Abstract
Background
Vitamin D is a likely candidate for treatment as its immune modulating characteristics have effects on coronavirus disease 2019 (COVID-19) patients. It was sought herein, to summarize the studies published to date regarding the vitamin D supplementation to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive patients.
Methods
A systematic review and meta-analysis were performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The primary outcome were 14-day and in-hospital mortality reported as an odds ratio (OR) with the associated 95% confidence interval (CI).
Results
Eight articles were included in the review with a combined total of 2,322 individual patients, 786 in the vitamin D supplementation group and 1,536 in the control group. The use of vitamin D compared to the group without vitamin D supplementation was associated with a lower 14-day mortality (18.8% vs. 31.3%, respectively; OR = 0.51; 95% CI: 0.12–2.19; p = 0.36), a lower in-hospital mortality (5.6% vs. 16.1%; OR = 0.56; 95% CI: 0.23–1.37; I2 = 74%; p = 0.20), the rarer intensive care unit admission (6.4% vs. 23.4%; OR = 0.19; 95% CI: 0.06–0.54; I2 = 77%; p = 0.002) as well as rarer mechanical ventilation (6.5% vs. 18.9%; OR = 0.36; 95% CI: 0.16–0.80; I2 = 0.48; p = 0.01).
Conclusions
Vitamin D supplementation in SARS-CoV-2 positive patients has the potential to positively impact patients with both mild and severe symptoms. As several high-quality randomized control studies have demonstrated a benefit in hospital mortality, vitamin D should be considered a supplemental therapy of strong interest. Should vitamin D prove to reduce hospitalization rates and symptoms outside of the hospital setting, the cost and benefit to global pandemic mitigation efforts would be substantial.
Keywords: COVID-19, SARS-CoV-2, vitamin D, calciferol, systematic review, meta-analysis
Introduction
In March of 2020, the respiratory disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) — coronavirus disease 2019 (COVID-19) was declared a worldwide pandemic by the World Health Organization (WHO). Since then, COVID-19 has infected hundreds of millions of people and pushed hospital systems to the brink of collapse. Now, more infectious variants of COVID-19 are threatening to cause surges in hospitalizations and again put pressure on hospitals systems [1]. As the WHO has issued masking, social distancing, vaccination and other preventative measures, some countries have even required their citizens to wear N95 respirators in public, as this has been found to dramatically reduce the risk of getting infected by SARS-CoV-2 [2–4]. While these measures serve as a model for what can be done, this measure is likely to be impractical for many countries and governments to implement and enforce. As the immediate goal of these interventions has been to decrease hospitalizations, identifying a biologically active agent that could reduce or shorten hospitalizations, limit severity of disease, or alleviate symptoms would be similarly important [5]. As the vast majority of hospitalizations for COVID-19 are due to acute respiratory symptoms leading to acute respiratory distress syndrome (ARDS) and respiratory failure [6–8], known immunomo dulating candidates that interact with respiratory monocytes are of particular interest [9]. Vitamin D is likely the best studied candidate as its immune modulating characteristics and effects on pulmonary parenchyma have been well documented [10]. Studies have also indicated that there is a correlation between the susceptibility to COVID-19 and lower vitamin D levels [11]. Additionally, the incidence of vitamin D toxicity is almost non-existent, and this over-the-counter supplement has been shown to specifically stimulate type II pneumocytes [12], which are a prime target of the SARS-CoV-2 [13]. This meta-analysis was conducted in order to investigate the possibility of adding vitamin D supplementation to the existing recommended COVID-19 prevention and mitigation strategies.
Methods
The present study involved a systematic literature review and meta-analysis of the impact of vitamin D supplementation in SARS-CoV-2 positive patients. The focus was on measuring the impact this intervention has had on mortality outcomes according to the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) statement [14].
Search methods
Applying a predetermined search strategy, two independent reviewers (L.S. and M.P.) searched PubMed, EMBASE, Web of Science, Cochrane Collaboration Databases and Scopus electronic databases from databases inception till July 10th 2021. The search was performed using the following terms: “vitamin D” OR “25-hydroxyvitamin D” OR “calcifediol” AND “SARS-CoV-2” OR “COVID-19”.
Inclusion and exclusion criteria
Studies included in this meta-analysis met the following PICOS criteria: (1) Participants; patients > 18 years of age with SARS-CoV-2 positive result, (2) Intervention; vitamin D supplementation, (3) Comparison; non-vitamin D supplementation, (4) Outcomes; detailed information for mortality, (5) Study design; randomized controlled trials and observational studies. Excluded reviews were simulation trials, animal studies, letters, conference papers and case studies. Studies were also excluded if the full paper was not available in English.
Data extraction
Two independent reviewers (L.S. and M.P.) performed data extraction. All disagreements were resolved by referral to a third author (F.C.) as necessary. From all eligible studies, extracted the following information: the name of the first author, year of publication, country of research, study design, patient characteristics, and mortality characteristics. Data from included studies were recorded using a Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) specific report form. When data about the primary outcomes were missing, contacting the corresponding author of the original study was planned.
Outcomes
Primary end points were 14-day and in-hospital mortality. Secondary end points were intensive care unit (ICU) admission, need of mechanical ventilation, radiological improvement and secondary infection incidence.
Assessment for risk of bias
The risk of bias (RoB) of the included studies was independently assessed by all three authors (L.S., K.B. and A.G.) according to the Cochrane risk-of-bias tool for randomized trials (RoB 2 tool) and the Risk of Bias In Non-randomized Studies — of the Interventions (ROBINS-I). All disagreements were resolved by referral to the third author (M.J.J.) if necessary. The overall RoB 2 and ROBINS-I judgment at domain and study level was attributed according to the criteria specified in the ROBVIS tool.
Statistical analysis
All analyses were performed with the Review Manager software version 5.4 (Nordic Cochrane Center, Cochrane Collaboration), and Stata software, version 15.0 (College Station, TX, USA). The significance level for all statistical tests was p < 0.05 (two-tailed). For dichotomous data, odds ratios (ORs) were used as the effect measure with 95% confidence intervals (CIs) and for continuous data mean differences (MDs) were used with 95% CI. When the continuous outcome was reported in a study as median, range, and interquartile range, estimated means and standard deviations were used using the formula described by Hozo et al. [15]. For meta-analysis the random effects model (assuming a distribution of effects across studies) was used to weigh estimates of studies in proportion to their significance [16]. Heterogeneity was interpreted as not observed when I2 = 0%, low when I2 = 25%, medium when I2 = 50% and high when I2 = 75%.
Results
Search results and study selection
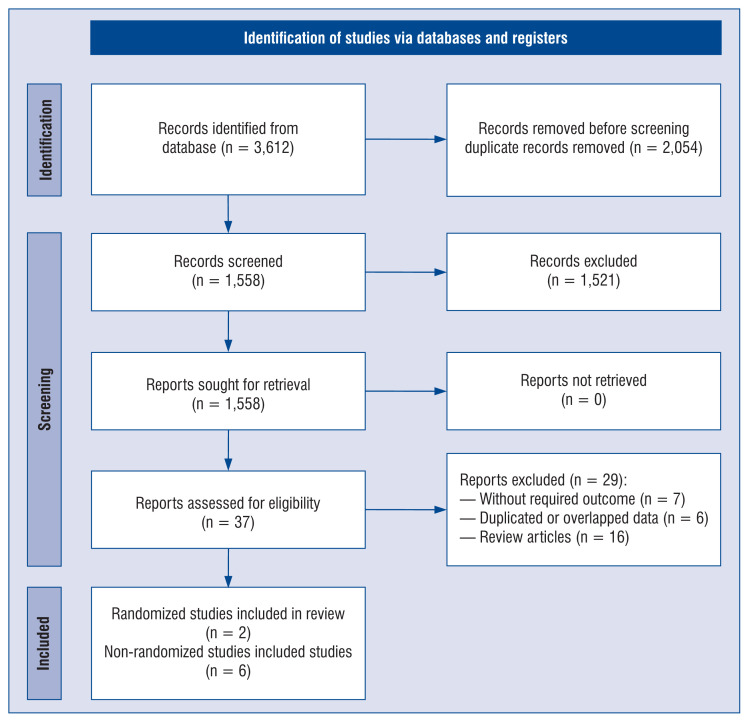
The systematic research, selection and reasons for exclusion are summarized in Figure 1. The literature search yielded 3,612 articles. After the removal of duplicated articles, 1,558 were included in the analysis. After excluding articles based on predetermined criteria, 8 articles were included in the review with a combined total of 2,322 individual patients, 786 in the vitamin D supplementation group and 1,536 in the control group. These studies originated in Spain (n = 4), France (n = 1), Italy (n = 1), Brazil (n = 1) and Singapore (n = 1). Of those, 2 articles were randomized clinical trials [17, 18], and 5 of them were non-randomized trials [19–24]. Mean age of COVID-19 patients treated with vitamin D was 62 (15.2) years compared to 64.8 (15.4) years for COVID-19 patients treated without vitamin D (MD = −0.29; 95% CI: −2.33 to 1.74; I2 = 78%; p = 0.78; Suppl. Table 1). Detailed characteristics of the studies included in the meta-analysis are presented in Table 1.
Figure 1.
Flow diagram showing stages of the database search and study selection as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Table 1.
Characteristics of included studies.
| Study | Country | Study design | Vitamin D supplementation group | Non-vitamin D supplementation group | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No. | Age | Sex, male | No. | Age | Sex, male | |||
| Alcala-Diaz et al., 2021 | Spain | Retrospective, multicenter cohort study | 79 | 69 ± 15 | 42 (53.2%) | 458 | 67 ± 16 | 275 (60.0%) |
| Annweiler et al., 2020 | France | Quasi-experimental study | 16 | 58.4 ± 7 | 11 (68.8%) | 32 | 64.1 ± 7.9 | 19 (59.4%) |
| Castillo et al., 2020 | Spain | Parallel pilot randomized open label, double-masked clinical study | 50 | 56.8 ± 14.2 | 27 (54.0%) | 26 | 55.8 ± 15 | 18 (69.2%) |
| Cereda et al., 2021 | Italy | Cohort observational study | 38 | 68.8 ± 10.6 | 16 (42.1%) | 286 | 70.5 ± 13.1 | 141 (49.3%) |
| Hernández et al., 2020 | Spain | Retrospective case–control study | 19 | 63.5 ± 4.6 | 7 (36.8%) | 197 | 59.9 ± 3.8 | 123 (62.4%) |
| Murai et al., 2021 | Brazil | Multicenter, double-blind, randomized, placebo-controlled study | 120 | 53.1 ± 10.8 | 70 (58.3%) | 120 | 52.8 ± 9.4 | 65 (54.2%) |
| Nogues et al., 2021 | Spain | Observational cohort study | 447 | 61.8 ± 15.5 | 264 (59.1%) | 391 | 62.4 ± 17.2 | 231 (59.1%) |
| Tan et al., 2020 | Singapore | Cohort observational study | 17 | 85.8 ± 1.5 | 11 (64.7%) | 26 | 88 ± 2.3 | 15 (57.7%) |
Assessment of risk of bias
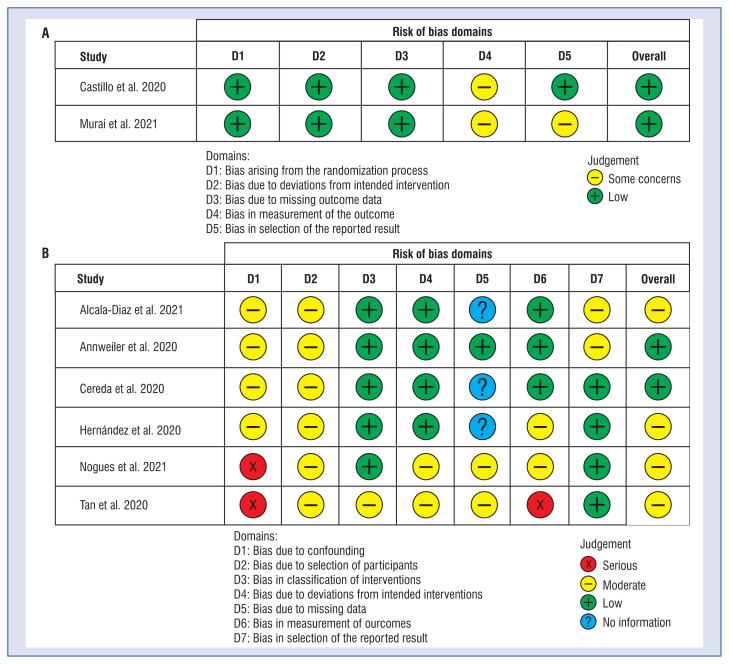
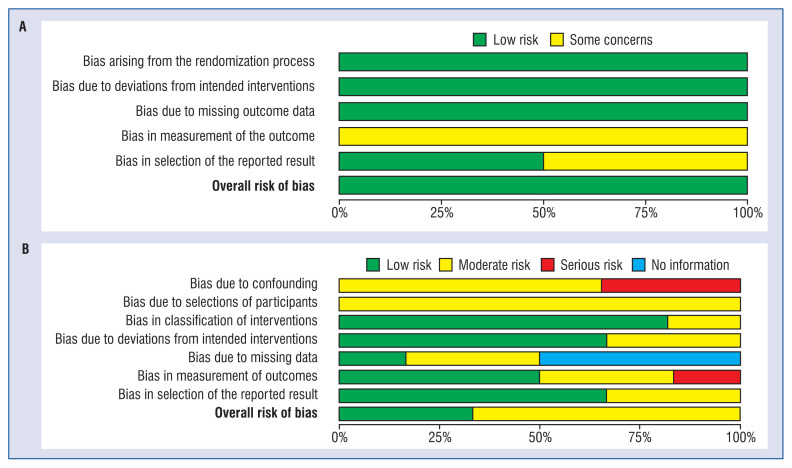
The detailed methodological description and risk of bias can be found in Figure 2. The risk of bias judgements summary is presented in Figure 3. In both randomized trials, overall risk of bias was rated as low. In the non-randomized trial two of them were rated as low, and three as moderate risk of bias.
Figure 2.
A plot of the distribution of review authors’ judgements across; randomized controls trials (A) and non-randomized controls trials (B) studies for each risk of bias item.
Figure 3.
A summary table of review authors’ judgements for each risk of bias item for each randomized controls trials (A) and non-randomized controls trials (B).
Meta-analysis outcomes
Detailed characteristics of the meta-analysis outcomes are presented in Table 2. 14-day mortality was reported in only 1 study and was 18.8% for patients with vitamin D supplementation compared to 31.3% for the group without vitamin D supplementation (OR = 0.51; 95% CI: 0.12–2.19; p = 0.36). Seven studies stated in-hospital mortality. Pooled analysis of in-hospital mortality in the vitamin D vs. non-vitamin D groups show a significant difference in mortality rate, 5.6% vs. 16.1%, respectively (OR = 0.56; 95% CI: 0.23–1.37; I2 = 74%; p = 0.20).
Table 2.
Study outcomes.
| Parameter | No. of studies | Events/participants | Events | Heterogeneity between trials | P-value for differences across groups | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Vitamin D supplementation | Non-vitamin D supplementation | Odds ratio | 95% CI | P-value | I2 statistic | |||
| Mechanical ventilation | 2 | 9/139 (6.5%) | 60/317 (1.9%) | 0.38 | 0.17 to 0.86 | 0.48 | 0% | 0.02 |
| Radiological worsening | 1 | 2/19 (10.5%) | 56/197 (28.4%) | 0.30 | 0.07 to 1.32 | NA | NA | 0.11 |
| Secondary infection | 1 | 2/19 (10.5%) | 44/197 (22.3%) | 0.41 | 0.09 to 1.84 | NA | NA | 0.24 |
| Thrombotic events | 1 | 1/19 (5.3%) | 10/197 (5.1%) | 1.04 | 0.13 to 8.58 | NA | NA | 0.97 |
| ICU admission | 5 | 42/653 (6.4%) | 178/760 (23.4%) | 0.19 | 0.06 to 0.54 | 0.002 | 77% | 0.002 |
| Mortality: | ||||||||
| 14 days mortality | 1 | 3/16 (18.8%) | 10/32 (31.3%) | 0.51 | 0.12 to 2.19 | NA | NA | 0.36 |
| In-hospital mortality | 7 | 42/750 (5.6%) | 220/1,370 (16.1%) | 0.56 | 0.23 to 1.37 | 0.002 | 74% | 0.20 |
CI — confidence interval; ICU — intensive care unit; NA — not applicable
The need for ICU care was statistically lower in the group in which vitamin D was administered orally compared to the control group without vitamin D (6.4% vs. 23.4%; OR = 0.19; 95% CI: 0.06–0.54; I2 = 77%; p = 0.002).
The implementation of vitamin D supplementation in patients with COVID-19 compared to patients who did not receive vitamin D was associated with less frequent use of mechanical ventilation (6.5% vs. 18.9%; OR = 0.36; 95% CI: 0.16–0.80; I2 = 0.48; p = 0.01).
The use of vitamin D was also associated with radiological improvement (10.5% vs. 28.4%; OR = 0.30; 95% CI: 0.07–1.32; p = 0.11) and secondary infection incidence (10.5% vs. 22.3%; OR = 0.41; 95% CI: 0.09–1.84; p = 0.24).
Discussion
Though global vaccination against the SARS-CoV-2 virus has been ongoing since late 2020 and the various vaccines continue to be effective at preventing hospitalizations [25], more infectious variants of SARS-CoV-2 are fueling a rebound in infections among the unvaccinated [26]. As most countries will not achieve herd immunity from vaccination efforts until well into 2022, COVID-19 will likely continue to occupy hospital systems in countries all over the world [27]. Treatment for hospitalized COVID-19 patients will also limit access to essential medical services for people suffering from chronic and degenerative diseases [28]. As a consequence, research into potential therapeutic agents such as azithromycin and chloroquine have made headlines [29, 30], however these strategies proved futile and even dangerous [31, 32]. Additionally, the use of lopinavir, ritonavir, remdesivir, oseltamivir, ribavirin to treat COVID-19 also proved not to be effective [33, 34].
At this time, vitamin D, which has immunomodulating characteristics and has been shown to be associated with better outcomes in upper respiratory tract infections, should be a candidate of interest in mitigating COVID-19 [35, 36]. This inexpensive and readily available supplement could be rapidly and widely implemented with minimal risk of detriment to the general public. The implementation of which could result in decreased ICU admissions that could reduce the number of occupied ICU beds and result in better clinical outcomes [36]. In one randomized control ICU study, supplemental vitamin D administered to COVID-19 patients, alongside existing therapy, was associated with lower ICU admission and mortality [21]. The inclusion criteria included COVID-19 positive patients with clinical and radiological findings of ARDS and resulted in a reduction in ICU treatment and a reduction of symptoms. It must be noted that the groups did not differ at the baseline with the control group presenting more often with hypertension while the clinical group was slightly older [37].
It has been hypothesized that the benefits of vitamin D in patients suffering from ARDS are due to the activation of the vitamin D receptor pathway, resulting in a decrease of cytokine expression [38], a central cause of rapid deterioration [39]. Additionally, vitamin D deficiency in ICU patients is common [40] and may indicate that other complications in COVID-19 infections are the result of this deficiency [13]. When a combination of vitamin D/magnesium/vitamin B12 were administered the older patients, this combination was found to reduce the need for the more advanced procedures without adding significant costs. The rationale for this combination lies in the fact that magnesium enhances vitamin D activity and plays a pivotal role in the immune system [41, 42]. Additionally, vitamin B12 stabilizes the gut microbiota, which has also played a pivotal role in a patient’s overall health [43, 44]. These observations are reinforced by other studies where vitamin D administered in frail elderly patients was associated with better survival rate and less severe COVID-19 course [45].
However, other studies have found that the administration of vitamin D in COVID-19 patients conveyed no clinical benefit in terms of severity of disease, while also being associated with a twofold increase in mortality rate [21]. It can be hypothesized that late administration of vitamin D in the presence of severe inflammation could impair the metabolism of vitamin D [46], resulting in a buildup of the metabolites. The last study included in this review found that the administration of vitamin D administration had no effect on the severity of the course of COVID-19 infections [47]. It should be noted that the protocol of this trial included the administration of a onetime dose of 200,000 IU of vitamin D among hospitalized patients with moderate or severe disease. It is not clear if this one dose regiment is sufficient as many patients with upper respiratory tract conditions display, e.g., asthma, impaired function of the CYP2R1 (vitamin D 25-hydroxylase) [48] which is an enzyme that catalyzes the formation of vitamin D3 to 25-hydroxyvitamin D3 (25(OH)D3), which reduces the biologically active form of vitamin D.
Conclusions
Vitamin D supplementation in SARS-CoV-2 positive patients has the potential to positively impact patients with both mild and severe symptoms. As a number of high-quality randomized control studies have demonstrated a benefit in hospital mortality, vitamin D should be considered a supplemental therapy of strong interest. At the same time, should vitamin D prove to reduce hospitalization rates and symptoms outside of the hospital setting, the cost and benefit to global pandemic mitigation efforts would be substantial. It can be concluded that further multicenter investigation of vitamin D in SARS-CoV-2 positive patients is urgently warranted at this time.
Supplementary Information
Acknowledgments
The study was supported by the ERC Research Net and by the Polish Society of Disaster Medicine.
Footnotes
This paper was guest edited by Prof. Togay Evrin
Funding
This work was supported by the Ministry of Science and Higher Education (project No. 024/RID/2018/19 entitled “Regional Initiative Excellence in 2019–2022”).
Conflict of interest: None declared
References
- 1.da Silva FC, Barbosa CP. The impact of the COVID-19 pandemic in an intensive care unit (ICU): Psychiatric symptoms in healthcare professionals. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110299. doi: 10.1016/j.pnpbp.2021.110299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect. 2020;105(1):104–105. doi: 10.1016/j.jhin.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barycka K, Szarpak L, Filipiak KJ, et al. Comparative effectiveness of N95 respirators and surgical/face masks in preventing airborne infections in the era of SARS-CoV2 pandemic: A meta-analysis of randomized trials. PLoS One. 2020;15(12):e0242901. doi: 10.1371/journal.pone.0242901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szarpak L, Smereka J, Filipiak KJ, et al. Cloth masks versus medical masks for COVID-19 protection. Cardiol J. 2020;27(2):218–219. doi: 10.5603/CJ.a2020.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saban M, Shachar T. The silent effect of COVID-19 on emergency departments: How to avoid complacency? Disaster Emerg Med J. 2020;5:224–226. doi: 10.5603/demj.a2020.0035. [DOI] [Google Scholar]
- 6.Alfano V, Ercolano S. The efficacy of lockdown against COVID-19: a cross-country panel analysis. Appl Health Econ Health Policy. 2020;18(4):509–517. doi: 10.1007/s40258-020-00596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213(2):54–56e1. doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruetzler K, Szarpak L, Filipiak K, et al. The COVID-19 pandemic — a view of the current state of the problem. Disaster Emerg Med J. 2020;5:106–107. doi: 10.5603/demj.a2020.0015. [DOI] [Google Scholar]
- 9.Nurminen V, Seuter S, Carlberg C. Primary vitamin D target genes of human monocytes. Front Physiol. 2019;10 doi: 10.3389/fphys.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medrano M, Carrillo-Cruz E, Montero I, et al. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018;19(9) doi: 10.3390/ijms19092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szarpak L, Rafique Z, Gasecka A, et al. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol J. 2021;28(5):647–654. doi: 10.5603/CJ.a2021.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehan VK, Torday JS, Peleg S, et al. 1Alpha,25-dihydroxy-3-epivitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab. 2002;76(1):46–56. doi: 10.1016/s1096-7192(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 13.Quesada-Gomez JM, Entrenas-Castillo M, Bouillon R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J Steroid Biochem Mol Biol. 2020;202:105719. doi: 10.1016/j.jsbmb.2020.105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane. Cochrane Handbook for Systematic Reviews of Interventions. 2019. [Access: 1 July 2019]. www.training.cochrane.org/handbook .
- 17.Castillo ME, Costa LE, Barrios JV, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murai I, Fernandes A, Sales L, et al. Effect of vitamin D3 supplementation vs placebo on hospital length of stay in patients with severe COVID-19: a multicenter, double-blind, randomized controlled trial. medRxiv. 2021 doi: 10.1101/2020.11.16.20232397. [DOI] [Google Scholar]
- 19.Alcala-Diaz JF, Limia-Perez L, Gomez-Huelgas R, et al. Calcifediol treatment and hospital mortality due to COVID-19: a cohort study. Nutrients. 2021;13(6) doi: 10.3390/nu13061760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annweiler G, Corvaisier M, Gautier J, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cereda E, Bogliolo L, Lobascio F, et al. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition. 2021;82:111055. doi: 10.1016/j.nut.2020.111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández JL, Nan D, Fernandez-Ayala M, et al. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J Clin Endocrinol Metab. 2021;106(3):e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PubMed] [Google Scholar]
- 23.Nogues X, Ovejero D, Pineda-Moncusí M, et al. Calcifediol treatment and COVID-19-related outcomes. J Clin Endocrinol Metab. 2021;106(10):e4017–e4027. doi: 10.1210/clinem/dgab405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing K, Tu XY, Liu M, et al. Efficacy and safety of COVID-19 vaccines: a systematic review. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23(3):221–228. doi: 10.7499/j.issn.1008-8830.2101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. 2021;595(7865):17–18. doi: 10.1038/d41586-021-01696-3. [DOI] [PubMed] [Google Scholar]
- 27.Li R, Rivers C, Tan Qi, et al. The demand for inpatient and ICU beds for COVID-19 in the US: lessons from Chinese cities. medRxiv. 2020 doi: 10.1101/2020.03.09.20033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Lancet. Rheumatology.Too long to wait: the impact of COVID-19 on elective surgery. Lancet Rheumatol. 2021;3(2):e83. doi: 10.1016/s2665-9913(21)00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz RA, Suskind RM. Azithromycin and COVID-19: Prompt early use at first signs of this infection in adults and children, an approach worthy of consideration. Dermatol Ther. 2020;33(4):e13785. doi: 10.1111/dth.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borba MG, Val FF, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 31.Kow CS, Hasan SS. Azithromycin in patients with COVID-19: Friend or foe? Clin Microbiol Infect. 2021;27(1):136–137. doi: 10.1016/j.cmi.2020.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho TC, Wang YH, Chen YL, et al. Chloroquine and hydroxychloroquine: efficacy in the treatment of the COVID-19. Pathogens. 2021;10(2) doi: 10.3390/pathogens10020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perveen RA, Nasir M, Talha KA, et al. Systematic review on current antiviral therapy in COVID-19 pandemic. Med J Malaysia. 2020;75(6):710–716. [PubMed] [Google Scholar]
- 34.Szarpak Ł, Dzieciątkowski T, Jaguszewski MJ, et al. Is remdesivir important in clinical practice as a treatment of COVID-19? A study based on meta-analysis data. Pol Arch Intern Med. 2021;131(1):96–97. doi: 10.20452/pamw.15686. [DOI] [PubMed] [Google Scholar]
- 35.Bradley R, Schloss J, Brown D, et al. The effects of vitamin D on acute viral respiratory infections: A rapid review. Adv Integr Med. 2020;7(4):192–202. doi: 10.1016/j.aimed.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Town JA, Churpek MM, Yuen TC, et al. Relationship between ICU bed availability, ICU readmission, and cardiac arrest in the general wards. Crit Care Med. 2014;42(9):2037–2041. doi: 10.1097/CCM.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh AK, Gupta R, Ghosh A, et al. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14(4):303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szarpak Ł, Nowak B, Kosior D, et al. Cytokines as predictors of COVID-19 severity: evidence from a meta-analysis. Pol Arch Intern Med. 2021;131(1):98–99. doi: 10.20452/pamw.15685. [DOI] [PubMed] [Google Scholar]
- 39.Tang Lu, Yin Z, Yu Hu, et al. Controlling cytokine storm is vital in COVID-19. Front Immunol. 2020;11:570993. doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, et al. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol. 2010;121(1–2):452–455. doi: 10.1016/j.jsbmb.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 41.Dai Qi, Zhu X, Manson JE, et al. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr. 2018;108(6):1249–1258. doi: 10.1093/ajcn/nqy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11) doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lurz E, Horne RG, Määttänen P, et al. Vitamin B12 deficiency alters the gut microbiota in a murine model of colitis. Front Nutr. 2020;7:83. doi: 10.3389/fnut.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annweiler C, Hanotte B, Grandin de l’Eprevier C, et al. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reijven PLM, Soeters PB, Reijven PLM, et al. Vitamin D: A magic bullet or a myth? Clin Nutr. 2020;39(9):2663–2674. doi: 10.1016/j.clnu.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 47.Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jolliffe DA, Stefanidis C, Wang Z, et al. Vitamin d metabolism is dysregulated in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202(3):371–382. doi: 10.1164/rccm.201909-1867OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.