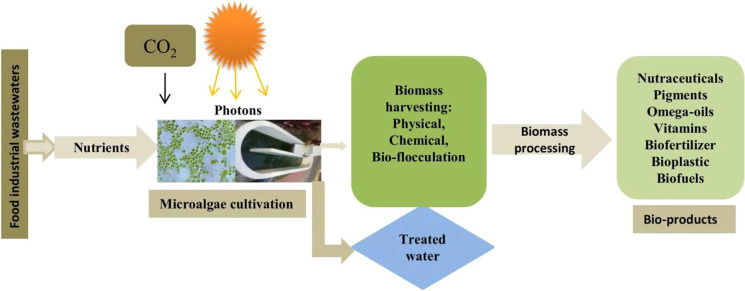
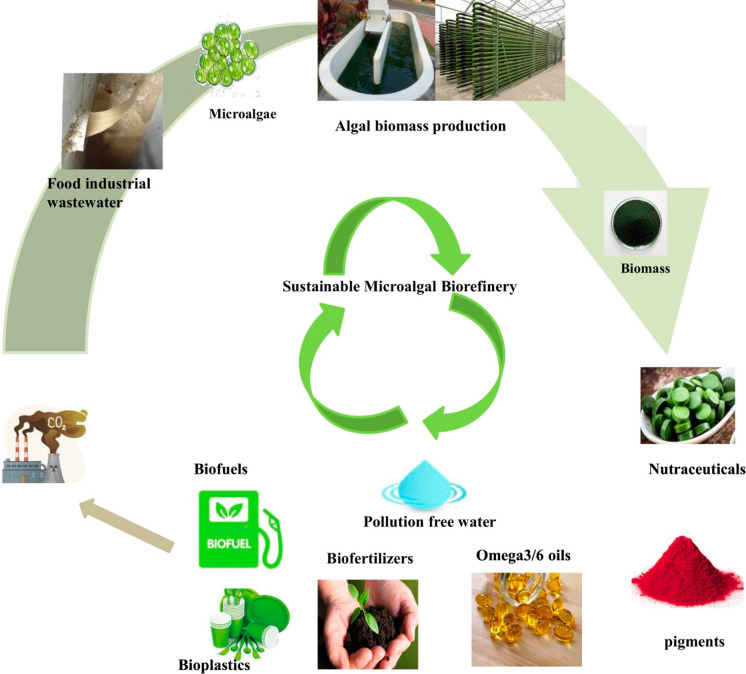
Abstract
Microalgae are recognized as cell factories enriched with biochemicals suitable as feedstock for bio-energy, food, feed, pharmaceuticals, and nutraceuticals applications. The industrial application of microalgae is challenging due to hurdles associated with mass cultivation and biomass recovery. The scale-up production of microalgal biomass in freshwater is not a sustainable solution due to the projected increase of freshwater demands in the coming years. Microalgae cultivation in wastewater is encouraged in recent years for sustainable bioeconomy from biorefinery processes. Wastewater from the food industry is a less-toxic growth medium for microalgal biomass production. Traditional wastewater treatment and management processes are expensive; hence it is highly relevant to use low-cost wastewater treatment processes with revenue generation through different products. Microalgae are accepted as potential biocatalysts for the bioremediation of wastewater. Microalgae based purification of wastewater technology could be a universal alternative solution for the recovery of resources from wastewater for low-cost biomass feedstock for industry. This review highlights the importance of microalgal biomass production in food processing wastewater, their characteristics, and different microalgal cultivation methods, followed by nutrient absorption mechanisms. Towards the end of the review, different microalgae biomass harvesting processes with biorefinery products, and void gaps that tend to hinder the biomass production with future perspectives will be intended. Thus, the review could claim to be valuable for sustainable microalgae biomass production for eco-friendly bioproduct conversions.
Graphical abstract
Keywords: Microalgae, Food industry wastewater, Biomass production, Harvesting process, Biorefinery chemicals, Biofuels
Introduction
Fresh water is the most demanding and valuable commodity resource for the future. Escalating population and widening industrialization utilizes a large amount of freshwater for different purposes and generates a huge volume of wastewater. The release of untreated wastewater into the environment creates a severe danger to the ecosystem (Ummalyma et al. 2021). Qadir et al. (2020) showed that presently approx. 380 trillion L/y of wastewater is produced throughout the world and Asian counties are the leading producer of wastewater (~ 159trillion L/y), followed by Europe and North America. A report from UNESCO, United Nations (2017) showed that approx. 80% of the untreated wastewater is released into the water bodies. A study showed that developed and developing countries treat 70% of wastewater, whereas medium-income countries 38% treatment and appro.8% in low-income countries. Meanwhile, 40% of the world will face a freshwater crisis in 2030, which will be a serious social and economic challenge in the coming years (Sun et al. 2016; Sato et al. 2013). Among different food processing industry generates huge amounts of waste and wastewater. According to the report of Boland et al. (2013), Food and Agricultural Organization showed that from 2000–2050 milk and meal production would increase globally by 82 and 100% respectively. Reports highlighted that beer production in many of the top countries reached 199 million kiloliters. For one liter of beer production approx. 4.5L of water is consumed (Arantes et al. 2017).
Food processing is the crucial part of food supply chains and its water footprint is greater considerations, for significant water usage in manufacturing and a huge volume of wastewater (Menese et al. 2017). Food processing wastewaters are organic and it enhances the growth of microorganisms and causes eutrophication of freshwater bodies if discharged without treatment. This wastewater does not contain toxic metals such as heavy metals and is non-toxic compared with other industrial effluents (Ghimpusan et al. 2017). Treatment for nutrients and pollutants removal from food processing wastewater can be performed by a physical and chemical process. The drawback of the traditional method is the usage of strong chemicals; secondary pollution to the atmosphere, the high operating costs could reduce the efficiency during commercial applications (Li et al. 2019). To overcome these existing challenges, there is an increasing attraction towards biological green processes for wastewater treatments and pollutants remediation all over the world. To deal with the emerging concerns about environment and biodiversity preservation, there are stringent regulations on safe discharge, which has directed the need for alternative innovative and sustainable processes for waste mitigation (Hussain et al. 2021; Ummalyma et al. 2021, Singh et al. 2020; Zhuang et al. 2020; Saravanan et al. 2018; Sharma et al. 2021). In this regard, the biological wastewater treatment option based on the microalgae-based process offers potential promises (Sirohi et al. 2021a, 2021b; Yu et al. 2021; Joun et al. 2021).
Microalgae are attractive biocatalysts to meet the wastewater treatment process and energy crisis. Microalgae can grow faster, remove nutrients, and treat wastewater simultaneously produce biomass suitable for biofuels and biorefinery products (Ummalyma et al. 2021; Hussain et al. 2021). Microalgae can able to grow different trophic modes of nutrition. Photoautotrophic algae trap CO2 and sunlight from the atmosphere for energy and carbon for their growth and reproduction meantime helping in the mitigation of CO2 and valuable products. Heterotrophic microalgae take organic nutrients for carbon and energy sources from the wastewater and produce biomass for biorefineries whereas mixotrophic microalgae can grow by trapping CO2 and organic carbon available from the wastewater as energy and carbon source for their multiplication and produce biomass for different applications (Hussain et al. 2021; Ummalyma et al. 2021). Several kinds of research have been performed for the purification of wastewater using different microalgal species. A recent report highlighted that microalgae have the potential for effective removal of high concentrations of organic carbon, phosphorous, and nitrogen from food processing wastewater and produced different value-added products (polysaccharides, biofuels, pigments, and amino acids (Nur and Buma 2019; Ummalyma et al. 2021). In this present review, discussing the importance of food processing wastewater as a nutrient medium for low-cost biomass for circular bioeconomy biorefinery products, and characterization of various food processing wastewaters are highlighted. Following different bioreactors used for microalgae, cultivations are detailed with mechanisms of nutrient absorption. In the end, harvesting processes of microalgal biomass, possible biorefinery products derived from wastewater-grown microalgae are ascribed and void gaps in this field for future research outlook are discussed.
Different sources of food industrial effluents and their composition
Food processing wastewaters (FPWWs) are generated mainly from dairy processing, meat processing, edible oil processing, starch, and brewery processing units. FPWWs are characterized by high organic contents in the form of total dissolved solids (TDSs), total suspended solids (TSSs), biological oxygen demand (BOD) and, Chemical oxygen demand (COD), oils, fats, and grease. The composition and contamination loads of the FPWWs depend on the type of industry, raw materials, and processing technologies (Amin et al. 2021; Subha et al. 2020; Li et al. 2019). It is non –toxic due to traces of chemicals such as antibiotics and heavy metals; however, microalgae biomass production in FPWWs is suitable for high-value chemicals such as proteins, polymers, pigments, polysaccharides, and DHA (Gecim et al. 2021; Humaidah et al. 2020). The difference in nutrient composition of FPWWs also influences microalgae growth and biochemicals. Different types of FPWWs and their compositions are described in the flowing section.
Starch processing wastewater
Starch processing wastewater (SPWW) is a typical waste stream from FPWW, which is rich in nutrients, is non-toxic, and an ideal medium for microalgal biomass production. The starch processing industry consumes a huge volume of water and hence produces 6–10m3 of wastewater per ton of starch processing. SPWW discharged from the starch processing industry are characterized by high organic nutrients in the form of COD (6000–30,000 mg/L) with a high amount of dissolved organic matter (Tan et al.2019; Wang et al. 2017). Cassava is the important staple food crop in the world; its processing generates 600L of wastewater to treat per ton of root. This wastewater is rich in high starch with BOD, COD, and ammonium nitrogen content with total dissolved solids with cyanoglycosides (de Carvalho et al. 2018). In addition to this, it contains high potassium salts, protein, organic acids, minerals, dissolved starch, oils, and fats. These components act as essential nutrients that support microalgal biomass production (Li et al. 2020).
Meat processing wastewater
Meat processing wastewater (MPWW) characteristics are depending on the factors such as the size of the processing unit, type of animal processing, the volume of water consumed per animal, and the washing process. Meat production reached approx.330million tons in the case of bovine, pig, and poultry. The leading meat processing countries are Russia, United States, Brazil, Mexico, and India (Food and Agricultural Organization 2018). Escalating demands for meat products, the amount of MPWW generated through slaughter, washing, and packing process. Wastewater produced as a result of meat processing units are enriched with a high quantity of nutrients in the form of organic matter, volatile fatty acids (VFA), detergents, pathogens, antibiotics, and heavy metals, intense coloration, and odor (Bethi et al. 2020; Aziz et al. 2019).MPWW has high concentrations of total organic carbon, total nitrogen, phosphorus, and these nutrients are essential macronutrients required for microalgae growth and biomass production. Aziz et al. (2019) reported that meat and slaughterhouse wastewater has 1100–15,000 mg/L of COD, 600-3900 mg/L of BOD, 50-800 mg/L TN, 20-300 mg/L ammonia, 15-200 mg/L TP,220–6400 mg/L of TSS, 350-1340 mg/L alkalinity, 175-797 mg/LVFA, 40–1385 mg/L of oil and grease respectively. A recent report showed that the cultivation of Chlorella vulgaris in acid precipitated poultry slaughterhouse wastewater removed 83% COD in batch mode. The maximum biomass produced from this method of cultivation was 1.2 g/L (Hilares et al. 2021). Phycoerythrin was purified from the biomass of Porphyrium cruentium cultivated from the MPWW highlighted that this medium is suitable for obtaining bioproducts from microalgae (Balaraman et al. 2021).
Dairy wastewater
Dairy processing is one of the key sources of wastewater generated all over the world. The dairy processing factories convert' raw milk to condensed milk, pasteurized milk, produce products such as creams, whey, cheese, butter, yogurt, etc. The generated wastewater mainly consists of milk product residues by-product of unit operations and wastewater generated by cleaning of milk equipment, washing of containers, quality control, and laboratory analysis. Every liter of milk processing approximately 0.2 to 10L of wastewater discharged (Li et al. 2019; Ummalyma et al. 2014). Wastewater quality and characteristics may fluctuate subject to the type of processing methods adopted, final products, and size of the factory (Zkeri et al. 2021). There are two different types of wastewater is generated from this industry; high-strength concentrated wastewater originated from cheese, whey, and milk permeate characterized by the high amount of COD (100 g/l) due to the high content of organic matter, fats, and lactose. The medium-strength dairy wastewater does not contain COD higher than 5 g/L (Zkeri et al. 2021). In general dairy, wastewater is characterized by high COD, BOD, and volatile solids. Major components present in this wastewater are soluble milk proteins, lactose, lipids, fatty acids, minerals, salts and detergents content, nitrogen, and phosphorous. Hence, this wastewater is rich in carbon, nitrogen, and phosphorous and it could be an excellent nutrient medium for microalgae biomass production for low-cost biorefinery products (Sawin et al. 2020; Li et al. 2019).
Winery and brewery wastewater
Winery wastewater (WWW) is produced as a result of different process activities such as washing during grape harvesting, pressing, fermentation process, bottling, and filtrations. It has been reported that 0.5- 14L of wastewaters are generated for 1L of wine production. WWW is organic due to the sugars, organic acids, polyphenols, esters aldehydes, soaps, and other detergents with low pH (Senapathi et al. 2020).
The brewery waste industry produces a huge volume of wastewater during the brewing process. It has been reported that 4-8m3 of wastewater is produced per m3 of beer production (Kebede2018). Reports showed that this wastewater has high organic loads (2000–6000 mg/L) in the form of COD with total nitrogen (25–80 mg/L) and phosphorous (10–15 mg/L). Brewery wastewater (BWW) is the suitable nutrient medium for microorganisms due to less heavy metals content and provides optimum growth conditions with neutral pH (Amenorfenyo et al. 2019; Farook et al. 2013). BWW contains sugars, volatile fatty acids, soluble starch, alcohols, and celluloses. BWW pH levels are variable depending on the concentrations and type of chemicals cleaning and sanitizing purpose (Amenorfenyo et al. 2019). Recently this wastewater is given attraction as a growth medium for microalgae biomass production for biomolecule recovery. Co-cultivation of Scenedesmus sp., and Chlorella sorokiniana in BWW, showed that 78% organic pollutant removal with 90–97% removal of ammonia, total nitrogen, and phosphorous. Produced biomass recovered 16 mg/l of chlorophyll, 9.57 mg/L of carotenoids, and 30.4 mg/L of carbohydrates respectively (Han et al. 2021). Song et al. (2020) reported that the cultivation of Chlorella sp.,Spirulina, and Scenedesmus sp., in the BWW prove that Scenedesmus sp., are fast-growing and adapted organisms in BWW with 1.02 g/L of biomass production with nutrients removal of COD (73%), ammonia (89%), total nitrogen (75%), and phosphorous (95%), respectively.
Oil mill wastewater
Oil mill effluents are mainly originated from the processing of olive oil and palm oil. Palm oils mill is mainly found in Southeast Asian countries like Thailand, Malaysia, Nigeria, and Columbia. The blooming of oil mill agribusiness generates enormous waste and effluents. Releasing of Palm oil mill effluents (POME) are tenfold higher than crude palm oil (Cheng et al. 2020). During the production of palm oil, approx.1.5m3freshwater is required for the processing of one-ton fresh palm fruit bunches, and concurrently generate 0.75 m3 of POME.
POME is unpleasant, greasy brownish, organic liquor with high chemical and biological oxygen demands. POME is a dark brown, viscous liquid consisting of 95–96 wt% of water, total solids of 4–5%, TSS of 2–4%, and high turbidity of 65, 590-69410NIU (Cheng et al. 2020).
The olive oil industry is active in European country mainly Serbia, France, Turkey, and Mediterranean Basin. Huge voluminous freshwater is used in the olive mill industry and finally discharged in the form of olive mill effluents (OME). Oil mill effluents are characterized by complex substrates composed of amino acids, inorganic nutrients such as calcium, magnesium, potassium, sodium, organic acids, and carbohydrates such as simple sugars and hemicelluloses (Li et al. 2019). Further characterization of POME showed that BOD and COD range from 15,000 to 30, 000 mg/L, and 40,000–90,000 mg/L respectively. TSS in the range of 20,000–40,000 mg/L, TDS between 15–30,000 mg/L, VSS in the range of 15–35,000 mg/L, respectively (Nur and Buma2019). Microalgae species are used for the phytoremediation of pollutants present in this wastewater and produce low-cost biomass for biorefinery products. Microalgae such as Chromochloris zofingiensis, and Haematococcus pluvialis were exploited to produce high-value molecules such as carotenoids and astaxanthin from biomass produced through the cultivation in POME (Fernando et al. 2021). Microalgae such as Coelastrella sp., Chlamydomonas sp., and Scenedesmus sp., were cultivated in POME showed 80% of pollutants removal by using raw and sterilized effluents (Udaiyappan et al. 2020). Phycoremediation and CO2 fixation from POME was studied with three microalgae species Chlorella sorokiniana UKM2, Chlorella pyrenoidosa UKM7, and Coelastralla. The result showed that these microalgae can be able to remove inorganic nutrients up to 100%. Two Chlorella sp. were cultivated in pretreated POME for biomass production, lipid content, and pollutants remediation. Results showed that 2.2 g/L of biomass with 11.2% lipid and 62% nitrogen removal, 47% COD reduction, and 30.7% total phosphorous reductions (Cheah et al. 2018). Table 1 represents different microalgae strains utilized for cultivation in food industrial wastewater for biorefinery products.
Table 1.
Microalgal strains used for cultivation in various food industrial wastewaters for biorefinery products
| Source of food industrial wastewater | Microalgae | Cultivation system | Target product | References |
|---|---|---|---|---|
| Alcohol wastewater | Chlorella pyrenoidosa | Photobioreactor | Lipid | Tan et al. (2018) |
| Starch Processing wastewater | Chlorella pyrenoidosa | Circulation photobiorecator | Lipid | Chu et al. (2015) |
| Cassava processing wastewater | Arthrospiraplatensis | Fed batch cultivation | Biomass | Araujo et al. (2020) |
| Dairy wastewater | Chlorococcum sp., RAP13 | Batch mode | Biomass and lipids | Ummalyma et al. (2014) |
| Dairy wastewater | Chlorella sorokiniana | Moving bed biofilm reactor | Biomass, protein, starch and lipids | Zkeri et al. (2021) |
| Dairy wastewater | Tetraselmis sp. | Batch | Lipid | Swain et al. (2020) |
| Dairy wastewater | A. protothecoides C. reinhardtii | Batch | Lipid and recombinant proteins | Gramegna et al. (2020) |
| Dairy wastewater | C. protothecoides | Batch | Biomass and fatty acids | Patel et al. (2020) |
| Food processing industrial wastewater | C.sorokiniana S. obliquus | Batch | Biomass, lipid and PUFA | Gupta et al. (2018) |
| Food processing industrial wastewater | Aurantiochytrium sp. | Batch | PUFA | Humaidah et al. (2020) |
| Molasses wastewater | Scenedesmus sp. | Batch | Lipid | Ma et al. (2017) |
| Meat processing wastewater | C. protothecoides, S. obliquus and C. vulgaris | Batch | Biomass | Hu et al. (2019) |
| Soybean processing wastewater | Chlorella sp. | Batch | Polysaccharide and lipids | Qiu et al. (2019) |
| Soybean processing wastewater | S. obliquus | Batch | Fatty acids | Shen et al. (2020) |
| Soybean processing wastewater | Aurantiochytrium sp. | Batch | Docosahexaenoic acid (DHA) | Lee et al. (2020) |
| Sugar cane wastewater | Scenedesmus sp. | Batch | Biomass and biomass | Zewdie et al. (2021) |
| Starch wastewater | Chlorella pyrenoidosa | Batch | Biomass Lipids | Tan et al. (2019) |
| Brewery wastewater | Chlorella sp., Scenedesmus sp., Spirulina sp. | Batch | Lipids, pigments and carbohydrates | Song et al. (2020) |
| Slaughterhouse wastewater | Chlorella vulgaris | Batch | Biomass | Hilares et al. (2021) |
| Slaughterhouse wastewater | Chlamydomonas subcaudata, sp., Nitzschiasp | High rate Algal pond | Fatty acids and Lipids | Hernández et al. 2016 |
| Palm oil mill effluent | Haematococcus pluvialis Chromochloris zofingiensis | Batch | carotenoids astaxanthin | Fernando et al. (2021) |
Biorefinery of food industry wastewater for production of value-added products
Low-cost microalgal biomass production for biorefinery products in food industrial wastewater is advantageous due to low toxicity in nature. Mass production of microalgae biomass in wastewater is possible for biomolecule extractions for different applications due to the absorption of the different organic and inorganic nutrients present in the wastewater streams that assist fast growth and biomass production (Ummalyma et al. 2021). A challenging issue in microalgae biomass production in the wastewater stream is the harvesting of biomass from the growth medium and biomolecules extractions. Selection of cultivation system for mass production of biomass is depending on the characteristics of wastewaters, type of microalgae chosen, and final applications. A suitable culture system should be easy to handle, low cost of construction, provision for adequate light supply, be capable of effective transfers of liquid and gas, no risk for contaminations (Tan et al. 2020).
Cultivation methods
Microalgae have the evolutionary capability to adapt to the harsh environment, rapid growth, and high photosynthetic capacity along with the accumulation of bioproducts within their cells makes them suitable industrial feedstock. Microalgae large-scale cultivation does not require fertile land, freshwater, pesticides, and herbicide compared to its terrestrial counterpart. Many reports are highlighted that microalgal biomass is successfully produced from different wastewater resources (Ummalyma et al. 2021; Fernando et al. 2021; Singh et al. 2020; Tan et al. 2020; Li et al. 2019). At the research level, microalgae produce biomass with a high growth rate in laboratory conditions, but when it comes to large-scale production still more challenge is there to recover high biomass productivity. Microalgae cultivation methods are generally categorized into the open pond, photobioreactor, and hybrid cultivation systems. The selection of cultivation methods is further reliant on the growth mode of microalgae such as phototrophic, heterotrophic, and mixotrophic nature.
Open cultivation systems
An open cultivation system is preferred for the large-scale cultivation of microalgae for low-cost biorefinery products. The open cultivation system is perhaps the oldest and most simple experimental setup to grow microalgae, most frequently used in outdoor conditions using sunlight. This cultivation has usually given importance in the industrial-scale production of biomass mainly due to simple operation, ease of construction, and low energy requirements (Bhatia et al. 2021). There are several open cultivation systems are utilized for microalgal biomass production such as natural ponds, lakes, specially designed reactors such as raceways, and circular ponds. The circular pond is the first artificially fabricated having a depth of 30 to 70 cm and a depth of 45 m with a rotating agitator for mixing and preventing biomass sedimentations. This system is larger and causes water resistance which leads to mechanical stress on the agitations. The design of this pond is limited by its bigger size, high construction cost, and energy requirement for the agitation process (Tan et al. 2020). A raceway reactor consists of a series of closed-loop channels, 30 cm deep and is fitted with a paddle wheel to avoid sedimentations and enable recirculation of microalgae culture broth for proper nutrients and CO2 supply to enhance the growth rate and biomass production (Bhatia et al. 2021). Outdoor open raceways were used for the commercial of production cyanobacteria and microalgae biomass. Raceways are the best open pond cultivation design available mainly due to energy efficiency and a single paddlewheel is sufficient for proper agitations of a 5-hectare raceway pond (Rogers et al. 2014). The benefits of open systems were easy to maintain and cost-efficient due to using solar energy as primary energy input.A circular open pond system is used in Taiwan and Japan for cultivating Chlorella sp., (Shen et al. 2009). Sapphire Energy Columbus, an algal biomass farm located in the United States successfully utilized a raceway pond for the production of 520 metric tons of dry microalgae biomass for continuously 2 years of operation without any technical problems (White and Ryan 2015). High rate algal ponds are preferred for wastewater treatment with shallow depth helps microalgae to proliferate and produce high biomass. Chlorella zofingiensis was cultivated in an open plastic pond by using dairy wastewater to produce biomass. Tan et al. (2017) utilized closed rectangular tanks of 175L capacity used for biomass production from Chlorella pyrenoidosa using alcohol wastewater. Food industrial effluents are treated in the outdoor MaB-floc raceway pond showed 20% removal of phosphorous with biomass productivity of 31.3 tons total suspended solids ha/pond/year (Hende et al. 2016). Rose oil processing wastewater is used for the cultivation of Chlorella sp., in raceway reactor showed that better removal of organic and inorganic nutrients. The component analysis of the resulting biomass showed suitable for application as a biofertilizer (Uysal and Ekinci 2021). It has been reported that open raceway pond cultivation of Nanochloropsis in different geographical locations under outdoor conditions showed that biomass productivity is varied from 2000 to 700 t km−2 year −1. The study showed that microalgae growth robustly depends on local climatic factors like direct and indirect solar radiations, wind velocity, ambient air temperature, and relative humidity (Banerjee and Ramaswamy 2019). The limitation of the open system is contamination due to microorganisms, and protozoa along with unfavorable weather conditions that could hamper the biomass productivity even total loss of desired strains. The presence of rotifers in the open pond system causes a culture crash and eventually reduced the biomass yields. The shading effects due to low penetration light, ineffective photosynthesis also affect biomass production in these cultivation systems. Growth relevant parameters such as temperature, pH, and light availability cannot be regulated. Summer periods evaporations are still challenging issues in open systems (Ummalyma et al. 2021). Large open outdoor systems are used for the cultivation of many microalgae species for resource recovery from wastewater for sustainable biorefinery products.
Closed cultivation systems
Closed cultivation systems are potential for the controlling and physiological conditions of microalgae. The bioreactor used for controlled growing of microalgae is a photobioreactor (PBR). PBR is a commonly used closed system for biomass production and construction and design are determined based on the microalgae strain selected for cultivation. The PBR explored for microalgae cultivation required high operating and capital costs. However, this system is efficient for high biomass production with proper control of culture parameters, which assist in preventing contamination problems compared with an open system. In PBR, the intensity of irradiation, the flow rate of air and CO2, agitation, and pH can be controlled according to the ideal conditions suitable for microalgae strain. In photobioreactor, microalgae can be cultivated under the desired mode of growth (heterotrophic, mixotrophic, and phototrophic), for that external supply of carbon source and inorganic carbon could be provided for better microalgae growth and biomass production. The limitation of the system is biofouling and cleaning. There are many types of closed PBR are available depending on the shape, geometry, and material used for the construction such as flat-plate, tubular, bubble column, and hybrid PBR. Commonly used PBR is horizontal tubular PBR for microalgae cultivation due to high biomass productivity; bubble column and airlift are normally used in large-scale industries (Yin et al. 2020, Bhatia et al. 2021; Ummalyma et al. 2021). For the low-value products (biofuel), utilization of PBR is not cost-effective, but it is useful for the making of high-value lucrative products. Fluidized bed photobioreactor used for the biomass production and nutrients removal in palm oil mill effluent showed that 90% removal of phosphorous and nitrogen. The maximum biomass production of 3.2 g/L with 0.36 g/L of lipid from Nannochloropsis sp (Cheirsilp et al. 2017). Pilot-scale tubular photobioreactors are used for the cultivation of Chlorella pyrenoidosa using food processing wastewater. Biomass production ranges between 1.8–2.1 g/L with 15% of lipid with effective pollutants removal 55% COD, 67% total phosphorous, and 88% of total nitrogen (Tan et al. 2021). Utilization of photo bioreactor containing shrimp wastewater for the cultivation of Synechocystis sp. PCC6803 was used for biomass production, and conversion of bioplastic poly-β-hydroxybutyrate (PHB). The study showed that 96% phosphate, 98% ammonia, 80% nitrates, and 67% nitrite were removed from the wastewater, and biomass is accumulated with 32% DCW of PHB (Krasaesueb et al. 2019). Tubular photobioreactor operated continuously for the cultivation Tetraselmis suecica in fish processing industry showed the biomass production of 1 g/L with 49% removal of nitrogen and 99% removal of phosphorous respectively. The final biomass is applied for feed application for juvenile shellfish (Michiel et al. 2014). Photobioreactors are used for the cultivation of monoalgae, and consortium for biomass production using dairy wastewater. Result showed that microalgae consortium consists of Chlorella and Scenedusmus sp., produced higher biomass of 5.1–5.4 g/L with removal of 62.86% COD and phosphorous of 95.96%. The monoculture of Chlorella sp., produced 4.72 g/L (Qin et al. 2016).
Hybrid system
A hybrid cultivation system (HCS) is designed for the amalgamation of two or more cultivation systems to produce biomass and resource recovery from wastewater. The HCS is integrated with an open system, photobioreactor, and certain cases with algal turf scrubber (Bhatia et al. 2021; SundarRajan et al. 2019). In this system, optimum cultivation condition is maintained in the first stage for accelerating biomass production yield and the second stage expose biomass into the stressful environment to enhance the desired product. It has been reported that average areal biomass productivity in HCS is higher than the non-hybrid systems. It is 46 to 74% higher than open systems and 12.5% enhancement of biomass than PBRs (Liu et al. 2018; Tan et al. 2017b). HCS can be considered as a better approach for monoculture biomass production of oleaginous microalgae. The report showed that 47 days of HCS cultivation of microalgae efficiently produced biomass from the target microalgal strains (Yun et al. 2018). This system is suitable for wastewater coupling with microalgae for getting the maximum benefit of each reactor, which helps in the reduction of the production cost of each kilogram of microalgal biomass (Salama et al. 2017). Cultivation of Tetraselmis sp. was used in HCS containing closed photobioreactor and open pond for enhancing the lipid yield. First microalgae are cultivated in 1200L PBR followed by second cultivated in 1000L open raceway pond with nutrients deficient media. The study showed that HCS produced more lipid yield compared to single-phase cultivation either in PBR or open ponds used as a control. Maximum biomass productivity obtained in a hybrid system is 0.5 gm-2/days per solar irradiation of 1 kWhm−2 (Narala et al. 2016). Production of biomass from target microalgae strains from the algal feeders in the open pond is a challenge in the industry. In such a case, the hybrid system is advantageous to produce biomass from desired microalgae. Yun et al. (2018) utilized a PBR-open raceway pond hybrid system for the cultivation of microalgae. Here PBR act as a continuous supply of inoculum of target microalgae to sustain microalgae growth and biomass production in an open raceway pond. Cultivation of Scenedesmus obtusiusculus in hybrid photobioreactors showed that biomass concentration of 1.2 g/L with a 0.38/day of specific growth rate, and biomass productivity of 41.8 mg/L/day using flue gas supplementation (Estrada-Graf et al. 2020).
Mechanisms of nutrients absorption by microalgae
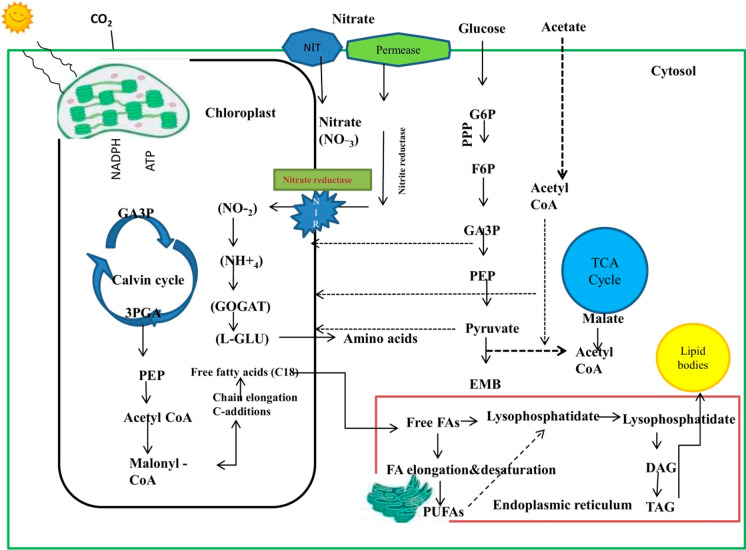
Wastewater contains both inorganic and organic nutrients. The mechanism of nutrient absorption is not well understood in microalgae. Food industrial wastewater is enriched with inorganic nutrients of nitrate, nitrite, and organic nitrogen, phosphates, and CO2.These are the essential nutrients for the growth, and reproduction of microalgae. Microalgae can consume both CO2 and HCO3– ions for their photosynthetic process. Microalgae can able to fix CO2 to produce organic matter by utilizing NADPH and ATP generated through the light reaction of photosynthesis. The detailed mechanism of the Calvin cycle and CO2 concentration mechanism is previously reported by Su et al. (2021).
Nitrogen is the crucial micronutrient required for the growth and reproduction and vital components of many microalgae cellular compounds. It is required for the synthesis of organic nitrogenous compounds inside the cell such as ATP, DNA, and RNA (Barsanti and Gualtieri 2006). During the assimilation process, microalgae convert inorganic nitrogen into organic nitrogen. The oxidized forms of inorganic nitrogen are translocated from the plasma membranes and undergo reduction to produce ammonia and finally incorporated into amino acids. The nitrogen metabolisms are linked with carbon metabolism inside the cell. The incorporation of ammonium ions to amino acids required a carbon backbone in the form of keto acid originated from oxaloacetate, energy in the form of ATP, NDPH are used as reducing agents sfor the synthesis of amino acid aspartate, glutamine, and glutamate (Lea and Mifin 2003). The nitrate and nitrates are reduced to ammonium ions by two enzymes such as nitrate reductase and nitrite reductases, respectively. Nitrate reductase converts nitrate to nitrite by utilizing NADH and pyrimidine nucleotides for transferring two electrons. Later nitrite reductase reduces nitrite to ammonium ions, by utilizing ferredoxin as an electron donor. Finally, the glutamine synthase enzyme catalyzes the transfer of the ammonium ions to glutamate using ATP molecules to convert glutamine (Fernandez and Galvan 2007). Another less energy expensive nitrogen source is ammonium preferred by microalgae. Transport proteins belonging to the ammonium transporter family are involved in the transport of ammonia across the membranes. These protein families are detected in diatoms and Chlamydomonas (Maestrini et al. 1986). Phosphorous also play an important role in the metabolic process and is found in proteins, lipids, and nucleic acids. Phosphorous fund in wastewater is in the form of polyphosphate, organic phosphate, and orthophosphate. The phosphate is assimilated by microalgae and incorporated in the nucleic acids, proteins, and lipids during the biosynthetic process through phosphorylation and synthesis of ATP (Martinez et al. 1999). The excess available in the form of orthophosphate can be stored as polyphosphate granules within the cells for future use.
Organic carbons present in the food industrial wastewater are mainly consisting of fructose, galactose, glucose, alcohol, lactose, and organic acids. Responds to these compounds are depend on the species-specific and concentration (Perez-Garcia et al. 2011). It has been reported that the carbohydrate transport system is involved in the metabolism which is energy-dependent and stimulated by irradiance (Morales-Sánchez et al. 2015). Glucose is an organic carbon substrate commonly consumed among microalgae (Ummalyma and Sukumaran 2015). In wastewater, microalgae are mostly undergoing a mixotrophic mode of growth. The glucose is metabolized in the cytosol by two different metabolic pathways such as the pentose phosphate pathway (PPP) in the absence of light and Embden Meyerhof Pathway (EMP) in the presence of lights (Perez-Garcia et al. 2011). Metabolism of carbon by microalgae mainly undergoes glycolysis, pentose phosphate pathways (PP), phosphorylation, and TCA cycle. Glucose utilizing microalgae metabolize glucose through glycolysis, TCA cycle, and NADPH synthesizing pool and assist the biosynthesis of the product via the acetyl-CoA route. Fructose is utilized in glycolysis pathway catalyzed by hexokinase (Su et al. 2021). Microalgae utilizing C5 sugars are metabolized by PP pathways. The report showed that Botryococcus braunii consumed mannose by PP pathways in presence of light (Tanoi et al. 2011). Mannose utilized by Schizochytrium sp. for biomass production. It has been reported that EMP pathway, oxidative phosphorylation, and TCA cycle are continued in mitochondria with the presence of glucose under different growth mode (phototrophic, heterotrophic, and mixotrophic) in Chlorella pyrenoidosa and Synechocystis sp., (Yang et al. 2000; Hong et al. 2007).
Acetate is another carbon found in wastewaters. Assimilation of acetate is mediated by acetylation of coenzyme- A assisted by acetyl-CoA synthetase to form acetyl-CoA with the help of ATP molecule (Droop 1974; Boyle and Morgan 2009). Acetate metabolism occurs in mitochondria and glyoxysomes through TCA cycle and glyoxylate cycle for the incorporation of acetyl clusters into the carbon skeleton.
Alcohols present in the winery wastewaters undergo oxidations to acetaldehyde and acetate by two enzymes such as aldehyde dehydrogenase and alcohol dehydrogenase present in the cytosol and mitochondria. Later acetate is converted to acetyl coenzyme A via acetyl-CoA synthetase, which enters into glyoxylate cycle and TCA cycle. Acetyl-CoA is used for NADH biosynthesis in the TCA cycle and converted to succinate and malate by isocitrate lyase and malate synthase (Ono et al. 1995; Yoval-Sanchez et al. 2011). Microalgae such as Arthrospira sp., Scenedesmus sp., Chlorella sp., and diatoms are reported microalgae species for the assimilation of alcohols from the growth medium for biomass and bioproduct accumulation (Bezerra et al. 2014; Matsudo et al. 2016). Amino acids present in the wastewater are absorbed via the glutamate-glutamine pathway and stored as amino acids and intermediate molecules which are used in the other biochemical process (Chen et al. 2017). The simplest representation of nutrient uptake by microalgae are represented in Fig. 1.
Fig. 1.
Simple representation of nutrient uptake metabolism by microalgae from wastewater
Biomass harvesting strategies
Algal biomass harvesting refers to the detachment of algae from its nutrient/growth medium using suitable techniques such as flocculation, centrifugation, floatation, filtration, and a combination of these techniques. The selection of a suitable method of harvesting depends on certain harvesting traits or requirements that deal with the cost, processing time, biomass species and quality, separation efficiency, and toxicity (Singh and Patidar 2018). Microalgae have a negative surface charge and density is also equal to the growth medium and hence always remains cells are dispersed in the medium. However, several harvesting methods are adopted for neutralizing the microalgal surface change for effective dewatering of microalgal biomass from the media (Ummalyma et al. 2017).
Floatation, flocculation, filtration, sedimentation, and centrifugation are the most commonly used methods for algae harvesting (Xu et al. 2021). In the floatation technique, small bubbles are produced using either dissolved air or dispersed air mechanisms (Ndikubwimana et al. 2016). The generated bubbles attach themselves to the microalgae cells and float them to the surface due to lower density that enables easy algal biomass harvesting at low energy demands. Although the floatation technique is time saving, efficient, requires low space and can be used for large-scale harvesting, it has certain disadvantages. For instance, if the bubbles produced are oversized, it could break up the floc and therefore, surfactants are usually needed for a stabile floc. In recent studies, there has been a discussion on ballasted floatation that would use a bubble less system generated from low-density materials (LDMs) for harvesting free-floating microalgae (Xu et al. 2021). The said LDMs in the newly developed ballasted floatation could include microspheres made of sodium borosilicate glass, fly ash or waste cooking oil that would increase the solid–liquid separation efficiency at the harvesting surface (Zhang and Zhang 2019). In flocculation, chemical salts, aluminium, and iron are added to the algal biomass media to promote the aggregation of microalgal cells to form a floc. Caetano et al. reported that modulation of pH or the addition of CaCl2 for the harvesting of Arthrospira maxima showed that alkaline pH and CaCl2 concentrations improved the efficiency of biomass harvesting (Caetano et al. 2020). The chemical flocculants are generally toxic and need an additional treatment process for their removal that contributes to the processing cost (Rinanti and Purwadi 2018). To tackle this problem, several bioflocculants made from chitosan and acrylic acid based biopolymers were developed which were more eco-friendly which could attain 90% cell recovery as compared to 95% in chemical flocculants (Zhu et al. 2018).
Bioflocculation or auto-flocculations happens mainly due to the secretion of biopolymers of extracellular polymeric substances. Reports showed that biopolymers produced by microbes and microalgae are used for biomass harvesting from the wastewater for low-cost microalgal biorefineries. Utilization of poly-γ-glutamic acids produced from B.licheniformis for the harvesting of microalgae Desmodesmis sp., showed 98% flocculation efficiency (Ndikubwimana et al.2016). EPS produced by Scenedesmus acuminatus are used for the flocculation of the same microalgae and the harvesting efficiency > 80%. Low molecular weight EPS composed of mannose and glucose influences the microalgal biomass harvesting process (Yang et al. 2020). Detailed bio-flocculation techniques and their flocculation mechanisms are discussed in a review reported by Ummalyma et al. (2017).
Electromembrane filtration and ultrafiltration are also used for the harvesting of microalgae biomass. The huge advantage of these methods is to support the separation process based on charge and molecular weight. Electromembrane filtration is a very useful method for harvesting Chlorella sp. KR-1 improves four fold (6.47) concentration factors (Kim et al. 2014).
Biomass quantity, biomass quality, operational cost, processing time, species specific and toxicity are the main criteria to choose the suitable harvesting techniques (Singh and Patidar 2018). According to these criterions, coagulation/ flocculation, centrifugation and filtration are the widely used techniques for microalgae harvesting. These techniques can be used alone or in combinations for raising the harvesting efficiency. Filtration and coagulation are effective techniques for high quality and quantity of cells but economically not feasible because of high operating and capital cost. Flotation and flocculation are fast, easy and can be used at large scale with low space requirement but use of chemical may be expensive and chances of contamination is also very high. Centrifugation harvesting is fast, convenient and suitable for all strain of microalgae but operating and maintenance cost make the process costly for large scale harvesting. Flocculation followed by gravity sedimentation is best and low cost operation among different combination of harvesting techniques.
Products from microalgae
Microalgae are rich in high-value metabolites like carbohydrates, proteins, lipids, pigments, essential fatty acids, vitamins, minerals, etc. (Udayan et al. 2021). Microalgae have been considered as a potential feedstock for biofuels, food supplements, and pharmaceuticals because of their high nutrient profile (Udayan et al. 2017). Microalgal cells consist of a high amount of lipids, proteins, and carbohydrates which favors the production of biofuels (Culaba et al. 2020). But the industrialization of microalgal metabolites is still in early stages because of the high cost of large-scale cultivation. Microalgal biomass production together with industrial wastewater management can be considered as a cost-effective method to reduce the needed for freshwater and fertilizers needed for microalgal growth (Jayaseelan et al. 2021). Cultivation of algae in wastewater increases the biomass and secondary metabolite production suitable for biorefinery product conversions because of the high nutrient load in the wastewater and also helps in wastewater treatment (Ummalyma et al. 2020; Ren et al. 2018).
Fatty acids and oils
The processing of wastewater using microalgae leads to the accumulation of fatty acids and oils and this bioprocess has gained much attraction from both industrial and research areas for the production of nutraceuticals, pharmaceuticals, biofuels, and oleo-chemical industries (Leong et al. 2021). Microalgae can able to accumulate 30–80% of lipids in their cells (Yong et al. 2020). Lipids can be polar and non-polar. Polar lipids like phospholipids, glycolipids, etc., are used as integral constituents of cell membranes and non-polar lipids like triglycerides, sterols, etc., are stored as energy reserves (D’Allessandro and Antoniosi 2016). Depending upon the cultivation conditions, microalgae accumulate TAG as lipid droplets. In terms of fatty acid composition, microalgae contain different types of fatty acids like saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) (Udayan et al. 2017). PUFAs are a nutritionally important class of fatty acids with proven health benefits (Udayan et al. 2021). Omega 3 PUFAs gained more attention recently especially during the Coronavirus pandemic conditions, due to their antioxidant activities, immune regulation, inflammation reduction, prevention of cardiovascular, and neurological disease conditions (Udayan et al. 2017, 2021). Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are the two important essential omega-3 fatty acids derived from microalgae.
When microalgae are subjected to different types of environmental stress and cultivation conditions, they will accumulate fatty acids and oils. Wastewaters released from different industries are rich in nutrients that support microalgal growth and metabolite production. Co-cultivation of microalgae with other bacteria will also help to increase lipid productivity (Sun et al. 2019). Different stress factors increase the microalgal lipid and fatty acid accumulation by changing the carbon flow conversion, redox balance, and stress hormone levels, which regulate lipid biosynthesis and conversion (Yew et al. 2019). Microalgae achieve high biomass content in a nutrient-rich wastewater medium which supports lipid productivity in later stages of growth.
For biodiesel properties, SFAs are more effective because PUFAs are not suitable for the stability of the fuel. PUFAs can be considered as edible oil which helps to maintain the health and balance of the human body (Udayan et al. 2021). Different strategies are adopted to increase the PUFA content in microalgae like treatment with wastewater, nutrient stress, plant growth regulators, temperature, salinity, UV radiation, etc. (Udayan et al. 2018; Udayan and Arumugam 2017; Udayan et al. 2020; Yew et al. 2019; Sun et al.2019). Table 2 represents the different lipid yield (%) obtained from microalgae cultivated in wastewaters.
Table 2.
Lipid production of different microalga species cultivated in wastewater
| Microalgae | Type of wastewater used | Lipid content (%) | References |
|---|---|---|---|
| Chlorella sp. | Swine industry | 1.77–3.55 | Min et al. (2011) |
| Desmodesmus sp. | Oil refinery | 21.95 | Mar et al. (2016) |
| Chlorella vulgaris | Pulp and aquaculture industry | 9.07 | Daneshvar et al. (2018) |
| Mix consortium | Poultry industry | 12.2 | Chinnasamy et al. (2010) |
| Desmodesmus sp. | Muncipal | 3.3 | Komolafe et al. (2014) |
| Chlorella sorokiniana | Domestic wastewater with urea supplementation | 61.52 | Ramanna et al. (2014) |
| Scenedesmus obliquus | Poultry industry | 33 | Hernández-García et al. (2019) |
| Chlorella sorokiniana CY | Palm oil mill effluent | 11.2 | Cheah et al. (2018) |
| Scenedesmus sp. | Meat processing | 41.7 | Apandi et al. (2018) |
| Microalgae consortium | Dairy wastewater | 22 | Hemalatha et al. (2019) |
Pigments
Cultivation of different microalgae and cyanobacteria in wastewater has been considered as an effective method for biomass and natural pigments production while improving the quality of water (Acien Fernandez et al. 2018). Microalgae can recycle the nutrients present in the wastewater, reduce energy consumption and provide oxygen also which has specific environmental benefits (Acien et al. 2016). Natural pigments from microalgae and cyanobacteria have attracted industries such as textile, cosmetics, pharmaceutical, and food (Pagels et al. 2019). The main photosynthetic pigments from microalgae are chlorophylls, carotenoids, and phycobilins (Udayan and Arumugam 2017a). Recently many researchers have been focused on the production of these natural pigments from microalgae using wastewater because of its pharmaceutical and industrial needs (Ajijah et al. 2020). Chlorella vulgaris grown in tofu wastewater has shown increased carotenoid content of 72.20 mg/L (Ajijah et al. 2020). Phormidium autumnale cultivated in slaughterhouse wastewater in a bubble column reactor has increased the lutein, beta carotene, violaxanthin, zeaxanthin, and canthaxanthin production (Rodrigues et al.2014). Palm oil municipal effluent (1%) used for the commercial outdoor cultivation of Spirulina platensis increased biomass content to 1.8 g/L and phycocyanin content to 12.01% after 7 days of cultivation (Sukumaran et al. 2014). Chlorella vulgaris, cultivated in synthetic wastewater showed high biomass production (1.087 g/L) (Wang et al. 2015). In another study, C.zofingensis cultivated in wastewater from the molasses production unit, showed increased astaxanthin productivity (1.7 mg/L) (Liu et al. 2012). Mixotrophic cultivation of Chlamydomonas acidophila in wastewater conducted in a 1L batch reactor increased the production of lutein (9 mg/g) and zeaxanthin (7 mg/g) (Cuaresma et al. 2011).
For large-scale production of pigments from microalgae, some challenges must be addressed, such as the high cost associated with upstream and downstream processing. Currently, the use of wastewater in the cultivation media is shown to reduce the cost of cultivation. However, the bacterial contamination and presence of toxic metals in the wastewater make it unsuitable for food applications directly (Acien et al. 2016).
Exopolysaccharides
Exopolysaccharides (EPS) are group of high molecular weight biopolymers secreted by microalgae to the external environment during their growth and metabolism (Xiao et al. 2016). EPS can be seen either weakly attached to the microalgal cell wall or secreted into the external environment (Trabelsi et al. 2016). Microalgae are the potential producers of EPS, especially red algae and cyanobacteria. The major function of EPS is to protect the cells from stress conditions, cell to cell communication, adhesion, and biofilm formation (Dertli et al. 2015). In the food industry, EPS has been used as thickeners and gelling agents which helps to improve the texture and quality of food (Feldmane et al. 2013). EPS also possesses potential antibacterial, antiviral, anticancer, and antioxidant properties which help in the development of novel pharmaceuticals (Table 3). Because the EPS are secreted externally to the culture medium, it can be easily extracted and purified (Bafanaa et al. 2013).
Table 3.
EPS producing microalgae and its potential applications
| Microalgae | Yield | Applications | References |
|---|---|---|---|
| Porphyridium sp. | 37% dry weight | Maintenance and modification of intestinal morphology, Hypocholesterolemic effect | Dvir et al. (2009) |
| Porphyridium cruentum | 50% dry weight | Anticancer, antiviral, antibacterial effects, immunomodulation | Sun et al. (2012) |
| Anabaena spiroides | 40% dry weight | Antithrombogenic, antiatherogenic, anticoagulant, metal binding, antibacterial, antioxidant, immunomodulation | Mona et al. (2015); Rafika et al. (2011) |
| Rhodella reticulata | Not available | Antioxidant, free radical scavenging effect | Chen et al. (2010) |
| Chlorella stigmatophora, Spirulina platensis, Nostoc sp. | 38% dry cell weight | Antibacterial, antiviral, metal binding, emulsification, flocculation, antioxidant | Yim et al. (2004); Freire-Nordi et al. (2005); Mona et al. (2015), Rafika et al. (2011); Han et al. (2016) |
Even though, EPS has many potential applications, the yield from microalgae is the major limitation in the scale-up industry. The type and concentration of EPS obtained from microalgae mainly depend on cultivation system design, nutrient and cultivation conditions, as well as extraction and purification process. Therefore, the usage of wastewater can solve this problem up to one extent.
Bioethanol
Bioethanol has been considered an alternative to conventional petroleum because of the same chemical and physical properties (Jayaseelan et al. 2021). Microalgal biomass has been attracted and great interest as a renewable and sustainable source for the production of bioenergy. Bioethanol made from microalgal biomass (3rd generation biofuel) is also an environmentally friendly beneficial fuel. Microalgae can store large amount of carbohydrates within the cells as triacylglycerol and starch under different conditions. The carbohydrates can be effectively used as a carbon source or substrate during fermentation for the production of bioethanol (Dexter et al. 2015). Microalgae are also able to accumulate protein along with carbohydrates and lipids under unfavorable conditions. Microalgae breakdown the complex nitrogen molecules into proteins. The difference in salinity, light intensity, temperature, and nutrient load can also accumulate carbohydrates. The absence of lignin and low hemicellulose levels makes hydrolysis and fermentation yields in microalgae more efficient (Gonzalez et al. 2018). Microalgae like Chlorella, Dunaliella, Scenedesmus, Arthrospira, and Spirulina have been used for the production of bioethanol (Schneidner et al. 2013). These microalgae are considered as potential candidates because of the high amount of starch and glycogen which are the important factors for the production of bioethanol.
In bioethanol production, the processes depend upon the type of biomass and selection of pretreatment, saccharification, fermentation, and product recovery. The pretreatment of the biomass is a crucial process because it is essential for the recovery of sugars used in the fermentation process. However, it is necessary to develop a well-designed and efficient system for the cultivation of microalgae that can remove compounds that cause impurities in the final product. Moreover, studies are needed to develop an efficient biorefinery system using wastewater for the production of bioethanol from microalgae.
Biochar
Microalgae are considered as the potent candidate for biochar production because of their sustainable and renewable biomass properties (Yu et al. 2017). Biochar conversion from microalgae is a promising approach that is associated with the development of value-added products after the production of biofuel from microalgae. Many reports are available on the pyrolysis of microalgae but studies that focus on the byproducts are very few. Algal biochar developed from the treatment of wastewater can be considered as a future technology by using biomass for the generation of carbon-negative energy with environmental applications (Yu et al. 2017). But the high nutrient content in microalgae is a major disadvantage in pyrolysis. One possible solution for this problem is the specific extraction of lipids from algae for bio-oil production and the remaining residues can be used for the production of biochar in the biorefinery. Microalgae have been considered as the potential feedstock for future sustainable energy sources without depending on fossil fuels and the cultivation of microalgae can be efficiently used to reduce the emission of greenhouse gases (Vassilev and Vassileva 2016).
Biochar derived from microalgae is gaining more importance because of its long-term advantage in carbon sequestration and soil amendment properties in agriculture. Photosynthesis helps in the carbon dioxide uptake in biochar technology and the captured carbon undergoes pyrolysis to synthesis biochar with specific long-term carbon storage using soil amendment (Yu et al. 2017). The biochar derived from biomass has more than 90% carbon. Moreover, the biochar derived from microalgae is rich in nitrogen and other nutrients, which can be used as a fertilizer in agriculture. All these advantages make possibilities of algal biochar in an economically feasible manner soon. However, till now, there is limited research data is available on the production of algal biochar and its utilization. Various possible bioproducts recovered from the sustainable wastewater-based microalgal biorefinery are represented in Fig. 2.
Fig. 2.
Sustainable microalgal biorefinery products coupled with food industrial wastewater
Research needs and perspectives
Mass production of microalgae for biorefinery must join with centralized food industrial wastewater for addressing sustainability. Microalgae cultivation system with wastewater treatment needs to be improved for the proper nutrient absorption and uptake mechanism also unraveled for better understanding of microalgae nutrients absorption from wastewater for target product for biorefinery. Biomass harvesting from the wastewater is still a challenging issue, bio-flocculation process needs to be focused on the possibilities of lucrative products and full-scale operation of microalgae for wastewater treatment. Microalgae research needs to be focused towards the possibility of self-flocculation of microalgae for harvesting of biomass and organic matter released into the medium as flocculation enhancing molecule and its mechanism of biomass flocculation need to be explore. Wastewater contains may contain toxic molecules that can be can be resolved by focusing the isolation of robust microalgal strains resistant to infection by other algal feeders and other microorganisms, capable of aggregate formation, the potential for auto-flocculations helps protection from microalgae feeders. Another challenging issue by the cultivation of microalgae in wastewater is their non-sterile environments stimulate the growth of algal feeders, protozoans, and others. Research could be directed towards co-culturing of microalgae obtained from food industrial wastewater could produce more biomass with stable growth, meanwhile, adapts to the ecological niches that avoids the possibilities of culture crash and contamination problems that helps in reducing the production cost and will improve wastewater treatment efficiently (Mohsenpour et al. 2021). Application study for microalgae strain improvement using the technology of gene editing would solve several existing issues with algal biomass and metabolites. Challenges of microalgae are need to be addressed by progress in the technological innovations at many supply chains to produce enough biomass feedstock and target products at profitable production costs to increase competitiveness in industry.
Conclusion
Microalgae cultivation in food industrial wastewater is advantageous to achieve the dual role of nutrients removal and low-cost biomass production. This review thoroughly summarized the different sources of food industrial effluents, with mass production of microalgae in various cultivation systems. The biomass harvesting process and processing of different bio-products conversions from the biomass were also discussed elaborately. Therefore, the selection of robust species could be very crucial for wastewater treatment, resistance to tolerate adverse environmental conditions, self-flocculation, and algal feeders with biomass generation for multi-biorefinery products. Integration of microalgae biomass production with wastewater generates renewable bioproducts and offers multiple benefits to the environment and energy sector.
Acknowledgements
SBU would like to thank the Director, IBSD for his motivation and support for contributing to this work.
Authors' contributions
SBU: Data collection, formal analysis, visualization, and writing the original manuscript. RS: Data collection, formal analysis, visualization, and writing the original manuscript. AU: Manuscript writing and correction. PY: Review and editing. AR: Review and editing. SJS: Conceptualization, review, and editing. AP: Conceptualization, review, Editing.
Funding
No funding was received to assist with the preparation of the manuscript.
Declarations
Competing interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors are mutually agreed to publish the work in this journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sabeela Beevi Ummalyma, Ranjna Sirohi have contributed equally and share co-first authors.
Contributor Information
Sang Jun Sim, Email: simsj@korea.ac.kr.
Ashok Pandey, Email: ashok.pandey1@iitr.res.in.
References
- Acien FG, Gomez-Serrano C, Morales-Amaral MM, Fernandez-Sevilla JM, Molina-Grima E. Wastewater treatment using microalgae How realistic a contribution might it be to significant urban wastewater treatment? Appl Microbiol Biotechnol. 2016;100(21):9013–9022. doi: 10.1007/s00253-016-7835-7. [DOI] [PubMed] [Google Scholar]
- Acien-Fernandez FG, Gomez-Serrano C, Fernandez-Sevilla JM. Recovery of nutrients from wastewaters using Microalgae. Front. Sustain Food Syst. 2018;2:1–13. doi: 10.3389/fsufs.2012.00059. [DOI] [Google Scholar]
- Ajijah N, Tjandra B, Hamidah U, Sintawardani N (2020) Utilization of tofu wastewater as a cultivation medium for Chlorella vulgaris and Arthrospira platensis In: IOP Conference Series: Environ Earth Sci IOP Publishing: 012027. 10.1088/1755-1315/483/1/012027
- Amenorfenyo DK, Huang X, Zhang Y, Zhang Y, Zeng Q, Zhang N, Ren J, Huang Q. Microalgae brewery wastewater treatment: Potentials benefits and the challenges. Int J Environ Res Public Health. 2019;16(11):191. doi: 10.3390/ijerph16111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A, Al Bazedi G, Abdel-Fatah MA. Experimental study and mathematical model of coagulation/sedimentation units for treatment of food processing wastewater. Ain Shams Eng J. 2021;12(1):195–203. doi: 10.1016/jasej.2020.08.001. [DOI] [Google Scholar]
- Apandi N, Mohamed RMSR, Al-Gheethi A, Gani P, Ibrahim A, Kassim AHM. Scenedesmus biomass productivity and nutrient removal from wet market wastewater. A Bio-Kinetic Study Waste Biomass Valori. 2018;10(10):2783–2800. doi: 10.1007/s12649-018-0313-y. [DOI] [Google Scholar]
- Arantes MK, Alves HJ, Sequinel R, da Silva EA. Treatment of brewery wastewater and its use for biological production of methane and hydrogen. Int J Hydrogen Energ. 2017;42:26243–26256. doi: 10.1016/j.ijhydene.2017.08.206. [DOI] [Google Scholar]
- Araujo GS, Santiago CS, Moreira RT, DantasNeto MP, Fernandes FAN. Nutrient removal by Arthrospira platensis cyanobacteria in cassava processing wastewater. Water Process Eng. 2020;40:101826. doi: 10.1016/j.jwpe.2020.101826. [DOI] [Google Scholar]
- Aziz A, Basheer F, Sengar A, Irfanullah KSU, Farooqi IH. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci Total Environ. 2019;286:681–708. doi: 10.1016/j.scitotenv.2019.05.295. [DOI] [PubMed] [Google Scholar]
- Bafanaa A. Characterization and optimization of production of exopolysaccharide from Chlamydomonas reinhardtii. Carbohydr Polym. 2013;95:746–752. doi: 10.1016/j.carbpol.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Balaraman HB, Sivasubramanian A, Rathnasamy SK. Sustainable valorization of meat processing waste water with synergetic eutectic mixture based purification of R-Phycoerythrin from Porphyrium cruentium. Bioresour Technol. 2021;336:125357. doi: 10.1016/j.biortech.2021.125357. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Ramaswamy S. Comparison of productivity and economic analysis of microalgae cultivation in open raceways and flat panel photobioreactor. Bioresour Technol Rep. 2019;8:100328. doi: 10.1016/j.biteb.2019.100328. [DOI] [Google Scholar]
- Barsanti L, Gualtieri P. Algae: anatomy, biochemistry, and biotechnology. CRC Press Boca Raton J Phycol. 2006;43(2):412–414. doi: 10.1111/j.1529-8817.2007.00335.x. [DOI] [Google Scholar]
- Bethi CMS, Narayan B, Martin A, Kudre TG. Recovery, physicochemical and functional characteristics of proteins from different meat processing wastewater streams. Environ Sci Pollut Res. 2020;27(20):25119–25131. doi: 10.1007/s11356-020-08930. [DOI] [PubMed] [Google Scholar]
- Bezerra RP, Matsudo MC, Perez-Mora LS, Sato S, Carvalho JCM. Ethanol effect on batch and fed-batch Arthrospira platensis growth. J Ind Microbiol Biotechnol. 2014;41:687–692. doi: 10.1007/s10295-014-1404-9. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Mehariya S, Bhatia RK, Kumar M, Pugazhendhi A, Awasthi MK, Atabani AE, Kumar G, Kim W, Seo SO, Yang YH. Wastewater based microalgal biorefinery for bioenergy production: progress and challenges. Sci Total Environ. 2021;751:141599. doi: 10.1016/j.scitotenv.2020.141599. [DOI] [PubMed] [Google Scholar]
- Boland MJ, Rae AN, Vereijken JM, Meuwissen MPM, Fischer ARH, Van Boekel MAJS, Rutherfurd SM, Gruppen H, Moughan PJ, Hendriks WH. The future supply of animal-derived protein for human consumption. Trends Food Sci Tech. 2013;29:62–73. doi: 10.1016/j.tifs.2012.07.002. [DOI] [Google Scholar]
- Boyle NR, Morgan JA. Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii. BMC SystBiol. 2009;3(1):4. doi: 10.1186/1752-0509-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano NS, Martins AA, Gorgich M, Gutiérrez DM, Ribeiro TJ, Mata TM. Flocculation of Arthrospira maxima for improved harvesting. Energ Rep. 2020;6:423–428. doi: 10.1016/j.egyr.2019.08.083. [DOI] [Google Scholar]
- Cheah WY, Show PL, Juan JC, Chang JS, Ling TC. Microalgae cultivation in palm oil mill effluent (POME) for lipid production and pollutants removal. Energ Convers Manage. 2018;174:430–438. doi: 10.1016/j.enconman.2018.08.057. [DOI] [Google Scholar]
- Cheirsilp B, Thawechai T, Prasertsan P. Immobilized oleaginous microalgae for production of lipid and phytoremediation of secondary effluent from palm oil mill in fluidized bed photobioreactor. Bioresour Technol. 2017;241:787–794. doi: 10.1016/j.biortech.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Chen B, You W, Huang J, Yu Y, Chen W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J Microbiol Biotechnol. 2010;26:833–840. doi: 10.1007/s11274-009-0240-y. [DOI] [Google Scholar]
- Chen H, Zheng Y, Zhan J, He C, Wang Q. Comparative metabolic profiling of the lipid-production green microalga Chlorella reveals that nitrogen and carbon metabolic pathways contribute to lipid metabolism. Biotechnol Biofuels. 2017;10(1):153. doi: 10.1186/s13068-017-0839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YW, Chong CC, Lam MK, Leong WH, Chuah LF, Yusup S, Setiabudi HD, Tang Y, Lim JW. Identification of microbial inhibitions and mitigation strategies towards cleaner bioconversions of palm oil mill effluent (POME): a review. J Clean Prod. 2020;280(1):124346. doi: 10.1016/j.jclepro.2020.124346. [DOI] [Google Scholar]
- Chinnasamy S, Bhatnagar A, Hunt RW, Das KC. Microalgae cultivation in a from poultry litter anaerobic digestion. BioresourTechnol. 2010;102:10841–10848. [Google Scholar]
- Chu HQ, Tan XB, Zhang YL, Yang LB, Zhao FC, Guo J. Continuous cultivation of Chlorella pyrenoidosa using anaerobic digested starch processing wastewater in the outdoors. Bioresour Technol. 2015;185:40–48. doi: 10.1016/j.biortech.2015.02.030. [DOI] [PubMed] [Google Scholar]
- Cuaresma M, Casal C, Forjan E, Vílchez C. Productivity and selective accumulation of carotenoids of the novel extremophile microalga Chlamydomonas acidophila grown with different carbon sources in batch systems. J IndMicrobiol Biotechnol. 2011;38(1):167–177. doi: 10.1007/s10295-010-0841-3. [DOI] [PubMed] [Google Scholar]
- Culaba AB, Ubando AT, Ching PML, Chen WH, Chang JS. Biofuel from microlage: sustainable pathways. Sustainability. 2020;12(19):8009. doi: 10.3390/su12198009. [DOI] [Google Scholar]
- D’Alesandro EB, Antoniosifilho NR. Concepts and studies on lipid and pigments of microalgae: a review. Renew SustEnerg Rev. 2016;58:832–841. doi: 10.1016/j.rser.2015.12.162. [DOI] [Google Scholar]
- Daneshvar E, Antikainen L, Koutra E, Kornaros M, Bhatnagar A. Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: treatment of wastewater and lipid extraction. Bioresour Technol. 2018;255:104–110. doi: 10.1016/j.biortech.2018.01.101. [DOI] [PubMed] [Google Scholar]
- de Carvalho JC, Borghetti IA, Cartas LC, Woiciechowski AL, Soccol VT, Soccol CR. Biorefinery integration of microalgae production into cassava processing industry: potential and perspectives. Bioresour Technol. 2018;247:1165–1172. doi: 10.1016/j.biortech.2017.09.213. [DOI] [PubMed] [Google Scholar]
- Dertli E, Mayer MJ, Narbad A. Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillu sjohnsonii FI9785. BMC Microbiol. 2015;15:8. doi: 10.1186/s12866-015-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter J, Armshaw P, Sheahan C, Pembroke JT. The state of autotrophic ethanol production in cyanobacteria. J Appl Microbiol. 2015;119:11–24. doi: 10.1111/jam.12821. [DOI] [PubMed] [Google Scholar]
- Ding GT, Mohd Yasin NH, Takriff MS, Kamarudin KF, Salihon J, Yaakob Z, Nazashida Mohd Hakimi NI. Phycoremediation of palm oil mill effluent (POME) and CO2 fixation by locally isolated microalgae: Chlorella sorokiniana UKM2, Coelastrella sp. UKM4 and Chlorella pyrenoidosa UKM7. J Water Process Eng. 2020;35:101202. doi: 10.1016/j.jwpe.2020.101202. [DOI] [Google Scholar]
- Droop MR. Heterotrophy of carbon. In: Stewart WDP, editor. Algal physiology and biochemistry. Oxford: Blackwell Scientific; 1974. pp. 530–559. [Google Scholar]
- Dvir I, Stark AH, Chayoth R, Madar Z, Arad SM. Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients. 2009;1(2):156–167. doi: 10.3390/nu1020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Graf A, Hernandez S, Morales M. Biomitigation of CO2 from flue gas by Scenedesmus obtusiusculus AT-UAM using a hybrid photobioreactor coupled to a biomass recovery stage by electro-coagulation-flotation. Environ Sci Pollut Res. 2020;27:28561–28574. doi: 10.1007/s11356-020-08240-2. [DOI] [PubMed] [Google Scholar]
- Farooq W, Lee YC, Ryu BG, Kim BH, Kim HS, Choi YE, Yang JW. Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. Bioresour Technol. 2013;132:230–238. doi: 10.1016/j.biortech.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Feldmane J, Semjonovs P, Ciprovica I. Potential of exopolysaccharides in yoghurt production. Int J BiolBiomol Agric Food Biotechnol Eng. 2013;7:8. doi: 10.5281/zenodo.1086547. [DOI] [Google Scholar]
- Fernandez E, Galvan A. Inorganic nitrogen assimilation in Chlamydomonas. Exp Bot. 2007;58:2279–2287. doi: 10.1093/jxb/erm106. [DOI] [PubMed] [Google Scholar]
- Fernando JSR, Premaratne M, Dinalankara DMSD, Perera JLNJ, Ariyadasa TU. Cultivation of microalgae in palm oil mill effluent (POME) for astaxanthin production and simultaneous phycoremediation. Environ Chem Eng. 2021;9(4):105375. doi: 10.1016/j.jece.2021.105375. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations (2018) Meat Market Review http://www.fao.org/3/I9286EN/i9286en
- Freire-Nordi CS, Vieira AAH, Nascimento OR. The metal binding capacity of Anabaena spiroides extracellular polysaccharide. An EPR study. Proc Biochem. 2005;40:2215–2224. doi: 10.1016/j.procbio.2004.09.003. [DOI] [Google Scholar]
- Gecim G, Aydin G, Tavsanoglu T, Erkoc E, Kalemtas A. Review on extraction of polyhydroxyalkanoates and astaxanthin from food and beverage processing wastewater. Water Process Eng. 2021;40:101775. doi: 10.1016/j.jwpe.2020.101775. [DOI] [Google Scholar]
- Ghimpusan M, Nechifor G, NechiforA DSO, Passeri P. Case studies on the physical-chemical parameters' variation during three different purification approaches destined to treat wastewaters from food industry. J Environ Manage. 2017;203:811–816. doi: 10.1016/j.jenvman.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Gonzalez LM, Correa DF, Ryan S, Jensen PD, Pratt S, Schenk PM. Integrated biodiesel and biogas production from microalgae. Towards a sustainable closed loop through nutrient recycling. Renew Sustain Energy Rev. 2018;82:1137–1148. doi: 10.1016/j.rser.2017.09.091. [DOI] [Google Scholar]
- Gramegna G, Scortica A, Scafati V, Ferella F, Gurrieri L, Giovannoni M, Bassi R, Sparla F, Mattei B, Benedetti M. Exploring the potential of microalgae in the recycling of dairy wastes. Bioresour Technol Rep. 2020;12:100604. doi: 10.1016/j.biteb.2020.100604. [DOI] [Google Scholar]
- Gupta S, Pawar SB. An integrated approach for microalgae cultivation using raw and anaerobic digested wastewaters from food processing industry. Bioresour Technol. 2018;259:571–576. doi: 10.1016/j.biortech.2018.08.113. [DOI] [PubMed] [Google Scholar]
- Han PP, Sun Y, Wu XY, Yuan YJ, Dai YJ, Jia SR. Emulsifying flocculating and physicochemical properties of exopolysaccharide produced by cyanobacterium Nostoc flagelliforme. Appl Biochem Biotechnol. 2016;172:36–49. doi: 10.1007/s12010-013-0505-7. [DOI] [PubMed] [Google Scholar]
- Han X, Hu X, Yin Q, Li S, Song C. Intensification of brewery wastewater purification integrated with CO2 fixation via microalgae co-cultivation. J Environ ChemEng. 2021;9(4):105710. doi: 10.1016/j.jece.2021.105710. [DOI] [Google Scholar]
- Hemalatha M, Sravan JS, Min B, Venkata Mohan S. Microalgae-biorefinery with cascading resource recovery design associated to dairy wastewater treatment. Bioresour Technol. 2019;284:424–429. doi: 10.1016/j.biortech.2019.03.106. [DOI] [PubMed] [Google Scholar]
- Hende SVD, Beelen V, Julien L, Lefoulon A, Vanhoucke T, Coolsaet C, Sonnenholzner S, Vervaeren H, Rousseau DPL. Technical potential of microalgal bacterial floc raceway ponds treating food-industry effluents while producing microalgal bacterial biomass: An outdoor pilot-scale study. Bioresour Technol. 2016;218:969–979. doi: 10.1016/j.biortech.2016.07.065. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Riano B, Coca M, Solana M, Bertucco A, Garcia-Gonzalez MC. Microalgae cultivation in high rate algal ponds using slaughterhouse wastewater for biofuel applications. Chem Eng. 2016;285:449–458. doi: 10.1016/j.cej.2015.09.072. [DOI] [Google Scholar]
- Hernández-García A, Velásquez-Orta SB, Novelo E, Yáñez-Noguez I, Monje-Ramírez I. Ledesma MTO (2019) Wastewaterleachate treatment by microalgae: Biomass, carbohydrate and lipid production. Ecotoxicol Environ Saf. 2019;174:435–444. doi: 10.1016/j.ecoenv.2019.02.052. [DOI] [PubMed] [Google Scholar]
- Hilares RT, Bustos KAG, Vera FPS, Andrade GJC, Tanaka DAP. Acid precipitation followed by Microalgae (Chlorella vulgaris) cultivation as a new approach for poultry slaughter house wastewater treatment. Bioresour Technol. 2021;335:125284. doi: 10.1016/j.biortech.2021.125284. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Lee CG. Evaluation of central metabolism based on a genomic database of SynechocystisPCC6803. Biotechnol Bioprocess Eng. 2007;12:165–173. doi: 10.1007/BF03028644. [DOI] [Google Scholar]
- Hu X, Meneses YE, Stratton J, Wang B. Acclimation of consortium of micro-algae help removal of organic pollutants from meat processing wastewater. Clean Prod. 2019;214:95–102. doi: 10.1016/j.jclepro.2018.12.255. [DOI] [Google Scholar]
- Humaidah N, Nakai S, Nishijima W, Gotoh T, Furuta M. Application of Aurantiochytrium sp. L3Wfor food-processing waste water treatment in combination with polyunsaturated fatty acids production for fish aquaculture. Sci Total Environ. 2020;743:140735. doi: 10.1016/j.scitotenv.2020.140735. [DOI] [PubMed] [Google Scholar]
- Hussain F, Shah SZ, Ahmad H, Abubshait SA, Abubshait HA, Laref A, Manikandan A, Kusuma HS, Iqbal M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production: a review. Renew Sustain Energy Rev. 2021;137:110603. doi: 10.1016/j.rser.2020.110603. [DOI] [Google Scholar]
- Jayaseelan M, Usman M, Somanathan A, Palani S, Muniappan G, Jeyakumar RB. Microalgal production of biofuels integrated with wastewater treatment. Sustainability. 2021;13(16):8797. doi: 10.3390/su13168797. [DOI] [Google Scholar]
- Joun J, Hong ME, Sirohi R, Sim SJ. Enhanced biomass production through a repeated sequential auto-and heterotrophic culture mode in Chlorella protothecoides. Bioresour Technol. 2021;338:125532. doi: 10.1016/j.biortech.2021.125532. [DOI] [PubMed] [Google Scholar]
- Kebede TB. Waste water treatment in brewery industry Review. Int J Eng Dev Res. 2018;6:716–722. [Google Scholar]
- Kim D, Hwang T, Oh Y, Han J. Harvesting Chlorella sp. KR-1 using cross-flow electro-filtration. Algal Res. 2014;6:170–174. doi: 10.1016/j.algal.2014.10.004. [DOI] [Google Scholar]
- Komolafe O, Orta SBV, Monje-Ramirez I, Noguez IY, Harvey A, Ledesma MTO. Biodiesel production from indigenous microalgae grown in wastewater. Bioresour Technol. 2014;154:297–304. doi: 10.1016/j.biortech.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Krasaesueb N, Incharoensakdi A, Khetkorn W. Utilization of shrimp wastewater for poly-β-hydroxybutyrate production by Synechocystis sp. PCC 6803 strain ΔSphU cultivated in photobioreactor. Biotechnol Rep. 2019;23:00345. doi: 10.1016/j.btre.2019.e00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea PJ, Miflin BJ. Glutamate synthase and the synthesis of glutamate in plants. Plant PhysiolBiochem. 2003;41:555–564. doi: 10.1016/S0981-9428(03)00060-3. [DOI] [Google Scholar]
- Lee GI, ShinWS Jung SMG, Kim W, LeeC KwonJH. Effects of soybean curd wastewater on growth and DHA production in Aurantiochytrium sp. LWT. 2020;134:110245. doi: 10.1016/j.lwt.2020.110245. [DOI] [Google Scholar]
- Leong HY, Chang CK, Khoo KS, Chew KW, Chia RS, Lim JW, Show PL. Waste biorefinery towards a sustainable circular bioeconomy: a solution to global issues. Biotechnol Biofuels. 2021;14(1):1–15. doi: 10.1186/s13068-021-01939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shi W, Du Q, Zhou R, Zeng X, Zhang H, Qin X. Recovery and purification of potato proteins from potato starch wastewater by hollow fiber separation membrane integrated process. Innov Food Sci Emerg Technol. 2020;63:102380. doi: 10.1016/j.ifset.2020.102380. [DOI] [Google Scholar]
- Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W, Huo S, Cheng P, Liu J, Addy M, Chen P, Chen D, Ruan R. Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour Technol. 2019;291:121934. doi: 10.1016/j.biortech.2019.121934. [DOI] [PubMed] [Google Scholar]
- Liu J, Huang J, Jiang Y, Chen F. Molasses-based growth and production of oil and astaxanthin by Chlorella zofingiensis. Bioresour Technol. 2012;107:393–398. doi: 10.1016/j.biortech.2011.12.047. [DOI] [PubMed] [Google Scholar]
- Liu W, Chen Y, Wang J, Liu T. Biomass productivity of Scenedesmus dimorphus (Chlorophyceae) was improved using an open pond-photobioreactor systems. Eur J Phycol. 2018 doi: 10.1080/09670262.2018.1519601. [DOI] [Google Scholar]
- Ma C, Wen H, Xing D, Pei X, Zhu J, Ren N, Liu B. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol Biofuels. 2017;10:111. doi: 10.1186/s13068-017-0797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrini SY, Robert JM, Leftley JW, Collos Y. Ammonium thresholds for simultaneous uptake of ammonium and nitrate by oyster-pond algae. Exp Mar Biol Ecol. 1986;102:75–98. doi: 10.1016/0022-0981(86)90127-9. [DOI] [Google Scholar]
- Mar CC, Fan Y, Li FL, Hu GR. Bioremediation of wastewater from edible oil refinery factory using oleaginous microalga Desmodesmus sp. S1. Int J Phytoremediation. 2016;18:1195–1201. doi: 10.1080/15226514.2016.1193466. [DOI] [PubMed] [Google Scholar]
- Martinez ME, Jimenez JME, Yousfi F. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour Technol. 1999;67:233–240. doi: 10.1016/S0960-8524(98)00120-5. [DOI] [Google Scholar]
- Matsudo MC, Sousa TF, Perez-Mora LS, Bezerra RP, Sato S, Carvalho JCM. Ethanol as complementary carbon source in Scenedesmus obliquus cultivation. J Chem Technol Biotechnol. 2016;92(4):781–786. doi: 10.1002/jctb.5059. [DOI] [Google Scholar]
- Meneses YE, Stratton J, Flores RA. Water reconditioning and reuse in the food processing industry: current situation and challenges. Trends Food Sci Tech. 2017;61:72–79. doi: 10.1016/j.tifs.2016.12.008. [DOI] [Google Scholar]
- Michiel MHA, Vaskoska M, Vermuë MH, Wijffels RH. Growth of Tetraselmis suecica in a tubular photobioreactor on wastewater from a fish farm. Water Res. 2014;65:290–296. doi: 10.1016/j.watres.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Min M, Wang L, Li Y, Mohr MJ, Zhou HuBW, Chen P, Ruan R. Cultivating Chlorella sp. in a pilot-scale photobioreactor using centrate wastewater for microalgae biomass production and wastewater nutrient removal. Appl Biochem Biotechnol. 2011;165:123–137. doi: 10.1007/s12010-011-9238-7. [DOI] [PubMed] [Google Scholar]
- Mohd Udaiyappan AF, Hasan HA, Takriff MS, Abdullah SRS, Maeda T, Mustapha NA, Mohd Yasin NH, NazashidaMohd Hakimi NI. Microalgae-bacteria interaction in palm oil mill effluent treatment. J Water Process Eng. 2020;35:101203. doi: 10.1016/j.jwpe.2020.101203. [DOI] [Google Scholar]
- Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T. Integrating microalgae into wastewater treatment: a review. Sci Total Environ. 2021;752:142168. doi: 10.1016/j.scitotenv.2020.142168. [DOI] [PubMed] [Google Scholar]
- Mona S, Kaushik A. Chromium and cobalt sequestration using exopolysaccharides produced by freshwater cyanobacterium Nostoclinckia. Ecol Eng. 2015;82:121–125. doi: 10.1016/j.ecoleng.2015.04.037. [DOI] [Google Scholar]
- Morales-Sánchez D, Martinez-Rodriguez OA, Kyndt J, Martinez A. Heterotrophic growth of microalgae: metabolic aspects. World J Microbiol Biotechnol. 2015;31:1–9. doi: 10.1007/s11274-014-1773-2. [DOI] [PubMed] [Google Scholar]
- Narala RR, Garg S, Sharma KK, Thomas-Hall SR, Deme M, Li Y, Schenk PM. Comparison of microalgae cultivation in photobioreactor open raceway pond and a two-stage hybrid system. Front Energ Res. 2016 doi: 10.3389/fenrg.2016.00029. [DOI] [Google Scholar]
- Ndikubwimana T, Zeng X, Murwanashyaka T, Manirafasha E, He N, Shao W, Lu Y. Harvesting freshwater microlage with microbial bioflocculant: a pilotscale study. Biotechol Biofuels. 2016;9:47. doi: 10.1186/s13068-016-0458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur MMA, Buma AGJ. Opportunities and challenges of microalgal cultivation on wastewater, with special focus on palm oil mill effluent and the production of high value compounds. Waste Biomass Valori. 2019;10:2079–2097. doi: 10.1007/s12649-018-0256-3. [DOI] [Google Scholar]
- Ono K, Kawanaka Y, Izumi Y, Inui H, Miyatake K, Kitaoka S, Nakano Y. Mitochondrial Alcohol Dehydrogenase from Ethanol-Grown Euglena gracilis. J Biochem. 1995;117:1178–1182. doi: 10.1093/oxfordjournals.jbchem.a124841. [DOI] [PubMed] [Google Scholar]
- Pagels F. Guedes AC, Amaro HM. Kijjoa A, Vasconcelos V (2019) Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. BiotechnolAdv37(3): 422– 443.10.1016/j.biotechadv.2019.02.010 [DOI] [PubMed]
- Patel AK, Joun J, Sim SJ. A sustainable mixotrophic microalgae cultivation from dairy wastes for carbon credit, bioremediation and lucrative biofuels. Bioresour Technol. 2020;313:123681. doi: 10.1016/j.biortech.2020.123681. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia O, Escalante FME, deBashan LE, Bashan Y. Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 2011;45:11–36. doi: 10.1016/j.watres.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Qadir M, Drechsel P, Cisneros BJ, Kim Y, Pramanik A, Mehta P, Olaniyan O. Global and regional potential of wastewater as a water nutrient and energy source. Nat Resour Forum. 2020;44:40–51. doi: 10.1111/1477-8947.12187. [DOI] [Google Scholar]
- Qin L, Wang Z, Sun Y, Shu Q, Feng P, Zhu L, Xu J, Yuan Z. Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ Sci Pollut Res. 2016;23:8379–8838. doi: 10.1007/s11356-015-6004-3. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zu Y, Song C, Xie M, Qi Y, Kansha Y, Kitamura Y. Soybean processing wastewater purification via Chlorella L166 and L38 with potential value-added ingredients production. Bioresour Technol Rep. 2019;7:100195. doi: 10.1016/j.biteb.2019.100195. [DOI] [Google Scholar]
- Rafika C, Lamia T, Rym BD, Omeya EA, Ali Y, Khamissa G, Jihen BA, Hela O, Ouada HB. Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by Cyanobacterium Arthrospira platensis. Braz Arch Biol Technol. 2011;54:831–838. doi: 10.1590/S1516-89132011000400024. [DOI] [Google Scholar]
- Ramanna L, Guldhe A, Rawat I, Bux F. The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol. 2014;168:127–135. doi: 10.1016/j.biortech.2014.03.064. [DOI] [PubMed] [Google Scholar]
- Ren HY, Liu BF, Kong F, Zhao L, Ma L, Ren NQ. Favorable energy conversion efficiency of coupling dark fermentation and microalgae production from food waste. Energ Conver Manag. 2018;166:156–162. doi: 10.1016/j.enconman.2018.04.032. [DOI] [Google Scholar]
- Rinanti A, Purwadi R (2018) Harvesting of freshwater microalgae biomass by Scenedesmus sp. as bioflocculant. In: IOP conference series: earth and environmental science, vol 106, no 1. IOP Publishing, p 012087
- Rodrigues DB, Flores EM, Barin JS, Mercadante AZ, Jacob-Lopes E, Zepka LQ. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res Int. 2014;65:144–148. doi: 10.1016/j.foodres.2014.06.037. [DOI] [Google Scholar]
- Rogers JN, Rosenberg JN, Guzman BJ, Oh VH, Mimbela LE, Ghassemi A, Betenbaugh MJ, Oyler GA, Donohuge MD. A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res. 2014;4:76–88. doi: 10.1016/j.algal.2013.11.007. [DOI] [Google Scholar]
- Salama ES, Kurade MB, Abou-Shanab RAI, El-Dalatony MM, Yang IS, Min B, Jeon BH. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew SustEnerg Rev. 2017;79:1189–1211. doi: 10.1016/j.rser.2017.05.091. [DOI] [Google Scholar]
- Sato T, Qadir M, Yamamoto S, Endo T, Zahoor A. Global regional and country level need for data on wastewater generation treatment and use. Agric Water Manag. 2013;130:1–13. doi: 10.1016/j.agwat.2013.08.007. [DOI] [Google Scholar]
- Schneider RC, Bjerk TR, Gressler PD, Souza MP, Corbellini VA, Lobo EA (2013) Potential production of biofuel from microalgae biomass produced in wastewater. Biodiesel–feedstocks production and applications. 10.5772/52439
- Sharma P, Gaur VK, Sirohi R, Varjani SJ, Kim SH, Wong JWC. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour Technol. 2021;325:124684. doi: 10.1016/j.biortech.2021.124684. [DOI] [PubMed] [Google Scholar]
- Shen Y, Yuan W, Pei Z, Wu Q, Mao M (2009) Microalgae mass production methods. Trans ASABE 52. 10.13031/2013.27771
- Shen XF, Gao LJ, Zhou SB, Huang JL, Wu CZ, Qin QW, Zeng RJ. High fatty acid productivity from Scenedesmus obliquus in heterotrophic cultivation with glucose and soybean processing wastewater via nitrogen and phosphorus regulation. Sci Total Environ. 2020;708:134596. doi: 10.1016/j.scitotenv.2019.134596. [DOI] [PubMed] [Google Scholar]
- Singh A, Ummalyma SB, Sahoo D. Bioremediation and biomass production of microalgae cultivation in river water contaminated with pharmaceutical effluent. Bioresour Technol. 2020;307:123233. doi: 10.1016/j.biortech.2020.123233. [DOI] [PubMed] [Google Scholar]
- Singh G, Patidar SK. Microalgae harvesting techniques: a review. Environ Manhage. 2018;217:499–508. doi: 10.1016/j.envman.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Sirohi R, Joun J, Hong ME, Gaur V, Sim SJ. Algal glycol biotechnology: omics approaches for strain improvement. Microbial Cell Fact. 2021;21(1):163. doi: 10.1186/s12934-021-01656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi R, Lee JS, Yu BS, Roh H, Sim SJ. Sustainable production of polyhydroxybutyrate from autotrophs using CO2 as feedstock: challenges and opportunities. Bioresour Technol. 2021;341:125751. doi: 10.1016/j.biortech.2021.125751. [DOI] [PubMed] [Google Scholar]
- Song C, Hu X, Liu Z, Li S, Kitamura Y. Combination of brewery wastewater purification and CO2 fixation with potential value-added ingredients production via different microalgae strains cultivation. J Clean Prod. 2020;68:122332. doi: 10.1016/j.jclepro.2020.122332. [DOI] [Google Scholar]
- Spennati E, Casazza AA, Converti A. Winery wastewater treatment by microalgae to produce low-cost biomass for energy production purposes. Energies. 2020;13(10):2490. doi: 10.3390/en13102490. [DOI] [Google Scholar]
- Su Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci Total Environ. 2021;762:144590. doi: 10.1016/j.scitotenv.2020.144590. [DOI] [PubMed] [Google Scholar]
- Subha C, Kumar MD, Kannah RY, Kavitha S, Gunasekaran M, Banu JR (2020) Bioenergy recovery from food processing wastewater microbial fuel cell In: Banu RJ, Kumar G, Gunasekaran M, Kavitha (Eds) Food Waste to Valuable Resources. Academic press, pp. 251–274.10.1016/B978-0-12-818353-3.00012-2
- Sukumaran P, Nulit R, Zulkifly S, Halimoon N, Omar H, Ismail A. Potential of fresh POME as a growth medium in mass production of Arthrospira platensis. Int J Curr Microbiol App Sci. 2014;3(4):235–250. [Google Scholar]
- Sun L, Wang L, Zhou Y. Immunomodulation and antitumor activities of different molecular weight polysaccharides from Porphyridium cruentum. Carbohydr Polym. 2012;87:1206–1210. doi: 10.1016/j.carbpol.2011.08.097. [DOI] [Google Scholar]
- Sun X, Ren L, Zhao Q, Zhang L, Huang H. Application of chemicals for enhancing lipid production in microalgae a short review. Bioresour Technol. 2019;293:122135. doi: 10.1016/j.biortech.2019.122135. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen Z, Wu G, Wu Q, Zhang F, Niu Z, Hu HY. Characteristics of water quality of municipal wastewater treatment plants in China: implications for resources utilization and management. J Clean Prod. 2016;131:1–9. doi: 10.1016/j.jclepro.2016.05.068. [DOI] [Google Scholar]
- SundarRajan P, Gopinath KP, Greetham D, Antonysamy AJ. A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. J Clean Prod. 2019;210:445–458. doi: 10.1016/j.jclepro.2018.11.010. [DOI] [Google Scholar]
- Swain P, Tiwari A, Pandey A. Enhanced lipid production in Tetraselmis sp. by two stage process optimization using simulated dairy wastewater as feedstock. Biomass Bioenerg. 2020;139(2):105643. doi: 10.1016/j.biombioe.2020.105643. [DOI] [Google Scholar]
- Tan JS, Lee SY, Chew KW, Lam MK, Lim JW, Ho SH, Show PL. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioeng. 2020;11:116–129. doi: 10.1080/21655979.2020.1711626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Zhao X, Zhang Y, Zhou Y, Yang L, Zhang W. Enhanced lipid and biomass production using alcohol wastewater as carbon source for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour Technol. 2017;247:784–793. doi: 10.1016/j.biortech.2017.09.152. [DOI] [PubMed] [Google Scholar]
- Tan XB, Wan XP, Yang LB, Wang X, Meng J, Jiang MJ, Pi HJ. Nutrients recycling and biomass production from Chlorella pyrenoidosa culture using anaerobic food processing wastewater in a pilot-scale tubular photobioreactor. Chemosphere. 2021;270:129459. doi: 10.1016/j.chemosphere.2020.129459. [DOI] [PubMed] [Google Scholar]
- Tan XB, Zhao XC, Yang LB. Strategies for enhanced biomass and lipid production by Chlorella pyrenoidosa culture in starch processing wastewater. J Clean Prod. 2019;236:117671. doi: 10.1016/j.jclepro.2019.117671. [DOI] [Google Scholar]
- Tan XB, Zhao XC, Zhang YL, Zhou YY, Yang LB, Zhang WW. Enhanced lipid and biomass production using alcohol wastewater as carbon source for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour Technol. 2018;247:784–793. doi: 10.1016/j.biortech.2017.09.152. [DOI] [PubMed] [Google Scholar]
- Tanoi T, Kawachi M, Watanabe MM. Effects of carbon source on growth and morphology of Botryococcusbraunii. Appl Phycol. 2011;23:25–33. doi: 10.1007/s10811-010-9528-4. [DOI] [Google Scholar]
- Trabelsi L, Chaieb O, Mnari A, Abid-Essafi S, Aleya L. Partial characterization and antioxidant and antiproliferative activities of the aqueous extracellular polysaccharides from the thermophilic microalgae Graesiella sp. BMC Complement. Altern Med. 2016;16:210. doi: 10.1186/s12906-016-1198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayan A, Arumugam M. Selective enrichment of Eicosapentaenoic acids (20:5n–3) in N.oceanica CASA CC201 by natural auxin supplementation. Bioresour Technol. 2017;310:123437. doi: 10.1016/j.biortech.2017b.03.149. [DOI] [PubMed] [Google Scholar]
- Udayan A, Arumugam M, Pandey A (2017) Nutraceuticals from algae and cyanobacteria. In: Rastogi RP, Madamwar D, Pandey A (eds) Algal green chemistry. Elsevier, pp 65–89. 10.1016/B978-0-444-63784-0.00004-7
- Udayan A, Kathiresan S, Arumugam M. Kinetin and Gibberellic acid (GA3) act synergistically to produce high value polyunsaturated fatty acids in Nannochloropsis oceanica CASACC201. Algal Res. 2018;32:182–192. doi: 10.1016/j.algal.2018.03.007. [DOI] [Google Scholar]
- Udayan A, Pandey AK, Sharma P, Sreekumar N, Kumar S. Emerging industrial applicaions of algae: challenges and future perspectives. Sys Microbiol and Biomanuf. 2021 doi: 10.1007/s43393-021-00038-8. [DOI] [Google Scholar]
- Udayan A, Sabapathy H, Arumugam M. Stress hormones mediated lipid accumulation and modulation of specific fatty acids in Nannochloropsis oceanica CASA CC201. Bioresour Technol. 2020;310:123437. doi: 10.1016/j.biortech.2020.123437. [DOI] [PubMed] [Google Scholar]
- Ummalyma SB, Sukumaran RK. Cultivation of microalgae in dairy effluent for oil production and removal of organic pollution load. Bioresour Technol. 2014;165:295–301. doi: 10.1016/j.biortech.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Ummalyma SB, Sukumaran RK. Cultivation of freshwater microalga Chlorococcum sp. RAP13 in sea water for producing oil suitable for biodiesel. Appl Phycol. 2015;27:141–147. doi: 10.1007/s10811-014-0340-4. [DOI] [Google Scholar]
- Ummalyma SB, Gnansounou E, Sukumaran RK, Sindhu R, Pandey A, Sahoo D. Bioflocculation: an alternative strategy for harvesting of microalgae. an overview. Bioressour Technol. 2017;242:227–235. doi: 10.1016/j.biortech.2017.02.097. [DOI] [PubMed] [Google Scholar]
- Ummalyma SB, Sahoo D, Pandey A. Microalgae biorefineries for industrial product. In: Yousuf A, editor. Microalgae cultivation for biofuels production. Ny: Elsevier; 2020. pp. 187–195. [Google Scholar]
- Ummalyma SB, Sahoo D, Pandey A. Resource recovery through bioremediation of wastewaters and waste carbon by microalgae: a circular bioeconomy approach. Environ Sci Pollut Res. 2021;28:58837–58856. doi: 10.1007/s11356-020-11645-8. [DOI] [PubMed] [Google Scholar]
- UNESCO, WWAP (United Nations World Water Assessment Programme), The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource, 2017, UNESCO, Paris, 2017, www.unwater.org.
- Uysal O, Ekinci K. Treatment of rose oil processing effluent with Chlorella sp. using photobioreactor and raceway. Environ Manag. 2021;295:113089. doi: 10.1016/j.jenvman.2021.113089. [DOI] [PubMed] [Google Scholar]
- Vassilev SV, Vassileva CG. Composition properties and challenges of algae biomass for biofuel application. An overview. Fuel. 2016;181:1–33. doi: 10.1016/j.fuel.2016.04.106. [DOI] [Google Scholar]
- Wang Q, Wei X, Chen S. Production and application of poly-g-glutamic acid. In: Pandey A, Negi S, Soccol CR, editors. Current developments in biotechnology and bioengineering. Elsevier; 2017. pp. 693–717. [Google Scholar]
- Wang Y, Yang Y, Ma F, Xuan L, Xu Y, Huo H, Zhou D, Dong S. Optimization of Chlorella vulgaris and bioflocculant producing bacteria co-culture enhancing microalgae harvesting and lipid content. Lett ApplMicrobiol. 2015;60(5):497–503. doi: 10.1111/lam.12403. [DOI] [PubMed] [Google Scholar]
- White RL, Ryan RA. Long-term cultivation of algae in raceway ponds: lessons from the field. Int Biotechnol. 2015;11(4):213–220. doi: 10.1089/ind.2015.0006. [DOI] [Google Scholar]
- Xiao R, Zheng Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv. 2016;34(7):1225–1244. doi: 10.1016/j.biotechadv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Xu K, Zou X, Chang W, QuY LiY. Microalgae harvesting technique using ballasted flotation: a review. Sep Purif Technol. 2021;276:119439. doi: 10.1016/j.seppur.2021.119439. [DOI] [Google Scholar]
- Yang C, Hua Q, Shimizu K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic dark-heterotrophic conditions. Biochem Eng. 2000;6:87–102. doi: 10.1016/S1369-703X(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang H, Cheng S, Zhang W, Zhang X. Enhanced microalgal harvesting using microalgae-derived extracellular polymeric substances as flocculation aid. ACS Sustain Chem Eng. 2020;8:4069–4075. doi: 10.1021/acssuschemeng.9b06156. [DOI] [Google Scholar]
- Yew GY, Lee SY, Show PL, Tao Y, Law CL, Nguyen TTC. Recent advances in algae biodiesel production from upstream cultivation to downstream processing. Bioresour Technol Rep. 2019;7:100227. doi: 10.1016/j.biteb.2019.100227. [DOI] [Google Scholar]
- Yim JH, Kim SJ, Ahn SH, Lee CK, Rhie KT, Lee HK. Antiviral effects of sulfated exopolysaccharide from the marine microalga Gyrodiniumim pudicum strain KG03. Mar Biotechnol. 2004;6:17–25. doi: 10.1007/s10126-003-0002-z. [DOI] [PubMed] [Google Scholar]
- Yin Z, Zhu L, Li S, Hu T, Chu R, Mo F, Hu D, Liu C, Li B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions. Bioresour Technol. 2020;301:122804. doi: 10.1016/j.biortech.2020.122804. [DOI] [PubMed] [Google Scholar]
- Yong JJY, Chew KW, Khoo PL, Chang JS. Prospects and development of algal-bacterial biotechnology in environmental management and protection. Biotechnol Adv. 2020 doi: 10.1016/j.biotechadv.2020.107684. [DOI] [PubMed] [Google Scholar]
- Yoval-Sanchez B, Jasso-Chavez R, Lira-Silva E, Moreno-Sanchez R, Rodríguez-Zavala JS. Novel mitochondrial alcohol metabolizing enzymes of Euglena gracilis. J BioenergBiomembr. 2011;43:519–530. doi: 10.1007/s10863-011-9373-4. [DOI] [PubMed] [Google Scholar]
- Yu BS, Sung YJ, Choi H, Sirohi R, Sim SJ. Concurrent enhancement of CO2 fixation and productivities of omega-3 fatty acids and astaxanthin in Haematococcus pluvialis culture via calcium-mediated homeoviscous adaptation and biomineralization. Bioresour Technol. 2021;340:125720. doi: 10.1016/j.biortech.2021.125720. [DOI] [PubMed] [Google Scholar]
- Yu KL, Lau BF, Show PL, Ong HC, Ling TC, Chen WH, Ng EP, Chang JS. Recent developments on algal biochar production and characterization. Bioresour Technol. 2017;246:2–11. doi: 10.1016/jbiortech.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Yun JH, Cho DH, Lee S, Heo J, Tran QG, Chang YK, Kim HS. Hybrid operation of photobioreactor and wastewater fed open raceway ponds enhances the dominance of target algal species and algal biomass production. Algal Res. 2018;29:319–329. doi: 10.1016/j.algal.2017.11.037. [DOI] [Google Scholar]
- Zewdie DT, Ali AY. Utilization of sugarcane factories wastes as inexpensive source of nutrients and CO2 for microalgal biomass production process coupling and potential evaluation. SN Appl Sci. 2021;3:297. doi: 10.1007/s4252-021-04311-2. [DOI] [Google Scholar]
- Zhang H, Zhang X. Microalgal harvesting using foam flotation: a critical review. Biomass Bioenerg. 2019;120:176–188. doi: 10.1016/j.biombioe.2018.11.018. [DOI] [Google Scholar]
- Zhu L, Li Z, Hiltunen E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol Biofuels. 2018;11(1):1–10. doi: 10.1186/s13068-018-1183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang LL, Li M, Ngo HH. Non suspended microalgae cultivation for wastewater refinery and biomass production. Bioresour Technol. 2020;308:123320. doi: 10.1016/j.biortech.2020.123320. [DOI] [PubMed] [Google Scholar]
- Zkeri E, Iliopoulou A, Katsara A, Korda A, Aloupi M, Gatidou G, Fountoulakis MS, Stasinakis AS. Comparing the use of a two stage MBBR system with a methanogenic MBBR coupled with a microalgae reactor for medium strength dairy wastewater treatment. Bioresour Technol. 2021;323:124629. doi: 10.1016/j.biortech.2020.124629. [DOI] [PubMed] [Google Scholar]