Abstract
The treatment of post-acute sequelae of Covid-19 (PASC) has been informed primarily by symptomatic parallels with other chronic inflammatory syndromes. This manuscript takes a more systemic approach by examining how a marginal deficiency of tetrahydrobiopterin (BH4) resulting from mutations of the GCH1 (GTP cyclohydrolase 1) gene may result in the uncoupling of inducible Nitric Oxide Synthase (iNOS) early in the initial response of the innate immune system to SARS-CoV-2. The resulting production of superoxide instead of nitric oxide leads to a self-perpetuating cycle of oxidative stress with the potential to impair numerous metabolic processes and damage multiple organ systems. This marginal deficiency of BH4 may be exhibited by 30% or more of the patient population that have heterozygous or homozygous mutations of GCH1. As the cycle of oxidative stress continues, there is less BH4 available for other metabolic needs such as 1) resisting increased ferroptosis with its damage to organs, and 2) regulating the deactivation of the hyperinflammatory state. Finally, possible steps are proposed for clinical treatment of the hypothesized oxidative stress involved with PASC.
Keywords: Post-acute sequelae of Covid-19 (PASC), Uncoupling iNOS, Marginally deficient BH4, Oxidative stress, GCH1 mutations, AGMO, Ferroptosis
Background
Patients who have survived the acute phase of Covid-19 often experience a wide range of variable symptoms that are officially designated as “post-acute sequelae of Covid-19” (PASC) but are colloquially called “long-haul” Covid. Physicians and researchers have found it difficult to account for how PASC afflicts not just those patients who had been seriously ill with Covid-19 but also those who had been only mildly ill. Patients who had initially expected to recover in a span of 2–3 weeks have been plagued by a variety of chronic symptoms that include fatigue, shortness of breath, loss of smell/taste, migraine headaches, brain fog, memory loss, sleep disorders, postural orthostatic tachycardia syndrome (POTS), chest pain, muscle pain and weakness, joint pain, gastrointestinal difficulties, depression, skin rashes, and even “covid toe” [1], [2], [3], [4]. These long-term symptoms have debilitated the lives of an increasing number of patients, some of whom recovered from acute Covid-19 in early 2020 and are still suffering from PASC. Sadly, there are still no acknowledged treatments available for these patients.
Thus far, most of the research on PASC has employed a monotherapy perspective by searching for a single cause that 1) is distinctively associated with the SARS-CoV-2 virus, and 2) may be cured by a single medication or treatment protocol. However, Proal and VanElzakker [5] have recently argued that PASC may be a post-viral recovery syndrome that is best understood as a complex systemic interaction involving multiple factors beyond the nature of the virus itself. Similar recovery syndromes have been previously reported for other viral threats including MERS, SARS, and even various seasonal flus [1]. Additionally, many of the symptoms of PASC closely resemble the symptoms of other syndromes such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), POTS, dysautonomia, Ehlers-Danlos syndrome, etc. [3]. After an extensive review of the literature, Proal and VanElzakker [5] concluded: “It is likely that individual patients with a PASC diagnosis have different underlying biological drivers of their symptoms, none of which are mutually exclusive. Potential contributors to PASC include injury to one or multiple organs, persistent reservoirs of SARS-CoV-2 in certain tissues, re-activation of neurotrophic pathogens such as herpesviruses under conditions of COVID-19 immune dysregulation, SARS-CoV-2 interactions with host microbiome/virome communities, clotting/coagulation issues, dysfunctional brainstem/vagus nerve signaling, ongoing activity of primed immune cells, and autoimmunity due to molecular mimicry between pathogen and host proteins.” In conclusion, they took the additional step of calling for the identification of “individual human genome variants” that could further add to this set of biological drivers underlying PASC [5].
Hypotheses
The purpose of this paper is to extend Proal and VanElzakker’s [5] systemic approach to PASC by identifying and exploring the adverse impact of a marginal deficiency of tetrahydrobiopterin (BH4) due to mutations of the GCH1 (GTP cyclohydrolase 1) gene. More specifically, the paper examines the joint impact of the following hypotheses:
-
1.
Insufficient BH4 leads to the uncoupling of the inducible Nitric Oxide Synthase (iNOS) and thereby initiates self-perpetuating oxidative stress with excess superoxide and peroxynitrite.
-
2.
Insufficient BH4 impedes and may even prevent the downregulation of this hyperinflammatory oxidative stress and the return to oxidative homeostasis.
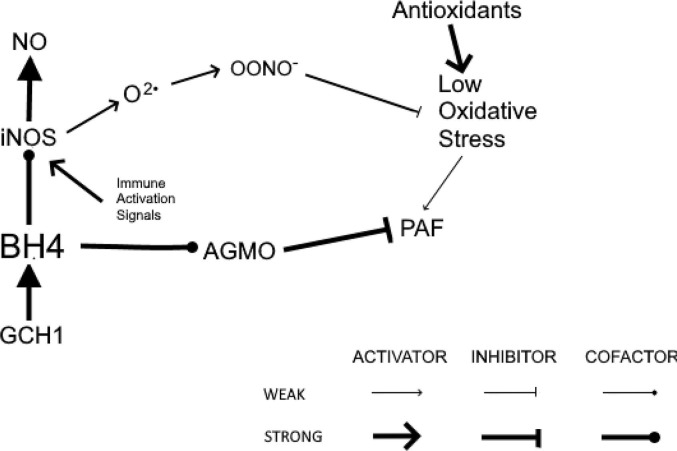
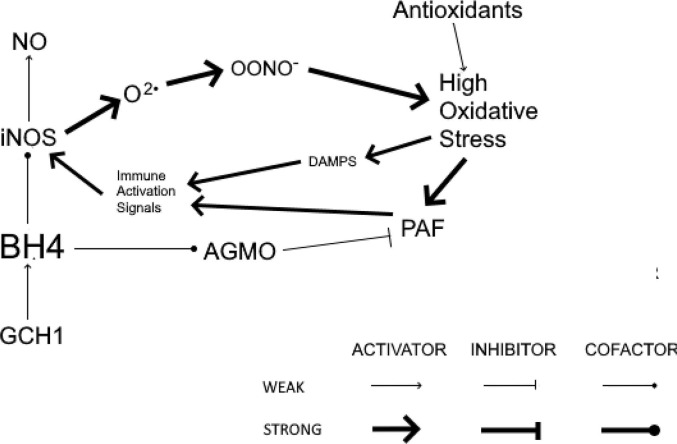
Fig. 1 illustrates sufficient BH4 to serve as the cofactors for both iNOS and AGMO. Fig. 2 exhibits the self-perpetuating cycle of hyperinflammation that results from insufficient BH4 that uncouples iNOS and prevents AGMO from reducing immune activation. The result is the production of optimal levels of NO and the prevention of ongoing oxidative stress. Finally, suggestions will be offered for treating the hypothesized cycle of self-perpetuating oxidative stress operative in PASC patients with GCH1 mutations.
Fig. 1.
After being induced by the innate immune system, coupled iNOS produces maximal NO and minimal O2•. Adequate downregulation of inflammation prevents a self-perpetuating cycle of hyperinflammation. GCH1, GTP cyclohydrolase 1; BH4, tetrahydrobiopterin; iNOS, inducible nitric oxide synthase; NO, nitric oxide; O2•, superoxide; OONO−, peroxynitrite; PAF, platelet-activating factor; AGMO, alkylglycerol monooxygenase.
Fig. 2.
After being induced by the innate immune system, uncoupled iNOS produces excessive O2• and OONO−. Inadequate BH4 impairs AGMO and associated downregulation of inflammation to allow for the development of a self-perpetuating cycle of hyperinflammation. GCH1, GTP cyclohydrolase 1; BH4, tetrahydrobiopterin; iNOS,inducible nitric oxide synthase; NO, nitric oxide; O2•, superoxide; OONO−, peroxynitrite; PAF, platelet-activating factor; AGMO, alkylglycerol monooxygenase; DAMPs, damage-associated molecular patterns.
Support for hypotheses
BH4 and the initiation of oxidative stress
Because nitric oxide (NO) is the simplest bioactive signaling molecule in the body, NO is an important component of numerous signaling cascades that regulate a wide variety of essential metabolic processes [6]. The amounts of NO required for these regulatory purposes are produced in small pulses by endothelial Nitric Oxide Synthase (eNOS) and neuronal Nitric Oxide Synthase (nNOS). A third form of NOS, called inducible Nitric Oxide Synthase (iNOS), is specialized for continuous production of large amounts of NO for the purpose of directing its powerful cytotoxic effect against invading bacteria and viruses. Normally then, as an invading virus is detected, a subset of macrophages is activated to produce a complex configuration of messenger molecules (cytokines) that instruct iNOS to produce NO in large amounts. This step helps to establish an appropriately active inflammatory response to battle the invading virus or bacteria. If these activated macrophages continue to perceive immunological threat in some form, they will prompt iNOS to continue producing NO in large amounts and the hyperactive inflammatory response will continue [6].
Insofar as high levels of NO also have a strong cytotoxic effect over time on healthy cells, iNOS ultimately needs to be deactivated by a subset of deactivating macrophages that have sensed the weakening or termination of the immunological threat. At this point, activation of iNOS should cease and nitric oxide (NO) should return to the normal level produced by eNOS and nNOS. However, if a false immunological threat has been sensed, the deactivating macrophages will fail to send out the appropriate set of deactivating cytokines and the hyperinflammatory response will continue in an inappropriate fashion to damage a wide range of body systems.
All three forms of nitric oxide synthase require the cofactor tetrahydrobiopterin (BH4) to oxidize l-arginine into NO and l-citrulline. When there is insufficient BH4 and/or arginine to produce the NO required, the 3 forms of NOS are said to be “uncoupled” and convert l-arginine into superoxide instead of NO [7]. Sporadic uncoupling of eNOS and nNOS can lead to relatively unnoticed metabolic consequences that may only become serious with the passage of considerable time. For example, the formation of atherosclerotic plaque has been connected to inflammation of the endothelium over time caused by a deficiency of NO and a corresponding excess of superoxide and derivative free radicals [8]. In contrast, the continuous activation of iNOS requires much greater levels of BH4 to produce the large quantities of NO required to attack invading viruses and bacteria. If this activated iNOS is significantly uncoupled, large amounts of superoxide are produced with profound metabolic consequences that may manifest rather quickly.
The first such consequence of the uncoupling of iNOS is the continuing accumulation of superoxide at ever higher levels. The second metabolic consequence derives from the high affinity of superoxide for oxidizing NO into peroxynitrite, another cytotoxic substance that increases oxidative stress along with superoxide. At this point, if there is some BH4 momentarily available to allow iNOS to produce NO, the newly produced NO likely will be exposed to the excess superoxide and quickly converted into peroxynitrite [9]. In this manner, high levels of oxidative stress cause the hyperinflammatory response to continue beyond the defeat of the virus by the adaptive immune system [10]. The cytotoxic effect of oxidative stress is sensed by the activating macrophages as a continuing threat. Consequently, the activating macrophages keep iNOS in a vicious cycle of producing more superoxide and peroxynitrite while the deactivating macrophages fail to turn off iNOS activity [11]. If this cycle of oxidative stress persists, multiple body systems can be severely damaged, some even permanently [12].
Antioxidants and the reduction of oxidative stress
Normally, oxidative stress is returned to oxidative homeostasis by antioxidants that catabolize superoxide and peroxynitrite for recycling or elimination. For example, superoxide dismutase (SOD) is the enzyme that breaks down superoxide into ordinary oxygen and hydrogen peroxide (which is further broken down by catalase). Similarly, peroxynitrite is broken down primarily by the gamma tocopherol form of vitamin E [13]. Other important antioxidants that reduce oxidative stress include vitamin C and various forms of glutathione [14]. This mix of antioxidants is usually sufficient to reduce the reactive oxidant species (ROS) no longer needed by the innate immune system once a virus has been defeated. However, if there are systemic factors (such as genetics, environment, and diet) that lead to lower levels of these antioxidants, the hyperinflammatory oxidative stress hypothesized for PASC may remain active, uncontrolled, and self-perpetuating.
Genetic factors underlying oxidative stress
The initiating event in this hypothesized account of PASC is the uncoupling of iNOS due to a deficiency of BH4. Previous research has established that BH4 deficiencies are primarily caused by genetic mutations of the GCH1 gene (sometimes named GTPCH1 or DYT5). The GCH1 gene encodes the enzyme GTP cyclohydrolase 1, which accomplishes the first of three steps in the production of BH4. Initial research into BH4 deficiency has primarily focused on rare loss of function mutations of GCH1 that cause dopa responsive dystonia and GCH-deficient hyperphenylalaninemia [15], [16]. Both loss of function conditions are serious ailments that require ongoing treatment with major medication and/or extensive dietary management [16]. Subsequent research has started to focus on discovery of other GCH1 variants with links to more common disease states [17].
BH4 has also been identified as a required cofactor for 3 genes in the neurotransmitter pathways (PAH, TH, and TPH) that are ultimately responsible for the production of serotonin, dopamine, norepinephrine, and epinephrine [18]. Yet neuropsychiatric conditions resulting from decreased levels of these neurotransmitters have not been identified as loss of function conditions. Put very simply, BH4 deficiencies are not “all or nothing”. There can be varying levels of deficiency that may lead to varying degrees of symptomatology in various body systems. Which GCH1 mutations lead to which symptoms is a contextual matter based on the state of the surrounding metabolic system and the functions required by that system at that point in time.
Most recently, two studies have established that patients with a haplotype of three heterozygous mutations of the GCH1 gene produce significantly less BH4 than patients without that haplotype. Wolkow et al. [19] identified a haplotype consisting of rs10483639, rs841, and rs3783641, and estimated it was associated with 30% to 50% less BH4 production. Antoniades et al. [20] identified a very similar haplotype consisting of rs10483639, rs8007267, and rs3783641. In addition, they reported the frequency of genotypes for this haplotype: OO normal haplotype (70% of their subject population), XO heterozygous haplotype (27.4%), and XX homozygous haplotype (2%). Furthermore, the XX homozygous haplotype had 80% lower BH4 than the OO normal haplotype. Presumably, the XO heterozygous haplotype had an intermediate level of BH4 reduction associated with it, similar to the intermediate BH4 level reported by Wolkow et al. [19].
Yet no evidence has linked these two closely related haplotypes to any loss of functions or severe symptoms, possibly because the BH4 deficiency associated with the more common heterozygous haplotype is not major enough to disrupt normal metabolism with its ability to utilize alternative pathways as needed. Such a deficiency might best be termed “marginal” because under normal metabolic demands the body is able to adjust its use of limited BH4 to keep adverse effects manageable. This hypothesized marginal BH4 deficiency may become a serious problem under the stressful metabolic conditions imposed by battling Covid-19. In this case, the activation of iNOS by the innate immune system creates a major demand for BH4 that far exceeds the marginally available supply, thereby initiating the persistent uncoupling of iNOS and the self-perpetuating cycle of oxidative stress as hypothesized above [21], [22].
Alkaitis and Crabtree [11] have previously described a self-perpetuating cycle of oxidative stress involving the uncoupling of eNOS due to low BH4. However, such a self-perpetuating cycle of eNOS is considerably more limited in its strength and range of effect than the self-perpetuating cycle of iNOS just proposed for several reasons.
-
•
eNOS produces NO in small pulses whereas iNOS is turned on or “induced” for continuous production of large quantities of NO. Consequently, the uncoupling of iNOS is liable to produce far more oxidative stress than the uncoupling of eNOS by itself.
-
•
The uncoupling of iNOS is also likely to entail the uncoupling of eNOS and nNOS as well because the demand for large amounts of BH4 by iNOS will decrease the overall availability of BH4 to eNOS and nNOS.
-
•
The initiation of iNOS by the immune system occurs in the context of major immunological threat which can only amplify the negative impact of the uncoupling of iNOS, eNOS, and nNOS.
Thus, the self-perpetuating cycle of oxidative stress centered around the activation and uncoupling of iNOS is liable to be far stronger and more serious than the oxidative stress derived from the uncoupling of eNOS alone.
Gantzer [23] has noted that the uncoupling of NOS by either too little BH4 or too little arginine produces not only superoxide and but also BH2 (a partially oxidized pterin). Both BH4 and BH2 bind to iNOS with equal affinity but only BH4 provides the desired enzymatic effect of producing NO. In this manner, BH2 can block BH4 from binding to iNOS. Any unbound BH4 is exposed to superoxide which converts the BH4 to peroxynitrite. Thus, BH2 contributes to even more oxidative stress. To maintain BH4 homeostasis, BH2 is normally recycled back into BH4 by quinoid dihydropteridin reductase (QDPR) using dihydrofolate (DHF). This recycling pathway is also important because the use of BH4 as a cofactor in the production of neurotransmitters (serotonin, dopamine, norepinephrine, and epinephrine) always produces BH2 as an output [19]. Consequently, this recycling pathway is a major source of BH4 and must remain reliably functional. However, there are two types of genetic mutations that reduce its efficiency. First, mutations of QDPR have been shown to impair this recycling pathway. Second, folate mutations (chiefly MTHFR C677T and MTHFR A1298C) and/or a diet deficient in fresh green vegetables can reduce the availability of dihydrofolate for the recycling pathway [24].
Thus far, it has been hypothesized that significant combinations of GCH1, QDPR and MTHFR mutations can lead to oxidative stress triggered by insufficient BH4 to maintain the coupling of iNOS when it has been induced. But a significant increase in oxidative stress is also likely to occur in patients with already existent oxidative stress resulting from ongoing exposure to various immunological threats such as other viruses, bacteria, mold, etc. Such ongoing mild to moderate exposure may not have been sufficient to trigger the activation of iNOS. But it may have led to a slow accumulation of superoxide, peroxynitrite and other forms of ROS that could increase quickly once Covid-19 exposure leads to the activation of iNOS. Put somewhat more simply, genetic mutations and/or preexisting oxidative stress could cause the activation of iNOS to build quickly into “massive oxidative stress” [25] even if the initial acute Covid-19 episode was just mild or moderate in intensity. In a similar manner, it must be noted that a deficiency of antioxidants needed to offset the hypothesized increase in oxidative stress can also be rooted endogenously in mutations or exogenously in deficient diet.
If oxidative stress continues, the ongoing deficiency of BH4 can impair other metabolic processes beyond those involving eNOS, nNOS, and iNOS. BH4 is also an essential cofactor for phenylalanine hydroxylase (PAH), tyrosine hydroxylase (TH), and tryptophan hydroxylase (TPH) in the production of serotonin, epinephrine, norepinephrine, and dopamine. Deficiencies of these neurotransmitters have been tied to numerous dysfunctions of the CNS and PNS associated with PASC, including brain fog, anosmia/dysgeusia, insomnia, dysautonomia, essential tremor, POTS, Ehlers Danlos syndrome, and intestinal dysmotility. While some of these symptoms might be treated for the short term with a SSRI, SNRI or NDRI, it seems reasonable given this analysis that long term treatment is best based on reducing the overall deficiency of BH4 in the body and thereby returning oxidative stress to normal homeostatic levels.
BH4 and the failure to deactivate the innate immune response
In the broadest sense, self-perpetuating oxidative stress is what happens when the innate immune system activates iNOS in the face of an immune threat and then fails to deactivate it when that threat has dissipated. The specific details of how these two essential functions are accomplished are far from fully understood – especially for the deactivation function. One essential question that needs to be answered is how can deactivating macrophages fail to initiate the reduction of self-perpetuating oxidative stress to a normal homeostasis.
Recent research has presented some evidence that BH4 functions to directly control important processes in addition to functioning as a cofactor in the NOS and neurotransmitter pathways. For example, there are reports that BH4 is a critical regulator of T cells because 1) “GCH1 induction and BH4 synthesis are required for effective proliferation of CD4+ and CD8+ T cells” [26], and 2) BH4 helps to modulate T cell functions once activated [27]. A deficiency of BH4 would not only impair the functions of T cells in battling SARS-CoV-2, but also would possibly allow for reactivation of lifelong viruses such as EBV during PASC [28].
Other research has focused on platelet-activating factor (PAF), a powerful endogenous pro-inflammatory mediator [29]. Because PAF is an alkylglycerol, its regulation involves alkylglycerol monooxygenase (AGMO), the only enzyme capable of irreversibly cleaving the O-alkyl bond of PAF [30]. When macrophages are activated by inflammatory stimuli, they produce increased levels of PAF and decreased levels of AGMO. Similarly, when macrophages are activated by anti-inflammatory stimuli, they produce decreased levels of PAF and increased levels of AGMO [30], [31], [32], [33]. However, this homeostatic control is dependent upon sufficient BH4 to serve as the required cofactor for AGMO [34]. Consequently, reduced levels of BH4 due to GCH1 mutations may prevent AGMO from downregulating heightened levels of PAF to restore oxidative homeostasis.
A third line of research focuses on the body’s need to eliminate the cells that have been severely damaged by the SARS-CoV-2 virus. One of the forms of regulated cell death accomplishing this purpose is ferroptosis, which uses rapid iron accumulation and lipid peroxidation of membrane cells to cause cell death. BH4 helps to prevent excessive ferroptosis by functioning as an antioxidant that promotes the survival of some membrane cells. If this process becomes dysregulated by a shortage of BH4, ferroptotic cells release damage-associated molecular pattern molecules (DAMPs) which can signal the need for continued pro-inflammatory activity [35], [36]. In this regard, “the GCH1- BH4- phospholipid axis acts as a master regulator of ferroptosis resistance” [37]. If BH4 is in short supply, resistance to ferroptosis may be diminished, multiple organ systems may be damaged, and the immune system may stay in an inflammatory mode [38].
Other aspects of BH4 and PASC
It is important to note at this point that the effects of oxidative stress (attributed thus far to GCH1 mutations, marginal deficiencies of BH4, and the activation of iNOS) can be increased as well by mutations in genes which do not require BH4 as a cofactor, but which operate in the same pathways as the genes discussed above. For example, the production of L-dopa and 5-hydroxytryptophan (5-HTP) is accomplished by the TH enzyme that requires BH4 as a cofactor. Subsequently, the Dopa Decarboxylase (DDC) enzyme converts L-dopa into dopamine, and 5-HTP into serotonin without requiring BH4 as a cofactor. Mutations in DDC impede these conversions and further reduce the production of dopamine and serotonin. The extent of such associated mutations can greatly complicate what must be accomplished to restore homeostatic balance once the SARS-CoV-2 virus has been defeated. Thus, the negative impact of GCH1 mutations in PASC may be amplified by subsequent mutations operative in the metabolic pathways undergirding PASC.
Another important caveat is appropriate at this point. This account of PASC has not been proposed as the sole cause of this syndrome. Rather it describes a chain of events that may be problematic for 30% or more of the patient population with a specified set of metabolic/genetic deficiencies [20] leading to decreased BH4 and ongoing oxidative stress. It is entirely possible that another distinctive pattern of metabolic difficulties may be identified for another cluster of patients. Or these different patterns of metabolic difficulties may be layered over each other in a potentially synergistic interaction. Certainly, treatment should be considerably more successful when based on the diagnosis of these patterns of systemic deficiencies than when based on the treatment of individual symptoms in separate “silos.”
Research hypotheses
-
1.
Mean BH4 level will be significantly lower for patients with active PASC than for patients recovered from Covid-19 without PASC and for patients who never contracted Covid-19.
-
2.
Mean oxidative stress will be significantly higher for patients with active PASC than for patients recovered from Covid-19 without PASC and for patients who never contracted Covid-19.
-
3.
The extent and frequency of GCH1 mutations will be significantly higher for patients with PASC than for patients recovered from Covid-19 without PASC and for patients who never contracted Covid-19.
-
4.
Mean levels of neurotransmitters (serotonin, dopamine, epinephrine, norepinephrine) will be significantly lower for patients with active PASC than for patients recovered from Covid-19 without PASC and for patients who never had Covid-19.
Possible treatment
The final issue concerns how this hypothesized account of PASC can be translated into clinical treatment that allows the syndrome’s self-perpetuating oxidative stress to return to a healthier oxidative homeostasis. Because this restoration of homeostasis is essentially a widespread recalibration of a complex system rather than the focused repair of a single point in the system, therapeutic reliance on one medication or supplement is liable to be less effective than the balanced therapeutic provision of a range of essential endogenous or exogenous elements that are used by the system to self-regulate.
-
1.
Employ laboratory tests to confirm some aspects of oxidative stress in patients diagnosed with PASC. Such confirmation will most likely be partial in nature insofar as there is no validated test of oxidative stress that is widely accepted and available for clinical practice. The best available tests yield a ratio that represents the current balance between oxidants and antioxidants. For example, the ratio of BH4 to BH2 is often used in research because BH2 is the oxidized byproduct of BH4. Higher values of this ratio therefore represent lower oxidative stress. Another possible ratio is based on the widespread antioxidant function of reduced glutathione (GSS), which when oxidized is subsequently converted to GSSG. A higher ratio of GSS to GSSG indicates lower oxidative stress. Sometimes this ratio is expressed as the percent of reduced glutathione. The higher the percent of reduced glutathione, the less glutathione has been used to counteract oxidative stress.
A final testing approach, which can provide partial confirmation of oxidative stress, employs test panels that either focus on the overall balance of pro-inflammatory vs. anti-inflammatory cytokines or focus on total antioxidant capacity. Some test panels may provide measures of individual antioxidants (glutathione peroxidase, superoxide dismutase, CoQ-10).
-
2.
Neurotransmitter test panels are also appropriate when the patient’s symptoms may be based on insufficient BH4 to serve as the essential cofactor for the PAH, TH, and TPH enzymes in the production of serotonin, epinephrine, norepinephrine, and dopamine.
-
3.
Genetic testing may be appropriate to identify possible problems with GCH1, MTHFR, QDPR, iNOS, eNOS, nNOS, PAH, TH, TPH, DDC, SOD1, SOD2, and various glutathione genes.
-
4.Restore oxidative balance through the supplemental provision of antioxidants:
-
-vitamins C, D3, E (mixed tocopherols; especially gamma tocopherol)
-
-balanced diet with fresh vegetables and fruit
-
-OTC antioxidants: superoxide dismutase, catalase, alpha-lipoic acid, CoQ-10, melatonin, glutathione (liposomal or sublingual spray; or N-acetyl-cystine and glycine)
-
-prescribe tramadol to increase superoxide dismutase and glutathione peroxidase [39].
-
-
-
5.
Supplement with quercetin and resveratrol (which are also antioxidants) to stimulate immune regulators to downregulate iNOS [40], [41], [42], [43], [44], [45].
-
6.
Restore methylation cycle with l-methylfolate and hydroxycobalamin (B12) to increase BH4 through de novo production and recycling.
-
7.
Control or reduce other sources of oxidative stress (allergens, mold, smoking, and volatile organic chemicals) that increase superoxide.
-
8.
Provide sufficient exogenous BH4 to recouple all three forms of NOS (eNOS, iNOS, nNOS) by supplementing with Pteridin-4 (OTC form of sapropterin, 2.5 mg) or by prescribing low dose Kuvan (sapropterin) in powder form.
Conclusion
In response to a call by Proal and VanElzakker [5] for the identification and exploration of genetic components undergirding PASC, this manuscript has proposed that mutations of GCH1 and related genes lead to marginal deficiencies of BH4 that are typically unremarkable when iNOS has not been induced. However, upon exposure to SARS-CoV-2, the innate immune system quickly induces iNOS, thereby causing production of excessive superoxide because iNOS is uncoupled by the marginal deficiency of BH4. The resulting oxidative stress becomes self-perpetuating, even when the virus has been cleared, because the deficiency of BH4 that initially triggered the oxidative stress now also directly impairs the immune regulators responsible for deactivating that ongoing oxidative stress. Suggestions were made for testing and treating this conception of PASC. Indeed, this approach may also be extended to recovery syndromes that have been previously noted for SARS, MERS, and other viruses.
Clinical research is needed to investigate 1) the frequency and extent of this self-perpetuating cycle of oxidative stress among PASC patients, 2) the association of GCH1 and related mutations with greater severity of PASC, 3) the extent of PASC symptoms directly or indirectly grounded in GCH1 and related mutations, and 4) the nature and efficacy of treatment protocols.
No funding was received for this study.
Consent statement/Ethical approval: Not required.
Declaration of Competing Interest
The author declares that there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Greenhalgh T., Knight M., A’Court C., Buxton M., Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;11 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 2.Salamanna F., Veronesi F., Martini L., Landini M.P., Fini M. Post-COVID-19 Syndrome: The persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med. 2021;8:392. doi: 10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proal A.D., VanElzakker M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12:1494. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue Q, Yan Y, Zhang R, Xiong H. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci [Internet]. 2018 Nov 29 [cited 2021 Mar 22];19(12). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6320759/. [DOI] [PMC free article] [PubMed]
- 7.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 9.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc Natl Acad Sci. 2018;115(23):5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkham P. Oxidative stress and macrophage function: a failure to resolve the inflammatory response. Biochem Soc Trans. 2007;35(Pt 2):284–287. doi: 10.1042/BST0350284. [DOI] [PubMed] [Google Scholar]
- 11.Alkaitis M.S., Crabtree M.J. Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep. 2012;9(3):200–210. doi: 10.1007/s11897-012-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura S., Shimosawa T. Oxidative stress and organ damages. Curr Hypertens Rep. 2014;16(8):452. doi: 10.1007/s11906-014-0452-x. [DOI] [PubMed] [Google Scholar]
- 13.Wolf G. σ-tocopherol: an efficient protector of lipids against nitric oxide—initiated peroxidative damage. Nutr Rev. 1997;55(10):376–378. doi: 10.1111/j.1753-4887.1997.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 14.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa Y., Kish S.J., Bebin E.M., Jacobson R.D., Fryburg J.S., Wilson W.G., et al. Dystonia with motor delay in compound heterozygotes for GTP-cyclohydrolase I gene mutations. Ann Neurol. 1998;44(1):10–16. doi: 10.1002/ana.410440107. [DOI] [PubMed] [Google Scholar]
- 16.Rudakou U, Ouled Amar Bencheikh B, Ruskey JA, Krohn L, Laurent SB, Spiegelman D, et al. Common and rare GCH1 variants are associated with Parkinson’s disease. Neurobiol Aging 2019;73:231.e1-231.e6. [DOI] [PMC free article] [PubMed]
- 17.Zhang L., Rao F., Zhang K., Khandrika S., Das M., Vaingankar S.M., et al. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117(9):2658–2671. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opladen T., López-Laso E., Cortès-Saladelafont E., Pearson T.S., Sivri H.S., Yildiz Y., et al. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies. Orphanet J Rare Dis. 2020;15(1):126. doi: 10.1186/s13023-020-01379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolkow PP, Kosiniak-Kamysz W, Osmenda G, Wilk G, Bujak-Gizycka B, Ignacak A, et al. GTP Cyclohydrolase I gene polymorphisms are associated with endothelial dysfunction and oxidative stress in patients with Type 2 Diabetes Mellitus. PLOS ONE 2014;9(11):e108587. [DOI] [PMC free article] [PubMed]
- 20.Antoniades C., Shirodaria C., Van Assche T., Cunnington C., Tegeder I., Lötsch J., et al. GCH1 haplotype determines vascular and plasma biopterin availability in Coronary Artery Disease. J Am Coll Cardiol. 2008;52(2):158–165. doi: 10.1016/j.jacc.2007.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felger J.C., Miller A.H. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felger J.C., Treadway M.T. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2017;42(1):216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gantzer J. eNOS and BH4; endothelial function or dysfunction. Importance of tetrahydrobiopterin (BH4). J Neurol Clin Neurosci [Internet]. 2018 Nov 12 [cited 2021 Mar 7];2(4). Available from: https://www.pulsus.com/abstract/enos-and-bh4-endothelial-function-or-dysfunction-importance-of-tetrahydrobiopterin-bh4-4879.html.
- 24.Crabtree M.J., Channon K.M. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide Biol Chem. 2011;25(2):81–88. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavoie J.-C., Chessex P. Consider optimizing glutathione levels in COVID-19 symptomatic patients. Free Radic Biol Med. 2020;1(159):S44. [Google Scholar]
- 26.Cronin S.J.F., Seehus C., Weidinger A., Talbot S., Reissig S., Seifert M., et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature. 2018;563(7732):564–568. doi: 10.1038/s41586-018-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W., Li L.i., Brod T., Saeed O., Thabet S., Jansen T., et al. Role of increased guanosine triphosphate cyclohydrolase-1 expression and tetrahydrobiopterin levels upon T cell activation. J Biol Chem. 2011;286(16):13846–13851. doi: 10.1074/jbc.M110.191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T., Song J., Liu H., Zheng H., Chen C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci Rep. 2021;25(11):10902. doi: 10.1038/s41598-021-90351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomikos T., Fragopoulou E., Antonopoulou S., Panagiotakos D.B. Mediterranean diet and platelet-activating factor; a systematic review. Clin Biochem. 2018;1(60):1–10. doi: 10.1016/j.clinbiochem.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Tokuoka S.M., Kita Y., Shindou H., Shimizu T. Alkylglycerol monooxygenase as a potential modulator for PAF synthesis in macrophages. Biochem Biophys Res Commun. 2013;436(2):306–312. doi: 10.1016/j.bbrc.2013.05.099. [DOI] [PubMed] [Google Scholar]
- 31.Watschinger K., Keller M.A., McNeill E., Alam M.T., Lai S., Sailer S., et al. Tetrahydrobiopterin and alkylglycerol monooxygenase substantially alter the murine macrophage lipidome. Proc Natl Acad Sci U S A. 2015;112(8):2431–2436. doi: 10.1073/pnas.1414887112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sailer S., Keller M.A., Werner E.R., Watschinger K. The Emerging Physiological Role of AGMO 10 Years after Its Gene Identification. Life. 2021;11(2):88. doi: 10.3390/life11020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waqas SFH, Hoang AC, Lin Y-T, Ampem G, Azegrouz H, Balogh L, et al. Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages. J Clin Invest 127(7):2842–54. [DOI] [PMC free article] [PubMed]
- 34.Bendall J.K., Douglas G., McNeill E., Channon K.M., Crabtree M.J. Tetrahydrobiopterin in Cardiovascular Health and Disease. Antioxid Redox Signal. 2014;20(18):3040–3077. doi: 10.1089/ars.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X., Lin D., Tu S., Gao S., Shao A., Sheng J. Is ferroptosis a future direction in exploring Cryptococcal Meningitis? Front Immunol. 2021;19(12) doi: 10.3389/fimmu.2021.598601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vénéreau E., Ceriotti C., Bianchi M.E. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraft V.A.N., Bezjian C.T., Pfeiffer S., Ringelstetter L., Müller C., Zandkarimi F., et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6(1):41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edeas M., Saleh J., Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Ashmawy N.E., Lashin A.-H.-A., Okasha K.M., Abo Kamer A.M., Mostafa T.M., El-Aasr M., et al. The plausible mechanisms of tramadol for treatment of COVID-19. Med Hypotheses. 2021;1(146) doi: 10.1016/j.mehy.2020.110468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh C.K., Chhabra G., Ndiaye M.A., Garcia-Peterson L.M., Mack N.J., Ahmad N. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28(8):643–661. doi: 10.1089/ars.2017.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan X., Garg N.J. Sirtuin control of mitochondrial dysfunction, oxidative stress, and inflammation in Chagas Disease models. Front Cell Infect Microbiol. 2021;11:505. doi: 10.3389/fcimb.2021.693051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hori Y.S., Kuno A., Hosoda R., Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS ONE. 2013 11;8(9) doi: 10.1371/journal.pone.0073875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon J., Lee S., Kim Y.-N., Lee I.H. Deacetylation of CHK2 by SIRT1 protects cells from oxidative stress-dependent DNA damage response. Exp Mol Med. 2019;51(3):1–9. doi: 10.1038/s12276-019-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hung C.-H., Chan S.-H., Chu P.-M., Tsai K.-L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol Nutr Food Res. 2015;59(10):1905–1917. doi: 10.1002/mnfr.201500144. [DOI] [PubMed] [Google Scholar]
- 45.Heger V., Tyni J., Hunyadi A., Horáková L., Lahtela-Kakkonen M., Rahnasto-Rilla M. Quercetin based derivatives as sirtuin inhibitors. Biomed Pharmacother Biomedecine Pharmacother. 2019;111:1326–1333. doi: 10.1016/j.biopha.2019.01.035. [DOI] [PubMed] [Google Scholar]