Highlights
-
•
Controlled germination of wheat grain using low-frequency ultrasonic treatment.
-
•
Germinating exposed to ultrasound intensifies the accumulation of γ-aminobutyric acid and increases the AOA of wheat grain products.
-
•
Whole-wheat flour made from germinated wheat when exposed to ultrasound showed a high nutritional value.
Keywords: Ultrasound, Wheat grain, Germination, γ-Aminobutyric acid, Antioxidant activity, Grain products
Abstract
The use of ultrasound to intensify the germination process of Triticum aestivum L. wheat was studied. This method of controlled germination can be used in several sectors of food industry, in particular in bakery. The effect of low-frequency ultrasound (20 kHz) at different intensities and duration on the germination process of Triticum aestivum L. wheat was systematically studied. We have found that 3-minute processing at 227 W/l output reduces the duration of wheat grain germination by 25% (12 ± 2 h) compared to the control samples. The use of ultrasound stimulated γ-aminobutyric acid (GABA) synthesis (18.9 ± 0.5 mg/100 g), increased the antioxidant activity (AOA) (2.86 ± 0.2 mg/g Trolox equivalents) and the amount of flavonoids (0.19 ± 0.03 mg QE/g). The SEM analysis of powder particles of whole-wheat flour made from wheat germinated with ultrasound exposure showed densely packed aggregates of protein matrix. To sum up, controlled ultrasound during wheat grain germination increases the amount of GABA and AOA. The whole-wheat flour is useful for food enrichment.
1. Introduction
Grain crops germination is actively used in food technology as an effective way to increase their nutritional value. The process of grain germination is known to activate enzymatic systems [1], increase the availability of reducing sugars, free amino acids, including lysine [2], stimulate the accumulation of γ-aminobutyric acid (GABA) [3] and increase antioxidant activity (AOA) [4].
AOA of germinated grain is closely related to the complex of mitochondrial redox reactions [5]. Release of reactive oxygen species is observed in grain cells at germination, with protection provided by the use of a highly active antioxidant system in the composition of low and high-molecular weight compounds [6].
Among the chemical components synthesized during grain germination, GABA occupies a special place as a substance recommended for the prevention of neurological disorders [7]. GABA attracted more attention due to its biological activities such as lowering blood pressure [8], stimulating immunity enhancement [9], improving brain function and enhancing intelligence [10]. Consumption of food enriched with GABA helps to reduce anxiety [11], [12].
In this regard, the search for ways to enhance the amount of substances possessing antioxidant activity and GABA synthesis in plant materials is getting more attention.
The use of ultrasonic processing in food industry is growing [13], [14]. Chemat showed the beneficial effects of ultrasound in combination with mechanical kneading on the extraction of flavonoids from plant raw materials [15]. Yang et al. showed that the amount of GABA increased by 43.4% due to ultrasonic treatment (40 kHz, 300 W) during the process of soybean seeds steeping for 30 min [16]. Some studies have shown the possibility of obtaining GABA from germinated grain [7]. Other studies have considered the use of germinated wheat in bakery products as a source of GABA [12]. It is reported that grain products obtained using germinated raw materials are of a softer consistency and sweeter [17].
At the same time, when obtaining food products based on whole-wheat flour from germinated grain, there is a high probability of changing the technological application of the obtained raw materials. The article shows that the increase in enzymatic activity caused by germination has a negative effect on the quality of wheat processing for grinding and baking [18]. However, controlled grain germination yields flour with improved functional properties, including greater bread volume, better texture and other improved sensory bread characteristics and higher pasta elasticity and plasticity [19].
This study reports on the enhancement of bioactive substances, including GABA during Triticum aestivum L. germination by low-frequency ultrasound stimulation. The properties of products derived from germinated grains were also studied.
2. Materials and methods
2.1. Materials
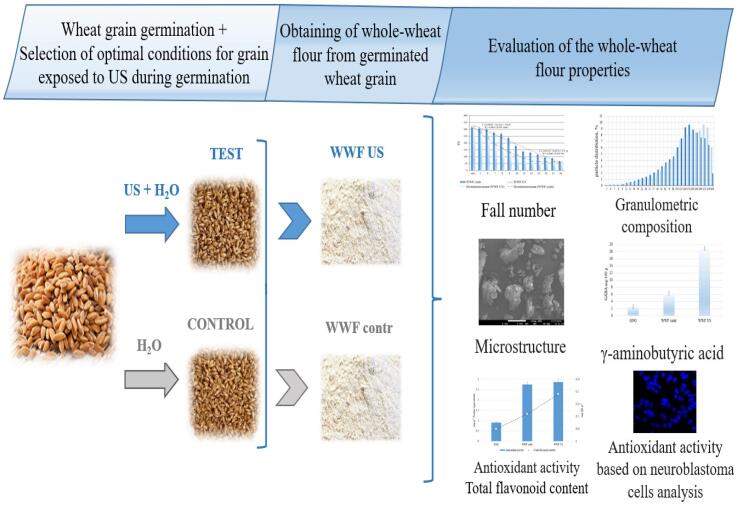
The experimental procedure is presented in Fig. 1. In this study, we used grain of soft spring white wheat (Triticum aestivum L.), “Lubava” variety. The grain was grown and harvested (2018) at the experimental farm of Bredinsky district, Chelyabinsk region, Russia (grains non-germinated (GNG)). The protein content was 12.5 ± 0.03 g/100 g in terms of humidity. All grains used in the studies were pre-selected and sorted by length (8.6 ± 0.6 mm) and width (4.8 ± 0.4 mm) using the program for grain phenotyping SeedCounter v. 1.9.5, developed by Novosibirsk State University [20].
Fig. 1.
The experimental procedure.
All other chemicals used were of analytical grade.
2.2. Obtaining of whole-wheat flour from germinated wheat grain (whole-wheat flour (WWF))
2.2.1. Wheat grain germination
At the preparatory stage the wheat grain was washed five times in running water at a temperature of 20 ± 2 C to remove contamination products and foreign substances.
The washed grain (500 g) was soaked in water (water to grain ratio of 1:1):
Control – the wheat grain was kept in water at a temperature of 22 ± 2 °C for 6 h;
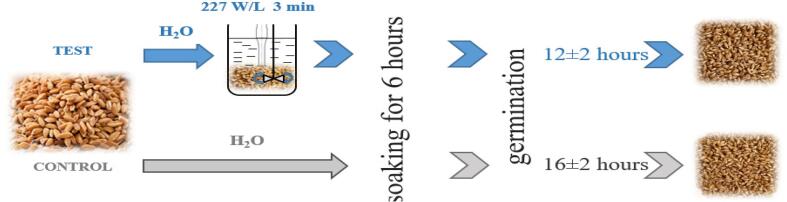
Test – the wheat grain before the soaking stage was exposed to ultrasound (see 2.2.3) and constant kneading (Fig. 2). Ultrasonic treatment (22 ± 1.25 kHz) was performed by means of changing power and duration (75, 113, 151, 189, 227, 265, 303, 341, 379 W/L, intensity for 3, 5, 7 min). After ultrasonic exposure the soaking process was carried out according to the above method.
Fig. 2.
The ultrasonic vibrating system.
The samples prepared for germination were placed in chambers (germination box SHPZ, Russia). Germination was carried out at a temperature of 22 ± 2 °C and relative humidity of 95 ± 3%, the germination period was from 16 to 24 h. When the sprout reached the size of 1.5–2 mm in more than 90% of grains, the grain was removed from the chamber and dried (drying box M 720, Russia) at a temperature of 35–40 °C for 10 h until a final moisture content of 8–14% was reached.
2.2.2. Production of whole wheat flour from germinated wheat grain
Whole-wheat flour was made by grinding germinated grain in the laboratory mill Perten 3100, at a fixed rate of 20,000 vol./min. Perten Instruments, Sweden, equipped with a metal mesh of 0.8 mm. The grain was exposed to grinding for 180 sec until the flour particles were stable in size. To equalize the particle size distribution, according to the proposed by Wang et al method, the whole-wheat flour was additionally sifted through a sieve with a cell size of 0.6 mm [21].
2.2.3. Ultrasonic treatment
Volna-M UZTA-0.63/22-OM as ultrasound generator was used for ultrasonic treatment.
Technical specifications of Volna-M UZTA-0.63/22-OM device are the following: ultrasound oscillation frequency is (22 ± 1.65 kHz); power is 630 W; intercity of ultrasound, not less than 10 W/cm2; power supply voltage is (220 ± 22 V AC); maximum continuous operation time is 8 h; diameter of emitting surface is 30 mm; overall dimensions: electronic generator is (300 × 280 × 120 mm), oscillating system is ∅ (80 × 150 mm).
The ultrasonic vibrating system uses annular piezoelectric elements and is made of BT5 titanium alloy. Its operating principle is based on high-intensity ultrasonic waves propagating in fluid and in fluid-dispersed substances. The engineering solutions used are protected by Russian Federation patent № 2141386 [22].
2.3. Selection of optimal conditions for grain exposed to US during germination
To establish the optimal modes of ultrasonic effect on the rate of wheat germination the following controlled parameters were determined: germination energy (E) and germination capacity (N). Mathematical planning was carried out using the Statistica 13 program.
2.3.1. Calculation of germination energy and capacity of grain
The germination energy and germination capacity of grains were calculated according to the methodology described in GOST 10968-88. To measure these parameters, 500 grains were taken from the average grain sample, then each sample was soaked at 20 ± 2 °C, so that the water level was 1–1.5 cm above the grain level. The rest of moisture was drained 4 h later and the sample was left in a closed container for 16 h. This operation was repeated for 120 h. Grain germination energy (%) was calculated as the ratio of the number of grains germinated after 72 h to the total number of the analyzed grains. Grain germination capacity (%) was calculated as the ratio of the number of grains germinated after 120 h to the total number of the analyzed grains.
2.4. Evaluation of the whole-wheat flour properties
2.4.1. The Hagberg falling number (FN)
The value of the Hagberg falling number (FN) was calculated using the international method AACC 56-81.03 with the FN 1500 system (Perten Instruments, Sweden) with a flour sample size of 7 g (14% of moisture) in 25 ml of water. Grain samples were selected every hour during the germination process from 5 to 16 h, as was suggested by Ding et al [23].
2.4.2. The distribution of the average particle
The distribution of the average particle size of flour samples was measured using laser dynamic light scattering device Microtrac S3500 (AASS 55-40.01, 2010) [1].
3. Scanning electron microscopy (SEM)
To obtain micrographs, flour and dough samples were fixed on a slide table, sprayed with platinum and examined using a scanning electron microscope (JSM-7001F (JEOL), Japan) at 20 kV and magnification 250×, 1000×, 1500×, 2000×.
3.1. Physical and chemical analysis of whole-wheat flour
The moisture content was calculated using the international method AACC 44-15.02. The protein content of whole-wheat flour was calculated using the Kjeldahl micro-method (AACC 46-13.01, 2010). Lipids in whole-wheat flour were measured by 30-25.01, the ash content - according to AACC 08-12.01, total starch – AACC 76-13.01, index and gluten content – AACC 38-12.02 (AACCI, 2010), the dough was kneaded in accordance with the method AACC 54-21.02 (2010) by the Farinograph-AT (Brabender, Germany) [1].
3.2. Measuring the content of γ-aminobutyric acid (GABA)
Germinated wheat grain (1.00 g) was ground with 6 ml of 4% acetic acid. The homogenate was left on the shaker for 1 h and centrifuged at 6000g for 15 min. The supernatant was collected and treated with 4 ml of ethanol, then centrifuged at 17,000g for 20 min. Purified supernatant was boiled out (0.1 MPa, 45 °C). The residues were dissolved in 0.5 ml of distilled water and centrifuged at 3000g for 10 min.
The resulting suspension was filtered through a 0.45 µm membrane filter. 100 µl of filtered supernatant was analyzed using HPLC (Shimadzu Prominence LC-20 Automated liquid chromatography system, Japan) with a reversed phase column Prodigy C 18 (5 µm), an internal diameter of 4.6 × 250 mm, as described by Rossetti and Lombard [24] and Bai et al. [25].
3.3. Total flavonoid content
The total flavonoid content was measured using the procedure described by Shafii et al. [26]. 0.5 ml of sample extract was mixed with 0.1 ml of 10% (w/v) ethanol solution of aluminum chloride, 0.1 ml of 1 M sodium acetate and 4.3 ml of distilled water. After 30 min in the dark, absorption at 415 nm was measured using a Jenway spectrophotometer (6405 UV/Vis, England). Quercetin (0.01–0.5 mg L−1; R2 = 0.997) was used as the standard, and the results were expressed in mcg-1-quercetin equivalents.
3.4. Antioxidant activity measuring
Radical analysis (DPPH). Activity of radical absorption in the samples was measured using 2,2-diphenyl-1-picrilhydrazyl (DPPH) [27]. The extracts (0.5 ml) were mixed with 3.6 ml of radical solution (0.025 g DPPH in 100 ml ethanol). The absorbance of the extract sample was measured using a Jenway spectrophotometer (6405 UV/Vis, England) at 515 nm. Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) (10–100 mg/l; R2 = 0.988) was used as standard, and the results were expressed in mg.-1 Trolox equivalents.
3.5. Evaluation of antioxidant activity by the intensity of AOF generation
A cell model of induced generation of active oxygen forms (AOF) in human undifferentiated neuroblastoma SH-SY5Y cell culture was used to evaluate the antioxidant activity of the studied samples.
DMEM (Gibco, UK) medium with addition of 10% embryonic bovine serum (Gibco, New Zeland) was used for cell cultivation. A fluorescent dye 2,7-dichlorodihydrofluorescein-diacetate (DCFH2-DA) was used to evaluate the intensity of the AOF generation in neuroblastoma cells (bovine embryonic serum Gibco, New Zeland). The dye was loaded into the cells for 30 min at a temperature of 37 °C. The dye concentration was 10 µm. Incubation period with samples was 2 h, after that the cells were washed. Then a phorbol ester (PMA, phorbol-12-myristate-13-acetate) was added to the cell culture to induce the active oxygen forms generation. All experiments were performed in HBSS (Hank's balanced salt solution) with the addition of HEPES to maintain buffer capacity.
Fluorescence was recorded using a fluorescent tablet reader Tecan Spark 10 M with a thermostatic camera. To visualize cell cultures with fluorescence microscopy, we used a system based on an inverted motorized fluorescent microscope Leica DMI6000B, with a monochrome CCD camera HAMMAMATSU C9100, a light source Leica EL6000 with a high-pressure mercury lamp HBO 103 W/2 with a lens Leica HCX PL APO lambda blue 63.0 × 1.40 Oil.
3.6. Statistical processing of results
The studies were carried out in threefold repetition. Grain germination, whole-wheat flour and bread samples production were carried out under the same conditions to ensure the accuracy of the results. Experimental data were processed by methods of mathematical statistics using Microsoft Excel, MathCad and Statistica 13. The obtained data are presented with a confidence factor of 0.95.
4. Results
4.1. Analysis of ultrasonic effect on the germination rate
The germination rate of grain is important for the technological process of production as an economic factor. Since it is important to get a product of high quality, the process of grain germination should be under control. The use of ultrasound for grain processing requires a clear dosage to minimize the risks of negative changes. The power (P) and duration (t) of ultrasonic exposure (USE) are determining factors that can stimulate the vitality inherited by nature in every cultivated crop.
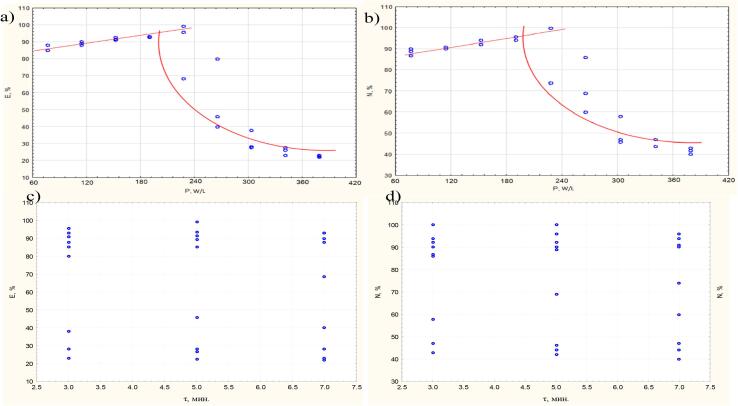
As part of the research, we compiled a large amount of data with successive variations in power (P – 76, 113, 151, 189, 227, 265, 302, 340, 378 W/L) and duration of ultrasonic exposure (t – 3, 5 and 7 min). We used germination energy (E) and germination capacity (N) as evaluation criteria to establish applicable ultrasound modes. The graphs of the obtained dependences (Fig. 3) showed that the dependence curve is divided into 2 sections. E and N increase in the first section and decrease in the second. The dependences pass through the maximum at ultrasonic power of ∼227 W/l. It should be noted that the responses of E and N correlate well with each other (R = 0.994).
Fig. 3.
Piecewise continuous dependences: a) E from P; b) N from P; c) E from t; d) N from t.
Processing with two piecewise continuous functions gives Eq. (1) for the first section:
| (1) |
Eq. (2) for the second section:
The intersection point corresponds to P = 202 ± 15 W/l and E = 94.5 ± 6.9%. The correlation coefficient of the calculated and experimental values is 0.975. The standard deviation is 6.9%.
For value N, Eq. (3) of the first section has the form:
| (3) |
Eq. (4) for the second section:
| (4) |
The intersection point is P = 194 ± 10 W/l and E = 96.4 ± 5.2%.
The correlation coefficient of the calculated and experimental values is 0.973. The standard deviation is 5.2%.
We have not found a statistically significant dependence of germination energy and germination capacity on the time of ultrasonic exposure (Fig. 3c and d). Taking into account the technological capabilities of the device, in further experiments we used the mode 227 W/l with 3 min of exposure.
This mode was used to study the effect of ultrasound on the activity of the enzyme α-amylase (the Hagberg falling number) in order to determine the preferable duration of Triticum aestivum L. germination.
As the objects of the study, we chose:
WWFcontr – whole-wheat flour from germinated wheat grains obtained by soaking for 6 h at a water temperature of 22 ± 2 °C and germinating for 16 h at a relative humidity of 95 ± 3%, followed by drying to a moisture content of not more than 14%.
WWFus – whole-wheat flour from germinated wheat grain after preliminary US exposure, followed by soaking for 6 h at a water temperature of 22 ± 2° C, germinating for 16 h at a relative humidity of 95 ± 3% and drying to a moisture content of not more than 14%.
4.2. Change in the falling number (FN)
The dough should contain a certain amount of α-amylase to break down starch into amylopectin, but in a case of an excessive amount of this enzyme, starch can completely dissolve, which causes defects in the final product. α-amylase can react with starch throughout the entire chain of molecules and create smaller chains of various lengths, which leads to a sharp decrease in viscosity and inverse curvilinear relationship between its enzymatic activity and the FN value [28]. According to the scientific data, the FN value ∼60 is characteristic of the α-amylase high activity (bread with sticky crumb, of smaller size, dark in color, contains large cavities); FN ∼ 250 – normal activity (bread of good quality); FN ∼ 400 – low activity (dry bread, of smaller size, perishable). Therefore, the determination of the FN grinding of grain batches will help to improve their technological properties.
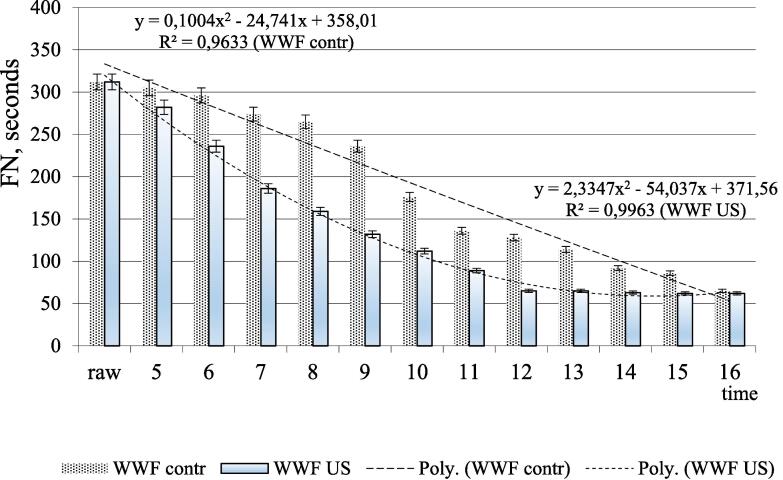
In this research, the FN value change was determined during the wheat grain germination in order to control the rate of this process. The results obtained (Fig. 4) describe a decrease in the FN value during germinating. The initial FN values of wheat samples were 312 ± 4 s; during germination these values decreased multiple times. The ultrasonic exposure provides a high germination rate; the FN value for WWFus was steadily minimal (65 ± 3 s) after 12 h, for WWFcontr – after 16 h. US treatment provides rapid penetration of moisture to the embryo and endosperm [29].
Fig. 4.
Changes in falling-number value (FN) (in terms of 14% humidity): whole-wheat flour made from germinated wheat at 22 ± 2 °C during 16 h at a relative humidity of 95 ± 3%. *Abbreviations: falling number, FN; whole-wheat flour made from germinated wheat (WWFcontr); whole-wheat flour made from germinated wheat exposed to US treatment (WWFUS).
The nature of the decrease in the FN value for wheat grain demonstrates an inverse curvilinear relationship between germination time and the FN value. This is consistent with studies by Singh et al. who found a similar decrease in FN values during germination [30].
Changes in the FN values during germination can be described by a second-order polynomial equation with high determination coefficients, especially for WWFus samples. The use of polynomial curves will help to determine the FN of wheat grains and control the duration of germinating for processing (baking industry, technology for the production of biologically active substances, etc.).
We have determined the germination time of Triticum aestivum L wheat grain for whole-wheat flour technology (Fig. 5). For WWFcontr it is no more than 18 h, for WWFus – 14 h.
Fig. 5.
Methodology of Triticum aestivum L germination for obtaining whole-wheat flour.
The use of ultrasonic influence allows us to reduce the term of the germination process by 4 h and control this process. The mechanism of ultrasonic influence is described in the works of several authors [14]. The researchers prove that ultrasonic vibrations cause formation, growth and intense destruction of microbubbles in the treated liquid medium, where acoustic cavitation is observed. This phenomenon implies alternate compressing and stretching of the molecular structure of the medium. During each phase of stretching (rarefaction) phase, the liquid medium can literally rupture, forming tiny cavities (microbubbles). During the exposure cycle, these cavities can collapse, releasing large amounts of energy in the immediate proximity of the microbubbles.
Bubbles collapse near the surface asymmetrically and fluid flow is impeded by the hard surfaces of the wheat grain in the hydromodule. A jet of water falls on the surface of the wheat grain, thus collapsing the cavitation bubble. Such a water jet can (i) damage the shell walls of the grain, (ii) increase water absorption by the shell parts, and (iii) increase heat and mass transfer from the surface.
Note that the mechanical and chemical effects of collapse work in two different areas: (i) within the bubble, which can be seen as a high-pressure and high-temperature microreactor, and (ii) in the immediate vicinity of the bubble, where the shock wave generated by collapse creates enormous shear forces that alter the physical and chemical properties of water. The shock waves of the cavitation field break hydrogen bonds in the water structure [14], [31]. High adsorption activity of hydroxyl ions due to ultrasonic influence accelerates soaking of the wheat grain surface.
The obtained results agree with the data of the researchers Ding, Hou et al. [23], who found a change in the surface microstructure of red rice kernels and microcracks in their shells. Miano, Pereira et al. [32] in their studies also describe similar effects, which can accelerate the process of hydration of bean cultures when germinating by 25%.
Then, we have studied the effect of ultrasonic treatment on the particle size of whole-wheat flour synthesis of GABA (γ-aminobutyric acid), flavonoids and a change in the total AOA.
4.3. Granulometric composition and microstructural characteristics of whole-wheat flour
The main parameter of suitability in bread technology is obtaining flour with particles of a fixed size. The processes that occur in the grain during germination affect the strength properties of the grains; therefore, special conditions are required for grinding. It was not recommended in some studies to grind the germinated grain with classical grinding, since high plasticity of the grains reduces the yield of flour and increases the ash content in the flour [33]. According to Ariyama and Khan [34], germination significantly reduces the yield of flour (by about 6% after 40 h of wheat germinating). Biologically active compounds in the grain are mainly located in the shell parts. Therefore, the production of flour by milling for this type of grain raw materials is most preferable. For dry grinding, the moisture content of the germinated grain should be no higher than 14% [35]. Grinding should lead to determining the particle size distribution which is important from a technological point of view, since it affects the properties of flour and subsequent processing steps [36]. In studies such parameters as the particle size distribution (Fig. 6) and the average-weighted particle size (d) were used to characterize the granulometric composition of whole-wheat flour.
Fig. 6.
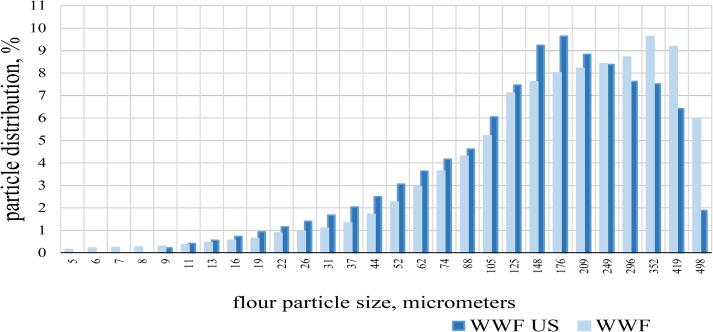
Profile of distributing size particles of whole-wheat flour. *Abbreviations: falling number, FN; whole-wheat flour made from germinated wheat (WWFcontr); whole-wheat flour made from germinated wheat exposed to US treatment (WWFUS).
The results showed that the ground product obtained from germinated wheat grain (WWFcontr and WWFus) contained a higher proportion of large particles (more than 104 ± 6 μm) and a low proportion of small particles (4.5 ± 1.2–104 ± 4.5 μm). The average-weighted particle size of germinated samples was WWFcontr – 176 ± 15 μm and WWFus – 170 ± 15 μm.
The grinding process, which depends on the degree of adhesion between the starch granules and the protein matrix, is significant for flour produced from germinated wheat grains [37]. The results of scanning electron microscopy (SEM) (Fig. 7) characterize the internal packing of particles, their distribution and aggregation.
Fig. 7.
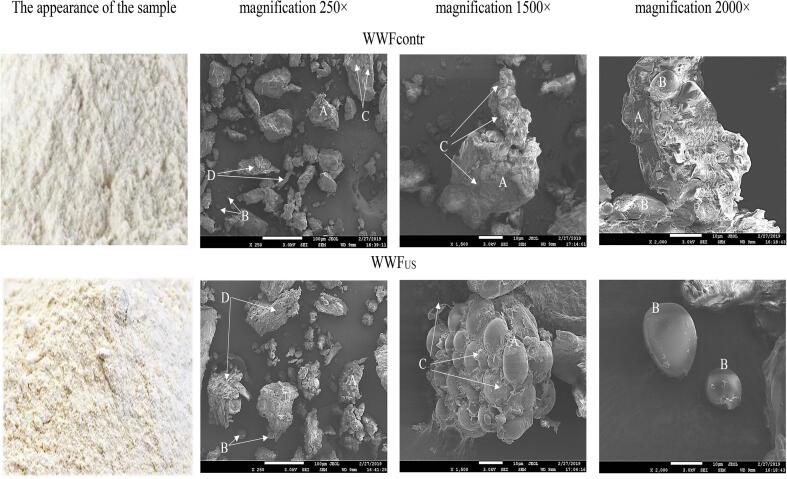
Scanning electron micrographs of whole-wheat flour made from germinated wheat: A – protein matrix aggregates, B – large starch grains, C – protein matrix, D – shell particles of grain, magnification 250×, 1500×, 2000×.
SEM micrographs indicate large compactly packed discrete particles [38] (protein matrix aggregates) with undamaged swollen starch grains (A) in whole-ground WWFus flour. In addition, we can see a large amount of undamaged starch grains (B) and protein matrix (C). In the WWFcontr whole-wheat flour sample, we can find large aggregates of the protein matrix (A) in large quantities with non-swollen starch grains and a dense protein matrix (C); occasionally, large starch grains (B) are found. Moreover, in whole-wheat flour, both WWFcontr and WWFUS, we can find separate large shell particles of grain (D) with individual inclusions of starch grains. An additional decrease in grain hardness resultant from US exposure led to the formation of particles of a more uniform size, rounded shape, with a minimum number of damaged starch grains.
4.4. Technological characteristics of whole-wheat flour
For the technology of products based on whole-wheat flour, important indicators of its suitability are physical and chemical characteristics (Table 1), based on the quantity and quality of nutrients, especially gluten complex. The formation of a protein network in dough making is due to the presence of hydrophobic interactions between nonpolar groups of protein molecules, the occurrence of redox reactions due to the oxidation of sulfhydryl groups by oxygen with the formation of disulfide bonds, including cross between individual protein polypeptide chains, as well as hydrogen bonds (see Table 2).
Table 1.
Technological characteristics of whole-wheat flour made from germinated wheat.
| Indicator | Whole-wheat flour made from non-germinated wheat grain GNG |
Whole-wheat flour made from germinated wheat WWFcontr |
Whole-wheat flour made from germinated wheat exposed to US WWFUS |
|---|---|---|---|
| Modes of exposure: | – | Soaking –6 h Germinating – 16 h |
US: 227 W/L 3 min Soaking –6 h Germinating – 12 h |
| Crude protein content (% d.b.) | 11.6 ± 0.3 | 13.8 ± 0.3 | 13.9 ± 0.3 |
| Lipids content (% d.b.) | 1.4 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 |
| Starch content, % | 60.3 ± 0.6 | 42.9 ± 0.6 | 41.6 ± 0.5 |
| Moisture (% w.b.) | 13.2 ± 0.4 | 13.9 ± 0.4 | 13.6 ± 0.4 |
| Ash (% d.b.) | 2.1 ± 0.2 | 2.0 ± 0.2 | 2.1 ± 0.2 |
| Wet gluten content (% w.b.) | 20.3 ± 2.0 | 22.3 ± 2.0 | 22.6 ± 1.5 |
| Gluten index | 65.3 ± 0.2 | 73.8 ± 1.2 | 73.1 ± 1.2 |
Table 2.
Micrographs of neuroblastoma cells.
 |
In all whole-flour samples, the moisture content was 13.5 ± 0.8% with almost constant Ash (% d.b.). The protein content in WWFcontr and WWFus is higher than GNG by 18.9% and 19.8%, respectively. The values of the gluten index and the content of wet gluten in WWFcontr and WWFus flour was significantly different (p ≤ 0.05) from GNG.
On one hand, changes in the gluten index can be due to the relaxing effect of proteolytic enzymes that get activated during germination. On the other hand, they can occur due to the formation of phenolic compounds capable of binding sulfides available for the formation of disulfide bridges. When the structure of gluten is formed, protein oxidation and gluten strengthening can also occur [39], [40]. This process compensates for the effects of proteolytic enzymes.
US intensification of the germinating process reduces the amount of starch by 18.7%. Such changes can have a negative effect on the strength of the porous structure of the crumb after baking. SEM micrographs of the whole-wheat flour dough (Fig. 8) prove a change in the structure. Therefore, dosage adjustment of the component is necessary.
Fig. 8.
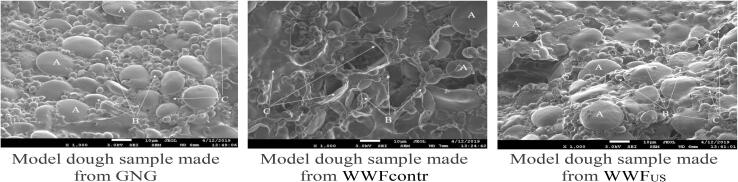
Scanning electron micrographs of whole-wheat flour dough: A – large starch grains, B – starch grains of small and medium size, C – protein matrix, magnification 1000×.
In the GNG dough we can visualize starch grains of round or elliptic form of small and medium size (B), which are fixed in a moderately developed protein matrix (C). In model WWFcontr and WWFus dough samples, protein granules and large starch grains (A) represent a single, excessively liquefied amorphous structure.
According to the data obtained, we believe that the usage of WWFus obtained in the production of bakery products should be balanced. These structural changes during dosed application will make it possible to obtain a more elastic and extensible dough, which will increase the porosity and specific volume of final products.
4.5. Synthesis of GABA in whole-wheat flour from germinated wheat
An important substance that is accumulated during germinating is GABA, a protein free amino acid, which is mainly formed as a result of γ-decarboxylation of 1-glutamic acid, which is catalyzed by the enzyme glutamate decarboxylase [41]. The effect of GABA synthesis activation in germinated wheat has been found and described [42], [43].
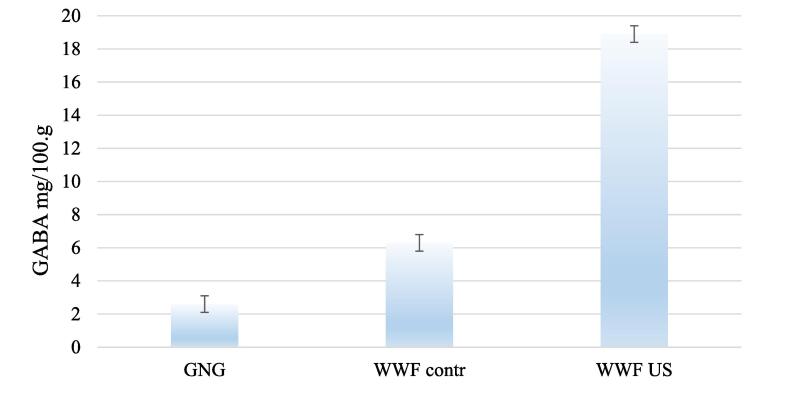
An HPLC analysis showed (Fig. 9) that each wheat grain sample contained GABA (Fig. 7), but the GABA content in WWFus was significantly higher (18.9 mg/100 g) than in WWFcontr (6.3 mg/100 g) and in non-germinated grain (2.6 mg/100 g).
Fig. 9.
GABA changes in wheat grain germination. *Abbreviations: non-germinated grain (GNG), control wheat grain (WWFcontr); wheat grain exposed to US (WWF US).
Junzhou Ding, Gary G. Hou, Boris V. Nemzer and other authors [32] found that the use of ultrasound can activate GABA formation during germination of cereal crops. Since the stress provoked by ultrasound activates endogenous enzymes (in particular, glutamate decarboxylase), which leads to sharp increase in GABA in germinated grains.
Baranzelli J., Kringel D.H., Colussi R. et al. established that increase in GABA during wheat germination under abiotic stress produced by ultrasound is explained by the increased activity of glutamate decarboxylase, which decarboxylates L-glutamate, activates glutamate decarboxylase enzyme and releases GABA [9]. Junzhou Ding and his colleagues [21] name this process a short pathway of GABA synthesis or a GABA stunt.
According to the results of their studies, Junzhou Ding, Gary G.Houb, Shanbai Xiong and their colleagues [32] determined that ultrasound treatment increased the content of succinic acid with simultaneous increase in metabolites associated with GABA shunt, such as glutamic acid, alanine and tricarboxylic acid, in the grain after ultrasound treatment. This suggests that increased polyamine degradation and inhibition of glutamate transamination may contribute to the accumulation of GABA in the grain during ultrasonic treatment. Thus, ultrasound exposure (as a stress) increases the energy metabolism of wheat grain germination. It leads to increased GABA accumulation by enhancing the activity of glutamate decarboxylase and providing more L-glutamate [44]. Whereas traditional methods of germination do not allow activating the above mentioned processes and obtaining high values of GABA in the final product [42].The obtained results showed a potential for the production of bakery products enriched with GABA and other functional components.
4.6. AOA of whole-wheat flour
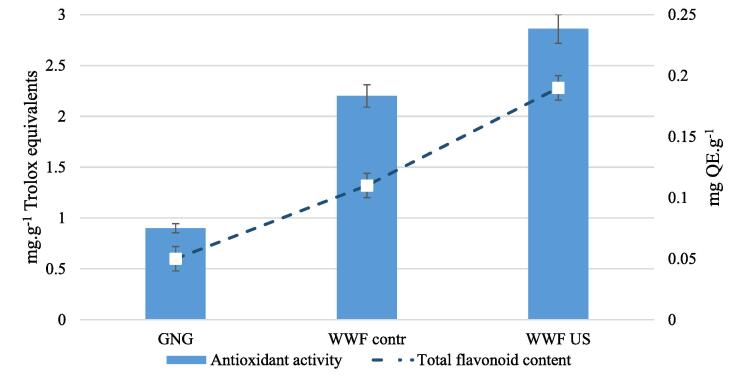
Germinating leads to the activation of enzymatic processes. These processes are aimed at the release of phytochemicals from the embryo, endosperm and shell parts, including flavonoids which determine antioxidant activity. The US intensification of the germination process affected (Fig. 10) the value of the total antioxidant activity, growth for WWFus was 73%, the number of flavonoids increased by 160%. A similar dynamics was noted during the germination of Canadian wheat [4].
Fig. 10.
Change of antioxidant activity and number of flavonoids in the process of wheat grain germination. *Abbreviations: non-germinated grain (GNG), control wheat grain (WWFcontr); wheat grain exposed to US (WWFUS).
Researchers of the germination process of Romaine lettuce, red rice [23] and other cereal crops established that ultrasonic exposure can act as an abiotic stress factor for plants. The studies describe that ultrasonic exposure triggers the protective response of the plant organism and stimulates the production of secondary metabolites such as phenolic compounds, thus enhancing the antioxidant activity of wheat grain during germination.
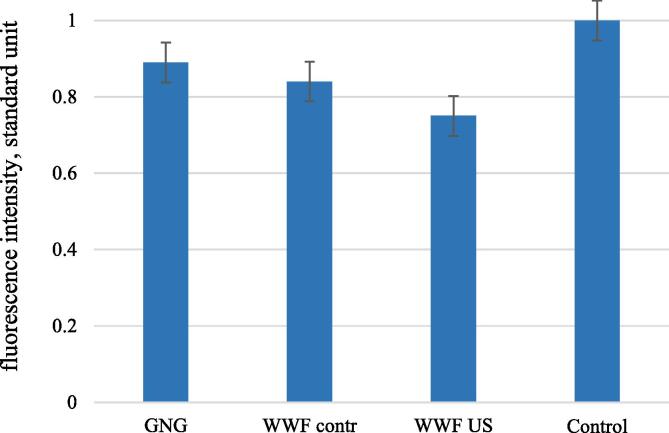
The utility assessment of the components of whole-wheat flour from germinated wheat grains for consumers was performed on the cell model of an undifferentiated human neuroblastoma SH-SY5Y. The results of measuring the dye fluorescence intensity proportional to the amount of AOF are shown in Fig. 11. Fluorescence intensity with PMA was taken as a standard unit.
Fig. 11.
Intensity of AOF generation: three hours later (the control sample with PMA is taken as a standard unit, all the rest values are given in reference to the control sample).
The data given in Fig. 11 show that the WWFus sample is the most active in blocking (AOF) 0.75 with respect to the PMA control), which is generally expected and consistent with previous results of our AOA studies (DPPH). In the WWFcontr sample antioxidant activity was lower (fluorescence intensity 0.84 with respect to the PMA control).
Micrographs visualizing changes in the fluorescence of cell cultures of undifferentiated neuroblastoma during the study of inhibition of AOF flour samples demonstrate some degree of antioxidant activity.
According to the data obtained, the fluorescence intensity at the end of the study was minimal for the WWFus sample, which corresponds to the data in Fig. 11.
Thus, studies of antioxidant activity using a neuroblastoma cell model show an increase in the antioxidant properties of wheat during germinating. The growth of the antioxidant activity of the germinated grain samples in relation to non-germinated was 5.6% and 15.7% for WWFus and WWFcontr, respectively. The AOA gets minimal values in traditional germination methods (WWFcontr) [45] due to the lack of an abiotic stress factor and the absence of an organism response.
5. Conclusions
The ultrasonic treatment can be one of the effective factors stimulating the intensity of grain germination in the production of healthy products. If we take into account the proposed modes of a controlled germinating process exposed to ultrasound, it will be possible to provide an effective and fast way to increase GABA and AOA in grain-based products. We plan to continue the research in order to study the synthesis of GABA and AOA increase in WWFus during baking processes, as well as to assess the utility of WWFus in final products for increasing their nutrient value.
CRediT authorship contribution statement
N. Naumenko: Methodology, Investigation, Writing (original draft). I. Potoroko: Conceptualization. I. Kalinina: Investigation, Writing (review & editing).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: N. Naumenko reports a relationship with South Ural State University National Research University that includes: employment. I. Potoroko reports a relationship with South Ural State University National Research University that includes: employment. I. Kalinina reports a relationship with South Ural State University National Research University that includes: employment.
Acknowledgements
This article is supported by a grant from the Russian Science Foundation (RSF) under Project 22-26-00079.
References
- 1.AACC International . 11th ed. AACC International; St. Paul, MN, USA: 2010. 2010 AACC International Approved methods of analysis. [Google Scholar]
- 2.Tian B., Xie B., Shi J., Wu J., Cai Y., Xu T., Xue S., Deng Q. Physicochemical changes of oat seeds during germination. Food Chem. 2010;119(3):1195–1200. doi: 10.1016/j.foodchem.2009.08.035. [DOI] [Google Scholar]
- 3.Ding J., Yang T., Feng H., Dong M., Slavin M., Xiong S., Zhao S. Enhancing contents of γ-aminobutyric acid (GABA) and other micronutrients in dehulled rice during germination under normoxic and hypoxic conditions. J. Agric. Food. Chem. 2016;64(5):1094–1102. doi: 10.1021/acs.jafc.5b0485910.1021/acs.jafc.5b04859.s001. [DOI] [PubMed] [Google Scholar]
- 4.P.V. Hung, D.W. Hatcher, W. Barker, Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities, Food. Chem. 126 (4) (2011), 1896–1901. https://doi.org/10.1016/j.foodchem.2010.12.015. [DOI] [PubMed]
- 5.Wu Z., Song L., D Huang Food grade fungal stress on germinating peanut seeds induced phytoalexins and enhanced polyphenolic antioxidants. J. Agric. Food. Chem. 2011;59:5993–6003. doi: 10.1021/jf200776w. [DOI] [PubMed] [Google Scholar]
- 6.Akram N.A., Nudrat A.A., Fahad S., et al. Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance Front. Plant Sci. 2017:8. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.J. Ohm, C.W. Lee, K. Cho, Germinated wheat: phytochemical composition and mixing characteristics, Cereal Chem. 93 (6) (2016), 612–617. https://doi.org/10.1094/CCHEM-01-16-0006-R.
- 8.K. Inoue, T. Shirai, H. Ochiai, M. Kasao, K. Hayakawa, M. Kimura, H. Sansawa Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives, Eur. J. Clin. Nutr., 57 (2003), 490–495. https://doi.org/ 10.1038/sj.ejcn.1601555. [DOI] [PubMed]
- 9.Abdou Adham M., Higashiguchi S., Horie K., Kim Mujo, Hatta H., Yokogoshi H. Relaxation and immunity enhancement effects of γ-aminobutyric acid (GABA) administration in humans. BioFactors. 2006;26(3):201–208. doi: 10.1002/biof.v26:310.1002/biof.5520260305. [DOI] [PubMed] [Google Scholar]
- 10.L.M. Palmer, J.M. Schulz, S.C. Murphy, D. Ledergerber, M. Murayama, M.E. Larkum The cellular basis of GABAB-mediated interhemispheric inhibition, Science (80), 335 (2012), 989-993. https://doi.org/10.1126/science.1217276. [DOI] [PubMed]
- 11.Chalermchaiwat P., Jangchud K., Jangchud A., Charunuch C., Prinyawiwatkul W. Antioxidant activity, free gamma-aminobutyric acid content, selected physical properties and consumer acceptance of germinated brown rice extrudates as affected by extrusion process. Lebensmittel-Wissenschaft Technol. Food Sci. Technol. 2015;64:490–496. doi: 10.1016/j.lwt.2015.04.066. [DOI] [Google Scholar]
- 12.Cornejo F., Cáceres P.J., Villaluenga C.M., Rosell C.M., Frias J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015;173:298–304. doi: 10.1016/j.foodchem.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Chandrapala J., Leong T. ultrasonic processing for dairy applications: recent advances. Food Eng. Rev. 2015;7(2):143–158. doi: 10.1007/s12393-014-9105-8. [DOI] [Google Scholar]
- 14.Ashokkumar M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015;25:17–23. doi: 10.1016/j.ultsonch.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 15.M. Boukroufa, C. Boutekedjiret, F. Chemat, Development of a green procedure of citrus fruits waste processing to recover carotenoids, Resource-Efficient Technol. 3 (3) (2017) 252–262. https://doi.org/10.1016/j.reffit.2017.08.007.
- 16.Yang H., Gao J., Yang A., Chen H. The ultrasound-treated soybean seeds improve edibility and nutritional quality of soybean sprouts. Food Res. Int. 2015;77:704–710. doi: 10.1016/j.foodres.2015.01.011. [DOI] [Google Scholar]
- 17.F. Hübner, T. O’Neil, K.D. Cashman, E.K. Arendt, The influence of germination conditions on beta-glucan, dietary fibre and phytate during the germination of oats and barley, Eur. Food Res. Technol., 231 (1) (2010), 27-35. https://doi.org/10.1007/s00217-010-1247-1.
- 18.H.J. Chung, S.H. Jang, H.Y. Cho, S.T. Lim, Effects of steeping and anaerobic treatment on GABA (γ-aminobutyric acid) content in germinated waxy hull-less barley, LWT - Food Sci. Technol. 42 (10) (2009), 1712–1716. https://doi.org/10.1016/j.lwt.2009.04.007.
- 19.S. Shafqat, Effect of different sprouting conditions on Alpha smylase activity, functional properties of wheat flour and on shelf-Life of bread supplemented with sprouted wheat University of Guelph, Guelph, Ontario (2013).
- 20.E. Komyshev, M. Genaev, D. Afonnikov, Evaluation of the SeedCounter, A Mobile Application for Grain Phenotyping, Front Plant Sci. 2016; 7: 1990. Published online 2017 Jan 4. doi: 10.3389/fpls.2016.01990. https://doi.org/10.3389/fpls.2016.01990. [DOI] [PMC free article] [PubMed]
- 21.Wang N., Hou G.G., Kweon M., Lee B. Effects of particle size on the properties of whole-grain soft wheat flour and its cracker baking performance. J. Cereal Sci. 2016;69:187–193. doi: 10.1016/j.jcs.2016.03.010. [DOI] [Google Scholar]
- 22.Khmelev V.N., Popov O.V. Altai State Technical University; 1997. Multifunctional Ultrasonic Devices and Their Application in Small Industries, Agriculture and at Home: A Monograph Barnaul Univ. (in Russian) [Google Scholar]
- 23.J. Ding, G.G. Hou, B.V. Nemzer, S. Xiong, A. Dubat, H. Feng, Effects of controlled germination on selected physicochemical and functional properties of whole-wheat flour and enhanced γ-aminobutyric acid accumulation by Ultrasonication, Food Chem. 243 (2018), 214–221. https://doi.org/10.1016/j.foodchem.2017.09.128. [DOI] [PubMed]
- 24.Rossetti V., Lombard A. Determination of glutamate decarboxylase by high performance liquid chromatography. J. Chromatogr. B. 1996;681:63–67. doi: 10.1016/0378-4347(96)88202-8. [DOI] [PubMed] [Google Scholar]
- 25.Q.Y. Bai, M.Q. Chai, Z.X. Gu, X.H. Cao, Y. Li, K.L. Liu, Effects of components in culture medium on glutamate decarboxylase activity and γ-aminobutyric acid accumulation in foxtail millet (Setaria italica L.) during germination, Food Chem., 116 (1) (2009), 152–157. https://doi.org/10.1016/j.foodchem.2009.02.022.
- 26.Shafii Z.A., Basri M., Malek E.A., Ismail M. Phytochemical and antioxidant properties of Manilkara zapota (L.) P Royen fruit extracts and its formulations for cosmceuetical application. Asian J. Plant Sci. Res. 2017;7(3):29–41. [Google Scholar]
- 27.Sánchéz-Moreno C., Larrauri A., Saura-Calixto F. A procedure to measure the antioxidant efficiency of polyphenols. J. Sci. Food Agric. 1998;76(2):270–276. doi: 10.1002/(sici)1097-0010(199802)76:2. [DOI] [Google Scholar]
- 28.D. Mares, K. Mrva, Late-maturity α-amylase: Low falling number in wheat in the absence of preharvest sprouting, J. Cereal Sci., 47 (1) (2008), 6-17. https://doi.org/10.1016/j.jcs.2007.01.005.
- 29.Khmelev V.N., Lebedev A.N., Khmelev M.V. Ultrasonic drying and pre sowing treatment of seeds, International Workshop and Tutorials on Electron Devices and Materials, EDM - Proceedings 7th Annual Inter-national Workshop and Tutorials on Electron Devices and Materials 2006, EDM. EDM - Proceedings» Novosibirsk; 2006. pp. 251–253. [Google Scholar]
- 30.H. Singh, N. Singh, L. Kaur, S.K. Saxena, Effect of sprouting conditions on functional and dynamic rheological properties of wheat, J. Food Eng. 47 (1) (2001), 23–29. https://doi.org/10.1016/S0260-8774(00)00094-7.
- 31.Krasulya O., Bogush V., Trishina V., et al. Impact of acoustic cavitation on food emulsions. Ultrasonics Sonochem. 2016;30:98–102. doi: 10.1016/j.ultsonch.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Junzhou Ding, Alexander V. Ulanov, Mengyi Dong, Tewu Yang, Boris V. Nemzer, Shanbai Xiong, Siming Zhao, Hao Feng Enhancement of gama-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication Ultrasonics Sonochemistry Volume 40, Part A, January 2018, pp. 791-797. [DOI] [PubMed]
- 33.H. Gąsiorowski Rye. - Chemistry and Technology, 83-09-01609-3, PWRiL Poznań (1993) (in Polish).
- 34.Ariyama T., Khan K. Effect of laboratory sprouting and storage on physicochemical and bread making properties of hard red spring wheat. Cereal Chem. 1990;67(1):53–58. [Google Scholar]
- 35.Warechowska M., Markowska A., Warechowski J., Miś A., Nawrocka A. Effect of tempering moisture of wheat on grinding energy, middlings and flour size distribution, and gluten and dough mixing properties. J. Cereal Sci. 2016;69:306–312. doi: 10.1016/j.jcs.2016.04.007. [DOI] [Google Scholar]
- 36.Beecher B.A., Bettge E., Smidansky E., Giroux M.J. Expression of wild type pin B sequence in transgenic wheat complements a hard phenotype. Theoret.Appl. Genet. 2002;105:870–877. doi: 10.1007/s00122-002-1034-x. [DOI] [PubMed] [Google Scholar]
- 37.Greffeuille V., Abecassis J., Barouh N., Villeneuve P., Mabille F., Bar C., L’Helgouac’h, et al. Analysis of the milling reduction of bread wheat farina: physical and biochemical characterization. J. Cereal Sci. 2006;45:97–105. doi: 10.1016/j.jcs.2006.07.003. [DOI] [Google Scholar]
- 38.R.C. Hoseney Principles of cereal science and technology AACC Inc., MN, USA (1994). https://doi.org/10.1016/j.jcs.2010.01.001.
- 39.Sullivan B., Dahle L.K., Schipke J.H. The oxidation of wheat flour. Cereal Chem. 1962;40:515–531. [Google Scholar]
- 40.Rosell C.M., Wang J., Aja S., Bean S., G Lookhart Wheat flour proteins as affected by transglutaminase and glucose oxidase. Cereal Chem. 2003;80:52–55. doi: 10.1094/CCHEM.2003.80.1.52. [DOI] [Google Scholar]
- 41.Park J.K., Jin S.H., Ko J.H., Baek N.I., Choi S.Y., Cho S.W., et al. Influence of ginsenosided on the kainic acid-induced seizure activity in immature rats. J. Biochem. Mol. Biol. 1999;32:339–344. [Google Scholar]
- 42.H Nagaoka Treatment of germinated wheat to increase levels of GABA and IP6 catalyzed by endogenous enzymes. Biotechnol. Prog. 2005;21:405–410. doi: 10.1021/bp0496777. [DOI] [PubMed] [Google Scholar]
- 43.Oh S.H., Choi W.G. Changes in the levels of gamma-aminobutyric acid and glutamate decarboxylase in developing soybean seedlings. J. Plant Res. 2001;114:309–313. doi: 10.1007/PL00013992. [DOI] [Google Scholar]
- 44.Lieve Lamberts, Iris J. Joye, Tim Beliën, Jan A. Delcour, Dynamics of γ-aminobutyric acid in wheat flour bread making, Food Chem. 130 (4) (2012) 896–901.
- 45.N. Rosa-Sibakov, K. Poutanen, V. Micard, How does wheat grain, bran and aleurone structure impact their nutritional and technological properties? Trends Food Sci. Technol., 41 (2) (2015), pp. 118-134, 10.1016/j.tifs.2014.10.003.