Abstract
Background
Tissue adhesives have been used for many years to close simple lacerations as an alternative to standard wound closure (sutures, staples, adhesive strips). Potential advantages over standard wound closure include ease of use, decrease in pain, time to apply and not requiring a follow‐up visit for removal. Whilst studies have compared tissue adhesives with standard wound closure to determine the cosmetic outcome and other secondary outcomes no systematic review was previously available, so that no generalizable, definitive answers about the effectiveness of tissue adhesives existed.
Objectives
To summarize the best available evidence for the effects of tissue adhesives on the healing of traumatic lacerations in children and adults.
Search methods
We searched the Cochrane Wounds Group Specialised Trials Register (October 2007), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 3, 2007), MEDLINE (1950 to October Week 1 2007), EMBASE (1980 to 2007 Week 41), CINAHL (1982 to October Week 2 2007), Web of Science ‐ Science Citation Index (1975 to April 29, 2007), seven clinical trial registries, and reference lists of articles. We also contacted manufacturers and researchers in the field.
Selection criteria
Randomised controlled trials comparing tissue adhesives with standard wound closure or one tissue adhesive compared with another tissue adhesive for acute, linear, low tension, traumatic lacerations in an emergency or primary care setting.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. Study authors were contacted for additional information. Information on adverse effects was collected from the trials. Outcomes of cosmesis (subgroups of age, wound location and need for deep sutures), pain, procedure time, ease of use, and complications were analysed separately for two comparisons: 1) tissue adhesive compared with standard wound closure; and 2) tissue adhesive compared with another tissue adhesive.
Main results
Thirteen studies were included in this review. Eleven studies compared a tissue adhesive with standard wound closure. No significant difference was found for cosmesis at any time point examined, using either Cosmetic Visual Analogue Scale (CVAS) or Wound Evaluation Score (WES). Pain scores (Parent VAS weighted mean difference (WMD) ‐13.4 mm; 95% CI ‐20.0 to ‐6.9) and procedure time (WMD ‐4.7 minutes; 95% CI ‐7.2 to ‐2.1) significantly favoured tissue adhesives. Only one study reported on ease of use, favouring standard wound closure. Small but statistically significant risk differences were found for dehiscence (favouring standard wound care, Number Needed to Harm (NNH) 40; 95% CI 20 to 1168) and erythema (favouring tissue adhesive, NNH 10; 95% CI 5 to 239). Other complications were not significantly different between treatment groups.
Two studies compared tissue adhesives. One study compared two different tissue adhesives, butylcyanoacrylate with octylcyanoacrylate, for pediatric facial lacerations and found no significant difference for cosmesis, pain, procedure time, or complications. Another study compared two different formulations (viscosities) of octylcyanoacrylate to assess the incidence of product migration as a proxy for complications of application; the high‐viscosity product migrated on significantly fewer participants.
Authors' conclusions
Tissue adhesives are an acceptable alternative to standard wound closure for repairing simple traumatic lacerations. They offer the benefit of decreased procedure time and less pain, when compared to standard wound closure. A small but statistically significant increased rate of dehiscence with tissue adhesives is observed.
Plain language summary
Tissue adhesives for traumatic lacerations in children and adults
Cuts (lacerations) often need to be closed to ensure proper healing, and prevent infection or unattractive scarring. Wounds may be closed with stitches (sutures), staples, tapes or glue (tissue adhesive). The review found that glue is an excellent substitute for stitches, staples or tapes to close simple cuts. Glue causes less pain, is quicker and needs no follow up for removal. A slightly higher number of cuts may break open (dehisce) after being glued, compared to cuts closed with stitches, staples or tapes. Though there are a few different types of glue available, no one glue seems to be superior.
Background
Traumatic wounds, including lacerations, are one of the most common reasons for people presenting to the Emergency Department (ED) (Sibert 1981). The management of these lacerations involves cleaning the wound and then re‐approximating the wound edges until natural healing occurs. Without proper closure, the patient is at increased risk of infection and excessive scar formation, which results in a poor cosmetic outcome (Hollander 1999).
Traditionally, approximation of these lacerations has been accomplished using sutures, which either dissolve after a number of days, or require another visit for removal. Insertion of sutures requires a local anaesthetic agent to reduce the pain associated with the procedure. Infiltration of the wound with a local anaesthetic can be quite painful. Small children with lacerations pose further challenges, as they may require sedation to reduce pain, emotional distress and movement during the procedure. This adds time and complexity to the patient's ED visit (Osmond 1999a; Osmond 2000). Other standard wound closure (SWC) options include staples and adhesive strips.
For many years, tissue adhesive compounds (glue) made from cyanoacrylates have been available to close simple lacerations. These compounds are supplied as liquid monomers that quickly form a strong bond over the approximated wound. This keeps the edges together until healing has occurred. Practitioners experienced in suturing find them quick and easy to use following a brief orientation to the product and its limitations. They are relatively painless for the patient and provide an excellent cosmetic outcome (Osmond 1999a; Osmond 2000; Sells 1999). No follow‐up appointment for suture removal is required and one study has shown them to be a cost‐effective alternative to sutures (Osmond 1995).
There is an increasing amount of literature supporting the use of tissue adhesives for various minor lacerations (Applebaum 1993; Barnett 1998; Bruns 1998; Elmasalme 1995; Goktas 2002; Holger 2004; Mattick 2002a; Mizrahi 1988; Morton 1988; Osmond 1999a; Perron 2000; Quinn 1993; Quinn 1998a; Saxena 1999; Schultz 1979; Simon 1997; Singer 1998; Singer 2003; Watson 1989; Zempsky 2001). Initially, the evidence was in the form of successful case series that lacked controls or blinded outcome assessments. A number of randomised controlled trials have since been reported which advocate tissue adhesives in their respective study populations. However, the evidence has not been summarised and interpreted in a systematic and rigorous review.
Objectives
The objective of this review was to summarize the best available evidence for the effects of tissue adhesives on the healing of traumatic lacerations in children and adults.
Specific Aims
To compare the relative effectiveness of:
tissue adhesives compared with standard wound closure (i.e., sutures, staples or adhesive strips); and
comparison of different tissue adhesives for repair of lacerations in subgroups of patients of differing age (pediatric versus adult), location of the laceration (face versus body) and type of laceration (requiring versus not requiring deep sutures).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing tissue adhesives with standard wound closure (i.e., sutures, staples, adhesive strips) or one tissue adhesive compared with another tissue adhesive.
Types of participants
We considered studies that recruited people of any age in an ED, outpatient clinic, walk‐in clinic or other primary care setting. The wounds had to be acute, linear lacerations, less than 12 hours old, resulting from blunt or sharp trauma, excluding stellate lacerations, puncture wounds, and mammalian bites. Wounds of any length, width and depth were included, provided that the edges could be approximated with minimal tension after deep sutures were placed, if required. Also excluded were studies of wounds which were infected, heavily contaminated or devitalized, those crossing joints or mucocutaneous junctions, those in hair‐bearing areas, and those in people with keloid formation or chronic illness which could impair healing (e.g., peripheral vascular disease, diabetes).
We did not consider any trials that evaluated tissue adhesives in the operating room for closure of surgical incisions (see Coulthard 2004 for data on surgical incisions), or trials dealing with other types of wounds (e.g., ulcers, skin grafts, dental repair).
Types of interventions
We considered studies in which participants were randomised to closure of their laceration by one of two or more methods, one of which was a tissue adhesive. The comparison arms were either another form of skin closure (i.e., sutures, staples, adhesive strips) or a second tissue adhesive.
Types of outcome measures
Primary outcomes
We considered all patient outcomes reported by the included studies. The primary measure was cosmetic outcome, as determined by one or more blinded evaluators, using one or more validated cosmetic scores, such as the Cosmetic Visual Analogue Scale or Wound Evaluation Score.
Secondary outcomes
Other outcomes that we included when available were: patient pain during the procedure; time needed to complete the procedure; the ease of the procedure; and the occurrence of any complications (e.g., infection, wound dehiscence, erythema, or tissue adhesive migration).
Search methods for identification of studies
Electronic searches
For this update we searched the databases: Cochrane Wounds Group Specialised Register (October 2007); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 3, 2007); Ovid MEDLINE (1950 to October Week 1 2007); Ovid EMBASE (1980 to 2007 Week 41); Ovid CINAHL (1982 to October Week 2 2007); Web of Science ‐ Science Citation Index (1975 to April 29, 2007)
The following search strategy was used in The Cochrane Central Register of Controlled Trials (Online Version): #1 MeSH descriptor Lacerations explode all trees #2 lacerat* #3 traumatic NEXT (wound* or injur*) #4 (#1 OR #2 OR #3) #5 MeSH descriptor Tissue Adhesives explode all trees #6 MeSH descriptor Acrylates explode all trees #7 tissue NEXT adhesive* #8 acrylate* or bucrylate* or cyanoacrylate* or enbucrilate* #9 glu or glue or glues #10 (#5 OR #6 OR #7 OR #8 OR #9) #11 (#4 AND #10) For the Ovid MEDLINE search strategy see Appendix 1; for Ovid EMBASE see Appendix 2; for Ovid CINAHL see Appendix 3 and for Web of Science see Appendix 4. Ovid MEDLINE was searched using the following strategy in combination with the Cochrane highly sensitive search strategy for identifying reports of randomised controlled trials (Higgins 2005). The Ovid EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN). We did not restrict the search by language or publication status.
The following clinical trials registers were searched in June 2007:
ClinicalTrials.gov (US).
CenterWatch Clinical Trials Listing Service (US).
Current Controlled Trials (International).
National Research Register (UK).
Trials Central (International).
WHO International Clinical Trials Registry Platform (International).
CRISP (Computer Retrieval of Information on Scientific Projects) Database (US)
Searching other resources
Citation list searches of all selected articles were undertaken. In addition we contacted the primary author of all selected articles and the manufacturers of tissue adhesive products (B. Braun Melsungen AG, Closure Medical Corp, GluStitch Inc, Loctite Inc, Sherwood‐Davis & Geck).
Data collection and analysis
Selection of studies
Two authors independently examined the title and abstracts of trials generated by the search to identify potentially relevant trials (KF, KR). With the full text of these articles, two authors independently assessed each study for inclusion using a standardized form with eligibility criteria (KF, KR). We resolved disagreements by consensus or by a third author when necessary.
Data extraction and management
One author (KF) extracted data and a second author (KR) checked for accuracy. We used a standard data form to capture the following information:
characteristics of the study (design, method of randomisation, withdrawals/dropouts, funding source);
study participants (age, wound location, wound characteristics);
intervention (type of tissue adhesive, wound preparation, wound dressing);
comparison intervention (suture or other tissue adhesive);
outcome measures (type of scoring, timing of assessment, complications); and
results.
If two unique publications reported data on the same study, we extracted all data. If discrepancies were detected, clarification was sought from the author, or we included the more conservative results. We requested additional unpublished data from primary authors and included if available.
Assessment of risk of bias in included studies
Two authors independently assessed the quality of each selected study for quality using two established methods. First, each study was evaluated using the previously validated Jadad 5‐point scale to assess randomisation, blinding, and withdrawals/dropouts (Jadad 1996). Next, concealment of allocation was assessed as adequate, inadequate or unclear using the methodology described by Schulz 1995. Any discrepancies were resolved through discussion.
Data synthesis
Medians were substituted for means where means were not reported (Bruns 1998; Mattick 2002a; Osmond 1999a; Simon 1997). Where there were two observers rating cosmetic wound appearances (CVAS), their summary measures were pooled (Bruns 1998; Holger 2004; Simon 1997; Zempsky 2001). Where standard deviations or any variations thereof (e.g., standard error, variance) were not reported, P values from t‐tests were inverted to obtain standard deviations (Quinn 1993; Quinn 1998a). If not stated, p‐values were assumed to be two‐sided. Upper bound p‐values were also inverted giving a conservative estimate in some cases (Quinn 1993; Quinn 1998a). Non‐parametric confidence intervals (CI) were used to obtain standard deviations where not reported (Bruns 1998). Therein a robustness to the 'normality' assumption was assumed. In cases where only p‐values from non‐parametric tests were reported, an average of the standard deviations from other trials with that particular outcome was substituted (Mattick 2002a; Simon 1997).
Two comparisons were established a priori for separate analyses: (1) tissue adhesive compared with standard wound closure (SWC); and (2) tissue adhesive compared with tissue adhesive. Continuous data (i.e., CVAS, pain scores, time to complete, ease of the procedure) were converted to a mean difference and an overall weighted mean difference (WMD) with 95% confidence intervals (95% CI) was calculated. The Wound Evaluation Score (WES) was reported as a dichotomous outcome in each study (i.e., optimal or suboptimal cosmesis), and thus we expressed it as a relative risk (RR) and a pooled relative risk with 95% CI. Complications (i.e., dehiscence, infection, erythema, delayed closure, discharge, tissue adhesive migration) were presented as risk differences (RD); since some studies had zero events in both treatment groups a relative risk could not be calculated. The number needed to harm (NNH) was also calculated for further clarification where the results were significant. The baseline SWC risks were combined using the same weights used in the corresponding meta‐analysis.
Almost all pain VAS and time to complete the procedure data were skewed with a right tail (Altman 1996), however, almost all treatment groups were sufficiently large to meet the requirements of the central limit theorem. Thus, the distribution of the means, not the singular data points, were approximately normal and we could use the WMD to summarize this data.
Results for tissue adhesives compared with standard wound closure were calculated using random effects models (DerSimonian 1986), since two tissue adhesives were used (e.g., butylcyanoacrylate (Histoacryl TM), octylcyanoacrylate (Dermabond TM)) and treatments varied for standard wound closures (e.g., suturing, staples, adhesive strips). Statistical heterogeneity was quantified using the I2 statistic (Higgins 2003). The I2 statistic indicates the per cent variability due to between study variability as opposed to within study variability. An I2 greater than 50% may be considered large. Possible sources of heterogeneity were explored by subgroup and sensitivity analyses using the primary outcomes at the most popular time point (i.e., CVAS at one to three months and WES at one to three months). Subgroup analyses examined the effect of different age groups (pediatric, less than 18 years versus adult, greater than or equal to 18 years), wound location (face versus body), type of tissue adhesive, and methodological quality criteria (components of the Jadad score, funding source) using a subgroup chi squared test (Deeks 2001). Subgroups of extent of laceration (lacerations requiring deep sutures versus not) and allocation concealment could not be analysed due to insufficient data. Sensitivity analyses assessed for the choice of statistical model (i.e., fixed‐effect versus random‐effects model).
Results for the tissue adhesive compared with another tissue adhesive comparisons were calculated using fixed‐effect models, since there was only one study included for each unique pair of tissue adhesives compared.
Publication bias was tested visually using the funnel plot and quantitatively using weighted regression (Egger 1997).
Results
Description of studies
Using the various search strategies, we identified more than 850 unique articles. From these, 45 were selected as potentially relevant, including one abstract (Symeonoglou 1998) only identified through the Cochrane Wounds Group trials register, and one trial (Stuart 1999) only identified through the clinical trials registers. No additional articles were identified by citation searches, or through correspondence with authors and tissue adhesive manufacturers.
Two authors (KF and KR) assessed the abstracts, and full text when available, of the 45 potential articles for relevance using a standard inclusion/exclusion form. Unanimous agreement was attained on all relevance decisions. Fifteen unique studies were selected, represented by 25 of the 45 articles. Six of the unique studies were represented by more than one identified publication, due to the presence of abstracts, separate reporting of early and late cosmetic outcomes, or analysis of the results sub grouped by other variables. One study (Zempsky 2001) was initially represented by the interim results published in abstract format only; the completed results have now been published and these data have been updated.
Three studies, despite meeting inclusion for relevance, have been subsequently excluded. The first study (Singer 2002), a multi‐centre trial that included both traumatic lacerations and operative wounds, reported combined results for both populations. Four of the 10 sites within the multi‐centre trial dealt with lacerations. Two of these sites have independently reported their results and are included in our review (Bruns 1998; Singer 1998). Multiple attempts to obtain the unpublished data from the remaining two sites have been unsuccessful. The second excluded study (Symeonoglou 1998) was an abstract of a RCT evaluating sutures and two tissue adhesives for closure of facial lacerations. No results were presented in the abstract, no subsequent publication of results has been found, and multiple attempts to contact the authors have been unsuccessful. The last study (Stuart 1999) was identified through a clinical trials register. Attempts to contact the investigator for the status of the study have been unsuccessful and no published results have been found.
The remaining 20 excluded articles were comprised of four review articles (Brown 1997; Liebelt 1997; Mattick 2002b; Sportsmed 1997), five studies dealing with operative incisions or other wounds (Alamouti 1998; Bernard 2001; Greene 1999; Ludlow 2000; Qureshi 1997), one study dealing with high tension lacerations across joints (Saxena 1999), two studies that were not randomised (Charters 2000; Doraiswamy 2003), one observational study without a comparison arm (Giri 2004), one study that used tissue adhesives in a non‐traditional method for scalp lacerations (Hock 2002), and six studies that did not include tissue adhesives (Eaglstein 2002; Farris 1981; Fatovich 1995; Giovannacci 2002; Quinn 2003; Sutton 1985).
Thirteen studies met the inclusion criteria for this review. Eleven studies compared a tissue adhesive with standard wound closure, five with butylcyanoacrylate (Histoacryl TM)(Barnett 1998; Goktas 2002; Quinn 1993; Schultz 1979; Simon 1997) and six with octylcyanoacrylate (Dermabond TM) (Bruns 1998; Holger 2004; Mattick 2002a; Quinn 1998a; Singer 1998; Zempsky 2001). The standard wound closure method was sutures in seven studies (Barnett 1998; Goktas 2002; Holger 2004; Quinn 1993; Quinn 1998a; Schultz 1979; Simon 1997), adhesive strips in two studies (Zempsky 2001; Mattick 2002a), and a mixture of closure methods in the remaining two studies (Bruns 1998; Singer 1998), though the majority of participants received sutures. In one of the studies comparing tissue adhesive with sutures (Holger 2004), the tissue adhesive was actually compared with two types of suture (i.e., absorbable and non‐absorbable) in a three‐arm trial; additional data from the author allowed us to combine these two standard wound closure arms.
Seven of the 11 studies were limited to children (Barnett 1998; Bruns 1998; Holger 2004; Mattick 2002a; Quinn 1993; Simon 1997; Zempsky 2001), two were limited to adults (Goktas 2002; Quinn 1998a), and the remaining two studies included all ages (Schultz 1979; Singer 1998). Three of the pediatric studies (Holger 2004; Quinn 1993; Zempsky 2001) and one of the studies without age restriction (Schultz 1979) were limited to facial lacerations. Four studies (Barnett 1998; Mattick 2002a; Quinn 1993; Schultz 1979) excluded deep suture lacerations. The remaining studies did not stratify their results by extent of laceration.
Two studies compared two tissue adhesives. The first study (Osmond 1999a) compared butylcyanoacrylate and octylcyanoacrylate for the closure of pediatric facial lacerations not requiring deep sutures. The other study (Singer 2003) compared high‐viscosity octylcyanoacrylate and low‐viscosity (standard) octylcyanoacrylate for the closure of any laceration amenable to tissue adhesive closure in children and adults.
Two studies allowed participants with more than one laceration to be enrolled; each laceration was assessed independently. The first study (Quinn 1998a) randomised each laceration independently, while the other study (Bruns 1998) randomised the participant and all lacerations were assigned to that treatment arm. This non‐independence occurred in only two participants, each with two lacerations, and would not affect the results significantly. All other studies randomised and assessed one laceration per participant.
All studies were published in English, except Schultz 1979 (Danish). All studies were conducted in an ED setting.
Outcome measures
Cosmesis was the primary outcome reported by all selected studies except one (Singer 2003). Most researchers used the Cosmetic Visual Analogue Scale, the Wound Evaluation Score, or a combination of these two measures of cosmesis. The timing for cosmesis assessments varied somewhat between studies, but three distinct time periods became evident allowing grouping: (1) suture/staple removal and complication follow up at 5 to 14 days; (2) early healing at 1 to 3 months; and (3) late healing at 9 to 12 months. Early cosmesis scores (7 to 14 days) have been shown to poorly correlate with late scores (6 to 9 months) (Hollander 1995b).
The Cosmetic Visual Analogue Scale (CVAS) is a 100 mm line, with zero being "worst scar imaginable" and 100 being the "best scar imaginable". A blinded assessor, usually a plastic surgeon, rates the appearance of each laceration, either in person or from a standardized photograph, by placing a mark along the line. The CVAS was developed to assess laceration repairs in clinical trials. It has been shown to be a reliable and valid outcome measure of long‐term cosmesis with excellent intra‐ and inter‐rater agreement. A measure of 12 to 15 mm has been shown to be the minimum clinically important difference (MCID) between optimal and sub‐optimal scars using the WES scoring method (Quinn 1995; Quinn 1998a). A modified version of the CVAS was used in one study with the participant rating their satisfaction with their scar (Singer 1998). Since cosmesis ultimately deals with the patient's perception of how well the laceration has healed, these results were combined with other CVAS values.
The Wound Evaluation Score (WES) assesses six clinical variables of each scar: absence of step‐off, contour irregularities, wound margin separation greater than two millimetres, edge inversion, excessive distortion, and overall cosmetic appearance. Each variable is given a score of zero or one, with a total score of six considered "optimal" (Hollander 1995a). Results are reported as dichotomous data, the number of optimal scars in each group. Though determining this score requires that the evaluator be able to assess the scar in person, it offers the advantage of providing specific feedback of the imperfections that have resulted in a suboptimal scar. One study (Barnett 1998) used a non‐validated five‐point scale (i.e. 1 = poor, 5 = excellent) to assess cosmesis. Following communication with the author, we converted those participants with "excellent" scores to "optimal" to combine with other WES assessments. Another study (Quinn 1993) used both CVAS and a non‐validated three‐point categorical scale (i.e., unacceptable, acceptable, excellent) to assess cosmesis. We could not determine a justifiable way to combine the three‐point scale with WES, so these data were excluded. Finally, Schultz 1979 used a non‐validated rating of "acceptable" versus "unacceptable"; "acceptable" was translated to "optimal" to be combined with other categorical WES assessments.
The primary outcome in the remaining study (Singer 2003) was the incidence of migration of the tissue adhesive greater than 1 cm from the wound edges. This outcome was chosen as a proxy for complications during application (i.e., for wounds adjacent to the eyes where excessive migration could cause the eyelids to become stuck together).
We assessed secondary outcomes of pain associated with the procedure and ease of the procedure using visual analogue scales. The time to complete the procedure, when included in the results, was reported as a mean number of minutes for each study group.
The remaining secondary outcomes considered were complications: incidents of infection, dehiscence, induration or erythema, discharge, and the need for delayed closure.
Risk of bias in included studies
No studies were double‐blind due to the nature of the interventions (impossible to insert "placebo" sutures or staples), thus Jadad scores ranged from one to three. Four studies scored a three (two points on randomisation and one point for reporting withdrawals) (Goktas 2002; Holger 2004; Osmond 1999a; Quinn 1998a). Three studies scored a two (one point for randomisation and one point for withdrawals) (Bruns 1998; Quinn 1993; Schultz 1979). Two studies scored two points for randomisation (Mattick 2002a; Singer 2003). The remaining four studies scored a one (randomisation) (Barnett 1998; Simon 1997; Singer 1998; Zempsky 2001). Two studies (Osmond 1999a; Singer 2003) reported adequate allocation concealment.
The 'Characteristics of Included Studies' table provides further information on the sample size, population/wound type studied, intervention, outcome measures, funding source, and assessment of the methodological quality/allocation concealment for each of the studies included in this review.
Effects of interventions
Tissue adhesive compared with standard wound closure
Cosmetic scores
There were nine studies with 889 lacerations that compared tissue adhesives with standard wound closure using the CVAS outcome measure. Overall, there were no significant differences between tissue adhesives and standard wound closure for CVAS at any of the time points examined. Only one study measured CVAS between 5 to 14 days; the WMD showed no difference (0.0 mm; 95% CI ‐4.8 to 4.8; 52 lacerations (Analysis 1.1)). At 1 to 3 months, the pooled WMD was 1.6 mm (95% CI ‐3.2 to 6.4; seven studies with 549 lacerations (Analysis 1.2). The I2 statistic was large and indicated 57% heterogeneity due to between‐study heterogeneity. The CVAS pooled WMD at 9 to 12 months was 1.5 mm (95% CI ‐3.1 to 6.1; four studies with 237 lacerations; (Analysis 1.3)). Between‐study heterogeneity was negligible (I2 = 0%).
1.1. Analysis.
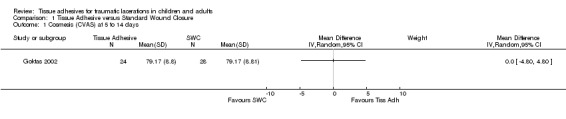
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 1 Cosmesis (CVAS) at 5 to 14 days.
1.2. Analysis.
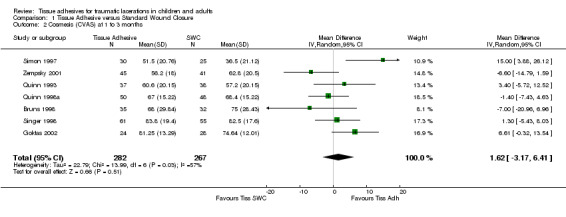
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 2 Cosmesis (CVAS) at 1 to 3 months.
1.3. Analysis.
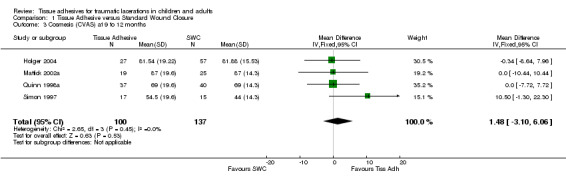
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 3 Cosmesis (CVAS) at 9 to 12 months.
Four studies (with 364 lacerations) used the WES; there were no significant differences between treatment groups at any of the time points examined. At 5 to 14 days, the pooled RR for an optimal wound was 0.97 (95% CI 0.87 to 1.09; two studies with 195 lacerations (Analysis 1.4)). The pooled RR for an optimal wound at 1 to 3 months was 0.99 (95% CI 0.89 to 1.11; four studies with 364 lacerations; (Analysis 1.5)). The pooled RR for an optimal wound at 9 to 12 months was 1.08 (95% CI 0.89 to 1.30; two studies with 140 lacerations; (Analysis 1.6)). Between‐study heterogeneity was absent at all these time points.
1.4. Analysis.
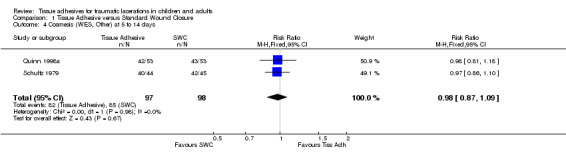
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 4 Cosmesis (WES, Other) at 5 to 14 days.
1.5. Analysis.
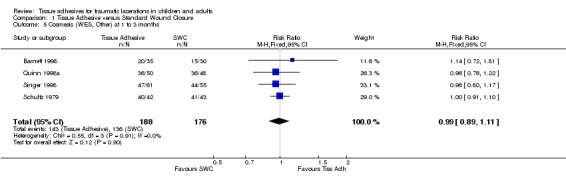
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 5 Cosmesis (WES, Other) at 1 to 3 months.
1.6. Analysis.
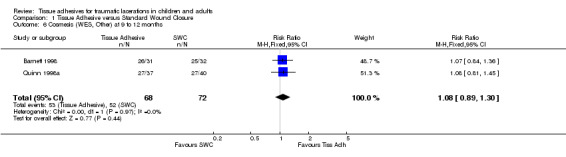
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 6 Cosmesis (WES, Other) at 9 to 12 months.
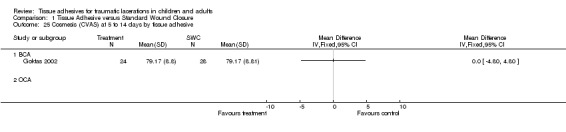
All subgroup analyses (i.e., age, location, need for deep sutures, tissue adhesive, Jadad score and its components, funding) except two gave insignificant findings (Analysis 01.07 through 01.30). For the subgroup by age using CVAS at 5 to 14 days, the one trial of adults found a statistically significant superiority of BCA over SWC (WMD 7.74, 95% CI 2.71 to 12.77; (Analysis 1.7)). For the subgroup by tissue adhesive using CVAS at 1 to 3 months, BCA was found to be superior to SWC (WMD 7.50, 95% CI 1.75 to 13.25; three studies with 182 lacerations; (Analysis 1.26)).
1.7. Analysis.
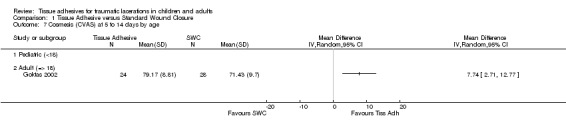
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 7 Cosmesis (CVAS) at 5 to 14 days by age.
1.26. Analysis.
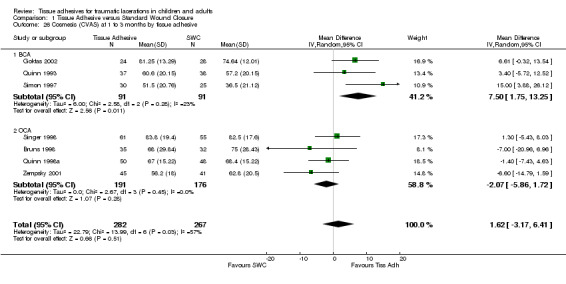
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 26 Cosmesis (CVAS) at 1 to 3 months by tissue adhesive.
The funnel plot for CVAS at one to three months did not indicate publication bias. The weighted regression bias coefficient was insignificant (P = 0.83). WES at one to three months had too few studies (N = 4) to assess publication bias.
Secondary outcomes
Pain
Six studies measured pain scores (VAS) with 570 lacerations. All VAS results were significant and favoured the tissue adhesive interventions. The pain outcome with the most studies, the parent‐reported VAS, had a pooled WMD of ‐13.4 mm (95% CI ‐20.0 to ‐6.9; five studies with 434 lacerations; (Analysis 1.31)). Between‐study heterogeneity was minimal (I2=15%). The remaining pain outcomes contained data from only one study each. The patient‐reported VAS WMD was ‐10.8 mm (95% CI ‐17.1 to ‐4.5). The physician‐reported VAS WMD was ‐12.6 mm (95% CI ‐20.1 to ‐5.1). The nurse‐reported VAS WMD was ‐14.9 (95% CI ‐22.5 to ‐7.3).
1.31. Analysis.
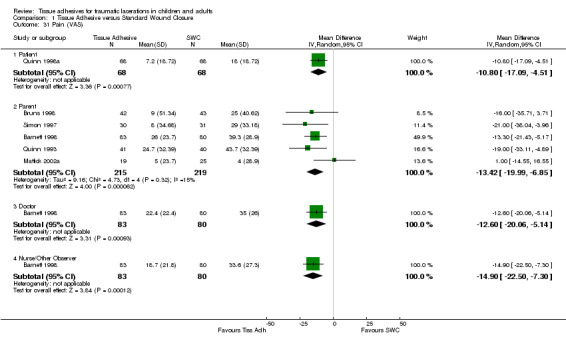
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 31 Pain (VAS).
Time to complete the procedure
Time to complete the procedure also favoured the tissue adhesive interventions (pooled WMD ‐4.7 min, 95% CI ‐7.2 to ‐2.1; six studies with 584 lacerations)(Analysis 1.32). Although between‐study heterogeneity was large (I2=84%), the six studies were consistent in that they all showed that tissue adhesives were much less time consuming. The large heterogeneity seems to be due to the inconsistent SDs of the various studies; the heterogeneity disappeared completely (I2=0%) if we considered a standardized mean difference, while the result was still statistically significant.
1.32. Analysis.
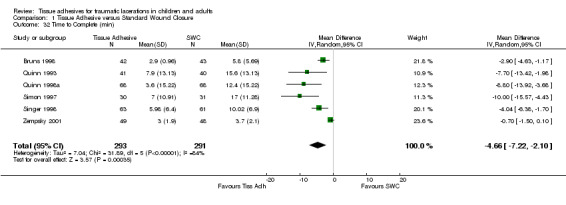
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 32 Time to Complete (min).
Ease of procedure
Only one study (Mattick 2002a) reported ease of the procedure for the physician. These data were quite skewed; they reported a p‐value of 0.07 from a Mann‐Whitney test and showed greater ease for the SWC procedure (medians five versus nine mm)(Analysis 1.33).
1.33. Analysis.
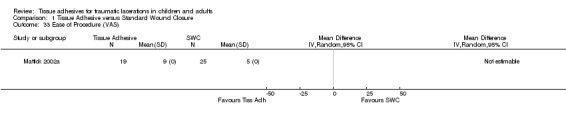
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 33 Ease of Procedure (VAS).
Complications
Nine studies recorded complications on 834 lacerations. All complications were insignificant using the random‐effects (RE) model, except for the incidence of dehiscence and erythema. Fewer cases of dehiscence occurred using standard wound care (Risk Difference (RD) 2.4%, 95% CI 0.1 to 4.9)(Analysis 1.34), while fewer incidences of erythema occurred when using tissue adhesives (RD ‐10%, 95% CI ‐19 to ‐0.4). The baseline risk of dehiscence with tissue adhesives was 3.7% and 40 (95% CI 20 to 1168) patients would need to be treated with SWC to prevent one case of dehiscence when using tissue adhesives. The baseline risk of erythema with SWC was 18.3% and 10 (95% CI 5 to 239) patients would need to be treated with tissue adhesives to prevent one incident of erythema when using SWC. Risk differences for infection, delayed closure and discharge were not significant. The baseline SWC risks were 1.1, 0, and 8.2% respectively.
1.34. Analysis.
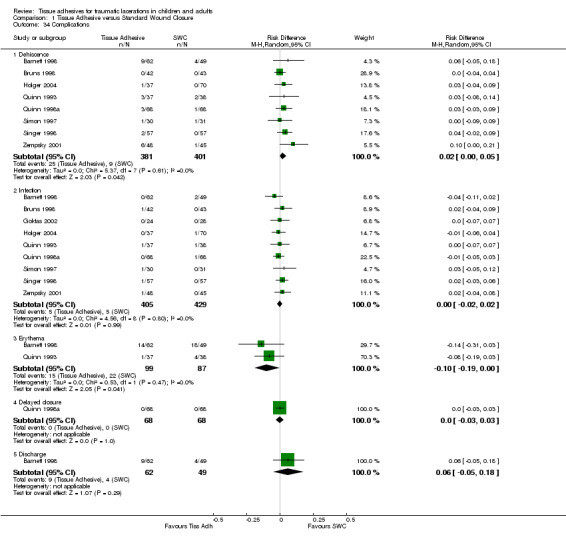
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 34 Complications.
Tissue adhesive compared with another tissue adhesive
Two studies compared tissue adhesives. No subgroup or sensitivity analyses were possible as they compared unique pairs of tissue adhesive and with different primary outcomes. As such, they are reported as unique comparisons.
Butylcyanoacrylate compared with octylcyanoacrylate
Osmond 1999a compared butylcyanoacrylate (Histoacryl TM) with octylcyanoacrylate (Dermabond TM) for 97 lacerations.
Cosmetic scores
There was no significant difference between butylcyanoacrylate and octylcyanoacrylate using CVAS at 1 to 3 months. The WMD was 2.5 mm (95% CI ‐3.6 to 8.6; 83 lacerations; (Analysis 2.1)). There was no significant difference in the WES at 5 to 14 days or at 1 to 3 months. The relative risks were 1.1 (95% CI, 0.9 to 1.3; (Analysis 2.2)) and 0.9 (95% CI, 0.7 to 1.2; (Analysis 2.3)), respectively.
2.1. Analysis.
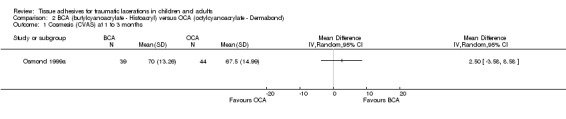
Comparison 2 BCA (butylcyanoacrylate ‐ Histoacryl) versus OCA (octylcyanoacrylate ‐ Dermabond), Outcome 1 Cosmesis (CVAS) at 1 to 3 months.
2.2. Analysis.
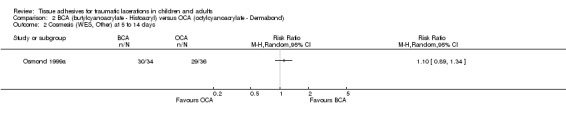
Comparison 2 BCA (butylcyanoacrylate ‐ Histoacryl) versus OCA (octylcyanoacrylate ‐ Dermabond), Outcome 2 Cosmesis (WES, Other) at 5 to 14 days.
2.3. Analysis.
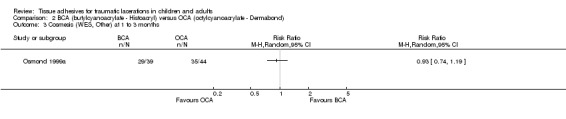
Comparison 2 BCA (butylcyanoacrylate ‐ Histoacryl) versus OCA (octylcyanoacrylate ‐ Dermabond), Outcome 3 Cosmesis (WES, Other) at 1 to 3 months.
Secondary outcomes
Pain
There was no statistically significant difference in the combined patient and parent‐reported pain scores (VAS) (WMD 5.0 mm, 95% CI ‐14.8 to 24.7)(Analysis 2.4).
2.4. Analysis.
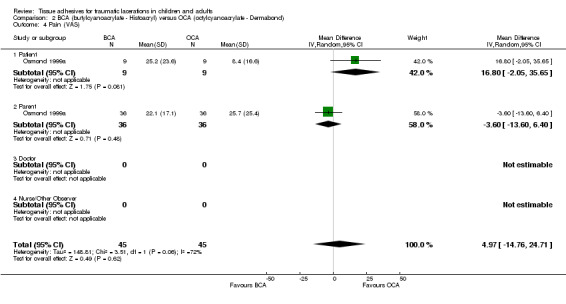
Comparison 2 BCA (butylcyanoacrylate ‐ Histoacryl) versus OCA (octylcyanoacrylate ‐ Dermabond), Outcome 4 Pain (VAS).
Time to complete the procedure
There was also no significant difference in the time required to complete the procedure (WMD 0.2 min, ‐1.1 to 1.5)(Analysis 2.5).
2.5. Analysis.
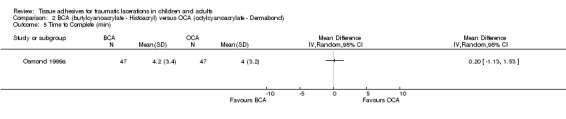
Comparison 2 BCA (butylcyanoacrylate ‐ Histoacryl) versus OCA (octylcyanoacrylate ‐ Dermabond), Outcome 5 Time to Complete (min).
Ease of procedure
The study reported no significant difference for ease of procedure; however, data were incomplete and not included in the review.
Complications
Two complications were measured (Analysis 2.6): there was no significant difference between treatment groups in either dehiscence (RD 4%, 95% CI ‐3 to 11) or infection (RD 0%, 95% CI ‐4 to 4).
2.6. Analysis.
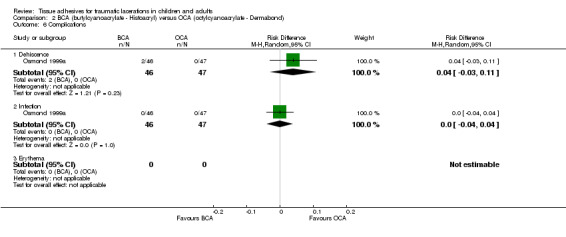
Comparison 2 BCA (butylcyanoacrylate ‐ Histoacryl) versus OCA (octylcyanoacrylate ‐ Dermabond), Outcome 6 Complications.
High‐viscosity octylcyanoacrylate compared with low‐viscosity octylcyanoacrylate
Singer 2003 compared high‐viscosity octylcyanoacrylate (Dermabond HV TM) with low‐viscosity (standard) octylcyanoacrylate (Dermabond TM)(Analysis 3.1). No efficacy estimates were available as the main outcome related to the complication of tissue adhesive migration.
3.1. Analysis.
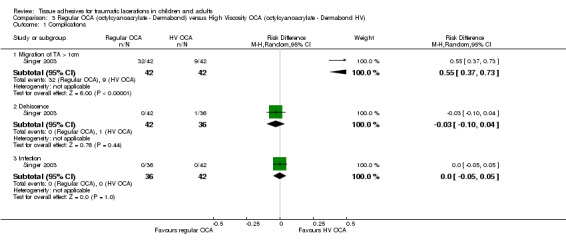
Comparison 3 Regular OCA (octylcyanoacrylate ‐ Dermabond) versus High Viscosity OCA (octylcyanoacrylate ‐ Dermabond HV), Outcome 1 Complications.
The study reported a significantly higher incidence of migration with the low‐viscosity product (RD 55%, 95% CI 37 to 73). There were no significant differences in the incidence of dehiscence (RD ‐3%, ‐10 to 4) or infection (RD 0%, ‐5 to 5)(Analysis 3.1).
Discussion
This systematic review has shown that tissue adhesives are an acceptable alternative to standard wound closure (SWC) for repairing simple traumatic lacerations.
No significant difference was found in the short or long‐term cosmetic outcome between lacerations closed with tissue adhesives and SWC. Indeed, the range of plausible values (the 95% CI) are all smaller than the range of minimum clinically important differences (12 to 15 mm) suggested (Goodman 1994). No further studies simply comparing a tissue adhesive to SWCs for cosmetic outcome are necessary. No subgroup differences were found when participants were grouped for age or for location. No comparisons could be made within the included studies for the extent of lacerations.
While cosmesis is an important outcome for both patients and providers, other outcome measures must be considered before declaring tissue adhesives an appropriate alternative to sutures and other SWC methods. The application of tissue adhesives is significantly faster (average 4.7 minutes less) and less painful than SWC. In clinical practice, this is particularly important to consider when treating young children. Suturing wounds in this age group can be emotionally traumatic for the child (unless sedation is used) as well as the parent. Though sedation is safe and uncomplicated for the majority of patients, this adds time and increases cost and complexity.
No individual study has been able to show a significant difference in complication rates between tissue adhesives and SWC. This is due to insufficient power to detect rare outcomes, such as infection and dehiscence. However, when pooling the results of the nine studies comparing tissue adhesives to SWC, significant random‐effects differences were found for erythema (favouring tissue adhesive) and dehiscence (favouring SWC). No differences were found for infection, delayed closure or discharge. This may still be due to insufficient pooled numbers of participants, calling for further clinical trials that are designed to assess complication rates rather than cosmesis, and thus are sufficiently powered to detect these rare events.
A small, but significant increased risk of dehiscence was found with tissue adhesives. The estimate of this risk difference is 2.4% (95% CI 0.1 to 4.9; Number Needed to Harm (NNH) 40, 95% CI 20 to 1168). The clinical significance of this result is difficult to interpret without further research to identify whether wound characteristics, patient characteristics, practitioner skill level/training, or different tissue adhesives influence this result. Also, it is not known whether dehisced wounds, including those that require secondary closure, have a different cosmetic outcome than those without dehiscence. Until these questions are answered, this complication should be discussed with the patient (and parent) as a potential, although infrequent, complication that would require a repeat visit for a second closure. Research to identify patients' tolerance for this potential second visit may also influence how practitioners approach this issue.
Erythema was found more often in participants treated with standard wound closure. The random‐effects estimate of this risk difference is ‐10% (95% CI ‐19 to ‐0.4; NNH 10, 95% CI 5 to 239). It is difficult to determine the clinical significance of this finding without further data to correlate this early complication with long‐term cosmesis.
While there are theoretical differences between the two tissue adhesive products currently available (butylcyanoacrylate (BCA), octylcyanoacrylate (OCA)), only one study with available data was identified that compared them directly. No significant difference was found between the two tissue adhesives for the outcomes of cosmesis, procedure time, pain, infection, or dehiscence. One other study was identified, but no data were available to include it in the review at this time. In an indirect (between study) comparison using a chi squared test (Deeks 2001), we found a significant difference that favoured BCA (WMD 7.5 versus ‐2.1 mm, p= 0.003) on CVAS at one to three months. Direct evidence (Oxman 1992), obtained through further tissue adhesive versus tissue adhesive trials, is required to make a definitive conclusion.
The lack of other studies comparing the two products may be due to a number of possible factors. In the United States, only octylcyanoacrylate (Dermabond TM) is available under FDA sanction. Without FDA approval of the research protocol, other tissue adhesives cannot be studied. In other countries where both products are available, there is a significant per‐patient cost savings of Histoacryl TM, when used as a multi‐dose vial, over Dermabond TM (Quinn 1998b). This cost difference may deter researchers from further comparisons between the products, when each has shown to be an equally acceptable alternative to standard wound care.
The final study compared a newer formulation of octylcyanoacrylate (high‐viscosity) with the standard formulation to assess the incidence of migration away from the wound. The authors chose this outcome as a proxy for complications during use, where the patient's eye may become inadvertently stuck shut by adhesive migrating from a nearby wound. While this complication does rarely occur and may be theoretically prevented using a higher‐viscosity tissue adhesive, migration can easily be prevented with appropriate patient selection, preparation and positioning (Farion 2003).
Two outcomes showed significant heterogeneity in the results of the included studies. The first, tissue adhesive versus SWC using CVAS at one to three months, is the outcome with the largest number of included studies. Each study relied on its own raters (plastic surgeons) to make cosmetic assessments using CVAS, which does not include a reference point of "adequate" or any other qualifier. Thus, each rater is able to set their own reference point, leading to heterogeneity in the results among studies. The other outcome, time to complete the procedure, may be explained by the various SWC methods used in the comparison arms. For example, two trials used adhesive strips, which take very little time to apply compared to trials in which sutures were used. As well, few studies clearly reported how the time was measured (start and end points), making it impossible to know if the various studies were assessing the same time period.
Two economic analyses of tissue adhesives have been published. The first analysis (Osmond 1995) compared the personnel time, supply costs and parental costs between non‐absorbable percutaneous sutures, absorbable percutaneous sutures and multi‐use Histoacryl TM in a Canadian ED setting, using 1993 costs. The ED overhead costs and costs associated with complications were not included, as they were thought to be equal across all three groups, with the assumption that cosmesis was equivalent. Absorbable sutures were found to be 2.4 times more costly than tissue adhesives, while non‐absorbable sutures were 6.8 times more costly, due to the need for a repeat visit. The other analysis (Zempsky 2005) compared three closure methods (adhesive strips, sutures and tissue adhesives) for simple wounds closed without complication, with subsequent dehiscence, or with subsequent infection. The authors used the literature to estimate the costs of the products used (primary closure +/‐ secondary closure if dehiscence occurred) and the physician's time (minutes to repair the wound multiplied by mean physician's labour rate/minute). This US‐based analysis only considered octylcyanoacrylate (Dermabond TM) and did not account for costs associated with removal of non‐absorbable sutures. In the end, they concluded that adhesive strips had the lowest cost per laceration overall, the lowest cost per laceration with subsequent dehiscence, and the lowest cost per laceration with subsequent infection. Tissue adhesives were found to be the most expensive alternative in all three scenarios.
An updated cost analysis is needed to compare tissue adhesives, considering these scenarios as well as the cost of procedural sedation, which is being utilized more often for lacerations repaired with sutures.
The evidence is limited by the overall low methodological quality of the included studies, as assessed by the Jadad score. Due to the types of treatments being compared, it was impossible to blind the participant or the physician, though blinded individuals did the later cosmetic assessments. Most studies failed to adequately describe the randomisation process and half did not fully report the management of dropouts or withdrawals. Similarly, only two studies adequately described their allocation concealment practices. The primary outcome of most studies is also a potential limitation, given the subjective nature of cosmesis scoring. Three studies assessed cosmesis with non‐validated scoring systems.
Several factors may have affected our results. Our use of imputed values and combining parametric and non‐parametric statistics may have lead to less precise results. Subgroup analyses were a necessary part of our review; unfortunately, these are indirect comparisons and are also underpowered due to lack of data. Finally, further data are required to determine whether variables such as the training level of the treating physician influence the results.
Authors' conclusions
Implications for practice.
· Tissue adhesives are an acceptable alternative to standard wound closure for repairing simple traumatic lacerations. · Tissue adhesives offer the benefit of rapid application and less pain. This has greatest implication for children with lacerations. · A small but significant increased rate of dehiscence with tissue adhesives must be considered when choosing the closure method (Number Needed to Harm 40).
Implications for research.
· Research is needed to determine the characteristics (patient, wound, product, operator) that result in an increased rate of dehiscence with tissue adhesives and whether dehiscence results in poor cosmetic outcome. This will require a very large RCT powered to detect these possible differences leading to rare events. · Additional research is needed to compare different tissue adhesives and the relative costs of tissue adhesives versus other forms of wound closure. · Further studies are needed to evaluate the use of tissue adhesives for more complex lacerations, or use in locations which have been excluded in prior research (over joints, scalp).
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 2 April 2007 | New search has been performed | For this second update, new searches were carried out in April 2007. Six studies were identified of which two (Holger 2004, Singer 2003) were included. One additional study identified through the search represents publication of the final results previously included from an abstract (Zempsky 2001). The remaining three studies were excluded. The reviewers' conclusions remain unchanged. |
| 26 May 2004 | New search has been performed | A first update was published in the Cochrane Library, Issue 3, 2004 with 11 included studies. |
| 2 May 2002 | New citation required and conclusions have changed | Substantive amendment. This review was originally published in the Cochrane Library, Issue 3, 2002 with 9 included studies. |
Acknowledgements
The reviewers would like to thank * Natasha Wiebe and Ellen Crumley for their work on prior versions of this review. * E. Andrea Nelson, Sally Bell‐Syer, Wendy Taylor and Ruth Foxlee from the Cochrane Review Wounds Group for their assistance. * Kirsten Lone Jensen (Trials Search Coordinator, Administrator, The Nordic Cochrane Centre) for translation of the Danish study (Schultz 1979). * Cochrane Review Wounds Group referees (Peter Moore, Liz Scanlon, Annette Wysocki, Sheila Benton‐Jones), Editors (Nicky Cullum & David Margolis), and Statistician (Seokyung Hahn) for their comments to improve the review.
Appendices
Appendix 1. Ovid MEDLINE Search Strategy
Ovid MEDLINE was searched using the following strategy in combination with the Cochrane highly sensitive search strategy for identifying reports of randomised controlled trials (Higgins 2005) 1 exp Lacerations/ 2 lacerat$.ti,ab. 3 (traumatic adj (wound$ or injur$)).ti,ab. 4 or/1‐3 5 exp Tissue Adhesives/ 6 exp Acrylates/ 7 tissue adhesive$.ti,ab. 8 (crylate$ or bucrylate$ or cyanoacrylate$ or enbucrilate$).ti,ab. 9 (glu or glue or glues).ti,ab. 10 or/5‐9 11 4 and 10
Appendix 2. Ovid EMBASE Search Strategy
Ovid EMBASE was searched using the following strategy in combination with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) 1 exp Laceration/ 2 lacerat$.ti,ab. 3 (traumatic adj (wound$ or injur$)).ti,ab. 4 or/1‐3 5 exp Tissue Adhesive/ 6 exp Acrylic Acid Derivative/ 7 tissue adhesive$.ti,ab. 8 (crylate$ or bucrylate$ or cyanoacrylate$ or enbucrilate$).ti,ab. 9 (glu or glue or glues).ti,ab. 10 or/5‐9 11 4 and 10
Appendix 3. Ovid CINAHL Search Strategy
Ovid CINAHL was searched using the following strategy in combination with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) 1 exp "Tears and Lacerations"/ 2 lacerat$.ti,ab. 3 (traumatic adj (wound$ or injur$)).ti,ab. 4 or/1‐3 5 exp Fibrin Tissue Adhesive/ 6 tissue adhesive$.ti,ab. 7 (crylate$ or bucrylate$ or cyanoacrylate$ or enbucrilate$).ti,ab. 8 (glu or glue or glues).ti,ab. 9 or/5‐8 10 4 and 9
Appendix 4. Web of Science Search Strategy
Web of Science ‐ Science Citation Index was searched using the following strategy: #1 TS=(lacerat* OR skin injur* OR wound*) #2 TS=(tissue adhesive* OR acrylate* OR cyanoacrylate* OR octylcyanoacrylate* OR enbucrilate* OR bucrylate* OR glue* OR suture*) #3 #1 AND #2 #4 TS=(controlled clinical trial OR meta analysis OR multicenter study OR randomized controlled trial OR random* OR blind* OR placebo*) #5 #3 AND #4
Data and analyses
Comparison 1. Tissue Adhesive versus Standard Wound Closure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cosmesis (CVAS) at 5 to 14 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Cosmesis (CVAS) at 1 to 3 months | 7 | 549 | Mean Difference (IV, Random, 95% CI) | 1.62 [‐3.17, 6.41] |
| 3 Cosmesis (CVAS) at 9 to 12 months | 4 | 237 | Mean Difference (IV, Fixed, 95% CI) | 1.48 [‐3.10, 6.06] |
| 4 Cosmesis (WES, Other) at 5 to 14 days | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.09] |
| 5 Cosmesis (WES, Other) at 1 to 3 months | 4 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 6 Cosmesis (WES, Other) at 9 to 12 months | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.30] |
| 7 Cosmesis (CVAS) at 5 to 14 days by age | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Pediatric (<18) | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adult (=> 18) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Cosmesis (CVAS) at 1 to 3 months by age | 6 | 433 | Mean Difference (IV, Random, 95% CI) | 1.71 [‐4.20, 7.63] |
| 8.1 Pediatric (<18) | 4 | 283 | Mean Difference (IV, Random, 95% CI) | 1.25 [‐8.58, 11.08] |
| 8.2 Adult (=> 18) | 2 | 150 | Mean Difference (IV, Random, 95% CI) | 2.41 [‐5.43, 10.26] |
| 9 Cosmesis (CVAS) at 9 to 12 months by age | 4 | 237 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [‐2.18, 6.51] |
| 9.1 Pediatric (<18) | 3 | 92 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐4.78, 9.78] |
| 9.2 Adult (=> 18) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 1.98 [‐3.43, 7.39] |
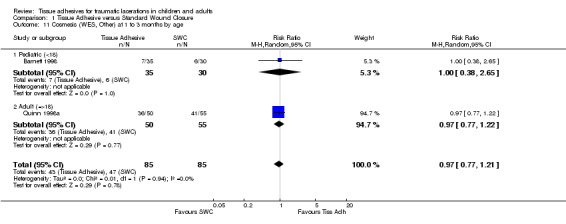
| 10 Cosmesis (WES, Other) at 5 to14 days by age | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Pediatric (<18) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adult (=>18) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
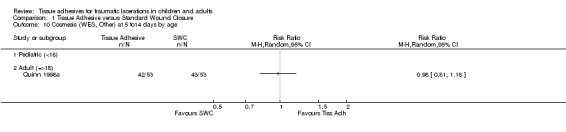
| 11 Cosmesis (WES, Other) at 1 to 3 months by age | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.21] |
| 11.1 Pediatric (<18) | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.38, 2.65] |
| 11.2 Adult (=>18) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 12 Cosmesis (WES, Other) at 9 to 12 months by age | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.42] |
| 12.1 Pediatric (<18) | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.70, 1.98] |
| 12.2 Adult (=>18) | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.81, 1.45] |
| 13 Cosmesis (CVAS) at 5 to 14 days by location | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Face | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Body | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Cosmesis (CVAS) at 1 to 3 months by location | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
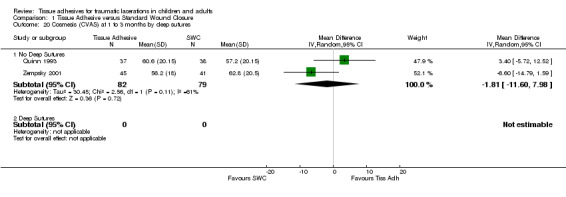
| 14.1 Face | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐11.60, 7.98] |
| 14.2 Body | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Cosmesis (CVAS) at 9 to 12 months by location | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Face | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Body | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Cosmesis (WES, Other) at 5 to 14 days by location | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 16.1 Face | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Body | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Cosmesis (WES, Other) at 1 to 3 months by location | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 17.1 Face | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 17.2 Body | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.61, 3.67] |
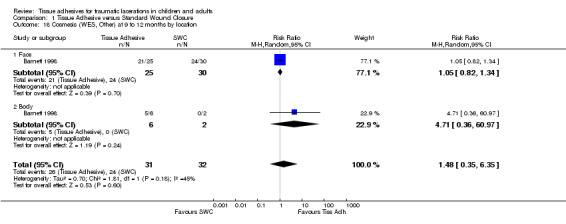
| 18 Cosmesis (WES, Other) at 9 to 12 months by location | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.35, 6.35] |
| 18.1 Face | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.82, 1.34] |
| 18.2 Body | 1 | 8 | Risk Ratio (M‐H, Random, 95% CI) | 4.71 [0.36, 60.97] |
| 19 Cosmesis (CVAS) at 5 to 14 days by deep sutures | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19.1 No Deep Sutures | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Deep Sutures | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Cosmesis (CVAS) at 1 to 3 months by deep sutures | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 No Deep Sutures | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐11.60, 7.98] |
| 20.2 Deep Sutures | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
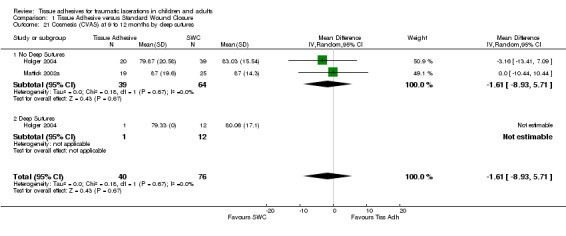
| 21 Cosmesis (CVAS) at 9 to 12 months by deep sutures | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐8.93, 5.71] |
| 21.1 No Deep Sutures | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐8.93, 5.71] |
| 21.2 Deep Sutures | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Cosmesis (WES, Other) at 5 to 14 days by deep sutures | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 22.1 No Deep Sutures | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Deep Sutures | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
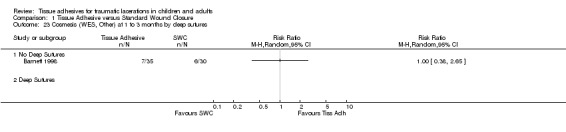
| 23 Cosmesis (WES, Other) at 1 to 3 months by deep sutures | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.1 No Deep Sutures | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Deep Sutures | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 Cosmesis (WES, Other) at 9 to 12 months by deep sutures | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 24.1 No Deep Sutures | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Deep Sutures | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Cosmesis (CVAS) at 5 to 14 days by tissue adhesive | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25.1 BCA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 OCA | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 Cosmesis (CVAS) at 1 to 3 months by tissue adhesive | 7 | 549 | Mean Difference (IV, Random, 95% CI) | 1.62 [‐3.17, 6.41] |
| 26.1 BCA | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 7.50 [1.75, 13.25] |
| 26.2 OCA | 4 | 367 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐5.86, 1.72] |
| 27 Cosmesis (CVS) at 9 to 12 months by tissue adhesive | 4 | 237 | Mean Difference (IV, Fixed, 95% CI) | 1.48 [‐3.10, 6.06] |
| 27.1 BCA | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 10.5 [‐1.30, 22.30] |
| 27.2 OCA | 3 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐5.09, 4.85] |
| 28 Cosmesis (WES, Other) at 5 to 14 days by tissue adhesive | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.89] |
| 28.1 BCA | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.15, 3.39] |
| 28.2 OCA | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.34, 2.31] |
| 29 Cosmesis (WES, Other) at 1 to 3 months by tissue adhesive | 4 | 364 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.58, 1.62] |
| 29.1 BCA | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.52, 3.03] |
| 29.2 OCA | 2 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.60] |
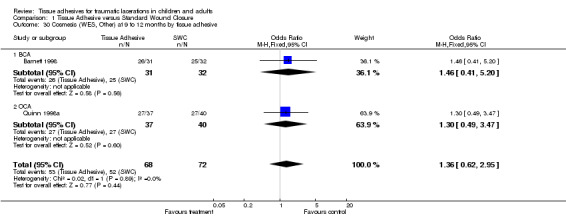
| 30 Cosmesis (WES, Other) at 9 to 12 months by tissue adhesive | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.62, 2.95] |
| 30.1 BCA | 1 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.41, 5.20] |
| 30.2 OCA | 1 | 77 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.49, 3.47] |
| 31 Pain (VAS) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 31.1 Patient | 1 | 136 | Mean Difference (IV, Random, 95% CI) | ‐10.80 [‐17.09, ‐4.51] |
| 31.2 Parent | 5 | 434 | Mean Difference (IV, Random, 95% CI) | ‐13.42 [‐19.99, ‐6.85] |
| 31.3 Doctor | 1 | 163 | Mean Difference (IV, Random, 95% CI) | ‐12.60 [‐20.06, ‐5.14] |
| 31.4 Nurse/Other Observer | 1 | 163 | Mean Difference (IV, Random, 95% CI) | ‐14.90 [‐22.50, ‐7.30] |
| 32 Time to Complete (min) | 6 | 584 | Mean Difference (IV, Random, 95% CI) | ‐4.66 [‐7.22, ‐2.10] |
| 33 Ease of Procedure (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 34 Complications | 9 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 34.1 Dehiscence | 8 | 782 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.05] |
| 34.2 Infection | 9 | 834 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 34.3 Erythema | 2 | 186 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.19, ‐0.00] |
| 34.4 Delayed closure | 1 | 136 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 34.5 Discharge | 1 | 111 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.05, 0.18] |
1.8. Analysis.
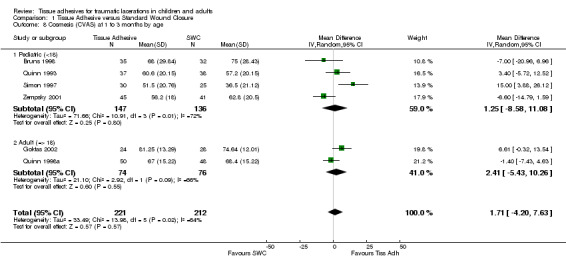
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 8 Cosmesis (CVAS) at 1 to 3 months by age.
1.9. Analysis.
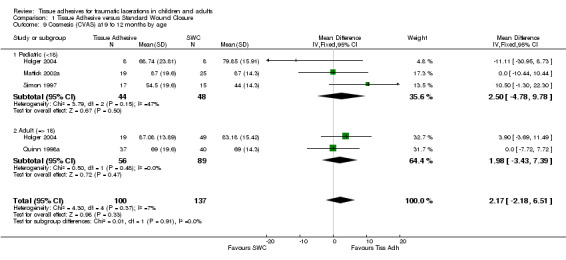
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 9 Cosmesis (CVAS) at 9 to 12 months by age.
1.10. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 10 Cosmesis (WES, Other) at 5 to14 days by age.
1.11. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 11 Cosmesis (WES, Other) at 1 to 3 months by age.
1.12. Analysis.
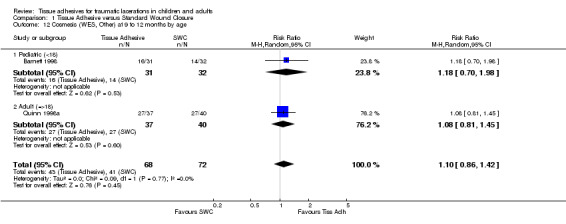
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 12 Cosmesis (WES, Other) at 9 to 12 months by age.
1.14. Analysis.
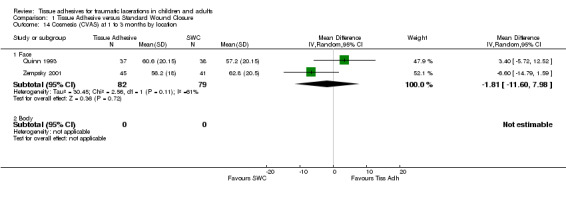
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 14 Cosmesis (CVAS) at 1 to 3 months by location.
1.15. Analysis.
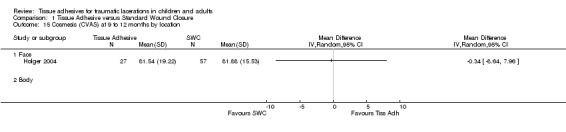
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 15 Cosmesis (CVAS) at 9 to 12 months by location.
1.16. Analysis.
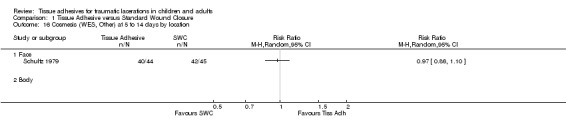
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 16 Cosmesis (WES, Other) at 5 to 14 days by location.
1.17. Analysis.
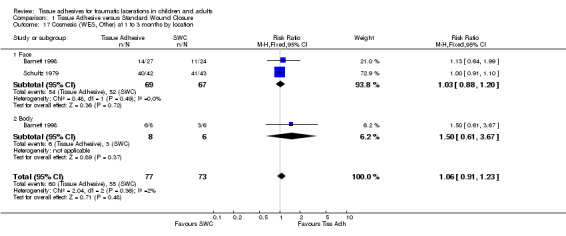
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 17 Cosmesis (WES, Other) at 1 to 3 months by location.
1.18. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 18 Cosmesis (WES, Other) at 9 to 12 months by location.
1.20. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 20 Cosmesis (CVAS) at 1 to 3 months by deep sutures.
1.21. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 21 Cosmesis (CVAS) at 9 to 12 months by deep sutures.
1.23. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 23 Cosmesis (WES, Other) at 1 to 3 months by deep sutures.
1.24. Analysis.
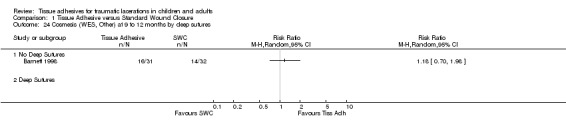
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 24 Cosmesis (WES, Other) at 9 to 12 months by deep sutures.
1.25. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 25 Cosmesis (CVAS) at 5 to 14 days by tissue adhesive.
1.27. Analysis.
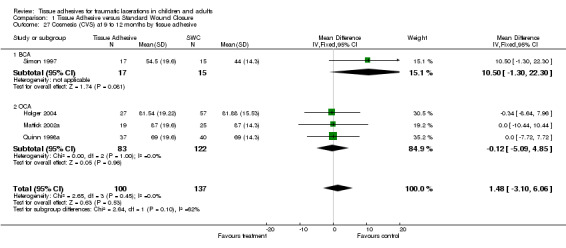
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 27 Cosmesis (CVS) at 9 to 12 months by tissue adhesive.
1.28. Analysis.
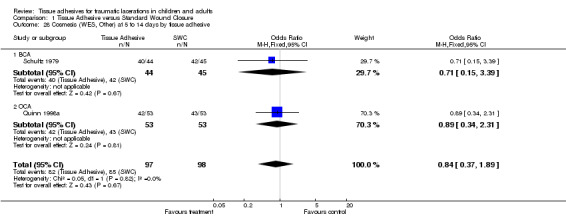
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 28 Cosmesis (WES, Other) at 5 to 14 days by tissue adhesive.
1.29. Analysis.
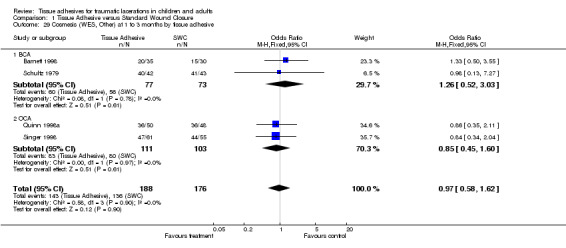
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 29 Cosmesis (WES, Other) at 1 to 3 months by tissue adhesive.
1.30. Analysis.
Comparison 1 Tissue Adhesive versus Standard Wound Closure, Outcome 30 Cosmesis (WES, Other) at 9 to 12 months by tissue adhesive.
Comparison 2. BCA (butylcyanoacrylate ‐ Histoacryl) versus OCA (octylcyanoacrylate ‐ Dermabond).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cosmesis (CVAS) at 1 to 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Cosmesis (WES, Other) at 5 to 14 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Cosmesis (WES, Other) at 1 to 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Pain (VAS) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 4.97 [‐14.76, 24.71] |
| 4.1 Patient | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 16.80 [‐2.05, 35.65] |
| 4.2 Parent | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐13.60, 6.40] |
| 4.3 Doctor | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Nurse/Other Observer | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Time to Complete (min) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Complications | 1 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Dehiscence | 1 | 93 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 6.2 Infection | 1 | 93 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 6.3 Erythema | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Regular OCA (octylcyanoacrylate ‐ Dermabond) versus High Viscosity OCA (octylcyanoacrylate ‐ Dermabond HV).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Migration of TA > 1cm | 1 | 84 | Risk Difference (M‐H, Random, 95% CI) | 0.55 [0.37, 0.73] |
| 1.2 Dehiscence | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 1.3 Infection | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barnett 1998.
| Methods | RCT of 163 enrolled lacerations. Cosmetic follow‐up in 40% at 3 months and 39% at 1 year. | |
| Participants | Children > 4 yrs with acute lacerations < 5 cm in length, not requiring deep sutures. All body areas except eyelids, mucous membranes, and joints. | |
| Interventions | BCA (n = 83) versus sutures (n = 80). | |
| Outcomes | Cosmesis: photographs at 3 and 12 months evaluated by 2 blinded observers using 5‐point categorical scale. Pain: VAS by MD, RN, and parent with categorical FACES score by patient (not used). Complications: redness, discharge,dehiscence. | |
| Notes | Jadad = 1; Funding = B. Braun Company Pty Ltd.; 5‐point categorical scale combined with WES by using 4/5 and 5/5= 6/6 "optimal" scoring; Breakdown of scores by location and clarification of reported complication rates provided by the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bruns 1998.
| Methods | RCT of 85/113 eligible lacerations on 83 patients (randomized by patient). Cosmetic follow‐up in 79% at 3 months. | |
| Participants | Children > 1 yr with acute lacerations, including those requiring deep sutures. All areas except scalp and mucous membranes. | |
| Interventions | OCA (n = 42) versus standard wound care (n = 43, with 42 receiving sutures and 1 stapled). | |
| Outcomes | Cosmesis: photograph at 3 months rated by 2 blinded plastic surgeons using CVAS. Pain: VAS by parent. Complications: infection, dehiscence. | |
| Notes | Jadad = 2; Funding = Closure Medical Corp; Breakdown of cosmesis by locations, need for deep sutures not available from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Goktas 2002.
| Methods | RCT of 92 patients with lacerations. Cosmetic follow‐up reported for 57% of patients at 3 months. | |
| Participants | Adults with lacerations < 5cm not crossing joints or high‐tension areas. | |
| Interventions | BCA versus standard wound care (sutures). | |
| Outcomes | Cosmesis: assessment at 10 days and 90 days by blinded plastic surgeon using CVAS. Satisfaction: patient and MD asked if satisfied. Costs: estimated. Complications: infection. | |
| Notes | Jadad = 3; Funding = Unknown; n in each original group, location of wounds, use of deep sutures not known. Results only for those completing 90 day follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Holger 2004.
| Methods | Three‐arm RCT of 150/230 eligible patients. 5 patients withdrew. Cosmetic follow‐up in 56% at 9‐12 months. | |
| Participants | Patients >5yrs with acute facial lacerations, including those determined before randomization to require deep sutures. Excludes scalp, ear, mucous membranes. | |
| Interventions | OCA (n = 49) versus absorbable suture (n = 47) versus non absorbable suture (n = 49). Two suture arms combined with data provided by author. | |
| Outcomes | Cosmesis: CVAS by 2 blinded MD's in person at 9‐12 months; patient/parent completed CVAS on satisfaction with wound. Complications: dehiscence and infection. | |
| Notes | Jadad = 3; Funding = Health Partners Research Foundation; Author correspondence provided combined data for 2 suture arms as well as subgroup data for patient age and deep sutures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mattick 2002a.
| Methods | RCT of 60 enrolled patients. 6 patients failed study enrolment (4 OCA failures, 1 adhesive strip failure, 1 camera failure). Cosmetic follow‐up in 73% between 3 and 12 months. All results limited to those for patients that completed follow‐up. | |
| Participants | Children 1‐14 yrs with acute lacerations < 5 cm in length, not requiring deep sutures or local anaesthetic for cleaning. All body areas except mucous membranes, scalp or areas of high tension. | |
| Interventions | OCA (n = 30) versus adhesive strips (n = 30). | |
| Outcomes | Cosmesis: photographs at 3‐12 months, compared to pre‐repair photos, by blinded plastic surgeon using CVAS. Parents also provided assessment of scar with CVAS. Pain/Distress: VAS by parent . Ease: VAS by MD/RN doing procedure. Complications: screened for at 7‐day patient review. | |
| Notes | Jadad = 2; Funding = Unknown; Camera and Dermabond adhesive supplied by Ethicon; Parental CVAS not used as blinded assessment at the same time available. Breakdown of location not available. All CVAS scores from 3‐12 months combined with other studies with late (9‐12 months) assessments, as no indication of when the majority of assessments were made was given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Osmond 1999a.
| Methods | RCT of 94/124 eligible lacerations. Cosmetic follow‐up in 88% at 3 months. | |
| Participants | Children < 18 yrs with acute facial lacerations < 4cm, not requiring deep sutures. Excludes scalp, ear, mucous membranes. | |
| Interventions | OCA (n = 47) versus BCA (n = 47). | |
| Outcomes | Cosmesis: WES by blinded RN at 10‐14 days and 3 months. Photograph at 3 months evaluated by blinded plastic surgeon on 2 occasions using CVAS. Time: recorded by MD. Ease: VAS by MD. Pain: VAS by patient (>7 yrs) or parent (<7 yrs). Complications: infection, dehiscence. | |
| Notes | Jadad=3; Funding= Closure Medical Corp; Ease not used due to insufficient data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Quinn 1993.
| Methods | RCT of 81/90 eligible lacerations. Cosmetic follow‐up in 93% at 3 months. | |
| Participants | Children <18 yrs with facial lacerations < 4 cm, not requiring deep sutures. Excludes mucous membranes, hair‐covered areas. | |
| Interventions | BCA (n = 41) versus sutures (n = 40). | |
| Outcomes | Cosmesis: Photograph taken at 3 months evaluated by 2 blinded plastic surgeons on 2 occasions using 3‐point categorical scale and CVAS. Time: recorded by MD. Pain: VAS by parent. Complications: infection, dehiscence, erythema. | |
| Notes | Jadad= 2; Funding= Children's Hospital of Eastern Ontario Research Institute; 3‐point scale data not combined with WES. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Quinn 1998a.
| Methods | RCT (computer generated, stratified for facial versus non‐facial) of 136/179 eligible lacerations on 130 patients (randomized by laceration allowing cross‐over). Cosmetic follow‐up in 72% at 3 months and 57% at 1 year. | |
| Participants | Adults with lacerations of any length, including those requiring deep sutures. All body areas except ear, scalp, mucous membrane, joints, hands or feet. | |
| Interventions | OCA (n = 68) versus sutures (n = 68). | |
| Outcomes | Cosmesis: WES by 2 RN's (one blinded) at 3‐10 days, 3 and 12 months. Photos at 3 and 12 months rated by blinded plastic surgeon on 2 occasions using CVAS. Time: recorded by MD. Pain: VAS by patient. Complications: infection, dehiscence, need for delayed closure or revision. | |
| Notes | Jadad = 3; Funding = Closure Medical Corp; Breakdown of cosmesis by location, need for deep sutures not available from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schultz 1979.
| Methods | 100 consecutive patients randomized by sealed envelope method. 11 patients withdrew. | |
| Participants | Patients of all ages with acute simple lacerations to the face < 5 cm in length, not requiring deep sutures. | |
| Interventions | BCA (n = 50) versus sutures (n = 50). | |
| Outcomes | Cosmesis: Acceptable (good healing without diastasis or infection) versus Unacceptable as judged at 7 and 30 days. Complications: diastasis, infection, allergic reaction or skin discoloration | |
| Notes | Jadad = 2; Funding = unknown; Translated from Danish; Acceptable/Unacceptable scoring combined with WES with Acceptable = 6/6 "optimal"; Breakdown of cosmesis by age, need for deep sutures not available from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Simon 1997.
| Methods | RCT of 61 eligible lacerations. Cosmetic follow‐up in 90% at 2 months and 52% at 1 year. | |
| Participants | Children 1‐18 yrs with acute lacerations < 5 cm in length, including those requiring deep sutures. All body areas except ear, mucous membranes and joints. | |
| Interventions | BCA (n = 30) versus sutures (n = 31). | |
| Outcomes | Cosmesis: photograph at 2 and 12 months rated by 2 blinded plastic surgeons using CVAS. Time: recorded by MD. Pain: VAS by parent. Complications: infection, dehiscence. | |
| Notes | Jadad = 1; Funding = unknown; Breakdown of cosmesis by location, need for deep sutures not available from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Singer 1998.
| Methods | RCT (block randomized for deep suture vs no deep suture) of 124 eligible lacerations. Cosmetic follow‐up in 94% at 3 months. | |
| Participants | Patients of all ages (>1 yr) with acute lacerations, including those requiring deep sutures. All body areas except mucous membrane and hair‐covered areas. | |
| Interventions | OCA (n = 63) versus standard wound care (n = 61 with 54 receiving sutures, 1 stapled and 6 closed with adhesive strips). | |
| Outcomes | Cosmesis: WES by another blinded MD at 3 months; patient completed CVAS on satisfaction with wound. Complications: infection, dehiscence. | |
| Notes | Jadad = 1; Funding = Closure Medical Corp; Patient satisfaction CVAS combined with other blinded CVAS since it is the true "Gold Standard" for cosmetic outcome. Breakdown of cosmesis by age, location, need for deep sutures not available from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Singer 2003.
| Methods | RCT of 84 patients. No cosmesis outcome measures. | |
| Participants | Patients >1yr with acute lacerations amenable to tissue adhesive closure. Excludes scalp and mucocutaneous areas. | |
| Interventions | High‐viscosity OCA (n = 42) versus regular OCA (n = 42). | |
| Outcomes | Complications: migration of tissue adhesive >1cm from the wound margins. | |
| Notes | Jadad = 2; Funding = Closure Medical Corp.; No subgroup data for age or location is available. No other outcomes were recorded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zempsky 2001.
| Methods | RCT of 100 lacerations. 3 patients later excluded as non‐facial lacerations. Cosmetic follow‐up in 85% at 2 months. | |
| Participants | Children (1‐18 yrs) with simple facial lacerations not requiring deep sutures. Wounds in high‐tension or mobile areas were avoided. | |
| Interventions | OCA (n = 49) versus adhesive strips (n = 48) | |
| Outcomes | Cosmesis: photograph at 2 months evaluated by 2 blinded plastic surgeons using CVAS. Pain: VAS by either parent or child. Complications: infection, dehiscence. | |
| Notes | Jadad = 3; Funding = 3M Inc.; Initially included abstract data updated to data reported in full publication; Breakdown of pain scores for those from parent versus those from child not available from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
BCA: Butylcyanoacrylate or Histoacryl Blue CVAS: Cosmetic Visual Analogue Scale ENB: Enbucrylate or Indermil FACES: a categorical pain scale (see reference Bieri 1990) MD: Doctor OCA: Octylcyanoacrylate or Dermabond RCT: Randomised Controlled Trial RN: Registered Nurse VAS: Visual Analogue Scale WES: Wound Evaluation Score n=number of lacerations in each treatment arm
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alamouti 1998 | Study of wound closure after surgical excision of skin cancers, no traumatic lacerations. |
| Bernard 2001 | Study of incisions, not lacerations. |
| Brown 1997 | Journal Club review of Simon's article reporting 1 yr follow‐up on BCA versus suture. |
| Charters 2000 | Study not randomized ("Consecutively assigned"). |
| Doraiswamy 2003 | Study not described as randomized ; study outcomes very subjective or not combinable with other outcomes. |
| Eaglstein 2002 | Study deals with abrasions, not lacerations and compares a liquid bandage product to traditional band‐aids. |
| Farris 1981 | Study is not randomized, nor does it deal with lacerations or tissue adhesives. |
| Fatovich 1995 | Study deals with sedation/analgesia for laceration repair, not the product used for the repair. |
| Giovannacci 2002 | Study does not deal with lacerations or tissue adhesives. |
| Giri 2004 | Observational study with no comparison arm. |
| Greene 1999 | Study deals with surgical incisions. |
| Hock 2002 | Study deals with tissue adhesive for scalp lacerations, though the method that the adhesive is used is not standard. As well, because the laceration was in an area covered with hair, no cosmetic assessment was made beyond 7 days, and all assessments of healing/complications were not completed in a blinded fashion. |
| Liebelt 1997 | Summary article on tissue adhesives in children |
| Ludlow 2000 | Study deals with aphthous ulcers. |
| Mattick 2002b | Review article. |
| Quinn 2003 | Study deals with suturing versus conservative treatment of hand lacerations. |
| Qureshi 1997 | Study is not randomized and deals with surgical incisions. |
| Saxena 1999 | All patients with high tension lacerations across joints. Lacerations treated with tissue adhesive or suture, then splinted to reduce tension. |
| Singer 2002 | Multi‐centered study has 10 sites, of which 4 sites deal with lacerations. Results from 2 of the sites have previously been independently published and included in the original review. (See BRUNS 1998 and SINGER 1998). Attempts to obtain unpublished data from the remaining 2 sites have been unsuccessful. |
| Sportsmed 1997 | Highlights of Simon's article reporting 1 yr follow‐up on BCA versus suture. |
| Stuart 1999 | Trial listed in on‐line trial register. All attempts to contact author for more information about the status of the trial/data were unsuccessful. No subsequent publication has been found. |
| Sutton 1985 | Study deals with suture versus adhesive strips. |
| Symeonoglou 1998 | Abstract of study, but without any results. All attempts to contact the authors to determine the status of the trial/results have been unsuccessful. |
Contributions of authors
Ken Farion (Primary Reviewer): protocol development, study selection, data extraction, data entry, development of final review and updates (original and all updates). Ellen Crumley: literature searching, quality assessment (original and 1st update). Lisa Hartling: protocol development, quality assessment, methodological expertise, development of final review and updates (original and all updates). Terry Klassen: protocol development, content and methodological expertise, development of final review and updates (original and all updates). Martin Osmond: protocol development, content expertise, development of final review and updates (original and all updates). Kelly Russell: study selection, data extraction, data entry, development of final review and updates (original and all updates). Natasha Wiebe: protocol development, statistical analyses, development of final review (original and 1st update). Tamara Durec: literature searching, development of update (2nd update). Ben Vandermeer: statistical analyses, development of update (2nd update).
Sources of support
Internal sources
Alberta Research Centre for Child Health Evidence (ARCHE), University of Alberta, Edmonton, Canada.
External sources
Alberta Heritage Foundation for Medical Research, Canada.
Declarations of interest
Dr. Martin Osmond received funding from Closure Medical Corp. for his study (Osmond 1999a).
Edited (no change to conclusions)
References
References to studies included in this review
Barnett 1998 {published and unpublished data}
- Barnett P, Jarman FC, Goodge J, Silk G, Aickin R. Randomised trial of histoacryl blue tissue adhesive glue versus suturing in the repair of paediatric lacerations. Journal of Paediatrics and Child Health 1998;34(6):548‐50. [DOI] [PubMed] [Google Scholar]
- Jarman FC, Holmes A, Aickin R, Goodge G, Silk G, Barnett P. Randomised Trial of n‐Butyl‐2‐Cyanoacrylate tissue adhesive glue vs suturing for the repair of paediatric lacerations. Emergency Medicine 1994;6(4):339. [Google Scholar]
- Jarman FC, Holmes A, Aickin R, Goodge G, Silk G, Barnett P. Randomised Trial of n‐Butyl‐2‐Cyanoacrylate tissue adhesive glue vs suturing for the repair of paediatric lacerations. Journal of Paediatrics and Child Health 1994;30:A17. [DOI] [PubMed] [Google Scholar]
Bruns 1998 {published data only}
- Bruns TB, Robinson BS, Smith RJ, Kile DL, Davis TP, Sullivan KM, Quinn JV. A new tissue adhesive for laceration repair in children. Journal of Pediatrics 1998;132(6):1067‐70. [DOI] [PubMed] [Google Scholar]
Goktas 2002 {published data only}
- Goktas N, Karcioglu O, Coskun F, Karaduman S, Menderes A. Comparison of tissue adhesive and suturing in the repair of lacerations in the emergency department.[erratum appears in Eur J Emerg Med. 2002 Dec;9(4):360.]. European Journal of Emergency Medicine 2002;9(2):155‐8. [DOI] [PubMed] [Google Scholar]
Holger 2004 {published and unpublished data}
- Holger JA, Wandersee SC, Hale DB. Cosmetic outcomes of facial lacerations repaired with tissue‐adhesive, absorbably, and nonabsorbable sutures. American Journal of Emergency Medicine 2004;22(4):254‐7. [DOI] [PubMed] [Google Scholar]
Mattick 2002a {published data only}
- Mattick A, Clegg G, Beattie T, Ahmad T. A randomised, controlled trial comparing a tissue adhesive (2‐octylcyanoacrylate) with adhesive strips (Steristrips) for paediatric laceration repair. Emergency Medicine Journal 2002;19(5):405‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osmond 1999a {published and unpublished data}
- Osmond MH, Quinn JV, Sutcliffe T, Jarmuske M, Klassen TP. A randomized, clinical trial comparing butylcyanoacrylate with octylcyanoacrylate in the management of selected pediatric facial lacerations. Academic Emergency Medicine 1999;6(3):171‐7. [DOI] [PubMed] [Google Scholar]
Quinn 1993 {published data only}
- Quinn JV, Drzewiecki A, Li MM, Stiell IG, Sutcliffe T, Elmslie TJ, et al. A randomized, controlled trial comparing a tissue adhesive with suturing in the repair of pediatric facial lacerations. Annals of Emergency Medicine 1993;22(7):1130‐5. [DOI] [PubMed] [Google Scholar]
Quinn 1998a {published data only}
- Quinn J, Wells G, Sutcliffe T, Jarmuske M, Maw J, Stiell I, Johns P. A randomized trial comparing octylcyanoacrylate tissue adhesive and sutures in the management of lacerations [see comments]. JAMA 1997;277(19):1527‐30. [PubMed] [Google Scholar]
- Quinn J, Wells G, Sutcliffe T, Jarmuske M, Maw J, Stiell I, Johns P. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3‐month, and 1‐year cosmetic outcome. Annals of Emergency Medicine 1998;32(6):645‐9. [DOI] [PubMed] [Google Scholar]
Schultz 1979 {published data only}
- Schultz A, Olesgaard P. [Tissue glue in minor skin lesions. A prospective controlled comparison between tissue glue and the suturing of skin minor lesions]. Ugeskrift for Laeger 1979;141(45):3106‐7. [PubMed] [Google Scholar]
Simon 1997 {published data only}
- Bruns TB, Simon HK, McLario DJ, Sullivan KM, Wood RJ, Anand KJ. Laceration repair using a tissue adhesive in a children's emergency department. Pediatrics 1996;98(4 Pt 1):673‐5. [PubMed] [Google Scholar]
- Simon HK, McLario DJ, Bruns TB, Zempsky WT, Wood RJ, Sullivan KM. Long‐term appearance of lacerations repaired using a tissue adhesive. Pediatrics 1997;99(2):193‐5. [DOI] [PubMed] [Google Scholar]
- Simon HK, Zempsky WT, Bruns TB, Sullivan KM. Lacerations against Langer's lines: to glue or suture?. Journal of Emergency Medicine 1998;16(2):185‐9. [DOI] [PubMed] [Google Scholar]
Singer 1998 {published data only}
- Hollander JE, Singer AJ. Application of tissue adhesives: rapid attainment of proficiency. Stony Brook Octylcyanoacrylate Study Group. Academic Emergency Medicine 1998;5(10):1012‐7. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Hollander JE, Valentine SM, Turque TW, McCuskey CF, Quinn JV. Prospective, randomized, controlled trial of tissue adhesive (2‐octylcyanoacrylate) vs standard wound closure techniques for laceration repair. Stony Brook Octylcyanoacrylate Study Group. Academic Emergency Medicine 1998;5(2):94‐9. [DOI] [PubMed] [Google Scholar]
Singer 2003 {published data only}
- Singer AJ, Giordano P, Fitch JL, Gulla J, Ryker D, Chale S. Evaluation of a new high‐viscosity octylcyanoacrylate tissue adhesive for laceration repair: A randomized, clinical trial. Academic Emergency Medicine 2003;10(10):1134‐7. [DOI] [PubMed] [Google Scholar]
Zempsky 2001 {published data only}
- Zempsky WT, Grem C, Nichols J, Parrotti D. Prospective Comparison of Cosmetic Outcomes of Simple Facial Lacerations Closed with Steri‐Strips or Dermabond. Academic Emergency Medicine 2001;8(5):438. [DOI] [PubMed] [Google Scholar]
- Zempsky WT, Parrotti D, Grem C, Nichols J. Randomized controlled comparison of cosmetic outcomes of simple facial lacerations closed with Steri Strip skin closures or Dermabond tissue adhesive. Pediatric Emergency Care 2004;20(8):519‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alamouti 1998 {published data only}
- Alamouti D, Kobyiciski G, Herde M, Allard P, HoffmannK, Altmeyer P. Octylcyanoacrylate tissue adhesive in the management of laceration closure in skin cancer. Journal of the European Academy of Dematology and Venereology 1998;11:S213. [Google Scholar]
Bernard 2001 {published data only}
- Bernard L, Doyle J, Friedlander SF, Eichenfield LF, Gibbs NF, Cunningham BB. A prospective comparison of octyl cyanoacrylate tissue adhesive (dermabond) and suture for the closure of excisional wounds in children and adolescents. Archives of Dermatology 2001;137(9):1177‐80. [DOI] [PubMed] [Google Scholar]
Brown 1997 {published data only}
- Brown V. Laceration repair with tissue adhesive in children. Journal of Family Practice 1997;44(5):445‐6. [PubMed] [Google Scholar]
Charters 2000 {published data only}
- Charters A. Wound glue: a comparative study of tissue adhesives. Accident and Emergency Nursing 2000;8(4):223‐7. [DOI] [PubMed] [Google Scholar]
Doraiswamy 2003 {published and unpublished data}
- Doraiswamy NV, Baig H, Hammett S, Hutton M. Which tissue adhesive for wounds?. Injury, International Journal of the Care of the Injured 2003;34(8):564‐7. [DOI] [PubMed] [Google Scholar]
Eaglstein 2002 {published data only}
- Eaglstein WH, Sullivan TP, Giordano PA, Miskin BM. A liquid adhesive bandage for the treatment of minor cuts and abrasions. Dermatologic Surgery 2002;28(3):263‐7. [DOI] [PubMed] [Google Scholar]
Farris 1981 {published data only}
- Farris RS, Hays LV. Controlled trial of the effectiveness of randomized wound closures. Missouri Medicine 1981;78(1):17‐20. [PubMed] [Google Scholar]
Fatovich 1995 {published data only}
- Fatovich DM, Jacobs IG. A randomized, controlled trial of oral midazolam and buffered lidocaine for suturing lacerations in children (the SLIC Trial). Annals of Emergency Medicine 1995;25(2):209‐14. [DOI] [PubMed] [Google Scholar]
Giovannacci 2002 {published data only}
- Giovannacci L, Eugster T, Stierli P, Hess P, Gurke L. Does fibrin glue reduce complications after femoral artery surgery? A randomised trial. European Journal of Vascular and Endovascular Surgery 2002;24(3):196‐201. [DOI] [PubMed] [Google Scholar]
Giri 2004 {published data only}
- Giri P, Kanti Das M, Majumdar A. Management of different types of wound by cyanoacrylate glue fixation: A random study of 213 patients. Journal of the Indian Medical Assocation 2004;102(11):625‐6. [PubMed] [Google Scholar]
Greene 1999 {published data only}
- Greene D, Koch RJ, Goode RL. Efficacy of octyl‐2‐cyanoacrylate tissue glue in blepharoplasty. A prospective controlled study of wound‐healing characteristics. Archives of Facial Plastic Surgery 1999;1(4):292‐6. [DOI] [PubMed] [Google Scholar]
Hock 2002 {published data only}
- Hock MO, Ooi SB, Saw SM, Lim SH. A randomized controlled trial comparing the hair apposition technique with tissue glue to standard suturing in scalp lacerations (HAT study). Annals of Emergency Medicine 2002;40(1):19‐26. [DOI] [PubMed] [Google Scholar]
Liebelt 1997 {published data only}
- Liebelt EL. Current concepts in laceration repair. Current Opinion in Pediatrics 1997;9(5):459‐64. [DOI] [PubMed] [Google Scholar]
Ludlow 2000 {published data only}
- Ludlow JB, Kutcher MJ, Samuelson A. Intraoral digital imaging documenting recurrent aphthous ulcer healing in 2‐octyl cyanoacrylate versus sham‐treated lesions. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2000;89(4):425‐31. [DOI] [PubMed] [Google Scholar]
Mattick 2002b {published data only}
- Mattick A. Use of tissue adhesives in the management of paediatric lacerations. Emergency Medicine Journal 2002;19(5):382‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 2003 {published data only}
- Quinn J, Cummings S, Callaham M, Sellers K. Conservative treatment reduced pain and was as effective as sutures in hand lacerations. The Journal of Bone and Joint Surgery. American Volume 2003;85‐A(5):974. [DOI] [PubMed] [Google Scholar]
Qureshi 1997 {published data only}
- Qureshi A, Drew PJ, Duthie GS, Roberts AC, Monson JR. n‐Butyl cyanoacrylate adhesive for skin closure of abdominal wounds: preliminary results. Annals of the Royal College of Surgeons of England 1997;79(6):414‐5. [PMC free article] [PubMed] [Google Scholar]
Saxena 1999 {published data only}
- Saxena AK, Willital GH. Octylcyanoacrylate tissue adhesive in the repair of pediatric extremity lacerations. American Surgeon 1999;65(5):470‐2. [PubMed] [Google Scholar]
Singer 2002 {published data only}
- Singer AJ, Quinn JV, Clark RE, Hollander JE, TraumaSeal Study Group. Closure of lacerations and incisions with octylcyanoacrylate: a multicenter randomized controlled trial. Surgery 2002;131(3):270‐6. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Quinn JV, Hollander JE. Comparison of Octylcyanoacrylate and Standard Wound Closure Methods for Lacerations and Incisions: A Multi‐center Trial. Academic Emergency Medicine 2001;8(5):538. [Google Scholar]
Sportsmed 1997 {published data only}
- Skin Wounds: To Glue or Not to Glue?. The Physician and Sportsmedicine 1997; Vol. 25, issue 5:18, 22.
Stuart 1999 {unpublished data only}
- Stuart, J. PRCT comparing staples with glue for scalp lacerations. http://www.nrr.nhs.uk/ViewDocument.asp?ID=N0155009284 1999.
Sutton 1985 {published data only}
- Sutton R, Pritty P. Use of sutures or adhesive tapes for primary closure of pretibial lacerations. British Medical Journal (Clinical research ed.) 1985;290(6482):1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Symeonoglou 1998 {published data only}
- Symeonoglou G, Songra A. Comparison of sutures and tissue adhesives in the treatment of facial lacerations: a clinical trial [abstract]. Journal of Cranio Maxillo Facial Surgery 1998;26(Suppl 1):187. [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Statistics Notes: Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Applebaum 1993
- Applebaum JS, Zalut T, Applebaum D. The use of tissue adhesion for traumatic laceration repair in the emergency department. Annals of Emergency Medicine 1993;22(7):1190‐2. [DOI] [PubMed] [Google Scholar]
Coulthard 2004
- Coulthard P, Worthington HV, Esposito M, Elst M, Waes OJF. Tissue adhesives for closure of surgical incisions. Cochrane Database of Systematic Reviews 2002, Issue 3. [Art. No.: CD004287. DOI: 10.1002/14651858.CD004287.pub2] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care. 2nd Edition. London, UK: BMJ Book, 2001:300. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmasalme 1995
- Elmasalme FN, Matbouli SA, Zuberi MS. Use of tissue adhesive in the closure of small incisions and lacerations. Journal of Pediatric Surgery 1995;30(6):837‐8. [DOI] [PubMed] [Google Scholar]
Goodman 1994
- Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Annals of Internal Medicine 1994;121(3):200‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, eds. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005] Appendix 5b. http://www.cochrane.org/resources/handbook/hbook.htm (accessed October 17 2007).
Hollander 1995a
- Hollander JE, Singer AJ, Valentine S, Henry MC. Wound registry: development and validation [published erratum appears in Annals of Emergency Medicine 1995;26(4):532]. Annals of Emergency Medicine 1995;25(5):675‐85. [DOI] [PubMed] [Google Scholar]
Hollander 1995b
- Hollander JE, Blasko B, Singer AJ, Valentine S, Thode HC, Jr, Henry MC. Poor correlation of short‐ and long‐term cosmetic appearance of repaired lacerations. Academic Emergency Medicine 11‐1‐1995;2(11):983‐7. [DOI] [PubMed] [Google Scholar]
Hollander 1999
- Hollander JE, Singer AJ. Laceration management. Annals of Emergency Medicine 1999;34(3):356‐67. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Mizrahi 1988
- Mizrahi S, Bickel A, Ben‐Layish E. Use of tissue adhesives in the repair of lacerations in children. Journal of Pediatric Surgery 1988;23(4):312‐3. [DOI] [PubMed] [Google Scholar]
Morton 1988
- Morton RJ, Gibson MF, Sloan JP. The use of histoacryl tissue adhesive for the primary closure of scalp wounds. Archives of Emergency Medicine 1988;5(2):110‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osmond 1995
- Osmond MH, Klassen TP, Quinn JV. Economic comparison of a tissue adhesive and suturing in the repair of pediatric facial lacerations. Journal of Pediatrics 1995;126(6):892‐5. [DOI] [PubMed] [Google Scholar]
Osmond 2000
- Osmond MH. Wound repair and tissue adhesives. In: Moyer VA, Elliott EJ, Davis RL, Gilbert R, Klassen T, Logan S, Mellis C, Williams K editor(s). Evidence Based Pediatrics and Child Health. 1st Edition. London: BMJ Books, 2000. [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116:78‐84. [DOI] [PubMed] [Google Scholar]
Perron 2000
- Perron AD, Garcia JA, Parker Hays E, Schafermeyer R. The efficacy of cyanoacrylate‐derived surgical adhesive for use in the repair of lacerations during competitive athletics. American Journal of Emergency Medicine 2000;18(3):261‐3. [DOI] [PubMed] [Google Scholar]
Quinn 1995
- Quinn JV, Drzewiecki AE, Stiell IG, Elmslie TJ. Appearance scales to measure cosmetic outcomes of healed lacerations. American Journal of Emergency Medicine 1995;13(2):229‐31. [DOI] [PubMed] [Google Scholar]
Quinn 1998b
- Quinn JV. Tissue Adhesives in Wound Care. Hamilton: B.C. Decker Inc., 1998. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Sells 1999
- Sells L, Mihalov L. Topical anesthetics and tissue adhesives: a new generation in pediatric wound management. Pediatric Emergency Medicine Reports 1999;4(9):1‐10. [Google Scholar]
Sibert 1981
- Sibert JR, Maddocks GB, Brown BM. Childhood accidents‐‐an endemic of epidemic proportion. Archives of Disease in Childhood 1981;56(3):225‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 17 October 2007).
Watson 1989
- Watson DP. Use of cyanoacrylate tissue adhesive for closing facial lacerations in children [see comments]. British medical Journal (Clinical Research Ed ) 1989;299(6706):1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zempsky 2005
- Zempsky WT, Zehrer CL, Lyle CT, Hedbloom EC. Economic comparison of methods of wound closure: would closure strips vs. sutures and wound adhesives. International Wound Journal 2005;2(3):272‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Farion 2003
- Farion KJ, Osmond MH, Hartling L, Russell KF, Klassen TP, Crumley E, Wiebe N. Tissue adhesives for traumatic lacerations: A systematic review of randomized controlled trials. Academic Emergency Medicine 2003;10(2):110‐8. [DOI] [PubMed] [Google Scholar]


