Abstract
Background:
The association between vitamin D status and breast cancer risk is equivocal. No systematic reviews or meta-analyses have examined this association stratified by receptor status. Our objective is to conduct a systematic review to answer the question, “Is there a relationship between lower serum/plasma vitamin D levels and increased risk of triple negative breast cancer (TNBC) specifically?”
Methods:
We systematically searched Embase and PubMed databases for published original research studies examining the risk of a breast cancer diagnosis according to vitamin D status. We excluded studies that did not provide risk estimates stratified by receptor status.
Results:
Fourteen studies met our criteria, including case-control, nested case-control, and case-series studies, reflecting the cumulative results of 13,135 breast cancer cases. When grouped by relevancy to TNBC, the proportion of analyses across all study types showing a significant association between vitamin D status and breast cancer diagnosis was 37% for non-TNBC analyses, 48% for analyses that included some TNBC cases, and 88% for TNBC analyses.
Conclusions:
Our results suggest that low vitamin D status may particularly increase the risk of TNBC, although more research is needed to determine if this association is causative. Women should be routinely screened for 25(OH)D deficiency.
Introduction
One out of every eight women in the United States will develop invasive breast cancer over the course of her lifetime. Breast cancer is the most common cancer among women, excluding skin cancers, and the second leading cause of cancer death (1). In particular, triple negative breast cancer (TNBC) is a subtype that carries the worst prognosis of all breast cancer diagnoses. The incidence of TNBC is higher in younger women, women of African or Hispanic descent, and women with the BRCA1 genetic variant (2). TNBC is less likely to be found on a mammogram, more likely to be aggressive, and lacks targeted therapies—all of which contribute to its poorer prognosis (2). Identifying modifiable risk factors for TNBC is an important step toward reducing its prevalence.
In 2011, the St Gallen International Breast Cancer Conference Expert Panel adopted a classification system for breast cancer based on intrinsic biologic subtypes, which continues to be used for recommending treatment protocols (3,4). Summarized in Table 1, the groupings include four intrinsic subtypes: Luminal A, Luminal B, HER2 overexpression, and Basal-like (3). The immunohistochemistry markers for estrogen receptor (ER), progesterone receptor (PR), Human epidermal growth factor receptor 2 (HER2), and Ki-67 (a proliferation index) are used as prognostic indicators and to approximate the intrinsic subtype (5).
Table 1.
Breast Cancer Classification.a
| Intrinsic Subtype | Clinico-pathologic definition | Immunohistochemistry Status |
Prognosisc | ||
|---|---|---|---|---|---|
| ER and/or PR | HER2 overexpression | Ki-67 | |||
|
| |||||
| Luminal A | Luminal A | Positive | No | Low | Good |
| Luminal B | Luminal B (HER2 negative) | Positive | No | High | Intermediate |
| Luminal B (HER2 positive) | Positive | Yes | Any | Poor | |
| HER2 overexpressionb | HER2 positive (nonluminal) | Absent | Yes | Not defined | Poor |
| Basal-like | Triple negative (ductal) | Absent | No | Not defined | Very Poor |
Table adapted from St Gallen International Expert Consensus Panel (3).
Listed in original source as "Erb-B2 overexpression".
Prognoses adapted from Dai 2015 (5).
TNBC is characterized by the absence of ER and PR receptors and normal HER2 expression. Up to 90% of incident basal-like breast cancers are TNBC (6) and approximately 86% of TNBC diagnoses are basal-like (4). “Basal-like” and “triple-negative” are often used interchangeably because of the high degree of overlap between these classifications.
Preclinical data suggest that basal-like cancers arise from different cell types of origin, compared to other types of breast cancer. Basal-like cancers are characterized by a high expression of keratins and genes related to proliferation, intermediate expression of HER2 genes, and low expression of genes related to luminal cancers (4).
Vitamin D, a fat-soluble vitamin, can be obtained via two routes. Ultraviolet-B rays from the sun react with 7-dehydrocholesterol in the skin to form vitamin D3 and vitamin D3 is absorbed from dietary intake and supplements. In the liver, the 25-hydroxylase enzyme converts vitamin D3 to 25-hydroxyvitamin D3 (25(OH)D). Primarily in the kidneys but also in the breast and other tissues, the 1α-hydroxylase enzyme converts 25(OH)D to the biologically active 1,25-hydroxyvitamin D3 (1,25 (OH)D) (7). The half-life of plasma l,25(OH)D is only 4–6 hours. In contrast, the half-life of plasma 25(OH)D is 2–3 weeks which makes it a more useful clinical measure of vitamin D status (8).
Among the many functions of vitamin D are its modulation of cell proliferation, angiogenesis, cell differentiation, and apoptosis (9). These antineoplastic properties have prompted research into the relationship between 25 (OH)D status and the risk of breast cancer. Several molecular signaling pathways, including Notch, Hedgehog, Wnt/B-catenin, and TGF-B, are implicated in promoting the growth of TNBC (2). For all of these pathways, gene expression is modulated by vitamin D (10).
Two recent meta-analyses have examined the relationship between 25(OH)D and overall breast cancer risk. One of these meta-analyses found an overall significant association, and the other did not (11,12). Thus, the question as to whether lower 25(OH)D status increases the risk of breast cancer continues to lack consensus. No systematic reviews or meta-analyses have examined if the association between 25(OH)D and breast cancer risk varies with receptor status. The present hypothesis is that the risk of developing TNBC, in particular, is significantly increased with low 25(OH)D status. Thus, our objective was to conduct a systematic review of published original research studies that specifically report the association between 25(OH)D and breast cancer with risk estimates stratified by receptor status.
Methods
Search Strategy
We conducted a systematic review following PRISMA guidelines (13). The first author (JT) searched the Embase database on May 29th, 2017 using the search terms “(25 hydroxyvitamin D OR vitamin D) AND (breast cancer OR breast tumor)” and PubMed on June 3rd, 2017, using the MeSH terms “Vitamin D” AND “Breast Neoplasms.” The first author combined the results from the two searches and removed duplicate titles.
Inclusion and Exclusion Criteria
We included original research studies that reported the association between serum/plasma 25(OH)D and breast cancer diagnosis when the risk estimates were reported separately by receptor status or intrinsic subtype. We excluded studies published before January 1, 2007 because receptor status was rarely reported prior to this date. We also excluded studies that: 1) were not original research reports, 2) were not written in English, 3) were predominately focused on male breast cancer or juvenile breast cancer, 4) did not base vitamin D exposure on measured values of 25(OH)D in serum or plasma, 5) evaluated free 25(OH)D instead of total 25(OH)D (as results would not be comparable).
Exposure Assessment
Reporting units.
Individual studies reported 25(OH)D levels in either ng/mL or nmol/L. We converted values reported in nmol/L to ng/mL by dividing by a conversion factor of 2.5.
Star rating of exposure.
We developed a 5-point star rating system to gauge the quality of assessment of 25 (OH)D status. We awarded one star for each of the following: 1) listing the quantile or cut-point values for categories of 25(OH)D, 2) accounted for the month or season of 25(OH)D assessment, 3) obtained the blood sample for 25(OH)D assay prior to the initiation of any cancer treatment, 4) 25(OH)D assessed from blood samples obtained at multiple points in time, and 5) 25 (OH) D was assayed using a chromatography method, or if assayed using an immunoassay method, the intra-assay coefficient of variation (CV) was reported and less than 10% for all batches. The two main methodologies for assessing 25(OH)D status are chromatography and competitive binding assays. The isotope-dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) is commonly used as a comparative standard and is generally considered the method with highest attainable accuracy. Chromatography methods can distinguish between D2 and D3, whereas most binding assays cannot (14).
Lower limit of detection (LOD).
Most studies did not address the LOD for 25(OH)D, thus we contacted the corresponding author for each study via email and asked if a lower limit of detection was established and how they handled cases that fell below the limit. Authors who did not respond to the initial contact were each contacted one additional time to solicit a response.
Data Extraction
We extracted the adjusted risk ratio point estimate and its 95% confidence interval of the lowest reported 25 (OH)D category versus the highest for all breast cancer diagnosis types reported in each study. To aid in visual representation across studies, we created three forest plots to summarize the results according to study design: case-control, nested case-control, or case-series. Within each forest plot, we grouped the results by TNBC relevance: 1) diagnoses that do not include TNBC, 2) diagnoses that partially represent TNBC, and 3) diagnoses that are TNBC. See Table 2 for a summary of the receptor combinations and intrinsic sub-types that were included in each TNBC relevancy group. The results were then ordered by increasing risk ratio point estimate. In constructing the forest plots, we transposed the results that were expressed with the lowest 25(OH)D category as the reference so that all data are presented in a uniform way. Regarding group 2 (diagnoses that partially represent TNBC), we could not definitively classify all results as TNBC or not TNBC because sometimes only the status for one or two receptor biomarkers was considered in the analysis. For example, if results were reported for cases with ER−/PR− cancer, some of these cancers will be TNBC and some will be HER2-enriched, hence the intermediate classification. For the purposes of this study, we assumed all basal cancers are TNBC.
Table 2.
Grouping and classification of breast cancer diagnoses.
| TNBC relevancya | Intrinsic subtypesb | Receptor statusb |
|---|---|---|
|
| ||
| Does not include TNBC diagnoses | Luminal A | ER+ |
| Luminal B | PR+ | |
| HER2-enriched | ER+ and PR+ ER+ and PR+ and HER2− ER+ and PR+ and HER2+ HER2+ |
|
|
| ||
| May include TNBC diagnosesc | ER− PR− ER− and PR− HER2− |
|
|
| ||
| TNBC diagnoses | Basald | ER−PR−HER2− |
Abbreviations: ER – Estrogen Receptor, PR – Progesterone Receptor, TNBC – Triple Negative Breast Cancer, HER2 – Human Epidermal growth factor Receptor 2.
Breast cancer diagnoses grouping according to relevancy of TNBC.
Ways in which breast cancer diagnoses were categorized in results included in this review.
Diagnoses which did not report all three receptor statuses (ER, PR, HER2) prevent definitive determination of TNBC status.
Cancers identified as basal are assumed to be TNBC.
Bias
We evaluated studies for selection bias and confounding. To evaluate the potential for selection bias, we noted the criteria for selecting controls and examined if studies matched for age, race/ethnicity, menopausal status, use of hormone replacement therapy, and season or date of blood sample donation. We also noted studies which excluded cases or controls with a history of any cancer or history of breast cancer as part of their selection criteria. To evaluate adequacy of addressing confounding, we examined if the variables that are associated with both breast cancer and 25(OH)D status were considered in the final adjusted models. Figure 1 is a Venn diagram of well-established factors associated with breast cancer and 25(OH)D deficiency. The factors shared by both conditions include body mass index (BMI), menopausal status, age, and race/ethnicity (7,15). Thus, we particularly examined if studies took into consideration potential confounding by these four variables.
Figure 1.
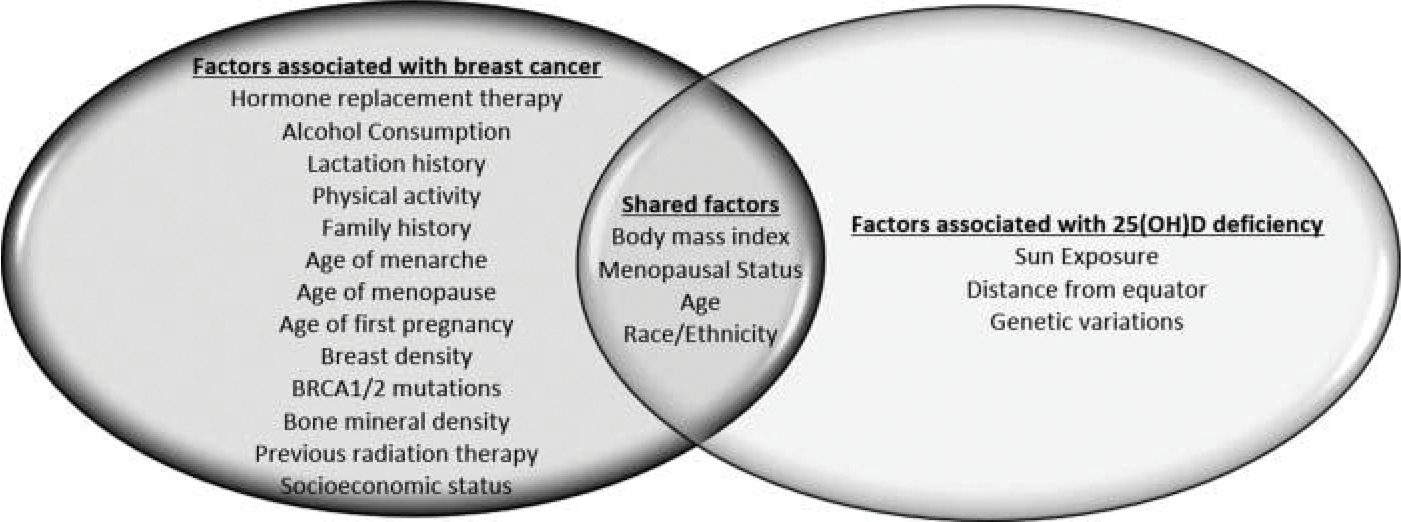
Venn diagram of risk factors.
Results
Literature Search
Figure 2 summarizes the results of the literature search. Searching Embase and PubMed returned 634 and 587 articles, respectively. After we removed duplicates, 656 articles published within the last 10 years remained. The first author excluded 609 articles that were clearly not relevant to the research question based on title and abstract content. The first author then examined 47 full text articles for eligibility, consulting the senior author for clarification as required, and excluded 34 for the following reasons: risk estimates were not stratified by receptor status or intrinsic subtype (n = 24), no risk estimates were reported at all (n = 8) or not for 25(OH)D specifically (n = 1), and free 25(OH)D rather than total 25(OH)D was evaluated (n = 1). This yielded 13 eligible studies (16–28). We identified one additional study via hand searching (29). Thus, we included 14 original research studies published between 2009 and 2016 in this review. The papers identified for the systematic review are summarized in Table 3.
Figure 2.
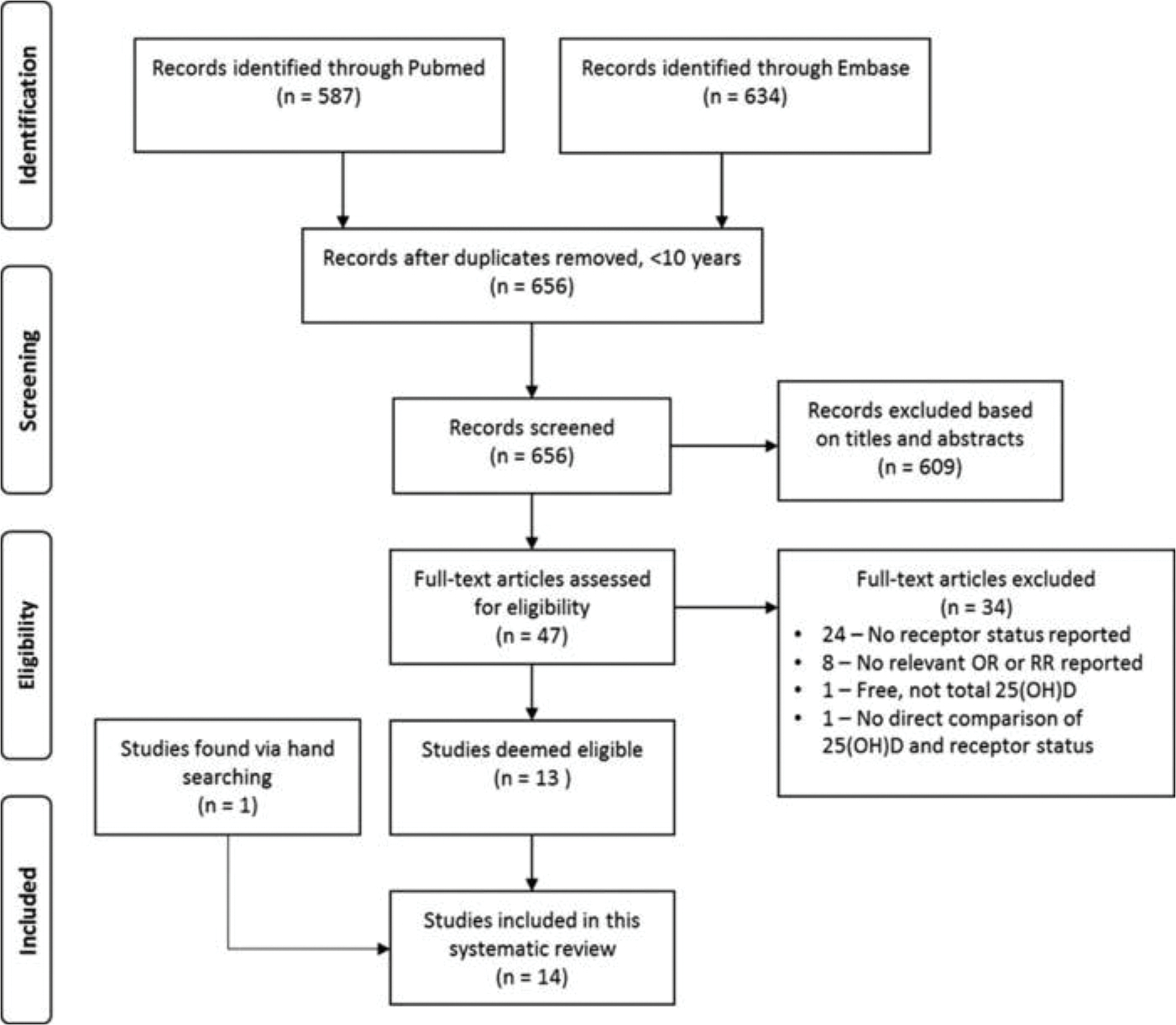
Flowchart for study selection.
Table 3.
Summary of study and participant characteristics.
| Authors | Design | Geographic region | Cohort or participant source | Cases | Controls | Statistical covariates |
|---|---|---|---|---|---|---|
|
| ||||||
| Abbas – 2009 | NCC | Germany | 38 hospitals in southern Germany |
N = 289 German speaking women aged 30–50, diagnosed with breast cancer by age 50 (premenopausal) between January 1992 and December 1995 |
N = 595 Selected from random list of residents. 2 controls matched to each case on age and study region. |
Age, season of blood collection, number of births, family history, age at menarche, duration of breast-feeding, BMI, alcohol consumption Not significant: Age at first birth, smoking, education |
| Abulkhair – 2015 | CS | Saudi Arabia | Oncology Department of King Abdulaziz Medical City |
N = 406 (404 women, 2 men) Newly diagnosed cases of breast cancer. 87% Saudi nationality, median age 48.5 |
N/A | None |
| Crew – 2009 | NCC | New York | Long Island Breast Cancer Study Project |
N = 1026 English speaking women, average age 58.6, 94.4% white, living in Nassau and Suffolk counties, diagnosed with in situ or invasive cancer between August 1996 and July 1997 |
N = 1075 Frequency matched to the expected age distribution of case women by 5 year age groups. <65 years identified by random digit dialing. ≥65 identified from Health Care Financing Administration records. |
Age, race, BMI, season of blood draw |
| Eliassen – 2011 | NCC | US | Nurses Health Study II |
N = 613 Cancer free cohort members from US women aged 32–54 who provided blood and urine samples between 1996–1999 and later developed breast cancer |
N = 1218 2 controls matched to each case on age, menopausal status at diagnosis, ethnicity, luteal day, time of day and fasting status for blood draw |
Age at menarche, BMI at age 18, parity, age at first birth, BMI at blood collection, family history, history of benign breast disease, age at blood collection, season, fasting status at blood collection, time of blood collection, luteal day, race, menopausal status |
| Eliassen − 2016 | NCC | US | Nurses Health Study |
N = 1506 Cancer free cohort members from US women aged 43–69 who donated blood samples from 1989–1990 and/or 2000–2002 and later developed breast cancer |
N = 1506 One control for each case matched for age at donation, menopausal status and HRT use at blood collection and diagnosis, month, time of day and fasting status of blood collection |
Fasting status, time of blood draw, age at blood draw, menopausal status and HRT, age at menarche, BMI at age 18, weight change from age 18 to blood draw, parity and age at first birth, family history of breast cancer, history of benign breast disease. Stratified by season. |
| Kuhn – 2013 | NCC | Denmark, France, Germany, Greece, Italy, Norway, Spain, Sweden, The Netherlands, UK | European Prospective Investigation into Cancer and Nutrition (EPIC) – Includes cases from French E3N cohort, excludes Malmo Diet and Cancer Study participants |
N = 1391 Women from the EPIC cohort diagnosed with breast cancer, but no previous cancer. Selection targeted premenopausal women and equal numbers of ER+ and ER− postmenopausal women. |
N = 1391 No history of any cancer, matched for study center, age, menopausal status, oral contraceptive or HRT use at blood draw, time of day of blood collection, fasting status, phase of menstrual cycle |
BMI, age at menarche, age at first full-term pregnancy, number of full-term pregnancies, breastfeeding, alcohol consumption, smoking status, education level, physical activity |
| McCullough – 2009 | NCC | US | Cancer Prevention Study II (CPS-II) |
N = 516 Postmenopausal women from the CPS-II cohort who reported a new incidence of breast cancer on a biennial follow up in June 2005. Age range 47–85, 96.7% white women. |
N = 516 Matched on date of birth, race/ethnicity, date of blood collection |
Adjusted for birth year, year of blood draw, race, season, parity and age at first birth, BMI at blood collection, weight change from age 18 to blood collection |
| Park – 2015 | CC | Korea | Cases: Breast Cancer Center registry, Yonsei University College of Medicine Controls: KNHANES IV-2 -KNHANES V-3 |
N = 3634 Korean women, average age 50.7, treated for breast cancer between January 2006 and December 2012 |
N = 17,133 Matching criteria not stated |
Age, residential regions, BMI, marriage status, age at menarche, menopausal status, age at first birth, oral contraceptives, HRT Age, laboratory, race, month of blood draw |
| Peppone – 2012 | CC/CS | US | University of Rochester Medical Center |
N = 194 US women with no previous cancer history diagnosed with breast cancer between January 2009 and November 2010 |
N = 194 Individuals with no history of cancer who had 25(OH)D measured for the first time between January 2009 and October 2010. Matched on age and date of blood collection |
|
| Rejnmark – 2009 | NCC | Denmark | Women who received a diagnostic mammogram between May 2003 and July 2007 in Denmark |
N = 142 Women, average age 58, from cohort who developed in situ or invasive breast cancer during the follow-up period and had no previous history of breast cancer. |
N = 420 3 controls per case, when possible, with no history of breast cancer. Women matched on age, menopausal status, and season of blood sampling. |
None stated |
| Scarmo – 2013 | NCC | New York and Sweden | New York University Women’s Health Study (NYUWHS) and Sweden Mammary screening Cohort (NSMSC) |
N = 1585 Women who were part of the NYUWHS cohort who developed breast cancer before January 2007 or part of the NSMSC cohort, who developed breast cancer before January 2010. Average age 58, 90% Caucasian, 6% African American. |
N = 2940 2 controls per case, matched on age, date of blood donation, number and dates of subsequent blood donations. Additionally, NYUWHS cases were also matched for menopausal status and race/ethnicity |
Age at menarche, family history, age at first birth/parity/nulliparous, BMI, HRT, alcohol consumption |
| Shirazi – 2016 | NCC | Sweden | Malmo Diet and Cancer Study (MDCS) |
N = 764 Women, average age 57, from MDCS diagnosed with breast cancer before December 31, 2006 |
N = 764 Matched on calendar time at inclusion, menopausal status, age at inclusion, and time of year of blood donation. |
Age at baseline, month and year at baseline, menopausal status, HRT, socio-economic index |
| Yao – 2011 | CC/CS | US | Data Bank and Biorepository at Roswell Park Cancer Institute (RPCI) |
N = 579 Non-Hispanic white women with no previous history of cancer, enrolled from 2003–2008 |
N = 574 Family and friends of RCPI patients and visitors, but not family and friends of cases. Matched on age and month of blood draw. |
Age at diagnosis, BMI. Stratified by menopausal status. Not significant: Physical activity |
| Yao – 2014 | CS | US | Data Bank and Biorepository at Roswell Park Cancer Institute (RPCI) |
N = 490 Non-Hispanic white women, average age 56.4, with no previous history of cancer, diagnosed between December 2003 and June 2009 |
N/A | Age at diagnosis, specimen storage time, season of blood draw, timing of blood draw in relation to receipt of treatment, tumor stage. Stratified by menopausal status. Not significant: family history, BMI, tumor grade, smoking status |
Abbreviations: NCC – Nested case-control, CC – Case-control, CS – Case series, BMI – Body Mass Index, HRT – Hormone Replacement Therapy, BMI – Body Mass Index, ER – Estrogen Receptor, PR – Progesterone Receptor, TNBC – Triple Negative Breast Cancer, HER2 – Human Epidermal growth factor Receptor 2.
Exposure Assessment
Star Rating
Exposure assessment results, including 25(OH)D cut-points, lower level of detection, and star rating criteria, are summarized in Table 4. All studies, except for Scarmo 2013, reported the 25(OH)D value that corresponds to the cut-point for each quantile or a priori-defined category. We contacted the authors for the Scarmo 2013 paper, who provided us with the cut-point values for each quintile category of 25(OH)D. Eleven of the 14 studies adjusted for the month or season in which blood was collected for 25(OH)D assessment. Seven studies defined month or season as a statistical covariate, 3 studies used season-standardized 25(OH)D cut-points, and one study stratified the results by season. We elected to extract summer values from the latter study as these values are the most likely to Abbas 2009, collected samples for 25(OH)D analysis an average of 189 days after diagnosis and Crew 2009, also collected samples after diagnosis, with 20% of the patients receiving some chemotherapy before samples were taken. The remaining nested case-controls studies assessed 25(OH)D from a blood sample taken upon enrollment into the cohort. In these latter studies, the time between assessment and breast cancer diagnosis ranged from 1 month to 20 years. Eliassen 2016, and Scarmo 2013, included subsets of cases with two values of 25(OH)D available for analysis. The remaining 12 studies based their analysis on the 25(OH)D result from a single blood sample. Multiple 25(OH)D assay methods were used within and between studies. Coefficients of variation between duplicate assays ranged from 3.1% to 21.8% among the studies that reported this value. After taking all of the above quality of exposure assessment factors into consideration, we awarded five stars to Scarmo 2013; four stars to Eliassen 2016, McCullough 2009, Shirazi 2016, Yao 2011, and Yao 2016; three stars to Abbas 2009, Eliassen 2011, Kuhn 2009, Peppone 2012, and Rejnmark 2009; and two stars to Abulkhair 2015, Crew 2009, and Park 2015.
Table 4.
Exposure Assessment.
| Author year | Serum 25(OH)D Reference Cut Points | Level of Detection (LOD) | Adjustments made for season at time of 25(OH)D sample donation | Timing of 25(OH)D sample donation in cases | Multiple 25(OH)D assessments | Method of 25(OH)D assessment | Star rating |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Abbas 2009 | Apriori cut points <30 nmol/L 30–34 nmol/L 45–60 nmol/L ≥60 nmol/L <12 ng/mL 12–18 ng/mL 18–24 ng/mL ≥24 ng/mL |
No data | Yes – covariate in statistical analysis | Average 189 days after diagnosis | No | Immunodiagnostic Systems Limited enzyme immunoassay 1 batch CV = 3.1% intra-assay CV = 3.3% interassay 10% duplicate measures with average absolute deviation = 1.7% |
*** |
| Abulkhair 2015 | Apriori cut points ≤25 nmol/L >25 nmol/L ≤10 ng/mL >10 ng/mL |
No data | No | Initial visit with oncologist | No | DiaSorin Liaison CLIA (No CV data provided) |
** |
| Crew 2009 | Apriori cut points <20 ng/mL 20–29 ng/mL 30–39 ng/mL ≥40 ng/mL |
Range of measures in study was 0–103 ng/mL. Only one subject had a value of 0 ng/mL. | Yes – covariate in statistical analysis | Variable – average of 60 days between time of diagnosis and blood draw. 20% of cases received chemotherapy before blood collection. | No | DiaSorin RIA Cases and controls assayed in each of 8 batches Respective accuracy and precision: @ 17 ng/mL = +4%, 15% @48 ng/mL = +7, 18% @57 ng/mL = +15%, 15% |
** |
| Eliassen 2011 | Quartile cut points <18.4 ng/mL 18.4–< 24.6 ng/mL 24.6–<30.6 ng/mL ≥30.6 ng/mL |
No cases below LOD | Yes – covariate in statistical analysis | Mean 57 months (Range 1–127) before diagnosis |
No | RIA CV = 10.7% or 6.0%, based on sample batch |
*** |
| Eliassen 2016 | Apriori cut points <30 ng/mL ≥30 ng/mL |
No cases below LOD | Yes – stratified statistical analysis by season (summer/winter) of blood draw | Mean 7.9 years (Range 1 month to 20 years) before diagnosis | Yes – 413 cases and controls contributed two measures of 25(OH)D | Analyzed in 7 batches Batches 1 & 2 – PBA Batch 3 – RIA Batches 4–7 CLIA CV for batches 1–3 = 18.1%–21.8% CV for batches 4–7 = 6.5%–9.4% |
**** |
| Kuhn 2013 | Season standardized apriori cut points ≤39.3 nmol/L >39.3 – 50.9 nmol/L >50.9 – 63.0 nmol/L >63.0 nmol/L ≤15.7 ng/mL >15.7 – 20.4 ng/mL >20.4 – 25.2 ng/mL >25.2 ng/mL |
Lower LOD <6.7 nmol/L (2.7 ng/mL) 5 cases excluded with 25(OH) D below LOD |
Yes – used season standardized 25(OH)D values | Mean 4.1 years (+/−2.8 years) before diagnosis | No | OCTEIA 25(OH)D enzyme immunoassay CV @ 23.0 nmol/L = 3.7–6.6% CV@ 42.6 nmol/L = 5.6–10.9% |
*** |
| McCullough 2009 | Tertile cut points <45.9 nmol/L 45.9–64.2 nmol/L ≥64.2 nmol/L <18.4 ng/mL 18.4–25.7 ng/mL ≥25.7 ng/mL |
No Lower LOD. No cases excluded for 25(OH)D level. Lowest case reported was 7.5 nmol/L (3 ng/mL) and lowest control was 7.8 nmol/L (3.1 ng/mL). | Yes – covariate in statistical analysis | Range <1 month to 6.9 years before diagnosis | No | DiaSorin Liaison CLIA CV range = 6.4–8.8% |
**** |
| Park 2015 | Apriori cut points <20 ng/mL ≥20 ng/mL |
No lower LOD established. No cases excluded on this basis. | No | Time of diagnosis | No | DiaSorin RIA used for both cases and controls. However, cases and controls were analyzed at different laboratories. (No CV data provided) |
** |
| Peppone 2012 | Apriori cut points <20 ng/mL 20–31 ng/mL <32 ng/mL ≥32 ng/mL |
Lower LOD = 5 ng/mL One case was below LOD and assigned a value of 5 ng/mL |
Yes – covariate in statistical analysis | Blood sample taken prior to surgery, mean 30.1 days | No | January – June 2009 – CLIA After June 24, 2009 – LC-MS/MS LC-MS/MS levels were 14% higher than CLIA levels, controlling for age, race, and month of test. (No CV data provided) |
*** |
| Rejnmark 2009 | Tertile cut points <60 nmol/L 60–84 nmol/L >84 nmol/L 24 ng/mL 24–33.6 ng/mL 33.6 ng/mL |
Lower LOD <10 nmol/L (4 ng/mL). Persons with levels below the LOD were assigned a value of 9 nmol/L (3.6 ng/mL) | No | Prior to mammogram/study enrollment – average time unclear | No | ID-LC-MS/MS CV@ 41.2 nmol/L = 8.8% CV@ 25.3 nmol/L = 9.4% |
*** |
| Scarmo 2013 | NSMSC season-adjusted quintiles+ <40.09 nmol/L 40.09–49.57 nmol/L 49.58–57.37 nmol/L 57.38–67.95 nmol/L >67.95 nmol/L <16.0 ng/mL 16.0–19.8 ng/mL 19.8–22.9 ng/mL 23.0–27.2 ng/mL >27.2 ng/mL NYUWHS season-adjusted quintiles+ <38.18 nmol/L 38.18–50.14 nmol/L 50.15–60.77 nmol/L 60.78–73.64 nmol/L >73.64 nmol/L <15.3 ng/mL 15.3–20.1 ng/mL 20.1–24.3 ng/mL 24.3–29.5 ng/mL >29.5 ng/mL |
No cases below LOD | Yes – used season standardized 25(OH)D values | At study enrollment. Mean 8.7 years before diagnosis. | Yes – 678 cases and 1208 controls contributed two measures of 25(OH)D, at least 1 year apart. Average time between sample donations was 2.1 years in the NYUWHS cohort and 4.4 years in the NSMSC cohort. | DiaSorin Liaison CLIA CV (NYUWHS) Intra-assay = 9.5% Inter-assay = 11.4% CV (NSMSC) Intra-assay = 7.4% Inter-assay = 9.0% |
***** |
| Shirazi 2016 | Tertile cut points ≤ 76 nmol/L ≥77–≤97 nmol/L ≥98 nmol/L ≤ 30.4 ng/mL ≥ 30.8–≤38.8 ng/mL ≥39.2 ng/mL |
No data | Yes – covariate in statistical analysis | Mean 7.0 years (SD = 3.8) before diagnosis | No | HPLC CV@ 70 nmol/L = 8.5% CV@ 210 nmol/L = 7.1% |
**** |
| Yao 2011 | Apriori cut points <26.2 ng/mL >26.2 ng/mL |
No cases below LOD | Yes – used season standardized 25(OH)D values | Prior to surgery or any adjuvant treatment (27-day average between diagnosis and blood draw) | No | DiaSorin Liaison CLIA CV = 8.8% |
**** |
| Yao 2014 | Apriori cut points <20 ng/mL 20–29.9 ng/mL ≥30 ng/mL |
No cases below LOD | Yes – covariate in statistical analysis | Prior to surgery or any adjuvant treatment | No | DiaSorin Liaison CLIA CV = 8.8% |
**** |
Abbreviations: CLIA – Chemiluminescent Immunoassay, CV – Coefficient of Variation, HPLC – High Performance Liquid Chromatography, ID-LC-MS/MS – Isotope Dilution Liquid Chromatography tandem Mass Spectrometry, LC-MS/MS – Liquid Chromatography tandem Mass Spectrometry, PBA – Protein Binding Assay, RIA – Radioimmunoassay.
Quintile values were provided through communication with the author and did not appear in the published article.
Cut-Point Evaluation
The 25(OH)D cut-point ranges among the 14 studies, along with reference ranges (7,30), are illustrated in Figure 3. One of 2 case-control and 2 of 4 case-series studies presented in this review used a priori cut-points where <20 ng/ml was considered deficient. Abulkhair 2015 considered ≤10 ng/ml deficient and Yao 2011, which used a season-standardized binary cut-point, considered ≤26.2 ng/mL and lower deficient. Five of the nested case control studies used quantiles for comparison of 25(OH)D levels and the remaining four used a priori-determined values. Among the nested case-control studies, the cut-point for the lowest category ranged from 12 ng/mL to 30.4 ng/mL and the cut-point for the highest category ranged from 24 ng/mL to 40 ng/mL.
Figure 3.
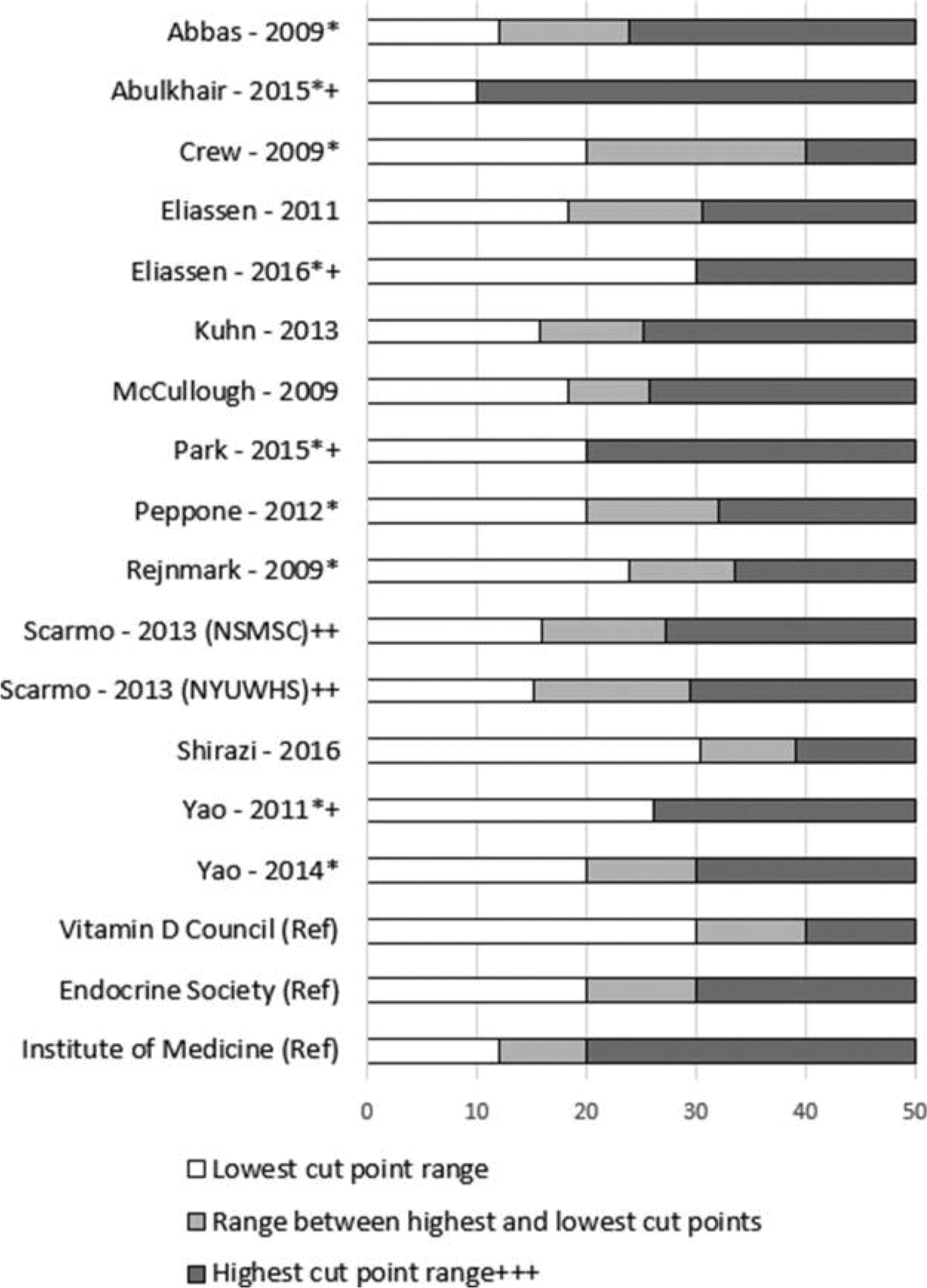
Cut-Point Ranges. * Denotes study found at least one significant relationship between 25(OH)D status and risk of breast cancer by any receptor status. + Denotes study which used binary cut-points. ++ Quintile values were provided through communication with the author and did not appear In the published article. Each cohort used a slightly different range of values, so results are stratified in this figure. +++ Graph shows highest cut-point range though 50 ng/ml. Upper limits in studies are not known and could be higher or lower. (Ref) Reference standards. From left to right, colored sections indicate deficiency, insufficiency, and sufficiency. High ranges were truncated at 50 ng/mL for clarity. Upper levels of sufficiency established by the Institute of Medicine, Endocrine Society, and Vitamin D Council are 50 ng/mL, 100 ng/mL, and 80 ng/mL, respectively.
Cut-Point Generalizability to 25(OH)D Deficiency and Sufficiency
The Endocrine Society cut points for 25(OH)D classification are commonly cited. Sufficiency is defined as 25 (OH)D levels above 30 ng/mL and deficiency is defined as 25(OH)D levels below 20 ng/mL.(7) Using the Endocrine Society classifications as a guide, cut points established by Eliassen 2011, Peppone 2012, and Yao 2014 approximate these standards. Abbas 2009, Abulkhair 2015, Kuhn 2013, McCullough 2009, Park 2015, Scarmo 2013, and Yao 2011 established cut-point ranges in which the highest 25(OH)D category may include individuals who are insufficient or deficient. Conversely, Eliassen 2016, Shirazi 2016, and Yao 2011 established cut-point ranges in which the lowest 25(OH)D category may include individuals who are insufficient, as opposed to deficient.
Lower Level of Detection
The lower limit of detection (LOD) for 25(OH)D ranges from 0.8 to 1.6 ng/mL in chromatographic separation methods and is around 4 ng/mL for binding assays (14). Eleven of the 14 corresponding authors for each study replied to our e-mail query regarding the lower LOD for their method of 25(OH)D assay. Seven of these 11 studies did not have any cases below their assay lower LOD. Kuhn 2013, established a lower LOD at 6.7 nmol/L (2.7 ng/mL) and excluded 5 cases (out of 1391 cases in the analysis) which fell below this level. Peppone 2012 established a lower LOD of 5 ng/mL; one record fell below this level and was assigned a value of 5 ng/mL. Rejnmark 2009, established a lower LOD of 10 nmol/L (4 ng/mL); persons with levels below the LOD were assigned a value of 9 nmol/L (3.6 ng/mL).
Outcome Assessment
We evaluated outcome assessment according to study design. Results are summarized in forest plots (Figures 3–5). Supplemental Table S1 summarizes the raw values as reported by the individual studies.
Figure 5.
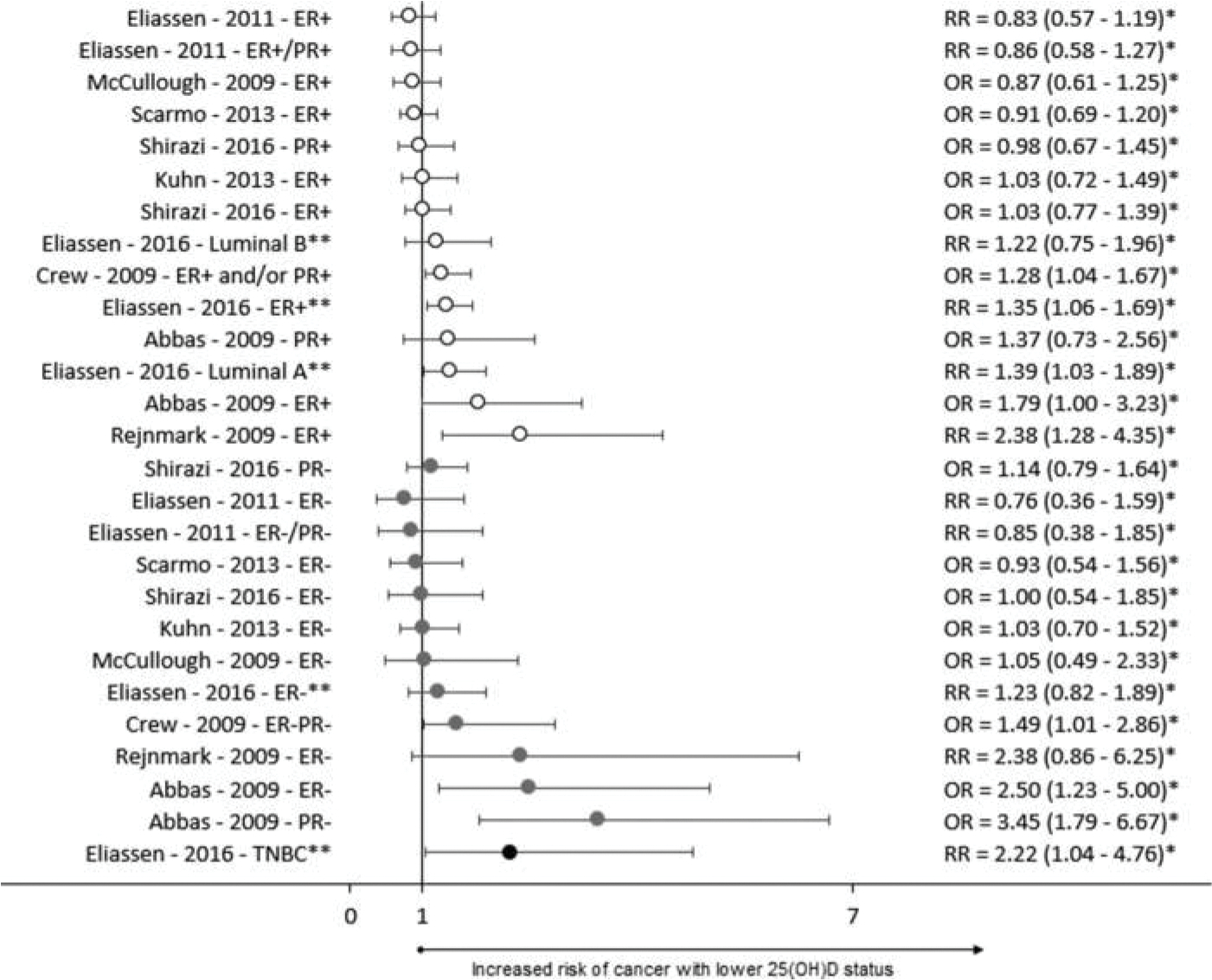
Forest plot of nested case-control studies. ER – Estrogen Receptor, PR – Progesterone receptor, HER2 – Human Epidermal growth factor Receptor 2, TNBC – Triple Negative Breast Cancer, * Inverse of reported value, ** Summer levels for 25(OH)D assessment.
Case-Control Studies
Results from case-control studies are summarized in Figure 4. Park 2015 and Yao 2011 provided results stratified by a comprehensive combination of receptor statuses. Additionally, Yao 2011 stratified results by menopausal status. Eight of 14 analyses examining the risk of non-TNBC cancers, 5 of 5 analyses examining the risk of cancers that include TNBC, and 3 of 3 analyses examining the risk of TNBC specifically, resulted in significant inverse relationships with 25(OH)D status.
Figure 4.
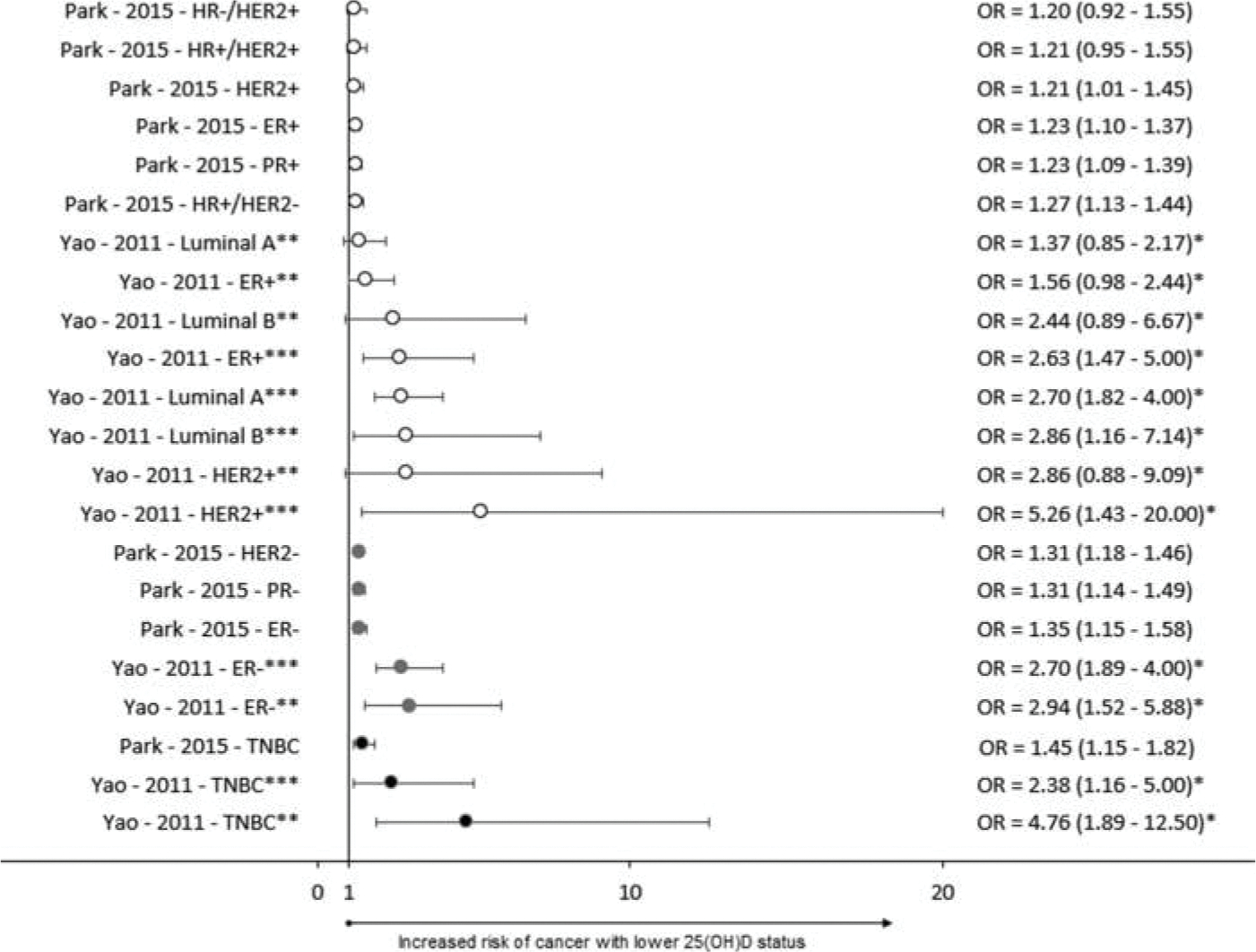
Forest plot of case-control studies. ER – Estrogen Receptor, PR – Progesterone receptor, HER2 – Human Epidermal growth factor Receptor 2, TNBC – Triple Negative Breast Cancer, * Inverse of reported value, ** Premenopausal, ***Postmenopausal.
Nested Case-Control Studies
Results from nested case-control studies are summarized in Figure 5. Most studies only looked for the presence or absence of hormone receptor expression. None of the nested case-control studies specifically evaluated the relationship between 25(OH)D status and HER2 overexpression and only one study evaluated TNBC status. Four of 14 analyses found a significant inverse relationship between 25(OH)D status and the risk of non-TNBC cancers. Three of 12 analyses which examined receptor statuses that partially represent TNBC found a significant inverse relationship with 25(OH)D status. Eliassen 2016 showed that deficient summer levels of 25(OH)D were significantly associated with an increased risk of TNBC.
Case-Series Studies
Results from case-series studies are summarized in Figure 6. There was more heterogeneity among the case-series studies, possibly because the comparison groups varied and did not consist of healthy controls. One out of 7 analyses comparing the risk of non-TNBC cancers to other cancers had a significant outcome. Three of 6 analyses which compared ER− or PR− cases to ER+ or PR+ cases showed a significant association. Of the analyses which compared TNBC to other types of cancer, 3 of 4 found a significant inverse association between vitamin D status and risk of TNBC (relative to other types of breast cancer diagnoses). The single analysis that was not significant was a comparison between TNBC and luminal A breast cancer among menopausal women.
Figure 6.
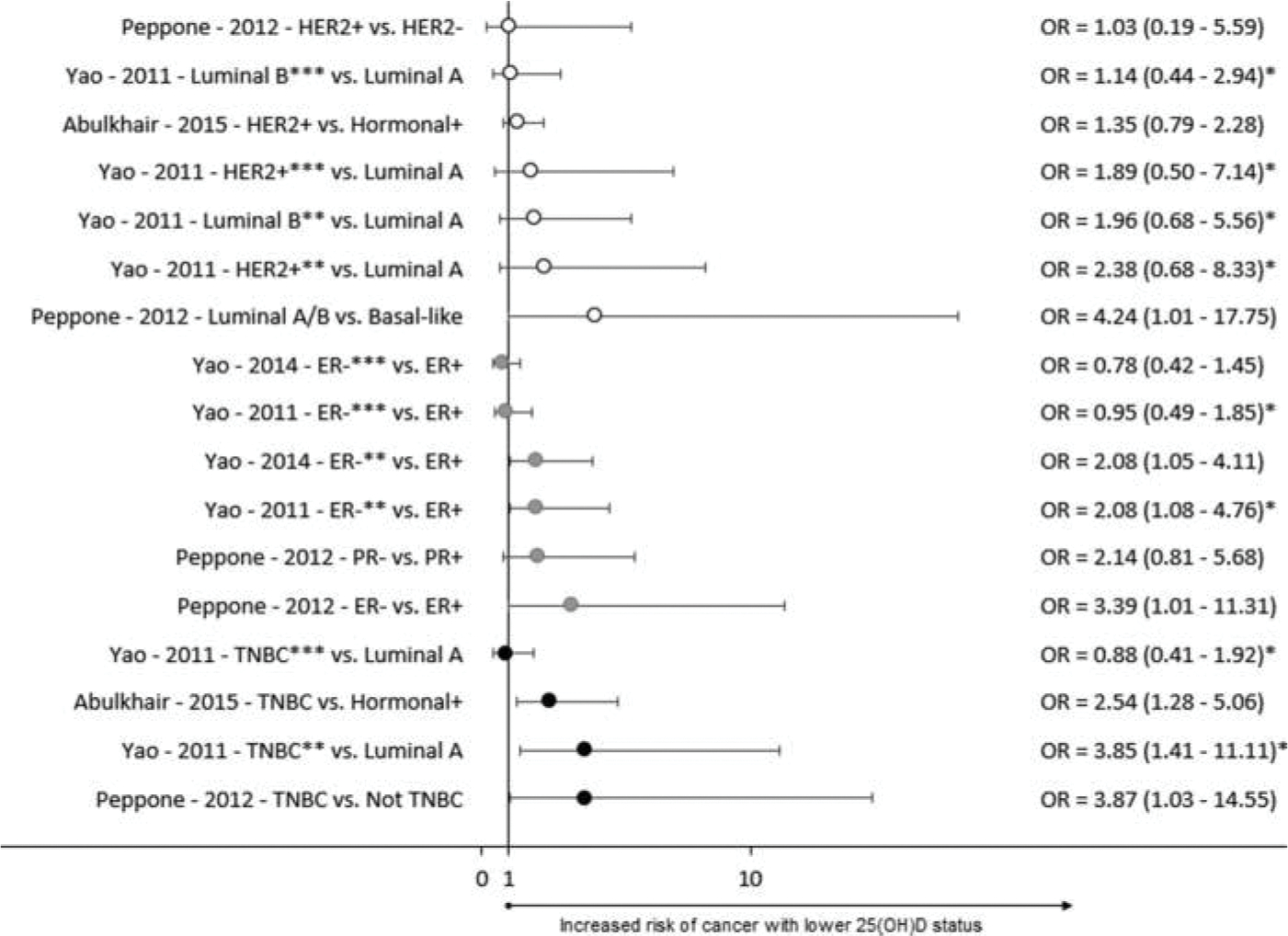
Forest plot of case-series studies. ER – Estrogen Receptor, PR – Progesterone receptor, HER2 – Human Epidermal growth factor Receptor 2, TNBC – Triple Negative Breast Cancer, * Inverse of reported value, ** Premenopausal, ***Postmenopausal.
Bias
Selection Bias
Nine of 9 nested case-control and 1 of 2 case-control studies stated criteria on which controls were matched to cases (the exception was Park 2015). All 10 of these studies matched cases and controls using age as a criterion. Additional matching criteria included date or season of blood collection, menopausal status, race and/or ethnicity, and/or hormone replacement therapy use. Kuhn 2013 and Peppone 2012 explicitly excluded both cases and controls with a previous diagnosis of any cancer. Crew 2009 and Rejnmark 2009 excluded cases and controls with a history of breast cancer. Case series studies lack a true control group and can only be used to discern the increased risk of one outcome group over another.
Confounding
Eliassen 2011, McCullough 2009, Park 2015, and both studies by Yao addressed all four of our a priori-identified potential confounders (menopausal status, BMI, age, and race/ethnicity) either by statistical adjustment, stratification of results, or through their selection criteria. Crew 2009 did not adjust for menopausal status. Abbas 2009 and Eliassen 2016 failed to adjust for race/ethnicity. Pep-pone 2012 did not adjust for BMI or menopausal status. Shirazi 2016 did not adjust for BMI or race/ethnicity. Kuhn 2013 and Scarmo 2013 failed to adjust for age, race/ethnicity, or menopausal status. Abulkhair 2015 and Rejnmark 2009 did not make any statistical adjustments.
Discussion
To our knowledge, this systematic review is the first to examine the relationship between plasma/serum 25 (OH) D levels and breast cancer risk when study results are grouped by TNBC relevance. The study types identified by the systematic review included case-control, nested case-control, and case-series analyses. Across all study types, 13 of 35 analyses for non-TNBC cancers showed a significant association with 25(OH)D status. Progressing to groups that partially represent TNBC, 11 of 23 analyses, or about half of the analyses, yield a significant association with 25(OH)D status. However, among TNBC-specific analyses, we consistently found significant relationships between lower 25(OH)D levels and increased risk of TNBC. Seven of 8 analyses involving TNBC-specific diagnoses showed a significant association with 25(OH)D. No study showed any detriment to having adequate 25(OH)D levels.
Five studies failed to find a significant effect between 25(OH)D status in any of their stratified analyses. All five of these studies were nested case-control studies that did not look specifically at TNBC. Of these 5 studies, in Kuhn 2013, McCullough 2009, and Scarmo 2013 established quantiles where the highest 25(OH)D category may have included individuals that are deficient; and conversely, in Shirazi 2016 included individuals with sufficient 25(OH) D may have been included in the lowest 25(OH)D category, which limits the conclusions that can be drawn from these studies. After excluding the results from these 4 studies, 13 of 30 non-TNBC results and 11 of 18 results partially representing TNBC were significant.
There are 5 important limitations to our review. 1) We were not able to cleanly group results according to TNBC versus not TNBC. Many studies only stratified by estrogen receptor status, or estrogen and progesterone receptor status, requiring us to create an intermediary category of studies where some, but not all, cases are TNBC. 2) Cut-points for lowest and highest categories of 25(OH)D status varied between studies, making comparison problematic. 3) Temporal associations between the duration of 25(OH)D deficiency and breast cancer risk cannot be determined. For example, the case-control studies represented here all assessed 25(OH)D around the time of diagnosis, so it is not possible to discern if 25 (OH)D status is a cause or effect of the diagnosis. On the other hand, the nested case-control studies assessed 25 (OH)D at enrollment which may have been 1 month before a diagnosis or 20 years. This type of study assumes that an individual’s 25(OH)D levels remain relatively constant over time. Only two studies had a subset of participants for which two assessments for 25(OH)D were available, and two assessments are not enough to establish a pattern within an individual over time. 4) The studies overwhelmingly represent women of white/Cau-casian race/ethnicity. This is unfortunate because women of African ancestry are at the highest risk for both 25 (OH)D deficiency and TNBC. According to NHANES 2005–2006 data, over 80% of African-American in the U.S. are vitamin D-deficient (defined as ≤20 ng/mL) (31); and according to U.S. breast cancer statistics from 2012, 22% of breast cancer diagnoses in African-American are TNBC cases compared to 11% of breast cancer diagnoses in white women (1). 5) Last, there are several barriers to accurately assessing plasma/serum 25(OH)D status. There is no accepted gold standard for assessment. Vitamin D has a high affinity for vitamin D binding protein, which must be overcome in all assay methods. Matrix effects, such as the presence of other lipid compounds, other vitamin D metabolites, and C-3 epimers can all interfere with assessment. Results from binding assays could overestimate 25(OH)D levels in individuals supplementing with D2. Standard chromatography methods may not resolve C-3 epimers, whereas most binding assays are not affected (8,14). One study in an adult population showed that C-3 epimers increase with increasing levels of serum 25(OH)D and the mean concentration in the study cohort was 1.9 ng/mL (32). This suggests the contribution of C-3 epimers to overall 25(OH)D levels are nominal and predictable, thus supporting chromatography as a preferred analytical method.
Despite these limitations, we were able to discern a clear pattern where the more likely the results represented TNBC diagnoses, the more frequently the results were significant. Yao 2011 provides the strongest evidence in terms answering our specific research question. This study reported case-control and case-series results evaluating the risk of ER+, ER−, luminal A, luminal B, nonluminal HER2+, and TNBC cancer diagnoses, stratified by menopausal status. Four out of 5 stars were awarded for its assessment of exposure criteria, and all potential con-founders and most criteria for selection bias were addressed. The results reported by Yao 2011 are consistent with the overall pattern that we observed in this review. The most striking finding by Yao 2011 was that in a case control analysis, premenopausal women who were deficient in 25(OH)D had a nearly 5 fold greater risk of TNBC than healthy controls, OR = 0.21 (0.08–0.53).
The two most recent meta-analyses to investigate associations between 25(OH)D status and overall breast cancer risk differed in their findings. Wang 2013 found an overall significant inverse relationship between 25(OH)D levels and the risk of breast cancer, RR = 0.845 (0.750–0.951) (11), whereas Kim and Je 2014 failed to reach significance, RR = 0.95 (0.88 – 1.01) (12). Twelve of the 14 studies identified by Kim and Je overlapped with those used by Wang. Scarmo 2013 and Kuhn 2013 were included in the Kim and Je meta-analysis, but not in the Wang meta-analysis, despite these papers being published before the systematic search date established by Wang. The Scarmo 2013 and Kuhn 2013 studies did not reach significance, lowering the overall risk estimate in the Kim and Je 2014 study. None of the studies used in the analyses by Wang or Kim and Je stratified by TNBC receptor status.
Eliassen 2016 was the only nested case-control study to report a risk estimate specifically for TNBC diagnoses (19). Reporting of HER2 status was not required by cancer registries until 2010 (1), thus analyses based on longitudinal cohorts (such as nested-case control studies) are limited in TNBC-specific analyses. Now that reporting HER2 status is standard, future analyses based on prospective cohorts will be able to stratify their analyses by TNBC-specific diagnosis. The quality of future nested case-control studies will be improved by the parent cohort study including more frequent assessments of plasma/serum 25(OH)D so that temporal associations between 25 (OH)D status and sub-types of breast cancer diagnoses may be more clearly elucidated.
In conclusion, findings from this systematic review support the hypothesis that the risk of developing TNBC in particular is increased in those with lower versus higher levels of 25(OH)D. Vitamin D is a routine clinical assay and its deficiency is easily remedied with supplements. Thus, women should be routinely screened for vitamin D deficiency, particularly those in vulnerable populations, such as women with darker skin, older women, and women with minimal sun exposure. There are no targeted therapies available to treat TNBC, so this potential avenue of prevention should be incorporated into patient care. While the association between 25(OH) D status and risk of TNBC, or breast cancer in general, cannot be conclusively stated, and incident cancer likely stems from multiple factors, there are no obvious detriments to correcting a 25(OH)D deficiency.
Supplementary Material
Acknowledgments
The first author would like to express thanks to the instructors in the Department of Nutritional Sciences, University of Cincinnati for their guidance in the development of this research.
Funding
This systemic review was conducted as part of a master’s thesis. There is no direct funding or financial support related to this article.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, et al. : Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 66, 31–42, 2016. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 2.Jamdade VS, Sethi N, Mundhe NA, Kumar P, Lahkar M, et al. : Therapeutic targets of triple-negative breast cancer: A review. Br J Pharmacol 172, 4228–4237, 2015. doi: 10.1111/bph.l3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, et al. : Strategies for subtypes–dealing with the diversity of breast cancer: Highlights of the st. gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22, 1736–1747, 2011. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, et al. : Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 24(Suppl 2), S26–35, 2015. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Dai X, Li T, Bai Z, Yang Y, Liu X, et al. : Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 5,2929–2943,2015. [PMC free article] [PubMed] [Google Scholar]
- 6.Cheang MC, Martin M, Nielsen TO, Prat A, Voduc D, et al. : Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 20, 474–482, 2015. doi: 10.1634/theoncologist.2014-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linus Pauling Institute: Vitamin D. 2014. [Google Scholar]
- 8.Enko D, Fridrich L, Rezanka E, Stolba R, Ernst J, et al. : 25-hydroxy-vitamin D status: Limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab 60, 1541–1550, 2014. doi: 10.7754/Clin.Lab.2014.131114. [DOI] [PubMed] [Google Scholar]
- 9.Lopes N, Paredes J, Costa JL, Ylstra B, Schmitt F: Vitamin D and the mammary gland: A review on its role in normal development and breast cancer. Breast Cancer Res 14, 211, 2012. doi: 10.1186/bcr3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.So JY, Suh N: Targeting cancer stem cells in solid tumors by vitamin D. J Steroid Biochem Mol Biol 148, 79–85, 2015. doi: 10.1016/j.jsbmb.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW: Serum 25-hydroxyvitamin D and breast cancer risk: A meta-analysis of prospective studies. Tumour Biol 34, 3509–3517, 2013. doi: 10.1007/s13277-013-0929-2. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Je Y: Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br J Cancer 110, 2772–2784, 2014. doi: 10.1038/bjc.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 6, e1000100, 2009. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen MJ, Wielders JP, Bekker CC, Boesten LS, Buijs MM, et al. : Multicenter comparison study of current methods to measure 25-hydroxyvitamin D in serum. Steroids 77, 1366–1372, 2012. doi: 10.1016/j.steroids.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Peairs KS, Choi Y, Stewart RW, Sateia HF: Screening for breast cancer. Semin Oncol 44, 60–72, 2017. doi: 10.1053/j.seminoncol.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Abbas S, Chang-Claude J, Linseisen J: Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a german case-control study. Int J Cancer 124, 250–255, 2009. doi: 10.1002/ijc.23904. [DOI] [PubMed] [Google Scholar]
- 17.Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, et al. : Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res 2, 598–604, 2009. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, et al. : Plasma 25-hydroxyvitamin D and risk of breast cancer in the nurses’ health study II. Breast Cancer Res 13, 2011. doi: 10.1186/bcr2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliassen AH, Warner ET, Rosner B, Collins LC, Beck AH, et al. : Plasma 25-hydroxyvitamin D and risk of breast cancer in women followed over 20 years. Cancer Res 76, 5423–5430, 2016. doi: 10.1158/0008-5472.CAN-16-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, et al. : Plasma 25-hydroxyvitamin D and the risk of breast cancer in the european prospective investigation into cancer and nutrition: A nested case-control study. Int J Cancer 133, 1689–1700, 2013. doi: 10.1002/ijc.28172. [DOI] [PubMed] [Google Scholar]
- 21.McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, et al. : Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: A nested case control study in the cancer prevention study-II nutrition cohort. Breast Cancer Res 11, 2009. doi: 10.1186/bcr2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S, Lee DH, Jeon JY, Ryu J, Kim S, et al. : Serum 25-hydroxyvitamin D deficiency and increased risk of breast cancer among korean women: A case-control study. Breast Cancer Res Treat 152,147–154, 2015. doi: 10.1007/s10549-015-3433-0. [DOI] [PubMed] [Google Scholar]
- 23.Peppone LJ, Rickies AS, Janelsins MC, Insalaco MR, Skinner KA: The association between breast cancer prognostic indicators and serum 25-OH vitamin D levels. Ann Surg Oncol 19, 2590–2599, 2012. doi: 10.1245/s10434-012-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, et al. : Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: A nested case-control study. Cancer Epidemiol Biomarkers Prev 18, 2655–2660, 2009. doi: 10.1158/1055-9965.EPI-09-0531. [DOI] [PubMed] [Google Scholar]
- 25.Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, et al. : Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: A nested case-control study. Breast Cancer Res 15, R15,2013. doi: 10.1186/bcr3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirazi L, Almquist M, Borgquist S, Malm J, Manjer J: Serum vitamin D (250HD3) levels and the risk of different subtypes of breast cancer: A nested case-control study. Breast 28, 184–190 2016. doi: 10.1016/j.breast.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Yao S, Sucheston LE, Millen AE, Johnson CS, Tramp DL, et al. : Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: A case-control and a case-series study. PLoS One 6, el7251, 2011. doi: 10.1371/journal.pone.0017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao S, Hong C-, Mccann SE, Zirpoli G, Quan L, et al. : Combined effects of circulating levels of 25-hydroxyvitamin D and Thl and Th2 cytokines on breast cancer estrogen receptor status. Cancers 6,211–225,2014. doi: 10.3390/cancers6010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abulkhair O, Saadeddin A, Makram O, Gasmelseed A, Pasha T, et al. : Vitamin D levels and breast cancer characteristics: Findings in patients from saudi arabia. J Steroid Biochem Mol Biol 164, 106–109, 2016. doi: 10.1016/j.jsbmb.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Vitamin D Council: Testing for vitamin D. 2017. [Google Scholar]
- 31.Forrest KY, Stuhldreher WL: Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31, 48–54, 2011. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Lensmeyer G, Poquette M, Wiebe D, Binkley N: The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. J Clin Endocrinol Metab 97, 163–168, 2012. doi: 10.1210/jc.2011-0584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


