Abstract
Background:
Novel influenza viruses continue to pose a potential pandemic threat worldwide. In recent years, plants have been used to produce recombinant proteins, including subunit vaccines. A subunit influenza vaccine, HAC1, based on recombinant hemagglutinin from the 2009 pandemic A/California/04/2009 (H1N1) strain of influenza virus, has been manufactured using a plant virus-based transient expression technology in Nicotiana benthamiana plants and demonstrated to be immunogenic and safe in pre-clinical studies (Shoji et al., 2011).
Methods:
A first-in-human, Phase 1, single-center, randomized, placebo-controlled, single-blind, dose escalation study was conducted to investigate safety, reactogenicity and immunogenicity of an HAC1 formulation at three escalating dose levels (15μg, 45μg and 90μg) with and without Alhydrogel®, in healthy adults 18–50 years of age (inclusive). Eighty participants were randomized into six study vaccine groups, a saline placebo group and an approved monovalent H1N1 vaccine group. Recipients received two doses of vaccine or placebo (except for the monovalent H1N1 vaccine cohort, which received a single dose of vaccine, later followed by a dose of placebo).
Results:
The experimental vaccine was safe and well tolerated, and comparable to placebo and the approved monovalent H1N1 vaccine. Pain and tenderness at the injection site were the only local solicited reactions reported following vaccinations. Nearly all adverse events were mild to moderate in severity. The HAC1 vaccine was also immunogenic, with the highest seroconversion rates, based on serum hemagglutination-inhibition and virus microneutralization antibody titers, in the 90 g non-adjuvanted HAC1 vaccine group after the second vaccine dose (78% and 100%, respectively).
Conclusions:
This is the first study demonstrating the safety and immunogenicity of a plant-produced subunit H1N1 influenza vaccine in healthy adults. The results support further clinical investigation of the HAC1 vaccine as well as demonstrate the feasibility of the plant-based technology for vaccine antigen production.
Keywords: Influenza A, H1N1, Recombinant vaccine, Hemagglutinin, Plant-produced
1. Introduction
Influenza is a serious respiratory disease caused by influenza viruses. During annual epidemics, three to five million cases of severe influenza illness are recorded worldwide, resulting in 250,000–500,000 deaths [1]. In addition to seasonal influenza outbreaks, occasional influenza pandemics can arise at any time when influenza A virus containing a novel hemagglutinin (HA) subtype is introduced and spreads efficiently among humans. In 2009, a novel, swine-origin influenza A H1N1 virus [A(H1N1)pdm09] emerged and infected humans. The virus, a triple reassortant with genes acquired from swine, avian and human influenza viruses [2], was first detected in people in the U.S. in April 2009 [3] and spread rapidly across the globe, primarily in children and younger adult populations with little pre-existing serologic immunity to the novel HA [4], causing the World Health Organization to declare a pandemic on June 11, 2009 [5].
Currently licensed influenza vaccines are made in embryonated eggs [6]. For the past four decades, these vaccines have been successfully used and proven to be safe and effective [7–9]. Recently, safety and immunogenicity of several 2009 H1N1 influenza A vaccines in healthy volunteers have been demonstrated [10–13]. However, the 2009 H1N1 pandemic demonstrated that egg-based technologies fall short of satisfying the global need for an emerging pandemic influenza vaccine in a timely manner. Therefore, a number of countries, including the U.S., are developing alternative economic and scalable platforms for production of large amounts of safe and effective recombinant, or subunit, vaccines in a short time frame. Some of these alternative manufacturing approaches are based on mammalian and insect cell expression systems [14–17]. Over the past ten years, plants have emerged as a highly promising approach to economically manufacture subunit vaccines and therapeutic proteins [18–22]. The immunogenicity and protective efficacy of plant-produced vaccine candidates against a variety of pathogens has been demonstrated in numerous pre-clinical studies [21,23–28]. More recently, safety and biological relevance of plant-produced therapeutic proteins and subunit vaccines, including H1N1 and H5N1 influenza vaccines, have been demonstrated in humans in Phase 1–3 clinical trials [29–33].
Fraunhofer USA Center for Molecular Biotechnology (FhCMB) has developed a transient expression system using Nicotiana benthamiana plants agroinfiltrated with a plant virus-based ‘launch’ vector encoding target sequence [34] and demonstrated its utility for producing vaccine antigens [23–27]. A recombinant HA influenza vaccine, HAC1, based on the A/California/04/2009 (H1N1) strain, for the prevention of disease caused by a novel A(H1N1)pdm09 virus, was developed and produced in N. benthamiana at pilot plant scale under current Good Manufacturing Practice (cGMP) guidelines [35]. Pre-clinical studies demonstrated safety and protective immunogenicity of HAC1 in animals [35] and prompted further investigation of this vaccine candidate in humans. A Phase 1 study was conducted to determine the safety, reactogenicity and immunogenicity of HAC1 delivered intramuscularly at three escalating dose levels in healthy adults.
2. Materials and methods
2.1. Study design
This study was a first-in-human, Phase 1, single-center, randomized, placebo-controlled, single-blind, dose-escalation clinical study conducted at the Walter Reed Army Institute of Research (WRAIR) in Silver Spring, Maryland. The protocol was approved by the Human Use Review Committee of the WRAIR and by the U.S. Army Medical Research and Material Command’s Human Subjects Research Board, Fort Detrick, Maryland. The study was conducted in accordance with the principles of the Declaration of Helsinki, the standards of Good Clinical Practice (as defined by the International Conference on Harmonization) and federal regulations. All participants provided written informed consent prior to screening and enrollment into the study.
The primary objective was to evaluate the safety, reactogenicity and tolerability of an HAC1 vaccine formulation delivered unadjuvanted or adjuvanted with Alhydrogel® intramuscularly at doses of 15 μg, 45 μg or 90 μg in a two-dose regimen delivered 21 days apart. The secondary objective was to evaluate and compare immunogenicity of two injections of this formulation with a single dose of a licensed monovalent H1N1 vaccine by measuring hemagglutination-inhibition (HAI) and virus microneutralization (MN) antibody titers.
This study was registered at www.clinicaltrials.gov under reference identifier NCT01177202.
2.2. Vaccine
The HAC1 vaccine, developed by FhCMB, is a formulated recombinant HA monomer based on A/California/04/2009 (H1N1) influenza virus containing a poly-histidine (6 × His) affinity purification tag and the endoplasmic reticulum retention signal, KDEL, at the C-terminus. The HA antigen was cloned, expressed in N. benthamiana, purified and characterized as reported previously [35]. The concentration of HAC1 was 360 μg/mL in an aqueous formulation of normal saline with trace amounts of PBS (45 mM NaCl, 0.9 mM KCl, 3 mM Na2HPO4 and 0.6 mM KH2PO4) for intramuscular administration.
The active control was a licensed Influenza A (H1N1) 2009 monovalent inactivated influenza virus vaccine that was administered per prescribing information as a single 0.5 mL intramuscular dose. No adjuvant was used with this licensed vaccine.
2.3. Study population and treatment
Healthy, non-pregnant adults 18–50 years of age (inclusive) were eligible for enrollment if they had a screening H1N1 HAI titer ≤40, no prior vaccination with 2010–2011 seasonal influenza vaccine containing A/California/04/2009-like virus, no medical condition that may be associated with impaired immune responsiveness, including diabetes mellitus, and no cancer or treatment for cancer within the previous three years, excluding basal cell carcinoma or squamous cell carcinoma. Subjects were excluded if they did not meet any of these criteria or had a history of anaphylactic type reaction to injected vaccines, positive serology for HIV-1, HIV-2, HBsAg or HCV antibodies or any acute or chronic pulmonary, cardiovascular, hepatic, neurologic or renal disease that might confound evaluation of the vaccine, or had recently taken or planned to take any other experimental vaccine within 30 days prior to vaccination.
A total of 80 subjects were enrolled and randomized into 8 groups of 10 subjects, to receive two doses of the vaccine formulation, placebo, or active control (an FDA-approved monovalent H1N1 vaccine). Subjects in the study vaccine groups received two doses of HAC1 at 15μg, 45μg or 90 μg, administered either nonadjuvanted (Group A [15 μg], Group C [45 gμ] and Group E [90 μg]) or with 0.3% aluminum hydroxide (Alhydrogel®) adjuvant (Brenntag Biosector, Denmark) (Group B [15 μg], Group D [45 μg] and Group F [90 μg]). The placebo control group received two doses of saline (0.9% Sodium Chloride, USP, Hospira, Lake Forrest, IL). The subjects in the active control group received an approved monovalent H1N1 vaccine on Day 0 and saline on Day 21 (the approved vaccine had a single-dose vaccine regimen for study subjects). All doses were administered in a volume of 0.5 mL. Subjects were blinded as to the group to which they were assigned. Vaccinations were administered in the deltoid muscle of the same non-dominant arm at Day 0 and Day 21. Dose escalation was staggered by at least 7 days to assess adverse events (AEs) before proceeding to higher doses.
2.4. Safety assessments
Safety was assessed by recording medical history, monitoring AEs and vital signs, and performing physical examinations and laboratory tests for chemistry and hematology. The safety endpoint was the proportion of subjects in each treatment group reporting one or more local or systemic solicited and unsolicited reactions within 7 days after vaccination (Days 0–6 and 21–27), and the occurrence of any serious AEs (SAEs), defined as any untoward events resulting in hospitalization or death, during the study. Clinical safety laboratory assessments were performed at baseline, day of vaccination, and 7 days after each vaccination. The presence of solicited local and general signs and symptoms, including measurement of oral temperature, were assessed after each vaccination and at 1, 2, 3, 7 and 14 days post-vaccination. The solicited injection site AEs were pain, tenderness, erythema, and induration. Solicited general AEs were fever, fatigue, headache, malaise, myalgia, nausea, and vomiting. Also, the investigators recorded any other AEs occurring on the first administration of the vaccine through Day 56 as unsolicited AEs. AEs were assessed for intensity. Injection site pain was graded as 1 = mild pain not interfering with function, 2 = moderate pain; repeated use of non-narcotic pain reliever >24 h or interferes with activity, 3 = severe pain; any use of narcotic pain reliever or prevents normal activity, and 4 = hospitalization. Solicited systemic AEs were graded as 1 = no interference with normal activity, 2 = some interference with normal activity, 3 = significant; prevents daily activity, and 4 = hospitalization. Additional grading scales were applied to visible swelling or redness at the injection site: 1 = 2.5–5 cm, 2 = 5.1–10 cm, 3 = >10 cm, and 4 = necrosis or exfoliative dermatitis and to oral temperature: 1 = 37–38.4°C, 2 = 38.5–38.9°C, 3 = 39–40°C, and 4 = >40°C. Unsolicited AEs were graded as 1 = easily tolerated with no interference with everyday activities, 2 = sufficiently discomforting to interfere with everyday activities, 3 = preventing everyday activities, and 4 = ER visit or hospitalization required. Vaccine-related AEs were those that the investigator judged as having a reasonable possibility that the vaccine contributed to the AE.
2.5. Immunogenicity assessments
Sera for immunogenicity assessments were collected on Day 0 (pre-vaccination), Day 21 (pre-vaccination) and Day 56, and sent to the Influenza Division, CDC for analysis by HAI and MN assays using previously described methods and an A/California/04/2009-like virus [36]. Immunogenicity assessments included determination of (1) geometric mean titer (GMT) of HAI and MN antibody titers; (2) the proportion of subjects in each group who seroconverted on Days 21 and 56; and (3) the proportion of subjects in each group who achieved an HAI titer of ≥40 or an MN titer of ≥80 or ≥160 on Days 21 and 56. Seroconversion was defined for HAI and MN titers according to the FDA guidance as the percentage of subjects with either a pre-vaccination titer of <10 and a post-vaccination titer of ≥40 or a pre-vaccination titer of >10 and a minimum 4-fold rise in a post-vaccination antibody titer.
2.6. Statistical analysis
Statistical analyses were performed by Statistics Collaborative, Inc. (SCI). The safety population included all subjects who received at least one dose of HAC1. Only subjects who received both doses of HAC1 were included in the immunogenicity analyses.
For clinical laboratory tests, shift and grade summaries were based on the FDA’s guidelines [37]. All AEs were coded for intensity and vaccine-relatedness and summarized by MedDRA System Organ Class. Incidence of any AE, any solicited AE (overall, local and systemic) and any unsolicited AE in each HAC1 vaccine group were compared against the recipients of the approved H1N1 vaccine, as well as against the recipients of placebo control. For analyses of AEs, Fisher’s exact test was used to compare each investigational vaccine group to the placebo control group and to the active control group.
Standard statistical analyses were used to distinguish significant changes in antibody titers. For each post-vaccination assessment, natural log-transformed HAI and MN titers between dose groups and between adjuvanted and non-adjuvanted groups were compared using the analysis of variance (ANOVA) method. The proportion of subjects who seroconverted and/or had an HAI titer achievement of ≥40 was determined using Fisher’s exact test. The relationship between the percentages of subjects with each endpoint and dose group was determined using the Cochran–Armitage trend test. No adjustment of P-values was made for multiple analyses.
3. Results
3.1. Study population
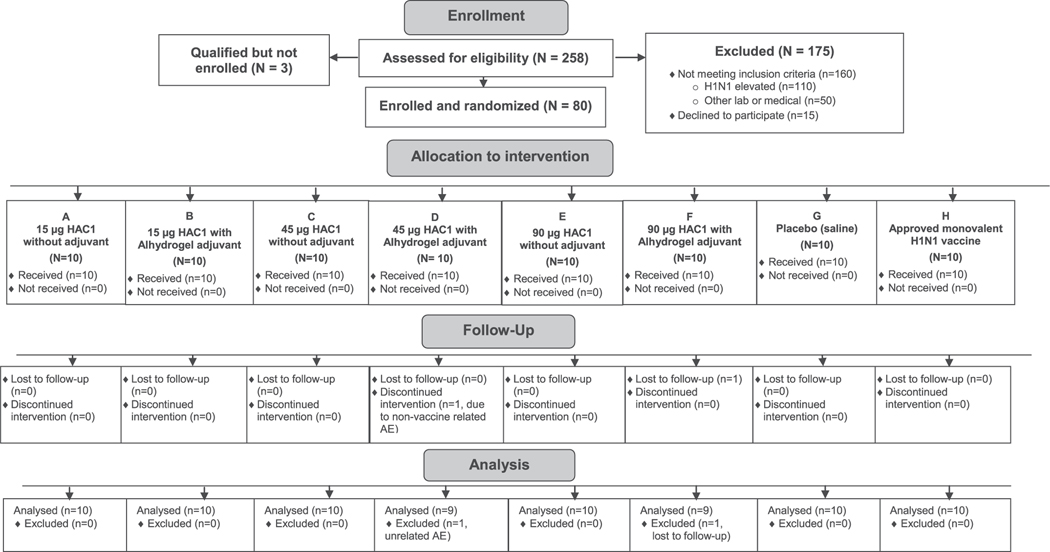
Eighty subjects were enrolled, randomized and received the first scheduled vaccination; 78 subjects received both vaccinations (two doses of the vaccine formulation, two doses of placebo, or one dose of the FDA-approved monovalent H1N1 vaccine and one dose of saline) and were included in the immunogenicity analysis (Fig. 1). The patient demographic characteristics are summarized in Table 1.
Fig. 1.
Subject disposition.
Table 1.
Demographic characteristics.
| Group A (15 μg) N = 10a |
Group B (15 μg + A)b N = 10 |
Group C (45 μg) N = 10 |
Group D (45 μg + A) N = 10 |
Group E (90 μg) N = 10 |
Group F (90 μg + A) N = 10 |
Group G (saline) N = 10 |
Group H (H1N1)c N = 10 |
Overall N = 80 |
|
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female, n (%) | 6 (60) | 7 (70) | 8 (80) | 9 (90) | 5 (50) | 2 (20) | 6 (60) | 2 (20) | 45 (56) |
| Age (years) | |||||||||
| Mean (SD) | 32.3 (8.2) | 33.2 (7.5) | 35.1 (8.7) | 31.9 (7.4) | 28.3 (6.4) | 32.5 (7.9) | 30.8 (7.9) | 28.1 (8.7) | 31.5 (7.8) |
| Median | 32 | 33 | 36 | 34 | 27 | 31 | 30 | 27 | 30 |
| Range (min, max) | 20, 48 | 22, 45 | 22, 47 | 22, 44 | 22, 39 | 23, 46 | 23, 45 | 19, 49 | 19, 49 |
| Ethnicity, n (%) | |||||||||
| Not Hispanic or Latino | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 10 (100) | 9 (90) | 10 (100) | 9 (90) | 78 (98) |
| Hispanic or Latino | 0 | 0 | 0 | 0 | 0 | 1 (10) | 0 | 1 (10) | 2 (3) |
| Race, n (%) | |||||||||
| Black or African American | 6 (60) | 6 (60) | 6 (60) | 2 (20) | 4 (40) | 4 (40) | 5 (50) | 5 (50) | 38 (48) |
| White | 4 (40) | 4 (40) | 4 (40) | 6 (60) | 4 (40) | 4 (40) | 3 (30) | 4 (40) | 33 (41) |
| Asian | 0 | 0 | 0 | 2 (20) | 2 (20) | 1 (10) | 2 (20) | 1 (10) | 8 (10) |
| Other | 0 | 0 | 0 | 0 | 0 | 1 (10) | 0 | 0 | 1 (1) |
N: number of subjects.
A: alhydrogel® adjuvant.
Approved monovalent vaccine containing an A/California (H1N1)-like strain.
3.2. Safety and reactogenicity
Vaccinations were safe and well tolerated in all groups. No deaths, SAEs, clinically significant laboratory abnormalities, or AEs of special interest were reported during the trial. No subject withdrew due to an adverse event.
Pain and/or tenderness at the injection site were the only local solicited AEs noted, being reported in 37 subjects (46%) (Tables 2 and 3). All such AEs were considered vaccine-related, and all but one were Grade 1 intensity, with a single subject in Group D (45 μg + adjuvant) reporting a Grade 2 reaction (Table 2). The occurrence of local solicited AEs increased with increasing dose levels, in both adjuvanted and non-adjuvanted groups, as well as with the addition of Alhydrogel® (Table 2).
Table 2.
Summary of all AEs after any vaccination.
| AEs, n (%) | Group A (15 μg) N = 10a |
Group B (15 μg + A)b N = 10 |
Group C (45 μg) N = 10 |
Group D (45 μg + A) N = 10 |
Group E (90 μg) N = 10 |
Group F (90 μg + A) N = 10 |
Group G (saline) N = 10 |
Group H (H1N1)c N = 10 |
Overall N = 80 |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Any AEs | 9/10 (90) | 9/10 (90) | 10/10 (100) | 10/10 (100) | 8/10 (80) | 10/10 (100) | 7/10 (70) | 10/10 (100) | 73/80 (91) |
| Solicited AEs | 6/10 (60) | 7/10 (70) | 7/10 (70) | 9/10 (90) | 7/10 (70) | 8/10 (80) | 4/10 (40) | 5/10 (50) | 53/80 (66) |
| Local | 2/10 (20) | 6/10 (60) | 6/10 (60) | 8/10 (80) | 6/10 (60) | 7/10 (70) | 1/10 (10) | 1/10 (10) | 37/80 (46) |
| Grade 1 | 2/10 (20) | 6/10 (60) | 6/10 (60) | 8/10 (80) | 6/10 (60) | 7/10 (70) | 1/10 (10) | 1/10 (10) | 37/80 (46) |
| Grade 2 | 0 | 0 | 0 | 1/10 (10) | 0 | 0 | 0 | 0 | 1/80 (1) |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Systemic | 5/10 (50) | 6/10 (60) | 3/10 (30) | 5/10 (50) | 2/10 (20) | 5/10 (50) | 3/10 (30) | 4/10 (40) | 33/80 (41) |
| Grade 1 | 5/10 (50) | 6/10 (60) | 3/10 (30) | 5/10 (50) | 2/10 (20) | 5/10 (50) | 3/10 (30) | 4/10 (40) | 33/80 (41) |
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unsolicited AEs | 8/10 (80) | 9/10 (90) | 10/10 (100) | 9/10 (90) | 6/10 (60) | 7/10 (70) | 7/10 (70) | 10/10 (100) | 66/80 (83) |
| Grade 1 | 8/10 (80) | 9/10 (90) | 10/10 (100) | 8/10 (80) | 6/10 (60) | 7/10 (70) | 7/10 (70) | 10/10 (100) | 65/80 (81) |
| Grade 2 | 0 | 2/10 (20) | 3/10 (30) | 3/10 (30) | 1/10 (10) | 0 | 4/10 (40) | 2/10 (20) | 15/80 (19) |
| Grade 3 | 0 | 0 | 0 | 1/10 (10) | 0 | 0 | 0 | 0 | 1/80 (1) |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
N: number of subjects
A: alhydrogel® adjuvant.
Approved monovalent vaccine containing an A/California (H1N1)-like strain.
Table 3.
Summary of solicited AEs after any vaccination.
| Group A (15 μg) N=10a |
Group B (15 μg + A)b N=10 |
Group C (45 μg) N= 10 |
Group D (45 μg + A) N=10 |
Group E (90 μg) N= 10 |
Group F (90 μg + A) N=10 |
Group G (saline) N=10 |
Group H (H1N1)c N=10 |
Overall N=80 |
|
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Solicited AEs, n (%) | 6/10(60) | 7/10(70) | 7/10(70) | 9/10(90) | 7/10(70) | 8/10(80) | 4/10(40) | 5/10(50) | 53/80 (66) |
| Local | 2/10(20) | 6/10(60) | 6/10(60) | 8/10(80) | 6/10(60) | 7/10(70) | 1/10(10) | 1/10(10) | 37/80 (46) |
| Pain | 2/10(20) | 6/10(60) | 3/10(30) | 6/10(60) | 3/10(30) | 3/10(30) | 1/10(10) | 0 | 24/80 (30) |
| Tenderness | 0 | 4/10(40) | 4/10(40) | 3/10(30) | 6/10(60) | 7/10(70) | 0 | 1/10(10) | 25/80(31) |
| Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Induration/swelling | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Systemic | 5/10(50) | 6/10(60) | 3/10(30) | 5/10(50) | 2/10(20) | 5/10(50) | 3/10(30) | 4/10(40) | 33/80 (41) |
| Fever | 0 | 0 | 0 | 1/10(10) | 0 | 0 | 0 | 0 | 1/80(1) |
| Gastrointestinal | 1/10(10) | 0 | 1/10(10) | 1/10(10) | 2/10(20) | 2/10(20) | 0 | 1/10(10) | 8/80(10) |
| Headache | 4/10(40) | 5/10(50) | 3/10(30) | 3/10(30) | 0 | 3/10(30) | 2/10(20) | 3/10(30) | 23/80 (29) |
| Fatigue | 2/10(20) | 2/10(20) | 2/10(20) | 1/10(10) | 0 | 2/10(20) | 0 | 2/10(20) | 11/80(14) |
| Myalgia | 0 | 1/10(10) | 0 | 1/10(10) | 0 | 0 | 1/10(10) | 1/10(10) | 4/80 (5) |
N: number of subjects.
A: alhydrogel®adjuvant.
Approved monovalent vaccine containing an A/California (H1N1)-like strain.
Systemic solicited AEs occurred in 33 subjects (41%), evenly distributed across study groups (Table 2). The most common systemic solicited AE was headache (23 subjects; 29%), which occurred in all study groups with similar incidence ranging from 30 to 50%, except for Group E (90 μg) where no headaches were reported (Table 3). Only four of these headache AEs (5%) were considered vaccine related.
Unsolicited AEs were more common in all study groups, with 66 subjects (83%) experiencing at least one unsolicited AE (Table 2). Unsolicited AEs had a larger range of intensity, with 15 subjects (19%) experiencing at least one Grade 2 AE, and one subject (1%) experiencing a Grade 3 AE (Table 2). Four of the unsolicited AEs (5%) were considered vaccine related and all four of these were Grade 1 intensity. The occurrence of unsolicited AEs was similar across all groups following each vaccination.
3.3. Immunogenicity
All vaccine cohorts demonstrated and maintained HAI and MN titers above the placebo cohort throughout Day 56 (Tables 4 and 5). The HAI titers were highest in Group H (H1N1 active control group) on Day 21 (GMT: 101.5; 95% confidence interval [CI]: 26.2, 393.1) and Day 56 (GMT: 75.4; 95% CI: 19.4, 292.9), followed by Group E (90 μg; GMT: 73.9; 95% CI: 36.5, 149.5), Group D (45 μg + adjuvant; GMT: 53.4; 95% CI: 182, 156.3) and Group F (90 μg + adjuvant; GMT: 36.4; 95% CI: 11.1, 119.2) on Day 56 (Table 4). The MN titers were highest in Group E (90 μg) on Day 56 (GMT: 436.6; 95% CI: 196.4, 970.7), followed by Group H (H1N1 active control group) on Day 21 (GMT: 342.5; 95% CI: 82.9, 1414.2), Group D (45 μg + adjuvant; GMT: 307.2; 95% CI: 123.3, 765.2) on Day 56, Group H (H1N1 active control group; GMT: 186.5; 95% CI: 44.9, 775.1) on Day 56, and Group F (90 μg + adjuvant; GMT: 124.5; 95% CI: 36.5, 424.9) on Day 56 (Table 5).
Table 4.
HAI antibody titer, seroconversion, and proportion of subjects achieving titer ≥40.
| Treatment Group | N a | HAI titer | Seroconvertedc | Titer ≥40 | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| GMT | 95% CIb | n (%) | 95% CId | n (%) | 95% CI | ||
|
| |||||||
| Group A (15 μg) | |||||||
| Day 0 | 10 | 6.3 | 4.7, 8.5 | – | – | ||
| Day 21 | 10 | 20.9 | 6.8, 63.9 | 2 (20) | 3, 56 | 2 (20) | 3, 56 |
| Day 56 | 10 | 22.9 | 7.7, 67.1 | 3 (30) | 7, 65 | 3 (30) | 7, 65 |
| Group B (15 μg + Ae) | |||||||
| Day 0 | 10 | 6.6 | 5.2, 8.4 | – | – | ||
| Day 21 | 10 | 23.4 | 9.8, 55.9 | 4(40) | 12, 74 | 4(40) | 12, 74 |
| Day 56 | 10 | 28.8 | 12.8, 64.9 | 5 (50) | 19, 81 | 5 (50) | 19, 81 |
| Group C (45 μg) | |||||||
| Day 0 | 10 | 5.9 | 4.0, 8.8 | – | – | ||
| Day 21 | 10 | 20.3 | 6.6, 62.4 | 3 (30) | 7, 65 | 3 (30) | 7, 65 |
| Day 56 | 10 | 24.5 | 8.9, 67.9 | 5 (50) | 19, 81 | 5 (50) | 19, 81 |
| Group D (45 μg + A) | |||||||
| Day 0 | 9 | 5.6 | 4.4, 7.1 | – | – | ||
| Day 21 | 9 | 42.5 | 12.3, 146.5 | 5 (56) | 21, 86 | 5 (56) | 21, 86 |
| Day 56 | 9 | 53.4 | 18.2, 156.3 | 4 (44) | 14, 79 | 4 (44) | 14, 79 |
| Group E (90 μg) | |||||||
| Day 0 | 10 | 5.2 | 4.8, 5.6 | – | – | ||
| Day 21 | 10 | 33.6 | 11.4, 98.9 | 5(50) | 19, 81 | 5(50) | 19, 81 |
| Day 56 | 9 | 73.9 | 36.5, 149.4 | 7(78) | 40, 97 | 7(78) | 40, 97 |
| Group F (90 μg + A) | |||||||
| Day 0 | 9 | 5.0 | – | – | – | ||
| Day 21 | 9 | 27.2 | 6.9, 107.2 | 4(44) | 14, 79 | 4(44) | 14, 79 |
| Day 56 | 9 | 36.4 | 11.1, 119.2 | 5(56) | 21, 86 | 5(56) | 21, 86 |
| Group G (saline) | |||||||
| Day 0 | 10 | 7.4 | 3.3, 16.4 | – | – | ||
| Day 21 | 10 | 7.6 | 3.5, 16.4 | 0 | 0, 31 | 1 (10) | 0, 45 |
| Day 56 | 10 | 6.7 | 3.8, 11.7 | 0 | 0, 31 | 1 (10) | 0, 45 |
| Group H (H1N1f) | |||||||
| Day 0 | 10 | 5.4 | 4.6, 6.3 | – | – | ||
| Day 21 | 10 | 101.5 | 26.2, 393.1 | 7 (70) | 35, 93 | 7 (70) | 35, 93 |
| Day 56 | 10 | 75.4 | 19.4, 292.9 | 6 (60) | 26, 88 | 6 (60) | 26, 88 |
N: number of subjects.
95% upper and lower confidence limits of the GMT, based on the t distribution with n −1 degrees of freedom.
Subjects have achieved seroconversion if they have either a pre-vaccination HAI titer <10 and a post-vaccination HAI titer ≥40 or a pre- vaccination HAI titer ≥10 and a minimum 4-fold rise in a post-vaccination HAI antibody titer.
Exact (Clopper–Pearson) confidence limits were computed for each binomial proportion
A: alhydrogel® adjuvant.
Approved monovalent vaccine containing an A/California (H1N1)-like strain.
Table 5.
MN antibody titer, seroconversion, and proportion of subjects achieving titer ≥80 and ≥160.
| Treatment Group | N a | MN Titer | Seroconvertedc | Titer ≥80 | Titer ≥160 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| GMT | 95% CIb | n (%) | 95% CId | n (%) | 95% CId | n (%) | 95% CI | ||
|
| |||||||||
| Group A (15 μg) | |||||||||
| Day 0 | 10 | 7.2 | 4.5, 11.4 | – | – | – | |||
| Day 21 | 10 | 45.9 | 9.4, 223.4 | 5 (50) | (19, 81) | 2 (20) | (3, 56) | 2 (20) | (3, 56) |
| Day 56 | 10 | 65.8 | 15.8, 273.1 | 6 (60) | (26, 88) | 3 (30) | (7, 65) | 2 (20) | (3, 56) |
| Group B (15 μg + Ae) | |||||||||
| Day 0 | 10 | 13.0 | 7.4, 22.9 | – | – | – | |||
| Day 21 | 10 | 83.9 | 28.6, 246.5 | 5(50) | (19, 81) | 5(50) | (19, 81) | 5(50) | (19, 81) |
| Day 56 | 10 | 110.1 | 40.4, 300.0 | 6(60) | (26, 88) | 6(60) | (26, 88) | 5(50) | (19, 81) |
| Group C (45 μg) | |||||||||
| Day 0 | 10 | 8.4 | 4.1, 17.4 | – | – | – | |||
| Day 21 | 10 | 67.8 | 14.6, 313.8 | 5(50) | (19, 81) | 4(40) | (12, 74) | 3(30) | (7, 65) |
| Day 56 | 10 | 93.6 | 22.7, 387.0 | 7(70) | (35, 93) | 5(50) | (19, 81) | 5(50) | (19, 81) |
| Group D (45 μg + A) | |||||||||
| Day 0 | 9 | 9.5 | 4.3, 20.9 | – | – | – | |||
| Day 21 | 9 | 244.4 | 64.8, 922.1 | 7 (78) | (40, 97) | 6 (67) | (30, 93) | 5 (56) | (21, 86) |
| Day 56 | 9 | 307.2 | 123.3, 765.2 | 9 (100) | (66, 100) | 7 (78) | (40, 97) | 7 (78) | (40, 97) |
| Group E (90 μg) | |||||||||
| Day 0 | 10 | 7.1 | 5.0, 10.2 | – | – | – | |||
| Day 21 | 10 | 171.2 | 55.8, 525.2 | 8 (80) | (44, 97) | 7 (70) | (35, 93) | 5 (50) | (19, 81) |
| Day 56 | 9 | 436.6 | 196.4, 970.7 | 9 (100) | (66, 100) | 9 (100) | (66, 100) | 8 (89) | (52, 100) |
| Group F (90 μg + A) | |||||||||
| Day 0 | 9 | 6.1 | 4.8, 7.7 | – | – | – | |||
| Day 21 | 9 | 86.4 | 14.6, 510.3 | 6(67) | (30, 93) | 5(56) | (21, 86) | 5(56) | (21, 86) |
| Day 56 | 9 | 124.5 | 36.5, 424.9 | 8(89) | (52, 100) | 5(56) | (21, 86) | 5(56) | (21, 86) |
| Group G (saline) | |||||||||
| Day 0 | 10 | 20.3 | 7.5, 55.0 | – | – | – | |||
| Day 21 | 10 | 17.5 | 7.6, 40.6 | 0 | (0, 31) | 1 (10) | (0, 45) | 1 (10) | (0, 45) |
| Day 56 | 10 | 13.3 | 7.4, 24.0 | 0 | (0, 31) | 1(10) | (0, 45) | 0 | (0, 31) |
| Group H (H1N1f) | |||||||||
| Day 0 | 10 | 7.3 | 4.3, 12.4 | – | – | – | |||
| Day 21 | 10 | 342.5 | 82.9, 1414.2 | 7 (70) | (35, 93) | 7 (70) | (35, 93) | 6 (60) | (26, 88) |
| Day 56 | 10 | 186.5 | 44.9, 775.1 | 7 (70) | (35, 93) | 5 (50) | (19, 81) | 5 (50) | (19, 81) |
N: number of subjects.
95% upper and lower confidence limits of the GMT, based on the t distribution with n −1 degrees of freedom.
Subjects have achieved seroconversion if they have either a pre-vaccination MN titer <10 and a post-vaccination MN titer ≥40 or a pre-vaccination MN titer ≥10 and a minimum 4-fold rise in a post-vaccination MN antibody titer.
Exact (Clopper–Pearson) confidence limits were computed for each binomial proportion.
A: alhydrogel® adjuvant.
Approved monovalent vaccine containing an A/California (H1N1)-like strain.
HAI seroconversion rates (and the proportions of subjects achieving HAI titers of ≥40) among the non-adjuvanted HAC1 vaccinated cohorts increased with dose escalation, ranging from 20% in Group A (15 μg) to 50% in Group E (90 μg) on Day 21 and from 30% in Group A (15 μg) to 78% in Group E (90 g) on Day 56, indicative of a dose response (Table 4). Seroconversion rates for MN titers in non-adjuvanted vaccine cohorts also increased with dose escalation ranging from 50% in Group A (15 μg) to 80% in Group E (90 μg) on Day 21 and from 60% in Group A (15 μg) to 100% in Group E (90 μg) on Day 56 (Table 5). A dose response was also observed with the proportions of subjects achieving MN antibody titers of ≥80 and ≥160 (Table 5). Analysis of both HAI and MN seroconversion rates in the groups receiving adjuvanted HAC1 demonstrated variable results (Tables 4 and 5). No subject in the placebo control group seroconverted; in contrast, 7 (70%) subjects seroconverted on Day 21 in the H1N1 active control group (Tables 4 and 5).
In all study vaccine groups, MN seroconversion rates were significantly different compared to the placebo control group on both Days 21 (p < 0.05 for Groups A–C, p ≤ 0.003 for Groups D–F) and 56 (p = 0.011 for Groups A and B, p ≤ 0.003 for Groups C–F). Seroconversion rates for HAI were statistically different (p ≤ 0.033) compared to placebo control in Group D (45 μg + adjuvant), Group E (90 μg) and Group F (90 μg + adjuvant) on Day 21 and in all groups except Group A (15 μg) on Day 56.
4. Discussion
This first-in-human, Phase 1 clinical study assessed safety and immunogenicity of HAC1, a plant-produced recombinant HA influenza vaccine candidate developed by FhCMB for the prevention of disease caused by a novel A (H1N1)pdm09 virus. FhCMB engineered and produced HAC1 in less than one month and subsequently scaled it up for cGMP manufacturing in FhCMB’s pilot facility [35].
At the doses tested in this Phase 1 study, the vaccine was generally shown to be safe in healthy volunteers, with no reported SAEs and no evidence of any dose-limiting or dose-related toxicity. As expected, the most frequent AE was local injection site reaction after either dose, which was generally mild and self-limited. In addition, the vaccine was shown to be highly immunogenic. HAI and MN GMTs were enhanced with increasing doses of non-adjuvanted HAC1 vaccine. Responses detected post-1st dose of HAC1, showed seroconversion rates between 20 and 56% by HAI and 50–80% by MN. These responses were enhanced following a second immunization with the highest seroconversion rates seen in the 90 μg non-adjuvanted HAC1 group at 78% and 100% for HAI and MN, respectively. Additionally, responses elicited by the second 90 μg dose of non-adjuvanted HAC1 were comparable (by HAI) to or greater (by MN) than immune responses elicited by a single dose of the licensed, control H1N1 vaccine. The extent of the impact the second dose of HAC1 had on the immune responses should be evaluated further in the next stage of product development. Inclusion of a single dose group as a comparator control that enables the kinetics of the antibody responses to be followed post first dose could provide valuable insight on the utility of a booster dose.
It has been shown in extensive preclinical testing that the development of anti-HA antibody responses were not affected by the presence of the 6 × His tag and the KDEL sequence present at the Cterminus of HAC1 and that this C-terminal tag did not itself induce any detectable antibody titers [35]. In addition, we have previously shown that the plant-produced HAC1 is a glycosylated protein with six of the seven potential N-linked glycosylation sites being glycosylated [35]. No apparent safety concerns were noted as a result of the presence of the C-terminal tags or plant glycans in a repeat dose toxicology study in New Zealand White rabbits [35]. Data from this clinical trial confirms the safety profile of this vaccine in humans.
It was previously demonstrated in pre-clinical studies that Alhydrogel® adjuvant enhanced antibody responses elicited by HAC1 and provided a dose-sparing effect in animal models [35]. In addition, in a randomized, Phase 1 clinical trial, alum-adjuvanted whole-virus avian A/Hong Kong/1073/99 (H9N2) vaccine was shown to be more immunogenic than the unadjuvanted vaccine [38]. Therefore, we chose to evaluate the HAC1 vaccine in the clinic with and without Alhydrogel®. In contrast to the effects of Alhydrogel® in the indicated pre-clinical and clinical studies, the present clinical study demonstrated variable results with regard to enhancement of antibody responses to different doses of HAC1 when the vaccine was adsorbed on Alhydrogel®. These results, however, are consistent with the findings from other randomized, controlled influenza vaccine clinical studies in different populations of human volunteers. Indeed, several groups evaluating split-virion 2009 pandemic influenza A H1N1 vaccines [11,39,40] as well as split-virion or whole-virus influenza A H5N1 vaccines have reported that alum either failed to enhance or even decreased antibody production [41–47]. The reason for Alhydrogel®’s inefficiency is not known, since potency of this adjuvant in stimulating both T helper 1 and 2 type responses and antibody production has been well documented [48]. As suggested by Liang et al. [24], the absence of benefit may be due to a delayed antigen release from alum-adjuvanted vaccine formulations.
In conclusion, the plant-produced experimental, recombinant HAC1 vaccine demonstrated an acceptable safety profile with a dose-dependent immune response in healthy adults. These results validate FhCMB’s plant-based transient expression system as an alternative platform for manufacturing influenza vaccines and support further clinical development of HAC1.
Acknowledgements
The authors would like to thank Heather Tatum, Peter Browning and Leilani Thomas for specimen management, Eno Ekong and Monique Woodruff for technical support, Eric Gillis for data management, and Zainab V. Nanji and Dr. Natasha Kushnir for editorial assistance. The authors would also like to thank Dr. William Warren and his group of Sanofi Pasteur VaxDesign Corporation for assistance in pre-screening.
The HAC1 clinical trial and serologic testing were supported by the Defense Advanced Research Projects Agency (DARPA). The cGMP manufacture of HAC1 was supported by DARPA under contract number # HR0011–10-C-0051.
Dr. Jacqueline M. Katz has received funding for research not related to the present study from GlaxoSmithKline and Juvaris, Inc. (now Colby Pharmaceuticals).
The findings, conclusions and opinions expressed herein are those of the authors and do not represent official positions of the Departments of the Army or Defense or ofthe Centers for Disease Control and Prevention.
Footnotes
ClinicalTrials.gov identifier: NCT01177202.
Conflict of interest: Other authors have no conflict of interest to declare.
References
- [1].World Health Organization Media Centre. Influenza (Seasonal). Fact sheet № 211; 2009. http://www.who.int/mediacentre/factsheets/fs211/en/index.html[accessed 26.3.12].
- [2].Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009;325:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;360(25):2605–15. [DOI] [PubMed] [Google Scholar]
- [4].Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009;361:1945–52. [DOI] [PubMed] [Google Scholar]
- [5].WHO D-G statement; 2009. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html [accessed 27.03.12].
- [6].Matthews JT. Egg-based production of influenza vaccine: 30 years of commercial experience. The Bridge (Nat Acad Eng) 2006;36(3):17–24. [Google Scholar]
- [7].Schultz-Cherry S, Jones JC. Influenza vaccines: the good, the bad, and the eggs. Adv Virus Res 2010;77:63–84. [DOI] [PubMed] [Google Scholar]
- [8].Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 2011;29(49):9159–70. [DOI] [PubMed] [Google Scholar]
- [9].Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12(1):36–44. [DOI] [PubMed] [Google Scholar]
- [10].Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med 2009;361:2405–13. [DOI] [PubMed] [Google Scholar]
- [11].Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2010;375:56–66. [DOI] [PubMed] [Google Scholar]
- [12].Plennevaux E, Sheldon E, Blatter M, Reeves-Hoché MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 2010;375:41–8. [DOI] [PubMed] [Google Scholar]
- [13].Talaat KR, Greenberg ME, Lai MH, Hartel GF, Wichems CH, Rockman S, et al. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J Infect Dis 2010;202: 1327–37. [DOI] [PubMed] [Google Scholar]
- [14].Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009;361:2424–35. [DOI] [PubMed] [Google Scholar]
- [15].Pushko P, Kort T, Nathan M, Pearce MB, Smith G, Tumpey TM, et al. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine 2010;28(30): 4771–6. [DOI] [PubMed] [Google Scholar]
- [16].López-Macías C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, ArteagaRuiz O, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine 2011;29(44):7826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Protein Sciences Corp. White Paper; 2011. http://www.proteinsciences.com/News/index.html#/16/ [accessed 27.03.12].
- [18].Yusibov V, Streatfield SJ, Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccin 2011;7:313–21. [DOI] [PubMed] [Google Scholar]
- [19].Yusibov V, Rabindran S. Recent progress in the development of plant derived vaccines. Expert Rev Vaccines 2008;7(8):1173–83. [DOI] [PubMed] [Google Scholar]
- [20].Sharma AK, Sharma MK. Plants as bioreactors: recent developments and emerging opportunities. Biotechnol Adv 2009;27(6):811–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].D’Aoust MA, Couture MM, Charland N, Trépanier S, Landry N, Ors F, et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J 2010;8:607–19. [DOI] [PubMed] [Google Scholar]
- [22].Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J 2010;8(5):620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chichester JA, Musiychuk K, de la Rosa P, Horsey A, Stevenson N, Ugulava N, et al. Immunogenicity of a subunit vaccine against Bacillus anthracis. Vaccine 2007;25(16):3111–4. [DOI] [PubMed] [Google Scholar]
- [24].Mett V, Lyons J, Musiychuk K, Chichester JA, Brasil T, Couch R, et al. A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine 2007;25:3014–7. [DOI] [PubMed] [Google Scholar]
- [25].Shoji Y, Chichester JA, Bi H, Musiychuk K, de la Rosa P, Goldschmidt L, et al. Plant-expressed HA as a seasonal influenza vaccine candidate. Vaccine 2008;26:2930–4. [DOI] [PubMed] [Google Scholar]
- [26].Shoji Y, Bi H, Musiychuk K, Rhee A, Horsey A, Roy G, et al. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 2009;27:1087–92. [DOI] [PubMed] [Google Scholar]
- [27].Shoji Y, Farrance CE, Bautista J, Bi H, Musiychuk K, Horsey A, et al. A plantbased system for rapid production of influenza vaccine antigens. Influenza Other Respi Viruses 2012;6:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].D’Aoust MA, Lavoie PO, Couture MM, Trépanier S, Guay JM, Dargis M, et al. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J 2008;6(9):930–40. [DOI] [PubMed] [Google Scholar]
- [29].De Leede LG, Humphries JE, Bechet AC, Van Hoogdalem EJ, Verrijk R, Spencer DG. Novel controlled-release Lemna-derived IFN-alpha2b (Locteron): pharmacokinetics, pharmacodynamics, and tolerability in a phase I clinical trial. J Interferon Cytokine Res 2008;28(2):113–22. [DOI] [PubMed] [Google Scholar]
- [30].McCormick AA, Reddy S, Reinl SJ, Cameron TI, Czerwinkski DK, Vojdani F, et al. Plant-produced idiotype vaccines for the treatment of non-Hodgkin’s lymphoma: safety and immunogenicity in a phase I clinical study. Proc Natl Acad Sci U S A 2008;105(29):10131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 2010;5(12):e15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Medicago Inc News release; 2011. http://www.medicago.com/English/news/News-Releases/News-ReleaseDetails/2011/Medicago-to-present-additional-positive-clinical-data-at-the-2011ESWI-influenza-conference1126562/default.aspx [accessed 26.01.12].
- [33].Zimran A, Brill-Almon E, Chertkoff R, Petakov M, Blanco-Favela F, Muňoz ET, et al. Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood 2011;118(22):5767–73. [DOI] [PubMed] [Google Scholar]
- [34].Musiychuk K, Stephenson N, Bi H, Farrance CE, Orozovic G, Brodelius M, et al. A launch vector for the production of vaccine antigens in plants. Influenza Other Respi Viruses 2007;1:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shoji Y, Chichester JA, Jones M, Manceva SD, Damon E, Mett V. Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum Vaccin 2011;7(Suppl.):41–50. [DOI] [PubMed] [Google Scholar]
- [36].World Health Organization WHO Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza; 2011. http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf [accessed 28.06.12].
- [37].Guidance for industry toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials; 2007. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf [accessed 12.04.12]. [DOI] [PubMed]
- [38].Nicholson KG, Thompson CI, Klap JM, Wood JM, Batham S, Newman RW, et al. Safety and immunogenicity of whole-virus, alum-adjuvanted whole-virus, virosomal, and whole-virus intradermal influenza A/H9N2 vaccine formulations. Vaccine 2010;28:171–8. [DOI] [PubMed] [Google Scholar]
- [39].Wu J, Li W, Wang HQ, Chen JT, Lv M, Zhou JC, et al. A rapid immune response to 2009 influenza A(H1N1) vaccines in adults: a randomized, double-blind, controlled trial. J Infect Dis 2010;202:675–80. [DOI] [PubMed] [Google Scholar]
- [40].Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 2009;361:2414–23. [DOI] [PubMed] [Google Scholar]
- [41].Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008;197:667–75. [DOI] [PubMed] [Google Scholar]
- [42].Brady RC, Treanor JJ, Atmar RL, Keitel WA, Edelman R, Chen WH, et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine 2009;27:5091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 2006;367:1657–64. [DOI] [PubMed] [Google Scholar]
- [44].Ehrlich HJ, Müller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008;358:2573–84. [DOI] [PubMed] [Google Scholar]
- [45].Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, He F, et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I–II randomized clinical trial. J Infect Dis 2008;198:1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Keitel WA, Dekker CL, Mink C, Campbell JD, Edwards KM, Patel SM, et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine 2009;27:6642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nolan TM, Richmond PC, Skeljo MV, Pearce G, Hartel G, Formica NT, et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine 2008;26:4160–7. [DOI] [PubMed] [Google Scholar]
- [48].Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin4-deficient mice, alum not only generates T helper 1 responses equivalent to Freud’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol 1996;26:2062–6. [DOI] [PubMed] [Google Scholar]