Abstract
Background
It is unclear whether patients with type 2 diabetes who have poor glycaemic control despite maximal oral hypoglycaemic agents (OHAs) should be commenced on insulin as monotherapy, or insulin combined with oral hypoglycaemic agents (insulin‐OHA combination therapy).
Objectives
To assess the effects of insulin monotherapy versus insulin‐OHA combinations therapy.
Search methods
Eligible studies were identified by searching MEDLINE, EMBASE, and The Cochrane Library.
Selection criteria
Randomised controlled trials (RCTs) with 2 months minimum follow‐up duration comparing insulin monotherapy (all schemes) with insulin‐OHA combination therapy.
Data collection and analysis
Data extraction and assessment of study quality were undertaken by three reviewers in pairs.
Main results
Twenty RCTs (mean trial duration 10 months) including 1,811 participants, with mean age 59.8 years and mean known duration of diabetes 9.6 years. Overall, study methodological quality was low. Twenty‐eight comparisons in 20 RCTs were ordered according to clinical considerations. No studies assessed diabetes‐related morbidity, mortality or total mortality. From 13 studies (21 comparisons), sufficient data were extracted to calculate pooled effects on glycaemic control. Insulin‐OHA combination therapy had statistically significant benefits on glycaemic control over insulin monotherapy only when the latter was applied as a once‐daily injection of NPH insulin. Conversely, twice‐daily insulin monotherapy (NPH or mixed insulin) provided superior glycaemic control to insulin‐OHA combination therapy regimens where insulin was administered as a single morning injection. In more conventional comparisons, regimens utilising OHAs with bedtime NPH insulin provided comparable glycaemic control to insulin monotherapy (administered as twice daily, or multiple daily injections). Overall, insulin‐OHA combination therapy was associated with a 43% relative reduction in total daily insulin requirement compared to insulin monotherapy. Of the 14 studies (22 comparisons) reporting hypoglycaemia, 13 demonstrated no significant difference in the frequency of symptomatic or biochemical hypoglycaemia between insulin and combination therapy regimens. No significant differences in quality of life related issues were detected. Combination therapy with bedtime NPH insulin resulted in statistically significantly less weight gain compared to insulin monotherapy, provided metformin was used ± sulphonylurea. In all other comparisons no significant differences with respect to weight gain were detected.
Authors' conclusions
Bedtime NPH insulin combined with oral hypoglycaemic agents provides comparable glycaemic control to insulin monotherapy and is associated with less weight gain if metformin is used.
Plain language summary
Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus
Simple application of a single daily insulin injection in addition to oral hypoglycaemic agents may facilitate the initiation of insulin therapy in type 2 diabetes mellitus.This review examined 20 trials including 1,811 participants which compared insulin monotherapy with insulin in combination with oral hypoglycaemic agents (OHA) in insulin‐requiring patients with type 2 diabetes. The results suggest that a bedtime NPH insulin‐oral hypoglycaemic agent combination therapy regimen provides comparable glycaemic control to insulin monotherapy. Due to lack of studies it remains unclear whether insulin‐OHA combination regimens with metformin alone are superior to those with metformin plus a sulphonylurea. In most cases no significant differences in hypoglycaemic events were observed between insulin mono‐ and OHA combination therapy. No study assessed diabetes‐related morbidity or mortality.
Background
Description of the condition
Diabetes mellitus is a chronic metabolic disorder resulting from a fundamental defect in insulin secretion, insulin action, or both. Consequential chronic hyperglycaemia (i.e. elevated levels of plasma glucose) with associated disturbances of carbohydrate, fat and protein metabolism ensues. Long‐term (microvascular) complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is also increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group on The Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups‐CRGs'). For an explanation of methodological terms, see the main Glossary on The Cochrane Library.
Description of the intervention
In the United Kingdom Prospective Diabetes Study (UKPDS) of newly diagnosed type 2 diabetes patients, compared with conventional therapy, intensive glucose control (mean HbA1c 7.0% versus 7.9%) resulted in a statistically significant 25% relative risk reduction of microvascular complications, and in a non‐significant 16% risk reduction of myocardial infarction (UKPDS 33). Consequently, most glycaemic management guidelines for type 2 diabetes recommend a target HbA1c less than 7%. Insulin therapy is recommended for patients who are unable to reach this target with oral hypoglycaemic agents alone.
Initial treatment of patients with type 2 diabetes mellitus should be in the form of diet and education. Weight reduction in obese patients and exercise to improve insulin sensitivity and glucose tolerance (Agurs 1997; Bosello 1997). If non‐pharmacological measures are insufficient, additional therapy with oral hypoglycaemic agents is indicated. Later, as oral agents become less efficacious, exogenous insulin, given either as a monotherapy or in combination with (an) oral hypoglycaemic agent(s), may be required.
The UKPDS also demonstrated that despite treatment with a combination of oral agents, a substantial number of patients require insulin therapy to maintain strict glycaemic control (Turner 1999). The UKPDS did not investigate the use of insulin‐oral hypoglycaemic agents (OHA) combination therapy although when patients require insulin, benefit may be obtained from combining insulin with oral hypoglycaemic agents.
Historically, the effects of insulin have been controversial (Zavaroni 1989; Stout 1990). The side effects of weight gain and hypoglycaemia are well known, though it was also long thought that exogenous insulin was a causative risk factor for cardiovascular complications. The UKPDS and other studies have found no evidence for this (Ruige 1998). It is also uncertain if, and how, insulin therapy may influence 'quality of life' and patient treatment satisfaction. Improving glycaemia per se may improve general well‐being, however, daily injections with insulin, home monitoring of blood glucose, episodes of hypoglycaemia and referral from primary to secondary care can interfere with the daily functioning of patients (de Sonnaville 1998, Goddijn 1999, van der Does 1996).
Three previous reviews comparing insulin monotherapy to insulin / oral hypoglycaemic agent combination therapy have focused on insulin combined with sulphonylureas or placebo, excluding other groups of oral agents (Peters 1991; Pugh 1992; Johnson 1996). These reviews included studies where either insulin‐treated patients were randomised to the addition of a sulphonylurea or placebo, or where insulin‐requiring patients with poor glycaemic control despite oral hypoglycaemic agents were randomised to receive insulin combined with sulphonylurea therapy, or insulin alone. These reviews were of limited design and did not explicitly address the aim of the present study, namely to determine the optimum initial insulin treatment strategy for insulin‐requiring type 2 patients. Despite the apparent similarities of these reviews, the authors' conclusions differed. Peters 1991concluded that combination therapy should not be used in insulin‐treated patients with type 2 diabetes since improvement was only slight and blood glucose values were not normalised with this therapy. The later reviews of Pugh 1992 and Johnson 1996 however, recommended insulin / sulphonylurea combination therapy, finding it to be more efficacious than insulin alone.
Why it is important to do this review
Yki‐Järvinen 2001 published a fairly comprehensive overview of studies on insulin‐OHA combination therapies, though this review did not meet the criteria of the Cochrane Collaboration. Therefore, an up‐to‐date systematic review conforming to the methods of the Cochrane Collaboration was undertaken to clarify the potential benefits of combination therapy compared to insulin monotherapy.
Objectives
To assess the effects of insulin monotherapy versus insulin‐oral hypoglycaemic agents combination therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs of any design) with a minimum follow‐up duration of two months.
Types of participants
Patients with type 2 diabetes mellitus (according to appropriate diagnostic criteria of the time) and inadequate glycaemic control despite oral hypoglycaemic agents. To be consistent with changes in classification and diagnostic criteria of the disease, the diagnosis should have been established using the standard criteria valid at the outset of the trial (NDDG 1979; WHO 1980; WHO 1985; ADA 1997; ADA 1999; WHO 1999). Since changes in diagnostic criteria may produce significant variability in the clinical characteristics of the patients included as well as in the results obtained, these differences were considered and later explored in a sensitivity analysis.
Types of interventions
The following possible types of interventions and comparisons were included: Insulin monotherapy compared to combinations of insulin with single or multiple oral hypoglycaemic agents.
Types of outcome measures
Primary outcomes
any diabetes‐related morbidity: myocardial infarction, angina, heart failure, stroke, renal failure, amputation (of at least one digit), vitreous haemorrhage, retinal photocoagulation, blindness in one eye, or cataract extraction;
Glycaemic control (fasting blood glucose, HbA1, HbA1c).
Secondary outcomes
quality of life (ideally using validated scales);
patient satisfaction (ideally using validated scales);
amount of insulin necessary for good glycaemic control;
adverse effects: incidence of hypoglycaemia, weight gain, gastrointestinal symptoms.
Timing of outcome measurement
short‐term: 2 ‐ 6 months;
intermediate‐term: greater than 6 to 12 months;
long‐term: more than 12 months.
Search methods for identification of studies
Electronic searches
Electronic search strategies were used to identify relevant trials (as specified under 'types of studies') and reviews/meta‐analyses (for identification of additional trials). The following databases were searched:
The Cochrane Library (issue 2, 2004; including the Cochrane Controlled Trials Register (CCTR) and the Database of Reviews of Effectiveness (DARE);
MEDLINE (1966 to 05/2004);
EMBASE (1974 to 05/2004).
We also searched databases of ongoing trials:
Current Controlled Trials (www.controlled‐trials.com);
The National Research Register (www.update‐software.com/National/nrr‐frame.html).
One reviewer (ANG) searched the following computerised bibliographic databases: The Cochrane Library, MEDLINE and EMBASE, with no language restriction. For detailed search strategies please see under Appendix 1. Relevant published studies of any language were included. The reference lists of relevant trials and reviews identified were also scrutinised to identify other potentially relevant studies.
Data collection and analysis
Selection of studies
References identified from searches were entered into Reference Manager 10. To determine the studies to be assessed further, two independent reviewers (ANG, GDV) scanned titles, abstract and keywords of every record retrieved. Full articles were retrieved for further assessment if the information given suggested that the study:
included patients with type 2 diabetes mellitus;
compared insulin with a combination of insulin with (an) oral hypoglycaemic agent(s);
assessed one or more relevant clinical outcome measure;
used random allocation to comparison groups.
Where details regarding these criteria were inadequate from the information given in the title and abstract, the full article was retrieved for clarification. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). Differences in opinion were discussed with a third party (RPS). Where resolution of disagreement was not possible, the article was added to those 'awaiting assessment' and the authors contacted for clarification. When no clarification was provided, the review group editorial base would have been consulted. If the results of a trial were reported in separate articles data were appropriately combined and analysed as one study.
Data extraction and management
Three reviewers (ANG, GDV, NF) independently extracted the data including:
general information: published/unpublished, title, authors, reference/source, contact address, country, urban/rural etc., language of publication, year of publication, duplicate publications, sponsoring, setting.
trial characteristics: design, duration, randomisation (and method), allocation concealment (and method), blinding (patients, people administering treatment, outcome assessors), assessment of blinding.
intervention(s): placebo included, interventions(s) (dose, route, timing), comparison intervention(s) (dose, route, timing).
patients: sampling (random/convenience), exclusion criteria, total number and number in comparison groups, sex, age, duration of diabetes, similarity of groups at baseline (including any co‐morbidity), assessment of compliance, withdrawals / losses to follow‐up (reasons/description), subgroups.
outcomes: outcomes specified above (also: what was the main outcome assessed in the study?), any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes.
results: for outcomes and times of assessment (including a measure of variation), if necessary converted to measures of effect specified below; intention‐to‐treat analysis.
A template data extraction form was developed, piloted and approved by the Metabolic and Endocrine Disorders Group Editorial Base before final data extraction commenced. Data extraction and data entry were performed independently in pairs (ANG / GDV and ANG / NF). Differences in data extraction were resolved by consensus with the fourth reviewer (RPS), with referral to the original article. Where the published report contained incomplete (or absent) data (see data extraction list), the reviewers contacted the first author using the standard letter from the Editorial Base.
Assessment of risk of bias in included studies
Each trial was independently assessed by two reviewers (ANG, GDV). Interrater agreement was calculated using the kappa statistic. In cases of disagreement, the Cochrane Metabolic and Endocrine Disorders Group would have been consulted and a judgement made based on consensus.
The trials were assessed on methodological quality using a selection of the 17‐item Maastricht‐ Amsterdam Criteria List (Van Tulder 1997), which includes criteria of Jadad (Jadad 1996) and Schulz (Schulz 1995). The following factors were scored (total score range from 0 ‐ 7): 1. Minimisation of selection bias ‐ a) was the randomisation procedure adequate? b) was the allocation concealment adequate? 2. Minimisation of performance bias ‐ were a) the patients and b) people administering the treatment blind to the intervention? 3. Minimisation of attrition bias ‐ a) were withdrawals and dropouts completely described? b) was analysis by intention‐to‐treat? 4. Minimisation of detection bias ‐ were a) outcome assessors blind to the intervention?
Based on these criteria, studies were subdivided into three categories: A ‐ all quality criteria met: low risk of bias. B ‐ one or more of the quality criteria only partly met: moderate risk of bias. C ‐ one or more criteria not met: high risk of bias. This classification was used as the basis for a sensitivity analysis. The influence of individual quality criteria were also used in sensitivity analyses. Studies were not excluded on the basis of methodological criteria alone.
Assessment of reporting biases
Small study bias was evaluated by using a funnel plot method (Egger 1997).
Data synthesis
Available data were included in a meta‐analysis if they were sufficiently similar and of sufficient quality. For dichotomous data the results are expressed as odds ratios (OR) with corresponding 95% confidence intervals (95% CIs), and continuous data as weighted mean difference with 95% CIs, or as standardised weighted mean difference where outcomes were conceptually the same but measured in different ways (Rosenthal 1994; Mulrow 1997; Lau 1997). Where studies that did not provide HbA1c change‐from‐baseline values, these data were computed from baseline and post‐treatment values, eventually distracted from graphs. When standard deviations of mean differences for the main outcome HbA1c were not provided in the publications, these data were computed assuming a general correlation coefficient that was derived from baseline and post‐treatment outcomes for HbA1c in studies that presented accompanying SDs (see below). Glycated haemoglobin values determined with different methodologies were standardized to a reference range of 4.0 to 6.0 % (Little 1986; DCCT 1993). Insulin requirement in combination therapy regimens was expressed as a relative reduction in insulin dose compared to monotherapy, expressed as percentage (unweighted mean; 95% CI). Differences underlying the results of studies (statistical heterogeneity) were assessed using both the Q‐test (with a P‐value less than 0.1 considered as significant) and by I‐squared (Higgins 2003). Clinical heterogeneity was surveyed by comparing the studies with regard to different clinical parameters: patient characteristics (e.g. previous treatment), disease duration, interventions and outcome. Where significant clinical or statistical heterogeneity was found, it was considered unreasonable to assume one 'true' effect underlying the data constant across different populations, necessitating a random‐effects model to pool data. (DerSimonian 1986).
For each study the mean changes from baseline and standard deviations of the outcome HbA1[c] were extracted, if available. If not available, mean change scores of HbA1[c] were calculated by subtracting baseline from post‐treatment values. Matching standard deviations were computed in SPSS 11.0 with a formula (formula 1), which included a general correlation coefficient between baseline and post‐treatment values of HbA1[c] of 0.5. This figure was set 0.1 point lower than the correlation coefficient that was calculated from studies that provided information on change scores inclusive standard deviations, and which appeared to be 0.6 in most studies (formula 2) (Armitage 2002).
Formula 1: SPSS syntax for computing standard deviations of changes from baseline values of HbA1[c] SD = sqrt(sd_tr_b**2 + sd_tr_p**2 ‐ 2 * corr * sd_tr_b * sd_tr_p).
abbreviations: sd = standard deviation Sqrt = square root sd_tr_b = standard deviation of mean baseline HbA1[c] sd_tr_p = standard deviation of mean post treatment HbA1[c] corr = correlation coefficient between baseline and post‐treatment values of HbA1[c]
Formula 2: SPSS syntax for computing correlation coefficient between baseline and post‐treatment values of HbA1[c] corr_tr = (sd_tr_b**2 + sd_tr_p**2 ‐ sddiff_tr**2) / (2*sd_tr_b*sd_tr_p).
Abbreviations: corr_tr = correlation coefficient between baseline and post‐treatment values of HbA1[c] sd_tr_b = standard deviation of mean baseline HbA1[c] sd_tr_p = standard deviation of mean post treatment HbA1[c] sddiff_tr = standard deviation of change from baseline HbA1[c]
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned for the following variables: different oral hypoglycaemic agent(s) and different types of insulin, timing and frequency of insulin injections.
Sensitivity analysis
Sensitivity analyses were planned to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies.
repeating the analysis taking account of study quality, as specified above.
repeating the analysis excluding any very long or large studies to establish how much they dominate the results.
Results
Description of studies
Results of the search
The search strategy provided 1,709 citations. After exclusion of doubles and studies not related to the objective of the review, two reviewers independently assessed the remaining 192 abstracts. Full text was obtained of 127 potentially relevant studies, of which 22 fulfilled the inclusion criteria of the review. Three studies found after the last search are waiting for assessment and will be included in the first update of this review (Olsson 2002; Stehouwer 2003; Goudswaard 2004).
Interrater agreement
The observed agreement in trial selection was 94% (kappa = 0.71; 95% CI 0.56 to 0.86). For unclear cases agreement was reached by reading the article together, followed by discussion. The observed overall agreement in the extraction of the data was 95%. After discussion all disagreements were resolved.
Missing data
We contacted Chow, Fövényi, Holman, and Pontiroli for further details regarding their studies; all provided further information (Chow 1995; Fövényi 1997; Holman 1987; Pontiroli 1990).
Included studies
Twenty RCTs described in 22 articles met the inclusion criteria. The results of two RCTs were reported in four separate articles. Data from these duplicate publications were appropriately combined (Gutniak 1987; Karlander 1991, Yki‐Järvinen 1999; Mäkimattila 1999) and thus analysed as two studies (Gutniak 1987; Yki‐Järvinen 1999). Fifteen articles (75%) were published in English, three in German (Bachmann 1988; Lotz 1988; Lundershausen 1987), one in Dutch (Wolffenbuttel 1991), one in Hungarian (Fövényi 1997), and two in Chinese (Sun 1995, Xu 2001). No eligible trials were found before the year 1987. At least 50% of the studies were sponsored by the pharmaceutical industry.
Studies and participants
All 20 included studies were randomised controlled studies, of which 16 had a parallel design, and four a crossover design (Holman 1987; Riddle 1998; Pontiroli 1990; Ravnik‐Oblak 1995). Weighted mean trial duration was 10.0 months (range 2 to 36 months). A total of 1811 participants (mean per study 91; range 10 to 432) were included in these studies, with 46% men (range 29% to 64%). Gender was not reported in five trials (Gutniak 1987; Lotz 1988; Ravnik‐Oblak 1995; Riddle 1992; Shank 1995). Participants had mean age of 59.8 years (95% CI 57.6 to 62.1), and mean known duration of diabetes was 9.6 years (95% CI 8.3 to 10.9). All studies provided information on oral hypoglycaemic therapy at baseline. Further details and criteria for entry into the individual studies are listed in the Table "Characteristics of included studies".
Study settings
In one study patients were recruited in primary care (Holman 1987), all other studies were conducted in secondary care. In three studies patients were admitted to hospital for baseline measurements and initiation of insulin therapy (Gutniak 1987; Ravnik‐Oblak 1995; Yki‐Järvinen 1992).
Study characteristics
Twenty studies providing 28 comparisons between insulin monotherapy and insulin‐oral hypoglycaemic agent combination regimens were evaluated. In both monotherapy and combination therapy groups, insulin was applied as a once‐daily (morning or bedtime), twice‐daily, or a multiple‐daily injection regimen. Oral hyperglycaemic agents utilised included sulphonylureas (75%), metformin (4%) or both (21%). Comparisons were initially categorised according to mode of insulin monotherapy, and subsequently sub‐categorised according to combination therapy regimen used, to provide clinically relevant comparisons: 1) Insulin monotherapy (once‐daily injection) versus combination regimens (Holman 1987; Lundershausen 1987; Pontiroli 1990; Riddle 1989; Riddle 1992; Riddle 1998; Shank 1995; Sun 1995; Xu 2001). 2) Insulin monotherapy (twice‐daily injection) versus combination regimens (Bachmann 1988; Chow 1995; Fövényi 1997; Gutniak 1987; Lotz 1988; Ravnik‐Oblak 1995; Wolffenbuttel 1991; Wolffenbuttel 1996; Yki‐Järvinen 1992; Yki‐Järvinen 1999). 3) Insulin monotherapy (multiple‐daily injections) versus combination regimens (Bastyr 1999; Holman 1987; Yki‐Järvinen 1992).
Outcome measures
No studies assessed diabetic complications, diabetes‐related mortality or total mortality. All except three studies (Lundershausen 1987, Bachmann 1988; Ravnik‐Oblak 1995) reported glycaemic control as mean values of HbA1 (Holman 1987; Lotz 1988; Riddle 1989; Riddle 1992) or HbA1c. Five studies provided change‐from‐baseline values for glycated haemoglobin with standard deviations (Bastyr 1999; Riddle 1992; Riddle 1998; Yki‐Järvinen 1992; Yki‐Järvinen 1999). Fasting blood glucose values were not reported in two studies (Fövényi 1997; Yki‐Järvinen 1992). Three studies did not provide the method of analysis for glycated haemoglobin (Sun 1995; Bastyr 1999; Xu 2001). Seven studies (13 comparisons) provided change‐from‐baseline values for body weight with standard deviations (Bastyr 1999; Chow 1995; Fövényi 1997; Gutniak 1987; Riddle 1992; Yki‐Järvinen 1992; Yki‐Järvinen 1999). Insulin requirement was reported in all but three studies (Ravnik‐Oblak 1995; Sun 1995; Xu 2001). Patient satisfaction, general well‐being or quality of life was assessed in three studies (Chow 1995; Riddle 1989; Yki‐Järvinen 1992). All but seven studies (Fövényi 1997; Lundershausen 1987; Pontiroli 1990; Ravnik‐Oblak 1995; Riddle 1989; Wolffenbuttel 1991; Xu 2001) in some way provided information on hypoglycaemic events, although only three (Riddle 1992; Yki‐Järvinen 1992; Yki‐Järvinen 1999) provided number of hypoglycaemic events with standard deviations. Other adverse effects were reported in two studies (Bastyr 2000; Riddle 1998).
Excluded studies
Reasons for exclusion of studies are given in 'Table of excluded studies'. Main reasons for exclusion were: Patients were previously treated with insulin (n = 47), absence of a treatment arm with either monotherapy with insulin or with a combination of insulin with oral hypoglycaemic agents (n = 32), a non‐appropriate study design (n = 12), and "other reasons" (n=14).
Risk of bias in included studies
The methodological quality scores of the included studies (scale range 0 (min) to 7 (max)) were assigned using the criteria described above, and are listed in the table of included studies. Only information published in the trials was used to determine a quality score. Inter‐observer calculation of the items of study quality revealed a substantial observed agreement of 82% (kappa = 0.62; 95% CI 0.48 to 0.76). Mean study quality was 2.6 (95% CI 1.5 to 3.7). Mean patients' drop‐out rate was 5.5%. Disregarding one study with a drop‐out rate of 51% (Bachmann 1988), mean drop‐out rate was only 1.4%. None of the studies reported a power calculation. Of the four cross‐over studies none had a wash‐out period, and two analysed data for carryover and period effects. Inclusion criteria were not described in four studies (Holman 1987; Lundershausen 1987; Riddle 1992; Xu 2001). In most studies patients with co‐morbidity and diabetes complications were excluded.
Allocation
Eight studies detailed the method of randomisation (Bastyr 2000; Chow 1995; Lotz 1988; Riddle 1989; Riddle 1992; Shank 1995; Yki‐Järvinen 1992; Yki‐Järvinen 1999), although in two trials the method could not be considered as adequate (Chow 1995; Lotz 1988). Fifteen studies (75%) had inadequate or unclear allocation concealment, and in five studies allocation concealment was adequate (Riddle 1989; Riddle 1992; Riddle 1998; Yki‐Järvinen 1992; Yki‐Järvinen 1999).
Blinding
Stated method of blinding was open in eleven studies, single‐blinding in two, double‐blinding in three, and triple‐blinding in four. None of the studies reported checked blinding conditions in patients and health care providers.
Incomplete outcome data
Seventy per cent of studies reported drop‐outs in some detail. Intention‐to‐treat analyses were described in six studies.
Effects of interventions
For details see Data and analyses.
Glycaemic control (glycosylated haemoglobin)
Comparisons were initially categorised according to mode of insulin monotherapy (once‐daily, twice‐daily, or multiple‐daily injections), and subsequently sub‐categorised according to combination therapy regimen used, to provide clinically useful subgroups as pre‐planned. From thirteen studies (21 comparisons) sufficient data were extracted to calculate pooled effects on glycaemic control.
Once‐daily insulin monotherapy regimens
In nine comparisons, insulin monotherapy applied as either a single morning (Pontiroli 1990) or evening injection (Holman 1987; Riddle 1989; Riddle 1992; Riddle 1998; Shank 1995; Sun 1995; Xu 2001) was compared with a matching insulin injection combined with a sulphonylurea (SU). One study (Lundershausen 1987) provided no information on timing of insulin injections. Data from five comparisons comparing a single evening insulin injection to evening insulin combined with daytime sulphonylurea were pooled in a meta‐analysis (Riddle 1992; Riddle 1998; Shank 1995; Sun 1995; Xu 2001). Insulin‐oral hypoglycaemic agents (OHA) combination therapy was associated with a significant mean (pooled weighted mean difference) lowering of HbA1c of 0.3% (95% CI 0.0 to 0.6; P = 0.03) compared to insulin monotherapy. Heterogeneity was low (I2 = 16,3%; Chi2 = 4.8; P = 0.31) Four comparisons were not included in the meta‐analysis. Lundershausen 1987 reported no outcome data for HbA1(c), and three cross‐over studies with heterogeneous design had potential carryover effect for HbA1(c) (Holman 1987; Pontiroli 1990; Riddle 1989). Pontiroli 1990 and Riddle 1989 reported better glycaemic control with combination therapy, whereas Holman 1987 and Lundershausen 1987 found no difference compared with insulin monotherapy.
Twice‐daily insulin monotherapy regimens
Bedtime neutral protamine Hagedorn (NPH) insulin plus oral hypoglycaemic agents
In seven comparisons, twice‐daily insulin monotherapy was compared with bedtime NPH combined with either SU (1) (Fövényi 1997; Wolffenbuttel 1996; Yki‐Järvinen 1999), metformin (2) (Yki‐Järvinen 1999), or SU plus metformin (3) (Chow 1995; Yki‐Järvinen 1992; Yki‐Järvinen 1999).
Bedtime NPH plus SU (three comparisons)
Insulin‐OHA combination therapy was associated with a non‐significant mean (pooled weighted mean difference) lowering of HbA1c of 0.1% (95% CI ‐0.9 to 1.1; P = 0.87) compared to insulin monotherapy. Heterogeneity was high (I2 = 90.4%; Chi2 = 20.9; P < 0.0001). After elimination of one large study of poor quality (Fövényi 1997) insulin monotherapy was associated with a non‐significant mean (pooled weighted mean difference) lowering of HbA1c of 0.4% (95% CI ‐0.9 to 0.1; P = 0.08) compared to insulin‐OHA combination therapy. There was no statistically significant heterogeneity (I2 = 0%; Chi2 = 0.81; P = 0.37).
Bedtime NPH plus metformin (one comparison)
Insulin‐OHA combination therapy was associated with a significant mean lowering of HbA1c of 0.6% (P < 0.05) compared to insulin monotherapy.
Bedtime NPH plus SU plus metformin (three comparisons)
Insulin monotherapy was associated with a non‐significant mean (pooled weighted mean difference) lowering of HbA1c of 0.2% (95% CI ‐0.7 to 0.4; P = 0.54) compared to insulin‐OHA combination therapy. Heterogeneity was moderate (I2 = 33.1%; Chi2 = 2.99; P = 0.22). Elimination of one study of poor quality (Chow 1995) did not change this result.
Morning NPH insulin plus oral hypoglycaemic agents
In four comparisons, twice‐daily insulin monotherapy was compared with morning NPH insulin combined with SU (Lotz 1988; Wolffenbuttel 1991; Wolffenbuttel 1996) or SU plus metformin (Yki‐Järvinen 1992). Insulin monotherapy was associated with a significant mean (pooled weighted mean difference) lowering of HbA1c of 0.4% (95% CI 0.1 to 0.8; P = 0.03) compared to insulin‐OHA combination therapy. There was no statistically significant heterogeneity (I2 = 0%; Chi2 = 1.5; P = 0.68).
Twice‐daily insulin plus oral hypoglycaemic agents
In three comparisons, insulin monotherapy was compared with twice‐daily (morning plus bedtime) premixed insulin 30/70 combined with SU (Bachmann 1988; Gutniak 1987; Ravnik‐Oblak 1995). Bachmann 1988 and Ravnik‐Oblak 1995 reported HbA1(c) as median values. Gutniak 1987 and Bachmann 1988 found no statistically significant difference between monotherapy and combination therapy, and Ravnik‐Oblak 1995 found a significant lower HbA1c for combination therapy (P < 0.05).
Multiple daily insulin injections
In two comparisons (Bastyr 1999; Yki‐Järvinen 1992), a multiple insulin injection regimen (pre‐meal soluble insulin with bedtime NPH) was compared to bedtime NPH insulin combined with SU or SU plus metformin. Insulin‐OHA combination therapy was associated with a non‐significant mean (pooled weighted mean difference) lowering of HbA1c of 0.2% (95% CI ‐0.4 to 0.1; P = 0.30) compared to insulin monotherapy. There was no statistically significant heterogeneity (I2 = 0%; Chi2 = 0.42; P = 0.48). In two comparisons, similar multiple injection regimens were compared with morning ultralente (Holman 1987) or NPH insulin (Yki‐Järvinen 1992) combined with SU. In both studies mean decrease of HbA1 was not significantly different between regimens. One study compared a multiple insulin injection regimen with a matching multiple injection regimen combined with SU (Bastyr 1999). Mean decrease of HbA1c did not significantly differ between regimens.
Subgroup analyses
In a subgroup analysis we combined studies that included metformin (± SU) in insulin‐OHA combination therapy (Chow 1995; Yki‐Järvinen 1992; Yki‐Järvinen 1999). Of Yki‐Järvinen 1999 we included the most successful of three comparisons, for which analysis was not pre‐planned. No significant difference was found of insulin‐OHA combination therapy over insulin monotherapy. This did not change after excluding one study of lower quality (Chow 1995).
Sensitivity analyses
Since only published studies were included in this review pre‐planned analyses excluding unpublished trials were not performed. Repeating analyses excluding one large trial with poor quality and another trial of poor quality did not significantly alter the results.
Small study bias
For the outcome glycaemic control (HbA1(c)) we graphically evaluated a funnel plots of comparison 02.01. Visual assessment indicates small study bias (Funnel plot Figure 1). Other comparisons included too few studies for assessment of bias by funnel plots.
1.
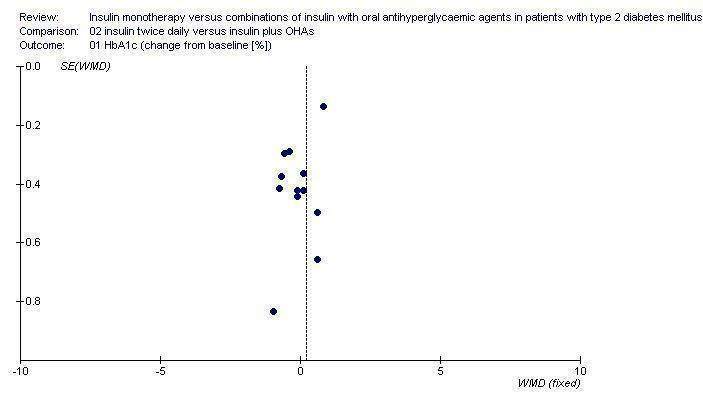
Funnel plot for the outcome HbA1c
Hypoglycaemia
Hypoglycaemia was reported quantitatively or qualitatively in all but six studies (Fövényi 1997; Lundershausen 1987; Pontiroli 1990; Ravnik‐Oblak 1995; Riddle 1989; Xu 2001). Heterogeneity in the definitions used between studies, and the quality of reporting of hypoglycaemia precluded the pooling of data. Of the fourteen studies (22 comparisons) that reported hypoglycaemia, in all but one comparison (Yki‐Järvinen 1999), no significant difference in the frequency of hypoglycaemic events (symptomatic or biochemical) between insulin monotherapy and insulin‐OHA combination therapy was demonstrated. Overall, only one episode of severe hypoglycaemia (requiring third party assistance) was reported (Wolffenbuttel 1996).
Once‐daily insulin monotherapy regimens
Hypoglycaemia rates were reported in some detail in six studies comparing a single daily injection of insulin applied in the evening (Holman 1987; Riddle 1989; Riddle 1992; Riddle 1998; Shank 1995; Sun 1995) to a matching insulin injection plus oral hypoglycaemic agents. No episodes of severe hypoglycaemia (requiring third party help) occurred. Riddle 1998 reported more frequent symptoms compatible with hypoglycaemia (though not confirmed biochemically) with combination therapy (51% of patients) compared to insulin monotherapy (37%) (P < 0.05), though the quicker rate of decline of HbA1c seen in the combination therapy group was considered an important factor by trial investigators. Non‐statistically significant increases in hypoglycaemia with combination therapy were reported in two studies (Riddle 1992 ((mean ± SD) 8.8 ± 6.6 versus 6.9 ± 6.6 symptomatic hypoglycaemic events per patient), Shank 1995 (0.1 ± 0.2 versus 0.2 ± 0.3 hypoglycaemic episodes (blood glucose less than 3.89 mmol/l) per patient per week. Sun 1995 reported one subject experiencing hypoglycaemic symptoms in each of the monotherapy and combination therapy groups. Riddle 1989 qualitatively reported similar rates of hypoglycaemia for both regimens. Holman 1987 reported a non‐significant similar frequency of hypoglycaemia "sufficient to interrupt normal daily activities", occurring in six (40%) of patients on once daily basal insulin and five (33%) of those treated with sulphonylurea plus insulin.
Twice‐daily insulin monotherapy regimens
Bedtime NPH insulin plus oral hypoglycaemic agents.
Hypoglycaemia was reported in four studies with compared twice‐daily insulin monotherapy to bedtime NPH insulin plus either sulphonylurea (1) (Wolffenbuttel 1996; Yki‐Järvinen 1999), metformin (2) (Yki‐Järvinen 1999), or sulphonylurea plus metformin (3) (Chow 1995; Yki‐Järvinen 1992; Yki‐Järvinen 1999).
Bedtime NPH insulin plus sulphonylurea
Yki‐Järvinen 1999 reported a similar frequency of symptomatic hypoglycaemic episodes over 12 months of therapy, affecting (mean ± SD) 3.4 ± 4.7 patients treated with combination therapy versus 3.9 ± 7.8 treated with insulin alone. Fasting hypoglycaemic episodes (self‐monitored fasting glucose less than 3.5 mmol/l) were less common with twice daily insulin monotherapy (1.2% of fasting glucose readings) than with sulphonylurea plus bedtime insulin (2.2%) (P < 0.05). Wolffenbuttel 1996 reported only severe hypoglycaemia, which did not occur in either treatment arm.
Bedtime NPH insulin plus metformin
One trial (Yki‐Järvinen 1999) reported significantly less symptomatic ((mean ± SD episodes per patient) 1.8 ± 1.7 versus 3.9 ± 7.8) hypoglycaemic episodes with insulin plus metformin compared to insulin alone (P < 0.05). Biochemical fasting hypoglycaemic episodes (less than 3.5 mmol/l) were similar 1.1% versus 1.2 % of measurements for insulin plus metformin and insulin monotherapy respectively.
Bedtime NPH insulin plus sulphonylurea and metformin
Symptomatic hypoglycaemia rates were similar in three studies comparing twice daily insulin monotherapy to regimens with sulphonylurea plus metformin plus bedtime NPH insulin; Chow 1995 (mean ± SD hypoglycaemic events per patient): 1.4 versus 1.0; Yki‐Järvinen 1992: 4.0 ± 5.5 versus 1.0 ± 5.3; Yki‐Järvinen 1999: 3.9 ± 7.8 versus 3.3 ± 7.7, for monotherapy versus combination therapy respectively (non‐significant for each comparison). The frequency of low blood capillary glucose measurements was reported to be similar in one study (Yki‐Järvinen 1992 (less than 4.0 mmol/l), though Yki‐Järvinen 1999 reported significantly more fasting hypoglycaemia (less than 3.5 mmol/l) with combination therapy compared to insulin monotherapy (1.8% versus 1.2% respectively, for all fasting glucose measurements (P < 0.01).
Morning NPH plus sulphonylurea
Yki‐Järvinen 1992 reported non‐significant similar rates of symptomatic hypoglycaemic events with monotherapy and combination therapy (mean ± SD per patient) 4 ± 5.5 versus 3 ± 5.7 respectively . Wolffenbuttel 1996 reported a non‐significant frequency of severe hypoglycaemia (requiring third party help), which over the study duration affected only one individual in the combination therapy group.
Twice‐daily insulin plus sulphonylurea
Bachmann 1988 reported similar hypoglycaemia rates with both regimens (17% versus 20% of patients affected respectively (non‐significant). Gutniak 1987 reported more patients experiencing hypoglycaemia (glucose less than 3.5 mmol/l) with combination therapy than monotherapy in the first two weeks of the study (8.8% versus 4.1%, P < 0.002) though hypoglycaemia rates declined during the course of the study to 1.2% and 2.6% per week at three months (non‐significant between groups). Overall hypoglycaemia rates for the entire study were not reported.
Multiple‐daily insulin injections
Three studies comparing a multiple insulin injection regimen (pre‐meal soluble insulin with bedtime NPH) with bedtime NPH insulin plus sulphonylurea (Bastyr 1999; Holman 1987) or sulphonylurea plus metformin (Yki‐Järvinen 1992) reported frequency of hypoglycaemia.
Symptomatic (Holman 1987; Yki‐Järvinen 1992) and biochemical (Bastyr 1999 (blood glucose < 3.0 mmol/l) hypoglycaemia was non‐significantly less with combination therapy in all three studies; Holman 1987: 33% versus 47% of patients affected; Yki‐Järvinen 1992: (mean ± SD) 1 ± 5.3 versus 2 ± 5.6 episodes per patient; Bastyr 1999: 0.9 ± 2.3 versus 1.2 ± 2.4 events per patient per 30 days, for combination therapy and insulin monotherapy respectively (non‐significant for each comparison).
Yki‐Järvinen 1992 reported a similar frequency of biochemical hypoglycaemia (< 4.0 mmol/l) with both regimens. Yki‐Järvinen 1992 also compared the same multiple injection regimen to a combination of morning NPH insulin plus sulphonylurea. Symptomatic hypoglycaemia ((mean ± SD) 3 ± 5.7 versus 2 ± 5.6 episodes per patient, for monotherapy and combination therapy respectively) and self monitored glucose values < 4.0 mmol/l were not significantly different between groups. No significant differences in 30‐day hypoglycaemia rates were found by Bastyr 1999 who compared a multiple insulin injection regimen with sulphonylurea plus pre‐meal soluble insulin. Mean ± SD episodes of hypoglycaemia per patient per 30 days were 1.2 ± 2.4 versus 1.0 ± 1.7, respectively.
Insulin dose
Insulin doses were titrated to predetermined glycaemic targets based on fasting (± post prandial) or diurnal mean glucose values in fourteen studies; median fasting glucose target was less than 7.0 mmol/l (range: less than 5.6 to less than 10.1 mmol/l) (Bachmann 1988; Chow 1995; Fövényi 1997; Gutniak 1987; Holman 1987; Lundershausen 1987; Riddle 1992; Riddle 1998; Shank 1995; Wolffenbuttel 1991; Wolffenbuttel 1996; Xu 2001; Yki‐Järvinen 1992; Yki‐Järvinen 1999). Six studies did not formally report glucose targets to which insulin doses were titrated (Bastyr 1999; Lotz 1988; Pontiroli 1990; Ravnik‐Oblak 1995; Riddle 1989; Sun 1995). Three studies utilised structured insulin titration algorithms based on self‐measured fasting capillary glucose levels. Riddle 1992 commenced insulin at 30 units/day and increased the dose weekly by 20 units for mean fasting capillary glucose more than 10 mmol/l, by 15 units for 7.8 to 10 mmol/l, by 10 units 6.7 to 7.8 mmol/l and by 5 units for mean capillary glucose 5.6 to 6.7 mmol/l. A reduction in insulin dose of 5 to 10 units was permitted for recurrent symptomatic or biochemical hypoglycaemia (less than 3.3 mmol/l). Riddle 1998 commenced insulin at 10 units/day and increased the dose weekly by 10 units until fasting glucose was less than 7.8 mmol/l for two consecutive days, then by 5 units weekly until fasting glucose was less than 6.7 mmol/l, aiming for a target fasting glucose 5.5 to 6.7 mmol/l. Yki‐Järvinen 1999 used a patient‐led insulin self‐titration titration regimen based on daily fasting glucose measurements. Starting insulin dose equalled capillary fasting glucose (mmol/l) and insulin doses were subsequently increased by 2 or 4 units, if three successive fasting glucose measurements were above 6 or 8 mmol/l, respectively. Three studies restricted the maximum dose of insulin in combination regimens using once daily NPH insulin. Fövényi 1997 considered combination therapy unsuccessful if insulin requirements exceeded 40 units at bedtime, such patients were converted to twice daily insulin monotherapy. Chow 1995 limited the maximum daily dose of insulin for combination therapy and insulin monotherapy regimens to 26 and 84 units (or less than 1 unit/kg bodyweight) respectively. Furthermore, where the bedtime insulin dose exceeded 24 units, the insulin dose was apportioned between two daily injections. Lotz 1988 similarly limited the maximum dose of a single daily NPH insulin injection to 28 units. Overall, insulin‐oral hypoglycaemic agent combination therapy was associated with a weighted mean relative reduction in total daily insulin requirement of 46% (range: ‐5 to 74%) compared to insulin monotherapy. Compared with a single daily insulin injection, regimens combining a sulphonylurea with a matched daily insulin injection were associated with a 29% relative reduction in total daily insulin dose. Compared with twice daily insulin, combination regimens with bedtime NPH insulin were associated with relative reductions of 57%, 29% and 64%, for sulphonylurea, metformin or both oral agents, respectively. Similarly, regimens combining morning NPH insulin with a sulphonylurea ± metformin, and regimens utilizing twice daily insulin with sulphonylurea, were associated with relative reductions in total daily insulin dose of 43%, and 42% respectively, compared to twice daily insulin monotherapy. In comparison with multiple daily insulin injections, combination regimens were also associated with a relative reduction in daily insulin requirement of 48%.
Well‐being, quality of life and treatment satisfaction
Two studies objectively assessed well‐being, quality of life or treatment satisfaction. Chow 1995 used a visual analogue score (VAS) based, structured well‐being questionnaire to assess subjective well‐being and acceptability of insulin injections. Similar significant improvements in subjective well‐being following the initiation of insulin therapy were noted with both insulin monotherapy and insulin‐OHA combination therapy groups. However, significantly more patients in the combination therapy group wanted to continue insulin therapy at the end of the study (89% versus 76% for insulin monotherapy, P < 0.0001). Yki‐Järvinen 1992 also assessed subjective well‐being with questionnaires. Insulin therapy with all insulin treatment regimens was associated with significantly greater improvement in the subjective sense of well‐being (74%, 84%, 100% and 86% for the multiple insulin injection, OHA + morning NPH, OHA + evening NPH and twice‐daily insulin mixture groups respectively) compared to the control group (41% improvement)(P<0.001). Wolffenbuttel 1991 and Wolffenbuttel 1996 qualitatively reported "improved well‐being in nearly all patients", though methods for measuring well‐being were not stated and no between‐group comparisons were made.
Adverse effects
Weight gain
Fifteen studies provided information on body weight outcomes (body weight or body mass index). Due to the heterogeneity of reported data and the absence of standard deviations of mean differences in most studies only the results of studies in three subgroups (Comparison 02.02.01, Comparison 02.02.03 and Comparison 02.03.01) were pooled statistically.
Once‐daily insulin monotherapy regimens
Mean within‐group change in weight from baseline was reported in four studies. Riddle 1992; Riddle 1998; Shank 1995 each found a non‐significant trend to greater weight gain with insulin‐sulphonylurea combination therapy compared to evening insulin alone (3.3 versus 3.9 kg, 4 versus 4.3 kg, 0.6 versus 4.5 kg, insulin monotherapy versus combination therapy for each study, respectively). One cross‐over study (Riddle 1989) reported significantly greater weight gain for patients when treated with insulin‐OHA combination therapy (mean ± SD) 2.6 ± 1.8 kg) compared to insulin alone (0.6 ± 2.2 kg, P < 0.01), though a significant confounding carry‐over effect was observed, suggesting that the weight gain associated with each therapeutic intervention was affected by the order of treatment. Holman 1987 found no significant difference in weight between groups at the end of each treatment period. Four studies (Lundershausen 1987; Pontiroli 1990; Sun 1995; Xu 2001) did not provide weight gain data.
Twice‐daily insulin monotherapy regimens
Bedtime NPH insulin plus oral hypoglycaemic agents
Bedtime NPH plus sulphonylurea (three comparisons)
Compared with insulin monotherapy, insulin‐OHA combination therapy was associated with a non‐significant (pooled weighted mean difference) 0.2 kg less weight gain (95% CI: ‐0.2 to 0.6; P=0.3) (Fövényi 1997; Yki‐Järvinen 1999). There was no statistically significant heterogeneity (I2 = 0%; P = 0.69). Wolffenbuttel 1996 reported a non‐significant mean weight gain of 4 kg and 4.4 kg for insulin monotherapy and insulin‐OHA combination therapy, respectively.
Bedtime NPH plus metformin (one comparison)
Insulin‐OHA combination therapy resulted in a significant mean 3.7 kg less weight gain compared to insulin monotherapy (P < 0.01) (Yki‐Järvinen 1999).
Bedtime NPH plus sulphonylurea and metformin (three comparisons)
Compared with insulin monotherapy, combination therapy was associated with a non‐significant (pooled weighted mean difference) 1.5 kg less weight gain (95% CI: ‐0.1 to 3.2; P = 0.07). The test for heterogeneity was not significant (Chi2 = 4.69; P = 0.1), although I2 test showed notable heterogeneity (I2 = 57.3%) (Chow 1995; Yki‐Järvinen 1992; Yki‐Järvinen 1999).
Morning NPH insulin plus oral hypoglycaemic agents
In one comparison, weight gain with insulin monotherapy was 0.4 kg less with monotherapy compared to combination therapy (P = 0.57) (Yki‐Järvinen 1992). Wolffenbuttel 1991 also reported similar weight gain with both regimens (mean weight gain 4.2 kg with monotherapy versus 3.9 kg with insulin/sulphonylurea (non significant). Lotz 1988 expressed weight data as percentage change in BROCA index, no significant between‐group differences were found.
Twice‐daily insulin plus oral hypoglycaemic agents
Gutniak 1987 reported non‐significantly greater weight gain with insulin‐OHA combination therapy compared to insulin monotherapy. Ravnik‐Oblak 1995 reported similar significant increases in (median) bodyweight (expressed absolute change in BMI) with both therapies. Data from Bachmann 1988 were disregarded since patients were withdrawn from this study if weight gain exceeded 3 kg.
Multiple‐daily insulin injections
Compared with insulin monotherapy, insulin‐OHA combination therapy (Bastyr 1999; Yki‐Järvinen 1992) was associated with a significant (pooled weighted mean difference) 1.1 kg less weight gain (95% CI: 0.5 to 1.7; P<0.001). Holman 1987 reported similar weight gain with both therapies. Yki‐Järvinen 1992 also compared multiple‐daily insulin injections to sulphonylurea plus metformin and morning NPH insulin. Weight gain was non‐significantly less with combination therapy. Bastyr 1999 reported non‐significantly less weight gain with sulphonylurea combined with pre‐meal soluble insulin compared to basal‐bolus insulin therapy.
Other adverse effects
No studies assessed diabetes‐related morbidity, mortality or total mortality, though two studies reported adverse events in some detail (Bastyr 1999; Riddle 1998). These studies reported no significant differences in frequency, or severity, of adverse events with insulin or combination therapy regimens. Gastrointestinal symptoms were not reported as an outcome measure in any study, though in one study (Yki‐Järvinen 1999), side effects of metformin (diarrhoea, metallic taste, abdominal discomfort) necessitated study discontinuation for four patients randomised to receive metformin in conjunction with insulin (3 of 24) or insulin and sulphonylurea (1 of 24 patients).
Discussion
This review was performed to assess the effects of insulin monotherapy compared with combinations of insulin with oral hypoglycaemic agents in patients with inadequate glycaemic control despite treatment with oral hypoglycaemic agents. Of 127 potentially relevant studies, 20 randomised clinical trials met the inclusion criteria for this review. These studies included a total of 1811 participants, and mean trial duration was 10 months. Participants had mean age of 59.8 years and mean known duration of diabetes of 9.6 years. Twenty‐eight relevant comparisons were evaluated and categorised according to clinically relevant treatment schemes, based on both daily frequency and timing of insulin injections and class of oral hypoglycaemic agents used.
Significant clinical heterogeneity was observed in the inclusion criteria of individual studies with respect to oral hypoglycaemic agent(s) (and doses) used and the level of glycaemia required to determine oral agent failure (see table of included studies), reflecting a change in the use of oral hypoglycaemic agents over time, drug licensing regulations in different countries, and a more aggressive modern approach to the glycaemic management of type 2 diabetes.
Of the oral agents used in combination therapy regimens sulphonylureas were most frequently utilised (75% of all comparisons), then sulphonylurea with metformin (21%), and in one study (4%) a combination regimen of metformin with insulin was used.
Overall study quality was poor (mean score 2.6 of maximal 7 points), and only five studies had adequate concealment of allocation. Most studies (85%) had a follow‐op time of less than one year, so the long‐term effects on glycaemic control, diabetes‐related complications, and other relevant outcomes are unclear. Except in one subcategory (comparison 02.01.01) statistically heterogeneity was low or moderate. The results of this review should be interpreted against the background of these limitations.
Glycaemic control
Glycaemic control was the main outcome measure in all studies. Of thirteen studies (21 comparisons) sufficient data could be extracted to calculate pooled effects on glycaemic control. The four cross‐over studies were not used for the analyses since phase‐specific data were not available and wash‐out periods were not used.
The results of this systematic review demonstrate no statistically significant benefits on glycaemic control with insulin monotherapy (two or more daily injections) versus oral hypoglycaemic agents combined with a single bedtime injection of neutral protamine Hagedorn (NPH) insulin, except for one study that included a combination therapy arm with insulin‐metformin (Yki‐Järvinen 1999). This study reported significantly better glycaemic control with insulin‐metformin compared with other insulin‐OHA combination regimens and also insulin monotherapy. Compared with insulin‐sulphonylurea, insulin‐metformin combination therapy resulted in a significantly greater improvement in HbA1c of 0.6% (‐1.9±1.4 versus ‐2.5±1.7, P<0.05). This is also of clinical significance in light of the UKPDS (UKPDS 33) which, with a 0.9% difference in HbA1c between intensively‐treated and conventionally‐treated patients, reported a statistically significant relative risk reduction in microvascular disease of 25%. It should be noted however that in this study (Yki‐Järvinen 1999), analysis was not as per intention‐to‐treat and only 19 of 24 (79%) patients randomised to receive insulin‐metformin completed the study. Besides, the insulin saving effect of sulphonylurea was lost. Therefore the results of this study should be interpreted with caution.
Insulin‐OHA combination therapy provided statistically significantly lower HbA1c (pooled difference of 0.3%) compared with insulin monotherapy when the latter was applied as a once‐daily injection of NPH insulin. Conversely, twice‐daily insulin monotherapy (NPH or mixed insulin) provided lower HbA1c (pooled difference 0.4%) to insulin‐OHA combination therapy only if insulin was given as a single morning injection. Since these regimens are infrequently currently used in the management of type 2 diabetes, conclusions drawn from these results are limited. Besides, in both cases the weighted mean differences between the two insulin schemes were small and, although statistically significant, less clinically relevant.
These results do suggest that present‐day combination therapy regimens provide at least comparable glycaemic control compared to insulin monotherapy. This is relevant for daily diabetes care, since the simple addition of bedtime NPH insulin to oral hypoglycaemic agents (metformin ± sulphonylurea) may allow physicians and patients to overcome a possible resistance to the use of insulin. Moreover, from a clinical standpoint the beneficial insulin sparing effects of oral agents could be maintained. In this respect the continuation of sulphonylurea is more beneficial than the continuation of metformin. Opponents to bedtime NPH insulin‐OHA combination therapy suggest that glycaemic control remains sub‐optimal with this approach (Westphal 2003), however, the results of this review demonstrate that insulin monotherapy fares no better with respect to glycaemic control. This is of particular relevance to elderly patients where the inherent risk of hypoglycaemia may outweigh the benefits of tight glycaemic control.
Quality of life
Quality of life related issues were investigated in only four studies, so this review could not be conclusive regarding quality of life. In general, these studies reported improved well‐being with both insulin monotherapy and insulin‐OHA combination regimens, with no significant differences between groups.
Insulin dose
In seventy percent of the studies insulin doses were titrated to predetermined glycaemic targets based on fasting or diurnal mean glucose values (median fasting glucose target less than 7.0 mmol/l; range 5.6 to 10.1 mmol/L). In less than half (45%) of the studies were patients instructed to measure blood glucose levels at home. Three studies restricted the maximum dose of insulin in combination regimens using once‐daily NPH insulin (range 26 to 40 IU). Overall, insulin‐OHA combination therapy was associated with a relative reduction in total daily insulin requirement of 46% compared to insulin monotherapy (all schemes). This figure reflects the insulin saving capacity of oral hypoglycaemic agents when combined with insulin. However, compared with twice‐daily or multiple injection monotherapy regimens, the insulin‐sparing effect of a sulphonylurea whether or not combined with metformin seemed to be superior to that of metformin alone (˜50% versus 29%), although the latter figure was based on data from a solitary study (Yki‐Järvinen 1999).
Hypoglycaemia
Hypoglycaemia was reported quantitatively or qualitatively in all but five studies (Fövényi 1997; Lundershausen 1987; Pontiroli 1990; Ravnik‐Oblak 1995; Xu 2001) Heterogeneity in the definitions used between studies, and the quality of reporting of hypoglycaemia precluded the pooling of data. Of the fourteen studies (22 comparisons) that reported hypoglycaemia, all but one (comparison 02.01.02) (Yki‐Järvinen 1999) demonstrated no statistically significant difference in the frequency of hypoglycaemic events (symptomatic or biochemical) between insulin monotherapy and insulin‐OHA combination therapy. Overall, only one episode of severe hypoglycaemia (requiring third party assistance) was reported (Wolffenbuttel 1996).
Weight gain
Of 10 studies (13 comparisons) sufficient data could be extracted to calculate pooled effects on body weight. Overall, the results of this review suggest that insulin‐OHA combination therapy resulted in statistically significant less weight gain compared with insulin monotherapy provided that NPH insulin was applied at bedtime and metformin was used as a single agent or in combination with a sulphonylurea. In all other comparisons no significant differences with respect to weight gain were detected between monotherapy and combination therapy regimens. Metformin reduces insulin requirement and may also prevent weight gain, even in combination with a sulphonylurea or intensive insulin treatment. However, only one study included a treatment arm with insulin in combination with metformin alone. Whether metformin should be used as a single agent, or applied in conjunction with other oral agents in insulin combination regimens remains unclear.
Adverse effects
Very few studies in this review systematically reported adverse effects of oral agents or insulin in detail. Withdrawal of patients due to side‐effects of oral medication (e.g. gastrointestinal symptoms of metformin use) appeared minimal.
Limitations
Overall, study quality was low (mean score 2.6 (range 0 to 7), and no study included a power calculation. The majority of studies had small sample size and limited follow‐up and were therefore unable to report on hard end‐points. Of the studies included in this review, combination regimens utilising oral hypoglycaemic agents with a single bedtime injection of NPH insulin provided comparable glycaemic control to any insulin monotherapy regimen, though the long‐term success of such regimens remains unclear.
Wolffenbuttel 1996 reported that after six months of treatment, 32% of patients in the sulphonylurea‐bedtime neutral protamine Hagedorn (NPH) insulin arm required a second injection at morning time to control glycaemia. Similarly, Fövényi 1997 reported that 40.5% of patients at 12 months, and 58.2% of patients at three years, required conversion to conventional insulin therapy because of inadequate glycaemic control, though combination therapy was considered unsuccessful if the dose of bedtime NPH insulin required to suppress fasting glucose to less than 7.0 mmol/L exceeded 40 units. Chow 1995 divided the bedtime insulin between two daily injections when the total daily insulin requirement exceeded 24 units. Yki‐Järvinen 1999 however, using a patient led structured insulin dose titration regimen targeting fasting glycaemia, reported that individual bedtime NPH insulin dose required to achieve fasting glucose values less than 6.0 mmol/L ranged from 8 to 168 units, suggesting that some patients in other studies may have been unnecessarily converted to conventional insulin regimens. This regimen was associated with a low drop‐out rate; over one year of treatment no patients withdrew due to lack of efficacy of treatment.
Three studies attempted to identify criteria that predicted a good response to combination therapy. Ravnik‐Oblak 1995 reported that those who responded well to combination therapy had a shorter duration of diabetes, greater bodyweight and a higher basal C‐peptide at baseline, to those who responded poorly. However, age, diabetes duration, BMI, glycaemia, peripheral insulin resistance or ß‐cell insulin secretory capacity were not useful predictors. Chow 1995 and Riddle 1989 also found no correlation between these baseline values and future success of combination therapy, in addition to prior oral hypoglycaemic therapy and initial lipid sub‐fractions.
No published studies have directly compared bedtime insulin regimens with oral hypoglycaemic agents combined with long‐acting insulin analogues to insulin monotherapy, although two large studies (Riddle 2003 (n = 756); Yki‐Järvinen 2000 (n = 426)) have compared combination regimens with oral hypoglycaemic agents and bedtime NPH insulin versus insulin glargine over 24 and 52 weeks respectively. In both studies, similar levels of glycaemic control were achieved with both regimens, though with significantly less nocturnal hypoglycaemia with insulin glargine. Using a forced insulin titration regimen, Riddle 2003 systematically titrated insulin doses on a weekly basis to achieve fasting plasma glucose levels of less than 5.5 mmol/L. Both regimens achieved a mean HbA1c at 24 weeks of less than 7.0% (6.96% (glargine) versus 6.97% (NPH), reference range 4 to 6%).
Authors' conclusions
Implications for practice.
MI, glycaemia, peripheral insulin resistance or ß‐cell insulin secretory capacity were not useful predictors. Chow 1995 and Riddle 1989 also found no correlation between these baseline values and future success of combination therapy, in addition to prior oral hypoglycaemic therapy and initial lipid sub‐fractions.
Implications for research.
More studies are required to determine the optimal combination of antidiabetic agents for this category of patients. These studies should focus on hard endpoints as (diabetes‐related) morbidity and mortality, treatment satisfaction, quality of life and general well‐beingincluding, and safety aspects of the different combination regimens (e.g. sulphonylureas and metformin), should be larger with respect to number of patients, and have longer follow‐up. These studies should also include newer oral agents (e.g. meglitinides, thiazolidinediones). Further research on this issue should assess also the possible long‐term benefits over NPH insulin of recently introduced long‐acting insulin analogues (Riddle 2003).
Further research should address the following questions:
Is insulin‐OHA combination therapy with metformin preferable to sulphonylurea plus metformin?
Is there a ceiling effect for insulin dose in insulin‐OHA combination therapy, above which there is little / no benefit?
Can failure on insulin‐OHA combination therapy be predicted from patient characteristics at baseline?
What are the effects of long‐acting insulin analogues (glargine, detemir) versus NPH insulin when combined with oral hypoglycaemic agents?
What insulin schemes are preferred by patients, and do they affect quality of life and general well‐being?
What's new
| Date | Event | Description |
|---|---|---|
| 3 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the authors Francis Chow, József Fövényi, Rury Holman, and Antonio Pontiroli who kindly provided unpublished information.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. 1. exp Drug Combinations/ 2. (drug therap$ or drug combination$).tw. 3. ((combination$ or oral or multiple) adj (therap$ or agent$ or drug$ or treatment$)).tw. 4. monotherap$.tw. 5. or/1‐4 6. exp SULFONYLUREA COMPOUNDS/ 7. exp BIGUANIDES/ 8. exp ACARBOSE/ 9. (biguanid$ or sulfonylurea$ or sulphonylurea$ or acarbose).tw. 10. (gliglacid$ or glibornurid$ or gliguidon$ or glisoxepid$ or glipizid$ or gliburid$ or 11. glyburid$ or tolazamid$).tw. 12. (tolbutamid$ or carbutamid$ or chlorpropamid$ or acetohexamid$ or glibenclamid$ or 13. glimepirid$).tw. 14. (metformin$ or buformin$ or chlorhexidin$ or chlorguanid$ or phenformin$).tw. 15. (miglitol$ or nateglinid$ or glucobay).tw. 16. (troglitazon$ or rosiglitazon$ or pioglitazon$ or thioazolidinedion$ or glitazon$).tw. 17. repaglinid$.tw. 18. exp INSULIN/ 19. insulin$.tw. 20. ((antidiabet$ or anti diabet$) adj (drug$ or herb$ or agent$ or compound$)).tw. 21. (hypoglyc?emic adj (drug$ or herb$ or agent$ or compound$)).tw. 22. or/6‐21 23. 5 and 22 24. exp diabetes mellitus, non‐insulin‐dependent/ 25. exp insulin resistance/ 26. impaired glucose toleranc$.tw. 27. glucose intoleranc$.tw. 28. insulin$ resistanc$.tw. 29. exp obesity in diabetes/ 30. (obes$ adj diabet$).tw. 31. (MODY or NIDDM).tw. 32. (non insulin$ depend$ or noninsulin$ depend$ or noninsulin?depend$ or non 33. insulin?depend$).tw. 34. ((typ$ 2 or typ$ II) adj diabet$).tw. 35. ((keto?resist$ or non?keto$) adj diabet$).tw. 36. ((adult$ or matur$ or late or slow or stabl$) adj diabet$).tw. 37. (insulin$ defic$ adj relativ$).tw. 38. pluri?metabolic$ syndrom$.tw. 39. or/24‐38 40. exp diabetes insipidus/ 41. diabet$ insipidus.tw. 42. 40 or 41 43. 39 not 42 44. randomized controlled trial.pt. 45. controlled clinical trial.pt. 46. randomized controlled trials.sh. 47. random allocation.sh. 48. double‐blind method.sh. 49. single‐blind method.sh. 50. or/44‐49 51. limit 50 to animal 52. limit 50 to human 53. 51 not 50 54. 50 not 53 55. clinical trial.pt. 56. exp clinical trials/ 57. (clinic$ adj25 trial$).tw. 58. ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw. 59. placebos.sh. 60. placebo$.tw. 61. random$.tw. 62. research design.sh. 63. (latin adj square).tw. 64. or/55‐63 65. limit 64 to animal 66. limit 64 to human 67. 65 not 66 68. 64 not 67 69. comparative study.sh. 70. exp evaluation studies/ 71. follow‐up studies.sh. 72. prospective studies.sh. 73. cross‐over studies.sh. 74. exp Intervention Studies/ 75. or/69‐74 76. limit 75 to animals 77. limit 75 to human 78. 76 not 77 79. 75 not 78 80. 54 or 68 or 79 81. 23 and 43 and 80 |
Data and analyses
Comparison 1. Insulin once daily versus insulin once daily plus oral antihyperglycaemic agents (OHAs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c (change from baseline [%]) | 5 | 297 | Mean Difference (IV, Random, 95% CI) | 0.33 [0.03, 0.62] |
| 1.1 insulin once daily versus insulin nph bedtime plus sulphonylurea (SU) | 5 | 297 | Mean Difference (IV, Random, 95% CI) | 0.33 [0.03, 0.62] |
1.1. Analysis.
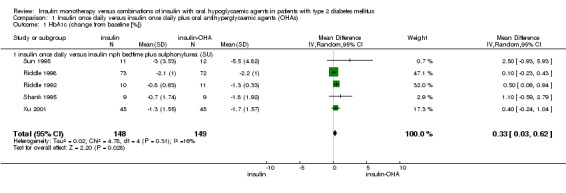
Comparison 1 Insulin once daily versus insulin once daily plus oral antihyperglycaemic agents (OHAs), Outcome 1 HbA1c (change from baseline [%]).
Comparison 2. insulin twice daily versus insulin plus OHAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c (change from baseline [%]) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 insulin twice daily versus insulin nph bedtime plus sulphonylurea (SU) | 3 | 391 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.93, 1.09] |
| 1.2 insulin twice daily versus insulin nph bedtime plus sulphonylurea (SU) + metformin | 3 | 157 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.72, 0.38] |
| 1.3 insulin twice daily versus insulin nph morning plus sulphonylurea (SU) or SU+metformin | 4 | 191 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.82, ‐0.05] |
| 2 weight gain (change from baseline[kg]] | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 insulin twice daily versus insulin nph bedtime plus sulphonylurea (SU) | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.15, 0.58] |
| 2.2 insulin twice daily versus insulin nph bedtime plus sulphonylurea (SU) + metformin | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 1.54 [‐0.10, 3.18] |
2.1. Analysis.
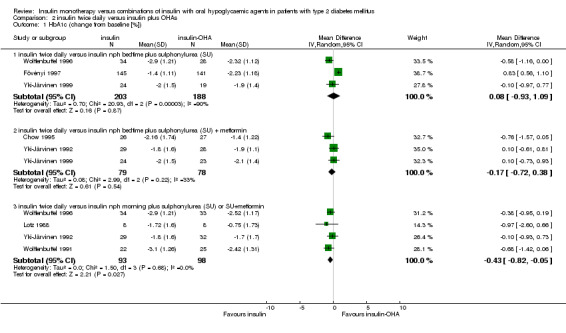
Comparison 2 insulin twice daily versus insulin plus OHAs, Outcome 1 HbA1c (change from baseline [%]).
2.2. Analysis.
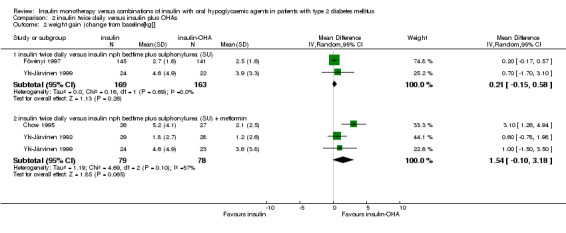
Comparison 2 insulin twice daily versus insulin plus OHAs, Outcome 2 weight gain (change from baseline[kg]].
Comparison 3. insulin basal/bolus versus insulin plus OHA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c (change from baseline [%]) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 insulin basal/bolus versus insulin nph bedtime plus sulphonylurea (SU) +/‐ metformin | 2 | 346 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| 2 weight gain (change from baseline[kg]] | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 insulin basal/bolus versus insulin nph bedtime plus sulphonylurea (SU) +/‐ metformin | 2 | 342 | Mean Difference (IV, Random, 95% CI) | 1.09 [0.53, 1.66] |
3.1. Analysis.
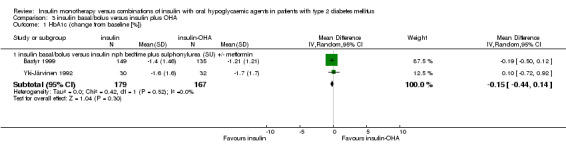
Comparison 3 insulin basal/bolus versus insulin plus OHA, Outcome 1 HbA1c (change from baseline [%]).
3.2. Analysis.
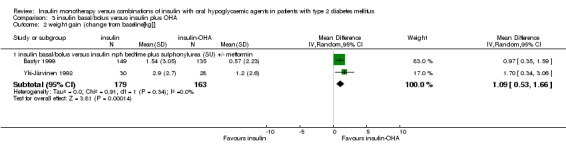
Comparison 3 insulin basal/bolus versus insulin plus OHA, Outcome 2 weight gain (change from baseline[kg]].
Comparison 4. Insulin monotherapy versus insulin plus metformine +/‐ sulphonylurea (SU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c (change from baseline [%]) | 3 | 153 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.78, 0.62] |
4.1. Analysis.
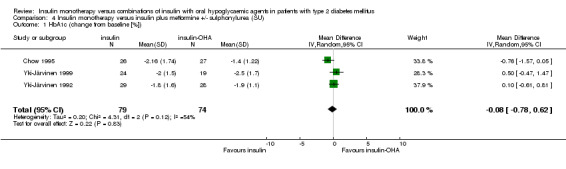
Comparison 4 Insulin monotherapy versus insulin plus metformine +/‐ sulphonylurea (SU), Outcome 1 HbA1c (change from baseline [%]).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bachmann 1988.
| Methods | Design: randomised placebo controlled trial Duration: 6 months Randomisation procedure: unclear Blinding: patients yes; care provider yes; outcome assessor unclear Intention to treat: no | |
| Participants | Country: Germany Setting: secondary care outpatient Inclusion criteria: > 40 years; > 3 year SU therapy; > 3 months max. SU therapy; FBG > 12.2 mmol/l or post‐prandial BG > 15.5 mmol/l; bodyweight < 150% of ’ideal bodyweight’ Exclusion criteria: unclear Patients randomised: 140 Nr of patients/group: unclear Drop‐outs / loss to follow‐up: 72 Nr of patients/group analysed: 37 / 31 Age (years, median): 66 / 69 Sex (% male): 38 / 19 Diabetes duration (years, median): 10 / 12 Diabetes therapy: glibenclamide 15 mg | |
| Interventions | Group 1: mixed insulin (25% regular / 75% protamine insulin) + glibenclamide 15 mg Group 2: mixed insulin (25% regular / 75% protamine insulin) + placebo Glucose targets to which insulin doses were titrated: FBG <= 10 mmol/l and post‐prandial BG £ 12.2 mmol/l | |
| Outcomes | Glycaemia: FBG, post‐prandial BG, HbA1 Weight: Weight Insulin amount (E): mean daily insulin dose at final visit Hypoglycaemia: hypoglycaemic episodes Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 2 Characteristics only available for analysed patients; presented as median values Sponsoring: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bastyr 1999.
| Methods | Design: randomised controlled trial Duration: 2 months Randomisation procedure: computer generated Blinding: patients no; care provider no; outcome assessor no Intention to treat: yes | |
| Participants | Country: USA, Europe Setting: secondary care outpatient, 58 centers, 11 countries Inclusion criteria: 40‐85 year; type 2 diabetes according to WHO; secondary failure on SU; FBG > 7.8 mmol/l or AMBG > 10.0 mmol/l or HbA1c > 150% of the upper limit of the non‐diabetic range at the local laboratory Exclusion criteria: unclear Patients randomised: 423 Nr of patients/group: 139 / 149 / 135 Drop‐outs / loss to follow‐up: 27 Nr of patients/group analysed: 139 / 149 / 135 Age (years, median): 60.1 / 59.6 / 60.7 Sex (% male): 44 / 58 / 54 Diabetes duration (years): 10 / 9 / 9 Diabetes therapy: unclear | |
| Interventions | Group 1: pre‐prandial insulin Lispro + glibenclamide 15 mg (Europe) or glyburide 20 mg (USA) Group 2: pre‐prandial insulin Lispro + bedtime NPH insuline Group 3: bedtime NPH insulin + glibenclamide 15 mg (Europe) or 20 mg (USA) Glucose targets to which insulin doses were titrated: not available | |
| Outcomes | Glycaemia: FBG, post‐prandial BG, HbA1c Weight: body weight, BMI Insulin amount (E): mean daily insulin dose at final visit Hypoglycaemia: hypoglycaemic episodes, Well‐being: not reported Treatment Satisfaction: not reported Adverse events: reported | |
| Notes | Quality score: 3 Sponsoring: pharmaceutical | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Chow 1995.
| Methods | Design: randomised controlled trial Duration: 8 months Randomisation procedure: “consecutively and alternately” Blinding: patients no ; care provider no ; outcome assessor no Intention to treat: no | |
| Participants | Country: Hong Kong Setting: secondary care outpatients Inclusion criteria: age > 20 year and maximum dose of SU and/or metformin and FPG > 7.8 mmol/l Exclusion criteria: MI, CCF, PVD, renal failure, hepatic disease, proliferative retinopathy, severe maculopathy, insulin‐dependent diabetes, previous treatment with insulin, excessive alcohol consumption, night‐shift work. Patients randomised: 55 Nr of patients/group: 28 / 27 Drop‐outs / loss to follow‐up: 1 / 1 Nr of patients/group analysed: 27 / 26 Age (years): 57 / 51 Sex (% male): 33 / 35 Diabetes duration (years): 9.9 / 8.0 Diabetes therapy: SU (10), SU+metformin (17) / SU (9), SU+metformin (17) | |
| Interventions | Group 1: OHA continued + intermediate‐acting insulin (NPH) before bedtime Group 2: intermediate‐acting insulin (NPH) before breakfast (a dinner injection was added when > 24 U were needed) (1 patient received NPH/regular insulin 70/30) Glucose targets to which insulin doses were titrated: FPG < 7.8 mmol/l (both groups) and post‐prandial PG < 11.1 mmol/l (group 2) | |
| Outcomes | Glycaemia: FPG, HbA1c Weight: body weight, BMI Insulin amount (E): insulin doses at 6 months Hypoglycaemia: hypoglycaemia Well‐being: well‐being questionnaire Treatment Satisfaction: injection pain and problems questionnaire Adverse events: not reported | |
| Notes | Quality score: 1 Sponsoring: pharmaceutical | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Fövényi 1997.
| Methods | Design: Randomised controlled trial Duration: 3 years Randomisation procedure: unclear Blinding: patients no; care provider no; outcome assessor no Intention to treat: no | |
| Participants | Country: Hungary Setting: Secondary care outpatient, single centre Inclusion criteria: HbA1c > 7.5% (normal range not given, HPLC assay) despite max. Sulphonylurea therapy (± acarbose, ±biguanide) Exclusion criteria: unclear Patients randomised: 286 Nr of patients/group: 141 / 145 Drop‐outs / loss to follow‐up: 82 (58.2%) subjects from group 1 switched to twice daily conventional insulin treatment because of insufficient glycaemic control Nr of patients/group analysed: 141 / 145 Age (years, mean): 59.8 yrs / 60.5 yrs Sex (% male): 41.8% / 40.7% Diabetes duration (years): 10.2 / 10.5 Diabetes therapy: Glibenclamide 96%, Gliclazide 4% | |
| Interventions | Group 1: Sulphonylurea (dose unchanged) + bedtime NPH insulin (6‐10 units initially) Group 2: Twice daily conventional insulin (not specified) Glucose targets to which insulin doses were titrated: Fasting blood glucose < 7.0 mmol/l Pre‐prandial / bedtime <10.0 mmol/l Max dose of bedtime NPH allowed = 40 units, above this converted to twice daily insulin. If FBG <7.0 mmol/l, but daytime >10.0 mmol/l, NPH changed to long‐acting insulin (Humulin U or Ultratard). Max dose of long acting insulin allowed = 28 units, above this converted to twice daily insulin. | |
| Outcomes | Glycaemia: HbA1c (method and normal range not given) Weight: Weight gain Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: not reported Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 1 (drop‐outs described) Sponsoring: not stated No predetermined time for end‐point analyses. Patients randomised to one or other treatment and analysed 3.5 years after recruitment commenced. Duration of follow‐up therefore variable (expressed as mean ± SD). Analysis not intention to treat. Large drop‐out of subjects from combination therapy group. Subjects analysed as 3 separate groups. 1. SU/insulin. 2. Twice daily insulin. 3. Converted from SU/insulin to twice daily insulin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Gutniak 1987.
| Methods | Design: double‐blind placebo controlled trial Duration: 10½ months Randomisation procedure: unclear Blinding: patients yes; care provider yes; outcome assessor unclear Intention to treat: unclear | |
| Participants | Country: Sweden Setting: secondary care outpatient Inclusion criteria: pre‐prandial BG > 11 mmol/l in 50% of the samples during 1 months unless diet, exercise, and 28 mg glyburide Exclusion criteria: unclear Patients randomised: 20 Nr of patients/group: 10 / 10 Drop‐outs / loss to follow‐up: 0 Nr of patients/group analysed: 10 / 10 Age (years): 57 Sex (% male): unclear Diabetes duration (years): 14.1 Diabetes therapy: glyburide 20 mg / day | |
| Interventions | Group 1: mixed insulin (intermediate‐acting (NPH) plus regular insulin) twice daily + glyburide 10,5 mg Group 2: mixed insulin (intermediate‐acting (NPH) plus regular insulin twice daily + placebo tablets Glucose targets to which insulin doses were titrated: FBG < 8 mmol/l and post‐prandial BG < 10 mmol/l | |
| Outcomes | Glucose profile: FBG, HbA1c Other: body weight, insulin amount Adverse effects: hypoglycaemia | |
| Notes | Quality score: 2 Sponsoring: pharmaceutical SD calculated from SE; data in text don’t correspond with graphs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Holman 1987.
| Methods | Design: cross‐over study Duration: 5 x 8 weeks Randomisation procedure: unclear Blinding: patients no; care provider no; outcome assessor no Intention to treat: no | |
| Participants | Country: United Kingdom Setting: primary care Inclusion criteria: maximal SU therapy, asymptomatic diabetes type 2 Exclusion criteria: retinopathy, cardiovascular disease Patients randomised: 17 Drop‐outs / loss to follow‐up: 2 Nr of patients analysed: 15 Age (years): 57 Sex (% male): 50 Diabetes duration (years): 8 Diabetes therapy: ‘maximal SU therapy’ | |
| Interventions | Group 1: maximal SU Group 2: maximal SU + metformin Group 3: maximal SU + long‐acting insulin once daily Group 4: long‐acting insulin once daily Group 5: long‐acting insulin once daily + short‐acting insulin twice daily Glucose targets to which insulin doses were titrated: FPG < 6 mmol/l | |
| Outcomes | Glycaemia: basal PG, HbA1 Weight: body weight Insulin amount (E): insulin amount Hypoglycaemia: hypoglycaemia reported Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 1 Sponsoring: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Karlander 1991.
| Methods | see Gutniak 1987 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Lotz 1988.
| Methods | Design: randomised controlled trial Duration: 2 year Randomisation procedure: ‘randomised order’ (=alternately?) Blinding: patients no ; care provider no ; outcome assessor no Intention to treat: yes | |
| Participants | Country: Germany Setting: secondary care outpatient Inclusion criteria: age 45‐80; maximal OHAs > 2 year; FBG > 11.1 mmol/l; post‐prandial BG > 13.9 mmol/l; HbA1 > 11.0%; weight < 130% BROCA (length (cm) – weight (kg)) Exclusion criteria: unclear Patients randomised: 16 Nr of patients/group: 8 / 8 Drop‐outs / loss to follow‐up: 0 / 0 Nr of patients/group analysed: 8 / 8 Age (years): 65 / 59 Sex (% male): unclear Diabetes duration (years): 15 / 11 Diabetes therapy: SU (not specified) | |
| Interventions | Group 1: insulin (not specified), twice daily Group 2: intermediate‐insulin once a day + glibenclamide 7 mg Glucose targets to which insulin doses were titrated: unclear | |
| Outcomes | Glycaemia: FBG, HbA1 (Biorad) Weight: weight (% BROCA) Insulin amount (E): daily insulin dose at final visit Hypoglycaemia: qualitatively reported Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 1 Sponsoring: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Lundershausen 1987.
| Methods | Design: randomised controlled trial Duration: 6 months Randomisation procedure: not reported Blinding: patients yes; care provider yes; outcome assessor unclear Intention to treat: yes | |
| Participants | Country: Germany Setting: unclear Inclusion criteria: maximal SU (glibenclamide 15 mg) and FBG > 10 mmol/l, Exclusion criteria: infections, kidney failure, liver disease, neoplasia, other metabolic disorders, co‐medication interfering with glucose metabolism Patients randomised: 79 Nr of patients/group: 39 / 40 Drop‐outs / loss to follow‐up: 0 / 0 Nr of patients/group analysed: 39 / 40 Age (years): 62 / 62 Sex (% male): 38 / 33 Diabetes duration (years): 11 / 11 Diabetes therapy: glibenclamide 15 mg | |
| Interventions | Group 1: insulin + glibenclamide 10 mg Group 2: insulin + placebo Glucose targets to which insulin doses were titrated: mean glucose value 12 mmol/l | |
| Outcomes | Glycaemia: “glucose value according to Michaelis” (normal values good: < 11 mmol/l; acceptable >11 < 16.5 mmol/l; poor > 16.5 mmol/l) Weight: weight change; BMI change (only reported for all patients) Insulin amount: daily insulin dose Hypoglycaemia: qualitatively reported Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 3 Sponsoring: not reported Type of insulin not specified One year follow‐up study, however at six months 41 patients discontinued oral medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mäkimattila 1999.
| Methods | see Yki‐Järvinen 1999 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pontiroli 1990.
| Methods | Design: Randomised cross‐over controlled trial Duration: 2x 3 month treatment periods Randomisation procedure: unclear Blinding: patients no; care provider no; outcome assessor unclear Intention to treat: no | |
| Participants | Country: Italy Setting: not stated Inclusion criteria: “Poor metabolic control” despite glibenclamide 15 mg/day, normal bodyweight (IBW ±10%, Metropolitan Life Insurance tables) Exclusion criteria: Ischaemic heart disease, congestive cardiac failure, “renal impairment”, “hepatic impairment”, “dyslipidaemia” Patients randomised: 10 Nr of patients/group: 5 / 5 Drop‐outs / loss to follow‐up: 1 non‐completer Nr of patients/group analysed: 9 (total) Age (years, mean): 61 yrs (all subjects) Sex (% male): 60% male (all subjects) Diabetes duration (years): 12.8 yrs (all subjects) Diabetes therapy: Glibenclamide 15 mg/day (3‐month run‐in period) | |
| Interventions | Group 1: Glibenclamide 5mg tds + am ultralente insulin
Group 2: am ultralente insulin alone
Cross‐over after 3 months, no washout period, carry‐over effect not described Glucose targets to which insulin doses were titrated: not stated |
|
| Outcomes | Glycaemia: HbA1c (HPLC method, normal range not given) Weight: Weight gain (comment only, no data) Hypoglycaemia: not reported Insulin amount (E): Mean daily insulin dose at final visit Well‐being: not reported Treatment Satisfaction: not reported Adverse effects: not reported | |
| Notes | Quality score: 1 (drop‐outs (n=1) described) Sponsoring: not stated Outcome data extracted from figures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ravnik‐Oblak 1995.
| Methods | Design: Randomised crossover controlled trial Duration: 2x 3 month treatment periods Randomisation procedure: unclear Blinding: patients no; care provider no; outcome assessor no. Intention to treat: Yes | |
| Participants | Country: Slovenia Setting: Secondary care outpatient Inclusion criteria: HbA1c > 9.0% despite glibenclamide 10 mg bd, FBG > 10 mmol/l (for 3 months), Age >35 years, Diabetes duration > 3 years, BMI < 30 kg/m2, Fasting C‐peptide > 0.3 mmol/l Exclusion criteria: Liver disease, renal disease, heart failure, myocardial infarction within 6 months, medication with potential to interact with oral hypoglycaemic agents or insulin. Patients randomised: 27 Nr of patients/group: 14 / 13 Drop‐outs / loss to follow‐up: nil Nr of patients/group analysed: 27 (total) Age (years, median): 58 yrs (all subjects) Sex (% male): 56% (all subjects) Diabetes duration (years, median): 10.5 / 8 Diabetes therapy: glibenclamide 10 mg bd (no run‐in period) | |
| Interventions | Group 1: Glibenclamide 10 mg bd + insulin (combination of short and intermediate acting insulin ( Actrapid HM, Protophane HM (Novo Nordisk) once or twice daily) Group 2: Insulin alone Cross‐over after 3 months, no wash‐out period, carry‐over effect not described. Glucose targets to which insulin doses were titrated: not stated | |
| Outcomes | Glycaemia: HbA1c (normal range <6.5%, HPLC assay) Weight: BMI Insulin amount (E): Median daily insulin dose at final visit Hypoglycaemia: not reported Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 2 (no drop‐outs, therefore also ITT analysis) Sponsoring: not stated Outcome data extracted from figures. Data expressed as median values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Riddle 1989.
| Methods | Design: Randomised crossover controlled trial Duration: 2x 4 month treatment periods Randomisation procedure: Performed centrally by drug manufacturer (sequentially numbered supplies of study drug matched to subjects place in sequence of enrolment). Blinding: patients yes; care provider yes; outcome assessor yes. Intention to treat: No | |
| Participants | Country: Oregon, USA Setting: Secondary care outpatient Inclusion criteria: Age 40‐75 yrs, diabetes (gradual) onset > 35 yrs of age, diabetes duration >1 but <15 yrs, weight < 160% ideal bodyweight (Metropolitan Life Insurance tables, 1983), sub‐optimal glycaemic control on current therapy ‐ fasting plasma glucose >7.8 mmol/l. Exclusion criteria: Major systemic illness other than diabetes, alcoholism, pancreatitis, pancreatic resection, use of corticosteroids, any disability likely to interfere with adherence to the trial protocol. Patients randomised: 21 Nr of patients/group: 10 / 11 Drop‐outs / loss to follow‐up: 0 / 1 Nr of patients/group analysed: 10/10 Age (years, mean): 61 yrs (all subjects) Sex (% male): 40% male (all subjects) Diabetes duration (years, mean): 6 years Diabetes therapy: Glibenclamide 10 mg bd (2‐8 week run‐in period) | |
| Interventions | Group 1: Glibenclamide 10 mg + evening porcine NPH (Insulatard, Novo Nordisk) Group 2: Placebo + evening porcine NPH Crossover after 4 months, no washout period, treatment effect described Glucose targets to which insulin doses were titrated: Insulin increased at the physicians’ discretion aiming for “excellent glycaemic control”. | |
| Outcomes | Glycaemia: HbA1 (thiobarbituric acid method, normal range 5.3‐8.9%), FPG Weight: Weight gain Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: Mentioned in text, no data Well‐being: not reported Treatment Satisfaction: not reported Adverse events: Reported | |
| Notes | Quality score: 6 (not ITT) Sponsoring: Financial support from Upjohn and the American Diabetes Association Oregon Affiliate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Riddle 1992.
| Methods | Design: Double‐blind randomised placebo‐controlled trial Duration: 16 weeks Randomisation procedure: Randomised at time of entry into the treatment protocol by assignment of a study number corresponding to a treatment code determined by the drug manufacturer. Blinding: patients yes; care provider yes; outcome assessor yes. Intention to treat: Yes | |
| Participants | Country: Oregon, USA Setting: Secondary care outpatient Inclusion criteria: Diabetes of gradual onset > 40 yrs of age, diabetes duration >1 yr, fasting plasma glucose >7.8 mmol/l despite glibenclamide 10 mg bd. Exclusion criteria: Major systemic illness other than diabetes. Use of corticosteroids, oestrogen, thyroxine, Adrenergic blockers or diuretics within 1 month of study entry. Patients randomised: 21 Nr of patients/group: 11 / 10 Drop‐outs / loss to follow‐up: nil Nr of patients/group analysed: 11/10 Age (years, mean): 55 / 52 yrs Sex (% male): not stated Diabetes duration (years): 6 / 4 Diabetes therapy: glibenclamide 10 mg bd (3‐week run‐in period) | |
| Interventions | Group 1: Glibenclamide 10 mg pre‐breakfast + suppertime Novolin 70:30 insulin (Novo Nordisk human 70% NPH, 30% soluble) Group 2: Placebo + suppertime 70:30 insulin Glucose targets to which insulin doses were titrated: Subjects asked to measure capillary blood glucose (CPG) daily before breakfast and supper Insulin starting dose + 30 units, increased weekly, If mean CBG > 10 mmol/l, insulin increased by 20 units CBG 7.8‐10, insulin increased by 15 units CBG 6.7‐7.8 mmol/l, insulin increased by 10 units CBG 5.6‐ 6.7 mmol/l, insulin increased by 5 units If recurrent hypoglycaemic symptoms, or repeated CBG < 3.3 mmol/l, reduce by 5‐10 units | |
| Outcomes | Glycaemia: HbA1 (thiobarbituric acid method, normal range 5.3‐8.9%), FPG Weight: Weight gain Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: Symptomatic hypoglycaemic episodes Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 7
Sponsoring: Support by a research grant from Hoechst‐Roussel Pharmaceuticals Structured insulin titration regimen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Riddle 1998.
| Methods | Design: Double‐blind randomised placebo‐controlled trial Duration: 24 weeks Randomisation procedure: not stated Blinding: patients yes; care provider yes; outcome assessor yes. Intention to treat: Yes | |
| Participants | Country: Oregon, USA Setting: Secondary care outpatient Inclusion criteria: Age 45‐70 yrs, weight 130‐170% IBW, FPG 10.0‐16.7 mmol/l with glimepiride 8mg bd, adequate contraception Exclusion criteria: Pregnancy, breast feeding, DM duration > 15 yrs, history of ketoacidosis, autoimmune disease, any major systemic illness other than diabetes, allergy or intolerance to sulphonylureas, use of glucocorticoids, phenytoin, nicotinic acid, sympathomimetics, phenothiazines, isoniazid. Serum creatinine or serum alanine aminotransferase >1.5 times upper limit of normal; fasting C‐peptide < 0.4 pmol/l. Patients randomised: 145 Nr of patients/group: 72 / 73 Drop‐outs / loss to follow‐up: 2 / 11 Nr of patients/group analysed: 72 / 73 Age (years, mean): 58 / 58 yrs Sex (% male): 63% / 55% Diabetes duration (years): 7 / 7yrs Diabetes therapy: Glimepiride 8 mg bd (8‐week run‐in period) | |
| Interventions | Group 1: Glimepiride 8mg bd + suppertime 70:30 insulin (70%NPH / 30% regular human insulin) Group 2: Placebo + suppertime 70:30 insulin Glucose targets to which insulin doses were titrated: FBG 5.5‐6.7 mmol/l Subjects asked to measure capillary blood glucose (CPG) daily before breakfast and supper Insulin starting dose = 10 units for 2 weeks, then Increased weekly by 10 units until FBG < 7.8 mmol/l for 2 consecutive days then, by 5 units until FBG < 6.7 mmol/l for 2 consecutive days then Small reductions allowed if hypoglycaemic symptoms occurred | |
| Outcomes | Glycaemia: HbA1c (HPLC assay, normal range 4‐6%), FPG Weight: Weight gain Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: Symptomatic hypoglycaemic episodes Well‐being: not reported Treatment Satisfaction: not reported Adverse events: Reported | |
| Notes | Quality score: 6 (randomisation method not stated) Sponsoring: Support by a research grant from Hoechst Marion Roussel Pharmaceuticals Structured insulin titration regimen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Shank 1995.
| Methods | Design: Double‐blind randomised placebo‐controlled trial Duration: 6 months Randomisation procedure: Randomisation code Blinding: patients yes; care provider yes; outcome assessor yes. Intention to treat: No | |
| Participants | Country: Texas, USA Setting: Secondary care outpatient Inclusion criteria: FPG >7.77 mmol/l with max. Dose of sulphonylurea, FPG< 15.54 mmol/l without sulphonylurea. Exclusion criteria: Other medication known to affect glucose metabolism, prior insulin treatment , regular vigorous exercise, other major illness other than diabetes. Patients randomised: 30 Nr of patients/group: 10 / 10 / 10 Drop‐outs / loss to follow‐up: 1 / 1 / 0 Nr of patients/group analysed: 9 / 9 / 10 Age (years, mean): 53 yrs (all subjects) Sex (% male): not given Diabetes duration (years): not stated Diabetes therapy: Glipizide 20 mg bd (2‐month run‐in period) | |
| Interventions | Group 1: Glipizide 20 mg bd + bedtime NPH insulin (Novolin‐N, Novo Nordisk) Group 2: Placebo + bedtime NPH insulin Group 3: Glipizide 20 mg bd Glucose targets to which insulin doses were titrated: first 3 months, insulin given as 5 units / 1.73 m2 and titrated to 20 units / 1.73 m2 (low‐dose) Second 3 months, target FPG 3.89‐6.66 mmol/l | |
| Outcomes | Glycaemia: HbA1c (microcollumn affinity chromatography, normal range 3.1‐6.1%), FPG Weight: Weight gain Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: Symptomatic (and asymptomatic <3.89 mmol/l) hypoglycaemic episodes Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 6 (no ITT) Sponsoring: Support by grants from roerig‐Pfizer, Novo Nordisk, Geriatric research and clinical centre, Veterens Affairs Medical Research Service. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sun 1995.
| Methods | Design: Randomised placebo controlled trial Duration: 4 months Randomisation procedure: not stated Blinding: patients yes; care provider unclear; outcome assessor unclear. Intention to treat: yes | |
| Participants | Country: China Setting: Secondary care outpatient Inclusion criteria: Age >40 yrs, type 2 diabetes >5 yrs duration, treatment with max. Sulphonylurea > 3 weeks, FBG > 7.8 mmol/l, 2hr post‐prandial > 11.1 mmol/l. Exclusion criteria: not stated Patients randomised: 33 Nr of patients/group: 12 / 11 / 10 Drop‐outs / loss to follow‐up: 0 / 0 / 0 Nr of patients/group analysed: 12 / 11 / 10 Age (years, mean): 53.6 / 54.4 /54.5 yrs Sex (% male): 50% / 45% / 60% male Diabetes duration (years): not stated Diabetes therapy: Gliquidone 60 mg tds, 3 weeks run‐in | |
| Interventions | Group 1: Gliquidone 60 mg tds + bedtime NPH (0.4 units/kg, Novo Nordisk) Group 2: Placebo + NPH (0.4 units/kg) Group 3: Gliquidone 60 mg tds Glucose targets to which insulin doses were titrated: not stated | |
| Outcomes | Glycaemia: HbA1c (method not given, normal range < 6%), FBG Weight: not reported Insulin amount (E): not given Hypoglycaemia: Symptomatic hypoglycaemic episodes Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 3 Sponsoring: Supported by Novo Nordisk and Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wolffenbuttel 1991.
| Methods | Design: randomised controlled trial Duration: 6 months Randomisation procedure: “aselect assignment” Blinding: patients no; care provider no; outcome assessor no Intention to treat: unclear | |
| Participants | Country: Netherlands Setting: secondary care outpatient Inclusion criteria: FBG > 8.0 mmol/l; maximal dosage SU (glibenclamide) and/or metformin Exclusion criteria: unclear Patients randomised: 47 Nr of patients/group: 22 / 25 Drop‐outs / loss to follow‐up: 0 / 0 Nr of patients/group analysed: 22 / 25 Age (years): 70 / 68 Sex (% male): 83 / 47 Diabetes duration (median; years): 9 / 10 Diabetes therapy: glibenclamide 15 mg (27 patients); glibenclamide 15 mg + metformin (dose not reported) (20 patients) | |
| Interventions | Group 1: intermediate‐acting insulin (NPH) before breakfast and dinner; eventually replaced by mixed insulin (30% short‐acting and 70% intermediate‐acting) in case of post‐prandial BG > 10.0 mmol/l Group 2: intermediate‐acting insulin (NPH) before breakfast or bedtime + glibenclamide 15 mg; eventually a second injection was added in case of post‐prandial BG > 10.0 mmol/l Glucose targets to which insulin doses were titrated: FBG < 7.0 mmol/l; post‐prandial BG < 10.0 mmol/l; HbA1c < 8.0% | |
| Outcomes | Glycaemia: FBG; HbA1c Weight: weight Insulin amount (E): daily dose Hypoglycaemia: reported qualitatively Well‐being: reported qualitatively Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 0 Sponsoring: Novo Nordisk Pharmaceuticals, Diabetes Research Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wolffenbuttel 1996.
| Methods | Design: Randomised controlled trial Duration: 6 months Randomisation procedure: not stated Blinding: patients no; care provider no; outcome assessor no. Intention to treat: no | |
| Participants | Country: Netherlands Setting: Secondary care outpatient Inclusion criteria: Fasting blood glucose (mean of 3 measurements) >8.0 mmol/l, HbA1c > 8.9% despite diet & max. oral hypoglycaemic agents (glibenclamide 15 mg / day ±metformin). Exclusion criteria: Intercurrent illness, cardiac, hepatic, renal or other endocrine disease. Severe untreated hypertension (diastolic BP > 110 mm Hg), impaired renal function (creatinine > 140 micromol/l), or treatment with corticosteroids. Patients randomised: 102 Nr of patients/group: 34 / 28 / 33 Drop‐outs / loss to follow‐up: 7 (unclear which group(s)) Nr of patients/group analysed: 34 / 28 / 33 Age (years, mean): 68 yrs (completers) Sex (% male): 39% male (completers) Diabetes duration (years): median 9 yrs (completers) Diabetes therapy: Glibenclamide 15 mg/day (n=66), Glibenclamide 15 mg/day + metformin (n=29) | |
| Interventions | Group 1: Twice‐daily insulin mixture (Mixtard 30/70, 30% short acting, 70% NPH, Novo Nordisk) Group 2: Glibenclamide 10 mg +5mg + bedtime NPH insulin Group 3: Glibenclamide 10 mg +5mg + NPH insulin before breakfast Glucose targets to which insulin doses were titrated: Fasting blood glucose < 7.0 mmol/l, pre‐prandial glucose < 10 mmol/l, HbA1c <8.0%. If daytime/evening (group 2) or bedtime (group 3) blood glucose exceeded 10 mmol/l, subjects were switched to a regimen consisting of NPH insulin before breakfast and at bedtime with continued glibenclamide | |
| Outcomes | Glycaemia: HbA1c (HPLC assay, normal range 4.4‐6.2%), FBG Weight: Weight gain Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: Severe hypoglycaemic episodes reported Well‐being: not formally reported Treatment Satisfaction: not formally reported Adverse events: Reported | |
| Notes | Quality score: 1 ? ( withdrawals & dropouts described?) Sponsoring: Supported by Novo Nordisk and Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Xu 2001.
| Methods | Design: Randomised controlled trial Duration: 6 months Randomisation procedure: unclear Blinding: unclear Intention to treat: unclear | |
| Participants | Country: China Setting: Sec care outpat and sec inpat Inclusion criteria: data missing Exclusion criteria: data missing Patients randomised: 90 Nr of patients/group: 45 / 45 Drop‐outs / loss to follow‐up: 0 / 0 Nr of patients/group analysed: 45 / 45 Age (years, mean): 51.4 / 52.1 Sex (% male): 47 / 51 Diabetes duration (years;(SD)): 7.3 (4.5) / 7.4 (4.8) Diabetes therapy: OHAs | |
| Interventions | Group 1: insulin once daily 24 IU/day Group 2: metformin 1500 daily + insulin once daily 24U/day Glucose targets to which insulin doses were titrated: 3.5 ‐ 7.0 mmol/l | |
| Outcomes | Glycaemia: HbA1c (method and normal range not given), FBG Weight: data missing Insulin amount (IU): fixed insulin dose in both groups Hypoglycaemia: not reported Well‐being: not reported Treatment Satisfaction: not reported Adverse events: not reported | |
| Notes | Quality score: 0 Sponsoring: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yki‐Järvinen 1992.
| Methods | Design: Randomised controlled trial Duration: 3 months | |
| Participants | Country: Finland Setting: Secondary care outpatient Inclusion criteria: Age 40‐70 yrs, type 2 diabetes > 3 yrs, BMI <35 kg/m2, FPG > 8.0 mmol/l despite max. sulphonylurea ±metformin. Fasting C‐peptide > 0.33 mol/l. Exclusion criteria: Congestive cardiac failure, myocardial infarction or stroke within 6 months; epilepsy or other severe disease; liver disease; nephropathy (serum creatinine > 120 micromol/l or albuminuria > 300 mg/24 hrs; proliferative diabetic retinopathy or severe maculopathy; previous insulin therapy for more than 2 weeks; excess alcohol consumption; night shift work; serum triglycerides > 5mmol/l; presence of islet cell antibodies. Patients randomised: 153 Nr of patients/group: 32 / 28 / 30 / 31 / 32 Drop‐outs / loss to follow‐up: 0 / 0 / 1 / 1 / 2 Nr of patients/group analysed: 32 / 28 / 29 / 30 / 30 Age (years, mean): 59 / 60 / 59 / 60 / 59 yrs (completers) Sex (% male): 38 / 54 / 41 / 60 / 37% (completers) Diabetes duration (years): 11 / 10 / 10 / 11 / 10 (completers) Diabetes therapy: Glibenclamide (mg/day) 11 / 11 / 12 / 11 / 11 Glipizide (mg/day) 18 / 19 / 17 / 22 / 18 Metformin (g/day) 1.3 / 1.3 / 1.2 / 1.4 / 1.4 | |
| Interventions | Group 1: Oral hypoglycaemic agent therapy (unchanged: SU +/‐ metformin) + NPH insulin before breakfast (Novo Nordisk)Group 2: Oral hypoglycaemic agent therapy (unchanged) + 9 pm NPH insulin Group 3: NPH and regular insulin (70:30) before breakfast and dinner Group 4: Basal‐bolus regimen (actrapid soluble insulin before meals, 9pm NPH)Group 5: Oral hypoglycaemic agent therapy (unchanged) (control group)(add groups if appropriate) Glucose targets to which insulin doses were titrated:FBG < 7.0 mmol/l, post‐prandial <10 mmol/lInsulin starting doses Groups 1 & 2: Insulin dose equalled mean diurnal blood glucose concentration (mmol/l)Group 3: 0.25 units/kg + 4 units for each mmol/l mean diurnal blood glucose concentration exceeded 10 mmol/l. Two thirds given at 7am, one third at 4pm.Group 4: As for group 3. Two thirds given at before meals, one third at 9pm. | |
| Outcomes | Glycaemia: HbA1c (HPLC assay, 4‐6%), FBG Weight: Weight gain Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: Symptomatic and biochemical (<4.0 mmol/l) hypoglycaemia Well‐being: Reported though method not stated Treatment Satisfaction: not reported Adverse events: Those resulting in withdrawal reported | |
| Notes | Quality score: 4 Sponsoring: Supported by grants from Novo Nordisk, Finnish State Medical Research Council, Sigrid Juselius research foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yki‐Järvinen 1999.
| Methods | Design: Randomised placebo controlled trial Duration: 1 year Randomisation procedure: “minimization of differences” Blinding: patients yes; care provider no; outcome assessor no. Intention to treat: no | |
| Participants | Country: Finland Setting: Secondary care outpatient Inclusion criteria: Age 40‐70 yrs, BMI <35 kg/m2, FBG >8.0 mmol/l, diabetes duration >3 yrs, previous oral therapy with max. SU (glipizide >15 mg / day, glibenclamide >10 mg / day. Fasting C‐peptide >0.33nmol/l. Exclusion criteria: NYHA grade III/IV heart failure, MI or stroke within 6 months, epilepsy or other severe disease, liver disease unrelated to diabetes, nephropathy (macroalbuminuria or serum creatinine >120 micromol/l, proliferative retinopathy or severe maculopathy, insulin theraspy for more than 2 weeks, excessive alcohol consumption, night shift work. Patients randomised: 96 Nr of patients/group: 24 / 24 / 24 / 24 Drop‐outs / loss to follow‐up: 2 / 5 / 1 / 0 Nr of patients/group analysed: 22 / 19 / 23 / 24 Age (years, mean): 61 / 57 / 55 / 58 yrs (completers) Sex (% male): 59 / 58 / 61 / 67 % (completers) Diabetes duration (years): not stated Diabetes therapy: glipizide >15mg / day, glibenclamide >10 mg / day | |
| Interventions | Group 1: Glibenclamide 3.5mg + 7mg + bedtime NPH insulin (+ metformin placebo)
Group 2: Metformin 1g bd + bedtime NPH insulin (+ glibenclamide placebo)
Group 3: Glibenclamide (3.5mg + 7mg) + metformin (1g bd) + bedtime NPH insulin
Group 4: BD NPH insulin Glucose targets to which insulin doses were titrated: FPG <6.0mmol/l Starting dose of NPH = FPG (mmol/l), FPG measured daily. Insulin increased by 4 units if 3 successive FPG readings > 8 mmol/l, and 2 units if > 6.0 mmol/l |
|
| Outcomes | Glycaemia: HbA1c (HPLC method, normal range not given), FPG Weight: Weight gain, BMI Insulin amount (E): Mean daily insulin dose at final visit Hypoglycaemia: not reported Well‐being: not reported Treatment Satisfaction: not reported Adverse events: Reported | |
| Notes | Quality score: 4 Sponsoring: Supported by grant from the Acadamy of Finland Structured patient‐led insulin titration regimen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Abbreviations: BMI = body mass index, CCF = congestive cardiac failure, CPG = capillary blood glucose, (F)BG = (fasting) blood glucose, FPG = fasting plasma glucose, HPLC = high performance liquid chromatography, IBW = ideal bodyweight, MI = myocardial infarction, NPH = neutral protamine hegedorn, OHA = oral hypoglycaemic agents, PVD = peripheral vascular disease, SU = sulphonylurea
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1985 | previously insulin‐treated |
| Aviles 1999 | previously insulin‐treated |
| Bastyr 2000 | no insulin monotherapy arm |
| Bieger 1984 | previously insulin‐treated |
| Birkeland 1994 | no insulin / oha combination therapy arm |
| Birkeland 1996 | no insulin / oha combination therapy arm |
| Bruns 1988 | not a RCT; follow‐up < 2 months |
| Calle 1995 | no insulin / oha combination therapy arm |
| Camerini 1994 | previously insulin‐treated (62% of patients) |
| Carta 1984 | not a RCT |
| Casner 1988 | previous insulin‐treated |
| Castillo 1987 | previously insulin‐treated |
| Chazan 2001 | not a RCT |
| Chiasson 1994 | no insulin monotherapy arm |
| Clauson 1996 | previously insulin‐treated |
| Cortes 1993 | patients well‐controlled at inclusion |
| Diehl 1985 | no insulin / oha combination therapy arm |
| Elgrably 1991 | not a RCT |
| Falko 1985 | previously insulin‐treated |
| Feinglos 1997 | no insulin monotherapy arm |
| Feinglos 1998 | previously insulin‐treated |
| Firth 1986 | no insulin /oha combination therapy arm |
| Firth 1987 | no insulin /oha combination therapy arm |
| Fonseca 2000 | previously insulin‐treated |
| Fritsche 2000 | previously insulin‐treated |
| Giugliano 1993 | previously insulin‐treated |
| Groop 1984 | previously insulin‐treated |
| Groop 1985 | previously insulin‐treated |
| Groop 1989 | no insulin / oha combination therapy arm |
| Groop 1991 | no insulin /oha combination therapy arm |
| Groop 1992 | no insulin monotherapy arm |
| Guvener 1999 | previously insulin‐treated |
| Hamelbeck 1982 | follow‐up< 2 months |
| Hirsch 1999 | previously insulin‐treated |
| Josse 1995 | previously insulin‐treated |
| Kasim 1986 | not a RCT |
| Kelley 1998 | previously insulin‐treated |
| Kitabchi 1987 | previously insulin‐treated |
| Klein 1991 | no insulin monotherapy arm |
| Kyllastinen 1985 | previously insulin‐treated |
| Landstedt 1995 | previously insulin‐treated |
| Landstedt 1999 | previously insulin‐treated |
| Lardinois 1985 | previously insulin‐treated |
| Lawrence 1988 | not a RCT |
| Lebovitz 1990 | not a RCT |
| Lewitt 1989 | previously insulin‐treated |
| Liedtke 1990 | follow‐up < 2 months |
| Lindstrom 1992 | no insulin /oha combination therapy arm |
| Lindstrom 1999 | previously insulin‐treated |
| Lins 1988 | previously insulin‐treated |
| Longnecker 1986 | previously insulin‐treated |
| Lopez 1999 | no insulin /oha combination therapy arm |
| Martin 1986 | not a RCT |
| Mauerhoff 1986 | previously insulin‐treated |
| Mezitis 1992 | previous insulin‐treated |
| Mohan 1990 | previously insulin‐treated |
| Nathan 1988 | patients on diet alone |
| Niazi 1998 | no insulin monotherapy arm |
| Niskanen 1992 | no insulin / oha combination therapy arm |
| Okada 1996 | not a RCT |
| Osei 1984 | previously insulin‐treated |
| Panahloo 1998 | patients on diet alone |
| Pasmantier 1990 | study on human pro‐insulin |
| Peacock 1984 | no insulin / oha combination therapy arm |
| Polo 1998 | study on combination nicotinamide with insulin |
| Ponssen 2000 | previously insulin‐treated |
| Quatraro 1986 | previously insulin‐treated |
| Raskin 2001 | previously insulin‐treated |
| Reich 1987 | previously insulin‐treated |
| Relimpio 1998 | previously insulin‐treated |
| Rivellese 2000 | no insulin / oha combination therapy arm |
| Robinson 1998 | previously insulin‐treated |
| Rodier 1995 | no insulin / oha combination therapy arm |
| Romano 1997 | no insulin / oha combination therapy arm |
| Rosak 1985 | previously insulin‐treated |
| Samanta 1987 | newly diagnosed type 2 diabetes patients |
| Sanchez 1999 | previously insulin‐treated |
| Sane 1992 | no insulin monotherapy arm |
| Sangiorgio 1996 | no insulin monotherapy arm |
| Schade 1987 | previously insulin‐treated |
| Schwartz 1997 | no insulin monotherapy arm |
| Schwartz 1998 | previously insulin‐treated |
| Simonson 1987 | previously insulin‐treated |
| Simpson 1990 | previously insulin‐treated |
| Sinagra 1998 | previously insulin‐treated |
| Soneru 1993 | no insulin monotherapy arm |
| Sotaniemi 1990 | follow‐up < 2 months |
| Standl 1999 | previously insulin‐treated |
| Stenman 1988 | previously insulin‐treated |
| Stocks 1988 | previously insulin‐treated |
| Stradner 1990 | no insulin monotherapy arm |
| Thompson 1998 | previously insulin‐treated |
| Tovi 1998 | no insulin / oha combination therapy arm |
| Trischitta 1992 | no insulin monotherapy arm |
| Trischitta 1998 | no insulin monotherapy arm |
| Trznadel 1997 | not a RCT |
| Turner 1999 (2) | no insulin / oha combination therapy arm |
| UKPDS 13 1995 | no insulin / oha combination therapy arm |
| UKPDS 24 1998 | no insulin / oha combination therapy arm |
| UKPDS 33 1998 | no insulin / oha combination therapy arm |
| Vigneri 1991 | no insulin monotherapy arm |
| Wolffenbuttel 1989 | no insulin / oha combination therapy arm |
| Yki‐Jarvinen 2000 | no insulin monotherapy arm |
| Yu 1999 | previously insulin‐treated |
| Yudkin 2000 | previously insulin‐treated |
Contributions of authors
AN GOUDSWAARD: Protocol development, searching for trials, trial selection, quality assessment of trials, data extraction, review development.
NJ FURLONG: Quality assessment of trials, data extraction, review development.
GD VALK: Protocol development, trial selection, quality assessment of trials, data extraction, review development.
RP STOLK: Methodology, statistics.
GEHM RUTTEN: Review development.
Sources of support
Internal sources
Julius Center for Health Science and Primary Care, Netherlands.
External sources
No sources of support supplied
Declarations of interest
In 1999 ANG has received an unrestricted research grant from Novo Nordisk for conducting studies on monitoring and treatment of patients with type 2 diabetes in primary care. Research conducted by NJF during 1999‐2001 was supported by an unrestricted research grant from Novo Nordisk.
Edited (no change to conclusions)
References
References to studies included in this review
Bachmann 1988 {published data only}
- Bachmann W, Lotz N, Mehnert H, Rosak C, Schoffling K. [Effectiveness of combined treatment with glibenclamide and insulin in secondary sulfonylurea failure. A controlled multicenter double‐blind clinical trial]. Deutsche Medizinische Wochenschrift 1988;113(16):631‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bastyr 1999 {published data only}
- Bastyr EJ, Johnson ME, Trautmann ME, Anderson JH, Vignati L. Insulin lispro in the treatment of patients with type 2 diabetes mellitus after oral agent failure. Clinical Therapeutics 1999;21(10):1703‐14. [DOI] [PubMed] [Google Scholar]
Chow 1995 {published and unpublished data}
- Chow CC, Tsang LW, Sorensen JP, Cockram CS. Comparison of insulin with or without continuation of oral hypoglycemic agents in the treatment of secondary failure in NIDDM patients. Diabetes Care 1995;18(3):307‐14. [DOI] [PubMed] [Google Scholar]
Fövényi 1997 {published and unpublished data}
- Fövényi J, Grosz A, Thaisz E, Lehotkai, L, Sallai T, Kocsis G. [Daytime sulfonyluyrea ‐ bedtime insulin combination therapy in Type II diabetes]. Magy Belorv Arch (Hungarian Archive of Internal Medicine) 1997;50:607‐13. [Google Scholar]
Gutniak 1987 {published data only}
- Gutniak M, Karlander SG, Efendic S. Glyburide decreases insulin requirement, increases beta‐cell response to mixed meal, and does not affect insulin sensitivity: effects of short‐ and long‐term combined treatment in secondary failure to sulfonylurea. Diabetes Care 1987;10(5):545‐54. [DOI] [PubMed] [Google Scholar]
Holman 1987 {published data only}
- Holman RR, Steemson J, Turner RC. Sulphonylurea failure in type 2 diabetes: treatment with a basal insulin supplement. Diabetic Medicine 1987;4(5):457‐62. [DOI] [PubMed] [Google Scholar]
Karlander 1991 {published data only}
- Karlander SG, Gutniak MK, Efendic S. Effects of combination therapy with glyburide and insulin on serum lipid levels in NIDDM patients with secondary sulfonylurea failure. Diabetes Care 1991;14(11):963‐67. [DOI] [PubMed] [Google Scholar]
Lotz 1988 {published data only}
- Lotz N, Bachmann W, Ladik T, Mehnert H. [Combination therapy with insulin/sulfonylurea in the long‐term therapy of type II diabetes following "secondary failure"]. [German]. Klinische Wochenschrift 1988;66(21):1079‐84. [DOI] [PubMed] [Google Scholar]
Lundershausen 1987 {published data only}
- Lundershausen R, Orban S, Pissarek D, Panzram G. [Long‐term effect of combination glibenclamide‐insulin treatment in the secondary failure of sulfonylurea therapy‐‐results of a one‐year double blind study]. [German]. Wiener Klinische Wochenschrift 1987;99(17):603‐8. [PubMed] [Google Scholar]
Mäkimattila 1999 {published data only}
- Mäkimattila S, Nikkila K, Yki‐Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with Type II diabetes mellitus. Diabetologia 1999;42(4):406‐12. [DOI] [PubMed] [Google Scholar]
Pontiroli 1990 {published and unpublished data}
- Pontiroli AE, Dino G, Capra F, Pozza G. Combined therapy with glibenclamide and ultralente insulin in lean patients with NIDDM with secondary failure of sulfonylureas. Follow up at two years. Diabete et Metabolisme 1990;16(4):323‐27. [PubMed] [Google Scholar]
Ravnik‐Oblak 1995 {published data only}
- Ravnik‐Oblak M, Mrevlje F. Insulin versus a combination of insulin and sulfonylurea in the treatment of NIDDM patients with secondary oral failure. Diabetes Research & Clinical Practice 1995;30(1):27‐35. [DOI] [PubMed] [Google Scholar]
Riddle 1989 {published data only}
- Riddle MC, Hart JS, Bouma DJ, Phillipson BE, Youker G. Efficacy of bedtime NPH insulin with daytime sulfonylurea for subpopulation of type II diabetic subjects. Diabetes Care 1989;12(9):623‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Riddle 1992 {published data only}
- Riddle M, Hart J, Bingham P, Garrison C, McDaniel P. Combined therapy for obese type 2 diabetes: suppertime mixed insulin with daytime sulfonylurea. American Journal of the Medical Sciences 1992;303(3):151‐56. [DOI] [PubMed] [Google Scholar]
Riddle 1998 {published data only}
- Riddle MC, Schneider J. Beginning insulin treatment of obese patients with evening 70/30 insulin plus glimepiride versus insulin alone. Glimepiride Combination Group. Diabetes Care 1998;21(7):1052‐57. [DOI] [PubMed] [Google Scholar]
Shank 1995 {published data only}
- Shank ML, Prato S, DeFronzo RA. Bedtime insulin/daytime glipizide. Effective therapy for sulfonylurea failures in NIDDM. Diabetes 1995;44:165‐72. [DOI] [PubMed] [Google Scholar]
Sun 1995 {published data only}
- Sun Y, Xiong Y, Yang J. [The effectiveness of combined insulin and sulfonylurea in treating non‐insulin dependent diabetic patients]. [Chinese]. Chung‐Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 1995;34(4):246‐49. [PubMed] [Google Scholar]
Wolffenbuttel 1991 {published data only}
- Wolffenbuttel BH, Rondas‐Colbers GJ, Menheere PP, Sels JP, Nieuwenhuijzen‐Kruseman AC. [The effects of insulin combined with glibenclamide on glucose and lipid metabolism in patients with Type II diabetes mellitus]. Ned Tijdschr Geneeskd 1991;135:1080‐84. [PubMed] [Google Scholar]
Wolffenbuttel 1996 {published data only}
- Wolffenbuttel BH, Sels JP, Rondas‐Colbers GJ, Menheere PP, Nieuwenhuijzen‐Kruseman AC. Comparison of different insulin regimens in elderly patients with NIDDM. Diabetes Care 1996;19:1326‐32. [DOI] [PubMed] [Google Scholar]
Xu 2001 {published data only}
- Xu WC, Chen CR, Chen YS. Combination therapy with bedtime insulin and daytime oral hypoglycaemic agents in type 2 diabetic patients [Chinese]. Hebei Medicine 2001;23:23‐24. [MEDLINE: ] [Google Scholar]
Yki‐Järvinen 1992 {published data only}
- Yki‐Järvinen H, Kauppila M, Kujansuu E, Lahti J, Marjanen T, Niskanen L, et al. Comparison of insulin regimens in patients with non‐insulin‐dependent diabetes mellitus. New England Journal of Medicine 1992;327(20):1426‐33. [DOI] [PubMed] [Google Scholar]
Yki‐Järvinen 1999 {published data only}
- Yki‐Järvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Annals of Internal Medicine 1999;130(5):389‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1985 {published data only}
- Allen BT, Feinglos MN, Lebovitz HE. Treatment of poorly regulated non‐insulin‐dependent diabetes mellitus with combination insulin‐sulfonylurea. Arch Intern Med 1985;145(10):1900‐03. [PubMed] [Google Scholar]
Aviles 1999 {published data only}
- Aviles‐Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin‐treated type 2 diabetes mellitus. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1999;131(3):182‐88. [DOI] [PubMed] [Google Scholar]
Bastyr 2000 {published data only}
- Bastyr EJ, Stuart CA, Brodows RG, Schwartz S, Graf CJ, Zagar A, et al. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. IOEZ Study Group. [see comments]. Diabetes Care 2000;23(9):1236‐41. [DOI] [PubMed] [Google Scholar]
Bieger 1984 {published data only}
- Bieger WP, Dlugosch R, Rettenmeier A, Holler HD, Bert H, Schwarz W, et al. Trial of sulfonylurea in combination with insulin in the therapy of diabetes type I and II. Evidence against a primary extrapancreatic receptor effect. Klinische Wochenschrift 1984;62(13):631‐39. [DOI] [PubMed] [Google Scholar]
Birkeland 1994 {published data only}
- Birkeland KI, Hanssen KF, Urdal P, Berg K, Vaaler S. A long‐term, randomized, comparative study of insulin versus sulfonylurea therapy in type 2 diabetes [see comments]. Journal of Internal Medicine 1994;236:305‐13. [DOI] [PubMed] [Google Scholar]
Birkeland 1996 {published data only}
- Birkeland KI, Rishaug U, Hanssen KF, Vaaler S. NIDDM: a rapid progressive disease. Results from a long‐term, randomised, comparative study of insulin or sulphonylurea treatment. Diabetologia 1996;39:1629‐33. [DOI] [PubMed] [Google Scholar]
Bruns 1988 {published data only}
- Bruns W, Willkommen G, Philipp A, Czeczatka W, Hildebrandt R, Jutzi E. Zur Behandlung von Typ II (non‐insulin‐dependent)‐Diabetikern mit Sulfonylharnstoff‐Sekundärversagen Kombinationstherapie von Glibenclamid und Insulin. Zeitschrift für Klinische Medizin 1988;43:625‐29. [MEDLINE: ] [Google Scholar]
Calle 1995 {published data only}
- Calle‐Pascual AL, Garcia HJ, Martin‐Alvarez PJ, Vara E, Calle JR, Munguira ME, et al. Comparison between acarbose, metformin, and insulin treatment in type 2 diabetic patients with secondary failure to sulfonylurea treatment. Diabetes and Metabolism 1995;21:256‐60. [PubMed] [Google Scholar]
Camerini 1994 {published data only}
- Camerini‐Davalos RA BJJV. Effect of insulin‐glipizide combination on skeletal muscle capillary basement membrane width in diabetic patients. Clinical Therapeutics 1994;16(6):952‐61. [PubMed] [Google Scholar]
Carta 1984 {published data only}
- Carta Q, Trovati M, Dani F, Caselle MT, Vitali S, Cavalot F, et al. [Insulin or insulin + oral hypoglycemic drugs in the treatment of type 2 diabetes difficult to compensate metabolically?]. [Italian]. Minerva Endocrinologica 1984;9(2):241‐45. [PubMed] [Google Scholar]
Casner 1988 {published data only}
- Casner PR. Insulin‐glyburide combination therapy for non‐insulin‐dependent diabetes mellitus: a long‐term double‐blind, placebo‐controlled trial. Clinical Pharmacology & Therapeutics 1988;44(5):594‐603. [DOI] [PubMed] [Google Scholar]
Castillo 1987 {published data only}
- Castillo M, Scheen AJ, Paolisso G, Lefebvre PJ. The addition of glipizide to insulin therapy in type‐II diabetic patients with secondary failure to sulfonylureas is useful only in the presence of a significant residual insulin secretion. Acta Endocrinologica 1987;116(3):364‐72. [DOI] [PubMed] [Google Scholar]
Chazan 2001 {published data only}
- Chazan AC, Gomes MB. Gliclazide and bedtime insulin are more efficient than insulin alone for type 2 diabetic patients with sulfonylurea secondary failure. Brazilian Journal of Medical & Biological Research 2001;34(1):49‐56. [DOI] [PubMed] [Google Scholar]
Chiasson 1994 {published data only}
- Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA. The efficacy of acarbose in the treatment of patients with non‐insulin‐dependent diabetes mellitus. A multicenter controlled clinical trial. Annals of Internal Medicine 1994;121(12):928‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clauson 1996 {published data only}
- Clauson P, Karlander S, Steen L, Efendic S. Daytime glibenclamide and bedtime NPH insulin compared to intensive insulin treatment in secondary sulphonylurea failure: a 1‐year follow‐up. Diabetic Medicine 1996;13(5):471‐77. [DOI] [PubMed] [Google Scholar]
Cortes 1993 {published data only}
- Cortes AH, Espinosa Lopez FR, Angulo Cervera JA, Diaz TJ. [A comparative study of insulin and glyburide versus glyburide or insulin in the chronic control of patients with type‐2 diabetes]. [Spanish]. Gaceta Medica de Mexico 1993;129(6):383‐86. [PubMed] [Google Scholar]
Diehl 1985 {published data only}
- Diehl AK, Sugarek NJ, Bauer RL. Medication compliance in non‐insulin‐dependent diabetes: a randomized comparison of chlorpropamide and insulin. Diabetes Care 1985;8:219‐23. [DOI] [PubMed] [Google Scholar]
Elgrably 1991 {published data only}
- Elgrably F, Costagliola D, Chwalow AJ, Varenne P, Slama G, Tchobroutsky G. Initiation of insulin treatment after 70 years of age: patient status 2 years later. Diabet Med 1991;8(8):773‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Falko 1985 {published data only}
- Falko JM, Osei K. Combination insulin/glyburide therapy in type II diabetes mellitus. Effects on lipoprotein metabolism and glucoregulation. American Journal of Medicine 1985;79(3B):92‐101. [DOI] [PubMed] [Google Scholar]
Feinglos 1997 {published data only}
- Feinglos MN, Thacker CH, English J, Bethel MA, Lane JD. Modification of postprandial hyperglycemia with insulin lispro improves glucose control in patients with type 2 diabetes. Diabetes Care 1997;20(10):1539‐42. [DOI] [PubMed] [Google Scholar]
Feinglos 1998 {published data only}
- Feinglos MN, Thacker CR, Lobaugh B, DeAtkine DD, McNeill DB, English JS. Combination insulin and sulfonylurea therapy in insulin‐requiring type 2 diabetes mellitus. Diabetes Research & Clinical Practice 1998;39(3):193‐99. [DOI] [PubMed] [Google Scholar]
Firth 1986 {published data only}
- Firth RG, Bell PM, Rizza RA. Effects of tolazamide and exogenous insulin on insulin action in patients with non‐insulin‐dependent diabetes mellitus. The New England Journal of Medicine 1986;314:1280‐86. [DOI] [PubMed] [Google Scholar]
Firth 1987 {published data only}
- Firth R, Bell P, Marsh M, Rizza RA. Effects of tolazamide and exogenous insulin on pattern of postprandial carbohydrate metabolism in patients with non‐insulin‐ dependent diabetes mellitus. Results of randomized crossover trial. Diabetes 1987;36:1130‐38. [DOI] [PubMed] [Google Scholar]
Fonseca 2000 {published data only}
- Fonseca V, Foyt HL, Shen K, Whitcomb R. Long‐term effects of troglitazone: open‐label extension studies in type 2 diabetic patients. Diabetes Care 2000;23(3):354‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fritsche 2000 {published data only}
- Fritsche A, Schmulling RM, Haring HU, Stumvoll M. Intensive insulin therapy combined with metformin in obese type 2 diabetic patients. Acta Diabetologica 2000;37(1):13‐18. [DOI] [PubMed] [Google Scholar]
Giugliano 1993 {published data only}
- Giugliano D, Quatraro A, Consoli G, Minei A, Ceriello A, Rosa N, et al. Metformin for obese, insulin‐treated diabetic patients: improvement in glycaemic control and reduction of metabolic risk factors. European Journal of Clinical Pharmacology 1993;44(2):107‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Groop 1984 {published data only}
- Groop L, Harno K, Tolppanen EM. The combination of insulin and sulphonylurea in the treatment of secondary drug failure in patients with type II diabetes. Acta Endocrinologica 1984;106(1):97‐101. [DOI] [PubMed] [Google Scholar]
Groop 1985 {published data only}
- Groop L, Harno K, Nikkila EA, Pelkonen R, Tolppanen EM. Transient effect of the combination of insulin and sulfonylurea (glibenclamide) on glycemic control in non‐insulin dependent diabetics poorly controlled with insulin alone. Acta Medica Scandinavica 1985;217(1):33‐39. [DOI] [PubMed] [Google Scholar]
Groop 1989 {published data only}
- Groop L, Widen E, Franssila‐Kallunki A, Ekstrand A, Saloranta C, Schalin, C, et al. Different effects of insulin and oral antidiabetic agents on glucose and energy metabolism in type 2 (non‐insulin‐dependent) diabetes mellitus. Diabetologia 1989;32(8):599‐605. [DOI] [PubMed] [Google Scholar]
Groop 1991 {published data only}
- Groop L, Widen E. Treatment strategies for secondary sulfonylurea failure. Should we start insulin or add metformin? Is there a place for intermittent insulin therapy?. Diabete et Metabolisme 1991;17(1 Pt 2):218‐23. [PubMed] [Google Scholar]
Groop 1992 {published data only}
- Groop LC, Widen E, Ekstrand A, Saloranta C, Franssila‐Kallunki A, Schalin‐Jantti C, et al. Morning or bedtime NPH insulin combined with sulfonylurea in treatment of NIDDM. Diabetes Care 1992;15(7):831‐34. [DOI] [PubMed] [Google Scholar]
Guvener 1999 {published data only}
- Guvener N, Gedik O. Effects of combination of insulin and acarbose compared with insulin and gliclazide in type 2 diabetic patients. Acta Diabetologica 1999;36(1‐2):93‐97. [DOI] [PubMed] [Google Scholar]
Hamelbeck 1982 {published data only}
- Hamelbeck H, Klein W, Zoltobrocki M, Schoffling K. [Glibenclamide‐insulin combination in the management of secondary failure of sulfonyl‐urea medication]. Deutsche Medizinische Wochenschrift 1982;107(42):1581‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hirsch 1999 {published data only}
- Hirsch IB. Metformin added to insulin therapy in poorly controlled type 2 diabetes. Diabetes Care 1999;22(5):854. [DOI] [PubMed] [Google Scholar]
Josse 1995 {published data only}
- Josse RG. Acarbose for the treatment of type II diabetes: the results of a Canadian multi‐centre trial. [erratum appears in Diabetes Res Clin Pract 1995 Sep;29(3):215]. Diabetes Research & Clinical Practice 1995;28 Suppl:S167‐S172. [DOI] [PubMed] [Google Scholar]
Kasim 1986 {published data only}
- Kasim SE, LeBoeuf RC, Rockett MJ, Page J, Keyser AJ. The effects of oral agent or insulin treatments on the plasma lipoproteins and the plasma lipoprotein lipase activator in diabetic patients. Hormone & Metabolic Research 1986;18(3):190‐93. [DOI] [PubMed] [Google Scholar]
Kelley 1998 {published data only}
- Kelley DE, Bidot P, Freedman Z, Haag B, Podlecki D, Rendell M, et al. Efficacy and safety of acarbose in insulin‐treated patients with type 2 diabetes. Diabetes Care 1998;21(12):2056‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kitabchi 1987 {published data only}
- Kitabchi AE, Soria AG, Radparvar A, Lawson‐Grant V. Combined therapy of insulin and tolazamide decreases insulin requirement and serum triglycerides in obese patients with noninsulin‐dependent diabetes mellitus. American Journal of the Medical Sciences 1987;294(1):10‐14. [DOI] [PubMed] [Google Scholar]
Klein 1991 {published data only}
- Klein W. Sulfonylurea‐metformin‐combination< /strong> versus sulfonylurea‐insulin‐combination</s trong> in secondary failures of sulfonylurea monotherapy. Results of a prospective randomized study in 50 patients. Diabete et Metabolisme 1991;17(1 Pt 2):235‐40. [PubMed] [Google Scholar]
Kyllastinen 1985 {published data only}
- Kyllastinen M, Groop L. Combination of insulin and glibenclamide in the treatment of elderly non‐insulin dependent (type 2) diabetic patients. Annals of Clinical Research 1985;17(3):100‐04. [PubMed] [Google Scholar]
Landstedt 1995 {published data only}
- Landstedt‐Hallin L, Adamson U, Arner P, Bolinder J, Lins PE. Comparison of bedtime NPH or preprandial regular insulin combined with glibenclamide in secondary sulfonylurea failure. Diabetes Care 1995;18(8):1183‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Landstedt 1999 {published data only}
- Landstedt‐Hallin L, Arner P, Lins PE, Bolinder J, Olsen H, Groop L. The role of sulphonylurea in combination therapy assessed in a trial of sulphonylurea withdrawal. Scandinavian Insulin‐Sulphonylurea Study Group Research Team. Diabetic Medicine 1999;16(10):827‐34. [DOI] [PubMed] [Google Scholar]
Lardinois 1985 {published data only}
- Lardinois CK, Liu GC, Reaven GM. Glyburide in non‐insulin‐dependent diabetes. Its therapeutic effect in patients with disease poorly controlled by insulin alone. Archives of Internal Medicine 1985;145(6):1028‐32. [DOI] [PubMed] [Google Scholar]
Lawrence 1988 {published data only}
- Lawrence AM, Abraira C. New modalities in diabetes treatment. The American journal of medicine 1988;85:153‐58. [DOI] [PubMed] [Google Scholar]
Lebovitz 1990 {published data only}
- Lebovitz HE, Pasmantier R. Combination insulin‐sulfonylurea therapy. Diabetes Care 1990;13(6):667‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lewitt 1989 {published data only}
- Lewitt MS, Yu VK, Rennie GC, Carter JN, Marel GM, Yue DK, et al. Effects of combined insulin‐sulfonylurea therapy in type II patients. [see comments]. Diabetes Care 1989;12(6):379‐83. [DOI] [PubMed] [Google Scholar]
Liedtke 1990 {published data only}
- Liedtke RK SMM. Transdermal insulin application in type II diabetic patients. Results of a clinical pilot study. Arzneimittel‐Forschung Drug Research 1990;40(8):884‐86. [PubMed] [Google Scholar]
Lindstrom 1992 {published data only}
- Lindstrom T, Arnqvist HJ, Ludvigsson J, Schenck HH. C‐peptide profiles in patients with non‐insulin‐dependent diabetes mellitus before and during insulin treatment. Acta Endocrinologica 1992;126(6):477‐83. [DOI] [PubMed] [Google Scholar]
Lindstrom 1999 {published data only}
- Lindstrom T, Nystrom FH, Olsson AG, Ottosson AM, Arnqvist HJ. The lipoprotein profile differs during insulin treatment alone and combination therapy with insulin and sulphonylureas in patients with Type 2 diabetes mellitus. Diabetic Medicine 1999;16(10):820‐26. [DOI] [PubMed] [Google Scholar]
Lins 1988 {published data only}
- Lins PE, Lundblad S, Persson‐Trotzig E, Adamson U. Glibenclamide improves the response to insulin treatment in non‐insulin‐dependent diabetics with second failure to sulfonylurea therapy. Acta Medica Scandinavica 1988;223(2):171‐79. [DOI] [PubMed] [Google Scholar]
Longnecker 1986 {published data only}
- Longnecker MP, Elsenhans VD, Leiman SM, Owen OE, Boden G. Insulin and a sulfonylurea agent in non‐insulin‐dependent diabetes mellitus. Archives of Internal Medicine 1986;146(4):673‐76. [PubMed] [Google Scholar]
Lopez 1999 {published data only}
- Lopez‐Alvarenga JC, Aguilar‐Salinas CA, Velasco‐Perez ML, Arita‐Melzer O, Guillen LE, Wong B, et al. Acarbose vs. bedtime NPH insulin in the treatment of secondary failures to sulphonylurea‐metformin therapy in type 2 diabetes mellitus. Diabetes Obesity & Metabolism 1999;1(1):29‐35. [DOI] [PubMed] [Google Scholar]
Martin 1986 {published data only}
- Martin DB. Type II diabetes: insulin versus oral agents. New England Journal of Medicine 1986;314(20):1314‐15. [DOI] [PubMed] [Google Scholar]
Mauerhoff 1986 {published data only}
- Mauerhoff T, Ketelslegers JM, Lambert AE. Effect of glibenclamide in insulin‐treated diabetic patients with a residual insulin secretion. Diabete et Metabolisme 1986;12(1):34‐38. [PubMed] [Google Scholar]
Mezitis 1992 {published data only}
- Mezitis NH, Heshka S, Saitas V, Bailey TS, Costa R, Pi‐Sunyer FX. Combination therapy for NIDDM with biosynthetic human insulin and glyburide. Diabetes Care 1992;15(2):265‐69. [DOI] [PubMed] [Google Scholar]
Mohan 1990 {published data only}
- Mohan V, Snehalatha C, Ramachandran A, Viswanathan M. Combination therapy of glibenclamide and insulin in NIDDM patients with secondary failure to oral drugs. [see comments]. Journal of the Association of Physicians of India 1990;38(8):537‐41. [PubMed] [Google Scholar]
Nathan 1988 {published data only}
- Nathan DM, Roussell A, Godine JE. Glyburide or insulin for metabolic control in non‐insulin‐dependent diabetes mellitus. A randomized, double‐blind study. Annals of Internal Medicine 1988;108(3):334‐40. [DOI] [PubMed] [Google Scholar]
Niazi 1998 {published data only}
- Niazi R, Muzaffar Z. Comparison of bedtime NPH insulin or metformin combined with glibenclamide in secondary sulphonylurea failure in obese type II (NIDDM) patients. JPMA ‐ Journal of the Pakistan Medical Association 1998;48(11):336‐38. [PubMed] [Google Scholar]
Niskanen 1992 {published data only}
- Niskanen L, Lahti J, Uusitupa M. Morning or bed‐time insulin with or without glibenclamide in elderly type 2 diabetic patients unresponsive to oral antidiabetic agents. Diabetes Res Clin Pract 1992;18:185‐90. [DOI] [PubMed] [Google Scholar]
Okada 1996 {published data only}
- Okada S. The effect of an alpha‐glucosidase inhibitor and insulin on glucose metabolism and lipid profiles in non‐insulin‐dependent diabetes mellitus. Journal of International Medical Research 1996;24(5):438‐47. [DOI] [PubMed] [Google Scholar]
Osei 1984 {published data only}
- Osei K, O'Dorisio TM, Falko JM. Concomitant insulin and sulfonylurea therapy in patients with type II diabetes. Effects on glucoregulation and lipid metabolism. American Journal of Medicine 1984;77(6):1002‐09. [DOI] [PubMed] [Google Scholar]
Panahloo 1998 {published data only}
- Panahloo A, Mohamed Ali V, Andres C, Denver AE, Yudkin JS. Effect of insulin versus sulfonylurea therapy on cardiovascular risk factors and fibrinolysis in type II diabetes. Metabolism: Clinical & Experimental 1998;47(6):637‐43. [DOI] [PubMed] [Google Scholar]
Pasmantier 1990 {published data only}
- Pasmantier R, Chaiken RL, Hirsch SR, Lebovitz HE. Metabolic effects of combination glipizide and human proinsulin treatment in NIDDM. Diabetes Care 1990;13 Suppl 3:42‐46. [DOI] [PubMed] [Google Scholar]
Peacock 1984 {published data only}
- Peacock I, Tattersall RB. The difficult choice of treatment for poorly controlled maturity onset diabetes: tablets or insulin?. British Medical Journal Clinical Research Ed 1984;288(6435):1956‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polo 1998 {published data only}
- Polo V, Saibene A, Pontiroli AE. Nicotinamide improves insulin secretin and metabolic control in lean type 2 diabetic patients with secondary failure to sulphonylureas. Acta Diabetologica 1998;35(1):61‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ponssen 2000 {published data only}
- Ponssen HH, Elte JW, Lehert P, Schouten JP, Bets D. Combined metformin and insulin therapy for patients with type 2 diabetes mellitus. Clinical Therapeutics 2000;22(6):709‐18. [DOI] [PubMed] [Google Scholar]
Quatraro 1986 {published data only}
- Quatraro A, Consoli G, Ceriello A, Giugliano D. Combined insulin and sulfonylurea therapy in non‐insulin‐dependent diabetics with secondary failure to oral drugs: a one year follow‐up. Diabete et Metabolisme 1986;12(6):315‐18. [PubMed] [Google Scholar]
Raskin 2001 {published data only}
- Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin‐treated type 2 diabetes. Diabetes Care 2001;24(7):1226‐32. [DOI] [PubMed] [Google Scholar]
Reich 1987 {published data only}
- Reich A, Abraira C, Lawrence AM. Combined glyburide and insulin therapy in type II diabetes. Diabetes Research 1987;6(2):99‐104. [PubMed] [Google Scholar]
Relimpio 1998 {published data only}
- Relimpio F, Pumar A, Losada F, Mangas MA, Acosta D, Astorga R. Adding metformin versus insulin dose increase in insulin‐treated but poorly controlled Type 2 diabetes mellitus: an open‐label randomized trial. Diabetic Medicine 1998;15(12):997‐1002. [DOI] [PubMed] [Google Scholar]
Rivellese 2000 {published data only}
- Rivellese AA, Patti L, Romano G, Innelli F, Marino L, Annuzzi G, et al. Effect of insulin and sulfonylurea therapy, at the same level of blood glucose control, on low density lipoprotein subfractions in type 2 diabetic patients. Journal of Clinical Endocrinology & Metabolism 2000;85(11):4188‐92. [DOI] [PubMed] [Google Scholar]
Robinson 1998 {published data only}
- Robinson AC, Burke J, Robinson S, Johnston DG, Elkeles RS. The effects of metformin on glycemic control and serum lipids in insulin‐treated NIDDM patients with suboptimal metabolic control. Diabetes Care 1998;21(5):701‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodier 1995 {published data only}
- Rodier M. Effects of insulin therapy upon plasma lipid fatty acids and platelet aggregation in NIDDM with secondary failure to oral antidiabetic agents. Diabetes Research and Clinical Practice 1995;28(1):19‐28. [DOI] [PubMed] [Google Scholar]
Romano 1997 {published data only}
- Romano G, Patti L, Innelli F, Marino L, Annuzzi G, Iavicoli M, et al. Insulin and sulfonylurea therapy in NIDDM patients. Are the effects on lipoprotein metabolism different even with similar blood glucose control?. Diabetes 1997;46:1601‐6. [DOI] [PubMed] [Google Scholar]
Rosak 1985 {published data only}
- Rosak C, Schwarz O, Althoff PH, Schoffling K, Schmidt FH. [Combined treatment of type‐2 diabetics with insulin and glibenclamide after secondary drug failure. Double‐blind, insulin‐placebo‐controlled crossover study]. [German]. Deutsche Medizinische Wochenschrift 1985;110(51‐52):1975‐80. [DOI] [PubMed] [Google Scholar]
Samanta 1987 {published data only}
- Samanta A, Burden AC, Kinghorn HA. A comparative study of sulphonylurea and insulin therapy in non insulin dependent diabetics who had failed on diet therapy alone. Diabetes Research 1987;4:183‐5. [PubMed] [Google Scholar]
Sanchez 1999 {published data only}
- Sanchez‐Barba Izquierdo MI, Ibarra Rueda JM, Ruiz de Adana PR. [The combination of insulin and metformin in obese patients with type‐2 diabetes mellitus]. [Spanish]. Atencion Primaria 1999;24(8):462‐67. [PubMed] [Google Scholar]
Sane 1992 {published data only}
- Sane T, Helve E, Yki‐Järvinen H, Taskinen MR. One‐year response to evening insulin therapy in non‐insulin‐ dependent diabetes. Journal of Internal Medicine 1992;231:253‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sangiorgio 1996 {published data only}
- Sangiorgio L, Rabuazzo MA, Cordaro G, Grasso G, Condorelli L, Lunetta M. [Comparative study of the efficiency of ultralente insulin and NPH insulin combined with sulfonylurea in type 2 diabetes patients with secondary tolerance to sulfonylurea. Possible selection criteria]. Minerva Endocrinol 1996;21(2):47‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Schade 1987 {published data only}
- Schade DS, Mitchell WJ, Griego G. Addition of sulfonylurea to insulin treatment in poorly controlled type II diabetes. A double‐blind, randomized clinical trial. JAMA 1987;257(18):2441‐45. [PubMed] [Google Scholar]
Schwartz 1997 {published data only}
- Schwartz SL, Fischer JS, Kipnes MS, Boyle M. Effects of short‐term insulin therapy upon therapeutic response to glipizide. American Journal of Medicine 1987;83(3A):22‐30. [DOI] [PubMed] [Google Scholar]
Schwartz 1998 {published data only}
- Schwartz S, Raskin P, Fonseca V, Graveline JF. Effect of troglitazone in insulin‐treated patients with type II diabetes mellitus. Troglitazone and Exogenous Insulin Study Group. [see comments]. New England Journal of Medicine 1998;338(13):861‐66. [DOI] [PubMed] [Google Scholar]
Simonson 1987 {published data only}
- Simonson DC, Delprato S, Castellino P, Groop L, DeFronzo RA. Effect of glyburide on glycemic control, insulin requirement, and glucose metabolism in insulin‐treated diabetic patients. Diabetes 1987;36(2):136‐46. [DOI] [PubMed] [Google Scholar]
Simpson 1990 {published data only}
- Simpson HC, Sturley R, Stirling CA, Reckless JP. Combination of insulin with glipizide increases peripheral glucose disposal in secondary failure type 2 diabetic patients. Diabetic Medicine 1990;7(2):143‐47. [DOI] [PubMed] [Google Scholar]
Sinagra 1998 {published data only}
- Sinagra D, Scarpitta AM, Amato M. Effects of insulin‐oral hypoglycemic agents combined therapy in outpatients with type 2 diabetes. European Review for Medical & Pharmacological Sciences 1998;2(5‐6):175‐79. [PubMed] [Google Scholar]
Soneru 1993 {published data only}
- Soneru IL, Agrawal L, Murphy JC, Lawrence AM, Abraira C. Comparison of morning or bedtime insulin with and without glyburide in secondary sulfonylurea failure. Diabetes Care 1993;16(6):896‐901. [DOI] [PubMed] [Google Scholar]
Sotaniemi 1990 {published data only}
- Sotaniemi EA, Vierimaa E, Huupponen R, Karvonen I, Vuoti MJ, Rytomaa K. Insulin and sulphonylurea in the therapy of type 2 diabetes. Diabetes Research and Clinical practice 1990;8:243‐51. [DOI] [PubMed] [Google Scholar]
Standl 1999 {published data only}
- Standl E, Baumgartl HJ, Fuchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obesity & Metabolism 1999;1(4):215‐20. [DOI] [PubMed] [Google Scholar]
Stenman 1988 {published data only}
- Stenman S, Groop PH, Saloranta C, Totterman KJ, Fyhrqvist F, Groop L. Effects of the combination of insulin and glibenclamide in type 2 (non‐insulin‐dependent) diabetic patients with secondary failure to oral hypoglycaemic agents. Diabetologia 1988;31(4):206‐13. [DOI] [PubMed] [Google Scholar]
Stocks 1988 {published data only}
- Stocks AE MAH. Lack of effect of glibenclamide on insulin requirements and diabetic control in persons with insulin‐dependent diabetes. Medical Journal of Australia 1988;149(9):472‐73. [DOI] [PubMed] [Google Scholar]
Stradner 1990 {published data only}
- Stradner F, Pieber T, Toplak H, Schreiber U, Pfeiffer KP. [Insulin‐sulfonylurea combination therapy in secondary therapy failure with sulfonylurea compounds. Randomized study between evening and morning intermediary insulin administration using the Novo Pen semi‐ automatic insulin injector]. Schweizerische medizinische Wochenschrift 1990;120(27‐28):989‐94. [MEDLINE: ] [PubMed] [Google Scholar]
Thompson 1998 {published data only}
- Thompson RG, Pearson L, Schoenfeld SL, Kolterman OG. Pramlintide, a synthetic analog of human amylin, improves the metabolic profile of patients with type 2 diabetes using insulin. The Pramlintide in Type 2 Diabetes Group. Diabetes Care 1998;21(6):987‐93. [DOI] [PubMed] [Google Scholar]
Tovi 1998 {published data only}
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes & Metabolism 1998;24(5):442‐47. [PubMed] [Google Scholar]
Trischitta 1992 {published data only}
- Trischitta V, Italia S, Mazzarino S, Buscema M, Rabuazzo AM, Sangiorgio, L, et al. Comparison of combined therapies in treatment of secondary failure to glyburide. Diabetes Care 1992;15(4):539‐42. [DOI] [PubMed] [Google Scholar]
Trischitta 1998 {published data only}
- Trischitta V, Italia S, Raimondo M, Guardabasso V, Licciardello C, Runello F, et al. Efficacy of combined treatments in NIDDM patients with secondary failure to sulphonylureas. Is it predictable?. Journal of Endocrinological Investigation 1998;21(11):744‐47. [DOI] [PubMed] [Google Scholar]
Trznadel 1997 {published data only}
- Trznadel‐Morawska I, Malecki M, Sieradzki J. [Estimation of different models of insulin therapy in noninsulin‐dependent diabetes mellitus]. Przeglad lekarski 1997;54(5):308‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Turner 1999 (2) {published data only}
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999;281(21):2005‐12. [DOI] [PubMed] [Google Scholar]
UKPDS 13 1995 {published data only}
- United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non‐insulin dependent diabetes followed for three years. [see comments]. BMJ 1995;310(6972):83‐88. [PMC free article] [PubMed] [Google Scholar]
UKPDS 24 1998 {published data only}
- United Kingdom Prospective Diabetes Study 24: a 6‐year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. [see comments]. Annals of Internal Medicine 1998;128(3):165‐75. [DOI] [PubMed] [Google Scholar]
UKPDS 33 1998 {published data only}
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. [see comments]. [erratum appears in Lancet 1999 Aug 14;354(9178):602]. Lancet 1998;352(9131):837‐53. [PubMed] [Google Scholar]
Vigneri 1991 {published data only}
- Vigneri R, Trischitta V, Italia S, Mazzarino S, Rabuazzo MA, Squatrito, S. Treatment of NIDDM patients with secondary failure to glyburide: comparison of the addition of either metformin or bed‐time NPH insulin to glyburide. Diabete et Metabolisme 1991;17(1 Pt 2):232‐34. [PubMed] [Google Scholar]
Wolffenbuttel 1989 {published data only}
- Wolffenbuttel BH, Weber RF, Koetsveld PM, Weeks L, Verschoor L. A randomized crossover study of sulphonylurea and insulin treatment in patients with type 2 diabetes poorly controlled on dietary therapy. Diabetic Medicine 1989;6:520‐25. [DOI] [PubMed] [Google Scholar]
Yki‐Jarvinen 2000 {published data only}
- Yki‐Jarvinen H, Dressler A, Ziemen M, HOE 901/300s Study, Group. Less nocturnal hypoglycemia and better post‐dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 2000;23(8):1130‐36. [DOI] [PubMed] [Google Scholar]
Yu 1999 {published data only}
- Yu JG, Kruszynska YT, Mulford MI, Olefsky JM. A comparison of troglitazone and metformin on insulin requirements in euglycemic intensively insulin‐treated type 2 diabetic patients. Diabetes 1999;48(12):2414‐21. [DOI] [PubMed] [Google Scholar]
Yudkin 2000 {published data only}
- Yudkin JS, Panahloo A, Stehouwer C, Emeis JJ, Bulmer K, Mohamed‐Ali V, Denver AE. The influence of improved glycaemic control with insulin and sulphonylureas on acute phase and endothelial markers in Type II diabetic subjects. Diabetologia 2000;43(9):1099‐1106. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Goudswaard 2004 {published data only}
- Goudswaard AN, Stolk RP, Zuithoff P, Valk HW, Rutten GE. Starting insulin in type 2 diabetes: Continue oral hypoglycemic agents? A randomized trial in primary care. Journal of Family Practice 2004;53(5):393‐399. [PubMed] [Google Scholar]
Olsson 2002 {published data only}
- Olsson PO, Lindstrom T. Combination‐therapy with bedtime nph insulin and sulphonylureas gives similar glycaemic control but lower weight gain than insulin twice daily in patients with type 2 diabetes. Diabetes Metab 2002;28(4 Pt 1):272‐277. [PubMed] [Google Scholar]
Stehouwer 2003 {published data only}
- Stehouwer MH, DeVries JH, Lumeij JA, Ader HJ, Engbers AM, Iperen AA, Snoek FJ, Heine RJ. Combined bedtime insulin‐daytime sulphonylurea regimen compared with two different daily insulin regimens in type 2 diabetes: effects on HbA1c and hypoglycaemia rate‐a randomised trial. Diabetes Metab Res Rev 2003;19(2):148‐152. [DOI] [PubMed] [Google Scholar]
Additional references
ADA 1997
- American Diabetic Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183‐97. [DOI] [PubMed] [Google Scholar]
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1999;22(Suppl 1):S5‐19. [DOI] [PubMed] [Google Scholar]
Agurs 1997
- Agurs Collins TD, Kumanyika SK, Have TR, Adams Campbell LL. A randomized controlled tria of weight reduction and exercise for diabetes management in older African‐American subjects. Diabetes Care 1997;20(10):1503‐11. [DOI] [PubMed] [Google Scholar]
Armitage 2002
- Armitage P, Berry G, Matthews JNS. Statistical methods in medical research, par. 5.3. 4th Edition. Blackwell Science, 2002:paragraph 5.3. [Google Scholar]
Bosello 1997
- Bosello O, Armellini F, Zamboni M, Fitchet M. The benefits of modest weight loss in type II diabetes. International Journal of Obesity and related Metabolic disorders 1997;21 Suppl 1:S10‐3. [PubMed] [Google Scholar]
Burgers 2002
- Burgers JS, Bailey JV, Klazinga NS, Bij AK, Grol R, Feder G. Inside guidelines: comparative analysis of recommendations and evidence in diabetes guidelines from 13 countries. Diabetes Care 2002;25(11):1933‐39. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and psychological measurement 1960;20:37‐46. [Google Scholar]
DCCT 1993
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine 1993;329:977‐86. [DOI] [PubMed] [Google Scholar]
de Sonnaville 1998
- Sonnaville de JJ, Snoek FJ, Colly LP, Deville W, Wijkel D, Heine RJ. Well‐being and symptoms in relation to insulin therapy in type 2 diabetes. Diabetes Care 1998;21(6):919‐24. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled clinical trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garber 2003
- Garber AJ. Benefits of combination therapy of insulin and oral hypoglycemic agents. Archives of internal medicine 2003;163(15):1781‐82. [DOI] [PubMed] [Google Scholar]
Goddijn 1999
- Goddijn PP, Bilo HJ, Feskens EJ, Groenier KH, Zee KI, Meyboom‐de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabetic Medicine 1999;16(1):23‐30. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Johnson 1996
- Johnson JL, Wolf SL, Kabadi UM. Efficacy of insulin and sulfonylurea combination therapy in type II diabetes. A meta‐analysis of the randomized placebo‐controlled trials. Archives of internal medicine 1996;156(3):259‐64. [PubMed] [Google Scholar]
Lau 1997
- Lau J, Loannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. Annals of internal medicine 1997;127:820‐6. [DOI] [PubMed] [Google Scholar]
Little 1986
- Little RR, England JD, Wiedmeyer HM, McKenzie EM, Mitra R, Erhart PM, Durham JB, Goldstein DE. Interlaboratory standardization of glycated hemoglobin determinations. Clinical chemistry 1986;32(2):358‐60. [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. updated September 1997. Vol. 4, Oxford: Update Software, 1997. [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
Peters 1991
- Peters AL, Davidson MB. Insulin plus a sulfonylurea agent for treating type 2 diabetes. Annals of internal medicine 1991;115(1):45‐53. [DOI] [PubMed] [Google Scholar]
Pugh 1992
- Pugh‐JA, Wagner‐ML, Sawyer‐J, Ramirez‐G, Tuley‐M, Friedberg‐SJ. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A metaanalysis [see comments]. Diabetes Care 1992;15:953‐9. [DOI] [PubMed] [Google Scholar]
Riddle 2002
- Riddle MC. Timely addition of insulin to oral therapy for type 2 diabetes. Diabetes Care 2002;25(2):395‐96. [DOI] [PubMed] [Google Scholar]
Riddle 2003
- Riddle MC, Rosenstock J, Gerich J. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26(11):3080‐86. [DOI] [PubMed] [Google Scholar]
Rosenthal 1994
- Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges HV editor(s). The handbook of research synthesis. New York: Sage Foundation, 1994:231‐44. [Google Scholar]
Ruige 1998
- Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta‐analysis. Circulation 1998;97(10):996‐1001. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Stout 1990
- Stout RW. Insulin and atheroma. 20‐yr perspective. Diabetes Care 1990;13(6):631‐54. [DOI] [PubMed] [Google Scholar]
Turner 1999
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999;281(21):2005‐12. [DOI] [PubMed] [Google Scholar]
UKPDS 33
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
van der Does 1996
- Does van der FE, Neeling JN, Snoek FJ, Kostense PJ, Grootenhuis PA, Bouter LM, Heine RJ. Symptoms and well‐being in relation to glycemic control in type II diabetes. Diabetes Care 1996;19(3):204‐10. [DOI] [PubMed] [Google Scholar]
Van Tulder 1997
- Tulder MW, Assendelft WJJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine 1997;22:2323‐30. [DOI] [PubMed] [Google Scholar]
Westphal 2003
- Westphal SA, Palumbo PJ. Insulin and oral hypoglycemic agents should not be used in combination in the treatment of type 2 diabetes. Archives of internal medicine 2003;163(15):1783‐85. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. Geneva. WHO, 1980. [PubMed]
WHO 1985
- WHO Expert Committee on Diabetes Mellitus. World Health Organization, 1985. Technical Report Series 727. [PubMed]
WHO 1999
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization, 1999:1‐59. [MEDLINE: ] [Google Scholar]
Yki‐Järvinen 2000
- Yki‐Järvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post‐dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 2000;23(8):1130‐36. [DOI] [PubMed] [Google Scholar]
Yki‐Järvinen 2001
- Yki‐Järvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care 2001;24(4):758‐67. [DOI] [PubMed] [Google Scholar]
Zavaroni 1989
- Zavaroni I, Bonora E, Pagliara M, Dall'Aglio E, Luchetti L, Buonanno G, et al. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. The New England Journal of Medicine 1989;320(11):702‐6. [DOI] [PubMed] [Google Scholar]


