Highlights
-
•
The efficacy of adjuvant therapy for periampullary carcinoma is controversial.
-
•
There is a trend of classification periampullary carcinoma into PB-type and IN-type, and the prognosis of different subtypes may be significantly different.
-
•
The PB-type patients who accepted gemcitabine based chemotherapy and IN-type patients who accepted 5-FU based chemotherapy, maybe improved the prognosis.
-
•
Chemoradiotherapy appears to be more effective in patients with advanced stages.
-
•
There are few related studies on targeted therapy and immunotherapy, and further research is needed.
Keywords: Periampullary carcinoma, Adjuvant therapy, Histopathological type, 5-FU, Gemcitabine
Abstract
Objective
This review investigates the role of adjuvant therapy (AT) and the importance of histopathological typing in periampullary carcinoma (PAC) treatment.
Background
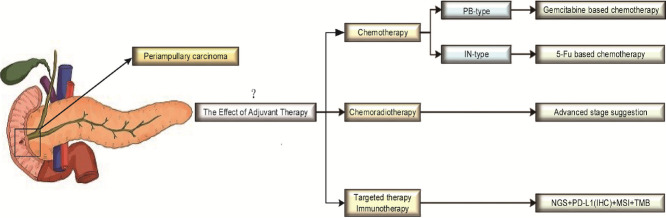
PAC is a relatively rare gastrointestinal malignancy. The regimen and effect of AT in PAC are still controversial. However, there is a treatment based on histopathological types (pancreaticobiliary-type, PB-type or intestinal-type, IN-type), but there are no clear guidelines indicating that typing can be used to guide the selection of AT drugs.
Methods
A literature search of PubMed and Web of Science databases was conducted for studies published from January 2001 to August 2021 on the use of AT in PAC.
Results
A total of 75 studies were included in this review. According to existing studies, AT for PAC is mostly based on 5-FU or gemcitabine, but the effect is unknown. However, when PAC is classified into different histopathological types, AT with gemcitabine is beneficial for patients with the PB-type of PAC, while 5-FU-based AT is beneficial for patients with the IN-type of PAC. In addition, the benefits of AT are more pronounced in patients with a high-risk disease, such as patients with stage II/III, T3/T4 tumors, or positive lymph node involvement. There are few studies on targeted therapy and immunotherapy for PAC.
Conclusions
This review suggests that AT has potential survival benefits, especially when based on the histopathologic type that helps the choice of drugs during AT in PAC patients.
Graphical abstract
Introduction
Periampullary carcinoma (PAC) is a malignant tumor originating from the area within 2 cm of the Ampulla of Vater [1,2] and comprises different types of cancer, including Ampulla of Vater cancer, pancreatic head cancer, distal common bile duct cancer, and duodenal papillary cancer. Most PAC are adenocarcinomas; however, they can occasionally present an adenosquamous, neuroendocrine, or mucinous carcinoma phenotype. PAC is relatively rare in clinic and constitutes 5% of all gastrointestinal tract malignancies [3]. It has the eighth to ninth most frequent incidence and is the fourth to fifth most common cause of death associated with gastrointestinal tract malignancies. The incidence is 11.7/10 5 people for pancreatic head cancer, 0.88/10 5 for the distal common bile duct cancer, 0.49/10 5 Ampulla of Vater cancer, and 0.01/10 5 for duodenal papillary cancer [4], [5], [6]. The age of most patients ranges from 40 to 70 with a slight male predominance [7].
Currently, surgical resection remains the main therapy option to improve long-term survival through local resection (ampullectomy), pancreaticoduodenectomy (PD), and pylorus-preserving pancreaticoduodenectomy (PPPD) [11], [12], [13]. PD is considered as the standard treatment for PACs with a resection rate of 20–50% [[14], [15], [16], [17]]. Due to the similar anatomical position and different histological sources, there is a significant variation in the survival rate after resection [9]. The 5-year survival rates after surgical resection are 33–68% for ampullary carcinoma, 23–30% for distal common bile duct carcinoma, 25–59% for duodenal adenocarcinoma, and 5–20% for pancreatic carcinoma [8,16,[18], [19], [20], [21], [22]]. Metastatic or advanced PACs are characterized by worse prognosis with 2-year survival rates ranging from 5 to 10% [23].
Surgery alone cannot provide a favorable long-term cure rate as 30–50% of patients experience recurrence and ultimately die from the disease [24]. The most common recurrence sites are the celiac lymph node, the liver, and the lungs [10,25]. Therefore, adjuvant therapy (AT) is recommended with the aim of improving the long-term survival [26], [27], [28], [29], [30]. However, there is no convincing evidence that AT is beneficial and no optimal treatment drugs for PACs’ AT.
Based on related studies that were published between 2001 and 2021, this review investigates the function of different AT strategies and the importance of histopathological subtyping in PACs’ treatment in guiding clinical treatments, associated with the lack of standard regimen that undermine the efficacy of treatment on PACs.
Methods
Search strategy
In this review, we define AT, including chemotherapy, radiotherapy, concurrent chemoradiotherapy, targeted therapy, immunotherapy and neoadjuvant therapy in PAC patients who accept surgery or not.
A literature search of PubMed and Web of Science databases was conducted for experimental studies published from January 2001 to February 2021 on the use of AT in PACs. Reviews and related background literature were also performed. The references of each article were manually checked to prevent omissions. The search was repeated in August 2021 to detect the most recently published studies. The primary objective of this review is to determine the efficacy and optimal treatment regimen of AT in patients with PACs.
The keywords used were: periampullary, ampullary, duodenal papillary, cancer, and all relevant key variations of these terms. The exclusion criteria were as follows: (1) Letters, and conference summaries with limited data; (2) Studies conducted using animals or cell lines; (3) Studies with no available English translation; (4) Periampullary carcinoma is not mentioned in title or abstract; and (5) No full-text available. The literature search was conducted by five authors, and discrepancies were discussed until a consensus on the relevance had been reached.
Result
Selection of studies
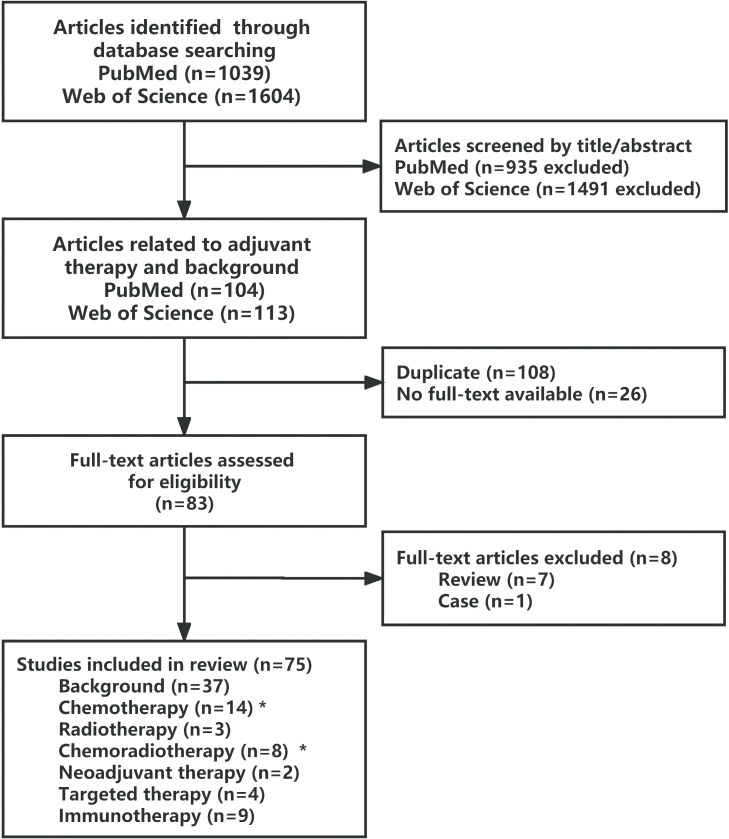
The flow chart in Fig. 1 shows the results of 75 analyzed studies. A preliminary search of PubMed and Web of Science resulted in 1039 and 1604 eligible studies, respectively. Since our main objective was to determine the efficacy and optimal regimen of AT for patients with PACs, the only relevant literature on the application of AT in PACs and with main introduction on PACs, was included. As a result, 83 publications were included by combining the results of the two databases. After reviewing the full text of the publications, 75 of these met the inclusion criteria, including 38 experimental studies and 37 as background support publications. The main studies of AT for PAC are listed in Tables 1 and 2.
Fig. 1.
Flowchart showing the selection process of the review articles. (⁎Two overlapping articles in these groups),
Table 1.
Characteristics of major adjuvant chemotherapy studies.
| Author | Trial type | Year | Country | N | Treatment arms | Primary endpoint | Result | |
|---|---|---|---|---|---|---|---|---|
| Regimen/Drug | Survival (Months) | |||||||
| Kim [25] | R | 2020 | Korea | 646 | 1. Fluoropyrimidine-based (123) 2. Ob (123) |
Median OS Median RFS |
Fluoropyrimidine-based vs Ob Fluoropyrimidine-based vs Ob |
41 vs 36 (P = 0.134) 18 vs 16 (P = 0.205) |
| Al Abbas [32] | R | 2019 | America | 121 | 1. 5‐FU (14) 2. Gem (33) 3. Ob (74) |
Median OS | ACT vs Ob 5-FU vs Ob Gem vs Ob 5-FU vs Gem |
45.6 vs 32.1 (P = 0.032) 87.4 vs 32.1 (P = 0.046) 38.0 vs 32.1 (P = 0.167) 87.4 vs 38.0 (P = 0.203) |
| 1. PB (58): 5-FU (6) Gem (24) Ob (28) 2. IN (39): 5-FU (4) Gem (6) Ob (29) 3. AM (24):5-FU (4) Gem (3) Ob (17) |
Median OS | PB: 5-FU vs Gem vs Ob IN: 5-FU vs Gem vs Ob AM: 5-FU vs Gem vs Ob |
40.4 vs 30.4 vs 29.4 (P. NS) 52.7 vs NR vs 32.4 (P. NS) 87.4 vs 23.5 vs 33.5 (P. NS) |
|||||
| 1. Stage Ⅰ + Ⅱ A: ACT (10) Ob (29) 2. Stage Ⅱ B + Ⅲ: ACT (37) Ob (26) |
Median OS | stage Ⅰ + Ⅱ A: ACT vs Ob stage Ⅱ B + Ⅲ: ACT vs Ob |
NS (P = 0.121) 38.6 vs 21.4 (P < 0.01) |
|||||
| Schiergens [33] | R | 2015 | Germany | 95 | 1. Gem (34) 2. Ob (60) |
OS | Gem vs Ob | NS (P = 0.832) |
| 1. PB (46): Gem (22) Ob (24) 2. IN (47): Gem (12) Ob (35) 3. Undifferentiated (2) |
Median OS | PB: Gem vs Ob IN: Gem vs Ob |
32 vs 13 (P = 0.013) 35 vs 112 (P = 0.193) |
|||||
| Moekotte [34] | R | 2020 | Six countries | 976 | 1. Gem-based (194) 2. Ob (194) |
Median OS | Gem vs Ob | NR vs 60 (P = 0.051) |
| 1. PB/AM (194): Gem-based (97) Ob (97) 2. IN (90): Gem-based (45) Ob (45) |
Median OS | PB/AM: Gem-based vs Ob IN: Gem-based vs Ob |
NR vs 32 (P = 0.020) NR vs NR (P = 0.719) |
|||||
| Neoptolemos [35] | RCT | 2012 | Europe Australia Japan Canada | 428 | 1. CF + FU (143) 2. Gem (141) 3. Ob (144) |
Median OS | ACT vs Ob CF + FU vs Ob Gem vs Ob |
43.1 vs 35.2 (P = 0.03) 38.9 vs 35.2 (P = 0.74) 45.7 vs 35.2 (P = 0.10) |
| Ramaswamy [36] | R | 2019 | India | 214 | 1. PB (105): Gem-based (64) Ob (16) 2. IN (109): Gem-based (50) Ob (9) |
Median OS | PB: Gem vs Ob IN: Gem vs Ob |
PB: 58.09 vs 18.46 (P < 0.001) IT: NR vs 28.62 P < 0.001) |
| 1. Stage Ⅰ + Ⅱ + Ⅲ (214): Gem-based (135) Ob (79) 2. Stage Ⅱ + Ⅲ (139): Gem-based (114) Ob (25) |
Median OS | Stage Ⅰ + Ⅱ + Ⅲ: Gem-based vs Ob Stage Ⅱ +Ⅲ: Gem-based vs Ob |
NS (P = 0.603) NR vs 22.28 (P = 0.036) |
|||||
| Author | Trial type | Year | Country | N | Treatment arms | Primary endpoint | Result | |
|---|---|---|---|---|---|---|---|---|
| Regimen/Drug | Survival (Months) | |||||||
| Ecker [39] | R | 2019 | Multinational | 357 | 1. FU-based (29) 2. Gem-based (57) 3. Ob (70) |
OS | ACT vs Ob FU-Based vs Ob Gem-Based vs Ob |
NS (P = 0.69) NS (P = 0.68) NS (P = 0.74) |
| 1. PB (65): 5-FU (9) Gem (29) Ob (27) 2. IN (78): 5-FU (19) Gem (18) Ob (41) |
OS | PB: 5-FU vs Ob Gem vs Ob IN: 5-FU vs Ob Gem vs Ob |
NS (P = 0.94) NS (P = 0.77) NS (P = 0.72) NS (P = 0.63) |
|||||
| 1. Stage Ⅰ (30): ACT (14) Ob (16) 2. Stage Ⅱ (19): ACT (12) Ob (7) 3. Stage Ⅲ (99): ACT (52) Ob (47) |
OS | stage Ⅰ: ACT vs Ob stage Ⅱ: ACT vs Ob stage Ⅲ: ACT vs Ob |
NS (P = 0.05) NS (P = 0.55) NS (P = 0.36) |
|||||
| Kim [40] | R | 2013 | Korea | 21 | XELOX (21) | ORR Median TTP Median OS |
38%⁎ 7.6 19.7 |
|
| 1. PB (10) 2. IN (7) |
Median TTP | PB vs IN | 6.4 vs 13.1 (P = 0.038) | |||||
| Overman [41] | P | 2009 | America | 30 SBA 18 AAC 12 |
CAPOX (30) | ORR Median TTP Median OS |
50%⁎(SBA 61%,AAC 33%) 11.3 20.4 |
|
| Kim [42] | R | 2010 | Korea | 29 | Platinum-based (29) | ORR Median TTP Median OS |
27.5%⁎ 4.9 12.5 |
|
| 1. FP (11) 2. XP (9) 3. GP (9) |
TTP OS |
FP vs XP vs GP FP vs XP vs GP |
NS (P = 0.79) NS (P = 0.85) |
|||||
Abbreviation: R retrospective; RCT randomized controlled trial; P prospective; Gem gemcitabine; 5-FU 5-fluorouracil; CF folinic acid; XELOX capecitabin + oxaliplatin; CAPOX capecitabin + oxaliplatin; Ob observation; FP 5-FU+cisplatin; XP capecitabine + cisplatin; GP gemcitabine + cisplatin; IN intestinal; PB pancreatobiliary; AM ambiguous; ACT adjuvant chemotherapy; OS overall survival; RFS recurrence-free survival; ORR objective response rate/overall response rate; TTP time to progression; PFS progression-free survival; AAC ampullary adenocarcinoma; SBA small bowel adenocarcinoma; NR not reached; NS not state. ⁎The value is a percentage
Table 2.
Characteristics of major adjuvant chemoradiotherapy studies.
| Author | Trial type | Year | Country | N | Treatment arms | Primary endpoint | Results | |
|---|---|---|---|---|---|---|---|---|
| Regimen/Drug | Survival (Months) | |||||||
| Nassour [37] | R | 2017 | USA | 4190 | 1. ACRT (568), Ob (568) 2. ACT (768), Ob (768) |
Median OS | ACRT vs Ob ACT vs Ob |
38.1 vs 31.0 (P = 0.02) 47.2 vs 35.5 (P < 0.01) |
| Bolm [43] | R | 2020 | Europe | 214 | 1. AT (75): ACT with Gem (35), ACT with Gem + Oxaliplatin (7), ACRT (7), Capecitabine (6), Folfox (5), Unknown (15) 2. Ob (139) |
Median OS | AT vs Ob PB/Mixed: AT vs Ob |
113 vs 168 (P = 0.608) 85 vs 65 (P = 0.005) |
| Zhou [54] | R | 2009 | USA | 111 | 1. ACRT (50): 5-FU (37), Capecitabine (10), Gem + 50.4 Gy (3) 2. Ob (61) |
Median OS | ACRT vs Ob node-positive: ACRT vs Ob |
33.4 vs 36.2 (P = 0.969) 21.6 vs 13.0 (P = 0.092) |
| Smeenk [55] | RCT | 2007 | Europe | 218 | 1. AT (110): ACRT with 5-FU + 40Gy 2. Ob (108) |
Median OS | ACRT vs Ob | 1.8 year vs 1.6 year (P. NS) |
| Turan [56] | R | 2015 | Turkey | 563 | 1. CRT-CT (231): Gem (151), 5-FU + Leucovorin (48), FU (30), Others (13) 2. CT (26) 3. CRT (215): Gem (71), Gem + Cisplatin (70), 5-FU + Leucovorin (41), Gem + Leucovorin + 5-FU (28), Cisplatin + 5-FU (5) |
Median OS | CRT-CT vs CT | NS (P = 0.003) |
| Kim [57] | R | 2008 | Korea | 118 | 1. ACRT (41): 5-FU (500 mg/m2/day.i.v.) + 40Gy 2. Ob (77) |
Median OS | node-positive: ACRT vs Ob | NS (P = 0.003) |
| Kim [58] | R | 2020 | South Korea | 651 | 1. AT (255): ACT with 5-FU/Gem, ACRT with 5-FU/Gem + 50.4Gy 2. Ob (396) |
5-year OS rate | T1/T2: ACRT vs Ob T3/T4: ACRT vs Ob node-positive: ACRT vs Ob |
57.6% vs 80.7% (P = 0.007) 62.4% vs 55.2% (P = 0.087) 46.9% vs 26.3% (P = 0.12) |
| Krishnan [59] | R | 2008 | USA | 96 | 1. ACRT (54): 5-FU (29)/Capecitabine (24)/Gem (1) + 45 Gy (25, preoperative) / 50.4 Gy (29, postoperative) 2. Ob (42) |
Median OS | T3/T4: ACRT vs Ob | 35.2 vs 16.5 (P = 0.06) |
Abbreviation: AT adjuvant therapy; ACRT adjuvant chemoradiotherapy; CRT chemoradiotherapy; CT chemotherapy; CRT-CT chemoradiotherapy with maintained chemotherapy; Ob observation; Gem gemcitabine; 5-FU 5-fluorouracil; OS overall survival; NS not state; PB pancreatobiliary.
Adjuvant chemotherapy for PACs
Condition of adjuvant chemotherapy
As PACs are relatively rare, the current application of adjuvant chemotherapy (ACT) for PACs mostly refers to that of pancreatic cancer, cholangiocarcinoma (gemcitabine-based), and colorectal cancer (5-FU-based) [32,33]. There are still controversies about the optimal regimen and optional time, and whether ACT is needed [3,25,[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]
Several trials have shown that ACT improves survival in patients with PACs [25,[34], [35], [36], [37]]. In an international multicenter cohort of 976 patients with PACs, patients receiving gemcitabine-based regimens had a better survival than those who did not (median OS not reached vs. 60 months, p = 0.051) [34]. Similarly, in the ESPAC-3 multicenter randomized phase III trial of ACT (combination of FU with folinic acid or gemcitabine) compared to 428 resected PACs, a benefit to ACT was observed after multiple regression analysis (median OS 43.1 vs. 35.2 months, p = 0.03) [35].
However, some studies have questioned the value of ACT [3,38,39]. In a retrospective study of 357 patients with PACs, the use of chemotherapy (FU-based or gemcitabine-based) was not associated with improved long-term survival (p = 0.69) [39]. Two other studies were consistent with the result, but it remains to be verified due to the small number of patients [3,38].
To explore the optimal regimen, some studies compared the efficacy of FU-based and gemcitabine-based regimens [32,35]. A US study of 121 patients showed that patients receiving 5-FU have longer median OS and improved survival (p = 0.046) than those receiving gemcitabine (87.4 vs. 38 months). In this study, gemcitabine did not show a significant effect (p = 0.167) [32]. However, in the ESPAC-3 study, 428 patients with PACs were enrolled, and gemcitabine showed a better survival benefit compared to that of the combination of FU with folinic acid (median OS 45.7 vs. 38.9 months) [35].
The role of histopathological typing in adjuvant chemotherapy
At present, there is no convincing evidence on the benefits of ACT and optimal treatment regimen for PACs. One main reason is the heterogeneity of the tumor tissue origin (duodenal, biliary, or ductal pancreatic epithelia), which shows different sensitivities to chemotherapeutic agents leading to inconsistent results in the above-mentioned trials [42]. Nevertheless, the effect of tissue heterogeneity in AT did not attract enough attention.
Therefore, there has been a recent tendency to vary the treatment of patients with PACs based on whether the tumor displayed an intestinal (IN-type) or pancreatobiliary (PB-type) histopathological phenotype [9]. The IN-type PAC originates from the intestinal epithelium overlying the ampulla, including duodenal carcinoma and some of the ampullary carcinomas. Whereas the PB-type PAC originates from the epithelium of the distal common bile duct and the head of pancreatic duct, including pancreatic cancer, distal bile duct cancer, and some of the ampullary carcinomas [3,44]. According to reports, the PB-type PAC has a worse prognosis compared to that of IN-type PAC (median OS 16.1 vs. 115.5 months, p < 0.001) [45]. Therefore, the histopathological phenotype has been reported as a factor that is independently associated with survival in patients with PACs and is used to guide the selection of relevant adjuvant regimens [39,46]. As PB-type tumors behave more like pancreatic cancer, gemcitabine-based regimens may be indicated. Conversely, due to the similarity between IN-type tumors and colorectal cancer, 5-FU-based regimens may be more effective [33,41].
At present, the main typing methods combine H&E staining with immunohistochemical (IHC) staining. H&E staining is mainly based on tissue morphology and structure, while IHC staining is mostly based on the detections of the markers, CK20, CDX2, MUC1, and MUC2 [47]. According to the system proposed by Ang et al., tumors that stain positive for CK20, CDX2, or MUC2, and negative for MUC1, or positive for all of three markers, regardless of MUC1, are classified as IN-type. Tumors that stain positive for MUC1 in the absence of both CDX2 and MUC2 are classified as PB-type, regardless of the status of CK20. Other combinations are classified as ambiguous (AM-type) [47].
Some studies reported the benefits of subtype classification in the treatment of PACs [33,34,36,39,40,43]. A retrospective study of 95 patients showed no efficacy with gemcitabine in the entire cohort (p = 0.832). However, after histopathological classification, the survival benefit of gemcitabine monotherapy was only seen in PB-type patients (median OS 32 vs. 13 months, p = 0.013), whereas gemcitabine tended to be associated with decreased OS in IN-type patients (median OS 35 vs. 112 months, p = 0.193) [33]. In a study of 976 patients with PAC, the survival benefit after gemcitabine therapy was only seen in patients with PB and AM types (median OS not reached vs. 32 months, p = 0.020). IN-type patients did not show any survival benefit from gemcitabine therapy (median OS not reached vs. not reached, p = 0.719) [34]. A study of 214 patients with PACs, who were treated with gemcitabine, showed that although both subtypes significantly benefited from ACT (median OS IN: not reached vs. 28.62 months, PB: 58.09 vs. 18.46 months, p < 0.001), there was no statistical difference in OS between IN-type and PB-type patients (p = 0.209). Due to the poor survival of PB-type patients compared with that of IN-type patients, this study suggest that the benefit of gemcitabine is more pronounced in PB-type patients [36]. Similarly, a Korean study of 17 patients with PAC concluded that the XELOX (Capecitabine and Oxaliplatin) regimen was better for patients with advanced IN-type PAC compared to that of patients with PB-type PAC (median TTP 13.1 vs. 6.4 months, p = 0.038). However, this difference may be attributed to the favorable prognosis of IN-type patients [40].
Existing studies mostly support the potential benefits of typing therapy, but a study had a different opinion [32]. In the PB-type cohort, patients receiving 5-FU had the longest median survival compared with that of gemcitabine or no treatment cohorts (40.4 vs. 30.4 vs. 29.4 months). For the AM subtype, 5-FU also contributed to the longest survival compared with that of gemcitabine or no treatment cohorts (87.4 vs. 23.5 vs. 33.5 months). The results showed that the combination of AT with 5-FU confers a survival benefit, regardless of the subtype. The lack of survival benefit from gemcitabine may be related to the limited sample size of the AT arm (n = 47), which limited robust analysis of the interactions between subtypes and different chemotherapy regimens [32].
Adjuvant radiotherapy and chemoradiotherapy for PACs
The role of adjuvant radiotherapy in the treatment of PACs
By searching the literature, we only found three publications on the role of adjuvant radiotherapy (ART) alone for PACs [48], [49], [50]. According to these studies, there is no survival advantage for ART for PAC patients.
A retrospective study evaluated the effect of ART on survival in resected PACs [48]. In PSM cohorts, 326 patients received surgery and ART and 236 patients received surgery alone. No significant difference in OS was observed (29 vs. 37 months, p = 0.119).
Another retrospective study that aimed at defining the role of external beam radiotherapy on resected PAC showed similar results [49]. After PSM, 606 patients were identified, of which 308 patients received ART. When compared to surgery alone, ART failed to improve OS (27 vs. 29 months, p = 0.58). Moreover, ART did not confer an OS benefit among high-risk patients in the PSM cohorts that were stratified by poor prognostic tumor characteristics, such as patients with T3/T4 tumors (26 vs. 24 months, p = 0.13) and presence of poor histology (26 vs. 25 months, p = 0.59). Adjuvant radiation in the setting of lymph node metastasis showed a trend towards improved survival but it was not statistically significant (26 vs. 22 months, p = 0.06).
The role of adjuvant chemoradiotherapy in the treatment of PACs
Currently, there is some practice to use a regimen based on combining ART with 5-FU, gemcitabine or a combination with cisplatin. ART often starts two weeks after chemotherapy, and some patients are treated with a total dose of 54 Gy and1.8 Gy per day, 5 days a week [51], [52], [53]. [But the results of adjuvant chemoradiotherapy (ACRT) are inconsistent and it is unclear whether there is a benefit [37,43,54,55].
From the National Cancer Database, PAC patients who underwent resection were identified [37]. After PSM, 568 observation patients were compared with 568 ACRT patients (group A) and 768 patients who had observation, were compared with 768 patients who received ACT (group B). In group A, ACRT was associated with improved OS compared with that of the observation group (38.1 vs. 31.0 months, p = 0.02). In group B, the application of ACT was also associated with improved OS (47.2 vs. 35.5 months, p < 0.01). In this retrospective study, the use of both ACT and ACRT for PACs, was associated with a significantly improved OS.
Another study analyzed 111 patients who underwent PD, of which 50 patients (45%) received ACRT [54]. Radiation was delivered using a four- or five-field coplanar beam arrangement (80%), intensity-modulated radiation therapy (IMRT) (14%), and 3-field (6%). The median total radiation dose was 50.4 Gy (range: 38.7–54.0 Gy). The median duration of radiotherapy was 41 days (range: 30–64 days), with a mean starting at 74 days after surgery (range: 36–145 days). There was one planned interruption (14 days) in radiotherapy at 20 Gy for 17 patients. Concurrent chemotherapy included continuous infusion 5-FU (76%), capecitabine (20%), and gemcitabine (4%). Univariate analysis showed that curative resections of PACs followed by ACRT does not lead to a statistically significant difference in OS when compared with surgery alone (33.4 vs. 36.2 months, p = 0.969).
Despite the controversial results, the benefits of ACRT are especially valuable in patients with high-risk disease such as patients with T3/T4 tumors and positive nodal involvement [56], [57], [58], [59].
A total of 563 patients who were curatively resected for PACS were retrospectively analyzed [56]. A total of 231 were given CRT with maintenance chemotherapy (CRT-CT), 26 with CRT alone, and 215 with CT alone. Although there was no significant difference in OS (p = 0.082) between the groups for patients with node-negative disease, CRT-CT provided improved RFS (p = 0.004) and OS (p = 0.003) compared to that of CT alone in node-positive patients, indicating that the addition of radiation to CT has a survival benefit in patients with node-positive disease.
Another study analyzed 118 PAC patients who underwent resection [57]. A total of 41 patients received ACRT, and 77 were in the observation group. Postoperative radiotherapy was delivered to the tumor bed and regional lymph nodes, for a total dose of up to 40 Gy delivered in 2-Gy fractions for 5 days a week, with a planned 2-week rest period after the first 20 Gy. Intravenous 5-FU (500 mg/m2/day) was given on the first 3 days of each week of radiotherapy. In subgroup analysis, patients with nodal metastasis showed evident improvement in OS (p = 0.0235).
Similar results were observed in a multicenter retrospective study [58]. A total of 396 out of 655 patients underwent surgery alone and 255 received AT (ACT or ACRT) after surgery. Patients who were treated with ACRT received 5-FU-based ACT and radiation with a main dose of 50.4 Gy. The 5-year OS rate was improved in AT-received patients and who had a high-risk of disease such as node-positive (any TN1, any TN2) (55.2% vs. 62.4%, p = 0.087) or advanced T stage (T3N0, T4N0) (29.5% vs. 33.7%, p = 0.229).
Neoadjuvant therapy for PACs
Neoadjuvant therapy is progressively being used in patients who are unable to undergo radical surgery and in patients with a high relapse rate [31]. However, the role of preoperative CRT for PACs, remains undefined [60,61].
A study analyzed 61 PAC patients who underwent resection and received adjuvant (n = 43) or neoadjuvant (n = 18) CRT [60]. The CRT regimen included a concurrent fluoropyrimidine-based treatment (91%), a mitomycin concurrent with 5-Fu (3%), and a concurrent Gem (6.5%). The median dose of radiation was 50 Gy. After neoadjuvant CRT, 12 patients were downstaged on final pathology and 5 had a pathologic complete response. A trend toward a 3-year OS rate (62% vs. 46%, p = 0.074) benefit in patients receiving CRT was identified.
There was similar research in USA which included 142 PAC patients who underwent PD, of which 43 patients (30.3%) underwent preoperative therapy [61]. The preoperative treatment consisted of CRT (65%), RT (7%), or both (28%). There was no significant difference in rates of loco-regional recurrence (7.0% vs. 9.1%, p > 0.05), median OS (146 vs. 107 months, p > 0.05) or 5-year OS rates (70.4% vs. 60.6%,p > 0.05).
Targeted therapy for PACs
Although research is focused on the study of potential targets, there is no clear and effective targeted drug for the treatment of PACs [62,63]. Therefore, we summarize the potential use of target therapy in some cases.
Currently, common gene mutations associated with PACs include oncogenic mutations of KRAS, BRAF, and HER2 [62], among which KRAS is the most common oncogenic mutant in PACs with common mutation sites including G12D, G12V, G13D, G12C, G12N, G12S, G12A, and G12R [62]. Trastuzumab can be used as a targeted therapy for HER2 positive mutations, and various studies have demonstrated the presence of HER2 amplification or overexpression in 0–23.0% of PAC patients [64]. One study showed that insertion mutations in BRCA2 at c.156_157ins Alu are found in 12.5% of Portuguese patients with PACs, indicating that these patients may benefit from a treatment with the PARP inhibitor [62].
There are only some reported cases of targeted therapy for PACs, and therefore, we discuss some cases with significant targeted therapy effects. One of the cases is associated with a 55-year-old man with a PB-type of PAC, who had metastasis after surgery [64]. The tumor was HER2(+), and therefore, he was treated with trastuzumab (318mg), cyclophosphamide (750mg), and methotrexate (1.5 gm) for 21 days per cycle, and for 11 cycles. Interestingly, all metastatic sites disappeared, and the patient achieved a PFS of 6.9 months and an OS of 20.3 months [64]. VEGF plays a key role in tumor-associated neo-angiogenesis. A study showed that the combination of Bevacizumab with Capecitabine and Oxaliplatin has a good impact on metastatic PAC patients with a high level of VEGF-A expression. Among 30 patients, there were 7 PAC patients, resulting in an ORR of 48.3%, a median PFS of 8.7 months, and a median OS of 12.9 months. Moreover, PAC patients with an intestinal type have a better response compared to that of the mixed type [65].
Immunotherapy for PACs
Immunotherapy is currently less efficient for the treatment of PACs, and there is no clear treatment drug or program. Most of the research is still in the laboratory stage and has not been clinically applied.
Some studies reported a positive response rate of PACs using the PD-L1 checkpoint inhibitor. One study found that 59/123 of cases were PD-L1 positive, and a higher positive rate was identified in PB-type compared to IN-type (39 vs. 20) [66]. Another study found that 7/26 of cases were PD-L1 positive, but a higher positive rate was observed in IN-type (6 vs. 1) [67]. Tumors expressing PD-L1 are associated with shorter DFS and OS [66]. The CD8+ T lymphocyte density of IN-type tumors is higher than PB-type tumors and is associated with a better DFS [66]. In the study of DNA mismatch repair protein deficiency (dMMR), the survival rate of PAC patients with dMMR was significantly better than that of PAC patients with intact MMR. Compared with women, the male MMR protein is more frequently lost [68]. dMMR is more common in IN-type and is associated with a significantly prolonged OS. In PB-type tumors, dMMR does not affect the prognosis, but there is a significant negative interaction with AT [46].
Furthermore, some studies have found that some PACs have characteristics of intestinal tumors, such as a high degree of microsatellite instability (MSI-H) or dMMR. The Food and Drug Administration (FDA) approved the PD-1 antibodies, nivolumab and pembrolizumab, in 2017 and 2020, respectively, for the treatment of colorectal cancer patients with MSI-H or dMMR [69]. Thus, these findings indicate that some PAC patients can be candidates for treatments with PD-L1 and CTLA-4 checkpoint inhibitors [70,71]. A 59-year-old woman with PAC had tumor enlargement after chemotherapy and because she was carrying MSI-H and a high tumor mutational burden (TMB), she accepted a combination treatment with nivolumab (1 mg/kg) and ipilimumab (3mg/ kg). After four cycles of treatments, the tumor shrank, and the CEA level decreased. Subsequently, the patient underwent pancreaticoduodenectomy [70].
In addition to PD-L1, a study found that other immunosuppressive molecules, such as Gal-9, HVEM, IDO, HLA-G, CD8+, and FoxP3+ tumor infiltrating lymphocytes (TIL), are commonly expressed in PACs [72]. Besides, there is also research on the detection of PACs’ tumor microenvironment [73]. One study showed that the infiltration of NK cells and NKT cells in malignant tissues is low, and that the infiltration of CD56+ NK cells and NKT cells is related to an extended OS. Therefore, researchers believe that the role of NK cells and NKT cells in PACs are worthy of further studies [73]. Another study on the clinical significance of dendritic cells (DCs) and tumor-associated macrophages in PAC showed that the high density of CD1a + DCs is an independent prognostic factor for decreased OS in PB-type tumors. In IN-type tumors, the high-density collagen structure macrophage receptor (MARCO) + DC is significantly associated with poor prognosis, and it is only obvious in patients receiving ACT, but the association is not obvious in PB-type tumors [74].
Toxicity
Recent studies showed that AT is mostly well tolerated and safe as most side effects were mild, and no treatment-related deaths were found [35,[40], [41], [42],51,75]. Severe hematologic toxicities (WHO grade 3-4), such as neutropenia and thrombocytopenia, were the most common side effects of AT, and grade 1-2 hematologic toxicity was mainly associated with anemia. Grade 3 or 4 non-hematologic toxicity was mainly peripheral neuropathy. Other toxicities, including diarrhea, fatigue, stomatitis, anorexia, nausea, vomiting, and hair loss were primarily grade 1-2 that are easily controlled [35,40–42,51,75].
Discussion
PACs have lower incidence compared with other types of digestive system tumors. They also have complicated surrounding structures and various tissue origins, which contribute to the low number of studies on PACs, various treatment regimens, and distinct results [3,25,[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43],[54], [55], [56], [57], [58], [59]]. Based on the diversity of tissue origin, classifying PACs by histopathological features and applying specific AT to different PAC subtypes are reasonable strategies that lead to remarkable effects [33,34,36,39,40,43].
Currently, the effect of AT on PACs remains undefined. By consulting related literature, the two possible reasons are as follows: (1) Most studies did not adopt appropriate chemotherapy regimen based on histopathological subtyping; and (2) Chemotherapy regimens and cycles are diverse even for the same PAC type [3,25,32,35,38,41,42]. However, analysis of the following histopathological subtyping demonstrate that chemotherapy does effectively prolong postoperative survival time of PAC patients [33,34,36,39,40,43]. The currently recognized histopathological typing method is a combination of H&E staining with IHC staining to divide PACs into IN-type and PB-type [47]. IN-type patients are supposed to have longer DFS and OS, while patients with PB-type are more likely to go through abdominal cavity and hepatic metastases [45]. Thus, 5-FU-based ACT is recommended for IN-type PAC patients and Gemcitabine-based ACT for PB-type patients [33,41]. These tailored chemotherapy regimens, that are based on histopathological subtyping, lead to prominent effects [33,34,36,39,40,43]. However, our conclusions are expected to be proven by more reliable evidence which will depend on additional RCT studies. This therapeutic approach is valuable as it could standardize the formulating process of ACT regimen in PAC.
In our study, we found that ART alone cannot bring survival benefit to patients, which may be due to the limited number of studies on ART in PAC treatment, the variance of the radiotherapy pattern (internal or external radiation), doses, and cycles [48], [49], [50]. However, the combination of chemotherapy with radiotherapy is deemed to improve postoperative survival of PAC patients with a high-risk disease [37,[56], [57], [58], [59]].
As for targeted therapy and immunotherapy, there are few existing studies and case reports. The combination of targeted therapy with immunotherapy, and targeted or immunotherapy with HAIC, have made a breakthrough in hepatocellular carcinoma treatment; however, there is no related study that mentioned this effect, which is still unknown in PAC [64,65,70,[76], [77], [78]]. Nevertheless, for other types of neoplasms, it was recommended to conduct an NGS sequencing and detect PD-L1, TMB, MSI, and MMR to forecast the potential use of targeted therapy and immunotherapy. Moreover, due to the large number of cases showing a high MSI in PAC patients, these latter may benefit from immunotherapy [66,68,70,71]. Our team is trying to use of a combination of immunotherapy with chemotherapy in some suitable (PD-L1(+), TMB-high, MSI) PAC patients, and due to the limited number of cases, the results are currently unclear.
The literature search changed our traditional understanding and treatment principles for PACs. Firstly, we are supposed to abandon the traditional notion that PAC patients have better prognosis than those with pancreatic cancer and cholangiocarcinoma. IN-type PACs are likely to have better prognosis than the PB-type, but this latter, pancreatic cancer, and cholangiocarcinoma, have equal prognoses [33,45]. Secondly, we need to hold the idea of histopathological subtyping. We are not supposed to take it for granted, as occupation in duodenal papilla is equivalent to duodenal papillary cancer, and the possibility of PB-type PAC should not be excluded. Thirdly, it is suggested to take notice of the curative effect of AT, based on subtyping. The classification would remove confounding factors and draw a more precise conclusion.
In the future, we believe that no matter what research is performed, PAC should be divided into PB-type and IN-type. A combination of targeted/immunotherapy with chemotherapy may be a promising study in PAC. There are still many questions that need to be studied in the future: (1) Whether neoadjuvant therapy could bring survival benefit? (2) Are there changes of surgical resection rate after neoadjuvant therapy? (3) What kind of patients need postoperative AT? (4) Whether postoperative AT could bring benefit to PAC patients at an early stage? (5) How many radiotherapy and chemotherapy cycles are reasonable? (6) How to treat recurrent patients after chemotherapy? (7) Is there a difference in survival benefit among patients with different PAC subtypes, receiving different chemotherapy regimens? (8) Is there a survival benefit in MSI-High patients who underwent immunotherapy? These are common but undetermined questions in clinics. Research based on histopathological subtyping is likely to provide more precise answers.
Our review still has limitations, which are reflected in the following aspects: (1) Most cited papers are retrospective studies and lack RCT studies, and therefore, the accuracy of some conclusions needs to be further confirmed; (2) Some studies mixed PAC with bile duct cancer, small bowel cancer or pancreatic cancer, which may affect the conclusions [35,41,51,62,72,75]; (3) Some of the points that were put forward in the study are based on the reading and analysis of the literature, and further studies are needed for confirmation.
Conclusion
This review suggests the potential survival benefits of AT for patients with PACs, especially for patients who are treated with a corresponding chemotherapy regimen, based on histopathological types. In addition, the benefits of AT appear to be more pronounced in patients with high-risk diseases. Further studies are needed to confirm our conclusions, which are of great value in standardizing the formulation of AT regimens for patients with PACs.
CRediT authorship contribution statement
Zhiqing Duan: Writing – original draft, Writing – review & editing. Yinuo Zhang: Writing – original draft, Writing – review & editing. Yajie Tang: Writing – original draft, Writing – review & editing. Ruqing Gao: Writing – original draft, Writing – review & editing. Jing Bao: Writing – original draft, Writing – review & editing. Bo Liang: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Funding
This research was funded by a grant from the National Natural Science Foundation of China (No. 82160578), the Natural Science Foundation of Jiangxi Province, China (No. 20212BCJ23024 and 20202BAB216029), the Health Department of Jiangxi Province, China (No. 20198020), and the Education Department of Jiangxi Province, China (No. GJJ190019).
References
- 1.Hester C.A., Dogeas E., Augustine M.M., Mansour J.C., Polanco P.M., Porembka M.R., Wang S.C., Zeh H.J., Yopp A.C. Incidence and comparative outcomes of periampullary cancer: a population-based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J. Surg. Oncol. 2019;119(3):303–317. doi: 10.1002/jso.25336. [DOI] [PubMed] [Google Scholar]
- 2.Hugenschmidt H., Labori K.J., Brunborg C., Verbeke C.S., Seeberg L.T., Schirmer C.B., Renolen A., Borgen E.F., Naume B., Wiedswang G. Circulating tumor cells are an independent predictor of shorter survival in patients undergoing resection for pancreatic and periampullary adenocarcinoma. Ann. Surg. 2020;271(3):549–558. doi: 10.1097/sla.0000000000003035. [DOI] [PubMed] [Google Scholar]
- 3.Romiti A., Barucca V., Zullo A., Sarcina I., Di Rocco R., D'Antonio C., Latorre M., Marchetti P. Tumors of ampulla of vater: a case series and review of chemotherapy options. World. J. Gastrointest. Oncol. 2012;4(3):60–67. doi: 10.4251/wjgo.v4.i3.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J., Liu D. Surgical treatment of periampullary carcinoma. Chin. J. Pract. Surg. 2005;25:571–573. [Google Scholar]
- 5.Albores-Saavedra J., Schwartz A.M., Batich K., Henson D.E. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5625 cases from the SEER program. J. Surg. Oncol. 2009;100(7):598–605. doi: 10.1002/jso.21374. [DOI] [PubMed] [Google Scholar]
- 6.Henson D.E., Schwartz A.M., Nsouli H., Albores-Saavedra J. Carcinomas of the pancreas, gallbladder, extrahepatic bile ducts, and ampulla of vater share a field for carcinogenesis: a population-based study. Arch. Pathol. Lab. Med. 2009;133(1):67–71. doi: 10.5858/133.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Liu X. Clinical analysis on 26 cases of pylorus preserving pancreaticoduodenectomy in periampullary carcinoma treatment. Guide Chin. Med. 2015;13:179. [Google Scholar]
- 8.O'Connell J.B., Maggard M.A., Manunga J., Tomlinson J.S., Reber H.A., Ko C.Y., Hines O.J. Survival after resection of ampullary carcinoma: a national population-based study. Ann. Surg. Oncol. 2008;15(7):1820–1827. doi: 10.1245/s10434-008-9886-1. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasegaram M.D., Gill A.J., Samra J., Price T., Chen J., Fawcett J., Merrett N.D. Ampullary cancer of intestinal origin and duodenal cancer-a logical clinical and therapeutic subgroup in periampullary cancer. World J. Gastrointest. Oncol. 2017;9(10):407–415. doi: 10.4251/wjgo.v9.i10.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hawary M.M., Kaza R.K., Francis I.R. Optimal imaging modalities for the diagnosis and staging of periampullary masses. Surg. Oncol. Clin. North Am. 2016;25(2):239–253. doi: 10.1016/j.soc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 11.El Nakeeb A., El Sorogy M., Ezzat H., Said R., El Dosoky M., Abd El Gawad M., Elsabagh A.M., El Hanafy E. Predictors of long-term survival after pancreaticoduodenectomy for peri-ampullary adenocarcinoma: a retrospective study of 5-year survivors. Hepatobiliary Pancreat. Dis. Int. 2018;17(5):443–449. doi: 10.1016/j.hbpd.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Zheng-Pywell R., Reddy S. Ampullary cancer. Surg. Clin. North Am. 2019;99(2):357–367. doi: 10.1016/j.suc.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Huttner F.J., Fitzmaurice C., Schwarzer G., Seiler C.M., Antes G., Buchler M.W., Diener M.K. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochr. Datab. Syst. Rev. 2016;2 doi: 10.1002/14651858.CD006053.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko A.H., Nakakura E.K. Adjuvant therapy for ampullary cancer. JAMA Surg. 2019;154(8):715. doi: 10.1001/jamasurg.2019.1171. [DOI] [PubMed] [Google Scholar]
- 15.Al-Jumayli M., Batool A., Middiniti A., Saeed A., Sun W., Al-Rajabi R., Baranda J., Kumer S., Schmitt T., Chidharla A., et al. Clinical outcome of ampullary carcinoma: single cancer center experience. J. Oncol. 2019 doi: 10.1155/2019/3293509. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riall T.S., Cameron J.L., Lillemoe K.D., Winter J.M., Campbell K.A., Hruban R.H., Chang D., Yeo C.J. Resected periampullary adenocarcinoma: 5-year survivors and their 6-to 10-year follow-up. Surgery. 2006;140(5):764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Rostain F., Hamza S., Drouillard A., Faivre J., Bouvier A.M., Lepage C. Trends in incidence and management of cancer of the ampulla of Vater. World J. Gastroenterol. 2014;20(29):10144–10150. doi: 10.3748/wjg.v20.i29.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feretis M., Wang T., Iype S., Duckworth A., Brais R., Basu B., Jamieson N.V., Huguet E., Balakrishnan A., Jah A., et al. Development of a prognostic model that predicts survival after pancreaticoduodenectomy for ampullary cancer. Pancreas. 2017;46(10):1314–1321. doi: 10.1097/mpa.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berberat P.O., Kunzli B.M., Gulbinas A., Ramanauskas T., Kleeff J., Muller M.W., Wagner M., Friess H., Buchler M.W. An audit of outcomes of a series of periampullary carcinomas. Eur. J. Surg. Oncol. 2009;35(2):187–191. doi: 10.1016/j.ejso.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Schnelldorfer T., Ware A.L., Sarr M.G., Smyrk T.C., Zhang L., Qin R., Gullerud R.E., Donohue J.H., Nagorney D.M., Farnell M.B. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann. Surg. 2008;247(3):456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 21.Woo S.M., Ryu J.K., Lee S.H., Yoo J.W., Park J.K., Kim Y.T., Jang J.Y., Kim S.W., Kang G.H., Yoon Y.B. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2007;14(11):3195–3201. doi: 10.1245/s10434-007-9537-y. [DOI] [PubMed] [Google Scholar]
- 22.Balachandran P., Sikora S.S., Kapoor S., Krishnani N., Kumar A., Saxena R., Kapoor V.K. Long-term survival and recurrence patterns in ampullary cancer. Pancreas. 2006;32(4):390–395. doi: 10.1097/01.mpa.0000220864.80034.63. [DOI] [PubMed] [Google Scholar]
- 23.Key C., Meisner A.L.W., Ries L.A.G., Young J.L., Keel G.E., Eisner M.P., Lin Y.D., Horner M.J. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988-2001, Patient and Tumor Characteristics. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD, USA: 2007. Cancers of the liver and biliary tract; pp. 49–58. ISBN 978-1-249-18244-3. [Google Scholar]
- 24.Zhou Y.M., Liao S., Wei Y.Z., Wang S.J. Prognostic factors and benefits of adjuvant therapy for ampullary cancer following pancreatoduodenectomy: a systematic review and meta-analysis. Asian J. Surg. 2020;43(12):1133–1141. doi: 10.1016/j.asjsur.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.H., Jeong J.H., Ryoo B.Y., Kim K.P., Chang H.M., Oh D., Song T.J., Lee S.S., Seo D.W., Lee S.K., et al. Adjuvant chemotherapy for resected ampulla of vater carcinoma: retrospective analysis of 646 patients. Cancer Res. Treat. 2020 doi: 10.4143/crt.2020.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tella S.H., Mahipal A. The future of adjuvant therapy in ampullary cancer: should we offer it to our patients? Hepatobiliary Surg. Nutr. 2020;9(3):368–370. doi: 10.21037/hbsn.2019.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo H.K., Hwang D.W., Lee J.H., Song K.B., Shin S.H., Kwon J., Lee Y.J., Kim S.C. Role of systemic inflammation in predicting the prognosis of ampulla of vater carcinoma. Surg. Oncol. 2019;29:33–40. doi: 10.1016/j.suronc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Rizzo A., Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021;15(5):547–554. doi: 10.1080/17474124.2021.1890031. [DOI] [PubMed] [Google Scholar]
- 29.Rizzo A., Brandi G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: reflections on a standard of care. Expert Rev. Gastroenterol. Hepatol. 2021;15(5):483–485. doi: 10.1080/17474124.2021.1864325. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo A., Ricci A.D., Brandi G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021;15(5):527–536. doi: 10.1080/17474124.2021.1853527. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.M., Eads J.R. Adjuvant and neoadjuvant therapy for resectable pancreatic and periampullary cancer. Surg. Clin. North Am. 2016;96(6):1287–1300. doi: 10.1016/j.suc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Al Abbas A.I., Falvello V., Zenati M., Mani A., Hogg M.E., Zeh H.J., Singhi A., Bahary N., Zureikat A.H. Impact of adjuvant chemotherapy regimen on survival outcomes in immunohistochemical subtypes of ampullary carcinoma. J. Surg. Oncol. 2019 doi: 10.1002/jso.25808. [DOI] [PubMed] [Google Scholar]
- 33.Schiergens T.S., Reu S., Neumann J., Renz B.W., Niess H., Boeck S., Heinemann V., Bruns C.J., Jauch K.W., Kleespies A. Histomorphologic and molecular phenotypes predict gemcitabine response and overall survival in adenocarcinoma of the ampulla of Vater. Surgery. 2015;158(1):151–161. doi: 10.1016/j.surg.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Moekotte A.L., Malleo G., van Roessel S., Bonds M., Halimi A., Zarantonello L., Napoli N., Dreyer S.B., Wellner U.F., Bolm L., et al. Gemcitabine-based adjuvant chemotherapy in subtypes of ampullary adenocarcinoma: international propensity score-matched cohort study. Br. J. Surg. 2020;107(9):1171–1182. doi: 10.1002/bjs.11555. [DOI] [PubMed] [Google Scholar]
- 35.Neoptolemos J.P., Moore M.J., Cox T.F., Valle J.W., Palmer D.H., McDonald A.C., Carter R., Tebbutt N.C., Dervenis C., Smith D., et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308(2):147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 36.Ramaswamy A., Bhandare M., Bal M., Shrirangwar S., Kataria P., Majumdar S., Swami R., Rohila J., Chaudhari V., Mandavkar S., et al. Clinico-pathological correlates and survival outcomes in 214 resected ampullary adenocarcinomas-are outcomes different in intestinal and pancreatobiliary subtypes with adjuvant gemcitabine? HPB. 2020;22(3):376–382. doi: 10.1016/j.hpb.2019.07.006. (Oxford) [DOI] [PubMed] [Google Scholar]
- 37.Nassour I., Hynan L.S., Christie A., Minter R.M., Yopp A.C., Choti M.A., Mansour J.C., Porembka M.R., Wang S.C. Association of adjuvant therapy with improved survival in ampullary cancer: a national cohort study. J. Gastrointest. Surg. 2018;22(4):695–702. doi: 10.1007/s11605-017-3624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan L., Cong M., Sun X. Chemotherapy for ampullary carcinoma. Chin. Gen. Pract. 2014;17(17):1994–1997. 2007. [Google Scholar]
- 39.Ecker B.L., Vollmer C.M., Behrman S.W., Allegrini V., Aversa J., Ball C.G., Barrows C.E., Berger A.C., Cagigas M.N., Christein J.D., et al. Role of adjuvant multimodality therapy after curative-intent resection of ampullary carcinoma. JAMA Surg. 2019;154(8):706–714. doi: 10.1001/jamasurg.2019.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H.S., Shin S.J., Kim J.H., Kim H., Choi H.J. Better outcome of XELOX chemotherapy in patients with advanced intestinal-type adenocarcinoma of the ampulla of vater. Tohoku J. Exp. Med. 2013;231(1):21–28. doi: 10.1620/tjem.231.21. [DOI] [PubMed] [Google Scholar]
- 41.Overman M.J., Varadhachary G.R., Kopetz S., Adinin R., Lin E., Morris J.S., Eng C., Abbruzzese J.L., Wolff R.A. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of vater. J. Clin. Oncol. 2009;27(16):2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 42.Kim S.T., Lee J., Lee K.T., Lee J.K., Lee K.H., Choi S.H., Heo J.S., Choi D.W., Park S.H., Park J.O., et al. The efficacy of frontline platinum-based combination chemotherapy in advanced adenocarcinoma of the ampulla of vater. Med. Oncol. 2010;27(4):1149–1154. doi: 10.1007/s12032-009-9351-4. [DOI] [PubMed] [Google Scholar]
- 43.Bolm L., Ohrner K., Nappo G., Rückert F., Zimmermann C., Rau B.M., Petrova E., Honselmann K.C., Lapshyn H., Bausch D., et al. Adjuvant therapy is associated with improved overall survival in patients with pancreatobiliary or mixed subtype ampullary cancer after pancreatoduodenectomy – a multicenter cohort study. Pancreatology. 2020;20(3):433–441. doi: 10.1016/j.pan.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Bronsert P., Kohler I., Werner M., Makowiec F., Kuesters S., Hoeppner J., Hopt U.T., Keck T., Bausch D., Wellner U.F. Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer. 2013;13:428. doi: 10.1186/1471-2407-13-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang D.K., Jamieson N.B., Johns A.L., Scarlett C.J., Pajic M., Chou A., Pinese M., Humphris J.L., Jones M.D., Toon C., et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J. Clin. Oncol. 2013;31(10):1348–1356. doi: 10.1200/jco.2012.46.8868. [DOI] [PubMed] [Google Scholar]
- 46.Heby M., Lundgren S., Nodin B., Elebro J., Eberhard J., Jirström K. Relationship between mismatch repair immunophenotype and long-term survival in patients with resected periampullary adenocarcinoma. J. Transl. Med. 2018;16(1):66. doi: 10.1186/s12967-018-1444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ang D.C., Shia J., Tang L.H., Katabi N., Klimstra D.S. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am. J. Surg. Pathol. 2014;38(10):1371–1379. doi: 10.1097/pas.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W., Wang B., Zhao A., Tian Q., Zhang L., Wang L., Zhao X., Yang J., Dong D. The role of radiotherapy in patients with resected ampullary carcinoma: findings based on the SEER database. HPB. 2019;21(11):1535–1540. doi: 10.1016/j.hpb.2019.03.369. (Oxford) [DOI] [PubMed] [Google Scholar]
- 49.Miura J.T., Jayakrishnan T.T., Amini A., Johnston F.M., Tsai S., Erickson B., Quebbeman E.J., Christians K.K., Evans D.B., Gamblin T.C., et al. Defining the role of adjuvant external beam radiotherapy on resected adenocarcinoma of the ampulla of vater. J. Gastrointest. Surg. 2014;18(11):2003–2008. doi: 10.1007/s11605-014-2629-7. [DOI] [PubMed] [Google Scholar]
- 50.Zaki Azzam A., Alqarni A., Mahmoud Amin T. The role of intraoperative radiotherapy (IORT) in the management of patients with pancreatic and periampullary cancer: a single center experience. J. Egypt. Natl. Cancer Inst. 2018;30(2):77–79. doi: 10.1016/j.jnci.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Morak M.J., van der Gaast A., Incrocci L., van Dekken H., Hermans J.J., Jeekel J., Hop W.C., Kazemier G., van Eijck C.H. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann. Surg. 2008;248(6):1031–1041. doi: 10.1097/SLA.0b013e318190c53e. [DOI] [PubMed] [Google Scholar]
- 52.Manne A., Hatic H., Li P., Jacob R., Williams G., Paluri R. The clinical benefit of adjuvant therapy in long-term survival of early-stage ampullary carcinoma: a single institutional experience. J. Clin. Med. Res. 2020;12(9):560–567. doi: 10.14740/jocmr4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parikh P., Waters J.A., Pitt H.A., Aguilar-Saavedra J.R., Cardenes H., Chiorean E.G., Helft P.R., Schmidt C.M., Nakeeb A., Lillemoe K.D. Ampullary carcinoma: adjuvant chemoradiation improves survival in node-positive patients. Gastroenterology. 2010;138(5) S875-S875. [Google Scholar]
- 54.Zhou J., Hsu C.C., Winter J.M., Pawlik T.M., Laheru D., Hughes M.A., Donehower R., Wolfgang C., Akbar U., Schulick R., et al. Adjuvant chemoradiation versus surgery alone for adenocarcinoma of the ampulla of vater. Radiother. Oncol. 2009;92(2):244–248. doi: 10.1016/j.radonc.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smeenk H.G., van Eijck C.H., Hop W.C., Erdmann J., Tran K.C., Debois M., van Cutsem E., van Dekken H., Klinkenbijl J.H., Jeekel J. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann. Surg. 2007;246(5):734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 56.Turan N., Benekli M., Unal O.U., Unek İ.T., Tastekin D., Dane F., Algın E., Ulger S., Eren T., Topcu T.O., et al. Impact of adjuvant treatment modalities on survival outcomes in curatively resected pancreatic and periampullary adenocarcinoma. Chin. J. Cancer Res. 2015;27(4):408–416. doi: 10.3978/j.issn.1000-9604.2015.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim K., Chie E.K., Jang J.Y., Kim S.W., Oh D.Y., Im S.A., Kim T.Y., Bang Y.J., Ha S.W. Role of adjuvant chemoradiotherapy for ampulla of vater cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(2):436–441. doi: 10.1016/j.ijrobp.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 58.Kim H.S., Jang J.Y., Yoon Y.S., Park S.J., Kwon W., Kim S.W., Han H.S., Han S.S., Park J.S., Yoon D.S. Does adjuvant treatment improve prognosis after curative resection of ampulla of vater carcinoma? A multicenter retrospective study. J. Hepatobiliary Pancreat. Sci. 2020;27(10):721–730. doi: 10.1002/jhbp.801. [DOI] [PubMed] [Google Scholar]
- 59.Krishnan S., Rana V., Evans D.B., Varadhachary G., Das P., Bhatia S., Delclos M.E., Janjan N.A., Wolff R.A., Crane C.H., et al. Role of adjuvant chemoradiation therapy in adenocarcinomas of the ampulla of vater. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(3):735–743. doi: 10.1016/j.ijrobp.2007.07.2327. [DOI] [PubMed] [Google Scholar]
- 60.Palta M., Patel P., Broadwater G., Willett C., Pepek J., Tyler D., Zafar S.Y., Uronis H., Hurwitz H., White R., et al. Carcinoma of the ampulla of vater: patterns of failure following resection and benefit of chemoradiotherapy. Ann. Surg. Oncol. 2012;19(5):1535–1540. doi: 10.1245/s10434-011-2117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cloyd J.M., Wang H., Overman M., Zhao J., Denbo J., Prakash L., Kim M.P., Shroff R., Javle M., Varadhachary G.R., et al. Influence of preoperative therapy on short- and long-term outcomes of patients with adenocarcinoma of the ampulla of vater. Ann. Surg. Oncol. 2017;24(7):2031–2039. doi: 10.1245/s10434-017-5777-7. [DOI] [PubMed] [Google Scholar]
- 62.Jayaramayya K., Balachandar V., Santhy K.S. Ampullary carcinoma-A genetic perspective. Mutat. Res. 2018;776:10–22. doi: 10.1016/j.mrrev.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Wang C.Y., Chao Y.J., Chen Y.L., Wang T.W., Nam Nhut P., Hsu H.P., Shan Y.S., Lai M.D. Upregulation of peroxisome proliferator-activated receptor-alpha and the lipid metabolism pathway promotes carcinogenesis of ampullary cancer. Int. J. Med. Sci. 2021;18(1):256–269. doi: 10.7150/ijms.48123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagarkar R., Patil D., Limaye S., Devhare P., Ghaisas A., Srivastava N., Apurwa S., Patil S., John J., Raazi Z., et al. Liquid biopsy and multi-analyte testing guided treatment of HER2 positive periampullary adenocarcinoma with durable complete response after trastuzumab based therapy. Oncotarget. 2020;11(45):4195–4200. doi: 10.18632/oncotarget.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gulhati P., Raghav K., Shroff R.T., Varadhachary G.R., Kopetz S., Javle M., Qiao W., Wang H., Morris J., Wolff R.A., et al. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: a single-center, open-label, phase 2 study. Cancer. 2017;123(6):1011–1017. doi: 10.1002/cncr.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M.H., Jang M., Kim H., Lee W.J., Kang C.M., Choi H.J. Distinct immunological properties of the two histological subtypes of adenocarcinoma of the ampulla of vater. Cancer Immunol. Immunother. 2019;68(3):443–454. doi: 10.1007/s00262-018-02293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saraggi D., Galuppini F., Remo A., Urso E.D.L., Bacchin D., Salmaso R., Lanza C., Bao R.Q., Fanelli G.N., Guzzardo V., et al. PD-L1 overexpression in ampulla of vater carcinoma and its pre-invasive lesions. Histopathology. 2017;71(3):470–474. doi: 10.1111/his.13254. [DOI] [PubMed] [Google Scholar]
- 68.Xue Y., Balci S., Aydin Mericoz C., Taskin O.C., Jiang H., Pehlivanoglu B., Muraki T., Memis B., Saka B., Kim G.E., et al. Frequency and clinicopathologic associations of DNA mismatch repair protein deficiency in ampullary carcinoma: routine testing is indicated. Cancer. 2020;126(21):4788–4799. doi: 10.1002/cncr.33135. [DOI] [PubMed] [Google Scholar]
- 69.Grothey A. Pembrolizumab in MSI-H-dMMR advanced colorectal cancer – a new standard of care. N. Engl. J. Med. 2020;383(23):2283–2285. doi: 10.1056/NEJMe2031294. [DOI] [PubMed] [Google Scholar]
- 70.Pothuri V., Herndon J., Ballentine S.J., Lim K.H., Fields R.C. A case of a pathological complete response to neoadjuvant nivolumab plus ipilimumab in periampullary adenocarcinoma. Oncologist. 2021;26(9):722–726. doi: 10.1002/onco.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gingras M.C., Covington K.R., Chang D.K., Donehower L.A., Gill A.J., Ittmann M.M., Creighton C.J., Johns A.L., Shinbrot E., Dewal N., et al. Ampullary cancers harbor ELF3 tumor suppressor gene mutations and exhibit frequent WNT dysregulation. Cell Rep. 2016;14(4):907–919. doi: 10.1016/j.celrep.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sideras K., Biermann K., Yap K., Mancham S., Boor P.P.C., Hansen B.E., Stoop H.J.A., Peppelenbosch M.P., van Eijck C.H., Sleijfer S., et al. Tumor cell expression of immune inhibitory molecules and tumor-infiltrating lymphocyte count predict cancer-specific survival in pancreatic and ampullary cancer. Int. J. Cancer. 2017;141(3):572–582. doi: 10.1002/ijc.30760. [DOI] [PubMed] [Google Scholar]
- 73.Lundgren S., Micke P., Elebro J., Heby M., Hrynchyk I., Nodin B., Leandersson K., Mezheyeuski A., Jirström K. Topographical distribution and spatial interactions of innate and semi-innate immune cells in pancreatic and other periampullary adenocarcinoma. Front. Immunol. 2020;(11) doi: 10.3389/fimmu.2020.558169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lundgren S., Karnevi E., Elebro J., Nodin B., Karlsson M.C.I., Eberhard J., Leandersson K., Jirström K. The clinical importance of tumour-infiltrating macrophages and dendritic cells in periampullary adenocarcinoma differs by morphological subtype. J. Transl. Med. 2017;15(1):152. doi: 10.1186/s12967-017-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nitsche M., Horstmann O., Christiansen H., Hermann R.M., Hess C.F., Becker H., Pradier O., Schmidberger H. Chemoradioimmunotherapy with 5-fluorouracil, cisplatin and interferon-alpha in pancreatic and periampullary cancer: results of a feasibility study. Cancer Radiother. 2008;12(8):817–821. doi: 10.1016/j.canrad.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 77.Li Q.J., He M.K., Chen H.W., Fang W.Q., Zhou Y.M., Xu L., Wei W., Zhang Y.J., Guo Y., Guo R.P., et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J. Clin. Oncol. 2022;40(2):150–160. doi: 10.1200/jco.21.00608. [DOI] [PubMed] [Google Scholar]
- 78.He M., Li Q., Zou R., Shen J., Fang W., Tan G., Zhou Y., Wu X., Xu L., Wei W., et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]