Summary
Complex mechanisms govern the sorting of membrane (cargo) proteins at endosomes to ensure that protein localization to the post-Golgi endomembrane system is accurately maintained. Endosomal retrieval complexes mediate sorting by recognizing specific motifs and signals in the cytoplasmic domains of cargo proteins transiting through endosomes. In this review, the recent progress in understanding the molecular mechanisms of how the retromer complex, in conjunction with sorting nexin (SNX) proteins, operates in cargo recognition and sorting is discussed. New data revealing the importance of different SNX proteins and detailing how post-translational modifications can modulate cargo sorting to respond to changes in the environment are highlighted along with the key role that endosomal protein sorting plays in SARS-CoV-2 infection.
Subject areas: Biological sciences, Cell biology, Functional aspects of cell biology
Graphical abstract
Biological sciences; Cell biology; Functional aspects of cell biology
The importance of endosomal protein sorting
Endosomal protein sorting is a highly dynamic process where integral membrane proteins, their associated lipids, along with proteins transiting endosomes are concentrated in the membrane, packaged into tubulo-vesicular carriers, and then delivered to distinct destinations (Burd and Cullen, 2014; Cullen and Steinberg, 2018; Wang et al., 2018) (Figure 1). Those membrane proteins, referred to as cargoes, could be either recycled back to trans-Golgi-network (TGN), the plasma membrane for reuse, or targeted to lysosomes for degradation. As a consequence, endosomal sorting not only determines the fate of cell surface proteins but also impacts a wide range of cellular and physiological activities, including signal transduction, cellular polarity, nutrient uptake and immune response. Endosomal retrieval complexes (ERCs) are responsible for recognizing specific signals in the cytoplasmic domains of cargo proteins, thereby preventing delivery into the endosomal degradation pathway (Figure 1B). Dysregulation of ERCs has been linked with a variety of diseases, most notably, neurodegenerative diseases (Small and Petsko, 2015; Vagnozzi and Pratico, 2019). Furthermore, multiple pathogenic bacteria and viruses, including the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have been shown to directly target ERCs for their survival and replication (Daniloski et al., 2020; Zhu et al., 2021; Yong et al., 2021b; Zhang et al., 2018; Personnic et al., 2016).
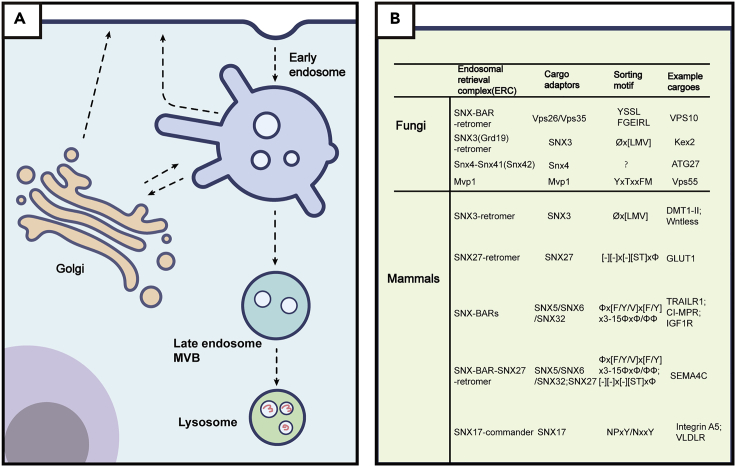
Figure 1.
Endosomal protein sorting and cargo recognition
(A) Model depicting endosomal protein sorting in mammals.
(B) Endosomal sorting. Motifs and their cargo adaptors in fungi and mammals.
Φ, hydrophobic amino acids; x, any amino acids; −, negatively charged residues.
Recognition of endosomal sorting signals
SNX-BAR-retromer
The best characterized ERC is retromer, which was identified in yeast S. cerevisiae over 20 years ago (Seaman et al., 1998). The yeast retromer is composed of the trimeric Vps26p-Vps29p-Vps35p complex, which can recognize cargo, and the Vps5p-Vps17p dimer, which binds the membrane and drives vesicle/tubule formation. Vps5p and Vps17p belong to the conserved sorting nexin (SNX) family and encompass a membrane-bending BAR (Bin-Amphiphysin-Rvs) domain, in addition to the phospholipid PI(3)P-interacting p40 phox-homology (PX) domain. All five genes of retromer are needed for the endosome-to-TGN delivery of cargoes, such as Vps10p, the receptor for vacuolar hydrolase carboxypeptidase Y (CPY), and in yeast, the retromer complex operates as a stable heteropentamer. Vps10p contains a bipartite recycling signal (1428-FGEIRL-1433 and 1492-YSSL-1495), which is recognized by Vps35p and Vps26p (Suzuki et al., 2019). Similar to Vps10p, another known cargo of retromer, Ear1p, also contains two separate motifs, although different regions of Vps26p are required to recognize Ear1p (Suzuki et al., 2019).
Cryo-EM tomography studies of retromer from the thermophilic fungus C. thermophilum, reported by Kovtun et al., have provided important new insights into how fungal retromer assembles on membranes (Kovtun et al., 2018). Recombinant proteins, purified and then incubated with artificial membranes in the form of liposomes, revealed that fungal retromer forms a two-layer coat, analogous to the classical clathrin and COP-II coats. The inner layer is formed by the SNX-BAR component Vps5p, which directly contacts the lipid membrane via both the PX and BAR domains; the Vps26p-Vps29p-Vps35p complex does not directly contact the membrane but forms an arch-like structure over the SNX-BAR layer. Two Vps35p molecules form a dimer and make up the legs of the arches. Vps26p, via dimerization, connects two neighboring SNX-BARs and anchors the arches. Such a conformation places Vps29p at the apex, making it accessible to diverse regulators. The study by Kovtun et al. employed retromer lacking Vps17p and created a homodimer of Vps5p instead (Kovtun et al., 2018). Therefore, the arrangement of the SNX-BAR dimer and retromer arch reported by Kovtun et al. may overemphasize the lack of membrane contact by Vps26p (see below). In yeast, the SNX-BAR dimer and retromer trimer form a stable pentameric complex due to interactions between Vps29p and the N-terminal region of Vps5p which are not conserved in mammals. The SNX-BAR dimer and retromer trimer only loosely associate in mammals, and therefore, it remains unclear how mammalian proteins assemble and arrange on cargo-containing membranes (Burd and Cullen, 2014).
SNX-BARs
Mammalian SNX-BARs consist of a heterodimer formed by SNX1 or SNX2 with either SNX5 or SNX6. Although these proteins share a similar domain architecture with yeast Vps5p and Vps17p, they differ from their fungal counterparts in several ways. First, yeast SNX-BARs and retromer form a stable pentameric complex, whereas mammalian SNX-BARs only loosely interact with the retromer trimer. Second, unlike yeast Vps17p, the PX domains of mammalian SNX5 and SNX6 contain a long insertion and do not to bind endosomal PI(3)P.
Recent studies reveal that mammalian SNX-BARs directly recognize a conserved bipartite motif, termed the SNX-BAR-binding motif (SBM), within the cytoplasmic tails of many cargo proteins, and retrieve them from endosomes to the TGN or the plasma membrane (Simonetti et al., 2017, 2019; Kvainickas et al., 2017; Yong et al., 2020). Structural studies have revealed how the PX domains of SNX5/SNX6 recognize SBMs (and why Vps17p does not) (Simonetti et al., 2019). Briefly, the SBM peptide forms a β-hairpin structure and binds to the extended PX domain of SNX5 (or SNX6). Residues from the upstream β-strand fit a strictly conserved Φx[F/Y/V]x[F/Y] consensus, where Φ and x represent aliphatic and any residues, respectively. Residues from the downstream β-strand (βB) fit a less conserved ΦxΦ or ΦΦ motif. Those aromatic and aliphatic residues from both strands dock into a complementary hydrophobic pocket of SNX5/SNX6. Experimentally confirmed cargoes transported by SNX-BARs include the cation-independent mannose-6-phosphate receptor (CI-MPR), semaphorin4C (SEMA4C), insulin-like growth factor 1 receptor (IGF1R), and TNF-related apoptosis-inducing ligand receptor 1 (TRAILR1) (Simonetti et al., 2017, 2019; Kvainickas et al., 2017; Yong et al., 2020). Interactions between SNX-BARs and many more potential cargoes, identified through proteomic or bioinformatics studies, remain to be confirmed experimentally (Simonetti et al., 2019; Yong et al., 2020).
SNX3-retromer
In addition to SNX-BARs, another member of the SNX family, SNX3 participates in endocytic recycling together with retromer in both yeast and mammals. SNX3 interacts with retromer and mediates endosome-to-TGN retrieval of a large set of cargoes, including CI-MPR, divalent metal transporter 1-II (DMT1-II), and Wntless, a critical regulator for the Wnt signaling pathway (Harrison et al., 2014; Harterink et al., 2011; Seaman, 2007). All these cargoes share a consensus sequence Øx[L/M/V], where Ø is a bulky aromatic residue. Thus, there are some notable similarities between the motifs recognized by the SNX-BAR-retromer and SNX3-retromer ERCs, and it is likely that there will be many cargoes that can be sorted by either complex—one such cargo is the CI-MPR (Cui et al., 2019). Structural studies reveal that SNX3, via its N-terminal flexible region, binds to both Vps26 and Vps35 (Lucas et al., 2016). As a result of the binding, a hydrophobic groove is formed by amino acids from both Vps26 and SNX3, which accommodates the Øx[L/M/V] motif.
Unlike SNX-BARs, SNX3 does not contain a BAR domain and thus does not bend membrane on its own. Recent in vitro cryo-EM tomography studies suggest that both metazoan and fungal SNX3-retromer form an arch-like structure on membranes, similar to the SNX-BAR-retromer (Leneva et al., 2021). Strikingly, the two structures display distinct modes of membrane interaction. In the SNX-BAR-retromer structure reported by Kovtun et al. (2018), SNX-BARs (represented by Vps5p) are solely responsible for contacting membrane; in contrast, in the SNX3-retromer structure, both SNX3 and Vps26 dock directly to the membrane, with Vps26 playing a dominant role. These observations indicate that SNX3-retromer is sufficient to induce membrane curvature and tubulation without classical membrane curvature drivers, such as BAR-domain-containing proteins, and suggest that retromer operates in a highly versatile manner by incorporating different types of membrane adaptors and cargoes. Currently however, it is not yet established that SNX3 with the retromer trimer is sufficient for tubule formation in vivo. The close association of Vps26p with the membrane in yeast retromer also will enable Vps26p to play a key role in cargo selection. Mammalian Vps26 can also directly interact with cargo consistent with the retromer trimer functioning to sort cargo at endosomes (Cui et al., 2019; Lucas et al., 2016; Fjorback et al., 2012).
SNX27-retromer
Metazoan-specific SNX27 is another member of the SNX family that associates with retromer (Steinberg et al., 2013). Unlike the SNX-BAR-retromer, or SNX3-retromer, SNX27-retromer selectively recycles numerous cargoes exclusively to the plasma membrane and is not required for endosome-to-TGN retrieval (Steinberg et al., 2013). Prominent cargoes include the glucose transporter GLUT1, SEMA4C, many G-protein-coupled receptors (GPCRs) such as the β2 adrenergic receptor (β2AR), and parathyroid hormone receptor (PTHR) (Temkin et al., 2011; Steinberg et al., 2013). Aside from the PX domain, SNX27 has two more domains: the PDZ (postsynaptic density 95-discs large-zonula occludens) and the FERM (band4.1-ezrinradixin-moesin) domains. The PDZ domain of SNX27 binds type-I PDZ-binding motifs (PDZbms), with the consensus sequence [S/T]xΦ, located at the C-terminus of many cargoes (Clairfeuille et al., 2016). Interaction with the Vps26 subunit of retromer enhances the binding with PDZbms (Gallon et al., 2014). As SNX27, like SNX3, does not have a BAR domain, it will be very interesting to determine how SNX27-retromer assembles on the membrane. It is possible that an arrangement similar to SNX3-retromer is adopted by SNX27-retromer.
SNX-BAR-SNX27-retromer
A key advance in the field is the recent observation that SNX-BARs directly interact with SNX27 (Yong et al., 2021c; Chandra et al., 2021; Simonetti et al., 2022). The interaction is mediated by the Asp-Leu-Phe (DLF) motifs located within the N-termini of SNX1/SNX2 and the FERM domain of SNX27 (Yong et al., 2021c; Chandra et al., 2021; Simonetti et al., 2022). Both SNX1 and SNX2 harbor more than one DLF motif, which could be used to promote and stabilize the formation of vesicular/tubular structures. As SNX27 is known to interact with retromer, it has been proposed that SNX-BARs, SNX27, and retromer form a “supercomplex” to promote the endosome-to-plasma membrane recycling of cargoes containing SBM, PDZbm, or both (Yong et al., 2020, 2021c) (Figure 2). The notion of an endosomal retrieval supercomplex could explain many contradictory observations in the field. For instance, GLUT1 contains a PDZbm, but its trafficking is regulated by not only SNX27 and retromer but also by SNX-BARs (Yong et al., 2020, 2021c). Conversely, SNX27 is critical for the endocytic retrieval of TRAILR1, which only contains an SBM (Yong et al., 2020, 2021c). The three types of SNX (SNX-BAR, SNX3, and SNX27) are only similar in their PX domains and their ability to associate with the retromer trimer, perhaps hinting at some form of convergent evolution of the SNX—retromer machinery. The different combinations of SNX and retromer trimer may facilitate the sorting of a diverse set of cargo proteins to multiple destinations and/or lead to kinetic differences in rates of recycling and retrieval that reflect how rapidly tubule-vesicular transport intermediates can form.
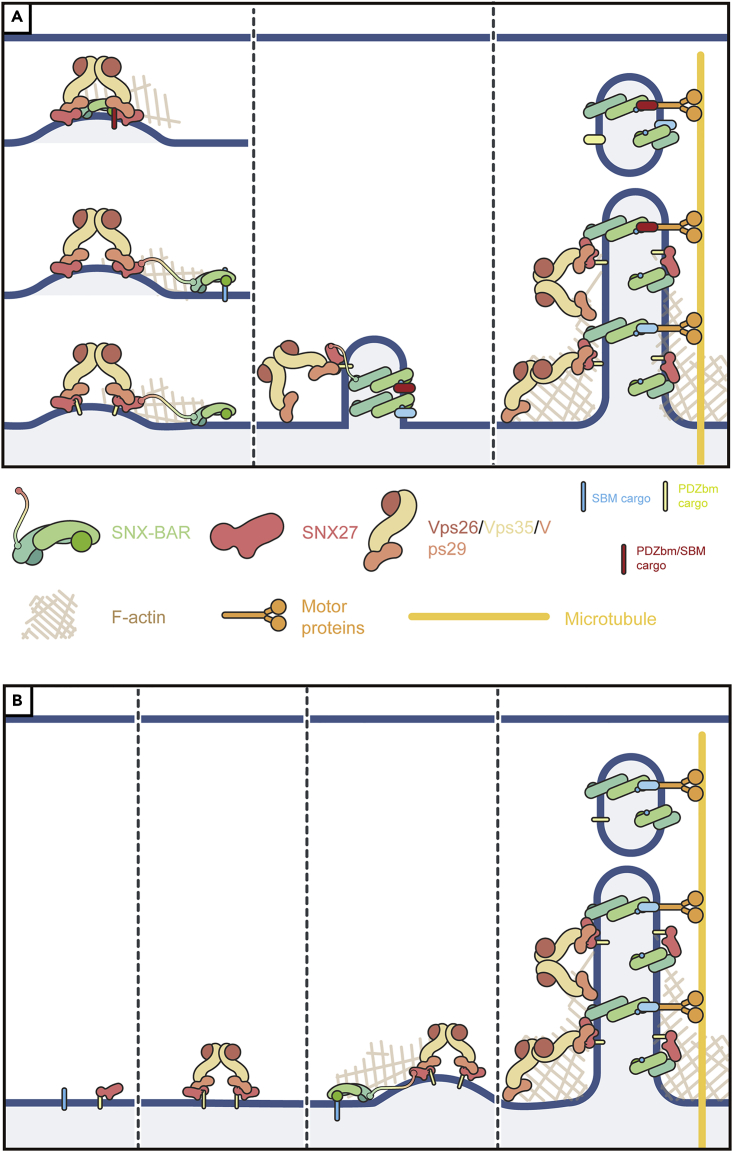
Figure 2.
Two different models explaining how SNX-BAR, SNX27, and retromer function together to mediate endosomal protein sorting
(A) The SNX-BAR-SNX27-retromer supercompex is recruited to membrane by phospholipid and membrane proteins containing SBM, PDZbm, or both motifs. Cargoes containing both SBM and PDZbm are most effective in recruiting the supercomplex as they contain multiple binding sites.
(B) SNX27 is first recruited to membrane by PDZbm-containing cargoes. Interaction with retromer further promotes the formation the retrieval sub-domain. SNX27 cargoes are handed over SNX-BAR-coated tubulovesicular carriers via the interaction between SNX27 and SNX1/SNX2. In both cases, the cargo-enriched tubulo-vesicular carriers are pinched off from endosomes and deliver to the plasma membrane with the aid of actin cytoskeleton and motor proteins.
Connections of SNX-BARs with SNX27-retromer
Why do SNX27-retromer, the complex responsible for transporting PDZbm-containing cargoes, associate with SNX-BARs, the complex for SBM-containing cargoes? Currently, two different—but not competing—models have been proposed. In the first model (concurrent model), we suggest that the formation of the SNX27-SNX-BAR-retromer supercomplex aids its membrane recruitment to different types of cargoes, which contain an SBM, PDZbm, or both (Yong et al., 2021c) (Figure 2A). In the second model (sequential model), Cullen & colleagues proposed that cargoes possessing a PDZbm are first recognized by SNX27-retromer and then “handed over” to SNX-BAR-decorated tubulovesicular carrier due to the SNX-BAR-SNX27 interaction (Simonetti et al., 2022) (Figure 2B). Tubule maturation and fission may be further aided by actin polymerization and recruitment of motor proteins (Figures 2A and 2B). Although the two models differ in details, the recent studies collectively provide new insights into the role of sequence-dependent endosome sorting and demonstrate the complexity of ERCs in higher eukaryotes.
SNX17-commander
SNX27 belongs to the PX-FERM subfamily which also contains SNX17. The two proteins share a FERM domain, along with the PX domain. However, the two proteins have distinct functions in regulating endosomal trafficking. SNX27 associates with retromer and regulates endosome-to-plasma membrane retrieval of PDZbm-containing cargoes; in contrast, SNX17 couples with the commander complex and mediates endosome-to-plasma membrane recycling of cargoes belonging to a different class (McNally et al., 2017). Commander is composed of two subcomplexes: the CCC (CCDC22-CCDC93-COMMD) complex and retriever (VPS35L-VPS26C-VPS29) which shares a structure similar to retromer (McNally et al., 2017; Mallam and Marcotte, 2017; Phillips-Krawczak et al., 2015). The association between commander and SNX17 is mediated by the disordered C-terminal tail of SNX17 and retriever subunit VPS26C (McNally et al., 2017).
SNX17-commander recognizes NPxY/NxxY-containing cargoes via the FERM domain of SNX17 (Ghai et al., 2013). Notable cargoes include integrins, lipoprotein receptor family members, and amyloid precursor protein (McNally et al., 2017; Bottcher et al., 2012; Steinberg et al., 2012; Stockinger et al., 2002). Recent studies have revealed why the FERM domains of SNX17 and SNX27 have distinct binding specificities (Yong et al., 2021c). Although the two FERM domains share a similar overall structure, they differ from each other in many key details (Yong et al., 2021c). Specifically, multiple residues in the NPxY/NxxY-binding pocket of SNX17 are not conserved in SNX27. Conversely, SNX27 possesses a unique hydrophobic “cave” surrounded by positively charged residues, which accommodates the DLF motif from SNX1/SNX2. Taken together, SNX17-commander represents a distinct type of ERC and transports a separate class of cargoes.
SNX4 and SNX8
SNX4 (Snx4p in yeast) and SNX8 (Mvp1p in yeast) are members of the SNX-BAR subfamily. Both genes are involved in retromer-independent membrane trafficking in yeast (Suzuki and Emr, 2018; Suzuki et al., 2021). Snx4p forms a complex with either Snx41p or Snx42p and is required for the vacuole-to-endosome recycling of autophagy protein Atg27p (Suzuki and Emr, 2018). Atg27p is a type-I transmembrane protein critical for autophagosome formation (Backues et al., 2015). Snx4p interacts with the cytoplasmic tail of Atg27p, although exact sequence constraints for the interaction remain to be established (Suzuki and Emr, 2018). Depletion of the Snx4p complex leads a defect in Atg27p trafficking and autophagy. Although the SNX4 complex is conserved in mammals, it remains to be determined whether mammalian SNX4 complex has a similar function.
A more recent study further identifies that Mvp1p mediates another endosomal recycling pathway, independent of retromer and Snx4p (Suzuki et al., 2021). Mvp1p binds to endosomal membrane protein Vps55p via its “YXTXXFM” motif and is responsible for Vps55p delivery to the Golgi (Suzuki et al., 2021). The sorting motif recognized by Mvp1p is distinct from those of retromer and Snx4p.SNX8, the mammalian homolog of Mvp1p, could mediate the formation of endosomal tubules. However, it remains elusive whether mammalian SNX8 functions in endosomal recycling as well, and if it does, what kinds of cargo it recognizes and transports.
Modulation of the interaction between cargoes and cargo adaptors
Another important aspect of cargo sorting is that both cargo and ERCs are subjected to a variety of post-translational modifications (PTMs), notably ubiquitination and phosphorylation. These modifications could regulate membrane recruitment of ERCs, alter the interaction between cargoes and cargo adaptors, and modulate the ERC assembly, thereby shifting the balance of recycling versus degradation of cargoes.
PTMs of cargoes
Cargoes destined for the degradative pathway are often subjected to Lys63-linked polyubiquitination, sorted by the ESCRT assemblies, and targeted to lysosomes (Cullen and Steinberg, 2018; Weeratunga et al., 2020). In addition to ubiquitination, phosphorylation could also modulate the interaction between cargoes and ERCs, e.g., the interaction between the SNX27 PDZ domain and PDZbm. Phosphorylation of serine/threonine residues within the PDZbm ([S/T]xΦ) impairs its interaction with SNX27 PDZ domain.On the other hand, phosphorylation of residues upstream of the [S/T]xΦ motif results in negatively charged residues which may pair with the positively charged residues in the SNX27-binding pocket with increased affinity (Clairfeuille et al., 2016).
PTMs of ERCs
Phosphorylation of a conserved serine within the PI(3)P-binding pocket is known to switch membrane localization of many SNX proteins (Lenoir et al., 2018). Phosphorylation could also modulate the dimerization of SNX5 (or SNX6) with SNX1 and SNX2, thus impacting endocytic recycling (Itai et al., 2018). More recently, it was shown that a variety of extracellular stimuli, including starvation, LPS, and IL-6 promote SNX27 phosphorylation at S51 via MAPK11 and MAPK14 (Mao et al., 2021). This alters the conformation of the SNX27 cargo-binding pocket, reducing its interaction with cargoes and thereby inhibiting endocytic recycling (Mao et al., 2021). These studies demonstrate that the endosomal sorting processes can be dynamically modulated in response to extracellular stimuli via PTMs of cargoes or ERCs. Additional regulation of endosomal protein sorting may occur through PTMs of associated machinery such as the WASH complex. The ubiquitination of the Wash1 protein modulates its activity at the membrane, and several components of the WASH complex, including Fam21, are phosphorylated at multiple sites (https://www.phosphosite.org/) (Hao et al., 2013; Jia et al., 2010; Tsarouhas et al., 2019).
Key roles of endosomal sorting in SARS-CoV-2 infection
Pathogenic bacteria and viruses often harness or hijack host endosomal sorting pathways to facilitate their survival and replication (Yong et al., 2021b). Emerging evidence shows that SARS-CoV-2 targets the endosomal sorting pathway during infection. Genome-scale CRISPR screens have identified SNX27, retromer, and several other key components of the endosomal sorting pathways as required for SARS-CoV-2 infection (Daniloski et al., 2020; Zhu et al., 2021). Proteomic studies have also identified extensive interactions between viral proteins and host proteins that regulate endosomal sorting, such as SNX27 and the WASH complex (Gordon et al., 2020; Stukalov et al., 2021).
Endosomal sorting complexes could regulate SARS-CoV-2 infection via multiple mechanisms. First, angiotensin-converting enzyme 2 (ACE2), the key host receptor for SARS-CoV-2, harbors a PDZbm in its cytoplasmic tail. The complex structure of ACE2-PDZbm/SNX27-PDZ reveals that the ACE2 peptide adopts an extended β strand and interacts with SNX27 via backbone hydrogen bonds, similar to other PDZbms (Yang et al., 2022). SNX27, retromer, and multiple components of the endosomal sorting pathways likely regulate the surface expression of ACE2 through endosomal recycling pathways (Zhu et al., 2021). Second, SNX27 directly interacts with cytoplasmic tail of the SARS-CoV-2 spike protein (Zhao et al., 2021; Cattin-Ortola et al., 2021). This interaction promotes surface localization of the spike protein and transduction efficiency of spike-bearing pseudovirus (Zhao et al., 2021). Remarkably, although the spike protein binds to the PDZ domain of SNX27—similar to many PDZbm-containing cargoes—the spike protein does not contain a canonical PDZbm. It will be interesting to determine the molecular basis of the spike protein-SNX27 interaction and determine whether SNX27 regulates the trafficking of the spike protein in a manner similar to host proteins.
Summary and outlook
There are a great many cargo proteins that traffic through endosomes to their destination, be it the cell surface, the TGN, or a lysosome. The different ERCs that operate at endosomes all contribute to ensuring the process occurs with efficiency and high fidelity. It is worth noting however that simple eukaryotes such as yeast employ a more modest array of ERCs which reflects the fact that there are both fewer cargoes and also fewer destinations as yeast do not recycle proteins directly from endosomes to the cell surface but retrieve first to the TGN/Golgi.
The strides made in understanding how cargoes are recognized have led to a much more profound knowledge of the mechanisms of endosomal protein sorting. Although SNX17-commander and SNX-BARs have been recently identified as direct cargo recognition modules, it remains to be determined whether there are additional complexes responsible for cargo recognition and retrieval. Another major challenge in the field is to dissect the molecular determinants for distinct sorting itineraries. For instance, as retromer-linked SNX-BARs mediate both endosome-to-TGN and endosome-to-plasma membrane trafficking of cargo proteins, how is the destination of a particular cargo determined? The association of SNX-BARs with SNX27 suggests a potential synergy between different ERCs. It will be a major challenge moving forward to dissect how the SNX-BAR-SNX27-retromer supercomplex is assembled on membrane and how the supercomplex differs from SNX-BARs and SNX27-retromer alone in regulating sequence-dependent sorting (Yong et al., 2021a). Finally, the modulation of sorting by PTMs is a new avenue opened up for exploration.
Acknowledgments
Research in the authors’ laboratory is supported by Natural Science Foundation of China (NSFC) grants (#91854121, #31871429), National Science Fund for Distinguished Young Scholars (32125012), National Key R&D Program of China (2018YFC1005004), and Sichuan Science and Technology Program (2018RZ0128).
Author contributions
All authors analyzed literature data and wrote the manuscript.
Declaration of interests
The authors declare no conflict of interest.
Contributor Information
Matthew N.J. Seaman, Email: mnjs100@cam.ac.uk.
Da Jia, Email: jiada@scu.edu.cn.
References
- Backues S.K., Orban D.P., Bernard A., Singh K., Cao Y., Klionsky D.J. Atg23 and Atg27 act at the early stages of Atg9 trafficking in S. cerevisiae. Traffic. 2015;16:172–190. doi: 10.1111/tra.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher R.T., Stremmel C., Meves A., Meyer H., Widmaier M., Tseng H.Y., Fassler R. Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat. Cell Biol. 2012;14:584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- Burd C., Cullen P.J. Retromer: a master conductor of endosome sorting. Cold Spring Harb. Perspect. Biol. 2014;6:a016774. doi: 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin-Ortola J., Welch L.G., Maslen S.L., Papa G., James L.C., Munro S. Sequences in the cytoplasmic tail of SARS-CoV-2 Spike facilitate expression at the cell surface and syncytia formation. Nat. Commun. 2021;12:5333. doi: 10.1038/s41467-021-25589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M., Collins B.M., Jackson L.P. Biochemical basis for an interaction between SNX27 and the flexible SNX1 N-terminus. Adv. Biol. Regul. 2021:100842. doi: 10.1016/j.jbior.2021.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairfeuille T., Mas C., Chan A.S.M., Yang Z., Tello-Lafoz M., Chandra M., Widagdo J., Kerr M.C., Paul B., Merida I., et al. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat. Struct. Mol. Biol. 2016;23:921–932. doi: 10.1038/nsmb.3290. [DOI] [PubMed] [Google Scholar]
- Cui Y., Carosi J.M., Yang Z., Ariotti N., Kerr M.C., Parton R.G., Sargeant T.J., Teasdale R.D. Retromer has a selective function in cargo sorting via endosome transport carriers. J. Cell Biol. 2019;218:615–631. doi: 10.1083/jcb.201806153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P.J., Steinberg F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2018;19:679–696. doi: 10.1038/s41580-018-0053-7. [DOI] [PubMed] [Google Scholar]
- Daniloski Z., Jordan T.X., Wessels H.H., Hoagland D.A., Kasela S., Legut M., Maniatis S., Mimitou E.P., Lu L., Geller E., et al. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell. 2020:92–105.e16. doi: 10.1016/j.cell.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjorback A.W., Seaman M., Gustafsen C., Mehmedbasic A., Gokool S., Wu C., Militz D., Schmidt V., Madsen P., Nyengaard J.R., et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J. Neurosci. 2012;32:1467–1480. doi: 10.1523/jneurosci.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallon M., Clairfeuille T., Steinberg F., Mas C., Ghai R., Sessions R.B., Teasdale R.D., Collins B.M., Cullen P.J. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc. Natl. Acad. Sci. U S A. 2014;111:E3604–E3613. doi: 10.1073/pnas.1410552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R., Bugarcic A., Liu H., Norwood S.J., Skeldal S., Coulson E.J., Li S.S.C., Teasdale R.D., Collins B.M. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc. Natl. Acad. Sci. U S A. 2013;110:E643–E652. doi: 10.1073/pnas.1216229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y.H., Doyle J.M., Ramanathan S., Gomez T.S., Jia D., Xu M., Chen Z.J., Billadeau D.D., Rosen M.K., Potts P.R. Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell. 2013;152:1051–1064. doi: 10.1016/j.cell.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M.S., Hung C.S., Liu T.T., Christiano R., Walther T.C., Burd C.G. A mechanism for retromer endosomal coat complex assembly with cargo. Proc. Natl. Acad. Sci. U S A. 2014;111:267–272. doi: 10.1073/pnas.1316482111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harterink M., Port F., Lorenowicz M.J., Mcgough I.J., Silhankova M., Betist M.C., Van Weering J.R., Van Heesbeen R.G., Middelkoop T.C., Basler K., et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat. Cell Biol. 2011;13:914–923. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itai N., Shimazu T., Kimura T., Ibe I., Yamashita R., Kaburagi Y., Dohi T., Tonozuka T., Takao T., Nishikawa A. The phosphorylation of sorting nexin 5 at serine 226 regulates retrograde transport and macropinocytosis. PLoS One. 2018;13:e0207205. doi: 10.1371/journal.pone.0207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D., Gomez T.S., Metlagel Z., Umetani J., Otwinowski Z., Rosen M.K., Billadeau D.D. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc. Natl. Acad. Sci. U S A. 2010;107:10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun O., Leneva N., Bykov Y.S., Ariotti N., Teasdale R.D., Schaffer M., Engel B.D., Owen D.J., Briggs J.A.G., Collins B.M. Structure of the membrane-assembled retromer coat determined by cryo-electron tomography. Nature. 2018;561:561–564. doi: 10.1038/s41586-018-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvainickas A., Jimenez-Orgaz A., Nagele H., Hu Z., Dengjel J., Steinberg F. Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J. Cell Biol. 2017;216:3677–3693. doi: 10.1083/jcb.201702137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leneva N., Kovtun O., Morado D.R., Briggs J.A.G., Owen D.J. Architecture and mechanism of metazoan retromer:SNX3 tubular coat assembly. Sci. Adv. 2021;7:eabf8598. doi: 10.1126/sciadv.abf8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M., Ustunel C., Rajesh S., Kaur J., Moreau D., Gruenberg J., Overduin M. Phosphorylation of conserved phosphoinositide binding pocket regulates sorting nexin membrane targeting. Nat. Commun. 2018;9:993. doi: 10.1038/s41467-018-03370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Gershlick D.C., Vidaurrazaga A., Rojas A.L., Bonifacino J.S., Hierro A. Structural mechanism for cargo recognition by the retromer complex. Cell. 2016;167:1623–1635.e14. doi: 10.1016/j.cell.2016.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallam A.L., Marcotte E.M. Systems-wide studies uncover commander, a multiprotein complex essential to human development. Cell Syst. 2017;4:483–494. doi: 10.1016/j.cels.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Liao C., Qin J., Gong Y., Zhou Y., Li S., Liu Z., Deng H., Deng W., Sun Q., et al. Phosphorylation of SNX27 by MAPK11/14 links cellular stress-signaling pathways with endocytic recycling. J. Cell Biol. 2021;220 doi: 10.1083/jcb.202010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K.E., Faulkner R., Steinberg F., Gallon M., Ghai R., Pim D., Langton P., Pearson N., Danson C.M., Nagele H., et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 2017;19:1214–1225. doi: 10.1038/ncb3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personnic N., Barlocher K., Finsel I., Hilbi H. Subversion of retrograde trafficking by translocated pathogen effectors. Trends Microbiol. 2016;24:450–462. doi: 10.1016/j.tim.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Phillips-Krawczak C.A., Singla A., Starokadomskyy P., Deng Z., Osborne D.G., Li H., Dick C.J., Gomez T.S., Koenecke M., Zhang J.S., et al. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol. Biol. Cell. 2015;26:91–103. doi: 10.1091/mbc.e14-06-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N.J. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J. Cell Sci. 2007;120:2378–2389. doi: 10.1242/jcs.009654. [DOI] [PubMed] [Google Scholar]
- Seaman M.N., Michael McCaffery J., Emr S.D. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti B., Danson C.M., Heesom K.J., Cullen P.J. Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J. Cell Biol. 2017;216:3695–3712. doi: 10.1083/jcb.201703015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti B., Guo Q., Giménez-Andrés M., Chen K.E., Moody E.R.R., Evans A.J., Chandra M., Danson C.M., Williams T.A., Collins B.M., Cullen P.J. SNX27-Retromer directly binds ESCPE-1 to transfer cargo proteins during endosomal recycling. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti B., Paul B., Chaudhari K., Weeratunga S., Steinberg F., Gorla M., Heesom K.J., Bashaw G.J., Collins B.M., Cullen P.J. Molecular identification of a BAR domain-containing coat complex for endosomal recycling of transmembrane proteins. Nat. Cell Biol. 2019;21:1219–1233. doi: 10.1038/s41556-019-0393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S.A., Petsko G.A. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat. Rev. Neurosci. 2015;16:126–132. doi: 10.1038/nrn3896. [DOI] [PubMed] [Google Scholar]
- Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J., Tavare J.M., Cullen P.J. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 2013;15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg F., Heesom K.J., Bass M.D., Cullen P.J. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J. Cell Biol. 2012;197:219–230. doi: 10.1083/jcb.201111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger W., Sailler B., Strasser V., Recheis B., Fasching D., Kahr L., Schneider W.J., Nimpf J. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J. 2002;21:4259–4267. doi: 10.1093/emboj/cdf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukalov A., Girault V., Grass V., Karayel O., Bergant V., Urban C., Haas D.A., Huang Y., Oubraham L., Wang A., et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 2021;594:246–252. doi: 10.1038/s41586-021-03493-4. [DOI] [PubMed] [Google Scholar]
- Suzuki S.W., Chuang Y.S., Li M., Seaman M.N.J., Emr S.D. A bipartite sorting signal ensures specificity of retromer complex in membrane protein recycling. J. Cell Biol. 2019;218:2876–2886. doi: 10.1083/jcb.201901019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S.W., Emr S.D. Membrane protein recycling from the vacuole/lysosome membrane. J. Cell Biol. 2018;217:1623–1632. doi: 10.1083/jcb.201709162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S.W., Oishi A., Nikulin N., Jorgensen J.R., Baile M.G., Emr S.D. A PX-BAR protein Mvp1/SNX8 and a dynamin-like GTPase Vps1 drive endosomal recycling. Elife. 2021;10:e69883. doi: 10.7554/elife.69883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P., Lauffer B., Jager S., Cimermancic P., Krogan N.J., Von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarouhas V., Liu D., Tsikala G., Fedoseienko A., Zinn K., Matsuda R., Billadeau D.D., Samakovlis C. WASH phosphorylation balances endosomal versus cortical actin network integrities during epithelial morphogenesis. Nat. Commun. 2019;10:2193. doi: 10.1038/s41467-019-10229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi A.N., Pratico D. Endosomal sorting and trafficking, the retromer complex and neurodegeneration. Mol. Psychiatry. 2019;24:857–868. doi: 10.1038/s41380-018-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Fedoseienko A., Chen B., Burstein E., Jia D., Billadeau D.D. Endosomal receptor trafficking: retromer and beyond. Traffic. 2018;19:578–590. doi: 10.1111/tra.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeratunga S., Paul B., Collins B.M. Recognising the signals for endosomal trafficking. Curr. Opin. Cell Biol. 2020;65:17–27. doi: 10.1016/j.ceb.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Yang B., Jia Y., Meng Y., Xue Y., Liu K., Li Y., Liu S., Li X., Cui K., Shang L., et al. SNX27 suppresses SARS-CoV-2 infection by inhibiting viral lysosome/late endosome entry. Proc. Natl. Acad. Sci. U S A. 2022;119 doi: 10.1073/pnas.2117576119. e2117576119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong X., Billadeau D.D., Jia D. All ways lead to Rome: assembly of retromer on membranes with different sorting nexins. Signal Transduct Target Ther. 2021;6:139. doi: 10.1038/s41392-021-00561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong X., Mao L., Shen X., Zhang Z., Billadeau D.D., Jia D. Targeting endosomal recycling pathways by bacterial and viral pathogens. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.648024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong X., Zhao L., Deng W., Sun H., Zhou X., Mao L., Hu W., Shen X., Sun Q., Billadeau D.D., et al. Mechanism of cargo recognition by retromer-linked SNX-BAR proteins. PLoS Biol. 2020;18:e3000631. doi: 10.1371/journal.pbio.3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong X., Zhao L., Hu W., Sun Q., Ham H., Liu Z., Ren J., Zhang Z., Zhou Y., Yang Q., et al. SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2105510118. e2105510118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Monteiro Da Silva G., Deatherage C., Burd C., Dimaio D. Cell-penetrating peptide mediates intracellular membrane passage of human papillomavirus L2 protein to trigger retrograde trafficking. Cell. 2018;174:1465–1476.e13. doi: 10.1016/j.cell.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhong K., Zhao J., Yong X., Tong A., Jia D. SARS-CoV-2 spike protein harnesses SNX27-mediated endocytic recycling pathway. MedComm. 2021;2:798–809. doi: 10.1002/mco2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Feng F., Hu G., Wang Y., Yu Y., Zhu Y., Xu W., Cai X., Sun Z., Han W., et al. A genome-wide CRISPR screen identifies host factors that regulate SARS-CoV-2 entry. Nat. Commun. 2021;12:961. doi: 10.1038/s41467-021-21213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]