Abstract
Purpose
Ketamine is a N-methyl-D-aspartate (NMDA) antagonist with strong analgesic properties. Its addition to the treatment of neuropathic pain may reduce pain intensity and improve overall quality of life. A systematic review and meta-analysis of randomized controlled trials was performed to investigate the addition of ketamine to the treatment of patients with neuropathic pain.
Patients and Methods
GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach was used to rate the overall certainty of the evidence for each outcome. Eighteen (18) randomized controlled trials including 706 participants were included for further analysis.
Results
Ketamine addition to standard treatment of neuropathic pain (NP) resulted in a statistically significant reduction of pain intensity at one week after the end of treatment with ketamine (MD −2.14, 95% CI −2.65 to −1.63; p<0.00001) and after 30 days after the end of treatment with ketamine (MD −1.68, 95% CI −2.25 to −1.12; p<0.00001) and a statistically significant increase in discomfort (RR 4.06; 95% CI 1.18 to 13.95; p=0.03), and psychedelic effects (RR 4.94; 95% CI 2.76 to 8.84; p<0.00001).
Conclusion
There is a statistically significant pain reduction by adding ketamine to the treatment of chronic NP when compared to the standard treatment. However, such pain reduction comes at the expense of adverse outcomes, especially psychedelic effects related to the administration of ketamine. However, the overall quality of certainty of evidence is low due to the clinical heterogeneity among the intervention characteristics of the trials analyzed (different administration routes, dosing regimen, therapy durations, different clinical characteristics of the population investigated). Future large multi-centered trials are necessary to confirm or not the results of the present review.
Keywords: ketamine, chronic pain, neuropathy, neuralgia, treatment
Introduction
Although data on neuropathic pain prevalence in the general population may not be accurate due to different definitions and evaluation methods, estimations indicate that between 6.9 and 10% of the global population are affected by some type of neuropathic pain.1 The World Health Organization (WHO) has estimated that 22% of the world’s primary care patients have chronic debilitating pain making chronic pain a problem to be addressed by all physicians and health professionals.2–4
Neuropathic pain (NP) may develop after a nerve injury or disease, with changes occurring downwards and upwards along the modulating pathways of the injured neuron.
Three factors distinguish NP from others types of pain: a) there is no transduction (conversion of noxious stimulus to electric signal); b) the prognosis is poor: pain from injuries in nervous tissues is more likely to become chronic; c) NP is refractory to therapy with conventional analgesics, such as non-steroidal anti-inflammatory drugs (NSAIDs) and opioids.5 A variety of substances are involved in the development and maintenance of NP, which may explain the high incidence of comorbidities associated with it and why drugs that are effective in other conditions can also be effective in NP.6–9 However, in case of NP etiological therapy is rarely effective. Thus, pain relief is the primary focus.7
N-Methyl-D-aspartate receptors (NMDARs) are ionotropic glutamate receptors that play a role in synaptic transmission, in neuroplasticity, and in learning and memory processes. Alterations on NMDARs functions are involved in some of the nervous system disorders, such as neuropathic pain. Therefore, they have been extensively investigated as possible therapeutic targets for pain management.
In such context ketamine has been receiving new attention and its role has expanded from general anesthesia to depression treatment,10 multimodal analgesia,11 as an anti-hyperalgesic, and in the treatment of NP.12,13
The purpose of the systematic review and meta-analysis is to assess the impact of the addition of ketamine to the treatment of patients suffering from chronic NP.
Materials and Methods
Methodology was followed The Cochrane Handbook for Intervention Reviews.14 This systematic review was registered in PROSPERO International Prospective Register of Systematic Reviews (number CRD42020203060) and is reported in accordance with PRISMA Preferred Reporting Items for Systematic Reviews and Meta-analysis statement (PRISMA) statement.15 (Figure 1 – PRISMA Checklist).
Figure 1.
Study selection PRISMA flow diagram.
Notes: Adapted from: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.
Search Strategy
The search was performed in the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE via PubMed (OvidSP), LILACS (Literatura Latino-Americana e do Caribe em Ciências da Saúde), Web of Science and the EMBASE (Excerpta Medica dataBASE). The databases were searched for published RCTs with no restrictions to date, from inception to the last search performed on November 18th, 2021.
The search was conducted using multiple combinations of the following keywords: “neuralgia” and “ketamine” (Appendix 1 – search strategy). No language or publication status restrictions were imposed. In addition, an online search for additional eligible studies was conducted in the Science Research website, and we also hand searched the reference lists of included studies.
Eligibility Criteria and Study Selection
We considered all RCTs evaluating the addition of ketamine, by any route of administration, to the standard treatment compared to standard treatment (ST) plus placebo, or ST alone, in adult patients with NP. (Appendix 2– eligibility criteria). Using standardized screening forms (Appendix 3 – data extraction form), two reviewers (JEGP, LFGP) independently screened all titles and abstracts identified by the literature search, obtained full-text articles of all potentially eligible studies, and evaluated these studies for eligibility. Reviewers resolved the disagreement through discussion, and with third-party adjudication if necessary.
Primary and Secondary Outcomes
The primary outcome of this review was pain measured by numerical rating scale (NRS) or visual analog scale (VAS). Secondary outcomes were the following: worst pain score measured by numerical rating scale, least pain score measured by numerical rating scale, pain interference on life appreciation, pain measured by multidimensional pain scales, quality of life, mood, impact on interpersonal interactions, quality of sleep, impact on general daily activities, impact on work, and adverse outcomes (eg, psychedelic effects, nausea, and vomiting) Eligible studies reported on one or more of the outcomes listed above.
Data Extraction and Quality Assessment
Two reviewers (JEGP, LFGP) independently extracted the following data using a pre-piloted, standardized data extraction form: characteristics of the study design; participants; interventions; outcomes and the length of follow-up. If eligible articles had missing data, we contacted authors for clarification.
Reviewers independently assessed the validity of included studies using the risk of bias approach for Cochrane reviews.14,16,17 Risk of bias was assessed using five separate criteria: adequacy of sequence generation, allocation sequence concealment, blinding (investigators, patients, collectors, statistician, outcome assessors), incomplete outcome data, and selective outcome reporting. For incomplete outcome data, we considered loss to follow-up enough to induce clinically relevant bias as high risk of bias.
Certainty of Evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to rate the certainty of evidence, in which a body of evidence based on randomized trials begins as high certainty evidence but may be rated down by one or more levels for each of five categories of limitations: risk of bias, inconsistency, indirectness, imprecision and reporting bias.18 Detailed GRADE guidance was used to assess the overall risk of bias, imprecision,19 inconsistency,16 indirectness20 and publication bias,21 and results were summarized in an evidence profile table.
Statistical Analysis
We calculated pooled risk ratios (RRs) for dichotomous outcomes, mean differences (MD) for continuous outcomes and standardized mean difference (SMD) for continuous outcomes measured by different scales, with the corresponding 95% confidence interval (CI). We used a random-effects model with the Mantel-Haenszel statistical method for the dichotomous outcomes and the Inverse Variance for the continuous outcomes. We addressed variability in results across studies using the I2 statistic and the P value (>0.10) obtained from the Cochrane chi-square test.
Risk-ratio does not incorporate zero-event trials, thereby excluding these trials and data from the combined estimate. A random-effect model was chosen because when dealing with a series of studies, subjects differ substantially from one study to another.22
Our primary analyses were based on all randomized patients who had reported outcomes for each study (complete case analysis). We used Review Manager (RevMan®) (version 5.3; Nordic Cochrane Centre, Cochrane Collaboration) for all analyses.23 We planned to perform sensitivity and pre-specified subgroup analyses according to different regimens of administration, but data was insufficient to perform those analyses. Publication biases were assessed via visual inspection of funnel plots for outcomes with 10 or more studies.24
Results
Search results
We identified a total of 1404 studies plus 4 additional studies through hand search. After independent screening by title, and then by abstract, we selected 39 studies, and after duplicate removal, 32 studies were deemed eligible for inclusion in the review. Of those, 14 did not fulfill our eligibility criteria and were excluded (Figure 1. PRISMA flow diagram). We, therefore, included 18 studies Max with a total of 706 participants in this review.25–42
Characteristics of the Included Studies
Among the 18 eligible randomized control studies (RCTs), they took place in a variety of settings (Table 1) including Brazil,29,32,41,42 Canada,36 Denmark,35 Egypt,27,28 France,34 Italy,40 Netherlands,25,30,37 Norway,38 South Korea,31 United Kingdom,33 United States of America.26,39 Sample sizes ranged from eight38 to 21435 participants (Table 1).
Table 1.
Study Characteristics According to Population and Setting
| Author, Year | Country | Number of Randomized Participants | Mean Age Per Studied Group | Sex (Male, n) | Inclusion Criteria | Exclusion Criteria | Follow-Up (Weeks) |
|---|---|---|---|---|---|---|---|
| Eide, 199438 | Norway | 8 (cross-over study) |
I: 71.9 P: 71.9 (cross-over study) |
I: 4 C: 4 (cross-over study) |
Patients with post-herpetic neuralgia attending the Pain Clinic, The National Hospital, Oslo, Norway, that were able to and willing to participate. | Patients not to use analgesic medication the last 2 days before each test session. | 3 weeks |
| Max, 199539 |
USA | 8 (cross-over study) |
I:40 P:40 (cross-over study) |
I: 0 C: 0 (cross-over study) |
Patients with chronic posttraumatic pain and widespread mechanical allodynia that need to demonstrate symptoms and signs suggesting altered central nervous system processing of sensory input. All patients were required to have mechanical allodynia to light stroking with a cotton gauze pad extending at least 5cm from site of injury. | Not reported | 3 days |
| Mercadante, 200040 | Italy | 10 (cross-over study) |
I: 57 C: 57 (cross-over study) |
I: 7 C: 7 (cross-over study) |
Patients with cancer and pain unrelieved by their dose of morphine and a Karnofsky status of 50 or more were selected for this study. No adjuvant drugs had been previously used. | Patients with coexisting liver or renal disease or with encephalopathy were excluded. | 3 days |
| Lauretti, 200241 | Brazil | 26 | I: 46 ± 12 C: 47 ± 10 |
I: 6 C: 8 |
Patients aged between 21 and 65years, with neuropathic chronic pain for more then six months, refractory to NSAID, physiotherapy, antidepressants, tramadol or intravenous meperidine, were included. | Not reported | 3 weeks |
| Kvarnstrom, 200335 | Denmark | 12 (cross-over study) | I: 47 C: 47 |
I: 3 C: 3 |
Patients should be affected by peripheral nerve or root lesions of traumatic origin, with spontaneous and evoked pain in the cutaneous territory supplied by the injured nerve together with clinically demonstrable sensory deficit or sensory hyperfunction. The age of the patients should be between 20 and 75 years | Patients with drug abuse, cardiovascular disease or previous treatment with intravenous ketamine or lidocaine were not considered for the study. | 1 week |
| Lynch, 200536 | Canada | 92 | Ketamine: 51 Amitriptyline: 51 Ketamine+Amitriptyline: 52 C: 52 |
Ketamine: 9 Amitriptyline: 11 Ketamine+Amitriptyline: 12 C: 15 |
Nonpregnant adult; Established diagnosis of postherpetic neuralgia, diabetic neuropathy, or postsurgical/post traumatic neuropathic pain; Moderate to severe pain all or most of the time persisting despite other treatment modalities; Pain has persisted for 3 months or longer; Presence of dynamic tactile allodynia or pinprick hyperalgesia in pain; Normal cognitive and communicative ability as judged by clinical assessment and ability to complete self-report questionnaires. | Evidence of another type of pain as severe as the pain understudy; Evidence of another type of neuropathic pain not included in this study; Major depression requiring treatment; Allergy to amitriptyline or ketamine; Ongoing use of a monoamine oxiDase inhibitor. | 3 weeks |
| Vranken, 200537 | Netherlands | 33 | I (50 mg): 58.4 ± 12.3 I (75mg): 51.2 ± 14.3 C: 51.8 ± 11 |
I (50 mg): 5 I (75mg): 6 C: 5 |
Age 18 years or older; patients suffering from neuropathic pain caused by lesion or dysfunction in the central nervous system, and insufficiently responding to conventional medical therapy (including opioids, anticonvulsants, antidepressants, baclofen, a-adrenergic agonists, oral anesthetic antiarrhythmic agents). Neuropathic pain was described by at least one of the following: burning pain, paroxysmal episodes of shooting pain, or pain on light touch. Additionally, patients had to score above 12 on the Leeds Assessment of Neuropathic Symptoms and Signs questionnaire. | Patients were excluded from the study if they: were pregnant; had a history of intolerance, hypersensitivity, or known allergy to ketamine; had a known history of significant hepatic, renal, or psychiatric disorder; had a history of cardiac events including arrhythmias, congestive heart failure, or unstable angina; had poorly controlled hypertension (systolic BP above180 mmHg, or diastolic BP above 90 mmHg despite anti-hypertensive therapy); had a history of substance abuse. | 1 week |
| Tonet, 200842 | Brazil | 30 | Not specified | Not specified | Adult patients with chronic neuropathic pain. | Not specified | 4 weeks |
| Sigtermans 200925 | Netherlands | 60 | I: 43.7 ± 11.5 C: 47.5 ± 13.1 |
I: 8 C: 4 |
Patients who were diagnosed with Complex Regional Pain Syndrome Type-1, that was based on the International Association for the study of pain criteria. | Pain score of less than 5 of 10, age < 18 years, pregnancy/lactation, increased intracranial pressure, a history of psychosis, a serious medical disease (eg, cardiovascular, renal, or liver disease) and use of strong opioid medication. |
12 weeks |
| Schwartzman 200926 | USA | 19 | I: 38 (mean) C: 45.5 (mean) |
I: 0 C: 1 |
Patients diagnosed with CRPS based on the revised IASP (International Association for the Study of Pain) criteria; whose condition was intractable for a minimum of 6 months and had failed at least three therapies. The patients were ketamine naive and were of either gender including all racial or minority groups. The patient’s age was between 18 and 65 years. | Patients who were pregnant or had known substance abuse issues, glaucoma or thyrotoxicosis were excluded. Any subject that was unable to provide consent due to cognitive difficulties was not enrolled in this study. Patients with active litigation, compensation or disability issues related to their CRPS, and subjects on calcium channel or beta blockers due to the need to utilize clonidine with ketamine were excluded. |
12 weeks |
| Amr, 201028 | Egypt | 40 | I: 48.6±10.1 C:48.7 ± 9.7 |
I: 16 C: 17 |
All patients had been exhibiting symptoms for over 6 months. The study’s inclusion process continued until the requested number of patients was reached. | Patients who had SCI at or above the C-4 level were excluded because of the risk of respiratory arrest. Other exclusion factors were: pre existing hypertension, angina, congestive cardiac failure, hepatic impairment, renal impairment, and an allergy to any drugs used in the study. | 4 weeks |
| Amr, 201127 | Egypt | 40 | I: 48.6±10.1 C:48.7 ± 9.7 |
I: 16 C: 17 |
Duration of symptoms was more than six months in all patients. The process of inclusion into the study went on until the target number of patients was reached. | Patients with previous chronic anticoagulation therapy, coagulation disorders, infection in the back, bed sores, spine deformity, hepatic or renal impairment were excluded from the study. | 8 weeks |
| Barros, 201229 | Brazil | 12 (cross-over study) |
I: 71.7 P: 71.7 (Cross-over study) |
I:4 C:4 |
Post Herpetic Neuralgia patients seen at the Pain Management Clinic (HC-FMB-Unesp, Botucatu-SP), older than 18 years old and able to understand the Numerical Verbal Scale (NVS: 0 to 10) were invited to take part in the study. | Those presenting abnormal biochemical blood tests and skin lesions in the area of pain were not included in the study. | 5 weeks |
| Niesters, 201330 | Netherlands | 10 (cross-over study) |
I: 54.4 ±4,2 C: 54.4 ±4,2 (cross-over study) |
I: 2 C: 2 (cross-over study) |
Patients were required to have at least two of the following symptoms in legs, arms, or both (in a stocking-glove distribution): (i) symmetrical dysesthesias or paresthesias; (ii) burning or painful feet with night-time worsening; or (iii) peripheral tactile allodynia. With respect to the QST, subjects were included if they had an abnormal warm and cold detection threshold, an abnormal warm and cold pain threshold, or allodynia. | Age18 or.80 yr; presence or history of a medical disease such as renal, cardiac, vascular (including hypertension), or infectious disease; presence or history of a neurological and psychiatric disease such as increased cranial pressure, epilepsy or psychosis; glaucoma; pregnancy; obesity (BMI.30); or use of strong opioid medication. | 1 day |
| Kim, 201531 | South Korea | 30 | I: 69 C: 69 |
I: Not specified C: Not specified |
Patients with reported pain resistant to conventional treatments, including stellate ganglion block, local anesthetic infiltration, epidural block, and systemic administration of anticonvulsants and antidepressants. Spontaneous pain with a visual analog scale (VAS) scoreN7 and lasting for≥6 months. | Patients were excluded if they had hypermagnesemia, hypercalcemia, abnormal electrocardiogram, asthma, any degree of heart block, or renal impairment (blood ureaN12 mmol/L and creatinineN150μmol/L) or were taking digoxin. | 2 weeks |
| Rigo, 201732 | Brazil | 42 | Ketamine: 54 ± 12.4 Methadone: 52 ± 13.6 Keta+Metha: 45 ± 8.5 |
Ketamine: 6 Methadone: 6 Keta+Metha: 5 |
Patients who had experienced neuropathic pain for more than 6 months and who were poorly responsive to drugs used to treat neuropathic pain who were 22 to 77 years old from the Clinical Care & Pain Management. (HUSM) | Patients with a history of severe psychiatric disorder, misuse of illegal drugs, or hepatic disease were excluded. | 12 weeks |
| Fallon, 201833 | UK | 214 | I: Not specified C: Not specified |
I: Not specified C: Not specified |
≥18 years old; histological cancer diagnosis; written informed consent; Index neuropathic pain related to underlying malignancy or resulting from treatment received for this; Index neuropathic pain (worst pain) ≥ 4 on 0–10 (VAS); McGill Sensory Scale Score > 5; Patient has had a trial of at least one adjuvant analgesic (gabapentin, pregabalin, amitriptyline) or has been offered these and declined; patient is able to comply with study procedures. | Patients who have received chemotherapy or radiotherapy in the preceding six weeks; who may have a change in tumoricidal treatment during the period of study; Diastolic pressure > 100 mmHg at screening; History seizures in last 2 years; currently taking class I-antiarrhythmic drugs; life expectancy less than two months; patient who are actively hallucinating; women of childbearing potential not using adequate contraception, patients with cerebrovascular disease; patients with psychotic disorders. | 4 weeks |
| Pickering, 201934 | France | 20 (cross-over study) |
I: 55 ± 12 C: 55 ± 12 (cross-over study) |
I:10 C: 10 (cross-over study) |
Patients with at least 18 year of age, chronic pain for more than 3 months, peripheral or central pain requiring IV ketamine infusion, and no previous ketamine treatment (naïve patients). | Previous IV ketamine treatment; contraindication (1) to ketamine (hypersensitivity, uncontrolled high blood pressure, severe heart failure), (2) to magnesium (severe kidney failure), or (3) to sodium chloride (water inflation, fluid retention); medical/surgical history or drug treatment judged by the investigator to be incompatible with the trial; women of childbearing age without effective contraceptive method; pregnancy or lactation; involvement in another clinical trial; and inability to comply with protocol requirements. | 35 days |
Abbreviations: I, intervention group; C, control; NSAIDS, nonsteroidal anti-inflammatory drugs; CRPS, Complex Regional Pain Syndrome; IASP, International Association for the Study of Pain; SCI, spinal cord injury; QST, quantitative sensory testing; BMI, body mass index; VAS, visual analogic scale.
A total of two trials with 20 participants,29,38 included exclusively patients with post-herpetic neuralgia, while only one trial included 8 patients exclusively suffering from post-traumatic neuralgia;39 two trials with 224 participants, included exclusively patients with cancer related neuropathic pain;33,40 two trials, with 45 participants, included patients with neuralgia from direct damage to either central or peripheral nervous system;35,37 two trials included 79 patients suffering from complex regional pain syndrome (CRPS);25,26 one trial with 92 participants, stated to encompass all types of neuropathic pain36 and eight trials, with 231 participants, did not specify the etiology of neuropathic pain.27,28,30–32,34,41,42 (Table 1).
All except one of our eligible trials included both male and female participants, with one trial including only female patients.39 Males represented 53.82% of the overall population studied (excluding the studies that are not specified). There were two studies that did not specify the gender distribution of the population.31,33 The mean age of the participants ranged from 4039 to 71.938 years (Table 1).
A set of diverse protocols has been adopted for ketamine administration across different trials. Control group in 13 RCTs (558 participants) received placebo.25–30,33,35–40 The duration of treatment with ketamine ranged from one day30 to 12 weeks.25,26,32
Different routes of treatment have been adopted for ketamine administration across the trials, with eleven trials adopting the intravenous route,25,26,28,30,31,33–35,38–40 two trials adopting the epidural route,27,41 two trials administering ketamine through the oral route32,42 and three trials adopting the topical route for treatment administration.29,36,37
A wide range of doses have been utilized, beginning at 0.1 mg/kg/day41 and going up until 0.75 mg/kg/day.39 (Table 2).
Table 2.
Study Characteristics Related to Description of Intervention, Control, and Outcomes
| Author, Year | No. of Randomized Patients in Intervention and Control | Description of Intervention | Dose | Description of Control | Measured Outcomes |
|---|---|---|---|---|---|
| Eide, 199438 | I:8 C:8 (cross-over study) |
Ketamine (0,15 mg/kg), morphine (0.075 mg/kg) or saline (9 mg/mL NaCl) were given IV | Intravenous - Ketamine 0.15 mg/kg injected in 10 minutes or Morphine. | Saline solution | Assessment of allodynia, wind-up-like pain, tactile and thermal sensibility and pain, using VAS. |
| Max, 199539 |
I:8 C:8 (cross-over study) |
For 3 days, patients were given 2 hours of intravenous ketamine, alfentanil or placebo. If no pain relief after 60 minutes, the infusion rates were doubled at this time and again at 90 minutes. | Intravenous - Ketamine 0.75 mg/kg/h, can get doubled. Alfentanil 1.5 mcg/kg/min, can get doubled. | Saline solution – 0.375 mL/kg/h | Background pain and mechanical allodynia, each rated every 10 minutes on a VAS. At 10 minutes intervals, the side-effects were asked. |
| Mercadante, 200040 | I:10 C:10 (cross-over study) |
On 3 separate days, patients received ketamine hydrochloride 0.25 mg/kg, 0.50 mg/kg, or saline solution as a slow intravenous bolus administered in 30 minutes. | Intravenous - Ketamine 0.25 mg/kg or 0.5 mg/kg, administered in 30 minutes. | Saline solution | Pain intensity; nausea and vomiting, drowsiness, confusion, and dry mouth; MMSE; arterial blood pressure and side effects. |
| Lauretti, 200241 | I: 10 (3 excluded) C:13 |
At intervention group was given 0.1 mg/kg ketamine (2 mL) in 1% lidocaine solution. At control Group was given 30 μg clonidine (2 mL) in 1% lidocaine solution. The epidural catheter was maintained for 3 consecutive weeks. The outcomes were assessed weekly. | Epidural catheter - 0.1 mg/kg racemic ketamine in 1% lidocaine solution, followed by 30 mg of 1% lidocaine. (Total dose – 0,3mg/kg/day) |
30 μg preservative-free clonidine (2 mL) in 1% lidocaine solution followed by 30 mg of 1% lidocaine (3mL) (Total dose - 80 μg/day) |
The pain intensity was assessed by a VAS in the days 1, 7, 14 and 21 by the beginning of the study. |
| Kvarnstrom, 200335 | I: 12 C:12 (cross-over study) |
Effects of ketamine 0.4 mg/kg and lidocaine 2.5 mg/kg were investigated. All substances were given intravenously. Two intravenous cannulas were applied, one for the infusion and one for blood sampling. | Intravenous – Ketamine 0.4 mg/kg for 40 minutes | Saline solution | Sensibility to touch, static sensibility, thermal sensitivity and intensity of continuous spontaneous pain using a VAS. Measurements were taken at T:0 and then at T:15, T:45, T:60, T:120, T:150. |
| Lynch, 200536 | Ketamine 22 Amitriptyline 22 Ketamine + Amitriptyline 23 C:25 |
Treatments consisted of four topical creams, containing placebo (vehicle only), 2% amitriptyline,1% ketamine, or a combination of 2% amitriptyline and 1% ketamine. | Topical cream - 1% Ketamine, or 2% amitriptyline + 1% ketamine, 3 times/day for 3 weeks | Topical placebo (vehicle only) | Average daily pain intensity using an 11-point NRS McGill Pain Questionnaire, measures of allodynia and hyperalgesia, and patient satisfaction. |
| Vranken, 200537 | Ketamine 11 (50 mg)/11 (75mg) C:11 |
First, S(C)-ketamine50 mg will be compared with placebo. If S(C)-ketamine 50 mg turns out to be more effective than placebo, S(C)-ketamine 75 mg will be compared to S(C)-ketamine 50 mg. | Iontophoretic administration - Ketamine 50mg or 75mg for 5 days. | Isotonic saline solution – 3mL | Pain intensity measured by VAS, health status (Pain Disability Index and EQ-5D) and quality of life (SF-36). |
| Tonet, 200842 | I: 10 amitriptyline + carbamazepine + ketamine C: 13 amitriptyline + carbamazepine. |
In the first group received amitriptyline (25 mg) + carbamazepine (600 mg) + ketamine (30 mg/day) patients in the second group amitriptyline (25 mg/day) + carbamazepine (600 mg/day). When there was a need for analgesic supplementation, codeine (30 mg) was administered. | Oral - Ketamine 30 mg/ day, for 4 weeks. | Amitriptyline 25 mg + carbamazepine 600mg | The patients were evaluated for pain intensity, weekly for four weeks, using the numerical pain scale. |
| Sigtermans 200925 | I: 30 C:30 |
Patients were given a 4.2-day intravenous infusion of low-dose ketamine or placebo using an individualized dosage based on effect (pain relief) and side effects (nausea/vomiting/psychomimetic effects). | Intravenous - Ketamine infusion rate started at 1.2 mcg/kg.min to a maximum of 7.2 mcg/kg.min | Normal saline solution | Spontaneous pain assessed by a NRS. Radboud Skills Questionnaire (RASQ) and the Walking Ability Questionnaire (WAQ); active range of motion, threshold for touch; skin temperature and volumetric measurements. |
| Schwartzman 200926 | I: 9 C: 10 |
Infusion of 100 mL of normal saline with or without ketamine for 4 h (25 mL/h) daily for 10 days (5 days on, 2 days off, 5 days on). First day, infusion was set to 50% of the maximum rate. Second day, the infusion was increased to 75% of the maximum rate. Third day, infusion was increased to the maximum rate and maintained. | Intravenous - Ketamine maximum dose - 0.35 mg/kg/h, not to exceed 25 mg/h. | Normal saline solution | Overall pain level, joint pain, pin hyperalgesia, touch allodynia, cold allodynia and deep pressure evoked pain, strength and facility of movement, McGill questionnaire, quality of life questionnaire and a pain questionnaire, sensory and motor tests were assessed. |
| Amr, 201028 | I: 20 C: 20 (cross-over study) |
Intervention group received 80 mg ketamine over a 5-hour daily for 7 days and 300 mg of gabapentin 3 times daily. Control group received a saline infusion over 5 hours daily for 7 days and 300 mg of gabapentin 3 times daily. | Intravenous -Ketamine 80 mg administered in 5 hours, for 7 days | Isotonic saline 0.9% | VAS for pain was assessed prior to treatment, daily following the infusions for 7 days and one week after infusion termination. Side effects, were reported. |
| Amr, 201127 | I: 20 C: 20 (cross-over study) |
Intervention group received 0.2 mg/Kg of ketamine (2 mL) through epidural injection. Control group received saline solution 0.9%(2 mL) through epidural injection. Both groups received gabapentin 300 mg 3 times/day. | Epidural infusion - 0.2 mg/Kg of preservative-free ketamine 2 mL. | Isotonic saline 0.9% 2mL | VAS for pain obtained pre-injection, 7, 15, 30, 45 and 60 days post injection. Patients were also asked to report any side-effects. |
| Barros, 201229 | I: 12 C: 12 (cross-over study) |
Divided into two groups instructed to apply the ointment on the site of pain four times a day. After 15 days of treatment - washout period of seven days. After the washout period, treatments were inverted and carried out for the same time. | Topical Oinment - Ketamine 1%, during 15 days | Placebo ointment | Numerical Verbal Scale, Measured at the times: M1 – First 15 days of treatment; M2 start of washout; M3 – start of 15 days of crossover treatment; M4 – End of treatment. |
| Niesters, 201330 | I: 10 C: 10 (cross-over study) |
Treatments were as follows: - 1h infusion of 0.57 mg/kg S(+) ketamine; - morphine bolus of 0.05 mg/kg followed by 0.015 mg/kg/h for 1 h; and a 1 h saline solution infusion. | Intravenous – Ketamine 0.57 mg/kg infusion duration of 1 hour | Isotonic saline 0.9% during 1 hour | Spontaneous pain scores were measured by NRS. Subjects were contacted after their treatment to determine the duration of pain relief. And conditioned pain modulation (CPM). |
| Kim, 201531 | I: 15 C: 15 |
Patients were randomly divided into 2 groups of 15 patients each, and ketamine 1 mg/kg or magnesium 30 mg/kg was administered intravenously for 1 hour after midazolam sedation. | Intravenous - Ketamine (1 mg/kg per hour) diluted in 0.9% normal saline to a final volume of 100 mL | Magnesium sulfate (30 mg/kg per hour) were diluted in 0.9% normal saline to a final volume of 100 mL | Pain was rated on a VAS during a 2-week follow-up. All patients also completed the Doleur Neuropathique 4 questionnaire at baseline and final visits. |
| Rigo, 201732 | I: 11 Methadone: 13 Methadone + Ketamine: 13 |
Patients were randomly allocated to receive one of the 3 treatments: 3 mg methadone, 30 mg ketamine, or 3 mg methadone plus 30 mg ketamine 3 times a day. | Oral – Ketamine 3mg during 3 months | Methadone 3mg or Methadone 3mg + Ketamine 30mg | During 90 days, we assessed pain scores using a 10-point VAS, allodynia, burning/shooting pain, and side effects |
| Fallon, 201833 | I: 107 C: 107 |
Randomized in two groups to receive ketamine or placebo across 2 weeks to an effective and tolerable dosage. The starting dosage was 40mg/d, with a maximum400 mg/d. Patients receive a stable dose for 16 days. | Oral – Ketamine 40–400 mg/d, during 2 weeks | Placebo | Duration of analgesic benefit using the Short Form McGill Pain Questionnaire. Mean and worst pain; Hospital Anxiety and Depression Score and serious adverse events. |
| Pickering, 201934 | I: 20 C: 20 (cross-over study) |
Each patient received: placebo /placebo, ketamine /placebo, and ketamine /magnesium, every 35 days. After this, patients returned for the second randomization. They were re-evaluated and randomized if their pain intensity on the day of randomization was like pain intensity at inclusion. The same assessment was done before the third period. | Intravenous – Ketamine 0.5 mg/kg diluted in 45 mL saline solution | Magnesium 3000 mg administered over 30 min | Primary endpoint - area under the curve of daily pain intensity for a period of 35 days after infusion. Secondary endpoints - pain (at 7, 15, 21 and 28 days) and health-related, emotional, sleep, and quality of life questionnaires. |
Abbreviations: I, intervention group; C, control; VAS, visual analogic scale; MMSE, Mini-Mental State Examination.
Risk of Bias in Individual Studies
The overall quality of all included studies was considered high. Random sequence generation, allocation concealment, blinding of participants and personnel and selective reporting were all considered of low risk of bias. Publication bias could not be evaluated since no single meta-analysis pooled more than 10 studies. Four studies presented high risk of bias derived from loss to follow-up.26,32,33,36 (Figure 2; Table 3)
Figure 2.
Risk of bias according to different domains.
Table 3.
Risk of Bias
| Author, Year | Was the Randomization Sequence Adequately Generated? | Was Allocation Adequately Concealed? | Was There Blinding of Participants? | Was There Blinding of Caregivers? | Was There Blinding of Data Collectors? | Was There Blinding of Staticians? | Was There Blinding of Outcome Assessors? | Was Loss to Follow-Up (Missing Outcome Data) Infrequent?* | Are Reports of the Study Free of Suggestion of Selective Outcome Reporting? | Was The Study Apparently Free of Other Problems That Could Put It at a Risk of Bias? |
|---|---|---|---|---|---|---|---|---|---|---|
| Amr 201028 | Probably yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Probably yes | Definetely yes | Probably yes | Definetely yes | Definetely yes |
| Amr 201127 | Probably yes | Definetely yes | Definetely yes | Definetely yes | Probably yes | Probably yes | Probably yes | Probably yes | Definetely yes | Definetely yes |
| Barros 201229 | Probably yes | Probably yes | Definetely yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Definetely yes | Probably yes |
| Eide, 199438 | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Definetely yes | Probably yes |
| Fallon, 201833 | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Definetely not | Definetely yes | Definetely yes |
| Kim, 201531 | Probably yes | Probably yes | Definetely yes | Probably yes | Probably yes | Probably yes | Definetely yes | Definetely yes | Probably yes | Probably yes |
| Kvarnstrom, 200335 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Probably yes |
| Lauretti, 200241 | Probably yes | Probably yes | Definetely yes | Probably yes | Probably yes | Probably yes | Probably yes | Definetely yes | Definetely yes | Probably yes |
| Lynch, 200536 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely not | Probably yes | Probably yes |
| Max, 199539 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Probably yes |
| Mercadante, 200040 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Probably yes |
| Niesters, 201330 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes |
| Pickering, 201934 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes |
| Rigo, 201732 | Probably yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Probably not | Definetely yes | Definetely yes |
| Scwartzman, 200926 | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably yes | Probably not | Probably yes | Probably yes |
| Sigtermans, 200925 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Probably yes |
| Vranken, 200537 | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes | Definetely yes |
Effectiveness of Interventions
Primary Outcome: Pain
Overall Pain Reduction Compared to Standard Treatment
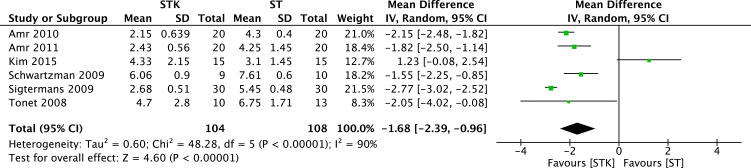
Results from six RCTs (212 patients)28–35,31,37 yielded a statistically significant reduction of pain between ketamine and standard treatment (ST) (MD −1.68, 95% CI −2.39 to −0.96; I2= 90%; p < 0.00001) (Figure 3). The certainty of evidence was rated as low because of imprecision (low number of patients (<400) and inconsistency. No publication bias was detected. We were unable to perform sensitivity analysis due to the small number of trials included in this analysis.
Figure 3.
Meta-analysis on the overall mean pain reduction. Forest plot is representing the comparison of the overall mean pain between group ketamine and ST.
Pain Reduction Compared to Standard Treatment at Different Time Periods
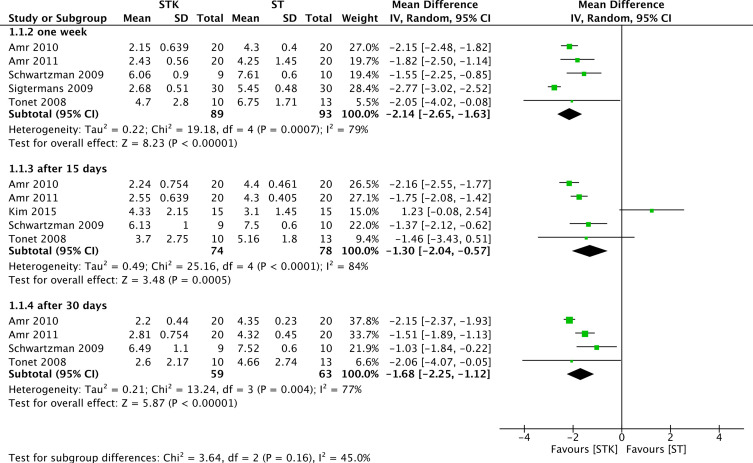
Results from five RCTs (182 patients)25–27,29,42 yielded a statistically significant reduction of pain until after one-week post-treatment between ketamine and ST (MD −2.14, 95% CI −2.65 to −1.63; I2= 79%; p < 0.00001) (Figure 4). A mean reduction of 46% compared to baseline pain. The certainty of evidence was rated as low because of imprecision (low number of patients: <400) and inconsistency (Table 4). No publication bias was detected.
Figure 4.
Meta-analysis on the average mean pain reduction. Forest plot is representing the comparison of the mean pain between group ketamine and ST according to time after the end of treatment.
Table 4.
GRADE Evidence Profile for Clinical Outcomes
| Quality Assessment | Summary of Findings | Certainty in Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Event Rates | Relative Risk or Average (CI 95%) | Anticipated Absolute Effects | |||||||||
| No of Participants (studies) Follow-Up in Days | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Control | Ketamine | Controla | Ketamine | ||
| Pain Average at one week after treatment (p < 0.00001) | |||||||||||
| 182 (5) 04–12 weeks |
No serious limitations | Serious limitationsa | No serious limitations | Serious imprecisionb | Undetected | Average 2.14 pain reduction (2.65 less to 1.63 less) compared to control | XXOO LOW |
||||
| Pain Average at two weeks after treatment (p = 0.0005) | |||||||||||
| 152 (5) 02–12 weeks |
No serious limitations | Serious limitationsa | No serious limitations | Serious imprecisionc | Undetected | Average 1.30 pain reduction (2.04 less to 0.57 less) compared to control | XXXO VERY LOW |
||||
| Pain Average at four weeks after treatment (p < 0.00001) | |||||||||||
| 122 (4) 04–12 weeks |
No serious limitations | Serious limitationsa | No serious limitations | Serious imprecisionc | Undetected | Average 1.68 pain reduction (2.25 less to 1.12 less) compared to control | XXXO VERY LOW |
||||
| Psychedelic effects (p=0.0007) | |||||||||||
| 266 (9) 01–90 days |
No serious limitations | No serious limitations | No serious limitations | Serious imprecisionc | Undetected | 8/134 | 56/132 | 4.94 (2.76–8.84) | 200 per 1000d | 788 more per 1000 | XXOO LOW |
| Discomfort (p=0.03) | |||||||||||
| 40 (2) 01–03 weeks |
No serious limitations | No serious limitations | No serious limitations | Serious imprecisionc | Undetected | 2/20 | 12/20 | 4.06 (1.18–13.95) | 167 per 1000e | 511 more per 1000 | XXOO LOW |
Notes: Table Representing the Quality of the Evidence and Summary of Findings. aSerious limitations due to high heterogeneity (I2>60%). bSerious imprecision due to less than 400 patients/events. cSerious imprecision due to wide confidence intervals and less than 400 patients/events. dBased on data from Niesters, 2013. eBased on data from Kvarnstrom, 2003.
Results from five RCTs (152 patients)26–28,31,42 yielded a statistically significant reduction in pain between ketamine and ST until up to two weeks post-treatment (MD −1.30, 95% CI −2.04 to −0.57; I2= 84%; p = 0.0005) (Figure 4). A mean reduction of 28% compared to baseline pain The certainty of evidence was rated as very low because of imprecision (low number of patients (<400) and wide confidence intervals) and inconsistency (Table 4). No publication bias was detected.
Results from 4 RCTs (122 patients)26–28,42 yielded a statistically significant reduction on pain after until 30 days post-treatment between ketamine and ST (MD −1.68, 95% CI −2.25 to −1.12; I2= 77%; p < 0.00001) (Figure 4). A mean reduction of 36% compared to baseline pain The certainty of evidence was rated as very low because of imprecision (low number of patients (<400) and wide confidence) and inconsistency (Table 4).
The test for subgroup differences yielded a I2=45% (Figure 4), thus revealing a consistent effect of ketamine compared to ST across the different time points after the treatment. No publication bias was detected.
Pain Reduction Compared to Baseline Pain Levels Over Time
Results from five RCTs (181 patients)25–28,42 yielded a statistically significant reduction of pain between baseline values and after one week of treatment with ketamine (MD −4.12, 95% CI −5.72 to −2.51; I2= 98%; p < 0.00001) (Figure 5). The certainty of evidence was rated as very low because of imprecision (low number of patients (<400) and wide confidence intervals) and inconsistency. No publication bias was detected.
Figure 5.
Meta-analysis on the average mean pain reduction over time. Forest plot is representing the comparison of the mean pain in the ketamine at different time points compared to baseline pain.
Results from other five RCTs (151 patients)26–28,31,42 yielded a statistically significant reduction of pain between baseline values and after two weeks of treatment with ketamine (MD −3.60, 95% CI −4.75 to −2.44; I2= 94%; p < 0.00001) (Figure 5). The certainty of evidence was rated as very low because of imprecision (low number of patients (<400) and wide confidence intervals) and inconsistency. No publication bias was detected.
Results from three RCTs (103 patients)27,28,42 yielded a statistically significant reduction of pain between baseline values and after four weeks of treatment with ketamine (MD −3.86, 95% CI −4.51 to −3.21; I2= 78%; p < 0.00001) (Figure 5). The certainty of evidence was rated as very low because of imprecision (low number of patients (<400) and wide confidence intervals) and inconsistency. No publication bias was detected.
Results from two RCTs (58 patients)26,27 yielded a statistically significant reduction of pain between baseline values and after two months of treatment with ketamine (MD −2.22, 95% CI −4.22 to −0.21; I2= 95%; p = 0.03) (Figure 5). The certainty of evidence was rated as very low because of imprecision (low number of patients (<400) and wide confidence intervals) and inconsistency. No publication bias was detected.
Results from two RCTs (40 patients)26,32 yielded a non-statistically significant difference in pain between baseline values and after three months of treatment with ketamine (MD −3.22, 95% CI −7.66 to 1.22; I2= 98%; p = 0.15) (Figure 5). The certainty of evidence was rated as very low because of imprecision (low number of patients (<400) and wide confidence intervals including both benefit and harm) and inconsistency.
The test for subgroup differences yielded a I2=0%, thus revealing a consistent effect of ketamine across the different time points after the treatment. No publication bias was detected.
Pain Reduction Measured by Multidimensional Scales
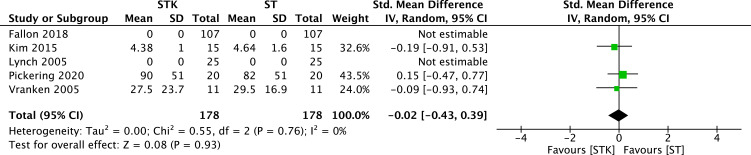
Results from five RCTs (356 patients)31,33,34,36,37 yielded a non-statistically significant difference between ketamine and ST on the reduction of pain at different multidimensional pain scales (MD −0.02, 95% CI −0.43 to 0.39; I2= 0%; p = 0.93) (Figure 6). The certainty of evidence was rated as low because of imprecision (low number of patients (<400) and confidence intervals including clinically important benefit and harm) and no publication bias was detected.
Figure 6.
Meta-analysis on the average standardized mean pain reduction. Forest plot is representing the comparison of the mean pain between group ketamine and ST according to different multidimensional pain scales.
Secondary Outcome
Adverse Outcomes
Psychedelic Effects
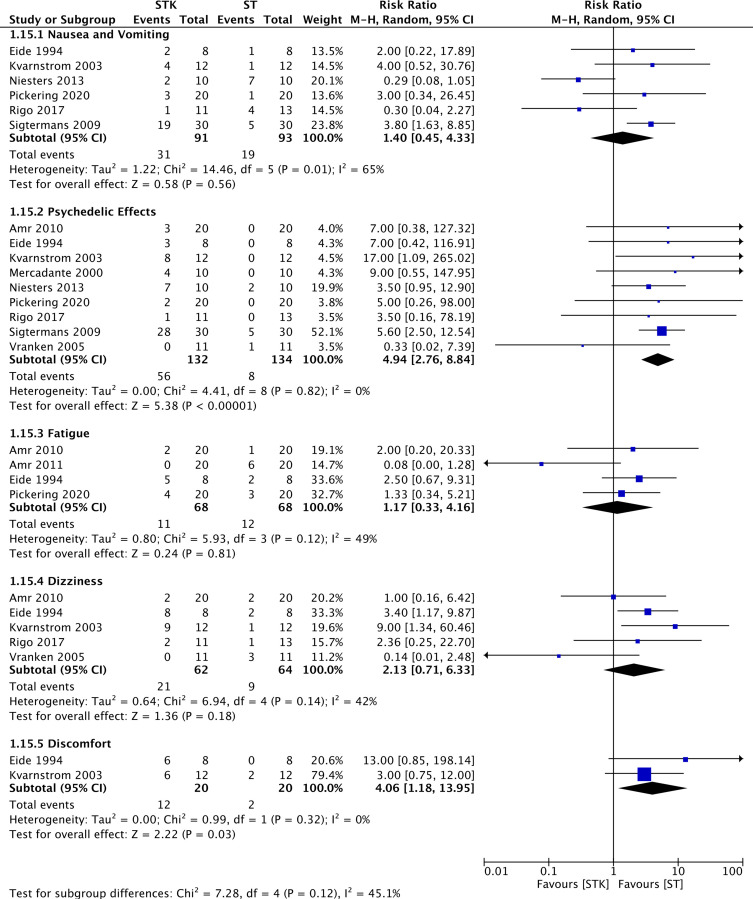
Results from nine RCTs (266 patients)25,28,30,32,34,35,37,38,40 yielded a statistically significant increase in the number of psychedelic effects when ketamine was used for the treatment of patients with NP compared to ST (RR 4.94, 95% CI 2.76 to 8.84; events (STK:56/132, ST:8/134); I2= 0%; p < 0.00001) (Figure 7). The certainty of evidence was rated as low because of imprecision (low number of events (<400) and wide confidence intervals) (Table 4) and no publication bias was detected (Table 4).
Figure 7.
Meta-analyses on the incidence of adverse outcomes. Forest plot is representing the comparison between group ketamine and ST according to different adverse outcomes.
Discomfort
Results from two RCTs (40 patients)35,38 yielded a statistically significant increase in the number of patients reporting general discomfort when ketamine was used for the treatment of NP compared to ST (RR 4.06, 95% CI 1.18 to 13.95; events (STK:12/20, ST:2/20); I2= 0%; p = 0.03) (Figure 7). The certainty of evidence was rated as low because of imprecision (low number of events (<400) and wide confidence intervals) (Table 4) and no publication bias was detected (Table 4).
Nausea and Vomiting, Fatigue and Dizziness
There was no difference between ketamine and ST regarding nausea and vomiting, fatigue, and dizziness (Figure 7). The certainty of evidence was rated as low for nausea and vomiting, fatigue, dizziness because of imprecision (low number of events (<400) and wide confidence intervals including both clinically important benefit and harm) and no publication bias was detected.
Adverse Outcomes (Composite Analysis)
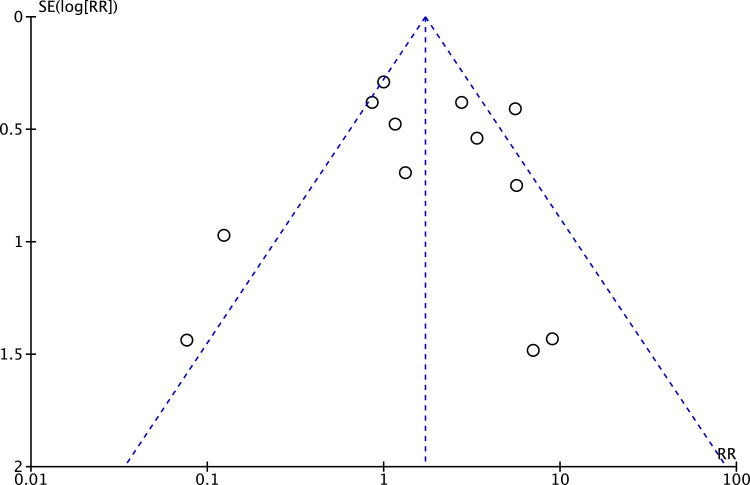
An overall analysis on adverse outcomes did not detect a significant difference between the two groups. The certainty of evidence was rated as low because of imprecision (low number of events (<400) and wide confidence intervals including both clinically important benefit and harm) and no publication bias was detected according to the characteristic Christmas tree shape of the Funnel Plot (Figure 8).
Figure 8.
Publication bias. Funnel Plot representing the distribution of studies according to their results.
We were unable to perform the following pre-specified analyses due to the lack of data from the included studies: Worst pain score measured by numerical rating scale; Least pain score measured by numerical rating scale; pain interference on general activities by numerical scale; pain interference on mood activities by numerical scale; pain interference on ability to walk by numerical scale; pain interference on work by numerical scale; pain interference on personal relationships by numerical scale; pain interference on sleep by numerical scale; pain interference on life appreciation by numerical scale.
We tried to contact authors to ask for further information regarding the missing data, but unfortunately no further information has been provided.
Discussion
Main Findings
There is a statistically significant pain reduction by adding ketamine to the treatment of chronic NP when compared to the standard treatment. However, such pain reduction comes at the expense of adverse outcomes, especially psychedelic effects related to the administration of ketamine. This raises questions such as whether these undesirable effects would make patients drop out of treatment and whether (S+) ketamine could provide the same amount of pain relief, but still sparing patients from experiencing such inconvenient side effects.
Strengths and Limitations
To our knowledge this is the most extensive search (no language or publication status restrictions) of the potential use of ketamine as an adjunct to NP treatment in the literature to date. We included studies in which ketamine was administered via different routes, dosing regimen, with different therapy duration. We independently rated the overall certainty of evidence (GRADE approach) for each outcome, extracted and analyzed data on the main outcomes deemed most important for stakeholders. We also assessed publication bias for the outcomes with ten or more studies included and the only funnel plot performed did not suggest the possibility of publication bias (Figure 7).
The primary limitation of our review is the low certainty of evidence due to study limitations, mostly due to inconsistency and imprecision. Thus, revealing the existence of great heterogeneity among ketamine administration protocols and the different clinical presentations and pathophysiology of neuropathic pain conditions. Ketamine was administered through the intravenous route in most of the studies included in this review. Amongst those studies the doses ranged from 0.5 to 1.5 mg/kg/day and duration of treatment lasted up to 10 days, and with pain reduction lasting up to one month.
Despite the moderate number of identified trials,17 the certainty of evidence is low to very low due to the low number of patients/events included in each individual meta-analysis, causing imprecision to be a major limiting factor to the quality of the yielded evidence.
Another limitation of this study is that we were unable to perform sensitivity analyses to explore the causes of high heterogeneity due to the small number of studies included in each meta-analysis.
Finally, due to a variety of outcomes, we could not pool the data of all eligible studies in all the proposed meta-analyses for this review, as this may limit the strength of evidence from yielded results.
Relation to Previous Studies
Six systematic reviews have been published in the recent past relevant to our study objectives.43–48. One review,43 although not performing meta-analysis, evaluated the administration of ketamine along with other NMDA receptor antagonist drugs. Its results suggested that introducing ketamine to the treatment of patients with neuropathic pain brought benefits to patients, but in this review, there were no meta-analysis performed and it also included studies in which patients were evaluated while still on ketamine infusion, therefore turning its conclusions very unlikely to be translated into everyday practice.
Another one limited itself to investigate the use of ketamine strictly through the intravenous route.48 Orhurhu et al48 found, like in our study, significant pain reduction at two weeks after the ketamine infusion in a meta-analysis including only two trials where patients suffered from neuropathic pain and no further benefits at time points thereafter.
Other reviews were less extensive in terms of the population, limiting their scope to only specific clinical conditions presenting neuropathic pain, such as complex regional pain syndrome (CRPS) without a broader and more extensive analysis encompassing several neuropathic pain conditions.43–46
Only one review had a similar approach to ours.47 Nonetheless, at the time (2010) limited itself to RCTs where ketamine was administered through the intravenous route in patients with neuropathic pain post limb amputation. And it is worth noting that neither one of these reviews used the Grading of Recommendations Assessment, and the Development and Evaluation (GRADE) approach to rate the certainty of evidence.
Clinical Implications of the Study
Results showed that ketamine reduced significantly (ranging from 28% to 46%), neuropathic pain compared to the baseline pain in the control group; and its effects can last for up to two months after the end of treatment. Thus, ketamine could be an attractive option to both patients and care providers for the management of breakthrough pain episodes and for cases refractory to standard care.
Although ketamine administration does not seem to cause serious adverse outcomes, our meta-analysis revealed a significant increase in psychedelic effects. Nevertheless, considering that intravenous ketamine administration takes place in Hospital facilities, those side effects would be safely manageable; hence moving the risk-benefit relation in favor of ketamine. In addition, those side effects could be decreased or avoided by choosing (S+) ketamine instead of the racemic ketamine. Yet, only in one study S(+) ketamine was the treatment of choice,37 and its results found a non-significant reduction in pain after the administration of ketamine through intravenous route.
Research Perspectives
Based on the data from this systematic review to determine the potential efficacy of ketamine for neuropathic pain treatment, we found statistically significant results on pain reduction, but we lacked quality of evidence due to imprecision from small sample size and clinical heterogeneity among included studies.
Some insights may be drawn from our results. Although it was not possible to perform the pre-specified meta-analyses based on different characteristics of ketamine therapy regimen, the most prevalent route of administration was the intravenous route, with 11 trials adopting such route doses ranged from 0.15 to 1.5 mg/Kg/day,25,26,28,30,31,33–35,37–40 with one trial using doses from 1.7 up to 10 mg/Kg/day.25 The duration of treatment was under 10 days in 8 out of these 11 trials that opted for the intravenous route.25,26,28,30,31,34,35,38 Plus, all studies from which we could retrieve data used racemic ketamine as the drug of choice for the study. Thus, we believe that more randomized clinical trials should be performed to determine the efficacy of ketamine for the treatment of neuropathic pain.
Conclusion
This comprehensive meta-analysis of 18 RCTs provides current evidence for the addition of ketamine to the standard treatment of patients suffering from neuropathic pain. It confirms previous observations that ketamine can be safely administered to patients, although revealing a significant increase in psychedelic effects amongst these patients. It also demonstrates with very low quality of evidence that the addition of ketamine to the standard treatment of patients with neuropathic pain may be more efficacious than the standard treatment alone on reducing neuropathic pain for as long as two months after the end of the treatment.
Acknowledgments
We would like to thank Dr. Per Kristian Eide, for replying to our queries.
Funding Statement
Centro de Estudos em Anestesia e Reanimação do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (FMUSP) (publication fees).
Abbreviations
CENTRAL, Central Register of Controlled Trials; CRPS, Complex Regional Pain Syndrome; EMBASE, Excerpta Medica database; FDA, Food and Drug Administration office; GRADE, Grading of Recommendations Assessment, Development and Evaluation; LILACS, Literatura Latino-Americana e do Caribe em Ciências da Saúde; MEDLINE, Medical Literature Analysis and Retrieval System Online; NMDA, N-methyl-D-aspartate; NMDARs, N-methyl-D-aspartate receptors; NP, neuropathic pain; NSAIDs, non-steroidal anti-inflammatory drugs; NRS, numerical rating scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; PROSPERO, International Prospective Register of Systematic Reviews; RCTs, randomized controlled trials; ST, standard of treatment; VAS, visual analog scale; WHO, World Health Organization.
Consent for Publication
The authors confirm that all the contents in this review can be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi: 10.1016/j.pain.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 2.Lépine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19(S1):1. doi: 10.1002/hup.618 [DOI] [PubMed] [Google Scholar]
- 3.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5(6):317–328. doi: 10.1016/j.jpain.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Yawn BP, Wollan PC, Weingarten TN, et al. The prevalence of neuropathic pain: clinical evaluation compared with screening tools in a community population. Pain Med. 2009;10(3):586–593. doi: 10.1111/j.1526-4637.2009.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656 [DOI] [PubMed] [Google Scholar]
- 6.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3(1):17002. doi: 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383. doi: 10.1177/2058738419838383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urits I, Li N, Berardino K, et al. The use of antineuropathic medications for the treatment of chronic pain. Best Prac Res Clin Anaesthesiol. 2020;34(1):493–506. doi: 10.1016/j.bpa.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 9.Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2016;37(1):29–42. doi: 10.1007/s00296-016-3481-8 [DOI] [PubMed] [Google Scholar]
- 10.Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- 11.Raeder JC, Stenseth LB. Ketamine: a new look at an old drug. Curr Opin Anaesthesiol. 2000;13(4):463–468. doi: 10.1097/00001503-200008000-00011 [DOI] [PubMed] [Google Scholar]
- 12.Kissin I, Bright CA, Bradley EL. The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesth Analg. 2000;91(6):1483–1488. doi: 10.1097/00000539-200012000-00035 [DOI] [PubMed] [Google Scholar]
- 13.Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730–1739. doi: 10.1213/01.ANE.0000086618.28845.9B [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829):d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence - Inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 17.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence - Study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence - Imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi: 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence - Indirectness. J Clin Epidemiol. 2011;64(12):1303–1310. doi: 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence - Publication bias. J Clin Epidemiol. 2011;64(12):1277–1282. doi: 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 22.Lane PW. Meta-analysis of incidence of rare events. Stat Methods Med Res. 2013;22(2):117–132. doi: 10.1177/0962280211432218 [DOI] [PubMed] [Google Scholar]
- 23.Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. Available from: https://training.cochrane.org/handbook/current. Accessed August 18, 2021.
- 24.Sun X, Ioannidis JPA, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis users’ guide to the medical literature. JAMA. 2014;311(4):405–411. doi: 10.1001/jama.2013.285063 [DOI] [PubMed] [Google Scholar]
- 25.Sigtermans MJ, van Hilten JJ, Bauer MCR, et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009;145(3):304–311. doi: 10.1016/j.pain.2009.06.023 [DOI] [PubMed] [Google Scholar]
- 26.Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo-controlled study. Pain. 2009;147(1–3):107–115. doi: 10.1016/j.pain.2009.08.015 [DOI] [PubMed] [Google Scholar]
- 27.Amr Y. Epidural ketamine in post spinal cord injury-related chronic pain. Anesth Essays Res. 2011;5(1):83–86. doi: 10.4103/0259-1162.84196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amr Y. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: a prospective, randomized, double blind trial. Pain Physician. 2010;13(3):245–249. doi: 10.36076/ppj.2010/13/245 [DOI] [PubMed] [Google Scholar]
- 29.Antonio Moreira de Barros G, Amante Miot H, Massarico Braz A, Ramos F, Aristoteles Borges M. Topical (S)-ketamine for pain management of postherpetic neuralgia * (S)-cetamina tópica no tratamento da dor da neuralgia pós-herpética. An Bras Dermatol. 2012;87(3):504–505. doi: 10.1590/s0365-05962012000300032 [DOI] [PubMed] [Google Scholar]
- 30.Niesters M, Aarts L, Sarton E, Dahan A. Influence of ketamine and morphine on descending pain modulation in chronic pain patients: a randomized placebo-controlled cross-over proof-of-concept study. BJA. 2013;110(6):1010–1016. doi: 10.1093/bja/aes578 [DOI] [PubMed] [Google Scholar]
- 31.Kim YH, Lee PB, Oh TK. Is magnesium sulfate effective for pain in chronic postherpetic neuralgia patients comparing with ketamine infusion therapy? J Clin Anesth. 2015;27(4):296–300. doi: 10.1016/j.jclinane.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 32.Ferreira J, Karine Rigo F, Trevisan G, et al. Management of neuropathic chronic pain with methadone combined with ketamine: a randomized, double blind, active-controlled clinical trial. Pain Physician. 2017;20(3):207–215. doi: 10.36076/ppj.2017.215 [DOI] [PubMed] [Google Scholar]
- 33.Fallon MT, Wilcock A, Kelly CA, et al. Oral ketamine vs placebo in patients with cancer-related neuropathic pain: a randomized clinical trial. JAMA Oncol. 2018;4(6):870–872. doi: 10.1001/jamaoncol.2018.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering G, Pickering G, Pereira B, et al. Ketamine and magnesium for refractory neuropathic pain: a randomized, double-blind, crossover trial. Anesthesiology. 2020;133(1):154–164. doi: 10.1097/ALN.0000000000003345 [DOI] [PubMed] [Google Scholar]
- 35.Kvarnstrom A, Karlsten R, Quiding H, Emanuelsson B-M, Gordh T. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand. 2003;47(7):868–877. doi: 10.1034/j.1399-6576.2003.00187.x [DOI] [PubMed] [Google Scholar]
- 36.Lynch ME, Clark AJ, Sawynok J, Sullivan MJL. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2005;103(1):140–146. doi: 10.1097/00000542-200507000-00021 [DOI] [PubMed] [Google Scholar]
- 37.Vranken JH, Dijkgraaf MGW, Kruis MR, van Dasselaar NT, van der Vegt MH. Iontophoretic administration of S(+)-ketamine in patients with intractable central pain: a placebo-controlled trial. Pain. 2005;118(1–2):224–231. doi: 10.1016/j.pain.2005.08.020 [DOI] [PubMed] [Google Scholar]
- 38.Eide P, Jorum E, Stubhaug A, Bremnes J, Brcivik H. Relief of post-herpetic neuralgia with the N-methyl-D-asp&c acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain. 1994;58(3):347–354. doi: 10.1016/0304-3959(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 39.Max M, Byas-Smith G, Gracely R, Bennett G. Intravenous infusion of the NMDA antagonist, Ketamine, in chronic posttraumatic pain with Allodynia: a double-blind comparison to alfentanil and placebo. Clin Neuropharmacol. 1995;18(4):360–368. doi: 10.1097/00002826-199508000-00008 [DOI] [PubMed] [Google Scholar]
- 40.Mercadante S, Arcuri E, Tirelli W, Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage. 2000;20(4):246–252. doi: 10.1016/s0885-3924(00)00194-9 [DOI] [PubMed] [Google Scholar]
- 41.Rocha Lauretti G, de Menezes Rodrigues A, Maria Alves Gomes J, Paulino Dos Reis M. Epidural Ketamine Versus Epidural Clonidine as Therapeutic for Refractory Neuropathic Chronic Pain [Avaliação Clínica Comparativa entre a Cetamina e a Clonidina por Via Peridural no Tratamento da Dor Crônica Neuropática]. Rev Bras Anestesiol. 2002;52(1):34–40. doi: 10.1590/S0034-70942002000100005 [DOI] [Google Scholar]
- 42.Tonet C, Sakata R, Issy A, Garcia J, Marcelino A. Evaluation of oral ketamine for neuropathic pain [Avaliação da cetamina oral para dor neuropática]. Rev Bras Med. 2008;65(7):214–218. [Google Scholar]
- 43.Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy, or mastectomy. Eur J Pain. 2015;19(4):451–465. doi: 10.1002/ejp.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Y, Song D, He A, Xu R, Xiu X. Systematic review efficacy of pain relief in different postherpetic neuralgia therapies: a network meta-analysis. Pain Physician. 2018;21:19–32. doi: 10.36076/ppj.2018.1.19 [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, Wang Y, Wang D. The effect of ketamine infusion in the treatment of complex regional pain syndrome: a systemic review and meta-analysis. Curr Pain Headache Rep. 2018;22:12. doi: 10.1007/s11916-018-0664-x. [DOI] [PubMed] [Google Scholar]
- 46.Aiyer R, Mehta N, Gungor S, Gulati A. A systematic review of NMDA receptor antagonists for treatment of neuropathic pain in clinical practice. Clin J Pain. 2018;34(5):450–467. doi: 10.1097/AJP.0000000000000547 [DOI] [PubMed] [Google Scholar]
- 47.Collins S, Sigtermans MJ, Dahan A, Zuurmond WW, Perez RS. NMDA receptor antagonists for the treatment of neuropathic pain. Pain Med. 2010;11(11):1142–1726. doi: 10.1111/j.1526-4637.2010.00981.x [DOI] [PubMed] [Google Scholar]
- 48.Orhurhu V, Orhurhu MS, Bhatia A, Cohen SP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241–254. doi: 10.1213/ANE.0000000000004185 [DOI] [PubMed] [Google Scholar]