Abstract
Background:
Household transmission contributes to SARS-CoV-2 spread, but the role of children in transmission is unclear. We conducted a study that included symptomatic and asymptomatic children and adults exposed to SARS-CoV-2 in their households with the objective of determining how SARS-CoV-2 is transmitted within households.
Methods:
In this case-ascertained antibody-surveillance study, we enrolled households in Ottawa, Ontario, in which at least 1 household member had tested positive for SARS-CoV-2 on reverse transcription polymerase chain reaction testing. The enrolment period was September 2020 to March 2021. Potentially eligible participants were identified if they had tested positive for SARS-CoV-2 at an academic emergency department or affiliated testing centre; people who learned about the study through the media could also self-identify for participation. At least 2 participants were required for a household to be eligible for study participation, and at least 1 enrolled participant per household had to be a child (age < 18 yr). Enzyme-linked immunosorbent assays were used to evaluate SARS-CoV-2-specific IgA, IgM and IgG against the spike-trimer and nucleocapsid protein. The primary outcome was household secondary attack rate, defined as the proportion of household contacts positive for SARS-CoV-2 antibody among the total number of household contacts participating in the study. We performed descriptive statistics at both the individual and household levels. To estimate and compare outcomes between patient subgroups, and to examine predictors of household transmission, we fitted a series of multivariable logistic regression with robust standard errors to account for clustering of individuals within households.
Results:
We enrolled 695 participants from 180 households: 180 index participants (74 children, 106 adults) and 515 of their household contacts (266 children, 249 adults). A total of 487 household contacts (94.6%) (246 children, 241 adults) had SARS-CoV-2 antibody testing, of whom 239 had a positive result (secondary attack rate 49.1%, 95% confidence interval [CI] 42.9%–55.3%). Eighty-eight (36.8%, 95% CI 29.3%–43.2%) of the 239 were asymptomatic; asymptomatic rates were similar for children (51/130 [39.2%, 95% CI 30.7%–48.5%]) and adults (37/115 [32.2%, 95% CI 24.2%–41.4%]) (odds ratio [OR] 1.3, 95% CI 0.8–2.1). Adults were more likely than children to transmit SARS-CoV-2 (OR 2.2, 95% CI 1.3–3.6). The odds of transmission from asymptomatic (OR 0.6, 95% CI 0.2–1.4) versus symptomatic (OR 0.9, 95% CI 0.6–1.4) index participants to household contacts was uncertain. Predictors of household transmission included household density (number of people per bedroom), relationship to index participant and number of cases in the household.
Interpretation:
The rate of SARS-CoV-2 transmission within households was nearly 50% during the study period, and children were an important source of spread. The findings suggest that children are an important driver of the COVID-19 pandemic; this should inform public health policy.
Household transmission is an important contributor to the spread of SARS-CoV-2 infection.1–5 Household exposure to an infected person is associated with a 10-fold higher risk of infection compared to exposure outside the home.6 However, the reported rate of SARS-CoV-2 spread within households depends on the surveillance method.6 Meta-analyses of household transmission studies in which viral swabs were primarily used for detection showed a secondary attack rate of 19%,1,2 but reliance on viral swabs may underestimate transmission, and experts have advocated for the addition of serologic testing to enhance studies of household transmission.7–9
Evaluating SARS-CoV-2-specific antibodies provides additional information about recent and past infection, as antibodies are detected reliably within 1–2 weeks after symptom onset.10–12 Previous studies using antibody detection suggested that the rate of household transmission may range from 20% to 80%.13–18 These studies were limited: some tested small proportions of household members, and others excluded children or focused on transmission by adults.9,13–15,17–21 Few investigated the proportion of household members who developed antibodies after exposure to an infected child.
The role of children in transmission warrants attention, since they are often asymptomatic or have mild symptoms. 16,22–28 Addressing this gap in the literature would allow a more evidence-based approach to public health initiatives, given that COVID-19 pandemic control strategies have often affected the lives of children and youth (e.g., school closures, suspension of recreational activities), as well as negatively affecting their overall well-being.29,30
Analysis of household transmission of SARS-CoV-2 is central to the development of health policies that protect vulnerable people while minimizing the impact on children. 29–31 We conducted an antibody surveillance study that included symptomatic and asymptomatic children and adults exposed to SARS-CoV-2 in their households with the objective of determining how SARS-CoV-2 infection is transmitted within households.
Methods
Study design and setting
We conducted a prospective, case-ascertained antibody-surveillance study of households in Ottawa, Ontario (population 1 million32) in which at least 1 member had SARS-CoV-2 infection from September 2020 to March 2021, when community cases ranged from 6 to 12 per 100 000 population.33 The study was reported according to the STROBE guideline for cohort studies.34
Participants
Households were eligible if an adult or child tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR) from a throat or nasopharyngeal swab. Polymerase chain reaction testing was voluntary and was performed in accordance with public health guidelines at the time (e.g., symptoms, history of travel, contact at high risk).35 In addition to the SARS-CoV-2-positive person, enrolment of at least 1 additional household member was required; an attempt was made to enrol all household members. At least 1 member of the household had to be a child (age < 18 yr).
We defined household members as people residing in the same dwelling as the index participant. If more than 1 person in a household tested positive within 14 days, the index participant was the person who first developed symptoms or, in the absence of symptoms, the first to test positive. There was no maximum allowable time between index participant infection and study enrolment. Households were excluded if any member had received a COVID-19 vaccine before enrolment or was not comfortable communicating in English or French.
We informed the Ottawa population about the study through media and social media campaigns. Self-identified potentially eligible participants were invited to contact the study team via a dedicated study email address. Potentially eligible participants (those who tested positive for SARS-CoV-2 at any of the 3 academic emergency departments or the affiliated regional COVID-19 testing centre) were also identified by the study team.
Study protocol
In-person enrolment visits were booked within 14 days of patient screening or consent, but a minimum of 14 days was required between a positive RT-PCR test result and the study visit. Enrolment visits were conducted by a trained study team at the Children’s Hospital of Eastern Ontario; all household members attended the same appointment.
Data collection
A questionnaire informed by the World Health Organization household transmission investigation protocol35 was administered by a trained research assistant to standardize and harmonize data collection in order to optimize accuracy; a questionnaire was completed for the index participant and each household member at the study visit. Data were collected on demographic characteristics, household composition and density (number of people per bedroom36), COVID-19 symptoms, distancing protocols in the home during the illness, medical and smoking histories, and all RT-PCR testing completed before enrolment. Blood samples for SARS-CoV-2 antibody testing were drawn from all participants at the study visit. Within 3 hours of phlebotomy, the serum was separated and stored at −80°C until ready for analysis at the Langlois Laboratory, University of Ottawa.
Automated chemiluminescent enzyme-linked immunosorbent assays (ELISAs), adapted and optimized from the assay described by Isho and colleagues,37 were used to evaluate SARS-CoV-2-specific IgA, IgM and IgG against the spike-trimer and nucleocapsid protein. This serologic testing platform was validated and compared with 10 commercial platforms by the National Microbiology Laboratory of Canada and was found to perform as well as the most accurate commercial tests, with sensitivity and specificity greater than 98% (Appendix 1, Figure S1, available at www.cmajopen.ca/content/10/2/E357/suppl/DC1).38
For each sample of a specific plate, the average background luminescence value from up to 8 negative control samples was removed. The resulting values were rescaled based on the luminescence value corresponding to the half-maximal inhibitory concentration value of a 4-parameter log-logistic standard curve also included on the plate. Signal-to-cut-off values were calculated with cut-offs corresponding to a 2% false-discovery rate for each antigen–antibody pair, derived from the scaled luminescence of 580 prepandemic serum samples calculated by means of the same approach.
Samples were considered antibody positive for a particular isotype (IgG, IgA or IgM) when both antispike and anti-nucleocapsid antibodies were detected above the cut-off values (signal-to-cut-off value ≥ 1) for that isotype. Samples were considered positive for SARS-CoV-2 antibody if they were positive for IgG or for both IgA and IgM.
Outcomes
The primary outcome was the household secondary attack rate — the proportion of infected household contacts (positive for SARS-CoV-2 antibody) among the total number of household contacts participating in the study.39 Household contacts were deemed infected if they met the criteria for SARS-CoV-2 antibody positivity. Secondary outcomes were the proportion of asymptomatic infections among household contacts, by age, and clinical risk factors for household transmission.
We defined asymptomatic infection as positivity for SARS-CoV-2 antibody in the absence of symptoms. We chose risk factors for transmission a priori, based on prior studies and clinical knowledge.1,2,28 We defined distancing as isolating from the SARS-CoV-2-positive household member(s) or always masking indoors in the presence of the SARS-CoV-2-positive member(s), or both.35
Statistical analysis
We described patient and household characteristics using summary statistics. We described variables with patients stratified by participant type (index participants or household contact) or age (child or adult) subgroups, or both.
To estimate and compare the proportion of household contacts who developed SARS-CoV-2 antibodies among patient subgroups based on age of the index/household contact, we fitted logistic regression models specifying index participant’s age alone; household contact’s age alone; an interaction between index (child v. adult) and household contact’s age (child v. adult); and an interaction between the index’s age (6 categories) and household contact’s age (child v. adult), with estimation of robust (Huber–White) standard errors to account for clustering of individuals within a household unit.40,41 We derived estimates of proportions, odds ratios (ORs) and 95% confidence intervals (CIs) from these models.
To examine predictors of SARS-CoV-2 antibody development among household contacts, we applied a multivariable logistic regression with estimation of robust standard errors with household as the clustering unit. Predictors specified included age, sex, total number of SARS-CoV-2-positive household members (including the index participant), household density, mask-wearing frequency, whether completely isolated from index participant, presence of underlying comorbidity, whether present smoker or vaper, relationship to index participant, whether the index participant was admitted to hospital and whether the index participant was symptomatic. We expressed associations as adjusted OR and associated 95% CI. We assessed the discriminative ability of the model using the C-statistic. Observations were excluded if there were any missing data.
We performed statistical analyses using R software, version 4.0.5 (R Foundation for Statistical Computing).
Ethics approval
The study received approval from the Children’s Hospital of Eastern Ontario Research Ethics Board (20/81X), the Ottawa Health Science Network Research Ethics Board (20200673-01K) and the University of Ottawa Health Sciences and Science Research Ethics Board (20200358). Informed written consent was obtained from all participants or their parent or guardian, and assent was obtained when appropriate.
Results
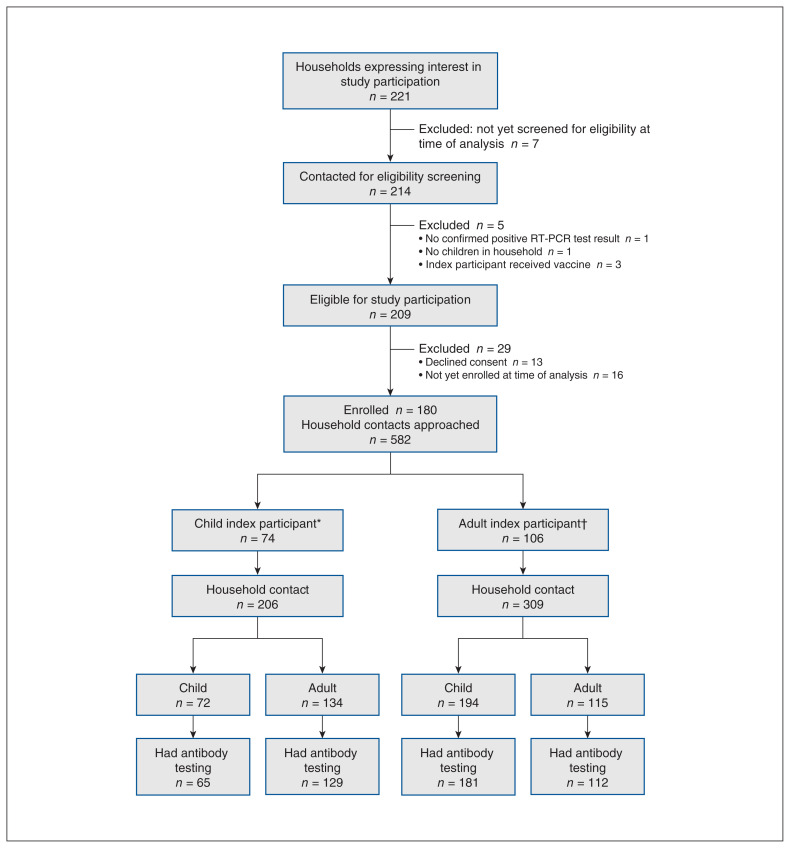
A total of 180 people (74 children, 106 adults) confirmed to be SARS-CoV-2-positive and 515 (266 children, 249 adults) of their household contacts were enrolled (Figure 1). Index participants and household contacts were enrolled a median of 46 days (interquartile range [IQR] 32–86 d) and 41 days (IQR 28–73 d), respectively, after infection was confirmed in the index participant. A median of 4 (IQR 3–4) members per household were enrolled; in 137 households (76.1%), all members were enrolled. In 156 households (87.2%), all children participated in the study, and in 147 households (82.1%), all adults participated. The median household density was 1.25 (IQR 1.0–1.3) people per bedroom. Of the 454 household contacts (88.1%) who reported being tested for SARS-CoV-2 before study enrolment, 158 (34.8%) had a positive result within 14 days of the index participant’s positive test result.
Figure 1:
Flow diagram showing screening and enrolment of study population. *Age less than 18 years. †Age 18 years or more. Note: RT-PCR = reverse transcription polymerase chain reaction.
Baseline characteristics of the index participants and household contacts are provided in Table 1 and Appendix 1, Table S1. Of the 106 adult index participants, 57 (54.3%) were female, and the median age was 40 (IQR 36–45) years. Of the 74 child index participants, 24 (32.4%) were female, and the median age was 8 (IQR 5–12) years. There were 19 asymptomatic infections among the index participants, 13 (68%) in children. Forty-five household contacts (8.7%) were completely isolated from the index participant, and 44 (8.5%) always wore a mask while indoors with the index participant.
Table 1:
Baseline characteristics of people who tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction (index participants) and household contacts
| Characteristic | Index participants, no. (%)* | Household contacts, no. (%)* | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Overall n = 180 |
Children n = 74 |
Adults n = 106 |
Overall n = 515 |
Children n = 266 |
Adults n = 249 |
|
| Age, median (IQR), yr | 33.9 (9.6–41.5) | 8.2 (5.4–12.5) | 40.5 (36.3–45.3) | 17.1 (8.7–41.4) | 8.9 (5.2–12.9) | 41.9 (36.6–47.0) |
|
| ||||||
| Age group, yr | ||||||
|
| ||||||
| ≤ 3 | 11 (6.1) | 11 (14.9) | – | 47 (9.1) | 47 (17.7) | – |
|
| ||||||
| 4–11 | 43 (23.9) | 43 (58.1) | – | 142 (27.6) | 142 (53.4) | – |
|
| ||||||
| 12–17 | 20 (11.1) | 20 (27.0) | – | 77 (15.0) | 77 (28.9) | – |
|
| ||||||
| 18–29 | 9 (5.0) | – | 9 (8.5) | 17 (3.3) | – | 17 (6.8) |
|
| ||||||
| 30–39 | 42 (23.3) | – | 42 (39.6) | 80 (15.5) | – | 80 (32.1) |
|
| ||||||
| 40–49 | 44 (24.4) | – | 44 (41.5) | 108 (21.0) | – | 108 (43.4) |
|
| ||||||
| ≥ 50 | 11 (6.1) | – | 11 (10.4) | 44 (8.5) | – | 44 (17.7) |
|
| ||||||
| Sex | ||||||
|
| ||||||
| Female | 81 (45.0) | 24 (32.4) | 57 (53.8) | 275 (53.4) | 139 (52.3) | 136 (54.6) |
|
| ||||||
| Male | 98 (54.4) | 50 (67.6) | 48 (45.3) | 238 (46.2) | 126 (47.4) | 112 (45.0) |
|
| ||||||
| Missing | 1 (0.6) | 0 (0.0) | 1 (0.9) | 2 (0.4) | 1 (0.4) | 1 (0.4) |
|
| ||||||
| Indigenous | ||||||
|
| ||||||
| Yes | 1 (0.6) | 0 (0.0) | 1 (0.9) | 17 (3.3) | 9 (3.4) | 8 (3.2) |
|
| ||||||
| No | 179 (99.4) | 74 (100.0) | 105 (99.0) | 494 (95.9) | 255 (95.9) | 239 (96.0) |
|
| ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.8) | 2 (0.8) | 2 (0.8) |
|
| ||||||
| Race or ethnicity† | ||||||
|
| ||||||
| Black | 15 (8.3) | 7 (9.5) | 8 (7.5) | 44 (8.6) | 27 (10.2) | 17 (6.9) |
|
| ||||||
| Asian | 3 (1.7) | 2 (2.7) | 1 (0.9) | 18 (3.5) | 10 (3.8) | 8 (3.2) |
|
| ||||||
| South Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.8) | 3 (1.1) | 1 (0.4) |
|
| ||||||
| West Asian/Arab | 15 (8.3) | 7 (9.5) | 8 (7.5) | 44 (8.6) | 25 (9.5) | 19 (7.7) |
|
| ||||||
| Latin American | 5 (2.8) | 5 (6.8) | 0 (0.0) | 8 (1.6) | 4 (1.5) | 4 (1.6) |
|
| ||||||
| White | 149 (82.8) | 62 (83.8) | 87 (82.1) | 415 (81.1) | 216 (81.8) | 199 (80.2) |
|
| ||||||
| Other‡ | 2 (1.1) | 1 (1.4) | 1 (0.9) | 3 (0.6) | 2 (0.8) | 1 (0.4) |
|
| ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.6) | 2 (0.8) | 1 (0.4) |
|
| ||||||
| Underlying comorbidity | ||||||
|
| ||||||
| Yes | 46 (25.6) | 7 (9.5) | 39 (36.8) | 93 (18.0) | 42 (15.8) | 51 (20.5) |
|
| ||||||
| No | 134 (74.4) | 67 (90.5) | 67 (63.2) | 419 (81.4) | 222 (83.4) | 197 (79.1) |
|
| ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.6) | 2 (0.8) | 1 (0.4) |
|
| ||||||
| Smoking or vaping | 46 (25.6) | 5 (6.8) | 41 (38.7) | 142 (27.6) | 14 (5.3) | 128 (51.4) |
|
| ||||||
| Daily/frequently | 6 (13.0) | 1 (20.0) | 5 (12.2) | 20 (14.1) | 0 (0.0) | 20 (15.6) |
|
| ||||||
| Sometimes/rarely | 12 (26.1) | 2 (40.0) | 10 (24.4) | 25 (17.6) | 6 (42.8) | 19 (14.8) |
|
| ||||||
| Past smoker/vaper | 28 (60.9) | 2 (40.0) | 26 (63.4) | 94 (66.2) | 6 (42.8) | 88 (68.8) |
|
| ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.1) | 2 (14.3) | 1 (0.8) |
|
| ||||||
| Working outside home§ | ||||||
|
| ||||||
| Yes | 52 (28.9) | 3 (4.1) | 49 (46.2) | 88 (17.1) | 4 (1.5) | 84 (33.7) |
|
| ||||||
| No | 128 (71.1) | 71 (95.9) | 57 (53.8) | 424 (82.3) | 260 (97.7) | 164 (65.9) |
|
| ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.6) | 2 (0.8) | 1 (0.4) |
|
| ||||||
| Student‡ | ||||||
|
| ||||||
| Yes | 76 (42.2) | 69 (93.2) | 7 (6.6) | 261 (50.7) | 245 (92.1) | 16 (6.4) |
|
| ||||||
| No | 104 (57.8) | 5 (6.8) | 99 (93.4) | 251 (48.7) | 19 (7.1) | 232 (93.2) |
|
| ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.6) | 2 (0.8) | 1 (0.4) |
|
| ||||||
| Symptom burden¶ | ||||||
|
| ||||||
| Asymptomatic | 19 (10.6) | 13 (17.6) | 6 (5.7) | 260 (50.5) | 140 (52.6) | 120 (48.2) |
|
| ||||||
| Mild | 154 (85.6) | 60 (81.1) | 94 (88.7) | 248 (48.2) | 124 (46.6) | 124 (49.8) |
|
| ||||||
| Moderate | 5 (2.8) | 1 (1.4) | 4 (3.8) | 3 (0.6) | 0 (0.0) | 3 (1.2) |
|
| ||||||
| Severe | 2 (1.1) | 0 (0.0) | 2 (1.9) | 1 (0.2) | 0 (0.0) | 1 (0.4) |
|
| ||||||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.6) | 2 (0.8) | 1 (0.4) |
Note: IQR = interquartile range.
Except where noted otherwise.
Self-reported. Participants could choose more than 1 category.
Unspecified selection of more than 1 category.
At the time the participant or a household member tested positive.
Mild = no hospital admission, moderate = hospital admission but not intensive care unit admission, severe = intensive care unit admission.
Incidence of household infection and transmission
Testing for SARS-CoV-2 antibody was done in 487 household contacts (94.6%), of whom 239 had a positive result (secondary attack rate 49.1%, 95% CI 42.9%–55.3%) (Table 2). Of the 239, 88 (36.8%, 95% CI 29.3%–43.2%) were asymptomatic. Asymptomatic rates were similar among child household contacts (51/130 [39.2%, 95% CI 30.7%–48.5%) and adult household contacts (37/115 [32.2%, 95% CI 24.2%–41.4%]) (OR 1.3, 95% CI 0.8–2.1). Positivity rates among those tested less than 30 days, 30–90 days or more than 90 days after a positive SARS-CoV-2 test result were similar (Appendix 1, Table S2).
Table 2:
Proportion of household contacts testing positive for SARS-CoV-2 antibodies by age of index participant
| Group; age, yr | Household contact, all ages | Child household contacts | Adult household contacts | OR (95% CI)† | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n/N* | % (95% CI) | n/N* | % (95% CI) | n/N* | % (95% CI) | ||
| Index participants, all ages | 239/487 | 49.1 (42.9–55.3) | 130/246 | 52.8 (45.3–60.2) | 109/241 | 45.2 (38.4–52.2) | 0.8 (0.6–1.1) |
|
| |||||||
| Child index participants | 72/194 | 37.1 (27.7–47.6) | 23/65 | 35.4 (23.1–50.0) | 49/129 | 38.0 (28.6–48.4) | 1.2 (0.8–2.0) |
|
| |||||||
| ≤ 3 | 6/32 | 18.8 (7.0–41.3) | 1/11 | 9.1 (1.0–50.3) | 5/21 | 23.8 (7.3–55.5) | 3.2 (0.2–48.0) |
|
| |||||||
| 4–11 | 47/113 | 41.6 (29.0–55.4) | 17/40 | 42.5 (26.7–60.0) | 30/73 | 41.1 (28.4–55.1) | 1.0 (0.6–1.7) |
|
| |||||||
| 12–17 | 19/49 | 38.8 (20.8–60.4) | 5/14 | 35.7 (12.4–68.5) | 14/35 | 40.0 (23.3–59.3) | 1.6 (0.7–3.7) |
|
| |||||||
| Adult index participants | 167/293 | 57.0 (49.5–64.2) | 107/181 | 59.1 (50.3–67.4) | 60/112 | 53.6 (44.8–62.1) | 0.9 (0.6–1.3) |
|
| |||||||
| 18–29 | 17/32 | 53.1 (32.3–72.9) | 7/15 | 46.7 (26.0–68.6) | 10/17 | 58.8 (34.0–79.9) | 1.6 (0.8–3.3) |
|
| |||||||
| 30–49 | 130/230 | 56.5 (47.7–64.9) | 90/152 | 59.2 (49.4–68.3) | 40/78 | 51.3 (40.7–61.8) | 0.8 (0.6–1.2) |
|
| |||||||
| ≥ 50 | 20/31 | 64.5 (43.9–80.9) | 10/14 | 71.4 (34.9–92.1) | 10/17 | 58.8 (39.0–76.1) | 0.7 (0.2–2.8) |
Note: CI = confidence interval, OR = odds ratio.
n/N = number of household contacts with SARS-CoV-2 antibodies in households in which the index participant was in a specified age category/total number of household contacts in households in which the index participant was in specified age category.
Odds that an adult versus a child in the household would become infected from index participant of a given age.
In 57/181 households (31.5%, 95% CI 25.2%–38.6%), all household members were positive for SARS-CoV-2 antibody; there was no secondary transmission in 27 households (14.9%, 95% CI 10.5%–20.8%).
Transmission by age
The proportion and associations of household contacts positive for SARS-CoV-2 antibody, by age of the index participant, are shown in Table 2 and Appendix 1, Table S3. Adult index participants were more likely than child index participants to transmit infection (OR 2.2, 95% CI 1.3–3.6). In households in which the index participant was a child, 72/194 members (37.1%, 95% CI 27.7%–47.6%) had SARS-CoV-2 antibody; the odds of transmission to other children versus adults was 1.2 (95% CI 0.8–2.0).
The youngest children (age ≤ 3 yr) transmitted to a lower proportion of household contacts (6/32 [18.8%, 95% CI 7.0%–41.3%]) than did those aged 4–11 (47/113 [41.6%, 95% CI 29.0%–55.4%]) and 12–17 (19/49 [38.8%, 95% CI 20.8%–60.4%]) (OR 3.0, 95% CI 0.9–10.0 and 2.7, 95% CI 0.7–10.5, respectively). In households in which the index participant was an adult, 167/293 members (57.0%, 95% CI 49.5%–64.2%) were positive for SARS-CoV-2 antibody; the odds of transmission to children versus adults was 0.9 (95% CI 0.6–1.3). For index participants of all ages, the secondary risk to child versus adult household contacts was similar (OR 0.8, 95% Cl 0.6–1.1).
Transmission by symptoms
The rate of transmission from asymptomatic index participants to household contacts was 35.3% (95% CI 18.5%–56.8%), and the rate of transmission from symptomatic index participants was 50.7% (95% CI 44.2%–57.1%) (OR 0.6, 95% CI 0.2–1.4) (Table 3).
Table 3:
Proportion of household contacts who tested positive for SARS-CoV-2 antibodies by age group and symptom status of index participant
| Age group; symptom status | Household contacts, all ages | Child household contacts | Adult household contacts | OR (95% CI)† | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n/N* | % (95% CI) | n/N* | % (95% CI) | n/N* | % (95% CI) | ||
| Index participants, all ages | |||||||
|
| |||||||
| Asymptomatic | 18/51 | 35.3 (18.5–56.8) | 9/21 | 42.9 (20.4–68.7) | 9/30 | 30.0 (14.3–52.4) | 0.6 (0.3–1.4) |
|
| |||||||
| Symptomatic | 221/436 | 50.7 (44.2–57.1) | 121/225 | 53.8 (46.0–61.4) | 100/211 | 47.4 (40.2–54.7) | 0.9 (0.6–1.2) |
|
| |||||||
| OR (95% CI)‡ | 0.6 (0.2–1.4) | 0.7 (0.3–1.9) | 0.5 (0.2–1.3) | ||||
|
| |||||||
| Child index participants | |||||||
|
| |||||||
| Asymptomatic | 10/34 | 29.4 (12.3–55.3) | 4/11 | 36.4 (15.7–63.7) | 6/23 | 26.1 (9.7–53.6) | 0.6 (0.3–1.3) |
|
| |||||||
| Symptomatic | 62/160 | 38.8 (28.3–50.4) | 19/54 | 35.2 (21.3–52.1) | 43/106 | 40.6 (30.0–52.1) | 1.4 (0.8–2.4) |
|
| |||||||
| OR (95% CI)§ | 0.7 (0.2–2.3) | 1.3 (0.4–4.4) | 0.6 (0.2–1.9) | ||||
|
| |||||||
| Adult index participants | |||||||
|
| |||||||
| Asymptomatic | 8/17 | 47.1 (13.1–84.0) | 5/10 | 50.0 (10.9–89.1) | 3/7 | 42.9 (11.0–82.0) | 0.9 (0.2–4.1) |
|
| |||||||
| Symptomatic | 159/276 | 57.6 (50.1–64.8) | 102/171 | 59.6 (50.8–67.9) | 57/105 | 54.3 (45.4–62.9) | 0.9 (0.6–1.3) |
|
| |||||||
| OR (95% CI)¶ | 0.7 (0.1–3.0) | 0.7 (0.1–3.6) | 0.7 (0.1–3.6) | ||||
Note: CI = confidence interval, OR = odds ratio.
n/N = number of household contacts with SARS-CoV-2 antibodies in households in which the index participant was in a specified age category/total number of household contacts in households in which the index participant was in specified age category.
Odds that a child versus an adult household contact would become infected from the index participant in the given category.
Odds that a given type of household contact would become infected from an asymptomatic versus a symptomatic index participant of any age.
Odds that a given type of household contact would become infected from an asymptomatic versus a symptomatic child index participant.
Odds that a given type of household contact would become infected from an asymptomatic versus a symptomatic adult index participant.
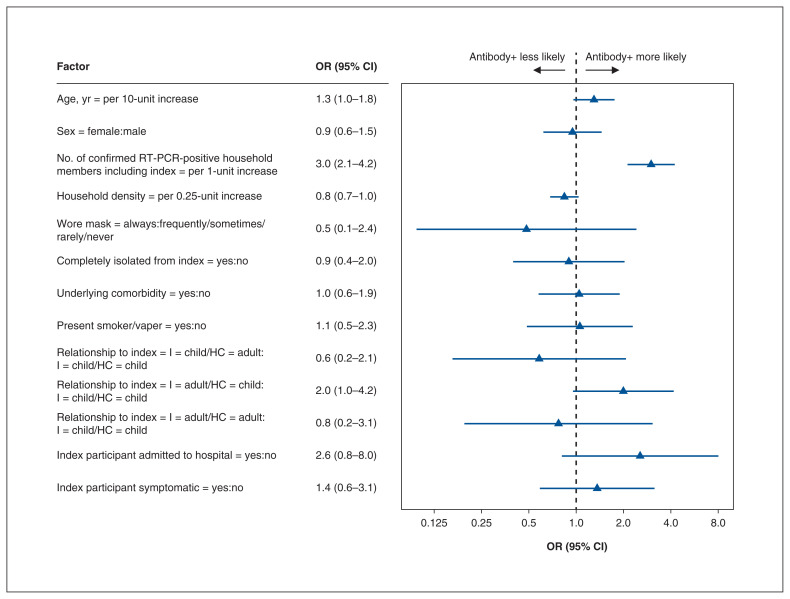
Risk factors for transmission
Results from the multivariable logistic regression analyses are summarized in Figure 2 and Appendix 1, Table S4. Compared to households in which the index participant was the only infected person, the odds of transmission increased with every additional infected member (OR 3.0, 95% CI 2.1–4.2). Lower-density households were associated with lower transmission rates (OR 0.8, 95% CI 0.7–1.0). The odds of an adult’s transmitting to a child were higher compared to the odds of a child’s transmitting to another child (OR 2.0, 95% CI 1.0–4.2). The odds of transmission was 2.6 (95% CI 0.8–8.0) if the index participant was admitted to hospital. Isolation of the index participant and indoor masking were not associated with decreased transmission. The odds of transmission from symptomatic and from asymptomatic index participants was uncertain (OR 1.4, 95% CI 0.6–3.1).
Figure 2:
Risk factors for household transmission. Note: CI = confidence interval, HC = household contact, I = index participant, OR = odds ratio.
Interpretation
Our prospective study used a highly sensitive laboratory ELISA for SARS-CoV-2-specific antibodies to determine SARS-CoV-2 spread in households with children. We enrolled the majority of members from participating households and found high transmission by both children and adults, with a secondary attack rate of about 50%. Although adults were more likely to spread infection than children, children transmitted infection to roughly one-third of their household members. Children and adults were equally likely to be infected from the infected adult or child in their home.
A meta-analysis of household transmission of SARS-CoV-2 including 87 studies published between March 2020 and June 2021 from 30 countries showed an overall secondary attack rate of 19%.1 The accuracy of this estimate may have been affected by a high degree of methodologic heterogeneity with respect to test type and frequency of testing for diagnosis of contacts, isolation practices of infected household members and duration of follow-up. Serologic testing to diagnose infection in household contacts was used in very few of the studies, and few looked at spread specifically from children. Serosurveillance can enhance the accuracy of household transmission estimates; however, most investigators using serosurveillance reported transmission from adult index cases only13–15,17,19,21,42 or from specialized populations,13–15,17,21 whereas others excluded child household contacts, which limits the generalizability of the results.16,22,43
Miller and colleagues43 used a commercial ELISA assay on day 35 to test for IgG antibodies in household contacts of 92 child index cases in England. Children aged 10 years or less and those aged 11–18 transmitted to 25% (95% CI 12%–38%) and 30% (95% CI 19%–41%) of contacts, respectively. Although these results are similar to ours, the spread may not have been attributable exclusively to transmission within the home, as, unlike in our study setting, there was high community prevalence of SARS-CoV-2 during the study period.
Paul and colleagues5 used a large provincial health administrative data set with viral swab results to examine household transmission among people with laboratory-confirmed SARS-CoV-2 infection in Ontario in 2020. They found that transmission occurred in only 27% of households; younger children (age ≤ 3 yr, 4–8 yr and 9–13 yr) were more likely to transmit SARS-CoV-2 within the home than those aged 14–17 (OR 1.4, 95% CI 1.2–1.7; OR 1.4, 95% CI 1.2–1.7; and OR 1.1, 95% CI 1.0–1.3, respectively). Although our results similarly show the important role of children as a source of viral spread, we found that the risk of transmission within households expanded as age increased within the pediatric age group (with the youngest children at lowest odds). Given that the omicron variant is more infectious than delta and that delta was at least twice as contagious as earlier SARS-CoV-2 strains,44 we suspect that household transmission is, and will continue to be, even greater than observed in our study.
To provide evidence-based public health guidance to households with infected members, it is essential to understand risk factors for transmission. Factors found in the current study to be associated with increased transmission such as household density and relationship to the index participant are not easily modifiable. We did not find reduced transmission when household members were isolated from those with confirmed infection within the home, although adherence to self-reported measures could not be verified. Consistent with the literature suggesting that household transmission is greater when illness is more severe in the index participant1,45 we observed higher transmission rates when the index participant was admitted to hospital, although the width of the confidence interval made this association uncertain. We also found that both symptomatic and asymptomatic people were responsible for transmission within the home. With the increasing recognition of spread both by asymptomatic people46 and from aerosolized droplets,47,48 the benefits of advising people to isolate within a household may be minimal, particularly if isolation is delayed until a positive test result.
In many cases, children are exempt from COVID-19 safety measures. For example, in many jurisdictions, unvaccinated children are permitted to circulate in the community even if they are a close contact of a person with COVID-19; children are often not required to abide by quarantine rules if travelling with fully vaccinated adults; in certain provinces, length of isolation after confirmed SARS-CoV-2 infection is shorter for certain categories of children; and mask mandates for children are highly variable depending on location.49–51 We found that children are sources of SARS-CoV-2 spread within the home, and we hypothesize that they will be an even greater source of spread with the emergence of more infectious variants. Children also have considerable potential to spread in settings such as school and daycare, where they congregate indoors for long periods and where mask wearing may not be consistent or continuous. More consistency in public health policies related to nonpharmacologic interventions such as mask wearing and ventilation is critically important to control spread.
Limitations
There was no maximum allowable time between confirmed infection and study enrolment, which led to highly varied times between infection and antibody testing. This may have affected our ability to detect infection; however, we measured 3 isotypes (IgM, IgA, IgG) to minimize the risk of missing very recent or remote infection. It may also have resulted in a falsely elevated estimation of transmission; however, COVID-19 case counts were very low (6–12 cases per 100 000) in Ottawa during the study period (compared to 250 cases per 100 000 in February 202252), which increases the certainty that transmission occurred in the home.
Our cohort was recruited before widespread vaccination and circulation of variants in our community. In the era before the omicron variant, decreased household transmission was observed when members had had at least 1 vaccine dose.53,54 However, given the immunity-evading properties of the omicron variant,55 our study population may mirror the current state of many Canadian households, with adults who have not received a booster vaccine, and unvaccinated or partially vaccinated children. The omicron variant, which is highly contagious, will likely further increase household transmission from all members.
We used a variety of strategies to identify eligible households, including self-identification. It is possible that characteristics of families who self-identified were different from those of families who were recruited through follow-up of a positive test result.
Finally, we defined the household member who first developed symptoms or, in the absence of symptoms, had the first positive RT-PCR test result for SARS-CoV-2 as the index participant. This assignment may have been erroneous if there was a co-primary infection or if the first-infected member was asymptomatic, as provincial guidelines at the time allowed PCR testing only for symptomatic people. Use of this definition may have underestimated the prevalence of asymptomatic spread and may have assigned directionality of transmission inaccurately.
Conclusion
People with SARS-CoV-2 infection transmitted extensively within their households, with a secondary attack rate of about 50%. Although children transmitted at a lower rate than adults, they still infected one-third of household members; this finding should inform public health policy. Asymptomatic spread occurred and should be accounted for in the formulation of public health and travel policies. Our findings have implications for in-person school or activities because the risk of transmission may be high when masks are not worn continuously or consistently in poorly ventilated settings. Nonpharmacologic interventions should continue to be considered for congregate settings.
Supplementary Material
Acknowledgements
The authors thank Ms. Dale Dalgleish, Mr. Tyrus Crawford and Ms. Taylor McNeely, as well as the research assistants and nurses, for their collaboration in patient recruitment and enrolment; Dr. Andrew Willmore for his important contributions to the system used to identify adult index participants; and Ms. Lynda Rocheleau for her assistance with data analysis in the Pelchat Laboratory, University of Ottawa.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Marc-André Langlois and Roger Zemek contributed equally as senior authors. Maala Bhatt, Roger Zemek, Marc-André Langlois, Amy Plint and Anne Pham Huy conceived and designed the study. Ken Tang, Martin Pelchat and Marc-André Langlois analyzed the data. Maala Bhatt, Jennifer Dawson, Ken Tang, Marc-André Langlois, Amy Plint and Roger Zemek drafted the manuscript. All of the authors acquired and interpreted the data, revised the manuscript critically for important intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was supported by grants from the Children’s Hospital Academic Medical Organization, the PSI Foundation (COV-6) and the Ontario COVID-19 Rapid Research Fund (C-741-1934-BHATT). The Langlois Laboratory was supported by a COVID-19 Rapid Response grant from the Canadian Institutes of Health Research (VR2-172722) and a grant supplement by the COVID-19 Immunity Task Force. Production of COVID-19 reagents was supported financially by the National Research Council Canada Pandemic Response Challenge program.
Data sharing: Aggregate data presented in this manuscript are accessible to interested parties by application to the corresponding author.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/2/E357/suppl/DC1.
References
- 1.Madewell ZJ, Yang Y, Longini IM, Jr, et al. Factors associated with household transmission of SARS-CoV-2. JAMA Netw Open. 2021;4:e2122240. doi: 10.1001/jamanetworkopen.2021.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh WC, Naing L, Chaw L, et al. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PLoS One. 2020;15:e0240205. doi: 10.1371/journal.pone.0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson HA, Mousa A, Dighe A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) setting-specific transmission rates: a systematic review and meta-analysis. Clin Infect Dis. 2021;73:e754–64. doi: 10.1093/cid/ciab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu VT, Yousaf AR, Chang K, et al. Georgia Camp Investigation Team. Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med. 2021;385:954–6. doi: 10.1056/NEJMc2031915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul LA, Daneman N, Schwartz KL, et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr. 2021;175:1151–8. doi: 10.1001/jamapediatrics.2021.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei H, Xu X, Xiao S, et al. Household transmission of COVID-19-a systematic review and meta-analysis. J Infect. 2020;81:979–97. doi: 10.1016/j.jinf.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallett S, Allen AJ, Graziadio S, et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18:346. doi: 10.1186/s12916-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hippich M, Holthaus L, Assfalg R, et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med (N Y) 2021;2:149–63e4. doi: 10.1016/j.medj.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyde Z. Difference in severe acute respiratory syndrome coronavirus 2 attack rate between children and adults may reflect bias. Clin Infect Dis. 2022;74:152–5. doi: 10.1093/cid/ciab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arkhipova-Jenkins I, Helfand M, Armstrong C, et al. Antibody response after SARS-CoV-2 infection and implications for immunity. Ann Intern Med. 2021;174:811–21. doi: 10.7326/M20-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020;15:e0244126. doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalf CJE, Farrar J, Cutts FT, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. 2016;388:728–30. doi: 10.1016/S0140-6736(16)30164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladhani SN, Andrews N, Aiano F, et al. RAPID-19 Investigation Team. Secondary attack rate and family clustering of SARS-CoV-2 infection in children of healthcare workers with confirmed COVID-19. Clin Infect Dis. 2021;73:e260–3. doi: 10.1093/cid/ciaa1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Méndez-Echevarría A, Sainz T, de Felipe B, et al. High rates of SARS-CoV-2 family transmission in children of healthcare workers during the first pandemic wave in Madrid, Spain. Pediatr Infect Dis J. 2021;40:e185–8. doi: 10.1097/INF.0000000000003088. [DOI] [PubMed] [Google Scholar]
- 15.Brotons P, Launes C, Buetas E, et al. Kids Corona Study Group. Susceptibility to severe acute respiratory syndrome coronavirus 2 infection among children and adults: a seroprevalence study of family households in the Barcelona metropolitan region, Spain. Clin Infect Dis. 2021;72:e970–7. doi: 10.1093/cid/ciaa1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwelker K, Zhou F, Blomberg B, et al. High attack rates of SARS-CoV-2 infection through household-transmission: a prospective study. medRxiv. 2020 Nov 4; doi: 10.1101/2020.11.02.20224485. [DOI] [Google Scholar]
- 17.McDade TW, McNally EM, Zelikovich AS, et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay. PLoS One. 2020;15:e0237833. doi: 10.1371/journal.pone.0237833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis. 2021;21:333–43. doi: 10.1016/S1473-3099(20)30833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reukers DFM, van Boven M, Meijer A, et al. High infection secondary attack rates of SARS-CoV-2 in Dutch households revealed by dense sampling. Clin Infect Dis. 2022;74:52–8. doi: 10.1093/cid/ciab237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angulo-Bazán Y, Solis-Sánchez G, Cardenas F, et al. Household transmission of SARS-CoV-2 (COVID-19) in Lima, Peru. Cad Saude Publica. 2021;37:e00238720. doi: 10.1590/0102-311X00238720. [DOI] [PubMed] [Google Scholar]
- 21.Buonsenso D, Valentini P, De Rose C, et al. Gemelli Against COVID-19 Post-Acute Care Study Group. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in children with household exposure to adults with COVID-19: preliminary findings. Pediatr Pulmonol. 2021;56:1374–7. doi: 10.1002/ppul.25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galow L, Haag L, Kahre E, et al. Lower household transmission rates of SARS-CoV-2 from children compared to adults. J Infect. 2021;83:e34–6. doi: 10.1016/j.jinf.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah K, Kandre Y, Mavalankar D. Secondary attack rate in household contacts of COVID-19 Paediatric index cases: a study from Western India. J Public Health (Oxf) 2021;43:243–5. doi: 10.1093/pubmed/fdaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Li YY, Liu MJ, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021;21:617–28. doi: 10.1016/S1473-3099(20)30981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugon P, Fuller T, Damasceno L, et al. SARS-CoV-2 infection dynamics in children and household contacts in a slum in Rio de Janeiro. Pediatrics. 2021;148:e2021050182. doi: 10.1542/peds.2021-050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40:e137–45. doi: 10.1097/INF.0000000000003043. [DOI] [PubMed] [Google Scholar]
- 27.Davies NG, Klepac P, Liu Y, et al. CMMID COVID-19 working group. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–11. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 28.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults. JAMA Pediatr. 2021;175:143. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadermann AC, Thomson KC, Richardson CG, et al. Examining the impacts of the COVID-19 pandemic on family mental health in Canada: findings from a national cross-sectional study. BMJ Open. 2021;11:e042871. doi: 10.1136/bmjopen-2020-042871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Vanderloo LM, Maguire JL, et al. TARGet Kids! Collaboration. Public health preventive measures and child health behaviours during COVID-19: a cohort study. Can J Public Health. 2021;112:831–42. doi: 10.17269/s41997-021-00549-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashikkali L, Carroll W, Johnson C. The indirect impact of COVID-19 on child health. Paediatr Child Health (Oxford) 2020;30:430–7. doi: 10.1016/j.paed.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Current population and household estimates. Ottawa: City of Ottawa; [accessed 2022 Feb. 16]. Available: https://ottawa.ca/en/living-ottawa/statistics-and-demographics/current-population-and-household-estimates. [Google Scholar]
- 33.Daily COVID-19 dashboard. Ottawa: Ottawa Public Health; [accessed 2021 Aug. 31]. Available: https://www.ottawapublichealth.ca/en/reports-research-and-statistics/daily-covid19-dashboard.aspx. [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epidemiological protocol: household transmission investigation protocol for 2019-novel coronavirus (COVID-19) infection. Geneva: World Health Organization; 2020. [accessed 2021 May 3]. Available https://www.who.int/publications/i/item/household-transmission-investigation-protocol-for-2019-novel-coronavirus-(2019-ncov)-infection. [Google Scholar]
- 36.Chambers EC, Schechter C, Tow A, et al. Household density and obesity in young black and white adults. Ethn Dis. 2010;20:366–9. [PubMed] [Google Scholar]
- 37.Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in patients with COVID-19. Sci Immunol. 2020;5:eabe5511. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cholette F, Mesa C, Harris A, et al. COVID-19 Immunity Task Force (CITF) Working Group. Dried blood spot specimens for SARS-CoV-2 antibody testing: a multi-site, multi-assay comparison. PLoS One. 2021;16:e0261003. doi: 10.1371/journal.pone.0261003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dicker R, Coronado F, Koo D, et al. Principles of epidemiology in public health practice. 3rd ed. Atlanta: Centers for Disease Control and Prevention; 2006. [accessed 2022 Feb. 4]. Lesson 3: Measure of risk; Section 2: Morbidity frequency measures. Available https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section2.html. [Google Scholar]
- 40.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Berkeley Symp Math Statist Prob. 1967;1:221–33. [Google Scholar]
- 41.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–30. [Google Scholar]
- 42.Cerami C, Popkin-Hall ZR, Rapp T, et al. Household transmission of SARS-CoV-2 in the United States: living density, viral load, and disproportionate impact on communities of color. Clin Infect Dis. 2021 Aug 12; doi: 10.1093/cid/ciab701. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller E, Waight PA, Andrews NJ, et al. Transmission of SARS-CoV-2 in the household setting: a prospective cohort study in children and adults in England. J Infect. 2021;83:483–9. doi: 10.1016/j.jinf.2021.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.What you need to know about variants. Atlanta: Centers for Disease Control and Prevention; [accessed 2022 Feb. 1]. updated 2022 Feb 2. Available https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html. [Google Scholar]
- 45.Bi Q, Lessler J, Eckerle I, et al. SEROCoV-POP Study Group. Insights into household transmission of SARS-CoV-2 from a population-based serological survey. Nat Commun. 2021;12:3643. doi: 10.1038/s41467-021-23733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu X, Nergiz AI, Maraolo AE, et al. Defining the role of asymptomatic and pre-symptomatic SARS-CoV-2 transmission: a living systematic review. Clin Microbiol Infect. 2021;27:511–9. doi: 10.1016/j.cmi.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scientific brief: SARS-CoV-2 transmission. Atlanta: Centers for Disease Control and Prevention; [accessed 2022 Feb. 1]. updated 2021 May 7. Available https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html. [Google Scholar]
- 48.Coronavirus disease (COVID-19): How is it transmitted? Geneva: World Health Organization; 2021. [accessed 2022 Jan. 27]. Available https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-how-is-it-transmitted. [Google Scholar]
- 49.COVID-19 case and contact management in schools. Government of Saskatchewan; [accessed 2022 Feb. 16]. Available: https://www.saskatchewan.ca/government/health-care-administration-and-provider-resources/treatment-procedures-and-guidelines/emerging-public-health-issues/2019-novel-coronavirus/about-covid-19/covid-19-case-and-contact-management-in-schools. [Google Scholar]
- 50.Staying safe and healthy this school year. Edmonton: Government of Alberta; [accessed 2022 Feb. 16]. Available: https://www.alberta.ca/k-12-learning-during-covid-19.aspx. [Google Scholar]
- 51.Wearing a mask or a face covering in public settings in the context of the COVID-19 pandemic. Gouvernement du Québec; [accessed 2022 Feb. 16]. Available: https://www.quebec.ca/en/health/health-issues/a-z/2019-coronavirus/mask-or-face-covering/wearing-a-face-covering-in-public-settings-covid-19. [Google Scholar]
- 52.Ontario COVID-19 Data Tool. Toronto: Ontario Agency For Health Protection and Promotion (Public Health Ontario); [accessed 2022 Feb. 16]. Available: https://www.publichealthontario.ca/en/data-and-analysis/infectious-disease/covid-19-data-surveillance/covid-19-data-tool?tab=maps. [Google Scholar]
- 53.Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med. 2021;385:1718–20. doi: 10.1056/NEJMc2106757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris RJ, Hall JA, Zaidi A, et al. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385:759–60. doi: 10.1056/NEJMc2107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–84e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.