Abstract
The accumulation of norfloxacin by Bacteroides fragilis NCTC 9343 was determined by the modified fluorescence method. The time required to achieve a steady-state concentration (SSC) after allowing B. fragilis to accumulate norfloxacin in an aerobic or an anaerobic environment was ∼2 min; the SSC achieved in air was 90.28 ± 9.32 ng of norfloxacin/mg (dry weight) of cells, and that achieved anaerobically was 98.45 ± 3.7 ng of norfloxacin/mg (dry weight) of cells. Initial rates of accumulation were determined with a range of external concentrations, as up to 8 μg/ml the concentration of norfloxacin accumulated increased proportionally to the external concentration, 12.13 ng/mg (dry weight) of cells per μg of exogenous norfloxacin per ml. At concentrations above 10 μg/ml no increase in the rate of norfloxacin accumulation was observed. From the kinetic data, a Lineweaver-Burk plot calculated a Km of 5.03 μg/ml and a Vmax of 25.1 ng of norfloxacin/s. With an increase in temperature of between 0 and 30°C, the concentration of norfloxacin accumulated also increased proportionally at 4.722 ng of norfloxacin/mg (dry weight) of cells/°C. At low concentrations of glucose (<0.2%; 11 mM), the concentration of norfloxacin accumulated was decreased. With the addition of 100 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) the mean SSC of norfloxacin was increased to 116 ± 7.01 ng of norfloxacin/mg (dry weight) of cells; glucose had no significant effect in the presence of CCCP. Magnesium chloride (20 mM) decreased the SSC of norfloxacin to 40.5 ± 3.76 ng of norfloxacin per mg (dry weight) of cells. These data suggest that the mechanism of accumulation of norfloxacin by B. fragilis is similar to that of aerobic bacteria and that the fluoresence procedure is suitable for use with an anaerobic bacterium.
The rate of antibiotic resistance among Bacteroides species has risen steadily over the last several years (28). Current treatment of anaerobic infections caused by Bacteroides fragilis relies mainly on the use of antibiotics such as metronidazole, cefuroxime, amoxicillin-clavulanate potassium, and clindamycin. In the United Kingdom metronidazole plus cefuroxime or amoxicillin-clavulanate potassium alone is used, whereas in the United States the use of clindamycin supersedes that of metronidazole (7). Since the mid-1980s workers have reported metronidazole-resistant B. fragilis (2, 11, 26). More recently, highly metronidazole resistant Bacteroides spp. have been isolated from patients in Kuwait (27). The numbers of strains resistant to clindamycin and most cephalosporins are also increasing (28).
Fluoroquinolones have not previously been considered suitable for the treatment of anaerobic infections due to their limited in vitro activity and lack of clinical efficacy (9). However, newer fluoroquinolones such as moxifloxacin, sparfloxacin, and sitafloxacin and a related naphthyridone, trovafloxacin, have been reported to have improved in vitro activities against anaerobes, especially B. fragilis (1, 9, 19, 30). Recent studies have demonstrated that these newer fluoroquinolones can be used as a single agent and appear to be as successful as metronidazole, clindamycin, and gentamicin for the treatment of polymicrobial infections in animals (25, 29). As well as their good in vitro activity against anaerobes, these new fluoroquinolones possess favorable pharmacokinetics, such as long half-lives and good oral bioavailabilities, allowing once-daily dosing and oral administration (J. A. Weigelt, Proc. 8th Int. Congr. Infect. Dis., abstr. 81.004, 1998).
However, the numbers of antibiotic-resistant anaerobic bacteria will continue to rise, and when and if the fluoroquinolones become an accepted alternative to the current anaerobic drugs, the mechanisms by which anaerobic bacteria such as B. fragilis develop resistance to such agents should be studied. Fluoroquinolone resistance in most bacterial species, including B. fragilis, has been attributed to one or more mutations in one or more of the genes that encode topoisomerase II and topoisomerase IV (20); L. J. V. Piddock and V. Ricci, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C180, p. 121, 1998). As well as mutations in genes that encode the target enzymes, it has also been shown that multidrug efflux pumps can confer fluoroquinolone resistance (17, 21). To investigate the role of fluoroquinolone efflux in resistance, it is important to establish a reproducible technique for measurement of the concentration accumulated by the test bacterial species. Several procedures have been used to measure fluoroquinolone accumulation by bacteria, including the bioassay and radiometric and fluoresence methods of detection. Most recent publications have used either the radiometric method, or the fluoresence method, as both produce reliable data. The fluoresence method is increasingly popular, as radiolabeled agents can be difficult to obtain. The aim of this study was to adapt the fluoresence method to study the accumulation of norfloxacin by B. fragilis.
MATERIALS AND METHODS
Bacterial strains, growth conditions, antimicrobial agents, and determination of activity.
B. fragilis NCTC 9343 and ATCC 25285 and Escherichia coli NCTC 10418 were obtained from the Public Health Laboratory Service (Colindale, United Kingdom) and were used throughout the study. Norfloxacin was obtained from Sigma Chemical Company (Poole, United Kingdom). B. fragilis was grown anaerobically in Wilkins-Chalgren media (Oxoid Ltd., Basingstoke, United Kingdom) at 37°C in an atmosphere of 80% nitrogen, 10% carbon dioxide, and 10% hydrogen, which was attained in an MKII anaerobic workstation (Don Whitley, Shipley, United Kingdom). All media were prewarmed and prereduced prior to use. Anaerobiosis in the anaerobic chamber was monitored by using a strict aerobe (Pseudomonas aeruginosa) as a biological indicator, and the chemical indicator was methylene blue. In addition, anaerobiosis is continually monitored electronically in the workstation. E. coli was grown anaerobically and aerobically at 37°C in Wilkins-Chalgren and Iso-Sensitest media (Oxoid Ltd.). MICs were determined by a routine agar doubling dilution method with an inoculum of 106 CFU/ml. The MIC was defined as the lowest concentration of drug at which no visible growth was observed.
Bacterial growth kinetics.
Bacterial growth was measured by two methods: the first by monitoring the viable cell count and the second by monitoring the optical density of the culture (22). When the viable cell count was determined anaerobically, at no time was the culture removed from the anaerobic cabinet. As the fluorescence method had been widely used in this laboratory prior to this study for measurement of fluoroquinolone accumulation by aerobic bacteria in air, it was hoped that it would be applicable to this investigation. Therefore, it was important to determine whether exposure of B. fragilis to aerobic conditions, such as those in an accumulation experiment, for up to 20 min would affect viability. A liquid culture was allowed to grow to the mid-logarithmic phase and was divided into two parts; one part was kept under anaerobic conditions and the other was kept in air and agitated as if in an accumulation experiment for 1 h, and the optical densities and viable cell counts of both cultures were monitored. To determine whether norfloxacin had a similar bactericidal effect for B. fragilis under anaerobic conditions as it did under aerobic conditions, B. fragilis was grown to the mid-logarithmic phase and norfloxacin was added to a final concentration of 10 μg/ml and was then divided into four parts. Two cultures remained in an anaerobic environment, and the other two were transferred to an aerobic environment. After 1 h samples from each culture were taken and the viable counts were determined.
Measurement of norfloxacin accumulation.
The accumulation of norfloxacin by B. fragilis was measured by the fluoresence assay (3, 18), but the assay was altered to accommodate the growth characteristics of B. fragilis. Cells were grown to the mid-exponential phase (A675 of 0.4 to 0.5 for B. fragilis and A660 of 0.7 to 0.8 for E. coli) in broth and harvested by centrifugation at 3,003 × g (Mistral centrifuge; MSE, Scientific Instruments, Crawley, United Kingdom) for 20 min at 4°C. The cells were washed in 10 ml of 50 mM sodium phosphate buffer (pH 7) and were concentrated with the same buffer to give a suspension of B. fragilis with an A675 of 20 and a suspension of E. coli with an A660 of 20. For all experiments with B. fragilis (unless indicated otherwise) the bacterial suspension was returned to the MKII anaerobic workstation and was left for 20 min to reequilibriate to the anaerobic environment. To confirm that this was sufficient to ensure anaerobiosis, a bottle of methylene blue indicator was removed from the cabinet, centrifuged as described above for a cell culture, and then returned to the anaerobic atmosphere; the indicator became slightly aerobic, as a very pale blue color was observed; this took ∼20 min to return to a colorless (anaerobic) state. For E. coli the suspension was placed in a stirring 37°C water bath in air and was left to equilibrate for 10 min. After withdrawal of a 500-μl sample at time zero and a sample for bacterial culture to confirm the organism's identity, norfloxacin was added to a final concentration of 10 μg/ml, and 500-μl samples were removed at timed intervals and placed in ice-cold buffer held on ice to prevent leakage of the fluoroquinolone from the cells (18). The cells were immediately centrifuged at 12,000 × g (Jouan MR1812 centrifuge) for 5 min at 4°C. The cell pellets were washed once with ice-cold sodium phosphate buffer (50 mM; pH 7) and were resuspended in 1 ml of 0.1 M glycine hydrochloride (pH 3) to lyse overnight at room temperature away from light. Cellular debris was removed by centrifugation at 12,000 × g (Jouan MR1812 centrifuge), and the fluorescence of the supernatants was determined at an excitation wavelength of 281 nm and an emission wavelength of 440 nm. The results were expressed as nanograms of norfloxacin per milligram (dry weight) of cells (see below). For E. coli, experiments were performed after growth in air and an anaerobic environment in both Wilkins-Chalgren and Iso-Sensitest broths. Accumulation was also assayed aerobically and anaerobically. To study the effect of glucose upon the concentration of norfloxacin accumulated, experiments were performed with B. fragilis as described above, except that the cell pellets were resuspended in sodium phosphate buffer containing a final concentration of glucose that ranged from 0.1 to 0.6% (wt/vol). To examine the effects of increasing exogenous concentrations of norfloxacin and possible saturation of transport, the accumulation of norfloxacin over a concentration range of 0 to 100 μg/ml was studied with B. fragilis. The cells were incubated anaerobically at 37°C for 10 min in the presence of each norfloxacin concentration. The data were analyzed with Enzpack software. The effect of temperature upon norfloxacin accumulation into B. fragilis was investigated over a range from 0 to 37°C. Temperatures were attained by incubating tubes containing a magnetic stirrer in a water bath over a heating block; for the lower temperatures, a combination of crushed ice and the heating block was used. A comparison of the buffer used by Miyamae et al. (17) and sodium phosphate buffer was performed by the method described above. Potassium phosphate buffer (20 mM; pH 7) containing 0.2% (wt/vol; 11 mM) glucose, 1 mM dithiothreitol, and lithium chloride (0.1 M) was used as a substitute for and in conjunction with sodium phosphate buffer (50 mM; pH 7). When studying the effects of the efflux inhibitors carbonyl cyanide m-chlorophenylhydrazone (CCCP) and reserpine and the effect of magnesium chloride, the inhibitor was added to a parallel set of tubes to the desired final concentration.
B. fragilis cell dry weight.
B. fragilis cell dry weight was determined as described previously (18).
Statistical analysis.
All experiments were performed at least three times, and differences in accumulation data between different studies were analyzed by the two-tailed Student's t test. A P value of <0.05 was considered significant.
RESULTS
Activity of norfloxacin against B. fragilis and E. coli.
The MIC of norfloxacin for B. fragilis was 32 μg/ml, and that for E. coli was 0.12 μg/ml. For E. coli the value remained constant irrespective of the incubation atmosphere.
Growth characteristics of B. fragilis.
The generation time of B. fragilis was 60 ± 9 min, and the mid-logarithmic phase occurred at an optical density at 675 nm of 0.4 to 0.6. Aerobic exposure for 1 h did not have a deleterious effect on the viability of B. fragilis; in fact, the number of cells increased from (6.7 ± 2.5) × 106 to (8.3 ± 3.7) × 106 CFU/ml, and under anaerobic conditions the number of cells increased to (8.4 ± 3.8) × 106 CFU/ml (no significant difference; P = 0.104). Likewise, norfloxacin had similar activity against B. fragilis in an anaerobic environment and an aerobic environment.
Accumulation of norfloxacin by B. fragilis compared with that by E. coli.
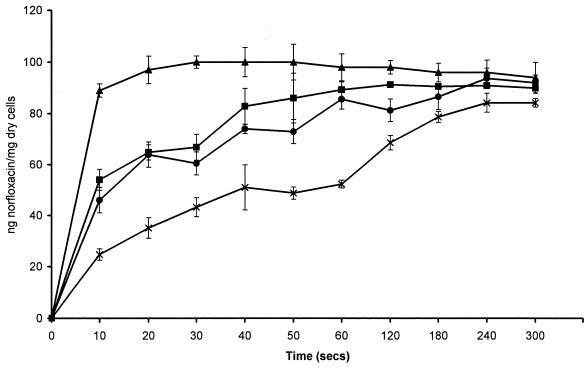
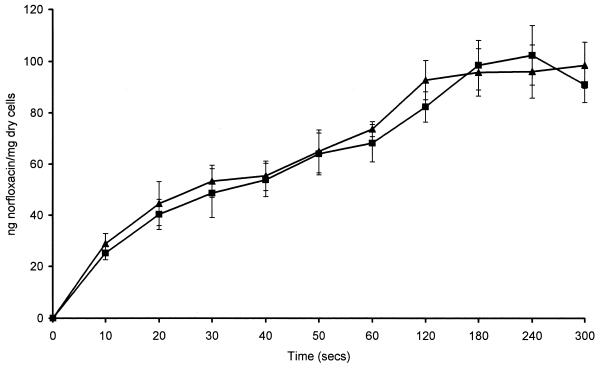
To determine whether accumulation of norfloxacin was affected by anaerobiosis, experiments were performed with E. coli NCTC 10418, which is a facultative anaerobe. E. coli was grown aerobically and anaerobically, and accumulation experiments were performed in air and within the MKII anaerobic workstation. When E. coli was grown and assayed aerobically the steady-state concentration (SSC) was achieved rapidly, within 30 s (Fig. 1). However, when E. coli was either grown or assayed anaerobically, the time required to achieve the SSC was much slower, approximately 2 min. The initial rates of accumulation were 2.61 ng/mg of cells/s for E. coli grown and assayed anaerobically, 4.64 ng/mg of cells/s for E. coli grown anaerobically and assayed aerobically, 5.32 ng/mg of cells/s for E. coli grown aerobically and assayed anaerobically, and 8.93 ng/mg of cells/s for E. coli grown and assayed aerobically. The final SSCs achieved between the different experiments were very similar. The SSC of norfloxacin achieved by E. coli grown aerobically and assayed aerobically was 94.3 ± 1.7 ng of norfloxacin/mg (dry weight) of cells, and when E. coli grown aerobically and assayed anaerobically the SSC was 89.2 ± 3.6 ng of norfloxacin/mg (dry weight) of cells (Fig. 1). These values are not significantly different (P = 0.126). The SSC of norfloxacin achieved by E. coli grown anaerobically and assayed aerobically was 84.2 ± 3.7 ng of norfloxacin/mg (dry weight) of cells, and when E. coli was grown anaerobically and assayed anaerobically the SSC was 91.9 ± 1.7 ng of norfloxacin/mg (dry weight) of cells (Fig. 1). These values are also not significantly different (P = 0.07). The kinetics of accumulation of norfloxacin by B. fragilis were similar whether the experiment was performed aerobically or anaerobically (Fig. 2). Compared with the accumulation of norfloxacin by E. coli in an aerobic environment, the accumulation by B. fragilis was slower, with an initial rate of accumulation of 1.083 ng of norfloxacin/mg (dry weight) of cells/s; it took B. fragilis about 3 min to reach an SSC of 98.45 ± 3.7 ng of norfloxacin/mg (dry weight) of cells, whereas it took E. coli 30 s. Accumulation of norfloxacin by B. fragilis ATCC 25285 gave data similar to those for B. fragilis NCTC 9343, with an SSC of 95.86 ± 4.2 ng of norfloxacin/mg (dry weight) of cells being achieved.
FIG. 1.
Accumulation of 10 μg of norfloxacin per ml by E. coli NCTC 10418 grown aerobically (▴) and anaerobically (■) and assayed aerobically, and accumulation of 10 μg of norfloxacin per ml by E. coli NCTC 10418 grown aerobically (●) and anaerobically (×) and assayed anaerobically.
FIG. 2.
Accumulation of 10 μg of norfloxacin per ml by B. fragilis NCTC 9343 assayed aerobically (■) and anaerobically (▴).
Characteristics of norfloxacin accumulation by B. fragilis.
With an increase in glucose concentration, between 0.1 and 0.6% (wt/vol), the concentration of norfloxacin accumulated after 20 min increased slightly from 78.34 to 86.81 ng of norfloxacin/mg (dry weight) of cells. Only for glucose concentrations of 0.1, 0.2 and 0.3% (wt/vol) were the data statistically different from those obtained without glucose (P = 0.00025, 0.0038, and 0.0038, respectively). For glucose concentrations higher than 0.4% P values were >0.05, suggesting that there was no effect at these higher concentrations.
To determine whether the data obtained in the present study were different from those of Miyamae et al. (17) due to their use of a different buffer in the accumulation assay, the accumulation of norfloxacin (10 μg/ml) by B. fragilis was determined with sodium phosphate buffer and was compared with that determined with potassium phosphate buffer containing glucose, dithiothreitol, and lithium chloride. The mean SSC achieved with the accumulation buffer of Miyamae et al. (17) was 87.08 ± 3.8 ng of norfloxacin/mg (dry weight) of cells, whereas it was 91.06 ± 4.21 ng of norfloxacin/mg (dry weight) of cells with sodium phosphate buffer alone. There was no significant difference between these two sets of data (P = 0.735). In addition, once an SSC had been attained no significant decrease was observed with either buffer (data not shown).
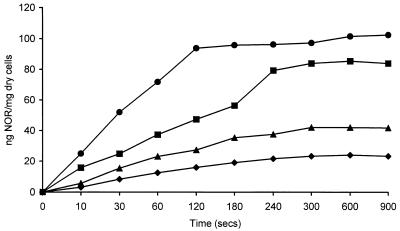
At norfloxacin concentrations of 1 to 8 μg/ml the concentration of norfloxacin accumulated increased proportionally by 12.13 ng of norfloxacin/mg (dry weight) of cells for each exogenous μg. At concentrations above 10 μg/ml there was no significant increase in the concentration of norfloxacin accumulated. The kinetics of accumulation were determined with several norfloxacin concentrations and by Michaelis-Menten kinetic analysis performed with the initial rate data derived from the slope of each curve of the mean data (Fig. 3). A Lineweaver-Burk plot calculated a Km of 5.03 μg/ml and a Vmax of 25.1 ng of norfloxacin/s. Other plots, including a Hanes-Woolf plot, Eadie-Hoftsee plot, and a direct linear plot, gave similar values (data not shown).
FIG. 3.
Kinetics of accumulation of norfloxacin (NOR) by B. fragilis NCTC 9343. Norfloxacin concentrations were 8 μg/ml (●), 6 μg/ml (■), 4 μg/ml (▴), 2 μg/ml (⧫). The data shown are the means of three experiments.
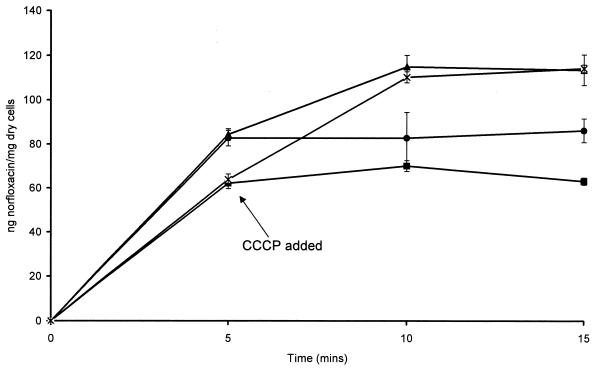
With an increase in temperature of between 0 and 37°C, the concentration of norfloxacin accumulated increased. Between 5 and 30°C, the concentration of norfloxacin accumulated at a constant rate of 4.722 ng of norfloxacin/mg (dry weight) of cells/°C. However, above 30°C the increase in the concentration of norfloxacin accumulated was reduced. Addition of 100 μM CCCP increased the mean SSC of norfloxacin to 116 ± 7.01 ng of norfloxacin/mg (dry weight) of cells (Fig. 4). Compared with the SSC of norfloxacin alone, this increase was statistically significant (P = 0.00099). With the addition of 0.1% glucose, the SSC of norfloxacin decreased to 62.13 ± 4.69 ng of norfloxacin/mg (dry weight) of cells. However, when 0.1% glucose and 100 μM CCCP were added together, the SSC increased to 110.18 ± 2.47 ng of norfloxacin/mg (dry weight) of cells. Compared with norfloxacin alone, the effect of glucose was significant (P = 0.013), as was that of glucose versus that of CCCP (P = 0.00009). There was no statistically significant difference between the SSCs of norfloxacin with CCCP with or without glucose.
FIG. 4.
Accumulation of 10 μg of norfloxacin per ml by B. fragilis NCTC 9343 with or without 100 μM CCCP and with or without 0.1% glucose. Norfloxacin alone (●), norfloxacin plus 100 μM CCCP (▴), norfloxacin plus 0.1% glucose (■), and norfloxacin plus 100 μM CCCP and 0.1% glucose (×) were tested.
In the presence of 20 μg of reserpine per ml, the mean SSC increased to 103.65 ± 4.55 ng of norfloxacin/mg (dry weight) of cells. This increase was not statistically significant (P = 0.0619).
In the presence of 20 mM magnesium chloride, the mean SSC of norfloxacin was reduced to 40.5 ± 3.76 ng of norfloxacin/mg (dry weight) of cells. This value was significantly different from that for norfloxacin alone (P = 0.0196).
DISCUSSION
There has been much interest in the role of efflux as a mechanism of bacterial resistance to fluoroquinolones and other agents. In order to determine the role of efflux in the intrinsic resistance of B. fragilis to antibiotics and also in antibiotic-resistant strains, this study sought to develop a method to study the accumulation of fluoroquinolones by B. fragilis. Although several methods have been used to measure accumulation, the “gold standard” has been the use of radiolabeled agents. In a previous study that compared the methods, it was shown that similar kinetics of accumulation were obtained for E. coli with radiolabeled and nonradiolabeled norfloxacin (18). Three procedures with radiolabeled agents were evaluated, and one gave data very similar to those obtained by the fluoresence method. Both methods diluted the cells immediately after sampling (1:20 for fluoresence; 1:40 for the method with radiolabeled agents) to remove exogenous antibiotic, followed by a further wash step. The other two methods with radiolabeled agents gave higher values, but the cells were washed only once. The advantage of the fluorescence method is that it is easily available and can be adapted to measure the accumulation of most fluoroquinolones, so it was carefully examined for use with B. fragilis. However, as this method had been used previously only to study aerobic bacteria and Lewin et al. (12, 13, 14) had suggested that oxygen was required for fluoroquinolones to be bactericidal, the present study first examined whether it was feasible for the accumulation assay to be performed with B. fragilis in air. It was found that short-term exposure of a B. fragilis suspension in phosphate buffer to air had no effect on its viability, confirming that B. fragilis is aerotolerant, presumably due to the expression of a virulence factor (7). It had already been noted that B. fragilis had reduced susceptibility to norfloxacin compared to that of E. coli. Therefore, to determine whether norfloxacin lost activity in the anaerobic environment, a facultative E. coli strain was used to examine whether activity in the anaerobic environment was reduced compared with that in air, and the observed MICs of norfloxacin for E. coli were identical in both atmospheres. These data compare well with those of Cooper et al. (5), who also used an anaerobic cabinet. Although it has been stated that anaerobiosis in an anaerobic chamber is not as strict as that obtained by Lewin et al. (12, 13, 14), it is clear that norfloxacin is equally bactericidal for E. coli under the standard conditions used in many clinical microbiology laboratories.
To determine whether the anaerobic environment affected the level of fluoroquinolone accumulation, E. coli was grown aerobically and anaerobically and norfloxacin accumulation was measured in both environments. It was found that if E. coli had been grown anaerobically or if the accumulation assay was performed in the anaerobic workstation, the initial rate of accumulation was far slower than that found when E. coli was grown aerobically or the accumulation assay was performed in air. Likewise, the rate of accumulation of norfloxacin by B. fragilis was shown to be slower than that by E. coli in air and not dissimilar to that by E. coli under anaerobic conditions. However, although the rate of accumulation was slower, the final concentrations achieved at steady state were similar to those attained in air. These data suggest that the environment influences the rate at which fluoroquinolones accumulate within the bacteria. It is hypothesized that the acidic nature of the anaerobic environment allows the fluoroquinolone to exist in a cationic state rather than the zwitterionic state in which it normally exists at neutral pH and that the passage of this charged molecule across the cell membrane is hindered due to electrostatic interactions between the fluoroquinolone and membrane components.
Glucose can act as an energy source for efflux pumps (8), and the data from the present study suggest that at low concentrations of glucose a putative efflux pump(s) is energized. However, as the concentration of glucose increases, the energizing activity of glucose is lost. This observation is similar to that of Zeller et al. (31) for Streptococcus pneumoniae. The addition of the efflux pump inhibitor CCCP increased the apparent concentration of norfloxacin accumulated, suggesting that the inhibition of an efflux pump allows higher levels of intracellular accumulation of the fluoroquinolone. Addition of 0.1% glucose (the concentration that gave the greatest effect) and CCCP had the same effect as addition of CCCP alone, suggesting that CCCP inhibition of the putative pump protein was not reversed by energizing the pump. Addition of reserpine also enhanced the concentration of norfloxacin accumulated (although not to statistically significant levels). Miyamae et al. (17) proposed a putative efflux pump for norfloxacin in a norfloxacin-resistant mutant of B. fragilis ATCC 25285, the phenotype of which resembled that of a norA and bmr mutant of gram-positive bacteria. Those investigators also observed enhanced accumulation of norfloxacin in the presence of CCCP and reserpine. However, in contrast to the present study, Miyamae et al. (17) did not report the same kinetic data, and after achieving a maximum concentration of norfloxacin, this concentration subsequently decreased over time. This phenomenon was not observed in the present study. Miyamae et al. (17) used radiolabeled norfloxacin, but it is unclear what atmosphere the assay was performed in. To determine whether the differences were due to the methods or the strain, the accumulation of norfloxacin by ATCC 25285 was examined as described for B. fragilis NCTC 9343. No detectable differences were observed between the kinetics of accumulation of the two strains. Therefore, a comparison of the buffers used in the accumulation assay was made. However, no significant difference in the data was observed with either buffer. The cause of the discrepancy is therefore not known, and as the norfloxacin-resistant mutant was not available for study (H. Nikaido, personal communication), further experiments could not be performed.
As has been found previously in the study of fluoroquinolone accumulation by other bacteria, lower concentrations of norfloxacin were accumulated at lower temperatures (6, 10, 23). It may be that accumulation of fluoroquinolones is dependent upon the rate of cell metabolism (4), and therefore, as cell metabolism decreases with a decrease in temperature, less fluoroquinolone is accumulated. Alternatively, the low temperatures will cause the cell membrane to become more rigid and less fluid. As it has been proposed that fluoroquinolones enter bacterial cells by passive diffusion, the rigidity of the cell membrane would prevent diffusion of norfloxacin across the membrane.
To further characterize the mechanism of accumulation of norfloxacin by B. fragilis experiments were performed with a range of norfloxacin concentrations. Michaelis-Menten kinetic analysis suggests that the accumulation of norfloxacin by B. fragilis was apparently saturable, thereby suggesting that the transport of norfloxacin into B. fragilis is mediated by saturable carrier protein. Similar data were obtained previously for some fluoroquinolones and P. aeruginosa (23). However, despite these data, as yet no carrier protein that mediates fluoroquinolone transport into any bacteria has been identified. An alternative explanation for these data is that at high fluoroquinolone concentrations, drug-mediated chelation of divalent cations could result in cell lysis with drug release, creating an artificial plateau. However, addition of magnesium ions did not negate the effect with P. aeruginosa (23). In addition, at the norfloxacin concentration examined, it is unlikely that cell lysis is occurring, particularly in the time frame of an accumulation experiment, as after 1.5 h of exposure to fluoroquinolone at concentrations below and above the optimal bactericidal concentrations, the bacterial cells became elongated and filamented (24). Another explanation could be the result of experimental artifacts, such as miscalculation of initial rates due to long periods of drug exposure (the sampling time plus the time required to remove the fluoroquinolone). However, Mortimer and Piddock (18) and more recently Ma et al. (15) have previously shown that dilution of the cells in ice-cold buffer and immediate centrifugation of the cells, as was performed in the experiments conducted for the present study, rapidly separated the drug from the cell. In addition, Mortimer and Piddock (18) showed that this procedure also prevented efflux.
As has been found for other fluoroquinolones and other bacteria, the level of accumulation of norfloxacin by B. fragilis in the presence of magnesium chloride was decreased. In a previous study, Marshall and Piddock (16) suggested that the cause of this decrease was chelation of the fluoroquinolone with magnesium, which forms a complex which is too bulky to enter the bacterial cell. An alternative proposal is that magnesium ions have a small effect on the membrane fusion protein of the putative efflux pump. Zgurskaya and Nikaido (32, 33) have suggested that the presence of magnesium ions causes the membrane fusion protein AcrA of E. coli to become less asymmetric, giving rise to a better link between the outer membrane pore and the inner membrane efflux pump protein, thereby giving improved extrusion of the antibiotic, and lower concentrations of antibiotic accumulated.
In conclusion, the present study describes a robust procedure for measurement of the accumulation of norfloxacin by B. fragilis. This procedure can be performed with cells grown anaerobically and assayed for accumulation either in an anaerobic environment, if available, or in an aerobic environment. As an anaerobic workstation was available and as B. fragilis is an obligate anaerobe (although with some aerobic tolerance), we chose to perform the remainder of our experiments under anaerobic conditions. The accumulation of norfloxacin by B. fragilis appears to be similar to the accumulation of this agent by aerobic bacteria. This method can now be applied to the analysis of fluoroquinolone-resistant B. fragilis and also to the investigation of other fluoroquinolones to determine whether the concentration of fluoroquinolone accumulated plays a role in the various activities of different fluoroquinolone antibiotics against this species. As the data support the presence of an efflux pump in wild-type B. fragilis, mutants with enhanced efflux could arise after exposure to fluoroquinolones.
ACKNOWLEDGMENT
We are grateful to Bayer AG for funding this study.
REFERENCES
- 1.Aldridge K E, Ashcraft D, Bowman K A. Comparative in vitro activities of trovafloxacin (CP 99,219) and other antimicrobials against clinically significant anaerobes. Antimicrob Agents Chemother. 1997;41:484–487. doi: 10.1128/aac.41.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brogan O, Garnett P A, Brown R. Bacteroides fragilis resistant to metronidazole, clindamycin and cefoxitin. J Antimicrob Chemother. 1989;23:660–662. doi: 10.1093/jac/23.4.660. [DOI] [PubMed] [Google Scholar]
- 3.Chapman J S, Georgopapadakou N H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988;32:438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen S P, Hooper D C, Wolfson J S, Souza K S, McMurry L M, Levy S B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988;32:1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper M A, Andrews J M, Wise R. Bactericidal activity of sparfloxacin and ciprofloxacin under anaerobic conditions. J Antimicrob Chemother. 1991;28:399–406. doi: 10.1093/jac/28.3.399. [DOI] [PubMed] [Google Scholar]
- 6.Diver J M, Piddock L J V, Wise R. The accumulation of five quinolone antibacterial agents by Escherichia coli. J Antimicrob Chemother. 1990;25:319–333. doi: 10.1093/jac/25.3.319. [DOI] [PubMed] [Google Scholar]
- 7.Finegold S M, Wexler H M. Anaerobic infections. In: Scholssberg D, editor. Current therapy of infectious disease. St. Louis, Mo: The C. V. Mosby Co.; 1996. pp. 369–375. [Google Scholar]
- 8.Georgopapadakou N H. Quinolone uptake and efflux. In: Georgopapadakou N H, editor. Drug transport in antimicrobial and anti-cancer chemotherapy. New York, N. Y: Marcel Dekker Inc.; 1995. pp. 245–267. [Google Scholar]
- 9.Goldstein E J C. Patterns of susceptibility to fluoroquinolones among anaerobic bacterial isolates in the United States. Clin Infect Dis. 1993;16(suppl. 4):5377–5381. doi: 10.1093/clinids/16.supplement_4.s377. [DOI] [PubMed] [Google Scholar]
- 10.Kotera Y, Watanabe M, Yoshida S, Inoue M, Mitsuhashi S. Factors influencing the uptake of norfloxacin by Escherichia coli. J Antimicrob Chemother. 1991;27:733–739. doi: 10.1093/jac/27.6.733. [DOI] [PubMed] [Google Scholar]
- 11.Lamothe F, Fijalkowski C, Malouin F, Bourgault A-M, Delorme L. Bacteroides fragilis resistant to both metronidazole and imipenem. J Antimicrob Chemother. 1986;18:642–643. doi: 10.1093/jac/18.5.642. [DOI] [PubMed] [Google Scholar]
- 12.Lewin C S, Morrissey I, Smith J T. Role of oxygen in the bactericidal action of the 4-quinolones. Rev Infect Dis. 1989;11(Suppl. 5):S913–S914. [Google Scholar]
- 13.Lewin C S, Morrissey I, Smith J T. The mode of action of quinolones: the paradox in activity of low and high concentrations and activity in the anaerobic environment. Eur J Clin Microbiol Infect Dis. 1991;10:240–248. doi: 10.1007/BF01966996. [DOI] [PubMed] [Google Scholar]
- 14.Lewin C S, Morrissey I, Smith J T. The bactericidal activity of sparfloxacin. J Antimicrob Chemother. 1992;30:625–632. doi: 10.1093/jac/30.5.625. [DOI] [PubMed] [Google Scholar]
- 15.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 16.Marshall A J H, Piddock L J V. Interaction of divalent cations, quinolones and bacteria. J Antimicrob Chemother. 1994;34:465–483. doi: 10.1093/jac/34.4.465. [DOI] [PubMed] [Google Scholar]
- 17.Miyamae S, Nikaido H, Tanaka Y, Yoshimura F. Active efflux of norfloxacin by Bacteroides fragilis. Antimicrob Agents Chemother. 1998;42:2119–2121. doi: 10.1128/aac.42.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer P G S, Piddock L J V. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1991;28:639–653. doi: 10.1093/jac/28.5.639. [DOI] [PubMed] [Google Scholar]
- 19.Nord C E. In vitro activity of quinolones and other antimicrobial agents against anaerobic bacteria. Clin Infect Dis. 1996;23(Suppl. 1):S15–S18. doi: 10.1093/clinids/23.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 20.Onodera Y, Sato K. Molecular cloning of the gyrA and gyrB genes of Bacteroides fragilis encoding DNA gyrase. Antimicrob Agents Chemother. 1999;43:2423–2429. doi: 10.1128/aac.43.10.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piddock L J V. Mechanisms of fluoroquinolone resistance: an update 1994–1998. Drugs. 1999;58(Suppl. 2):11–18. doi: 10.2165/00003495-199958002-00003. [DOI] [PubMed] [Google Scholar]
- 22.Piddock L J V, Wise R. Properties of the penicillin-binding proteins of four species of the genus Bacteroides. Antimicrob Agents Chemother. 1986;29:825–832. doi: 10.1128/aac.29.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piddock L J V, Jin Y-F, Ricci V, Asuquo A E. Quinolone accumulation by Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother. 1999;43:61–70. doi: 10.1093/jac/43.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Piddock L J V, Walters R N, Diver J M. Correlation of quinolone MIC and inhibition of DNA, RNA, and protein synthesis and induction of the SOS response in Escherichia coli. Antimicrob Agents Chemother. 1990;34:2331–2336. doi: 10.1128/aac.34.12.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano S L. Trovafloxacin in gynaecology. Medicina (Buenos Aires) 1999;59:55–61. [PubMed] [Google Scholar]
- 26.Rotimi V O, Duerden B I, Ede V, MacKinnon A E. Metronidazole-resistant Bacteroides from untreated patient. Lancet. 1979;i:833. doi: 10.1016/s0140-6736(79)91360-6. [DOI] [PubMed] [Google Scholar]
- 27.Rotimi V O, Khoursheed M, Brazier J S, Jamal W Y. Bacteroides species highly resistant to metronidazole: an emerging clinical problem? Clin Microbiol Infect. 1999;5:166–169. doi: 10.1111/j.1469-0691.1999.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 28.Snydman D R, Cuchural G J. Susceptibility variations in Bacteroides fragilis: a national survey. Infect Dis Clin Practice. 1994;3:S34–S43. [Google Scholar]
- 29.Thadepalli H, Reddy U, Chuah S K, Thadepalli F, Malilay C, Polzer R J, Hanna N, Esfandiari A, Brown P, Gollapudi S. In vivo efficacy of trovafloxacin (CP-99,217), a new quinolone in experimental intra-abdominal abscesses caused by Bacteroides fragilis and Escherichia coli. Antimicrob Agents Chemother. 1997;41:583–586. doi: 10.1128/aac.41.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeller V, Janoir C, Kitzis M-D, Gutmann L, Moreau N J. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1973–1978. doi: 10.1128/aac.41.9.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zgurskaya H I, Nikaido H. AcrA is a highly asymmetric protein capable of spanning the periplasm. J Mol Biol. 1999;285:409–420. doi: 10.1006/jmbi.1998.2313. [DOI] [PubMed] [Google Scholar]
- 33.Zgurskaya H I, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:7190–7195. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]