Abstract
Amphotericin B (AMB) remains the principal therapeutic choice for deep mycoses. However, its application is limited by toxicity and a route of administration requiring slow intravenous injection. An oral formulation of this drug is desirable to treat acute infections and provide prophylactic therapy for high-risk patients. Cochleates are a novel lipid-based delivery system that have the potential for oral administration of hydrophobic drugs. They are stable phospholipid-cation crystalline structures consisting of a spiral lipid bilayer sheet with no internal aqueous space. Cochleates containing AMB (CAMB) inhibit the growth of Candida albicans, and the in vivo therapeutic efficacy of CAMB administered orally was evaluated in a mouse model of systemic candidiasis. The results indicate that 100% of the mice treated at all CAMB doses, including a low dosage of 0.5 mg/kg of body weight/day, survived the experimental period (16 days). In contrast, 100% mortality was observed with untreated mice by day 12. The fungal tissue burden in kidneys and lungs was assessed in parallel, and a dose-dependent reduction in C. albicans from the kidneys was observed, with a maximum 3.5-log reduction in total cell counts at 2.5 mg/kg/day. However, complete clearance of the organism from the lungs, resulting in more than a 4-log reduction, was observed at the same dose. These results were comparable to a deoxycholate AMB formulation administered intraperitoneally at 2 mg/kg/day (P < 0.05). Overall, these data demonstrate that cochleates are an effective oral delivery system for AMB in a model of systemic candidiasis.
The opportunistic fungal pathogen Candida albicans causes life-threatening infections among cancer patients, organ or bone marrow transplant recipients, and patients with congenital and acquired immunodeficiencies (4, 14, 17, 24, 28, 29, 32, 43). Candida is the fourth leading cause of nosocomial bloodstream infections, accounting for 8% of all infections, and remains an important cause of morbidity and mortality in immunocompromised patients (7, 28, 31, 32, 43).
Parenteral administration of amphotericin B (AMB), a polyene antibiotic with strong antifungal activity, remains the therapy of choice for systemic mycoses. It is highly hydrophobic and is commonly administrated as desoxycholate amphotericin (DAMB), a detergent micelle complex. AMB binds preferentially to ergosterol in fungal plasma membranes, although it also interacts with animal cell sterols such as cholesterol, which accounts for known toxicity (10, 35). DAMB therapy is associated with nephrotoxicity, central nervous system and liver damage, and side effects such as nausea and fever (23, 34, 35).
AMB, with its inherent low solubility in water and many organic solvents, shows relatively poor bioavailability (11, 12). In order to increase the therapeutic index of AMB and reduce its associated toxicity, new lipid-based formulations have been developed (1, 3, 33). These drug delivery systems, such as liposomal formulations, lipid complexes, lipid emulsions, and colloidal dispersions, have been introduced into clinical practice. But their use remains limited by cost, stability, and toxicity (19, 21, 33, 39, 40, 42). Despite the improvement in therapeutic index for liposomal AMB formulations, the overall prognosis for patients with severe candidemia remains poor due to the inadequacy of treatments. The development of new, effective antifungal delivery systems for acute and prophylactic application remains an important objective.
AMB is not significantly absorbed across the gastrointestinal tract in either detergent-solubilized form or in liposomal preparations (41). AMB cochleates (CAMB) are a novel lipid-based delivery vehicle that have an advantage over existing formulations due to the stability of cochleates and their resistance to degradation in the gastrointestinal tract. Thus, cochleate preparations have the potential to delivery AMB orally.
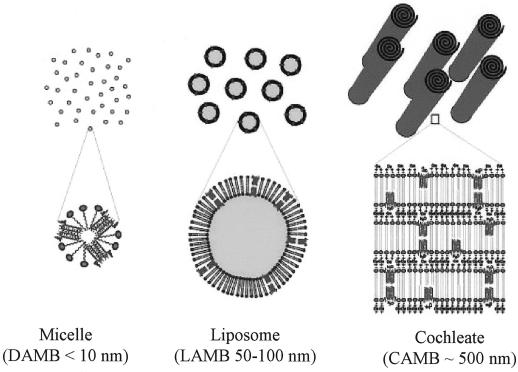
Cochleates are stable phospholipid-calcium precipitates comprised mainly of phosphatidylserine. They have a defined multilayered structure consisting of a continuous, solid, lipid bilayer sheet rolled up in a spiral, with no internal aqueous space (Fig. 1). Papahadjopoulos et al. first described cochleates in 1975 as an intermediate in the preparation of large unilamellar vesicles (30). Cochleates have been used to deliver protein, peptide, and DNA for vaccine and gene therapy applications (26, 44) and have been used recently as a drug delivery system (45, 46; L. Zarif, I. Segarra, T. Jin, D. Hyra, and R. J. Mannino, Proceedings of the 26th International Symposium on Controlled Release of Bioactive Materials, abstr. p. 964–965, 1999). CAMB have been shown to be highly protective in a mouse candidiasis model following parenteral administration (J. R. Graybill, L. K. Najvar, R. Bocanegra, A. Scolpino, R. J. Mannino, and L. Zarif, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2009, p. 583, 1999). Because of the hydrophobic nature of AMB molecules, it was hypothesized that AMB would be localized in the unique, rigid lipid bilayers of the cochleates. This unique association should provide protection for AMB from degradation when exposed to harsh environmental conditions or enzymes. CAMB should be an ideal system to deliver amphotericin B orally. Initial biodistribution studies of CAMB administered orally in a mouse model showed that cochleates delivered therapeutic levels of AMB to target organs (46).
FIG. 1.
Schematic representation of physical states of AMB delivery suspensions, including dispersed detergent micelles, ordered liposomal vesicles, and rolled crystalline cochleates.
In this study, we describe the in vivo efficacy of CAMB delivered by the oral route in a murine model of systemic candidiasis by assessing animal survival and fungal tissue burden in lungs and kidneys of infected animals. CAMB is compared to two commercially available AMB preparations, desoxycholate dispersed (DAMB; Fungizone) and liposomal amphotericin B (LAMB; AMBisome).
MATERIALS AND METHODS
Organism and culture conditions.
C. albicans ATCC 36082 (American Type Culture Collection, Rockville, Md.) was used throughout this study. Cells from this suspension were streaked onto YPD agar plates (1% Bacto yeast extract–2% Bacto Peptone–2% dextrose [adjusted to pH 5.7]–2% [wt/wt] Bacto agar), which were then incubated at 30°C for 48 h. A well-separated colony was resuspended in 10 ml of YPD broth and incubated at 30°C overnight. An aliquot (100 μl) of the overnight culture was transferred to 50 ml of YPD medium and incubated at 30°C for 18 h. The log-phase Candida suspension was centrifuged at 3,000 × g for 10 min and washed two times with an equal volume of sterile saline. Challenge organisms were quantified by hemocytometry and were diluted with sterile saline to a final concentration of 4 × 106 organisms per ml. The viable count was confirmed by serially diluting the yeast suspension and plating the inoculum onto YPD agar plates. The MIC of CAMB was determined by the microdilution method of the National Committee for Clinical Laboratory Standards (27). The end point was determined as the point at which growth was not observed.
Animals.
Female BALB/c mice (age, 10 to 12 weeks; weight, 20 to 25 g) (Charles River Laboratories, Wilmington, Mass.) were used throughout the experiments. The mice were housed in cages with five animals per group and had access to food and water ad libitum. All procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Public Health Research Institute (New York, N.Y.) and the United States Public Health Service.
CAMB preparation.
CAMB were prepared using an aqueous/aqueous hydrogel binary system (Zarif et al., Proceedings 26th Int. Symp. Controlled Release Bioactive Mater., abstr. p. 964–965). AMB in methanol (0.5 mg/ml) was added to l-α-phosphatidylserine (Avanti Lipids, Alabaster, Ala.) in 100% chloroform at a molar ratio of 10:1. The mixture was placed in a round-bottom solvent flask and was dried to a mixed drug-lipid film using a rotary evaporator. The film was hydrated with deionized water at a concentration of 10 mg of lipid/ml, and the crude AMB-lipid suspension was sonicated for 10 min at 80 W in alternate mode, with a temperature clamp at 4°C to form unilamellar vesicles. The liposome suspension was mixed with 40% (wt/wt) dextran (molecular weight, ∼500,000) in a suspension of 3/1 (vol/vol) dextran/liposome. This mixture was injected via an 18-gauge syringe into 15% (wt/wt) polyethylene glycol 8000 with stirring at 800 to 1,000 rpm. A CaCl2 solution (100 mM) was added to the suspension to reach a final concentration of 1 mM, and stirring was continued for 1 h. A washing buffer containing 1 mM CaCl2 and 150 mM NaCl was added to the suspension at a ratio of 1:1 (vol/vol). The suspension was vortexed and centrifuged at 3,000 × g at 4°C for 30 min. The sample was resuspended in washing buffer and recentrifuged, as above. The final pellet was reconstituted with the same buffer. Laser light scattering (weight analysis, Coulter N4 Plus) indicated that the CAMB mean diameter was 407.3 ± 233.8 nm. The amount of AMB in the cochleates was determined by high-pressure liquid chromatography (45).
Antifungal agents.
DAMB (Bristol-Myers Squibb, Princeton, N.J.), LAMB (AMBisome; NeXstar Pharmaceuticals, Cambridge, United Kingdom), and CAMB (BioDelivery Sciences Inc., Newark, N.J.) were used in this study. DAMB and LAMB were reconstituted according to the manufacturers' instructions and were diluted in sterile water.
Animal infection model and antifungal assessment.
Disseminated infection with C. albicans was induced by injection of 106 blastoconidia in 0.25 ml of sterile saline via the lateral tail vein of female BALB/c mice. Systemic infections were produced that caused complete mortality in nontreated control groups within 10 to 15 days. There were 10 mice per treatment group for survival and target organ studies; the mice were housed in groups of 5 in separate cages. Infected mice were treated with DAMB, LAMB, and CAMB as follows: DAMB (1 and 2 mg/kg of body weight/day) was injected intraperitoneally (IP), LAMB (10 mg/kg/day) and CAMB (0.5, 1, 2.5, 5, 10, and 20 mg/kg/day) were given by oral gavage (PO). Agent administration was begun 24 h following infection and continued as a daily single dose of drug for 15 consecutive days. The animals were fasted for 4 h before each drug dose administration, but water was available ad libitum. A group of 10 infected mice treated with empty cochleates by oral administration was included as a control. Survival and target organ studies were used to determine antifungal efficacy. Morbidity and mortality were recorded twice a day for 16 days. The surviving mice were killed by carbon dioxide asphyxiation 24 h after the administration of the last dose of drug. The animals were necropsied, and target organs, kidneys and lung, were removed aseptically, weighed, and homogenized in sterile saline. The microbiological response to antifungal treatment was evaluated by a determination of the concentration (in CFU per gram) of C. albicans in tissue. Serial dilutions of homogenized target organs were plated in duplicate onto YPD agar plates containing 80 mg of chloramphenicol/ml and 50 mg of ampicillin/ml to eliminate bacterial cross-contamination. Culture plates were incubated at 30°C for 48 h, after which the CFU were counted and the number of CFU per gram of tissue was calculated for each organ. The method was sensitive for detection of ≥10 CFU/g. The culture-negative plates were counted as having 0 CFU/g.
Statistical analysis.
Differences in survival after 16 days of observation were assessed by Kaplan-Meier analysis followed by the Wilcoxon test. Comparisons of colony counts among different treatment groups were performed by the Kruskal-Wallis test with multiple comparison. A P value of <0.05 was considered statistically significant. All statistical analysis was performed with the software package GBSTAT (Dynamic Microsystems, Inc., Silver Spring, Md.).
RESULTS
Therapeutic efficacy of CAMB in a survival mouse model of disseminated candidiasis.
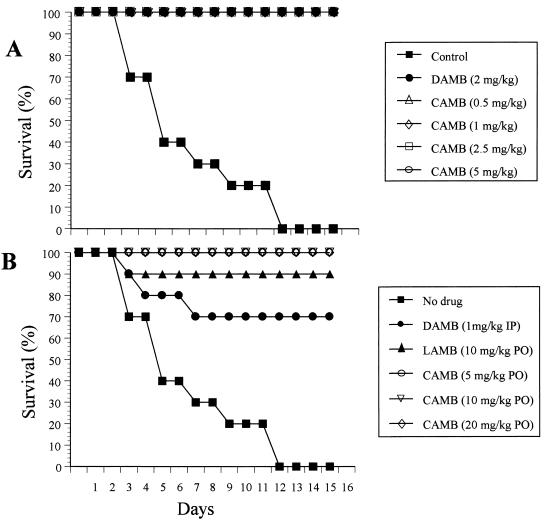
CAMB effectively inhibited growth of C. albicans in vitro with a MIC of <1 μg/ml (45). The in vivo therapeutic efficacy of oral CAMB was evaluated in two trials of a disseminated candidiasis model utilizing female BALB/c mice (Fig. 2A and B). Mice were treated daily for 15 days starting 24 h after infection. In trial 1, CAMB was given PO at doses of 0.5, 1, 2.5, and 5 mg/kg/day and was compared with DAMB at 2 mg/kg/day administered IP. In trial 2, CAMB administered PO was investigated at 5, 10, and 20 mg/kg/day and was compared with LAMB at 10 mg/kg/day administered PO and DAMB at 1 mg/kg/day administered IP. In both trials, empty cochleates were used as a negative control. Mice treated with empty cochleates rapidly became sick, and 100% mortality was observed by day 12. In contrast, even the lowest dose of CAMB, 0.5 mg/kg/day, provided 100% survival even after 16 days postinfection (Fig. 2A). Mice treated with DAMB at 1 mg/kg/day showed 30% mortality by day 7 of infection, although a dose of 2 mg/kg/day was completely protective (P < 0.05). Oral CAMB at 0.5 mg/kg/day was equivalent to IP DAMB at 2 mg/kg/day (Fig. 2A) (P < 0.01), and both were superior to LAMB at 10 mg/kg/day PO (P < 0.05), which showed 10% mortality (Fig. 2B). In each case, the difference in the survival between treated and untreated animals was significant (P < 0.001).
FIG. 2.
Survival of mice infected with C. albicans (106 yeast cells per mouse) was determined for mice treated with PO CAMB at 0.5, 1, 2.5, and 5 mg/kg/day and IP DAMB at 2 mg/kg/day (A) and with PO CAMB at 5, 10, and 20 mg/kg/day, IP DAMP at 1 mg/kg/day, and PO LAMB at 10 mg/kg/day (B). The mice were infected intravenously by administration of a single bolus of 0.25 ml of a C. albicans suspension in sterile saline. The treatment was started 24 h postinfection by IP DAMB, PO LAMB, and PO CAMB administration of a daily single dose (0.1 ml) of drug for 15 days. Empty cochleates were used for the control.
Efficacy of CAMB in the target organ assay of disseminated candidiasis.
An evaluation of tissue burden for C. albicans was conducted in parallel with the two survival studies in which CAMB was administered PO (Fig. 2A and B). Surviving animals were euthanized on day 17, which was 24 h after administration of the last dose of drug. Kidneys and lungs were removed and assayed for CFU of C. albicans. CAMB showed a dose-dependent reduction in C. albicans from both kidneys and lungs (Table 1). At the lowest dosage (0.5 mg/kg/day), CAMB was effective in reducing by 2 logs the CFU count from both kidneys and lungs relative to controls (P < 0.01). A maximum effect was obtained with CAMB at 2.5 mg/kg/day, which reduced CFU counts in the kidneys by 3.5 logs relative to controls (P < 0.05) and completely eradicated yeast cells from the lung. This activity was comparable to that of DAMB at 2 mg/kg/day administered IP (P < 0.05). AMB was found at comparable levels in tissues following CAMB (PO) at 10 mg/kg/day (kidneys: 0.374 ± 0.187 [n = 10] μg of AMB/g of tissue; lungs: 0.105 ± 0.306 [n = 10] μg of AMB/g of tissue) and DAMB (IP) at 2 mg/kg/day (kidneys: 0.440 ± 0.161 [n = 10] μg of AMB/g of tissue; lungs: 0.111 ± 0.093 [n = 10] μg of AMB/g of tissue).
TABLE 1.
Quantitative organ culture results from in vivo infection with C. albicans
| Drug | Dose (mg/kg/day) | Delivery route | No. of survivorsa | Organ load (CFU/g of tissue)e
|
|
|---|---|---|---|---|---|
| Kidneys | Lungs | ||||
| Trial 1 | |||||
| Controlb | 0 | PO | 0 | 110 ± 18 × 105 | 204 ± 45 × 102 |
| DAMB | 2 | IP | 10 | 20 ± 4 × 102 | 0 |
| CAMB | 0.5 | PO | 10 | 100 ± 16 × 103 | 15 ± 7 × 101 |
| CAMB | 1 | PO | 10 | 503 ± 96 × 102 | 3 ± 5 × 101 (7) |
| CAMB | 2.5 | PO | 10 | 26 ± 8 × 102 c,d | 0 |
| CAMB | 5 | PO | 10 | 12 ± 6 × 102 c,d | 0 |
| Trial 2 | |||||
| Control | 0 | PO | 0 | 121 ± 48 × 105 | 172 ± 87 × 102 |
| DAMB | 1 | IP | 7 | 496 ± 71 × 102 | 28 ± 57 × 102 (7) |
| LAMB | 10 | PO | 9 | 275 ± 216 × 102 | 6 ± 7 × 101 (5) |
| CAMB | 5 | PO | 10 | 13 ± 8 × 102 c,d (1) | 0 |
| CAMB | 10 | PO | 10 | 28 ± 31 × 102 c,d | 0 |
| CAMB | 20 | PO | 10 | 26 ± 48 × 102 c,d (2) | 0 |
Surviving animals, out of a total group of 10, on day 16.
Animals treated PO with empty cochleates.
P < 0.01 compared with results for the respective control mice.
P < 0.05 compared with results for the respective DAMB at 2 mg/kg/day.
Numbers in parentheses indicate the number of animals with undetectable colony counts.
At a lower dose of 1 mg/kg/day, both DAMB and CAMB were equally effective in reducing by 2 logs the CFU count from mouse kidneys. However, CAMB was somewhat more effective, since it reduced by 3 logs the yeast cell count from the lung (P < 0.05). LAMB at 10 mg/kg/day administered PO was 10-fold less effective relative to CAMB at 2.5 mg/kg/day PO in reducing the fungal density in kidneys (2.5 logs versus 3.5 for CAMB) (P < 0.05) and could not eradicate yeast cells from the lungs.
DISCUSSION
AMB remains the drug of choice for life-threatening invasive fungal infections, primarily due to its broad spectrum of activity (5, 15, 16, 38, 41), a paucity of inherent resistant species (Candida lusitaniae), and a low propensity for inducing resistance (28). AMB is a polyene antibiotic that binds to ergosterol present in the fungal cellular membrane. After binding, pore formation results in inhibition of metabolic activity due to the release of intracellular constituents, which leads to cell death. AMB binds with significant but reduced affinity to sterols such as cholesterol present in the mammalian cellular membrane, leading to increased cell membrane permeability to ions, which produces the toxic side effects of the drug (10, 35).
DAMB is a commercial micellar deoxycholate preparation of AMB that has proven efficacy in the treatment of invasive fungal infections. However, its narrow therapeutic index and adverse side effects, especially nephrotoxicity, limit its use (8, 34, 35). Numerous researchers have tried over the last two decades to overcome AMB toxicity problems and improve the safety of the drug by formulating AMB into lipid-based delivery systems (2, 9, 18, 20, 25, 33, 36, 39, 40, 42). Different lipid-based AMB systems were developed, including AMBisome, Amphotec, and Abelcet, which improve AMB's therapeutic drug index (22). The pharmacokinetics and pharmacodynamics of AMB incorporated into liposomes differ greatly from those of its micellar counterpart. Different studies suggest that drugs incorporated into liposomes or lipid formulations are selectively taken up into the reticuloendothelial system and concentrated in the liver, spleen, and lung (22). Lipid-rich particles are also ingested by phagocytic monocytes, which help to target sites of infection or inflammation (6). Moreover, the liposomal products have significantly less nephrotoxicity than conventional DAMB even at much higher doses (25, 39, 40). However, they are frequently utilized as second-line therapeutics because they are less efficacious, unstable, and more expensive than DAMB. At present, none of these formulations can be used for oral delivery.
Encapsulation of AMB into lipid-based cochleates results in stable, nontoxic, and highly efficacious AMB lipid particles that facilitate systemic delivery of AMB after oral administration. Cochleates are composed of safe products: phosphatidylserine (PS) and calcium. PS is used to nourish and protect the structure and function of brain cells, and numerous clinical trials on PS given orally demonstrate the safety of PS in treating dementia and age-associated memory and intellectual impairment (13, 37). In this study, mice treated with multiple (15) high oral doses (10 and 20 mg/kg/day) of CAMB showed no symptoms of toxicity when compared with mice treated with low IP doses (1 and 2 mg/kg/day) of DAMB.
Cochleates can be envisioned as membrane fusion intermediates which facilitate an interaction with biological membranes. AMB appears to be bound between the spiral layers of the cochleates, which protects it from the harsh environment of the gastrointestinal tract. It is readily released upon interaction of the cochleates with target cells (L. Zarif, unpublished data). CAMB were highly effective at blocking cell growth of C. albicans in vitro, at a level comparable to that of DAMB (45). More importantly, oral administration of CAMB in our mouse model of disseminated candidiasis resulted in 100% survival at all doses applied (0.5 to 20 mg/kg/day) (Fig. 2) and showed a significant dose-dependent reduction in organ colonization (Table 1). Low doses of CAMB, 0.5 mg/kg/day, were sufficient to achieve complete survival of the mice infected with 106 Candida cells and resulted in a 2-log reduction in kidney and lung colony counts. At 2.5 mg/kg/day, CAMB were able to reduce kidney colony counts by >3 logs and completely eradicated yeast cells from the lung (Table 1). Nearly identical results were found for DAMB at 2 mg/kg/day administrated IP. Tissue levels of AMB in kidneys and lungs following these treatments were also highly similar. However, DAMB at 1 mg/kg/day was less effective and oral administration of LAMB at 10 mg/kg/day was also less effective than CAMB in reducing the fungal load from the kidneys and lung (P < 0.05) (Table 1). The effectiveness of CAMB in clearing organisms from the lung may result from the propensity of cochleates to be taken up by alveolar macrophages and/or from concentrating the drug in lung tissue (Zarif, unpublished data).
These results demonstrate that CAMB, a formulation of cochleate lipid particles containing AMB, promotes the uptake of AMB from the gastrointestinal tract and suggest that this route of administration has the potential for therapeutic application.
ACKNOWLEDGMENTS
We thank Pierre Daublain and Jie Wang for technical assistance in preparing amphotericin B cochleate formulations. We give special acknowledgment to Chris Lambros from the NIAID for his support.
This research work was supported by NIH SBIR Grant R43-AI-46040 (to L.Z.).
REFERENCES
- 1.Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant. 1994;14(Suppl. 5):S3–S7. [PubMed] [Google Scholar]
- 2.Adler-Moore J P, Proffitt R T. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J Liposome Res. 1993;3:429–450. [Google Scholar]
- 3.Adler-Moore J P, Chiang S M, Satorius A, Guerra D, McAndrews B, McManus E J, Proffitt R T. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome) J Antimicrob Chemother. 1991;28(Suppl. B):63–71. doi: 10.1093/jac/28.suppl_b.63. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie E J, Rex J H, Uzun O, Vartivarian S. Predictors of adverse outcome in cancer patients with candidemia. Am J Med. 1998;104:238–245. doi: 10.1016/s0002-9343(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong D. Treatment of opportunistic fungal infections. Clin Infect Dis. 1993;16:1–7. doi: 10.1093/clinids/16.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Bangham A D. Liposomes: realizing their promise. Hosp Pract. 1992;27:51–56. doi: 10.1080/21548331.1992.11705537. [DOI] [PubMed] [Google Scholar]
- 7.Beck-Sague C, Jarvis W R. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 8.Bodey G P, Anaissie E J, Elting L S, Estey E, O'Brien S, Kantarjian H. Antifungal prophylaxis during remission induction therapy for acute leukemia fluconazole versus intravenous amphotericin B. Cancer. 1994;73:2099–2106. doi: 10.1002/1097-0142(19940415)73:8<2099::aid-cncr2820730814>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Brajtburg J, Elberg S, Kobayashi G S, Bolard J. Amphotericin B incorporated into egg lecithin-bile salt mixed micelles: molecular and cellular aspects relevant to therapeutic efficacy in experimental mycoses. Antimicrob Agents Chemother. 1994;38:300–306. doi: 10.1128/aac.38.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brajtburg J, Powderly W G, Kobayashi G S, Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ching M S, Raymond K, Bury R W, Mashford M L, Morgan D J. Absorption of orally administered amphotericin B lozenges. Br J Clin Pharmacol. 1983;16:106–108. doi: 10.1111/j.1365-2125.1983.tb02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collette N, van der Auwera P, Lopez A P, Heymans C, Meunier F. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob Agents Chemother. 1989;33:362–368. doi: 10.1128/aac.33.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel R R, Satzger W, Gunther W. Double-blind cross-over study of phosphatidylserine vs. placebo in patients with early dementia of the Alzheimer type. Neuropsychopharmacology. 1992;2:149–155. doi: 10.1016/0924-977x(92)90025-4. [DOI] [PubMed] [Google Scholar]
- 14.Fraser V J, Jones M, Dunkel J, Storfer S, Medoff G, Dunagan W C. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992;15:414–421. doi: 10.1093/clind/15.3.414. [DOI] [PubMed] [Google Scholar]
- 15.Gallis H A. Amphotericin B: a commentary on its role as an antifungal agent and as a comparative agent in clinical trials. Clin Infect Dis. 1996;22(Suppl. 2):S145–S147. doi: 10.1093/clinids/22.supplement_2.s145. [DOI] [PubMed] [Google Scholar]
- 16.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich J M, Reed E C, Mori M, Fisher L D, Skerrett S, Dandliker P S, Klis B, Counts G W, Meyers J D. Clinical features and analysis of risk factors for invasive candidal infection after marrow transplantation. J Infect Dis. 1991;164:731–740. doi: 10.1093/infdis/164.4.731. [DOI] [PubMed] [Google Scholar]
- 18.Groll A H, Muller F M, Piscitelli S C, Walsh T J. Lipid formulations of amphotericin B: clinical perspectives for the management of invasive fungal infections in children with cancer. Klin Paediatr. 1998;210:264–273. doi: 10.1055/s-2008-1043890. [DOI] [PubMed] [Google Scholar]
- 19.Gulati M, Bajad S, Singh S, Ferdous A J, Singh M. Development of liposomal amphotericin B formulation. J Microencapsul. 1998;15:137–151. doi: 10.3109/02652049809006844. [DOI] [PubMed] [Google Scholar]
- 20.Hay R J. Liposomal amphotericin B, AmBisome. J Infect. 1994;28(Suppl. 1):35–43. doi: 10.1016/s0163-4453(94)95956-0. [DOI] [PubMed] [Google Scholar]
- 21.Heinemann V, Kahny B, Debus A, Wachholz K, Jehn U. Pharmacokinetics of liposomal amphotericin B (AmBisome) versus other lipid-based formulations. Bone Marrow Transplant. 1994;14(Suppl. 5):S8–S9. [PubMed] [Google Scholar]
- 22.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22(Suppl. 2):S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 23.Kaloyanides G J. Antibiotic-related nephrotoxicity. Nephrol Dial Transplant. 1994;9(Suppl. 4):130–134. [PubMed] [Google Scholar]
- 24.Kauffman C A, Vazquez J A, Sobel J D, Gallis H A, McKinsey D S, Karchmer A W, Sugar A M, Sharkey P K, Wise G J, Mangi R, Mosher A, Lee J Y, Dismukes W E. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14–18. doi: 10.1086/313583. [DOI] [PubMed] [Google Scholar]
- 25.Leenders A C, Daenen S, Jansen R L, Hop W C, Lowenberg B, Wijermans P W, Cornelissen J, Herbrecht R, van der Lelie H, Hoogsteden H C, Verbrugh H A, de Marie S. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol. 1998;103:205–212. doi: 10.1046/j.1365-2141.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- 26.Mannino R J, Gould-Fogerite S. Antigen cochleate formulations for oral and systemic vaccination in new generation vaccines. In: Levine M M, editor. New generation vaccines. New York, N.Y: Marcel Dekker; 1997. pp. 1–9. [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 28.Nguyen M H, Peacock J E, Jr, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 29.Nieto-Rodriguez J A, Kusne S, Manez R, Irish W, Linden P, Magnone M, Wing E J, Fung J J, Starzl T E. Factors associated with the development of candidemia and candidemia-related death among liver transplant recipients. Ann Surg. 1996;223:70–76. doi: 10.1097/00000658-199601000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papahadjopoulos D, Vail W J, Jacobson K, Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975;394:483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- 31.Pfaller M A, Jones R N, Doern G V, Sader H S, Hollis R J, Messer S A. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. The SENTRY Participant Group. J Clin Microbiol. 1998;36:1886–1889. doi: 10.1128/jcm.36.7.1886-1889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller M A, Jones R N, Messer S A, Edmond M B, Wenzel R P. National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis. 1998;31:327–332. doi: 10.1016/s0732-8893(97)00240-x. [DOI] [PubMed] [Google Scholar]
- 33.Robinson R F, Nahata M C. A comparative review of conventional and lipid formulations of amphotericin B. J Clin Pharm Ther. 1999;24:249–257. doi: 10.1046/j.1365-2710.1999.00220.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabra R, Branch R A. Amphotericin B nephrotoxicity. Drug Saf. 1990;5:94–108. doi: 10.2165/00002018-199005020-00003. [DOI] [PubMed] [Google Scholar]
- 35.Sawaya B P, Briggs J P, Schnermann J. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J Am Soc Nephrol. 1995;6:154–164. doi: 10.1681/ASN.V62154. [DOI] [PubMed] [Google Scholar]
- 36.Singhal S, Hastings J G, Mutimer D J. Safety of high-dose amphotericin B lipid complex. Bone Marrow Transplant. 1999;24:116–177. doi: 10.1038/sj.bmt.1701829. [DOI] [PubMed] [Google Scholar]
- 37.Villardita C, Grioli S, Salmeri G, Nicoletti F, Pennisi G. Multicenter clinical trial of brain phosphatidylserine in elderly patients with intellectual deterioration. Clin Trials J. 1987;24:84–93. [Google Scholar]
- 38.Walsh T J, Gonzalez C, Lyman C A, Chanock S J, Pizzo P A. Invasive fungal infections in children: recent advances in diagnosis and treatment. Adv Pediatr Infect Dis. 1996;11:187–290. [PubMed] [Google Scholar]
- 39.Walsh T J, Hiemenz J W, Seibel N L, Perfect J R, Horwith G, Lee L, Silber J L, DiNubile M J, Reboli A, Bow E, Lister J, Anaissie E J. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26:1383–1396. doi: 10.1086/516353. [DOI] [PubMed] [Google Scholar]
- 40.Walsh T J, Yeldandi V, McEvoy M, Gonzalez C, Chanock S, Freifeld A, Seibel N I, Whitcomb P O, Jarosinski P, Boswell G, Bekersky I, Alak A, Buell D, Barret J, Wilson W. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother. 1998;42:2391–2398. doi: 10.1128/aac.42.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warnock D W. Amphotericin B: an introduction. J Antimicrob Chemother. 1991;28(Suppl. B):27–38. doi: 10.1093/jac/28.suppl_b.27. [DOI] [PubMed] [Google Scholar]
- 42.Wong-Beringer A, Jacobs R A, Guglielmo B J. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin Infect Dis. 1998;27:603–618. doi: 10.1086/514704. [DOI] [PubMed] [Google Scholar]
- 43.Wright W L, Wenzel R P. Nosocomial Candida. Epidemiology, transmission, and prevention. Infect Dis Clin N Am. 1997;11:411–425. doi: 10.1016/s0891-5520(05)70363-9. [DOI] [PubMed] [Google Scholar]
- 44.Zarif L, Mannino R J. Cochleates: lipid-based vehicles for gene delivery—concept, achievements and future development. In: Habib N, editor. Cancer gene therapy: past achievements and future challenges. London, England: Plenum Publishing Co.; 2000. pp. 83–94. [DOI] [PubMed] [Google Scholar]
- 45.Zarif L, Graybill J R, Perlin D, Najvar L, Bocanegra R, Mannino R J. Antifungal activity of amphotericin B cochleates against Candida albicans infection in a mouse model. Antimicrob Agents Chemother. 2000;44:1463–1469. doi: 10.1128/aac.44.6.1463-1469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarif L, Segarra L, Jin T, Scolpino A, Hyra D, Perlin D S, Graybill J R, Mannino R J. Oral and systemic delivery of AmB mediated by cochleates. AAPS PharmSci. 1999;1:S-453. [Google Scholar]