolster efforts to address specific concerns related to COVID-19 vaccines. Moreover, although we did not observe an effect for messaging that emphasizes social responsibility in light of racial/ethnic disparities in COVID-19 outcomes, it is possible that Black Americans interpret vaccine inequities as a structural problem in need of a structural solution. In other words, asking individuals to account for institutional and societal failures may not seem like an appropriate response.
Although our study provides insight on the potential of messages that emphasize past medical mistreatment to decrease vaccine hesitancy, these strategies should not be used at the exclusion of improving vaccine access. A substantial number of unvaccinated Americans face barriers to receiving the vaccine that are unrelated to hesitancy. That vaccination rates are consistently lower in communities that are socially vulnerable (Hughes et al., 2021) suggests that messaging alone will not overcome disparities in vaccination in the U.S. Important challenges remain, particularly among individuals who lack reliable transportation, are employed in jobs with limited flexibility to attend vaccination appointments or take time off due to side effects, or provide critical caregiving roles in which taking time off for vaccine side effects may not be possible (Zhang and Fisk, 2021, Kullgren et al., 2012). Our ad hoc analysis of sites where people would prefer to be vaccinated suggests that investing in a wide range of vaccination settings may be a critical complement to vaccine messages that aim to address specific concerns related to the COVID-19 vaccines. In particular, sites such as hospitals and primary care offices, which have not been adequately leveraged in the initial vaccine rollout, may help decrease disparities in vaccination rates across racial/ethnic groups.
6. Limitations
Our study has limitations that should be considered. First, while the outcome variables examined in this study are predictive of vaccine uptake (Quinn et al., 2019, Bish et al., 2011), we cannot conclude whether the effects of our messaging affected the number of people who subsequently received the COVID-19 vaccine. Second, we were unable to assess baseline vaccine attitudes and thus could not determine the degree to which vaccine attitudes changed as a result of exposure to the experimental stimuli. Third, data were collected in April 2021 when many states in the U.S. expanded vaccine eligibility to everyone. It is therefore possible that our sample might have higher levels of vaccine hesitancy than the general population because they remained unvaccinated. We posit this was appropriate given that unvaccinated populations are the intended target of our messaging. Finally, our messaging intervention was designed to be brief and easy to implement by public health agencies. It is possible that interventions which are lengthier, provide more depth, or provide repeated messaging would produce stronger effects on vaccine attitudes and may therefore be more advantageous. We assert that the pressing need to increase vaccination against COVID-19 warrants interventions of the nature addressed within our study but encourage future work to develop and test more detailed interventions for use with other vaccines.
7. Public health implications
The consequences of COVID-19 have taken shape in the context of pervasive structural racial inequalities which have left Black Americans uniquely vulnerable. Exacerbating these disparities, vaccination rates among Black Americans have continued to lag substantially behind those of White Americans. To reduce disparities, public health agencies must identify successful communication strategies that are tailored to specific, vaccine hesitant populations. Results from our study suggest that messages which acknowledge the harms of medical experimentation and the ethical safeguards in place to prevent further harm may hold promise for future messaging strategies. In our efforts to achieve widespread vaccination, we encourage public health agencies to adopt tailored strategies to alleviate the concerns most central to individuals who remain unvaccinated and eliminate structural barriers to vaccination.
CRediT authorship contribution statement
Lindsay Y. Dhanani: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Berkeley Franz: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Dr. Janet Simon for providing biostatistical support for the analyses included in the manuscript.
Footnotes
We conducted an ANCOVA to assess any differences in vaccine acceptance and hesitancy between White and Hispanic participants within each condition before combining these samples. Results indicated no racial differences within any condition for either acceptance or hesitancy.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101792.
Contributor Information
Lindsay Y. Dhanani, Email: dhanani@ohio.edu.
Berkeley Franz, Email: franzb@ohio.edu.
Appendix A.
Vaccine messaging strategies
Condition 1: The general information condition
Vaccines are one of the most effective tools to protect your health and prevent disease. The COVID-19 pandemic has affected Americans greatly, but vaccines will reduce the number of new cases and deaths in the U.S. Vaccines work with your body’s natural defenses so your body will be ready to fight the virus, if you are exposed. Studies show that COVID-19 vaccines are very effective at keeping you from getting COVID-19. Each of the initial vaccines developed has been shown to be highly effective. These vaccines cannot give you the disease itself. Several vaccines will be initially available, but all types of the vaccines will help protect you. All of the vaccines on the market have undergone rigorous clinical trials and testing prior to being approved for use. The vaccines may cause side effects in some people, like sore muscles, feeling tired, or mild fever. These reactions mean the vaccine is working to help teach your body how to fight COVID-19 if you are exposed. For most people, these side effects will last no longer than a day or two and do not mean that you have COVID-19. Only when the vast majority of Americans have accepted a vaccine, will we see the end of this pandemic.
Condition 2: The social justice condition
Vaccines are one of the most effective tools to protect your health and prevent disease. The COVID-19 pandemic has affected Americans greatly, but vaccines will reduce the number of new cases and deaths in the U.S. Vaccines work with your body’s natural defenses so your body will be ready to fight the virus, if you are exposed. Studies show that COVID-19 vaccines are very effective at keeping you from getting COVID-19. Each of the initial vaccines developed has been shown to be highly effective. These vaccines cannot give you the disease itself. Several vaccines will be initially available, but all types of the vaccines will help protect you. All of the vaccines on the market have undergone rigorous clinical trials and testing prior to being approved for use. The vaccines may cause side effects in some people, like sore muscles, feeling tired, or mild fever. These reactions mean the vaccine is working to help teach your body how to fight COVID-19 if you are exposed. For most people, these side effects will last no longer than a day or two and do not mean that you have COVID-19. Only when the vast majority of Americans have accepted a vaccine, will we see the end of this pandemic.
It is also important to recognize that Black and Hispanic communities have experienced more COVID-19 cases and deaths than other populations. Although life expectancy has declined for all Americans this year due to COVID-19, Black and Hispanic Americans have seen the biggest drop in life expectancy. These numbers demonstrate continued racial injustice in our society and in the U.S. healthcare system, but vaccines have the potential to change this. In addition to preventing you from getting seriously ill from COVID-19, vaccines will help drive cases down and will protect others in your community. This means that vaccines are a critical tool for improving health among Black and Hispanic Americans and ensuring that communities of color are no longer disproportionately affected by this pandemic.
Condition 3: The ethical oversight condition
Vaccines are one of the most effective tools to protect your health and prevent disease. The COVID-19 pandemic has affected Americans greatly, but vaccines will reduce the number of new cases and deaths in the U.S. Vaccines work with your body’s natural defenses so your body will be ready to fight the virus, if you are exposed. Studies show that COVID-19 vaccines are very effective at keeping you from getting COVID-19. Each of the initial vaccines developed has been shown to be highly effective. These vaccines cannot give you the disease itself. Several vaccines will be initially available, but all types of the vaccines will help protect you. All of the vaccines on the market have undergone rigorous clinical trials and testing prior to being approved for use. The vaccines may cause side effects in some people, like sore muscles, feeling tired, or mild fever. These reactions mean the vaccine is working to help teach your body how to fight COVID-19 if you are exposed. For most people, these side effects will last no longer than a day or two and do not mean that you have COVID-19. Only when the vast majority of Americans have accepted a vaccine, will we see the end of this pandemic.
The U.S. medical and public health systems make sure that all vaccines are as safe as possible before offering them to the general public. All of the COVID-19 vaccines that are currently being used have gone through the same safety tests and meet the same standards as any other vaccines produced through the years, such as the vaccines children regularly receive. Safety protocols are very important because in the past, racial minorities, particularly Black Americans, were unethically used as test subjects for medical research. This caused significant harm to Black communities and contributes to the enduring inequality in health outcomes between racial groups in the United States. It is important to make sure this type of mistreatment never happens again as part of medical research. For this reason, ethical oversights are now in place in the United States to ensure that all research and medical treatments do not cause harm to any populations, including racial and ethnic minorities. The COVID-19 vaccine was developed under these very strict safety protocols.
Appendix B. Supplementary data
The following are the Supplementary data to this article:
References
- Johns Hopkins University. Johns Hopkins Coronavirus Resource Center. Published 2020. Accessed June 25, 2020. https://coronavirus.jhu.edu/.
- CDC. COVID-19 Hospitalization and Death by Race/Ethnicity. CDC COVID-19 Cases, Data & Surveillance. Published August 2020. Accessed November 18, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html.
- Ndugga, N., Pham, O., Hill, L., Artiga, S., Alam, R., Parker, N. 2021. Latest Data on COVID-19 Vaccinations Race/Ethnicity. Kaiser Family Foundation. Published May 12, 2021. Accessed May 18, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/.
- Williams D.R. Miles to go before we sleep: racial inequities in health. J. Health Soc. Behav. 2012;53(3):279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T.J., Croft J.B., Liu Y., Lu H., Eke P.I., Giles W.H. Vital signs: racial disparities in age-specific mortality among blacks or African Americans — United States, 1999–2015. MMWR Morb. Mortal Wkly. Rep. 2017;66(17):444–456. doi: 10.15585/mmwr.mm6617e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster Pirtle W.N. Racial capitalism: a fundamental cause of novel coronavirus (COVID-19) pandemic inequities in the United States. Heal Educ Behav. 2020;47(4):504–508. doi: 10.1177/1090198120922942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaye I.M., Horney J.A. The impact of social vulnerability on COVID-19 in the U.S.: an analysis of spatially varying relationships. Am. J. Prev. Med. 2020;59(3):317–325. doi: 10.1016/j.amepre.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L., Hart L.H., Katz M.H. Racial and ethnic health disparities related to COVID-19. JAMA – J. Am. Med. Assoc. 2021;325(8):719–720. doi: 10.1001/jama.2020.26443. [DOI] [PubMed] [Google Scholar]
- Khazanchi R., Evans C.T., Marcelin J.R. Racism, not race, drives inequity across the COVID-19 continuum. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19933. [DOI] [PubMed] [Google Scholar]
- Hall W.J., Chapman M.V., Lee K.M., et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am. J. Public Health. 2015;105(12):e60–e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner A., Franz B., Henry B.J. We need to talk about Racism - in all of its forms - In understand COVID-19 disparities. Heal Equity. 2020;4(1) doi: 10.1089/heq.2020.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk for COVID-19 Infection, Hospitalization, and Death By Race/Ethnicity | CDC. Accessed May 18, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html.
- CDC. Flu Vaccination Coverage, United States, 2019–20 Influenza Season. CDC Flu Vax View. Published October 1, 2020. Accessed May 18, 2021. https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates.htm.
- Hall L.L., Xu L., Mahmud S.M., Puckrein G.A., Thommes E.W., Chit A. A map of racial and ethnic disparities in influenza vaccine uptake in the medicare fee-for-service program. Adv. Ther. 2020;37(5):2224–2235. doi: 10.1007/s12325-020-01324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A.E., Reither E.N., Mamelund S.E., Lim S. Black-white disparities in 2009 H1N1 vaccination among adults in the United States: a cautionary tale for the COVID-19 pandemic. Vaccine. 2021;39(6):943–951. doi: 10.1016/j.vaccine.2020.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels S.J.M., Marshall H.S., Watson M., Braunack-Mayer A.J., Reuzel R., Tooher R.L. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30(24):3546–3556. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- Winston, C.A., Wortley, P.M., Lees, K.A. 2006. Factors associated with vaccination of medicare beneficiaries in five U.S. communities: results from the racial and ethnic adult disparities in immunization initiative survey, 2003. J. Am. Geriatr. Soc. 54(2), 303-310. doi:10.1111/j.1532-5415.2005.00585.x. [DOI] [PubMed]
- Callaghan T., Moghtaderi A., Lueck J.A., et al. Correlates and disparities of intention to vaccinate against COVID-19. Soc. Sci. Med. 2021;272 doi: 10.1016/j.socscimed.2020.113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. Adults. Ann. Intern. Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent E.A., Persad G., Sangenito S., Glickman A., Boyle C., Emanuel E.J. US Public attitudes toward COVID-19 vaccine mandates. JAMA Netw Open. 2020;3(12):e2033324. doi: 10.1001/jamanetworkopen.2020.33324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, R. Black People Need Better Covid-19 Vaccine Access, Not Better Vaccine Attitudes. The New York Times. Published March 5, 20Accessed May 18, 20. https://www.nytimes.com/2021/03/05/opinion/us-covid-black-people.html.
- Yuko, E. 2021. Why Are Black Communities Being Singled Out as Covid Vaccine Hesitant? Rolling Stone. Published March 9, 2021. Accessed May 18, 2021. https://www.rollingstone.com/culture/culture-features/covid-19-vaccine-hesitant-black-communities-singled-out-1137750/.
- Freimuth V.S., Quinn S.C., Thomas S.B., Cole G., Zook E., Duncan T. African Americans’ views on research and the Tuskegee Syphilis study. Soc. Sci. Med. 2001;52(5):797–808. doi: 10.1016/S0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Gamble V.N. Under the Shadow of Tuskegee: African Americans and Health Care. Am. J. Public Health. 1997;87(11):1773–1778. doi: 10.2105/AJPH.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf D.P., Mathews K.J., Jackson P., Hofsuemmer J., Martin E., Edwards D. More than Tuskegee: understanding mistrust about research participation. J. Health Care Poor Underserved. 2010;21(3):879–897. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research.; 1979. Accessed May 18, 2021. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html. [PubMed]
- Best A.L., Fletcher F.E., Kadono M., Warren R.C. Institutional distrust among african americans and building trustworthiness in the covid-19 response: implications for ethical public health practice. J. Health Care Poor Underserved. 2021;32(1):90–98. doi: 10.1353/hpu.2021.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC NCIRD. COVID-19 Vaccines Are One of the Tools We Have to Fight the COVID-19 Pandemic.; 2021. Accessed May 18, 2021. www.cdc.gov/coronavirus/vaccines.
- Ruiz J.B., Bell R.A. Predictors of intention to vaccinate against COVID-19: results of a nationwide survey. Vaccine. 2021;39(7):1080–1086. doi: 10.1016/j.vaccine.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H.J., Jarrett C., Schulz W.S., et al. Measuring vaccine hesitancy: the development of a survey tool. Vaccine. 2015;33(34):4165–4175. doi: 10.1016/j.vaccine.2015.04.037. [DOI] [PubMed] [Google Scholar]
- Shapiro G.K., Tatar O., Dube E., et al. The vaccine hesitancy scale: psychometric properties and validation. Vaccine. 2018;36(5):660–667. doi: 10.1016/j.vaccine.2017.12.043. [DOI] [PubMed] [Google Scholar]
- Olejnik, S.F., Algina, J. 2016. Parametric ANCOVA and the Rank Transform ANCOVA When the Data are Conditionally Non-Normal and Heteroscedastic: http://dx.doi.org/103102/10769986009002129. 9(2), 129-149. doi:10.3102/10769986009002129.
- Stiger, T.R., Kosinski, A.S., Barnhart, H.X., Kleinbaum, D.G. 2011. Anova for repeated ordinal data with small sample size? a comparison of anova, manova, wls and gee methods by simulation. http://dx.doi.org/101080/03610919808813485. 27(2), 357-375. doi:10.1080/03610919808813485.
- Wu H., Leung S.O. Can likert scales be treated as interval scales?—A simulation study. J. Soc. Serv. Res. 2017;43(4):527–532. doi: 10.1080/01488376.2017.1329775. [DOI] [Google Scholar]
- Unroe K.T., Evans R., Weaver L., Rusyniak D., Blackburn J. Willingness of long-term care staff to receive a COVID-19 vaccine: a single state survey. J. Am. Geriatr. Soc. 2021;69(3):593–599. doi: 10.1111/jgs.17022. [DOI] [PubMed] [Google Scholar]
- Olagoke A.A., Olagoke O.O., Hughes A.M. Intention to vaccinate against the novel 2019 coronavirus disease: the role of health locus of control and religiosity. J. Relig. Health. 2021;60(1):65–80. doi: 10.1007/s10943-020-01090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. SPSS Statistics for Windows, Version 27. Published online 2020.
- Siddiqui S, Armour S. Biden Administration Plans Localized Approach to Promote Covid-19 Vaccine. The Wall Street Journal. https://www.wsj.com/articles/biden-administration-plans-localized-approach-to-promote-covid-19-vaccine-11616773642. Published March 26, 2021. Accessed May 19, 2021.
- Hughes M.M., Wang A., Grossman M.K., et al. County-level COVID-19 vaccination coverage and social vulnerability — United States, December 14, 2020-March 1, 2021. MMWR Surveill. Summ. 2021;70(12):431–436. doi: 10.15585/mmwr.mm7012e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Fisk R.J. Barriers to vaccination for coronavirus disease 2019 (COVID-19) control: experience from the United States. Glob. Heal. J. 2021;5(1):51–55. doi: 10.1016/j.glohj.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullgren J.T., McLaughlin C.G., Mitra N., Armstrong K. Nonfinancial barriers and access to care for U.S. adults. Health Serv. Res. 2012;47(1 PART 2):462–485. doi: 10.1111/j.1475-6773.2011.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn S.C., Jamison A.M., An J., Hancock G.R., Freimuth V.S. Measuring vaccine hesitancy, confidence, trust and flu vaccine uptake: results of a national survey of White and African American adults. Vaccine. 2019;37(9):1168–1173. doi: 10.1016/j.vaccine.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Bish A., Yardley L., Nicoll A., Michie S. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29(38):6472–6484. doi: 10.1016/j.vaccine.2011.06.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.