Abstract
Adequate nutrition is required to support productive honey bee colonies, therefore beekeepers supplement colonies with additional protein at targeted time points. We tested the effects of commercially available protein feeds in spring, in advance of colonies being used for hybrid canola pollination. The feed treatments across the three-year study included the following patty types: Global 15% pollen, Global 0% pollen, Bee Pollen-Ate, FeedBee, and Healthy Bees, as well as an unsupplemented control in year two of the study only. The amount of feed consumed varied among colonies, treatments, date, and year. Similarly, there were also differences in feed efficiency (bees reared per gram of feed consumed), likely due to the relative availability of external forage sources to supplement the feed provided. Unsupplemented colonies were able to rear less brood, and subsequently had fewer adult bees than supplemented colonies, in an apiary where pollen was not abundant. Differences in consumption among treatments often failed to translate in to differences in amount of brood reared or subsequent adult population. All the protein feed treatments contained all ten amino acids essential to honey bees, however lysine and arginine were below the optimal proportion required for growth in all patties except the FeedBee patty. The amount of protein and amount and types of sugars and fats in the products also varied among product type and batch. The results of this study demonstrate a benefit to supplementary spring protein feeding to increase honey bee colony populations in advance of a summer pollination market.
Keywords: honey bee, pollen supplement, nutrition, pollen patty, pollen substitute
The health, fecundity, and longevity of animals depend on the quantity and quality of the food they consume. The nutritional needs of Western honey bees (Apis mellifera L. [Hymenoptera: Apidae]; hereafter ‘honey bees’) are naturally met through the collection of pollen, nectar, and water by worker bees foraging in the environment. Nectar provides the colony with carbohydrates, and pollen supplies the protein, fats, and other nutrients required to rear brood (reviewed by Wright et al. 2018).
Nutritional need varies among honey bees of different ages and castes, with protein consumed most by young workers, with maximum consumption at day 5 posteclosion (Haydak 1970). It is over these first days of adult life that workers continue to develop the fat bodies, hypopharyngeal glands, and other internal structures that they require to complete the physiologically demanding tasks associated with nursing brood (Maurizio 1954). Many studies have demonstrated that the amount and type of protein a colony can access influence both the longevity and glandular development of individual workers, as well as their ability to rear brood (e.g., Maurizio 1954, Standifer 1967, Haydak 1970, Knox et al. 1971, Pernal and Currie 2000).
The quantity of protein in feed can be defined by the crude protein content, where 23–30% is the optimum for honey bee nutrition (Herbert et al. 1977). In addition to total protein, De Groot (1953) reported the essential amino acid requirements for honey bee growth and development. The ability of a protein source to sustain brood rearing is determined by a balance of these ten essential amino acids: threonine, valine, methionine, leucine, iso-leucine, phenylalanine, lysine, histidine, arginine, and tryptophan. While total dietary protein is correlated with longevity, high protein alone does not guarantee consumption or effective digestion (Schmidt et al. 1987), and protein levels that are too high relative to carbohydrate availability can have negative effects on longevity under experimental conditions (Pirk et al. 2010). While many studies have evaluated feed quality by measuring correlates of fitness such as glandular development and longevity on individuals (e.g., Standifer 1967, Knox et al. 1971, Pernal and Currie 2000, DeGrandi-Hoffman et al. 2010), studies on full sized free flying colonies provide the most pertinent insights into the effects of feed on colony fitness, especially as season and local environmental context are important determinants of colony health.
Meeting the nutritional needs of commercially managed honey bee colonies is critical to maximising their survival, health, and productivity (Farrar 1993, Mattila and Otis 2006). This can be challenging in temperate climates with long winters and short growing seasons, if land use does not provide sufficient amounts of diverse forage sources, in areas where colony density is high (such as colonies moved to pollination before bloom commences), in areas with periods of dearth or inclement weather that prevents foraging, or when there is a need for colony populations to grow faster than the nutrition offered on the landscape would normally allow. For example, beekeepers may supplement colonies with the goal of meeting early spring pollination requirements, ensuring maximum population coincides with peak nectar flow, or producing bees for sale or increase of colony numbers. In these cases, supplementation can make both nutritional and economic sense.
While the feeding of protein is a common spring management practice, the amount, delivery (dry versus patty form), pollen content, protein content, sugar content, and fat content all vary widely among beekeeping operations. Typically, these protein feeds are referred to as pollen substitutes when they contain no natural pollen and pollen supplements when they contain pollen (Saffari et al. 2010a), and a soft patty formulation is a common feeding method. Adult bees most likely use this type of supplemental feed in glandular secretions as they do not feed the product directly to larvae, store it as beebread, or eject it as debris (Noordyke et al. 2021).
Although pollen remains the most desirable and attractive protein source for honey bees, there are advantages to pollen substitutes. Pollen is expensive to source, can be difficult to obtain in quantity, and also carries a risk of introducing pathogens (e.g., Gochnauer and Corner 1974, de Sousa Pereira et al. 2019, Schittny et al. 2020) or pesticides (e.g., Mullin et al. 2010, Ostiguy et al. 2019) into the fed colonies. For this reason, pure pollen is rarely fed to colonies and instead, the protein component of feed is usually either a mix of pollen and other proteins or made entirely of pollen substitute ingredients. Many protein sources have been used as the basis of pollen-substitute diets, including: various yeast-based ingredients, egg products, dairy products, rye flour, oat flour, peanut meal, corn flour, pea flour, casein, cotton seed meal, peanut meal, soy products, meat and fish products, chickpea flour, potato and sweet potato powders, black gram, guar meal (as reviewed by Paray et al. 2021), and more recently spirulina algae. While most protein supplements and substitutes promote greater colony productivity and bee health than not feeding, they are generally less beneficial than natural pollen (Stanger and Laidlaw 1974, Doull 1980, Nabors 2000, DeGrandi-Hoffman et al. 2008, DeGrandi-Hoffman et al., 2010, DeGrandi-Hoffman et al. 2016). Problems associated with some pollen substitute diets include low adult longevity (Lamontagne-Drolet et al. 2019, Oskay 2021), unpalatability, inability to rear brood (Herbert et al. 1977, Amro et al. 2016), unavailability, and cost.
Across the prairie regions of Western Canada, beekeepers feed protein supplements in early spring to stimulate brood production and colony growth. This is an especially critical management practice in the grassland region of Southern Alberta, where there is little early spring forage, which is largely restricted to trees and shrubs in river bottoms. This region faces long winters and short growing seasons, requiring rapid build up of colony populations to maximise honey production and/or pollination rental fees. A commonly used protein supplement in this region is a 15% pollen patty (Global Patties Inc., Airdrie, Alberta).
The objective of this study is to evaluate the efficacy of commercially available protein supplements as part of beekeeping management practices in southern Alberta. Various protein substitutes in patty formulation were compared to an industry standard of Global 15% pollen as well as no protein supplementation (one year only) over three successive springs using colonies in a commercial beekeeping operation that pollinates hybrid canola seed production fields.
Methods
Experimental Design
The protein supplement patties used in the trial were either made in the laboratory or obtained from commercial sources and were kept frozen at −20°C until application. The patties varied in weight, area, and thickness according to source, but were standardised as much as possible within each study year by either trimming the patty if it was larger than the others, or adding in additional patty material between the layers of waxed paper if it was smaller. Table 1 shows the source, size, and formulation information of all protein patties used across the three-year study.
Table 1.
Protein patty treatments and source, size, and formulation used for each year of the trial
| 2018 | Global 15% | Commercial pollen supplement patties containing 15% bee-collected pollen by weight. Sold by Global Patties. Patty weight = 0.43 ± 0.002 kg (0.96 lbs), 311 cm2. |
| Bee Pollen-Ate | Commercial protein supplement for honey bees (yeast based with zinc proteinate and selenium yeast) made commercially by Alltech Inc. To produce the patty, Global Patties used 25% of Bee Pollen-Ate by weight. Patty weight(= 0.43 ± 0.002 kg (0.95 lbs), 284 cm2. | |
| FeedBee | Pollen substitute patties made by the researchers from (3.0 kg FeedBee powder: 1,869 ml of beet-derived sucrose syrup (Lantic Inc.): 129 ml flax seed oil). Patty weight ( = 0.44 ± 0.002 kg (0.97 lbs), 219 cm2. | |
| Trio | Colonies given small patties of each of the above types. Patty weight and area: Feedbee ( = 0.23 ± 0.0005 kg (0.50 lbs), 123 cm2; Global 15% ( = 0.22 ± 0.005 kg (0.49 lbs), 130 cm2; Pollen-Ate 25% = 0.21 ± 0.002 kg (0.47 lbs), 145 cm2. | |
| 2019 | Global 15% | Commercial pollen supplement patties containing 15% bee-collected pollen by weight. Sold by Global Patties Patty weight ( = 0.46 ± 0.008 kg (1.0 lbs), 226 cm2. |
| Bee Pollen-Ate | Commercial protein supplement for honey bees (yeast based with zinc proteinate and selenium yeast) made commercially by Alltech Inc. To produce the patty, Global Patties used 20% of Bee Pollen-Ate by weight. Patty weight ( = 0.58 ± 0.023 kg (1.27 lbs), 227 cm2. | |
| Global 0% | Commercial pollen substitute patties sold by Global Patties (contain no pollen). Patty weight ( = 0.66 ± 0.025 kg (1.45 lbs), 228 cm2. | |
| Control | No supplemental protein given | |
| 2020 | Global 15% | Commercial pollen supplement patties containing 15% bee-collected pollen by weight. Sold by Global Patties Patty weight ( = 1.31 ± 0.004 kg (2.9 lbs), 453 cm2. |
| Global 0% | Commercial pollen substitute patties sold by Global Patties (contain no pollen). Patty weight ( = 1.27 ± 0.006 kg (2.8 lbs), 453 cm2. | |
| Bee Pollen-Ate | Commercial protein supplement for honey bees (yeast based with zinc proteinate and selenium yeast) made commercially by Alltech Inc. To produce the patty, Global Patties used 15% of Bee Pollen-Ate by weight. Patty weight ( = 1.03 ± 0.003 kg (2.3 lbs), 453 cm2. | |
| Healthy Bees | Commercial pollen substitute patty (spirulina algae-based protein) sold by Healthy Bees. Patty weight = 0.91 ± 0.0006 kg (2.0 lbs), 406 cm2. |
After being moved from an indoor wintering facility outside to the apiary, the bees were allowed to acclimatise and orient for 2 d. There was variation in the experimental start date (Table 2), as the colonies remained inside the overwintering building until the weather was warm enough not to stress the small, uninsulated colonies. The 120 experimental colonies (30 colonies per treatment × 4 feed treatments) were kept together in a single apiary for the duration of the experiment in each year of the study (for more details on colony management, see Supplementary information).
Table 2.
Experimental timing for each year of the study
| Event/assessment | 2018 | 2019 | 2020 |
|---|---|---|---|
| Hives moved out of wintering building | April 18 | April 1 | April 7 |
| Cluster score Brood area Patty application |
April 20 | April 3 | April 9 |
| Two-week brood area | April 17–18 | April 23 and 27 | |
| Brood solidness | May 3 | May 5 | |
| Patty removal Cluster score Brood area Brood solidness |
May 23–25 | May 1,3 | May 5 |
| Prepollination cluster score | June 18 (59 d postinitial feed) | June 13–14 (71 d postinitial feed) | June 16 (68 d postinitial feed) |
The experimental patties were initially fed 2 d after the colonies were moved out of the wintering building (Table 2) and were replaced weekly for 4–5 wk. The amount of patty remaining in the colonies was checked midweek, and any patties that were likely to be completely consumed before the next weekly visit were replaced. In 2020, due to restrictions related to the COVID-19 pandemic, patties could not be replaced as frequently. Therefore, larger patties were used that were replaced less frequently. In this way, each hive always contained a pollen patty, and consumption by the colonies was unrestricted (ad libitum feeding) in all cases. The patties were weighed before being applied and after removal from the hives to calculate consumption by the colonies (consumption per time period = initial − final weight).
To estimate the population of adult bees, the size of the cluster (number of combs covered by adult bees) was evaluated three times during the project (Table 2): prior to the first treatment, mid-experiment after all protein treatments had ceased, and prior to the normal canola pollination/honey production season, which is analogous to pollination grading. Pollination grading in hybrid canola is performed by visually estimating the number of frames of bees, up to a maximum count of 18 frames. As beekeepers are paid according to the pollination grade, this measurement helps to determine whether patty treatments effect the economic output of the hive. However, this measurement is not the same as pollination grading as that would be done 2 wk later, at warmer temperatures, and typically looking at only the top brood chamber.
Cluster size was defined as the number of inter-frame spaces filled by bees found by averaging a visual assessment of the top and bottom of each box on a cool morning (0°C–15°C). In 2018 the study hives had attached bottom boards, so the bottom of the bottom box could not be viewed, and the bottom score was estimated by looking down through the box to the bottom. In 2019 and 2020 the bottom box was removable, so the bottom score could be directly evaluated. If the colony was housed in more than one box, the sum of the average of the top and bottom of every box was used as the cluster score.
The area of capped brood in each colony was evaluated by overlaying a one-inch2 grid over each frame with capped brood, and visually counting the area occupied by capped brood on each frame of each colony. Brood solidness (percent of cells filled with capped brood) was estimated by overlaying three patches of the most solid capped brood in each colony with a rhombus shape containing 100 cells and counting the number of cells with capped brood inside the rhombus. Different frames or different sides of the frame were used for each rhombus measurement, such that the patches were on two or three different frames per colony. These three measures were averaged to determine a brood solidness measurement for each colony.
Patty Analyses
One patty from each box of protein patties from all feeding dates was retained and kept frozen for subsequent nutritional analyses. Equal amounts of each representative patty were combined and homogenised to produce 800 g samples, which were sent to Central Laboratories (Winnipeg, MB) for analysis of fats, amino acids, proteins, and sugar content. The analysis methods were accredited by the Standards Council of Canada: Amino acid profiles were completed using acid hydrolysis (HPLC), sugar profiles were completed using AFVAV-SLMF-0018, the fatty acid components were analysed using reference method AOAC 996.06, moisture AOAC 922.02, and crude protein was analysed using CTL-PDSOP.
Statistical Analyses
The total weight of patty consumed was converted to grams consumed per day for each period between patty replacement. All data were checked for normality prior to analysis with ANOVA using the Shapiro–Wilks test. One date × treatment combination violated the assumption of normality: 2018 FeedBee Week 4 (Shapiro–Wilk test W = 0.900, P < W 0.02). However, ANOVA is robust to minor departures from normality, and transformation did not improve the normality, therefore untransformed data were used in the analysis as all other data were normal.
A one-way ANOVA was performed on cumulative total consumption across all dates among treatments, followed by repeated measures analysis of the weekly mean per day consumptions (date and treatment) with a Greenhouse-Geisser correction, then subsequent post hoc one-way ANOVAs for each date to find treatment differences. A Bonferroni correction was applied to alpha for these multiple one-way ANOVAs (e.g., 2018 α original = 0.05/5 tests therefore α altered = 0.01 required for significance). In 2019, consumption rates of each patty type within a single colony (Trio treatment only) were analysed with ANOVA using colony as a blocking variable, followed by post hoc Tukey's HSD test. Where the ANOVA revealed significance, means were subsequently compared using Tukey's Honestly Significant Difference test post hoc.
Treatment effects on cluster size, brood area, and brood patterns were analysed using a one-way ANOVA with feed treatment as a factor within each time point examined. Paired t-tests were used to compare the start versus end point for each colony (population, brood pattern, brood area). Colony population growth was estimated by subtracting the initial cluster score from the prepollination score, then feed treatment effects on population growth were compared using a one-way ANOVA by feed treatment. All statistical analyses were conducted in JMP Pro (Version 15.2.0, SAS Institute; Cary, NC). Data was considered statistically significant when P ≤ 0.05. All analyses were performed using JMP (2019) software.
Results
The average amount of protein feed consumed by colonies in each treatment over the three years of experiments ranged from 0.17 to 2.72 kg depending on year and treatment (Table 3).
Table 3.
Total protein feed consumption per colony for the duration of the experiment, for all colonies remaining queenright for the entire feeding period (April to May 2018–2020)
| Feed treatment | Sample size (no. colonies) | Mean consumption per colony (kg) ± SE | Minimum consumption per colony (kg) | Maximum consumption per colony (kg) |
|---|---|---|---|---|
| 2018 | ||||
| Global 15% | 28 | 1.38 ± 0.06 | 0.80 | 1.87 |
| FeedBee | 24 | 0.66 ± 0.06 | 0.17 | 1.30 |
| Bee Pollen-Ate 25% | 23 | 1.26 ± 0.09 | 0.36 | 1.97 |
| Trio | 26 | 1.79 ± 0.09 | 0.37 | 2.45 |
| 2019 | ||||
| Global 15% | 29 | 1.70 ± 0.05 | 1.19 | 2.18 |
| Global 0% | 29 | 1.94 ± 0.06 | 0.89 | 2.53 |
| Bee Pollen-Ate 20% | 28 | 1.66 ± 0.08 | 0.82 | 2.72 |
| Control | 28 | NA | NA | NA |
| 2020 | ||||
| Global 15% | 26 | 1.95 ± 0.07 | 1.12 | 2.62 |
| Global 0% | 25 | 1.53 ± 0.09 | 0.70 | 2.66 |
| Bee Pollen-Ate 15% | 26 | 1.44 ± 0.10 | 0.57 | 2.07 |
| Healthy Bees | 27 | 1.36 ± 0.09 | 0.58 | 2.13 |
2018
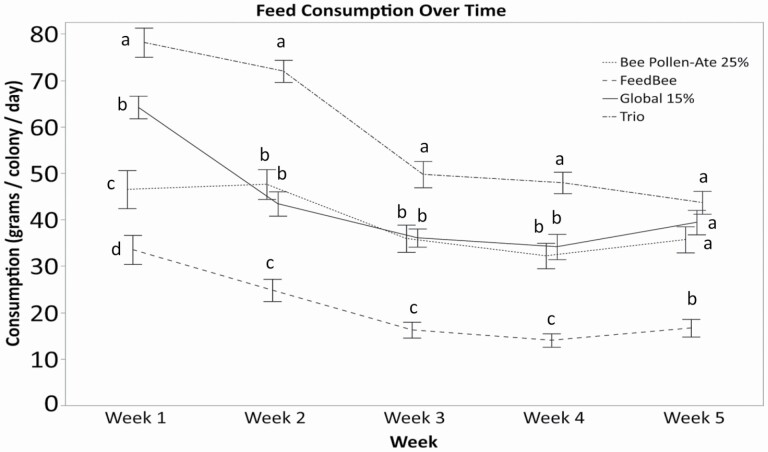
The experimental colonies consumed more of the Trio protein treatment than the other protein feed treatments (Fig. 1; cumulative consumption F = 47.38; df = 3, 101; P < 0.0001), despite all treatments being fed ad libitum. The cumulative consumption of the Trio and Global 15% feeds was more than double the consumption of the FeedBee feed. There was an effect of both treatment and date on patty consumption, as well as a significant interaction between feed treatment and date (repeated measures ANOVA; ANOVAtreatment F = 48.08; df = 3,98; P < 0.0001; ANOVAdate G-G correction F = 170.65; df = 3.01, 295.15; P < 0.0001; ANOVAtreatment*date G-G correction F = 11.75; df = 9.03, 295.15; P < 0.0001). Consumption differences among feed types were most evident early in the spring, and declined over time (Fig. 1; Table 4) as consumption generally declined. This makes intuitive sense, as pollen was not naturally available on the first date, and gradually increasing numbers of foragers were seen returning with pollen, until about week 3–4 when there was a strong pollen flow (personal observation). Trio was the most-consumed treatment in the first week, followed by Global 15%, then Bee Pollen-Ate, then FeedBee, however for weeks 2–5 there was no difference in the consumption of Global 15% and Bee Pollen-Ate. The FeedBee treatment had consistently lower consumption rates than the other treatments (Table 4).
Fig. 1.
Protein patty consumption by honey bee colonies over the spring of 2018 (g consumed per colony per day ± SE) for each feed treatments: Global 15% (containing 15% bee-collected pollen by weight), Bee Pollen-Ate (produced using 25% of Bee Pollen-Ate by weight of the dry feed), FeedBee, and Trio which was a combination of the three previous treatments. Significant differences (P ≤ 0.05) among treatments within each time point are represented by different letters according to a one-way ANOVA followed by a Tukey's HSD test (α ≤ 0.05). Differences in consumption among treatments were more apparent early in the spring compared to later in the spring when more natural pollen was available.
Table 4.
The mean consumption (g) of patty per colony per day within the treatment groups in 2018 (at each week and in total)
| Time point | Global 15% | Feedbee | Bee Pollen-Ate 25% | Trio | ANOVA | ||
|---|---|---|---|---|---|---|---|
| F | df | P | |||||
| Week 1 | 64.1b | 33.5d | 46.5c | 76.6a | 38.98 | 3, 101 | 0.0001 |
| Week 2 | 43.4b | 24.8c | 47.6b | 69.9a | 54.75 | 3, 101 | 0.0001 |
| Week 3 | 36.1b | 16.3c | 35.9b | 48.2a | 33.11 | 3, 101 | 0.0001 |
| Week 4 | 34.2b | 14.0c | 32.2b | 46.4a | 34.31 | 3, 101 | 0.0001 |
| Week 5 | 39.4a | 16.7b | 35.7a | 42.5a | 22.94 | 3, 101 | <0.0001 |
| Cumulative total | 1379.2b | 664.7c | 1259.4b | 1792.5a | 47.38 | 3, 101 | <0.0001 |
| Consumption Within Trio Treatment | |||||||
| Cumulative total | 807.5a | 302.3b | 735.3a | 125.18 | 2, 77 | <0.0001 | |
In the main experiment, each colony received only one patty type. Average consumption for each patty type within the Trio treatment in which each colony received all the feed types is also shown. Treatment means followed by different letters within each row vary significantly according to ANOVA followed by Tukey's HSD test.
The pattern of consumption of each individual patty type within the Trio treatment was similar to overall consumption (Table 4), with cumulative consumption of the Global 15% and Bee Pollen-Ate treatments higher than the FeedBee feed within the Trio fed-colonies. (Fig. 1; Table 4).
At the outset of the trial, the adult bee and brood populations were similar across all treatment groups (cluster score = 7.50 ± 0.13 frames, F = 1.02; df = 3, 100; P = 0.388; brood area = 34.7 ± 4.2 cm2, F = 1.33; df = 3, 100; P = 0.27). The differences in feed consumption were therefore not due to initial variance among treatments in colony population or brood food requirements.
The solidness of the sealed brood pattern did not differ between treatment groups at either two ( = 86.8 ± 1.0/100 cells filled with capped pupae, F = 1.27; df = 3, 100; P = 0.29) or 5 wk after the feed treatments began ( = 91.8 ± 0.87/100 cells filled, F = 0.31; df = 3, 100; P = 0.82). It is interesting to note that the brood pattern of the experimental colonies generally became more solid over the spring (paired t-test T = −6.09; df = 3, 100; P < 0.0001).
Despite differences in patty consumption, there were no differences in colony population or area of brood after 5 wk of receiving different feed treatments (cluster scores = 8.53 ± 0.15 frames, F = 0.65; df = 3, 100; P = 0.58; brood area = 3299.3 ± 77.7 cm2, F = 1.28; df = 3, 100; P = 0.28). Similarly, there were no differences among treatments in colony population at the prepollination cluster score performed in mid-June (cluster score = 8.50 ± 0.30 frames, F = 0.81; df = 3, 99; P = 0.48). Regardless of feed treatment, all colonies grew similarly (ANOVA on increase in number of frames covered by bees prepollination − initial F = 0.41; df = 3, 99; P = 0.74; cluster score increase = 11.0 ± 0.26 frames). This indicates that in 2018 differences in patty diet or the consumption of the diet did not affect pollination revenue, which is based on a modified cluster score estimation performed by seed production companies.
The FeedBee treatment resulted in a greater brood and adult population increase per gram of feed consumed by each colony (week 5 brood area (cm2)/total feed consumed (g) F = 31.48; df = 3, 99; P < 0.0001; (prepollination cluster (frames)/total feed consumed (g) F = 27.18; df = 3, 99; P < 0.0001).
2019
In the second year, the colonies receiving the Global 0% pollen feed consumed more than did the colonies receiving Global 15% or Bee Pollen-Ate 20% feeds (Table 5, cumulative consumption F = 5.49; df = 2, 85; P = 0.006). Similar to the results in 2018, there was an effect of both treatment and date on patty consumption, as well as a significant interaction between feed treatment and date (repeated measures ANOVA; ANOVAtreatment F = 5.49; df = 2, 83; P = 0.0058; ANOVAdate G-G correction F = 176.09; df = 2.28, 188.84; P < 0.0001; ANOVAtreatment*dateG-G correction F = 4.53; df = 4.55, 188.94; P < 0.001). In the first and second week, the colonies receiving the Global 0% feed consumed significantly more than those receiving Global 15% feeds, and in weeks 2 and 4 more Global 0% feed was consumed than Bee Pollen-Ate (Table 5).
Table 5.
The mean consumption (g) of patty per colony per day within the treatment groups in 2019 (at each week and in total)
| Time point | Global 15% | Global 0% | Bee Pollen-Ate 20% | ANOVA | ||
|---|---|---|---|---|---|---|
| F | df | P | ||||
| Week 1 | 60.2b | 72.6a | 65.5ab | 6.79 | 2, 85 | 0.002 |
| Week 2 | 74.3b | 85.6a | 71.9b | 5.93 | 2, 85 | 0.004 |
| Week 3 | 56.0 | 61.5 | 53.6 | 2.94 | 2, 85 | 0.058 |
| Week 4 | 57.2ab | 53.8a | 46.5b | 4.99 | 2, 85 | 0.009 |
| Cumulative total | 1709.95b | 1938.38a | 1662.65b | 5.49 | 2, 85 | 0.006 |
Each colony received only one patty type. Treatment means followed by different letters within each row vary significantly according to ANOVA followed by Tukey's HSD test. Control colonies did not receive protein feed and are therefore not included in analyses of feed consumption.
The initial population of adult bees (cluster score = 7.18 ± 0.14 frames; F= 1.23; df = 3, 113; P = 0.30) and initial capped brood area (93.8 ± 7.7 cm2; F = 0.62; df = 3, 113; P = 0.60) did not vary by treatment, indicating that at the start of the experiment, colonies in the different treatment groups were similarly sized.
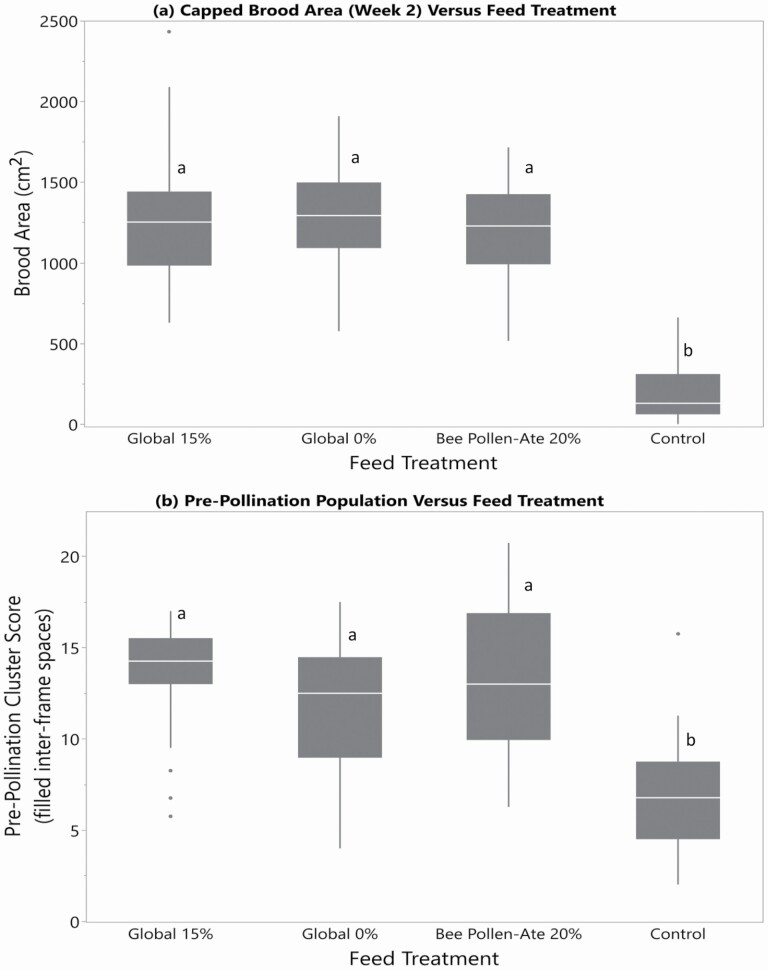
In contrast, 2 wk after the feed treatments began, significantly less capped brood was found in the control colonies receiving no supplemental protein, than was found in all other feed groups (F = 82.6; df = 3, 113; P < 0.0001; Fig. 2a). At this point, at the beekeepers’ request, beekeeper-made patties (0.7 kg containing Bee Pollen-Ate per colony) were added to feed the control colonies, which were in poor condition with very little brood, but this patty quickly dried out and very little of it was consumed by the control hives (personal observation). After 4 wk from the start of the experiment, the control colonies still had smaller brood areas (F = 34.3; df = 3, 113; P < 0.0001) than colonies receiving any of the treatment feeds (Global 0%, Global 15%, or Bee Pollen-Ate 20%). In addition, the solidness of the brood pattern was lower in the control colonies than those fed Global 0% or Global 15% at week 5 (F = 3.78; df = 3, 112; P = 0.0126).
Fig. 2.
The size of honey bee colonies versus feed treatment in spring 2019. (a) Amount of capped brood at 2 wk after the start of the feeding trial and (b) the prepollination cluster size (number of inter-frame spaces filled with adult bees) in June, after the supplemental feeding had stopped. The four feeding treatments were: Global 15% (containing 15% bee-collected pollen by weight), Global 0% (contain no pollen), Bee Pollen-Ate (produced using 20% of Bee Pollen-Ate by weight of the dry feed), and a control (no supplemental protein given). Different letters indicate significantly different colony sizes among feed treatments as indicated by Tukey's HSD test (α ≤ 0.05). Control colonies had both less brood at 2 wk, and smaller adult populations later in the summer than all other feed treatments. Within each box, horizontal white lines denote the median value. The boxes extend from the 25th to the 75th percentile with vertical extending lines denoting the adjacent values (1.5× the interquartile range of the 25th and 75th percentile of each group). Dots denote outliers.
In this region, standard management practice is to add a second brood chamber to single brood chamber colonies as needed, based on the strength of the colony. This was done by the beekeepers, based on their assessment of colony strength, and again differences resulting from the feed treatments were observed (χ 2 = 9.37, P = 0.0248) with 27% of control colonies still in one brood chamber versus 3% of Global 15%, 7% of Global 0%, and Bee Pollen-Ate fed colonies. The negative effect of the control treatment persisted, and was even more pronounced in the prepollination cluster assessment, when control colonies were observed to have smaller clusters than all other feed treatments (F = 16.5; df = 3, 99; P < 0.0001; Fig. 2b; Global 15% = 13.4 ± 0.6, Global 0% = 11.7 ± 0.7, Bee Pollen-Ate = 13.1 ± 0.8, control = 6.9 ± 0.7 frames). This would translate into large economic consequences for the beekeepers, as only 1/30 (3%) control colonies would have been large enough to be rented for pollination (vs 21–31% of the colonies in the other feed treatments).
Regardless of feed treatment, all colonies grew similarly, except the control treatment (ANOVA on prepollination-initial cluster F = 20.53; df = 3, 99; P < 0.0001; cluster score decrease for control = 0.5 ± 0.64 frames whereas other treatments increased Global 0% = 4.6 ± 0.63, Global 15% = 6.5 ± 0.64, Bee Pollen-Ate = 5.8 ± 0.80 frames). It is important to note that the 2019 colony growth was lower for all colonies than in 2018, likely because we deliberately selected an apiary with meagre spring floral resources to sustain the colonies.
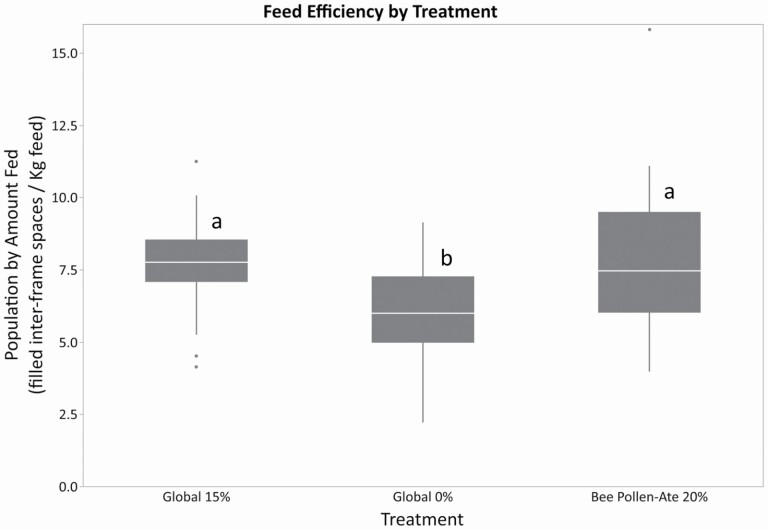
There was no difference among the treatments in the feed efficiency using capped brood as the unit of interest (week 4 brood area (cm2)/total feed consumed (g) F = 2.23; df = 2, 85; P = 0.1139), however the adult population produced per gram of feed consumed was lower for the Global 0% feed than Global 15% or Bee Pollen-Ate (Fig. 3; prepollination cluster (frames)/total feed consumed (g) F = 8.05; df = 2, 76; P = 0.0007). This makes intuitive sense as our previous results demonstrate increased consumption of the Global 0% treatment without differences among treatments in colony growth.
Fig. 3.
Feed efficiency (prepollination cluster score/total feed consumed) of the three feed treatments in 2019. The three feed treatments were: Global 15% (containing 15% bee-collected pollen by weight), Global 0% (contain no pollen), and Bee Pollen-Ate (produced using 20% of Bee Pollen-Ate by weight of the dry feed). Honey bee colonies fed the Global 15% and Bee Pollen-Ate feeds had higher populations for the amount of feed consumed compared to the Global 0% feed. Within each box, horizontal white lines denote the median value. The boxes extend from the 25th to the 75th percentile with vertical extending lines denoting the adjacent values (1.5× the interquartile range of the 25th and 75th percentile of each group). Dots denote outliers outside the range of adjacent values. Different letters indicate significantly feed efficiency among feed treatments as indicated by Tukey's HSD test (α ≤ 0.05).
2020
In the third year of the trials, the colonies receiving the Global 15% pollen feed consumed more feed than did the colonies receiving Global 0% or Bee Pollen-Ate 15% feeds (Table 6, cumulative consumption F = 8.61; df = 3, 103; P < 0.0001).
Table 6.
The mean consumption (g) of patty per colony per day within the treatment groups (for each two-week period and in total) in 2020
| Time point | Global 15% | Global 0% | Bee Pollen-Ate 15% | Healthy Bees | ANOVA | ||
|---|---|---|---|---|---|---|---|
| F | df | P | |||||
| Week 1–2 | 80.2a | 74.8a | 58.3b | 58.3b | 9.13 | 3,103 | <0.0001 |
| Week 3–4 | 71.4a | 46.8b | 53.0b | 48.2b | 10.21 | 3,103 | <0.0001 |
| Cumulative total | 1952.96a | 1525.06b | 1436.7b | 1364.96b | 8.61 | 3,103 | <0.0001 |
Each colony received only one patty type. Treatment means followed by different letters within each row vary significantly according to ANOVA followed by Tukey's HSD test.
As observed in 2018 and 2019, there was an effect of both treatment and date on patty consumption, as well as a significant interaction between feed treatment and date (repeated measures ANOVA; ANOVAtreatment F = 8.50; df = 3, 100; P < 0.0001; ANOVAdate G-G correction F = 143.85; df = 1, 100; P < 0.0001; ANOVAtreatment*dateG-G correction F = 21.31; df = 3, 100; P < 0.001). Colonies receiving the Global 15% feed consumed significantly more than those receiving Bee Pollen-Ate 15% or Healthy Bees at both dates.
Despite randomisation of treatments, the pretreatment population of adult bees differed among treatments, with colonies receiving the Healthy Bees treatment having larger cluster scores (7.1 ± 0.31 frames) than those receiving Global 15% (5.4 ± 0.23 frames) or Bee Pollen-Ate (6.0 ± 0.30 frames), and those receiving Global 0% (6.9 ± 0.28 frames) having larger cluster than Global 15% (cluster score F = 7.97; df = 3, 103; P < 0.0001). Therefore, population growth was used in subsequent analyses rather than absolute population size estimates.
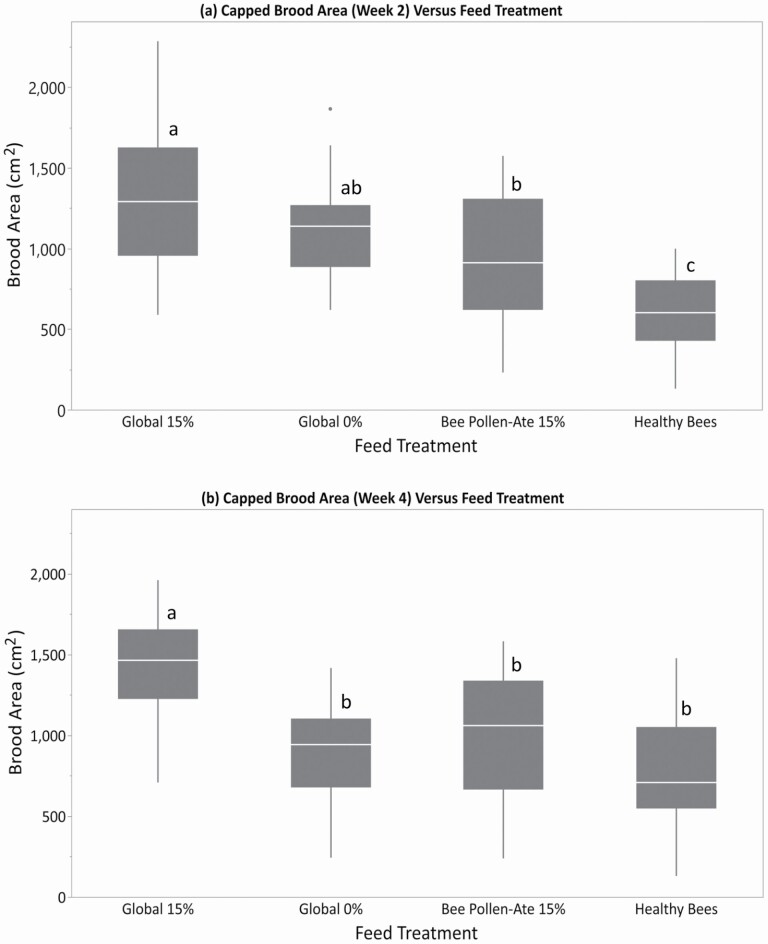
Initial capped brood area did not vary by treatment (21.06 ± 3.5 cm2; F = 2.53; df = 3, 103; P = 0.061). However, within 2 wk after the feed treatments began, differences in the amount of capped brood among the feed treatments were apparent, with colonies fed Global 15% having more capped brood than those fed Bee Pollen-Ate 15% or Healthy Bees patties, and colonies fed the Healthy Bees patties producing less capped brood than all other feed treatments (Fig. 4a, F = 19.51; df = 3, 103; P < 0.0001). By week 4, the brood area difference was only apparent in the Global 15%-fed colonies, which had more capped brood than all other treatments (Fig. 4b, F = 17.71; df = 3, 103; P < 0.0001). Colonies fed the Global 15% patties also had the most solid brood patterns at week 4 (F = 12.03; df = 3, 103; P < 0.0001) compared to all other treatments.
Fig. 4.
Size of honey bee colonies versus feed treatment in spring 2020. The amount of capped brood (pupae) at (a) 2 wk and (b) 4 wk after the start of the feeding trial. The four feed treatment groups were: Global 15% (containing 15% bee-collected pollen by weight), Global 0% (contain no pollen), Bee Pollen-Ate (produced using 15% of Bee Pollen-Ate by weight of the dry feed), and Healthy Bee (produced with spirulina algae-based protein). Different letters indicate significantly different colony sizes among feed treatments as indicated by Tukey's HSD test (α ≤ 0.05). Colonies fed the Global 15% feed had the greatest amount of capped brood at both time points, but differences among the other feed treatments were apparent only at 2 wk. Within each box, horizontal white lines denote the median value. The boxes extend from the 25th to the 75th percentile with vertical extending lines denoting the adjacent values (1.5× the interquartile range of the 25th and 75th percentile of each group).
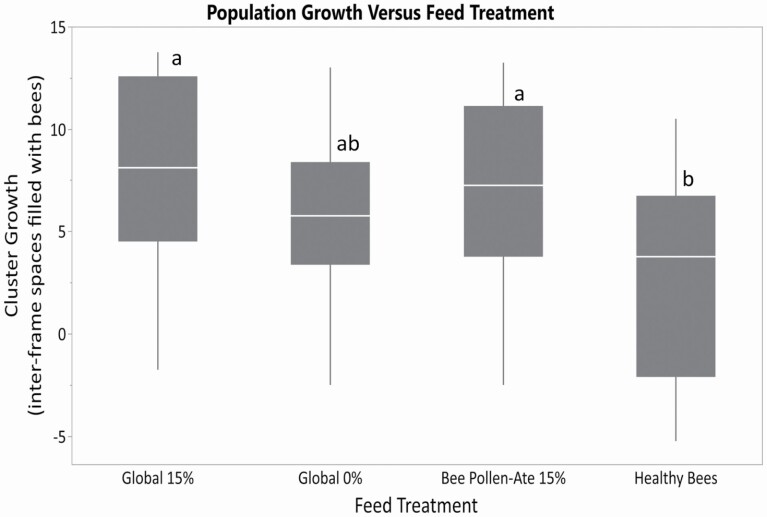
Population growth from pretreatment cluster scores to prepollination cluster scores was higher for the colonies fed Global 15% and Bee Pollen-Ate 15% than those fed Healthy Bees, with growth in colonies fed Global 0% intermediate (Fig. 5; F = 5.29; df = 3, 80; P = 0.0023). When consumption rates are incorporated ([prepollination cluster score-initial cluster score]/[total feed consumption]), the same trend is evident, with colonies fed Global 15% or Bee Pollen-Ate 15% growing more per gram of feed consumed than those fed Healthy Bees (F = 4.75; df = 3, 80; P = 0.0043).
Fig. 5.
Population growth of honey bee colonies versus feed treatment (prepollination cluster in June minus initial cluster score in April) in 2020. The four feed treatment groups were: Global 15% (containing 15% bee-collected pollen by weight), Global 0% (contain no pollen), Bee Pollen-Ate (produced using 15% of Bee Pollen-Ate by weight of the dry feed), and Healthy Bee (produced with spirulina algae-based protein). Colonies fed Global 15% and Bee Pollen-Ate 15% feeds had higher population growth than those fed Healthy Bees. Within each box, horizontal white lines denote the median value. The boxes extend from the 25th to the 75th percentile with vertical extending lines denoting the adjacent values (1.5× the interquartile range of the 25th and 75th percentile of each group). Different letters indicate significantly different colony sizes among feed treatments as indicated by Tukey's HSD test (α ≤ 0.05).
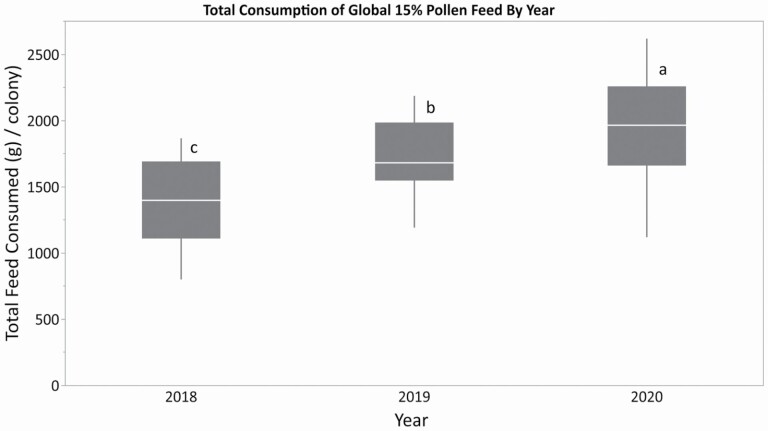
As the Global 15% patties were fed in all three experimental years, the compare consumption and growth can be compared across years for this treatment. Total feed consumption of the Global 15% patty was lowest in 2018, followed by 2019, and highest in 2020 (Fig. 6; F = 20.43; df = 2, 83; P < 0.0001). As the colonies were fed for different amounts of time each year depending on when temperatures permitted them to be moved outdoors, the consumption of Global 15% patties per day for each year was also analysed. Consumption per day fed was highest in 2020 (F = 8.52; df = 2, 83; P < 0.004).
Fig. 6.
Consumption of Global 15% protein patty supplements by honey bee colonies across three years (grams per colony). The patties were fed ad libitum. A different apiary was used in 2018 versus the same apiary used in 2019 and 2020. Within each box, horizontal white lines denote the median value. The boxes extend from the 25th to the 75th percentile with vertical extending lines denoting the adjacent values (1.5× the interquartile range of the 25th and 75th percentile of each group). Different letters indicate significantly different amounts of feed consumed among feed treatments as indicated by Tukey's HSD test (α ≤ 0.05).
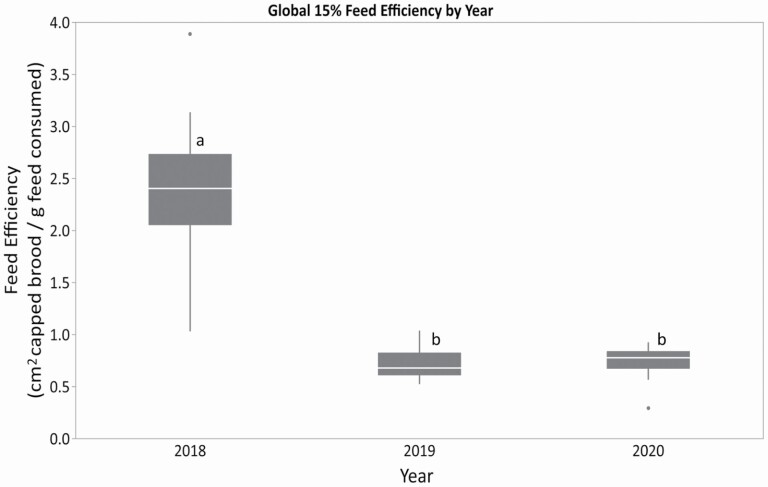
If instead of consumption rates, the feed efficiency (area of capped brood produced per gram of feed consumed) is examined, it becomes evident that the ‘good’ spring apiary used in 2018 had a higher apparent feed efficiency (cm2 capped brood in May/grams feed consumed Global 15%) than those in the ‘poor’ apiary used in 2019 and 2020 (Fig. 7, F = 104.73; df = 2, 82; P < 0.0001), likely because of the utilisation of external forage sources to supplement the feed provided to produce brood.
Fig. 7.
Feed efficiency per year for honey bee colonies fed the Global 15% pollen feed treatment in the spring (cm2 capped brood in May per gram of feed consumed in 2018, 2019, and 2020). The patties were fed ad libitum. Colonies also had access to natural forage, as available depending on year and time of season. A different apiary was used in 2018 versus the same apiary used in 2019 and 2020. Within each box, horizontal white lines denote the median value. The boxes extend from the 25th to the 75th percentile with vertical extending lines denoting the adjacent values (1.5× the interquartile range of the 25th and 75th percentile of each group). Dots denote outliers outside the range of adjacent values. Different letters indicate significant differences among years as indicated by Tukey's HSD test (α ≤ 0.05).
Feed Analyses
The protein content of all the feeds fell within the range observed from natural pollen (Table 7; 14–21%). However, protein above 20% is recommended for brood rearing (Herbert et al. 1977), which was only observed in the FeedBee (2018) and Bee Pollen-Ate 20% patty (2019). All the protein feed treatments contained all ten amino acids essential to honey bees (De Groot 1953), in various amounts (Table 7). The amino acid levels varied among feeds, even among years within the same feed type, likely due to variation in the original ingredients used to make the patties. Two amino acids, lysine and arginine, were below the optimal proportion required for growth in all patties except the FeedBee patty (and dry FeedBee, Table 8) (DeGroot 1953). Due to relatively high tryptophan levels in the Global 15%, Global 0%, and Bee Pollen-Ate 15% patties in 2020, these patties had proportionately low values of several other essential amino acids. The percent fat and the fatty acids also varied among feed treatments, with the Healthy Bees product having the highest overall fat content, followed by the FeedBee product either dried or as fed (Table 9).
Table 7.
The moisture, protein, essential amino acids, and sugar components of the protein patty feed diets used in the experiment, on an as fed basis
| As Fed | 2018 | 2019 | 2020 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global 15 | FeedBee Patty | Bee Pollen-Ate 25 | Dry FeedBee | Global 15 | Global 0 | Bee Pollen-Ate 20 | Global 15 | Global 0 | Bee Pollen-Ate 15 | Healthy Bees | ||
| Moisture (%) | Dry Matter | 81.14 | 89.8 | 80.3 | 94.58 | 82.15 | 83.09 | 79.02 | 79.94 | 81.71 | 82.64 | 85.66 |
| Protein (%) | Crude Protein | 17.83 | 21.28 | 16.57 | 31.77 | 18.45 | 17.58 | 20.43 | 14.48 | 14.02 | 20.1 | 18.11 |
| Essential Amino Acids (%) | Arginine | 0.13 | 0.6 | 0.14 | 1.58 | 0.14 | 0.2 | 0.14 | 0.07 | 0.09 | 0.07 | 0.07 |
| Histidine | 0.33 | 0.43 | 0.3 | 0.8 | 0.29 | 0.4 | 0.36 | 0.24 | 0.22 | 0.26 | 0.25 | |
| Isoleucine | 0.69 | 0.81 | 0.69 | 1.22 | 0.64 | 0.76 | 0.87 | 0.7 | 0.34 | 0.73 | 0.7 | |
| Leucine | 1.26 | 2.2 | 1.01 | 3.48 | 1.07 | 1.38 | 1.41 | 1.09 | 1.07 | 1.49 | 1.43 | |
| Lysine | 0.16 | 0.36 | 0.21 | 1.45 | 0.16 | 0.26 | 0.23 | 0.31 | 0.15 | 0.19 | 0.18 | |
| Methionine | 0.27 | 0.35 | 0.23 | 0.51 | 0.29 | 0.27 | 0.28 | 0.16 | 0.23 | 0.24 | 0.32 | |
| Phenylanine | 0.72 | 0.99 | 0.58 | 1.62 | 0.68 | 0.88 | 0.79 | 0.64 | 0.71 | 0.87 | 0.84 | |
| Threonine | 0.64 | 0.68 | 0.67 | 1.09 | 0.54 | 0.71 | 0.8 | 0.33 | 0.51 | 0.75 | 0.72 | |
| Tryptophan | 0.12 | 0.12 | 0.1 | 0.26 | 0.14 | 0.14 | 0.12 | 0.64 | 0.36 | 0.31 | 0.14 | |
| Valine | 0.82 | 0.98 | 0.88 | 1.45 | 0.74 | 0.9 | 1.04 | 0.77 | 0.46 | 0.93 | 0.89 | |
| Sugars (g/100g) | Total Sugar | 41.9 | 36.2 | 39.4 | 26.5 | 42.5 | 43.3 | 38 | 41.7 | 49.5 | 42.8 | 58.9 |
| Fructose | 21.7 | <0.2 | 13.4 | <0.2 | 22.3 | 11.1 | 17.7 | 20.9 | 12.0 | 17.9 | 7.1 | |
| Glucose | 20.2 | <0.2 | 13.4 | <0.2 | 20.2 | 10.9 | 17.2 | 19.9 | 12.4 | 18.0 | 37.9 | |
| Sucrose | <0.2 | 36.2 | 12.5 | 26.5 | <0.2 | 21.4 | 3 .0 | <0.2 | 25.5 | 7.0 | 13.8 | |
Table 8.
The proportional essential amino acid complement (amino acids with less than proportion recommended by DeGroot 1953; bold) found in the experimental patty feed treatments, on an as fed basis
| As Fed | 2018 | 2019 | 2020 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global 15 | FeedBee Patty | Bee Pollen-Ate 25 | Dry FeedBee | Global 15 | Global 0 | Bee Pollen-Ate 20 | Global 15 | Global 0 | Bee Pollen-Ate 15 | Healthy Bees | Required (DeGroot 1953) | ||
| Essential Amino Acids (As Proportion of Tryptophan) | Arginine | 1.1 | 5.0 | 1.4 | 6.1 | 1.0 | 1.4 | 1.2 | 0.1 | 0.3 | 0.2 | 0.5 | 3 |
| Histidine | 2.8 | 3.6 | 3.0 | 3.1 | 2.1 | 2.9 | 3.0 | 0.4 | 0.6 | 0.8 | 1.8 | 1.5 | |
| Isoleucine | 5.8 | 6.8 | 6.9 | 4.7 | 4.6 | 5.4 | 7.3 | 1.1 | 0.9 | 2.4 | 5.0 | 4.0 | |
| Leucine | 10.5 | 18.3 | 10.1 | 13.4 | 7.6 | 9.9 | 11.8 | 1.7 | 3.0 | 4.8 | 10.2 | 4.5 | |
| Lysine | 1.3 | 3.0 | 2.1 | 5.6 | 1.1 | 1.9 | 1.9 | 0.5 | 0.4 | 0.6 | 1.3 | 3.0 | |
| Methionine | 2.3 | 2.9 | 2.3 | 2.0 | 2.1 | 1.9 | 2.3 | 0.3 | 0.6 | 0.8 | 2.3 | 1.5 | |
| Phenylanine | 6.0 | 8.3 | 5.8 | 6.2 | 4.9 | 6.3 | 6.6 | 1.0 | 2.0 | 2.8 | 6.0 | 2.5 | |
| Threonine | 5.3 | 5.7 | 6.7 | 4.2 | 3.9 | 5.1 | 6.7 | 0.5 | 1.4 | 2.4 | 5.1 | 3.0 | |
| Tryptophan | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Valine | 6.8 | 8.2 | 8.8 | 5.6 | 5.3 | 6.4 | 8.7 | 1.2 | 1.3 | 3.0 | 6.4 | 4.0 | |
Table 9.
The fat components of the protein patty feed diets used in the experiment, on an as fed basis
| As Fed | 2018 | 2019 | 2020 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global 15 | FeedBee Patty | Bee Pollen-Ate 25 | Dry FeedBee | Global 15 | Global 0 | Bee Pollen-Ate 20 | Global 15 | Global 0 | Bee Pollen-Ate 15 | Healthy Bees | ||
| Fats (g/100g |
Fat (GC/FID) | 1.02 | 3.56 | 0.4 | 2.8 | 1.17 | 0.84 | 0.840 | 1.8 | 1.4 | 1.3 | 5.7 |
| C16 Palmitic | 0.281 | 0.327 | 0.103 | 0.379 | 0.315 | 0.233 | 0.252 | 0.5 | 0.4 | 0.4 | 0.8 | |
| C16:1 Palmitoleic | <0.001 | 0.02 | 0.074 | 0.026 | <0.001 | <0.001 | 0.033 | <0.1 | <0.1 | <0.1 | <0.1 | |
| C18 Stearic | 0.054 | 0.106 | 0.031 | 0.061 | 0.061 | 0.051 | 0.06 | 0.1 | <0.1 | 0.1 | 0.1 | |
| C18:1 Oleic | 0.105 | 0.688 | 0.054 | 0.646 | 0.114 | 0.095 | 0.094 | 0.2 | 0.2 | 0.1 | 1.6 | |
| C18:2 Linoleic | 0.38 | 1.17 | 0.056 | 1.5 | 0.467 | 0.404 | 0.338 | 0.8 | 0.7 | 0.6 | 1 | |
| C18:3 Linolenic | 0.144 | 1.16 | 0.011 | 0.073 | 0.156 | 0.045 | 0.029 | 0.2 | <0.1 | <0.1 | 0.2 | |
| C20 Arachidic | 0.006 | 0.012 | <0.001 | 0.015 | 0.009 | <0.001 | <0.001 | <0.1 | <0.1 | <0.1 | <0.1 | |
| C22 :0 Behenic | 0.007 | 0.006 | <0.001 | 0.012 | 0.005 | <0.001 | <0.001 | <0.1 | <0.1 | <0.1 | <0.1 | |
| C8 Caprylic | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.1 | <0.1 | <0.1 | 0.2 | |
| cis-Monounsaturated Fatty Acids | 0.13 | 0.72 | 0.13 | 0.69 | 0.14 | 0.11 | 0.14 | 0.2 | 0.2 | 0.2 | 1.6 | |
| cis-Polyunsaturated Fatty Acids | 0.5 | 2.23 | 0.07 | 1.51 | 0.6 | 0.43 | 0.35 | 0.9 | 0.7 | 0.6 | 1.3 | |
| Omega-3 Polyunsaturated Fatty Acids | 0.14 | 1.11 | 0.01 | 0.07 | 0.15 | 0.04 | 0.03 | 0.2 | <0.1 | <0.1 | 0.2 | |
| Omega-6 Polyunsaturated Fatty Acids | 0.36 | 1.12 | 0.06 | 1.44 | 0.45 | 0.39 | 0.32 | 0.7 | 0.7 | 0.5 | 1.1 | |
| Saturated Fatty Acids | 0.34 | 0.45 | 0.18 | 0.48 | 0.38 | 0.27 | 0.31 | 0.6 | 0.5 | 0.5 | 2.5 | |
The Global 15% patties were consistent across all three years with total protein content of 14–18% and total sugar content of 41–42%, which was predominantly fructose and glucose. The Global 0% patties also had protein in the 14–17% range, but contained a mix of glucose, fructose, and sucrose, with sucrose as the largest sugar component in both years (2019 and 2020). The Bee Pollen-Ate patties (made by Global Patties using dry feed from AllTech Inc.) also contained a mix of sucrose, fructose, and glucose, however the glucose/fructose components were larger than the sucrose component. There may be variation in the sugar syrups used to make all protein feeds depending on the source. For example, the Lantic factory in southern Alberta used to source syrup for beekeepers in Alberta sells both sucrose and invert syrups, sourced from both sugar cane and sugar beet, depending on availability. In addition, patty manufacturers must vary the liquid components of the recipe depending on the solubility of the dry ingredients and may include sucrose to assist in solubility and forming patties with appropriate texture. The Feedbee patty fat and sucrose contents are much higher than the other patties due to the recipe for this feed containing flaxseed oil and sugar syrup made from table sugar.
Discussion
The results of this study clearly demonstrate the potential for benefit of spring protein supplementation to honey bee colonies in temperate regions, especially in environments depauperate of pollen. We found that supplemented colonies had a larger amount of capped brood than unsupplemented colonies after only 2 wk. This effect persisted into prepollination adult populations, providing both a direct economic benefit (increased pollination fees) to beekeepers, as well as potentially increasing winter survival though the accumulation of increased stored food resources and increased population.
Colonies fed the three-patty type ‘Trio’ treatment consumed significantly more patty overall than colonies fed the three single-patty type treatments (Fig. 1). The reason for this is not clear, but perhaps this indicates a preference for variety, or an ability to compensate for specific deficiencies in food sources by including other sources. Specifically, recent evidence suggests that bees can balance essential fatty acid and amino acid intake at the colony level (Hendriksma and Shafir 2016, Zarchin et al. 2017), and it is possible that this extends to other essential nutrients. However, we did not detect any benefit of this increased feed consumption in terms of brood production or colony population growth. If the bees were compensating for a nutritional deficiency of one patty type by consuming additional feed types in the Trio treatment, the colonies fed single patties could have been doing so through foraging for pollen as it became available. Given that the bees exhibited increased consumption of the other single patty types over the Feedbee patty, one might expect them to completely ignore the Feedbee when offered with alternatives in the Trio treatment. However, the consumption of the individual patties within the Trio treatment reflected overall consumption patterns across individual colonies with no choice. Saffari et al. (2010b) also found similar patterns of consumption in both choice and no choice feeding experiments. Rather than suggesting that the contents of feeds that are consumed less are less preferred, this may suggest that they are required in lower quantity.
In general, more feed of all types was consumed early in each study year, with consumption decreasing as the natural pollen flow increased (personal observation). Standifer et al. (1973) concluded that while supplemental protein feeding during a dearth can increase brood production, there is no benefit to protein feeding during a pollen flow. Similarly, other authors have found that supplementing colonies with pollen or protein substitutes is only (or more) beneficial during a dearth or inclement weather that prevents foraging (Sheesley and Poduska 1968, Stanger and Laidlaw 1974, Mattila and Otis 2006, DeGrandi-Hoffman et al. 2008), and consumption of supplements decreases when natural forage is available (e.g., Nabors 2000). DeGrandi-Hoffman et al. (2008) found that when the supplemental diet was the only available food, the diet treatments resulted in differences in brood production and adult population, but in a second trial when natural pollen forage was available, there was no difference in the ability of colonies fed different diets to rear brood. In their three-year study, Mattila and Otis (2006) found that in years of good forage availability, differences in colony productivity due to feeding were short-lived, whereas in a year with a cold spring, the feeding resulted in long term productivity benefits to the supplemented colonies. The lower consumption of Global 15% patties in 2018 versus subsequent years was therefore expected, as the apiary used in 2018 was considered by the beekeeper to be a good location to grow bee colonies in the spring, with ample natural forage, whereas the forage in the apiary used in subsequent years was considered poor. However, the difference between 2019 and 2020, within the same apiary, highlights the need for beekeepers to monitor the availability of forage both among apiaries as well as among years, as weather patterns affect resource availability as well as appropriate foraging weather.
Brodschneider and Crailsheim (2010) present a review of feed consumption and show that the consumption varies widely among studies, and that comparability among studies is low due to differences in design (e.g., caged bees versus colonies), colony size, environmental access to forage, and time of year. Large consumption can be an indicator of feed palatability; however, it could also indicate that a large volume of feed is required to meet the nutritional demands of the bees. For example, in 2018, colonies in our study consumed less of the FeedBee supplement than the other feed treatments. One could conclude that the patty was less palatable, however the FeedBee patty had the highest protein levels of the tested patty diets in 2018, and all the essential amino acids in the relative amounts recommended by DeGroot (1953). Colonies in the FeedBee treatment had equivalent brood production and adult bee populations to the other treatment groups, despite consuming less of the feed, so it could also be concluded that less feed was required to rear an equivalent amount of brood. However, it is critical to note that the experiments in 2018 were conducted in an apiary with good spring forage, and after the bloom started our treatments were a supplement to the naturally available forage. It is equally possible that the colonies were largely relying on external protein from floral protein to sustain brood rearing by the end of the feeding period, as opposed to the beginning of the experimental period when no natural forage was available. While many studies have investigated worker preferences and effects of diet by measuring consumption, and acceptability is an essential feature of artificial diets, it is critical that beekeepers look beyond measures of consumption as indicators of the value of a feed product and additionally evaluate measures of feed efficiency, population increase, and bee health.
In 2019, the colonies fed Global 0% patties consumed significantly more feed than those fed Global 15% patties. In 2020, however, the opposite trend occurred and more of the Global 15% feed was consumed than all other feed types, in agreement with previous studies demonstrating greater consumption of supplements containing pollen (Lamontagne-Drolet et al. 2019). Pernal and Currie (2000) argue that consumption may be unrelated to protein content as they also found that pollen was consumed more readily than a pollen substitute (Bee-Pro) with a similarly high protein content. Other non-protein components of bee feed such as sugars, lipids, vitamins, and minerals have received far less study and may play an important phagostimulatory role in addition to their role in bee nutrition (Manning 2001, Wright et al. 2018). For example, the importance of 24-methylenecholesterol in honey bee survival and diet consumption has recently been demonstrated (Chakrabarti et al. 2020); however, this and other critical micronutrients have not been deliberately incorporated into bee feed (Bonoan et al. 2018).
While the fatty acid component of pollen varies, the three most common fatty acids in pollen are palmitic, linoleic (omega-6), and alpha-linolenic (omega-3) acids, with linoleic and alpha-linolenic acid being especially nutritionally critical (Wright et al. 2018). The addition of flax seed oil to the FeedBee recipe increases the lipid content of that treatment, however the ideal lipid content for bee feed remains uncertain. Herbert et al. (1980) demonstrated the importance of dietary lipids to brood production, as colonies fed diets supplemented with 2–4% lipids reared as much brood as colonies fed pollen, and more brood than colonies fed diets without pollen. Our feed treatments contained 1%–3.6% lipids, with the FeedBee patty (as formulated) containing the highest amount of lipids. More recent work has demonstrated the importance of lipids to digestion and longevity (Ma et al. 2015), as well as learning and glandular development (Arien et al. 2015). As bumble bees have been shown to overeat protein when fed low-fat diets to reach their ideal protein to lipid ratio (Vaudo et al. 2016), further research into the lipid component of honey bee diets, and the role it plays in consumption rates, is warranted.
Similarly, the importance of the amount and composition of the sugar component of protein feed has also received inadequate attention. Early studies demonstrated that a mixture of glucose and fructose increased the attractiveness of pollen to bees, and more bees chose to feed on a supplement containing sucrose in addition to glucose and fructose than glucose and fructose alone (Doull 1974). Honey bees prefer single sugar solutions of sucrose solutions to solutions of other single sugars such as glucose or fructose, but a mix of the three sugars is highly attractive (Wykes 1952, Waller 1972). In our study, we found that protein supplements containing a mix of sugars were consumed more than those containing only sucrose (Global 15% and BeePollen-Ate 25% both contained multiple sugars versus only sucrose in FeedBee in 2018). In one year (2019) we found that feeds with a mix of three sugars (fructose, glucose, and sucrose in the Global 0% patty) were consumed more than those with a mix of only two sugars (fructose and glucose in the Global 15% and BeePollen-Ate 20% patties), however the opposite was observed in 2020 (when Global 15% patties [containing only glucose and fructose]) were consumed more than the other patty types containing all three sugars (Global 0%, BeePollen-Ate 15%, and Healthy Bees). Sucrose syrup was available for bees to forage on in open feeding barrels in all three years, and it is possible this affected their attraction to the sugars in the protein patty feeds. It seems likely that in some cases invert syrup (containing glucose and fructose) or partially hydrolysed syrup (containing sucrose, glucose, and fructose) was used to make the patties; this may be to enhance solubility of other ingredients during production or to assist in retaining a palatable moist texture.
The optimal protein level in feed for brood production is approximately 23% (Herbert et al. 1977), with moderately higher or lower protein levels than this ideal (30% or 10%) resulting in less brood than optimal levels. Commercial diets are often lower in total protein content than optimal (Chakrabarti et al. 2020), and the patties tested in this study ranged from 14.02% to 21.28% protein (as fed). All the protein patties contained all ten amino acids essential to brood rearing, however they were not always in the ideal relative quantities. It is common for the amounts of these amino acids to vary among sources of both natural pollen and bee feed (e.g., DeGrandi-Hoffman et al. 2016). Arginine and lysine were the most commonly deficient in the feeds tested in the present study, and commercial feeds may benefit from supplementation with these amino acids. Haydak 1970 suggested that these two amino acids were of particular value, and could be used to enhance the nutritive value of stored pollen (Dietz and Haydak 1965 as cited in Haydak 1970). In 2020, the three patty products made by Global Inc. had high tryptophan levels, resulting in relatively low values of the other nine essential amino acids relative to tryptophan. Inconsistencies in the nutritional value of feed products among years would reduce the reliability of the effects of these feed products, further complicating both experimental evaluations and the management decisions facing beekeepers.
Our results clearly demonstrate a potential benefit to supplemental protein feeding on brood production and prepollination adult bee population. Many other studies have demonstrated a positive effect of protein supplementation on brood production in honey bee colonies (Sheesley and Poduska 1968, Standifer et al. 1973, Stanger and Laidlaw 1974, Nabors 2000, Mattila and Otis 2006, DeGrandi-Hoffman et al. 2008); however, there are critical seasonal time points and specific circumstances under which the cost of feed and labour associated with supplemental feeding are warranted. As supplemental protein feed is not stored in the colony, the timing of feeding should coincide with colony need for a protein source (Noordyke et al. 2021).
The current study found: 1) a clear benefit to supplementary spring protein feeding to increase honey bee colony populations in advance of a summer pollination market when natural pollen availability was limited, 2) that differences in consumption among feed treatments were more evident early in the season and diminished as consumption decreased as natural pollen forage became available, 3) that more feed was consumed in a ‘poor’ spring apiary with little natural forage (2019 and 2020) than the in an apiary with abundant spring pollen (2018), which resulted in a greater apparent feed efficiency in the apiary with abundant natural pollen, and 4) our results suggest that when there is abundant natural pollen, there may be little difference in the productivity resulting from different feed treatments, whereas in conditions with less natural forage increasing differences among feed treatments become evident. Such a difference was observed in 2020, when there was reduced growth in colonies fed Healthy Bees compared to the other feed types. We caution against the use of consumption alone as an indicator of feed quality, as differences in consumption do not always translate into differences in productivity, and feed efficiency and population increases must also be considered by beekeepers that must pay for feed and labour. However, feed efficiency may be inflated in areas with ample natural forage, as the colonies will be able to utilise pollen in addition to the supplemental feed. To be economical, effects of feeding on brood production and adult population must persist and translate into economic gains. This study also serves to highlight the differences among apiaries and years in feed consumption and the benefit of supplemental protein feeding.
Supplementary Material
Acknowledgments
The authors extend their sincere appreciation to Poelman Apiaries Ltd. for their cooperation with the project, input, and advice, and to Rhonda Thygesen and Christian Sprinkhuysen for assistance in the field. The authors also thank Global Patties Inc. for custom patties, discussion and input, Healthy Bees LLC for donation of protein patties, and AllTech Inc for cooperation and discussion. We also extend our gratitude to Dr. John McLean for stimulating discussions about fats and feeding, and especially for his Feed-Bee patty recipe. Dr. McLean, Kayla Price, and two anonymous reviewers provided valuable comments that improved the manuscript. This research was financially supported by the Alberta Ministry of Agriculture, Forestry, and Rural Economic Development.
References Cited
- Amro, A., Omar M., and Al-Ghamdi A... 2016. Influence of different proteinaceous diets on consumption, brood rearing, and honey bee quality parameters under isolation conditions. Turkish J. Vet. Anim. Sci. 40: 468–475. [Google Scholar]
- Arien, Y., Dag A., Zarchin S., Masci T., and Shafir S... 2015. Omega-3 deficiency impairs honey bee learning. Proc. Natl. Acad. Sci. U. S. A. 112: 15761–15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonoan, R. E., O’Connor L. D., and Starks P. T... 2018. Seasonality of honey bee (Apis mellifera) micronutrient supplementation and environmental limitation. J. Insect Physiol. 107: 23–28. [DOI] [PubMed] [Google Scholar]
- Brodschneider, R., and Crailsheim K... 2010. Nutrition and health in honey bees. Apidologie. 41: 278–294. [Google Scholar]
- Chakrabarti, P., Lucas H. M., and Sagili R. R... 2020. Evaluating effects of a critical micronutrient (24-methylenecholesterol) on honey bee physiology. Ann. Entomol. Soc. Am. 113: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot, A. P. 1953. Protein and amino acid requirements of the honey bee (Apis mellifera L.). Physiol. Comp. Oecol. 3: 197–285. [Google Scholar]
- DeGrandi-Hoffman, G., Wardell G., Ahumada-Segura F., Rinderer T., Danka R., and Pettis J... 2008. Comparisons of pollen substitute diets for honey bees: consumption rates by colonies and effects on brood and adult populations. J. Apic. Res. 47: 265–270. [Google Scholar]
- DeGrandi-Hoffman, G., Chen Y., Huang E., and Huang M. H... 2010. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Physiol. 56: 1184–1191. [DOI] [PubMed] [Google Scholar]
- DeGrandi-Hoffman, G., Chen Y., Rivera R., Carroll M., Chambers M., Hidalgy G., and de Jong E. W... 2016. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie. 47.2: 186–196. [Google Scholar]
- Dietz, A. H., and Haydak M... 1965. Causes of nutrient deficiency in stored pollen for development of newly emerged honey bees, pp. 222–225. InProc. Intern. Jubilee Beekeeping Congr., 20th, Bucharest. As cited in Haydak 1970. [Google Scholar]
- Doull, K. M. 1974. Effects of attractants and phagostimulants in pollen and pollen supplement on the feeding behaviour of honeybees in the hive. J. Apic. Res. 13: 47–54. [Google Scholar]
- Doull, K. M. 1980. Relationships between consumption of a pollen supplement, honey production and brood rearing in colonies of honeybees Apis mellifera L. II. Apidologie. 11: 367–374. [Google Scholar]
- Farrar, C. L. 1993. Productive management of honey bee colonies part IV. Am. Bee J. April: 261–263. [Google Scholar]
- Gochnauer, T. A., and Corner J... 1974. Detection and identification of Bacillus larvae in a commercial sample of bee-collected pollen. J. Apicult. Res. 13: 265–267. [Google Scholar]
- Haydak, M. H. 1970. Honey bee nutrition. Ann. Rev. Entomol. 15: 143–156. [Google Scholar]
- Hendriksma, H. P., and Shafir S... 2016. Honey bee foragers balance colony nutritional deficiencies. Behav. Ecol. Sociobiol. 70.4: 509–517. [Google Scholar]
- Herbert, E. W.Jr, Shimanuki H., and Caron D... 1977. Optimum protein levels required by honey bees (Hymenoptera, Apidae) to initiate and maintain brood rearing. Apidologie. 8: 141–146. [Google Scholar]
- Herbert, E. W.Jr, Shimanuki H., and Shasha B. S... 1980. Brood rearing and food consumption by honeybee colonies fed pollen substitutes supplemented with starch-encapsulated pollen extracts. J. Apic. Res. 19: 115–118. [Google Scholar]
- JMP® . 2019. Version 15Pro. SAS Institute Inc., Cary, NC. www.jmp.com [Google Scholar]
- Knox, D. A., Shimanuki H., and Herbert E. W... 1971. Diet and the longevity of adult honey bees. J. Econ. Entomol. 64: 1415–1416. [Google Scholar]
- Lamontagne-Drolet, M., Samson-Robert O., Giovenazzo P., and Fournier V... 2019. The impacts of two protein supplements on commercial honey bee (Apis mellifera L.) colonies. J. Apic. Res. 58: 800–813. [Google Scholar]
- Ma, L., Wang Y., Hang X., Wang H., Yang W., and Xu B... 2015. Nutritional effect of alpha-linolenic acid on honey bee colony development (Apis mellifera L.). J. Apic. Sci. 59.2: 63–72. [Google Scholar]
- Manning, R. 2001. Fatty acids in pollen: a review of their importance for honey bees. Bee World. 82: 60–75. [Google Scholar]
- Mattila, H. R., and Otis G. W... 2006. Influence of pollen diet in spring on development of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 99: 604–613. [DOI] [PubMed] [Google Scholar]
- Maurizio, A. 1954. Pollenernährung und Lebensvorgänge bei der Honigbiene (Apis mellifica L.), Landwirtsch. Jahrb. Schweiz. 62: 115–182. [Google Scholar]
- Mullin, C. A., Frazier M., Frazier J. L., Ashcraft S., Simonds R., Vanengelsdorp D., and Pettis J. S... 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One. 5: e9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors, R. 2000. The effects of spring feeding pollen substitute to colonies of Apis mellifera. Am. Bee J. 140: 322–323. [Google Scholar]
- Noordyke E. R., van Santen E., and Ellis J. D... 2021. Tracing the fate of pollen substitute patties in Western honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 114: 1421–1430. [DOI] [PubMed] [Google Scholar]
- Oskay, D. 2021. Effects of diet composition on consumption, live body weight and life span of worker honey bees (Apis mellifera L.). Appl. Ecol. Environ. Res. 19: 4421–4430. [Google Scholar]
- Ostiguy, N., Drummond F. A., Aronstein K., Eitzer B., Ellis J. D., Spivak M., and Sheppard W. S... 2019. Honey bee exposure to pesticides: a four-year nationwide study. Insects. 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paray, B. A., Kumari I., Hajam Y. A., Sharma B., Kumar R., Albeshr M. F., Farah M. A., and Khan J. M... 2021. Honeybee nutrition and pollen substitutes: a review. Saudi J. Biol. Sci. 28: 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernal, S. F., and Currie R. W... 2000. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie. 31: 387–409. [Google Scholar]
- Pirk, C. W., Boodhoo C., Human H., and Nicolson S. W... 2010. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie. 41: 62–72. [Google Scholar]
- Saffari, A., Kevan P. G., and Atkinson J. L... 2010a. Palatability and consumption of patty-formulated pollen and pollen substitutes and their effects on honeybee colony performance. J. Apic. Sci. 54: 63–71. [Google Scholar]
- Saffari, A., Kevan P. G., and Atkinson J. L... 2010b. Consumption of three dry pollen substitutes in commercial apiaries. J. Apic. Sci. 54: 5–12. [Google Scholar]
- Schittny, D., Yañez O., and Neumann P... 2020. Honey bee virus transmission via hive products. Vet. Sci. 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, J. O., Thoenes S. C., and Levin M. D... 1987. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. 80: 176–183. [Google Scholar]
- Sheesley, B. and Poduska B... 1968. Supplemental feeding of honey bees: colony strength and pollination results. Am. Bee J. 108: 357–359. [Google Scholar]
- de Sousa Pereira, K., Meeus I., and Smagghe G... 2019. Honey bee-collected pollen is a potential source of Ascosphaera apis infection in managed bumble bees. Sci. Rep. 1: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standifer, L. N. 1967. A comparison of the protein quality of pollens for growth-stimulation of the hypopharyngeal glands and longevity of honey bees, Apis mellifera L. (Hymenoptera: Apidae). Insectes Sociaux. 14: 415–425. [Google Scholar]
- Standifer, L. N., Haydak M. H., Mills J. P., and Levin M. D... 1973. Influence of pollen in artificial diets on food consumption and brood production in honey bee colonies. Am. Bee J. 113: 94–95. [Google Scholar]
- Stanger, W., and Laidlaw H. H... 1974. Supplemental feeding of honeybees (Apis mellifera Linnaeus). Am. Bee J. 114: 138–141. [Google Scholar]
- Vaudo, A. D., Stabler D., Patch H. M., Tooker J. F., Grozinger C. M., and Wright G. A... 2016. Bumble bees regulate their intake of essential protein and lipid pollen macronutrients. J. Exp. Biol. 219: 3962–3970. [DOI] [PubMed] [Google Scholar]
- Waller, G. D. 1972. Evaluating responses of honey bees to sugar solutions using an artificial-flower feeder. Ann. Entomol. Soc. Am. 65.4: 857–862. [Google Scholar]
- Wright, G. A., Nicolson S. W., and Shafir S... 2018. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 63: 327–344. [DOI] [PubMed] [Google Scholar]
- Wykes, G. R. 1952. The preferences of honeybees for solutions of various sugars which occur in nectar. J. Exp. Biol. 29.4: 511–519. [Google Scholar]
- Zarchin, S., Dag A., Salomon M., Hendriksma H. P., and Shafir S... 2017. Honey bees dance faster for pollen that complements colony essential fatty acid deficiency. Behav. Ecol. Sociobiol. 71: 172. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.