Abstract
Objective:
There is an interrelationship between thrombosis and inflammation. Previously, we have shown the importance of P-selectin in thrombogenesis and thrombus resolution in many preclinical animal models. The role of E-selectin has been explored in rodent models and in a small pilot study of clinical calf vein deep vein thrombosis (DVT). The purpose of this study is to determine the role of E-selectin in thrombosis in a primate model of proximal iliac vein thrombosis, a model close to the human condition.
Methods:
Iliac vein thrombosis was induced with a well characterized primate model. Through a transplant incision, the hypogastric vein and iliac vein branches were ligated. Thrombus was induced by balloon occlusion of the proximal and distal iliac vein for 6 hours. The balloons were then deflated and primates recovered. Starting on post-occlusion Day 2, animals were treated (TRT) with the E-selectin inhibitor (GMI-1271), 25 mg/kg, SC, once daily until Day 21 (n=4). Non-treated control (CTR) animals received no treatment (n=5). All animals were evaluated by magnetic resonance venography (MRV), vessel area by ultrasound, protein analysis, hematology (CBC), coagulation tests (bleeding time, PT, aPTT, fibrinogen, and thromboelastography) at baseline, Day 2, Day 7, Day14, and Day 21 with euthanasia. Additionally, platelet function and CD44 expression on leukocytes was determined.
Results:
E-selectin inhibition by GMI-1271 significantly increased vein recanalization by MRV versus CTR animals on Day 14 (*P<0.05) and Day 21(#P<0.0001). GMI-1271 significantly decreased vein wall inflammation by MRV with gadolinium vein wall enhancement verses CTR also on Day 14 (#P<0.0001) and Day 21 (#P<0.0001). The thromboelastographic measure of clot strength (MA), showed significant decreases in animals treated with GMI-1271 versus CTRs at Day 2 (*P<0.05) and Day 7 (*P<0.05). Animals treated with GMI-1271 had significant vessel area increase by Day 21 verses CTRs (*P<0.05). Vein wall intimal thickening (***P<0.001) and intimal fibrosis (*P<0.05) scoring were significantly decreased in GMI-1271 treated animals versus controls. Importantly, no significant differences in hematology or coagulation tests were noted between all groups, suggesting that E-selectin inhibition carries no bleeding potential. GMI-1271 did not affect platelet function or aggregation, or CD44 expression on leukocytes. Additionally, no episodes of bleeding were noted in either groups.
Conclusions:
The current study suggests that E-selectin modulates venous thrombus progression and its inhibition will increase thrombus recanalization and decrease vein wall inflammation, without affecting coagulation. The use of an E-selectin inhibitor such as GMI-1271 could potentially change how we treat DVT.
Keywords: Venous Thrombosis, E-selectin inhibition, Inflammation, E-selectin inhibition, Glycomimetic, Animal models, Nonhuman primate, Coagulation
Table of Contents Summary
This basic science study in a primate model of iliac vein thrombosis found that E-selectin inhibition by GMI-1271 decreased venous thrombus progression, increased thrombus recanalization, and decreases vein wall inflammation at 21 days after treatment, without affecting coagulation.
Introduction
Venous thromboembolism (VTE), which includes DVT and pulmonary embolism (PE), may affect up to 900,000 patients per year, with over 300,000 deaths per year in our country. The incidence has remained constant over the past 30 years and may actually be increasing. We have previously identified E-selectin as an important regulator of thrombus formation and fibrin content in a mouse gene-deletion venous thrombosis model. In preliminary data, we have shown that E-selectin inhibition leads to a 50% to 60% reduction in thrombus weight. The current agents that we have to treat VTE are all based on targeting some part of the coagulation pathway and as such, they all have bleeding potential (1, 2). Therefore, new therapeutics that are effective in limiting thrombosis, decreasing inflammation, and having lower bleeding potential remains an unmet need. E-selectin also appears to be important for thrombogenesis. We have identified E-selectin as an important regulator of thrombus formation and fibrin content in a mouse venous thrombosis model (3, 4). Endotoxin induced tissue factor (TF) mediated coagulation is enhanced in humans carrying the S128R E-selectin allele (5). Patients homozygous for the S128R E-selectin allele have an increased risk for VTE recurrence, highlighting the importance of E-selectin in venous thrombosis (6). E-selectin has been shown to be efficient at raising the affinity and avidity of CD18 (MAC-1) integrins which support neutrophil trafficking to sites of acute inflammation and recruit platelets and red blood cells (7). In studies using an E-selectin inhibitor (GMI-1271) using a mouse flow model of thrombosis, we discovered that GMI-1271 in a dose dependent fashion was equivalent to enoxaparin for inhibiting thrombosis, but with a significant decrease in tail vein bleeding time (8). In a recent small clinical pilot study using GMI-1271 to assess its safety in normal volunteers and efficacy in the treatment of calf vein DVT, two patients were treated for 5 days with GMI-1271. In the normal volunteers, GMI-1271 was found safe when given once or once daily 5 days in a row. Both patients had an excellent clinical response with immediate relief of pain and significant increase in vein recanalization over time.
The purpose of this study was to evaluate the effects of a specific E-selectin inhibitor, GMI-1271 on thrombosis in a primate model of iliac vein thrombosis, a model close to the human condition, in preparation for future studies on combining this inhibitor with standard therapy at lower doses.
Methods
E-selectin inhibition
GMI-1271 as previously described (Molecular Weight = 1326.5) was provided by GlycoMimetics, Inc., (Rockville, MD, USA) (8). GMI-1271 is a glycomimetic that binds to E-selectin about 1000 times stronger than the native carbohydrate ligand sialyl Le x/a determined by either IC50 values or KD determinations (ave. KD = 450 nM). GMI-1271 functions by binding this domain on E-selectin (expressed on endothelial cells) and therefore blocking the binding of the native carbohydrate ligand sialyl Le x/a expressed on inflammatory cells such as neutrophils and monocytes. This blockage prevents the adhesion of inflammatory cells to endothelial cells and subsequent infiltration. GMI-1271 was solubilized in 0.3% NaCL – 10mM Tris buffer, pH adjusted to 7.3 – 7.6. All solutions were sterile filtered with a 0.2micron filter. A concentration of 100 mg/ml was utilized for subcutaneous administration.
The GMI-1271 25mg/kg subcutaneous dose was chosen based upon comparison of human Phase 1 VITA pharmacokinetic data utilizing GMI 1271 (2, 5, 20, and 40 mg/kg dose) compared to pre-clinical pharmacokinetic safety studies of GMI-1271 in rodents and nonhuman primates. A non-compartmental pharmacokinetic study of GMI-1271 (IV and SC) was performed in baboons (n=3) using 25mg/kg and 50mg/kg doses. The 25mg/kg subcutaneous dose in baboon demonstrated comparable area under the curve (AUC) values to a human 20mg/kg IV dose (128,700 h*ng/ml in baboon with 2.2 hour half-life versus 153,522 h*ng/ml in human with 1.42 hour half-life, respectively). AUC values for baboon were linear within a 25–50mg/kg range. Mean residual times for baboon 25 and 50 mg/kg subcutaneous doses were similar. Based upon these findings a 25mg/kg subcutaneous dose was utilized.
Baboon Model of Iliac Vein Thrombosis and Experimental Design
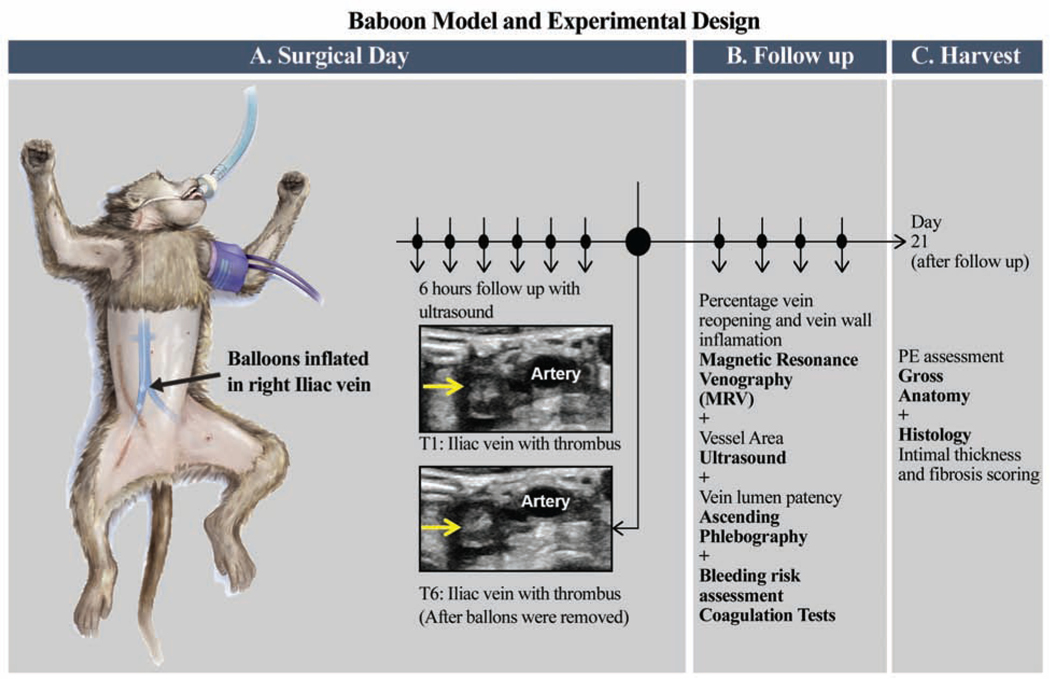
This model used a 6 hour temporary balloon occlusion in juvenile baboons. On surgical Day 0, standard contrast venography and ultrasound was performed in order to acquire baseline information regarding the vein to be thrombosed. First, a retroperitoneal incision was made and the hypogastric vein and other large draining side-branches to the iliac vein were divided and ligated. Then, a thrombus was created in the iliac vein by threading one balloon catheter via the internal jugular vein caudally down the inferior vena cava by fluoroscopy, to just below the iliac bifurcation. Another catheter was moved cranially up the femoral vein near the pelvic crest. This isolated a vein segment of approximately 2.5 cm in length. During the 6 hours of venous occlusion, the area between balloons were monitored hourly for absence of flow and increasing echogenicity representative of thrombus formation, using ultrasonography. After 6 hours, the balloon catheters were removed, incisions closed, an ultrasound performed to confirm the presence of a thrombus within the iliac vein and the animal monitored for post-operative recovery. Animals were evaluated on days 2, 7, 14, and 21 with venous duplex ultrasound imaging, standard contrast venography, and magnetic resonance venography (MRV), hematology and coagulation test were also performed on these days. Pre-anesthetic body weights, capillary refill time, mucous membrane color and skin turgor were assessed at these intervals in efforts to monitor alterations in hydration status. No overt difference in body weight or skin turgor were noted that could be attributed to detectable clinical alterations in body hydration. On Day 21, the animals were humanely euthanized and vein samples harvested from the thrombosed iliac (experimental samples) and from the non-thrombosed iliac (control samples) for histology. A total of nine baboons were divided into 2 experimental groups [Figure 1].
Figure 1: Baboon model of iliac vein venous thrombosis and experimental design.
A. This model used a 6 hour temporary balloon occlusion in juvenile baboons. On surgical day 0, standard contrast venography and ultrasound was performed in order to acquire baseline information regarding the vein to be thrombosed. A thrombus is created in the iliac vein by threading one balloon catheter via the internal jugular vein caudally down the inferior vena cava by fluoroscopy, to just below the iliac bifurcation. Another catheter was moved cranially up the femoral vein near the pelvic crest. This isolated a vein segment of approximately 3.0 cm in length. During the 6 hours of venous occlusion, the area between balloons were monitored hourly for absence of flow and increasing echogenicity representative of thrombus formation, using ultrasonography. After 6 hours, the balloon catheters were removed, incisions closed, an ultrasound performed to confirm the presence of a thrombus within the iliac vein and the animal monitored for post-operative recovery.
B. Animals were evaluated on Days 2, 7, 14, and 21; hematology and coagulation tests, venous duplex ultrasound imaging, magnetic resonance venography (MRV), and standard contrast venography.
C. On Day 21, the animals were humanely euthanized and vein samples harvested from the thrombosed iliac (experimental samples) and from the non-thrombosed iliac (control samples) for histology.
On day two post thrombus induction, animals were treated (TRT) with the E-selectin inhibitor (GMI-1271), 25 mg/kg, SC, once daily until Day 21 end of study (n=4). Non-treated control (CTR) animals received no treatment (n=5). All animals were evaluated at baseline, Day 2, Day 7, Day14, and Day 21 with euthanasia.
Animal Health
The health status of all animals was monitored. All animals were free of pathogens and received pre- and post-operative pain management. All non-human primates were housed and cared for by the University of Michigan Unit for Laboratory Animal Medicine in accordance to Guide for the Care and Use of Laboratory Animals (9). The University of Michigan is an AAALAC International accredited facility and this research protocol was approved by the university’s Institutional Animal Care and Use Committee (IACUC) and in the spirit of The Arrive Guidelines (11).
Contrast Venography/Thrombus Analysis
On Days 0 (baseline and T+6 hours), 2, 7, 14 and 21, bilateral 24-gauge intravenous catheters were placed in the saphenous veins. Five mLs of diatrizoate meglumine, (Hypaque®, USP 60%, Nycomed, Inc., Princeton, NJ, USA) was injected into both veins simultaneously. Images were recorded with a BV29 C-arm fluoroscopy unit (Phillips Medical, Philips Varadius Release 1.1, System, Cincinnati, OH, USA). At all-time points, subtracted and un-subtracted anterior-posterior (AP) and lateral (LAT) images were obtained and electronically stored (10–12).
Magnetic Resonance Venography- Time of Flight and Gadolinium (Gd) Enhanced
Magnetic resonance venography (MRV) was performed on a GE Healthcare 3T imaging system (GE Healthcare, Discovery MR750–60cm, Chicago, IL) with and without gadolinium. Thrombus resolution and lumen size was quantified by time-of-flight (TOF) imaging. The percent vein lumen size was determined by drawing a region of interest (ROI) around the flow channel in the affected iliac vein and comparing it to the contralateral unaffected vein. A position matched proximal, middle, and distal slice was evaluated for every animal on Days 2, 7, 14, and 21. Gadolinium (Gd), a heavy metal chelate that prolongs T1 relaxation and extravasates selectively into areas of capillary leakage was used as a noninvasive marker of inflammation (10–12). Statistical analysis was performed using values obtained from the combined proximal, middle, and distal iliac vein segments of each primate. The evaluation for PE was assessed in all cases using a Gd enhanced MRI technique.
Duplex Ultrasound Imaging
Ultrasound (Zonare system; Mindray Medical USA Corp, New Jersey, USA) was utilized to determine the presence of venous thrombus and to calculate the affected iliac vein area change over time. On Days 0 (baseline and T+6 hours), 2, 7, 14, and 21 (euthanasia; end of study), duplex ultrasonography was performed to evaluate the presence of thrombus and ultrasound analysis-open lumen. Ultrasound Doppler analysis was also used to evaluate iliac valve function as previously described (13). A proximal abdominal compression technique combined with M-mode ultrasound of the iliac vein determined time of valve closure. Normal valve closure was defined as ≤1000 milliseconds (ms) after compression (14).
Ultrasound Analysis-Open Lumen
The average area of the anterior posterior (AP) radius and transverse (T) radius of ultrasound measurements of the proximal (Prox.), middle (Mid.), and distal (Dist.) segments of the right iliac vein undergoing venous thrombosis were evaluated for each animal. Formulas: A=area, π=pi 3.14, a=Anterior Posterior Radius, b=Transverse Radius
The average area in cm square were calculated for each animal.
Thromboelastography
The thromboelastogram (TEG) parameters evaluated were r (clotting time), k (clot formation time) and ma (or MCF, maximum clot firmness). Clotting time (r) represents the time to initial clot formation, whereas clot formation time (k) represents the time between increases in amplitude in the thromboelastrogram from 2 to 20mm. Maximum clot firmness correlates with fibrinogen concentration and platelet count/function. Blood was obtained by venipuncture in a 2.7mL sodium citrate tube. One mL of the NaCi whole was mixed in a preloaded Kaolin tube (Heamonetics, Braintree Mass). Samples were run in duplicate on a Haemoscope Thromboelastograph Analyzer (Niles, Illinois). 20uL of 0.2M CaCl2 was added to each cup and pin (2). Next 320μL of NaCi whole blood/Kaolin mix was forcefully added to each cup. Results were printed out in the form of a thromboelastograph and analyzed.
Vein Wall Morphometrics
Standard methods for tissue fixation. Paraffin embedded IVCs were sectioned (3–5μm), stained with hematoxylin and eosin (H&E), and examined under oil immersion light microscopy (1000X). Five representative high power fields (Cell/5HPFs; one HPF is 210 μm in diameter) of the IVC wall were examined, and the inflammatory cells at points corresponding to positions 12, 2, 5, 7, and 9 on the face of a clock were counted manually (8, 10). Results from the five HPFs were added together for each section of vein wall studied and the mean ± significant error was noted. Cells were identified as neutrophils (NEU), monocyte/macrophages (MON), or lymphocytes (LYM) based on the nuclear and cytoplasmic characteristics of the cell. TC=total cells (TC).
Vein wall Fibrosis
Intimal thickness scoring criteria:
0: Intima appears as just a potential space with space occupied only by endothelial cells.
1: Very small spaces between endothelial cells and the internal elastic lamina. Intima still appears generally as thick as the nuclei of normal endothelial cells.
2: Intima is at least twice as thick as an endothelial nucleus at its widest point in the HPF.
3. Intima is at least 5 times the thickness of a red blood cell diameter. Intimal thickness tends tobe highly variable and may contain cells other than endothelial cells.
4. Intima is greatly thickened and contains either fibroblasts, white blood cells and/orhemorrhage at its widest point.
Intimal fibrosis scoring criteria:
0: No fibrosis evident.
1: Intima contains a small amount of dense eosinophilic or amphiphilic material. Fibroblasts may or may not be evident.
2: Intima contains fibroblasts and some small dense bundle of eosinophilic or amphiphilic collagenous connective tissue.
3. Intima contains numerous fibroblasts and is irregularly thickened by large amounts of collagenous connective tissue. White blood cells and/or red blood cells usually present.
Enzyme-linked immunosorbent assay (ELISA)
Blood was collected by venipuncture in 0.109M sodium citrate, centrifuged for 15 minutes at 2500 × g. The clear plasma drawn off and stored at −80°C. Serum was also collected by venipuncture in redtop serum separator tubes, allowed to clot for 30 minutes then spun down for 10 minutes at 1500xg. Commercial ELISA kits were used for Monkey sE-selectin, Monkey sP-selectin (Thermo Fisher Scientific, Waltham, MA) and Human D-Dimer (Hyphen-Biomed Neuville-Sur-Oise, France). Samples were run in duplicate according to manufacturer’s instructions. Plates were read on an Elx808 plate reader (Biotek, Vermont) at 450 nm wavelength.
Coagulation and Hematological Tests
Coagulation tests and hematological analysis were performed at all time points. Activated partial thromboplastin time (aPTT), prothrombin clotting time (PT), fibrinogen was measured using an i-STAT handheld analyzer (Abaxis, Union City, California, USA). Complete blood counts were measured and analyzed at all time points using the Element HT5 Hematology Analyzer (Heska Corporation, Loveland, Colorado, USA). Template bleeding times (BT) (Allegiance Health Care Corp, McGraw Park, IL, USA) were run at all time points according to manufacturer’s instructions. All samples were run in duplicate within a 5% error margin.
Preparation of Washed Baboon Platelets
Blood was drawn from the left iliac vein using a 22-G needle into a vacutainer containing 3.8% sodium citrate on Day 0 at baseline, 6 hours, Day 7, Day 14, and Day 21 post-surgery as described. The whole blood samples were centrifuged at 200 g for 10 minutes and platelet-rich-plasma (PRP) was transferred to a tube containing 10x acid citrate dextrose solution (ACD) and apyrase (0.02 U/mL) then centrifuged at 2000 g. Platelet pellets were resuspended in Tyrode’s buffer at 2.5×108 platelets/mL and kept in 37°C.
Platelet Aggregation and Dense Granule Secretion
Platelet aggregation was performed under stirring conditions (1100 rpm) at 37°C for 6 minutes using a Chrono-log Model 700D lumi-aggregometer (Havertown, PA). Prior platelet activation washed baboon platelets were incubated with Chrono-Lume reagent, an ATP-sensitive dye, for 1 minute. Then, platelet activation was induced by a varying dose of thrombin (1– 2nM). Platelet aggregation and dense granule secretion at base line (unstimulated) and in response to thrombin over the period of 21 days was measured in real-time by recording the changes in light transmission and fluorescence following the ATP release as surrogate for dense granule secretion.
Flow Cytometry
Washed baboon platelets were stimulated with thrombin (0, 2 and 3nM) and incubated with PE-conjugated mouse anti-human CD62P and FITC- conjugated anti-human CD41/61 (Clone PAC-1) antibodies for 10 minutes followed by fixation using 2% PFA solution. EDTA-anticoagulated fresh whole blood was collected by venipuncture from the baboon and incubated with PE-conjugated mouse anti-human CD41 and APC-conjugated anti human CD44 antibodies for 30 minutes. Platelet- leukocyte aggregates and CD44 expression on monocytes were determined by flow cytometry respectively.
Statistical Analysis
Statistical consultation was obtained at the University of Michigan Consulting for Statistics, Computing and Analytics Research (CSCAR). All animal numbers (n) were based on current protocols and known numbers needed for significance. For example, for comparison of percent vein re-opening between control and treated groups, a 50 % difference in means on average has been noted. Using an alpha of 0.05 and power of 80%, 3 animals per group were needed. For most immunological assays, to observe at least a 25 to 30% difference between the groups, n4 animals per group was sufficient (10–13). Statistical analysis included mean ± standard error of mean (SEM). Statistical significance (defined as *P<0.05, **P<0.01, ***P<0.001, or #P<0.0001) was calculated using an unpaired t-test with Welch’s correction. A two-sided Fischer’s Exact Test was used to evaluate iliac valve reflux data. A one-way analysis of variance (ANOVA) was utilized for comparison between the groups at their individual time points and appropriate controls using Prism version 8.0.2 (GraphPad Software Inc., La Jolla, CA, USA). Mixed model analysis was performed for ELISAs using R-Studies software to analysis treatment effects. Direct comparisons between groups were made for MRV, TEG, average vessel area, vein intimal thickness, intimal fibrosis, and vein wall inflammatory cell populations.
Results
Vein Recanalization and Vein Wall Inflammation
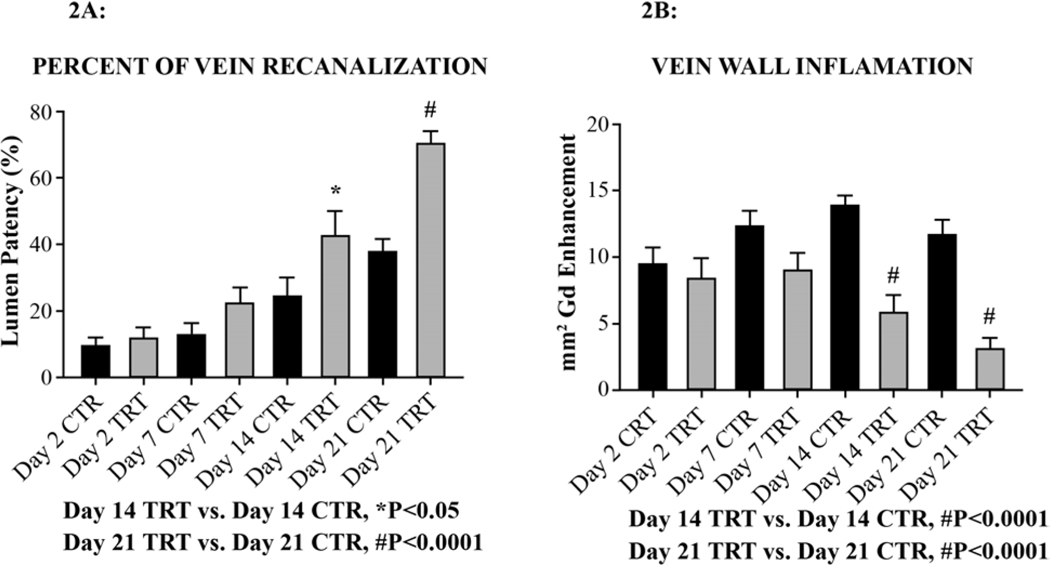
E-selectin inhibition by GMI-1271 significantly increased vein recanalization by MRV versus CTR animals on Day 14 (*P<0.05) and Day 21(#P<0.0001) [Figure 2A]. GMI-1271 significantly decreased vein wall inflammation by MRV with gadolinium vein wall enhancement verses CTR also on Day 14 (#P<0.0001) and Day 21 (#P<0.0001) [Figure 2B].
Figure 2: Magnetic resonance venography (MVR).
A) Figure 2A demonstrates vein recanalization by magnetic resonance venography, time offlight imaging, and percent vein recanalization analysis.
B) Figure 2B demonstrates vein wall inflammation as determined by capillary leakage in the vein wall using gadolinium (Gd) contrast.
Venous Clot Strength
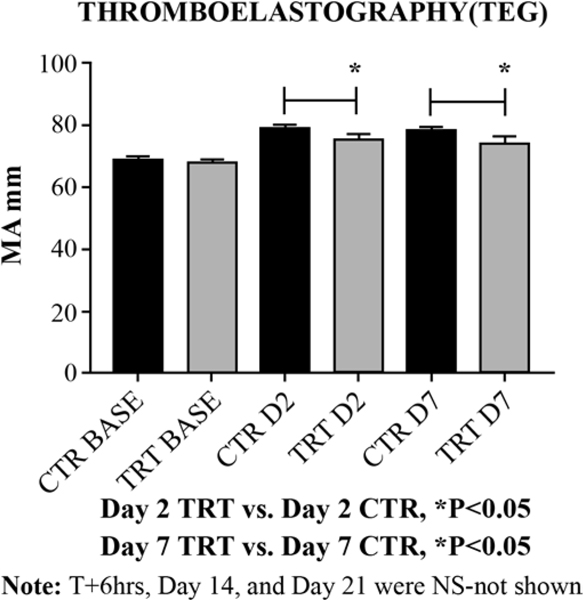
Maximum amplitude (MA mm); represents the ultimate strength of the fibrin clot; i.e. overall stability of the clot. The thromboelastographic measure of clot strength (MA), showed significant decreases in animals treated with GMI-1271 versus CTRs at Day 2 (*P<0.05) and Day 7 (*P<0.05) [Figure 3].
Figure 3:
Thromboelastography measure of clot strength (MA).
Ultrasound Analysis
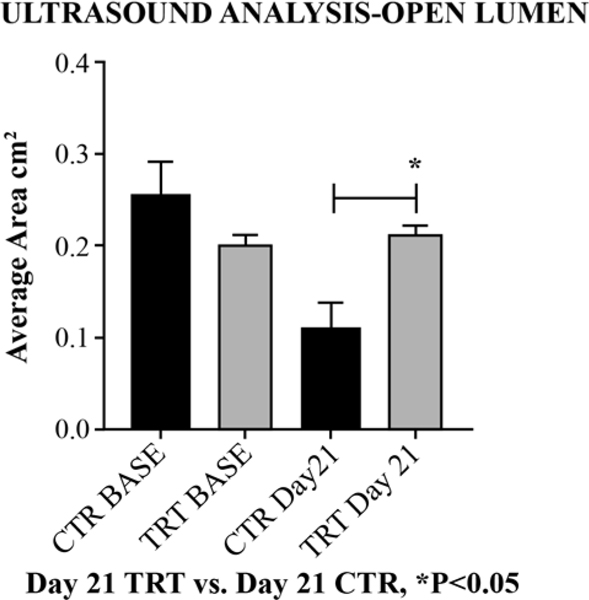
By Day 21 post thrombosis, animals treated with GMI-1271 showed significantly increased right iliac open lumen area (close to baseline) compared to non-treated control animals (1 Way ANOVA, ***P<0.001, CTR vs GMI-1271, Area cm2, *P<0.05) [Figure 4]. The evaluation of iliac vein valve reflux at Day 21 showed no significant differences between non-treated controls and animals treated with GMI-1271 by Fisher’s exact test P=0.17.
Figure 4:
Ultrasound Analysis-Open Lumen on day 21.
Vein Wall Analysis
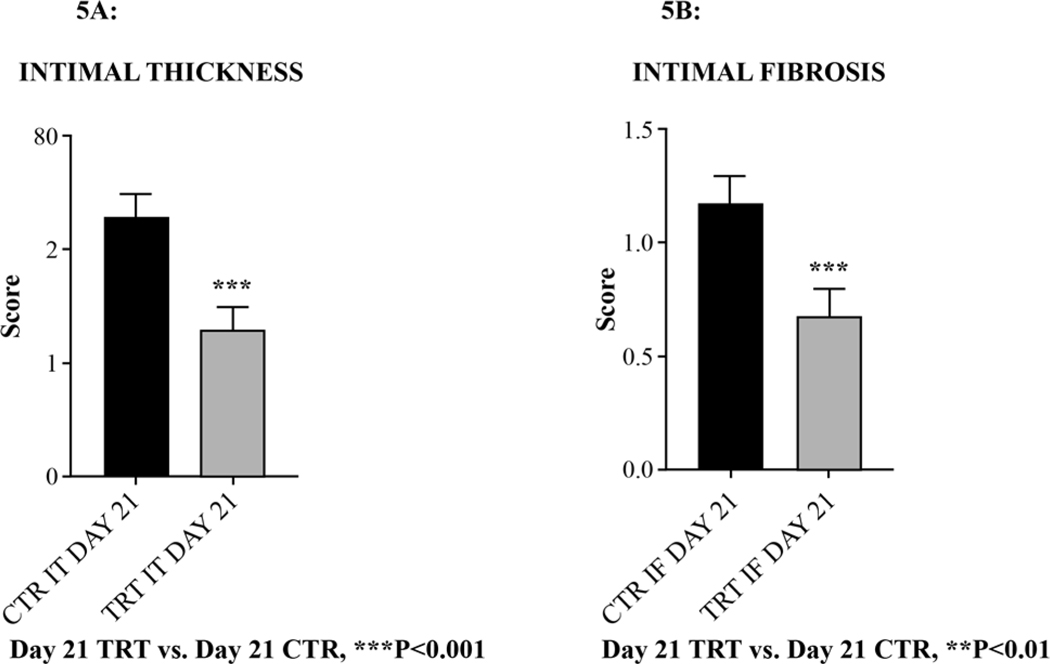
Notably, scoring of the intimal thickness (IT) and intimal fibrosis (IF), GMI-1271 significantly decreased IT (***P<0.001) [Figure 5A], and IF (*P<0.05) [Figure 5B] versus non treated controls as scored by a pathologist blind to the study protocol. E-selectin inhibition with GMI-1271 did not affect vein wall inflammatory cell extravasation (migration) compared to non-treated control animals. Both groups demonstrated the approximate inflammatory cell migration into the vein wall on Day 21 post venous thrombosis.
Figure 5:
Scoring of the right iliac vein wall intimal thickness (IT) and intimal fibrosis (IF) on Day 21 post venous thrombosis.
Circulating Protein Analysis
Circulating E-selectin, P-selectin and D-Dimer were evaluated by commercial ELISA kits at each time point. The protein levels of circulating E-selectin trended to be lower over tine versus non-treated controls. Animals treated with GMI-1271 trended to have higher circulating levels of P-selectin and D-dimer which may suggest higher thrombolytic activity in these animals compared to controls.
Coagulation and Hematology
Coagulation data from all experimental groups are summarized in Table I. Measured hematologic indices were within normal clinical range for nonhuman primates (baboons, data not shown). Importantly, no significant differences in hematology or coagulation tests between the groups, suggesting that E-selectin inhibition carries no bleeding potential. Additionally, no clinical episodes of bleeding were noted in either groups.
Table 1: Coagulation Test.
Template bleeding time (BT), Prothrombin time (PT), activated partial thromboplastin time (aPTT) and, Fibrinogen were assessed in all groups at baseline, T6, and Days 2, 7, 14 and 21. Control: control group (n5 animals); GMI-1271 TRT: E-selectin inhibitor treatment group (n4 animals). Baseline; T6: 6 hours after thrombosis, Day 2: two days after thrombosis and start of treatments; Day &: seven days after thrombosis; Day 14: fourteen days after thrombosis; Day 21: twenty-one days after thrombosis and end of study. In parenthesis are standard error of mean (SEM) values. Direct comparisons between the groups showed no significant differences in all coagulation parameters measured.
| BT (seconds) | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 120 (16.4) | 150 (42.4) |
| T6 | 192 (18.0) | 202.5 (60.5) |
| Day 2 | 276.0 (60.0) | 277.5 (14.36) |
| Day 7 | 132 (15.3) | 172.5 (25.62) |
| Day 14 | 156 (24) | 217 (22.5) |
| Day 21 | 210 (85.4) | 172 (30.9) |
| PT (seconds) | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 21.3 (0.6) | 20.7 (0.4) |
| T6 | 20.4 (0.5) | 20.7 (0.1) |
| Day 2 | 21.8 (0.5) | 21.2 (0.7) |
| Day 7 | 20.1 (0.4) | 19.8 (0.3) |
| Day 14 | 20.7 (0.4) | 20.7 (0.4) |
| Day 21 | 21 (0.4) | 20.2 (0.3) |
| aPTT (seconds) | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 108.1 (1.6) | 106.8 (2.4) |
| T6 | 109.7 (3.1) | 120 (5.4) |
| Day 2 | 117.4 (4.8) | 111.3 (5.5) |
| Day 7 | 109 (1.6) | 107.1 (2.8) |
| Day 14 | 110.7 (1.8) | 103.9 (2.7) |
| Day 21 | 106 (2.0) | 100.8 (3.5) |
| Fibrinogen (g/L) | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 2.1 (0.04 | 2.2 (0.1) |
| T6 | 2.1 (0.1) | 2.2 (0.03) |
| Day 2 | 4.9 (0.8) | 3.2 (0.4) |
| Day 7 | 3.2 (0.4) | 3.3 (0.2) |
| Day 14 | 2.4 (0.1) | 2.4 (0.1) |
| Day 21 | 2.2 (0.1) | 2.4 (0.1) |
Both percent platelet aggregation and dense granule secretion assays were performed in low shear stress conditions mimicking venous return. GMI-1271 did not affect platelet aggregation [Table II], CD44 expression on leukocytes, or dense granule secretion [Table III]. E- selectin inhibition treatment had no detectable effect on platelet function and hemostasis consistent with coagulation data.
Table 2: Platelet Aggregation Analysis.
The percentage of platelet aggregation were assessed in all groups at baseline, T6, and days 2, 6, 14 and 21. Control: control group (n5 animals); GMI-1271 TRT: E-selectin inhibitor treatment group (n4 animals). Baseline; T6: 6 hours after thrombosis, Day 2: two days after thrombosis and start of treatments; Day 7: seven days after thrombosis; Day 14: fourteen days after thrombosis; Day 21: twenty-one days after thrombosis and end of study. In parenthesis are standard error of mean (SEM) values. Direct comparisons between the groups showed no significant differences.
| Dense Granule Secretion (nM ATP release) 1nM Thrombin | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 0.29 (0.2) | 0.20 (0.2) |
| T6 | 0.12 (0.1) | 0.23 (0.2) |
| Day 2 | 0.28 (0.1) | 0.16 (0.1) |
| Day 7 | 0.16 (0.1) | 0.03 (0.0) |
| Day 14 | 0.03 (0.0) | 0.07 (0.1) |
| Day 21 | 0.27 (0.2) | 0.42 (0.3) |
| Dense Granule Secretion (nM ATP release) 2nM Thrombin | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 0.67 (0.2) | 0.34 (0.2) |
| T6 | 0.19 (0.1) | 0.34 (0.1) |
| Day 2 | 0.47 (0.1) | 0.42 (0.1) |
| Day 7 | 0.34 (0.2) | 0.07 (0.1) |
| Day 14 | 0.23 (0.1) | 0.19 (0.1) |
| Day 21 | 0.56 (0.3) | 0.68 (0.2) |
Table 3: Dense Granule Secretion Analysis.
Platelet dense granule secretion were assessed in all groups at baseline, T6, and days 2, 6, 14 and 21. Control: control group (n5 animals); GMI-1271 TRT: E-selectin inhibitor treatment group (n4 animals). Baseline; T6: 6 hours after thrombosis, Day 2: two days after thrombosis and start of treatments; Day 7: seven days after thrombosis; Day 14: fourteen days after thrombosis; Day 21: twenty-one days after thrombosis and end of study. In parenthesis are standard error of mean (SEM) values. Direct comparisons between the groups showed no significant differences.
| Platelet Aggregation (%) 1nM Thrombin | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 32 (17.1) | 43 (13.7) |
| T6 | 44 (15.5) | 45 (18.0) |
| Day 2 | 72 (10.3) | 67 (21.6) |
| Day 7 | 62 (24.3) | 30 (12.5) |
| Day 14 | 23 (12.3) | 34 (13.3) |
| Day 21 | 41 (19.2) | 43 (21.3) |
| Platelet Aggregation (%) 2nM Thrombin | ||
|---|---|---|
| CONTROL | GMI-1271 TRT | |
| BASELINE | 72 (3.7) | 68 (6.2) |
| T6 | 58 (21.5) | 76 (6.3) |
| Day 2 | 88 (4.5) | 87 (3.9) |
| Day 7 | 76 (15.7) | 51 (21.7) |
| Day 14 | 56 (23.5) | 60 (10.4) |
| Day 21 | 63 (14.7) | 67 (11.8) |
Discussion
In the current study, we found that inhibition of E-selectin significantly increased vein recanalization when used therapeutically after DVT, significantly decreased vein wall inflammation, and decreased vein wall intimal thickening and fibrosis when compared to non-treated controls. These effects occurred without a demonstrated bleeding potential or actual bleeding episodes.
Previously, we evaluated a P-selectin inhibitor and a VWF inhibitor in both prophylaxis and treatment protocols (10). In the current study, based on previous studies indicating that E-selectin mRNA is expressed later after vascular endothelial cell activation (3), we decided to test GMI-1271 E-selectin inhibition in a treatment protocol alone and eventually in combination with lower doses of LMWH. Additionally, most patients do not present immediately after thrombus initiation, but often they come in hours to days later, thinking that their pain or swelling relates to something more common (such as a muscle strain or pull) and wait to see if their symptoms improve before seeking medical attention.
GMI-1271 is a small molecule E-selectin antagonist. It is designed to mimic the bioactive conformation of the sialyl-Le x/a carbohydrate ligand, which functions in early recognition and binding events of cell extravasation from the bloodstream (15). Selectins, E-selectin and P-selectin, are glycoproteins that facilitate and augment thrombosis, directly modulating neutrophil (PMN) and monocyte activity. We and others have shown the importance of these molecules in venous thrombogenesis (3, 4, 8, 10, 11, 16–19). However, the mechanisms of E-selectin mediated responses are not well known and although P-selectin inhibition has been tested in various animal models (3, 11, 16, 17, 20–23), E-selectin inhibition has not been tested in a large animal model. GMI-1271 binding to E-selectin, prevents it from binding to its natural sialyl-Le x/a ligand which facilitates leukocyte-endothelial cell binding. Thus, GMI-1271 binding to E-selectin decreases leukocyte activation and binding to activated vascular endothelium, decreasing fibrin formation and thrombus propagation. Support for this mechanism has been documented in the literature (3, 4, 8). We hypothesized that E-selectin inhibition will decrease vein wall inflammation and should result in decreased thrombus amplification and improved vein recanalization after thrombus initiation in an animal model closest to humans.
Therapeutically, GMI-1271 mimics the natural sialyl-Le x/a ligand that enables leukocytes to bind to E-selectin on the activated endothelium. E-selectin binding to GMI-1271 instead of it natural ligand promotes decreased leukocyte-endothelium interactions and leukocyte activation, which reduces fibrin formation and thrombus amplification (3, 4). The rolling and adhesion of leukocytes on E-selectin signals an up-regulation of CD11b/CD18 (Mac-1) into a high affinity conformation capable of binding RBCs or platelets (24, 25). This model is congruent with the findings that elimination of either E-selectin or activated Mac-1 in knockout mice reduces the formation of RBC or platelet/leukocyte aggregates (26). As early as 3 to 6 hours post thrombosis, E-selectin inhibition limits thrombogenesis. We have shown that combined E- and P-selectin inhibition produces greater inhibition of thrombogenesis than P-selectin inhibition alone in a rodent model, and that inhibition of E-selectin alone leads to a 50% to 60% reduction in thrombus weight (3). GMI-1271 is a specific only E-selectin inhibitor. In mouse studies, we have shown that this agent demonstrated decreased thrombosis and promoted vein recanalization without bleeding side effects.
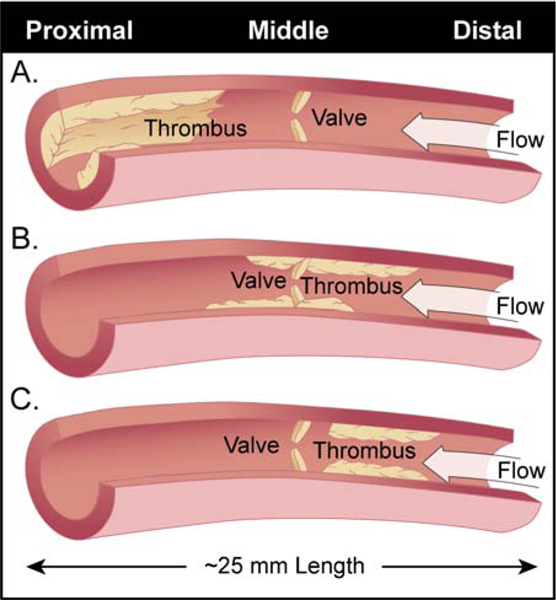
In the current study, animals treated with GMI-1271 had significantly decreased fibrin clot strength (MA) as measured by thromboelastography. This supports the increased vein recanalization we noted in animals with E-selectin inhibition compared to controls. Sullivan et al. showed that mice gene deleted for E-selectin had decreased thrombosis and fibrin deposition within the venous thrombi (4). Also in the current study, ultrasound analysis of vessel area demonstrated that animals treated with GMI-1271 showed significantly increased right iliac vein flow versus control animals on Day 21 post venous thrombosis. In this study, valve reflux times in the iliac vein were similar between treated and control animals despite higher recanalization and less inflammation in E-selectin GMI-1271 treated animals. We suggest that there are several clinical scenarios for treated and control animals having similar reflux times, but with treated animals having increased recanalization and decreased vein wall inflammation (Figure 6).
Figure 6: Clinical scenarios that can affect iliac valve reflux times in this study.
All animals were evaluated for thrombus burden and valve reflux times via duplex ultrasound imaging. The study area of the right iliac vein consisted of a proximal (Prox.), middle (Mid.) and distal (Dist.) segments for each animal which all together made up an iliac vein segment approximately 25 mm in length.
Panel A. Control animal number 5 had a large thrombus burden in the proximal iliac segment that did not incorporate the valve yielding a viable reflux time with a small flow channel present. These animals had decrease lumen patency and increased vein wall inflammation.
Panel B. Control animals 3 and 4 had non-occlusive middle iliac segment thrombus that incorporated the iliac valve through day 14 post thrombosis. However, by day 21, these animals had chronic anterior and posterior thrombus present with competent valves via duplex ultrasound. Treated animals 3 and 4 had non-occlusive middle iliac thrombus that incorporated the iliac valve through day 7 and 14 post thrombosis respectively. However, by day 21, these animals had complete resolution of thrombus burden but non-competent valves via duplex ultrasound.
Panel C. Control animals 1 and 2; Treated animals 1 and 2 had venous thrombi in the distal iliac segment that did not incorporate the iliac valve. The treated animals however had lower thrombus burden than the controls animals leading to increased lumen patency and decreased vein wall inflammation.
Note: Secondary venous valves above and/or below interrogated segment and/or technical error should be considered.
Taken together, this supports that GMI-1271 decreases venous fibrin clot strength which is supportive of natural vessel recanalization. Finally, GMI-1271 provided for less vein wall thickening and intimal fibrosis at Day 21, demonstrating the positive effect of the drug not only on the thrombus, but also on the vein wall. The fact that vein wall inflammatory cell extravasation did not show significant differences to control is consistent with previous studies with selectin inhibition in the primate. It is not just the number of inflammatory cells in the vein wall which is important, but the activation state of these cells. For example, activated neutrophils can produce prothrombotic neutrophil extracellular traps (NETs), and activated monocytes are involved in thrombus resolution and vein wall fibrosis. We hypothesize that inhibition of E-selectin is able to decrease the activation state of these cells, both those in the lumen and those in the vein wall. The decrease of leukocyte activation by GMI-1271 has been determined by immunohistochemically staining after acute venous thrombosis has been previously determined in a mouse model of venous thrombosis (8).
Limitations of the current animal study include the translatability of the findings to clinical DVT, the fact that the comparator group was not a group administered heparin or LMWH, and the limited ability to really evaluate well venous reflux in a supine animal under anesthesia. Regarding the model, we do believe that our primate model is as close to clinical DVT as possible as the hematology and physiology of the baboon is similar to human, and the animal spends a great amount of its time upright (27–32). In the next phase of the current study, we will be treating animals with LMWH at two different doses as part of our study combining the E-selectin inhibitor with lower doses of lovenox. Finally, there is no feasible way to study an upright primate with tests of venous reflux, so our best approximation has been to perform a proximal compression with a Doppler probe caudal to the iliac vein and determine if the iliac vein valve is functional and competent.
Although we have shown in the current study that GMI-1271 is effective when used alone, we have also shown in mice that GMI-1271, when combined with standard LMWH, will result in a lower effective dose of LMWH producing an equivalent antithrombotic response to higher dose LMWH. This is important as we know that it is the dose of LMWH (and other anticoagulants) which is correlated to bleeding potential. With this knowledge and the current study, the use of E-selectin inhibition with GMI-1271 may confer great benefit thus in DVT prophylaxis and treatment, allowing for its use either as a standalone agent or as an agent to augment current clinical therapy, in both instances improving vein recanalization, limiting or eliminating bleeding potential, and protecting the vein wall from intimal thickening and fibrosis. The use of the primate model, the model closest to clinical DVT, to test these concepts allows for a pre-clinical study that is a critical step towards testing the efficacy of GMI-1271 for the treatment of acute proximal DVT or PE in future clinical trials. Future nonhuman primate studies will test the concept of GMI-1271 augmenting LMWH in proximal iliofemoral DVT.
The importance of this study is the demonstration that inhibition of E-selectin, as in previous studies with inhibition of P-selectin, is not only anti-inflammatory but also anti-thrombotic. An agent that decreases thrombosis, adverse vein wall changes, and inflammation with limited or no bleeding potential has the possibility of changing the landscape of anticoagulant treatment of DVT and PE.
Conclusion
The current study suggests that E-selectin limits venous thrombus progression and its inhibition will increase thrombus recanalization and decrease vein wall inflammation, without affecting coagulation.
Clinical Relevance.
Venous thromboembolism (VTE), which includes DVT and pulmonary embolism (PE), is a common problem in clinical medicine. At present, agents for treatment, although effective, still demonstrate bleeding risks and do not prevent long-term pain, swelling and vein wall scarring. We demonstrate a new drug to treat thrombosis in an animal model that is both antithrombotic, and leads to less vein wall thickening with no bleeding potential.
Article Highlights.
Type of Research primate model of iliac vein thrombosis
Key Findings.
E-selectin inhibition by GMI-1271 significantly increased vein recanalization versus control animals on Day 14 (P<0.05) and Day 21 (P<0.0001), as imaged by magnetic resonance venography (MRV). GMI-1271 significantly decreased vein wall inflammation by enhancement of vein wall on MRV with gadolinium versus controls also on Day 14 (P<0.0001) and Day 21 (P<0.0001). No adverse coagulation events were noted.
Take Home Message:
E-selectin inhibitors such as GMI-1271 could potentially change treatment of deep venous thrombosis.
Acknowledgments
Sponsored by the NIH Vascular Interventions/Innovations and Therapeutic Advances (VITA)
Presented in part at the annual meeting of the American Venous Forum on Wednesday, February 20, 2019. Rancho Mirage, CA.
Disclosures
Dr. William Folger and Dr. John Magnani are employed by GlycoMimetic, Inc., Gaithersburg, MD. No financial support, provided GMI-1271 compound, professional consultation. Dr. Thomas Wakefield is an unpaid consultant to Selexys Corporation.
Abbreviations
- DVT
deep venous thrombosis
- PE
pulmonary embolism
- MRV
magnetic resonance venography
- TOF
time of flight
- Gd
gadolinium
- ROI
region of interest
- CBC
complete blood count
- BT
bleeding time
- PT
prothrombin time
- aPTT
activated partial thromboplastin time
- HPF
high powered field
- SC
subcutaneous
- IV
intravenous
- PROX
proximal
- MID
middle
- DIST
distal
- IT
intimal thickness
- IF
intimal fibrosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson I, Cifu AS. Management of Bleeding in Patients Taking Oral Anticoagulants. JAMA. 2018;319(19):2032–3. [DOI] [PubMed] [Google Scholar]
- 2.Chai-Adisaksopha C, Hillis C, Isayama T, Lim W, Iorio A, Crowther M. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost. 2015;13(11):2012–20. [DOI] [PubMed] [Google Scholar]
- 3.Myers D Jr., Farris D, Hawley A, Wrobleski S, Chapman A, Stoolman L, et al. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J Surg Res. 2002;108(2):212–21. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan VV, Hawley AE, Farris DM, Knipp BS, Varga AJ, Wrobleski SK, et al. Decrease in fibrin content of venous thrombi in selectin-deficient mice. J Surg Res. 2003;109(1):1–7. [DOI] [PubMed] [Google Scholar]
- 5.Jilma B, Marsik C, Kovar F, Wagner OF, Jilma-Stohlawetz P, Endler G. The single nucleotide polymorphism Ser128Arg in the E-selectin gene is associated with enhanced coagulation during human endotoxemia. Blood. 2005;105(6):2380–3. [DOI] [PubMed] [Google Scholar]
- 6.Jilma B, Kovar FM, Hron G, Endler G, Marsik CL, Eichinger S, et al. Homozygosity in the single nucleotide polymorphism Ser128Arg in the E-selectin gene associated with recurrent venous thromboembolism. Arch Intern Med. 2006;166(15):1655–9. [DOI] [PubMed] [Google Scholar]
- 7.Chase SD, Magnani JL, Simon SI. E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Ann Biomed Eng. 2012;40(4):849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culmer DL, Dunbar ML, Hawley AE, Sood S, Sigler RE, Henke PK, et al. E-selectin inhibition with GMI-1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model. Thromb Haemost. 2017;117(6):1171–81. [DOI] [PubMed] [Google Scholar]
- 9.Council NR. Guide for the care and use of laboratory animals. Eighth ed. ed. Washington, D.C.: The National Academies Press; 2011. 2011. 220 p. [Google Scholar]
- 10.Diaz JA, Wrobleski SK, Alvarado CM, Hawley AE, Doornbos NK, Lester PA, et al. P-selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von Willebrand factor. Arterioscler Thromb Vasc Biol. 2015;35(4):829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier TR, Myers DD Jr., Wrobleski SK, Zajkowski PJ, Hawley AE, Bedard PW, et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99(2):343–51. [DOI] [PubMed] [Google Scholar]
- 12.Myers DD Jr., Wrobleski SK, Longo C, Bedard PW, Kaila N, Shaw GD, et al. Resolution of venous thrombosis using a novel oral small-molecule inhibitor of P-selectin (PSI-697) without anticoagulation. Thromb Haemost. 2007;97(3):400–7. [PubMed] [Google Scholar]
- 13.Myers D, Wrobleski S, Londy F, Fex B, Hawley A, Schaub R, et al. New and effective treatment of experimentally induced venous thrombosis with anti-inflammatory rPSGL-Ig. Thromb Haemost. 2002;87(3):374–82. [PubMed] [Google Scholar]
- 14.Malgor RD LN. Duplex ultrasound scanning for chronic venous obstruction and valvular incompetence. In: Gloviczki P DM, Eklof B, Lurie F, and Wakefield TW editor. Handbook of Venous and Lymphatic Disorders. 4th edition ed. New York, New York: CRC Press, Taylor and Francis Group; 2017. p. 161. [Google Scholar]
- 15.Magnani JL. The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch Biochem Biophys. 2004;426(2):122–31. [DOI] [PubMed] [Google Scholar]
- 16.Myers DD, Hawley AE, Farris DM, Wrobleski SK, Thanaporn P, Schaub RG, et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38(5):1075–89. [DOI] [PubMed] [Google Scholar]
- 17.Hrachovinova I, Cambien B, Hafezi-Moghadam A, Kappelmayer J, Camphausen RT, Widom A, et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9(8):1020–5. [DOI] [PubMed] [Google Scholar]
- 18.Wakefield TW, Strieter RM, Downing LJ, Kadell AM, Wilke CA, Burdick MD, et al. P-selectin and TNF inhibition reduce venous thrombosis inflammation. J Surg Res. 1996;64(1):26–31. [DOI] [PubMed] [Google Scholar]
- 19.Wakefield TW, Strieter RM, Schaub R, Myers DD, Prince MR, Wrobleski SK, et al. Venous thrombosis prophylaxis by inflammatory inhibition without anticoagulation therapy. J Vasc Surg. 2000;31(2):309–24. [DOI] [PubMed] [Google Scholar]
- 20.Frenette PS, Wagner DD. Insights into selectin function from knockout mice. Thromb Haemost. 1997;78(1):60–4. [PubMed] [Google Scholar]
- 21.Wagner DD. P-selectin knockout: a mouse model for various human diseases. Ciba Found Symp. 1995;189:2–10; discussion −6, 77–8. [DOI] [PubMed] [Google Scholar]
- 22.Furie B. P-selectin and blood coagulation: it’s not only about inflammation any more. Arterioscler Thromb Vasc Biol. 2005;25(5):877–8. [DOI] [PubMed] [Google Scholar]
- 23.Furie B, Furie BC. Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol Med. 2004;10(4):171–8. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo A, Peired AJ, Wild M, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26(4):477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dongen CJ, van den Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004(4):CD001100. [DOI] [PubMed] [Google Scholar]
- 26.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abildgaard CF, Harrison J, Johnson CA. Comparative study of blood coagulation in nonhuman primates. J Appl Physiol. 1971;30(3):400–5. [DOI] [PubMed] [Google Scholar]
- 28.Callow AD, Ledig CB, O’Donnell TF, Kelly JJ, Rosenthal D, Korwin S, et al. A primate model for the study of the interaction of 111In-labeled baboon platelets with Dacron arterial prostheses. Ann Surg. 1980;191(3):362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly CA, Gleiser CA. Selected coagulation reference values for adult and juvenile baboons. Lab Anim Sci. 1986;36(2):173–5. [PubMed] [Google Scholar]
- 30.Todd ME, McDevitt E, Goldsmith EI. Blood-clotting mechanisms of nonhuman primates. Choice of the baboon model to simulate man. J Med Primatol. 1972;1(3):132–41. [DOI] [PubMed] [Google Scholar]
- 31.Feingold HM, Pivacek LE, Melaragno AJ, Valeri CR. Coagulation assays and platelet aggregation patterns in human, baboon, and canine blood. Am J Vet Res. 1986;47(10):2197–9. [PubMed] [Google Scholar]
- 32.Myers DD Jr., Nonhuman primate models of thrombosis. Thromb Res. 2012;129 Suppl 2:S65–9. [DOI] [PubMed] [Google Scholar]