Abstract
Several types of drugs currently used in clinical practice were screened in vitro for their potentiation of the antifungal effect of the fungistatic agent fluconazole (FLC) on Candida albicans. These drugs included inhibitors of multidrug efflux transporters, antimicrobial agents, antifungal agents, and membrane-active compounds with no antimicrobial activity, such as antiarrhythmic agents, proton pump inhibitors, and platelet aggregation inhibitors. Among the drugs tested in an agar disk diffusion assay, cyclosporine (Cy), which had no intrinsic antifungal activity, showed a potent antifungal effect in combination with FLC. In a checkerboard microtiter plate format, however, it was observed that the MIC of FLC, as classically defined by the NCCLS recommendations, was unchanged when FLC and Cy were combined. Nevertheless, if a different reading endpoint corresponding to the minimal fungicidal concentration needed to decrease viable counts by at least 3 logs in comparison to the growth control was chosen, the combination was synergistic (fractional inhibitory concentration index of <1). This endpoint fitted to the definition of MIC-0 (optically clear wells) and reflected the absence of the trailing effect, which is the result of a residual growth at FLC concentrations greater than the MIC. The MIC-0 values of FLC and Cy tested alone in C. albicans were >32 and >10 μg/ml, respectively, and decreased to 0.5 and 0.625 μg/ml when the two drugs were combined. The combination of 0.625 μg of Cy per ml with supra-MICs of FLC resulted in a potent antifungal effect in time-kill curve experiments. This effect was fungicidal or fungistatic, depending on the C. albicans strain used. Since the Cy concentration effective in vitro is achievable in vivo, the combination of this agent with FLC represents an attractive perspective for the development of new management strategies for candidiasis.
Candida infections represent an increasing challenge for clinicians. The epidemiology of the last decade shows that these infections are continuously increasing (1), owing to more aggressive management of medical and surgical cases. Fluconazole (FLC) has been shown to be as effective as amphotericin B in the treatment of candidemia in non-neutropenic patients (35). Since amphotericin B is toxic in its conventional form and very expensive in its new lipidic forms, azole antifungal agents are currently used as first-line drugs (6, 11). Nevertheless, the emergence of Candida albicans strains with decreased susceptibility to FLC and the epidemiologic shift to other Candida species complicate the management of these infections (5, 28, 45). Among azole antifungal agents, FLC offers several advantages, such as an excellent oral bioavailability, a stable parenteral formulation, and minimal drug interactions. However, FLC, like the other azole derivatives, is only fungistatic. Its efficacy relies on the function of the cellular host defenses (10) and is limited in cases of profound neutropenia (4, 6) or by severe depletion of CD4 cells (36). Thus, the potentiation of the antifungal effect of FLC by its combination with partner drugs would be very useful. The classical strategy against severe or resistant bacterial infections has been the combination of different classes of antimicrobial agents. By analogy, the combination of azole derivatives with other antifungal or antimicrobial agents could represent a possible approach. Sugar et al. (43) described an increased antifungal effect in vivo when combining FLC with quinolone antibiotics. Interestingly this combination was ineffective in vitro, and the mechanisms underlying this important observation have yet to be elucidated.
The recent discovery of drug-efflux-mediated resistance mechanisms in yeasts opens up a new therapeutic concept. In the last few years, it has been recognized that C. albicans expresses multidrug efflux transporter (MET) genes belonging to two different classes, the ATP-binding-cassette transporters and the major facilitators. METs mediate the efflux of a broad range of compounds, including FLC. Different MET genes have been identified in C. albicans (CDR1, CDR2, CaMDR1, and FLU1). The basal expression of these genes and their targeted deletion determine the level of azole susceptibility (37). The upregulation of some of these genes, particularly CDR1, CDR2, and CaMDR1, results in a decrease of the intracellular level of FLC in azole-resistant cells (38, 39).
In mammalian cells, similar efflux mechanisms have been described. In particular, the upregulation of P glycoproteins, which belong to METs of the ABC superfamily, is a characteristic of cancer cells resistant to cytotoxic agents. Several authors have reported the inhibition of these proteins with different classes of drugs in multiresistant cancer cells (32). Promising experimental results found a partial confirmation in clinical trials in the treatment of different types of solid tumors (25, 44) and hematologic malignancies (17, 19, 20, 41, 42). A similar experimental principle had already been investigated to reverse the chloroquine resistance in Plasmodium falciparum (3) or the resistance to quinolone antibiotics in Staphylococcus aureus (15). The rationale of the combination of FLC with inhibitors of mammalian METs currently used in clinical practice against FLC-susceptible C. albicans was based on the strong evolutionary structural conservation of these transporter proteins (13). The present study sought to investigate the effectiveness of several combinations of FLC with MET inhibitors, antifungal drugs, and antimicrobial agents and with nonantimicrobial membrane-active compounds, such as antiarrhythmic agents, proton pump inhibitors, and platelet aggregation inhibitors. We show here that some of these compounds shows potent antifungal effects when used in combination with FLC.
MATERIALS AND METHODS
Strains and media.
The following C. albicans strains were used in this study: the clinical isolate 731 originating from the collection of the University Hospital of Lausanne, the laboratory strain CAF2-1 (8), and the reference strain ATCC 90028. The strains were maintained at 4°C on Sabouraud dextrose agar plates and subcultured twice at 35°C before each experiment to ensure viability and purity. Liquid cultures were performed in Sabouraud medium (Diagnostics Pasteur, Marnes La Coquette, France). Sabouraud dextrose with 2% agar (Difco Laboratories, Basel, Switzerland) was used to conserve and subculture the test strains and for colony counting studies. YEPD agar consisting of 0.5% yeast extract (Difco), 0.5% peptone (Difco), 2% glucose, and 2% agar (Difco) was used for agar disk diffusion testing.
Drug susceptibility testing by disk diffusion assay.
To allow the rapid screening of a large number of compounds for their intrinsic antifungal activity and for their antifungal activity in combination with FLC, an agar diffusion method was chosen. A single colony of the strain to be tested was grown overnight in Sabouraud liquid medium in a rotary shaker (200 rpm) at 35°C. The inoculum was prepared by diluting the overnight culture with 0.9% NaCl to a 0.5 McFarland standard at a 530-nm wavelength (Densimat; Biomérieux, Marcy l'Etoile, France). This optical density corresponded to 1 × 106 to 5 × 106 CFU/ml. The yeast suspension was applied onto YEPD agar plates using cotton swabs. To test the antifungal activity of each combination of partner drugs and FLC, cellulose disks (Sensi Disc; Becton-Dickinson Europe, Meylan Cedex, France) impregnated with 1, 10, and 100 μg of the solubilized drugs and the control disk impregnated with the corresponding solvent were placed onto YEPD agar plates containing supra-MICs of FLC (25 μg/ml for C. albicans 731 and ATCC 90028 and 20 μg/ml for C. albicans CAF2-1). To assess the intrinsic antifungal activity of the partner drugs, the disks were placed onto plain YEPD agar plates. After 48 h of incubation at 35°C, the horizontal and vertical diameters of the growth inhibition areas were recorded and averaged. The assays were performed in duplicate. On the basis of the lowest partner drug amount resulting in growth inhibition, an antifungal index, calculated as the mean diameter of the corresponding growth inhibition zone (in millimeters)/the minimal drug amount resulting in growth inhibition (in micrograms), was determined for each tested drug, either alone or combined with FLC. The antifungal activity was expressed semiquantitatively with the following cutoff values: low, <1 mm/μg; intermediate, ≥1 to <10 mm/μg; and high, ≥10 mm/μg. This screening allowed the selection of a restricted number of drug combinations with intermediate or high antifungal activities that were studied further.
MIC testing.
MIC testing was performed according to the NCCLS approved standard M27-A for the reference method for broth dilution antifungal susceptibility testing of yeasts (27). To test the MIC of amphotericin B, RPMI was compared to Antibiotic Medium 3 (Difco) supplemented with 2% glucose. C. albicans ATCC 90028 was used in both liquid media as a quality control strain. The MICs of the reference strain were in the expected range for both FLC (0.25 to 1.0 μg/ml) and amphotericin B (0.5 to 2.0 μg/ml) when tested in RPMI. However, the MIC of amphotericin B tested in ABM 3, was repeatedly eightfold lower, i.e., 0.0625 μg/ml. This finding was verified comparing micro- and macrobroth dilution testing results and using different batches of broth medium and of amphotericin B. Furthermore, external laboratory controls confirmed these MIC values. Since ABM 3 was used for the time-kill curves, the corresponding MICs were also reported.
Checkerboard microtiter plate testing.
To characterize and quantify the antifungal activity of FLC, partner drugs, and their combinations, the compounds selected by agar disk diffusion were tested in a checkerboard microtiter plate format (Costar 96-well polystyrene cell culture cluster with flat bottom; Corning Inc., Corning, N.Y.). All experiments were performed according to NCCLS approved standard M27-A (28). RPMI 1640 with l-glutamine without bicarbonate (Difco) was used as broth medium buffered with 0.165 M MOPS (morpholine propanesulfonic acid; Fluka, Basel, Switzerland). The inoculum was prepared by adjusting the optical density of an overnight culture to the 0.5 McFarland standard at 530 nm. The obtained suspension of 1 × 106 to 5 × 106 CFU/ml was further diluted in the broth medium to a final inoculum ranging between 0.5 × 103 and 2.5 × 103 CFU/ml. The viable counts of each inoculum were verified by subcultures of a volume of 100 μl in serial dilutions on Sabouraud agar plates. The stock solution of FLC (2 mg/ml) was prepared and further diluted in RPMI. FLC was tested by using twofold dilutions at concentrations ranging from 0.03 to 32 μg/ml. The stock solutions of chlorpromazine, fluphenazine, amitriptyline, and clomipramine (5 mg/ml) were also prepared and further diluted in RPMI. These compounds were tested by using fourfold dilutions at concentrations ranging from 0.025 to 100 μg/ml. Cyclosporine (Cy) and FK506 were tested by fourfold dilutions at concentrations ranging from 0.01 to 10 μg/ml. These drugs were solubilized in 100% dimethyl sulfoxide (DMSO) to constitute a stock solution (1 mg/ml) that was further diluted in the broth medium. To test a concentration of Cy and FK506 of 10 μg/ml, a final DMSO concentration of 1% (vol/vol) was needed. Preliminary studies showed that DMSO has a toxic activity against C. albicans at concentrations higher than 8% (vol/vol) when used alone and higher than 4% (vol/vol) when combined with supra-MICs of FLC. The incubation lasted 48 h at 35°C. The MIC endpoint was defined as the lowest FLC concentration at which a prominent decrease in turbidity (score of 2 according to the NCCLS guidelines) was observed. This corresponded spectrophotometrically to an optical density of ≤50% of that of the growth control measured at 540 nm in a microplate reader (Microplate Reader 3550-UV; Bio-Rad) and to a growth inhibition of >90% (>1 log) when the viable counts were compared to those of the growth control. To characterize the interaction of each combination tested, the fractional inhibitory concentrations (FICs) of each drug tested and their sum, the so-called FIC index (2), were calculated on the basis of the MIC endpoint. As classically defined, an FIC index of <1 is expression of a drug synergism, whereas an FIC index of >1 represents an antagonism. The so-called trailing, the residual turbidity observed at supra-MICs of FLC, indicates an incomplete inhibition of growth. It corresponds spectrophotometrically to an optical density of between 5 and 50% of that of the growth control. CFU were counted in wells with or without this residual turbidity by subculturing 100 μl of their contents in serial dilutions (10−1, 10−2, 10−3, and 10−5) on Sabouraud agar plates. The dilution 10−2 was added to detect drug carryover (33, 34). With a starting inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml and a limit of detection for the subcultures of 101 CFU/ml, it was not possible to obtain a fungicidal effect, as classically defined by a >99.9% (>3-log) decrease in the viable counts of the starting inoculum. The minimal fungicidal concentration could therefore not be used as an endpoint in this experimental setting. However, the observed absence of trailing made it possible to use the MIC-0 (i.e., optically clear wells corresponding to a score of 0 according to the NCCLS guidelines) as an endpoint. This corresponded to the antifungal concentration needed to decrease the optical density by >95% and growth expressed in viable counts by >99.9% (>3 logs), when these parameters were compared to those of the growth control after 48 h of incubation. Thus, the MIC-0 reflected the expression of a powerful fungistatic effect. Based on this different endpoint, FICs and the FIC index were recalculated for each drug combination. Each experiment was performed in triplicate, and the results were reported as mean values.
Time-kill curves.
An inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml of C. albicans 731, CAF2-1, and ATCC 90028 was used in a macrobroth dilution under experimental conditions identical to those described for the checkerboard microtiter plate testing. The effect on viable counts of in vivo achievable concentrations of FLC (10 μg/ml), Cy (0.625 μg/ml), and their combination (10 and 0.625 μg/ml) after 12, 24, and 48 h of incubation was studied. For this purpose, 100 μl of the content of each test tube was subcultured in serial dilutions (10−1, 10−2, 10−3, and 10−5) on Sabouraud dextrose agar plates. A fungicidal effect was defined as a >99.9% (>3-log) decrease of the viable counts of the starting inoculum (24). However, for a limit of detection of 101 CFU/ml, the criteria for a fungicidal effect could not be fulfilled using a starting inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml. Confirmatory experiments were therefore performed with a starting inoculum of 0.5 × 103 to 2.5 × 105 CFU/ml of the laboratory strain CAF2-1. Each experiment was performed in triplicate, and the results were reported as mean values. Each experiment included as control the time-kill curve of amphotericin B at a concentration of 0.5 μg/ml under the same experimental conditions, except the antibiotic medium 3 supplemented with 2% glucose was used instead of RPMI.
Chemicals and drugs.
Nonantimicrobial inhibitors of mammalian METs were as follows: chlorpromazine-hydrochloride (Sigma, Buchs, Switzerland), haloperidol-decanoate (Janssen-Cilag, Baar, Switzerland), penfluridol (Janssen-Cilag), fluphenazine-dihydrochloride (Sigma), cyproheptadine-hydrochloride (Merck Sharp & Dohme-Chibret, Glattbrugg, Switzerland), amitriptyline-hydrochloride (Sigma), clomipramine-hydrochloride (Sigma), cyclosporine (Sigma), FK506 (kindly provided by Fujisawa, Tokyo, Japan), PSC833 (kindly provided by Novartis Pharma, Basel, Switzerland), reserpine (Sigma), verapamil-hydrochloride (kindly provided by Knoll, Liestal, Switzerland), and loperamide-hydrochloride (Janssen-Cilag). Nonantimicrobial membrane-active compounds were as follows: lidocaine-hydrochloride (Sintetica, Mendrisio, Switzerland), amiodarone-hydrochloride (Sigma), omeprazole-Na (Astra, Dietikon, Switzerland), and dipyridamole (Boehringer Ingelheim, Basel, Switzerland). Antimicrobial agents were as follows: amoxicillin-Na plus clavulanate K (SmithKline-Beecham, Thörishaus, Switzerland), cefamandole-nafate (Eli Lilly, Vernier, Switzerland), meropenem-trihydrate (Zeneca, Luzern, Switzerland), sulfamethoxazole plus trimethoprim (Roche, Basel, Switzerland), vancomycin-hydrochloride (Eli Lilly), levofloxacin (Hoechst Marion Roussel, Zürich, Switzerland), norfloxacin (kindly provided by Merck Sharp & Dohme-Chibret), sparfloxacin (kindly provided by Rhône-Poulenc Rorer, Thalwil, Switzerland), trovafloxacin (Pfizer, Zürich, Switzerland), ciprofloxacin-hydrochloride monohydrate (Bayer, Zürich, Switzerland), erythromycin-lactobionate (Abbott, Cham, Switzerland), clarithromycin-lactobionate (Abbott), metronidazole (Rhône-Poulenc Rorer, Thalwil, Switzerland), clindamycin-2-phosphate (Pharmacia & Upjohn, Dübendorf, Switzerland), amikacin-sulfate (Bristol-Myers Squibb, Baar, Switzerland), streptomycin-sulfate (Sigma), doxycycline-hyclate (Pfizer), chloramphenicol (Sigma), rifampin-Na (Novartis Pharma, Basel, Switzerland), fusidic acid-Na (Leo, Zürich, Switzerland), spectinomycin-dihydrochloride (Pharmacia & Upjohn), spiramycin (kindly provided by Rhône-Poulenc Rorer), d-cycloserine (Sigma), and quinine sulfate (Sigma). Antifungal agents were as follows: FLC (kindly provided by Pfizer, Sandwich, United Kingdom), terbinafine-hydrochloride (kindly provided by Novartis Pharma), amorolfine-hydrochloride (kindly provided by Roche), amphotericin B (kindly provided by Bristol-Myers Squibb, Paris, France), and flucytosine (kindly provided by Roche). The following solvents were used to solubilize to dilute the above-listed compounds: (i) DMSO (Sigma) for Cy, FK506, reserpine, PSC 833, amphotericin B, spiramycin, norfloxacin, penfluridol, cyproheptadine-hydrochloride, loperamide-hydrochloride, and haloperidol-decanoate; (ii) Macrogolum 400 and citric acid, as delivered in the original package, for omeprazole-Na; (iii) Macrogolum 600, tartaric acid, and HCl, as delivered in the original package, for dipyridamole; and (iv) Na2HPO4, citric acid, and sodium-EDTA, as delivered in the original package, for fusidic acid. All the other compounds were diluted in H2O.
RESULTS
Agar disk diffusion testing.
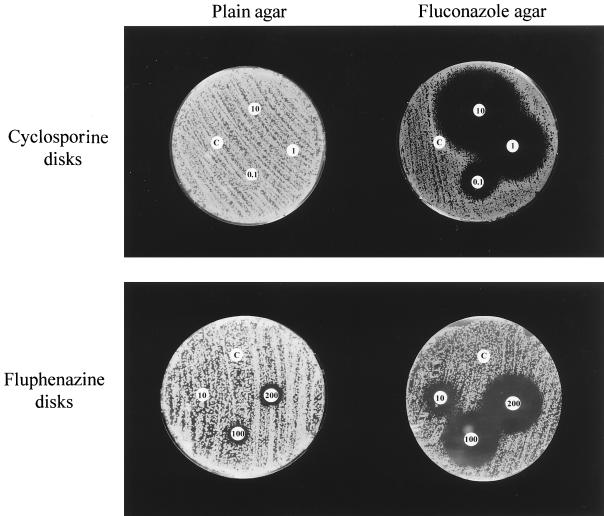
Several types of drugs, including MET inhibitors, antimicrobials, antifungals, and membrane-active compounds were tested for their potentiation of the antifungal activity of FLC. Combinations of these drugs with FLC were tested by an agar disk diffusion assay in which an azole-susceptible C. albicans isolate (clinical strain 731) was plated onto plain and FLC-containing YEPD agar plates. Cellulose disks containing different amounts of the tested substances were disposed onto the inoculated plates and, after incubation, the corresponding zones of growth inhibition were recorded. To quantify the antifungal activities obtained with these drugs alone and in combination with FLC, antifungal indices were calculated. An increase or a decrease of the antifungal index of a given drug when it was tested in combination with FLC reflected a synergistic or antagonistic effect, respectively. Representative results of these experiments are shown in Fig. 1, where different amounts of Cy and fluphenazine were tested on plain and FLC-containing agar plates. On FLC-free agar plates, Cy had no antifungal activity, whereas fluphenazine showed a weak intrinsic antifungal activity, as expressed by an antifungal index of 0.11 mm/μg. On FLC agar plates, Cy exhibited a high antifungal activity (antifungal index of 22 mm/μg), whereas the antifungal index of fluphenazine increased only to 1.7 mm/μg, corresponding to an intermediate antifungal activity. Table 1 summarizes the most significant results of these experiments.
FIG. 1.
Agar disk diffusion assay testing the combination of FLC with the partner drugs Cy and fluphenazine in C. albicans 731. (Upper panel) Combination of FLC with Cy. (Lower panel) Combination of FLC with fluphenazine. C. albicans 731 (FLC MIC of 0.5 μg/ml) was applied onto plain YEPD agar (left plates) and YEPD agar containing a supra-MIC of FLC (right plates). Disks impregnated with 0.1, 1, and 10 μg of Cy or with 10, 100, and 200 μg of fluphenazine and control disks (C) impregnated with the corresponding solvents were placed onto the inoculated agar plates. Cy alone had no antifungal activity (left upper plate), whereas fluphenazine showed a weak intrinsic antifungal activity (left lower plate). The combination of Cy with a supra-MIC of FLC poured into the agar (25 μg/ml) resulted in a powerful antifungal activity (right upper plate). The antifungal activity of fluphenazine was also increased, but to a lesser extent, when this compound was combined with FLC (right lower plate). The replacement of YEPD by RPMI in agar gave similar results (data not shown).
TABLE 1.
Agar disk diffusion testing: antifungal indices of the partner compounds tested alone and in combination with FLC against C. albicans 731a
| Partner compound | Antifungal index (mm/μg) of:
|
|
|---|---|---|
| Partner compound aloneb | Partner compound plus FLCc | |
| Mammalian MET inhibitors | ||
| Cy | 0 | 22 |
| FK506 | 0 | 34 |
| Chlorpromazine | 0.15 | 1.2 |
| Fluphenazine | 0.11 | 1.7 |
| Amitriptyline | 0.11 | 1.8 |
| Clomipramine | 0.08 | 1.5 |
| Antifungal agents | ||
| Amorolfine | 21 | 1.2 |
| Flucytosine | 1.7 | 1.7 |
| Amphotericin B | 0.8 | 0 |
| Terbinafine | 0 | 2.3 |
FLC MIC = 0.5 μg/ml.
Disks impregnated with the partner compounds were tested on plain YEPD agar.
Disks impregnated with the partner compounds were tested on YEPD agar containing a supra-MIC of FLC (25 μg/ml).
Among the other partner drugs belonging to the group of MET inhibitors, FK506, which had, like Cy, no intrinsic antifungal activity at the concentrations tested, exerted a powerful antifungal activity in combination with FLC. As was the case for fluphenazine, the drugs chlorpromazine, clomipramine, and amitriptyline had weak intrinsic antifungal activities, with indices ranging between 0.08 and 0.15 mm/μg. When these compounds were combined with FLC, intermediate antifungal activities, with indices ranging from 1.2 to 1.8 mm/μg, were obtained. All of these findings were confirmed against the laboratory isolate C. albicans CAF2-1 and the reference isolate C. albicans ATCC 90028 (data not shown). The remaining MET inhibitors listed in Materials and Methods had no intrinsic antifungal activity, and the antifungal activity of the combination of these compounds with FLC was negligible (indices of ≤0.2 mm/μg).
As expected, the activities of the antifungals listed in Table 1 could be easily detected by the disk diffusion assay on FLC-free medium, except for terbinafine. This compound had in this system no intrinsic antifungal activity, and its combination with FLC had an intermediate antifungal activity with an index of 2.3 mm/μg. The antifungal activity of flucytosine was intermediate (index of 1.7 mm/μg), and in combination with FLC its behavior was neutral, leaving this activity unchanged. With an index decrease from 21 to 1.2 mm/μg, amorolfine lost most of its intrinsic antifungal activity when combined with FLC, suggesting an important antagonism. Amphotericin B alone had in this experimental setting a low intrinsic antifungal activity (index of 0.8 mm/μg), and this weak activity was completely lost when this agent was combined with FLC, also suggesting an antagonism between the two drugs.
Antimicrobial agents and nonantimicrobial membrane-active compounds had no intrinsic antifungal activities, and the antifungal activities of the combination of these compounds with FLC were negligible (antifungal indices of ≤0.2 mm/μg). Cy, FK506, chlorpromazine, fluphenazine, clomipramine, and amitriptyline were selected for further quantification studies because of their remarkable antifungal activity in combination with FLC. The screening of antifungals gave interesting preliminary results with the combination of terbinafine and FLC, but since antifungal combinations were not the principal focus of the present work, their investigation were not pursued.
Checkerboard microtiter plate testing.
The drugs selected above had intrinsic MICs values in C. albicans 731 exceeding largely their in vivo achievable concentrations (Table 2). FLC MICs were tested in the presence of increasing concentrations of the selected partner drugs. Surprisingly, the combination of the immunosuppressive agents Cy or FK506 with FLC left the MICs of FLC unchanged, i.e., at 0.5 μg/ml. MICs were even increased by two- to eightfold when FLC was combined to given concentrations of partner drugs such as the neuroleptic agents chlorpromazine and fluphenazine or the tricyclic antidepressants amitriptyline and clomipramine (Table 2). In contrast, the MICs of all tested partner drugs were decreased when these compounds were combined with supra-MICs of FLC. Nevertheless, taking the MICs as an endpoint, the FIC indices of all the tested combinations were >1. There was therefore no synergism, according to its classical microbiological definition.
TABLE 2.
MIC results of checkerboard microtiter plate format testing the combinations of FLC with partner compounds against C. albicans 731
| Partner compound | MIC (μg/ml) with:
|
||
|---|---|---|---|
| Partner compound alone (intrinsic) | Partner compounda combined with FLC | FLCb (in combination) | |
| None | 0.5 | ||
| Cy | >10 | 0.625 | 0.5 |
| FK506 | >10 | 1.25 | 0.5 |
| Fluphenazine | >25 | 6.25 | 2 |
| Chlorpromazine | >100 | 25 | 2 |
| Clomipramine | >100 | 25 | 4 |
| Amitriptyline | >100 | 25 | 1 |
The MICs of the partner compounds listed were obtained when these drugs were combined with FLC at concentrations of >0.5 μg/ml. All MICs were decreased, and the corresponding FICs were <1.
The MICs of FLC indicated were obtained in combination with the following concentrations of the partner drugs: Cy, 0.625 μg/ml; FK506, 1.25 μg/ml; fluphenazine, 6.25 μg/ml; and chlorpromazine, clomipramine, and amitriptyline, 25 μg/ml. The MIC of FLC combined with Cy or FK506 was unchanged, and the resulting FICs were equal to 1. When FLC was combined with the four Ca2+-calmodulin antagonists, its MIC increased and the corresponding FICs were >1. The FIC indices, resulting from the sum of the FICs of the listed partner compounds and of the FLC, tested in combination, were all >1. Taking the MICs as the endpoint, the different drug associations were therefore not synergistic.
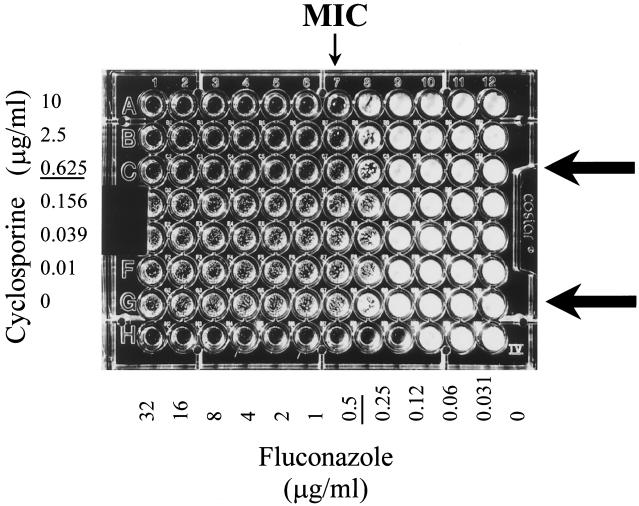
However, above a given minimal concentration, each of the partner drugs tested eliminated the residual turbidity, the so-called trailing, indicator of an incomplete growth inhibition at supra-MICs of FLC when this agent was used alone. In the microtiter plate shown in Fig. 2, one can observe that, at Cy concentrations of >0.625 μg/ml, the wells containing supra-MICs of FLC were optically clear and the corresponding residual growth was much less prominent than in Cy-free medium. The plotting of FLC concentrations to optical densities and viable counts measured in Cy-free medium and Cy-containing medium confirmed this observation (Fig. 3 and 4). After 48 h of incubation, a difference of only 1 log separated the viable counts of the growth control from those in the medium with supra-MICs of FLC alone, i.e., the wells where a residual turbidity was observed (wells G1 to G7 in Fig. 2). With a starting inoculum of 103 CFU/ml, increases of 3 and 4 logs in viable counts were measured with and without FLC, respectively. On the other hand, the elimination of the residual turbidity in Cy-containing medium was associated with a powerful fungistatic effect (wells C1 to C6 in Fig. 2): the viable counts after 48 h of incubation were comparable to those of the starting inoculum and were 3 logs lower compared to those in Cy-free medium (Fig. 4).
FIG. 2.
Checkerboard microtiter plate assay testing the combination of FLC with Cy in C. albicans 731. The MIC of Cy was >10 μg/ml when the drug was tested alone and decreased to 0.625 μg/ml in the presence of FLC concentrations of >0.5 μg/ml. The MIC of FLC, indicated by the thin arrow, was unchanged despite increasing concentrations of Cy. However, Cy at concentrations of >0.625 μg/ml combined with an FLC supra-MIC (i.e., >0.5 μg/ml) eliminated the residual turbidity (trailing) in the incubation wells. This observation made it possible to use a different endpoint, the MIC-0. The MIC-0 values of FLC and Cy are underlined in the concentration scales. For the wells in rows C and G (shown by thick arrows), the FLC concentrations are plotted to the corresponding optical densities and viable counts in Fig. 3 and 4.
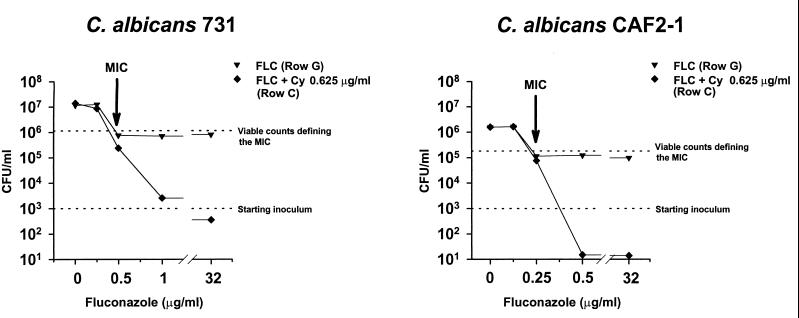
FIG. 3.
Effect of the combination of FLC with Cy on the spectrophotometrically measured growth of C. albicans 731. For the wells in rows G and C of the microtiter plate shown in Fig. 2, the FLC concentrations are plotted to the optical density (OD) values measured after 48 h of incubation. Row G was Cy-free, and row C contained Cy at the concentration of 0.625 μg/ml. Mean OD values of three separate experiments are shown.
FIG. 4.
Effect of the combination of FLC with Cy on the viability of the C. albicans strains 731 and CAF2-1. The combination of FLC and Cy was tested in C. albicans 731 (FLC MIC of 0.5 μg/ml) and CAF2-1 (FLC MIC of 0.25 μg/ml) in a checkerboard microtiter plate format. For the wells of row G (not containing FLC) and or row C (containing Cy at the concentration of 0.625 μg/ml), the FLC concentrations are plotted against the viable counts after 48 h of incubation. Mean CFU values of three separate experiments are shown.
These results were confirmed by testing the laboratory isolate C. albicans CAF2-1 (FLC MIC of 0.25 μg/ml). FLC alone was only weakly fungistatic in this strain. After 48 h of incubation, an inhibition of growth of only 1 log was obtained when the viable counts were compared to those of the growth control. The combination of FLC with Cy at a concentration of 0.625 μg/ml reduced the viable counts by 2 logs compared to those of the starting inoculum of 103 CFU/ml and reached the limit of detection of 101 CFU/ml (Fig. 4). Killing of C. albicans CAF2-1 had been therefore obtained by combining FLC with Cy. However, the 3-log decrease in the viable counts of the starting inoculum required by the definition of a fungicidal effect was not reached with the inoculum used in this experimental setting. Nevertheless, the absence of residual growth observed at supra-MIC concentrations of FLC in the presence of Cy concentrations of >0.625 μg/ml made it possible to use a different endpoint. This endpoint, the MIC-0, described very well the antifungal effect of FLC in combination with partner drugs. For example, the MIC-0 values of FLC and of Cy tested alone in C. albicans were >32 and >10 μg/ml, respectively. When the two drugs were tested in combination, the MIC-0 values of FLC and Cy were reduced to 0.5 and 0.625 μg/ml, respectively (Table 3). Based on this different endpoint, the recalculated FIC indices of all the combinations tested fit the definition of synergism (Table 3). Since the minimal concentration of partner drugs needed to eliminate trailing, i.e., to obtain a synergism according to the MIC-0 endpoint, was achievable in vivo only for Cy (i.e., 0.625 μg/ml), this compound was retained for further time-kill curve studies.
TABLE 3.
MIC-0 of FLC and of the partner compounds used alone and in combination with the corresponding FICs and FIC indicesa
| PC | FLC
|
PC
|
FIC index (FLC + PC) | ||||
|---|---|---|---|---|---|---|---|
| MIC-0 (FLC alone [μg/ml]) | MIC-0 (FLC combined with PC [μg/ml]) | FIC index | MIC-0 (PC alone [μg/ml]) | MIC-0 (PC combined with FLC [μg/ml]) | FIC index | ||
| Cy | >32 | 0.5 | 0.016 | >10 | 0.625 | 0.0625 | 0.0785 |
| FK506 | >32 | 0.5 | 0.016 | >10 | 1.25 | 0.125 | 0.141 |
| Fluphenazine | >32 | 2 | 0.0625 | >25 | 6.25 | 0.25 | 0.3125 |
| Chlorpromazine | >32 | 2 | 0.0625 | >100 | 25 | 0.25 | 0.3125 |
| Clomipramine | >32 | 4 | 0.125 | >100 | 25 | 0.25 | 0.375 |
| Amitriptyline | >32 | 1 | 0.0313 | >100 | 25 | 0.25 | 0.375 |
PC, partner compound.
Time-kill curves.
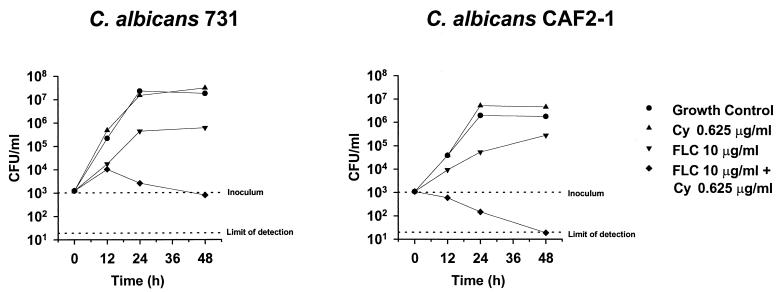
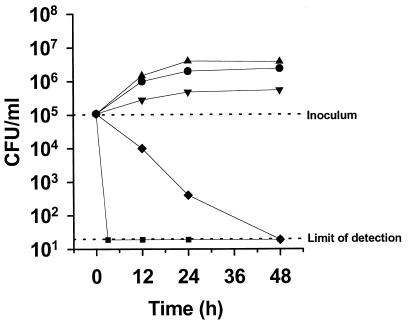
The synergisms observed in the checkerboard microtiter plate testing combining FLC and Cy in C. albicans 731 and CAF2-1 were confirmed in time-kill curves experiments. As shown in Fig. 5, FLC alone had a weak fungistatic effect on both strains that limited the increase in viable counts at 48 h of incubation by 1 to 1.5 logs compared to those of the drug-free growth control. As already mentioned above, Cy alone had no antifungal activity at the concentration tested, and therefore the viable counts at 48 h of incubation were identical to those of the growth controls. In contrast, the combination of FLC with Cy had in C. albicans 731 a potent fungistatic activity which left the viable counts of the starting inoculum unchanged at 103 CFU/ml after 48 h of incubation (Fig. 5). The same combination in C. albicans CAF2-1 resulted in a 2-log decrease in viable counts to the limit of detection (101 CFU/ml) within 48 h of incubation (Fig. 5). The killing effect observed in the checkerboard microtiter plate testing on this strain was therefore confirmed. This striking finding was confirmed in the reference strain ATCC 90028 (data not shown). Amphotericin B was used as a control (in antibiotic medium 3, the MICs of amphotericin B were 0.0625 μg/ml for all the three strains tested) in all experiments. This drug induced a decrease in viable counts of all the strains tested to the limit of detection within 90 min of incubation (data not shown). To verify whether the criteria required for a fungicidal effect (decrease in viable counts of 3 logs compared to the starting inoculum) were fulfilled, an inoculum of 105 CFU of C. albicans CAF2-1 per ml was tested. As shown in Fig. 6, a 4-log decrease in the viable counts of the starting inoculum could be observed after 48 h of incubation, thus demonstrating that the combination of FLC and Cy was fungicidal. Amphotericin B was fungicidal after 3 h in this experimental setting.
FIG. 5.
Time-kill curves of C. albicans strains 731 and CAF2-1. The strains were exposed to in vivo-achievable concentrations of FLC and Cy using starting inocula of 103 CFU/ml. CFU were determined after 12, 24, and 48 h of incubation. Mean CFU values of three separate experiments are shown.
FIG. 6.
Time-kill curves of the C. albicans CAF2-1. Experimental conditions were identical to those used in Fig. 5, except that a starting inoculum of 105 CFU/ml was used. CAF2-1 was exposed to amphotericin B to compare the extent and the timing of its killing effect to that of the combination of FLC and Cy. Symbols: ●, growth control; ▴, Cy (0.625 μg/ml); ▾, FLC (10 μg/ml); ⧫, FLC (10 μg/ml) plus Cy (0.625 μg/ml); ■, amphotericin B (0.5 μg/ml).
DISCUSSION
The recent discovery of MET-mediated active azole efflux in C. albicans initiated the search for partner drugs which, by interfering with this mechanism, could potentiate the antifungal activity of FLC against this yeast. For this purpose, mammalian MET inhibitors with no antimicrobial activity currently used in clinical practice were screened. Three other types of compounds also currently used, i.e., antimicrobial and antifungal agents and membrane-active compounds such as antiarrhythmic drugs, proton pump inhibitors, and platelet aggregation inhibitors were included in this screening. With agar disk diffusion testing, a striking interaction between FLC and two classes of mammalian MET inhibitors was identified. Combining FLC with the Ca2+-calmodulin antagonists chlorpromazine and fluphenazine (both neuroleptics) and clomipramine and amitriptyline (both tricyclic antidepressants), which themselves showed only a weak intrinsic antifungal activity, resulted in an increased antifungal effect expressed by a newly defined antifungal index. Yet the most significant results were observed with the combination of FLC with the immunosuppressive agents Cy and FK506. These drugs had no intrinsic antifungal activity at the concentrations tested, but their interaction with FLC resulted in an antifungal effect 10 to 20 times greater than that observed with the combination of FLC with chlorpromazine or its other parent compounds. All other drugs screened showed no or only a negligible interaction with FLC, with the exception of the antifungal compound terbinafine, whose antifungal index increased when this compound was combined with FLC. The disk diffusion method used in this screening procedure is subject to different limitations, in particular to unpredictable differences in stability and diffusion properties of the tested drugs, which could explain, for example, the weak intrinsic antifungal activity observed with amphotericin B and with terbinafine. Therefore, the results presented in Table 1 should be carefully interpreted. Nevertheless, this test seemed appropriate for the semiquantitative screening of a large number of compounds. After the selection of compounds with a strong interaction with FLC, further studies were performed to better characterize and quantify the antifungal activities of the different combinations.
By checkerboard microtiter testing it was observed that supra-MIC concentrations of FLC had only a weak fungistatic effect, and this finding correlated well with the measured residual turbidity, the so-called trailing. All selected partner drugs tested had at best a negligible intrinsic antifungal activity. Surprisingly, the MIC of FLC was unchanged when it was combined with Cy or FK506 and was even increased when the partner drug was chlorpromazine or its related compounds. On the other hand, the MICs of the partner compounds were all decreased when these drugs were combined with supra-MICs of FLC. Nevertheless, on the basis of the MICs and of the resulting FIC indices, none of the tested combination was synergistic, as classically defined. However, above a given minimal concentration, all of the tested partner drugs eliminated completely the trailing effect when they were combined with supra-MICs of FLC, leaving the contents of the corresponding wells optically clear. A trailing inhibition in Candida spp. has already been reported in other experimental settings. Marr et al. (23) obtained a significant trailing decrease without influence on the MIC by adjusting the pH of the medium to ≤5.0. Odds et al. (29) described an MIC reduction and an elimination of trailing caused by combining azoles with different antibiotics inhibiting protein synthesis, such as doxycycline and gentamicin. The reduction of trailing observed in the present study was explained by a strain-dependent potent fungistatic or fungicidal effect resulting from the combination of FLC with partner drugs. Since this effect decreased the viable counts by >99.9% (>3 logs) compared to those of the growth control, a different endpoint, the MIC-0, was chosen. Upon recalculating FICs and FIC indices according to this endpoint, we found all of the combinations tested to be synergistic. Of all the partner compounds tested, only Cy was effective at in vivo-achievable concentrations, and it was therefore object of further studies. These findings were confirmed by time-kill curves, which showed a strain-dependent potent fungistatic or fungicidal effect on the three strains investigated. The combination required 48 h to be fungicidal, whereas amphotericin B was fungicidal after 3 h of incubation. This striking and surprising synergism of FLC with an immunosuppressive agent raises questions about its underlying mechanisms. The difference of efficacy of the combination of FLC with Cy observed in the three FLC-susceptible isolates tested, i.e., fungistatic against C. albicans 731 on the one hand and fungicidal against C. albicans CAF2-1 and ATCC 90028 on the other hand, is still unexplained and may reflect different genetic backgrounds. The tested strains had never been preexposed to FLC or Cy. It is still not known if FLC and Cy are also synergistic in azole-resistant C. albicans strains or in other yeast species, in particular in non-C. albicans and non-Candida species with decreased susceptibility to azoles. Although our preliminary data show that a C. albicans strain with intermediate azole resistance behaves as described for strain 731 in this study, more work is needed to address this question.
The mechanisms of action of Cy are very complex (14) and, beside METs, there are other recognized molecular targets of this compound, such as cyclophilins and calcineurin (9, 18, 21, 22). The inhibition of these targets may profoundly affect different steps of the yeast cell metabolism. Moreover, it has been determined that Cy can alter the cell membrane architecture and therefore its functional properties (12). Furthermore, it has been shown that Cy has a toxic activity against different parasites and fungi (16, 26, 30, 31, 40). Interestingly, in the present experiments, Cy had no intrinsic antifungal activity at the concentrations tested. Despite the potent fungistatic or fungicidal effect obtained combining FLC with Cy, the MIC of FLC, as classically defined, remained unchanged. Together with the observation that the combination required 48 h to be fungicidal, this suggested that the action of Cy on the C. albicans cells is to some extent dependent on the slow effect of supra-MICs of FLC on the synthesis of ergosterol constituting the cell membrane. Cy may also interact directly with multidrug efflux transporters present in C. albicans and thus, by inhibiting their activity, this drug could increase the susceptibility of C. albicans to FLC. Although the close homologue of Cy, FK506, is known to inhibit the function of multidrug transporters of the ABC superfamily (7), it is not exactly established whether Cy has the same effect on multidrug transporters in yeasts. Clearly, the effect of Cy in combination with FLC needs to be more closely investigated. For example, it has been shown recently in a rat model of experimental endocarditis due to C. albicans CAF2-1 that the FLC-Cy combination is also fungicidal in vivo (O. Marchetti, J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-50, 1998). This new concept of combining FLC, and perhaps other azoles as well, with Cy or with its parent compounds lacking immunosuppressive activity (40) might open a new therapeutic approach for the management of fungal infections.
ACKNOWLEDGMENTS
We thank Josè Manuel Entenza, Marlyse Giddey, Françoise Ischer, and Marlies Knaup for their outstanding technical assistance.
D.S. is supported by a grant from the Swiss Research National Foundation (3100-055901).
REFERENCES
- 1.Beck-Sagué C M, Jarvis W R the National Nosocomial Infection Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 2.Berenbaum M C. A method of testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 3.Bitonti A J, Sjoerdsma A, McCann P P, Kyle J E, Oduola A M J, Rossan R N, Milhous M K, Davidson D E. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science. 1988;242:1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw B E, Anaissie E. Controversies in the management of candidiasis in neutropenic patients treated for malignant diseases: new versus old or better versus worse. Int J Infect Dis. 1997;1(Suppl. 1):S32–S36. [Google Scholar]
- 5.Denning D W, Baily G G, Hood S V. Azole resistance in Candida. Eur J Clin Microbiol Infect Dis. 1997;16:261–280. doi: 10.1007/BF01695630. [DOI] [PubMed] [Google Scholar]
- 6.Edwards J E., Jr International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997;25:43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 7.Egner R, Rosenthal F E, Kralli A, Sanglard D, Kuchler K. Genetic separation of FK 506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resistance transporter. Mol Biol Cell. 1998;9:523–543. doi: 10.1091/mbc.9.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruma D A, Klee C B, Bierer B E, Burakoff S J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci USA. 1992;89:3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcha B K, Brummer E, Stevens D A. Synergy of fluconazole with human monocytes or monocyte-derived macrophages for killing of Candida species. J Infect Dis. 1995;172:1620–1623. doi: 10.1093/infdis/172.6.1620. [DOI] [PubMed] [Google Scholar]
- 11.Graybill J R. Editorial response: can we agree on the treatment of candidiasis? Clin Infect Dis. 1997;25:60–62. doi: 10.1086/514503. [DOI] [PubMed] [Google Scholar]
- 12.Haynes M, Fuller L, Haynes D H, Miller J. Cyclosporin partitions into phospholipid vesicles and disrupts membrane architecture. Immunol Lett. 1985;11:343–349. doi: 10.1016/0165-2478(85)90118-x. [DOI] [PubMed] [Google Scholar]
- 13.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 14.Ho S, Clipstone N, Timmermann L, Northrp J, Graef I, Fiorentino D, Nourse J, Crabtree G R. The mechanism of action of cyclosporin A and FK 506. Clin Immunol Immunopathol. 1996;80(Suppl. 3):S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 15.Kaatz G W, Seo S M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkland T N, Fierer J. Cyclosporin A inhibits Coccidioides immitis in vitro and in vivo. Antimicrob Agents Chemother. 1983;24:921–924. doi: 10.1128/aac.24.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornblau S M, Estey E, Madden T, Tran H T, Zhao S, Consoli U, Snell V, Sanchez-Williams G, Kantarjian H, Keating M, Newman R A, Andreeff M. Phase I study of mitoxanthrone plus etoposide with multidrug blockade by SDZ PSC-833 in relapsed or refractory acute myelogenous leukemia. J Clin Oncol. 1997;15:1796–1802. doi: 10.1200/JCO.1997.15.5.1796. [DOI] [PubMed] [Google Scholar]
- 18.Lea J P, Sands J M, McMahon S J, Tumlin J A. Evidence that the inhibition of Na+/K+ ATPase activity by FK 506 involves calcineurin. Kidney Int. 1994;46:647–652. doi: 10.1038/ki.1994.317. [DOI] [PubMed] [Google Scholar]
- 19.List A F. Role of multidrug resistance and its pharmacological modulation in acute myeloid leukemia. Leukemia. 1996;10:937–942. [PubMed] [Google Scholar]
- 20.List A F, Spier C, Greer J, Wolff S, Hutter J, Dorr R, Salmon S, Futscher B, Baier M, Dalton W. Phase I/II trial of cyclosporine as a chemotherapy-resistance modifier in acute leukemia. J Clin Oncol. 1993;11:1652–1660. doi: 10.1200/JCO.1993.11.9.1652. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, Schreiber S L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 22.Marks A R. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 23.Marr K A, Rustad T R, Rex J H, White T C. The trailing endpoint phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43:1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGinnis M R, Rinaldi M G. Antifungal drugs: mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biologic fluids. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 176–211. [Google Scholar]
- 25.Mickisch G H, Kössig J, Tschada R K, Keilhauer G, Schlick E, Alken P M. Circumvention of multidrug resistance mediated by P-170 glycoprotein using calcium antagonists in primary human renal cell carcinoma. Urol Int. 1991;47:118–125. doi: 10.1159/000282204. [DOI] [PubMed] [Google Scholar]
- 26.Mody C H, Toews G B, Lipscomb M F. Treatment of murine cryptococcosis with cyclosporin A in normal and athymic mice. Am Rev Respir Dis. 1989;139:8–13. doi: 10.1164/ajrccm/139.1.8. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Clinical and Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. 17, no. 9. Approved standard M27-A. Villanova, Pa: National Committee for Clinical and Laboratory Standards; 1997. [Google Scholar]
- 28.Nguyen M H, Peacock J E, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 29.Odds F C, Abbott A B, Pye G, Troke P F. Improved method for estimation of azole antifungal inhibitory concentrations against Candida species, based on azole/antibiotic interactions. J Med Vet Mycol. 1986;24:305–311. doi: 10.1080/02681218680000461. [DOI] [PubMed] [Google Scholar]
- 30.Odom A, Muir S, Lim E, Toffaletti D L, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osato M S, Roussel K R, Wilhelmus K R, Jones D B. In vitro and in vivo antifungal activity of cyclosporine. Transplant Proc. 1983;15:2927–2930. [Google Scholar]
- 32.Pastan I, Gottesman M. Multiple drug resistance in human cancer. N Engl J Med. 1987;316:1388–1393. doi: 10.1056/NEJM198705283162207. [DOI] [PubMed] [Google Scholar]
- 33.Pearson R D, Steigbigel R T, Davis H T, Chapman S W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson L R, Shanholtzer C J. Tests for bactericidal effects of antimicrobial agents: technical performance and clinical relevance. Clin Microbiol Rev. 1992;5:420–432. doi: 10.1128/cmr.5.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rex J H, Bennett J E, Sugar A M, Pappas P G, van der Horst C M, Edwards J E, Jr, Washburn R G, Scheld W M, Karchmer A W, Dine A P, Levenstein M J, Douglas Webb C The Candidemia Study Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994;331:1325–1330. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 36.Sangeorzan J A, Bradley S F, He X, Zarins L T, Ridenour G L, Tiballi R N, Kauffman C A. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–346. doi: 10.1016/0002-9343(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 37.Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 39.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silverman J A, Hayes M L, Luft B J, Joiner K A. Characterization of anti-Toxoplasma activity of SDZ 215-918, a cyclosporin derivative lacking immunosuppressive and peptidyl-prolyl-isomerase-inhibiting activity: possible role of a P glycoprotein in Toxoplasma physiology. Antimicrob Agents Chemother. 1997;41:1859–1866. doi: 10.1128/aac.41.9.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonneveld P, Nooter K. Reversal of drug-resistance by cyclosporin-A in a patient with acute myelocytic leukemia. Br J Haematol. 1990;75:208–211. doi: 10.1111/j.1365-2141.1990.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 42.Sonneveld P, Wiemer E. Inhibitors of multidrug resistance. Curr Opin Oncol. 1997;9:543–548. doi: 10.1097/00001622-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Sugar A M, Liu X-P, Chen R-J. Effectiveness of quinolone antibiotics in modulating the effects of antifungal drugs. Antimicrob Agents Chemother. 1997;41:2518–2521. doi: 10.1128/aac.41.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tolcher A W, Cowan K H, Solomon D, Ognibene F, Goldspiel B, Chang R, Noone M H, Denicoff A M, Barnes C S, Gossard M R, Fetsch P A, Berg S L, Balis F M, Venzon D J, O'Shaughnessy J A. Phase I crossover study of placlitaxel with r-verapamil in patients with metastatic breast cancer. J Clin Oncol. 1996;14:1173–1184. doi: 10.1200/JCO.1996.14.4.1173. [DOI] [PubMed] [Google Scholar]
- 45.Wingard J R, Merz W G, Rinaldi M G, Johnson T R, Karp J E, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]