Abstract
Klebsiella pneumoniae K6 (ATCC 700603), a clinical isolate, is resistant to ceftazidime and other oxyimino-β-lactams. A consistent reduction in the MICs of oxyimino-β-lactams by at least 3 twofold dilutions in the presence of clavulanic acid confirmed the utility of K. pneumoniae K6 as a quality control strain for extended-spectrum β-lactamase (ESBL) detection. Isoelectric-focusing analysis of crude lysates of K6 demonstrated a single β-lactamase with a pI of 7.8 and a substrate profile showing preferential hydrolysis of cefotaxime compared to ceftazidime. PCR analysis of total bacterial DNA from K6 identified the presence of a blaSHV gene. K6 contained two large plasmids with molecular sizes of approximately 160 and 80 kb. Hybridization of plasmid DNA with a blaSHV-specific probe indicated that a blaSHV gene was encoded on the 80-kb plasmid, which was shown to transfer resistance to ceftazidime in conjugal mating experiments with Escherichia coli HB101. DNA sequencing of this blaSHV-related gene revealed that it differs from blaSHV-1 at nine nucleotides, five of which resulted in amino acid substitutions: Ile to Phe at position 8, Arg to Ser at position 43, Gly to Ala at position 238, and Glu to Lys at position 240. In addition to the production of this novel ESBL, designated SHV-18, analysis of the outer membrane proteins of K6 revealed the loss of the OmpK35 and OmpK37 porins.
Extended-spectrum β-lactamases (ESBLs) are enzymes that can hydrolyze oxyimino-β-lactams (e.g., cefotaxime, ceftazidime, and ceftriaxone) and the monobactam aztreonam, resulting in resistance to these drugs (10, 17). ESBLs, predominantly derivatives of plasmid-mediated TEM or SHV β-lactamases (10, 17), arise through mutations that result in one or more amino acid substitutions that alter the configuration or binding properties of the active site, thereby expanding the hydrolytic spectrum of the enzyme (17, 22, 30). Though these enzymes are most commonly detected in Klebsiella pneumoniae and Escherichia coli (17, 24), they have been found in other members of the family Enterobacteriaceae (11, 38, 40). Clinical isolates that produce ESBLs are frequently associated with nosocomial outbreaks (37, 46).
ESBL-producing Enterobacteriaceae, which are being identified worldwide (49, 54), are probably more prevalent than currently recognized because they are often undetected by routine susceptibility testing methods (18, 21). K. pneumoniae K6 (ATCC 700603) was selected by the National Committee for Clinical Laboratory Standards (NCCLS) as an ESBL quality control (QC) strain for confirmation tests that clinical laboratories can use to improve detection of ESBLs in K. pneumoniae, Klebsiella oxytoca, and E. coli (33). Here we report the molecular characterization of K. pneumoniae K6, which produces the novel β-lactamase SHV-18.
MATERIALS AND METHODS
Bacterial strains.
K. pneumoniae K6 (ATCC 700603) is a clinical isolate that was obtained from a patient at the Medical College of Virginia (Richmond, Va.) in 1994. E. coli HB101 [F− supE44 lacY1 ara-14 galK2 xyl-5 mtl-1 leuB6 Δ(mcrC-mrr) recA13 rpsL20 thi-1 Δ(gpt-proA)62 hsdSB20 λ−] (6) was used as a recipient in conjugal mating experiments. E. coli C600(pFCT3103) (52), which produces aminoglycoside-2"-O-nucleotidyltransferase [ANT(2")], was used as a positive control in the PCR detection of the aadB gene. K. pneumoniae ATCC 13883, which expresses OmpK35 and OmpK36 porins, was used for comparison in the isolation and analysis of the outer membrane proteins (OMPs).
Determination of susceptibility of K. pneumoniae K6 to selected antimicrobial agents.
MICs were determined by broth microdilution with cation-adjusted Mueller-Hinton broth (Difco Laboratories) using NCCLS methods (32). E. coli ATCC 25922, E. coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 29213, and Enterococcus faecalis ATCC 29212 were used for quality control.
Isolation and analysis of OMPs.
Strains were grown in Luria-Bertani (LB) broth (47) or in nutrient broth (low-osmolarity growth medium [51 mosmol kg−1]) (14). OmpK35 expression is enhanced in low-osmolarity medium (14).
Bacterial cell envelopes containing cytoplasmic and outer membranes were obtained by cell lysis and centrifugation. OMPs were isolated as sodium lauryl sarcosinate-insoluble material. Electrophoretic analysis of OMPs was performed in 11% acrylamide–0.2% bisacrylamide–0.1% sodium dodecyl sulfate gels. Samples were boiled for 5 min in Laemmli's sample buffer before electrophoresis. Gels were visualized by staining with Coomassie blue.
OmpK35, OmpK36, and OmpK37 porin expression was also analyzed by Western blotting (12, 14). For this purpose, sodium dodecyl sulfate gels were transferred to Immobilon P filters (Millipore Corporation, Bedford, Mass.), essentially using the buffers and conditions described by Towbin et al. (51). Filters were blocked in 1% bovine serum albumin in phosphate-buffered saline (PBS). After washing, the filters were incubated with anti-OmpK37 (12), anti-OmpK36, or anti-OmpK35 (14) diluted 1:100, 1:1,000, and 1:5,000, respectively, and then with alkaline phosphatase-labeled goat anti-rabbit immunoglobulin G (1:5,000) (Sigma, St. Louis, Mo.). The filters were developed as previously described (12). All the incubations were carried out at room temperature for 1 h in 1% bovine serum albumin–0.05% Tween 20–PBS, and after incubations with the antiserum, washing steps with 0.05% Tween 20–PBS were performed.
IEF and β-lactamase assays.
Crude preparations of β-lactamases were obtained by subjecting cells to a freeze-thaw procedure (9). Isoelectric focusing (IEF) was performed by the method of Matthew et al. (29). Crude extracts were spotted onto commercially prepared polyacrylamide gel plates (pH 3.5 to 9.5; Pharmacia LKB, Piscataway, N.J.) and electrophoresed using an LKB Multiphor II apparatus (Pharmacia LKB). Enzymes were visualized by staining with a 0.05% (500 μg/ml) solution of nitrocefin (Becton Dickinson Microbiology Systems, Cockeysville, Md.) following IEF. The isoelectric point (pI) of SHV-18 was estimated by comparison with SHV-3 (pI 7.0), SHV-2 (pI 7.6), SHV-4 (pI 7.8), SHV-5 (pI 8.2), and MIR-1 (pI 8.4) β-lactamases.
The following β-lactams were obtained for hydrolysis assays from the sources indicated parenthetically: cephaloridine, penicillin G, and cefotaxime from Sigma Chemical Co.; ceftazidime and clavulanic acid from U.S. Pharmacopeia (Rockville, Md.); aztreonam from ICN Biomedicals, Inc. (Aurora, Oh.); and tazobactam from Lederle (Pearl River, N.Y.). All substrates were prepared daily as 1-mg/ml stocks in 50 mM phosphate buffer, pH 7.0.
For kinetic analysis, the SHV-18 β-lactamase was purified from the E. coli HB101 transconjugant, TC-K6/1, which was shown by IEF to contain a single enzyme of pI 7.8. Cultures for purification of SHV-18 were grown overnight at 37°C in 3 liters of trypticase soy broth supplemented with 1 μg of ceftazidime per ml. Bacteria were harvested by centrifugation and washed with 50 mM phosphate buffer, pH 7.0. The pellet was resuspended in 5 ml of 0.2 M sodium acetate, pH 5.5, and subjected to five freeze-thaw cycles (9). β-Lactamase activity was enriched by chromatography through Sephacryl S-100 in 50 mM phosphate buffer, pH 7.0. Protein in peak fractions containing nitrocefin-hydrolyzing activity was precipitated with 90% ammonium sulfate; pellets were resuspended in 50 mM phosphate buffer, pH 7.0, and dialyzed in four 1-liter aliquots of the same buffer at 4°C over 15 h. The protein concentration of the partially purified SHV-18 β-lactamase was determined with the BCA protein assay (Pierce, Rockford, Ill.) to be 0.12 mg/ml. IEF analysis of the partially purified enzyme confirmed the presence of only one β-lactamase of pI 7.8.
For kinetic studies, initial hydrolysis rates were measured on a Shimadzu UV-1601 spectrophotometer at 25°C in 50 mM phosphate buffer, pH 7.0. Km and Vmax values were obtained by averaging results from Lineweaver-Burk, Eadie-Hofstee, Hanes-Woolf, and direct linear plot analyses. Substrates were assayed on two separate days. Aztreonam was hydrolyzed too slowly to reliably determine Km and Vmax values. Vmax for aztreonam was estimated as two times the highest hydrolysis rate obtained. Inhibition of hydrolysis was measured after a 5-min preincubation of 2.5 μl of enzyme with inhibitor in 20 μl of phosphate buffer, pH 7.0, at 25°C. Cephaloridine (24 μM) was prewarmed to 25°C and used as the substrate for the inhibition studies in a total volume of 1.0 ml. Fifty percent inhibitory concentrations were determined from inhibition graphs of percent control activity versus concentration of inhibitor.
Isolation and hybridization analysis of plasmid DNA.
Plasmid DNA was isolated from K. pneumoniae K6, its E. coli HB101 transconjugant (TC-K6/1), and three unrelated E. coli strains harboring plasmids pDK9 (165 kb), R1 (97.6 kb), and V517 (56.4, 7.6, 5.8, 5.3, 4.1, 3.2, 2.8, and 2.2 kb) (26) employing the method of Portnoy et al. (43). Plasmids were separated in a 0.85% agarose gel prepared with 0.5× TBE buffer (1× TBE buffer contains 100 mM Tris, 90 mM boric acid, and 1 mM EDTA [pH 8.4]) and electrophoresed at 90 V for 15 h at 4°C.
For DNA sequencing, plasmid DNA was isolated using a QIAGEN Plasmid Midi kit (QIAGEN, Chatsworth, Calif.) essentially according to the instructions provided by the vendor. However, in order to enhance the yield of large, low-copy plasmids, DNA was eluted from QIAGEN-tip 100 columns using five 1-ml aliquots of elution buffer that had been prewarmed to 65°C.
DNA was transferred from agarose gels (50) to positively charged nylon membranes (Zeta-Probe; Bio-Rad Laboratories, Hercules, Calif.) and fixed by baking for 2 h at 80°C. The DNA on the filters was hybridized with a 275-bp digoxigenin-labeled blaSHV DNA probe whose synthesis was described earlier (45). Hybridization, using the Genius nonradioactive nucleic acid labeling and detection system (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), was performed at 65°C overnight (5, 45).
Transfer of resistance.
Conjugal transfer of extended-spectrum β-lactam resistance was performed utilizing a filter mating method using K. pneumoniae K6 as the donor and streptomycin-resistant E. coli HB101 as the recipient. Aliquots of exponentially growing cultures of donor and recipient were mixed (10:1 ratio), placed on a 0.20-μm-pore-size sterile filter, and allowed to mate on LB agar at 37°C for 18 h. The filter was vortexed in saline and transconjugants were selected on LB agar containing 100 μg of streptomycin and 1.5 μg of ceftazidime per ml.
Amplification and DNA sequence analysis.
The presence of the genes encoding OmpK35, OmpK36, and OmpK37 porins was determined by using oligonucleotides and PCR conditions described by Doménech-Sánchez et al. (12).
The aadB gene encoding the ANT(2") was detected using previously described oligonucleotide primers (52). Amplification conditions were essentially as described, except that the annealing temperature was raised to 62°C.
For detection of blaSHV, an 867-bp gene fragment was amplified using forward (5′-GGTTATGCGTTATATTCGCC-3′) and reverse (5′-TTAGCGTTGCCAGTGCTC-3′) oligonucleotide primers whose first 5′ bases correspond to positions 121 and 988, respectively, within the coding sequence of SHV-1 (31, 45).
Amplification of the 867-bp blaSHV fragment was performed in a 100-μl reaction mixture containing 1 μl of crude cellular lysate, 10 pmol of each primer, a 200 μM concentration of each deoxynucleoside triphosphate, 1× reaction buffer containing 1.5 mM MgCl2 (Perkin-Elmer, Applied Biosystems Division [PE-ABI], Foster City, Calif.), and 2.5 U of native Taq polymerase (PE-ABI) using a GeneAmp PCR system 9600 thermal cycler (PE-ABI). Cycling parameters included a 5-min initial denaturation at 96°C followed by 35 cycles of denaturation (96°C for 1 min), annealing (60°C for 1 min), and extension (72°C for 1 min), ending in a final extension period of 72°C for 10 min. A 275-bp digoxigenin-labeled blaSHV probe was prepared under the same conditions, but an alternate deoxynucleoside triphosphate mix containing substituted nucleosides was used (45).
The 275-bp PCR product amplified from blaSHV-1 (45) and a 351-bp fragment amplified from blaTEM-1 were used as controls in hybridization experiments. The blaTEM-1 fragment was generated with forward (5′-ATGAGTATTCAACATTTCCG-3′) (45) and reverse (5′-TTACTGTCATGCCATCC-3′) (25) oligonucleotide primers using cycling parameters that were similar to those used for blaSHV-1, except that the annealing temperature was 55°C.
The nucleotide sequence of the blaSHV-18 gene was initially determined from an 80-kb plasmid purified from the E. coli HB101 transconjugant to which ceftazidime resistance had been transferred. The DNA sequence of most of the blaSHV-18 gene, including its upstream regulatory region, was determined for both strands using previously described oligonucleotide primers (45). To confirm and complete the sequence of both strands of the entire gene, forward (5′-AGAATAGCGCTGAGGTCTG-3′) and reverse (5′-AGCGCGAGAAGCATCCTG-3′) oligonucleotide primers, identified outside of the blaSHV-18 coding region, were used to generate a 1,369-bp PCR product from K. pneumoniae K6 and its transconjugant. Amplification conditions were as described above but with an annealing temperature of 63°C. Direct sequencing of these PCR products followed purification on QIAquick spin columns (QIAGEN).
Cycle sequencing reactions were performed in a GeneAmp PCR system 9600 thermal cycler with the ABI Prism dRhodamine terminator cycle sequencing ready reaction kit according to instructions provided by the vendor (PE-ABI). Products from sequencing reactions were purified on Centri-Sep spin columns (Princeton Separations, Adelphia, N.J.) before analysis on an ABI Prism 377 DNA sequencer (PE-ABI).
In order to eliminate errors that may have been introduced during amplification, the DNA sequences of leading and lagging strands were determined for independent PCR products. DNA sequencing data were analyzed using DNASIS for Windows (Hitachi Software Genetic Systems, San Francisco, Calif.).
Nucleotide sequence accession number.
The nucleotide sequence of blaSHV-18 reported in this study will appear under the GenBank accession number AF132290.
RESULTS AND DISCUSSION
Antimicrobial susceptibility patterns.
Using NCCLS interpretive criteria (32), K. pneumoniae K6 was resistant to ampicillin, aztreonam, cefoxitin, cefpodoxime, ceftazidime, chloramphenicol, piperacillin, and tetracycline; intermediate to ceftriaxone and gentamicin; and susceptible to amoxicillin-clavulanate, cefepime, cefotaxime, ciprofloxacin, imipenem, piperacillin-tazobactam, tobramycin, and trimethoprim-sulfamethoxazole (Table 1).
TABLE 1.
MICs of selected antimicrobial agents for various strains
| Antimicrobial agent | MIC (μg/ml) for:
|
||
|---|---|---|---|
| K. pneumoniae K6 | E. coli HB101 | E. coli HB101 TC-K6/1 | |
| Ampicillin | >64 | 4 | >64 |
| Amoxicillin-clavulanatea | 8 | 4 | 4 |
| Aztreonam | 64 | 0.12 | 8 |
| Aztreonam-clavulanate | ≤0.25 | 0.12 | ≤0.25 |
| Cefotaxime | 8 | 0.06 | 1 |
| Cefotaxime-clavulanate | ≤0.25 | 0.06 | ≤0.25 |
| Cefpodoxime | 16 | 0.5 | 8 |
| Cefpodoxime-clavulanate | 1 | 0.5 | ≤0.25 |
| Ceftazidime | 32 | 0.25 | 8 |
| Ceftazidime-clavulanate | 1 | 0.25 | ≤0.25 |
| Ceftriaxone | 16 | ≤0.06 | 4 |
| Ceftriaxone-clavulanate | ≤0.25 | ≤0.06 | ≤0.25 |
| Cefepime | 1 | ≤0.5 | ≤0.5 |
| Cefoxitin | 32 | 8 | 4 |
| Chloramphenicol | >32 | 8 | 8 |
| Ciprofloxacin | 0.5 | ≤0.06 | ≤0.06 |
| Gentamicin | 8 | ≤0.25 | 2 |
| Imipenem | ≤1 | ≤1 | ≤1 |
| Piperacillin | >128 | ≤2 | 32 |
| Piperacillin-tazobactam | 16 | 2 | ≤1 |
| Tetracycline | 16 | 2 | ≤1 |
| Tobramycin | 4 | ≤0.25 | 2 |
| Trimethoprim-sulfamethoxazole | 2 | ≤0.12 | ≤0.12 |
Clavulanic acid was used at a final concentration of 4 μg/ml.
A reduction in the MICs of aztreonam, cefotaxime, cefpodoxime, ceftazidime, and ceftriaxone by 3 two-fold dilutions or more in the presence of clavulanic acid was indicative of ESBL production by K. pneumoniae K6 (Table 1).
β-Lactamase characterization.
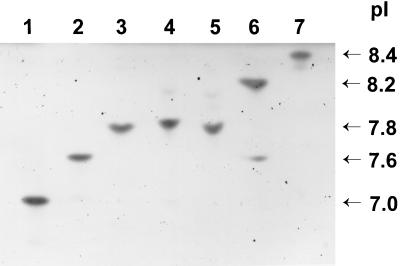
IEF of crude lysates of K. pneumoniae K6 (Fig. 1, lane 3) and the E. coli HB101 transconjugant TC-K6/1 (Fig. 1, lane 4) revealed a single β-lactamase in each with a pI of 7.8.
FIG. 1.
IEF patterns of β-lactamases produced by K. pneumoniae K6, its E. coli transconjugant, and controls. Lane 1, SHV-3 (pI 7.0); lane 2, SHV-2 (pI 7.6); lane 3, K. pneumoniae K6; lane 4, TC-K6/1 (E. coli HB101 transconjugant); lane 5, SHV-4 (pI 7.8); lane 6, SHV-5 (pI 8.2); lane 7, MIR-1 (pI 8.4).
The kinetic parameters for purified SHV-18 are summarized in Table 2. The highest Vmax was obtained for cephaloridine, which was hydrolyzed two times faster than penicillin. The rate of hydrolysis of penicillin was approximately four times the rate for cefotaxime. For the two extended-spectrum cephalosporins tested, the Vmax for cefotaxime was approximately two times greater than that for ceftazidime. The lowest hydrolysis rate of the five substrates tested was for aztreonam.
TABLE 2.
Kinetic properties of purified SHV-18 β-lactamase
| Substrate | Mean Vmaxa ± SD | Relative Vmax (%) | Mean Km ± SD (μM) | Relative Vmax/Km |
|---|---|---|---|---|
| Penicillin G | 27 ± 1.5 | 100 | 3.15 ± 1.15 | 100 |
| Cephaloridine | 54 ± 0.4 | 200 | 12 ± 0.7 | 53 |
| Ceftazidime | 3.7 ± 0.08 | 13.5 | 28.1 ± 4.1 | 1.5 |
| Cefotaxime | 7.3 ± 0.05 | 26.9 | 3.45 ± 0.02 | 24 |
| Aztreonamb | <1 | <1 | NDc | ND |
Nanomoles of substrate hydrolyzed per minute per milligram of protein.
Values based on estimated Vmax.
ND, not determined. The rate of hydrolysis of aztreonam was too slow to obtain an accurate Km value.
As shown in Table 3, the relative rates of hydrolysis obtained for SHV-18 were most consistent with the rates for the SHV-5 and SHV-7 β-lactamases. One minor difference in the hydrolytic profiles is that the relative rate of hydrolysis of cephaloridine compared to penicillin for SHV-18 was approximately twofold higher than that for SHV-7. SHV-18 differs from SHV-7 by a single amino acid substitution, a replacement of alanine for serine at position 238 (1, 8). The relative rates of hydrolysis of cephaloridine and cefotaxime for SHV-18 were very similar to the rates for SHV-13, which also contains an alanine substitution at position 238 (55).
TABLE 3.
Relative hydrolysis rates for purified SHV β-lactamases
The SHV-18 enzyme had high affinities for both penicillin and cefotaxime, based on the Kms of 3.2 and 3.5 μM, respectively (Table 2). Although aztreonam hydrolysis was observed, the rate of hydrolysis was too low to obtain an accurate Km under these assay conditions. Relative hydrolytic efficiencies, measured by Vmax/Km, revealed that penicillin was hydrolyzed approximately two times as efficiently as cephaloridine. The hydrolytic efficiency of penicillin was approximately 4 and 65 times faster than the values for cefotaxime and ceftazidime, respectively. Cefotaxime was hydrolyzed approximately 15 times as efficiently as ceftazidime.
Clavulanic acid was a fivefold-better inhibitor for SHV-18 than tazobactam, with 50% inhibitory concentrations of 4.7 and 23.9 nM, respectively (data not shown). As expected for serine-based β-lactamases, no inhibition was observed when the enzyme was preincubated with 10 mM EDTA at pH 7.0.
Characterization of OMPs.
While the genes encoding the porins (OmpK35, OmpK36, and OmpK37) were present in each of the strains tested as shown by PCR, not all of them were expressed. Two porins, corresponding to OmpK36 and OmpK35, were identified, via Western blots, in the reference strain K. pneumoniae ATCC 13883. However, K. pneumoniae K6, grown in LB broth or nutrient broth (low osmolarity), expressed only one porin which was confirmed by Western blot analysis to be OmpK36. K6 did not express the OmpK35 or OmpK37 porins (data not shown).
Plasmid profile, mating experiments, PCR analysis, and hybridization studies.
Plasmids encoding ESBLs are typically large (80 to 300 kb) and carry multiple resistance determinants (17, 19). K. pneumoniae K6 contained two plasmids with molecular sizes of approximately 160 and 80 kb (data not shown). When K. pneumoniae K6 was mated with E. coli HB101, transconjugants selected on streptomycin (100 μg/ml) and ceftazidime (1.5 μg/ml) were obtained. Plasmid analysis of transconjugants revealed that ceftazidime resistance transferred with the 80-kb plasmid. An 867-bp gene fragment was amplified from K. pneumoniae K6 and the transconjugant, TC-K6/1, using blaSHV-specific PCR primers, and the blaSHV gene in both was localized on the 80-kb plasmid by hybridization of plasmid DNA with a blaSHV-specific digoxigenin-labeled probe (data not shown). Resistance to cefoxitin, chloramphenicol, and tetracycline were not cotransferred with ceftazidime resistance, although aminoglycoside resistance, mediated by the aadB gene, was also transferred (Table 1). The aadB gene encoding ANT(2") was detected by PCR in both K6, which showed intermediate levels of resistance to gentamicin (Table 1), and the transconjugant, TC-K6/1 (data not shown).
DNA sequence and inferred amino acid analysis.
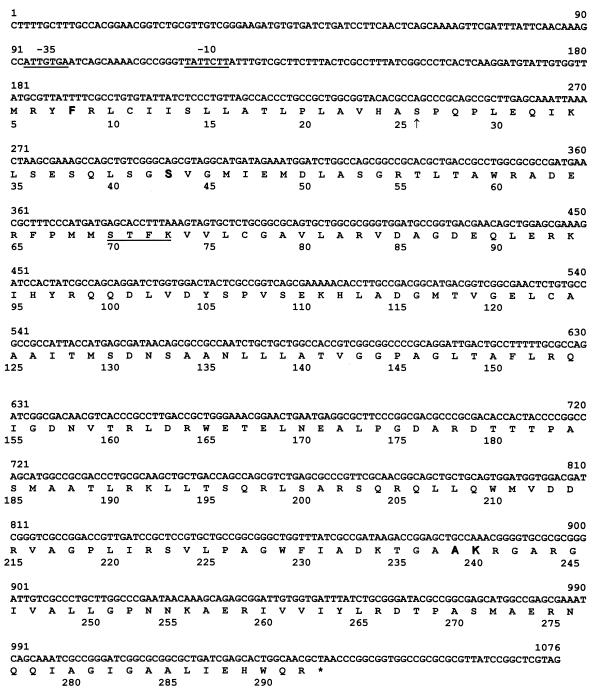
The nucleotide sequence of the gene encoding the novel ESBL was determined by using the 80-kb plasmid and a 1,369-bp PCR product, amplified from TC-K6/1, which encompassed both the structural gene and its upstream regulatory region. Both strands of the entire blaSHV-related gene were sequenced using a set of nested oligonucleotide primers (45). The nucleotide sequence and predicted amino acid sequence for this novel gene, designated blaSHV-18, are shown in Fig. 2.
FIG. 2.
Nucleotide sequence and predicted amino acid sequence of the SHV-18 β-lactamase gene. Amino acid numbering is according to the consensus numbering of Ambler et al. (1). Amino acids 238 and 240 are adjacent because of a deletion observed within the class A consensus sequence. An arrow indicates the start of the mature protein, as determined previously (2, 39). Underlined segments of the nucleotide sequence represent the putative −35 and −10 consensus sequences. The underlined region of the amino acid sequence indicates the active site Ser-X-X-Lys motif. Amino acids represented by letters in boldface type indicate significant changes from the amino acid sequence of SHV-1 (3).
The coding region of blaSHV-18 differed from that of blaSHV-1 (GenBank accession number AF148850) (7) at nine nucleotides, five of which resulted in four amino acid substitutions in the inferred protein: phenylalanine for isoleucine at position 8 in the leader peptide region, serine for arginine at position 43, alanine for glycine at position 238, and lysine for glutamate at position 240 (1) (Fig. 2). The four additional nucleotides that differed from blaSHV-1 (7) were silent point mutations, a C-to-T exchange at nucleotide 537, an A-to-G substitution at nucleotide 582, and C-to-G substitutions at nucleotides 939 and 966 of blaSHV-18 (Fig. 2).
Analysis of the nucleotide sequence of the upstream noncoding region of blaSHV-18 (Fig. 2) shows that it is nearly identical to blaSHV-7 (8) from position 180 through the −35 consensus sequence to position 77, with only a single difference at position 86, i.e., an A instead of a C in blaSHV-7. Except for the codon difference resulting in an alanine at position 238 of SHV-18, blaSHV-18 differs from blaSHV-7 within the coding region at only two nucleotides, silent G-for-C substitutions at positions 939 and 966 (Fig. 2). This high degree of identity between blaSHV-18 and blaSHV-7 in both noncoding and coding regions of the genes may suggest a common lineage.
A comparison of amino acid sequences at key positions for SHV-18 and related β-lactamases is shown in Table 4. Two of the four substitutions in the SHV-18 β-lactamase, phenylalanine at position 8 and serine at position 43, are shared only by SHV-7 (8) and OHIO-1 (48) but not by other SHV-type variants as shown in the Jacoby and Bush website (http://www.lahey.org/studies/webt.htm). The substitution of serine at position 43 is also present in a recently described SHV-type ESBL expressed in K. pneumoniae and Enterobacter cloacae isolates (P. L. Winokur, D. L. Desalvo, R. N. Jones, and M. A. Pfaller, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2045, 1999).
TABLE 4.
Comparative amino acid sequences of SHV-type β-lactamases at selected positions
| β-Lactamase | pI | Amino acid at positiona:
|
Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|
| 8 | 35 | 43 | 205 | 238 | 240 | |||
| SHV-1 | 7.6 | Ile | Leu | Arg | Arg | Gly | Glu | 3, 7 |
| SHV-2 | 7.6 | Ile | Leu | Arg | Arg | Serb | Glu | 2, 13, 15, 41 |
| SHV-2A | 7.6 | Ile | Gln | Arg | Arg | Ser | Glu | 42 |
| SHV-3 | 7.0 | Ile | Leu | Arg | Leu | Ser | Glu | 34 |
| SHV-4 | 7.8 | Ile | Leu | Arg | Leu | Ser | Lys | 39 |
| SHV-5 | 8.2 | Ile | Leu | Arg | Arg | Ser | Lys | 4 |
| SHV-7 | 7.6 | Phe | Leu | Ser | Arg | Ser | Lys | 8 |
| SHV-12 | 8.2 | Ile | Gln | Arg | Arg | Ser | Lys | 35 |
| SHV-13 | 7.6 | Ile | Gln | Arg | Arg | Ala | Glu | 55 |
| OHIO-1 | 7.0 | Phe | Leu | Ser | Arg | Gly | Glu | 48 |
| SHV-18 | 7.8 | Phe | Leu | Ser | Arg | Ala | Lys | This study |
Substitution of alanine for glycine at position 238 in SHV-18 and SHV-13 (55) β-lactamases is unique among both SHV- and TEM-related derivatives (http://www.lahey.org/studies/webt.htm). With the exception of these two variants, the only substitution at position 238 observed among natural isolates resistant to oxyimino-cephalosporins has been a serine-for-glycine change which has been correlated with resistance to cefotaxime (16, 23). It is now clear from the kinetic properties of SHV-18 and SHV-13 (55) (Table 3) that an alanine at position 238 can also confer cefotaxime-hydrolyzing activity.
Substitution of lysine for glutamate at position 240 of SHV-18, also seen in a number of other variants, including SHV-4 (39), SHV-5 (4), SHV-7 (8), and SHV-12 (35), is thought to have little effect on the hydrolysis of cefotaxime (16) but is necessary for resistance to ceftazidime and aztreonam (4, 16, 39). A high-level of resistance to ceftazidime, however, is achieved only by strains producing an SHV enzyme containing both serine at position 238 and lysine at position 240 (16). Substitutions at positions 238 and 240, which are adjacent residues, closely follow the highly conserved box VII triad (lysine [histidine]-threonine [serine]-glycine) described by Joris et al. (20) which is associated with cephalosporinase activity.
The influence of the modification at position 8 in the leader peptide on targeting the β-lactamase to the periplasm where it resides, or the significance of the substitution of serine for arginine at position 43, a location that very closely precedes the conserved box I motif described by Joris et al. (20), remains unclear (8).
The inability to cotransfer resistance to cefoxitin with ceftazidime resistance suggested that a combination of resistance mechanisms may be occurring simultaneously in K. pneumoniae K6. For example, an increase in MICs of cephamycins in other ESBL-producing strains of K. pneumoniae has been attributed to porin loss (27, 28, 36). Examination of the outer membrane components of K. pneumoniae K6 revealed a loss of expression of the OmpK35 porin, the putative homologue of the E. coli OmpF porin, which is consistent with an increase in the MIC of cefoxitin and increased resistance to extended-spectrum cephalosporins (14, 27, 28). Although OmpK36, the homologue of the E. coli OmpC porin (14), was expressed in K6, the OmpK37 porin (12) was not. OmpK37 is thought to be a narrower pore and is usually expressed under conditions which result in loss of the other two porins (i.e., antimicrobial pressure).
Reduced permeability of K. pneumoniae K6 due to porin loss may help explain why the MIC of ceftazidime is higher than that of cefotaxime (Table 1), yet the kinetic data show cefotaxime to be the preferred substrate of purified SHV-18 β-lactamase (Table 2). Studies of clinical isolates of K. pneumoniae with variable porin expression indicate that the MIC of cefotaxime reverts back to that of a porin-sufficient strain if either OmpK35 or OmpK36 is present (12, 27), suggesting that cefotaxime can diffuse efficiently through either porin. On the other hand, the MIC of ceftazidime in an OmpK35-deficient strain remains elevated even if expression of OmpK36 is restored, indicating that ceftazidime enters the cell through the OmpK35 porin (27). It has also been shown, in reconstituted proteoliposome studies, that the relative diffusion rate of ceftazidime through E. coli OmpF porin channels is considerably lower than that of cefotaxime, which could also account for the higher MIC of ceftazidime in the HB101 transconjugant (53).
The susceptibility and kinetic profiles of K. pneumoniae K6 and SHV-18 are consistent with those of K. pneumoniae 803 and the enzyme it produces, SHV-13, an ESBL which also displays cefotaxime-hydrolyzing activity despite containing an alanine rather than a serine substitution at position 238 (55).
The continuing evolution of genes encoding ESBLs such as SHV-18, reflected in the increasingly large number of derivatives of TEM and SHV β-lactamases (http://www.lahey.org/studies/webt.htm), and their widespread dissemination on multiresistant plasmids significantly limit therapeutic choices. Perhaps improved detection of ESBLs will provide a more accurate assessment of their prevalence and lead to a more focused use of antimicrobial agents (44), which in turn will reduce the selection and spread of organisms producing these enzymes.
ACKNOWLEDGMENTS
We thank Christine Steward and Portia Williams for antimicrobial susceptibility testing. We also thank Linda Weigel for helpful discussions. We are grateful to Sebastián Albertí for help regarding the porin profile analysis and for kindly reviewing the manuscript.
Work in G.A.J.'s laboratory was supported in part by grants from Merck and the VA/DOD Collaborative Research Program on Mechanisms of Emerging Pathogens.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frère J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthélémy M, Péduzzi J, Ben Yaghlane H, Labia R. Single amino acid substitution between SHV-1 β-lactamase and cefotaxime-hydrolyzing SHV-2 enzyme. FEBS Lett. 1988;231:217–220. doi: 10.1016/0014-5793(88)80734-8. [DOI] [PubMed] [Google Scholar]
- 3.Barthélémy M, Péduzzi J, Labia R. Complete amino acid sequence of p453-plasmid-mediated PIT-2 β-lactamase (SHV-1) Biochem J. 1988;251:73–79. doi: 10.1042/bj2510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billot-Klein D, Gutmann L, Collatz E. Nucleotide sequence of the SHV-5 β-lactamase gene of a Klebsiella pneumoniae plasmid. Antimicrob Agents Chemother. 1990;34:2439–2441. doi: 10.1128/aac.34.12.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehringer Mannheim Biochemicals. The Genius system user's guide for filter hybridization. Indianapolis, Ind: Boehringer Mannheim Biochemicals; 1995. [Google Scholar]
- 6.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 7.Bradford P A. Automated thermal cycling is superior to traditional methods for nucleotide sequencing of blaSHV genes. Antimicrob Agents Chemother. 1999;43:2960–2963. doi: 10.1128/aac.43.12.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford P A, Urban C, Jaiswal A, Mariano N, Rasmussen B A, Projan S J, Rahal J J, Bush K. SHV-7, a novel cefotaxime-hydrolyzing β-lactamase, identified in Escherichia coli isolates from hospitalized nursing home patients. Antimicrob Agents Chemother. 1995;39:899–905. doi: 10.1128/aac.39.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K, Singer S B. Effective cooling allows sonication to be used for liberation of β-lactamases from Gram-negative bacteria. J Antimicrob Chemother. 1989;24:82–84. doi: 10.1093/jac/24.1.82. [DOI] [PubMed] [Google Scholar]
- 10.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coudron P E, Moland E S, Sanders C C. Occurrence and detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae at a veterans medical center: seek and you may find. J Clin Microbiol. 1997;35:2593–2597. doi: 10.1128/jcm.35.10.2593-2597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doménech-Sánchez A, Hernández-Allés S, Martínez-Martínez L, Benedí V J, Albertí S. Identification and characterization of a new porin gene of Klebsiella pneumoniae: its role in β-lactam antibiotic resistance. J Bacteriol. 1999;181:2726–2732. doi: 10.1128/jb.181.9.2726-2732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbarg-Chenon A, Godard V, Labia R, Nicolas J-C. Nucleotide sequence of SHV-2 β-lactamase gene. Antimicrob Agents Chemother. 1990;34:1444–1446. doi: 10.1128/aac.34.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernández-Allés S, Albertí S, Álvarez D, Doménech-Sánchez A, Martínez-Martínez L, Gil J, Tomás J M, Benedí V J. Porin expression in clinical isolates of Klebsiella pneumoniae. Microbiology. 1999;145:673–679. doi: 10.1099/13500872-145-3-673. [DOI] [PubMed] [Google Scholar]
- 15.Huletsky A, Couture F, Levesque R C. Nucleotide sequence and phylogeny of SHV-2 β-lactamase. Antimicrob Agents Chemother. 1990;34:1725–1732. doi: 10.1128/aac.34.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huletsky A, Knox J R, Levesque R C. Role of Ser-238 and Lys-240 in the hydrolysis of third-generation cephalosporins by SHV-type β-lactamases probed by site-directed mutagenesis and three-dimensional modeling. J Biol Chem. 1993;268:3690–3697. [PubMed] [Google Scholar]
- 17.Jacoby G A. Genetics of extended-spectrum beta-lactamases. Eur J Clin Microbiol Infect Dis. 1994;13:S2–S11. doi: 10.1007/BF02390679. [DOI] [PubMed] [Google Scholar]
- 18.Jacoby G A, Han P. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 1996;34:908–911. doi: 10.1128/jcm.34.4.908-911.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacoby G A, Sutton L. Properties of plasmids responsible for production of extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joris B, Ghuysen J-M, Dive G, Renard A, Dideberg O, Charlier P, Frère J-M, Kelly J A, Boyington J C, Moews P C, Knox J R. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988;250:313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsanis G P, Spargo J, Ferraro M J, Sutton L, Jacoby G A. Detection of Klebsiella pneumoniae and Escherichia coli strains producing extended-spectrum β-lactamases. J Clin Microbiol. 1994;32:691–696. doi: 10.1128/jcm.32.3.691-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knox J R. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob Agents Chemother. 1995;39:2593–2601. doi: 10.1128/aac.39.12.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K-Y, Hopkins J D, O'Brien T F, Syvanen M. Gly-238-Ser substitution changes the substrate specificity of the SHV class A β-lactamases. Proteins Struct Funct Genet. 1991;11:45–51. doi: 10.1002/prot.340110106. [DOI] [PubMed] [Google Scholar]
- 24.Mabilat C, Courvalin P. Development of “oligotyping” for characterization and molecular epidemiology of TEM β-lactamases in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1990;34:2210–2216. doi: 10.1128/aac.34.11.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabilat C, Goussard S. PCR detection and identification of genes for extended-spectrum β-lactamases. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 553–559. [Google Scholar]
- 26.Macrina F L, Kopecko D J, Jones K R, Ayers D J, McCowen S M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978;1:417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Martínez L, Hernández-Allés S, Albertí S, Tomás J M, Benedí V J, Jacoby G A. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 1996;40:342–348. doi: 10.1128/aac.40.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Martínez L, Pascual A, Hernández-Allés S, Alvarez-Díaz D, Suárez A I, Tran J, Benedí V J, Jacoby G A. Roles of β-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob Agents Chemother. 1999;43:1669–1673. doi: 10.1128/aac.43.7.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthew M, Harris A M, Marshall M J, Ross G W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 30.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin Infect Dis. 1997;24:S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 31.Mercier J, Levesque R C. Cloning of SHV-2, OHIO-1, and OXA-6 β-lactamases and cloning and sequencing of SHV-1 β-lactamase. Antimicrob Agents Chemother. 1990;34:1577–1583. doi: 10.1128/aac.34.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, fifth ed. Approved standard M7-A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Tenth information supplement. Approved standard M100-S10. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 34.Nicolas M-H, Jarlier V, Honore N, Philippon A, Cole S T. Molecular characterization of the gene encoding SHV-3 β-lactamase responsible for transferable cefotaxime resistance in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1989;33:2096–2100. doi: 10.1128/aac.33.12.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nüesch-Inderbinen M T, Kayser F H, Hächler H. Survey and molecular genetics of SHV β-lactamases in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob Agents Chemother. 1997;41:943–949. doi: 10.1128/aac.41.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pangon B, Bizet C, Buré A, Pichon F, Philippon A, Regnier B, Gutmann L. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 β-lactamase. J Infect Dis. 1989;159:1005–1006. doi: 10.1093/infdis/159.5.1005. [DOI] [PubMed] [Google Scholar]
- 37.Peña C, Pujol M, Ardanuy C, Ricart A, Pallares R, Liñares J, Ariza J, Gudiol F. Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1998;42:53–58. doi: 10.1128/aac.42.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perilli M, Felici A, Franceschini N, De Santis A, Pagani L, Luzzaro F, Oratore A, Rossolini G M, Knox J R, Amicosante G. Characterization of a new TEM-derived β-lactamase produced in a Serratia marcescens strain. Antimicrob Agents Chemother. 1997;41:2374–2382. doi: 10.1128/aac.41.11.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Péduzzi J, Barthélémy M, Tiwari K, Mattioni D, Labia R. Structural features related to hydrolytic activity against ceftazidime of plasmid-mediated SHV-type CAZ-5 β-lactamase. Antimicrob Agents Chemother. 1989;33:2160–2163. doi: 10.1128/aac.33.12.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitout J D D, Thomson K S, Hanson N D, Ehrhardt A F, Moland E S, Sanders C C. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob Agents Chemother. 1998;42:1350–1354. doi: 10.1128/aac.42.6.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podbielski A, Melzer B. Nucleotide sequence of the gene encoding the SHV-2 β-lactamase (blaSHV-2) of Klebsiella ozaenae. Nucleic Acids Res. 1990;18:4916. doi: 10.1093/nar/18.16.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podbielski A, Schönling J, Melzer B, Warnatz K, Leusch H-G. Molecular characterization of a new plasmid-encoded SHV-type β-lactamase (SHV-2 variant) conferring high-level cefotaxime resistance upon Klebsiella pneumoniae. J Gen Microbiol. 1991;137:569–578. doi: 10.1099/00221287-137-3-569. [DOI] [PubMed] [Google Scholar]
- 43.Portnoy D A, Moseley S L, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahal J J, Urban C, Horn D, Freeman K, Segal-Maurer S, Maurer J, Mariano N, Marks S, Burns J M, Dominick D, Lim M. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA. 1998;280:1233–1237. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 45.Rasheed J K, Jay C, Metchock B, Berkowitz F, Weigel L, Crellin J, Steward C, Hill B, Medeiros A A, Tenover F C. Evolution of extended-spectrum β-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother. 1997;41:647–653. doi: 10.1128/aac.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice L B, Willey S H, Papanicolaou G A, Medeiros A A, Eliopoulos G M, Moellering R C, Jr, Jacoby G A. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990;34:2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 48.Shlaes D M, Currie-McCumber C, Hull A, Behlau I, Kron M. OHIO-1 β-lactamase is part of the SHV-1 family. Antimicrob Agents Chemother. 1990;34:1570–1576. doi: 10.1128/aac.34.8.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sirot D. Extended-spectrum plasmid-mediated β-lactamases. J Antimicrob Chemother. 1995;36:19–34. doi: 10.1093/jac/36.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 50.Southern E. Detection of species specific sequences among DNA fragments by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 51.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanhoof R, Content J, Van Bossuyt E, Dewit L, Hannecart-Pokorni E. Identification of the aadB gene coding for the aminoglycoside-2"-O-nucleotidyltransferase, ANT(2"), by means of the polymerase chain reaction. J Antimicrob Chemother. 1992;29:365–374. doi: 10.1093/jac/29.4.365. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura F, Nikaido H. Diffusion of β-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985;27:84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan M, Aucken H, Hall L M C, Pitt T L, Livermore D M. Epidemiological typing of Klebsiellae with extended-spectrum β-lactamases from European intensive care units. J Antimicrob Chemother. 1998;41:527–539. doi: 10.1093/jac/41.5.527. [DOI] [PubMed] [Google Scholar]
- 55.Yuan M, Hall L M C, Savelkoul P H M, Vandenbroucke-Grauls C M J E, Livermore D M. SHV-13, a novel extended-spectrum β-lactamase, in Klebsiella pneumoniae isolates from patients in an intensive care unit in Amsterdam. Antimicrob Agents Chemother. 2000;44:1081–1084. doi: 10.1128/aac.44.4.1081-1084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]