Abstract
Sarcoidosis is a multisystemic inflammatory chronic disease characterized by the presence of noncaseating granulomas most frequently in lungs and in intrathoracic lymph nodes. The nasopharyngeal form is unusual and noncommon in the ENT practice.
Background and objectives:
In order to establish a correct knowledge about this rare disease, we report two different cases of nasopharyngeal sarcoidosis moreover all the available literature is reviewed.
Materials and Methods:
A systematic literature review was made through PubMed databases, according to the PRISMA guidelines (1), combining the following key words: Nasopharyngeal, Rhinopharynx, Sarcoidosis, in publications between 1951 and 2020. In addition, we reported our personal experience on the disease by describing two clinical cases that occurred at our clinic in November 2018 and June 2019.
Results:
16 articles reported 27 cases of nasopharyngeal sarcoidosis. The number of males was 13 (48,2%) and the number of females was 14 (51,8%) with a mean age at the diagnosis of 35,28 ± 13.05 years old (range 5 - 64). In 16 (59,3%) cases nasopharyngeal sarcoidosis was associated with lungs and/or intrathoracic lymph nodes involvement; nasal obstruction was the most frequently reported symptom (51,8% of subjects).
Conclusions:
Nasopharyngeal sarcoidosis can mimic several disorders of the upper airway respiratory tract and it must therefore be considered in the differential diagnosis. A biopsy of nonspecific lesions in the nasopharynx is advisable to permit several early diagnosis of upper airway respiratory tract disorders including sarcoidosis.
Keywords: sarcoidosis, nasopharyngeal sarcoidosis, nasopharyngeal biopsy
Introduction
Sarcoidosis is an inflammatory chronic disease that affects one or more organs in the body, most commonly lungs and intrathoracic lymph nodes. It occurs at all ages, with the incidence peaking at 20-39 years and the highest annual incidence has been observed in northern European countries (2). In Italy a mean prevalence of 49 cases per 100.000 individuals has been described, with a 58% of female incident cases (3). The etiology is still unknown and the diagnosis is not standardized but it’s based on three major criteria: a compatible clinical and radiological presentation, the presence of noncaseating granulomatous inflammation in one or more tissue samples and exclusion of alternative causes of granulomatous disease (4). Sarcoidosis in the head and neck district is unusual disease and the most common localization is the parotid gland (6% of all prevalence) as an isolated clinical form or associated with Heerfordt Syndrome (5). The nasopharyngeal form is very uncommon and rare and it can occur as isolated sarcoidosis upper respiratory tract involvement (SURT) or in association with others systemic findings, almost always lungs involvement (6). For his rarity and hard diagnosis, the nasopharyngeal sarcoidosis could represent a pitfall for otorhinolaryngology. We report two cases and a literature review, the aim of this study is to evaluate the correct management of nasopharyngeal sarcoidosis.
Materials and Methods
A systematic literature review was made through PubMed databases, according to the PRISMA guidelines (1), combining the following key words: Nasopharyngeal, Rhinopharynx, Sarcoidosis, in publications between January 1951 and June 2020.
We included studies according to the following criteria: studies on established nasopharyngeal sarcoidosis involvement both in adult and pediatric population where it is clearly specified that the diagnosis was performed with a histological nasopharyngeal finding. Case Reports and studies written in English. The data from each study was transcribed in tabular forms and these were summarized using descriptive statistics. Dichotomous variables were reported as counts and percentages, while continuous variables as mean ± standard deviation.
In addition, we reported our personal experience on the disease by describing two clinical cases that occurred at our clinic in November 2018 and June 2019.
Presentation of case reports
Case 1
A 41-year-old man presented to our ENT (ear, nose and throat) clinic in Pisa in November 2018 with bilateral nasal obstruction, rhinorrhea and snoring. The patient was non-smoker, non-allergic and non-asthmatic. He presented a familiarity for autoimmune disorders. A flexible fiberoptic endoscopy evaluation of the nasal cavity revealed bilateral inferior hypertrophic turbinates and a nasal septal deviation with a prominent nasal septal left ridge. The patient presented a nonspecific lesion of the posterior wall near the right torus tubarius in rhinopharynx. The nasopharyngeal lesion appeared to be inflammatory and similar to adenoid hypertrophy with post nasal drip secretions (Figure 1A). We performed an endoscopic biopsy of the lesion under local anesthesia with the following histopathological report (Figure 2): similar sarcoid tissue with non-necrotizing granulomatous inflammation composed with epithelioid cells (CD68 PGM1 +) and occasional multinucleated giant cells. The Ziehl-Neelsen stain used to identify acid-fast organisms resulted negative. A CT scan of the head, neck and thorax revealed a volumetric enlargement of nasopharyngeal tonsil especially at right side and multiple mediastinal lymph nodes with the largest (2x6 cm) in subcarinal position. The PET scan confirmed a higher uptake in rhinopharynx (SUV 8,7 max), oro-hypopharynx and mediastinal lymph nodes (SUV 7,1 max) compatible with the diagnosis of sarcoidosis (Figure 3). He has been also submitted to other investigations such as pulmonary function tests tests and cardiological tests which resulted normal. The patient was treated only with a local corticosteroid therapy which led to a good control of nasal symptoms. Considering the good functional pulmonary and cardiological status, the pulmonologist preferred not to start a systemic therapy but to make only a careful observation with a follow up every 6 months.
Figure 1.

Lesions in the rhinopharynx in case 1 (A) and case 2 (B).
Figure 2.

Nasopharyngeal tissue with numerous, diffuse non-caseating epithelioid granulomas, composed by epithelioid cells (A-D) with rare Langhans giant cells (highlighted by arrows in B) (H&E: A-B, x40; C, x100; D, x200).
Figure 3.
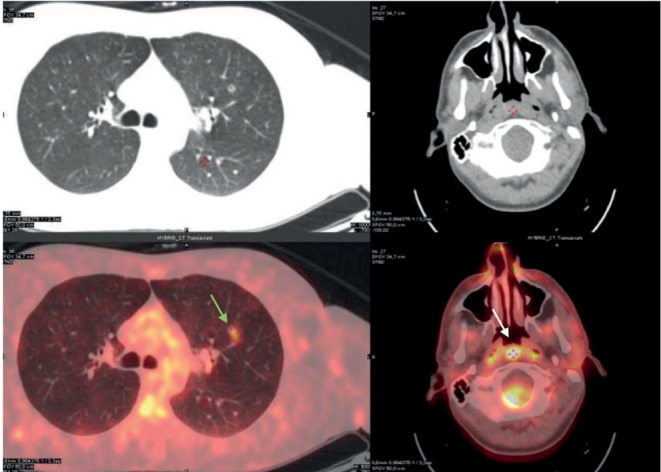
PET-CT examination shows partially excavated micronodule with moderate metabolic hyperactivity (SUV 7,1 max) in the anterior segment of the left upper lobe (green arrow), concomitant with some slightly hypermetabolic lymph nodes in the bilateral and subcarinal hilar area. Presence of glucose hypermetabolism (SUV 8,7 max) in the nasopharyngeal region (white arrow).
Case 2
A 32 year-old-woman with symptoms of fatigue and night sweats was admitted to our ENT department in June 2018 with a suspected cervical adenopathy. She had a medical history for Hashimoto’s thyroiditis in euthyroidism and thalassemia trait. Suspecting a lymphoma, we performed an excisional biopsy of the lymph node under general anesthesia. Unexpectedly the histopathological report revealed a chronic non-necrotizing granulomatous lymphadenitis with multinucleated giant cells in appearance similar to sarcoidosis. The CT scan revealed multiple pulmonary lymph nodes with the largest (8 mm) in the lower right lobe and multiple supra and infra-diaphragmatic lymph nodes. Blood chemistry tests reported lymphopenia and increased erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and angiotensin-converting enzyme (ACE). QuantiFERON-Tb Gold (QFT-G) and other blood tests were normal. Functional pneumological tests and cardiological tests resulted normal. She has been diagnosed with pulmonary sarcoidosis with a supra and infra-diaphragmatic lymph nodes involvement. The pulmonologist started a close follow-up. One year later in June 2019 the patient presented to our medical office with bilateral nasal obstruction, rhinorrhea and anosmia of recent onset. A flexible fiberoptic endoscopy evaluation of the nasal cavity revealed a bilateral inferior hypertrophy of turbinates with nasal secretions and mild swelling of tissues. The patient presented a nonspecific lesion of the posterior wall in the rhinoparyx with an adenoid-like appearance (Figure 1B). Suspecting a new localization of sarcoidosis, we performed an endoscopic biopsy of the lesion under local anesthesia that confirmed our suspect. Given the new localization of the disease, and new onset nasal symptoms, the patient started a systemic therapy with hydroxychloroquine and prednisone. At the present time, the patient has a good clinical status with a complete resolution of her anosmia and nasal congestion.
Results and Discussion
A review of the literature revealed a total of 16 articles describing 27 cases of nasopharyngeal sarcoidosis, the first article was published in 1951 while the last one in 2016. We identified only case reports, we didn’t find any type of design study. We excluded some cases from our review due to lack of sufficient information and according to our eligibility criteria (Figure 4). Table 1 summarizes the clinical data of these 27 cases, plus our two cases. Considering all the literature cases together, the number of males was 13 (48,2%) and the number of females was 14 (51,8%) with a mean age at the diagnosis of 35,28 ± 13.05 years old (range 5 - 64). A noted pre-existing condition of systemic sarcoidosis was already present in 4 subjects while for the other 23 (85,2%) patients the nasopharyngeal localization was a first diagnosis of sarcoidosis. Among these patients, in 16 (59,3%) cases the nasopharyngeal sarcoidosis was associated with lungs and/or intrathoracic lymph nodes involvement, in 2 (16,4%) cases no pulmonary involvement was reported by the authors while 5 (18,5%) cases resulted an isolated form of nasopharyngeal sarcoidosis. In all cases except for one, it was possible to find a nonspecific nasopharyngeal lesion; in the only case where no lesions were found, the authors performed random biopsies in a subject with a medical history of sarcoidosis and a new onset deafness which followed an ear infection (7). Nasal obstruction was the most frequently reported symptom (51,8% of subjects). In 2 cases there were a severe complications: an acquired nasopharyngeal stenosis occurred in one subject (8) and a destruction of the sphenoid bone in another subject (9). The 11 subjects of the first case reported in 1951 were treated with Roentgen therapy that nowadays represents only a historical value (10). 8 (29,6%) subjects were treated with systemic corticosteroids alone or in association with immunosuppressant drugs; in 1 of these subjects these therapies failed and he was treated with low-dose external beam radiotherapy (11). 1 (3,7%) subject was treated with local corticosteroid therapy that failed with subsequent conversion to systemic therapy. 2 (7,4%) subjects were treated with surgery (adenotonsillectomy and nasopharyngeal dilation). 1 (3,7%) subject with systemic sarcoidosis was not treated for the nasopharyngeal involvement but the other involvement were treated (12). In 1 (3,7%) subject no therapies were performed but only follow-up. In 3 (11,1%) subjects it was not possible to establish a therapy because this was not reported by the authors.
Figure 4.
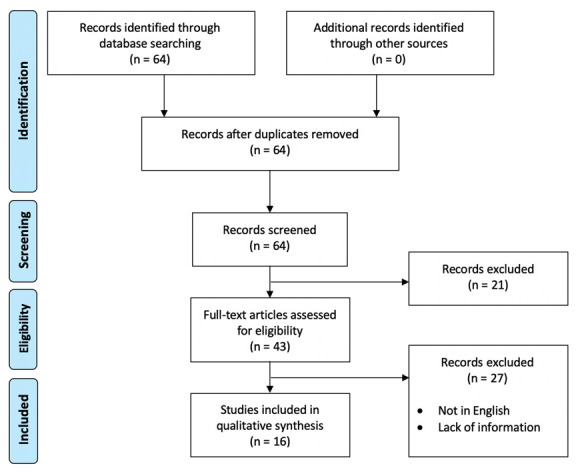
PRISMA 2009 flow diagram: selection of cases.
Table 1.
Clinical and pathologic characteristics of 27 reported cases with nasopharyngeal sarcoidosis plus our 2 cases.
| References | Year | N patients (M/F) | Mean Age (range) | Symptoms | Lungs and/or intrathoracic involvement | Treatment |
|---|---|---|---|---|---|---|
| Tuğrul S et al (23) | 2016 | 1 1/0 |
32 | Nasal obstruction Snoring |
Yes | / |
| El Bousaadani A et al (24) | 2015 | 1 0/1 |
43 | Nasal obstruction Epistaxis |
No | Systemic therapy (prednisone 20mg daily) |
| Akin S et al (25) | 2012 | 1 0/1 |
51 | Dryness of mouth and throat | No | Systemic therapy (100 mg/day azathioprine, 200 mg/day hydroxychloroquine, and 40 mg/day prednisolone) |
| Brodsky JR et al (8) | 2012 | 1 1/0 |
40 | Nasal obstruction Snoring Restless sleep |
/ | Surgery (serial dilatation to 15 mm with a balloon catheter and injection with 20 mg of topical triamcinolone) |
| Gil Calero MM et al (26) | 2011 | 1 0/1 |
45 | Nasal obstruction Rhinorrhea Bilateral hypoacusis Asthenia Fever Pretibial erythema nodosum |
No | Initial topical corticosteroid regime that failed. Then they started treatment with oral deflazacort at doses of 1 mg/(kg day) in a descending pattern for 12 days |
| Rottoli P et al (6) | 2005 | 1 0/1 |
39 | / | Yes | Systemic therapy (steroids) |
| Sugisaki K et al (27) | 2000 | 1 0/1 |
64 | Hearing loss Tinnitus |
Yes | Systemic therapy (steroids) |
| Osinubi OA et al (7) | 2000 | 1 1/0 |
54 | Hearing loss | Yes | / |
| Onishi Y et al (12) | 1998 | 1 1/0 |
27 | Low grade fever Malaise Headache Dysesthesia of the toes |
Yes | Nasopharyngeal involvement was not treated while other sarcoidosis involvements were treated |
| Douds AC et al (11) | 1997 | 1 1/0 |
39 | Nasal obstruction Stridor |
Yes | Low-dose external beam radiotherapy to nasopharynx and larynx (20 Gys in 10 fractions over 2 weeks) |
| Roger G et al (28) | 1994 | 1 1/0 |
13 | Nasal obstruction Snoring Sleep apnea |
Yes | Adenotonsillectomy but no specific treatment for sarcoidosis |
| Erwin SA et al (29) | 1989 | 1 0/1 |
12 | Nasal obstruction Snoring |
No | No therapy |
| Oluboyo PO et al (30) | 1987 | 1 0/1 |
37 | Hemoptysis (to be due to ulcerations of nasopharyngeal granulomas) | / | / |
| Wilson R et al (31) | 1986 | 2 1/1 |
10 (15-5) |
Gritty red eyes and painful Diminishing visual acuity Gritty red eyes Fever Lethargy and dyspnea Lacrimal gland enlargement |
Yes (1 subject) |
Systemic therapy (steroids) |
| Herberts G (9) | 1957 | 1 1/0 |
35 | Attacks of visual disturbance | No | Systemic therapy (steroids, PAS and ACTH) |
| Larsson L-G (10) | 1951 | 11 6/5 |
36,45 (23-48) |
No subjective symptoms 27,27% Nasal obstruction 63,64% Pain radiating towards ear 9,09% |
Yes (8 subjects) |
Roentgen therapy |
| Our cases | / | 2 1/1 |
36,5 (32-41) |
Nasal obstruction Rhinorrhea Snoring Anosmia |
Yes | Local therapy Systemic therapy (hydroxychloroquine and prednisone) |
Our two subjects (mean age 36.5) on CT both showed intrathoracic involvement but in the absence of pulmonary symptoms. They show only local symptoms (nasal congestion, rhinorrhea, snoring and anosmia) which were treated with local corticosteroid therapy in one subject while the other required systemic therapy with hydroxychloroquine and prednisone.
It is known that Sarcoidosis can involve several organs included the upper airway tract, but lungs and intrathoracic lymph nodes remains the most involved sites. A large epidemiological study about subjects affected by sarcoidosis reported the ear, nose, and throat (ENT) involvement in 51 (6,9%) out of 736 patients; with 29 cases interesting the parotid or salivary gland (3,9%) and only 22 cases all the others ENT site. Among these 22 cases, one of the districts less interested from the disease was the nasopharynx. In fact, only in 3 of all the 736 patients (0,4%), the disease was limited to the nasopharynx (13). The etiopathogenesis is still not clear and studies suggest that the exposure to environmental risk factors of patients with a genetic predisposition could lead to the disease (14). Newman et al conducted A Case Controlled Etiologic Study of Sarcoidosis (15) where they concluded that sarcoidosis was associated with exposures to “insecticides, agricultural employment, and microbial bioaerosols.” Kosikowska et al (16) studied nasopharyngeal swabs and sputum specimens and suggested that the qualitative and quantitative changes in the respiratory microbiota concerning the overall prevalence of H. influenzae together with the dropped number of H. parainfluenzae types and the decreased rate of H. parainfluenzae biofilm-producing may be associated with sarcoidosis. It is unknow how and why disease can develop on alternative sites of the body. The difficulty to make a diagnosis and treatment of nasopharyngeal sarcoidosis is also indirectly underlined by the WASOG Organ Assessment Instrument (17) (an update of the original ACCESS (18)) and the recent ERS(19) clinical practice guidelines on treatment of sarcoidosis where nasopharyngeal clinical manifestations do not differ from the common nasal symptoms and the committee found insufficient information to make recommendations for the treatment. In a recent review about Sinonasal and Laryngeal Sarcoidosis Edriss et al (20) analyzed selected case series published in the last 15 years and underlined how sarcoidosis should always be considered in the differential diagnosis during the evaluation of patients with inflammatory nonspecific upper airway symptoms. Patients with a known diagnosis of sarcoidosis and new-onset upper airway symptoms should be submitted to endoscopy evaluation, CT of head and sinuses and biopsy to confirm a new localization of the disease. In the same way patients with a new diagnosis of sarcoidosis with upper airway symptoms will also need an accurate medical history and clinical examination with the adequate tests as chest x-ray and pulmonary function test. The diagnosis of nasopharyngeal sarcoidosis is very difficult especially when the upper respiratory involvement is the first manifestation. It is important to collaborate with others specialists such as pneumologists because early identification of sarcoidosis upper respiratory involvement can helps to prevent severe complications (6). The treatment is not standardized: corticosteroids remain the core of therapies and systemic treatments are the best choice, with also a direct benefit on the upper airway tract (21). However, local treatments may be helpful. Sometimes these patients require only local measures: saline irrigation and topical steroids may be sufficient to control the disease (22).
Conclusions
In conclusion sarcoidosis is a multisystemic inflammatory chronic disease characterized by the presence of noncaseating granulomas most frequently in lungs and in intrathoracic lymph nodes and that can involve the upper airway respiratory tract. The nasopharyngeal form is unusual and noncommon in the ENT practice. It’s important to keep in mind that this should be considered in the differential diagnosis of the disorders of upper airway respiratory tract (adenoid hypertrophy, Tornwaldt cyst, nasopharyngeal carcinoma, granulomatosis with polyangioiitis etc)(4). A biopsy of nonspecific lesions in the nasopharynx is advisable because it can allow an early diagnosis of Sarcoidosis. In the same way, patients with a known pulmonary sarcoidosis that report a new onset of upper airway respiratory symptoms should be submitted to ENT evaluation and the specialists should consider a secondary extrapulmonary sarcoidosis localization.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. https://doi.org/10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. New England Journal of Medicine. 2007;357:2153–65. doi: 10.1056/NEJMra071714. https://doi.org/10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 3.Beghè D, Dall’Asta L, Garavelli C, Pastorelli AA, Muscarella M, Saccani G, et al. Sarcoidosis in an Italian province. Prevalence and environmental risk factors. PLoS One. 2017:12. doi: 10.1371/journal.pone.0176859. https://doi.org/10.1371/journal.pone.0176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201:e26–51. doi: 10.1164/rccm.202002-0251ST. https://doi.org/10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto-Gomez N, Peters JI, Nambiar AM. Diagnosis and Management of Sarcoidosis. Am Fam Physician. 2016;93:840–8. [PubMed] [Google Scholar]
- 6.Rottoli P, Bargagli E, Chidichimo C, Nuti D, Cintorino M, Ginanneschi C, et al. Sarcoidosis with upper respiratory tract involvement. Respiratory Medicine. 2006;100:253–7. doi: 10.1016/j.rmed.2005.04.018. https://doi.org/10.1016/j.rmed.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Osinubi OA. A rare cause of unilateral hearing loss. Postgraduate Medical Journal. 2000;76:584–5. doi: 10.1136/pmj.76.899.584. https://doi.org/10.1136/pmj.76.899.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodsky JR, Tatum SA, Kelley RT. Acquired nasopharyngeal stenosis in a patient with sarcoidosis. J Laryngol Otol. 2012;126:1182–5. doi: 10.1017/S0022215112001776. https://doi.org/10.1017/S0022215112001776. [DOI] [PubMed] [Google Scholar]
- 9.Herberts G. Benign lymphogranulomatosis (sarcoidosis) in the nasopharynx with destruction of the base of the skull; report of a case. Acta Otolaryngol. 1957;48:333–8. doi: 10.3109/00016485709126888. https://doi.org/10.3109/00016485709126888. [DOI] [PubMed] [Google Scholar]
- 10.Larsson L-G. Nasopharyngeal Lesions in Sarcoidosis. Acta Radiologica. 1951;36:361–73. doi: 10.3109/00016925109176986. https://doi.org/10.3109/00016925109176986. [DOI] [PubMed] [Google Scholar]
- 11.Douds AC, Campbell S, Young DW. Radiotherapy for nasal congestion. Lancet. 1997;350:786. doi: 10.1016/S0140-6736(05)62572-X. https://doi.org/10.1016/S0140-6736(05)62572-X. [DOI] [PubMed] [Google Scholar]
- 12.Onishi Y, Imai Y, Tojima H, Nakajima K, Takahashi A. Systemic Sarcoidosis with Significant Granulomatous Swelling of the Pharyngeal Tonsil. Intern Med. 1998;37:157–60. doi: 10.2169/internalmedicine.37.157. https://doi.org/10.2169/internalmedicine.37.157. [DOI] [PubMed] [Google Scholar]
- 13.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, et al. Clinical Characteristics of Patients in a Case Control Study of Sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–9. doi: 10.1164/ajrccm.164.10.2104046. https://doi.org/10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus A. Sarcoidosis: Epidemiology, Etiology, Pathogenesis, and Genetics. Disease-a-Month. 2009;55:649–60. doi: 10.1016/j.disamonth.2009.04.008. https://doi.org/10.1016/j.disamonth.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, et al. A Case Control Etiologic Study of Sarcoidosis: Environmental and Occupational Risk Factors. Am J Respir Crit Care Med. 2004;170:1324–30. doi: 10.1164/rccm.200402-249OC. https://doi.org/10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 16.Kosikowska U, Rybojad P, Stępień–Pyśniak D, Żbikowska A, Malm A. Changes in the prevalence and biofilm formation of Haemophilus influenzae and Haemophilus parainfluenzae from the respiratory microbiota of patients with sarcoidosis. BMC Infect Dis. 2016;16:449. doi: 10.1186/s12879-016-1793-7. https://doi.org/10.1186/s12879-016-1793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis VDLD. 2014;31:19–27. [PubMed] [Google Scholar]
- 18.Ma J, Rp B, As T, Ml T, H Y. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases: Official Journal of WASOG. 1999:16. https://pubmed.ncbi.nlm.nih.gov/10207945/ (accessed February 20, 2021) [PubMed] [Google Scholar]
- 19.Baughman RP, Valeyre D, Korsten P, Mathioudakis AG, Wuyts WA, Wells A, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58:2004079. doi: 10.1183/13993003.04079-2020. https://doi.org/10.1183/13993003.04079-2020. [DOI] [PubMed] [Google Scholar]
- 20.Edriss H, Kelley JS, Demke J, Nugent K. Sinonasal and Laryngeal Sarcoidosis—An Uncommon Presentation and Management Challenge. The American Journal of the Medical Sciences. 2019;357:93–102. doi: 10.1016/j.amjms.2018.11.007. https://doi.org/10.1016/j.amjms.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Braun JJ, Gentine A, Pauli G. Sinonasal Sarcoidosis: Review and Report of Fifteen Cases. The Laryngoscope. 2004;114:1960–3. doi: 10.1097/01.mlg.0000147928.06390.db. https://doi.org/10.1097/01.mlg.0000147928.06390.db. [DOI] [PubMed] [Google Scholar]
- 22.Baughman RP, Lower EE, Tami T. Upper airway.4: Sarcoidosis of the upper respiratory tract (SURT) Thorax. 2010;65:181–6. doi: 10.1136/thx.2008.112896. https://doi.org/10.1136/thx.2008.112896. [DOI] [PubMed] [Google Scholar]
- 23.Tuğrul S, Göktaş SS, Özücer B, Sönmez FC, Özturan O. A clinically unsuspected nasopharyngeal sarcoidosis. Kulak Burun Bogaz Ihtis Derg. 2016;26:169–71. doi: 10.5606/kbbihtisas.2016.02256. https://doi.org/10.5606/kbbihtisas.2016.02256. [DOI] [PubMed] [Google Scholar]
- 24.El Bousaadani A, Eljahd L, Benbakh M, Mahtar M. Nasopharyngeal mass filling the choanae revealing sarcoidosis. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132:309–10. doi: 10.1016/j.anorl.2015.08.037. https://doi.org/10.1016/j.anorl.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Akin S, Akin S, Karadag O, Kalyoncu U, Balcı S, Ozgen B. Nasopharyngeal sarcoidosis: a rare involvement. Rheumatol Int. 2012;32:1407–9. doi: 10.1007/s00296-011-1866-2. https://doi.org/10.1007/s00296-011-1866-2. [DOI] [PubMed] [Google Scholar]
- 26.Gil Calero MM, García López M, Carrasco-Gómez A, García-Fernández-De Sevilla T. Sarcoidosis in the nasopharynx, a rare location. Acta Otorrinolaringol Esp. 2011;62:323–4. doi: 10.1016/j.otorri.2010.05.002. https://doi.org/10.1016/j.otorri.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Sugisaki K, Miyazaki E, Fukami T, Sawabe T, Kumamoto T, Tsuda T, et al. A case of sarcoidosis presenting as multiple pulmonary nodules, nasopharyngeal and cerebellopontine tumors. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:82–5. [PubMed] [Google Scholar]
- 28.Roger G, Gallas D, Tashjian G, Baculard A, Tournier G, Garabedian EN. Sarcoidosis of the upper respiratory tract in children. Int J Pediatr Otorhinolaryngol. 1994;30:233–40. doi: 10.1016/0165-5876(94)90065-5. https://doi.org/10.1016/0165-5876(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 29.Erwin SA. Unsuspected sarcoidosis of the tonsil. Otolaryngol Head Neck Surg. 1989;100:245–7. doi: 10.1177/019459988910000314. https://doi.org/10.1177/019459988910000314. [DOI] [PubMed] [Google Scholar]
- 30.Oluboyo PO, Awotedu AA, Onadeko BO, Ukoli CO. Sarcoidosis presenting with severe haemoptysis. Trop Geogr Med. 1987;39:196–8. [PubMed] [Google Scholar]
- 31.Wilson R, Sweatman M, Mackay IS, Mitchell DN. Adenoidal tissue as an aid to the diagnosis of sarcoidosis in childhood. Thorax. 1986;41:66–7. doi: 10.1136/thx.41.1.66. https://doi.org/10.1136/thx.41.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]


