Abstract
Context
Injections with intramuscular (IM) testosterone esters have been available for almost 8 decades and not only result in predictable serum testosterone levels but are also the most inexpensive modality. However, they are difficult to self-administer and associated with some discomfort. Recently, subcutaneous (SC) administration of testosterone esters has gained popularity, as self-administration is easier with this route. Available data, though limited, support the feasibility of this route. Here we review the pharmacokinetics and safety of SC testosterone therapy with both long- and ultralong-acting testosterone esters. In addition, we provide guidance for clinicians on how to counsel and manage their patients who opt for the SC route.
Evidence Acquisition
Systematic review of available literature on SC testosterone administration including clinical trials, case series, and case reports. We also review the pharmacology of testosterone absorption after SC administration.
Evidence Synthesis
Available evidence, though limited, suggests that SC testosterone therapy in doses similar to those given via IM route results in comparable pharmacokinetics and mean serum testosterone levels. With appropriate training, patients should be able to safely self-administer testosterone esters SC with relative ease and less discomfort compared with the IM route.
Conclusion
Although studies directly comparing the safety of SC vs IM administration of testosterone esters are desirable, clinicians should consider discussing the SC route with their patients because it is easier to self-administer and has the potential to improve patient adherence.
Keywords: hypogonadism, transgender, androgen deficiency, testosterone replacement therapy, androgens
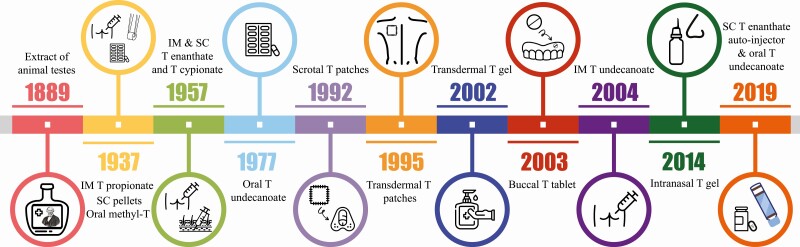
Testosterone is the main male sex hormone and is essential for the development and maintenance of male secondary sexual characteristics. Currently, testosterone therapy is indicated for men with unequivocal, organic, or pathologic androgen deficiency to alleviate symptoms and maintain secondary sexual characteristics by raising testosterone into the normal male range (1). In addition, testosterone therapy is used for gender-affirming (hormone) therapy for transgender men to induce masculinization (and suppress endogenous estradiol concentrations in patients with intact ovaries) (2). In both clinical scenarios, testosterone therapy is intended to be long term. Thus, it is desirable to have various formulation options available to ensure patient satisfaction and adherence. We have come a long way since the days of Brown-Séquard, who self-administered an extract of animal testes by subcutaneous (SC) injection in 1889 (Fig. 1) (3). Four decades after Brown-Séquard’s experiments, testosterone was isolated in 1935, and subsequently chemically synthesized (4-6); it took an additional 2 years for it to be introduced into clinical medicine for the treatment of male hypogonadism with SC or intramuscular (IM) injections of short-acting ester testosterone propionate, crystalline testosterone compressed into subcutaneous pellets, and oral methyltestosterone (7, 8). In the mid-1950s, long-acting testosterone esters (enanthate and cypionate) were introduced, and have since been the preferred testosterone formulation thanks to their affordability, longer half-life compared to propionate, and predictable pharmacokinetics (9). More recently, newer formulations of testosterone replacement have become available, which include ultralong-acting testosterone undecanoate for IM injection, transdermal patches and gels, buccal tablets, intranasal sprays, and oral testosterone undecanoate (Table 1), thus providing a range of options to choose from.
Figure 1.
Timeline of various testosterone formulations available since Brown-Sequard’s experiments in 1889.
Table 1.
Advantages and disadvantages of available testosterone formulations
| Route | Formulation | Advantages | Disadvantages |
|---|---|---|---|
| IM | T enanthate or cypionate | Relatively inexpensive, self-administered; predictable levels | Requires IM injection; peaks and valleys in serum T concentrations that may be associated with fluctuations in symptoms |
| T undecanoate | Infrequent administration | Requires IM injection of a large volume (3 or 4 mL); coughing episodes after injection in some men | |
| Transdermal | Gels (1%, 1.62%, or 2%) | Ease of application, good skin tolerability | Potential of transfer by skin contact; T concentrations may be variable from application to application; skin irritation in some men; moderately high DHT concentrations (of unknown significance) |
| Patch | Ease of application, predictable levels | High rate of skin irritation at application site; reduced adherence with sweating | |
| T axillary solution | Good skin tolerability | Potential transfer to others by contact; T concentrations may be variable from application to application; skin irritation in a small proportion of patients; moderately high DHT concentrations (of unknown significance) | |
| Transmucosal | Buccal tablets | Convenient | Gum irritation; dysgeusia; twice-daily dosing |
| Nasal gel | Rapid absorption; avoidance of first-pass metabolism | Multiple daily dosing; cannot be used in men with nasal disorders | |
| SC implant | Pellets | Infrequent administration | Requires surgical insertion; pellets may extrude spontaneously; risk of hematoma and infection |
| Oral | T undecanoate | Ease of administration | Requires twice-daily dosing; unfavorable effect on lipids and blood pressure |
Adapted from (1).
Abbreviations: DHT, 5-dihydrotestosterone; IM, intramuscular; SC, subcutaneous; T, testosterone.
Selection of the administration route of testosterone is influenced by patient preference, product availability, and the cost of the formulation. Each formulation has certain advantages and disadvantages (see Table 1) that can affect the patient’s choice and adherence (10-12). Patches result in skin irritation in a substantial number of patients, and sweating during the summer can affect patch adherence (13). Topical gels require daily application, can be messy, and carry the risk of exposure to those who come in contact with the patient’s application site (14). Nasal and buccal formulations require greater frequency of application and can cause local irritation (15-17). Long-acting SC pellets are costly, require surgical insertion, and are associated with the risk of infection and spontaneous extrusion (12). IM injections of long-acting testosterone esters (cypionate or enanthate) are cost-effective and result in physiological and predictable on-treatment serum testosterone levels, particularly when smaller doses are administered weekly (18). However, IM injections are associated with discomfort, patients experience difficulty with self-injection, and they often require assistance from family members to administer the drug. To mitigate the discomfort associated with frequent IM injections, they are commonly administered in large doses every 2 weeks to decrease the frequency of administration, resulting in large peaks and troughs (19, 20). The ultralong-acting ester testosterone undecanoate was developed to reduce these peaks and troughs, but the large volume injected has been rarely associated with a risk of pulmonary oil microembolism, necessitating administration of the drug by trained medical personnel (self-injection is not allowed) and observation of the patients in the clinic for 30 minutes thereafter (21, 22).
Despite the formal recommendation for oil-based testosterone formulations to be administered via the IM route, recent data suggest that SC administration of testosterone esters results in pharmacokinetics and serum testosterone concentrations that are similar to the IM route (23-27) and associated with less discomfort (24, 28). Recently, after assessing its safety and efficacy, the Food and Drug Administration approved an autoinjector device for weekly SC self-administration of testosterone enanthate (27, 29). However, this device is expensive compared to administration of ester with conventional syringe and needles.
Owing to the convenience of self-administration of testosterone esters, the SC route has recently gained popularity. The viability of using SC route for sex steroid administration was also shown in an elegant pharmacokinetic study in which nandrolone decanoate was administered to healthy male volunteers (30). Interestingly, previous data that used imaging (computed tomography or ultrasound) to estimate SC fat thickness and compared it with the length of the needle (or placement of the injectate) estimated that 12% to 85% of IM injections administered to men were actually SC (31-33). Indeed, this might explain the observation that IM injections are less painful in overweight and obese men (34). In this review, we summarize the published data on the pharmacokinetics and safety of SC administration of both long-acting (enanthate and cypionate) and ultralong-acting (undecanoate) testosterone esters in hypogonadal and transgender men. Last, we provide some guidance for clinicians regarding SC testosterone therapy.
Absorption of Injectable Testosterone
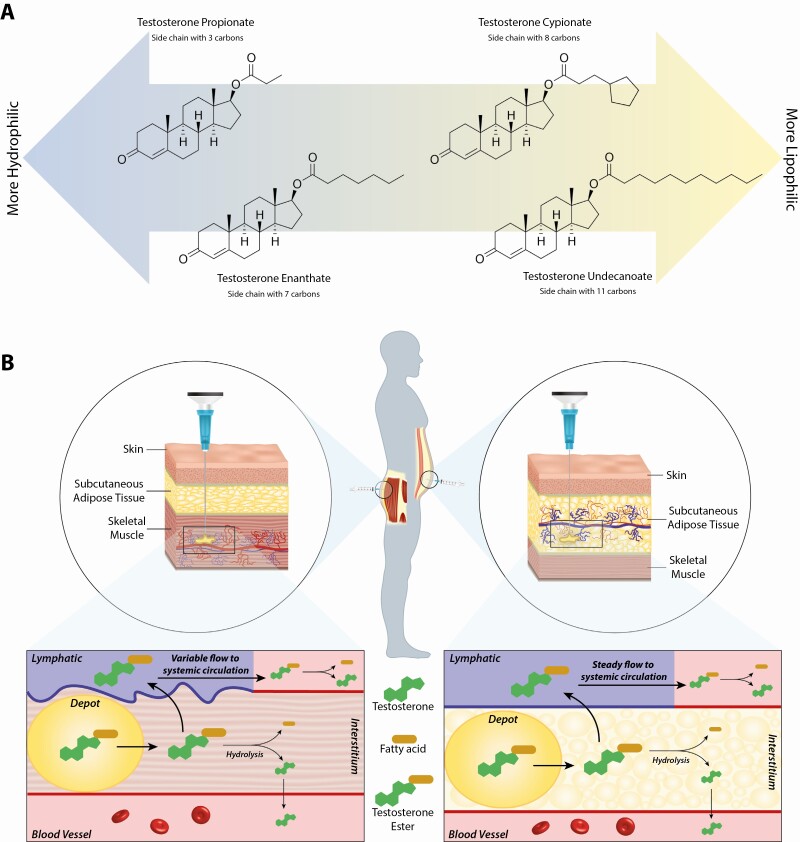
Unmodified testosterone has a half-life of 10 minutes; to overcome this limitation, testosterone is esterified and then dissolved in oil to allow for sustained release into the circulation after injection. These oily solutions contain a testosterone ester dissolved in vegetable oil (usually sesame seed, tea seed, castor seed, or cottonseed oil) with some benzyl alcohol. Benzyl alcohol is soluble not only in the oily phase, but also in the aqueous phase, thus facilitating the release of testosterone ester from the depot into the surrounding interstitial fluid (35). On release from the depot, the testosterone ester undergoes hydrolysis into testosterone and the ester-specific fatty acid (35, 36). Various testosterone esters have different absorption kinetics, with absorption time increasing with longer esterified side chains (fatty acids) because of the increased hydrophobicity of the molecule (Fig. 2A) (37). Commonly used testosterone esters include testosterone enanthate (7 carbons side chain), cypionate (8 carbons), and undecanoate (11 carbons). In the past, propionate (3 carbons) was widely used, but it is not in common use currently among adults. Absorption kinetics are affected by the viscosity of the oily vehicle, concentration of the ester (the higher the concentration in the depot, the higher the driving diffusion force for release), the volume of the product, and the site of the injection (35, 38).
Figure 2.
A, Illustration of the progressive increase in lipophilicity of testosterone esters with increase in number of carbons in the side chain. B, Schematic illustration of the absorption steps of testosterone esters after intramuscular (left) or subcutaneous (right) injection. With administration using either route, the ester exits the depot via diffusion into the interstitium, from where it enters the lymphatics and subsequently reaches the circulation where it undergoes hydrolysis by intracellular esterases. Testosterone ester is also partly hydrolyzed within the interstitium, with free testosterone entering the circulation directly.
Subcutaneous vs Intramuscular Routes
The IM and SC routes present a defined phase of absorption, in which the serum concentration of the drug administered progressively increases to a maximum (Cmax) and then decreases according to its elimination half-life. For testosterone esters, the time corresponding from administration to the Cmax, that is, time of maximum concentration (tmax), is determined by the rate at which absorption occurs, since the systemic elimination of testosterone is the same regardless of the route of administration. Therefore, the formulation and the injection site influence the speed and magnitude of absorption.
After IM or SC administration of a testosterone ester, absorption occurs first by diffusion from the depot into the interstitium (Fig. 2B). The physiology of the IM and SC milieu determines the patterns of absorption after administration. Molecules smaller than 1 kDa, such as testosterone, are preferentially absorbed by the blood capillaries because of the high rate of filtration and reabsorption of fluid across vascular capillaries (39). However, the hydrolysis of testosterone esters by tissue esterases is a slow process because of their high lipophilicity, with negligible spontaneous hydrolysis in water (40). This results in some of the esterified testosterone entering the lymphatics, thus prolonging the secondary absorption phase.
The interstitial fluid consists of plasma ultrafiltrate and proteins derived from tissue metabolism, and is drained by the lymphatics (41). Because of their lipophilicity, testosterone esters are unlikely to have significant diffusion into the tissues; they likely are associated with small proteins and are drained via the lymphatics into the central circulation, with hydrolysis of these esters likely occurring in the central circulation (40). Therefore, the pharmacokinetics of testosterone esters administered via IM vs SC route will vary according to the lymphatic circulation of the tissue. Lymphatic drainage is dependent on intrinsic and extrinsic pumping. Intrinsic pumping is dependent on the contraction of lymphangions (muscular unit of the lymphatics with unidirectional valves) that transport lymph by mechanisms analogous to that occurring in the cardiac chambers (42). Extrinsic pumping results from intermittent external pressure exerted by skeletal muscle contractions on the lymphatics (42). As the lymphatic drainage from SC tissue is largely dependent on intrinsic pumping, while IM lymphatic flow is also substantially influenced by extrinsic pumping during physical activity (43), these drainage patterns suggest that testosterone esters administered SC likely have more stable absorption kinetics compared to IM administration.
Similar to lymphatics, the hemorheological differences of the vascular compartments of the SC and IM tissues play a role in the pharmacokinetics of testosterone esters. As different muscle groups have variable blood flow (eg, the blood flow to the deltoids is higher than the glutei) (44), which further varies with physical activity (45), serum on-treatment testosterone concentrations after IM injections are dependent on these characteristics. To the contrary, after SC administration, the drug is delivered to the hypodermis (adipose tissue underlying the dermis), which is not only less vascularized compared to skeletal muscles, but the flow in this region does not increase significantly with physical activity. Since the blood flow at the site of drug administration influences the pharmacokinetics of the administered drug, SC injections display more stable vascular absorption patterns compared to IM injection.
Pharmacokinetics of Testosterone Esters Injected Subcutaneously
As discussed, SC administration of testosterone esters should result in a more stable absorption and release of testosterone into the circulation due to less fluctuation of lymphatic flow in the hypodermis with physical activity. This was confirmed by pharmacokinetic studies that assessed the Cmax and tmax of testosterone in the serum, and the average serum total testosterone concentration during the steady state. These data are summarized as follows.
Testosterone Enanthate and Testosterone Cypionate
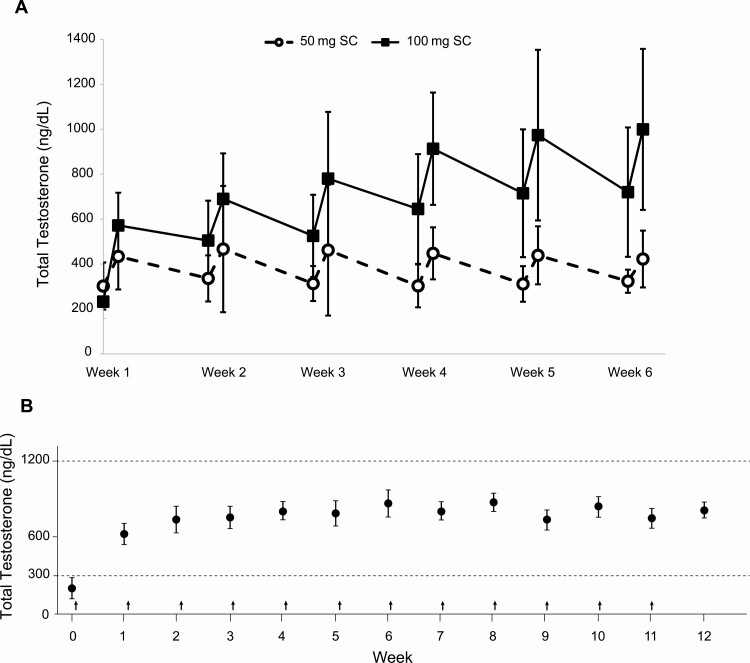
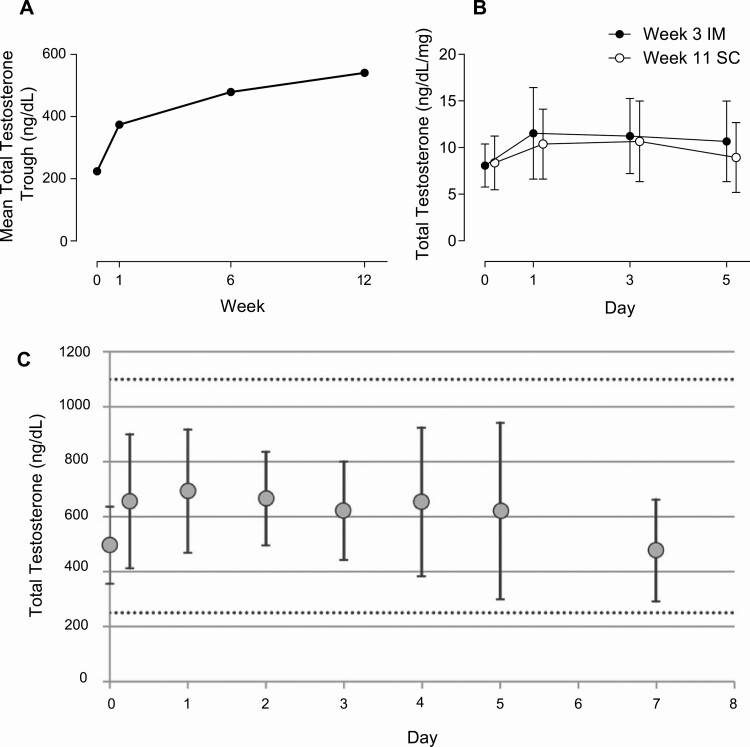
In 2006, a pilot study demonstrated the feasibility of the SC route as an effective option for testosterone therapy with testosterone esters (23). In this study, weekly SC injections of 25 to 100 mg of testosterone enanthate were administered to 22 hypogonadal men and, after weekly dose adjustments based on peak and trough levels, successfully restored serum total testosterone concentrations into the normal range (23). Almost a decade later, a study comparing the pharmacokinetics of testosterone esters administered via IM or SC route to hypogonadal men was performed (25). In this study, testosterone enanthate was administered via IM (single 200-mg dose) or SC injection (50 or 100 mg/week for 6 weeks) to 39 hypogonadal men (serum total testosterone < 300 ng/dL) (25). Participants who received SC weekly doses of either 50 mg or 100 mg achieved steady-state on-treatment serum total testosterone concentrations that were within the reference range (300-1100 ng/dL) (Fig. 3A) (25), similar to concentrations reported in a study of hypogonadal men receiving testosterone enanthate IM 100 mg/week (Fig. 3B) (46). To the contrary, the group receiving the 200-mg IM injection achieved supraphysiologic levels during the first week after the injection. The area under the concentration-time curve for testosterone during the last 2 weeks of the study (weeks 5 and 6 combined) in the 100-mg SC group was similar to that of the 200-mg IM group, suggesting that at steady state the bioavailability of testosterone enanthate is similar irrespective of the administration route (25). In another study, 150 hypogonadal men were started on SC testosterone enanthate 75 mg/week for 52 weeks, which was administered via a novel SC autoinjector (27). During week 7 of the study, the dose of testosterone was either reduced to 50 mg/week or increased to 100 mg/week with the aim of maintaining on-treatment serum testosterone levels within the normal range (27). At week 12, 92.7% of participants achieved average total testosterone levels within the desired range of 300 to 1100 ng/dL (mean ± SD = 553 ± 127 ng/dL); at week 52, mean serum total testosterone concentration was 487 ± 153 ng/dL (27). In a follow-up study by the same investigators, 21 men (aged 18-75 years) with symptomatic testosterone deficiency self-administered weekly SC testosterone enanthate at a dose of 75 mg for 12 weeks via SC autoinjector (29). Mean total testosterone concentrations gradually increased from predose values of 224 ng/dL to 374 ng/dL, 479 ng/dL, and 541 ng/dL at weeks 1, 6, and 12, respectively (29) (Fig. 4A).
Figure 3.
A, Mean serum total testosterone concentrations in men on 50 and 100 mg subcutaneous (SC) testosterone enanthate measured predose (0 hour) and 24 hours post dose. Adapted with permission from (25). B, Mean serum testosterone concentrations with weekly 100 mg intramuscular administration of testosterone enantathe to men with primary hypogonadism (vertical arrows represent injections, error bars represent SEM, and dashed lines represent normal range. Adapted with permission from (46).
Figure 4.
A, Mean trough concentrations of testosterone in hypogonadal men on weekly 75 mg subcutaneous (SC) testosterone enanthate (29). B, Total testosterone concentrations after intramuscular (IM) and SC administration of testosterone enanthate in 14 transgender men (24). C, Trough total testosterone concentrations on SC testosterone cypionate in 11 transgender men. Adapted with permission from (47).
The role of SC testosterone therapy has also been assessed in transgender men. In a prospective study, the effect of switching the route of testosterone therapy (with testosterone enanthate or cypionate) from the IM to the SC route was evaluated in 14 transgender men who had been on gender-affirming hormone therapy for at least 8 weeks (24). The mean age of the participants was 30 years and mean weekly dose was 68 mg (range, 30-110 mg; dose previously adjusted to achieve gonadotropin suppression). IM testosterone therapy was maintained for 3 weeks after enrollment before switching to self-administration of the same dose via the SC route for 8 weeks. Mean serum testosterone concentrations did not change significantly after switching administration routes (Fig. 4B) (24), confirming similar bioavailability after SC administration.
Another study in 11 transgender men on 75 mg (range, 50-100 mg) of weekly SC testosterone cypionate showed consistency in circulating total and free serum testosterone concentrations, which remained within the desired range (Fig. 4C) (47). Serum total testosterone levels measured at 6 hours (mean ± SD = 656 ± 244 ng/dL) and 5 days post injection (621 ± 321 ng/dL) were similar (47).
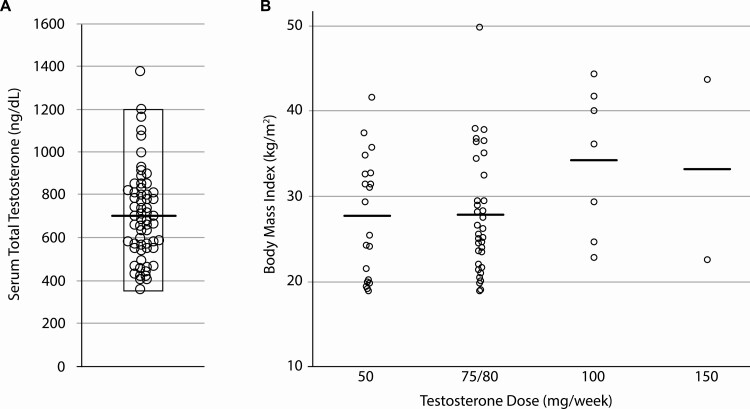
In a study of weekly SC testosterone enanthate or testosterone cypionate (50-150 mg) in 63 transgender men, 20 participants achieved goal serum total testosterone concentrations (348-1197 ng/dL) with 50 mg/week, 34 with 75 to 80 mg/week, 7 with 100 mg/week, and 2 with 150 mg/week (28). Mean serum total testosterone was 702 ± 212 ng/dL with a range of 357 to 1377 ng/dL (Fig. 5A). Interestingly, the optimal dose required to maintain serum total testosterone concentration within the desired range was not influenced by participant body mass index (Fig. 5B) (28).
Figure 5.
A, Serum total testosterone concentrations in 63 transgender men on weekly subcutaneous testosterone enanthate or cypionate. The bar represents mean value and the rectangle demarcates total testosterone range. Adapted with permission from (28). B, Optimal doses needed to maintain serum total testosterone concentration within the desired range were not influenced by participant’s body mass index (bars indicate mean values). Adapted with permission from (28).
Testosterone Undecanoate
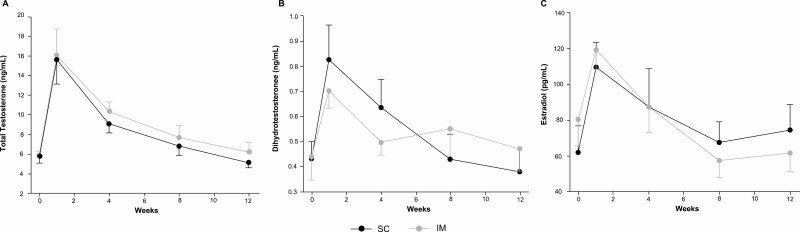
The ultralong-acting ester testosterone undecanoate has been available for IM injection in Europe and Australia for almost 2 decades, and in the United States since 2014. Because of the longer absorption time, it was introduced as an option to minimize peaks and troughs in serum testosterone levels after dosing, as well as to reduce the frequency of injections in men with organic androgen deficiency who require long-term testosterone therapy. The formulation contains 250 mg/mL of the ester dissolved in castor oil and is supplied in 3-mL vials in the United States, and 4-mL vials in other parts of the world. The recommended dose and administration interval differ by regulatory agencies; in the United States, after the loading dose at week 4 of therapy, the recommended maintenance dose is 750 mg (3 mL) given IM every 10 weeks (1), while in other countries, the loading dose is 1000 mg, which is administered at week 6 followed by 1000 mg (4 mL) every 12 weeks (48, 49). However, the higher injected volume of testosterone undecanoate, compared to enanthate or cypionate, carries a potential risk of pulmonary oil microembolism (22), an acute condition (onset < 60 minutes) that has been observed after its injection. These adverse reactions are rare and occur with approximately 1.9% of injections (21), appear to be related to introduction of the drug directly into the systemic circulation, and are associated with a transient cough (50). Therefore, the Food and Drug Administration has recommended that IM administration of testosterone undecanoate be performed slowly by trained personnel in the clinic, and the patient should be observed for at least 30 minutes after injections. In this context, SC administration of testosterone undecanoate could potentially be a safer route, because the SC compartment is less vascularized, thus reducing the chance of introducing the drug directly into the systemic circulation. As previous studies had demonstrated comparable pharmacokinetics after IM or SC administration of long-acting testosterone esters (enanthate and cypionate), Turner et al (26) sought to compare the pharmacokinetics of 1000 mg (4 mL) of testosterone undecanoate after a single dose via SC and IM administration in a crossover study of 20 men (11 hypogonadal, 9 transgender). Participants were randomly assigned to IM or SC injections and followed for 12 weeks before they crossed over to the other route without any washout. Serum testosterone profile after SC injection displayed a slower time to peak concentration (8.0 vs 3.3 days) with no significant differences in model-predicted peak concentration compared with the IM route (26). The duration of action was 104 days for the SC and 101 days for the IM route. Serum testosterone concentrations (Fig. 6A) did not differ according to route of administration after adjustment for age, body mass index, and clinical diagnosis (26).
Figure 6.
Serum total A, testosterone; B, 5-dihydrotestosterone; and C, estradiol concentrations after subcutaneous (SC) or intramuscular (IM) administration of 1000 mg of testosterone undecanoate. Adapted with permission from (26).
In summary, the stable and consistent serum testosterone concentrations after SC route of administration of testosterone enanthate and cypionate suggest that the SC route is a feasible option and can be self-administered by patients after appropriate training. SC testosterone undecanoate also appears feasible, though available data are limited.
Serum Concentrations of Testosterone Metabolites After Subcutaneous Administration
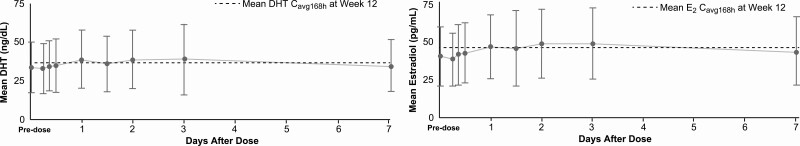
Few studies have evaluated serum concentrations of 5-dihydrotestosterone (DHT) and estradiol after SC injection compared to the standard IM route. Data suggest that serum concentrations of both DHT and estradiol increase in a similar manner regardless of the administration route or ester, that is, enanthate (25) (Table 2) or undecanoate (Fig. 6B and 6C) (26). Additionally, serum DHT and estradiol concentrations remain stable with few fluctuations after SC injections of testosterone enanthate (Fig. 7) (27).
Table 2.
Ratio of 5-dihydrotestosterone and estradiol to testosterone (T) by dose and route of administration during treatment with T enanthate
| Metabolite to T ratio at wk 6a | 50 mg/wk SC | 100 mg/wk SC | 200 mg IM |
|---|---|---|---|
| Mean DHT/T ratio | 0.0750 | 0.0609 | 0.0732 |
| Mean estradiol/T ratio | 0.0063 | 0.0055 | 0.0032 |
Data from (25).
a Ratio of AUC0-168h of DHT and estradiol to AUC0-168h of serum total T at week 6 of treatment.
Abbreviations: AUC, area under the curve; DHT, 5-dihydrotestosterone; IM, intramuscular; SC, subcutaneous; T, testosterone.
Figure 7.
Mean A, 5-dihydrotestosterone (DHT) and B, estradiol (E2) concentrations on weekly subcutaneous (SC) injections of 75 mg testosterone enanthate. Adapted with permission from (27).
Safety of Subcutaneous Testosterone Esters
The main benefit of using the SC route for administration of testosterone esters over the traditional IM route is the ease of self-administration. In addition, there is no risk of sciatic injury, administration can be accomplished using smaller needles, and the pain evoked during SC administration is usually lower. However, concerns remain regarding local reactions, such as scarring and infections. Although the published studies of SC testosterone administration have not observed serious local adverse reactions (23, 47), mild local reactions were common. As on-treatment serum testosterone concentrations after SC administrations are similar to IM, systemic adverse effects that have been associated with testosterone therapy were also reported (25, 27-29, 51). Table 3 summarizes the local and systemic adverse effects reported by studies that administered testosterone esters via SC.
Table 3.
Local and systemic adverse events during subcutaneous administration of testosterone esters (number of events in parenthesis)
| Study | Ester | Frequency | Dose range | Duration | Population | Sample | Local site-related adverse effects | Systemic adverse effects |
|---|---|---|---|---|---|---|---|---|
| Olson et al, 2014 (51) | TC | Weekly | 25-75 mg | 6 mo | Transgender men | 36 | Erythema (2), swelling (2) and pain (2) | – |
| Kaminetsky et al, 2015 (25) | TE | Weekly | 50-100 mg | 6 wk | Hypogonadal men | 29 | Ecchymosis (1) | 4 (not specified) |
| Spratt et al, 2017 (28) | TC or TE | Weekly | 50-150 mg | Up to 43 mo | Transgender men | 63 | Nodules (4), urticaria (2), inflammation (2) | Acne (37) |
| Kaminetsky et al, 2019 (27) | TE | Weekly | 50-100 mg | 52 wk | Hypogonadal men | 150 | Erythema (31), induration (11), hematoma (11), bleeding (10), ecchymosis (9), itching (9), pain (7) | Erythrocytosis (18), hypertension (19), polycythemia (3), 3 acne (3), prostate enlargement (2) |
| Gittelman et al, 2019 (29) | TE | Weekly | 50-100 mg | 26 wk | Hypogonadal men | 133 | Hemorrhage (8), bruising (5), pain (1) | Prostatitis (4), polycythemia (3), hypertension (3), fatigue (3), insomnia (3), nausea (3), deep vein thrombosis (1) |
| Turner et al, 2019 (26) | TU | Once | 1000 mg | 14 wk | Hypogonadal and transgender men | 20 | Pain (19) | Not reported |
Abbreviations: TC, testosterone cypionate; TE, testosterone enanthate; TU, testosterone undecanoate.
Local Adverse Effects
In a study of 63 transgender men (who were trained by an experienced nurse on self-administration) receiving weekly doses of SC testosterone enanthate or cypionate at doses of 50 to 150 mg for up to 43 months, 10 injection site reactions were reported by 9 participants (28). Four participants reported small, painless nodules that resolved within 2 days, while 2 participants developed urticaria at the injection site within a few hours that persisted for up to 3 days. Two individuals reported transient local inflammation, while one patient experienced a self-limited episode of cellulitis (28).
In another study of SC administration of testosterone enanthate (50 or 100 mg/week) with a SC autoinjector for 6 weeks in 29 hypogonadal men, only 1 participant developed ecchymosis at the injection site (25). In a larger, 26-week study of 133 men by the same investigators, weekly SC doses of testosterone enanthate (50-100 mg) with an autoinjector resulted in injection-site hemorrhage in 8, bruising in 5, and pain in 1 participant (29).
Systemic Adverse Effects
Systemic adverse effects associated with SC administration of testosterone are generally similar to those observed when testosterone is administered via other parenteral routes (see Table 3). A study of transgender men receiving SC weekly doses of testosterone enanthate or cypionate (28) showed that 37 of 67 participants developed acne; 2 of these individuals needed a referral to a dermatologist, while no participant chose to decrease their testosterone dose.
In a large study that used an SC autoinjector to administer weekly doses of testosterone enanthate (50-100 mg/week) for 26 weeks, 87 of 133 participants experienced a treatment-emergent adverse event (an adverse event that started or worsened after the first dose) during the study (29). The majority of these events were mild to moderate, although 5 patients experienced severe events. Three patients developed erythrocytosis that resulted in their discontinuation from the study (29). In a similar study by the same investigators in 150 hypogonadal men, 125 participants experienced a treatment-emergent adverse event, with 30 discontinuing therapy as a result of these events (27). The most frequent events were erythrocytosis (21 men; 7 discontinued), hypertension (19 men; 1 discontinued), and increase in serum prostate-specific antigen of 1.4 ng/mL or greater from baseline (18 men; 13 discontinued) (27). Though erythrocytosis and increase in prostate-specific antigen levels are known adverse effects of testosterone therapy (1), the incidences of such events after SC administration appear to be higher than those reported in studies of transdermal testosterone (52, 53). Because studies of SC testosterone therapy are limited, this needs to be verified in future studies. As for hypertension, approximately half of the participants had a history of hypertension at enrollment, and increases in systolic and diastolic blood pressures during testosterone therapy were considered to be of small magnitude (4.1 mm Hg for systolic and 1.4 mm Hg for diastolic blood pressure) (27); the implications of these changes on cardiovascular risk remain unclear.
Patient Preference
Data suggest that, in general, medications that require long-term administration have compliance rates between 40% and 50% (54). Thus, drugs that are easier to administer and are relatively inexpensive result in greater compliance, particularly among patients who require lifelong therapy (54, 55), such as men with organic hypogonadism. Indeed, long-term compliance among men who are prescribed testosterone therapy with IM injections is low; approximately 69% of men on long-acting esters discontinue treatment within 3 months of therapy, and 95% discontinue it within 12 months (56). Therefore, patient participation and engagement in the selection of testosterone formulation is likely to promote adherence (57). In this regard, self-administration of testosterone esters via SC injections is convenient, easy to learn, associated with less discomfort, obviates the need for office visits, and is inexpensive.
Studies that have assessed patient preference regarding the route of administration of testosterone esters (enanthate and cypionate) suggest that patients generally prefer the SC route compared to the IM route (24, 28, 51). Among transgender men, patients who had previously used IM testosterone therapy with long-acting esters did not want to revert back to IM injections after they were started on SC testosterone therapy (24, 28, 51). The only study that assessed patient preference with ultralong-acting testosterone undecanoate (larger volume of 4 mL) showed that 11 of 20 participants preferred IM injection, 6 preferred SC injection, and 3 did not have a preference (26); however, the 12-week postinjection acceptability scores were not significantly different between the 2 routes (26).
Guidance Regarding Subcutaneous Testosterone Therapy
Technique
All patients should receive training from medical personnel on how to self-inject testosterone. Studies involving SC administration of testosterone cypionate or enanthate have used 1-mL Luer-Lok syringes with a 20- or 25-gauge 5/8-inch needle to inject testosterone into the SC tissue of the abdomen or thigh (28, 47). A Luer-Lok syringe is preferred to prevent the needle from disengaging from the syringe during injection considering the viscosity of the solution. Testosterone should be injected 3 to 5 cm lateral to the umbilicus or in the SC tissue of the thigh. For testosterone undecanoate, limited published data suggest that slower injection (over 2-3 minutes) can be safely administered into the subcutaneous tissue of the abdomen using a 21-gauge 25-mm needle (26). In the authors’ clinical experience, a 23-gauge needle can be used without difficulty both for long- and ultralong-acting testosterone esters.
Therapy Initiation and Monitoring
Once a patient qualifies for testosterone therapy (1, 2), risks and benefits of therapy as well as pros and cons of each formulation should be discussed (see Table 1). Patients should be informed that currently, data and experience with SC testosterone therapy both are limited. This discussion should also include cost considerations because the SC autoinjector is more expensive compared to conventional SC injections with testosterone esters. After this discussion, should the patient decide on injectable testosterone and opt for the SC route, we suggest starting the patient at the dose of 75 mg/week of testosterone enanthate or cypionate because studies suggest that this slightly lower dose is sufficient to achieve therapeutic serum levels of testosterone compared with the IM route (24, 25, 27-29). On-treatment serum testosterone concentrations should be measured midpoint between the injections, and the dose can be reduced to 50 mg/week or increased to 100 mg/week aiming for the midrange serum total testosterone concentrations for healthy young men (1). Clinicians should continue to assess testosterone levels periodically. For testosterone undecanoate, the only study that assessed pharmacokinetics after SC injection suggests that the same dose should be used that is used for IM injection (26); however, more studies with testosterone undecanoate will shed further light regarding the optimal dose for SC administration. Additionally, though data on this ultralong-acting formulation are available with the 1000-mg dose regimen (26), studies with the 750-mg dose (the approved dose in the United States) are also needed. Similar to IM injections, periodic monitoring of the patients for risks and benefits should continue as recommended by clinical practice guidelines (1).
Conclusion
Administration of testosterone ester via the SC route has been gaining popularity. To date, limited data suggest that SC administration of testosterone enanthate and cypionate results in stable and predictable on-treatment concentrations, has good acceptability among patients, and can be self-administered more easily than IM injections. Furthermore, localized adverse effects at the injection site are mild and transient. Although long-term studies with larger numbers of patients are needed to evaluate the safety and compliance of SC testosterone (in particular for testosterone undecanoate), clinicians should be aware of this route of testosterone administration, as it has the potential to increase patient adherence to therapy of a formulation that is relatively inexpensive and results in comparable on-treatment serum testosterone concentrations.
Glossary
Abbreviations
- Cmax
serum concentration
- DHT
5-dihydrotestosterone
- IM
intramuscular
- SC
subcutaneous
- tmax
time of maximum concentration.
Contributor Information
Maria Gabriela Figueiredo, Research Program in Men’s Health: Aging and Metabolism, Division of Endocrinology and Metabolism, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Thiago Gagliano-Jucá, Research Program in Men’s Health: Aging and Metabolism, Division of Endocrinology and Metabolism, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA; Northwestern Medicine McHenry Hospital, Chicago Medical School, Rosalind Franklin University of Medicine and Science, McHenry, Illinois, USA.
Shehzad Basaria, Research Program in Men’s Health: Aging and Metabolism, Division of Endocrinology and Metabolism, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Financial Support
This work was supported in part by the Mid-Career Mentoring Award K24AG070078 to Dr Basaria.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715-1744. [DOI] [PubMed] [Google Scholar]
- 2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903. [DOI] [PubMed] [Google Scholar]
- 3. Brown-Séquard CE. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet. 1889;134(3438):105-107. [Google Scholar]
- 4. David K, Dingemanse E, Freud J, Laqueur E. Über krystallinisches männliches Hormon aus Hoden (Testosteron), wirksamer als aus Harn oder aus Cholesterin bereitetes Androsteron. Hoppe Seylers Z Physiol Chem. 1935;233(5-6):281-283. [Google Scholar]
- 5. Butenandt A, Hanisch G. Über Testosteron. Umwandlung des Dehydro-androsterons in Androstendiol und Testosteron; ein Weg zur Darstellung des Testosterons aus Cholesterin. Hoppe Seylers Z Physiol Chem. 1935;237(1-3):89-97. [Google Scholar]
- 6. Ruzicka L, Wettstein A.Sexualhormone VII. Über die künstliche Herstellung des Testikelhormons Testosteron (Androsten-3-on-17-ol). Helv Chim Acta. 1935;18(1):1264-1275. [Google Scholar]
- 7. Hamilton JB. Treatment of sexual underdevelopment with synthetic male hormone substance. Endocrinol. 1937;21(5):649-654. [Google Scholar]
- 8. Deanesly R, Parkes AS. Further experiments on the administration of hormones by the subcutaneous implantation of tablets. Lancet. 1938;232(6002):606-609. [Google Scholar]
- 9. Junkmann K. Long-acting steroids in reproduction. Recent Prog Horm Res. 1957;13:389-419; discussion 419-428. [PubMed] [Google Scholar]
- 10. Pfeil E, Dobs AS. Current and future testosterone delivery systems for treatment of the hypogonadal male. Expert Opin Drug Deliv. 2008;5(4):471-481. [DOI] [PubMed] [Google Scholar]
- 11. Kovac JR, Rajanahally S, Smith RP, Coward RM, Lamb DJ, Lipshultz LI. Patient satisfaction with testosterone replacement therapies: the reasons behind the choices. J Sex Med. 2014;11(2):553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith RP, Khanna A, Coward RM, et al. Factors influencing patient decisions to initiate and discontinue subcutaneous testosterone pellets (Testopel) for treatment of hypogonadism. J Sex Med. 2013;10(9):2326-2333. [DOI] [PubMed] [Google Scholar]
- 13. McGriff NJ, Csako G, Kabbani M, Diep L, Chrousos GP, Pucino F. Treatment options for a patient experiencing pruritic rash associated with transdermal testosterone: a review of the literature. Pharmacotherapy. 2001;21(11):1425-1435. [DOI] [PubMed] [Google Scholar]
- 14. Voelker R. Children’s exposure to testosterone gel spurs FDA to order boxed label warning. JAMA. 2009;301(23):2428. [DOI] [PubMed] [Google Scholar]
- 15. Parker S, Armitage M. Experience with transdermal testosterone replacement therapy for hypogonadal men. Clin Endocrinol (Oxf). 1999;50(1):57-62. [DOI] [PubMed] [Google Scholar]
- 16. Mattern C, Hoffmann C, Morley JE, Badiu C. Testosterone supplementation for hypogonadal men by the nasal route. Aging Male. 2008;11(4):171-178. [DOI] [PubMed] [Google Scholar]
- 17. Yin AY, Htun M, Swerdloff RS, et al. Reexamination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self-emulsifying formulation. J Androl. 2012;33(2):190-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Basaria S. Male hypogonadism. Lancet. 2014;383(9924):1250-1263. [DOI] [PubMed] [Google Scholar]
- 19. Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84(10):3469-3478. [DOI] [PubMed] [Google Scholar]
- 20. Partsch CJ, Weinbauer GF, Fang R, Nieschlag E. Injectable testosterone undecanoate has more favourable pharmacokinetics and pharmacodynamics than testosterone enanthate. Eur J Endocrinol. 1995;132(4):514-519. [DOI] [PubMed] [Google Scholar]
- 21. Middleton T, Turner L, Fennell C, et al. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol. 2015;172(5):511-517. [DOI] [PubMed] [Google Scholar]
- 22. Pastuszak AW, Hu Y, Freid JD. Occurrence of pulmonary oil microembolism after testosterone undecanoate injection: a postmarketing safety analysis. Sex Med. 2020;8(2):237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Futaisi AM, Al-Zakwani IS, Almahrezi AM, Morris D. Subcutaneous administration of testosterone. A pilot study report. Saudi Med J. 2006;27(12):1843-1846. [PubMed] [Google Scholar]
- 24. Wilson DM, Kiang TKL, Ensom MHH. Pharmacokinetics, safety, and patient acceptability of subcutaneous versus intramuscular testosterone injection for gender-affirming therapy: a pilot study. Am J Health Syst Pharm. 2018;75(6):351-358. [DOI] [PubMed] [Google Scholar]
- 25. Kaminetsky J, Jaffe JS, Swerdloff RS. Pharmacokinetic profile of subcutaneous testosterone enanthate delivered via a novel, prefilled single-use autoinjector: a phase II study. Sex Med. 2015;3(4):269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner L, Ly LP, Desai R, et al. Pharmacokinetics and acceptability of subcutaneous injection of testosterone undecanoate. J Endocr Soc. 2019;3(8):1531-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaminetsky JC, McCullough A, Hwang K, Jaffe JS, Wang C, Swerdloff RS. A 52-week study of dose adjusted subcutaneous testosterone enanthate in oil self-administered via disposable auto-injector. J Urol. 2019;201(3):587-594. [DOI] [PubMed] [Google Scholar]
- 28. Spratt DI, Stewart II, Savage C, et al. Subcutaneous injection of testosterone is an effective and preferred alternative to intramuscular injection: demonstration in female-to-male transgender patients. J Clin Endocrinol Metab. 2017;102(7):2349-2355. [DOI] [PubMed] [Google Scholar]
- 29. Gittelman M, Jaffe JS, Kaminetsky JC. Safety of a new subcutaneous testosterone enanthate auto-injector: results of a 26-week study. J Sex Med. 2019;16(11):1741-1748. [DOI] [PubMed] [Google Scholar]
- 30. Singh GK, Turner L, Desai R, Jimenez M, Handelsman DJ. Pharmacokinetic-pharmacodynamic study of subcutaneous injection of depot nandrolone decanoate using dried blood spots sampling coupled with ultrapressure liquid chromatography tandem mass spectrometry assays. J Clin Endocrinol Metab. 2014;99(7):2592-2598. [DOI] [PubMed] [Google Scholar]
- 31. Cockshott WP, Thompson GT, Howlett LJ, Seeley ET. Intramuscular or intralipomatous injections? N Engl J Med. 1982;307(6):356-358. [DOI] [PubMed] [Google Scholar]
- 32. Haramati N, Lorans R, Lutwin M, Kaleya RN. Injection granulomas. Intramuscle or intrafat? Arch Fam Med. 1994;3(2):146-148. [DOI] [PubMed] [Google Scholar]
- 33. Chan VO, Colville J, Persaud T, Buckley O, Hamilton S, Torreggiani WC. Intramuscular injections into the buttocks: are they truly intramuscular? Eur J Radiol. 2006;58(3):480-484. [DOI] [PubMed] [Google Scholar]
- 34. Sartorius G, Fennell C, Spasevska S, Turner L, Conway AJ, Handelsman DJ. Factors influencing time course of pain after depot oil intramuscular injection of testosterone undecanoate. Asian J Androl. 2010;12(2):227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalicharan RW, Schot P, Vromans H. Fundamental understanding of drug absorption from a parenteral oil depot. Eur J Pharm Sci. 2016;83:19-27. [DOI] [PubMed] [Google Scholar]
- 36. Fujioka M, Shinohara Y, Baba S, Irie M, Inoue K. Pharmacokinetic properties of testosterone propionate in normal men. J Clin Endocrinol Metab. 1986;63(6):1361-1364. [DOI] [PubMed] [Google Scholar]
- 37. van der Vies J. Implications of basic pharmacology in the therapy with esters of nandrolone. Acta Endocrinol Suppl (Copenh). 1985;271:38-44. [DOI] [PubMed] [Google Scholar]
- 38. Minto CF, Howe C, Wishart S, Conway AJ, Handelsman DJ. Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and injection volume. J Pharmacol Exp Ther. 1997;281(1):93-102. [PubMed] [Google Scholar]
- 39. McLennan DN, Porter CJ, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2(1):89-96. [DOI] [PubMed] [Google Scholar]
- 40. Kalicharan RW, Bout MR, Oussoren C, Vromans H. Where does hydrolysis of nandrolone decanoate occur in the human body after release from an oil depot? Int J Pharm. 2016;515(1-2):721-728. [DOI] [PubMed] [Google Scholar]
- 41. Hansen KC, D’Alessandro A, Clement CC, Santambrogio L. Lymph formation, composition and circulation: a proteomics perspective. Int Immunol. 2015;27(5):219-227. [DOI] [PubMed] [Google Scholar]
- 42. Moore JE Jr, Bertram CD. Lymphatic system flows. Annu Rev Fluid Mech. 2018;50:459-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Havas E, Parviainen T, Vuorela J, Toivanen J, Nikula T, Vihko V. Lymph flow dynamics in exercising human skeletal muscle as detected by scintography. J Physiol. 1997;504(Pt 1):233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Evans EF, Proctor JD, Fratkin MJ, Velandia J, Wasserman AJ. Blood flow in muscle groups and drug absorption. Clin Pharmacol Ther. 1975;17(1):44-47. [DOI] [PubMed] [Google Scholar]
- 45. Ruiz ME, Scioli Montoto S. Routes of drug administration. In: Talevi A, Quiroga PAM, eds. ADME Processes in Pharmaceutical Sciences. Springer; 2018:97-133. [Google Scholar]
- 46. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51(6):1335-1339. [DOI] [PubMed] [Google Scholar]
- 47. McFarland J, Craig W, Clarke NJ, Spratt DI. Serum testosterone concentrations remain stable between injections in patients receiving subcutaneous testosterone. J Endocr Soc. 2017;1(8):1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dimopoulou C, Ceausu I, Depypere H, et al. EMAS position statement: testosterone replacement therapy in the aging male. Maturitas. 2016;84:94-99. [DOI] [PubMed] [Google Scholar]
- 49. Yeap BB, Grossmann M, McLachlan RI, et al. Endocrine Society of Australia position statement on male hypogonadism (part 2): treatment and therapeutic considerations. Med J Aust. 2016;205(5):228-231. [DOI] [PubMed] [Google Scholar]
- 50. Svendsen O, Aaes-Jørgensen T. Studies on the fate of vegetable oil after intramuscular injection into experimental animals. Acta Pharmacol Toxicol (Copenh). 1979;45(5):352-378. [DOI] [PubMed] [Google Scholar]
- 51. Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M. Subcutaneous testosterone: an effective delivery mechanism for masculinizing young transgender men. LGBT Health. 2014;1(3):165-167. [DOI] [PubMed] [Google Scholar]
- 52. Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2018;103(5):1745-1754. [DOI] [PubMed] [Google Scholar]
- 53. Cunningham GR, Ellenberg SS, Bhasin S, et al. Prostate-specific antigen levels during testosterone treatment of hypogonadal older men: data from a controlled trial. J Clin Endocrinol Metab. 2019;104(12):6238-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035. [DOI] [PubMed] [Google Scholar]
- 56. Donatucci C, Cui Z, Fang Y, Muram D. Long-term treatment patterns of testosterone replacement medications. J Sex Med. 2014;11(8):2092-2099. [DOI] [PubMed] [Google Scholar]
- 57. Dwyer AA, Tiemensma J, Quinton R, Pitteloud N, Morin D. Adherence to treatment in men with hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf). 2017;86(3):377-383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.