Abstract
Background: It is sometimes difficult to distinguish between asthma and bronchiectasis as their symptoms overlap, and these two diseases are associated with pulmonary tuberculosis (PTB) or pneumonia.
Objective: The purpose of this study is to determine the effects of bronchodilator drugs, steroids, antidepressants drugs, and antianxiety drugs on the risks of PTB or pneumonia in patients with bronchiectasis–asthma combination or bronchiectasis–asthma–chronic obstructive pulmonary disease combination—BCAS cohort.
Methods: After propensity score matching, we retrospectively studied patients with BCAS (N = 620) and without BCAS (N = 2,314) through an analysis. The cumulative incidence of PTB or pneumonia was analyzed through Cox proportional regression. After adjustment for sex, age, comorbidities, and medications [including long-acting beta2 agonist/muscarinic antagonists (LABAs/LAMAs), short-acting beta2 agonist/muscarinic antagonists (SABAs/SAMAs), leukotriene receptor antagonist, montelukast, steroids (inhaled corticosteroids, ICSs; oral steroids, OSs), anti-depressants (fluoxetine), and anti-anxiety drugs (benzodiazepines, BZDs)], we calculated the adjusted hazard ratios (aHR) and their 95% confidence intervals (95% CI) for these risks. Similar to OSs, ICSs are associated with an increased risk of PTB or pneumonia, lumping these two as steroids (ICSs/OSs).
Results: For the aHR (95% CI), with non-LABAs/non-OSs as the reference 1, the use of LABAs [0.70 (0.52–0.94)]/OSs [0.35 (0.29–0.44)] was associated with a lower risk of PTB or pneumonia. However, the current use of LABAs [2.39 (1.31–4.34)]/SABAs [1.61 (1.31–1.96)], steroids [ICSs 3.23 (1.96–5.29)]/OSs 1.76 (1.45–2.14)], and BZDs [alprazolam 1.73 (1.08–2.75)/fludiazepam 7.48 (1.93–28.9)] was associated with these risks. The current use of LAMAs [0.52 (0.14–1.84)]/SAMAs [1.45 (0.99–2.11)] was not associated with these risks.
Conclusion: The current use of LAMAs/SAMAs is relatively safe with respect to PTB or pneumonia risks, but LABAs/SABAs, steroids, and BZDs could be used after evaluation of the benefit for the BCAS cohort. However, we must take the possible protopathic bias into account.
Keywords: pulmonary tuberculosis, pneumonia, bronchiectasis‐asthma combination, beta2 agonist/muscarinic antagonists, steroids, and benzodiazepines
Introduction
Asthma is an inflammatory and immune-related disease. Bronchiectasis is a chronic, progressive, infective, immune-related, and inflammatory obstructive airway disease (Martínez-García et al., 2018). Meanwhile, bronchiectasis, in general, results from airway damage from previous infection(s), perhaps mediated by elastase activity. Bronchiectasis complicating asthma is associated with the above-stated asthma phenotypic expression. There also is a more proximal, cylindrical bronchiectasis associated with allergic bronchopulmonary aspergillosis (ABPA) complicating asthma and with an eosinophilic phenotype. Moreover, asthma may be linked with bronchiectasis (BCAS), especially in cases of steroid-resistant asthma (Polverino et al., 2018). Certainly, the treatment of patients with BCAS is related somewhat to the treatment of asthma without bronchiectasis, and the treatment of bronchiectasis without asthma differs considerably.
BCAS is associated with frequent hospitalizations, concomitant gastroesophageal reflux disease, hypersensitivity to non-steroidal anti-inflammatory drugs, and high blood eosinophil counts and represents an additional phenotypic feature of severe eosinophilic asthma (Truong, 2013; Coman et al., 2018). In precision and personalized medicine, BCAS is considered a different entity from asthma (Polverino et al., 2018). One study indicated that the coronavirus predisposed patients to bronchiectasis or the exacerbation of asthma (Zhang et al., 2020). The coronavirus may play a role in the development of BCAS (Zhang et al., 2020). Thus, BCAS is an essential topic in the era of the coronavirus.
One study indicated that previous pneumonia, pulmonary tuberculosis (PTB), and non-tuberculous mycobacterium (NTM) increased the risk of bronchiectasis (Hill et al., 2019). Similar to oral steroid (OS) users, inhaled corticosteroid (ICS) users have a higher risk of PTB or pneumonia in asthma or chronic obstructive pulmonary disease (COPD) (Chang et al., 2016; Lee et al., 2019; Yeh et al., 2020). Meanwhile, PTB or pneumonia may coexist when steroids (ICSs/OSs) are used among BCAS (Feng et al., 2012; Chang et al., 2016; Yeh, 2019; Yeh et al., 2020). Few studies have investigated the relationship between PTB, pneumonia, bronchodilators, steroids, leukotriene receptor antagonists, and montelukast among a BCAS cohort. Moreover, no study focuses on the impact of antidepressants and benzodiazepines (BZDs) for PTB or pneumonia among the BCAS cohort. We wanted to evaluate the effects asthma therapies have on risk of PTB or pneumonia among the BCAS cohort by examining the general population. Meanwhile, protopathic bias occurs when a drug is prescribed for initial signs/symptoms of an outcome not yet diagnosed, reflecting a reversal of cause and effect (Arfè and Corrao, 2016). This type of bias is likely to arise in studies on the associations between drug use and PTB risk (studying outcomes with long latencies). We added this bias into account in this study.
Materials and Methods
Data Source
Data for this retrospective, population-based cohort study were obtained from the National Health Insurance Research Database (NHIRD), which was launched in 1995 and covers almost 99% of the 23 million people in Taiwan. We used the Longitudinal Health Insurance Database 2000, which maintains the registration data of everyone who was a beneficiary of the National Health Insurance program during the period of 1996–2000. Data on the medical services provided by programmers are collected by the National Health Insurance Administration and entered into the NHIRD. This longitudinal database contains population-level demographic and administrative information, including sex, birth year, region of residence, dates of admission and discharge, prescription drugs, surgical procedures, and diagnostic codes. The International Classification of Diseases Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes are also listed, with a maximum of five codes. In the NHIRD, the ICD-9-CM and procedure coding system are used to define the diagnostic and procedure codes, respectively. In accordance with the Personal Information Protection Act, individual identifiers are encrypted before release for research. The NHIRD has been used for various studies and provides high-quality information on diagnoses, hospitalizations, and prescriptions.
Ethics Statement
The NHIRD encrypts personal information to protect patients’ privacy. It provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. The study protocol was approved by the Institutional Review Board of China Medical University (CMUH104-REC2-115-AR4), which also specifically waived the informed consent requirement.
Study Population
Supplementary Figure S1 shows the process of selecting participants for study cohorts. We identified patients diagnosed with new bronchiectasis (ICD-9-CM code 494) or with new COPD (ICD-9-CM codes 491, 492, and 496) from claims data for 2000 to 2012. Patients aged ≧18 years having new bronchiectasis + new asthma (ICD-9-CM code 493) or new bronchiectasis + new asthma + new COPD were selected for the BCAS cohort. The comparison cohort (non-BCAS cohort) was randomly selected from the rest of the bronchiectasis, COPD, asthma, or patients with immunosuppressants such as steroid use who are without a diagnosis of BCAS.
To evaluate the risk of PTB or pneumonia in patients within the BCAS cohort, we selected a cohort of patients who had two outpatient visits or an inpatient visit for the BCAS cohort and a comparable non-BCAS cohort (Redondo et al., 2016; Vanfleteren, 2016; Hung et al., 2018; Mihălț;an and Constantin, 2019; Veith et al., 2020).
The BCAS cohort includes six bronchiectasis + asthma (pure BCAS) and seven bronchiectasis + asthma + COPD (BCAOS). The non-BCAS cohort (without bronchiectasis–asthma combination or without bronchiectasis–asthma–COPD combination) includes 1 pure bronchiectasis = (1 + 4 + 6 + 7, new bronchiectasis) − 4 BCOS − 6 BCAS − 7 BCAOS; 2 pure COPD = (2 + 4 + 5 + 7, new COPD) − 4 BCOS − 5 ACOS − 7 BCAOS; 3 pure asthma = (3 + 5 + 6 + 7, new asthma) − 5 ACOS − 6 BCAS − 7, BCAOS; 4 bronchiectasis + COPD (BCOS); 5 COPD + asthma (ACOS); and 8 others—such as patients with steroids use. The exclusion criteria include 1) diagnoses of PTB (ICD-9-CM codes 010–012) and pneumonia (codes 480–486) before entry into the study, wash out period 1996–1999; 2) age <18 years; and 3) incomplete demographic information (Supplementary Figure S1) (Shantakumar et al., 2018). We identified patients in the newly diagnosed BCAS cohort (N = 664) and the newly diagnosed non-BCAS cohort (N = 694,576) between 1 January 2000 and 31 December 2012.
These patients in the BCAS cohort were matched to patients in the non-BCAS cohort by age (5-year range), gender, comorbidities, smoking status, medications, and year of index date according to incidence density sampling. After 1:4 matching, the BCAS cohort (N = 620) and the non-BCAS cohort (N = 2,314) were found (Supplementary Figure S1) (Redondo et al., 2016; Vanfleteren, 2016; Veith et al., 2020).
The primary study outcome was the occurrence of PTB or pneumonia (outpatient and inpatient settings: PTB: ICD-9-CM codes 010–012; pneumonia: codes 480–486). Both cohorts were observed from the index date to the date of diagnosis of (PTB or pneumonia), withdrawal from the National Health Insurance program, or to the end of 2013, depending on whichever occurred first. Patients were followed up until death or the end of the study period (31 December 2013).
The incidence of PTB or pneumonia was defined as a new diagnosis and at least one visit with inpatient claims data or two visits with outpatient claims data after the index date. The ICD-9 CM, Anatomical Therapeutic Chemical (ATC), and full names list are presented in Supplementary Table S1. This study adjusted for the demographic factors of gender and age, comorbidities, and medications. Those comorbidities were calculated on the basis of the participants’ status before the index date. We defined the index date of the case cohort by the first date of drug prescription after a diagnosis of BCAS, and we restricted the case cohort to patients who used drugs for more than 28 days.
Statistical Analysis
Propensity score matching was used to balance the groups with respect to the known variables and to increase their comparability. Although some unmeasurable confounders may exist disproportionally between the study groups, propensity score matching can optimally balance the distributions of measured covariates as much as a randomized trial does. We estimated the patients’ propensity scores through a non-parsimonious multivariable logistic regression, with receipt of patients with or without BCAS as the independent variable. We incorporated clinically relevant covariates (comorbidities, medications, etc.) into our analysis—the primary analysis. PTB or pneumonia was the dependent variable (Table 1; Supplementary Figure S2). We used Student’s t test and the chi-square test to compare differences in baseline characteristics and comorbidities between the BCAS cohort and non-BCAS cohort. The incidence density rate (per 1,000 person-years) was analyzed to estimate the incidence of PTB or pneumonia among the cohorts stratified by gender, age, comorbidities, and medications. The annual incidence density rate was calculated by dividing the number of new diagnoses of PTB or pneumonia by the number of person-years for patients at risk for PTB or pneumonia for each year between 2000 and 2013. The risks of PTB or pneumonia between these two cohorts were compared through Cox proportional hazard regression models. The analysis was adjusted for gender, age, comorbidities, and medications for the BCAS cohort. We set our significance threshold at α = 0.05 for a priori hypotheses. Significance in subsequent explanatory analyses was defined as p < 0.01. Statistical analysis was performed using SAS (version 9.4 for Windows; SAS Institute, Inc., Cary, NC, United States).
TABLE 1.
Baseline characteristics of study population.
| Variable | Original population | p-value a | PS-matching population | p-value a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCAS cohort (n = 664) | Non-BCAS cohort (n = 694,576) | BCAS cohort (n = 620) | Non-BCAS cohort (n = 2,314) | |||||||
| N | % | N | % | N | % | N | % | |||
| Gender | 0.0003 | 0.40 | ||||||||
| Female | 372 | 56.0 | 340,724 | 49.1 | 345 | 55.6 | 1,244 | 53.8 | ||
| Male | 292 | 44.0 | 353,852 | 50.9 | 275 | 44.4 | 1,070 | 46.2 | ||
| Age at baseline, years | <0.0001 | 0.36 | ||||||||
| <35 | 69 | 10.4 | 339,290 | 48.9 | 65 | 10.5 | 215 | 9.29 | ||
| ≥35 | 595 | 89.6 | 355,286 | 51.1 | 555 | 89.5 | 2099 | 90.7 | ||
| Mean (SD) b | 59.1 (17.7) | 36.8 (18.2) | <0.0001 | 58.8 (17.7) | 60.9 (18.0) | 0.001 | ||||
| Comorbidity | ||||||||||
| Non-tuberculous mycobacterium | 1 | 0.15 | 139 | 0.02 | 0.01 | 1 | 0.16 | 9 | 0.39 | 0.38 |
| Rheumatoid arthritis | 24 | 3.61 | 7,269 | 1.05 | <0.0001 | 19 | 3.06 | 92 | 3.98 | 0.29 |
| Diffuse connective disease and Sjogren’s Syndrome | 21 | 3.16 | 5,946 | 0.86 | <0.0001 | 18 | 2.90 | 83 | 3.59 | 0.40 |
| COPD | 364 | 54.8 | 26,154 | 3.77 | <0.0001 | 336 | 54.1 | 1,271 | 54.9 | 0.74 |
| Diabetes | 186 | 28.0 | 66,474 | 9.57 | <0.0001 | 171 | 27.5 | 696 | 30.0 | 0.22 |
| Aspergillosis | 1 | 0.15 | 19 | 0.002 | <0.0001 | 1 | 0.16 | 4 | 0.17 | 0.95 |
| Candidiasis | 0 | 0 | 10 | 0.001 | 0.92 | 0 | 0 | 0 | 0 | — |
| Endemic mycoses | 0 | 0 | 43 | 0.01 | 0.83 | 0 | 0 | 0 | 0 | — |
| Mounier-Kuhn | 0 | 0 | 37 | 0.01 | 0.85 | 0 | 0 | 0 | 0 | — |
| Cystic fibrosis | 0 | 0 | 4 | 0.001 | 0.95 | 0 | 0 | 0 | 0 | — |
| Hypertension | 328 | 49.4 | 95,776 | 13.8 | <0.0001 | 298 | 48.0 | 1,206 | 52.1 | 0.07 |
| Hyperlipidemia | 163 | 24.5 | 61,673 | 8.88 | <0.0001 | 151 | 24.3 | 613 | 26.4 | 0.28 |
| Pulmonary embolism | 0 | 0 | 173 | 0.02 | 0.68 | 0 | 0 | 0 | 0 | — |
| Depression | 14 | 2.11 | 4,608 | 0.66 | <0.0001 | 13 | 2.10 | 56 | 2.42 | 0.63 |
| Stroke | 62 | 9.34 | 14,928 | 2.15 | <0.0001 | 54 | 8.71 | 245 | 10.5 | 0.16 |
| Heart disease | 271 | 40.8 | 59,964 | 8.63 | <0.0001 | 247 | 39.8 | 1,008 | 43.5 | 0.09 |
| Anxiety | 219 | 32.9 | 70,332 | 10.1 | <0.0001 | 196 | 31.6 | 770 | 33.2 | 0.43 |
| Smoking | ||||||||||
| Tobacco dependence | 3 | 0.45 | 747 | 0.11 | 0.006 | 3 | 0.48 | 13 | 0.56 | 0.81 |
| Tobacco use disorder complicating pregnancy | 0 | 0 | 0 | 0 | — | 0 | 0 | 0 | 0 | — |
| Medication | ||||||||||
| LABA | 131 | 19.7 | 806 | 0.12 | <0.0001 | 117 | 18.8 | 278 | 12.0 | <0.0001 |
| LAMA | 38 | 5.72 | 595 | 0.09 | <0.0001 | 32 | 5.16 | 81 | 3.50 | 0.05 |
| SABA | 231 | 34.8 | 16,880 | 2.43 | <0.0001 | 216 | 34.8 | 748 | 32.3 | 0.23 |
| SAMA | 150 | 22.6 | 11,876 | 1.71 | <0.0001 | 140 | 22.5 | 510 | 22.0 | 0.77 |
| ICSs | 196 | 29.5 | 1,250 | 0.18 | <0.0001 | 177 | 28.5 | 437 | 18.8 | <0.0001 |
| OSs | 608 | 91.6 | 510,508 | 73.5 | <0.0001 | 567 | 91.4 | 2,140 | 92.4 | 0.39 |
| Leukotriene antagonist | 57 | 8.58 | 1,426 | 0.21 | <0.0001 | 52 | 8.39 | 100 | 4.32 | <0.0001 |
| Montelukast | 55 | 8.28 | 1,401 | 0.20 | <0.0001 | 50 | 8.06 | 99 | 4.28 | 0.0001 |
| Alprazolam | 224 | 33.7 | 98,484 | 14.1 | <0.0001 | 203 | 32.7 | 848 | 36.6 | 0.07 |
| Fluoxetine | 0 | 0 | 271 | 0.04 | 0.61 | 0 | 0 | 0 | 0 | — |
| Fludiazepam | 112 | 16.9 | 39,967 | 5.75 | <0.0001 | 103 | 16.6 | 393 | 16.9 | 0.82 |
p-value using chi-square for the comparisons between with and without BCAS cohort.
BCAS cohort: bronchiectasis–asthma combination cohort; comparison cohort: non-BCAS cohort.
Average age using Wilcoxon rank-sum test for verification.
COPD, chronic obstructive pulmonary disease; LABAs/LAMAs: long-acting beta2 agonist/muscarinic antagonist; SABAs/SAMAs, short-acting beta2 agonist/muscarinic antagonist; ICSs, inhaled corticosteroids; OSs, oral steroids.
Results
Baseline Characteristics
No significant difference in the distribution of age groups was observed between the two cohorts because of the success of the incidence density sampling (Table 1). The mean age (standard deviation) of the participants was 58.8 (±17.7) years for the BCAS cohort and 60.9 (±18.0) years for the non-BCAS cohort (Student’s t test: p < 0.001). The majority of patients were more than 35 years old. Several significant differences in gender, age, grouped comorbidities, and grouped medications were observed between the BCAS and non-BCAS cohorts; the use of LABAs, ICSs, OSs, leukotriene receptor antagonist, and montelukast was more frequent in the BCAS cohort than in the non-BCAS cohort.
Comparison of PTB or Pneumonia Risks Among Groups With Non-Comorbidity and Non-Medicine Use as Reference
The incidence density rate of PTB or pneumonia was higher in the BCAS cohort than in the non-BCAS cohort (57.7 and 46.7 per 1,000 person-years, respectively) (Table 2). These results reveal that the risk of developing PTB or pneumonia was 1.54 times higher for the BCAS cohort [95% confidence interval (CI) = 1.31–1.80) than for the non-BCAS cohort. Age of over 35 years, male sex, diabetes, stroke, and heart disease were significantly and positively associated with PTB or pneumonia. Comorbidities of hyperlipidemia and depression and the use of LABAs, OSs, and BZDs were significantly and negatively associated with PTB or pneumonia. The risks of PTB or pneumonia were 1.18-fold and 2.64-fold higher for patients older than 35 years of age and men, respectively, than for patients younger than 35 years of age (95% CI = 1.03–1.36 and 1.90–3.68). The risks of PTB or pneumonia were significantly higher for those with diabetes [adjusted hazard ratio (aHR) = 1.19, 95% CI = 1.03–1.37), stroke (aHR = 1.36, 95% CI = 1.13–1.64), and heart disease (aHR = 1.19, 95% CI = 1.03–1.38). The risks were significantly lower for those with hyperlipidemia (aHR = 0.75, 95% CI = 0.64–0.88); depression (aHR = 0.53, 95% CI = 0.30–0.92); and who use LABAs (aHR = 0.70, 95% CI = 0.52–0.94), OSs (aHR = 0.35, 95% CI = 0.29–0.44), alprazolam (aHR = 0.73, 95% CI = 0.63–0.85), and fludiazepam (aHR = 0.79, 95% CI = 0.66–0.95). However, the effects of other variables (Table 2) were not found to be significant.
TABLE 2.
Cox model-measured hazard ratios and 95% confidence interval of pulmonary tuberculosis or pneumonia associated with gender, age, comorbidity, and medications after propensity matching of both the BCAS and non-BCAS cohorts.
| Pulmonary tuberculosis or pneumonia | Crude HR (95% CI) | Adjusted HR (95% CI) | |||
|---|---|---|---|---|---|
| Event | PY | IR | |||
| Bronchiectasis–asthma | |||||
| No | 743 | 15,909 | 46.7 | 1 (reference) | 1 (reference) |
| Yes | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Gender | |||||
| Female | 470 | 10,515 | 44.7 | 1 (reference) | 1 (reference) |
| Male | 481 | 8,996 | 53.5 | 1.19 (1.05–1.36)** | 1.18 (1.03–1.36)* |
| Age | |||||
| <35 | 41 | 2,558 | 16 | 1 (reference) | 1 (reference) |
| ≥35 | 910 | 16,953 | 53.7 | 3.46 (2.53–4.74)*** | 2.64 (1.90–3.68)*** |
| Comorbidity | |||||
| Non-tuberculous mycobacterium | |||||
| No | 950 | 19,451 | 48.8 | 1 (reference) | 1 (reference) |
| Yes | 1 | 60 | 16.7 | 0.34 (0.04–2.45) | 0.63 (0.08–4.51) |
| Rheumatoid arthritis | |||||
| No | 915 | 18,852 | 48.5 | 1 (reference) | 1 (reference) |
| Yes | 36 | 659 | 54.6 | 1.13 (0.81–1.58) | 1.03 (0.73–1.45) |
| Diffuse connective disease and Sjogren’s syndrome | |||||
| No | 919 | 19,003 | 48.4 | 1 (references) | 1 (reference) |
| Yes | 32 | 508 | 63 | 1.32 (0.93–1.88) | 1.18 (0.82–1.70) |
| COPD | |||||
| No | 372 | 9,990 | 37.2 | 1 (reference) | 1 (reference) |
| Yes | 579 | 9,521 | 60.8 | 1.67 (1.46–1.90)*** | 1.15 (0.99–1.32) |
| Diabetes | |||||
| No | 613 | 14,439 | 42.5 | 1 (reference) | 1 (reference) |
| Yes | 338 | 5,072 | 66.6 | 1.59 (1.39–1.82)*** | 1.19 (1.03–1.37)* |
| Aspergillosis | |||||
| No | 950 | 19,485 | 48.8 | 1 (reference) | 1 (reference) |
| Yes | 1 | 26 | 38.5 | 0.79 (0.11–5.65) | 1.05 (0.14–7.52) |
| Candidiasis | |||||
| No | 951 | 19,511 | 48.7 | 1 (reference) | 1 (reference) |
| Yes | 0 | 0 | 0 | — | — |
| Endemic mycoses | |||||
| No | 951 | 19,511 | 48.7 | 1 (reference) | 1 (reference) |
| Yes | 0 | 0 | 0 | — | — |
| Mounier-Kuhn | |||||
| No | 951 | 19,511 | 48.7 | 1 (reference) | 1 (reference) |
| Yes | 0 | 0 | 0 | — | — |
| Cystic fibrosis | |||||
| No | 951 | 19,511 | 48.7 | 1 (reference) | 1 (reference) |
| Yes | 0 | 0 | 0 | — | — |
| Hypertension | |||||
| No | 341 | 10,573 | 32.3 | 1 (reference) | 1 (reference) |
| Yes | 610 | 8,938 | 68.2 | 2.15 (1.88–2.46)*** | 1.05 (0.90–1.24) |
| Hyperlipidemia | |||||
| No | 736 | 14,999 | 49.1 | 1 (reference) | 1 (reference) |
| Yes | 215 | 4,512 | 47.7 | 0.98 (0.84–1.14) | 0.75 (0.64–0.88)*** |
| Pulmonary embolism | |||||
| No | 951 | 19,511 | 48.7 | 1 (reference) | 1 (reference) |
| Yes | 0 | 0 | 0 | — | — |
| Depression | |||||
| No | 938 | 19,143 | 49 | 1 (reference) | 1 (reference) |
| Yes | 13 | 368 | 35.3 | 0.73 (0.42–1.26) | 0.53 (0.30–0.92)* |
| Stroke | |||||
| No | 803 | 17,989 | 44.6 | 1 (reference) | 1 (reference) |
| Yes | 148 | 1,522 | 97.2 | 2.21 (1.85–2.64)*** | 1.36 (1.13–1.64)** |
| Heart disease | |||||
| No | 476 | 12,370 | 38.5 | 1 (reference) | 1 (reference) |
| Yes | 475 | 7,141 | 66.5 | 1.77 (1.55–2.01)*** | 1.19 (1.03–1.38)* |
| Anxiety | |||||
| No | 652 | 13,810 | 47.2 | 1 (reference) | 1 (reference) |
| Yes | 299 | 5,701 | 52.4 | 1.12 (0.98–1.29) | 1.04 (0.89–1.21) |
| Smoking | |||||
| Tobacco dependence | |||||
| No | 949 | 19,430 | 48.8 | 1 (reference) | 1 (reference) |
| Yes | 2 | 81 | 24.7 | 0.52 (0.13–2.10) | 0.66 (0.16–2.67) |
| Tobacco use disorder complicating pregnancy | |||||
| No | 951 | 19,511 | 48.7 | 1 (reference) | 1 (reference) |
| Yes | 0 | 0 | 0 | — | — |
| Medication | |||||
| LABA | |||||
| Non-use | 845 | 16,762 | 50.4 | 1 (references) | 1 (reference) |
| Use | 106 | 2,749 | 38.6 | 0.76 (0.62–0.93)** | 0.70 (0.52–0.94)* |
| LAMA | |||||
| Non-use | 914 | 18,744 | 48.8 | 1 (reference) | 1 (reference) |
| Use | 37 | 767 | 48.2 | 0.99 (0.71–1.38) | 0.85 (0.59–1.22) |
| SABA | |||||
| Non-use | 569 | 13,103 | 43.4 | 1 (reference) | 1 (reference) |
| Use | 382 | 6,408 | 59.6 | 1.37 (1.20–1.56)*** | 0.98 (0.79–1.21) |
| SAMA | |||||
| Non-use | 657 | 15,329 | 42.9 | 1 (reference) | 1 (reference) |
| Use | 294 | 4,182 | 70.3 | 1.64 (1.43–1.88)*** | 1.22 (0.98–1.53) |
| ICSs | |||||
| Non-use | 775 | 15,117 | 51.3 | 1 (reference) | 1 (reference) |
| Use | 176 | 4,394 | 40.1 | 0.77 (0.66–0.91)** | 0.88 (0.69–1.11) |
| OSs | |||||
| Non-use | 120 | 747 | 160.6 | 1 (reference) | 1 (reference) |
| Use | 831 | 18,764 | 44.3 | 0.26 (0.22–0.32)*** | 0.35 (0.29–0.44)*** |
| Leukotriene antagonist | |||||
| Non-use | 920 | 18,440 | 49.9 | 1 (reference) | 1 (reference) |
| Use | 31 | 1,071 | 28.9 | 0.57 (0.40–0.82)** | 0.79 (0.19–3.24) |
| Montelukast | |||||
| Non-use | 922 | 18,466 | 49.9 | 1 (reference) | 1 (reference) |
| Use | 29 | 1,045 | 27.8 | 0.55 (0.38–0.80)** | 1.09 (0.25–4.65) |
| Alprazolam | |||||
| Non-use | 646 | 11,677 | 55.3 | 1 (reference) | 1 (reference) |
| Use | 305 | 7,834 | 38.9 | 0.70 (0.61–0.80)*** | 0.73 (0.63–0.85)*** |
| Fluoxetine | |||||
| Non-use | 951 | 19,511 | 48.7 | 1 (reference) | 1 (reference) |
| Use | 0 | 0 | 0 | — | — |
| Fludiazepam | |||||
| Non-use | 797 | 15,575 | 51.2 | 1 (reference) | 1 (reference) |
| Use | 154 | 3,936 | 39.1 | 0.75 (0.63–0.90)** | 0.79 (0.66–0.95)* |
BCAS cohort, bronchiectasis–asthma combination cohort; COPD, chronic obstructive pulmonary disease; LABAs/LAMAs, long-acting beta2 agonist/muscarinic antagonist; SABAs/SAMAs, short-acting beta2 agonist/muscarinic antagonist; ICSs, inhaled corticosteroids; OSs, oral steroids; PY, person-years; IR, incidence rate per 1,000 person-years; HR, hazard ratio; CI, confidence interval; HR, adjusted for BCAS cohort, gender, age, comorbidities, and medication use.
—Unable to calculate because there are few or no events in the with and without BCAS cohort.
*p < 0.05, **p < 0.01, ***p < 0.001.
Comparison of Non-BCAS and BCAS Cohorts
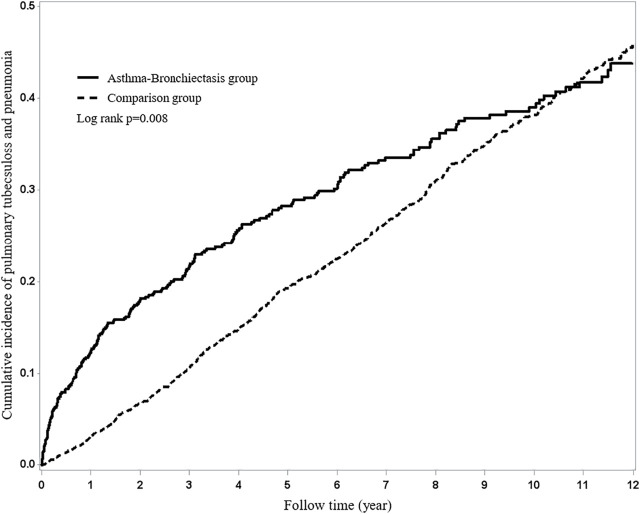
The results of the study revealed that 208 patients in the BCAS cohort and 743 patients in the non-BCAS cohort received a diagnosis of PTB or pneumonia (Table 3). After adjustment for age, comorbidities, and medications, women (aHR = 1.67, 95% CI = 1.33–2.09), those who were less than 35 years (aHR = 1.47, 95% CI = 1.25–1.72), those with COPD (aHR = 1.65, 95% CI = 1.35–2.02), those with diabetes (aHR = 1.64, 95% CI = 1.25–2.15), those with hypertension (aHR = 1.53, 95% CI = 1.25–1.87), those with heart disease (aHR = 1.64, 95% CI = 1.31–2.06), those with LABAs (aHR = 2.54, 95% CI = 1.63–3.69), those with ICSs (aHR = 2.00, 95% CI = 1.41–2.82), and those with OS (aHR = 1.38, 95% CI = 1.16–1.64) in the BCAS cohort were significantly more associated with risks of PTB or pneumonia than those in the non-BCAS cohort (Table 3). For current, recent, and past use, relative to the non-BCAS cohort, the risks of PTB or pneumonia were higher for those with SABAs (≤30 days), ICSs (≤30 days), and OSs (≤30 days), by 1.61 (95% CI = 1.31–1.96), 3.23 (95% CI = 1.96–5.29), and 1.76 (95% CI = 1.45–2.14), respectively. The risks of PTB or pneumonia were not associated with LAMAs (≤30 days) and SAMAs ≤30 days); the aHRs were 0.52 (0.14–1.84) and 1.45 (0.99–2.11), respectively (Table 4). A Kaplan–Meier analysis revealed statistically significant differences in the cumulative incidence of PTB or pneumonia between the BCAS and non-BCAS cohorts (log rank test: p = 0.008) (Figure 1). The area under curve for BCAS applied to predict PTB or pneumonia occurrence was 0.7195 (95% CI = 0.70–0.74) (Figure 2).
TABLE 3.
Incidence rate and hazard ratio of pulmonary tuberculosis or pneumonia between the two cohorts stratified by gender, age, comorbidities, and medications after propensity matching.
| BCAS cohort | Crude HR (95% CI) | Adjusted HR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | IR | Event | PY | IR | |||
| Gender | ||||||||
| Female | 363 | 8,528 | 42.6 | 107 | 1987 | 53.9 | 1.26 (1.01–1.56)* | 1.67 (1.33–2.09)*** |
| Male | 380 | 7,381 | 51.5 | 101 | 1,615 | 62.5 | 1.21 (0.97–1.50) | 1.44 (1.15–1.80)** |
| Age | ||||||||
| <35 | 31 | 2,014 | 15.4 | 10 | 544 | 18.4 | 1.19 (0.58–2.43) | 1.45 (0.65–3.26) |
| ≥35 | 712 | 13,895 | 51.2 | 198 | 3,058 | 64.7 | 1.26 (1.07–1.47)** | 1.47 (1.25–1.72)*** |
| Comorbidity | ||||||||
| Non-tuberculous mycobacterium | ||||||||
| No | 742 | 15,851 | 46.8 | 208 | 3,599 | 57.8 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Yes | 1 | 58 | 17.2 | 0 | 3 | 0 | — | — |
| Rheumatoid arthritis | ||||||||
| No | 715 | 15,363 | 46.5 | 200 | 3,489 | 57.3 | 1.22 (1.05–1.43)* | 1.53 (1.30–1.79)*** |
| Yes | 28 | 546 | 51.3 | 8 | 133 | 60.2 | 1.34 (0.61–2.96) | 2.37 (0.88–6.35) |
| Diffuse connective disease and Sjogren’s syndrome | ||||||||
| No | 716 | 15,469 | 46.3 | 203 | 3,535 | 57.4 | 1.23 (1.05–1.44)** | 1.56 (1.33–1.83)*** |
| Yes | 27 | 440 | 61.4 | 5 | 67 | 74.6 | 1.21 (0.46–3.19) | 0.70 (0.22–2.21) |
| COPD | ||||||||
| No | 291 | 8,098 | 35.9 | 81 | 1893 | 42.8 | 1.18 (0.92–1.51) | 1.54 (1.19–1.98)*** |
| Yes | 452 | 7,811 | 57.9 | 127 | 1709 | 74.3 | 1.28 (1.05–1.56)* | 1.65 (1.35–2.02)*** |
| Diabetes | ||||||||
| No | 478 | 11,706 | 40.8 | 135 | 2,733 | 49.4 | 1.20 (0.99–1.46) | 1.50 (1.23–1.83)*** |
| Yes | 265 | 4,203 | 63.1 | 73 | 869 | 84 | 1.32 (1.02–1.72)* | 1.64 (1.25–2.15)*** |
| Aspergillosis | ||||||||
| No | 742 | 15,890 | 46.7 | 208 | 3,596 | 57.8 | 1.23 (1.05–1.44)** | 1.54 (1.31–1.80)*** |
| Yes | 1 | 19 | 52.6 | 0 | 6 | 0 | — | — |
| Candidiasis | ||||||||
| No | 743 | 15,909 | 46.7 | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Endemic mycoses | ||||||||
| No | 743 | 15,909 | 46.7 | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Mounier-Kuhn | ||||||||
| No | 743 | 15,909 | 46.7 | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Cystic fibrosis | ||||||||
| No | 743 | 15,909 | 46.7 | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Hypertension | ||||||||
| No | 258 | 8,484 | 30.4 | 83 | 2089 | 39.7 | 1.31 (1.02–1.67)* | 1.58 (1.22–2.04)*** |
| Yes | 485 | 7,425 | 65.3 | 125 | 1,513 | 82.6 | 1.25 (1.03–1.52)* | 1.53 (1.25–1.87)*** |
| Hyperlipidemia | ||||||||
| No | 566 | 12,143 | 46.6 | 170 | 2,857 | 59.5 | 1.27 (1.07–1.50)** | 1.53 (1.29–1.83)*** |
| Yes | 177 | 3,766 | 47 | 38 | 745 | 51 | 1.09 (0.77–1.55) | 1.56 (1.08–2.25)* |
| Pulmonary embolism | ||||||||
| No | 743 | 15,909 | 46.7 | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Depression | ||||||||
| No | 734 | 15,603 | 47 | 204 | 3,540 | 57.6 | 1.22 (1.04–1.42)* | 1.52 (1.30–1.78)*** |
| Yes | 9 | 306 | 29.4 | 4 | 62 | 64.5 | 1.98 (0.61–6.44) | 16.1 (0.95–27.5) |
| Stroke | ||||||||
| No | 618 | 14,627 | 42.3 | 185 | 3,362 | 55 | 1.30 (1.10–1.53)** | 1.55 (1.31–1.84)*** |
| Yes | 125 | 1,282 | 97.5 | 23 | 240 | 95.8 | 0.96 (0.62–1.51) | 1.55 (0.95–2.52) |
| Heart disease | ||||||||
| No | 364 | 9,913 | 36.7 | 112 | 2,457 | 45.6 | 1.24 (1.00–1.53)* | 1.48 (1.19–1.84)*** |
| Yes | 379 | 5,996 | 63.2 | 96 | 1,145 | 83.8 | 1.33 (1.06–1.67)* | 1.64 (1.31–2.06)*** |
| Anxiety | ||||||||
| No | 507 | 11,197 | 45.3 | 145 | 2,613 | 55.5 | 1.22 (1.01–1.47)* | 1.55 (1.28–1.87)*** |
| Yes | 236 | 4,712 | 50.1 | 63 | 989 | 63.7 | 1.27 (0.96–1.68) | 1.61 (1.21–2.14)** |
| Smoking | ||||||||
| Tobacco dependence | ||||||||
| No | 741 | 15,849 | 46.8 | 208 | 3,581 | 58.1 | 1.23 (1.06–1.44)** | 1.54 (1.31–1.80)*** |
| Yes | 2 | 60 | 33.3 | 0 | 21 | 0 | — | — |
| Tobacco use disorder complicating pregnancy | ||||||||
| No | 743 | 15,909 | 46.7 | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Medication | ||||||||
| LABA | ||||||||
| Non-use | 673 | 13,817 | 48.7 | 172 | 2,945 | 58.4 | 1.19 (1.01–1.41)* | 1.45 (1.22–1.72)*** |
| Use | 70 | 2,092 | 33.5 | 36 | 657 | 54.8 | 1.68 (1.12–2.52)* | 2.54 (1.63–3.96)*** |
| LAMA | ||||||||
| Non-use | 716 | 15,351 | 46.6 | 198 | 3,392 | 58.4 | 1.24 (1.06–1.46)** | 1.57 (1.34–1.84)*** |
| Use | 27 | 558 | 48.4 | 10 | 210 | 47.6 | 0.92 (0.44–1.92) | 0.78 (0.33–1.82) |
| SABA | ||||||||
| Non-use | 441 | 10,799 | 40.8 | 128 | 2,304 | 55.6 | 1.35 (1.11–1.65)** | 1.61 (1.31–1.96)*** |
| Use | 302 | 5,110 | 59.1 | 80 | 1,298 | 61.6 | 1.03 (0.80–1.32) | 1.39 (1.07–1.80)* |
| SAMA | ||||||||
| Non-use | 506 | 12,567 | 40.3 | 151 | 2,762 | 54.7 | 1.35 (1.12–1.62)** | 1.65 (1.37–1.98)*** |
| Use | 237 | 3,342 | 70.9 | 57 | 840 | 67.9 | 0.94 (0.70–1.26) | 1.30 (0.96–1.77) |
| ICSs | ||||||||
| Non-use | 621 | 12,584 | 49.3 | 154 | 2,534 | 60.8 | 1.22 (1.02–1.46)* | 1.47 (1.22–1.75)*** |
| Use | 122 | 3,325 | 36.7 | 54 | 1,068 | 50.6 | 1.41 (1.02–1.94)* | 2.00 (1.41–2.82)*** |
| OSs | ||||||||
| Non-use | 85 | 672 | 126.5 | 35 | 75 | 466.7 | 2.81 (1.88–4.21)*** | 3.99 (2.54–6.27)*** |
| Use | 658 | 15,237 | 43.2 | 173 | 3,527 | 49.1 | 1.14 (0.96–1.34) | 1.38 (1.16–1.64)*** |
| Leukotriene antagonist | ||||||||
| Non-use | 724 | 15,167 | 47.7 | 196 | 3,273 | 59.9 | 1.25 (1.06–1.46)** | 1.55 (1.32–1.81)*** |
| Use | 19 | 742 | 25.6 | 12 | 329 | 36.5 | 1.37 (0.66–2.84) | 2.79 (1.07–7.27)* |
| Montelukast | ||||||||
| Non-use | 725 | 15,170 | 47.8 | 197 | 3,296 | 59.8 | 1.24 (1.06–1.46)** | 1.54 (1.31–1.80)*** |
| Use | 18 | 739 | 24.4 | 11 | 306 | 35.9 | 1.41 (0.66–3.00) | 2.92 (1.11–7.64)* |
| Alprazolam | ||||||||
| Non-use | 481 | 9,566 | 50.3 | 165 | 2,111 | 78.2 | 1.53 (1.28–1.83)*** | 1.86 (1.56–2.23)*** |
| Use | 262 | 6,343 | 41.3 | 43 | 1,491 | 28.8 | 0.68 (0.49–0.94)* | 0.89 (0.64–1.24) |
| Fluoxetine | ||||||||
| Non-use | 743 | 15,909 | 46.7 | 208 | 3,602 | 57.7 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Use | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Fludiazepam | ||||||||
| Non-use | 619 | 12,723 | 48.7 | 178 | 2,852 | 62.4 | 1.27 (1.07–1.50)** | 1.59 (1.34–1.89)*** |
| Use | 124 | 3,186 | 38.9 | 30 | 750 | 40 | 1.06 (0.71–1.58) | 1.27 (0.84–1.91) |
BCAS cohort, bronchiectasis–asthma combination cohort; COPD, chronic obstructive pulmonary disease; LABAs/LAMAs, long-acting beta2 agonist/muscarinic antagonist; SABAs/SAMAs, short-acting beta2 agonist/muscarinic antagonist; ICSs, inhaled corticosteroids; OSs, oral steroids; PY, person-years; IR, incidence rate per 1,000 person-years; HR, hazard ratio; CI, confidence interval; HR, adjusted for BCAS cohort, gender, age, comorbidities, and medication use.
—Unable to calculate because there are few or no events in the with and without BCAS cohort.
*p < 0.05, **p < 0.01, ***p < 0.001.
TABLE 4.
Incidence rate and hazard ratio of pulmonary tuberculosis or pneumonia between the two cohorts stratified by the current, recent, and past use days.
| BCAS cohort | Crude HR (95% CI) | Adjusted HR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | IR | Event | PY | IR | |||
| Drug-use days | ||||||||
| LABA | ||||||||
| Non-use | 673 | 13,817 | 4.87 | 172 | 2,945 | 5.84 | 1.19 (1.01–1.41)* | 1.45 (1.22–1.72)*** |
| Current use (≤30 days) | 38 | 615 | 6.18 | 25 | 180 | 13.89 | 2.22 (1.33–3.70)** | 2.39 (1.31–4.34)** |
| Recent use (30–90 days) | 6 | 113 | 5.31 | 2 | 22 | 9.09 | 2.82 (0.45–17.4) | -- |
| Past use (>90 days) | 26 | 1,364 | 1.91 | 9 | 455 | 1.98 | 1.12 (0.52–2.40) | 3.72 (1.48–9.31)** |
| LAMA | ||||||||
| Non-use | 716 | 15,351 | 4.66 | 198 | 3,392 | 5.84 | 1.24 (1.06–1.46)** | 1.57 (1.34–1.84)*** |
| Current use (≤30 days) | 15 | 219 | 6.85 | 6 | 87 | 6.9 | 0.98 (0.38–2.54) | 0.52 (0.14–1.84) |
| Recent use (30–90 days) | 1 | 23 | 4.35 | 1 | 12 | 8.33 | 2.44 (0.15–39.7) | — |
| Past use (>90 days) | 11 | 316 | 3.48 | 3 | 111 | 2.7 | 0.73 (0.20–2.68) | — |
| SABA | ||||||||
| Non-use | 79 | 3,281 | 2.41 | 20 | 880 | 2.27 | 0.96 (0.59–1.58) | 1.51 (0.90–2.55) |
| Current use (≤30 days) | 441 | 10,799 | 4.08 | 128 | 2,304 | 5.56 | 1.35 (1.11–1.65)** | 1.61 1.31–1.96)*** |
| Recent use (30–90 days) | 208 | 1,632 | 12.75 | 58 | 374 | 15.51 | 1.12 (0.84–1.51) | 1.31 (0.94–1.82) |
| Past use (>90 days) | 15 | 197 | 7.61 | 2 | 44 | 4.55 | 0.60 (0.13–2.64) | 11.1 (0.56–21.8) |
| SAMA | ||||||||
| Non-use | 506 | 12,567 | 4.03 | 151 | 2,762 | 5.47 | 1.35 (1.12–1.62)** | 1.65 (1.37–1.98)*** |
| Current use (≤30 days) | 170 | 1,289 | 13.19 | 42 | 257 | 16.34 | 1.20 (0.86–1.69) | 1.45 (0.99–2.11) |
| Recent use (30–90 days) | 9 | 105 | 8.57 | 1 | 26 | 3.85 | 0.44 (0.05–3.54) | — |
| Past use (>90 days) | 58 | 1,948 | 2.98 | 14 | 557 | 2.51 | 0.86 (0.48–1.55) | 1.42 (0.76–2.65) |
| ICSs | ||||||||
| Non-use | 621 | 12,584 | 4.93 | 154 | 2,534 | 6.08 | 1.22 (1.02–1.46)* | 1.47 (1.22–1.75)*** |
| Current use (≤30 days) | 63 | 740 | 8.51 | 35 | 198 | 17.68 | 2.04 (1.34–3.10)*** | 3.23 (1.96–5.29)*** |
| Recent use (30–90 days) | 5 | 127 | 3.94 | 1 | 30 | 3.33 | 0.89 (0.10–8.04) | — |
| Past use (>90 days) | 54 | 2,458 | 2.2 | 18 | 840 | 2.14 | 1.03 (0.60–1.77) | 1.77 (0.98–3.20) |
| OSs | ||||||||
| Non-use | 85 | 672 | 12.65 | 35 | 75 | 46.67 | 2.81 (1.88–4.21)*** | 3.99 (2.54–6.27)*** |
| Current use (≤30 days) | 441 | 4,529 | 9.74 | 152 | 1,039 | 14.63 | 1.52 (1.27–1.83)*** | 1.76 (1.45–2.14)*** |
| Recent use (30–90 days) | 45 | 1,307 | 3.44 | 4 | 381 | 1.05 | 0.30 (0.10–0.83)* | 0.66 (0.23–1.93) |
| Past use (>90 days) | 172 | 9,401 | 1.83 | 17 | 2,107 | 0.81 | 0.44 (0.26–0.72)** | 0.63 (0.38–1.04) |
| Leukotriene antagonist | ||||||||
| Non-use | 724 | 15,167 | 4.77 | 196 | 3,273 | 5.99 | 1.25 (1.06–1.46)** | 1.55 (1.32–1.81)*** |
| Current use (≤30 days) | 6 | 56 | 10.71 | 7 | 38 | 18.42 | 1.53 (0.51–4.58) | — |
| Recent use (30–90 days) | 2 | 35 | 5.71 | 1 | 6 | 16.67 | 1.81 (0.16–20.5) | — |
| Past use (>90 days) | 11 | 651 | 1.69 | 4 | 285 | 1.4 | 0.80 (0.25–2.52) | 1.56 (0.21–11.1) |
| Montelukast | ||||||||
| Non-use | 725 | 15,170 | 4.78 | 197 | 3,296 | 5.98 | 1.24 (1.06–1.46)** | 1.54 (1.31–1.80)*** |
| Current use (≤30 days) | 5 | 53 | 9.43 | 7 | 38 | 18.42 | 1.66 (0.52–5.27) | — |
| Recent use (30–90 days) | 2 | 35 | 5.71 | 1 | 6 | 16.67 | 1.81 (0.16–20.5) | — |
| Past use (>90 days) | 11 | 651 | 1.69 | 3 | 262 | 1.15 | 0.66 (0.18–2.39) | |
| Alprazolam | ||||||||
| Non-use | 481 | 9,566 | 5.03 | 165 | 2,111 | 7.82 | 1.53 (1.28–1.83)*** | 1.86 (1.56–2.23)*** |
| Current use (≤30 days) | 102 | 1,253 | 8.14 | 27 | 228 | 11.84 | 1.51 (0.98–2.31) | 1.73 (1.08–2.75)* |
| Recent use (30–90 days) | 16 | 371 | 4.31 | 0 | 100 | 0 | — | — |
| Past use (>90 days) | 144 | 4,719 | 3.05 | 16 | 1,163 | 1.38 | 0.44 (0.26–0.74)** | 0.59 (0.35–1.01) |
| Fluoxetine | ||||||||
| Non-use | 743 | 15,909 | 4.67 | 208 | 3,602 | 5.77 | 1.23 (1.05–1.43)** | 1.54 (1.31–1.80)*** |
| Current use (≤30 days) | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Recent use (30–90 days) | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Past use (>90 days) | 0 | 0 | 0 | 0 | 0 | 0 | — | — |
| Fludiazepam | ||||||||
| Non-use | 619 | 12,722 | 4.87 | 178 | 2,852 | 6.24 | 1.27 (1.07–1.50)** | 1.59 (1.34–1.89)*** |
| Current use (≤30 days) | 20 | 268 | 7.46 | 10 | 79 | 12.66 | 1.69 (0.79–3.64) | 7.48 (1.93–28.9)** |
| Recent use (30–90 days) | 5 | 67 | 7.46 | 2 | 48 | 4.17 | 0.56 (0.10–2.93) | — |
| Past use (>90 days) | 99 | 2,852 | 3.47 | 18 | 623 | 2.89 | 0.86 (0.52–1.42) | 0.97 (0.58–1.63) |
BCAS cohort: bronchiectasis–asthma combination cohort; COPD, chronic obstructive pulmonary disease; LABAs/LAMAs, long-acting beta2 agonist/muscarinic antagonist; SABAs/SAMAs: short-acting beta2 agonist/muscarinic antagonist; ICSs, inhaled corticosteroids; OSs, oral steroids; PY, person-years; IR, incidence rate per 100 person-years; HR, hazard ratio; CI, confidence interval; HR, adjusted for BCAS cohort, gender, age, comorbidities, and medication use.
—Unable to calculate because of there are few or no events in the with and without BCAS cohort.
p < 0.05, **p < 0.01, ***p < 0.001.
FIGURE 1.
Using Kaplan–Meier survival statistics, the crude overall survival curves with and without bronchiectasis–asthma cohort are shown (log-rank p < 0.0001).
FIGURE 2.
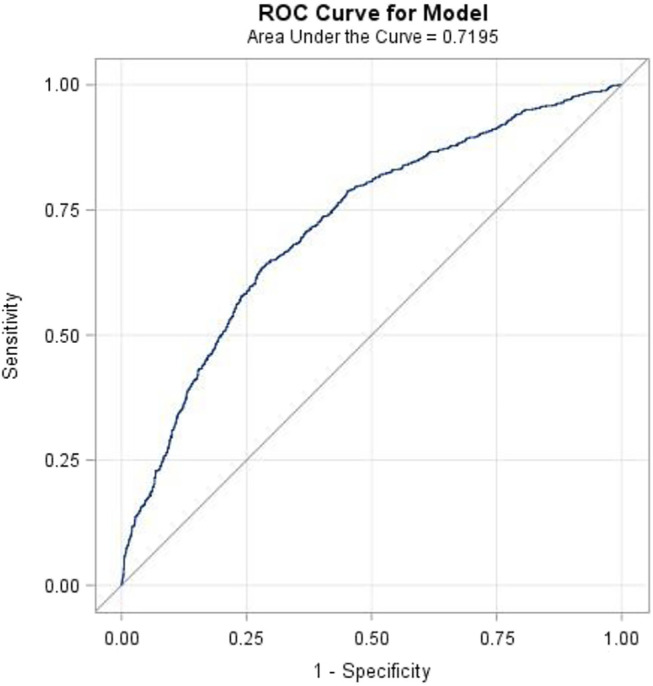
The area under the curve (AUC) for bronchiectasis-asthma cohort applied to predict pulmonary tuberculosis or pneumonia.
Propensity Matching
Before the experiment of propensity score matching, we performed a logistic regression to calculate the propensity score. Then, we estimated the probability of the BCAS cohort on the basis of the baseline variables, namely, index year, sex, age, history of comorbidities, and medicine. Finally, we performed multivariable Cox proportional hazards model stratification on the non-BCAS cohort to estimate the risks of PTB or pneumonia in the two cohorts.
Immortal Time Bias
Users of drugs were defined as those who received at least one prescription for drugs (including bronchodilators, steroids, leukotriene receptor antagonist, montelukast, anti-arrhythmic, antidepressants, and antianxiety drugs) between the BCAS cohort diagnosis date and index date. The date of prescription and number of days supplied were identified. The use of drugs was approved in Taiwan in January 2000 and was placed on the listing of NHI drugs for reimbursement in February 2001. The drug users were defined as patients who received prescription for >28 days and after index date, and those who did not receive drug prescription were classified as non-users of drugs. The immortal time bias is eliminated. Any drugs prescribed after index date and before the endpoints for the subjects were considered as exposure.
The Taiwan government launched the pay-for-performance of asthma (asthma P4P program) in 2001 to encourage clinics and hospitals to provide patient-centered care and focus on disease management. This program includes initial visit for new patients, outpatient care, hospitalization, first prescription, emergency visits, and drug refill prescription (codes for disease management and care fees: P1612C, P1613C, P1614B, and P1615C, https://www.nhi.gov.tw/Content-). The initial visit for new patients and first prescription could help us to confirm if the patients are alive or not. These strict policies help us to eliminate the immortal time bias in this study (Suissa and Ernst, 2020).
Validation of BCAS
One NHIRD study achieved high-sensitivity diagnosis of asthma with up to 92.0% accuracy on the basis of the clinical manifestation of asthma, family history of asthma, chest-X ray (CXR), and biochemistry and pulmonary function tests (Su et al., 2018). In Taiwan, the presence of bronchiectasis was confirmed based on the following high-resolution computed tomography (HRCT) criteria: 1) lack of tapering in the bronchi; 2) dilation of the bronchi where the internal diameter was larger than that of the adjacent pulmonary artery (e.g., broncho–arterial ratios >1); or 3) visualization of the peripheral bronchi within 1 cm of the costal pleural surface or the adjacent mediastinal pleural surface (Hill et al., 2019; Huang et al., 2020). Huang et al. (2020) used to diagnose bronchiectasis HRCT in 10,724 of the 15,729 patients (68.1%) and CXR in 5,005 of the 15,729 (31.9%) patients in a bronchiectasis cohort from the hospital database, and the average portion of patients having HRCT images increased annually (2002–2005: 43.1%, 2006–2010: 71.0%, and 2011–2016: 90.3%).
In this study, asthma was defined with ICD-9 CM code 493, combined with at least two prescriptions of anti-asthmatic drugs. Anti-asthmatic drugs include LABAs/LAMAs/SABAs/SAMAs/leukotriene receptor antagonist/montelukast/ICSs/OSs (Su et al., 2018; Yeh et al., 2019). Bronchiectasis was defined with ICD-9-CM code 494, combined with at least one CXR (including the posterior–anterior and lateral view) with computed tomography (CT) or two CXR with at least one inpatient visit or two outpatient visits. Moreover, the BCAS cohort receives procedures such as CXR (100%); CT-related test (83%); pulmonary function-related test (92.5%); asthma-related test and examination (96%); and medications such as the use of OSs (91.6%), SABA (34.8%), and ICSs (29.5%) (for code names, see Supplementary Table S1). These procedures, medications, and strategies help us to confirm the diagnosis of the BCAS cohort. Thus, subgroups of the BCAS cohort such as pure BCAS were derived from the bronchiectasis cohort presenting as patients having both components of the bronchiectasis and asthma in the general population (summary findings in Supplementary Table S2).
After Matching Study Asthma Therapies on BCAS and Non-BCAS Cohorts
In the non-BCAS cohort (1 + 2 + 3 + 4 + 5 + 8), only 3 and 5 have the asthma component. The BCAS cohort (6 + 7), including 6 and 7, all have the asthma component; thus, the frequency of asthma therapies (LABAs, ICSs, leukotriene receptor antagonist, and montelukast use) in the BCAS cohort was higher than the non-BCAS cohort (Supplementary Figure S1).
Validation of Tuberculosis and Use Interferon-Gamma for Early Detection of LTBI
During 1996–1999, tuberculosis and pneumonia were excluded before entry study, and only incident PTB or pneumonia were entered into the study (2000–2012, follow-up to 2013). The latency of the reactivation of the latent TB infection (LTBI) is about 2–5 years (Vynnycky and Fine, 1997). Thus, this wash out period (1996–1999) may be enough to help us to avoid these confounding factors.
Current methods for the diagnosis of LTBI are tuberculin skin test (TST) and interferon-gamma release assay (IGRA). The use of interferon for detecting LTBI was initiated from 2009 in Taiwan based on a published report (Chan et al., 2011; Lai et al., 2011). Also, like TST, IGRAs cannot distinguish between LTBI, active TB, or past infection. However, CXR, acid-fast stain, and culture for detecting active PTB were popular in Taiwan. Thus, the validation of PTB in NHIRD has high sensitivity (Su et al., 2016; Hsieh et al., 2019).
Epidemiologic Studies Can Indicate Strengths of Associations Between PTB Pneumonia and the BCAS Cohort
This is fundamentally the same methodology as for a prospective cohort study, except that the retrospective study is performed post-hoc, looking back. Owing to the data of CXR, TST, and IGRA, smear or culture for bacteria and TB was unavailable in the NHIRD. As mentioned before, we excluded PTB or pneumonia before the entry study. However, post-PTB or post-pneumonia was a predisposing factor of airway disease (Bashir et al., 2016; Basham et al., 2021). Thus, we used the terms such as “BCAS cohort is associated with PTB or pneumonia” and “bronchodilators and steroids are associated with PTB or pneumonia in BCAS cohort” in this study. Therefore, it is important to recognize that causality cannot be established definitively through in our study; however, this study could provide important evidence to suggest information regarding the strength of an association between the BCAS cohort, bronchodilators, steroids, and infection such as PTB or pneumonia.
Discussion
To the best of our knowledge, this study used the largest group to date to determine the effect of bronchodilators, steroids, antidepressant medicines, and BZDs on the risks of PTB or pneumonia among patients with the BCAS cohort. This study yielded three major findings as follows: 1) the risks of PTB or pneumonia were higher in the BCAS cohort than in the non-BCAS cohort; 2) compared to the non-BCAS cohort, in the BCAS cohort, bronchodilator (LABAs/SABAs) and steroid use was associated with risks of PTB or pneumonia, but BZDs and LAMAs/SAMAs were not; 3) current LABA/SABA and steroids use and BZDs use were associated with risks of PTB or pneumonia, but current LAMA/SAMA use was not associated with risks of PTB or pneumonia; and 4) the use of non-LABAs, non-OSs, and non-BZDs as reference 1 revealed that LABA, OS, and BZD use was associated with low risks of PTB or pneumonia.
The elevated neutrophil levels in sputum were associated with more exacerbations, low lung function, and greater duration and severity of bronchiectasis. Similarly, neutrophil elastase, derived from neutrophils, was found to be associated with an increased severity of bronchiectasis (Polverino et al., 2018; Mincham et al., 2021). The subgroups of the BCAS cohort such as BCAOS (7) include the asthma + bronchiectasis + COPD, and this triple component has complicated asthma, COPD, and bronchiectasis with high activity of the neutrophil (Polverino et al., 2018; Coman et al., 2018; Mihălț;an and Constantin, 2019; Mincham et al., 2021). The excessive activation of neutrophils results in the release of neutrophil elastase, leading to microenvironments that predispose to PTB or pneumonia among the BCAS cohort (Porto and Stein, 2016; Domon et al., 2018; Mihălț;an and Constantin, 2019). Thus, the BCAS cohort has a higher risk of PTB or pneumonia. Meanwhile, the subgroups of the BCAS cohort such as pure BCAS (6) with comorbidities such as diabetes, stroke, and heart disease also have higher risks (Porto and Stein, 2016; van der Meer et al., 2017; Domon et al., 2018).
Macrophage and monocytes expressed BZD-sensitive γ-aminobutyric acid type A (GABAA) receptors. The macrophage GABAA receptor expression was regulated by bacterial Toll-like receptor agonists and cytokines, indicating an endogenous role in the immune response (Falcón et al., 2021). One previous study suggested that BZDs may negatively influence immune function via the activation of GABAA receptors on immune cells such as activation or suppression of cytokine secretion and modification of cell proliferation, and GABAA can even affect migration of the cells, thus interfering with macrophages/monocytes and impairing cytokine release, phagocytosis, and bacterial-killing capabilities (Sanders et al., 2013; Falcón et al., 2021). In addition, BZDs may increase the risk of aspiration by decreasing lower esophageal sphincter pressure (Obiora et al., 2013; Chang et al., 2016). Therefore, BZDs might have predicted the outcome in the analysis, and these drugs were entered into the analysis to start with (Huang et al., 2021).
Patients with non-cystic fibrosis bronchiectasis are at increased risk for depression and anxiety. Untreated and undetected depressive/anxiety symptoms may increase physical disability, morbidity, and health care utilization (Wynne et al., 2020). BZDs play an auxiliary role in the management of bronchiectasis especially in the late course of this disease (Kapnadak et al., 2020). In this study, the use of non-LABAs, non-OSs, and non-BZDSs as reference 1 revealed that LABA, OS, and BZD use was associated with low risks of PTB or pneumonia. Another study reported that anxiety was associated with the risks of PTB or pneumonia in BCAS in combination with low HRQoL (Duko et al., 2015; Gao et al., 2018). Thus, relieving anxiety may have a benefit for the prevention of the risk of PTB or pneumonia in patients within the BCAS cohort (Lehrer et al., 2008; Patel et al., 2019). However, patients with current BZD use (≤30 days, BZDs) but not past use (>90 days) had increased risks of PTB or pneumonia.
As with any propensity analysis, hidden, impactful confounders are always a possibility, and these BZD drugs may simply point to a hidden confounding variable such as the comorbidity diabetes. It is to be noted that comorbid-related inflammatory cytokines and stimuli reduce GABAA receptor expression on alveolar macrophage (Antony et al., 2010; Obiora et al., 2013). A reduction in the expression of the receptor target, by comorbid inflammation, would be expected to reduce the immune effects of BZDs, leading to risk of PTB or pneumonia (Sanders et al., 2013).
These findings imply that 1) the primary effect of the BCAS cohort such as triplet (7, BCAOS) was a critical factor of the PTB or pneumonia; 2) the BCAS cohort such as pure BCAS (6) with comorbidities such as diabetes, stroke, and heart disease might be the relevant factor in subsequent PTB or pneumonia; and 3) perhaps steroids and BZDs have synergistic effects on these factors, leading to PTB or pneumonia.
As such, protopathic bias is a systematic error that occurs when measured exposure status medications may be affected by the latent onset of the target outcome—PTB or pneumonia. The lag-time approach consists in excluding from exposure assessment (PTB or pneumonia) the period immediately preceding the outcome detection date (≤30 days) (Arfè and Corrao, 2016). In this study, we excluded the patients with PTB or pneumonia before they enter into analysis. However, we found that the past use of medications (>90 days) is not associated with the risk of PTB or pneumonia except LABAs. These findings indicated that the protopathic bias may be an explanation of the association between the current use (≤30 days) of SABAs, steroids, and BZDs and PTB or pneumonia. If we take this possible protopathic bias into account, perhaps, the primary BCAS, especially its comorbidities, were key factors for the incident of PTB or pneumonia, and medications play a role on the synergistic effects.
In summary, because of the risks of PTB or pneumonia, LABAs/SABAs, steroids, and BZDs may be used in the BCAS cohort after benefit evaluation. However, LAMAs/SAMAs are safe options for the management of the BCAS cohort. Notably, we must take possible protopathic bias into account when we explain these findings.
Strength
The accuracy of medical records in the NHIRD is high, making it a valid resource for population-based research on PTB or pneumonia. Bronchodilator and steroid use in Taiwan follows international guidelines (Yeh et al., 2018). Patients who use steroids were associated with PTB. Because bronchiectasis, asthma, and COPD were validated in the previous study on the basis of the NHIRD, the BCAS cohort data in the NHIRD is reasonable (Su et al., 2018; Huang et al., 2020). Therefore, our method prevented potential bias. In addition, we followed-up with the patients from 2000 to 2013 to monitor the incidence of PTB or pneumonia in the BCAS cohort (Huang et al., 2020).
Limitations
Possible limitations of this study are bias and confounding variables, such as confounding by indication, protopathic bias, and surveillance bias. We used several strategies to control for bias and confounding variables, such as the new user design (entry after cohort) and the propensity matching method. With this framework, the results are not as accurate as those of randomized control trials. Data on lifestyle changes (exercise and diet) in low-adherence and high-adherence patients are unavailable in the NHIRD. Interferon use was associated with PTB infection. Owning to the higher frequency use of steroids than the interferon use in BCAS cohort and non-BCAS cohort, we replace the immunosuppressant (including the interferon) with steroids for analysis. However, this is another limitation. Finally, not all patients received HRCT in this study but at least two CXR for comparison of the irreversible change of bronchial tree (e.g., tram-track opacities) in outpatient visit or hospitalization. These confounding factors may have led to some bias in this study.
Conclusion
The LAMAs/SAMAs are relatively safe with respect to PTB or pneumonia risks, but LABAs/SABAs, steroids, and BZDs could be used after evaluation of the benefit for BCAS cohort. However, we must take the possible protopathic bias into account.
Summary
For pulmonary tuberculosis or pneumonia in predominant bronchiectasis–asthma, the long-acting/short-acting beta2 agonist, steroids, and benzodiazepines are to be used with caution; long-acting/short-acting muscarinic antagonists were relatively safe.
Data Availability Statement
The datasets presented in this article are not readily available because it is held by the Taiwan Ministry of Health and Welfare (MOHW). The Ministry of Health and Welfare must approve our application to access this data. Any researcher interested in accessing this dataset can submit an application form requesting access to the Ministry of Health and Welfare. Please contact the staff of MOHW (email: stcarolwu@mohw.gov.tw) for further assistance. The Taiwan Ministry of Health and Welfare is located in the following address: No.488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (R.O.C.) (phone: +886-2-8590-6848). All relevant data are within the paper.
Ethics Statement
This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115-AR4). The IRB also specifically waived the written informed consent for participation in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception and design: J-JY and H-CL. Administrative support: C-HK. Collection and assembly of data: J-JY and H-CL. Data analysis and interpretation: J-JY and H-CL. Manuscript writing: J-JY and H-CL. Final approval of manuscript: J-JY and H-CL. All authors contributed to the article and approved the submitted version.
Funding
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), the China Medical University Hospital (DMR-109-231, DMR-110-089, DMR-111-090, DMR-111-091, DMR-111-228), and the Ministry of Science and Technology (MOST 110-2321-B-039-003). The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.790031/full#supplementary-material
Abbreviations
aHR, adjusted hazard ratio; PTB, pulmonary tuberculosis; BCAS, bronchiectasis–asthma combination; LABA, long-acting beta2 agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting beta2 agonist; SAMA, short-acting muscarinic antagonists; CI, confidence interval; ICSs, inhaled corticosteroids; OSs, oral steroids; BZDs, benzodiazepines; NHIRD, National Health Insurance Research Database; LHID, Longitudinal Health Insurance Database; ICD-9-CM, International Classification of Diseases, Ninth revision, Clinical Modification.
References
- Antony S., Kumar P., George N., Chinthu R., Paulose C. (2010). Hypoglycemia Induced Behavioral Deficit and Decreased GABA Receptor, CREB Expression in the Cerebellum of Streptozoticin Induced Diabetic Rats. Brain Res. Bull. 83, 360–366. 10.1016/j.brainresbull.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Arfè A., Corrao G. (2016). The Lag-Time Approach Improved Drug-Outcome Association Estimates in Presence of Protopathic Bias. J. Clin. Epidemiol. 78, 101–107. 10.1016/j.jclinepi.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Basham C. A., Karim M. E., Cook V. J., Patrick D. M., Johnston J. C. (2021). Post-tuberculosis Airway Disease: A Population-Based Cohort Study of People Immigrating to British Columbia, Canada, 1985-2015. EClinicalMedicine. 33, 100752. 10.1016/j.eclinm.2021.100752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir A., Abdallah I. E., Musa O. (2016). TB Infection Decreases Asthma Prevalence and Severity of Symptoms. Eur. Respir. J. 48, PA2706. 10.1183/13993003.congress-2016.PA2706 [DOI] [Google Scholar]
- Chan P. C., Yang C. H., Chang F. Y. (2011). Scaling up of Latent Tuberculosis Infection Treatment for Close Contacts of Tuberculosis in Taiwan. J. Formos. Med. Assoc. 110, 733–736. 10.1016/j.jfma.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Chang T. M., Mou C. H., Shen T. C., Yang C. L., Yang M. H., Wu F. Y., et al. (2016). Retrospective Cohort Evaluation on Risk of Pneumonia in Patients with Pulmonary Tuberculosis. Medicine (Baltimore) 95, e4000. 10.1097/MD.0000000000004000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman I., Pola-Bibián B., Barranco P., Vila-Nadal G., Dominguez-Ortega J., Romero D., et al. (2018). Bronchiectasis in Severe Asthma: Clinical Features and Outcomes. Ann. Allergy Asthma Immunol. 120, 409–413. 10.1016/j.anai.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Domon H., Nagai K., Maekawa T., Oda M., Yonezawa D., Takeda W., et al. (2018). Neutrophil Elastase Subverts the Immune Response by Cleaving Toll-like Receptors and Cytokines in Pneumococcal Pneumonia. Front. Immunol. 9, 732. 10.3389/fimmu.2018.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duko B., Gebeyehu A., Ayano G. (2015). Prevalence and Correlates of Depression and Anxiety Among Patients with Tuberculosis at WolaitaSodo University Hospital and Sodo Health Center, WolaitaSodo, South Ethiopia, Cross Sectional Study. BMC Psychiatry. 15, 214. 10.1186/s12888-015-0598-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcón C. R., Hurst N. F., Vivinetto A. L., López P. H. H., Zurita A., Gatti G., et al. (2021). Diazepam Impairs Innate and Adaptive Immune Responses and Ameliorates Experimental Autoimmune Encephalomyelitis. Front. Immunol. 12, 682612. 10.3389/fimmu.2021.682612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J. Y., Fang W. F., Wu C. L., Yu C. J., Lin M. C., Ku S. C., et al. (2012). Concomitant Pulmonary Tuberculosis in Hospitalized Healthcare-Associated Pneumonia in a Tuberculosis Endemic Area: a Multi-center Retrospective Study. PLoS One 7, e36832. 10.1371/journal.pone.0036832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. H., Guan W. J., Zhu Y. N., Chen R. C., Zhang G. J. (2018). Anxiety and Depression in Adult Outpatients with Bronchiectasis: Associations with Disease Severity and Health-Related Quality of Life. Clin. Respir. J. 12, 1485–1494. 10.1111/crj.12695 [DOI] [PubMed] [Google Scholar]
- Hill A. T., Sullivan A. L., Chalmers J. D., De Soyza A., Elborn S. J., Floto A. R., et al. (2019). British Thoracic Society Guideline for Bronchiectasis in Adults. Thorax 74, 1–69. 10.1136/thoraxjnl-2018-212463 [DOI] [PubMed] [Google Scholar]
- Hsieh C. Y., Su C. C., Shao S. C., Sung S. F., Lin S. J., Kao Yang Y. H., et al. (2019). Taiwan's National Health Insurance Research Database: Past and Future. Clin. Epidemiol. 11, 349–358. 10.2147/CLEP.S196293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. Y., Chung F. T., Lo C. Y., Lin H. C., Huang Y. T., Yeh C. H., et al. (2020). Etiology and Characteristics of Patients with Bronchiectasis in Taiwan: a Cohort Study from 2002 to 2016. BMC Pulm. Med. 20, 45. 10.1186/s12890-020-1080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. H., Tai C. J., Kuan Y. H., Chang Y. C., Tsai T. H., Lee C. Y. (2021). Pneumonia Risk Associated with the Use of Individual Benzodiazepines and Benzodiazepine Related Drugs Among the Elderly with Parkinson's Disease. Int. J. Environ. Res. Public Health 18, 9410. 10.3390/ijerph18179410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C.-T., Li S. F., Chung W. S. (2018). Increased Risk of Acute Coronary Syndrome in Patients with Bronchiectasis: A Population-Based Cohort Study. Respirology 23, 828–834. 10.1111/resp.13298 [DOI] [PubMed] [Google Scholar]
- Kapnadak S. G., Dimango E., Hadjiliadis D., Hempstead S. E., Tallarico E., Pilewski J. M., et al. (2020). Cystic Fibrosis Foundation Consensus Guidelines for the Care of Individuals with Advanced Cystic Fibrosis Lung Disease. J. Cyst Fibros. 19, 344–354. 10.1016/j.jcf.2020.02.015 [DOI] [PubMed] [Google Scholar]
- Lai C. C., Tan C. K., Lin S. H., Liao C. H., Huang Y. T., Hsueh P. R. (2011). Diagnostic Performance of Whole-Blood Interferon-γ Assay and Enzyme-Linked Immunospot Assay for Active Tuberculosis'. Diagn. Microbiol. Infect. Dis. 71, 139–143. 10.1016/j.diagmicrobio.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Lee C. M., Heo J., Han S. S., Moon K. W., Lee S. H., Kim Y. J., et al. (2019). Inhaled Corticosteroid-Related Tuberculosis in the Real World Among Patients with Asthma and COPD: A 10-Year Nationwide Population-Based Study. J. Allergy Clin. Immunol. Pract. 7, 1197. 10.1016/j.jaip.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Lehrer P. M., Karavidas M. K., Lu S. E., Feldman J., Kranitz L., Abraham S., et al. (2008). Psychological Treatment of Comorbid Asthma and Panic Disorder: a Pilot Study. J. Anxiety Disord. 22, 671–683. 10.1016/j.janxdis.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García M. Á., Máiz L., Olveira C., Girón R. M., de la Rosa D., Blanco M., et al. (2018). Spanish Guidelines on Treatment of Bronchiectasis in Adults. Arch. Bronconeumol 54, 88–98. 10.1016/j.arbres.2017.07.016 [DOI] [PubMed] [Google Scholar]
- Mihălțan F., Constantin A. (2019). Asthma–COPD–Bronchiectasis Combination: an Unstudied Triplet. Pneumologia 68, 58–60. 10.2478/pneum-2019-0015 [DOI] [Google Scholar]
- Mincham K. T., Bruno N., Singanayagam A., Snelgrove R. J. (2021). Our Evolving View of Neutrophils in Defining the Pathology of Chronic Lung Disease. Immunology 164, 701–721. 10.1111/imm.13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiora E., Hubbard R., Sanders R. D., Myles P. R. (2013). The Impact of Benzodiazepines on Occurrence of Pneumonia and Mortality from Pneumonia: a Nested Case-Control and Survival Analysis in a Population-Based Cohort. Thorax 68, 163–170. 10.1136/thoraxjnl-2012-202374 [DOI] [PubMed] [Google Scholar]
- Patel S., Cole A. D., Nolan C. M., Barker R. E., Jones S. E., Kon S., et al. (2019). Pulmonary Rehabilitation in Bronchiectasis: a Propensity-Matched Study. Eur. Respir. J. 53, PA663. 10.1183/13993003.01264-2018 [DOI] [PubMed] [Google Scholar]
- Polverino E., Dimakou K., Hurst J., Martinez-Garcia M. A., Miravitlles M., Paggiaro P., et al. (2018). The Overlap between Bronchiectasis and Chronic Airway Diseases: State of the Art and Future Directions. Eur. Respir. J. 52, 1800328. 10.1183/13993003.00328-2018 [DOI] [PubMed] [Google Scholar]
- Porto B. N., Stein R. T. (2016). Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 7, 311. 10.3389/fimmu.2016.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo M., Keyt H., Dhar R., Chalmers J. D. (2016). Global Impact of Bronchiectasis and Cystic Fibrosis. Breathe (Sheff) 12, 222–235. 10.1183/20734735.007516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. D., Godlee A., Fujimori T., Goulding J., Xin G., Salek-Ardakani S., et al. (2013). Benzodiazepine Augmented γ-amino-butyric Acid Signaling Increases Mortality from Pneumonia in Mice. Crit. Care Med. 41, 1627–1636. 10.1097/CCM.0b013e31827c0c8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantakumar S., Pwu R. F., D'Silva L., Wurst K., Kuo Y. W., Yang Y. Y., et al. (2018). Burden of Asthma and COPD Overlap (ACO) in Taiwan: a Nationwide Population-Based Study. BMC Pulm. Med. 18, 16. 10.1186/s12890-017-0571-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su V. Y., Yang K. Y., Yang Y. H., Tsai Y. H., Perng D. W., Su W. J., et al. (2018). Use of ICS/LABA Combinations or LAMA Is Associated with a Lower Risk of Acute Exacerbation in Patients with Coexistent COPD and Asthma. J. Allergy Clin. Immunol. Pract. 6, 1927. 10.1016/j.jaip.2018.01.035 [DOI] [PubMed] [Google Scholar]
- Su V. Y., Yen Y. F., Pan S. W., Chuang P. H., Feng J. Y., Chou K. T., et al. (2016). Latent Tuberculosis Infection and the Risk of Subsequent Cancer. Medicine (Baltimore) 95, e2352. 10.1097/MD.0000000000002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S., Ernst P. (2020). Avoiding Immortal Time Bias in Observational Studies. Eur. Respir. J. 55, 2000138. 10.1183/13993003.00138-2020 [DOI] [PubMed] [Google Scholar]
- Truong T. (2013). The Overlap of Bronchiectasis and Immunodeficiency with Asthma. Immunol. Allergy Clin. North. Am. 33, 61–78. 10.1016/j.iac.2012.10.007 [DOI] [PubMed] [Google Scholar]
- van der Meer A. J., Zeerleder S., Blok D. C., Kager L. M., Lede I. O., Rahman W., et al. (2017). Neutrophil Extracellular Traps in Patients with Pulmonary Tuberculosis. Respir. Res. 18, 181. 10.1186/s12931-017-0663-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren L. E. (2016). Asymptomatic COPD, until You Take it to Exertion. Thorax. 71, 781–782. 10.1136/thoraxjnl-2016-208863 [DOI] [PubMed] [Google Scholar]
- Veith M., Tüffers J., Peychev E., Klemmer A., Kotke V., Janciauskiene S., et al. (2020). The Distribution of Alpha-1 Antitrypsin Genotypes between Patients with COPD/Emphysema, Asthma and Bronchiectasis. Int. J. Chron. Obstruct Pulmon Dis. 15, 2827–2836. 10.2147/COPD.S271810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vynnycky E., Fine P. E. (1997). The Natural History of Tuberculosis: the Implications of Age-dependent Risks of Disease and the Role of Reinfection. Epidemiol. Infect. 119, 183–201. 10.1017/s0950268897007917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne S. C., Patel S., Barker R. E., Jones S. E., Walsh J. A., Kon S. S., et al. (2020). Anxiety and Depression in Bronchiectasis: Response to Pulmonary Rehabilitation and Minimal Clinically Important Difference of the Hospital Anxiety and Depression Scale. Chron. Respir. Dis. 17, 1479973120933292. 10.1177/1479973120933292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. J., Lin C. L., Hsu C. Y., Shae Z., Kao C. H. (2019). Associations between Statins and Coronary Artery Disease and Stroke Risks in Patients with Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome: A Time-dependent Regression Study. Atherosclerosis 283, 61–68. 10.1016/j.atherosclerosis.2019.02.007 [DOI] [PubMed] [Google Scholar]
- Yeh J. J., Lin C. L., Hsu C. Y., Shae Z., Kao C. H. (2018). Statin for Tuberculosis and Pneumonia in Patients with Asthma–Chronic Pulmonary Disease Overlap Syndrome: A Time-dependent Population-Based Cohort Study. J. Clin. Med. 7, 381. 10.3390/jcm7110381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. J. (2019). Predictors of Initial Smear-Negative Active Pulmonary Tuberculosis with Acute Early Stage Lung Injury by High-Resolution Computed Tomography and Clinical Manifestations: An Auxiliary Model in Critical Patients. Sci. Rep. 9, 4527. 10.1038/s41598-019-40799-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. J., Lin C. L., Kao C. H. (2020). Associations Among Chronic Obstructive Pulmonary Disease with Asthma, Pneumonia, and Corticosteroid Use in the General Population. PLOS ONE 15, e0229484. 10.1371/journal.pone.0229484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Li J., Liu H., Han N., Ju J., Kou Y., et al. (2020). Long-term Bone and Lung Consequences Associated with Hospital-Acquired Severe Acute Respiratory Syndrome: a 15-year Follow-Up from a Prospective Cohort Study. Bone Res. 8, 8. 10.1038/s41413-020-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because it is held by the Taiwan Ministry of Health and Welfare (MOHW). The Ministry of Health and Welfare must approve our application to access this data. Any researcher interested in accessing this dataset can submit an application form requesting access to the Ministry of Health and Welfare. Please contact the staff of MOHW (email: stcarolwu@mohw.gov.tw) for further assistance. The Taiwan Ministry of Health and Welfare is located in the following address: No.488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (R.O.C.) (phone: +886-2-8590-6848). All relevant data are within the paper.