Abstract
Background
Since hypertension (HTN) is responsible for more than half of all deaths from cardiovascular disease, it is vital to understand the nutritional factors that reduce its risk. Little information, however, is known about it in the Kurdish population. This study was aimed to evaluate the healthy eating index (HEI) 2015 and major dietary patterns concerning incident HTN.
Methods
This case-cohort study was designed using Ravansar non-communicable diseases (RaNCD) cohort study data (294 participants with incident HTN and 1295 participants as representative random sub-cohort). HEI 2015 and major dietary patterns were extracted using data from their dietary intake, and three major dietary patterns were identified, including plant-based, high protein, and unhealthy dietary patterns. To analyses the association between HEI 2015 and major dietary patterns with incident HTN Cox proportional hazards regression models were applied.
Results
There was a significant positive correlation between HEI 2015 and plant-based diet (r = 0.492). The participants in the highest quartile of HEI-2015 had a 39% and 30% lower risk of incident HTN, compared to participants in the first quartile in both crude and adjusted model (HR: 0.61; 95% CI: 0.46–0.82) and (HR: 0.70; 95% CI: 0.51–0.97), respectively. Furthermore, participants with the highest tertile of the plant-based dietary pattern were at lower risk of incident HTN in both crude and adjusted models (HR: 0.69; 95% CI: 0.54–0.9) and (HR: 0.70; 95% CI: 0.53–0.94), respectively. However, the other two identified dietary patterns showed no significant association with incident HTN.
Conclusions
We found evidence indicating higher adherence to HEI 2015 and plant- based diet had protective effects on incident HTN. The HEI 2015 emphasizes limited sodium intake and adequate intake of vegetables and fruits.
Keywords: Diet, Hypertension, Incidence, Dietary sodium
Background
Hypertension (HTN) is a global health problem, the principal cause of cardiovascular diseases (CVDs) and premature death, affecting roughly one billion adults worldwide [1, 2]. By 2030, it is estimated that 41% of American adults will have been diagnosed with HTN [3]. In contrast to the trends reported in the USA and northern Europe, evidence indicates that CVDs mortality is rising in Iran, and 24% of females and 22% of males suffer from HTN [4, 2]. HTN is a multifactorial disease caused by a complex combination of dietary, lifestyle, and genetic risk factors [5]. The high prevalence of HTN can be attributed to sedentary behaviour, obesity, smoking, family history, and an unwholesome diet [2, 5, 6]. Interestingly, not only is it a curable and well-modifiable disease the controlled HTN but also improves the life quality and the prognosis and prevents relating clinical complications [2, 7]. The main approaches for preventing and managing HTN are lifestyle changes, involving physical activity, weight loss, and dietary modifications [2, 8, 9].
Dietary modifications play a crucial role in preventing and managing HTN[1]. Numerous studies have been conducted over the past few decades that highlight the importance of dietary modification in developing and controlling HTN [8–11]. Several indices have recently been developed to examine the healthiness of dietary patterns. Due to the interaction of nutrients in foods, it is often impossible to differentiate the effects of specific dietary ingredients. Accordingly, the usage of these indices and dietary patterns has evolved to assess the effects of overall dietary intake [10, 12, 13]. There is also the “food group effect”. Food intake is multidimensional exposure, namely food and component effect, contaminants, additives. Assessment of dietary patterns increases the ability to assess more substantial effects due to the cumulative effects of many diet factors (inter-correlations), and allow assessing of the interaction among synergistic components. Since 1995 the healthy eating index (HEI) has been a dietary index developed to evaluate the diet's quality in accordance with Dietary Guidelines for Americans (DGA). The HEI is updated every five years by the evidence-based recommendations of the United States Department of Agriculture (USDA) and Health and Human Services (HHS) [14, 15]. The latest version of this index is the HEI 2015 index noting two essential features in nutrition guidelines, adequacy moderation for dietary intakes. The first part consists of 9 food items, including total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and fatty acids and the second part that means limitation intake is related to following four food items, refined grains, sodium, added sugars, and saturated fats [14, 16].
Despite the tremendous increase in the prevalence of HTN in Iran [17], there are no standard quantitative dietary guidelines to evaluate dietary patterns and their relationships with HTN [5]. Additionally, little information about the relation between HTN and HEI 2015 and major dietary patterns in the Kurdish population is known. The Ravansar Non-Communicable Disease cohort study [18] provided this opportunity to estimate dietary patterns and HTN associations from an epidemiological standpoint. Therefore, the current study investigated the HEI 2015 and major dietary patterns in relation to incident HTN.
Methods
Study design and participants
This present case-cohort study was applied to data from the RaNCD cohort study in which the Kurdish population-based study was started as a prospective study for evaluating non-communicable diseases in October 2014 in Ravansar, Kermanshah Province, western Iran. The RaNCD cohort study is a part of the Prospective Epidemiological Research Studies in Iran (PERSIAN) mega cohort study with 10,047 participants (4764 men and 5283 women) aged 35–65 years in which recruitment phase commenced from October 2014 to January 2017 and followed until January 2021. Ethical approval for the RaNCD cohort study has been granted by the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran (No: KUMS.REC.1394.318), and written informed consent was completed from all participants prior to contribution. The protocols of these studies have already been published [18, 19].
Initially, 1295 participants without HTN incident were randomly considered as representative sub-cohort (20% of the base population). Among the rest, participants with hypertension were excluded (n = 1452), CVDs (n = 512), thyroid (n = 139), and cancer (n = 18) diseases, as well as pregnant women (n = 134) due to possible dietary changes. Additionally, incident CVDs (n = 134) were excluded from this study. Furthermore, the participants whose calories intake was not in the normal range, (800–4200 kcal/day for men and 600- 3500 kcal/day for women) (n = 796), were eliminated from the study. Finally, 294 participants with incident HTN remained and were selected as the case group.
Data sources/ measurements
We obtained data required for this analysis from the RaNCD cohort study, including demographics, socioeconomic factors and current lifestyles, such as physical activity (metabolic equivalent of task (MET) hour per day), educational level, smoking status (never, current, or former smoker), anthropometric indices, dietary intake, and medical history. The details of measurement methods are described in the study of the RaNCD cohort profile [18].
Anthropometric measurements
The height and weight of participants were measured with the least clothing and without shoes by the automatic stadiometer BSM 370 (Biospace Co., Seoul, Korea) and InBody 770 device (Inbody Co, Seoul, Korea), respectively in the study site in Ravansar in a standing position. Waist circumference (WC) measuring was applied using non-stretched and flexible tape in a standing position at the level of the iliac crest. Body mass index (BMI) was computed by dividing weight in kg into height square in meters.
Healthy eating index 2015
The study included adults with complete and reliable dietary data from national validated semi-quantitative 118 items food frequency questionnaire (FFQ) published in the previous studies [18, 20]. HEI 2015 was calculated based on RaNCD FFQ data using the method described by Krebs-Smith et al. [16]. HEI 2015 include 13 items, including whole fruits, total fruits, total protein foods, total vegetables, seafood and plant proteins, greens and beans, whole grains, dairy products, fatty acids, refined grains, sodium, added sugars and saturated fats. These food items were divided into two categories of adequacy and moderation food groups. Adequacy diet components contain whole fruits, total fruits, whole protein foods, total vegetables, seafood and plant proteins, greens and beans, whole grains, dairy products, and fatty acids, which is encouraged to increase intake, and a higher score reflects adequate intake. On the other hand, in HEI 2015, it is recommended to limit the intake of moderate diet components (the last four are refined grains, sodium, added sugars and saturated fats), and a higher score indicates that their intake has been limited. For scoring the first six, the amount consumed is given a point from zero to 5 and the rest of the range of points is from zero to ten. The score from each item, ultimately, is added together and the final HEI score is between zero and 100. (Table 1).
Table 1.
Healthy eating index—20151
| Component | Standard for maximum score 1 | Standard for minimum score of zero | Maximum points |
|---|---|---|---|
| Adequacy: | |||
| Total Fruits2 | ≥ 0.8 cup equivalent per 1,000 kcal | No Fruit | 5 |
| Whole Fruits3 | ≥ 0.4 cup equivalent per 1,000 kcal | No Whole Fruit | 5 |
| Total Vegetables4 | ≥ 1.1 cup equivalent per 1,000 kcal | No Vegetables | 5 |
| Greens and Beans4 | ≥ 0.2 cup equivalent per 1,000 kcal | No Dark-Green Vegetables or Legumes | 5 |
| Whole Grains | ≥ 1.5 cup equivalent per 1,000 kcal | No Whole Grains | 10 |
| Dairy5 | ≥ 1.3 cup equivalent per 1,000 kcal | No Dairy | 10 |
| Total Protein Foods4 | ≥ 2.5 cup equivalent per 1,000 kcal | No Protein Foods | 5 |
| Seafood and Plant Proteins4,6 | ≥ 0.8 cup equivalent per 1,000 kcal | No Seafood or Plant Proteins | 5 |
| Fatty Acids7 | (PUFAs + MUFAs)/SFAs ≥ 2.5 | (PUFAs + MUFAs)/SFAs ≤ 1.2 | 10 |
| Moderation: | |||
| Refined Grains | ≤ 1.8 oz equivalent per 1,000 kcal | ≥ 4.3 oz equivalent per 1,000 kcal | 10 |
| Sodium | ≤ 1.1 g per 1,000 kcal | ≥ 2.0 g per 1,000 kcal | 10 |
| Added Sugars | ≤ 6.5% of energy | ≥ 26% of energy | 10 |
| Saturated Fats | ≤ 8% of energy | ≥ 16% of energy | 10 |
| Total score | 100 | ||
1 Intakes between the minimum and maximum standards are scored proportionately
2 Includes 100% fruit juice
3 Includes all forms except juice
4 Includes legumes (beans and peas)
5 Includes all milk products, such as fluid milk, yogurt, and cheese, and fortified soy beverages
6 Includes seafood, nuts, seeds, soy products (other than beverages), and legumes (beans and peas)
7 Ratio of poly- and mono-unsaturated fatty acids (PUFAs and MUFAs) to saturated fatty acids (SFAs)
Dietary pattern
The major dietary patterns were identified by principal component analysis to energy-adjusted foods intake using data from the RaNCD FFQ. At the beginning, we categorized all food items considering nutrients similarity into 31 food groups. (Table 2) In the method of principal component analysis, the varimax rotation was applied to create a distinct and straightforward matrix and kept uncorrelated factor variables called the major pattern. The scree-plot was also drawn to determine the number of matrix components (the major dietary patterns)..The typical interpretation of the eigen values greater than 1 and the Scree diagram implied that three factor should remain. The extracted factors, dietary patterns, were identified based on the recent studies. The factor score of each dietary pattern was computed by calculating the factor load from every group dietary intake. Food groups with a factor loading exceeding 0.2 were used to correlate between food groups and the known dietary pattern. Participants individually received a score per pattern based on factor scores and then categorized into tertiles according to dietary model scores.
Table 2.
Food groupings used in the dietary pattern analyses
| Food groups | Dietary components |
|---|---|
| Leafy vegetables | Cauliflower, lettuce, cucumber, onion, green bean, mushroom, pepper, garlic, turnip, others |
| Fresh fruits | Melon, watermelon, honeydew melon, plums, prunes, apples, cherries, sour cherries, peaches, nectarine, pear, fig, date, grapes, kiwi, pomegranate, strawberry, banana, persimmon, berry, pineapple, oranges, others |
| Dried fruits | Dried apricots, Dried berries, raisins, and other type dried fruits |
| Dairy | Milk, yogurt, yogurt drink (doogh), cheese, chocolate milk, crud |
| Tomato | Tomato |
| Carotene-rich vegetables | Yellow squash, carrot |
| Condiments | Condiments |
| Pickles | Pickles |
| Legumes | All type beans, peas,lentils, mung bean, soy |
| Whole grain | Dark breads (Iranian), wheat, barley |
| Starchy vegetables | Corn, eggplant, green peas, green squash |
| Vegetable oil | Vegetable oil |
| Natural juices | All fruit juices |
| Butter | Butter, margarine, mayonnaise |
| Olive | Olive and olive oil |
| Organ meat | Heart, kidney, liver, tongue, brain, offal |
| Read meat | Beef, lamb, minced meat |
| Fish | All fish types |
| Processed meat | Hamburger, sausage, delicatessen meat, pizza |
| Soft drink | Soft drink |
| Nuts | Almond, peanut, walnut, pistachio, hazelnut, seeds |
| Egg | Egg |
| Poultry | Chicken |
| Snack | Corn puffs, potato chips, French fries |
| Sweets and desserts | Cookies, cakes, biscuit, muffins, pies, chocolates, ice, honey, jam, sugar cubes, sugar, candies, others |
| Tea and coffee | Tea and coffee |
| Hydrogenated fat | Hydrogenated fats, animal fats |
| Salt | Salt |
| Potato | Potato |
| Refined grain | White breads (lavash, baguettes), noodles, pasta, rice |
Blood pressure
The systolic and diastolic blood pressure (SBP and DBP) were measured after at least 4–5 min of rest by conventional sphygmomanometer and auscultation of the Korotkoff sounds in a sitting position two times with 10 min interval, and the mean of them was reported as the final blood pressure [18].
Hypertension (HTN) incidence
The incident HTN was defined based on the codes I10 of the International classification of diseases Tenth Edition (ICD-10), SBP/DBP ≥ 140/90 mmHg and/or using anti-hypertensive medications in the time interval between baseline (the first phase of Ravansar cohort has been conducted from 2014) and hypertension diagnosis (from 2015 to 2021), which the overall duration of the follow-up was 850.26 person-year, with a median duration of follow up of 2.32 years (min: 0.5, max: 4.8).
Statistical analysis
All statistical analysis was performed using Stata, version 14 (Stata Corp, College Station, TX) with P-value < 0.05 as a significant level. Differences in the baseline quantitative and qualitative variables across quartile of HEI 2015 were assessed using the One-way analysis of variance (ANOVA) and Chi -square tests, respectively. Correlation between major identified dietary patterns and HEI 2015 was determined by Person correlation and the difference of HEI 2015 across tertiles of major identified dietary patterns was evaluated by the One-way ANOVA.
Cox proportional hazards regression model were used to calculate hazard ratios (HRs) stratified by HEI 2015 and major identified dietary patterns, with hypertension as the event and the time interval between baseline (first phase of RaNCD cohort) and hypertension diagnosis as the time covariate. The fundamental assumption in the Cox model is that the hazards are proportional (proportional hazards), which means that the relative hazard remains constant over time with different predictor or covariate levels. The models were adjusted for confounding variables, including Modell I: unadjusted; Model II: adjusted for sex (categorical) and age (continuous); Model III: adjusted for Model II plus socioeconomic status (SES) (categorical), education levels (categorical), physical activity (continuous), diabetes (categorical); Model IV: adjusted for Model III plus BMI (continuous) and energy intake (continuous) and finally reported as HR with 95% confidence interval (CI). We also assessed the incidence of HTN per HEI-2015 components in crude and adjusted model for all mentioned adjusted variables in the previous analysis.
Results
The mean age was 46.5 ± 7.89 years and 48.83% were male. The representative random sub-cohort of 1295 participants was selected for this case-cohort study in the current prospective study. After exclusions, 294 incidents of HTN occurred. The mean weight, BMI, and WC in cases were significantly higher than in the sub-cohort group. In addition, the mean PA of cases was remarkably lower than in the sub-cohort group (P < 0.001). There was no significant difference between two studied groups regarding gender (P < 0.001). Baseline characteristics of the study population in both studied groups are shown in Table 3.
Table 3.
Baseline characteristics in cases and sub cohort groups
| Determinants | Total (n = 1587) | Cases (n = 294) | Sub cohort (n = 1291) |
|---|---|---|---|
| Age (year) | 46.5 ± 7.89 | 50.43 ± 7.34 | 45.59 ± 7.74 |
| Gender, male, % | 48.83 | 38.1 | 51.35 |
| SES, %1 | |||
| Weak | 33.18 | 36.52 | 32.38 |
| Moderate | 33.43 | 31.4 | 33.85 |
| Good | 33.37 | 32.08 | 33.77 |
| Weight (kg) | 72.25 ± 13.07 | 73.61 ± 13.3 | 71.94 ± 13 |
| BMI 2(kg/m2) | 27.23 ± 4.42 | 28.47 ± 4.35 | 26.95 ± 4.39 |
| WC3 (cm) | 96.67 ± 10.03 | 99.33 ± 9.39 | 96.06 ± 10.07 |
| PA 4 (MET/ day) | 40.99 ± 8.18 | 39.77 ± 7.2 | 41.27 ± 8.37 |
| Energy intake (Kcal/day) | 2518.97 ± 689.9 | 2416.42 ± 721.05 | 2541.25 ± 716.19 |
| Smoking, % | 18.54 | 19.59 | 18.31 |
| Diabetes, % | 6.5 | 5.34 | 11.64 |
| Components of HEI | |||
| Total Fruits | 2.96 ± 1.32 | 2.93 ± 1.31 | 3.07 ± 1.36 |
| Whole Fruits | 4.08 ± 1.23 | 4.07 ± 1.24 | 4.12 ± 1.17 |
| Total Vegetables | 3.63 ± 1.11 | 3.62 ± 1.11 | 3.68 ± 1.12 |
| Greens and Beans | 3.43 ± 1.27 | 3.41 ± 1.27 | 3.49 ± 1.26 |
| Whole Grains | 1.42 ± 1.05 | 1.41 ± 1.04 | 1.44 ± 1.12 |
| Dairy | 4.99 ± 2.81 | 4.97 ± 2.84 | 4.99 ± 2.80 |
| Total Protein Foods | 3.15 ± 1.12 | 3.04 ± 1.13 | 3.18 ± 1.12 |
| Seafood and Plant Proteins | 4.18 ± 0.69 | 4.17 ± 0.68 | 4.19 ± 0.72 |
| Fatty Acids | 4.97 ± 3.07 | 4.96 ± 3.06 | 5.01 ± 3.10 |
| Refined Grains | 0.20 ± 0.99 | 0.18 ± 0.94 | 0.30 ± 1.18 |
| Sodium | 2.42 ± 2.73 | 2.31 ± 2.68 | 2.45 ± 2.74 |
| Added Sugars | 8.84 ± 1.89 | 8.72 ± 2.13 | 8.87 ± 1.83 |
| Saturated Fats | 7.17 ± 2.68 | 7.13 ± 2.71 | 7.17 ± 2.67 |
| Total HEI score | 51.42 ± 7.38 | 51.41 ± 7.38 | 51.48 ± 7.40 |
1SES Socioeconomic status,2 BMI Body mass index, 3 WC Waist circumference, 4 PA Physical activity
The food groups intake results determined three major dietary patterns: plant-based, high protein, and unhealthy. The plant- based diet was characterized by higher adherence to leafy vegetables, starchy vegetables, carotene-rich vegetables, tomato, potato, legumes, nuts, olive, vegetable oil, fresh and dried fruits, and fruit juice. Another major dietary pattern, a high protein diet, tended to a higher intake of red and white meat, legumes, egg, whole, and refined grains. In addition, unhealthy dietary pattern was specified with a higher factor loading of salt, sweet, dessert, butter, hydrogenated fat, soft drink, refined grain, tea, and coffee. (Table 4).
Table 4.
Factor loading of food groups in all dietary patterns
| Food groups | Plant- based dietary pattern | High protein dietary pattern | Unhealthy dietary pattern |
|---|---|---|---|
| Leafy vegetables | .684 | - | - |
| Fresh fruits | .637 | .221 | |
| Pickles | .491 | - | .205 |
| Starchy vegetables | .445 | - | - |
| Condiments | .441 | - | .300 |
| Dried fruits | .436 | - | - |
| Tomato | .407 | - | - |
| Carotene-rich vegetables | .396 | .268 | |
| Nuts | .393 | .214 | - |
| Vegetable oil | .380 | - | -.341 |
| Dairy | .357 | - | - |
| Natural juices | .336 | .224 | - |
| Organ meat | - | .665 | - |
| Red meat | - | .637 | - |
| Fish | - | .545 | - |
| Processed meat | - | .448 | - |
| Legumes | .293 | .423 | - |
| Poultry | - | .373 | - |
| Soft drinks | - | .365 | .339 |
| Egg | - | .342 | - |
| Refined grains | - | .327 | .254 |
| Whole grains | .218 | .262 | - |
| Sweets and desserts | - | - | .658 |
| Hydrogenated fats | - | - | .561 |
| Tea and coffee | - | - | .544 |
| Salt | - | - | .353 |
| Potato | .246 | - | .311 |
| Butters | .234 | .231 | .300 |
| Olive | .272 | - | -.275 |
| Snack | .213 | - | .263 |
| Variance % | 13.72 | 6.55 | 5.4 |
Values < 0.2 have been removed for clarity
Table 5 shows the correlation between HEI 2015 and the major identified dietary patterns. According to it, there was a significant positive correlation between HEI 2015 with plant-based (r = 0.492) and high protein (r = 0.255) diets, while there was a notable inverse correlation between HEI 2015 and unhealthy diet (r = -0.473).
Table 5.
The correlation between healthy eating index 2015 and major identified dietary patterns
| Major dietary pattern | Categories | HEI | P* | r | P** |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Plant- based dietary pattern | T1 | 46.65 ± 6.06 | < 0.001 | 0.492 | < 0.001 |
| T2 | 51.91 ± 6.14 | ||||
| T3 | 55.68 ± 6.91 | ||||
| High protein dietary pattern | T1 | 48.8 ± 7.23 | < 0.001 | 0.255 | < 0.001 |
| T2 | 51.63 ± 6.68 | ||||
| T3 | 53.83 ± 7.34 | ||||
| Unhealthy dietary pattern | T1 | 55.5 ± 6.86 | < 0.001 | -0.473 | < 0.001 |
| T2 | 50.85 ± 6.63 | ||||
| T3 | 47.91 ± 6.56 |
P* was obtained one way ANOVA
P** was obtained Pearson correlation
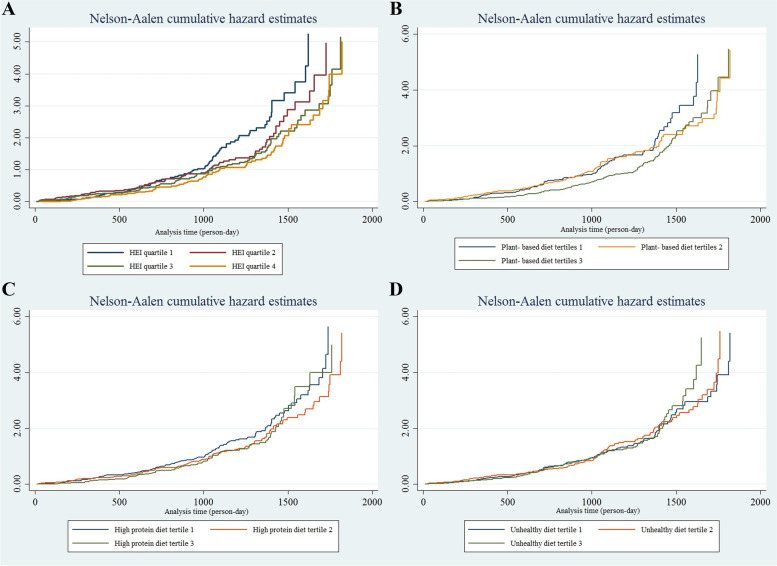
This present study revealed that the individuals in the highest quartile of HEI-2015 had a 39% lower risk of incident HTN than the participants in the first quartile (Model I: HR: 0.61; 95% CI: 0.46–0.82). After adjustment for potential confounders, including age, gender, SES, education, physical activity, diabetes, BMI, and energy intake, this association was remained (Model IV: HR: 0.7; 95% CI: 0.51–0.97). (Table 6) (Fig. 1, A) Similarly, among the major identified dietary patterns, persons who were the highest tertile of the plant-based dietary pattern were at lower risk of incident HTN in both crude and adjusted models (Model I: HR: 0.69; 95% CI: 0.54–0.9) and (Model IV: HR: 0.7; 95% CI: 0.53–0.94), respectively. (Table 6) (Fig. 1, B) However, the other two identified dietary patterns were not associated with incident HTN. (Table 6) (Fig. 1, C and D).
Table 6.
Hazard ratio of incident hypertension according to healthy eating index 2015
| Dietary pattern | categories | N | N. of cases | Hazard ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Model I | Model II | Model III | Model IV | ||||
| Quartiles of HEI 2015 | Q 1 | 479 | 84 | Ref | Ref | Ref | Ref |
| Q 2 | 337 | 59 | 0.84 (0.63, 1.12) | 0.94 (0.70, 1.27) | 0.98 (0.72, 1.34) | 0.97 (0.71, 1.34) | |
| Q 3 | 391 | 82 | 0.70 (0.53, 0.93) | 0.75 (0.57, 0.98) | 0.74 (0.55, 0.99) | 0.71 (0.52,0.95) | |
| Q 4 | 380 | 69 | 0.61 (0.46, 0.82) | 0.71 (0.52, 0.94) | 0.69 (0.51, 0.96) | 0.7 (0.51, 0.97) | |
| Tertiles of plant- based diet | T1 | 442 | 87 | Ref | Ref | Ref | Ref |
| T2 | 433 | 97 | 0.9 (0.69, 1.16) | 1.03 (0.78, 1.34) | 1 (0.76, 1.31) | 1 (0.76, 1.34) | |
| T3 | 420 | 110 | 0.69 (0.54, 0.9) | 0.71 (0.55, 0.92) | 0.68 (0.52, 0.9) | 0.7 (0.53, 0.94) | |
| Tertiles of high protein diet | T1 | 400 | 129 | Ref | Ref | Ref | Ref |
| T2 | 426 | 104 | 0.82 (0.67, 1.04) | 0.84 (0.66, 1.08) | 0.89 (0.69, 1.15) | 0.94 (0.72, 1.23) | |
| T3 | 469 | 61 | 0.83 (0.64, 1.09) | 0.95 (0.7, 1.29) | 1 (0.74, 1.37) | 1.1 (0.81, 1.57) | |
| Tertiles of unhealthy diet | T1 | 430 | 99 | Ref | Ref | Ref | Ref |
| T2 | 420 | 110 | 1.03 (0.81, 1.32) | 1.07 (0.84, 1.37) | 1.08 (0.83, 1.39) | 1.15 (0.89, 1.5) | |
| T3 | 444 | 85 | 1.06 (0.82, 1.38) | 1.04 (0.8, 1.35) | 1.02 (0.78, 1.34) | 1.25 (0.9, 1.73) | |
Model I: Unadjusted; Model II: Adjusted for sex and age; Model III: Adjusted for Model II plus SES, education level, physical activity, T2DM; Model IV: Adjusted for Model III plus BMI and energy intake
Fig. 1.
Estimates of the cumulative baseline hazard functions for the hypertension data by A: HEI; B: plant- based diet; C: high protein diet; D: unhealthy diet
Likewise, we observed that among the HEI- 2015 components, the participant who had higher scores of total fruits, fatty acids, and sodium were at lower risk of HTN incident (HR: 0.93; 95% CI: 0.85–0.99), (HR: 0.96; 95% CI: 0.92–0.99), and (HR: 0.96; 95% CI: 0.92–0.99), respectively. Other the HEI- 2015 components had no significant association with HTN incidents. (Table 7).
Table 7.
Relationship between the HEI- 2015 components and HTN incidence
| Components of HEI 2015 | Crude | Model 1 |
|---|---|---|
| HR (CI 95%) a | HR (CI 95%) a | |
| Total Fruits | 0.92 (0.85–0.99) | 0.93 (0.85–0.99) |
| Whole Fruits | 0.92 (0.84–1) | 0.94 (0.85–1.03) |
| Total Vegetables | 1.02 (0.93–1.12) | 1.02 (0.93–1.12) |
| Greens and Beans | 0.98 (0.91–1.07) | 0.96 (0.88–1.04) |
| Whole Grains | 1.07 (0.98–1.18) | 1.06 (0.96–1.17) |
| Dairy | 0.99 (0.95–1.02) | 0.99 (0.95–1.03) |
| Total Protein Foods | 0.95 (0.87–1.04) | 0.96 (0.87–1.06) |
| Seafood and Plant Proteins | 1.04 (0.9–1.21) | 1.13 (0.97–1.33) |
| Fatty Acids | 0.96 (0.93–0.99) | 0.96 (0.92–0.99) |
| Refined Grains | 0.93 (0.84–1.02) | 0.91 (0.83–1.01) |
| Sodium | 0.94 (0.91–0.98) | 0.96 (0.92–0.99) |
| Added Sugars | 0.98 (0.93–1.03) | 1 (0.95–1.05) |
| Saturated Fats | 1.00 (0.97–1.04) | 1.01 (0.97–1.05) |
aAdjusted for age, sex, SES, education level, physical activity, T2DM, BMI and energy intake
Discussion
This large prospective Kurdish population-based study extracted three major dietary patterns using principal component analyses methods, including plant-based, high protein, and unhealthy dietary patterns. We found that greater adherence to HEI 2015 and the plant-based dietary pattern has inversely correlated with the risk of incident HTN. Moreover, among the HEI- 2015 components, a higher score of total fruits, fatty acids, and sodium was associated with a reduced risk of incident HTN. There was no significant association between the other two identified dietary patterns (high protein and unhealthy dietary patterns) and incident HTN.
HTN contributes as one of the most critical risk factors for CVDs and their mortality estimated 874 million adults had an SBP of more than 140 mm Hg or higher in 2015 [21]. Diet modification is an effective strategy to prevent it [22]. Understanding national dietary patterns and their relationship to the risk of chronic non-communicable diseases overcomes the challenge of examining individual foods or nutrients and considering their common effects [23]. In this regard, limited data are available from Kurdish dietary patterns, and to the best of our knowledge, the major dietary patterns were identified and were compared to HEI 2015. The association between them and incident HTN was also evaluated.
This study indicated that higher adherence to HEI 2015 had a preventive effect on incident HTN. Similarly, greater adherence to the plant-based pattern that emphasized the higher intake of vegetables, especially leafy vegetables, fresh fruits, legumes, and whole grains, decreased incident HTN; the high protein diet, however, had no association with incident HTN. According to other studies, high protein diets are associated with higher blood pressure. Therefore, people with high protein diets may have higher blood pressure [1, 6, 24]. A systematic review and meta-analysis of 15 prospective cohort studies showed that higher diet quality characterized by HEI 2005, 2010, and the alternate healthy eating index (AHEI) was significantly related to decreasing all causes of mortality, including CVDs, cancer, and type 2 diabetes [25]. In three populated prospective cohort investigations, greater adherence to various wholesome eating patterns was consistently associated with lower cardiovascular disease risk [26]. A cross-sectional analysis of the Fasa PERSIAN study suggested that the HEI-2015 diet may prevent hypertension [2]. On the contrary, in our study Tehran Lipid and Glucose Study Increasing DASH score, the healthy and unhealthy dietary patterns were not associated with the risk of hypertension [5]. Hu et al. presented that greater following HEI 2015 was significantly lessened CVDs incidence (HR: 0.84; 95% CI: 0.76–0.93) and CVDs mortality (HR: 0.68; 95% CI: 0.58–0.8) [27]. However, HTN was not assessed in these studies. Another systematic review and meta-analysis of 15 randomized clinical trials demonstrated that the vegetarian diet lowered both systolic and diastolic blood pressure compared to the omnivorous diet [28]. A case-cohort study after 1.61 years follow up of 686 vegetarians showed that they were 34% less likely to develop HTN than people who did not follow a vegetarian diet (Odds Ratio(OR): 0.66; 95% CI: 0.5–0.87) [29]. A study by Song et al. showed that women who consumed higher legumes and whole-grain were lower at risk of HTN incidence (HR: 0.77; 95% CI: 0.59–1) [30]. In contrast, the results of Thai cohort study displayed that dietary pattern characterized by greater adherence to vegetables, fruits, soybean products, and milk was not associated with HTN (OR: 0.99; 95% CI: 0.86–1.15) [22]. Likewise, in the Mexican Teachers’ cohort study, there was no significant association between dietary patterns tended to vegetable, fruits, and legumes and HTN (OR: 0.94; 95% CI: 0.84,-1.05) [23].
Plant-based diets are generally higher in terms of diet quality than non-plant-based diets due to their high content of fiber, antioxidants, potassium, and low saturated fat and sodium [31–33]. A plant-based diet prevents incident HTN with beneficial effects on blood viscosity, vasodilation and reduced insulin resistance [28, 34]. Also, with its antioxidant and anti-inflammatory properties and the content of valuable fibres, it changes the colony and strains of the intestinal microflora and improves blood pressure by influencing the renin-angiotensin system and baroreceptors [33, 35]. According to the Lea Borgi study, consuming whole fruits for a more extended period and increasing consumption may reduce the risk of hypertension [36]. In A study conducted in Bangladesh, additionally, found that a higher intake of fruits and vegetables was associated with lower annual pulse pressure and systolic blood pressure, while a higher intake of meat was correlated with higher annual pulse pressure [37]. Based on a systematic review and network meta-analysis, the DASH diet may be the most effective dietary measure for lowering blood pressure between hypertensive and pre-hypertensive patients based on high-quality studies [1]. Hence, researchers have referred to it as the dietary approach to stop hypertension (DASH). To prevent hypertension, it emphasizes limiting sodium intake and increasing potassium intake through vegetables and fruits consumption [38, 39]. A systematic review and meta-analysis on 30 randomized clinical trials observed that adherence to the DASH diet can significantly lower blood pressure in hypertensive and healthy adults [40]. Francisco et al., in their study, reported that adherence to the DASH diet was associated with a significant reduction in blood pressure, even with a short follow-up period [41]. Legumes, fruits and vegetables are enriched antioxidants and essential vitamins, including fibre, vitamin C, potassium, folic acid, magnesium, flavonoids, and carotenoids, and have synergistic effects on lowering blood pressure by improving endothelial function and their antioxidative properties causing vasodilation [42, 43].
Our findings from the HEI 2015 showed that greater score of total fruits, fatty acids, and sodium was associated with a lower risk of incident HTN. In other words, limiting the intake of sodium and adequately unsaturated fatty acids compared to saturated fatty acids and sufficient fruits consumption, reduces the development of HTN. Moreover, in this study, higher adherence to an unhealthy diet was inversely related to HEI 2015 and raised the risk of incident HTN, although this association was insignificant even with controlling potential confounder variables. The study by Mendonça et al. on Spanish adults reported that higher adherence to diet with high content of saturated fats and salt and low content of fresh vegetables and fruits as ultra-processed foods was significantly increased the risk of incident HTN (HR: 1.21; 95% CI: 1.06- 1.37) [44]. The modern diet is associated with processed foods, also known as the Western diet. This pattern is associated with increased intake of calories, sodium, saturated fats, and decreased potassium [45, 46]. High sodium intake is the leading cause of increased hypertension, which inhibits the sodium pump and stimulates the sodium-calcium exchanger type 1 (NCX1), resulting in increased intracellular calcium concentration, which causes vasoconstriction [47, 48]. Additionally, high sodium intake reduces the synthesis of nitric oxide (NO) and increases the plasma level of the endogenous NO inhibitor, which reduces vasodilation [49, 50]. On the other hand, reducing potassium intake and affecting the sodium pump inhibit potassium channels in the cell membrane and increase intracellular calcium, which eventually leads to HTN[51, 52]. All of these factors contribute to the development of HTN [48].
The other two features of HEI 2015 had preventive effects of incident HTN were total fruits and fatty acids. Adequate intake of fruits along with high content of antioxidants, vitamins, minerals and fiber as nutritious diet items play a role in weight control and anthropometric indicators and reduce systemic blood pressure [42, 53]. Unsaturated fatty acids, especially omega-3 fatty acids, play an essential role in vascular vasodilation by increasing the bioavailability of NO and having anti-inflammatory effects [54].
Strengths and limitations
This study was the first conducted on a large Kurdish population-based study. This present study presented the major dietary pattern using the principal component analyses approach and compared it with HEI 2015. Using validated FFQ that trained nutritionists completed led to a likely reduction of dietary intake reminders compared to self-report dietary intake. We also considered potential confounding variables in all analyses, and we did not include some conditions because of possible changes in their diet. This present investigation, nevertheless, contained some limitations. Since the follow-up period was short, the incidence was low and we could not accomplish analyzes by gender. Likewise, the limited knowledge of food frequency questionnaires to accurately quantify food intake should be considered. Despite the inaccuracy in estimating food intake, a relative comparison between the three groups with distinct levels of adherence may reduce this error. As the final point, although we reckoned many potential confounders, we can not rule out the possibility of residual confounders, including alcohol consumption, which may not be reported due to religious issues.
Conclusions
To conclude, our findings supported the association between the HEI 2015 and incident HTN. Among the major identified dietary patterns, plant-based and high protein diets were positively correlated with HEI 2015, while the unhealthy diet was inversely correlated with HEI 2015. Similarly, the plant-based diet, and greater score of total fruits, fatty acids, and sodium were preventively associated with incident HTN. Furthermore, the other two dietary patterns had no significant association with incident HTN. Therefore, according to the HEI 2015 recommendations, our study recommends limiting sodium intake and adequate vegetables and fruits consumption.
Acknowledgements
RaNCD is part of PERSIAN national cohort and we would like to thank Research and Technology at the Ministry of Health and Medical Education of Iran and Director of the PERSIAN cohort, and also PERSIAN cohort for all their supports during design and running of RaNCD.
Authors’ contributions
S. Moradi and Y. Pasdar equally contributed to the conception and design of the research; F. Njafi, B. Hamzeh, and Y. Pasdar contributed to data collection; F. Najafi, R. Safari, and M. Darbandi contributed to the acquisition and analysis of the data; S. Moradi, R. Safari, and M. Darbandi contributed to the interpretation of the data; and S. Moradi, Y. Pasdar and E. Mohammadi contributed to draft the manuscript. S. Moradi, Y. Pasdar, S. Cheshmeh, and E. Mohammadi contributed to revising the manuscript. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere. The author(s) read and approved the final manuscript.
Funding
This study was supported by Ministry of Health and Medical Education of Iran and Kermanshah University of Medical Science (Grant No: 92472).
Availability of data and materials
Data will be available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval for the RaNCD cohort study has been granted by the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran (No: KUMS.REC.1394.318).
Written informed consent was obtained from each studied subject after explaining the purpose of the study. The right of subjects to withdraw from the study at any time and the subject's information is preserved and will not be published.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yahya Pasdar, Email: Yahya.pasdar@kums.ac.ir.
Behrooz Hamzeh, Email: behrooz.hamzeh@kums.ac.ir.
Shima Moradi, Email: Shima.moradi@kums.ac.ir.
Ehsan Mohammadi, Email: ehsan.mohammadi5592@gmail.com.
Sahar Cheshmeh, Email: Shr.cheshmeh@gmail.com.
Mitra Darbandi, Email: mitra.darbandi@yahoo.com.
Roya Safari Faramani, Email: roya.safari@kums.ac.ir.
Farid Najafi, Email: farid_n32@yahoo.com.
References
- 1.Schwingshackl L, Chaimani A, Schwedhelm C, Toledo E, Pünsch M, Hoffmann G, et al. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: a systematic review and network meta-analysis. Crit Rev Food Sci Nutr. 2019;59(16):2674–2687. doi: 10.1080/10408398.2018.1463967. [DOI] [PubMed] [Google Scholar]
- 2.Motamedi A, Ekramzadeh M, Bahramali E, Farjam M, Homayounfar R. Diet quality in relation to the risk of hypertension among Iranian adults: cross-sectional analysis of Fasa PERSIAN cohort study. Nutr J. 2021;20(1):57. doi: 10.1186/s12937-021-00717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kromhout D. Epidemiology of cardiovascular diseases in Europe. Public Health Nutr. 2001;4(2b):441–457. doi: 10.1079/PHN2001133. [DOI] [PubMed] [Google Scholar]
- 5.Ramezankhani A, Hosseini-Esfahani F, Mirmiran P, Azizi F, Hadaegh F. The association of priori and posteriori dietary patterns with the risk of incident hypertension: Tehran Lipid and Glucose Study. J Transl Med. 2021;19(1):44. doi: 10.1186/s12967-021-02704-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Liu A, Du M, Wu J, Wang W, Qian Y, et al. Diet quality is associated with reduced risk of hypertension among Inner Mongolia adults in northern China. Public Health Nutr. 2020;23(9):1543–1554. doi: 10.1017/S136898001900301X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2020;120(12):1998–2031.e15. doi: 10.1016/j.jand.2020.08.076. [DOI] [PubMed] [Google Scholar]
- 8.Khodarahmi M, Asghari-Jafarabadi M, AbbasalizadFarhangi M. A structural equation modeling approach for the association of a healthy eating index with metabolic syndrome and cardio-metabolic risk factors among obese individuals. PLoS One. 2019;14(7):e0219193. doi: 10.1371/journal.pone.0219193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shim J-S, Jung SJ, Kim HC. Self-reported diet management, dietary quality, and blood pressure control in Korean adults with hypertension. Clinical Hypertension. 2019;25(1):24. doi: 10.1186/s40885-019-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammadifard N, Talaei M, Sadeghi M, Oveisegharan S, Golshahi J, Esmaillzadeh A, et al. Dietary patterns and mortality from cardiovascular disease: Isfahan Cohort Study. Eur J Clin Nutr. 2017;71(2):252–258. doi: 10.1038/ejcn.2016.170. [DOI] [PubMed] [Google Scholar]
- 11.Zaribaf F, Mohammadifard N, Sarrafzadegan N, Karimi G, Gholampour A, Azadbakht L. Dietary patterns in relation to lipid profiles among Iranian adults. Journal of cardiovascular and thoracic research. 2019;11(1):19. doi: 10.15171/jcvtr.2019.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JE, Jung H, Lee JE. Dietary pattern and hypertension in Korean adults. Public Health Nutr. 2014;17(3):597–606. doi: 10.1017/S1368980013000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozaffari H, Ajabshir S, Alizadeh S. Dietary approaches to stop hypertension and risk of chronic kidney disease: a systematic review and meta-analysis of observational studies. Clin Nutr. 2020;39(7):2035–2044. doi: 10.1016/j.clnu.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Panizza CE, Shvetsov YB, Harmon BE, Wilkens LR, Le Marchand L, Haiman C, et al. Testing the predictive validity of the Healthy Eating Index-2015 in the multiethnic cohort: is the score associated with a reduced risk of all-cause and cause-specific mortality? Nutrients. 2018;10(4):452. doi: 10.3390/nu10040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasdar Y, Moradi S, Moradinazar M, Hamzeh B, Najafi F. Better muscle strength with healthy eating. Eating Weight Disord. 2021;26(1):367–374. doi: 10.1007/s40519-020-00863-1. [DOI] [PubMed] [Google Scholar]
- 16.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asgari S, Khaloo P, Khalili D, Azizi F, Hadaegh F. Status of Hypertension in Tehran: potential impact of the ACC/AHA 2017 and JNC7 Guidelines, 2012–2015. Sci Rep. 2019;9(1):6382. doi: 10.1038/s41598-019-42809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasdar Y, Najafi F, Moradinazar M, Shakiba E, Karim H, Hamzeh B, et al. Cohort profile: Ravansar Non-Communicable Disease cohort study: the first cohort study in a Kurdish population. Inter J Epidemiol. 2019;48(3):682–683. doi: 10.1093/ije/dyy296. [DOI] [PubMed] [Google Scholar]
- 19.Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–655. doi: 10.1093/aje/kwx314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moradi S, Pasdar Y, Hamzeh B, Najafi F, Nachvak SM, Mostafai R, et al. Comparison of 3 nutritional questionnaires to determine energy intake accuracy in Iranian adults. Clin Nutr Res. 2018;7(3):213–222. doi: 10.7762/cnr.2018.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 22.Shi Z, Papier K, Yiengprugsawan V, Kelly M, Seubsman SA, Sleigh AC. Dietary patterns associated with hypertension risk among adults in Thailand: 8-year findings from the Thai Cohort Study. Public Health Nutr. 2019;22(2):307–13. doi: 10.1017/S1368980018002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monge A, Lajous M, Ortiz-Panozo E, Rodríguez BL, Góngora JJ, López-Ridaura R. Western and Modern Mexican dietary patterns are directly associated with incident hypertension in Mexican women: a prospective follow-up study. Nutr J. 2018;17(1):1–10. doi: 10.1186/s12937-018-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng P-F, Shu L, Zhang X-Y, Si C-J, Yu X-L, Gao W, et al. Association between dietary patterns and the risk of hypertension among Chinese: A cross-sectional study. Nutrients. 2016;8(4):239. doi: 10.3390/nu8040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J Acad Nutri Diet. 2020;120(12):1998–2031.e15. [DOI] [PubMed]
- 26.Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern Med. 2020;180(8):1090–1100. doi: 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu EA, Steffen LM, Coresh J, Appel LJ, Rebholz CM. Adherence to the healthy eating index–2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr. 2020;150(2):312–321. doi: 10.1093/jn/nxz218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KW, Loh HC, Ching SM, Devaraj NK, Hoo FK. Effects of vegetarian diets on blood pressure lowering: a systematic review with meta-analysis and trial sequential analysis. Nutrients. 2020;12(6):1604. doi: 10.3390/nu12061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang S-Y, Chiu TH, Lee C-Y, Liu T-T, Tsao CK, Hsiung CA, et al. Vegetarian diet reduces the risk of hypertension independent of abdominal obesity and inflammation: a prospective study. J Hypertens. 2016;34(11):2164–2171. doi: 10.1097/HJH.0000000000001068. [DOI] [PubMed] [Google Scholar]
- 30.Song S, Kim J, Kim J. Gender differences in the association between dietary pattern and the incidence of hypertension in middle-aged and older adults. Nutrients. 2018;10(2):252. doi: 10.3390/nu10020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander S, Ostfeld RJ, Allen K, Williams KA. A plant-based diet and hypertension. Journal of geriatric cardiology: JGC. 2017;14(5):327. doi: 10.11909/j.issn.1671-5411.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soeters PB. Vegan diets: what is the benefit? Curr Opin Clin Nutr Metab Care. 2020;23(2):151–153. doi: 10.1097/MCO.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 33.Joshi S, Ettinger L, Liebman SE. Plant-based diets and hypertension. Am J Lifestyle Med. 2020;14(4):397–405. doi: 10.1177/1559827619875411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M-S, Wang A, Yu H. Link between insulin resistance and hypertension: what is the evidence from evolutionary biology? Diabetol Metab Syndr. 2014;6(1):1–8. doi: 10.1186/1758-5996-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 36.Borgi L, Muraki I, Satija A, Willett WC, Rimm EB, Forman JP. Fruit and vegetable consumption and the incidence of hypertension in three prospective cohort studies. Hypertension. 2016;67(2):288–293. doi: 10.1161/HYPERTENSIONAHA.115.06497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, Liu M, Parvez F, Wang B, Wu F, Eunus M, et al. Association of major dietary patterns and blood pressure longitudinal change in Bangladesh. J Hypertens. 2015;33(6):1193–1200. doi: 10.1097/HJH.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozemek C, Laddu DR, Arena R, Lavie CJ. The role of diet for prevention and management of hypertension. Curr Opin Cardiol. 2018;33(4):388–393. doi: 10.1097/HCO.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 39.Moradi S, Moloudi J, Moradinazar M, Sarokhani D, Nachvak SM, Samadi M. Adherence to healthy diet can delay Alzheimer’s diseases development: a systematic review and meta-analysis. Prev Nutri Food Sci. 2020;25(4):325. doi: 10.3746/pnf.2020.25.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, et al. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(5):1150–1160. doi: 10.1093/advances/nmaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francisco S, Araújo L, Griep R, Chor D, Molina M, Mil J, et al. Adherence to the Dietary Approaches to Stop Hypertension (DASH) and hypertension risk: results of the Longitudinal Study of Adult Health (ELSA-Brasil) Br J Nutr. 2020;123(9):1068–1077. doi: 10.1017/S0007114520000124. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen SM, Tran HTT, Tran BQ, Van Hoang M, Truong BD, Nguyen LT, et al. Compliance to dietary guidelines on fruit and vegetable intake and prevalence of hypertension among Vietnamese adults, 2015. Eur J Prev Cardiol. 2020;27(1):39–46. doi: 10.1177/2047487319867500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasdar Y, Hamzeh B, Moludi J, Mehaki B, Darbandi M, Moradi S. Dietary intake and risk of depression among male and female with HIV/AIDS. Eat Weight Disord. 2020;25(4):1029–1038. doi: 10.1007/s40519-019-00726-4. [DOI] [PubMed] [Google Scholar]
- 44.Mendonça RdD, Lopes ACS, Pimenta AM, Gea A, Martinez-Gonzalez MA, Bes-Rastrollo M. Ultra-processed food consumption and the incidence of hypertension in a Mediterranean cohort: the Seguimiento Universidad de Navarra Project. Am J Hypertens. 2017;30(4):358–66. doi: 10.1093/ajh/hpw137. [DOI] [PubMed] [Google Scholar]
- 45.Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK. The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ. 2017;357:j1794. doi: 10.1136/bmj.j1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samadi M, Moradi S, Moradinazar M, Mostafai R, Pasdar Y. Dietary pattern in relation to the risk of Alzheimer’s disease: a systematic review. Neurol Sci. 2019;40(10):2031–2043. doi: 10.1007/s10072-019-03976-3. [DOI] [PubMed] [Google Scholar]
- 47.Iwamoto T, Kita S, Katsuragi T. Salt-sensitive hypertension, Na+/Ca2+ exchanger, and vascular smooth muscle. Trends Cardiovasc Med. 2005;15(8):273–277. doi: 10.1016/j.tcm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Canale MP, Noce A, Di Lauro M, Marrone G, Cantelmo M, Cardillo C, et al. Gut dysbiosis and western diet in the pathogenesis of essential arterial hypertension: a narrative review. Nutrients. 2021;13(4):1162. doi: 10.3390/nu13041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101(8):856–861. doi: 10.1161/01.CIR.101.8.856. [DOI] [PubMed] [Google Scholar]
- 50.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. Functional foods for augmenting nitric oxide activity and reducing the risk for salt-induced hypertension and cardiovascular disease in Japan. J Cardiol. 2018;72(1):42–49. doi: 10.1016/j.jjcc.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+-ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R529–R535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 52.Nomura N, Shoda W, Uchida S. Clinical importance of potassium intake and molecular mechanism of potassium regulation. Clin Exp Nephrol. 2019;23(10):1175–1180. doi: 10.1007/s10157-019-01766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Li F, Wang L, Zhang D. Fruit and vegetables consumption and risk of hypertension: a meta-analysis. J Clin Hypertens. 2016;18(5):468–476. doi: 10.1111/jch.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bercea CI, Cottrell GS, Tamagnini F, McNeish AJ. Omega-3 polyunsaturated fatty acids and hypertension: a review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br J Pharmacol. 2021;178(4):860–877. doi: 10.1111/bph.15336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon request from the corresponding author.