To the Editor: In this open-label, nonrandomized clinical study, we assessed the immunogenicity and safety of a fourth dose of either BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna) administered 4 months after the third dose in a series of three BNT162b2 doses (ClinicalTrials.gov numbers, NCT05231005 and NCT05230953; the protocol is available with the full text of this letter at NEJM.org). Of the 1050 eligible health care workers enrolled in the Sheba HCW COVID-19 Cohort,1,2 154 received the fourth dose of BNT162b2 and, 1 week later, 120 received mRNA-1273. For each participant, two age-matched controls were selected from the remaining eligible participants (Fig. S1 in the Supplementary Appendix, available at NEJM.org).
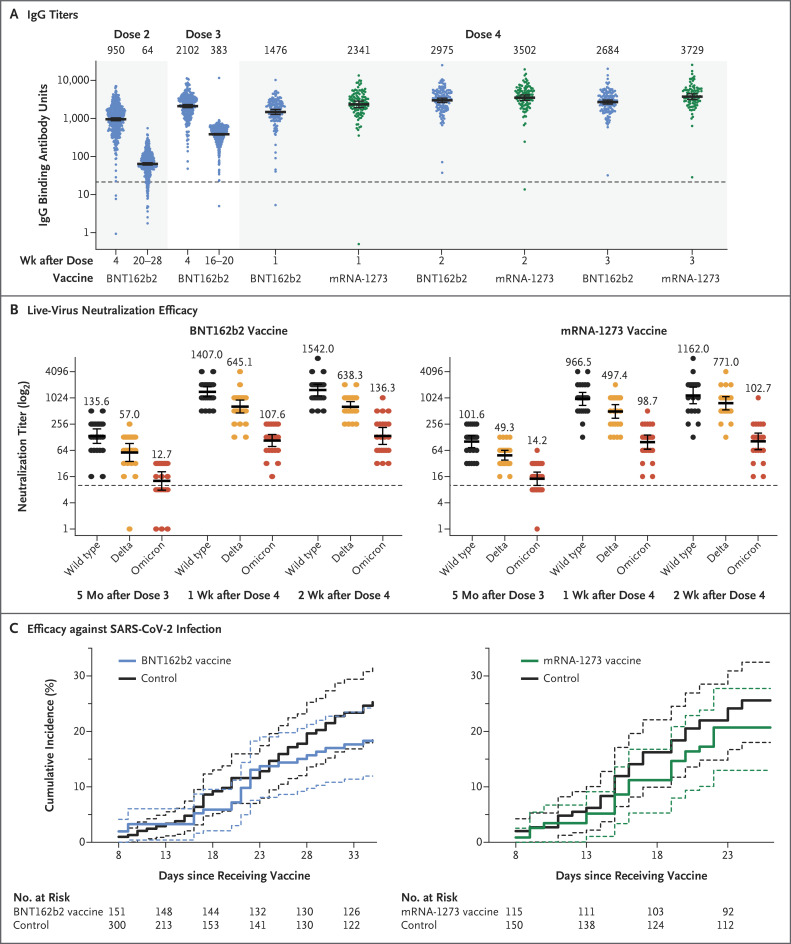
After the fourth dose, both messenger RNA (mRNA) vaccines induced IgG antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (Figure 1A) and increased neutralizing antibody titers (Fig. S3); each measure was increased by a factor of 9 to 10, to titers that were slightly higher than those achieved after the third dose, with no significant difference between the two vaccines. Concurrently, antibody levels in the control group continued to wane (Table S5). Both vaccines induced an increase in live neutralization of the B.1.1.529 (omicron) variant and other viral strains by a factor of approximately 10 (Figure 1B), similar to the response after the third dose.3 We found that the fourth dose did not lead to substantial adverse events despite triggering mild systemic and local symptoms in the majority of recipients (Fig. S2 and Table S4A and S4B).
Figure 1. Immunogenicity and Efficacy of a Fourth Dose of mRNA Vaccine.
Panel A shows IgG titers after three doses of BNT162b2 plus a fourth dose of a messenger RNA (mRNA) vaccine (either BNT162b2 or mRNA-1273). Panel B shows live-virus neutralization efficacy against different strains (Hu-1 [wild type], B.1.617.2 [delta], and B.1.1.529 [omicron]) at different time points. In Panels A and B, geometric mean titers are shown, and 𝙸 bars indicate the 95% confidence intervals; the dashed horizontal line indicates the cutoff for diagnostic positivity. Panel C shows the cumulative incidence of any severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among BNT162b2 and mRNA-1273 recipients and their matched controls. The dashed lines indicate 95% confidence intervals.
Because of the extremely high infection incidence and meticulous active surveillance with weekly SARS-CoV-2 polymerase-chain-reaction testing, we were also able to assess vaccine efficacy with a Poisson regression model (see the Supplementary Appendix). Overall, 25.0% of the participants in the control group were infected with the omicron variant, as compared with 18.3% of the participants in the BNT162b2 group and 20.7% of those in the mRNA-1273 group. Vaccine efficacy against any SARS-CoV-2 infection was 30% (95% confidence interval [CI], −9 to 55) for BNT162b2 and 11% (95% CI, −43 to 44) for mRNA-1273 (Figure 1C). Most infected health care workers reported negligible symptoms, both in the control group and the intervention groups. However, most of the infected participants were potentially infectious, with relatively high viral loads (nucleocapsid gene cycle threshold, ≤25) (Table S6). Vaccine efficacy was estimated to be higher for the prevention of symptomatic disease (43% for BNT162b2 and 31% for mRNA-1273) (Fig. S4).
Limitations of the study include its nonrandomized design and the 1-week difference between enrollment in the two intervention groups, generating potential biases. To overcome this, we assessed each intervention group separately and used a Poisson model accounting for calendar time. In addition, despite similar requests for weekly SARS-CoV-2 testing, adherence was slightly lower in the control group. We did not sequence the infecting virus and cannot be absolutely certain that all cases were caused by the omicron variant; however, during the study period, omicron accounted for 100% of the isolates that were typed. Finally, our cohort was too small to allow for accurate determination of vaccine efficacy. However, within the wide confidence intervals of our estimates, vaccine efficacy against symptomatic disease was 65% at most.
Our data provide evidence that a fourth dose of mRNA vaccine is immunogenic, safe, and somewhat efficacious (primarily against symptomatic disease). A comparison of the initial response to the fourth dose with the peak response to a third dose did not show substantial differences in humoral response or in levels of omicron-specific neutralizing antibodies. Along with previous data showing the superiority of a third dose to a second dose,4 our results suggest that maximal immunogenicity of mRNA vaccines is achieved after three doses and that antibody levels can be restored by a fourth dose. Furthermore, we observed low vaccine efficacy against infections in health care workers, as well as relatively high viral loads suggesting that those who were infected were infectious. Thus, a fourth vaccination of healthy young health care workers may have only marginal benefits. Older and vulnerable populations were not assessed.
Protocol
Supplementary Appendix
Disclosure Forms
This letter was published on March 16, 2022, at NEJM.org.
Deidentified data will be made available on request.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385(24):e84-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021;385:1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N Engl J Med 2022;386:492-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustig Y, Gonen T, Melzer L, et al. Superior immunogenicity and effectiveness of the 3rd BNT162b2 vaccine dose. December 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.19.21268037v1). preprint. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.