Abstract
We examined norovirus contamination on hands of ill patients during 12 norovirus outbreaks in 12 long-term care facilities (LTCFs). The higher frequency and norovirus titers on hands of residents compared to hands of heathcare workers highlights the importance of adhering to appropriate hand hygiene practices during norovirus outbreaks in LTCFs.
Norovirus outbreaks are common among vulnerable, elderly populations in long-term care facilities (LTCFs), and they are associated with a significant number of hospitalizations and deaths.1 In the United States, 63% of all norovirus outbreaks occur in LTCFs.2 Although hand hygiene is a key infection control measures, the role of hands in the transmission of norovirus during outbreaks remains poorly understood; few data on the frequency and level of hand contamination exist.
METHODS
A convenience sample of 35 ill patients (18 healthcare workers [HCWs] and 17 residents) who volunteered for the study were screened for eligibility during 12 norovirus outbreaks in 12 LTCFs (5 skilled nursing facilities, 4 assisted nursing facilities, and 3 dual-functional facilities) recruited by the Oregon Health Authority between 2013 and 2016 as part of an ongoing study on norovirus outbreaks in LTCFs.3 Written informed consent was obtained from each participant. A norovirus case was selected when the patient met the Kaplan criteria.3 Clinical data were collected from each case patient using a standardized questionnaire. The study was approved by the institutional review boards of the Oregon State Public Health Division (IRB 08–03) and the Centers for Disease Control and Prevention (CDC) (Protocol #5051). Residents who were impaired cognitively or in decision making were excluded from the study.
A stool specimen and a hand-rinse sample were collected from each patient within 10 days of onset of norovirus symptoms. Hand-rinse samples (dominant hand) were collected from patients immediately after enrollment using the glove juice technique (ASTM E1174-06).4 Norovirus particles were first concentrated from hand-rinse eluates by polyethylene glycol to 0.4 mL (Figure S1). Viral RNA was then extracted from the hand concentrates and clarified stool suspensions and was analyzed using real-time reverse-transcription polymerase chain reaction (RT-PCR) for GI and GII norovirus, as described previously.5 Norovirus-positive samples were reamplified using hemi-nested polymerase chain reaction (PCR) for sequence-based genotyping (Figure S1).5 Norovirus hand contamination rates of residents and HCWs were analyzed using the Fisher exact test. SPSS software version 21 (IBM, Armonk, NY) was used for statistical calculations. P values ≤.05 were considered statistically significant.
RESULTS
Of the 35 patients initially recruited, 4 patients (3 HCWs and 1 residents) were excluded from analysis because no stool sample was collected, and 1 resident withdrew voluntarily from the study. Data from 30 ill volunteer patients (7 direct-care HCWs, 8 non–direct-care HCWs, and 15 residents) were analyzed. Clinical symptoms included diarrhea (90%), vomiting (97%), nausea (90%), abdominal pain (87%), and fever (68%) (Table S1).
Norovirus was detected in 23 of 30 stool samples (77%); 2 samples were positive for GI and 21 samples were positive for GII (Table 1). The viral load in stool samples was 6.4 (range, 2.9–8.8) log10 RNA copies per gram. Genotypes included GI.4 (n = 2, 9%), GII.4 Sydney (n = 14, 61%), GII.3 (n = 1, 4%), GII.6 (n = 4, 17%), and GII.13 (n = 2, 9%). In addition, 10 of the 14 positive hand-rinse samples (71.4%) had an identical sequence as the corresponding stool sample.
TABLE 1.
Detection of Norovirus in Stool and Hand-Rinse Samples from Norovirus Outbreaks in Long-Term Care Facilities
| Outbreak ID |
Subject |
Stool Specimens |
Hand Rinse Samples |
Time intervald |
Clinical Status of Subject at the Time of Hand Sampling |
|||
|---|---|---|---|---|---|---|---|---|
| ID | Category | Genotypea | Norovirus Titer |
Genotypea | Norovirus Titer |
|||
| A | 1 | HCWD | – | ND | – | ND | +1 | Symptomatic |
| B | 2 | HCWD | GI.4 | 5.0 | – | ND | 0 | Symptomatic |
| B | 3 | HCWD | GI.4 | 4.6 | – | ND | +1 | Symptomatic |
| C | 4 | R | GII.13 | 6.9 | GII.13 | 6.3 | 0 | Symptomatic |
| C | 5 | R | GII.13 | 8.0 | GII.13 | 6.8 | 0 | Symptomatic |
| D | 6 | R | GII.4 Sydney | 8.2 | GII.4 Sydney | 7.9 | 0 | Symptomatic |
| D | 7 | R | GII.4 Sydney | 6.4 | – | ND | +1 | Symptomatic |
| E | 8 | HCWD | GII.4 Sydney | 6.7 | – | ND | 0 | Symptomatic |
| E | 9 | R | GII.4 Sydney | 5.3 | GIIe | 4.4 | 0 | Symptomatic |
| F | 10 | HCWN | GII.4 Sydney | 6.3 | – | ND | +2 | Postsymptomatic |
| F | 11 | HCWN | – | ND | GIIe | 3.4 | +2 | Postsymptomatic |
| F | 12 | R | – | ND | – | ND | +6 | Postsymptomatic |
| F | 13 | R | GII.4 Sydney | 8.8 | – | ND | 0 | Postsymptomatic |
| F | 14 | R | GII.4 Sydney | 8.2 | GII.4 Sydney | 7.7 | +2 | Symptomatic |
| F | 15 | R | GII.4 Sydney | 4.7 | GIIe | 5.3 | +3 | Postsymptomatic |
| G | 16 | HCWD | GII.4 Sydney | 4.6 | – | ND | 0 | Postsymptomatic |
| G | 17 | HCWN | GII.4 Sydney | 6.7 | GIIe | 4.9 | −4 | Postsymptomatic |
| G | 18 | HCWN | GII.4 Sydney | 4.3 | – | ND | −3 | Postsymptomatic |
| G | 19 | R | GII.4 Sydney | 7.2 | GII.4 Sydney | 5.8 | −2 | Postsymptomatic |
| G | 20 | HCWD | – | ND | – | ND | +6 | Postsymptomatic |
| H | 21 | R | GII.4 Sydney | 7.3 | GII.4 Sydney | 6.3 | −1 | Symptomatic |
| I | 22 | R | GII.4 Sydney | 6.5 | GII.4 Sydney | 6.1 | 0 | Symptomatic |
| J | 23 | HCWD | GII.6 | 3.9 | – | ND | +2 | Symptomatic |
| J | 24 | R | GII.6 | 5.7 | GII.6 | 2.4 | +2 | Symptomatic |
| J | 25 | HCWN | – | ND | – | ND | +2 | Symptomatic |
| K | 26 | R | GII.6 | 5.9 | – | ND | +7 | Symptomatic |
| K | 27 | HCWN | – | ND | – | ND | +5 | Symptomatic |
| K | 28 | HCWN | GII.6 | 6.2 | GII.6 | 2.4 | +3 | Postsymptomatic |
| L | 29 | R | GII.3 | 2.9 | GII.3 | 4.8 | +1 | Postsymptomatic |
| L | 30 | HCWN | – | ND | – | ND | +1 | Symptomatic |
note. HCWD, healthcare worker, direct care; HCWN, healthcare worker, non-direct care, R, nursing home resident; ND, not determined; −, sample was negative by RT-qPCR.
100% sequence identity between viruses in stool and hand-rinse sample of the same subject.
Log10 RNA copies per gram of stool.
Log10 RNA copies per hand.
Time period (in days) between collection of hand and stool samples after onset of clinical symptoms. The hand sample was collected later “+” or earlier “−” than the stool specimen.
GII positive by RT-qPCR, but hemi-nested PCR negative and therefore could not be genotyped.
Hand-rinse samples of 11 of the 15 residents (73.3%) tested positive (viral load of 6.1; range, 2.4–7.9) compared to 3 of the 15 samples (20.0%) from HCWs (viral load, 3.7 log10 RNA copies; range, 2.4–4.9) (Table 1). Residents had a higher positive rate than HCWs (73.3%, vs 20.0%; odds ratio, 11; 95% CI, 2.0–60.6; P = .009).
Of the 14 residents with a norovirus positive stool, 11 residents (8 symptomatic and 3 postsymptomatic) had a positive hand sample. Viral load on hands of symptomatic residents was 6.3 log10 RNA copies (range, 2.4–7.9) compared to 4.9 log10 RNA copies (range, 4.8–5.8) on hands of postsymptomatic residents (Table 1). Of the 9 HCWs with a norovirus-positive stool, hand samples from 4 symptomatic HCWs tested negative, whereas hand samples from 2 of the 5 postsymptomatic non–direct-care HCWs tested positive with a viral load of 3.7 log10 RNA copies (range, 2.4–4.9). The hand sample of 1 non–direct-care HCW tested positive for norovirus, while the stool sample tested negative. Of the 10 patients with a positive stool sample and a positive hand rinse sample collected within 2 days from each other, we found a strong correlation between the amount of virus in stool and on hands for 9 (8 symptomatic and 1 postsymptomatic) (r = 0.879; P = .002) (Figure 1).
FIGURE 1.
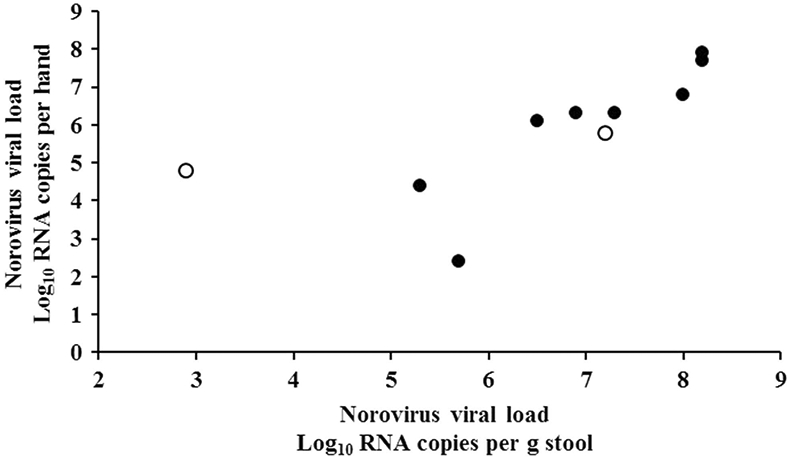
Correlation between viral load of norovirus on hand and in stool samples. The following symbols were used: symptomatic patients (•), and postsymptomatic patients (○), respectively.
DISCUSSION
We tested hand and stool samples of HCWs and residents during 12 norovirus outbreaks in 12 LTCFs. More hands from residents than from HCWs tested positive for norovirus. Furthermore, the amount of virus on hands of residents correlated with the amount detected in their stool samples, indicating that fecal contamination was the likely source of the hand contamination.
Recommended hand hygiene practices include washing hands with soap and water for 20 seconds.6 In particular, the higher risk of self-contamination of residents suggests that more active handwashing procedures should be enforced during norovirus outbreaks. Because published data suggest that 1–2 log10 RNA copies of virus is removed by hand washing,7,8 our findings of high levels (up to 8 log10) of norovirus contamination on hands, together with the fact that noroviruses have a low infectious dose,9 suggest that strict adherence to handwashing policies during norovirus outbreaks should be reinforced to interrupt further spread of norovirus in LTCFs. Alcohol-based hand sanitizer (ABHS) can be used in addition to hand washing, not as a substitute for washing with soap and water.6 However, the efficacy of ABHSs against human norovirus remains inconclusive because most data have been obtained using surrogate viruses.6 A recently published novel cell-culture system for human norovirus will allow testing of the efficacy of ABHS and other products,10 which will further help guide hygiene practices in LTCFs during norovirus outbreaks.
Our study has several limitations. The sample size was small, and the patients volunteered to participate. Therefore, it is unknown how many other norovirus-positive residents and HCWs may have had contaminated hands. Also, our results cannot be extrapolated to other LTCFs. We could not collect information on hygienic behaviors or functional status (eg, level of functional dependence, mobility, cognitive status, or continence) and handwashing practices (eg, use of ABHS) by patients that might have led to differences in hand contamination rates (HCWs vs residents). Furthermore, because environmental surface samples were not collected in this study, the interaction between hands and surfaces could not be evaluated. Finally, the detection of viral RNA does not necessarily indicate the presence of infectious norovirus and potential health risk.
In summary, our data suggest the potential role that hands contaminated with norovirus could play in the transmission of norovirus during outbreaks. Although the viability of the detected norovirus was not assessed, the high rates of norovirus on hands of infected residents suggests a role for HCWs in ensuring adequate hand hygiene in functionally dependent residents. Our findings highlight the importance of promoting and adhering to hand hygiene and other infection control practices during norovirus outbreaks in healthcare settings.6
Supplementary Material
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This study was supported in part by a grant from GoJo Industries to the Centers for Disease Control and Prevention.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.274
REFERENCES
- 1.Trivedi TK, DeSalvo T, Lee L, et al. Hospitalizations and mortality associated with norovirus outbreaks in nursing homes, 2009–2010. JAMA 2012;308:1668–1675. [DOI] [PubMed] [Google Scholar]
- 2.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinje J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol 2014;52:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costantini VP, Cooper EM, Hardaker HL, et al. Epidemiologic, virologic, and host genetic factors of norovirus outbreaks in long-term care facilities. Clin Infect Dis 2016;62:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu P, Escudero B, Jaykus LA, et al. Laboratory evidence of norwalk virus contamination on the hands of infected individuals. Appl Envir Microbiol 2013;79:7875–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park GW, Chhabra P, Vinjè J. Swab sampling method for the detection of human norovirus on surfaces. J Visual Exper 2017:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacCannell T, Umscheid CA, Agarwal RK, et al. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect Control Hosp Epidemiol 2011;32:939–969. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Envir Microbiol 2010;76:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sickbert-Bennett EE, Weber DJ, Gergen-Teague MF, Sobsey MD, Samsa GP, Rutala WA. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control 2005;33:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 2008;14:1553–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ettayebi K, Crawford SE, Murakami K, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016;353:1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


