Abstract
Background
Pathologic fracture of the long bones is a common complication of bone metastases. Intramedullary nail stabilization can be used prophylactically (for impending fractures) or therapeutically (for completed fractures) to preserve mobility and quality of life. However, local disease progression may occur after such treatment, and there is concern that surgical instrumentation and the intramedullary nail itself may seed tumor cells along the intramedullary tract, ultimately leading to loss of structural integrity of the construct. Identifying factors associated with local disease progression after intramedullary nail stabilization would help surgeons predict which patients may benefit from alternative surgical strategies.
Questions/purposes
(1) Among patients who underwent intramedullary nail stabilization for impending or completed pathologic fractures of the long bones, what is the risk of local progression, including progression of the existing lesion and development of a new lesion around the nail? (2) Among patients who experience local progression, what proportion undergo reoperation? (3) What patient characteristics and treatment factors are associated with postoperative local progression? (4) What is the difference in survival rates between patients who experienced local progression and those with stable local disease?
Methods
Between January 2013 and December 2019, 177 patients at our institution were treated with an intramedullary nail for an impending or completed pathologic fracture. We excluded patients who did not have a pathologic diagnosis of metastasis before fixation, who were younger than 18 years of age, who presented with a primary soft tissue mass that eroded into bone, and who experienced nonunion from radiation osteitis or an avulsion fracture rather than from metastasis. Overall, 122 patients met the criteria for our study. Three fellowship-trained orthopaedic oncology surgeons involved in the care of these patients treated an impending or pathologic fracture with an intramedullary nail when a long bone lesion either fractured or was deemed to be of at least 35% risk of fracture within 3 months, and in patients with an anticipated duration of overall survival of at least 6 weeks (fractured) or 3 months (impending) to yield palliative benefit during their lifetime. The most common primary malignancy was multiple myeloma (25% [31 of 122]), followed by lung carcinoma (16% [20 of 122]), breast carcinoma (15% [18 of 122]), and renal cell carcinoma (12% [15 of 122]). The most commonly involved bone was the femur (68% [83 of 122]), followed by the humerus (27% [33 of 122]) and the tibia (5% [6 of 122]). A competing risk analysis was used to determine the risk of progression in our patients at 1 month, 3 months, 6 months, and 12 months after surgery. A proportion of patients who ultimately underwent reoperation due to progression was calculated. A univariate analysis was performed to determine whether lesion progression was associated with various factors, including the age and sex of the patient, use of adjuvant therapies (radiation therapy at the site of the lesion, systemic therapy, and antiresorptive therapy), histologic tumor type, location of the lesion, and fracture type (impending or complete). Patient survival was assessed with a Kaplan-Meier curve. A p value < 0.05 was considered significant.
Results
The cumulative incidence of local tumor progression (with death as a competing risk) at 1 month, 3 months, 6 months, and 12 months after surgery was 1.9% (95% confidence interval 0.3% to 6.1%), 2.9% (95% CI 0.8% to 7.5%), 3.9% (95% CI 1.3% to 8.9%), and 4.9% (95% CI 1.8% to 10.3%), respectively. Of 122 patients, 6% (7) had disease progression around the intramedullary nail and 0.8% (1) had new lesions at the end of the intramedullary nail. Two percent (3 of 122) of patients ultimately underwent reoperation because of local progression. The only factors associated with progression were a primary tumor of renal cell carcinoma (odds ratio 5.1 [95% CI 0.69 to 29]; p = 0.03) and patient age (difference in mean age 7.7 years [95% CI 1.2 to 14]; p = 0.02). We found no associations between local disease progression and the presence of visceral metastases, other skeletal metastases, radiation therapy, systemic therapy, use of bisphosphonate or receptor activator of nuclear factor kappa-B ligand inhibitor, type of fracture, or the direction of nail insertion. There was no difference in survivorship curves between those with disease progression and those with stable local disease (= 0.36; p = 0.54).
Conclusion
Our analysis suggests that for this population of patients with metastatic bone disease who have a fracture or impeding fracture and an anticipated survival of at least 6 weeks (completed fracture) or 3 months (impending fracture), the risk of experiencing local progression of tumor growth and reoperations after intramedullary nail stabilization seems to be low. Lesion progression was not associated with the duration of survival, although this conclusion is limited by the small number of patients in the current study and the competing risks of survival and local progression. Based on our data, patients who present with renal cell carcinoma should be cautioned against undergoing intramedullary nailing because of the risk of postoperative lesion progression.
Level of Evidence
Level III, therapeutic study.
Introduction
A common complication of bone metastases is complete and impending pathologic fracture of long bones [14, 17, 24]. For patients with metastases of the long bones, an intramedullary nail can be used to stabilize the length of the long bone to provide durable support for the duration of the patient’s life [5, 9, 22]. The procedure is associated with reduced local pain and early postoperative mobilization [4].
One concern regarding intramedullary nail stabilization through a metastatic lesion is the potential for seeding tumor cells along the intramedullary tract, potentially causing local iatrogenic disease progression. In following the “one bone, one operation” philosophy, surgeons often choose implants that span almost the entire length of the bone. Although this method may offer stabilization if additional lesions develop, it also risks more potential seeding of the intramedullary cavity. Some surgeons advocate intralesional curettage and polymethyl methacrylate packing to reduce potential local progression and fixation failure [20].
Previous studies have evaluated the survival rate and postoperative complications of patients who underwent intramedullary nail stabilization in the femur or humerus [4, 6, 17, 23, 24, 27]. Known causes of intramedullary nail failure that required additional surgery include surgeon error, tumor progression, nonunion, and hardware failure [17, 27]. Additionally, patients who have short survival times (< 0.6 months after surgery) were unlikely to have intramedullary nail failure, whereas patients who lived more than 1 year after surgery had fewer failures [17]. Postoperative survival was generally low, ranging from 34% to 40% at 1 year, 7% to 25% at 2 years, and 4% to 15% at 3 years after surgery in various studies [17, 23, 24, 27].
Although the causes of intramedullary nail failure and survival have been studied, we do not know the factors associated with tumor progression, a specific cause of failure that may require additional surgery. It would be important to understand if there are patient or treatment characteristics that are associated with progression, and therefore reoperation, so that surgeons can better choose patients who undergo an initial intramedullary nailing.
We therefore asked: (1) Among patients who underwent intramedullary nail stabilization for impending or completed pathologic fractures of the long bones, what is the risk of local progression, including progression of the existing lesion and development of a new lesion around the nail? (2) Among patients who experience local progression, what proportion undergo a reoperation? (3) What patient characteristics and treatment factors are associated with postoperative local progression? (4) What is the difference in survival rates between patients who experienced local progression and those with stable local disease?
Patients and Methods
Study Design and Setting
This was a retrospective, comparative study performed at an urban tertiary care center. It involved the patients of three orthopaedic oncology subspecialists.
Participants
Between January 2013 and December 2019, 177 unique patients at our hospitals were treated with an intramedullary nail in the humerus, tibia, or femur for an impending or completed pathologic fracture. For all patients, we used reamed nails with interlocking screws proximally and distally. During the study period, the reamer-irrigator-aspirator was routinely used only for single-stage bilateral intramedullary nail procedures. The orthopaedic oncology surgeons (CDM, JAF, ASL) involved in the care of these patients treated an impending or pathologic fracture with an intramedullary nail when a long bone lesion either fractured or was deemed to be of at least 35% risk of fracture within 3 months, and in patients with an anticipated duration of overall survival of more than 6 weeks (fractured) or 3 months (lesion at risk of fracture) to yield palliative benefit during their lifetimes. We considered adult patients who presented with impending or completed pathologic fractures secondary to metastatic bone lesions as potentially eligible for this study. On that basis, we excluded 6% (10 of 177) of patients who did not have a pathologic diagnosis of metastasis before fixation, 0.5% (1 of 177) of patients who were younger than 18 years, 5% (9 of 177) of patients who presented with a primary soft tissue mass that eroded into bone, and 2% (3 of 177) of patients who experienced nonunion from radiation osteitis or an avulsion fracture rather than from metastasis. Overall, 87% (154 of 177) of patients were eligible for this study. Minimum follow-up time was 1 day after surgery. However, to ensure that we were able to follow patients radiographically after surgery and to accurately assess deaths among our patients, we excluded 18% (32 of 177) of patients without radiographic follow-up for at least 90 days but who were alive for at least 180 days after surgery. These patients were lost before the minimum study follow-up time and had incomplete data. If a patient had more than one intramedullary nail, the first nail placed was included. For patients who had multiple nails placed during the same surgery, the femoral nail was chosen. If both nails were placed in the femur, we alternated between choosing the left and right femur to ensure equal distribution. Of the 177 patients, 122 patients (treated with 122 nails) were included in our study (Fig. 1).
Fig. 1.
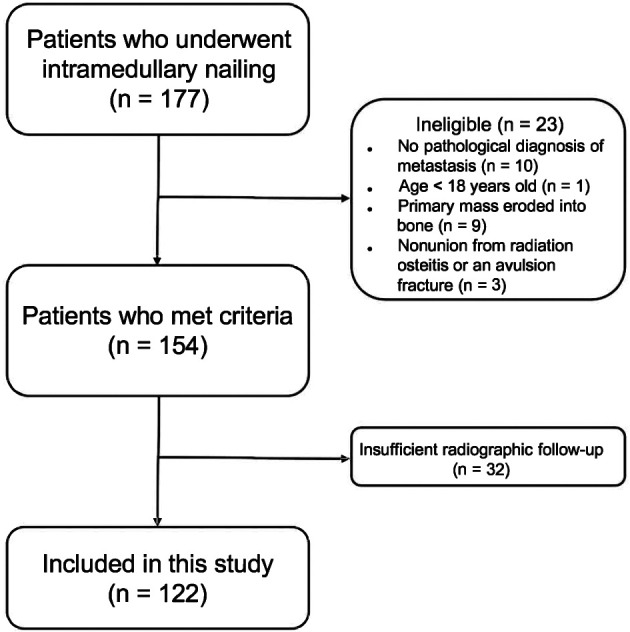
Flow diagram depicting which patients were included in this study.
Patient and Treatment Characteristics
Our cohort comprised 61 men and 61 women with a median (range) age of 63 years (39 to 91). The median follow-up period was 4 months (1 day to 61 months). Of 122 patients, 84 died at a median of 3 months (5 days to 40 months) postoperatively. If a patient had multiple nails, survival was determined from the time of the first intramedullary nail stabilization. The most common primary malignancies were as follows: 25% (31 of 122) of patients had multiple myeloma, 16% (20 of 122) had lung carcinoma, 15% (18 of 122) had breast carcinoma, and 12% (15 of 122) had renal cell carcinoma (Table 1). Retrograde (n = 14) and anterograde (n = 108) nails were included in our analysis. The femur was the most common bone treated (68% [83 of 122]), followed by the humerus (27% [33 of 122]) and the tibia (5% [6 of 122]) (Table 1).
Table 1.
Characteristics of 122 adults treated between January 2013 and December 2019 with intramedullary nail stabilization for an impending or completed pathologic fracture secondary to metastatic bone lesions
| Characteristic | Totals |
| Age | 63 ± 10 |
| Female sex | 50 (61) |
| Primary cancer | |
| Multiple myeloma | 25 (31) |
| Lung | 16 (20) |
| Breast | 15 (18) |
| Renal cell | 12 (15) |
| Prostate | 8 (10) |
| Other cancersa | 23 (28) |
| Bone | |
| Femur | 68 (83) |
| Humerus | 27 (33) |
| Tibia | 5 (6) |
| Site of metastasis | |
| Femur | |
| Head/neck | 5 (6) |
| Trochanter | 16 (20) |
| Diaphysis | 46 (56) |
| Diffuse | 1 (1) |
| Humerus | |
| Head/neck | 3 (3) |
| Diaphysis | 25 (30) |
| Tibia, diaphysis | 5 (6) |
| Nail insertion | |
| Anterograde | 89 (108) |
| Retrograde (femur only) | 11 (14) |
| Fracture | |
| Completed | 51 (62) |
| Impending | 60 (49) |
| Adjuvant therapyb | |
| Bisphosphonate use | 64 (78) |
| Radiation therapy | 59 (72) |
| Postoperative systemic therapy | 78 (95) |
| Metastasesc | |
| Concurrent visceral metastases | 25 (30) |
| Additional bony metastases | 74 (90) |
Data presented as mean ± SD or n (%).
Other cancers were acute myeloblastic leukemia, angiosarcoma, basal cell, cholangiocarcinoma, colon, esophageal, hemangioendothelioma, Hodgkin lymphoma, leiomyosarcoma, liposarcoma, liver, melanoma, Merkel cell, neuroblastoma, pancreatic, thyroid, and urothelial.
Multiple forms of adjuvant therapies were used for some patients.
Not all patients had visceral or other bone metastases.
We evaluated adjuvant therapies, including local radiation therapy of the affected bone, systemic therapy, and antiresorptive therapy. Overall, 59% (72 of 122) of nails were placed in sites that had received radiation therapy (6% [7 of 122] preoperatively and/or 47% [57 of 122] postoperatively). Systemic therapy included chemotherapy and immunotherapy, and 78% (95 of 122) of nails were inserted in patients who had undergone systemic therapy postoperatively. Sixty-four percent (78 of 122) of nails were inserted in patients who used bisphosphonates or receptor activator of nuclear factor kappa-B ligand inhibitors such as zoledronic acid and denosumab preoperatively.
Data Sources and Measurement
We reviewed patient records, radiology reports, and clinic notes to determine whether postoperative progression of a metastatic lesion in the long bone had occurred. Progression was defined according to the criteria developed by the University of Texas MD Anderson Cancer Center, which were adapted to focus on the involved bone only [7, 26]. The first author (PA) assessed progression using the treating surgeon’s medical records. Patients were typically followed daily by the orthopaedic oncology service during their hospital stay, then seen in clinic at 2 weeks, 6 weeks, and every 3 months postoperatively for up to 1 year. After 1 year, patients were typically evaluated once or twice per year for repeat radiographs, with increasing frequency as dictated by their clinical course. Since lesion progression can only be diagnosed at follow-up visits, our reported time until progression is the latest it could have occurred. It is highly likely that progression occurred before the patient was followed up in clinic. Patient date of death was found in electronic medical records and public obituaries.
Primary and Secondary Study Outcomes
Our primary study goal was to measure the risk of progression of skeletal metastases in the bone that underwent closed intramedullary nail stabilization and the risk of a newly recognized metastasis at the end of the nail in the same bone. We realize that death is a competing event because patients who die cannot have lesion progression. All instances of death before progression were considered a competing risk in our analysis. We calculated the cumulative incidence of patients who experienced progression at 1 month, 3 months, 6 months, and 12 months after surgery.
Our secondary study goals were to determine the proportion of patients who underwent reoperation after identifying progression or a new metastasis, identify risk factors associated with progression, and compare survival between patients with stable versus progressive metastases. Factors analyzed for their associations with progression or new metastases were age and sex of the patient, the use of adjuvant therapies (radiation therapy at the site of the lesion, systemic therapy, and antiresorptive therapy), histologic tumor type, fracture type (impending or complete), and location of the bone lesion.
Ethical Approval
Our institutional review board approved this study before data collection began.
Statistical Analyses
Cumulative incidence curves were used to estimate the cumulative risk of progression using the method of Fine and Gray. Any death that happened before progression was considered a competing risk. Time to progression was calculated from the date of initial surgery. The outcome was censored if the patient did not die or progress by the time of last follow-up. For an analysis of factors associated with disease progression, we used chi-square tests and calculated odds ratio for categorical variables and the Welch 2-tailed t-tests for continuous variables. A subdistribution hazard ratio with death as a competing risk was then calculated for variables that were found to be associated with progression by the initial test to confirm the association with progression. When comparing Kaplan-Meier survivorship curves, the log-rank test was used. A p value < 0.05 was considered significant. Cumulative risk analysis was performed using the cmprsk R package and the source code written by Scrucca et al. [25]. Stata/SE, version 15, software (StataCorp LLC) was used for all other analyses.
Results
Local Progression
The cumulative incidence of local tumor progression (with death as a competing risk) after surgery was 1.9% (95% confidence interval 0.3% to 6.1%) at 1 month, 2.9% (95% CI 0.8% to 7.5%) at 3 months, 3.9% (95% CI 1.3% to 8.9%) at 6 months, and 4.9% (95% CI 1.8% to 10.3%) at 12 months (Fig. 2).
Fig. 2.
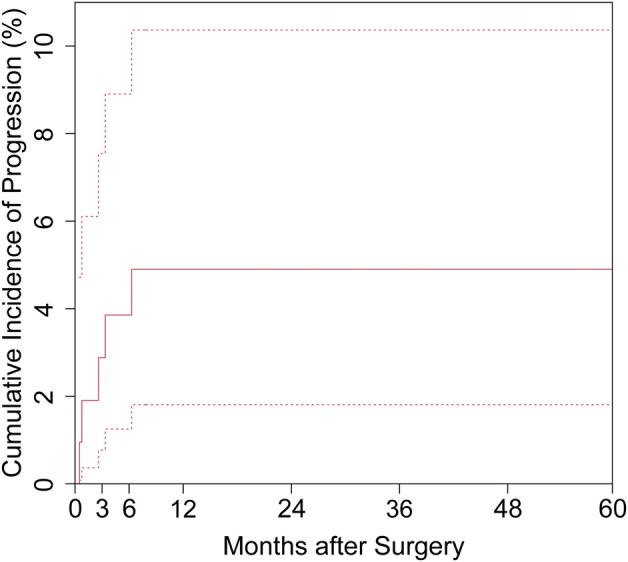
Cumulative incidence of progression for all patients against a competing risk of death is shown as a solid line, and the upper and lower 95% confidence intervals are shown as dotted lines.
The overall proportion of patients who experienced local tumor progression after intramedullary nail stabilization was 7% (8 of 122). Six percent (7 of 122) of patients had local progression of the original lesion, and 0.8% (1 of 122) of patients had a new lesion that developed at the end of the intramedullary nail (Table 2).
Table 2.
Patients with progression of metastatic lesions or a new distal lesion after intramedullary nail stabilization
| Patient number | Age in years | Sex (M/F) | Primary tumor | Lesion site | Fracture | Site | Time to progression in months | Additional surgery |
| 1 | 57 | M | Renal cell | Tibia, mid-shaft (two sites) | Completed | Expansion of both lesions | 0.5 | No |
| 2 | 46 | F | Renal cell | Humerus, proximal | Completed | Expansion of lesion | 12 | Forequarter amputation |
| 3 | 67 | F | Basal cell | Femur, proximal | Completed | Expansion of lesion | 2.6 | No |
| 4 | 49 | M | Renal cell | Femur, mid-shaft | Impending | Expansion of lesion | 9.9 | Resection and femur reconstruction |
| 5 | 47 | F | Angiosarcoma | Humerus, mid-shaft | Completed | Expansion of lesion | 0.8 | No |
| 6 | 62 | F | Breast | Femur, three sites | Impending | Expansion in distal lesion | 3.4 | No |
| 7 | 61 | F | Breast | Femur, proximal | Impending | Around nail | 2.6 | No |
| 8 | 56 | M | Lung | Femur, distal | Completed | New lesion | 0.1 | Hip ORIF |
M = male; F = female; ORIF = open reduction and internal fixation.
Reoperations
Two percent (3 of 122) of patients who progressed subsequently underwent a reoperation (two for progression of the existing lesion and one for a new lesion in the same bone). One patient with progression of the existing lesion initially underwent humeral intramedullary nail stabilization. After lesion progression was noted at 12 months postoperatively, this patient underwent forequarter amputation at 28 months after her initial surgery (Fig. 3). The other patient with progression of an existing lesion had intramedullary nail stabilization in the setting of renal cell carcinoma with multiple metastases. The patient was morbidly obese with a diaphyseal tumor along with numerous metastases and ultimately underwent total femur resection and reconstruction 20 months after the initial surgery (Fig. 4). The third patient who ultimately underwent additional surgery initially presented with a fracture in the distal right femur, along with non–small cell lung carcinoma. Follow-up images showed a new lesion in the proximal right femur, and this patient ultimately underwent right hip reduction and internal fixation 4 months after the initial surgery (Fig. 5). Among the remaining patients who did not undergo a reoperation, disease progression was evident in three patients soon after nail stabilization, but the size of the lesions plateaued and no additional surgery was performed. The remaining two patients died or entered hospice care soon after progression was found, and therefore, they did not undergo additional reoperations for progression.
Fig. 3.
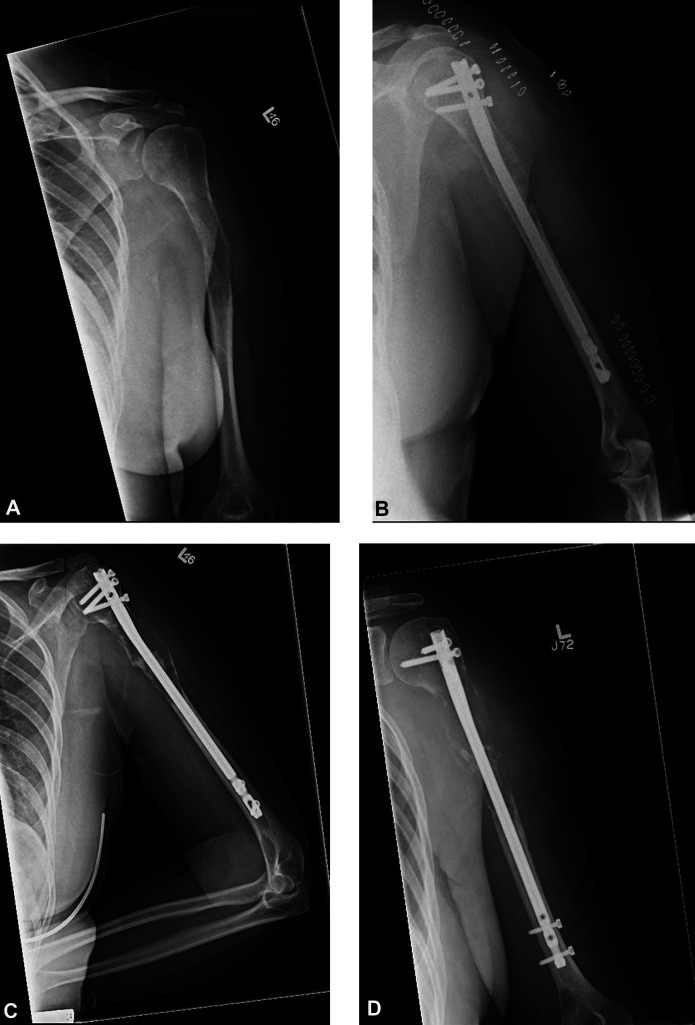
A-D These AP radiographs are from a 46-year-old woman with no history of metastatic disease who presented with (A) a complete pathologic fracture in the proximal shaft of the left humerus. (B) Another radiograph was taken 3 days after intramedullary nail stabilization. A biopsy performed at nail insertion showed metastatic disease with renal cell origin. (C) This radiograph was taken 5.7 months postoperatively. (D) This radiograph was taken 16 months postoperatively. Very little residual bone remained in the proximal humerus, prompting forequarter amputation.
Fig. 4.
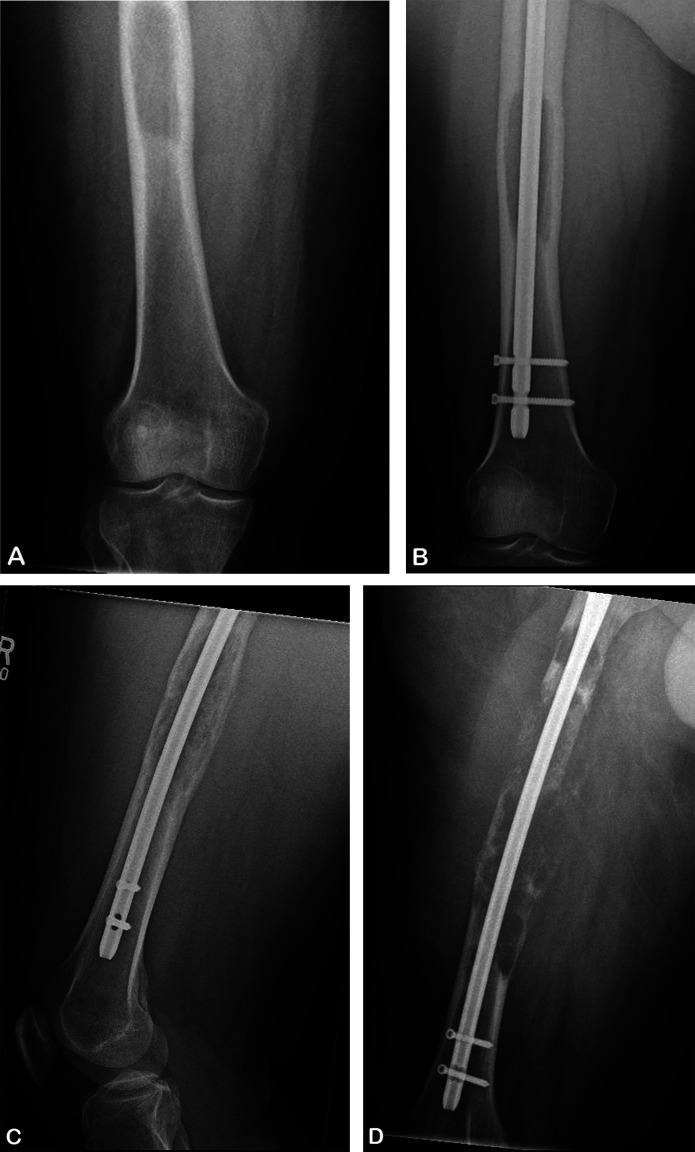
A-D These (A-B) AP and (C-D) lateral radiographs are from a 49-year-old man with a history of metastatic renal cell carcinoma who presented with (A) a lesion in his right femur. (B) A radiograph taken 25 days after intramedullary nail stabilization shows adequate nail placement. (C) A radiograph taken 11 months postoperatively shows expansion of the lesions with complete destruction of the lateral cortex of the midfemoral diaphysis. (D) Continued lesion expansion that was visible on a radiograph taken at 19 months postoperatively prompted resection and femur reconstruction.
Fig. 5.
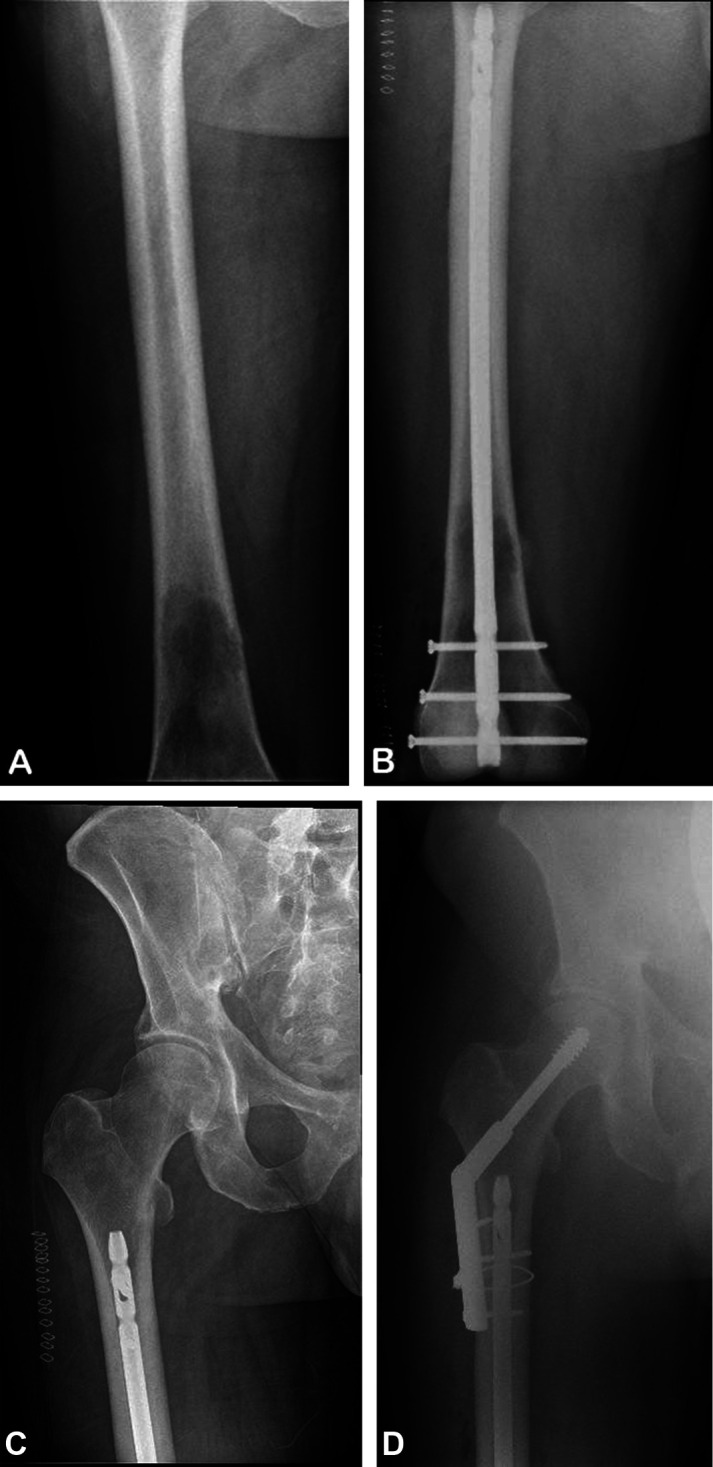
A-D These AP radiographs are from a 56-year-old man with a history of metastatic non–small cell lung carcinoma who presented with (A) a right femur lesion in the distal third of the diaphysis. (B) A radiograph taken 1 day after intramedullary nail stabilization shows adequate nail placement. (C) A radiograph taken 15 days postoperatively shows a slight lucency at the distal margin of the lesser trochanter, which may have caused the patient’s new-onset right groin pain. (D) Continued pain secondary to the proximal femur lesion, which was seen on a radiograph taken at 4.2 months postoperatively, prompted open reduction and internal fixation of the right hip.
Factors Associated with Local Progression
The two factors found to be associated with progression were a primary tumor of renal cell carcinoma (15 of 122 patients, OR 5.1 [95% CI 0.69 to 29.6]; p = 0.03) and age of the patient (difference in mean age 7.7 years [95% CI 1.2 to 14]; p = 0.02). Of the patients with renal cell carcinoma, 20% (3 of 15) experienced lesion progression compared with 5% (5 of 107) of patients with all other primary malignancies. The subdistribution hazard ratio (SHR) of patients with renal cell versus progression (controlling for death as a competing risk) was 4.4 (95% CI 1.1 to 18; p = 0.04). The SHR of patients older than 63 years and younger than 63 years (the mean age of all patients) was 6.1 (95% CI 0.75 to 49; p = 0.09). The mean age of patients who experienced progression was younger than the age of patients who did not (56 ± 8 years versus 63 ± 10 years; p = 0.02). Progression was not associated with the presence of visceral metastases or other skeletal metastases or the use of adjuvant therapies (Table 3). It was also not associated with patient sex, anatomic site of the lesion, fracture type (impending versus completed), or whether the nail was placed in an anterograde or retrograde manner (Table 4).
Table 3.
Odds ratio of progression of the metastatic lesion in the long bone in 122 patients after intramedullary nail insertion for a pathologic fracture
| Variable | Stable (n = 114) | Progression (n = 8) | OR (95% CI) | p value |
|
Renal cell carcinoma |
11 (12) |
38 (3) |
5.1 (0.69-29) |
0.03 |
|
Visceral metastases |
25 (28) |
25 (2) |
1.0 (0.96-6.1) |
0.98 |
|
Other skeletal metastases |
74 (84) |
75 (6) |
1.1 (0.18-11) |
0.93 |
|
Radiation therapy |
57 (65) |
88 (7) |
0.19 (0.004-1.6) |
0.09 |
|
Postoperative systemic therapy |
76 (87) |
100 (8) |
NAa |
0.12 |
|
Bisphosphonate use |
66 (75) |
38 (3) |
3.2 (0.58-21) |
0.11 |
Data presented as % (n).
Unable to calculate due to zero count cells; OR = odds ratio; CI = confidence interval.
Table 4.
Univariate chi-square analysis of progression of the metastatic lesion in the long bone in 122 patients after insertion of an intramedullary nail for a pathologic fracture
| Variable | Stable (n = 114) | Progression (n = 8) | X2 (df) | p value |
| Female sex | 51 (58) | 38 (3) | 0.54 (1) | 0.45 |
| Age | 63 ± 10 | 56 ± 8 | 2.1 (120)a | 0.02 |
| Bone | ||||
| Femur | 68 (78) | 63 (5) | 1.1 (2) | 0.59 |
| Humerus | 27 (31) | 25 (2) | ||
| Tibia | 4 (5) | 13 (1) | ||
| Primary tumor | ||||
| Breast | 14 (16) | 25 (2) | 8 (5) | 0.16 |
| Lung | 17 (19) | 13 (1) | ||
| Multiple myeloma | 27 (31) | 0 (0) | ||
| Renal cell | 11 (12) | 38 (3) | ||
| Prostate | 9 (10) | 0 (0) | ||
| Other | 23 (26) | 25 (2) | ||
| Impending fracture | 50 (57) | 38 (3) | 0.47 (1) | 0.49 |
| Anterograde nail | 89 (102) | 75 (6) | 1.54 (1) | 0.21 |
Data presented as mean ± SD or % (n).
Data presented as t-value; df = degrees of freedom.
Survival
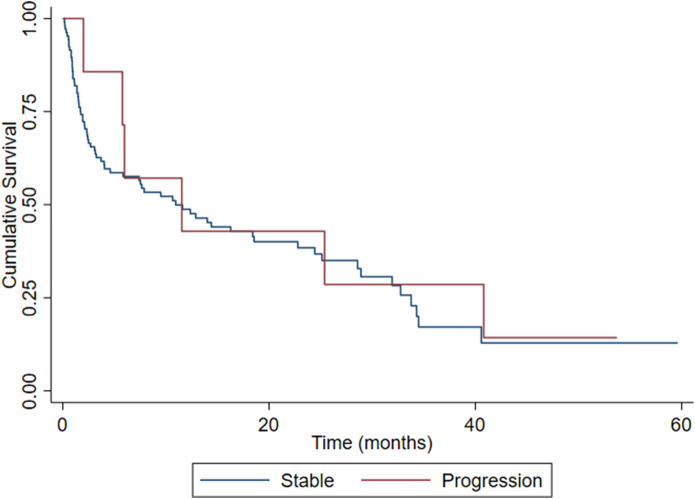
The median (range) time until diagnosis of progression was 3.0 months (0.5 to 12). The median survival was 11.5 months (2 to 41) for patients with tumor progression and 3.2 months (0 to 41) for patients who did not experience progression (those for whom the date of death was known). There was no difference in survivorship curves between those with disease progression and those with stable local disease (= 0.36; p = 0.54). The survival rate for all patients was 85% at 1 month after surgery and 38% at 1 year after surgery, with no difference (1 month, = 1.8; p = 0.18; 1 year, = 0.73; p = 0.40) between the progression and stable groups (Fig. 6).
Fig. 6.
These survival curves represent 122 patients according to the progression of bone lesions. Patients were treated between January 2013 and December 2019 with intramedullary nail stabilization for an impending or completed pathologic fracture because of metastatic bone lesions.
Discussion
The current guidelines for the treatment of metastases of the long bones advocate stabilizing a long bone at high risk of fracture, and the goal of intramedullary nailing is to stabilize the entire bone for the remainder of the patient’s life [2, 4, 6, 11, 17, 20, 23, 27, 29]. Previous studies have shown that local progression and the occurrence of new lesions at the end of implants, including intramedullary nails and femoral stems, in the setting of osseous metastases does exist. Our study seeks to expand this knowledge by focusing on one type of implant in a variety of long bones rather than limiting it to the femur as previous studies have done [2, 30]. Furthermore, although these previous analyses identified the incidence of progression, they did not consider death as a competing risk for progression or identify possible factors, such as patient and treatment characteristics, that could be related to progression. We found that the presence of visceral or other bone metastases and the fracture type were not associated with local disease progression. Because most patients who present with metastatic lesions use other adjuvant therapies, such as radiation therapy, systemic therapy, and antiresorptive therapy, we are unable to determine whether not using these therapies was associated with local progression around the nail. However, a primary tumor of renal cell carcinoma and younger patient age were associated with local progression. Overall, we recommend intramedullary nailing in patients with osseous metastases, but we urge caution in patients who are younger or present with renal cell carcinoma. For those patients, if they are candidates for surgery, we recommend very close radiographic follow-up after surgery within the first year.
Limitations
Our study has several limitations. First, the study was performed retrospectively and included a large variety of primary malignancies and comorbidities. Selection bias is likely present due to how patients were chosen for surgery at our institution. For pathologic long bone fractures of the lower extremity, patients typically undergo operative treatment if they are expected to survive longer than 6 weeks. However, periarticular lesions are more commonly treated with arthroplasty. Further, expansile destructive lesions and those with a large soft tissue component may be considered for resection and prosthetic reconstruction. For patients with impending pathologic fracture, we used similar operative considerations, although these patients would typically have an expected survival of more than 3 months, as estimated with PATHFx [3, 22]. Each patient’s treatment plan was individualized according to the histologic tumor type, responsiveness to treatments, and extent of metastases, which complicated our analysis of individual factors associated with local progression. Patients with renal cell carcinoma, in particular, were more commonly treated with wide resection and reconstruction rather than closed intramedullary nail insertion. Furthermore, some patients continued their care locally, which challenged the follow-up imaging review. Overall, 18% (32 of 177) of patients were lost to radiographic follow-up and were excluded from our analysis. This transfer bias decreased the size of the patient population, thereby decreasing the statistical power of our analysis. With incomplete records, we had no way of knowing how patients who were lost to follow-up fared. Because we were unable to capture a complete set of data within our study, our estimates of progression therefore are best-case estimates. Furthermore, with our small number of patients, our data may be underpowered to detect certain associations. It is likely that some of the patients who died soon after surgery would have experienced lesion progression.
Local Progression
The risk of local progression in our patient population was small, and all cases occurred by 1 year, although this would be a best-case estimate given the incomplete records of many patients. This finding is consistent with the findings of previous studies that analyzed the progression of metastatic disease in patients undergoing intramedullary nail stabilization [2, 29]. Interestingly, only one patient in our study had a new lesion at the end of the nail after intramedullary nail stabilization. This finding is similar to that of a retrospective study by Alvi and Damron [2], who analyzed 96 patients treated with long intramedullary nails or long cemented arthroplasty stems secondary to proximal femoral lesions and found only one patient with a new, previously unrecognized lesion. Because a new lesion after intramedullary nailing seems to be uncommon, there appears to be a minimal risk that seeding tumor cells after inserting the intramedullary nail results in recognizable local disease progression under current treatment paradigms. Patients who undergo intramedullary nailing should be followed radiographically for at least 1 year or until death, whichever occurs first.
Reoperations
In our study, the reoperation risk of intramedullary nail stabilization because of lesion progression was low. Our rate of failure is similar to those reported in other retrospective studies of intramedullary nails. Alvi and Damron [2] reported that 11 of 96 patients with metastatic osseous lesions experienced progressive disease, four of whom ultimately underwent surgical revision. In a similar study of patients who presented with primary bone and soft tissue sarcoma metastases and who underwent intramedullary nail stabilization, Moon et al. [18] reported that 12% (4 of 34) of patients underwent revision surgery because of local progression. In another study focusing on femoral neck metastases, Moon et al. [19] found that 4% (5 of 141) of patients underwent reoperation because of the development of additional metastases or nail failure. These findings reaffirm that the reoperation risk after intramedullary nail stabilization because of progression is low in patients with metastatic osseous tumors for whom surgery could yield benefit in their lifetimes. Most patients in this analysis were treated with radiation therapy and bisphosphonate treatment, although in the absence of expansile destruction or a renal cell carcinoma diagnosis, closed intramedullary nail treatment appears to provide a durable solution for long bone metastases without clinical local progression.
Factors Associated with Local Progression
Previous reports have analyzed factors associated with intramedullary nail failures in metastatic disease, even though these failures may not have been necessarily caused by lesion progression [17, 29]. Both the calculated odds ratio and the SHR (with death as a competing risk) showed that there is an associated risk of progression in patients with renal cell carcinoma. In a multicenter study with 245 patients, Willeumier et al. [29] reported that the presence of a complete pathologic fracture was associated with a lower risk of revision because of implant failure or breakage. However, our data suggest that fracture type (complete versus impending) was not associated with progression and resultant implant failure. The only tumor characteristic associated with local disease progression was a primary tumor diagnosis of renal cell carcinoma, which accounted for three of eight patients with progression. This finding is consistent with findings in other retrospective studies showing that bone metastases from renal cell carcinoma may be relatively refractory to local and systemic treatment [2, 13, 17]. Although data suggest that this response is not unique to osseous sites involving renal cell carcinoma [26], local control after intralesional treatment remains a concern. In our experience, patients with renal cell carcinoma are often not recommended for intramedullary nail stabilization because renal cell carcinoma responds poorly to radiation therapy [10, 28]. Additionally, zoledronic acid has been effective in delaying skeletal related events in many primary cancers, including breast, lung, and renal cell [15, 16, 21]. Although 47% (7 of 15) of our renal cell carcinoma patients received antiresorptive therapy and only 1 of 7 experienced progression, we do not have enough evidence in our cohort to suggest that the use of antiresorptive therapy influenced time to progression. Data suggest that excising osseous metastatic disease in some of these patients might be associated with a longer duration of survival, although it is unclear whether that is because of the surgery or the underlying nature of oligometastatic disease [1, 13]. Furthermore, we found that the risk of progression around or at the end of the nail was 3.5% for primary malignancies other than renal cell carcinoma. Importantly, most patients with bone metastases from renal cell carcinoma treated at our institution during the study period underwent tumor resection and were thus excluded from our analysis. The data in our study do not further clarify whether metastatic angiosarcoma, which is rare and highly angiogenic, should be treated similarly to renal cell carcinoma.
Younger age was associated with progression when a t-test was performed, but when controlling for death as a competing risk and calculating the SHR, younger age was not linked. However, our population size was small, and many patients were lost to follow-up, which may have prevented us from detecting a relationship. A study with a larger population may be warranted to investigate whether age is truly associated with progression, since our data provide a tenuous link between the two. Although there are no studies known to us that have compared progression of bone metastases with age of the patient, there is some evidence on metastatic disease progression in different types of cancer showing that younger age may be connected to worse outcomes. Cummings et al. [8] found that in 197 patients with breast cancer, younger age was associated with increased metastases to visceral and gynecological organs. Additionally, Bajard et al. [5] found that for patients with lung cancer, the progression of brain metastases was significantly higher in those younger than 62 years. Our data indicate that there may be an association with age, and that when surgeons consider patients younger than 63 for intramedullary nailing, they should plan to follow these patients closely radiographically because the possible higher risk of progression.
Only 57% of our patients underwent pre- and/or postoperative radiation therapy for their lesion of interest. We recognize that this number is low, as radiation therapy is a well-established treatment for metastatic bone pain. In our cohort, many patients were recommended surgery after diagnosis of metastases, followed by radiation. However, one-third of our patients died within 1 year and did not live long enough to complete treatment. Therefore, they did not receive radiation at the lesion site and thus lowered the percentage of patients who received radiation.
Survival
We also found that patients who experienced tumor progression after surgery lived longer than those who did not experience tumor progression, reflecting a tendency toward early death as a competing risk for local progression. However, the goal of surgery is typically to provide durable function and pain control, which was achieved in most patients. A previous study showed that longer survival was associated with increased incidence of complications from skeletal metastases [23]. Miller et al. [17] found that the median survival time for patients who experienced intramedullary nail failure was 3.2 years, whereas patients without failed implants had a median survival time of 0.6 months. It is unsurprising that longer survival is also associated with an increased incidence of progression. It is difficult to predict patient survival after intramedullary nail stabilization, although laboratory values such as hemoglobin and albumin concentrations, as well as the primary cancer type, may be useful in estimating the duration of survival [9, 12].
Conclusion
We found the type of primary malignancy was associated with the likelihood of local progression around the intramedullary nail used to treat long bone metastases, whereas the duration of survival and the use of adjuvant therapies were not. There may be an association between a younger patient age and progression, but our small study with significant loss to follow-up prevented us from detecting such an association. Only a small proportion of our patients underwent reoperation after intramedullary nailing, but we may have underestimated this risk because of loss to follow-up. Therefore, in properly selected patients with metastatic osseous disease, the risk of local disease progression or new adjacent lesions is low after intramedullary nail stabilization of the long bones for complete or impending pathologic fractures secondary to metastatic disease, with a special consideration for patients who present with renal cell carcinoma. For those patients, we recommend close radiographic follow-up during the first year after surgery.
Acknowledgments
We thank Jenni Weems MS, Kerry Kennedy BA, and Rachel Box MS in the Editorial Services group of the Johns Hopkins Department of Orthopaedic Surgery for their editorial assistance. For his statistical assistance, we thank Edgar Manriquez-Sandoval in the Biophysics Department of the Johns Hopkins Zanvyl Krieger School of Arts and Sciences.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from Johns Hopkins Medicine, Baltimore, MD, USA (number IRB00140712).
This work was performed at Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Contributor Information
Punthitra Arpornsuksant, Email: parporn1@jhmi.edu.
Carol D. Morris, Email: cmorri61@jhmi.edu.
Jonathan A. Forsberg, Email: jforsbe1@jh.edu.
References
- 1.Althausen P, Althausen A, Jennings LC, Mankin HJ. Prognostic factors and surgical treatment of osseous matastases secondary to renal cell carcinoma. J Urol. 1998;160:634-635. [PubMed] [Google Scholar]
- 2.Alvi HM, Damron TA. Prophylactic stabilization for bone metastases, myeloma, or lymphoma: do we need to protect the entire bone? Clin Orthop Relat Res. 2013;471:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson AB, Wedin R, Fabbri N, Boland P, Healey J, Forsberg JA. External validation of PATHFx version 3.0 in patients treated surgically and nonsurgically for symptomatic skeletal metastases. Clin Orthop Relat Res. 2020;478:808-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvinius C, Parra JLC, Mateo LS, Maroto RG, Borrego AF, Stern LLD. Benefits of early intramedullary nailing in femoral metastases. Int Orthop. 2014;38:129-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajard A, Westeel V, Dubiez A, et al. Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer. 2004;45:317-323. [DOI] [PubMed] [Google Scholar]
- 6.Choi ES, Han I, Cho HS, Park IW, Park JW, Kim HS. Intramedullary nailing for pathological fractures of the proximal humerus. Clin Orthop Surg. 2016;8:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings MC, Simpson PT, Reid LE, et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol. 2014;232:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsberg JA, Eberhardt J, Boland PJ, Wedin R, Healey JH. Estimating survival in patients with operable skeletal metastases: an application of a Bayesian belief network. PLoS One. 2011;6:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fottner A, Szalantzy M, Wirthmann L, et al. Bone metastases from renal cell carcinoma: patient survival after surgical treatment. BMC Musculoskelet Disord. 2010;11:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenbaum SL, Thornhill BA, Geller DS. Characterization and surgical management of metastatic disease of the tibia. Am J Orthop (Belle Mead NJ). 2017;46:E423-E428. [PubMed] [Google Scholar]
- 12.Kotian RN, Puvanesarajah V, Rao S, El Abiad JM, Morris CD, Levin AS. Predictors of survival after intramedullary nail fixation of completed or impending pathologic femur fractures from metastatic disease. Surg Oncol. 2018;27:462-467. [DOI] [PubMed] [Google Scholar]
- 13.Les KA, Nicholas RW, Rougraff B, et al. Local progression after operative treatment of metastatic kidney cancer. Clin Orthop Relat Res. 2001;390:206-211. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipton A, Colombo-Berra A, Bukowski RM, Rosen L, Zheng M, Urbanowitz G. Skeletal complications in patients with bone metastases from renal cell carcinoma and therapeutic benefits of zoledronic acid. Clin Cancer Res. 2004;10:6397S-6403S. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Olivo MA, Shah NA, Pratt G, Risser JM, Symanski E, Suarez-Almazor ME. Bisphosphonates in the treatment of patients with lung cancer and metastatic bone disease: a systematic review and meta-analysis. Support Care Cancer. 2012;20:2985-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller BJ, Carmody Soni EE, Gibbs CP, Scarborough MT. Intramedullary nails for long bone metastases: why do they fail? Orthopedics. 2011;34. [DOI] [PubMed] [Google Scholar]
- 18.Moon B, Lin P, Satcher R, Bird J, Lewis V. Intramedullary nailing of femoral diaphyseal metastases: is it necessary to protect the femoral neck? Clin Orthop Relat Res. 2015;473:1499-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon BS, Dunbar DJ, Lin PP, Satcher RL, Bird JE, Lewis VO. Is it appropriate to treat sarcoma metastases with intramedullary nailing? Clin Orthop Relat Res. 2017;475:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morishige M, Muramatsu K, Tominaga Y, Hashimoto T, Taguchi T. Surgical treatment of metastatic femoral fractures: achieving an improved quality of life for cancer patients. Anticancer Res. 2015;35:427-432. [PubMed] [Google Scholar]
- 21.O’Carrigan B, Wong MHF, Willson ML, Stockler MR, Pavlakis N, Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2017;10(10):CD003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overmann AL, Clark DRM, Tsagkozis P, Wedin R, Forsberg JA. Validation of PATHFx 2.0: an open-source tool for estimating survival in patients undergoing pathologic fracture fixation. J Orthop Res. 2020;38:2149-2156. [DOI] [PubMed] [Google Scholar]
- 23.Piccioli A, Rossi B, Scaramuzzo L, Spinelli MS, Yang Z, MacCauro G. Intramedullary nailing for treatment of pathologic femoral fractures due to metastases. Injury. 2014;45:412-417. [DOI] [PubMed] [Google Scholar]
- 24.Sarahrudi K, Hora K, Heinz T, Millington S, Vécsei V. Treatment results of pathological fractures of the long bones: a retrospective analysis of 88 patients. Int Orthop. 2006;30:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381-387. [DOI] [PubMed] [Google Scholar]
- 26.Tai KY, El Abiad JM, Morris CD, Markowski MC, Levin AS. Comparing the responses of osseous versus soft-tissue metastases of renal cell carcinoma to receptor tyrosine kinase inhibitors and immunotherapy. Kidney Cancer. 2020;4:151-158. [Google Scholar]
- 27.Tanaka T, Imanishi J, Charoenlap C, Choong PFM. Intramedullary nailing has sufficient durability for metastatic femoral fractures. World J Surg Oncol. 2016;14:4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umer M, Mohib Y, Atif M, Nazim M. Skeletal metastasis in renal cell carcinoma: a review. Ann Med Surg. 2018;27:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willeumier JJ, Kaynak M, Van Der Zwaal P, et al. What factors are associated with implant breakage and revision after intramedullary nailing for femoral metastases? Clin Orthop Relat Res. 2018;476:1823-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing Z, Moon BS, Satcher RL, Lin PP, Lewis VO. A long femoral stem is not always required in hip arthroplasty for patients with proximal femur metastases tumor. Clin Orthop Relat Res. 2013;471:1622-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]