To the Editor: Serum samples obtained from unvaccinated persons after infection with the B.1.1.7 (alpha), B.1.351 (beta), or B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been shown to neutralize the B.1.1.529 (omicron) variant only occasionally.1 Similarly, levels of neutralizing antibodies against the omicron variant are low and only short-lived after one or two doses of a coronavirus disease 2019 (Covid-19) vaccine but are enhanced in persons who have been vaccinated and have also been infected (i.e., those with hybrid immunity) or in vaccinated persons who have received a booster dose.2,3
Little is known about neutralization profiles in persons who have recovered from infection with the omicron variant.4,5 Studies have focused primarily on either vaccinated persons who have had breakthrough infections with the omicron variant or unvaccinated persons whose history of previous infection is unknown. Here, we report the results of an analysis of neutralization profiles against six SARS-CoV-2 variants in serum samples obtained from persons who had recovered from infection with the omicron BA.1 variant, with or without preexisting SARS-CoV-2 immunity.
In this retrospective study, we obtained serum samples 5 to 42 days after each person’s first positive polymerase-chain-reaction (PCR) assay during infection with the omicron BA.1 variant. We included persons who had one of four constellations of SARS-CoV-2 immunity before infection with the omicron BA.1 variant: vaccinated without previous SARS-CoV-2 infection (15 persons); unvaccinated without previous SARS-CoV-2 infection (18 persons); vaccinated, with previous infection with the D614G (wild-type), alpha, or delta variant (11 persons); or unvaccinated, with previous infection with the wild-type, alpha, or delta variant (15 persons). Details regarding demographic and clinical characteristics of the study population are provided in Tables S1 through S5 in the Supplementary Appendix, available with the full text of this letter at NEJM.org. We analyzed neutralizing antibodies against the following replication-competent SARS-CoV-2 variants: wild-type, alpha, beta, P.1 (gamma), delta, and omicron BA.1. We included the time point with the highest titers against BA.1 in the main analysis (Fig. S1).
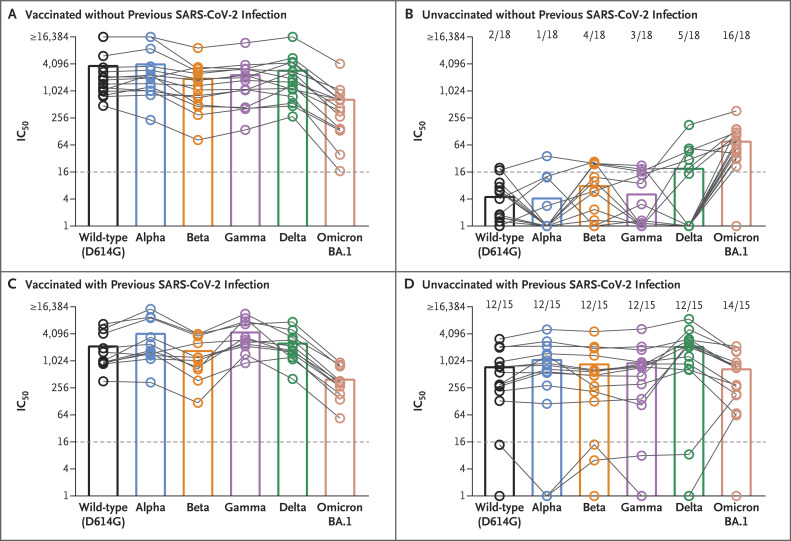
We found that neutralizing antibody titers against all the variants were high among vaccinated persons after omicron BA.1 breakthrough infection and among vaccinated or unvaccinated persons who had had previous infection with the wild-type, alpha, or delta variant before infection with the omicron BA.1 variant (Figure 1, Fig. S2, and Table S6). Mean neutralizing antibody titers against the omicron BA.1 variant were lower than those against the other variants among previously vaccinated persons but were similar to those against the other variants among unvaccinated persons who had had infection with the wild-type, alpha, or delta variant before infection with the omicron BA.1 variant. In contrast, samples obtained from unvaccinated persons who had not had previous SARS-CoV-2 infection before infection with the omicron BA.1 variant contained mainly neutralizing antibodies against omicron BA.1 but only occasionally contained neutralizing antibodies against the other variants. It is surprising that 2 unvaccinated persons who were thought to have been reinfected had neutralizing antibodies against only the omicron BA.1 variant and 1 unvaccinated person had no neutralizing antibodies against any variant, despite the fact that each of the 3 persons had a reported single previous positive PCR assay for the delta variant 2 months before reinfection (Figure 1D). Because serum samples obtained from these persons did not neutralize the delta variant nor any of the other variants except for omicron BA.1, it is possible that the previous PCR assay had yielded a false positive result.
Figure 1. Neutralization Capacity of Serum Samples Obtained from Patients Who Recovered from Infection with the Omicron BA.1 Variant.
Serum samples were obtained from 59 persons who had recovered from infection with the B.1.1.529 (omicron) BA.1 variant: 15 vaccinated persons without previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Panel A); 18 unvaccinated persons without previous SARS-CoV-2 infection (Panel B); 11 vaccinated persons with previous infection with the D614G (wild-type), B.1.1.7 (alpha), or B.1.617.2 (delta) variant (Panel C); and 15 unvaccinated persons with previous infection with the wild-type, alpha, or delta variant (Panel D). Serum samples were obtained from each person 5 to 42 days after the first positive polymerase-chain-reaction assay during infection with the omicron BA.1 variant. Samples were analyzed for 50% neutralization titers (IC50) with the use of replication-competent wild-type, alpha, beta, P.1 (gamma), delta, or omicron BA.1 SARS-CoV-2 isolates. Individual values (circles) and mean titers (bars) are shown. Samples from the same person are connected by lines. Titers below 1:16 (dotted lines) are regarded as negative. In Panels B and D, the numbers above the bars indicate the proportions of samples that were positive for neutralizing antibodies against the respective variant. In Panels A and C, all the samples were positive for neutralizing antibodies against all the variants.
Despite certain limitations of this study, including the small sample size and retrospective study design (Table S7), our data support the hypothesis that the omicron BA.1 variant is an extremely potent immune-escape variant that shows little cross-reactivity with the earlier variants. Therefore, unvaccinated persons who are infected with the omicron BA.1 variant only (without previous SARS-CoV-2 infection) might not be sufficiently protected against infection with a SARS-CoV-2 variant other than omicron BA.1; for full protection, vaccination is warranted.
Supplementary Appendix
Disclosure Forms
This letter was published on March 23, 2022, at NEJM.org.
Footnotes
Supported by the Institute of Virology of the Medical University of Innsbruck.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med 2022;386:698-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 omicron. Nature 2022;602:682-688. [DOI] [PubMed] [Google Scholar]
- 3.Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022;602:654-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan K, Karim F, Cele S, et al. Omicron infection of vaccinated individuals enhances neutralizing immunity against the delta variant. January 28, 2022. (https://www.medrxiv.org/content/10.1101/2021.12.27.21268439v2). preprint.
- 5.Suryawanshi RK, Chen IP, Ma T, et al. Limited cross-variant immunity after infection with the SARS-CoV-2 omicron variant without vaccination. February 9, 2022. (https://www.medrxiv.org/content/10.1101/2022.01.13.22269243v3). preprint. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.