Abstract
Therapeutic responses to clindamycin in combination with quinine were assessed in adult Thai patients with uncomplicated multidrug-resistant Plasmodium falciparum malaria. In total 204 patients were randomized to receive a 7-day oral treatment regimen of quinine (Q7) either alone (n = 68), in combination with clindamycin (Q7C7; n = 68), or in combination with tetracycline (Q7T7; n = 68). All patients had uncomplicated recoveries with no serious adverse effects. Fever clearance times for both of the two combination regimens (median of 47 h and range of 8 to 120 h for Q7C7 and median of 36 h and range of 8 to 117 h for Q7T7) were significantly shorter than that for the Q7-only regimen (median, 56; range, 4 to 152 h) (P = 0.002). Parasite clearance times (overall mean ± standard deviation, 78 ± 23 h) were not significantly different between the three treatment groups (P = 0.98). The cure rates assessed at 28 days of follow-up were 100% for Q7C7 and 98% for Q7T7, whereas the cure rate was 87% for the Q7-only regimen (P ≤ 0.04). Clindamycin in combination with quinine is a safe and effective treatment for multidrug-resistant P. falciparum malaria. This combination may be of particular value in children and pregnant women, in whom tetracyclines are contraindicated.
Multidrug-resistant Plasmodium falciparum malaria is of increasing public health concern in the tropics. For adult patients, combination treatments with quinine-tetracycline, artesunate-mefloquine, or artemether-lumefantrine are effective worldwide. These regimens yield high cure rates of 95 to 100% (3, 7, 14, 15, 17). When quinine is used for the treatment of children and pregnant women, it is often given alone, as the tetracyclines are contraindicated in these two important high-risk groups. Combinations with other antibiotics with antimalarial activity such as erythromycin or rifampin (9) have shown disappointing efficacies.
Clindamycin, usually combined with quinine, had been used extensively in South America and has also proved effective in adults and children with acute malaria in Africa (4–6, 13). The efficacy of clindamycin plus quinine has not been evaluated in the Southeast Asian region (12), where the most drug-resistant P. falciparum strains are found. We have recently assessed the antimalarial activity of clindamycin in adult patients with Plasmodium vivax malaria and found that it has antimalarial activity similar to that of tetracycline or doxycycline (9). The present study assessed the efficacy of clindamycin in combination with quinine in comparison with those of quinine-tetracycline and quinine alone for the treatment of adult patients with falciparum malaria in Thailand, which harbors the world's most drug-resistant P. falciparum.
MATERIALS AND METHODS
Patients.
The study was conducted with adult male patients with acute P. falciparum malaria admitted to the Bangkok Hospital for Tropical Diseases, Bangkok, Thailand, between 1995 and 1997. Fully informed consent was obtained from each subject. Exclusion criteria were severe malaria (18) or mixed malaria parasite infections. Patients who gave a history of drug hypersensitivity, patients who had taken any antimalarial drugs within the previous 48 h, or patients whose urine was positive in screening tests for sulfonamides (lignin test) or 4-aminoquinolines (Wilson-Edeson test) were also excluded. The study was approved by the ethics committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Management.
After clinical assessment and confirmation of the diagnosis from thick and thin blood smears, baseline blood samples were taken for routine hematology and biochemistry analyses. Patients were then allocated by simple randomization to a 7-day oral treatment regimen: quinine sulfate (10 mg of salt/kg of body weight three times a day; Thai Government Pharmaceutical Organization) either alone (Q7) or in combination with tetracycline (4 mg/kg four times a day; Thai Government Pharmaceutical Organization) (Q7T7) or with clindamycin (5 mg of base/kg four times a day; Dalacin C; Pharmacia & Upjohn Pharmaceuticals) (Q7C7).
Oral acetaminophen (0.5 to 1 g every 4 h) was given for a temperature of >38°C. Vital signs were recorded every 4 h until resolution of fever and thereafter every 6 to 12 h. Fever clearance times (FCTs) were expressed as FCTA, which was the time taken for the body temperature first to fall below 37.5°C, and FCTB, which was the time taken for the body temperature to fall below 37.5°C and remain below this value for >48 h. Patients who were subsequently unable to stay in the hospital until clearance of both fever and parasites were excluded from the study. Reappearance of infection was assessed in patients who remained in Bangkok either in the hospital or at home (i.e., outside the malaria transmission area) for at least 28 days. Patients with recrudescences were retreated with a 7-day course of quinine (10 mg of salt/kg three times a day) in combination with tetracycline (4 mg/kg four times a day), and those who had late vivax appearances were treated subsequently with the standard dose of chloroquine and primaquine.
Laboratory investigations.
Parasite counts were measured every 12 h in thin films or thick films until clearance and thereafter daily for 28 days. Parasite density was expressed as the number of parasites per microliter of blood, derived from the numbers of parasites per 1,000 red blood cells in a thin film stained with Giemsa or Field stain or was calculated from the white cell count and the numbers of parasites per 200 white blood cells in a thick film. The following variables were chosen prospectively to describe parasite clearance (PC): time taken from the start of antimalarial treatment until the asexual malaria parasite count fell to 50% (PC50) and 90% (PC90) of the value at the time of admission and the time taken for the parasite count to fall below detectable levels in a peripheral blood smear (PCT). The variables used to define the parasite reduction rates (PRRs) were the ratio of the parasite count before treatment to the counts at 24 h (PRR24) or 48 h (PRR48) and the ratio of the parasite count at 48 h to the count at 96 h (PRR48/96). Routine biochemical and hematological tests were repeated on days 7, 14, 21, and 28 after admission.
Statistical analysis.
The data for each treatment group were compared by one-way analysis of variance with post hoc multiple comparisons with the Bonferroni correction. Nonparametric data were compared by the Kruskal-Wallis test. The cumulative FCTs, PC rates, and cure rates were calculated by Kaplan-Meier survival analysis and were compared by the log-rank test. Associations between FCT, PC rate, and PRR were measured with Spearman's rank correlation coefficient. All statistical analyses were performed with the statistical computing package SPSS, version 8, for Windows (SSPS Inc.).
RESULTS
Patients.
The study included 204 male patients with P. falciparum malaria (age range, 15 to 64 years; mean ± standard deviation [SD] age 25.8 ± 9.5 years). These patients were randomized to three treatment groups (n = 68 each; Table 1). The majority of patients (n = 157; 77%) came from the western border of Thailand, where the most multidrug-resistant P. falciparum parasites are prevalent. Less than half the patients (n = 92; 45%) had a history of malaria. There were no significant differences in age or geographic distributions, parasite counts on admission, or incidence of previous malaria attacks between the three treatment groups, (P ≥0.09) (Table 1). Elevated serum bilirubin levels (total bilirubin concentration, ≥3 mg/dl) were noted in 26 patients (Q7 group, n = 13; Q7C7 group, n = 10; Q7T7 group, n = 3). None of these patients had any other complications, and all came from areas where malaria is endemic. The overall total bilirubin levels for the Q7C7 and Q7 groups on admission were not different (P = 0.68), but the levels for both groups were slightly higher than those for the Q7T7 group (P ≤ 0.004). Other baseline laboratory findings (Table 1) were not statistically significant among the groups studied. None of the patients had or developed severe anemia (hematocrit, >15% for all patients) or elevated serum creatinine levels (serum creatinine levels, <3 mg/dl for all patients) or other complications of malaria.
TABLE 1.
Laboratory findings on admission for patients with P. falciparum malaria
| Characteristic | Q7(n = 68) | Q7C7(n = 68) | Q7T7(n = 68) | All groups (n = 204) | P value |
|---|---|---|---|---|---|
| Age (yr)a | 24.6 ± 8.9 | 25.6 ± 9.3 | 27.3 ± 10.2 | 25.8 ± 9.5 | 0.23 |
| No. (%) of patients with previous malaria | 31 (46) | 29 (43) | 32 (47) | 92 (45) | 0.34 |
| No. (%) of patients from western Thailand | 57 (84) | 60 (88) | 40 (59) | 157 (77) | 0.12 |
| Parasite count (no./μl)b | 9,493 | 17,155 | 9,352 | 11,528 | 0.09 |
| Hematocrit (%)a | 35.1 ± 8.6 | 34.5 ± 9.0 | 34.3 ± 7.8 | 34.6 ± 8.5 | 0.84 |
| White blood cell count (no. [103]/μl)a | 6.29 ± 2.08 | 6.34 ± 2.49 | 6.01 ± 2.29 | 6.22 ± 2.29 | 0.69 |
| Platelet count (no. [109]/liter)a | 107 ± 77 | 104 ± 78 | 127 ± 82 | 112 ± 79 | 0.21 |
| Serum creatinine concn (mg/dl)a | 1.05 ± 0.29 | 1.04 ± 0.23 | 1.06 ± 0.35 | 1.06 ± 0.35 | 0.88 |
| Total bilirubin concn (mg/dl)d | 1.7 (0.1–9.4) | 1.3 (0.1–10.2) | 1.2 (0.1–7.0)c | 1.3 (0.1–10) | 0.007 |
| SGPTe level (U/liter)d | 30 (8.0–280) | 22 (8–194) | 26 (10–254) | 26 (8–280) | 0.19 |
Data are as mean ± SD.
Data are geometric means.
Value is significantly different from those for the other two groups.
Data are median (range).
SGPT, serum glutamic pyruvate transaminase.
Clinical response.
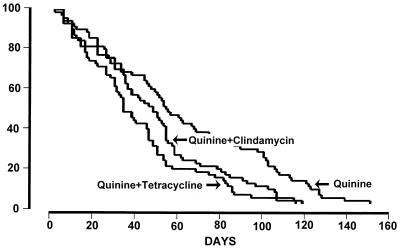
Clinical recovery following treatment occurred in all patients (Table 1). FCTAs ranged from 2 to 100 h (median, 8 h) and were not significantly different between the three treatment groups (P = 0.10). The overall median (range) FCTB was 48.0 h (4 to 152 h). As shown in Fig. 1, the FCTBs for the two combination regimens (Q7C7 group, 47 h; Q7T7 group, 36 h) were not significantly different (P = 0.08), but the FCTBs for both combination regimens were significantly shorter than the FCTB for the Q7-only regimen (56 h) (P ≤0.005). Patients admitted with hyperbilirubinemia had significantly longer FCTs (median, 72 h; range, 8 to 124 h) than those for the remaining patients (median, 48 h; range 7 to 152 h) (P = 0.014). After stratification for jaundice on admission, the FCTBs for the two combination regimens remained not significantly different (Q7C7 = 44 h, Q7T7 = 36 h [P = 0.32]), and the FCTBs for both combination regimens were significantly shorter than the FCTB for the Q7-only regimen (53 h) (P ≤ 0.012).
FIG. 1.
Cumulative fever clearance rates for the three treatment groups of patients with P. falciparum malaria.
PC and parasite reduction.
Following the start of treatment, the overall mean ± SD PCT was 77.7 ± 22.8 h. Between the treatment groups, there were no significant differences in PCTs (P = 0.98), and this remained after stratification for jaundice on admission (P = 0.82). There were also no significant differences in PRRs or clearance of parasitemia for the groups studied, as assessed from the PC50, the PC90, and the calculated PRRs (PRR24, PRR48, and PRR96) (P ≥0.65) (Table 2). The overall PCT for all patients correlated directly with the PC50 and PC90 (r = 0.34 and 0.68; respectively; P ≤ 0.001) and inversely with the calculated PRRs (PRR24 and PRR48; r = 0.31 and 0.42, respectively; P < 0.001). There were weak but statistically significant correlations between PCT and FCTs (r = 0.24 for FCTA and r = 0.33 for FCTB; P <0.01).
TABLE 2.
Clinical and parasitological responses in patients with P. falciparum malaria
| Response parameter | Q7(n = 68) | Q7C7(n = 68) | Q7T7(n = 68) | All groups (n = 204) | P value |
|---|---|---|---|---|---|
| FCTA (h)a | 10 (2–100) | 8 (2–95) | 8 (3–36) | 8 (2–100) | 0.10 |
| FCTB (h)a | 56 (4–152)b | 47 (8–120) | 36 (8–117) | 48 (4–152) | 0.004 |
| PC50 (h)c | 21 ± 17 | 21 ± 14 | 21 ± 14 | 21 ± 15 | 0.95 |
| PC90 (h)c | 57 ± 25 | 58 ± 19 | 61 ± 19 | 59 ± 21 | 0.65 |
| PCT (h)c | 77 ± 25 | 79 ± 20 | 77 ± 23 | 78 ± 23 | 0.98 |
| PRR24a | 4 (0–528) | 6 (<1–2,253) | 2 (<1–1,225) | 4 (<1–2,253) | 0.45 |
| PRR48a | 117 (<1–2,082) | 119 (<1–5,770) | 109 (<1–8,895) | 115 (<1–8,895) | 0.82 |
| PRR96a | 153 (6–2,412) | 39 (6–676) | 21 (2–977) | 45 (2–2,412) | 0.26 |
Data are median (range).
Value is significantly different from those for the other two groups.
Data are mean ± SD.
Clinical course.
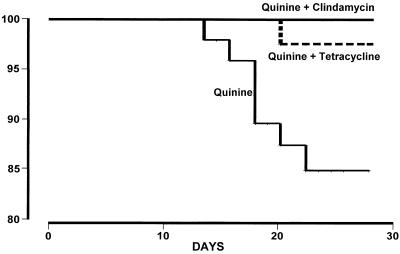
Overall, 161 (79%) of the recruited patients completed at least 28 days of follow-up or remained in the hospital until the appearance of vivax or falciparum malaria (Table 3). Of these 161 patients, 8 (5.0%) had a subsequent reappearance of falciparum malaria and another 33 (21%) had a delayed appearance of vivax malaria. Recrudescences of falciparum malaria were observed for patients who received the Q7-only regimen (n = 7; 13%) and in one patient treated with the Q7T7 regimen (2%) but not in patients treated with the Q7C7 regimen. The time to the onset of recrudescence ranged from 15 to 23 days (mean ± SD = 19.3 ± 2.5 days). The cure rates (i.e., no recrudescence of falciparum malaria) for the two combination regimens (98 and 100%) were both significantly higher than the cure rate for the Q7 regimen (87%) (P ≤ 0.04) (Fig. 2).
TABLE 3.
Clinical outcomes for monitored patients with subsequent appearances of malaria infection which occurred within 28 days after the start of treatment
| Treatment group | No. of patients who completed follow-up | No. (%) of patients with subsequent appearance of:
|
|
|---|---|---|---|
| P. falciparum | P. vivax | ||
| Q7 | 53 | 7 (13.2) | 12 (22.6) |
| Q7C7 | 60 | 0 | 12 (20.0) |
| Q7T7 | 48 | 1 (2.1) | 9 (18.8) |
| Total | 161 | 8 (5.0) | 33 (20.5) |
FIG. 2.
Cumulative cure rates for the three treatment groups of patients with P. falciparum malaria.
Cryptic infection with P. vivax was found in 33 (21%) patients. Despite the differences in the 28-day follow-up rates between the three groups, the rates of mixed infection among the three treatment groups were not significantly different (P = 0.9). The overall mean ± SD time taken for the appearance of vivax malaria was 23.7 ± 3.0 days and ranged from 16 to 28 days after the start of treatment. There was no significant difference in the time to the appearance of vivax malaria (within the 28-day follow-up period) between the two combination regimens (for Q7C7, 25.1 ± 1.4 days; for Q7T7, 24.8 ± 3.2 days [P = 1.0]), although the times to onset for both regimens were significantly delayed compared to the time to onset for the Q7 regimen (21.6 ± 3.0 days) (P ≤0.028). Of the 161 patients monitored, 16 patients returned for subsequent monthly follow-up and none had a reappearance of falciparum malaria, but five patients had later appearances of vivax malaria, between days 38 and 61. These late episodes of vivax malaria were found in all groups: Q7, n = 2; Q7C7, n = 2; Q7T7, n = 1.
Therapeutic responses in patients with subsequent recrudescences.
The eight patients with subsequent recrudescences had significantly longer FCTs (83.5 ± 44 versus 50.4 ± 31.1 h [P = 0.023]) and PCTs (95.4 ± 21.1 versus 77.2 ± 21.8 h [P = 0.005]) than those for patients with no recrudescence. Only one of these patients had elevated serum bilirubin levels on admission; there was no association between bilirubinemia and recrudescent infections. Parasite counts on admission between patients with and without recrudescences were not significantly different (geometric mean of 21,875 and range of 4,019 to 141,300 versus geometric mean of 14,475 and range of 30 to 276,948 [P = 0.32]). Between patients with and without mixed P. vivax and P. falciparum infection, there were no significant differences in parasite counts on admission, FCTs, or parasitological responses (P ≥ 0.14).
Clinical and laboratory findings following treatment.
On admission, gastrointestinal symptoms were noted in 93 (46%) patients. Of these, 85 (91%) had nausea, 31 (33%) had vomiting, 27 (29%) had abdominal pain, and 3 (3.2%) had diarrhea. There were no significant differences in the incidences of gastrointestinal symptoms among the three treatment groups (P = 0.45). Following treatment, only six additional patients developed any of the gastrointestinal symptoms (n = 3 for Q7, n = 2 for Q7C7, n = 1 for Q7T7). These patients complained of nausea (n = 6) with or without vomiting (n = 4) or abdominal pain (n = 3) or diarrhea (n = 2). After starting antimalarial treatment, all patients recovered and the gastrointestinal symptoms disappeared within 1 to 7 days (median, 2 days).
The majority of patients (193; 93.7%) developed transient tinnitus. The onset of tinnitus usually occurred after 3 days of treatment. There were no significant differences in the incidences of cinchonism among the three treatment groups (P = 0.8). All 26 jaundiced patients had normalization of bilirubin levels after 7 days (23 of 26; 88.5%) or 14 days (3 of 26; 11.5%) of treatment. None of the studied patients developed allergic rashes or other serious adverse effects, as monitored by clinical symptoms and laboratory data (data not shown).
DISCUSSION
The worsening of antimalarial drug resistance poses a considerable threat to human populations in the tropics. In Thailand, where the world's most drug-resistant malaria parasites are found, resistance to chloroquine, sulfadoxine-pyrimethamine, and more recently, mefloquine has limited the options for the treatment of falciparum malaria (8, 10, 16). Since the 1970s there has been a decline in the susceptibility to quinine, although there have been few recent data. The current trial provides reassuring results. The Q7 regimen was 87% effective, which is similar to that reported 15 years ago from the Bangkok Hospital for Tropical Diseases (2). Many had feared that mefloquine resistance would drive resistance to quinine. Although in some parts of Thailand mefloquine resistance has improved over the past 5 years with deployment of combination treatment that included an artemisinin derivative (8a), in other parts of the country mefloquine it is still used alone, providing selective pressure to the emergence of resistance. Whatever the explanation, quinine still retains good clinical activity against the multidrug-resistant P. falciparum parasites prevalent in Thailand.
The addition of a tetracycline, most commonly, doxycycline, to quinine consistently improves the cure rates for falciparum malaria (2, 7, 11, 14). Indeed, there has been no evident decline in the efficacy of this combination since it was first introduced nearly 20 years ago in this area (3). Quinine in combination with a tetracycline is a generally safe and effective regimen. Unfortunately, minor toxicity from quinine (cinchonism) is usual, and the combination of an extremely bitter taste and cinchonism compromises compliance, and thus efficacy. A major limitation of this combination is that tetracycline cannot be used in children less than 8 years old or during pregnancy. These two patient groups are both at high risk from falciparum malaria. In general, cure rates for children and pregnant women are also inferior to those for nonpregnant adults living in areas where malaria is endemic (1). Nevertheless, a 7-day course of quinine alone remains the treatment of choice for acute falciparum malaria in the first trimester of pregnancy in this area.
This study shows that clindamycin is an effective and well-tolerated alternative to tetracycline in combination antimalarial treatment. There were no treatment failures among the 60 patients treated with a 7-day course of quinine and clindamycin. Thus, estimated efficacy is 100%, and the lower 95% confidence interval for the true cure rate is equal to or greater than 95%. The addition of either doxycycline or clindamycin to quinine also delayed the appearance of P. vivax infections, suggesting additional activity against this parasite. The clindamycin was very well tolerated, and there were no adverse effects attributable to it. The principal adverse effect of clindamycin is diarrhea and, in extreme cases, pseudomembranous colitis caused by Clostridium difficile. This did not occur in the present trial. Unfortunately, clindamycin is significantly more expensive than tetracycline, and as cost is the major factor that determines the use of antimalarial drugs, this represents a significant drawback (US$15 for clindamycin versus <US$1 for doxycycline or tetracycline per adult treatment course). Nevertheless, clindamycin may be considered a safe and effective alternative to the tetracyclines in combination treatment of drug-resistant falciparum malaria. It may be of particular value for young children and pregnant women, as these two groups cannot receive tetracycline. Further studies with these high-risk groups should now be conducted.
ACKNOWLEDGMENTS
This study was part of the Wellcome-Mahidol University, Oxford Tropical Medicine Research Programme. We are grateful to Pharmacia & Upjohn and to R. X. Company Limited, Bangkok, Thailand, for providing us with antimalarials.
This study was supported by The Wellcome Trust of Great Britain.
REFERENCES
- 1.Chongsuphajaisiddhi T, Sabcharoen A, Attanath P. In vivo and in vitro sensitivity of falciparum malaria to quinine in Thai children. Ann Trop Paedatr. 1981;1:21–26. doi: 10.1080/02724936.1981.11748054. [DOI] [PubMed] [Google Scholar]
- 2.Harinasuta T, Bunnag D. Drug resistant malaria with special reference to chemotherapy. Mosquito Borne Dis Bull. 1984;1:23–30. [Google Scholar]
- 3.Harinasuta T, Bunnag D. Management of malaria with special reference to drug resistance. Jpn J Trop Med Hyg. 1988;16:121–130. [Google Scholar]
- 4.Kremsner P G, Wildling E, Jenne L, Graninger W, Biennzle U. Comparison of micronized halofantrine with chloroquine-antibiotic combinations for treating Plasmodium falciparum malaria in adults from Gabon. Am J Trop Med Hyg. 1994;50:790–795. doi: 10.4269/ajtmh.1994.50.790. [DOI] [PubMed] [Google Scholar]
- 5.Kremsner P G, Winkler S, Brandts C, Neifer S, Bienzle U, Graninger W. Clindamycin in combination with chloroquine or quinine is an effective therapy for uncomplicated Plasmodium falciparum malaria in children from Gabon. J Infect Dis. 1994;169:467–470. doi: 10.1093/infdis/169.2.467. [DOI] [PubMed] [Google Scholar]
- 6.Kremsner P G, Radlff P, Metzger W, Wildling E, Mordmuller B, Philipps J, Jenne L, Nkeyi M, Prada J, Bienzle U. Quinine plus clindamycin improves chemotherapy of severe malaria in children. Antimicrob Agents Chemother. 1995;39:1603–1605. doi: 10.1128/aac.39.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looareesuwan S, Wilairatana P, Vanijanonta S, Kyle D, Webster K. Efficacy of quinine-tetracycline for acute uncomplicated falciparum malaria in Thailand. Lancet. 1992;i:367–370. doi: 10.1016/0140-6736(92)91690-a. [DOI] [PubMed] [Google Scholar]
- 8.Nosten F, ter Kuile F, Chongsuphajaisiddhi T, Luxemburger C, Webster H K, Edstein M, Phaipun L, Thew K L T, White N J. Mefloquine-resistant falciparum malaria on the Thai-Burmese border. Lancet. 1991;337:1140–1143. doi: 10.1016/0140-6736(91)92798-7. [DOI] [PubMed] [Google Scholar]
- 8a.Nosten, F., M. van Vugt, R. Price, C. Luxemburger, K. L. Thway, A. Brockman, R. McGready, F. ter Kuile, S. Looareesuwan, and N. J. White. Plasmodium falciparum malaria incidence and mefloquine resistance in western Thailand: a prospective study. Lancet, in press. [DOI] [PubMed]
- 9.Pukrittayakamee S, Viravan C, Charoenlarp P, Yeamput C, Wilson R J M, White N J. Antimalarial effects of rifampin in Plasmodium vivax malaria. Antimicrob Agents Chemother. 1994;38:511–514. doi: 10.1128/aac.38.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pukrittayakamee S, Chantra A, Simpson J A, Vanijanonta S, Clemens R, Looareesuwan S, White N J. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother. 2000;44:1680–1685. doi: 10.1128/aac.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reacher M, Gampbell C C, Freeman J, Doberstyn E B, Brandling-Bennett A D. Drug therapy for Plasmodium falciparum malaria resistant to pyrimethamine-sulphadoxine (Fancidar7). A study of alternate regimens in eastern Thailand, 1980. Lancet. 1981;ii:1066–1069. doi: 10.1016/s0140-6736(81)91274-5. [DOI] [PubMed] [Google Scholar]
- 12.Salazar N P, Saniel M C, Estoque M H, Talao F A, Bustos D G, Palogan L P, Gabriel A I. Oral clindamycin in the treatment of acute uncomplicated falciparum malaria. Southeast Asian J Trop Med Public Health. 1990;21:397–340. [PubMed] [Google Scholar]
- 13.Vaillant M, Millet P, Luty A, Tshopamba P, Lekoulou F, Mayombo J, Georges A J, Deloron P. Therapeutic efficacy of clindamycin in combination with quinine for treating uncomplicated malaria in a village dispensary in Gabon. Trop Med Int Health. 1997;2:917–919. doi: 10.1046/j.1365-3156.1997.d01-411.x. [DOI] [PubMed] [Google Scholar]
- 14.Vanijanonta S, Chantra A, Phophak N, Chindanond D, Clemens R, Pukrittayakamee S. Therapeutic effects of chloroquine in combination with quinine in uncomplicated falciparum malaria. Ann Trop Med Parasitol. 1996;90:269–275. doi: 10.1080/00034983.1996.11813052. [DOI] [PubMed] [Google Scholar]
- 15.Watt G, Loesuttivibool L, Shanks G D, Boudreau E F, Brown A E, Pavanand K, Webster H K, Wechgritaya S. Quinine with tetracycline for the treatment of drug-resistant falciparum malaria in Thailand. Am J Trop Med Hyg. 1992;47:108–111. doi: 10.4269/ajtmh.1992.47.108. [DOI] [PubMed] [Google Scholar]
- 16.White N J. Antimalarial drug resistance: the pace quickens. J Antimicrob Chemother. 1992;30:571–585. doi: 10.1093/jac/30.5.571. [DOI] [PubMed] [Google Scholar]
- 17.White N J, Nosten F, Looareesuwan S, Watkins W M, Marsh K, Snow R W, Kokwaro G, Ouima J, Hien T T, Molyneux M E, Taylor T E, Newbold C I, Ruebush T K, Danis M, Greenwood B M, Anderson R M, Olliaro P. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization, Control of Tropical Diseases. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(Suppl. 2):1–65. [PubMed] [Google Scholar]