Abstract
Mycobacterium ulcerans inoculated into the footpads of mice at 6 × 103 CFU was shown to have a generation time of 6.5 days when estimated from weekly changes in microscopic counts of acid-fast bacilli (AFB) and 7.5 days when calculated from actual CFU enumerated on Lowenstein-Jensen egg medium incubated at 32°C. Footpads became swollen at week 10 (W10) after infection, and all infected control mice were dead at W15 after infection. Daily (5 days/week) treatment with 100 mg of clarithromycin (CLR)/kg of body weight beginning the day after infection prevented swelling of footpads at W10. When initiation of treatment was delayed until obvious footpad swelling was observed, there was a reduction in both the increase in AFB counts and deterioration of swollen footpads and also a prolonged survival of the mice to W18. Mice infected in the hind footpads with 5 × 105 CFU of M. ulcerans were divided into an untreated control group and six treatment groups that received one of the following therapies for 8 weeks: 100 mg of CLR/kg, 25 mg of minocycline (MIN)/kg, 50 mg of sparfloxacin (SPX)/kg, 10 mg of rifampin (RIF)/kg, 10 mg of rifabutin (RBT)/kg, or 100 mg of amikacin (AMK)/kg. After completion of therapy, treated animals were observed for an additional 17 weeks. All control mice and mice treated with CLR, MIN, or SPX exhibited swollen footpads during the observation period. In contrast, of those animals treated with RIF, RBT, or AMK, none had footpad swelling and all inoculated cultures done after the W17 observation remained negative. These results suggest that RIF, RBT, and AMK may be effective in the treatment of human infection with M. ulcerans.
Since its first description in Australia in 1948 by MacCallum and others (10), the Bairnsdale ulcer, subsequently named the Buruli ulcer, is currently reported with increasing prevalence in West and Central Africa. As described by van der Werf et al. (18), it is the third most common mycobacterial disease in humans after tuberculosis and leprosy. The usual clinical feature is a deep, necrotic, and painless ulcer with typically undermined edges resulting from coalescent necrosis of the subcutaneous fat with vascular occlusion. Large surgical excision of the necrotic tissue followed by skin grafting is, at present, the only treatment (18). The responsible organism is a slowly growing acid-fast and alcohol-fast mycobacterial species, Mycobacterium ulcerans. It grows at an optimal temperature of 32°C and is known to be resistant to most antituberculosis and antileprosy drugs (18).
Experimental growth of M. ulcerans in immunocompetent mice has been studied extensively by Fenner (3). After intravenous, intraperitoneal, or intranasal infection with 104 to 106 CFU of M. ulcerans, disseminated skin lesions developed within the ensuing 10 to 24 weeks. In contrast, when the organisms were injected into the hind footpads, the lesions remained localized in the footpads, which subsequently became inflamed, swollen, and finally ulcerated after 3 to 6 weeks, depending upon the size of the inoculum. Whatever the route of infection, the larger the inoculum, the more rapid the development of lesions. The division time of M. ulcerans was estimated by Fenner to be 3 to 4 days (3).
The objective of the present study was to apply the Fenner mouse footpad model to establish the kinetics of growth of M. ulcerans and to determine the impact of different antimicrobial agents on the growth of the organism. Because clarithromycin (CLR) recently has been demonstrated by Portaels et al. to have in vitro activity against M. ulcerans (13) and also has demonstrated activity against Mycobacterium avium and Mycobacterium leprae in mice and humans (1, 5, 7, 8, 9), priority was given to testing the in vivo activity of CLR.
When CLR exhibited only limited bacteriostatic activity against M. ulcerans in our first experiment, we decided to test the activity of two rifamycin derivatives (rifampin [RIF] and rifabutin [RBT]), amikacin (AMK), minocycline (MIN), and sparfloxacin (SPX) (the most active antimycobacterial fluoroquinolone). For this purpose, we used the kinetic method designed by C. C. Shepard for testing the activity of antileprosy drugs in the footpads of mice (14).
MATERIALS AND METHODS
First experiment.
The Cu001 strain of M. ulcerans we used was isolated from a mouse footpad that had been inoculated with material from a Buruli ulcer excised from a patient in Adzopé, Ivory Coast, in June 1996. The method of isolation was adapted from the methods developed by Shepard and McRae for the inoculation of M. leprae in the footpad of the mouse (16) and the counting of M. leprae organisms (15). Briefly, 5 × 104 acid-fast bacilli (AFB) harvested from the skin ulcer were inoculated into both hind footpads of Swiss mice. Three months later, M. ulcerans was harvested from the swollen footpads, and subcultured on Lowenstein-Jensen medium, and incubated at 32°C for 12 weeks. Colonies were subcultured in Dubos broth (Diagnostic Pasteur, Paris, France) at 32°C for 4 weeks. The turbidity of the resulting culture was adjusted with normal saline to match that of a 1-mg (wet weight)/ml suspension of Mycobacterium bovis BCG, equivalent to a McFarland number 1 standard (6), and was further diluted up to 10−2 mg/ml for mouse infection.
During the experiment, AFB counts were performed microscopically according to Shepard and McRae (15), and CFU counts were determined by plating appropriate dilutions onto Lowenstein-Jensen medium incubated at 32°C for 4 months.
CLR (provided as standard powder by Abbott France, Rungis, Val de Marne, France) was suspended in 0.05% agar-containing distilled water at a concentration of 10 mg/ml. The stock solutions were prepared weekly and stored at 4°C.
(i) MIC of CLR.
The MIC of CLR was determined on Lowenstein-Jensen medium (pH 7.2) containing twofold dilutions of CLR ranging from 64 to 0.125 μg/ml. The M. ulcerans suspension was adjusted to 1 mg/ml, serial 10-fold dilutions were made up to 10−5 mg/ml, and 0.1-ml volumes of the 10−1, 10−3, and 10−5-mg/ml suspensions were plated in triplicate on both drug-free (control) and drug-containing media. The MIC was defined as the lowest drug concentration that inhibited more than 99% of the bacterial growth observed on the drug-free medium after incubation at 32°C for 4 months (13).
(ii) Mouse model, infection, and treatment.
A total of 115 4-week-old female Swiss mice (Janvier Breeding Center, Le Genest-Saint-Isle, France) weighing 18 to 20 g were used. Before infection with M. ulcerans, the mice were housed in the animal facility for 1 week. Each mouse was infected in both hind footpads with 6 × 103 CFU of M. ulcerans in a volume of 0.03 ml (14).
The day after infection (D1), five mice were sacrificed (yielding 10 footpads) to establish baseline values of AFB and CFU counts. The remaining 110 mice were allocated randomly to three groups: a control group of 60 mice and two treatment groups of 25 mice each. CLR was administered orally to the first treatment group by esophageal cannula (gavage), five times weekly from D1 to week 10 (W10), in a dosage of 100 mg/kg (0.2 to 0.3 ml of the 10-mg/ml stock solution, according to body weight). The second treatment group began the same oral gavage with CLR only after the hind footpads became swollen (at W10) and were kept on therapy up to W18.
(iii) Assessment of M. ulcerans growth.
The growth of M. ulcerans in the footpads of mice was assessed in terms of footpad swelling and AFB and CFU counts. Mortality of infected mice and footpad swelling were recorded on a weekly basis. AFB and CFU counts were performed every 2 weeks on 6 to 10 footpads.
(iv) Statistical analysis.
Results were analyzed by the Student t test and Fischer's exact probability correlations. P values were two tailed, and a value of P ≤ 0.05 was considered statistically significant.
Second experiment.
In the second experiment, the activity of the following compounds was tested against strain Cu001 of M. ulcerans: CLR (Abbott), AMK (Bristol-Myers Squibb, Paris, France), RIF (Marion Merrel-Dow, Neuilly, France), RBT (Farmitalia-Carlo Erba, Rueil, France), MIN (Lederle, Oullins, France), and SPX (Rhone DPC Europe, Anthony, France). As in the first experiment, all drug solutions except AMK were made in 0.05% agar-containing distilled water at the desired concentrations. AMK was diluted in normal saline. The stock solutions were prepared weekly and stored at 4°C. All drugs were provided by their manufacturers.
(i) Infection, treatment, and assessment of effectiveness.
One hundred four female Swiss mice, aged 4 to 6 weeks, were infected in both hind footpads with 0.03 ml of a bacterial suspension containing 5 × 105 AFB of M. ulcerans. On D1 and D7 (W1) after infection in the second experiment, five mice were sacrificed (yielding 10 footpads) to establish baseline values of AFB counts in the footpads. The 94 remaining mice were allocated randomly to a control group of 22 mice and six treatment groups of 12 mice each to receive the following treatments given 5 days a week: CLR at 100 mg/kg; AMK at 100 mg/kg; RIF at 10 mg/kg; RBT at 10 mg/kg; MIN at 25 mg/kg; and SPX at 50 mg/kg. Drugs were given for 8 weeks by gavage, except AMK, which was injected subcutaneously. After cessation of treatment, the mice were kept under observation for 17 additional weeks. The activity of each drug was assessed in terms of inhibition of microbial growth as determined by comparing the growth curves of M. ulcerans in each of the treated groups with that in the untreated control group. If growth inhibition induced by treatment with a given drug was greater timewise than the duration of treatment itself, i.e., 8 weeks, the drug was considered to have bactericidal activity. The longer the period of inhibition, the more bactericidal the drug (14).
Every week for 26 weeks, the mice were examined for swelling of footpads. As soon as swelling was evident, the mouse was sacrificed, and an AFB count in the footpad was performed to confirm the growth of M. ulcerans. At the end of the experiment, i.e., 26 weeks, all surviving mice were sacrificed, and AFB and CFU counts were performed for each inoculated footpad.
(ii) Statistical analysis.
Survival analysis, with the swollen footpad as the measurement, was done by the Kaplan-Meier method (12). If a mouse had only a single swollen hind footpad, the companion normal footpad was censored at the time of the sacrifice. The log rank test was used to determine the level of statistical significance when comparing survival curves of the different treatment groups with the control group. P values were two tailed, and a value of P ≤ 0.05 was considered statistically significant.
RESULTS
First experiment. (i) MIC of CLR.
The MIC of CLR for the Cu001 M. ulcerans strain tested on Lowenstein-Jensen medium was 0.12 μg/ml, similar to that already reported (13).
(ii) Mortality.
Before W10, no control mice died. Between W10 and W12, 2 mice (5%) died, and between W12 and W14, 21 mice (67%) died. At W15, all control mice were dead.
Among mice treated with CLR from D1 to W10 there were no deaths, but as five animals were sacrificed every 2 weeks from W2 to W10 for AFB and CFU counts, there were no mice still alive on completion of treatment.
Among mice started on treatment with CLR at W10 after swelling of the footpads had occurred, there was no spontaneous death between W10 and W18. But 9 out of the 10 remaining mice died between W18 and W19. Compared to control mice, the mice started on CLR at W10 survived three additional weeks.
(iii) Footpad lesions.
All control mice had swollen footpads at W10. By W11, the swelling involved the entire foot, and by W12 the swelling was progressing up the leg.
No swelling developed in those mice treated with CLR from D1 to W10. In mice started on CLR only at W10, there was no improvement of the swollen footpads, but the ultimate deterioration of the foot and involvement of the leg was slowed compared to that in controls.
(iv) Enumeration of AFB in footpads.
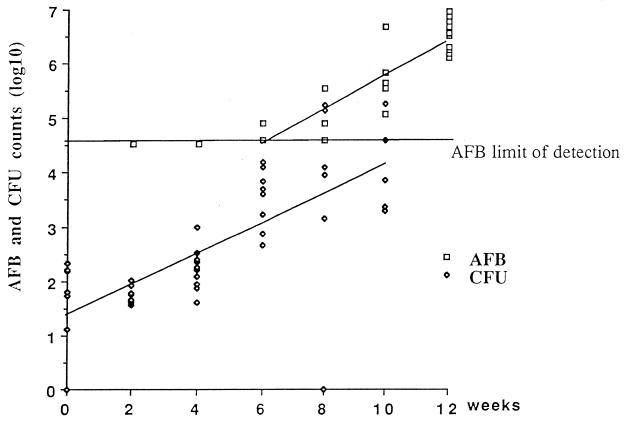
In the control mice, no AFB were detected before W4. At W6, the number of AFB was above the limit of detection (4.6 log10) in 2 out of 10 footpads (4.9 log10 in both footpads). At W8, the AFB counts were 4.9, 4.9, and 5.6 log10 in 3 footpads and below the limit of detection in 3 other footpads. At W10 and W12, all footpads were AFB positive, the median AFB count per footpad being 5.7 and 6.5 log10, respectively. At W14, the median AFB count was 6.2 log10, but the deteriorating footpads were massively superinfected with Staphylococcus aureus. Taking into account the AFB counts between W6 and W12, an exponential regression curve [AFB (log10) = 2.6 + 0.32 × W, where W is the week after infection] was drawn, of which the slope was 0.32 (95% confidence interval [CI95], 0.27 to 0.38). From this curve (Fig. 1), the generation time of M. ulcerans was estimated at 6.5 days (CI95, 5.5 to 7.7 days).
FIG. 1.
AFB and CFU counts in the footpads of mice infected with 6 × 103 CFU of M. ulcerans.
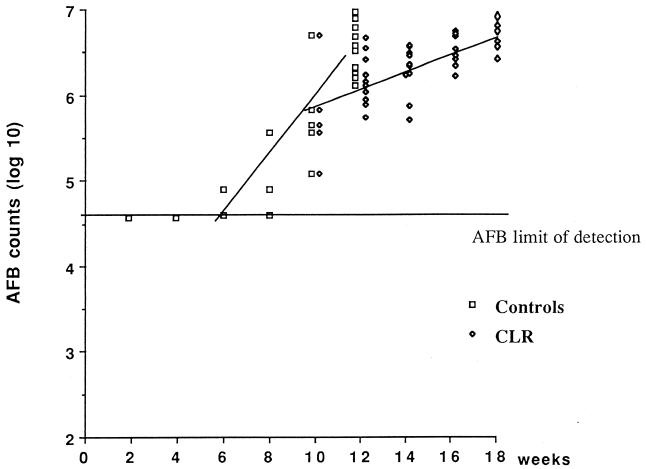
In mice treated with CLR from D1 to W10, the AFB counts remained under the detection limit from D1 to W8. At W10, only one of the six footpads was positive (4.9 log10 AFB). In the second group of mice started on CLR only at W10, the increase in the AFB counts was delayed compared to that in the control mice. The median AFB counts were 5.8 log10 at W10, 5.2 log10 at W12, 6.3 log10 at W14, 6.7 log10 at W16, and 6.8 log10 at W18. The slope of the growth curve drawn from these AFB counts was 0.12 (CI95, 0.08 to 0.16). Although the slope of this regression curve was inferior to that in the control mice (Fig. 2), the difference was not statistically significant (P = 0.15).
FIG. 2.
M. ulcerans AFB counts in mice treated with 100 mg of CLR/kg from W10 to W18 and in control mice.
(v) Enumeration of CFU in footpads.
In the control mice, visible colonies of M. ulcerans appeared by the third month, and the final CFU counts were performed after 4 months of incubation at 32°C. The median CFU counts did not increase between D1 and W2, 1.8 log10 (1.1 to 2.3) and 1.7 log10 (1.6 to 2.0), respectively. Thereafter the median CFU counts increased to 2.2, 3.7, 3.9, and 4.9 log10 at W4, W6, W8, and W10, respectively. After W10, all cultures were superinfected with S. aureus, and M. ulcerans CFU counts were no longer feasible. The slope of the growth curve drawn from the CFU counts between D1 and W10 was 0.28 (CI95, 0.19 to 0.35), which is not statistically different from the slope of the AFB curve of M. ulcerans in control mice (P = 0.75). From this curve (Fig. 1), the generation time of M. ulcerans was estimated at 7.5 days (CI95, 6 to 10.5 days), which is not statistically different from the estimated generation time as determined by the AFB curve.
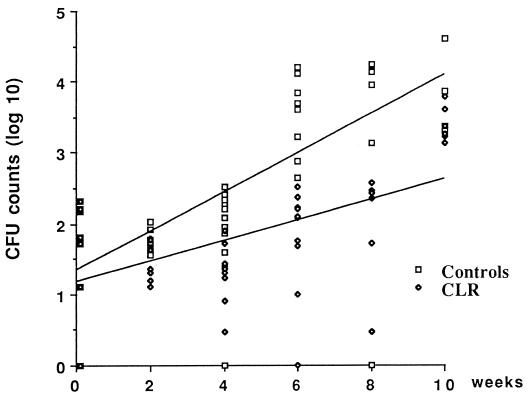
In mice treated with CLR from D1 to W10, the CFU counts did not increase during the first 4 weeks of treatment, but they slowly increased to reach 2.1, 2.3, and 3.5 log10 CFU per footpad at W6, W8, and W10, respectively. A comparison of the growth curve of M. ulcerans CFU in the control mice with the growth curve of M. ulcerans CFU in the CLR-treated mice is shown in Fig. 3. Although the slope of the curve was 0.14 (CI95, 0.08 to 0.21), suggesting a bacteriostatic activity of CLR against M. ulcerans, the difference between the slopes of the growth curves for control and in CLR-treated mice was not statistically significant (P = 0.45).
FIG. 3.
M. ulcerans CFU counts in mice treated with 100 mg of CLR/kg from D1 to W10 and in control mice.
In mice started on CLR treatment at W10, the median M. ulcerans CFU count was 4.3 log10 at W12. All cultures examined after W12 were superinfected with S. aureus.
Second experiment. (i) Footpad lesions.
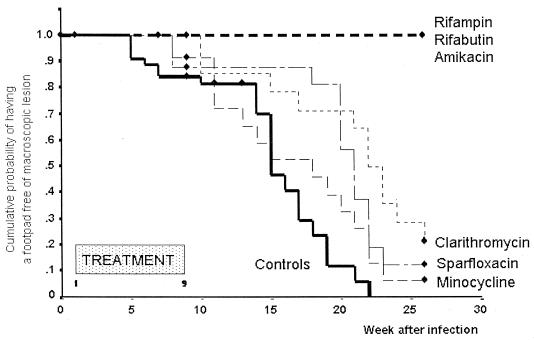
The footpads of mice were examined once weekly during the 8-week course of treatment as well as during the 17 weeks after completion of treatment. In the AMK-treated group, one mouse died from an accident caused by the injection of the drug. Swelling of footpads appeared at W5 in the controls, at W8 in the MIN- and SPX-treated mice, and at W10 in the CLR-treated mice. Swelling of 50% of footpads was recorded by 15 weeks (CI95, 13 to 17) in control mice, 18 weeks (CI95, 12 to 24) in MIN-treated mice, 21 weeks (CI95, 20 to 22) in the SPX-treated mice, and 22 weeks (CI95, 20 to 24) in the CLR-treated mice (Fig. 4). In the RIF-, RBT-, and AMK-treated mice, no footpad swelling occurred during the 26 weeks of the experiment. The difference between the mice treated with the latter three drugs and the control mice was highly significant (P < 0.001), and this difference suggested a bactericidal activity of these drugs against M. ulcerans. The temporal suppression of footpad swelling induced by CLR and SPX (compared to controls) was statistically significant (P = 0.002 and 0.008, respectively). But because the suppression was less than 8 weeks (the duration of treatment) of that observed in controls, it suggested that both drugs had only a bacteriostatic activity against M. ulcerans. For MIN, the suppression of growth was not significant (P = 0.18).
FIG. 4.
Cumulative probability (Kaplan-Meier method) of having no swollen footpad in mice infected with 5 × 105 M. ulcerans and treated with different antimicrobials from W1 to W9 and in control mice.
(ii) AFB and CFU counts in footpads.
For all swollen footpads from control mice and from mice treated with CLR, SPX, or MIN, the AFB counts were above 5 × 106, indicating that the inoculated organisms had actively multiplied. Since no footpad swelling occurred during the 26 weeks of the experiment in the RIF-, RBT-, and AMK-treated mice, AFB and CFU counts were performed for the still-normal-appearing footpads of these mice at W26. Microscopic AFB counts were negative in all footpads from AMK-treated mice, positive in only 2 (4.9 and 5.07 log10) of 14 footpads from RIF-treated mice, and positive in only 2 (5.07 and 5.3 log10) of 14 footpads from RBT-treated mice. All cultures performed from the normal-appearing footpads harvested at W26 remained negative.
DISCUSSION
After infection of the mouse footpad with 6 × 103 CFU of M. ulcerans, the generation time of M. ulcerans was estimated at 6.5 days (CI95, 5.5 to 7.7) from the AFB counts and at 7.5 days (CI95, 6 to 10.5) from the CFU counts. These times are a little longer than but consistent with that reported by Fenner, who estimated the generation time of M. ulcerans at 4 days (3). One possible explanation for the difference between our findings and Fenner's data might be the poor viability of the inoculated organisms in our experiment. This is suggested by the fact that the AFB counts were 10 times higher than the CFU counts.
Obstacles to the assessment of the M. ulcerans growth in mice were the low growth rate of the organism and the ultimate superinfection of the deteriorating footpads with S. aureus soon after the swelling had occurred. Although we tried, we were not successful in developing a selective culture medium for M. ulcerans that contained drugs active against S. aureus. Consequently, to overcome both obstacles, we focused our observations on the detection of the footpad swelling and on the AFB counts. The latter were always above 105 when the footpads began to swell and above 5 × 106 when the footpad swelling had progressed to the point that the corresponding legs became inflamed. The observation of footpad swelling combined with an AFB count greater than 5 × 106 might, therefore, be used as a surrogate marker of M. ulcerans growth in the mouse footpad.
Of clinical interest was the in vivo activity of CLR against M. ulcerans in the mouse. When administered at a daily dose of 100 mg/kg (that is equipotent to 1 g per day in humans [19]), CLR had an obvious bacteriostatic activity: the growth of M. ulcerans in mice treated with CLR was significantly delayed compared to that in control mice. However, since the activity was not bactericidal, one may question the potential or practical value of CLR in the treatment of M. ulcerans infection in humans.
More important from the point of view of treatment of human disease are the bactericidal activities exhibited by RIF, RBT, and AMK against M. ulcerans, as demonstrated in the second experiment. When mice infected with M. ulcerans were treated for 8 weeks with RIF alone, RBT alone, or AMK alone, no footpad swelling occurred either during treatment or during the 4-month period following completion of treatment. In contrast, swelling of footpads occurred during experimental treatment of some mice with SPX or MIN, and after completion of treatment in all mice treated with CLR, SPX, or MIN. In addition, at the end of the 4-month follow-up period, all footpads from mice treated with RIF, RBT, or AMK remained culture-negative, suggesting a strong bactericidal activity of all three drugs against M. ulcerans. These findings are in agreement with previous reports. Havel and Pattyn (4) showed that in mice infected with 103 M. ulcerans and then subjected to RIF given at different daily doses, frequencies, and durations of treatment, some were apparently cured by the treatments. No footpad swelling occurred during the 4-month period of observation following completion of 4 months of treatment with either RIF at 10 mg/kg given 5 days a week or 15 mg/kg given 5 or 3 days a week. Stanford and Phillips (17) also reported the value of RIF used alone or in combination and beginning 2 or 5 weeks after mice had been injected in the footpads and obvious swelling had occurred. Treatment was continued for 1 or 3 months. Each 1-month regimen resulted in local improvement, but the disease relapsed 6 to 8 weeks after completion of therapy. In contrast, all 3-month regimens produced apparent cure. The combination of RIF with clofazimine or pyrimethamine plus sulfadoxine was not more effective than RIF alone.
The activity of AMK against M. ulcerans has not been previously reported. However, another aminoglycoside, streptomycin (SM), given at the daily dose of 3 mg (150 mg/kg of body weight), has been tested against M. ulcerans in the footpads of mice (2, 11). Treatments were started the day after infection and given for 7 to 16 weeks. SM exhibited an antimicrobial activity comparable to that exhibited by AMK in our experiment.
In conclusion, both rifamycin derivatives (RIF and RBT) and AMK are likely to be of interest for the treatment of M. ulcerans infection in humans. SM is likely to be as active as AMK.
ACKNOWLEDGMENTS
This work was supported by the Association Française Raoul Follereau.
We thank Henri Asse and Henri Kouakou from the Raoul Follereau Institute, Adzopé, Ivory Coast, for providing the strain of M. ulcerans and George Kubica for his editorial assistance. We also thank Roselyne Marc and Micheline Rousselet for their technical assistance.
REFERENCES
- 1.Dautzenberg B, Truffot C, Legris S, Meyohas M C, Berlie H C, Mercat A, Chevret S, Grosset J. Activity of clarithromycin against Mycobacterium avium complex in patients with the acquired immune deficiency syndrome. Am Rev Respir Dis. 1991;144:564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- 2.Feldman W H, Karlson A G. Mycobacterium ulcerans infections: response to chemotherapy in mice. Am Rev Tuberc. 1957;75:266–279. doi: 10.1164/artpd.1957.75.2.266. [DOI] [PubMed] [Google Scholar]
- 3.Fenner F. Pathogenic behavior of Mycobacterium ulcerans and Mycobacterium balnei in mouse and developing chick embryo. Am Rev Tuberc. 1956;73:650–673. doi: 10.1164/artpd.1956.73.5.650. [DOI] [PubMed] [Google Scholar]
- 4.Havel A, Pattyn S R. Activity of rifampicin on Mycobacterium ulcerans. Ann Soc Belge Med Trop. 1975;55:105–108. [PubMed] [Google Scholar]
- 5.Heifets L B. Clarithromycin against Mycobacterium avium complex infections. Tuberc Lung Dis. 1996;77:19–26. doi: 10.1016/s0962-8479(96)90070-2. [DOI] [PubMed] [Google Scholar]
- 6.Inderlied C B, Salfinger M. Antimycobacterial agents and susceptibility tests. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 1601–1623. [Google Scholar]
- 7.Ji B, Lounis N, Truffot-Pernot C, Grosset J. Effectiveness of various antimicrobial agents against Mycobacterium avium complex in the beige mouse model. Antimicrob Agents Chemother. 1994;38:2521–2529. doi: 10.1128/aac.38.11.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji B, Jamet P, Perani E G, Bobin P, Grosset J H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J Infect Dis. 1993;168:188–190. doi: 10.1093/infdis/168.1.188. [DOI] [PubMed] [Google Scholar]
- 9.Ji B, Jamet P, Perani E G, Sow S, Lienhardt C, Petinon C, Grosset J H. Bactericidal activity of single dose of clarithromycin plus minocycline, with or without ofloxacin, against Mycobacterium leprae in patients. Antimicrob Agents Chemother. 1996;40:2137–2141. doi: 10.1128/aac.40.9.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacCallum P, Tolhurst J C, Buckle G, Sissons H A. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93–102. [PubMed] [Google Scholar]
- 11.Pattyn S R, Royackers J. Traitement de l'infection expérimentale de la souris par Mycobacterium ulcerans et Mycobacterium balnei. Ann Soc Belge Med Trop. 1965;45:31–38. [PubMed] [Google Scholar]
- 12.Peto R, Pike M C, Armitage P, Breslow N E, Cox D R, Howard S V, Mantel N, McPherson K, Peto J, Smith P G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portaels F, Traore H, De Ridder K, Meyers W M. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob Agents Chemother. 1998;42:2070–2073. doi: 10.1128/aac.42.8.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepard C C. A kinetic method for the study of the activity of drugs against Mycobacterium leprae. Int J Lepr. 1967;35:429–436. [Google Scholar]
- 15.Shepard C C, McRae D H. A method for counting acid-fast bacteria. Int J Lepr. 1968;36:78–82. [PubMed] [Google Scholar]
- 16.Shepard C C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J Exp Med. 1960;112:445–454. doi: 10.1084/jem.112.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanford J L, Phillips I. Rifampicin in experimental Mycobacterium ulcerans infection. J Med Microbiol. 1972;5:39–45. doi: 10.1099/00222615-5-1-39. [DOI] [PubMed] [Google Scholar]
- 18.Van der Werf T, van der Graaf W T A, Tappero J W, Asiedu K. Mycobacterium ulcerans infection. Lancet. 1999;354:1013–1018. doi: 10.1016/S0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 19.Xiong J H, Ji B, Perani E G, Petinon C, Grosset J H. Further study of the effectiveness of single dose of clarithromycin and minocycline against Mycobacterium leprae in mice. Int J Lepr. 1994;62:37–42. [PubMed] [Google Scholar]