Abstract
Background:
Newer “closed-loop” neurostimulation devices in development could, in theory, induce changes to patients’ personalities and self-perceptions. Empirically, however, only limited data of patient and family experiences exist. Responsive neurostimulation (RNS) as a treatment for refractory epilepsy is the first approved and commercially available closed-loop brain stimulation system in clinical practice, presenting an opportunity to observe how conceptual neuroethical concerns manifest in clinical treatment.
Methods:
We conducted ethnographic research at a single academic medical center with an active RNS treatment program and collected data via direct observation of clinic visits and in-depth interviews with 12 patients and their caregivers. We used deductive and inductive analyses to identify the relationship between these devices and patient changes in personality and self-perception.
Results:
Participants generally did not attribute changes in patients’ personalities or self-perception to implantation of or stimulation using RNS. They did report that RNS affected patients’ experiences and conceptions of illness. In particular, the capacity to store and display electrophysiological data produced a common frame of reference and a shared vocabulary among patients and clinicians.
Discussion:
Empirical experiences of a clinical population being treated with closed-loop neuromodulation do not corroborate theoretical concerns about RNS devices described by neuroethicists and technology developers. However, closed-loop devices demonstrated an ability to change illness experiences. Even without altering identify and self-perception, they provided new cultural tools and metaphors for conceiving of epilepsy as an illness and the process of diagnosis and treatment. These findings call attention to the need to situate neuroethical concerns in the broader contexts of patients’ illness experiences and social circumstances.
1. Introduction
Over the past decade, the U.S. BRAIN Initiative and related international initiatives have devoted intensive research efforts to developing new therapies drawing on emerging circuit-based models of brain function. While “open-loop” deep brain stimulation (DBS) devices have been used for decades to treat Parkinson’s disease and other disorders by applying consistent pre-programmed stimulation to targeted brain areas, “closed-loop” neuromodulation systems (also called “adaptive,” “bidirectional,” “responsive,” or “next-generation” brain stimulation) are now under development for several neurologic and psychiatric conditions. Closed-loop devices depart from established open-loop DBS by including the capacity to continuously monitor brain activity, to decode neural activity, and to modulate such activities based on internal software algorithms (Figure 1). Closed-loop devices have the potential to be more dynamic, personalized, and ultimately effective than open-loop systems (Priori et al. 2013; Parastarfeizabadi and Kouzani 2017). They have been proposed and tested for a number of neurological conditions for which prevailing treatments are often unsatisfactory, including drug-resistant epilepsy (Fountas et al. 2005), refractory depression (Scangos et al. 2021), anxiety (Widge et al. 2017), chronic pain (Shirvalkar et al. 2018), Alzheimer’s disease (Senova, Caillet, and Lozano 2018) and ischemic stroke (Gonzalez Andino et al. 2011). Such interventions hold promise for treating debilitating conditions, but these devices’ ability to modulate brain activity without direct human supervision also raises new ethical and policy questions (Kellmeyer 2018; Hegde, Chiong and Rao 2021).
Figure 1.
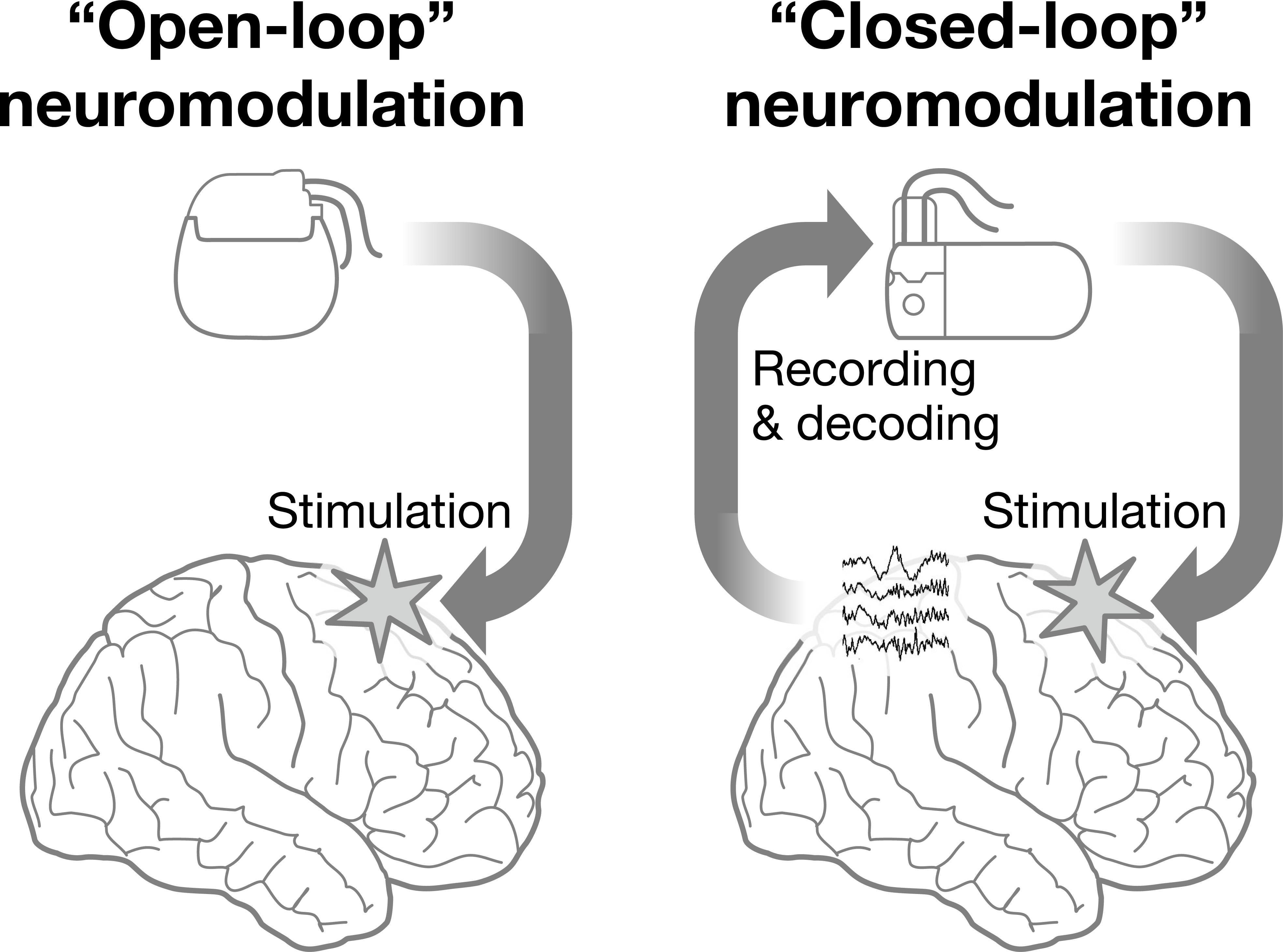
“Open-loop” and “Closed-loop” Neuromodulation.
Neuroethicists and other scholars have cautioned that closed-loop devices could have unintended and potentially destabilizing effects on individuals. Kellmeyer and colleagues (2016) argue that increasingly sophisticated closed-loop devices will exhibit “system autonomy,” a form of device agency and spontaneity that, depending on device design, could enhance or alternatively undermine users’ personal autonomy and accountability. Goering and colleagues (2017) note that closed-loop devices in psychiatry could yield unexpected and potentially alienating personality changes, experiences that might move us to adopt relational over individualistic philosophical accounts of agency. Gilbert, O’Brien and Cook (2018) argue that newer devices may produce “decisional vulnerability” in the user, reflecting overreliance on device outputs that undermines rather than supports agency.
To date these conceptual concerns have not been empirically examined with patients undergoing closed-loop neuromodulation for clinical indications. Goering and colleagues cite interviews with patients undergoing investigational open-loop DBS for psychiatric conditions, who were asked hypothetically about envisioned future closed-loop devices. Gilbert and colleagues present interview data from patients implanted with investigational seizure prediction devices (also see Gadhoumi et al. 2016), which they describe as “closed-loop brain-computer interfaces” although these devices do not have the automatic, algorithm-driven responsiveness to neural signals that we regard as characteristic of closed-loop neuromodulation.
In the present paper, we examine experiences of patients receiving clinical, non-investigational treatment with the NeuroPace Responsive Neurostimulation System (RNS) for refractory epilepsy. This device was approved in 2013 by the U.S. Food and Drug Administration and is the only approved and commercially-available closed-loop brain stimulation device in clinical practice (Sun and Morrell 2014). To date, over 3,000 patients have been implanted with RNS, and long-term data indicate sustained tolerability and increasing efficacy over 9 years of follow-up (Nair et al. 2020). This study focuses on the period surrounding RNS implantation including longitudinal follow-up during the first year post-surgery in order to gauge the impact of RNS on individual self-perception and experiences. As this is an exploratory empirical study, our conceptual target was open-ended and not explicitly reliant on any specific philosophical account of agency, autonomy, personal identity or related concepts (Pugh 2020). We examined patients’ experiences at the stage of initiating RNS treatment with the goal of exploring whether and how implantation of RNS shaped experiences of illness, identity, self-perception, or personality.
2. Methods
Design:
We used an ethnographic study design. Ethnography is a social science research approach that provides insights into everyday experiences of individuals, groups, and communities (Reeves, Kuper and Hodges 2008). Most ethnography involves in-depth longitudinal case study, mixed methods data collection including direct observation, and analysis of both pre-specified (deductive) and emergent (inductive) research questions (Lofland 1995; Charmaz and Olesen 1997).
Clinical Background and Study Setting:
Epilepsy affects approximately 3 million adults in the US (Tian et al. 2018). First-line treatment involves anti-seizure medication, but for more than one-third of patients medication fails to provide complete seizure control (Thijs et al. 2019). These patients may be considered for surgical resection, but this is generally contraindicated for patients who present with bilateral seizure foci or a seizure focus in close proximity to eloquent cortex (Yan and Ibrahim 2019; Brandy et al. 2020). RNS may be recommended for such patients as a third-line treatment strategy.
We conducted this study at a level 4 epilepsy center (Labiner et al 2010) in an academic medical institution. At the epilepsy center, a multi-disciplinary team (neurologists, neurosurgeons, neuropsychologists, nurses, and social workers) reviewed medication-refractory cases weekly to assess appropriateness for RNS among other treatment options. When patients undergo RNS, a neurosurgeon implants the device in the skull and places electrodes near the seizure focus. Following surgery, the device records neural activity, and patients regularly upload electrocorticography data to an internet cloud service maintained by device-manufacturer NeuroPace. This service includes a clinician interface allowing epileptologists to access, download and review electrocorticographic data, and to program the RNS device when patients are present. In most cases, 2–6 months after device implantation, sufficient data will have been collected to allow the patient and clinician to define thresholds for abnormal (epileptiform) activity. Once programmed and activated, the RNS device delivers electrical counter-stimulation when electrocorticography patterns suggest seizure activity (Fountas et al. 2005; Thomas and Jobst 2015; Geller 2018; Jarosiewicz and Morrell 2020), to reduce seizure frequency and severity. In general, patients for whom stimulation has been activated are counseled not to expect immediate and permanent seizure freedom. Rather, the RNS System tends to be presented to patients as a palliative treatment (Ma and Rao 2018). It is designed to become better at recognizing and inhibiting epileptiform activity over time as the recorded data reveal more about a patient’s condition, and their neurologist(s) can better tune the device’s settings. In clinical trials and open-label follow-up studies, median seizure reduction was 44% at 1 year, 53% at 2 years, 66% at 6 years and 75% at 9 years (Bergey et al. 2015; Nair et al. 2020).
Recruitment Procedures:
To recruit participants, we observed neurosurgical case conferences to identify adult patients at the study site who were recommended RNS treatment (see Setting and Clinical Background, above). We consulted treating epileptologists during case conference or via email to confirm study eligibility. Most patients were first encountered during a pre-operative clinical appointment; a treating epileptologist obtained permission from patients for a study researcher to observe these appointments.
Data Collection:
Given our research interest in personality changes and changes in self-perception related to device implantation and use, we followed patients longitudinally over multiple encounters during the dynamic early phases of RNS treatment. We collected data using direct observation of clinical appointments in the epilepsy clinic and also using in-depth interviews with patients and caregivers at a place of their choosing outside of the clinic environment (see interview guide in appendix). When feasible given constraints of clinic scheduling and variable individual clinical course, we performed one direct observation during pre-surgical workup (i.e., prior to device implantation) and followed patients for one year following implantation at intervals designed to capture experiences around early clinical milestones such as implantation, the post-operative period, device activation, and early calibration of stimulation parameters. During the interviews, the investigators raised the question of personality or self-perception change with each patient.
For direct observation, we obtained verbal informed consent from clinicians and patients to observe patients’ appointments preceding and following RNS implantation. Trained observers recorded notes in situ using standard fieldwork procedures (Emerson, Fretz, and Shaw 1995). Observers expanded these in situ notes into full ethnographic fieldnotes with word processing software upon leaving the field.
At the conclusion of the first observed visit, we invited patients and caregivers to participate in an in-depth interview and obtained contact information from willing respondents. We scheduled interviews for a time and place of participants’ choosing. In most cases, we conducted in-person interview in patients’ homes, but we conducted some interviews over Zoom in response to COVID-19 or, prior to COVID-19, given patient preference. Prior to conducting each interview, we obtained written informed consent either in person or via DocuSign. With participant permission, we audio-recorded interviews for later transcription. The study was approved by the Committee on Human Research at UCSF. In accordance with our approved protocol, in this manuscript we refer to participants using coded identifiers and do not identify the research site.
Data Analysis:
We entered observation fieldnotes and interview transcripts into Atlas.ti software to facilitate data analysis (Friese 2019). The study team developed a coding scheme to identify relevant sections of notes and transcripts. Prior to analysis, we established deductive codes to tag concepts of interest including patient experiences of epilepsy, treatment, and self-perceptions. During analysis, we remained open to identifying additional, inductive codes to note concepts that emerged during the course of data review. To enhance rigor, two study members double-coded two sets of fieldnotes and transcripts, compared codes, and resolved discrepancies (Cascio et al. 2019). A single team member then coded the remaining fieldnotes and transcripts. Our findings represent patterns of experiences and perceptions that occurred among multiple respondents. We illustrate findings with exemplary excerpts from observational fieldnotes and interview transcripts.
Addressing Bias:
Ethnography, like other descriptive and interpretive research methods, does not strive to eliminate bias by standardizing research processes but rather to acknowledge the potential for bias and address it using a variety of techniques. To reduce subjectivity bias, we used the technique of triangulation – using data from different investigators or stakeholders to enhance accuracy – by working within an interdisciplinary team including members from clinical, philosophical and sociological backgrounds. We also employed triangulation by having three researchers perform ethnographic observations and by comparing data acquired from the observations with interview responses to improve validity and minimize retrospection bias. Ethnography also includes techniques to address the potential of social desirability bias, i.e., the tendency of the interviewees to provide socially desirable responses in lieu of giving answers that reflect their true attitudes, experiences, and feelings. We established longitudinal relationships with participants, and we sought to interview them outside of the medical settings where they received care. The rapport derived from an ongoing relationship and creating a physical distance between our research interactions and those of the clinical team, were intended to reduce bias by improving the degree of rapport between participants and researchers and thus increase participants’ comfort in providing information and insights that was not tailored for social desirability.
3. Results
Fieldwork began in June 2018, and over the course of the next 24 months we recruited 12 patients to the study. Of 12 patients, we recruited 10 prior to RNS implantation; for the group recruited post RNS implantation, we observed at least one clinical appointment and conducted at least one in-depth interview. Table 1 illustrates substantial complexity and variability in patient clinical experiences. While most participants in the study followed the expected pathway of device implantation and activation, this was not universally the case. One participant unexpectedly experienced seizure freedom following device implantation, so the RNS device’s stimulation function was never activated. Two participants did not experience satisfactory seizure reduction from RNS stimulation, but chronic electrocorticography enabled the identification of unilateral seizure foci allowing for subsequent ablative surgery.
Table 1.
Participant characteristics including RNS implantation site and ethnographic data.
| Participant ID | Age (decade) | Gender | RNS Implantation Site | Ethnographic data |
|---|---|---|---|---|
| 1101 | 50s | Female | Bilateral hippocampi | Two interviews at 10 months post-op and 24 months post-op Four observations at 1 week pre-op, 2 weeks post-op, 7 months post-op, and 22 months post-op |
| 1104 | 30s | Female | Bilateral hippocampi | Two interviews at 6 months post-op and 12 months post-op Five observations at 6 months pre-op, 2 weeks post-op, 3 months post-op, 6 months post-op, and 10 months post-op |
| 1105 | 50s | Female | Left occipital pole, parietal cortex, and lateral occipital cortex | Two interviews at 3 months post-op and 15 months post-op Four observations at 1 month pre-op, 2 weeks post-op, 1 month post-op, and 12 months post-op |
| 1107 | 20s | Male | Bilateral hippocampi | Two interviews at 3 months post-op and 14 months post-op Four observations at 2 weeks post-op, 1 month post-op, 9 months post-op, and 12 months post-op |
| 1108 | 40s | Female | Left precentral and postcentral gyrus | One interview at 14 months post-op Two observations at 2 weeks post-op and 8 months post-op |
| 1109 | 30s | Female | Left hippocampus, amygdala, and occipital lobe | Two interviews at 3 months post-op and 12 months post-op Three observations at 2 months pre-op, 1 month post-op, and 2 months post-op |
| 1111 | 50s | Male | Bilateral hippocampi | (initial RNS implantation with follow up resection 35 months later) One interview at 38 months post-RNS, 3 months post-resection One observation at 36 months post-RNS, 1 month post-resection |
| 1112 | 30s | Male | Bilateral hippocampi | Two interviews at 1 month post-op and 10 months post-op Two observations at 2 months pre-op and 2 weeks post-op |
| 1113 | 50s | Female | Bilateral hippocampi | Two interviews at 1 month post-op and 12 months post-op One observation at 3 weeks post-op |
| 1114 | 70s | Female | Left hippocampus | (initial RNS implantation with follow up radiofrequency ablation 22 months later) One interview at 24 months post-RNS, 1.5 months post-radiofrequency ablation One observation at 24 months post-RNS, 1.5 months post-radiofrequency ablation |
| 1115 | 30s | Female | Bilateral hippocampi | One interview at 1 month post-op Two observations at 4 months pre-op and 2 months pre-op |
| 1116 | 30s | Female | Bilateral hippocampi | Two observations at 4 months pre-op and 1 month post-op |
Our observations and interviews yielded three major findings. First, patients and caregivers did not attribute changes in the patient’s personality or self-perception to the device itself. Second, several did attribute such changes to the patient’s underlying epilepsy and to medications used to manage epilepsy. Finally, the device (more specifically, recordings enabled by the device) did alter patients’ perceptions and understanding of their illness.
3.1. Self-Perception after RNS Device Implantation
Throughout the observed treatment trajectory, from neurosurgery and device programming to simulation activation and the continuing therapy, patients generally distinguished between themselves, their illness, and the device and maintained these notions across time. For example, during a first interview two years after RNS implantation, participant 1114 said, “No, that doesn’t really – doesn’t really affect me.” This sentiment was echoed by 1114’s caregiver, “I don’t think she felt like it was any – as far as an implant goes, any different than having an implant in her knee or hip or shoulder or whatever, it was – as long as there was a benefit or hope of a benefit.” And participant 1111 noted, “Actually I never felt anything. I mean, I’ve never felt, like, different. I mean, even with, you know, having the device, not sure I’ve ever felt any different.”
While some participants discussed their self-perceptions in relationship to RNS within the context of interactions with caregivers, co-workers, and others in their social network, these participants linked their ability to be themselves to how others perceived the significance of RNS implantation, but not to the device itself. Asked whether RNS precipitated changes in personality or identity, for example, a participant diagnosed with adult-onset epilepsy who was the primary earner in her household illustrated this dynamic in her response:
I mean, personality-wise, and emotional and all that, I think I’m basically the same person. I haven’t had any experiences where anybody is going, “What’s wrong with you today,” you know, or just thinking I’m being a real ass or, you know, anything. I haven’t really heard anybody make comments, so, including him [points at partner], so - and he’s the one I’m with most of the time. (participant 1101, 1st interview 10 months post-RNS implantation)
Her partner/caregiver added:
If I saw anything different, her and I would talk about it, but there has been nothing.
But while the participant and her partner were not concerned that RNS had changed her personality, the participant decided to conceal her implantation from her co-workers because of concerns about possible reactions.
Everybody at work needs to be competent and competent of what they do, but I guarantee you there’s going to be questions. Well, how does she know what medicines she’s giving? And, you know, there’s going to be this surrounding thing as to whether I can actually be physically okay to take care of the patients even though I was taking care of them way before this thing happened, but there’s this - you have something in your brain, you know?
By the time of her second interview, however, the situation at work had changed. Her co-workers now mostly knew she had been treated with RNS, and openness had not altered the participant’s self-perceptions or identity:
Most people know it now. I don’t know that they necessarily know exactly how it’s supposed to work and all that stuff. But most people know that I had brain surgery. It’s usually just all I call it. And if they want to know more, fine. If they don’t, fine. Because they can see my behavior. They can still see that I am who I used to be. And so I don’t think they question, going, oh, yeah, that’s why. Because she had brain surgery and that’s why she’s acting a little different. But I think that’s the main reason why I didn’t really want to tell people [… ] Those people you think, they really don’t need to know all of that about me. But I think most of them need to know now because I’m not really trying to hide it […] I think that’s the biggest thing, is just I don’t really care as much now because I still think I’m basically who I used to be. (participant 1101, 2nd interview 24 months post-RNS implantation)
One patient did report concerns about changed personality after implantation, though in this case these may have been attributable to other changes in her course of illness and treatment:
And so for a while I was just, like, so sure it was these electrical currents that were causing this huge change in my mood and my mental status. And the - or just having the surgery at all. But, you know, more specifically having brain surgery and having a new electrical current that isn’t normally there. And, I mean, one psychiatrist that I was going to for medicine, he said something like, ‘Yeah, you seem a little, you know, I don’t know? Testy, for lack of a better word.’ … But he, like, noticed it immediately and it was just around the same time. I just - the psychiatrist that I just started going to, he realized I increased one of the antiseizure drugs at the same time… I mean, I think the drugs make a huge difference. You know? That’s always been that way. […] And I was, like, really kind of worried that the new electrical currents could change things for better or for worse, you know? Could bring out new feelings, new ideas, change my personality, you know? I think there’s - I think it’s going to change - I think there’s been a change in my personality, somewhat. And it’s hard to not associate it with that, even though, you know, I have no evidence. So, really, it’s about the idea that there’s new electrical currents in my brain, you know? If I think about that too much, it’s a little creepy. (1113, 2nd interview, 12 months post-surgery)
Though changes in personality and self-perception were not attributed to the device, participants did note changes to other domains in their lives following device implantation. In one exchange, the interviewer asked whether the device had made the patient “a different person.”
Caregiver: For me, he’s a lot calmer. He’s a lot calmer. But I don’t know if that’s medication. But the other part of it is, [he] is way protective of me. So, I never know…I try not to evaluate him. But like I said, he’s - I feel like he’s feeling better. More confident.
Patient: I don’t feel changed. Like I said, the only thing I probably, I know it’s there. This little piece, I can feel…
Interviewer: It’s not that you think, oh, there’s a new [participant 1107] in the house?
Caregiver: Well there was a bad [participant 1107], yeah. And that one we think is gone.
Patient: That was the medication. Because I’d get angry pretty quick.
In this exchange, changes to the patient’s self-perception and the caregiver’s perception of the patient’s personality were attributed to medication, since discontinued, and not to RNS.
For another participant, RNS allowed for new possibilities in life. This patient achieved seizure control after stimulation was activated. She was cleared to drive and contemplated having children. In her second interview 12 months after surgery, she was asked to elaborate on these changes with the question, “Living on your own, how has that been, managing the seizures and having the device?”
I just live my normal life every day. I don’t really - this thing doesn’t really control how I live my life. It’s just there…Honestly, this device has given me a – what’s that – I’m thinking of the word, I can’t – a better outlook on life now because I can do the things now that I wanted to, but the seizures kept stopping me from doing. (participant 1109)
No participants reported a change in self-perception or identity when clinicians enabled the RNS stimulation function. For eight participants, clinic observation for our study coincided with the appointment at which stimulation was activated by clinicians, typically between three and six months post implantation. During this appointment, when the clinician activated the device stimulation he asked the patient if they experienced any change. In seven appointments, activation occurred without incident and patients denied any awareness of the device stimulation. During one appointment, however, device stimulation occurred under unusual circumstances, and this experience transiently restored a pre-epilepsy sense of self.
Three weeks after surgery participant 1105 had a clinic appointment to check the surgical wound and affirm the RNS device was successfully recording. In the exam room, the patient began to experience seizure symptoms. She had been experiencing frequent seizures post-surgery, and these disruptive events often began with auditory hallucinations. Earlier in the appointment, she described these as particularly upsetting: “this hearing stuff is horrible, it just goes on and on and on and on and on and on and on… it gets to the point that it’s making me crazy.” Later in the appointment, 1105, her caregiver, and an epileptologist with RNS expertise were comparing the seizure diary maintained by the caregiver with the recordings from the RNS device; a second epileptologist was present to consult on changes to the patient’s medication. Suddenly, the patient interrupted the conversation and with a distressed appearance asked if others in the room heard laughing voices. She cringed and covered her ears, and her caregiver and the medication-epileptologist comforted her. The medication-epileptologist asked the patient to repeat certain phrases to assess whether her speech was impaired and track the progress of the seizure. The RNS-epileptologist pointed out that he could activate the device stimulation to try to interrupt the seizure, and after a brief consultation with the medication-epileptologist proceeded to do so. Despite the exigency of active seizure, the RNS-epileptologist performed the usual routine of ensuring that the patient was not experiencing any side-effects from device activation such as flashes of light or tingling sensations. The patient assured him she was not even as her seizure distress continued. After the RNS-epileptologist activated stimulation, the exam room became quiet and shortly thereafter the patient announced that the seizure stopped. The medication-epileptologist asked if the voices had fully stopped, and the patient was unsure. As she struggled to find the right words to describe what she was hearing and feeling, the RNS-epileptologist continued to adjust device settings. After several minutes, she visibly calmed and then confidently and clearly announced that the seizure was over. Everyone in the room was relieved. The RNS-epileptologist acknowledged this was a remarkable event and reported that he had simply activated stimulation several times.
When we interviewed the patient six weeks later, we asked her to reflect on this experience. “it was so exciting,” she said. “We walked out and we - I mean, I was - I don’t even know how to explain how exciting that was. And it’s like the - the light turned on. And, so, that was really good. And it’s - it was exciting and wonderful.” But she reported that unfortunately this experience did not prove permanent or transformative in terms of her self-perceptions or personality.
1105: We had gone to dinner, and we had a really nice dinner and it was exciting, and it was almost like when you get engaged; you know? And, so, we were going home. And we were driving home, I was looking and then all of a sudden, there that pain, that little bit of pain started coming. And also this feeling that you get when you start to have that migraine - that migraine feel. And I thought, no, not yet. Not yet. It’s not coming back.
4105: Well, you started seeing hallucinations [crosstalk]
1105: Yeah, I think - and --
4105: - hallucinations started.
1105: And I said, I think it’s coming back or something.
4105: But by then your words were - your speech was really in the toilet.
1105: Yeah, and that was kind of - I was - I just thought, no, not already; you know? I - because I - because I thought it was going to stay for - forever; you know?...I said to 4105, but, you know, it’s like when Christmas is over. It’s like when Christmas is over. (participant 1105, 1st interview, 3 months post-RNS implantation).
3.2. Changes in Personality and Self-Perception Attributed to Disease and other Treatments
While participants generally did not attribute changes in self-perception to RNS implantation, many did describe earlier changes in self-perception in the context of their epilepsy illness and pharmacologic treatment. Participants universally described a self-perceived sense of agency and control that they enjoyed prior to developing epilepsy or that they experienced during extended periods of seizure-freedom post-diagnosis. Illness and pharmacologic treatment altered this self-perception in two ways.
First, epilepsy and its treatment with medication disrupted participants’ sense of individual self-control and agency. One participant noted that seizures tend to occur quickly without much time to prepare. This is followed by losing awareness over both self and environment:
I think, for me personally, it’s the lack of control because I don’t - I know it’s going to happen and what’s going to happen. I have no idea and no control over any of it, what I’m going to do, what I’m going to say, nothing, you know? (participant 1101, 1st interview conducted 10 months post-RNS implantation)
Losing control in this fashion has implications for self-perception beyond the moment at which the seizure occurs. In this interview, a participant described how the lack of seizure control engendered a broad loss of self-efficacy.
The lack of control and just not being present is just – it’s really horrible. I mean, it’s horrible for it to limit my life like it’s hard to work and it’s hard to – and I can’t drive and stuff, but it’s really horrible just when I’m doing nothing to have that. (participant 1113, 1st interview conducted 1 month post-RNS implantation)
As noted, RNS is offered to patients after attempts at seizure control with medication have failed, and all participants in our study had extensive prior experience with anti-seizure medications. Some reported that medications changed patients’ personalities in undesired ways such as aggressive/erratic behavior, suicidal thoughts and attempts at self-harm. One caregiver described how “too many of the pams” – the seizure control medications midazolam, lorazepam, clonazepam – “didn’t put her to sleep. It made her, like, a personality shift. I mean, it was dramatic.” (caregiver of participant 1105, 2nd interview conducted 15 months post-RNS implantation). Another caregiver described her son’s side effects of one medication as follows:
It was so bad because his behavior became so erratic. And like, he was going to kill everybody. I mean he literally cleared this whole dining room table. And that’s not like him. He can turn on a dime sometimes with his mood, but not to the point where like I was like, okay, that’s it. (caregiver of participant 1107, 1st post-op interview 3 months post-RNS implantation).
For many participants, personality and self-perception changes began prior to a formal diagnosis. They recalled years of feelings of déjà vu or “spacing out” that, in hindsight, were recognized as auras or absence seizures.
Second, epilepsy’s unpredictable seizures and the side-effects of treatment presented participants with barriers to becoming who they wanted to be or imagined themselves to be. Participants found that the illness and its treatment profoundly affected their sense of normalcy, of the body, and of the self, often leading to difficulties in forming and managing a cohesive life narrative. For one participant, both her seizures and medication have prevented her from becoming a mother:
I’ve always wanted kids. Always. Since I was probably 16. It’s always just been a goal of mine, but the seizures always just got in the way because then I have to talk to two doctors and, you know, will the baby be safe with the medication that I’m taking. From what my gynecologist said, the two that I am taking are safe to take when you are pregnant, but I’m still a long way from that. My goal is hopefully soon. (participant 1109, 1st post-op interview 3 months post-RNS implantation).
During her second interview, 12 months post-RNS implantation, the same participant mentioned that she was now contemplating children. Another participant, drawing comparisons between her current life and her life before the onset of her seizures, summarized it, “I mean, you do have to accept at some point that you’re not the person you were. You can’t do the things you could do.” (participant 1114, 1st post-op interview 1.5 months post-radio-frequency ablation surgery; two years post-RNS implantation)
While none of our participants wholeheartedly attributed substantive changes in their personality or self-perception to the RNS device and stimulation, many did attribute such changes either to the experience of epilepsy or the side-effects provoked by medication.
3.3. Effects of RNS Treatment on Conceptions of Illness
While RNS did not substantially change participants’ personalities or self-perceptions, we found that RNS did provide participants new ways of making sense of their illness. During clinic visits after device implantation, clinicians showed patients and caregivers the RNS device’s recordings of neural activity. Clinicians could display recordings that had been recorded and stored previously, when the patient was at home, as well as show real-time electrocorticography from the device during the appointment; such recordings are used to tune the device’s seizure-detection algorithms. In the clinic visits we observed, most patients and caregivers responded with great interest to seeing their neural data displayed on screen.
One participant and his parents experienced a variety of reactions during the appointment where they saw RNS neural data for the first time, 9 days after implantation. Early in the appointment, the patient’s mother (his primary caregiver) reported that she suspected the patient had a seizure a week earlier, and was interested in whether this event would be corroborated in the device data.
The clinician then pulls up the RNS recording data, and all the family huddles around the computer screen. This is the first time they are seeing device recordings…The patient’s mother pulls out her phone to take photos, explaining that the patient’s aunt is going to ask for pictures. The clinician laughs and says it isn’t a problem; patients take photos of their data all the time and some even post on Instagram.
The clinician explains that the waves represent discharges or spikes in brain activity and compares these to sparks in a forest which on their own do not equate to a seizure, but they have the potential to cause “wood to catch on fire.” The clinician tells the family not to worry that they are seeing these spikes in activity. The patient’s mother understood that detection of spikes means that the device leads were placed in the correct brain regions in order to glean information about the patient’s seizures…The clinician shows data from a Monday at 10am and points out the increase in wave height on the L hippocampal leads, explaining that this was a seizure. The mother is shocked by the time stamp, saying, “He was awake!” The clinician pointed out where the waves change from black to blue, explaining that the blue color represents activity that the device detects as abnormal. The mother compares the wave data to “watching a seismograph.”…
The clincian says it looks like the patient’s seizure activity is highest in the early morning hours i.e., 2am. The mother said the patient is asleep during that time, and she suspects this has been going on since childhood but because of the time of day, the seizures went undetected until adulthood…The patient said, “I’ll be damned! That’s crazy!” He recites his morning routine: wakes up ~6 or 7am with a lot on his mind, which he attributes to living in the country and having a great deal of responsibility over the animals, so he smokes. The patient attributes decline in sparks by ~9am to him smoking as a means of calming his racing thoughts. The mother confirmed that it’s not a good idea to talk to the patient in the morning because he is often irritable.
At a subsequent interview, the participant reflected upon the experience.
That was definitely something else. Creepy. A heartbeat? I can watch a heartbeat, and I can feel the pulse for that image. But brainwaves? Watching brainwaves grossed me out… It’s because it’s mine. It’s creepy because it’s mine. If it was somebody else’s, it wouldn’t be as creepy. But, it’s because it’s mine. (participant 1112, 1st post-op interview 1 month post-RNS implantation).
Other patients and families had similar responses upon seeing their RNS tracings for the first time. After this initial reaction, participants came to view RNS data as another source of information and insights into their seizures and illness. Clinicians, patients, and caregivers often compared seizure diaries to device recordings, and these sources of data did not always correspond. Some diary events appeared in the tracings while others did not, and the device also detected abnormal brain activity at times when participants did not experience symptoms. One participant described the accumulation of new information about their illness as “a puzzle that’s being slowly put together. That’s just how I kind of see it because this has been a very slow, a slow and long process…like literally putting a puzzle together. (participant 1109, 1st post-op interview 3 months post-RNS implantation).
Another participant, when asked how they felt when seeing their RNS data for the first time in clinic, noted:
Being able to look at that screen and figure out, you know, that there was legitimately something going on, which we had kind of seen before in the EEGs, but this one was so specific and he was able to explain what these different graphs and I felt very reassured in terms of, you know, not only what I had been doing but also what he had been doing in terms of, you know, reading this data and really making sure that they were getting what they needed. (participant 1115, 1st post-op interview 1 month post-RNS implantation).
RNS data did not affirm all patient experiences, which could call patients’ illness experiences into question. During a clinic appointment 2 weeks after implantation, another participant (1113) described an event she thought was a seizure, but the device had detected no unusual brain activity. The patient was disappointed that her experience had not been verified by RNS. The epileptologist responded that while “according to the device, no seizure had occurred,” given memory storage and anatomical coverage limitations of the device, this “does not necessarily mean” that the patient’s experience was not a seizure. Given the participant’s description, the epileptologist interpreted the event as a seizure, reassuring her with the observation that “computers are dumb” and that she knew her own body best.
New understandings of seizure activity extended beyond the question of whether or not a seizure had occurred. For some participants, access to RNS data led to a reconsideration of their seizure experiences, as this participant describes.
I used to think I could really tell the difference between an aura and a seizure. Especially if I didn’t have a blank out or anything… I have auras a lot and I don’t write them down, whereas I’ve written down every seizure that I’ve had, I think, for six years… I think maybe – and they call auras a seizure even though I feel like I can tell the difference. Now, I’m thinking that actually it can just be an aura and this device will record it as a seizure. So, probably I should write down every aura but I just have them a lot. (participant 1113, 1st interview conducted 1 month post-RNS implantation).
4. Discussion
This study empirically addresses concerns raised in the conceptual neuroethics literature on closed-loop neuromodulation, introducing experiences from a cohort of patients undergoing treatment with the first clinically approved and commercially available closed-loop brain modulation device. Three conclusions merit discussion. First, we found that this application of closed-loop technology did not transform patients’ sense of self or personality. Neither the chronic implantation of an electronic device in their brain nor the ensuing stimulation to modulate their brain function led to changes in patients’ self-perceptions, nor in the perceptions of the patient by family members and others around them. Our second finding is that potential impacts of closed-loop neuromodulation on personality and self-perception must be evaluated in relation to patients’ antecedent neurologic conditions and treatments received, which in our study were recognized by patients and family members as having had profound effects on personality and self-perception. Third, the capacity of these devices to record, store and display neural data offered patients new ways of understanding and making sense of their illness.
With regard to our first finding, our project responds to recent calls for fuller empirical investigation of neuroethical concerns about alteration of agency, autonomy, personal identity and related concepts (Gilbert, Viaña, and Ineichen 2018; Pugh 2020). While our findings do not substantiate concerns raised in earlier conceptual neuroethics literature regarding system autonomy, emerging forms of agency, or decisional vulnerability brought about by closed-loop neuromodulation (Kellmeyer et al. 2016; Goering et al. 2017; Gilbert, O’Brien and Cook 2018), it should be noted that the RNS device currently in clinical use does not have many more advanced functions envisioned by these authors and by neurotechnology developers. Furthermore, Goering et al. in particular had special concerns about implications of closed-loop neuromodulation in psychiatric disorders, where they envisioned that devices might exert effects specifically through programmed alterations to patients’ personality and behaviors. While our findings in epilepsy do not corroborate concerns about changes in personality or self-conception addressed to closed-loop brain devices as a class, some such concerns may depend on details of the neural/psychological mechanisms through which clinical effects of closed-loop neuromodulation are intended to occur. In the case of psychiatric closed-loop neuromodulation, however, such details have not been resolved and those concerns remain largely speculative. Though as noted in our second finding, we suggest that understanding the process of changes in personality or self-conception requires investigation into both the treatment’s medical and social context. Additionally, while epilepsy often has chronic manifestations, the cardinal clinical features of epilepsy are episodic in nature, and RNS stimulation is designed to respond episodically to detected events. Future applications of closed-loop neurotechnology directed at persisting rather than episodic clinical phenomena could have greater implications for personality or self-perception, either because they operate more continuously or are directed at clinical phenomena that are more stably integrated into patients’ self-concepts. Conceptual neuroethical concerns may require revisiting as newer closed-loop devices are developed that involve less direct human oversight and/or target other neural systems, such as systems for mood or behavioral control. Nonetheless, our research illustrates that the empirical search for such effects must be situated within the context of chronic illness (Gilbert et al. 2019; Papoutsi et al. 2021).
Methodologically, the question of whether closed-loop devices will have such unintended and potentially destabilizing effects begs for inductive, case-based research designs that allow patients to share their experiences in an unstructured way (see for instance Thomson et al. 2020). Contrary to a one-time curative therapy like resection, closed-loop neuromodulation is a chronic, long-term and continuous treatment. Adjustment to the therapy therefore represents a process that unfolds over time and within particular medical and social contexts. Moreover, how patients adapt to their treatment also depends on their psychological state before undergoing brain stimulation, on their familial relationships, and on their goals and hopes for the therapy (also see Bluhm, Cabrera and McKenzie 2020). Exploring whether and how RNS shapes patient’s self-perception and personality therefore requires longitudinal, experience-near accounts within and beyond the clinical setting. This may be particularly true for closed-loop devices, as they do not yet form a standard element of broadly shared or integrated historical patient narratives (also see Gaille 2019).
In contrast, our second finding was that participants did attribute changes in patients’ personalities and self-perceptions to their illness and to pharmacological treatments commonly used in epilepsy. This finding supports our largely negative first finding, providing assurance that our ethnographic methods were suited to detect changes in personality and self-perception due to RNS if they were present. Furthermore, this finding is a reminder that while the neuroethics literature sometimes treats such changes as discrete phenomena, the experience of undergoing neuromodulation is not an isolated event but is instead embedded in a long history linked to the neurobiological, psychological and social dimensions of patients’ illness, to unsuccessful previous treatments attempts, and to patients’ personal circumstances (also see Gilbert et al. 2017). Whereas seizures present recurrent episodic events causing temporary disruptions, they also lead to psychiatric, cognitive, and social comorbidities causing continued disruptions of the patients’ life narratives, which can challenge a person’s sense of self (Kılınç et al. 2018). Social isolation and stigmatization tend to follow (Ryan, Kempner and Emlen 1980; Fernandes et al. 2011). Indeed, recent research suggests that in patients with drug-resistant epilepsy, psychiatric symptomatology, depression, and cognition are stronger determinants of quality of life than seizure frequency (Johnstone et al. 2021). Antiepileptic drugs, in turn, control seizures but do not cure the underlying disease. And as opposed to invasive, targeted second-line treatments, neuropsychological side effects of pharmaceutical drugs may be an accepted part of the risk vs. benefit calculation.
Our study was initially motivated by the goal of exploring whether patients or family members attributed changes in personality or self-perception to the use of closed-loop neuromodulation. Based on current conceptual literature we had originally focused on broad effects of the stimulation “limb” of the device’s closed loop. However, in our third finding we found that the neural sensing “limb” of this loop (Figure 1) had its own effects on patients’ experiences of illness, which could be independent from the effects of stimulation. It has previously been noted that the use of pharmacological therapies can promote popular conceptions of disease in biochemical terms, e.g., of depression as due to a “chemical imbalance” (Read et al. 2015). With RNS, the experience of treatment but also of visualizing illness led patients to conceptualize their disease in electrophysiological terms where previously only directly observable manifestations were visible. The diagnostic and treatment process therefore provided them with new cultural tools and metaphors for conceiving of epilepsy as an illness. In some cases, the data helped clarify otherwise ambiguous sources of symptoms and provided external corroboration for patients’ personal seizure accounts. The visualizations could serve to validate the patients’ experience of their illness, in an intersubjective social context with their epileptologists with whom they could share a new frame of reference and vocabulary. Whereas patients generally valued the ability to view their brain data, the process could also lead to new uncertainties (Hegde, Chiong and Rao 2021). If a patient’s experience is not corroborated by the technology, patients and others around them could begin to question their understanding of their illness. Generating what appear as objective records of illness may be interpreted as discrediting patients’ own reports, and providers will need to reintegrate scientific and social concepts of disease and illness as a basis for a functional system of their care (see Eisenberg 1977). In light of ongoing research on RNS devices’ potential to provide indications on upcoming seizure risk or perform seizure prediction (Gadhoumi et al. 2016; Baud et al. 2018), the availability of personalized brain data may lead to new diagnostic expectations and an appeal for increased therapeutic engagement (Hegde, Chiong and Rao 2021). More broadly, neuroethicists and neurotechnologists developing closed-loop devices may need to pay greater attention to how patients/users of forthcoming devices will make meaning of the neural data stored and interpreted by such devices. Design choices about user interface, options for data use and portability, and the degree of anticipated interaction with clinicians many have unanticipated effects on how patients/users understand their brains and brain conditions.
5. Limitations
Our ethnographic method’s limits are related to our study design and aims of exploratory, open-ended inquiry. First, our data were collected from a small number of participants who were sampled at one clinical site. We lack analytical leverage to understand the impacts of this site’s institutional culture on patients’ experiences. Second, our recruitment process was based on patients discussed in neurosurgical case conferences and depended on the consultation with treating epileptologists. Future studies may benefit from adopting a more systematic enrollment process, potentially also including patients who do not undergo surgical treatment. Third, as our respondents’ responses are grounded on cultural norms and ways of life, different perspectives and views could emerge in other cultural and therapeutic contexts.
Supplementary Material
Footnotes
6. Disclosure Statement
All authors of this paper report no conflicts of interest with regard to the work presented.
Publisher's Disclaimer: This is an Accepted Manuscript of an article published by Taylor & Francis AJOB Neuroscience ahead of print, available online: https://doi.org/10.1080/21507740.2021.1958100
1. References
- Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, and Rao VR. 2018. Multi-day rhythms modulate seizure risk in epilepsy. Nature Communications 9, no. 88: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O’Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, and Seale CG. 2015. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84, no. 8: 810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R, Cabrera L, and McKenzie R. 2020. What we (Should) Talk about when we Talk about Deep Brain Stimulation and Personal Identity. Neuroethics 13: 289–301. [Google Scholar]
- Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, Smith BJ, Gwinn RP, Doherty MJ, Noe KH, Zimmerman RS, Bergey GK, Anderson WS, Heck C, Liu CY, Lee RW, Sadler T, Duckrow RB, Hirsch LJ, Wharen RE, Tatum W, Srinivasan S, McKhann GM, Agostini MA, Alexopoulos AV, Jobst BC, Roberts DW, Salanova V, Witt TC, Cash SS, Cole AJ, Worrell GA, Lundstrom BN, Edwards JC, Halford JJ, Spencer DC, Ernst L, Skidmore CT, Sperling MR, Miller I, Geller EB, Berg MJ, Fessler AJ, Rutecki P, Goldman AM, Mizrahi EM, Gross RE, Shields DC, Schwartz TH, Labar DR, Fountain NB, Elias WJ, Olejniczak PW, Villemarette-Pittman NR, Eisenschenk S, Roper SN, Boggs JG, Courtney TA, Sun FT, Seale CG, Miller KL, Skarpaas TL, and Morrell MJ. 2020. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 95, no. 9: e1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. 2015. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84, no.8: 810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandy BM, Fields MC, Knowlton RC, Chang EF, Szaflarski JP, Marcuse LV, and Rao VR. 2020. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia 61, no. 1: 96–106 [DOI] [PubMed] [Google Scholar]
- Cascio MA, Lee E, Vaudrin N, and Freedman DA. 2019. A Team-based Approach to Open Coding: Considerations for Creating Intercoder Consensus. Field Methods 31, no. 2: 116–30. [Google Scholar]
- Charmaz K, and Olesen V. 1997. Ethnographic research in medical sociology its foci and distinctive contributions. Sociological Methods & Research 25, no. 4:452–94. [Google Scholar]
- Eisenberg L 1977. Disease and illness Distinctions between professional and popular ideas of sickness. Culture, Medicine and Psychiatry 1: 9–23. [DOI] [PubMed] [Google Scholar]
- Emerson RM, Fretz RI, and Shaw LL. 1995. Writing Ethnographic Fieldnotes. Chicago: University of Chicago Press. [Google Scholar]
- Fernandes PT, Snape DA, Beran RG, and Jacoby A. 2011. Epilepsy stigma: What do we know and where next? Epilepsy & Behavior 22, no. 1: 55–62. [DOI] [PubMed] [Google Scholar]
- Fountas KN, Smith JR, Murro AM, Politsky J, Park YD, and Jenkins PD. 2005. Implantation of a Closed-Loop Stimulation in the Management of Medically Refractory Focal Epilepsy. Stereotactic and Functional Neurosurgery 83: 153–58. [DOI] [PubMed] [Google Scholar]
- Friese S 2019. ATLAS.ti 8 Mac - User Manual. Berlin: ATLAS.ti Scientific Software Development GmbH. [Google Scholar]
- Gaille M 2019. “Patient’s lived experience” - New insights from the “scene” of deep-brain stimulation medical care. Medicine, Health Care and Philosophy 22: 339–42. [DOI] [PubMed] [Google Scholar]
- Gadhoumi K, Lina JM, Mormann F, and Gotmana J. 2016. Seizure prediction for therapeutic devices: A review. Journal of Neuroscience Methods 260: 270–82. [DOI] [PubMed] [Google Scholar]
- Geller EB 2018. Responsive neurostimulation: review of clinical trials and insights into focal epilepsy. Epilepsy & Behavior Suppl no. 88: 11–20. [DOI] [PubMed] [Google Scholar]
- Gilbert F, Goddard E, Viaña JNM, Carter A, and Horne M. 2017. I Miss Being Me: Phenomenological Effects of Deep Brain Stimulation. AJOB Neuroscience 8, no. 2: 96–109. [Google Scholar]
- Gilbert F, Viaña JNM, and Ineichen C. 2018. Deflating the “DBS causes personality changes” bubble. Neuroethics. doi: 10.1007/s12152-018-9373-8 [DOI] [Google Scholar]
- Gilbert F, O’Brien T, and Cook M. 2018. The Effects of Closed-Loop Brain Implants on Autonomy and Deliberation: What are the Risks of Being Kept in the Loop? Cambridge Quarterly of Healthcare Ethics 27, no. 2: 316–25. [DOI] [PubMed] [Google Scholar]
- Gilbert F, Cook M, O’Brien T, and Illes J. 2019. Embodiment and Estrangement: Results from a First-in-Hum“Intelligent BCI” Trial. Science and Engineering Ethics 25, no. 1: 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering S, Klein E, Dougherty DD, and Widge AS. 2017. Staying in the Loop: Relational Agency and Identity in Next-Generation DBS for Psychiatry. AJOB Neuroscience 8, no. 2: 59–70. [Google Scholar]
- Gonzalez Andino SL, Herrera-Rincon C, Panetsos F, and Grave De Peralta R. 2011. Combining BMI stimulation and mathematical modeling for acute stroke recovery and neural repair. Frontiers in Neuroscience 25, no. 5: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M, Chiong W, and Rao VR. 2021. New Ethical and Clinical Challenges in “Closed-Loop” Neuromodulation. Neurology. Published online: 16 March 2021. 10.1212/WNL.0000000000011834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosiewicz B, and Morrell M. 2020. The RNS System: brain-responsive neurostimulation for the treatment of epilepsy. Expert Review of Medical Devices: 10.1080/17434440.2019.1683445. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Malpas CB, Velakoulis D, Kwan P, and O’Brien TJ. 2021. Psychiatric symptoms are the strongest predictors of quality of life in patients with drug-resistant epilepsy or psychogenic nonepileptic seizures. Epilepsy & Behavior 117: 107861. [DOI] [PubMed] [Google Scholar]
- Kellmeyer P 2018. Big Brain Data: On the Responsible Use of Brain Data from Clinical and Consumer-Directed Neurotechnological Devices. Neuroethics: 1–15. [Google Scholar]
- Kellmeyer P, Cochrane T, Müller O, Mitchell C, Ball T, Fins JJ, et al. 2016. The Effects of Closed-Loop Medical Devices on the Autonomy and Accountability of Persons and Systems. Cambridge Quarterly of Healthcare Ethics 25, no. 4: 623–33. [DOI] [PubMed] [Google Scholar]
- Kılınç S, Campbell C, Guy A, and van Werscha A. 2018. Epilepsy, identity, and the experience of the body. Epilepsy & Behavior 89: 42–47. [DOI] [PubMed] [Google Scholar]
- Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, and Gumnit RJ. 2010. National Association of Epilepsy Centers. Essential services, personnel, and facilities in specialized epilepsy centers—revised 2010 guidelines. Epilepsia 51, no. 11: 2322–33. [DOI] [PubMed] [Google Scholar]
- Lofland J 1995. Analytic ethnography: features, failings, and futures. Journal of Contemporary Ethnography 24, no. 1: 30–67. [Google Scholar]
- Ma BB, and Rao VR. 2018. Responsive neurostimulation: candidates and considerations. Epilepsy & Behavior 88: 388–95. [DOI] [PubMed] [Google Scholar]
- Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, Smith BJ, Gwinn RP, Doherty MJ, Noe KH, and Zimmerman RS. 2020. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 95, no. 9: e1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi C, Collins CDE, Christopher A, Shaw SE, and Greenhalgh T. 2021. Interrogating the promise of technology in epilepsy care: systematic, hermeneutic review. Sociology of Health & Illness 00: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parastarfeizabadi M, and Kouzani AZ. 2017. Advances in closed-loop deep brain stimulation devices. Journal of NeuroEngineering and Rehabilitation 14, no. 79: 10.1186/s12984-017-0295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Foffani G, Rossiad L, and Marceglia S. 2013. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Experimental Neurology 245 (July): 77–86. [DOI] [PubMed] [Google Scholar]
- Pugh J 2020. Clarifying the Normative Significance of ‘Personality Changes’ Following Deep Brain Stimulation. Science and Engineering Ethics 26, no. 3: 1655–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J, Cartwright C, Gibson K, Shiels C, and Magliano L. 2015. Beliefs of people taking antidepressants about the causes of their own depression. Journal of Affective Disorders 174: 150–56. [DOI] [PubMed] [Google Scholar]
- Reeves SA Kuper, and Hodges BD. 2008. Qualitative research methodologies: ethnography. BMJ 337: a1020. [DOI] [PubMed] [Google Scholar]
- Ryan R, Kempner K, and Emlen AC. 1980. The Stigma of Epilepsy as a Self-Concept. Epilepsia 21, no. 4: 433–444. [DOI] [PubMed] [Google Scholar]
- Scangos KW, Makhoul GS, Sugrue LP, Chang EF, and Krystal AD. 2021. State-dependent responses to intracranial brain stimulation in a patient with depression. Nature Medicine. Published online: 18 January 2021. 10.1038/s41591-020-01175-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senova S, Chaillet A, and Lozano AM. 2018. Fornical Closed-Loop Stimulation for Alzheimer’s Disease. Trends in Neurosciences 41, no. 7: 418–28. [DOI] [PubMed] [Google Scholar]
- Shirvalkar P, Veuthey TL, Dawes HE, and Chang EF. 2018. Closed-loop deep brain stimulation for refractory chronic pain. Frontiers in Computational Neuroscience 12 (March): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, and Morrell MJ. 2014. Device Profiles - The RNS System: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Review of Medical Devices 11, no. 6: 563–72. [DOI] [PubMed] [Google Scholar]
- Thijs RD, Surges R, O’Brien TJ, and Sander JW. 2019. Epilepsy in adults. The Lancet 393, no. 10172: 689–701. [DOI] [PubMed] [Google Scholar]
- Thomas GP, and Jobst BC. 2015. Critical review of the responsive neurostimulator system for epilepsy. Journal of Medical Devices (Auckl) 8: 405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CJ, Segrave RA, Racine E, Warren N, Thyagarajan D, and Carter A. 2020. “He’s Back so I’m Not Alone”: The Impact of Deep Brain Stimulation on Personality, Self, and Relationships in Parkinson’s Disease. Qualitative Health Research 30, no. 14: 2217–33. [DOI] [PubMed] [Google Scholar]
- Tian N, Boring M, Kobau R, Zack MM, and Croft JB. 2018. Active epilepsy and seizure control in adults — United States, 2013 and 2015. Morbidity and Mortality Weekly Report (MMWR) 67: 437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Ellard KK, Paulk AC, Basu I, Yousefi A, Zorowitz S, Gilmour A, Afzal A, Deckersbach T, Cash SS, Kramer MA, Eden UT, Dougherty DD, and Eskandar EN. 2017. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Experimental Neurology 287, pt. 4: 461–72. [DOI] [PubMed] [Google Scholar]
- Yan H, and Ibrahim GM. 2019. Resective epilepsy surgery involving eloquent cortex in the age of responsive neurostimulation: A value-based decision-making framework. Epilepsy & Behavior 99, no. 106479: 10.1016/j.yebeh.2019.106479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


