Abstract
Background and Aims
Petioles are important plant organs connecting stems with leaf blades and affecting light-harvesting ability of the leaf as well as transport of water, nutrients and biochemical signals. Despite the high diversity in petiole size, shape and anatomy, little information is available regarding their structural adaptations across evolutionary lineages and environmental conditions. To fill this knowledge gap, we investigated the variation of petiole morphology and anatomy of mainly European woody species to better understand the drivers of internal and external constraints in an evolutionary context.
Methods
We studied how petiole anatomical features differed according to whole-plant size, leaf traits, thermal and hydrological conditions, and taxonomic origin in 95 shrubs and trees using phylogenetic distance-based generalized least squares models.
Key Results
Two major axes of variation were related to leaf area and plant size. Larger and softer leaves are found in taller trees of more productive habitats. Their petioles are longer, with a circular outline and are anatomically characterized by the predominance of sclerenchyma, larger vessels, interfascicular areas with fibres and indistinct phloem rays. In contrast, smaller and tougher leaves are found in shorter trees and shrubs of colder or drier habitats. Their petioles have a terete outline, phloem composed of small cells and radially arranged vessels, fibreless xylem and lamellar collenchyma. Individual anatomical traits were linked to different internal and external drivers. Petiole length and vessel diameter increase with increasing leaf blade area. Collenchyma becomes absent with increasing temperature, and petiole outline becomes polygonal with increasing precipitation.
Conclusions
We conclude that species’ temperature and precipitation optima, plant height, and leaf area and thickness exerted a significant control on petiole anatomical and morphological structures not confounded by phylogenetic inertia. Species with different evolutionary histories but similar thermal and hydrological requirements have converged to similar petiole anatomical structures.
Keywords: Leaf area, petiole length, petiole anatomy, vessel diameter, supportive cells, temperate trees, temperate shrubs
Introduction
Petioles are one of the most efficient structures in plants and represent an essential connection between the stem and the plant’s photosynthetic machinery, the leaf blade (Niinemets and Fleck, 2002; Faisal et al., 2010; Levionnois et al., 2020). The primary function of a petiole is to provide mechanical support to self-hold and adjust leaf position towards the sun, improving light-harvesting ability. Apart from leaf position, they also play a key role in the hydraulic pathway throughout the plant, transporting water, nutrients and biochemical signals to the leaves, and photosynthates and other products towards the shoot (Niinemets and Fleck, 2002). Commonly green, and thus photosynthetically active, petioles may be stiff or flexible, long or short, but are usually wider at their base. In some woody taxa, they also can activate an abscission zone that ensures proper leaf shedding at senescence. Similar to variation of leaf blade size (Wright et al., 2017), the wide variation of petiole size, toughness and lifetime among plants must be considered when specific metabolic activities are evaluated. Vapour loss and hydraulic conductivity, for instance, change not only among species but also within the same taxa and are dependent on leaf position, sun exposure and vascular features (Sack et al., 2005; Poorter and Rozendaal, 2008).
Although petioles and leaves are highly diverse in size, shape and hence anatomical settings, comparative studies linking petiole structures with leaf parameters or whole-plant size across species and evolutionary lineages are rare. Most studies involve only one or a few closely related species (Niklas, 1992; Aasamaa and Sõber, 2010; Brocious and Hacke, 2016; Mahley et al., 2018), which provide a narrow view of potential variation. Petiole anatomical studies are mainly descriptive, focusing on the characterization of distinct taxa (Metcalfe and Chalk, 1950) or solving specific taxonomic problems (Ganem et al., 2019; Palacios-Rios et al., 2019; Karaismailoğlu, 2020). Nevertheless, anatomical traits are frequently combined with ecophysiological aspects (Niklas, 1992; Tadrist et al., 2014). Information about petiole biomechanics is commonly used to explain relationships between structure and anatomy, such as the size of epidermal cells and cross-sectional geometry compared with the size of the leaf blade, petiole stiffness, bending capacity or leaf angle (Niinemets and Fleck, 2002; Faisal et al., 2010; Levionnois et al., 2020). Additionally, distinct xylem traits such as vessel number, diameter and length have been used to examine physiological mechanisms of leaf hydraulic potential and vulnerability to xylem cavitation in petioles (Hacke and Sauter, 1996; Coomes et al., 2008; Aasamaa and Sõber, 2010; Hochberg et al., 2014; Brocious and Hacke, 2016; Gebauer et al., 2019).
Little information is available about the relative importance of internal (leaf and plant size) and external climatic (temperature, precipitation) drivers for interspecific variation in petiole anatomical and morphological structures. To our knowledge, only six studies providing an ecological interpretation that combines petiole morphoanatomy of one or a few species with abiotic conditions such as desiccation, light availability and wind gradient are available (Niklas, 1992; Hacke and Sauter, 1996; Niinemets and Fleck, 2002; Abrantes et al., 2013; Klepsch et al., 2016; Louf et al., 2018). Likewise, it is well known that large-statured and large-leaved plants predominate in wet, warm and productive environments, while short-statured and small-leaved plants occur in cold or arid environments, such as at high latitudes and elevations (Wright et al., 2017), but little is known about variation in petiole anatomy across these environmental and plant size gradients (Baird et al., 2021). Concerning vessel diameter variation, for instance, two explanations seem to complement each other, based on studies of roots, stems and branches (e.g. Zimmermann and Potter, 1982; Alder et al., 1996; Hacke and Sauter, 1996; Martinez-Vilalta et al., 2002). One hypothesizes that the variation in vessel diameter reflects environmental constraints operating at the whole-plant level (Hacke et al., 2017), while the other relates increasing vessel diameter along the plant stem to provide sap conduction requirements (Olson et al., 2013). However, to our knowledge, there are no studies that have combined petiole traits with whole-plant size, leaf traits and environmental factors in a phylogenetic context.
To fill this knowledge gap on petiole morphoanatomical trait variation and to better understand internal and external drivers and constraints in an evolutionary context, by using phylogenetic comparative models we investigated the variation of petiole morphology and anatomy among a census of mainly European woody species growing in contrasting environmental conditions. In particular, we studied how petiole anatomical features differed according to whole-plant size, leaf traits, thermal and hydrological conditions, and taxonomic origin for a wider group of temperate trees and shrubs. The study is based on 95 woody species and 18 morphoanatomical features, such as cross-sectional geometry, epidermis traits, and conductive, mechanical and storage tissue structures. Given that the leaves are the main photosynthetic apparatus of plants and that the petioles have a major role to support them mechanically and functionally, we assumed that the structure of the petiole will be more affected by leaf characteristics than by abiotic factors. We also expected that the supporting tissues (collenchyma and sclerenchyma) would be more developed in the larger leaves to optimize the ability to sustain leaves. Concomitantly, due to the effect of leaf size variation and hydraulic architecture being directly reflected in photosynthetic and water transport efficiency (Givnish, 1988; Terashima et al., 2011; Sack et al., 2012; Scoffoni et al., 2016), we expected vessel diameter to increase with leaf blade area.
MATERIALS AND METHODS
Plant species
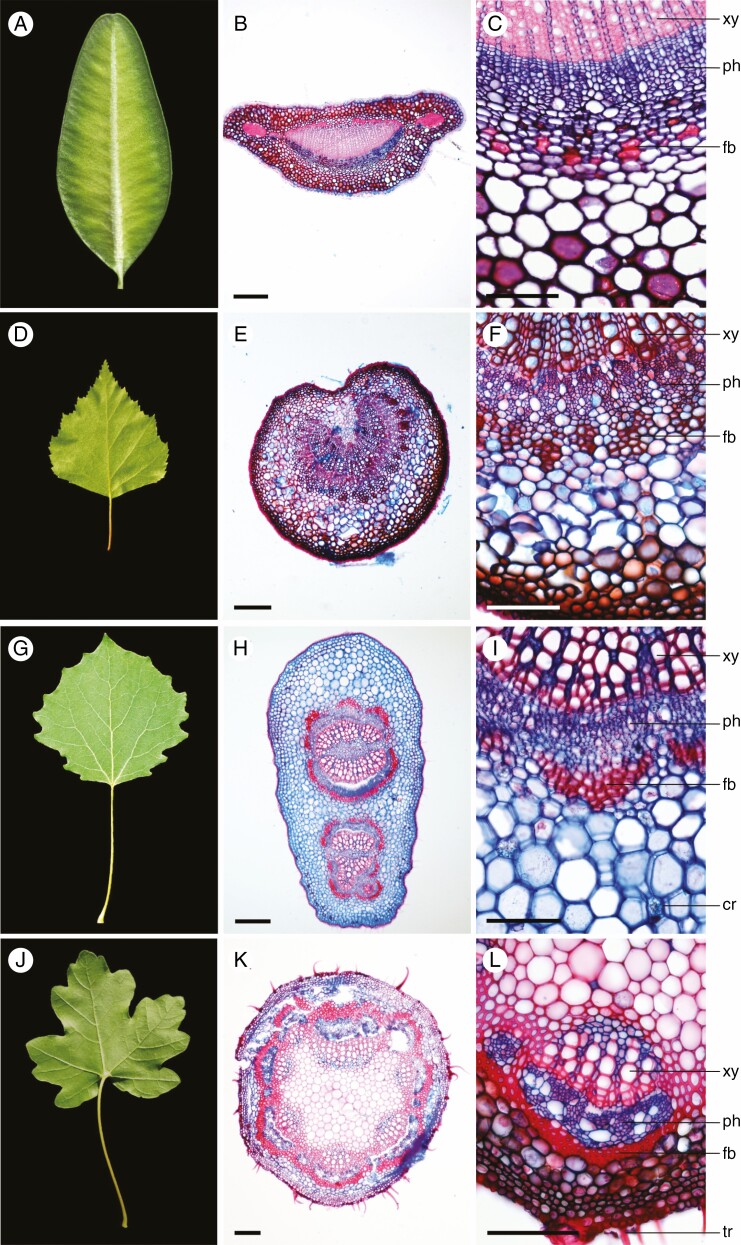
Our analysis was based on 95 woody species occurring in temperate and Mediterranean regions of Europe. The species belong to 72 genera and 35 families, with Rosaceae (14 taxa) as the most species-rich family, followed by Betulaceae (8), Salicaceae (7), Fabaceae (6), Fagaceae (6), Oleaceae (6), Ericaceae (5), Anacardiaceae (4) and other families (39). Prunus is the most represented genera (5), followed by Alnus (4), Quercus (4), Salix (4), Populus (4) and Acer (3). Most of the studied taxa are small trees (29), followed by large trees (25), large shrubs (19) and small shrubs (17), and woody lianas (5). Figure 1 shows examples of a few of the species analysed.
Fig. 1.
Buxus sempervirens (A–C), Betula pendula (D–F), Populus tremula (G–I) and Acer campestre (J–L). Petiole length is classified in < 2–5 cm (A), 5–10 cm (D, G) and > 10 cm (J). Cross-sections show petiole geometry: horizontally flattened (B), with an indentation (E), vertically flattened (H) and circular (K); and highlight trichomes (tr), fibre band (fb), phloem (ph), xylem (xy) and crystals (cr). Scale bars: 5 cm (A, D, J), 3 cm (G), 200 µm (B, E, H, K) and 100 µm (C, F, I, L).
Petiole anatomy
For each species, we randomly selected and sampled 3–5 fully expanded and uninjured, sunlit leaves from 3–5 canopy trees. Most samples were obtained during summer field seasons throughout a 5-year period (2012–2016) from trees growing in natural conditions in Switzerland. Six species were sampled in the Arboretum of Průhonice Park, Czech Republic, in summer 2016. Petioles were fixed and stored in 70 % ethanol. The mid-petiole region was selected in order to avoid the anatomical variation that may occur from the leaf base to the insertion point. Cross-sections 20–30 µm thick were made using a sledge microtome. We discoloured the sections with sodium hypochlorite, then double-stained them with a 1 : 1 aqueous Astrablue (0.5 %) and Safranin (1 %) blend, and finally mounted them on a permanent slide with Canada Balsam (Gärtner and Schweingruber, 2013; Schweingruber et al., 2020). The slides were examined using an Olympus BX53 microscope, Olympus DP73 camera and cellSense Entry 1.9 software.
We described a total of 18 anatomical features including cross-sectional geometry (outline), epidermis traits (cell and wall width) and cuticle thickness; the presence of trichomes, hypodermis, collenchyma, fibre band, crystals and secretory structures; and the arrangement of vascular bundles and interfascicular region, phloem (width of parenchyma cells) and xylem (vessel arrangement, vessel diameter and presence of fibres; Fig. 1). A complete characterization of the evaluated traits is presented in the Supplementary Data Figs S1–S50 and Tables S1–S4.
Quantitative anatomical measurements were also performed for each species using the software ImageJ. Cuticle thickness was obtained from an average of 20 measurements made along the periphery of the petiole. Evaluation of tissue predominance was focused on the vascular system (phloem and xylem) and fibre band (sclerenchyma); the total area of each tissue was measured followed by calculating their proportions in relation to the total cross-sectional area of the petiole. For average vessel diameter, we measured all the vessels present in several pre-defined representative 100 ×100 µm squares and average values were then calculated.
Morphology
For most of the species, we obtained data for plant height, leaf dry matter content (LDMC) and specific leaf area (SLA) from the LEDA database (Kleyer et al., 2008). We acquired leaf area (LA) values from the BIEN database (Enquist et al., 2016), TRY database (Kattge et al., 2020) and, in the case of Cercis siliquastrum, from the literature (Hatamian et al., 2019). All leaf trait measurements (LA, SLA and LDMC) included whole leaves (i.e. blades and petioles). For compound leaves, values for the whole leaves were used. For six species (i.e. Alnus cordata, Magnolia soulangeana, Pyrus communis, Rhododendron ferrugineum, Ulnus glabra and Vitex agnus) with no data available in databases or the literature, we measured leaf traits in 3–5 sunlit leaves collected from three canopy trees growing in the Arboretum of Průhonice Park, Czech Republic. Leaf area measurements were made using the ImageJ software (Dolezal et al., 2019). Mean values per species were used in further analyses.
Phylogeny
The phylogenetic relationships between the species studied were constructed based on three molecular markers: matK, rbcL and ITS (internal transcribed spacer). Combined, these three loci cover protein-coding, RNA-coding and non-coding sequences, as well as both plastid (matK, rbcL) and nuclear DNA (ITS). Varying mutational rates between loci maximizes the ability to discern major lineages together with species-level phylogenies; this threesome of markers has been suggested as a standard for phylogenetic analyses and barcoding of angiosperms (Li et al., 2017).
As a starting point, we acquired all relevant sequences from NCBI GenBank. All three combined matrices (one dataset per locus) were subsequently aligned in MAFFT 6 (Katoh and Toh, 2008), using the L-INS-i algorithm. Partial alignments were concatenated, manually adjusted in BioEdit (Hall, 1999), and subdued to the automated1 algorithm in trimAll software (Capella-Gutiérrez et al., 2009) to exclude highly divergent and gap-rich regions. The best-fit model for phylogenetic inference was selected according to the Bayesian information criterion (Schwarz, 1978) using the Baseml core of the Kakusan4 package (Adachi and Hagesawa, 1996; Tanabe, 2011), resulting in choice of the GTR model with rate variation across locations simulated by a discrete gamma distribution (Γ8), autocorrelated by the AdGamma rates prior, and unlinked for particular gene partitions. This model was subsequently submitted to MrBayes ver. 3.1.2 (Ronquist and Huelsenbeck, 2003) as the basis for Markov chain Monte Carlo (MCMC) analysis, encompassing two independent runs with four Metropolis-coupled MCMC chains of 107 generations sampled after every 1000th generation. In each run, one Markov chain was cold and three were incrementally heated by a parameter of 0.3. The first 25 % of entries were discarded as burn-in to eliminate trees sampled before reaching apparent stationarity, and the rest was used to compute the majority-rule consensus.
Data analyses
The estimation of species’ climatic preferences was obtained by calculating median values of annual mean temperature (bio1) and annual precipitation (bio12) using bioclimatic variables of the CHELSA climate database (Karger et al., 2016, 2017, 2018) and species coordinates of the GBIF database (Global Biodiversity Information Facility, www.gbif.org; see Supplementary Data Table S5 for DOIs of GBIF occurrence downloads). We used only GBIF coordinates without spatial issues and excluded occurrences that represented absences or were marked as managed. Species occurrences were downloaded from GBIF using the rgbif package for R (function occ_download with the predicates hasCoordinate equals TRUE and hasGeospatialIssue equals FALSE). Subsequently, climate data from the CHELSA layers were extracted for each species by the extract function of the raster package at all coordinates that represented presences (occurrenceStatus equals PRESENT) and no intentional cultivation (establishmentMeans not equal to MANAGED). To evaluate the effect of species’ temperature and precipitation preferences and plant morphology (height, LA, SLA and growth form) on petiole anatomy, we first performed ordination of petiole anatomical features, using non-metric multidimensional scaling, to find major axes of variation in anatomical features and to quantify the variation explained by environment and morphology.
Second, we used the phylogenetic distance-based generalized least squares model (D-PGLS, Adams, 2014b) to quantify how much variation in anatomical settings is explained by LA and other main predictors (plant height, SLA, precipitation and temperature) after accounting for LA while controlling for phylogenetic inertia. Our 18 anatomical variables were used to calculate the distance matrix among all 91 species (four species were excluded due to missing values) using a simple matching coefficient (Legendre and Legendre, 1998):
The distance matrix was used as the response; LA, SLA, height, annual precipitation and mean annual temperature were used as predictors. Since the distribution of most predictors was skewed or they had outlier values, the following transformations were performed: natural logarithm of LA, square root of height, and rank values for precipitation and temperature. The implementation of the model we used does not allow simultaneous estimation of the strength of the phylogenetic signal. We estimated and accounted for it with the appropriate degree by running the model with a phylogenetic tree transformed with the varying value of Pagel’s lambda (Pagel, 1999). We used lambda values from 0 (no phylogenetic signal) to 1 (phylogenetic signal corresponding to Brownian motion) varying by 0.01 and selected the model with the highest explained variability. Correlations among predictors were low (strongest being between LA and height, 0.41), and thus explained variability was not highly dependent on the order of the predictors. Therefore, we present explained variability for LA initially, followed by each predictor variable described twice, after accounting only for LA and after accounting for all the other predictors. We also explored the effect of each of our predictors on each anatomical trait separately using the same modelling approach.
Estimation of the phylogenetic signal in the anatomy of petioles alone was performed using the distance matrix that we had constructed for the main model. We also estimated multivariate generalization of Blomberg’s K (Blomberg et al., 2003; Adams, 2014a) and subsequently employed permutation tests to observe a statistical difference from the Brownian motion model of evolution.
The D-PGLS model was evaluated and the phylogenetic signal was estimated and tested using the package geomorph (ver. 3.3.0; Adams et al., 2019), and the phylogenetic tree was scaled using the package geiger (ver. 2.0.7; Harmon et al., 2008). All analyses were done in R (ver. 4.0.0; R Core Team, 2020).
RESULTS
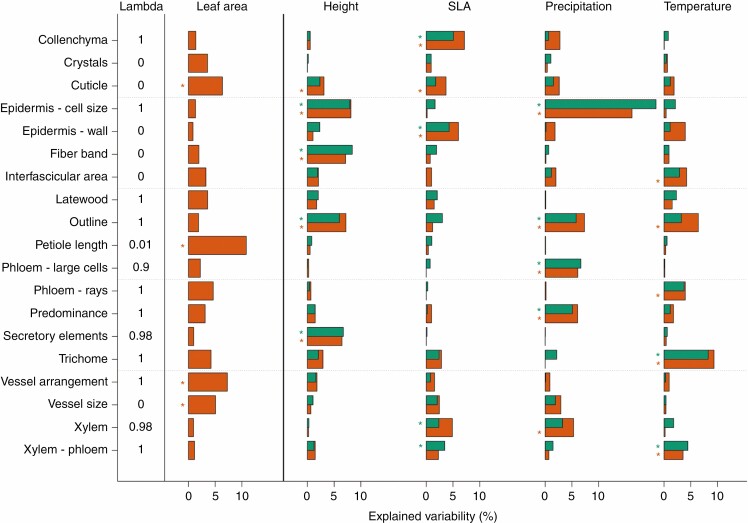
In analyses of individual petiole features, LA, plant height, temperature and precipitation were found to be linked to different anatomical traits (Fig. 2). For instance, variation in vessel diameter and vessel arrangement was mainly linked to LA, epidermis cell size to precipitation and plant height, petiole length to LA, cuticle thickness to SLA, and the presence of trichomes to temperature. Epidermal cell wall thickness was mainly related to precipitation. The presence of collenchyma was strongly determined by SLA, and tissue type proportion was linked to precipitation, phloem rays to temperature, and petiole outline to plant height and precipitation.
Fig. 2.
Explained variability in individual anatomical traits by LA, height, SLA, precipitation and temperature. Brown bars show explained variability accounting only for the covariate LA; green bars denote explained variability after accounting for the other predictors (including LA). *P < 0.05. In the left column is the estimated strength of the phylogenetic signal in each model (Pagel’s lambda). LA = leaf area, SLA = specific leaf area.
LA, height, SLA, precipitation and temperature each affected petiole anatomy (P-values after accounting for other predictors: 0.028, 0.015, 0.035, 0.006, 0.008, respectively) and together explained 12.19 % of the variability after controlling for phylogeny. The estimated phylogenetic signal of the main model was 1 (Pagel’s lambda), which corresponds to the Brownian motion model of evolution. The estimated strength of phylogenetic signal, Kmult, in anatomical traits only (without predictors) was 0.3983, which was significantly different from 1 (P = 0.001). Kmult < 1 indicates that the power of the phylogenetic signal was lower than expected under the Brownian motion model of evolution.
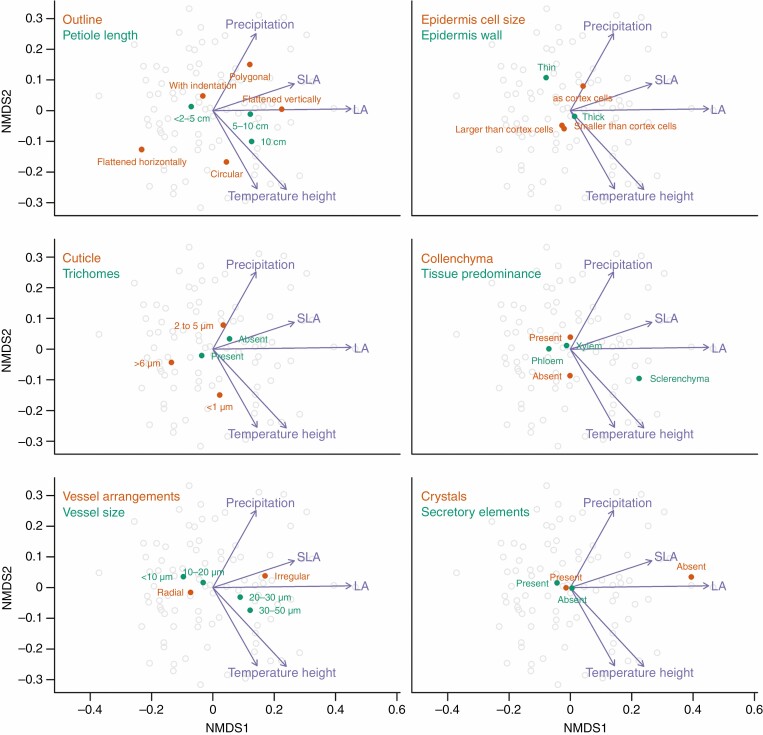
NMDS ordination (Fig. 3) showed that the main interspecific differences in petiole anatomy along the first axis were associated with variation in leaf area (LA is positively correlated with SLA), from soft leaves (i.e. composed of more parenchyma tissue and less schlerenchyma) with high SLA to tough leaves (i.e. composed of more schlerenchyma tissue) with low LA and high LDMC (SLA is negatively correlated with LDMC, a result not shown). The petiole anatomical changes along the second axis were associated with variation across temperature, precipitation and plant height gradients, from cold-adapted shrubs (i.e. families Cornaceae, Grossulariaceae and Aquifoliaceae) to warm-adapted tall trees (i.e. families Sapindaceae and Hippocastaneaceae; Fig. 3).
Fig. 3.
Ordination of species based on anatomical traits with a projection of predictors and selected anatomical traits. In all six panels, the same ordination is visualized only with projected centroids of levels of different selected traits. NMDS was performed on the distance matrix used in the main model (see Methods), with three axes (only the first two are visualized) and with stress 0.175 [using the package vegan (ver. 2.5-6; Oksanen et al., 2018)]. NMDS = non-metric multidimensional scaling, LA = leaf area, SLA = specific leaf area. (See also Supplementary Data Figs S1–S5 for the characterization of the cross-sectional geometry.).
Trees and shrubs with larger and softer leaves (high LA, high SLA, low LDMC) tended to have longer petioles (Figs 4 and 5) with epidermal cells of the same size as cortex cells, a thin cuticle without trichomes, irregularly arranged wide vessels, a predominance of sclerenchyma, phloem composed of large cells and interfascicular areas with fibres. Petioles of large trees were usually longer (5–10 cm), with almost circular or polygonal external outline, thin cuticle (< 1 µm), xylem fibres present, phloem rays indistinct, and prismatic crystals and druses (Figs 5 and 6). In contrast, trees with smaller leaves (low SLA and high LDMC) were generally smaller statured and from drier habitats with high precipitation seasonality. The petioles of these smaller trees were characterized by a thick cuticle (> 6 µm), simple and non-glandular trichomes, epidermal cells smaller than cortex cells, and phloem composed of small cells and radially arranged vessels (Fig. 3). Moreover, petioles of smaller trees and shrubs tended to have a circular external form, cuticle width of 2–5 µm, fibreless xylem, fibre band absent, an interfascicular area without fibres and lamellar collenchyma.
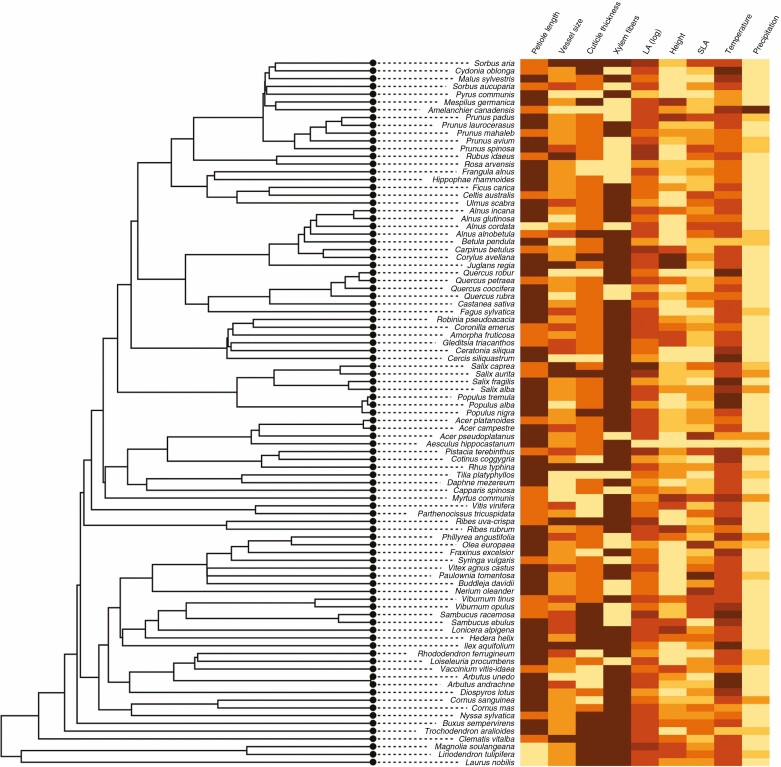
Fig. 4.
Phylogenetic tree with visualization of traits and environmental preferences of plants. The lowest values of each trait are in yellow and the highest values in brown.
Fig. 5.
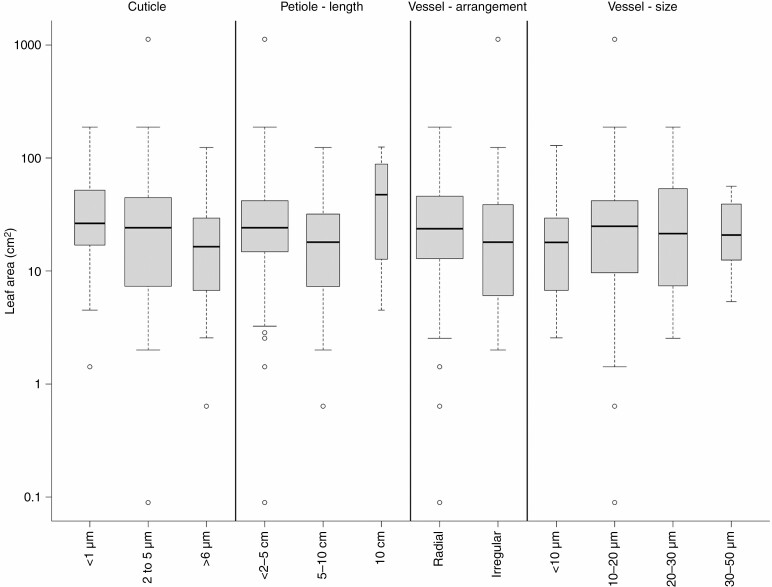
Relationship between leaf area and selected anatomical traits. The width of boxplots corresponds to the square root of the number of observations in a particular group.
Fig. 6.
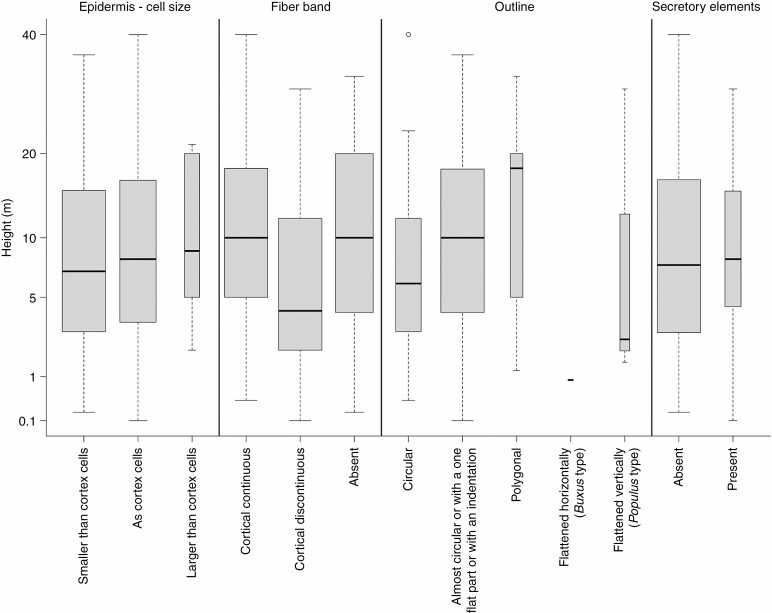
Relationship between height and selected anatomical traits. The width of boxplots corresponds to the square root of the number of observations in a particular group.
Stabilizing elements: petiole outline, epidermis, collenchyma and fibre bands
Plant height and precipitation accounted for the most variation in petiole outline (Fig. 3). A circular outline with flat section or indentation predominated among the studied species (68 %), typically in shrubs and smaller trees from Cornaceae, Aquifoliaceae, Rosaceae and Oleaceae. A circular petiole outline (22 % of taxa) tended to predominate in regions with lower precipitation (Fig. 3). A vertically flattened outline was found only in Populus spp. and horizontally flattened outline in Buxus sempervirens. A polygonal outline (5 %) occurred in unrelated taxa such as Clematis vitalba, Loiseleuria procumbens, Prunus laurocerasus, Ribes rubrum and Sambucus ebulus.
Unlike the petiole outline, the greatest variation in the petiole length was explained by leaf area (Figs 2 and 5). Shorter petioles (<2–5 cm) occurred in 65 % of species, followed by petioles 5–10 cm long (31 %), while longer petioles were found in only 4 % of species including Acer spp. and Ribes rubrum (Fig. 4). Variation in petiole cuticle thickness was primarily related to LA (Figs 2 and 5). Shrubs and small trees of Cornaceae, Aquifoliaceae, Rosaceae and Oleaceae, accounting for 49 % of all species within the study, had smaller leaves with a thick (2–5 µm) cuticle (Fig. 4). The largest cuticles (>6 µm) were associated with Mediterranean trees such as Phillyrea angustifolia, Quercus coccifera and Laurus nobilis (Fig. 4). Thin cuticles (<1 µm) predominated in large trees with bigger leaves such as Acer spp., Carpinus betulus, Castanea sativa, Celtis australis, Ceratonia siliqua, Cercis siliquastrum and Quercus robur (Supplementary Data Figs S6–S14 for the anatomical characterization of the cuticle).
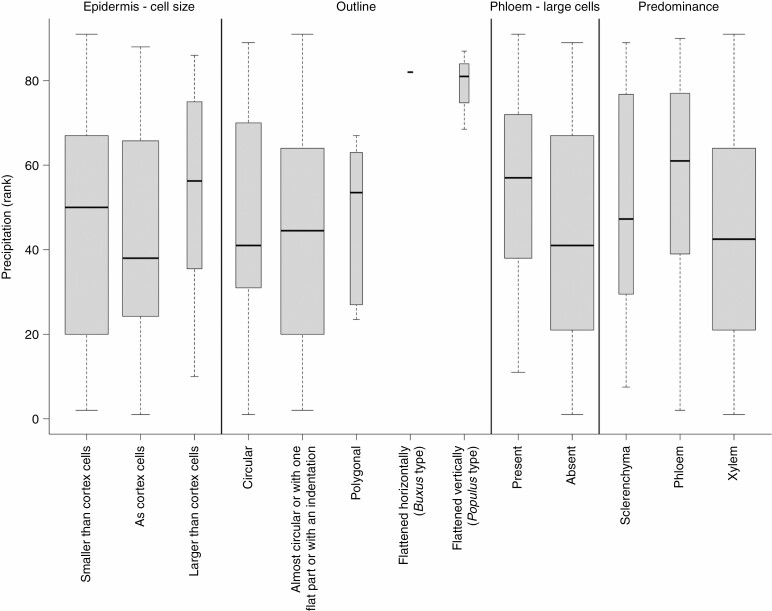
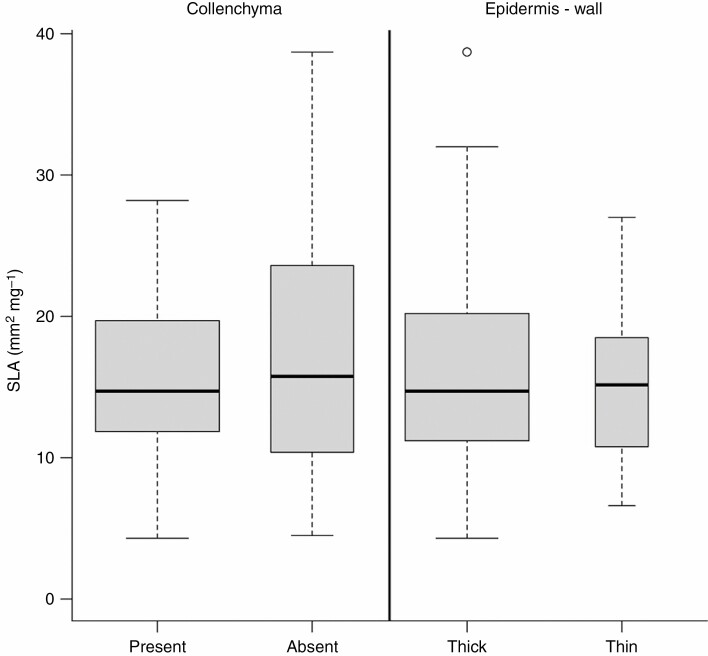
Plant height and precipitation accounted for the greatest variation in the petiole epidermal cells (Figs 2, 6 and 7). In 56 % of the species, epidermal cells were smaller than cortex cells, usually in smaller trees or shrubs with small SLA such as Salix, Prunus, Corylus, Vaccinium, Viburnum and Syringa (Fig. 4). Tall trees of the genera Quercus, Populus, Ulmus and Tilia had petiole epidermal cells of the same size as cortex cells. Petiole epidermal cells were larger than cortex cells in only 6 % of cases, such as in Aesculus hippocastanum and Prunus avium (see also Supplementary Data Figs S6–S14 for the anatomical characterization of the epidermis). SLA accounted for the greatest variation in epidermal cell-wall thickness (Fig. 8). Most of the tree species with larger SLA had thick-walled epidermal cells (84 %), while thin epidermal cell walls occurred among lianas (Vitex, Hedera) and shrubs (Ilex aquifolium, Capparis spinosa, Viburnum spp., Glyzirhyza glabra).
Fig. 7.
Relationship between precipitation and selected anatomical traits. The width of boxplots corresponds to the square root of the number of observations in a particular group.
Fig. 8.
Relationship between SLA and selected anatomical traits. The width of boxplots corresponds to the square root of the number of observations in a particular group.
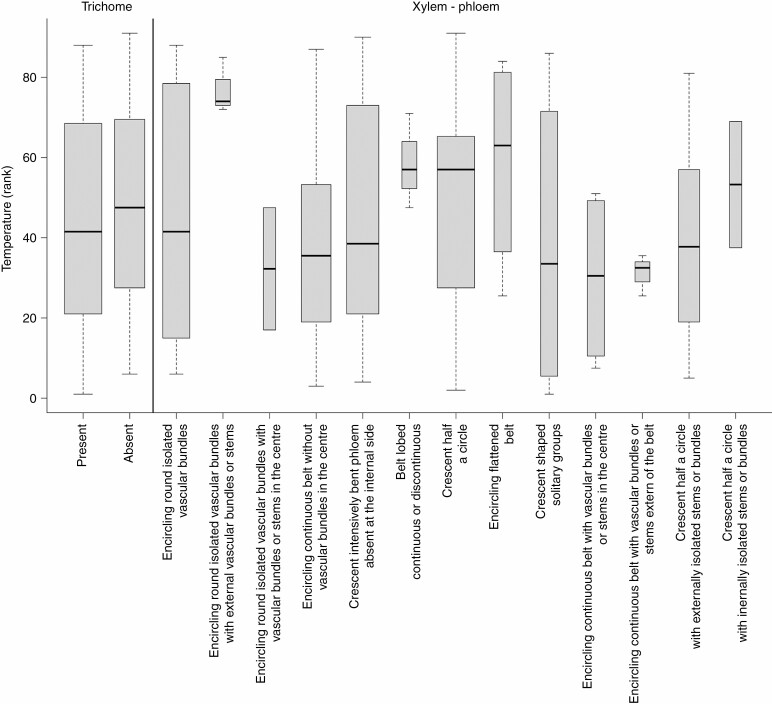
Temperature accounted for the greatest variation in epidermal trichomes (Figs 2 and 9), which are either unicellular (42 %), multicellular (19 %) or absent (39 %). Unicellular and mostly non-glandular trichomes predominated in small trees and shrubs from warmer regions and with high leaf thickness (high LDMC) such as Buxus sempervirens, Cydonia oblonga, Nerium oleander, Prunus spp. and Salix spp. (Fig. 4). Petiole trichomes were missing in large trees with large and softer leaves such as Fagus sylvatica, Aesculus hippocastanum and Robinia pseudoacacia. Less represented were branched non-glandular trichomes (Hippophae rhamnoides, Olea europaea, Quercus coccifera, Rhododendron ferrugineum), simple glandular trichomes (Corylus avellana, Rhus typhina, Vitex agnus-castus) and branched glandular trichomes (Buddleja davidii; see also Supplementary Data Figs S6–S14 for the anatomical characterization of the trichomes).
Fig. 9.
Relationship between temperature and selected anatomical traits. The width of boxplots corresponds to the square root of the number of observations in a particular group.
The main mechanical support was achieved through the formation of collenchyma at the periphery of the petioles and stabilizing cortical fibre bands (see Supplementary Data Figs S15–S21 for the anatomical characterization of the cortex). Variation in collenchyma was mainly related to SLA (Figs 2 and 8), with lamellar collenchyma being found in 70 % of species, mostly in shrubs and small trees with smaller leaves such as from Ericaceae, Cornaceae, Grossulariaceae and Aquifoliaceae, while collenchyma tended to be missing in taller trees with large leaves such as Populus tremula, Paulownia tomentosa and Juglans regia (Fig. 4). Variation in cortical fibre bands was related primarily to plant height (Figs 2, 3 and 6), with fibre bands identified as continuous (36 % of taxa), discontinuous (36 %) or absent (28 %). Fibre bands were largely absent in smaller trees and shrubs from Cornaceae, Aquifoliaceae, Rosaceae, Rhamnaceae, Grossulariaceae and Oleaceae, while continuous cortical fibre bands were highly developed in closely related large trees from Fagaceae (Quercus, Fagus, Castanea), Sapindaceae (Acer) and all Fabaceae (Amorpha fruticosa, Ceratonia siliqua, Cercis siliquastrum, Gleditsia triacanthos, Glycyrrhiza glabra and Robinia pseudoacacia; Fig. 4).
Structure of the conductive system
The structure of vascular bundles was mainly related to SLA and temperature (Figs 3 and 9). Three vascular bundle types were observed among the studied taxa: (1) crescent-shaped phloem and xylem (51 %) were found mostly in shrubs and smaller trees with tough leaves (low SLA), such as Laurus nobilis, Buxus sempervirens, Nerium oleander, Phillyrea angustifolia, Prunus mahaleb and Loiseleuria procumbens; (2) phloem and xylem encircling vascular bundles were found in 41 % of species, mostly large trees with softer leaves (Quercus, Acer, Gleditsia, Juglans, Robinia); and (3) multiple internal stems were found in (4 %) of the species, Populus spp. and Salix aurita (see Supplementary Data Figs S22–S33 for the anatomical characterization of the vascular system). In vascular bundles, xylem predominated (76 % of species) over phloem (15 % of species) and sclerenchymatous tissue (9 % of species). Phloem dominating over other tissue types was more frequently found in smaller trees (Malus, Prunus, Rhus, Salix) and shrubs (Lonicera alpigena, Rhododendron ferrugineum), while sclerenchyma predominated over xylem and phloem in unrelated groups of larger trees from warmer and drier habitats such as in Liriodendron tulipifera, Magnolia × soulangeana, Glycyrrhiza glabra and Quercus coccifera (see Figs S34–S48 for the anatomical characterization of the phloem and xylem and tissue proportion). Most of the studied species, including most tall trees, had lignified xylem with fibres composed of small cells (72 %; Fig. 4), while xylem without fibres tended to occur in smaller trees and shrubs across different families but all belonging to Rosopsida (Anacardiaceae, Araliaceae, Aquifoliaceae, Caprifoliaceae, Fabaceae, Rosaceae, Trochodendraceae) (see Figs S34–S41 for the anatomical characterization of the xylem).
The arrangement of vessels in the xylem was best explained by variation in LA (Figs 2 and 3). The vessels were primarily radially distributed among the studied species (71 %) and less frequently irregularly distributed (29 %; Fig. 2). Radially distributed vessels were mostly <20 µm in diameter and typically found in species with smaller and thicker leaves such as Nerium oleander, Phillyrea angustifolia, Buxus sempervirens and Arbutus unedo (see Supplementary Data Figs S34–S41 for the anatomical characterization of the xylem). Irregularly distributed vessels were often >20 µm in diameter and found in tall trees with softer leaves including Quercus and Populus, but also lianas such as Clematis vitalba and Hedera helix (Figs 2 and 4). Variation in vessel diameter was primarily related to leaf size variation (Fig. 5). Narrow vessels (10–20 µm) occurred in 56 % of taxa, generally in shorter trees and shrubs with smaller leaves, while higher trees with bigger leaves such as Quercus petraea, Juglans regia, Aesculus hippocastaneum and lianas (Parthenocissus tricuspidata and Vitis vinifera) had wider vessels (30–50 µm; Fig. 4). The narrowest vessels in petioles were typically found in small shrubs of cold and wet habitats such as Rhododendron ferrugineum and Vaccinium vitis-idaea or tree species from seasonally dry climates such as Phillyrea angustifolia, Quercus coccifera, Ilex aquifolium, Gleditsia triacanthos and Arbutus unedo (Fig. 4; see Figs S42–S48 for the anatomical characterization of the xylem vessels). Most of the studied species have phloem composed of small cells (72 %), while the proportion of species with large phloem cells is highest in small trees restricted to a few families such as Rosaceae (Malus sylvestris, Mespilus germanica, Pyrus communis, Sorbus aria, Sorbus aucuparia), Salicaceae, Sapindaceae and Grossulariaceae. Phloem rays are distinct (49 % of taxa) and indistinct (51 %), influenced by temperature (Fig. 2). The highest share of species with distinct phloem rays includes smaller trees from Betulaceae, Salicaceae and Rosaceae (Fig. 4).
Crystals and secretory elements
Variation in crystals and secretory elements was primarily related to plant height and LA, respectively (Figs 2 and 3). Distinct crystal types (prismatic, druses, acicular, sand) were found mainly in shorter taxa with smaller and thicker leaves (small SLA and high LDMC, Fig. 3). Species with both prismatic crystals and druses were the most common (46 %), followed by petiole with only druses (26 %), only prismatic crystals (9 %), acicular crystals (6 %), sand (4 %), and druses and sand (2 %). Acicular crystals were found in species from closely related families of the order Lamiales (Lamiaceae, Oleaceae, Paulowniaceae) such as Olea europaea, Syringa vulgaris, Paulownia tomentosa and Vitex agnus-castus. Sand crystals were found in Hippophae rhamnoides, Laurus nobilis, Phillyrea angustifolia and Sambucus spp. Secretory elements were found only in 12 % of the species. Secretory ducts occurred in 7 % of the species, including small trees (Myrtus communis, Rhus typhina), shrubs (Cotinus coggygria, Frangula alnus, Pistacia lentiscus), lianas (Hedera helix) and large trees (Tilia platyphyllos). Slime content was present in 5 % of taxa, exclusively in trees such as Betula pendula, Prunus avium, Prunus mahaleb and Ulmus glabra.
Discussion
We present here morphological and anatomical trends in petiole traits of woody species, as a first attempt to provide a broader view on the variation in petioles, which are a key organ supporting the main photosynthetic machinery in leaf blades and playing an essential role in the hydraulic pathway within the plant. Using D-PGLS models, we tested how petiole anatomical features differed according to whole-plant size, leaf traits, thermal and hydrological conditions, and phylogenetic origin in 95 shrubs and trees. We found that species’ temperature and precipitation optima, plant height, and leaf area and thickness exerted a significant control on petiole anatomical and morphological structures not confounded by phylogenetic inertia. Species with different evolutionary histories but similar thermal and hydrological requirements have converged to similar petiole anatomical and hence morphological structures. Our evaluation improves our understanding of how variation in petiole morphoanatomical traits is driven by plant height and leaf characteristics, as well as gradients of temperature and precipitation in an evolutionary context.
Longer petioles tended to be more frequent in larger leaves (higher LA) from taller tree species. The general plant advantage of this tendency is to provide radical changes in leaf orientation and optimize light-harvesting (Takenaka, 1994; Niinemets et al., 2004). Indeed, the combination of petiole length and leaf area directly influences leaf angle, which may reduce the shading of basal foliage and thus increase light-interception (Niklas, 1992; Takenaka, 1994; Niinemets and Fleck, 2002; Niinemets et al., 2004). The relationship between leaf size (leaf area and petiole length) and plant height also raised questions associated with wind interference. Considering that leaf size may vary with plant height (Niinemets and Kull, 1994), that longer petioles are more flexible to strong winds (Vogel, 1989) and that wind speed increases with height in the canopy (Niinemets and Fleck, 2002), we would expect that longer petioles would be found in leaves of taller trees in order to resist wind bending. Indeed, variation in petiole length is considered to be a leaf strategy to cope with the vertical gradient of wind speed on trees, reinforcing the tendency we observed here.
Additionally to petiole length, petiole cross-sectional geometry (outline) is also an important trait that influences leaf response to wind stresses and leaf self-holding (Niklas, 1992, 1996; Niinemets and Fleck, 2002; Faisal et al., 2012; Louf et al., 2018). In our observations, we identified five different petiole outlines: circular, with indentation (terete petiole), flattened horizontally, flattened vertically and polygonal. We found that those with an indentation and polygonal outline tend to occur in longer petioles from taller trees, while circular outlines tend to be more frequent in shorter petioles from smaller trees. This certainly represents an important morphological trait to understand trends in petiole biomechanics. However, because of the scarce information on this topic, it is difficult to evaluate in detail how outlines influence leaf torsion and bending under wind exposure. Nevertheless, a previous report showed that petioles with a vertically flattened outline, for instance, typical of Populus species, promote the reduction of leaf torsion by the wind when compared to petioles with indentation (Niklas, 1992). Our findings call for more experimental studies to better understand how petiole geometry influences leaf flexibility and support among trees.
Despite morphological trends, the main differences between petioles occurred anatomically and were directly influenced by leaf area. In general, larger leaves would require petioles with appropriate anatomical qualities to provide more leaf support and flexibility to handle more intense mechanical stresses (Vincent, 1982; Niklas, 1992). On the first layer of cells, the epidermis width and the thickness of its cell walls are considered to provide important tension-stiffening traits that, together with adjacent tissues, may strongly influence the flexural stiffness of the plant organ (Niklas and Paolillo, 1997; Niinemets and Fleck, 2002). In our observations, smaller leaves tended to present smaller epidermal cells and thin cell walls, which could represent a result of the lower effect of wind when compared to bigger leaves with wider epidermis. Towards the centre of the petiole, the increased frequency of sclerenchyma and collenchyma in larger leaves with longer petioles is consistent with their distinctive biomechanical properties. While collenchyma has soft cell walls, with viscoelastic properties that permit greater petiole bending without breakage, sclerenchyma has lignified and more rigid cell walls, which support and prevent mechanical damage to the adjacent, softer tissues. Indeed, we observed that collenchyma was located on the periphery of petioles, where bending is more intense, while sclerenchyma was associated with vascular bundles, generally located in the centre of the petiole. This tissue organization matches the typical distribution in petioles (Evert, 2006). Moreover, distinct parts of the leaf (midrib, leaf border and leaf apex) characteristically have well developed collenchyma and sclerenchyma to better hold the mechanical stress rate (Niklas, 1992; Evert, 2006). In the swollen base of petioles, for instance, which holds the leaf and prevents its breakage, the presence of collenchyma is abundant and promotes more plastic movements according to the wind (Niklas, 1992; Evert, 2006), while sclerenchyma is especially located next to the phloem, preventing rupture of the thin cell walls due to mechanical and drought stress (Evert, 2006).
Since leaf anatomy is extremely adaptable to environment conditions (Metcalf and Chalk, 1950; Kröber et al., 2015; Stojnić et al., 2016) and intimately linked to temperature and water gradients (Bussotti et al., 1995; Gravano et al., 1999; Doria et al., 2019), the presence of distinct tissues (epidermis, parenchyma, colenchyma, schlerenchyma) may indicate the conditions to which plants are exposed. However, anatomical variations have mainly described for the leaf blades and less information is available for petioles. We observed that shorter petioles from drier environments, for instance, tend to have smaller epidermal cells and thicker cuticles, which are typical traits to reduce water loss to the environment (Porsch, 1926; Ennajeh et al., 2010; De Micco and Aronne, 2012). Indeed, it is well known that the combination of these anatomical traits together with the reduction of leaf size and the higher proportion of schlerenchyma represent features that are frequently observed in leaves (leaf blade) exposed to water deficiency, high temperatures and light intensity (Castro Díez et al., 1998; Rotondi et al., 2003). However, our findings showed a distinct pattern for sclerenchyma in petioles. We observed that the proportion of sclerenchyma is higher in longer petioles from environments with higher temperatures but with no water deficiency. This tendency may indicate that the main role of sclerenchyma in petioles is to provide biomechanical support instead of preventing water loss, in agreement with the main petiole function of supporting the leaves.
Concerning xylem traits, our analysis showed that vessel diameter is also linked to leaf area, and again with petiole length. While vessels with larger diameter are found in longer petioles (with larger leaves), narrower vessels are developed on shorter petioles (with smaller leaves). Leaf vascular properties are commonly known to be strongly determined by leaf morphology (Salisbury, 1913; Coomes et al., 2008) but consistent responses are also connected to climatic variables (Sellin and Kupper, 2007; Scoffoni et al., 2008; Sack, 2012; Sanginés de Cárcer et al., 2017; Hacke et al., 2017; Kardošová et al., 2020). Even though we observed that xylem vessels tend to enlarge with higher levels of precipitation and temperature, the strongest relationship was still found with leaf size. This tendency agrees with the associations between vessel widening and increasing leaf area previously made in petioles from Fraxinus americana, Quercus robur, Acer pseudoplatanus and other trees (Nicklas, 1992; Coomes et al., 2008; Lechthaler et al., 2019; Levionnois et al., 2020). In these studies, the authors analysed single or a few species, but here we show that the same trend occurred when a larger group of species is considered. It is known that longer petioles can only maintain larger leaves with wider vessels (Coomes et al., 2008; Levionnois et al., 2020) as the reduction of vessel diameter would represent a diminished efficiency of water transport to the leaves (Sack et al., 2003; Brodribb, 2009; Jordan et al., 2013; Scoffoni et al., 2016; Levionnois et al., 2020). This is in line with previous observations of vessels widening within the leaf, from the very narrow vessels at the end of the sap path close to the stomata towards the wider vessels at the base of the leaf (Rosell et al., 2017, 2019). However, a decrease in vessel diameter in colder, drier climates provides a safer hydraulic structure, less vulnerable to embolisms induced by drought or freezing conditions, as narrow vessels are more resistant to cavitation (Zimmermann, 1983; Cochard, 2002).
Another interesting anatomical trend revealed here is the presence of different cell types in vascular tissues, which may be considered relevant for the taxonomy of certain groups. Despite the high plasticity of leaf anatomy, different studies have shown that vascular patterns and superficial traits (epidermis, cuticle, trichomes) in petioles have an important value for the taxonomy of distinct groups (Hare, 1944; Kocsis and Borhidi, 2003; Noraini et al., 2016; Talip et al., 2017; Anu and Dan, 2020; Karaismailoğlu, 2020). Our findings showed that in small trees and shrubs from some families (Anacardiaceae, Araliaceae, Aquifoliaceae, Caprifoliaceae, Fabaceae, Grossulariaceae, Rosaceae, Salicaceae, Sapindaceae, Trochodendraceae) the phloem has scattered larger parenchymatic cells and the xylem is fibreless. Even though neither of these traits has been considered to be axonomically relevant in petioles, in woody stems the patterns of parenchyma and the presence and absence of fibres are defined as the most conspicuous traits to characterize species (Chattaway, 1953; Roth, 1981; Archer and van Wyk, 1993; den Outer, 1993). Moreover, previous studies showed similar characteristics on the midrib of the same species (Săvulescu and Luchian, 2009; El-Alfy et al., 2011; Koçyiğit et al., 2015; Jušković et al., 2017). Even though anatomical changes occur along the petiole and at the beginning of the midrib (Sack and Holbrook, 2006), both traits seem to have the potential for taxonomic applications.
The structural traits of petioles we evaluated in this study included morphological and anatomical aspects that characterized their shape, stiffness and hydraulic potential. Our analysis was based on 95 major woody species from Europe representing one of the first attempts to find trends for the broad and diversified group of woody plants. Our results showed that leaf area has the strongest influence on petiole anatomical traits, more than temperature, precipitation and plant height, emphasizing the supportive and mechanical role of this part of the leaf. Petioles tend to be longer and have a circular outline in larger leaves. Anatomically, mechanically supportive cells (collenchyma and sclerenchyma) tend to be more predominant and xylem vessels tend to have a larger diameter in bigger leaves. These traits are aligned with our expectations, as bigger leaves represent more weight for self-holding and demand a more efficient vascular tissue. In the case of smaller leaves, petioles tend to be shorter, to have an outline with an indentation and have narrower vessels. Indeed, environmental factors may directly influence distinct plant organs, but our results appear to reveal a different pattern for petioles that relates to the leaf itself. Since this study is constrained geographically to Europe and hence is based on a limited set of woody species selected from otherwise highly species-rich functional groups of trees and shrubs, further investigations are needed to better understand the evolution of petiole structures in temperate and especially tropical woody species.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figures S1–S5: Characterization of the cross-sectional geometry. Figures S6–S14: Anatomical characterization of the epidermis. Figures S15–S21: Anatomical characterization of the cortex. Figures S22–S33: Anatomical characterization of the vascular system. Figures S34–S41: Anatomical characterization of the phloem and xylem. Figures S42–S48: Anatomical characterization of the xylem vessels and tissue proportion. Figure S49: Variability of petiole anatomy by LA, height, SLA, temperature and precipitation. Figure S50: Phylogenetic tree of species’ morphoanatomical variation. Table S1: List of anatomical features described for cross-sectional geometry and epidermis of petioles from each species. Table S2: List of anatomical features described for the cortical area and vascular bundles of petioles from each species. Table S3: List of anatomical features detailing vascular tissues in each species. Table S4: List of anatomical features described for tissue predominance and presence and absence of crystals and secretory structure from each species. Table S5: List of GBIF download DOIs for each species and climatic preferences of temperature and precipitation.
ACKNOWLEDGMENTS
We thank Fritz H. and Elisabeth Schweingruber for their support and generous donation to A.C.
FUNDING
This work was supported by Czech Science Foundation [grant no. 21-26883S] and Ministry of Education, Youth and Sport of the Czech Republic [grant no. LTAUSA18007].
LITERATURE CITED
- Aasamaa K, Sõber A. 2010. Sensitivity of stem and petiole hydraulic conductance of deciduous trees to xylem sap ion concentration. Biologia Plantarum 54: 299–307. [Google Scholar]
- Abrantes J, Campelo F, García-González I, Nabais C. 2013. Environmental control of vessel traits in Quercus ilex under Mediterranean climate: relating xylem anatomy to function. Trees 27: 655–662. [Google Scholar]
- Adachi J, Hagesawa M. 1996. MOLPHY version 2.3: Programs for molecular phylogenetics based on maximum likelihood. Computer Science Monographs, No. 28. Tokyo: Institute of Statistical Mathematics. [Google Scholar]
- Adams DC. 2014a. A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Systematic Biology 63: 685–697. [DOI] [PubMed] [Google Scholar]
- Adams DC. 2014b. A method for assessing phylogenetic least squares models for shape and other high-dimensional multivariate data. Evolution 68: 2675–2688. [DOI] [PubMed] [Google Scholar]
- Adams DC, Collyer ML, Kaliontzopoulou A. 2019. Geomorph: Software for geometric morphometric analyses. https://cran.r-project.org/package=geomorph. [Google Scholar]
- Alder NN, Sperry JS, Pockman WT. 1996. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia 105: 293–301. [DOI] [PubMed] [Google Scholar]
- Anu S, Dan M. 2020. Taxonomic significance on comparative petiole anatomy of twelve species of Curcuma L. (Zingiberaceae) from South India. Plant Archives 20: 35–41. [Google Scholar]
- Archer RH, van Wyk AE. 1993. Bark structure and intergeneric relationships of some southern African Cassinoideae (Celastraceae). IAWA Journal 14: 35–53. [Google Scholar]
- Baird AS, Taylor SH, Pasquet-Kok J, et al. 2021. Developmental and biophysical determinants of grass leaf size worldwide. Nature 592: 242–247. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T, Ives AR. 2003. Testing for signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- Brocious CA, Hacke UG. 2016. Stomatal conductance scales with petiole xylem traits in Populus genotypes. Functional Plant Biology 43: 553–562. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ. 2009. Xylem hydraulic physiology: The functional backbone of terrestrial plant productivity. Plant Science 177: 245–251. [Google Scholar]
- Bussotti F, Bottacci A, Bartolesi A, Grossoni P, Tani C. 1995. Morphoanatomical alterations in leaves collected from beech trees (Fagus sylvatica L.) in conditions of natural water stress. Journal of Environmental and Experimental Botany 35: 201–213. [Google Scholar]
- Capella-Gutierrez S, Silla-Martınez JM, Gabaldon T. 2009. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Díez P, Villar-Salvador P, Pérez-Rontomé C, Maestro-Martinez M, Montserrat-Martf G. 1998. Leaf morphology, leaf chemical composition and stem xylem characteristics in two Pistacia (Anacardiaceae) species along a climatic gradient. Flora 193: 195–202. [Google Scholar]
- Chattaway MM. 1953. The anatomy of bark. I. The genus Eucalyptus. Australian Journal of Botany 1: 402–433. [Google Scholar]
- Cochard H. 2002. Xylem embolism and drought-induced stomatal closure in maize. Planta 215: 466–471. [DOI] [PubMed] [Google Scholar]
- Coomes DA, Heathcote1 S, Godfrey ER, Shepherd JJ, Sack L, 2008. Scaling of xylem vessels and veins within the leaves of oak species. Biology Letters 4: 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Micco V, Aronne G. 2012. Morpho-anatomical traits for plant adaptation to drought. In: Aroca R, ed. Plant responses to drought stress. Berlin: Springer, 37–61. [Google Scholar]
- Den Outer RW. 1993. Evolutionary trends in secondary phloem anatomy of trees, shrubs and climbers from Africa (mainly Ivory Coast). Acta Botanica Neerlandica 42: 269–287. [Google Scholar]
- Dolezal J, Klimes A, Dvorsky M, Riha P, Klimesova J, Schweingruber F. 2019. Disentangling evolutionary, environmental and morphological drivers of plant anatomical adaptations to drought and cold in Himalayan graminoids. Oikos 128: 1576–1587. [Google Scholar]
- Dória LC, Podadera DS, Lima RS, Lens F, Marcati CR. 2019. Axial sampling height outperforms site as predictor of wood trait variation. IAWA Journal 40: 191–214. [Google Scholar]
- El-Alfy TSMA, El-Gohary HMA, Sokkar NM, El-Tawab SA, Al-Mahdy DAM. 2011. Botanical and genetic characteristics of Celtis australis L. and Celtis occidentalis L. grown in Egypt. Bulletin of Faculty of Pharmacy, Cairo University 49: 37–57. [Google Scholar]
- Ennajeh M, Vadel AM, Cochard H, Khemira H. 2010. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. The Journal of Horticultural Science and Biotechnology 85: 289–294. [Google Scholar]
- Enquist BJ, Condit R, Peet RK, Schildhauer M, Thiers BM. 2016. Cyberinfrastructure for an integrated botanical information network to investigate the ecological impacts of global climate change on plant biodiversity. PeerJ Preprints 4: e2615v2. [Google Scholar]
- Evert R. 2006. Parenchyma and collenchyma. In: Evert R, ed. Esau’s plant anatomy: meristems, cells and tissues of the plant body - their structure, function and development. Hoboken: Wiley, 175–190. [Google Scholar]
- Faisal TR, Abad EMK, Hristozov N, Pasim D. 2010. The impact of tissue morphology, cross-section and turgor pressure on the mechanical properties of the leaf petiole in plants. Journal of Bionic Engineering 7 Suppl.: S11–S23. [Google Scholar]
- Faisal TR, Rey AD, Pasini D. 2012. Hierarchical microstructure and elastic properties of leaf petiole tissue in Philodendron melinonii. MRS Online Proceedings Library 1420: 67–72. [Google Scholar]
- Ganem MA, Luna ML, Ahumada O, Giudice GE. 2019. Estudio morfo-anatómico comparado en pecíolos de las especies de Asplenium (Aspleniaceae) de Argentina. Boletin de la Sociedad Argentina de Botanica 54: 191–201. [Google Scholar]
- Gärtner H, Schweingruber FH. 2013. Microscopic preparation techniques for plant stem analysis, 1st edn. Remagen: Verlag Dr. Kessel. [Google Scholar]
- Gebauer R, Albrechtová P, Plichta R, Volařík D. 2019. The comparative xylem structure and function of petioles and twigs of mistletoe Loranthus europaeus and its host Quercus pubescence. Trees 33: 933–942. [Google Scholar]
- Givnish TJ. 1988. Adaptation to sun and shade: A whole-plant perspective. Australian Journal of Plant Physiology 15: 63–92. [Google Scholar]
- Gravano E, Bussotti F, Grossoni P, Tani C. 1999. Morpho-anatomical and functional modifications in beech leaves on the top ridge of the Apennines (central Italy). Phyton; Annales Rei Botanicae 39: 41–46. [Google Scholar]
- Hacke U, Sauter JJ. 1996. Drought-induced xylem dysfunction in petioles, branches, and roots of Populus balsamifera L. and Alnus glutinosa (L.) Gaertn. Plant Physiology 111: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke UG, Spicer R, Schreiber SG, Plavcová L. 2017. An ecophysiological and developmental perspective on variation in vessel diameter. Plant, Cell & Environment 40: 831–845. [DOI] [PubMed] [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hare CL. 1944. On the taxonomic value of the anatomical structure of vegetative organs of the dicotyledons: 5. The anatomy of the petiole and its taxonomic value. Proceedings of the Linnean Society of London 155: 223–229. [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: Investigating evolutionary radiations. Bioinformatics 24: 129–131. [DOI] [PubMed] [Google Scholar]
- Hatamian M, Nejad AR, Kafi M, Souri MK, Shahbazi K. 2019. Growth characteristics of ornamental judas tree (Cercis siliquastrum L.) seedlings under different concentrations of lead and cadmium in irrigation water. Acta Scientiarum Polonorum Hortorum Cultus 18: 87–96. [Google Scholar]
- Hochberg U, Degu A, Gendler T, Fait A, Rachmilevitch S. 2014. The variability in the xylem architecture of grapevine petiole and its contribution to hydraulic differences. Functional Plant Biology 42: 357–365. [DOI] [PubMed] [Google Scholar]
- Jordan GJ, Brodribb TJ, Blackman CJ, Weston PH. 2013. Climate drives vein anatomy in Proteaceae. American Journal of Botany 100: 1483–1493. [DOI] [PubMed] [Google Scholar]
- Jušković MZ, Vasiljević PJ, Savić AV, Jenačković DD, Stevanović MB. 2017. Comparative morphoanatomical analysis of the leaves and stems of Daphne (Thymelaeaceae) species. Biologia 72: 709–721. [Google Scholar]
- Karaismailoğlu MC. 2020. Petiole anatomy of 21 representatives of Tribe Alysseae (Brassicaceae) from Turkey. KSÜ Tarım ve Doğa Derg 23: 1535–1544. [Google Scholar]
- Kardošová M, Husárová H, Kurjak D, et al. 2020. Variation in leaf anatomy, vascular traits and nanomechanical cell-wall properties among European beech (Fagus sylvatica L.) provenances. Annals of Forest Science 77: 83. [Google Scholar]
- Karger DN, Conrad O, Böhner J, et al. 2016. CHELSA climatologies at high resolution for the earth’s land surface areas (Version 1.1). World Data Center for Climate (WDCC) at DKRZ. ; 10.1594/WDCC/CHELSA_v1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karger DN, Conrad O, Böhner J, et al. 2017. Climatologies at high resolution for the Earth land surface areas. Scientific Data 4: 170122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karger DN, Conrad O, Böhner J, et al. 2018. Data from: Climatologies at high resolution for the earth’s land surface areas. Dryad, Dataset. doi: 10.5061/dryad.kd1d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H. 2008. Improved accuracy of multiple ncRNA alignment by incorporating structural information into a MAFFT-based framework. BMC Bioinformatics 9: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattge J, Bönisch G, Díaz S, et al. 2020. TRY plant trait database – enhanced coverage and open access. Global Change Biology 26: 119–188. [DOI] [PubMed] [Google Scholar]
- Klepsch M, Lange A, Angeles G, Mehltreter K, Jansen S. 2016. The hydraulic architecture of petioles and leaves in tropical fern species under different levels of canopy openness. International Journal of Plant Sciences 177: 209–216. [Google Scholar]
- Kleyer M, Bekker RM, Knevel IC, et al. 2008. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. Journal of Ecology 96: 1266–1274. [Google Scholar]
- Kocsis M, Borhidi A. 2003. Petiole anatomy of some Rubiaceae genera. Acta Botanica Hungarica 45: 345–353. [Google Scholar]
- Koçyiğit M, Büyükkılıç B, Altınbaşak O, Ubul N. 2015. Comparative leaf anatomy of three food plants that are used medically; Mespilus germanica L., Malus sylvestris (L.) Mill. subsp. orientalis and Cydonia oblonga Mill. (Rosaceae). Journal of the Faculty of Pharmacy of İstanbul 46: 39–48. [Google Scholar]
- Kröber W, Heklau H, Bruelheide H. 2015. Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biology 17: 373–383. [DOI] [PubMed] [Google Scholar]
- Lechthaler S, Colangeli P, Gazzabin M, Anfodillo T. 2019. Axial anatomy of the leaf midrib provides new insights into the hydraulic architecture and cavitation patterns of Acer pseudoplatanus leaves. Journal of Experimental Botany 70: 6195–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Legendre L. 1998. Numerical ecology, 2nd edn. Amsterdam: Elsevier. [Google Scholar]
- Levionnois L, Coste S, Nicolini E, Stahl C, Morel H, Heuret P. 2020. Scaling of petiole anatomies, mechanics, and vasculatures with leaf size in the widespread Neotropical pioneer tree species Cecropia obtusa Trécul (Urticaceae). Tree Physiology 40: 245–258. [DOI] [PubMed] [Google Scholar]
- Li H, Xu Q, Lundgren MR, Ye Q. 2017. Different water relations between flower and leaf periods: a case study in flower-before-leaf-emergence Magnolia species. Functional Plant Biology 44: 1098–1110. [DOI] [PubMed] [Google Scholar]
- Louf J, Nelson L, Kang H, Song PN, Zehnbauer T, Jung S. 2018. How wind drives the correlation between leaf shape and mechanical properties. Scientific Reports-UK 8: 16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley JN, Pittermann J, Rowe N, et al. 2018. Geometry, allometry and biomechanics of fern leaf petioles: their significance for the evolution of functional and ecological diversity within the Pteridaceae. Frontiers in Plant Science 9: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Prat E, Oliveras I, Piñol J. 2002. Xylem hydraulic properties of roots and stems of nine Mediterranean woody species. Oecologia 133: 19–29. [DOI] [PubMed] [Google Scholar]
- Metcalfe CR, Chalk L. 1950. Anatomy of the dicotyledons, vol. II, 1st edn. Oxford: Clarendon Press. [Google Scholar]
- Niklas KJ. 1992. Petiole mechanics, light interception by lamina, and “economy in design”. Oecologia 90: 518–526. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. 1996. Differences between Acer saccharum leaves from open and wind-protected sites. Annals of Botany 78: 61–66. [Google Scholar]
- Niklas KJ, Paolillo DJ. 1997. The role of the epidermis asa stiffening agent in Tulipa (Liliaceae) stems. American Journal of Botany 84: 735–744. [PubMed] [Google Scholar]
- Niinemets U, Afas NA, Cescatti A, Pellis A, Ceulemans R. 2004. Petiole length and biomass investment in support modify light-interception efficiency in dense poplar plantations. Tree Physiology 24: 141–154. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Fleck S. 2002. Petiole mechanics, leaf inclination, morphology, and investment in support in relation to light availability in the canopy of Liriodendron tulipifera. Oecologia 132: 21–33. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Kull K. 1994. Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. Forest Ecology and Management 70: 1–10. [Google Scholar]
- Noraini T, Ruzi AR, Ismail BS, Hani BU, Salwa S, Azeyanty JA. 2016. Petiole vascular bundles and its taxonomic value in the tribe Dipterocarpeae (Dipterocarpaceae). Sains Malaysiana 45: 247–253. [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2018. vegan: community ecology package. R package. https://cran.r-project.org. [Google Scholar]
- Olson ME, Rosell JA. 2013. Vessel diameter–stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytologist 197: 1204–1213. [DOI] [PubMed] [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Palacios-Rios M, Galán JMG, Prada C, Rico-Gray V. 2019. Structure of the petioles and costae of Mexican and Central American species of Pteris (Polypodiopsida, Pteridaceae). Phytotaxa 401: 101–116. [Google Scholar]
- Poorter L, Rozendaal DMA. 2008. Leaf size and leaf display of thirty-eight tropical tree species. Oecologia 158: 35–46. [DOI] [PubMed] [Google Scholar]
- Porsch O. 1926. Zur physiologischen Bedeutung der Verholzung. Berichte der Deutschen Botanischen Gesellschaft 4: 137–142. [Google Scholar]
- R Core Team. 2020. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. Mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rosell JA, Olson ME, 2019. To furcate or not to furcate: the dance between vessel number and diameter in leaves. Journal of Experimental Botany 70: 5990–5993. [DOI] [PubMed] [Google Scholar]
- Rosell JA, Olson ME, Anfodillo T. 2017. Scaling of xylem vessel diameter with plant size: causes, predictions, and outstanding questions. Current Forestry Reports 3: 46–59. [Google Scholar]
- Roth I. 1981. Structural patterns of tropical barks. Berlin: Gebrüder Borntraeger. [Google Scholar]
- Rotondi A, Rossi F, Asunis C, Cesaraccio C. 2003. Leaf xeromorphic adaptations of some plants of a coastal Mediterranean macchia ecosystem. Journal of Mediterranean Ecology 4: 25–35. [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. 2003. The ‘hydrology’ of leaves: coordination of structure and function in temperate woody species. Plant, Cell & Environment 26: 1343–1356. [Google Scholar]
- Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology 57: 361–381. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C, McKown AD, et al. 2012. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nature Communications 3: 837. [DOI] [PubMed] [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. 2005. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist 167: 403–413. [DOI] [PubMed] [Google Scholar]
- Salisbury EJ. 1913. The determining factors in petiolar structure. New Phytologist 12: 281–289. [Google Scholar]
- Sanginés de Cárcer P, Signarbieux C, Schlaepfer R, Buttler A, Vollenweider P. 2017. Responses of antinomic foliar traits to experimental climate forcing in beech and spruce saplings. Environmental and Experimental Botany 140: 128–140. [Google Scholar]
- Săvulescu E, Luchian V. 2009. Comparative anatomy of the vegetative organs of the Hedera helix L. (Araliaceae). Scientific Papers, USAMV Bucharest, Series A, Vol. LII. [Google Scholar]
- Schwarz G. 1978. Estimating the dimension of a model. Annals of Statisti 6: 461–464. [Google Scholar]
- Schweingruber FH, Kucerova A, Adamec L, Dolezal J. 2020. Anatomic atlas of aquatic and wetland plant stems, 1st edn. Cham: Springer Nature Switzerland AG. [Google Scholar]
- Scoffoni C, Chatelet DS, Pasquet-kok J, et al. 2016. Hydraulic basis for the evolution of photosynthetic productivity. Nature Plants 2: 16072. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Pou A, Aasamaa K, Sack L. 2008. The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell & Environment 31: 1803–1812. [DOI] [PubMed] [Google Scholar]
- Sellin A, Kupper P. 2007. Temperature, light and leaf hydraulic conductance of little-leaf linden (Tilia cordata) in a mixed forest canopy. Tree Physiology 27: 679–688. [DOI] [PubMed] [Google Scholar]
- Stojnić S, Orlović S, Miljković D, von Wuehlisch G. 2016. Intra- and interprovenance variations in leaf morphometric traits in European beech (Fagus sylvatica L.). Archives of Biological Sciences 68: 781–788. [Google Scholar]
- Tadrist L, Saudreau M, Langre E. 2014. Wind and gravity mechanical effects on leaf inclination angles. Journal of Theoretical Biology 341: 9–16. [DOI] [PubMed] [Google Scholar]
- Takenaka A. 1994. Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecological Research 9: 109–114. [Google Scholar]
- Talip N, Cutler DF, Ahmad Puad AS, Ismail BS, Ruzi AR, Ahmad Juhari AA. 2017. Diagnostic and systematic significance of petiole anatomy in the identification of Hopea species (Dipterocarpaceae). South African Journal of Botany 111: 111–125. [Google Scholar]
- Tanabe AS. 2011. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Molecular Ecology Resources 11: 914–921. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets U. 2011. Leaf functional anatomy in relation to photosynthesis. Plant Physiology 155: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JFV. 1982. The mechanical design of grass. Journal of Materials Science 17: 856–860. [Google Scholar]
- Vogel S. 1989. Drag and reconfiguration of broad leaves in high winds. Journal of Experimental Botany 40: 941–948. [Google Scholar]
- Wright IJ, Dong N, Maire V, et al. 2017. Global climatic drivers of leaf size. Science 357: 917–921. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH. 1983. Xylem structure and the ascent of sap. Berlin: Springer-Verlag. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH, Potter D. 1982. Vessel-length distribution in branches, stem and roots of Acer rubrum L. IAWA Bulletin 3: 103–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.