Abstract
Objective
Lupus nephritis (LN) is a major complication and cause of death among patients with SLE. This research used in vivo and in vitro experiments to explore the therapeutic potential of metformin in kidney injury from LN-induced inflammation.
Methods
In vivo study, 8-week-old MRL/MpJ-Faslpr/J (MRL/lpr) mice were randomly divided into two groups (n=12 each): daily administration of 0.3 mg/mL metformin in drinking water and control (water only). Body weight and urinary samples were measured biweekly. Mice were sacrificed after 8-week treatment to harvest serum, lymph nodes, spleen and kidneys. In vitro study, human kidney-2 (HK-2) cells were pretreated with 1 mM metformin for 1 hour and then stimulated with 20 µg/mL lipopolysaccharides (LPS) or 10 ng/mL tumour necrosis factor-α (TNF-α) for another 48 hours. Protein was collected for subsequent analysis.
Results
We found that metformin administration improved renal function in MRL/lpr lupus-prone mice, measured by decreased urea nitrogen and urinary proteins. Metformin reduced immunoglobulin G and complement C3 deposition in glomeruli. The treatment also downregulated systemic and renal inflammation, as seen in decreased renal infiltration of F4/80-positive macrophages and reduced splenic and renal MCP-1 (monocyte chemoattractant protein-1) and TNF-α, and renal IL-1β (interleukin 1β) expression. Metformin administration decreased renal expression of necroptosis markers p-RIPK1 (phosphorylated receptor-interacting protein kinase 1) and p-MLKL, along with tubular injury marker KIM-1 (kidney injury molecule-1) in lupus mice. In addition, metformin alleviated the necroptosis of HK-2 cells stimulated by LPS and TNF-α, evidencing by a decrease in the expression of necroptosis markers p-RIPK1, p-RIPK3 and p-MLKL, and the inflammasome-related markers NLRP3 (NLR family pyrin domain containing 3), ASC (apoptosis-associated speck-like protein containing a CARD), caspase-1. Mechanistically, metformin treatment upregulated p-AMPK (phosphorylated AMP-activated protein kinase) and downregulated p-STAT3 (phosphorylated signal transducer and activator of transcription 3) expression in the kidneys. Moreover, AMPKα2 knockdown abolished the protective effects of metformin in vitro.
Conclusions
Metformin alleviated kidney injury in LN though suppressing renal necroptosis and inflammation via the AMPK/STAT3 pathway.
Keywords: Lupus Nephritis; Lupus Erythematosus, Systemic; Therapeutics
Key messages.
What is already known about this subject?
Effective therapeutic options for lupus nephritis (LN) are minimal. Metformin protects against renal inflammation and fibrosis in experimental models. However, the therapeutic potential of metformin in LN is still not explored.
What does this study add?
Our study demonstrated that metformin administration attenuated kidney injury, improved renal function and increased mouse survival ratio in experimental LN. The mechanism of action was mainly suppressing systemic and intrarenal inflammatory response in the kidneys, specifically necroptosis and inflammasome activation via AMPK (AMP-activated protein kinase)-mediated STAT3 (signal transducer and activator of transcription 3) inhibition.
How might this impact on clinical practice or future developments?
Metformin acts as a candidate drug for treating clinical LN.
Introduction
Lupus nephritis (LN) is a major complication of SLE, affecting about ~40% of adults and over 80% of children with SLE.1 2 More than 10% patients with LN develop into end-stage renal disease (ESRD) within 10 years of diagnosis, even with immunosuppressant and glucocorticoid treatment.3 The risk of progression from LN to ESRD has not decreased for nearly two decades, and LN remains a major cause of death of patients with SLE.4 Therefore, improved mechanistic understanding and novel therapies are urgently needed.
Patients with LN display elevated circulating autoreactive antibodies which then form circulating or in situ immune complexes with autoantigens.5 Build-up of immune complexes and complement components in glomeruli and tubulointerstitium causes intrinsic renal cell death, accompanied by reduced clearance of dying cells.6 7 These conditions then contribute to renal inflammation. Programmed cell death releases numerous damage-associated molecular patterns, such as ATP and high mobility group protein 1, which recruit and activate proinflammatory cells mainly via Toll-like receptors. In a positive feedback loop, proinflammatory cells then produce cytokines (eg, tumour necrosis factor-α, TNF-α) to induce more cell death.8 All of these processes combine to promote LN progression.
Metformin is a well-known hypoglycaemic drug that is widely used to treat diabetes. Growing evidence has revealed that metformin has antitumour, antiageing, cardiovascular protective, anti-inflammatory and immune regulatory effects though AMP-activated protein kinase (AMPK)-dependent and independent pathways.9 Metformin also protects against renal inflammation and fibrosis in experimental models.10–12 A registered clinical trial recently analysed metformin as an SLE treatment.13 However, no studies have examined the therapeutic potential of metformin for kidney injury due to autoimmune-driven inflammatory response in LN. Thus, this study aimed to test the protective effect of metformin in a mouse model of spontaneous LN.
Materials and methods
Agents and antibodies
Metformin (S5958) was obtained from Selleck Chemicals (Shanghai, China). rHuTNF-α (HY-P7058) and lipopolysaccharides (HY-D1056) were purchased from MedChemExpress (Shanghai, China). The antibody used in this study were as follows: mouse nephrin antibody (AF3159; R&D Systems, USA), monocyte chemoattractant protein-1 (MCP-1) antibody (sc-32771; Santa Cruz, USA), interleukin (IL)-1β antibody (sc12742; Santa Cruz, USA), TNF-α antibody (sc12744; Santa Cruz, USA), F4/80 antibody (sc-377009; Santa Cruz, USA), anti-mixed lineage kinase domain-like protein (MLKL) (phosphor S345) (ab196436; Abcam, USA), anti-receptor-interacting protein kinase 3 (RIP3) (phospho T231+S232) (ab205421; Abcam, USA), phospho-RIP (Ser166) (44 590S; Cell Signaling Technology, USA), ASC/TMS1 Antibody (NBP1-78977; Novus, USA), anti-caspase-1 antibody (ab1872; Abcam, USA), phospho-AMPKα (Thr172) (2535; Cell Signaling Technology, USA), AMPK α (D5A2) rabbit monoclonal antibody (5831S; Cell Signaling Technology, USA), NLRP3/NALP3 Antibody (NBP177080; Novus, USA), signal transducer and activator of transcription 3 (STAT3) (p Tyr705) antibody (NBP2-61588; Novus, USA), the fluorescein isothiocyanate (FITC)-conjugated complement component C3 (NB200-540 AF488; Novus, USA), the FITC-conjugated donkey anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor (A21202; Invitrogen, USA), kidney injury molecule-1 (KIM-1) antibody (AF1817; R&D Systems, USA). 3,30-Diaminobenzidine (DAB) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (abs830030) were purchased from Absin Technology (Shanghai, China), mouse ANAs (ANA/ENA) immunoglobulins (Ig) (total A+G+M) ELISA kit (5210; Alpha Diagnostic International, USA), mouse anti-double-stranded DNA (anti-dsDNA) Igs (total A+G+M) ELISA kit (5110; Alpha Diagnostic International).
Mice
The MRL/MpJ-Faslpr/J (MRL/lpr) mouse is a classical model of spontaneous SLE and LN.14 Mice were obtained from the Shanghai SLAC Laboratory Animal (000485; Shanghai, China,) and housed at the Animal Center of Guangdong Medical University. Eight-week-old female mice were randomly assigned to be treated with 0.3 mg/mL metformin in drinking water or control (regular water); both groups (n=12 each) were fed ad libitum. Mice were euthanised with a pentobarbital sodium injection after 8 weeks of treatment to harvest samples for analysis. All procedures were approved by the Animal Experimentation Ethics Committee of Guangdong Medical University (No. GDY1602005) and performed according to the guidelines of Animal Welfare and Ethics of the Institutional Animal Care and Use Committee.
Renal function
Serum creatinine and urea nitrogen were detected using creatinine assay kits (No: C011-2-1 and No: C013-2-1) purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Urinary protein levels were assayed with the Quick Start Bradford Protein Assay kit (1-800-424-6723) from Bio-Rad (Hercules, California, USA).
ELISA for ANAs and anti-double-stranded DNA (dsDNA) antibodies
The quantity of ANA and dsDNA antibodies in mouse serum was measured by mouse ANAs (ANA/ENA) Ig’s ELISA kit and mouse anti-dsDNA Ig’s (total A+G+M) ELISA kit, respectively, according to the manufacturer’s instructions.
Histology
Kidneys were fixed in 4% paraformaldehyde added to phosphate-buffered saline (pH 7.4), then dehydrated and embedded in paraffin. After dewaxing, 3 µm sections were stained with periodic acid-Schiff (PAS). Two pathologists, blinded to experimental conditions, used PAS staining results to evaluate kidney injury, as described previously.14
Immunofluorescence
To detect glomerular deposition of immune complexes, frozen kidney sections were blocked with 5% bovine serum albumin (BSA), and then incubated with FITC-conjugated donkey anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor or complement C3 antibody (11H9). Semiquantitative analysis of glomerular complement C3 deposition was performed using the following scale (0–3): 0=negative staining, 1=barely visible at high magnification, 2=moderately visible and 3=clearly visible, referring to a previous study.15
For the detection of other target proteins, frozen sections after blocking were also incubated with primary antibodies for analysis of target protein localisation and expression. After washing, sections were incubated with Alexa-594 or Alexa-488-conjugated secondary antibodies. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). Fluorescence signals were visualised under a confocal microscope (Leica, Germany). The mean fluorescence intensity was used to analyse target protein expression across 10 randomly selected fields as previous study.16
Immunohistochemistry (IHC)
Renal KIM-1, MCP-1 and macrophage marker F4/80 expression were determined using IHC. Paraffin-embedded kidney sections were deparaffinised, then treated with hydrogen peroxide for epitope retrieval and blockade of endogenous peroxidase. Next, sections were incubated with primary antibodies overnight before being washed. Sections were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody, followed by DAB immunostaining and haematoxylin counterstaining. For KIM-1 and MCP-1 analysis, the per cent area of positive staining for KIM-1 and MCP-1 was measured in Image J (NIH, USA). The number of infiltrated macrophages was counted in 15 random fields.
Cell culture and treatments
Human proximal tubular human kidney-2 (HK-2) cells (CRL-2190TM; ATCC) were cultured in DMEM (C11995500BT; Gibco) supplemented with 10% fetal bovine serum (10 270 106; Gibco) and 1% penicillin streptomycin at 37°C in an incubator with 5% CO2. HK-2 cells were pretreated with metformin (1 mM) for 1 hour and then exposed to TNF-α (10 ng/mL) or lipopolysaccharides (LPS, 40 µg/mL) to mimic inflammatory condition in lupus for another 48 hours. After the treatment, protein samples of cell lysis were collected for subsequent analyses, as described below.
To determine the regulatory role via the AMPK/STAT3 pathway in LN, HK-2 cells were treated with a specific AMPKα2 small interfering RNA (siRNA) (sense: GGCUUACACAGACCAAGAUTT, antisense: AUCUUGGUCUGUGUAAGCCTT). For the siRNA-mediated knockdown of AMPK, HK-2 cells were transfected with 60 nM AMPK siRNA for 24 hours or a negative control siRNA using a Lipofectamine 3000 Kit (L3000015; Invitrogen, USA) according to the manufacturer’s instructions. And then cells were pretreated with the metformin for 1 hour and then exposed to TNF-α (10 ng/mL) for another 48 hours. After the treatment of the cell, proteins were collected for subsequent analyses.
Western blotting
Western blots were used to detect renal expression of target proteins. Protein samples were subjected to 12% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), then transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% BSA in TBST solution, incubated with the primary antibody, washed and then incubated with HRP-conjugated secondary antibody. Chemiluminescent reactions with luminol were visualised in the Azure C500 Western Blot Imaging System and then analysed in Image J.
Quantitative real-time PCR (qRT-PCR)
Total RNA from cultured cells or kidney tissues was extracted using RNAiso Plus (9109; Takara, Japan). Complementary DNA was synthesised using a PrimeScript RT Reagent Kit (RR047A; Takara, Japan), and RT-PCR was performed as described previously, using the following primers: mouse MCP-1 (forward: 5′-CTTCTGGGCCTGCTGTTCA-3′, reverse: 5′-GCGGGCCAGCCTACTCATTGGGATCA-3′); TNF-α (forward: 5′-CATGAGCACAGAAAGCATGATCCG-3′, reverse: 5′-AAGCAGGAATGAGAAGAGGCTGAG-3′); IL-1β (forward: 5′-CTTCAGGCAGGCAGTATCACTCAT-3′, reverse: 5′-TCTAATGGGAACGTCACACACCAG-3′); Foxp3 (forward: 5′-CCCAGGAAAGACAGCAACCTT-3′, reverse: 5′-TTCTCACAACCAGGCCACTTG-3′); AMPKα2 (forward: 5′- TTGGCTGAAGCTGCCTTAAT-3′, reverse: 5′- CAACCAAATGGCTGTTTTCA-3′) and GAPDH (forward: 5′-GCATGGCCTTCCGTGTTC-3′; reverse: 5′-GATGTCATCATACTTGGCAGGTTT-3′). Primers were purchased from Sangon Biotech (Shanghai, China).
Statistical analysis
Data are presented as means±SEM from at least three independent experiments. Student’s t-test was used for between-group comparisons. Significance was set at p<0.05. Data analysis and generation of graphics (including survival curves) were performed in GraphPad Prism 5 (GraphPad Software, San Diego, California, USA).
Results
Metformin improves survival and renal function in experimental lupus nephritis
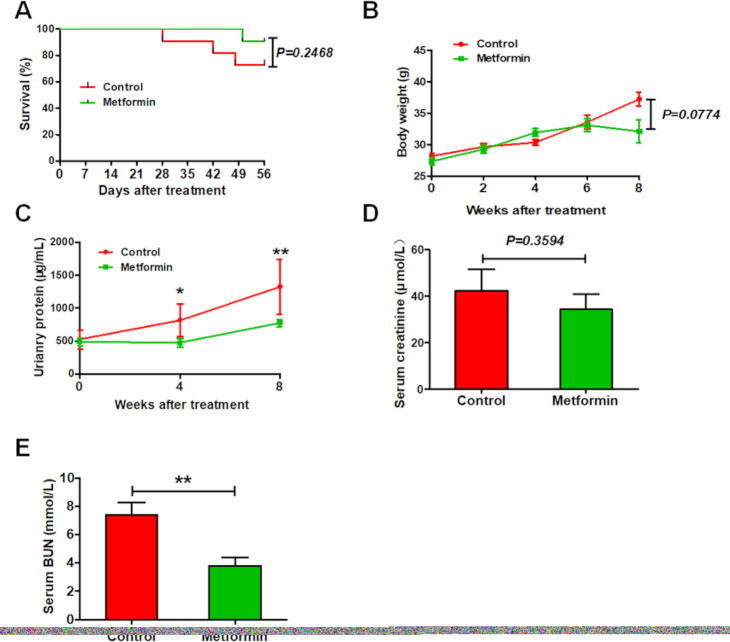
After 2 months of experimentation, 3 of 12 control MRL/lpr lupus mice were dead, compared with only one among metformin-treated lupus mice (figure 1A). Metformin-treated mice tended to have lower body weight than control mice, but this difference was not significant (figure 1B). In addition, metformin improved renal function among lupus mice, as evidenced by a remarked decrease in urinary proteins and urea nitrogen, but only trendy reduction in the level of serum creatinine (figure 1C–E).
Figure 1.
Metformin improves the survival and renal function in experimental lupus nephritis. (A) The survival ration of lupus mice with or without 8 weeks of metformin treatment. (B) The body weight of lupus mice. (C) The concentration of urinary proteins of lupus mice. (D) The level of serum creatinine of lupus mice. (E) The level of serum blood urea nitrogen (BUN) of lupus mice. Each bar represents the mean±SEM from at least three independent experiments versus control, **p<0.01.
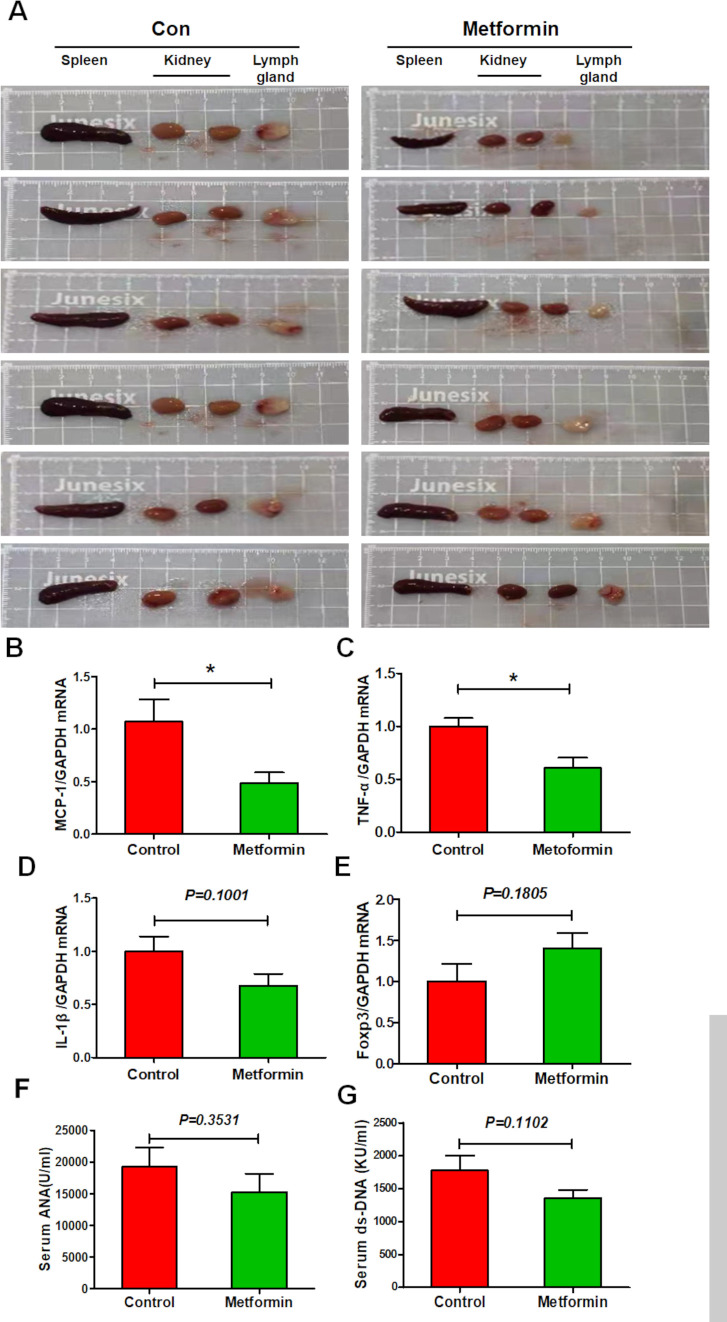
Metformin exhibits a slight inhibitory effect on splenic immune cell dysfunction in experimental lupus nephritis
Lupus is an autoimmune disease that affects multiple organs. Unsurprisingly, control lupus mice exhibited enlarged spleen, axillary lymph nodes and kidneys; the latter was also pale in colour. Metformin treatment seemed to reverse these effects (figure 2A). Moreover, splenic expression of proinflammatory cytokines MCP-1 and TNF-α was elevated in control lupus mice but suppressed in metformin-treated mice, while a little suppression on the expression of IL-1β (figure 2B–D). Metformin treatment also little upregulated splenic expression of anti-inflammatory transcription factor Foxp3 (figure 2E). In addition, metformin administration slightly reduced the serum level of ANA and ds-DNA antibodies (figure 2F, G). These data indicate that metformin treatment exhibits a slight inhibitory effect on splenic immune cell dysfunction in lupus mice.
Figure 2.
Metformin attenuates splenic immune cell dysfunction in experimental lupus nephritis (A) Representative photos of spleen, lymph node and kidneys from lupus mice with or without 8 weeks of metformin treatment. (B) The splenic mRNA level of monocyte chemoattractant protein-1 (MCP-1) in lupus mice. (C) The splenic mRNA level of tumour necrosis factor-α (TNF-α) in lupus mice. (D) The splenic messenger RNA (mRNA) level of interleukin 1β (IL-1β) in lupus mice. (E) The splenic mRNA level of Foxp3 in lupus mice. (F) The level of serum ANA of lupus mice. (G) The level of serum double-stranded DNA (dsDNA) of lupus mice. Each bar represents the mean±SEM from at least three independent experiments versus control, *p<0.05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Metformin suppresses renal pathohistological changes in experimental lupus nephritis
The results of PAS staining revealed that control mice exhibited glomeruli with diffuse endothelial cell proliferation, tuft-to-capsule adhesion, segmental sclerosis, tubular atrophy and perivascular infiltration of immune cells. Metformin administration attenuated all pathological changes in lupus mice, consistent with observed improvements to renal function (figure 3A–D). As a further assessment of glomerular lesions, immunofluorescence staining revealed that metformin treatment significantly downregulated lupus-induced elevation in glomerular IgG and C3 deposition (figure 3E, G, H). Notably, however, metformin did not affect the loss of glomerular nephrin expression, which is indicative podocyte injury (figure 3E, F).
Figure 3.
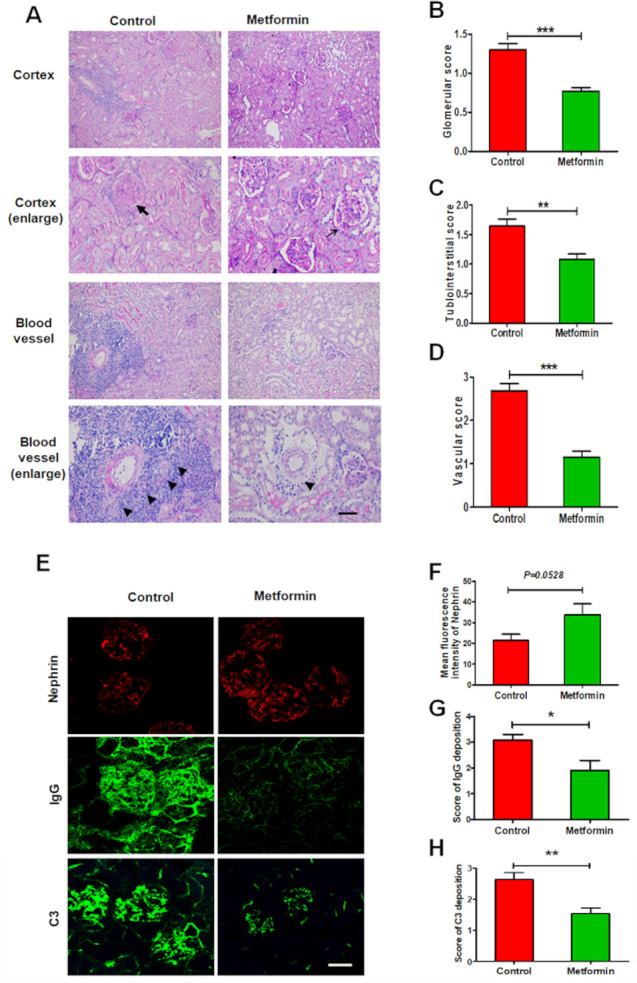
Metformin suppresses renal pathohistological changes in experimental lupus nephritis. (A) Representative histological images of the kidneys from lupus mice detected by periodic acid-Schiff staining. Arrow represents only a slight increase in mesangial matrix but no hypercellularity in glomeruli; bold arrow represents mesangial matrix expansion, glomerular capsule rupture, hypercellularity with intracapillary proliferation and influx of mononuclear cells. Triangle represents infiltration of inflammatory cells around perivascular. Scale bars=50 µm. (B) Quantitative analysis of the glomerular score. (C) Quantitative analysis of the tubulointerstitial score. (D) Quantitative analysis of the vascular score. (E) Representative images of the glomerular expression of nephrin, and the glomerular deposition of IgG and C3 in the kidneys from lupus mice. Scale bars=10 µm. (F) Quantitative analysis of the glomerular expression of nephrin. (G) Quantitative analysis of the glomerular deposition of IgG. (H) Quantitative analysis of the glomerular deposition of C3. Each bar represents the mean±SEM from at least three independent experiments versus control, *p<0.05, **p<0.01 and ***p<0.001.
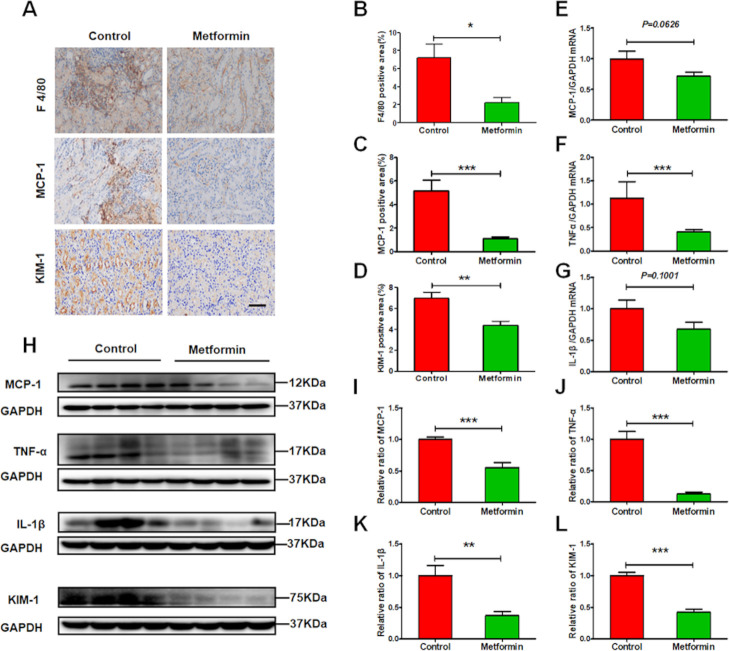
Metformin alleviates renal inflammation in experimental lupus nephritis
Immune cells mediate sustained renal inflammatory responses that eventually cause renal injury. Metformin administration greatly reduced lupus-induced increases to renal expression of chemokine MCP-1 and infiltration of F4/80-positive macrophages (figure 4A–C). Moreover, qRT-PCR and western blots revealed that metformin decreased the expression of proinflammatory cytokines IL-1β, TNF-α, and MCP-1 in the kidneys of lupus mice (figure 4E–K). Accompanying suppressed renal inflammation, tubular injury was also attenuated, as seen in the decreased expression of tubular injury marker KIM-1 (figure 4D, H, L).
Figure 4.
Metformin alleviates renal inflammation in experimental lupus nephritis. (A) Representative images of the expression of F4/80, monocyte chemoattractant protein-1 (MCP-1) and kidney injury molecule-1 (KIM-1) in the kidneys from lupus mice. Scale bars=50 µm. (B–D) Quantitative analysis of the renal expression of F4/80, MCP-1 and KIM-1 in lupus mice. (E–G) The renal messenger RNA (mRNA) level of MCP-1, tumour necrosis factor-α (TNF-α) and interleukin 1β (IL-1β). (H–L) Western blotting and quantitative analysis of the renal expression of MCP-1, TNF-α, IL-1β and KIM-1. Each bar represents the mean±SEM from at least three independent experiments versus control, *p<0.05, **p<0.01 and ***p<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
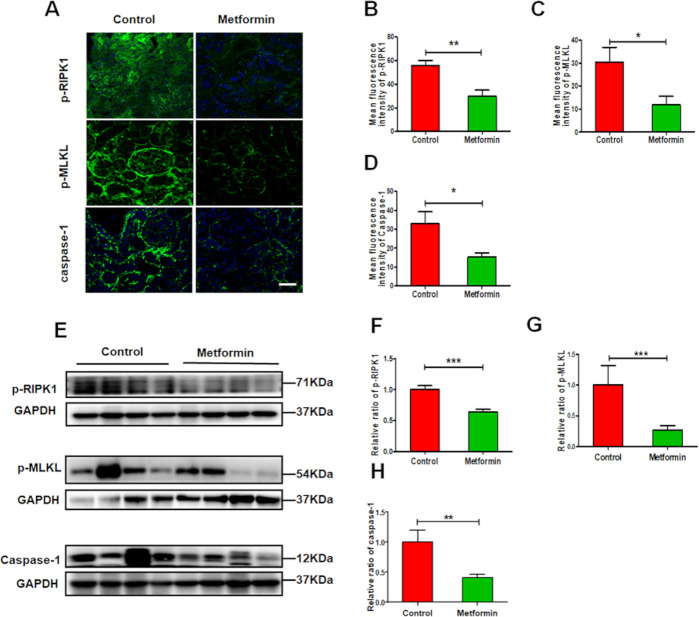
Metformin alleviates renal necroinflammation in experimental lupus nephritis
In vivo study, we found that metformin greatly reduced the renal expression of p-RIPK1, p-MLKL and caspase-1 (figure 5A–H), which related to renal necroinflammation in LN. While in vitro study, we also found that metformin suppressed the expression of NLRP3, ASC, caspase-1 and IL-1β (figure 6A–E) in HK-2 cells exposed to TNF-α. The similar findings were observed in metformin-treated HK-2 cells exposed to LPS. In addition, we found that metformin greatly reduced the renal expression of p-RIPK1, p-RIPK3 and p-MLKL in HK-2 cells stimulated by TNF-α or LPS that mimicking renal inflammatory condition in lupus (figure 6I–O). The data indicate that metformin treatment alleviates renal necroinflammation in experimental LN.
Figure 5.
Metformin alleviates renal necroinflammation in experimental lupus nephritis (A) Representative images of the expression of p-RIPK1, phosphorylated mixed lineage kinase domain-like pseudokinase (p-MLKL) and caspase-1 in the kidneys from lupus mice. Scale bars=10 µm. (B–D) Quantitative analysis of the renal expression of p-RIPK1, p-MLKL and caspase-1 in lupus mice. (E–H) Western blotting and quantitative analysis of the renal expression of p-RIPK1, p-MLKL and caspase-1. Each bar represents the mean±SEM from at least three independent experiments versus control, **p<0.01 and ***p<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 6.
Metformin alleviates the necroptosis and inflammasome activation in human kidney-2 (HK-2) cell under inflammatory condition. (A–E) Metformin (1 mM) pretreated HK-2 cells for 1 hour and then stimulated by tumour necrosis factor-α (TNF-α) (10 ng/mL) for another 48 hours. Western blotting and quantitative analysis of the expression of NLRP3, ASC, caspase-1 and interleukin 1β (IL-1β) in HK-2 cells. (F–H) Metformin (1 mM) pretreated HK-2 cells for 1 hour and then stimulated by lipopolysaccharides (LPS) (40 µg/mL) for another 48 hours. Western blotting and quantitative analysis of the expression of NLRP3 and caspase-1 in HK-2 cells. (I–K) Metformin (1 mM) pretreated HK-2 cells for 1 hour and then stimulated by TNF-α (10 ng/mL) for another 48 hours. Western blotting and quantitative analysis of the expression of p-RIPK1, p-RIPK3, phosphorylated mixed lineage kinase domain-like pseudokinase (p-MLKL) in HK-2 cells. (M–O) Metformin (1 mM) pretreated HK-2 cells for 1 hour and then stimulated by LPS (40 µg /mL) for another 48 hours. Western blotting and quantitative analysis of the expression of p-RIPK1 and p-RIPK3 in HK-2 cells. Each bar represents the mean±SEM from at least three independent experiments versus control, **p<0.01 and ***p<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
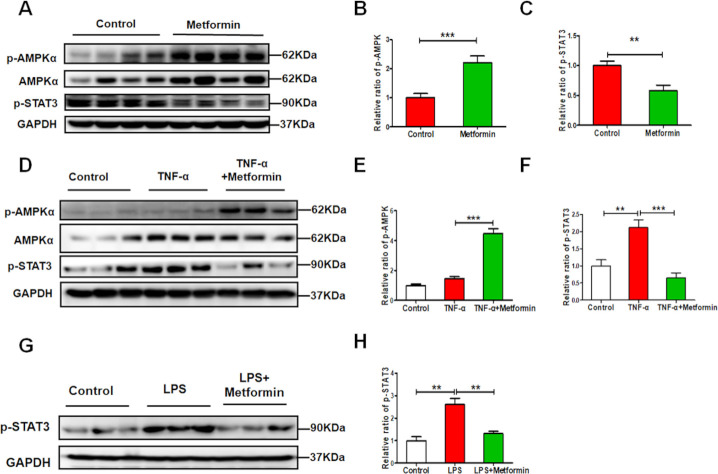
Metformin alleviates renal necroinflammation via AMPK/STAT3 pathway in experimental lupus nephritis
During lupus, necrotic renal cells release danger-associated molecular patterns to recruit immune cells. Immune cells infiltrating the kidneys produce proinflammatory cytokines to trigger renal cell necrosis, forming a feedback loop that promotes renal injury. In this study, we found the metformin treatment significantly increased p-AMPKα expression while decreasing p-STAT3 expression in the kidneys (figure 7A–C). The expression of p-STAT3 was increased in TNF-α- or LPS-treated HK-2 cells. In contrast, western blots revealed that the level of p-STAT3 was reduced by metformin combination, with a notable increase in p-AMPKα expression (figure 7D–H).
Figure 7.
Metformin alleviates renal injury via the AMPK/STAT3 (AMP-activated protein kinase/signal transducer and activator of transcription 3) pathway in vivo and in vitro experimental lupus nephritis. (A–C) Western blotting and quantitative analysis of the renal expression of p-AMPKα, AMPKα and p-STAT3. (D–F) Metformin (1 mM) pretreated human kidney-2 (HK-2) cells for 1 hour and then stimulated by tumour necrosis factor-α (TNF-α) (10 ng/mL) for another 48 hours. Western blotting and quantitative analysis of the expression of p-AMPKα, AMPKα and p-STAT3 in the HK-2 cells. (G–H) Metformin (1 mM) pretreated HK-2 cells for 1 hour and then stimulated by lipopolysaccharides (LPS) (40 µg/mL) for another 48 hours. Western blotting and quantitative analysis of the expression of p-STAT3 in HK-2 cells. Each bar represents the mean±SEM from at least three independent experiments versus control, **p<0.01 and ***p<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
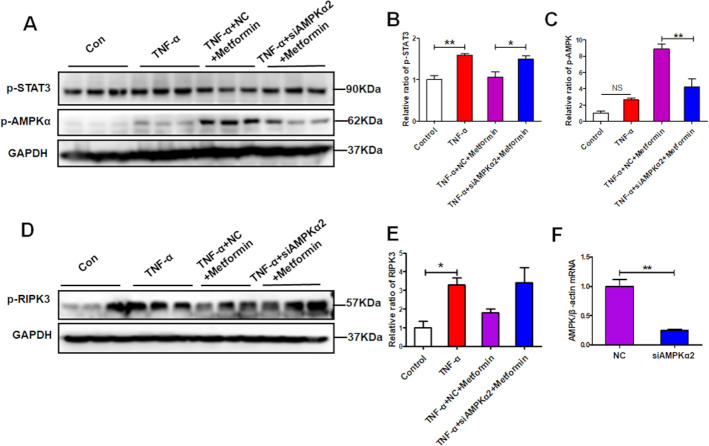
To confirm the protective effect of metformin on renal necroinflammation via AMPK/STAT3, we knocked down AMPKα2 in HK-2 cells (figure 8F). When inhibition of p-AMPK by siRNA-mediated AMPKα2 silencing, the suppression of metformin on p-STAT3 was abolished (figure 8A–C). As expected, the downregulated expression of p-RIPK3 by metformin was also reversed in the presence of AMPK inhibition (figure 8D, E). These data suggest that metformin suppresses renal necroinflammation via promoting AMPK-mediated STAT3 inhibition.
Figure 8.
AMP-activated protein kinase α2 (AMPKα2) knockdown abolishes the signal transducer and activator of transcription 3 (STAT3) suppression by metformin under inflammatory condition. Human kidney-2 (HK-2) cells were treated with a specific AMPK small-interfering RNA (siRNA) (60 nM) or a negative control (NC) (60 nM) for 24 hours and then cells were pretreated with the metformin for 1 hour and then exposed to tumour necrosis factor-α (TNF-α) (10 ng/mL)for another 48 hours. (A–C) Western blotting and quantitative analysis of the HK-2 cell expression of p-AMPKα and p-STAT3. (D–E) Western blotting and quantitative analysis of the HK-2 cell expression of p-RIPK3. (F) Real-time PCR (RT-PCR) detect the effectiveness of AMPKα2 siRNA.
Discussion
Our study demonstrated that metformin administration attenuated kidney injury, improved renal function and increased mouse survival ratio in experimental LN. The mechanism of action was mainly suppressing systemic and intrarenal inflammatory response in the kidneys, specifically necroptosis and inflammasome activation via AMPK-mediated STAT3 inhibition.
Necroptosis is a proinflammatory mode of cell death.17 Generally, proinflammatory cytokine TNF-α interacts with its receptors to induce necrosis.18 On inhibition of caspase activity, the receptors recruit RIPK1 and RIPK3, then promotes their autophosphorylation. This step causes RIPK1 and RIPK3 to form a complex that promotes the phosphorylation of mixed lineage kinase domain-like pseudokinase (MLKL). Entering into the plasma and organelle membranes, phosphorylated MLKL destroys membrane integrity to cause cell death.19 20 The RIPK1/RIPK3/p-MLKL pathway is activated in podocytes of renal biopsies from patients and in mice with LN, contributing to glomerular lesions.21 Inhibiting necroptosis through a RIP3-specific inhibitor attenuated LN progression. In addition to TNF-α, IgG can trigger necroptosis though the TNF receptor.21
Interesting, our study found that metformin administration suppressed renal expression of necroptosis markers p-RIPK1, p-RIPK3 and p-MLKL, probably ameliorating glomerular and tubular lesions. Elevated renal STAT3 activation may promote necroptosis via enhancing RIPK3 expression and MLKL phosphorylation, as previously described.22 We also know that metformin inhibits complex I of the mitochondrial respiratory chain to increase cellular AMP/ATP ratio, leading to AMPK activation.23 Upregulated AMPK then suppresses STAT3 phosphorylation and activation.24 25 Taken together, we speculate that metformin administration may suppress RIPK1/RIPK3/MLKL-mediated necroptosis in intrinsic renal cells via AMPK-mediated STAT3 inhibition.
In addition to necroptosis, renal inflammation can be promoted through inflammasome activation in intrinsic renal cells and proinflammatory cells.24 STAT3 activation also enhances caspase-1 and successive production of mature IL-1β and IL-18, even triggering pyroptosis, another type of cell death.26 27 Recent research indicates that RIPK3 is involved in activating the inflammasome.28 29
In our study, we also found that metformin administration suppressed renal expression of inflammasome marker NLRP3, ASC, caspase-1 and IL-1β. Thus, downregulation of the inflammasome may be another mechanism that allows metformin-mediated STAT3 suppression to reduce renal inflammation.
Metformin reversed both LN-induced enlargement of the spleen and the upregulation of splenic proinflammatory cytokine production. Therefore, metformin treatment improves systemic inflammatory response. A recent clinical trial at the Renji Hospital found that metformin administration reduced subsequent disease flare in patients with SLE,13 consistent with findings in animal studies.30 31 Overall, a lower systemic inflammatory response may also contribute to improved renal structure and function after metformin treatment.
Our study had two limitations. First, although reports are rare, metformin may have an anti-inflammatory effect via an AMPK-independent process.32 33 We did not examine how AMPK deficiency influences metformin protection against LN-induced kidney damage. Second, we have not yet explored whether metformin, as an LN treatment, improves kidney necroinflammation or systemic inflammation.
Nevertheless, taken as a whole, our findings allow us to conclude that metformin alleviates LN-induced kidney injury via suppressing renal necroptosis and inflammation.
Acknowledgments
We thank Editage (www.editage.cn) for English language editing and we also thank Ms Jing Zhang from Yuebin Medical Research Lab for providing technique support for this study.
Footnotes
X-cC, DW, H-lW and H-yL contributed equally.
Contributors: X-cC, NA and H-fL conceived and designed the experiment. X-cC, DW, H-lW, H-yL, CY, H-yS, Z-jL, X-rH, XL, L-fH and S-pZ were involved in the experiments. X-cC, NA and CY were involved in the data analysis and writing the original draft. Q-jP and H-fL reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript. H-fL is responsible for the overall content as guarantor.
Funding: This work was supported by the Funds for Science and Technology Innovation Strategy of Guangdong Province (grant nos: 2021A1515011581 and 2019A1515010678), the Science and Technology Planning Project of Zhanjiang City (grant nos: 2018A01040 and 2018A01034), the National Natural Science Foundation of China (grant nos: 81700627, 81670654 and 81974095), Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Non-communicable Diseases co-sponsored by province and city (2022B1212030003), and Discipline construction project of Guangdong Medical University (4SG21229G).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1.Brunner HI, Gladman DD, Ibañez D, et al. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 2008;58:556–62. 10.1002/art.23204 [DOI] [PubMed] [Google Scholar]
- 2.Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol 2017;12:825–35. 10.2215/CJN.05780616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoover PJ, Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist's perspective. Kidney Int 2016;90:487–92. 10.1016/j.kint.2016.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparotto M, Gatto M, Binda V, et al. Lupus nephritis: clinical presentations and outcomes in the 21st century. Rheumatology 2020;59:v39–51. 10.1093/rheumatology/keaa381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangou E, Georgakis S, Bertsias G. Update on the cellular and molecular aspects of lupus nephritis. Clin Immunol 2020;216:108445. 10.1016/j.clim.2020.108445 [DOI] [PubMed] [Google Scholar]
- 6.Mistry P, Kaplan MJ. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol 2017;185:59–73. 10.1016/j.clim.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenton K. The effect of cell death in the initiation of lupus nephritis. Clin Exp Immunol 2015;179:11–16. 10.1111/cei.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B-C, Tang T-T, Lv L-L, et al. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int 2018;93:568–79. 10.1016/j.kint.2017.09.033 [DOI] [PubMed] [Google Scholar]
- 9.Pan Q, Lu X, Zhao C, et al. Metformin: the updated protective property in kidney disease. Aging 2020;12:8742–59. 10.18632/aging.103095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Gui Y, Ren J, et al. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKα-regulated autophagy induction. Sci Rep 2016;6:23975. 10.1038/srep23975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y-C, Tang S-Q, Liu Y-T, et al. Ampk agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis 2021;12:925. 10.1038/s41419-021-04184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi H, Huang C, Shi Y, et al. Metformin attenuates renal fibrosis in a mouse model of adenine-induced renal injury through inhibiting TGF-β1 signaling pathways. Front Cell Dev Biol 2021;9:603802. 10.3389/fcell.2021.603802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun F, Geng S, Wang H, et al. Effects of metformin on disease flares in patients with systemic lupus erythematosus: post hoc analyses from two randomised trials. Lupus Sci Med 2020;7:e000429. 10.1136/lupus-2020-000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C, Xue J, An N, et al. Accelerated glomerular cell senescence in experimental lupus nephritis. Med Sci Monit 2018;24:6882–91. 10.12659/MSM.909353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieson MD, Gojmerac AM, Khan D, et al. Treatment with a selective histone deacetylase 6 inhibitor decreases lupus nephritis in NZB/W mice. Histol Histopathol 2017;32:1317–32. 10.14670/HH-11-885 [DOI] [PubMed] [Google Scholar]
- 16.Majumder S, Thieme K, Batchu SN, et al. Shifts in podocyte histone H3K27me3 regulate mouse and human glomerular disease. J Clin Invest 2018;128:483–99. 10.1172/JCI95946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney CJ, Martin SJ. An inflammatory perspective on necroptosis. Mol Cell 2017;65:965–73. 10.1016/j.molcel.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 18.Müller MB, Hoppe JM, Bideak A, et al. Exclusive expression of transmembrane TNF aggravates acute glomerulonephritis despite reduced leukocyte infiltration and inflammation. Kidney Int 2019;95:75–93. 10.1016/j.kint.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 19.Zhang D-W, Shao J, Lin J, et al. Rip3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009;325:332–6. 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- 20.Günther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 2011;477:335–9. 10.1038/nature10400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo C, Fu R, Zhou M, et al. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J Autoimmun 2019;103:102286. 10.1016/j.jaut.2019.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Zamel R, Bai X-H, et al. Ischemia-Reperfusion induces death receptor-independent necroptosis via calpain-STAT3 activation in a lung transplant setting. Am J Physiol Lung Cell Mol Physiol 2018;315:L595–608. 10.1152/ajplung.00069.2018 [DOI] [PubMed] [Google Scholar]
- 23.Ravindran S, Kuruvilla V, Wilbur K, et al. Nephroprotective effects of metformin in diabetic nephropathy. J Cell Physiol 2017;232:731–42. 10.1002/jcp.25598 [DOI] [PubMed] [Google Scholar]
- 24.Yue W, Zheng X, Lin Y, et al. Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2. Oncotarget 2015;6:21208–24. 10.18632/oncotarget.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori Y, Hattori K, Hayashi T. Pleiotropic benefits of metformin: macrophage targeting its anti-inflammatory mechanisms. Diabetes 2015;64:1907–9. 10.2337/db15-0090 [DOI] [PubMed] [Google Scholar]
- 26.Yao R, Chen Y, Hao H, et al. Pathogenic effects of inhibition of mTORC1/STAT3 axis facilitates Staphylococcus aureus-induced pyroptosis in human macrophages. Cell Commun Signal 2020;18:187. 10.1186/s12964-020-00677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao F, Tian X, Li Z, et al. Suppression of NLRP3 inflammasome by erythropoietin via the EPOR/JAK2/STAT3 pathway contributes to attenuation of acute lung injury in mice. Front Pharmacol 2020;11:306. 10.3389/fphar.2020.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paudel S, Jeyaseelan S. Kill two birds with one stone: role of the RIPK-3 in necroptosis and inflammasome activation. Am J Respir Cell Mol Biol 2021;64:525–7. 10.1165/rcmb.2021-0085ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Fang Y, Wu J, et al. RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis 2018;9:878. 10.1038/s41419-018-0936-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S-Y, Moon S-J, Kim E-K, et al. Metformin Suppresses Systemic Autoimmunity in Roquinsan/san Mice through Inhibiting B Cell Differentiation into Plasma Cells via Regulation of AMPK/mTOR/STAT3. J Immunol 2017;198:2661–70. 10.4049/jimmunol.1403088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin Y, Choi S-C, Xu Z, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 2015;7:274ra18. 10.1126/scitranslmed.aaa0835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen M, Jensen JB, Jakobsen S, et al. Renoprotective Effects of Metformin are Independent of Organic Cation Transporters 1 &2 and AMP-activated Protein Kinase in the Kidney. Sci Rep 2016;6:35952. 10.1038/srep35952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Y, Wang S, Zhang Y, et al. Metformin attenuates renal fibrosis in both AMPKα2-dependent and independent manners. Clin Exp Pharmacol Physiol 2017;44:648–55. 10.1111/1440-1681.12748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. The data that support the findings of this study are available from the corresponding author on reasonable request.