Summary
Background
Hearing loss is a common morbidity that requires a hearing device to improve quality of life and prevent sequelae, such as dementia, depression falls, and cardiovascular disease. However, conventional hearing aids have some limitations, including poor accessibility and unaffordability. Consequently, personal sound amplification products (PSAPs) are considered a potential first-line alternative remedy for patients with hearing loss. The main objective of this study was to compare the efficacy of PSAPs and conventional hearing aids regarding hearing benefits in patients with hearing loss.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Five databases and reference lists were searched from inception to January 12, 2022. Studies including randomised, controlled trials; nonrandomised, controlled trials; or observational studies comparing PSAPs and hearing aids with regard to hearing gain performance (e.g., speech intelligence) were considered eligible. The review was registered prospectively on PROSPERO (CRD42021267187).
Findings
Of 599 records identified in the preliminary search, five studies were included in the review and meta-analysis. A total of 124 patients were divided into the PSAP group and the conventional hearing aid group. Five studies including seven groups compared differences for speech intelligence in the signal-noise ratio (SNR) on the hearing in noise test (HINT) between PSAPs and conventional hearing aids. The pooled results showed nonsignificant differences in speech intelligence (SMD, 0.14; 95% CI, -0.19 to 0.47; P = .41; I2=65%), sound quality (SMD, -0.37; 95% CI, -0.87 to 0.13; P = .15; I2=77%) and listening effort (SMD 0.02; 95% CI, -0.24 to 0.29; P = .86; I2=32%). Nonsignificant results were also observed in subsequent analyses after excluding patients with moderately severe hearing loss. Complete sensitivity analyses with all of the possible combinations suggested nonsignificant results in most of the comparisons between PSAPs and conventional hearing aids.
Interpretation
PSAPs are potentially beneficial as conventional hearing aids are in patients with hearing loss. The different features among PSAPs should be considered for patients indicated for hearing devices.
Funding
This work was supported by grants from Ministry of Science and Technology (MOST-10-2622-8-075-001) and Veterans General Hospitals and University System of Taiwan Joint Research Program (VGHUST111-G6-11-2 and VGHUST111c-140).
Keywords: Personal sound amplification products, PSAP, Conventional hearing aids, Hearing impairment, Meta-analysis
Research in context.
Evidence before this study
Cochrane Library, PubMed, Embase, Web of Science, and Scopus were searched for studies from inception to January 12, 2021. Five observational studies comparing personal sound amplification products (PSAPs) and hearing aids in patients suffering from hearing loss were enrolled. In the meta-analysis, the pooled effect size showed nonsignificant results between interventions and comparators in speech intelligence (SMD, 0.14; 95% CI, -0.19 to 0.47; P = .41; I2=65%), sound quality (SMD, -0.37; 95% CI, -0.87 to 0.13; P = .15; I2=77%) and listening effort (SMD 0.02; 95% CI, -0.24 to 0.29; P = .86; I2=32%).
Added value of this study
We report a comparison of PSAPs and hearing aids. In the synthesis of current evidence, hearing aids did not show definite superiority over PSAPs. For patients with hearing loss, the use of PSAPs may be considered.
Implications of all the available evidence
There is a need for controlled trials that further compare the effectiveness of PSAPs and hearing aids within populations with different degrees of hearing loss and different settings of PSAPs. Additionally, considering the lack of safety information on PSAPs, further studies regarding this issue (e.g. the safe upper limit of hearing gain, the risk of noise-induced hearing injury by the PSAPs) should be reported.
Alt-text: Unlabelled box
Introduction
Hearing loss is one of the most common comorbidities that affects individual and public health.1 More than 5% of the global population is impacted by hearing loss, especially adults older than 65 years old.2, 3, 4 In an increasingly ageing society, the number of patients with hearing loss will inevitably increase.5 In addition to the high prevalence, the disproportionate 50% higher prevalence of hearing loss in low-income countries has rendered the condition much more complex.6 At the same time, hearing loss is also associated with multiple cognitive,7,8 physical,9,10 and even psychosocial11,12 outcomes and can result in serious comorbidities, including dementia, falls, stroke and cardiovascular disease.13, 14, 15 Since hearing loss has been suggested to be a modifiable risk factor for geriatric problems, such as dementia,14 correcting hearing loss has consistently been a global issue.1,14,16
Hearing aids serve as first-line management for patients with hearing loss. However, it is quite difficult to obtain suitable hearing aids for those in low-socioeconomic groups, especially those in undeveloped or underdeveloped countries, owing to the unavailability of hearing consultants and the unaffordability of hearing aids.17, 18, 19, 20 According to a report from the World Health Organization (WHO),21 there is usually a shortage of well-trained personnel for the provision of hearing aids in low- and middle-income countries, and only less than 3% of the need for hearing aid is actually provided in these countries, which is far less than the unsatisfied global supply.21,22 As a result, hearing aids remain out of reach for patients.23,24 In light of this fact, various types of hearing devices have been proposed as alternatives. Personal sound amplification products (PSAPs) are alternative hearing devices. As hearing devices sold to the public, PSAPs have become popular in recent years due to their availability, affordability, and rapidly growing functions.19,25 However, controversy regarding whether PSAPs are suitable as one of the first-line hearing devices for patients with hearing loss has persisted, partly due to the absence of regulations by regulatory agencies (e.g., the Food and Drug Administration (FDA) or Medicine & Healthcare products Regulatory Agency (MHRA)) and concerns regarding inconsistent quality of the devices. Evidence has demonstrated that PSAPs can provide benefits for patients with hearing loss when compared to patients without these devices. However, whether PSAPs provide comparable effectiveness to conventional hearing aids has remained unclear. Through a systematic review and meta-analysis, we aimed to provide more comprehensive and detailed evidence for the efficacy of PSAPs, in comparison with conventional hearing aids, for patients with hearing loss.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.26 The review was also registered prospectively on PROSPERO (CRD42021267187).
Information sources and search strategy
Databases including the Cochrane Library, PubMed, Embase, Web of Science, and Scopus were searched for studies published from database inception through January 12, 2022. We used a combination of Medical Subject Headings (MeSH) and text words to create three subsets: one subset included studies of personal sound production products (“personal sound amplification products,” “PSAP”) and one included studies of conventional hearing aids (“hearing aid,” “ear mould”). The detailed search strategy is shown in Supplemental Digital Content 1.
Study selection
The titles, abstracts, and keywords of the identified records were screened by two authors (C.-H. Chen and C.-Y. Chang). The full texts of records under review for eligibility criteria were then reviewed independently by the same two authors (C.-H. Chen and C.-Y. Chang). If disagreements developed during the screening and reviewing process, a discussion with the project team was held, and a final judgement was made by a third author (C.-Y. Huang).
Eligibility criteria
Included studies were selected based on the following criteria: the study compared PSAPs with conventional hearing aids regarding the outcome of interest (i.e., speech intelligence, sound quality and listening effort) in patients with hearing loss, and the study provided adequate information to quantify the effect estimates for meta-analysis. PSAPs were defined as hearing devices that one can buy directly and that are not regulated as medical devices by the FDA, while hearing loss was defined as hearing level >25 dBHL by 4-frequency averages (0.5 kHz, 1 kHz, 2 kHz and 4 kHz). Studies including randomised, controlled trials; nonrandomised, controlled trials; or observational studies were considered eligible for further review.
Data collection and data items
After review by two authors (C.-H. Chen and C.-Y. Chang), the effect estimates of interest were extracted. Primary data were analysed to evaluate the effectiveness regarding speech intelligence by the signal-noise ratio (SNR) in the hearing in noise test (HINT) in both the PSAP group and conventional hearing aid group. Other outcomes, including sound quality and listening effort, were also extracted for meta-analysis. SNR was represented by the noise status when a patient could correctly identify a sentence in the HINT. A lower SNR indicated that a patient could understand these sentences in a relatively noisy environment, which was an indication of better hearing gain in noise by either PSAPs or conventional hearing aids. Sound quality was measured by the patients based on the subjective experience of sound perception; a higher score indicated better hearing aid performance. Listening effort referred to the burden on the patient to listen clearly to a target sound; a higher score stood for better hearing aid performance. The data were divided into two arms in the present study during the meta-analysis; in one arm, patients wore PSAPs and underwent HINT; and in the other arm, the patients wore conventional hearing aids for the same test. For data reported as graphical outcomes, we used WebPlotDigitizer software27 to digitize the graphs and extract the data. The reliability of WebPlotDigitizer has been previously validated.28 In studies in which the continuous outcomes were presented as medians and interquartile ranges, means and standard deviations were estimated using Wan's method.29,30
Risk of bias in individual studies
The Risk of Bias in Non-randomised Studies of Interventions (ROBINS-I) tool31 was applied to evaluate the methodological quality of the included studies by two independent authors (C.-H. Chen and C.-Y. Chang). The grading of risk was divided into “low,” “moderate” and “serious” categories according to the reviewed item. Any disagreement was resolved by a project meeting and the judgement of a third author (C.-Y. Huang).
Statistical analysis
Random-effects models were used for effect size calculation under the assumption that a second source of error other than sampling error existed. Given that different versions of the HINT and different sound quality scales and listening effort scales were used across different countries, pooled standardised mean differences (SMDs) were calculated for comparisons between groups.30 Statistical heterogeneity was evaluated by the Cochran Q test and the I2 statistic. Heterogeneity was considered low, moderate, and high at I2 values of <50%, 50–74%, and ≥75%, respectively.32 In addition, sensitivity analyses were performed: (1) by excluding those with moderately severe sensorineural hearing loss enrolled in Cho et al.; (2) using either a basic hearing aid or premium hearing aid in Cho et al.; and (3) using one of the PSAPs in Brody et al. for each outcome to examine whether the outcomes remained robust in each combination mentioned above. Regarding potential publication bias, the graphical methods suggested by DOI and Furuya-Kanamori were performed.33 The threshold of the asymmetrical index, or LFK index, ranges from -1 to 1. An LFK index greater than 1 is regarded as positive publication bias, while an LFK index equal to 1 or less in the situation is not. All of the calculations for the meta-analysis were performed in R studio software with the metaphor package34 and Stata software, version 15.35
Ethics committee approval
Ethics committee approval was not applicable in this study.
Role of funding source
The funder of the study played no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
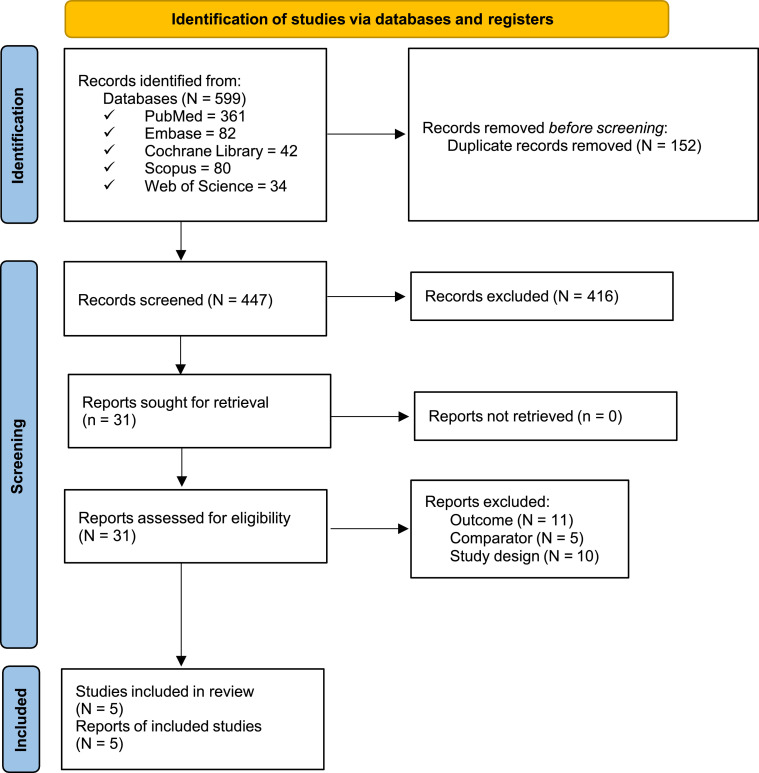
The present study identified 599 records in the preliminary search. After removing duplicates and screening titles and abstracts, 31 studies eventually underwent full-text review. Twenty-six studies were excluded due to unfavourable comparators, irrelevant outcomes and inadequate study designs. As a result, five eligible observational studies were included (Figure 1).17,25,36, 37, 38 A total of 124 patients were divided into the PSAP group and the conventional hearing aid group. One study enrolled patients with mild hearing loss, moderate hearing loss and moderately severe hearing loss25; three studies enrolled patients with mild hearing loss and moderate hearing loss17,36,38; and the other study enrolled patients with moderate hearing loss.37 Four studies used the Korean version of the hearing in noise test (K-HINT),25,36, 37, 38 and the other study used the original version of the HINT.17 Three studies reported sound quality comparisons between PSAPs and conventional hearing aids.17,25,36 Two studies reported listening effort comparisons between PSAPs and conventional hearing aids.17,25 Detailed information is presented in Table 1.
Table 2.
Comparison among conventional hearing aids, OTC hearing aids and PSAPs.
| Regulation | Requirement of professional hearing consultation | Cost | Targeted group | |
|---|---|---|---|---|
| Traditional hearing aid | FDA | Yes | High | Hearing loss |
| OTC hearing aid | FDA | No | Medium | Hearing loss |
| PSAP | FTA | No | Less | Normal hearing |
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. A total of 599 records were identified in the preliminary search. After removing duplicates and screening titles and abstracts, 31 studies eventually underwent a full-text review. Of those, 26 studies were excluded due to having unfavourable comparators, irrelevant outcomes or an inadequate study design. As a result, five eligible observational studies were included.
Table 1.
Study characteristics.
| Study | Country | Study type | Patients | Hearing loss* | Age (years, SD) | Intervention (PSAP/HA) | PSAP | HA | Evaluation of speech intelligence | Evaluation of sound quality | Evaluation of listening effort | Main result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cho et al., 201925,a | Republic of Korea | Observational study | 56 (27M/29 F) | Mild: 19 | 54.67 (8.81) | 19/19 | Ps2500amp | Basic HA: Ria2 Pro (Oticon) Premium HA: Opn1 (Oticon) |
SNR (K-HINT) | 5-point scalec | Dual-task paradigm | No difference among PSAP, basic HA and premium HA in speech perception, sound quality and listening effort |
| Moderate: 20 | 64.0 (5.98) | 20/20 | ||||||||||
| Moderately severe: 17 | 47.33 (21.02) | 17/17 | ||||||||||
| Brody et al., 201817,b | United States | Observational study | 25 (12M/13 F) | Mild to moderate | 69.6 (8.2) | 25/25 | Sound World Solutions CS50+ | ReSound LiNX2 5 | SNR (HINT) | 21-point scaled | 21-point scalef | Comparison of three types of PSAPs and a hearing aid showed improved speech recognition performance and reduced listening effort significantly toward the hearing aid. |
| FocusEar RS2 | ||||||||||||
| Tweak Focus | ||||||||||||
| Seol et al., 202143 | Republic of Korea | Observational study | 18 (11M/7 F) | Mild to moderate | 63.33 (6.03) | 18/18 | Etymotic Bean | ReSound LiNX Quattro | SNR (K-HINT) | NR | NR | Comparison found no statistical difference between PSAP and HA in speech intelligence. |
| Kim et al., 202137 | Republic of Korea | Observational study | 6 (1M/5 F) | Moderate | 59.83 (5.93) | 6/6 | Olive Smart Ear | ReSound LiNX 3D LT962-DRW | SNR (K-HINT) | NR | NR | Comparison found no statistical difference between PSAP and HA in speech intelligence. |
| Choi et al., 202036 | Republic of Korea | Observational study | 19 (4M/15 F) | Mild to moderate | 63.53 (10.44) | 19/19 | Ps2500amp | Audéo TM Q (Phonak AG) | SNR (K-HINT) | 5-level categorical scalese | NR | Comparison found no statistical difference between PSAP and HA in speech intelligence. |
Mild hearing loss: 26–40 dB hearing level (dB HL); moderate hearing loss: 41–55 dB HL; moderately severe hearing loss: 56–70 dB HL.
The study used two types of hearing aids (basic hearing aids and premium hearing aids) as comparators, which were further examined in the sensitivity tests.
The study performed a comparison between PSAPs and hearing aids using three types of PSAPs – Sound World Solutions CS50+, FocusEar RS2, and Tweak Focus – which were further examined in sensitivity tests.
The study categorized “very good” to “very bad” on a 5-point scale.
The 21-point scale rated sound quality from 0 to 100 using the following question: “How would you judge the overall sound quality?”.
The study categorized “excellent” to “bad” on a 5-level categorical scale.
The 21-point scale rated listening effort from 0 to 100 using the question: “How hard were you working to achieve your level of speech understanding?”.
Risk of bias assessment
Risk of bias was assessed for each of the included studies. Four studies were categorized as having moderate bias due to potential cofounding factors.17,25,36,38 Bias in the classification of interventions existed in one study due to lack of a definition of sound quality.36 Four studies might have contained moderate bias in the measurement of outcomes due to the difficulty of blinding both patients and outcome assessors.17,25,36,38 Four studies were categorized as having moderate to serious bias in the selection of the reported results.17,25,36,38 The detailed assessment is presented in the Supplemental Digital Content 2.
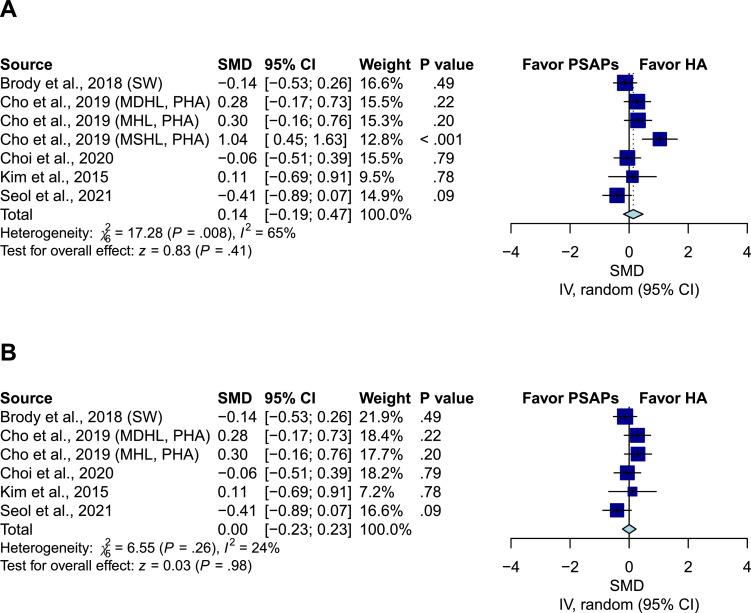
Comparison of speech intelligence between PSAPs and conventional hearing aids
Five studies including seven groups compared the SNR in the HINT between PSAPs and conventional hearing aids.39, 40, 41, 42 Overall, the pooled results showed a nonsignificant difference (SMD, 0.14; 95% CI, -0.19 to 0.47; P = .41; I2=65%) (Figure 2A). After excluding the patients with moderately severe hearing loss in Cho et al., the results remained nonsignificant (SMD, 0; 95% CI, -0.23 to 0.23; P = .98; I2=24%) (Figure 2B).
Figure 2.
Comparison of speech intelligence between personal sound amplification products (PSAPs) and conventional hearing aids. (A) The pooled result showed nonsignificant difference (SMD, 0.14; 95% CI, -0.19 to 0.47; P = .41; I2=65%). (B) After excluding the patients with moderately severe hearing loss in Cho et al., the result remained nonsignificant (SMD, 0; 95% CI, -0.23 to 0.23; P = .98; I2=24%). IV indicates the inverse variance method; SW indicates that the analysis adopted the Sound World Solutions CS50+ PSAP used in Brody et al.; MHL indicates mild hearing loss; MDHL indicates moderate hearing loss; MSHL indicates moderately severe hearing loss; PHA indicates premium hearing aid used in Cho et al.
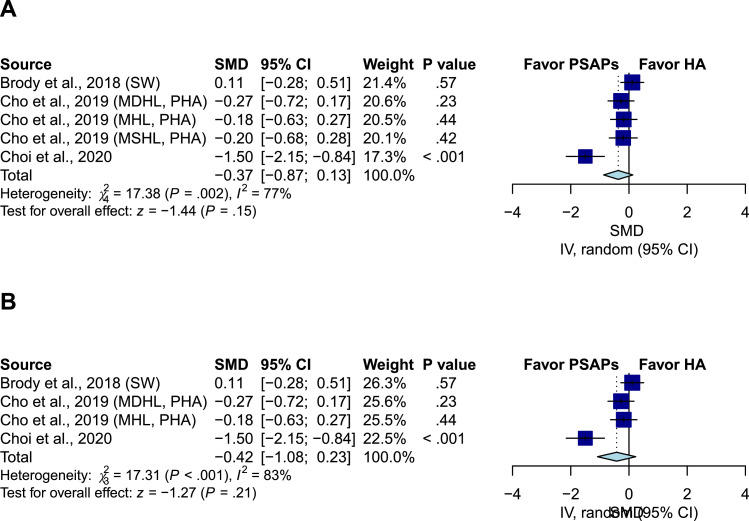
Sound quality comparison between PSAPs and conventional hearing aids
Three studies including five groups compared the sound quality between PSAPs and conventional hearing aids.17,25,36 The pooled results showed a nonsignificant difference (SMD, -0.37; 95% CI, -0.87 to 0.13; P = .15; I2=77%) (Figure 3A). After excluding the patients with moderately severe hearing loss in Cho et al., the results remained nonsignificant (SMD, -0.42; 95% CI, -1.08 to 0.23; P = .21; I2=83%) (Figure 3B).
Figure 3.
Comparison of sound quality between PSAPs and conventional hearing aids. (A) The pooled result showed nonsignificant difference (SMD, -0.37; 95% CI, -0.87 to 0.13; P = .15; I2=77%). (B) After excluding the patients with moderately severe hearing loss in Cho et al., the result remained nonsignificant (SMD, -0.42; 95% CI, -1.08 to 0.23; P = .21; I2=83%). IV indicates the inverse variance method; SW indicates that the analysis adopted Sound World Solutions CS50+ PSAP used in Brody et al.; MHL indicates mild hearing loss; MDHL indicates moderate hearing loss; MSHL indicates moderately severe hearing loss; PHA indicates premium hearing aid used in Cho et al.
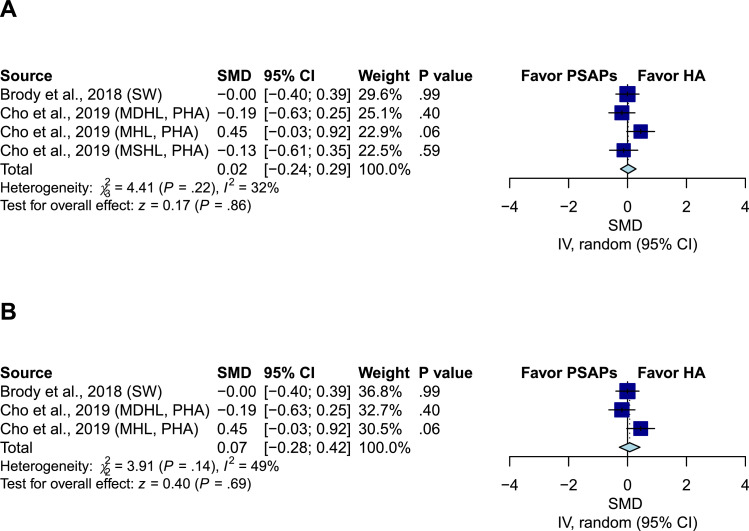
Listening effort comparison between PSAPs and conventional hearing aids
Two studies including four groups compared listening effort between PSAPs and conventional hearing aids.17,25 The pooled effect estimates demonstrated a nonsignificant difference (SMD 0.02; 95% CI, -0.24 to 0.29; P = .86; I2=32%) (Figure 4A). After excluding the patients with moderately severe hearing loss in Cho et al., the results remained nonsignificant (SMD 0.07; 95% CI, -0.28 to 0.42; P = .69; I2=49%) (Figure 4B).
Figure 4.
Comparison of listening effort between PSAPs and conventional hearing aids. (A) The pooled result showed nonsignificant difference (SMD 0.02; 95% CI, -0.24 to 0.29; P = .86; I2=32%). (B) After excluding the patients with moderately severe hearing loss in Cho et al., the result remained nonsignificant (SMD, 0.07; 95% CI, -0.28 to 0.42; P = .69; I2=49%). IV indicates the inverse variance method; SW indicates that the analysis adopted Sound World Solutions CS50+ PSAP used in Brody et al.; MHL indicates mild hearing loss; MDHL indicates moderate hearing loss; MSHL indicates moderately severe hearing loss; PHA indicates premium hearing aid used in Cho et al.
Sensitivity analysis and publication bias
We performed sensitivity tests by comparing basic hearing aids and premium hearing aids in Cho et al., with and without moderately severe hearing loss in Cho et al. and three different types of PSAPs used in Brody et al. The comparison of speech intelligence by the FocusEar PSAP in Brody et al. and basic hearing aids in Cho et al. was associated with a significant difference towards PSAPs when the moderately severe hearing loss population was included (Supplemental Digital Content 3, Figure 10A). Additionally, the comparison of listening effort by the FocusEar PSAP or Tweak Focus PSAP in Brody et al. and basic hearing aids in Cho et al. after exclusion of participants with moderately severe hearing loss were associated with a significant difference towards hearing aids (Supplemental Digital Content 3, Figures 12B and 15B). Otherwise, all of the results of the sensitivity analyses remained nonsignificant (Supplemental Digital Content 3, Figures 1–9, 10B, 11, 12A, 13–15A). Evaluation of publication bias by LFK index and DOI plot indicated a low risk of publication bias (Supplemental Digital Content 4, Figures 1–3).
Discussion
The paramount finding of the present study is that there were no significant differences between PSAPs and conventional hearing aids regarding speech intelligence, sound quality and listening effort. Further sensitivity analyses also suggested nonsignificant results, with the exception of one analysis that suggested favourable speech intelligence with the PSAPs.
In a previous meta-analysis,44 both PSAPs and conventional hearing aids demonstrated favourable effects on hearing gain in patients with hearing loss when compared to unaided patients. The study also provided evidence of differences between over-the-counter (OTC) hearing aids and conventional hearing aids. However, no direct comparison between PSAPs and conventional hearing aids has been performed, and a comparison between PSAPs and conventional hearing aids in comparison to unaided conditions was also unlikely because the study reported different estimates of the effects of PSAPs and hearing aids.44 The first evidence of a comparison between PSAPs and conventional hearing aids was provided by Reed et al.45 Five PSAPs were compared with conventional hearing aids and unaided conditions in the trial. Among those findings, we noted that the difference between particular PSAPs and hearing aids was not significant. As a consequence, we performed a systematic review and meta-analysis to evaluate whether PSAPs were comparable to conventional hearing aids. The present study not only compared the effectiveness of the PSAP hearing aid itself for speech intelligence but also compared the subjective perception of the sound quality by patients using the two types of hearing aids and the difference in listening effort through meta-analysis. To our knowledge, this study is the first meta-analysis to provide direct and comprehensive evidence of the noninferiority of PSAPs relative to conventional hearing aids.
Hearing aids are among the most important management strategies for patients with hearing loss.46,47 However, the selection of a hearing aid is a multifaceted process and is never a simple decision to make, as previous studies have identified approximately 30 factors that can affect the fit of a particular hearing aid.48 Among these factors, the price of hearing aids plays a very important role.18,20,49 With the development and advancement of technology, hearing aids have evolved from a simple sound amplifier in the past and are now easily compatible with personalized and telemedicine with equipped multifunctions.50,51 However, the price of hearing aids has not decreased as their manufacturing has matured. Instead, the complexity of hearing aids has resulted in greater costs for manufacturing, reflected in the price.18 As a result, the adoption rate of hearing aids might be further restricted.23 Subsidies within the social welfare system of a nation then become important for patients requiring hearing aids.20 Patients who do not have sufficient social welfare support might not be able to successfully adopt hearing aids.23 At the same time, hearing aids require certified professionals to fit them, while audiologic training is generally lacking in developing countries—there is less than one audiologist for every one million people according to previous studies.52, 53, 54 This fact reflects the low accessibility to hearing consultants in these countries, which would further impact the generality of fitting a hearing aid.24 Consequently, hearing loss coupled with the unavailability of a hearing aid could contribute to a decline in quality of life caused by inability to communicate with people and could result in serious comorbidities, including dementia, depression, falls, stroke and cardiovascular disease.13, 14, 15,55 Since previous evidence has identified hearing loss as a modifiable risk factor for dementia,14 the initiation of treating hearing loss becomes especially critical since the population with age-related hearing loss continues to grow with the increasing ageing of society.5,24,56
Regarding the unaffordability of conventional hearing aids, other hearing devices that are easier to obtain and more affordable are considered alternative choices to conventional hearing aids and include OTC hearing aids and PSAPs.57, 58, 59, 60 Differences exist among conventional hearing aids, OTC hearing aids and PSAPs. First, OTC hearing aids refer to hearing devices that can be sold by retailers (e.g., Costco, Carrefour) and are regulated by regulatory agencies (e.g., FDA or MHRA), the same as conventional hearing aids, while PSAPs specifically refer to hearing devices that people can obtain from the internet or from retailers and are not under regulation by these regulatory agencies.57, 58, 59, 60 Second, OTC hearing aids are designed for people with hearing loss as hearing aids, while PSAPs aim to promote the hearing ability of people with normal variations. Third, while conventional hearing aids require professional consultation for fitting and tuning, neither OTC hearing aids nor PSAPs require such consultations. Finally, the cost for the conventional hearing aids is significantly higher than that for OTC hearing aids and PSAPs since the latter two spare the expenses for licenced professionals and hearing services. In the real world, listeners perceive massive amounts of background noise, along with true speech voice. Correctly distinguishing this noise and speech voice is an essential aspect of hearing devices to help patients with hearing loss. A better hearing device would allow the user to accurately identify the smaller target voice in an environment with louder noise, which could be evaluated by the SNR. Currently, conventional hearing aids are usually equipped with a directional microphone or remote microphone systems to amplify the target voice and reduce perceived noise, thus lowering the SNR. Additionally, the internal chip of a hearing aid can provide advanced functions, such as compression, noise cancellation and feedback cancellation, to manage the electrical signal from the voice and noise; this process allows the sound output to more closely resemble to the original voice, consequently not only promoting speech discrimination for patients with hearing loss but also optimizing the sound quality and reducing the listening efforts of the users, resulting in a better user experience. With considerable advances in the technology for manufacturing PSAPs, PSAPs will also be equipped with these functions,58,61 along with the additional functions that improve user experience (e.g., compatibility with smartphones or after-sales service). Subsequently, the differences between PSAPs and conventional hearing aids will lessen, and the acceptance of PSAPs will increase with these cost-effective features.18,25,45,58
Regarding the absence of regulation by regulatory agencies, whether PSAPs are suitable for patients with hearing loss as a medical device remains controversial.62 The lack of regulation could suggest that the quality of PSAPs suffers from inconsistency. Previous research has shown that different PSAPs show a great diversity in price and incorporated functions.19,63 Inconsistent quality of PSAPs is also reflected in the user experience. The less expensive PSAPs with plain features were not as satisfying as the mid- to high-price PSAPs with multifunctional features.64 However, we believe that unfavoured PSAPs will be eliminated or forced to provide better functions under market pressure. In the foreseeable future, the quality of PSAPs should gradually improve and provide a better alternative for hearing devices.
Limitations remain in the present study. First, there was heterogeneity in the degree of hearing loss in the included studies, which ranged from mild to moderately severe hearing loss. Previous studies have indicated that those with mild hearing loss would benefit most from PSAPs.19,45,60,65 The majority of the included studies in this meta-analysis did not separately report data from patients with mild hearing loss. As a result, this study was unable to perform a subgroup analysis for the comparison of PSAPs and conventional hearing aids in patients with mild hearing loss. Conversely, for patients with moderately severe hearing loss, we performed a sensitivity test by excluding this subgroup, and the results remained nonsignificant. Second, several types of PSAPs and hearing aids were used in the included studies and could have contributed to the heterogeneity to some degree. We conducted a sensitivity test by pairing these PSAPs and hearing aids in the analysis to address heterogeneity and avoid miscalculation of the weights of the included studies. Except for one analysis of speech intelligence comparisons that showed PSAPs to be superior to hearing aids and two analyses of listening effort comparisons that showed PSAPs to be slightly inferior to hearing aids, all of the possible combinations of PSAP and hearing aid comparisons showed nonsignificant results, suggesting that different PSAPs could possibly bring about variations in the results. Third, different types of tools and scales for outcome evaluations across included studies (i.e., the HINT and sound quality scales) could have contributed to heterogeneity. Therefore, we used the SMD as the effect estimate to eliminate the influence of different scales. Fourth, limited by the number of included studies in this study and the sample sizes in the included studies, we were unable to perform additional analysis (i.e., meta-regression, subgroup analysis) for variables of interest or potential confounders, such as the cognitive status of patients, the price of PSAPs and the different incorporated functions mentioned in previous studies.63,66,67 Additionally, the discovery of publication bias was also limited under the circumstance. We alternatively sought publication bias by the method suggested by Furuya-Kanamori et al.,33 and the results demonstrated very limited publication bias in the present study. Fifth, since the included studies were not randomised, controlled trials, they were supposed to report potential confounders and the adjustment methods. However, the included studies did not report these issues that could have influenced their validity. Finally, a survey of the European Association of Hearing Aid Professionals (AEA) and the European Federation of Hard of Hearing People (EFHOH) reported that some PSAPs do not set an upper limit for sound amplification, which could easily lead to noise-induced hearing injury.68 Existing studies have rarely analysed and reported on the safety of PSAPs, so this study was not able to perform such a safety analysis. Based on the above, we look forward to more large-scale, randomised, controlled trials in the future to provide further information regarding safety and detailed characteristics for more comprehensive and solid results.
We compared the efficacy between PSAPs and conventional hearing aids by a systematic review and meta-analysis. The current study illustrated that PSAPs are potentially beneficial when compared to hearing aids regarding hearing gain, sound quality and listening effort; Given their availability and affordability, PSAPs could be considered for patients with hearing loss in the future. Nevertheless, as heterogeneity could develop from devices, the different features among PSAPs and hearing aids should be considered for patients indicated for hearing devices.
Contributors
C.-H. Chen, C.-Y. Huang, M.-C. Wang and Y.-F. Cheng were responsible to the conceptualization and design for the manuscript. All of the authors contributed to the acquisition, analysis, and interpretation of the data. C.-H. Chen drafted the manuscript. C.-H. Chen, C.-Y. Huang and M.-C. Wang performed critical revision of the manuscript for important intellectual content. C.-Y. Chang, M.-C. Wang, H.Y.-H. Lin, Y.-C. Chu and Y.-H. Lai performed the statistical analysis. Y.-F. Cheng obtained the funding. C.-Y. Huang and Y.-F. Cheng were supervisors of the manuscript.
Funding
This work was supported by grants from Ministry of Science and Technology (MOST-10-2622-8-075-001, MOST 110-2320-B-075-004-MY3) and Veterans General Hospitals and University System of Taiwan Joint Research Program (VGHUST111-G6-11-2 and VGHUST111c-140).
Data sharing statement
There is no restriction of the dataset employed in this study, and it is provided in Table 1 in this study.
Declaration of interests
We declare no competing interests.
Acknowledgments
We acknowledge Ms. Hsin-Yi Huang for providing consultation on statistical problems during the submission.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101378.
Appendix. Supplementary materials
References
- 1.Haile L.M., Kamenov K., Briant P.S., et al. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet. 2021;397(10278):996–1009. doi: 10.1016/S0140-6736(21)00516-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO global estimates on prevalence of hearing loss. 2012.
- 3.Stevens G., Flaxman S., Brunskill E., et al. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health. 2011;23(1):146–152. doi: 10.1093/eurpub/ckr176. [DOI] [PubMed] [Google Scholar]
- 4.Li L.Y.J., Wang S.Y., Wu C.J., Tsai C.Y., Wu T.F., Lin Y.S. Screening for hearing impairment in older adults by smartphone-based audiometry, self-perception, HHIE screening questionnaire, and free-field voice test: comparative evaluation of the screening accuracy with standard pure-tone audiometry. JMIR mHealth uHealth. 2020;8(10):e17213. doi: 10.2196/17213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis A., McMahon C.M., Pichora-Fuller K.M., et al. Aging and hearing health: the life-course approach. Gerontologist. 2016;56(Suppl 2):S256–SS67. doi: 10.1093/geront/gnw033. (Suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott C., Omoding S., Fermor J., Ogilvy D. An evaluation of the ‘voice test'as a method for assessing hearing in children with particular reference to the situation in developing countries. Int J Pediatr Otorhinolaryngol. 1999;51(3):165–170. doi: 10.1016/s0165-5876(99)00263-3. [DOI] [PubMed] [Google Scholar]
- 7.Deal J.A., Sharrett A.R., Albert M.S., et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol. 2015;181(9):680–690. doi: 10.1093/aje/kwu333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukla A., Reed N.S., Armstrong N.M., Lin F.R., Deal J.A., Goman A.M. Hearing loss, hearing aid use, and depressive symptoms in older adults—findings from the atherosclerosis risk in communities neurocognitive study (ARIC-NCS) J Gerontol Ser B. 2019;76(3):518–523. doi: 10.1093/geronb/gbz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deal J.A., Richey Sharrett A., Bandeen-Roche K., et al. Hearing impairment and physical function and falls in the atherosclerosis risk in communities hearing pilot study. J Am Geriatr Soc. 2016;64(4):906–908. doi: 10.1111/jgs.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin F.R., Metter E.J., O'Brien R.J., Resnick S.M., Zonderman A.B., Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewster K.K., Ciarleglio A., Brown P.J., et al. Age-related hearing loss and its association with depression in later life. Am J Geriatr Psychiatry. 2018;26(7):788–796. doi: 10.1016/j.jagp.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton D.S., Cruickshanks K.J., Klein B.E., Klein R., Wiley T.L., Nondahl D.M. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- 13.Deal J.A., Reed N.S., Kravetz A.D., et al. Incident hearing loss and comorbidity: a longitudinal administrative claims study. JAMA Otolaryngol Head Neck Surg. 2019;145(1):36–43. doi: 10.1001/jamaoto.2018.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla A., Reed N.S., Armstrong N.M., Lin F.R., Deal J.A., Goman A.M. Hearing loss, hearing aid use, and depressive symptoms in older adults—findings from the atherosclerosis risk in communities neurocognitive study (ARIC-NCS) J Gerontol Ser B. 2021;76(3):518–523. doi: 10.1093/geronb/gbz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Team . World report on hearing. World Health Organization; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brody L., Wu Y.H., Stangl E. A comparison of personal sound amplification products and hearing aids in ecologically relevant test environments. Am J Audiol. 2018;27(4):581–593. doi: 10.1044/2018_AJA-18-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundfast K.M., Liu S.W. What otolaryngologists need to know about hearing aids. JAMA Otolaryngol Head Neck Surg. 2017;143(2):109–110. doi: 10.1001/jamaoto.2016.3416. [DOI] [PubMed] [Google Scholar]
- 19.Mamo S.K., Reed N.S., Nieman C.L., Oh E.S., Lin F.R. Personal sound amplifiers for adults with hearing loss. Am J Med. 2016;129(3):245–250. doi: 10.1016/j.amjmed.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valente M., Amlani A.M. Cost as a barrier for hearing aid adoption. JAMA Otolaryngol Head Neck Surg. 2017;143(7):647–648. doi: 10.1001/jamaoto.2017.0245. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Preferred profile for hearing-aid technology suitable for low-and middle-income countries. 2017.
- 22.Smith A. Proceedings of the WHO/WW Hearing Fifth Workshop on the Provision of Hearing Aids and Services for Developing Countries Geneva. 2007. Update on burden of hearing impairment and progress of WHO/WWH hearing aids initiative. 2007. [Google Scholar]
- 23.Chien W., Lin F.R. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med. 2012;172(3):292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed N.S., Garcia-Morales E., Willink A. Trends in hearing aid ownership among older adults in the United States from 2011 to 2018. JAMA Intern Med. 2021;181(3):383–385. doi: 10.1001/jamainternmed.2020.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho Y.S., Park S.Y., Seol H.Y., et al. Clinical performance evaluation of a personal sound amplification product vs a basic hearing aid and a premium hearing aid. JAMA Otolaryngol Head Neck Surg. 2019;145(6):516–522. doi: 10.1001/jamaoto.2019.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohatgi A. WebPlotDigitizer. Austin, Texas, USA; 2017.
- 28.Drevon D., Fursa S.R., Malcolm A.L. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41(2):323–339. doi: 10.1177/0145445516673998. [DOI] [PubMed] [Google Scholar]
- 29.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J.P., Thomas J., Chandler J., et al. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 31.Sterne Jonathan, AC, Hernán Miguel, Reeves Barnaby, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuya-Kanamori L., Barendregt J.J., Doi S.A. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16(4):195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team (2020). R: A language and Environment For Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- 35.StataCorp. 2017. Stata statistical software: release 15. College Station, TX: StataCorp LLC.
- 36.Choi J.E., Kim J., Yoon S.H., Hong S.H., Moon I.J. A Personal sound amplification product compared to a basic hearing aid for speech intelligibility in adults with mild-to-moderate sensorineural hearing loss. J Audiol Otol. 2020;24(2):91–98. doi: 10.7874/jao.2019.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim G.Y., Kim J.S., Jo M., Seol H.Y., Cho Y.S., Moon I.J. Feasibility of personal sound amplification products in patients with moderate hearing loss: a pilot study. Clin Exp Otorhinolaryngol. 2021;15(1):60–68. doi: 10.21053/ceo.2020.02313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seol H.Y., Kim G.Y., Kang S., et al. Clinical comparison of a hearing aid, a personal sound amplification product, and a wearable augmented reality device. Clin Exp Otorhinolaryngol. 2021;14(3):359–361. doi: 10.21053/ceo.2021.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan K.N., Silverstein A., Bryan L.N., McCracken C.E., Little W.K., Shane A.L. Comparison of a smartphone otoscope and conventional otoscope in the diagnosis and management of acute otitis media. Clin Pediatr. 2019;58(3):302–306. doi: 10.1177/0009922818812480. (Phila) [DOI] [PubMed] [Google Scholar]
- 40.Kleinman K., Psoter K.J., Nyhan A., Solomon B.S., Kim J.M., Canares T. Evaluation of digital otoscopy in pediatric patients: a prospective randomized controlled clinical trial. Am J Emerg Med. 2021;46:150–155. doi: 10.1016/j.ajem.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Mousseau S., Lapointe A., Gravel J. Diagnosing acute otitis media using a smartphone otoscope; a randomized controlled trial. Am J Emerg Med. 2018;36(10):1796–1801. doi: 10.1016/j.ajem.2018.01.093. [DOI] [PubMed] [Google Scholar]
- 42.Schuster-Bruce J.R., Ali A., Van M., Rogel-Salazar J., Ofo E., Shamil E. A randomised trial to assess the educational benefit of a smartphone otoscope in undergraduate medical training. Eur Arch Otorhinolaryngol. 2021;278(6):1799–1804. doi: 10.1007/s00405-020-06373-1. [DOI] [PubMed] [Google Scholar]
- 43.Seol H.Y., Kim G.Y., Kang S., et al. Clinical comparison of a hearing aid, a personal sound amplification product, and a wearable augmented reality device. Clin Exp Otorhinolaryngol. 2021;14(3):359–361. doi: 10.21053/ceo.2021.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maidment D.W., Barker A.B., Xia J., Ferguson M.A. A systematic review and meta-analysis assessing the effectiveness of alternative listening devices to conventional hearing aids in adults with hearing loss. Int J Audiol. 2018;57(10):721–729. doi: 10.1080/14992027.2018.1493546. [DOI] [PubMed] [Google Scholar]
- 45.Reed N.S., Betz J., Kendig N., Korczak M., Lin F.R. Personal sound amplification products vs a conventional hearing aid for speech understanding in noise. JAMA. 2017;318(1):89–90. doi: 10.1001/jama.2017.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jerger J., Hayes D. Hearing aid evaluation: clinical experience with a new philosophy. Arch Otolaryngol. 1976;102(4):214–225. doi: 10.1001/archotol.1976.00780090056008. [DOI] [PubMed] [Google Scholar]
- 47.Cox R.M., Johnson J.A., Xu J. Impact of hearing aid technology on outcomes in daily life I: the patients’ perspective. Ear Hear. 2016;37(4):e224–ee37. doi: 10.1097/AUD.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vestergaard Knudsen L., Öberg M., Nielsen C., Naylor G., Kramer S.E. Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: a review of the literature. Trends Amplif. 2010;14(3):127–154. doi: 10.1177/1084713810385712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kochkin S. MarkeTrak VIII.: the key influencing factors in hearing aid purchase intent. Hear Rev. 2012;19(3):12–25. [Google Scholar]
- 50.Birlutiu A., Groot P., Heskes T. Multi-task preference learning with an application to hearing aid personalization. Neurocomputing. 2010;73(7):1177–1185. [Google Scholar]
- 51.Bush M.L., Sprang R. Management of hearing loss through telemedicine. JAMA Otolaryngol Head Neck Surg. 2019;145(3):204–205. doi: 10.1001/jamaoto.2018.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yimtae K., Israsena P., Thanawirattananit P., et al. A tablet-based mobile hearing screening system for preschoolers: design and validation study. JMIR mHealth uHealth. 2018;6(10):e186. doi: 10.2196/mhealth.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derin S., Cam O.H., Beydilli H., Acar E., Elicora S.S., Sahan M. Initial assessment of hearing loss using a mobile application for audiological evaluation. J Laryngol Otol. 2016;130(3):248–251. doi: 10.1017/S0022215116000062. [DOI] [PubMed] [Google Scholar]
- 54.Potgieter J.M., Swanepoel D.W., Smits C. Evaluating a smartphone digits-in-noise test as part of the audiometric test battery. S Afr J Commun Disord. 2018;65(1):574. doi: 10.4102/sajcd.v65i1.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arlinger S. Negative consequences of uncorrected hearing loss-a review. Int J Audiol. 2003;42:2S17–2S20. [PubMed] [Google Scholar]
- 56.Li-Korotky H.S. Age-related hearing loss: quality of care for quality of life. Gerontologist. 2012;52(2):265–271. doi: 10.1093/geront/gnr159. [DOI] [PubMed] [Google Scholar]
- 57.Almufarrij I., Munro K., Dawes P., Stone M.A., Dillon H. Direct-to-consumer hearing devices: capabilities, costs, and cosmetics. Trends Hear. 2019;23:1–18. doi: 10.1177/2331216519858301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casale M., Sabatino L., Salvinelli F. Personal sound amplification products for hearing loss. JAMA. 2017;318(18):1831. doi: 10.1001/jama.2017.14783. [DOI] [PubMed] [Google Scholar]
- 59.Maidment D.W., Barker A.B., Xia J., Ferguson M.A. A systematic review and meta-analysis assessing the effectiveness of alternative listening devices to conventional hearing aids in adults with hearing loss. Int J Audiol. 2018;57(10):721–729. doi: 10.1080/14992027.2018.1493546. [DOI] [PubMed] [Google Scholar]
- 60.Manchaiah V., Taylor B., Dockens A.L., et al. Applications of direct-to-consumer hearing devices for adults with hearing loss: a review. Clin Interv Aging. 2017;12:859–871. doi: 10.2147/CIA.S135390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powers T.A., Rogin C. MarkeTrak 10: history and methodology. Semin Hear. 2020;41(1):3–5. doi: 10.1055/s-0040-1701241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson E.E. Safety limit warning levels for the avoidance of excessive sound amplification to protect against further hearing loss. Int J Audiol. 2017;56(11):829–836. doi: 10.1080/14992027.2017.1346306. [DOI] [PubMed] [Google Scholar]
- 63.Lakshmi M.S.K., Rout A., Morris A., Smaldino J. Consumer opinion of personal sound amplification products: a preliminary sentiment analysis. Am J Audiol. 2019;28(2s):450–459. doi: 10.1044/2019_AJA-IND50-18-0103. [DOI] [PubMed] [Google Scholar]
- 64.Lakshmi M.S.K., Rout A., Morris A., Smaldino J. Consumer opinion of personal sound amplification products: a preliminary sentiment analysis. Am J Audiol. 2019;28(2S):450–459. doi: 10.1044/2019_AJA-IND50-18-0103. [DOI] [PubMed] [Google Scholar]
- 65.Reed N.S., Betz J., Lin F.R., Mamo S.K. Pilot electroacoustic analyses of a sample of direct-to-consumer amplification products. Otol Neurotol. 2017;38(6):804–808. doi: 10.1097/MAO.0000000000001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arehart K.H., Souza P., Baca R., Kates J.M. Working memory, age, and hearing loss: susceptibility to hearing aid distortion. Ear Hear. 2013;34(3):251–260. doi: 10.1097/AUD.0b013e318271aa5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dryden A., Allen H.A., Henshaw H., Heinrich A. The association between cognitive performance and speech-in-noise perception for adult listeners: a systematic literature review and meta-analysis. Trends Hear. 2017;21:1–21. doi: 10.1177/2331216517744675. 2331216517744675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.(EFHOH) EFoHoHP. The potential risks of using PSAPs – FDA Workshop 21st of April 2016. 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.