Abstract
Trogocytosis modulates immune responses, with still unclear underlying molecular mechanisms. Using leukemia mouse models, we found that lymphocytes perform trogocytosis at high rates with tumor cells. While performing trogocytosis, both Natural Killer (NK) and CD8+ T cells acquire the checkpoint receptor PD-1 from leukemia cells. In vitro and in vivo investigation revealed that PD-1 on the surface of NK cells, rather than being endogenously expressed, was derived entirely from leukemia cells in a SLAM receptor–dependent fashion. PD-1 acquired via trogocytosis actively suppressed NK cell antitumor immunity. PD-1 trogocytosis was corroborated in patients with clonal plasma cell disorders, where NK cells that stained for PD-1 also stained for tumor cell markers. Our results, in addition to shedding light on a previously unappreciated mechanism underlying the presence of PD-1 on NK and cytotoxic T cells, reveal the immunoregulatory effect of membrane transfer occurring when immune cells contact tumor cells.
Natural Killer cells are inhibited by PD-1 acquired from the surface of tumor cells via trogocytosis.
INTRODUCTION
During trogocytosis, immune cells acquire parts of the membrane of cells they interact with (1, 2). First characterized in aβ-T cells (3–8), it later became clear that virtually all immune cells perform trogocytosis (7, 9–16). This intercellular transfer of membranes results in the acquisition of proteins that would otherwise not be endogenously expressed by the cell performing trogocytosis, as in the case of Natural Killer (NK) cells that acquire viral proteins from infected cells (17, 18) or cancer antigens from tumor cells (19). Proteins transferred via trogocytosis are functional and influence the response of the accepting cell (11, 16, 18, 20–25). The pathophysiological relevance of trogocytosis is underscored by the high extent that immune cells perform it in the context of infections (26, 27), autoimmune diseases (28), and cancer (24, 29, 30). NK cells are important mediators of the response against intracellular pathogens and tumors (31, 32) and have been among the first immune cells shown to perform trogocytosis (10–12). Trogocytosis has been reported to contribute to the negative regulation of NK cell responses in different contexts. For example, acquisition of m157 or NKG2D ligands not only results in sustained and unproductive cross-linking of activating receptors leading to NK cell anergy (18, 33, 34) but also promotes NK fratricide (34, 35). On the other hand, acquisition of major histocompatibility complex (MHC) molecules from target cells engaged Ly49 receptors in cis, sustaining inhibitory signaling that dampened NK cell activation (11). Recently, CD9 was shown to be trogocytosed by NK cells from ovarian cancer cells, resulting in reduced killing capacity (36). Last, trogocytosis of HLA-G (Human Leukocyte Antigen G) from cancer cells resulted in the generation of NK cells with suppressive properties (37).
We recently reported that NK cells are suppressed by the checkpoint receptor PD-1 and contribute to the therapeutic efficacy of PD-1/L1 blockade in mouse models of cancer (38). These results, corroborated by others (39–44), were at least partially confuted by findings that murine and human NK cells fail to endogenously express Pdcd1 mRNA or PD-1 protein (45). In light of our results indicating that PD-1 is found on the surface of NK cells, and considering the high trogocytosis activity of NK cells, we propose that NK cells acquire PD-1 directly from tumor cells. Mechanistic experiments corroborated our hypothesis and revealed that SLAM receptors were important mediators of PD-1 trogocytosis. Functionally, trogocytosed PD-1 suppressed NK cell–mediated cancer immunosurveillance. Last, analysis of NK cells in patients with clonal plasma cell disorders suggests that PD-1 trogocytosis occurs in cancer patients. Together, our data shed light on a new mechanism that regulates NK cell function via acquisition of PD-1 from tumor cells.
RESULTS
NK cells acquire PD-1 from tumor cells
Consistent with what has been previously reported (38, 45), murine NK cells acutely stimulated ex vivo with a panel of inflammatory mediators failed to up-regulate PD-1 at the protein level (fig. S1). Lack of PD-1 induction was in line with epigenetic analysis of the Pdcd1 locus, which was not accessible in splenic NK cells, either before or after cytokine stimulation, in sharp contrast with the promoter of another checkpoint receptor (Tigit) in NK cells, or Pdcd1 locus in CD8+ T cells (fig. S2).
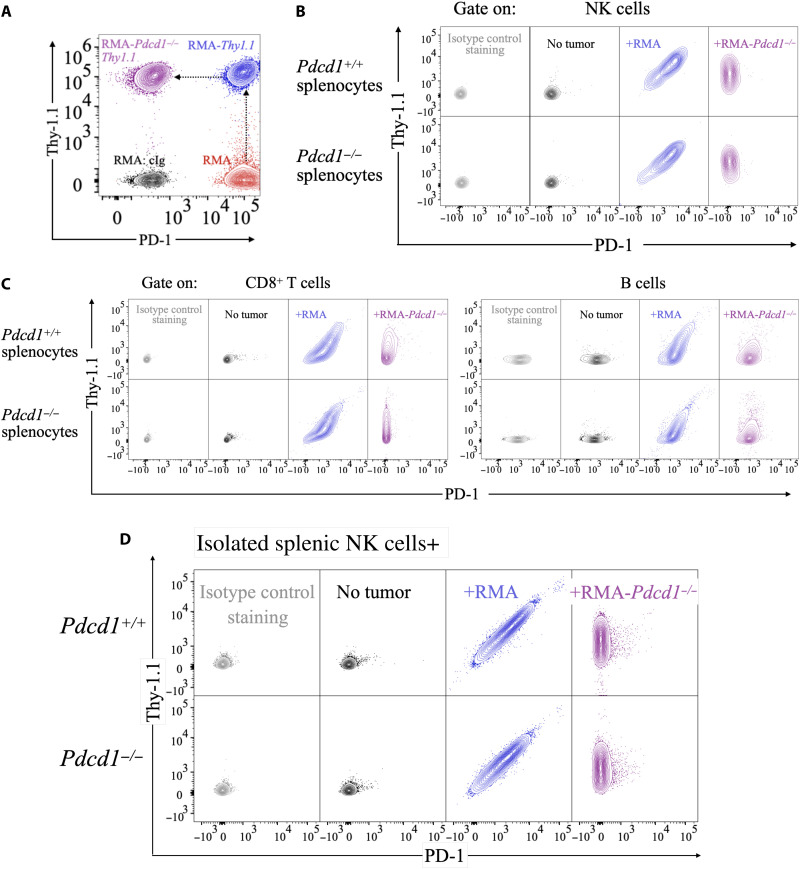
Considering the conflicting evidence regarding PD-1 expression on NK cells (38, 44, 45), we hypothesized that rather than endogenously expressing the protein, NK cells acquired PD-1 from other cells. To test this hypothesis, we initially used RMA cells, which derive from transformation of murine T cells (46), express high levels of PD-1 (Fig. 1A, in red), and were used extensively in our previous study (38). We generated RMA cells expressing the syngeneic marker Thy-1.1 (not expressed by C57BL/6 mice, which express the Thy-1.2 allelic variant) and targeted PD-1 with CRISPR-Cas9 (RMA-Pdcd1−/−Thy1.1) (Fig. 1A, in blue and purple, respectively). We then cocultured tumor cells with splenocytes from Pdcd1+/+ or Pdcd1−/− littermates, with RMA cells expressing PD-1 or not (gating strategy in fig. S3). In the absence of tumor cells, immune cells did not stain for PD-1 or Thy-1.1. In sharp contrast, NK, T, and B cells from both Pdcd1+/+ and Pdcd1−/− mice stained positively for PD-1 when incubated with RMA cells, but not RMA-Pdcd1−/− cells (Fig. 1, B and C, and fig. S4), indicating that PD-1 was not endogenously expressed by innate and adaptive lymphocytes, but acquired from tumor cells in these settings. Consistent results were obtained by using NK cells isolated from Pdcd1+/+ or Pdcd1−/− mice (purity of ~90%) (Fig. 1D and fig. S5A). Regardless of PD-1 expression on tumor cells, Thy-1.1 was detected in abundance on the surface of immune cells (Fig. 1, B and C, and fig. S5B). Acquisition of PD-1 and Thy-1.1 by NK cells was tightly correlated (fig. S5C), suggesting that the two molecules were transferred to NK cells as part of a unique phenomenon. Both PD-1 and Thy-1.1 were stable on the surface of NK cells, as we could detect both proteins 18 hours after we separated NK cells from tumor cells (fig. S6). To determine whether other proteins endogenously expressed by RMA cells were acquired by NK cells, we cocultured CD45.1-expressing NK cells with RMA cells (which express CD45.2). In addition to PD-1 and Thy-1.1, NK cells also acquired TCRvβ12 and CD45.2 (fig. S7), although the staining was weaker than for PD-1 and Thy-1.1. These data indicate that when interacting with RMA cells, NK cells acquire several proteins they would not endogenously express.
Fig. 1. Lymphocytes acquire PD-1 and Thy-1.1 from RMA cancer cells.
(A) RMA cells (red) were transduced with a retroviral vector encoding Thy-1.1 to generate RMA-Thy1.1 (blue), and then PD-1 was knocked out by CRISPR-Cas9 to generate RMA-Pdcd1−/−Thy1.1 (purple). A representative flow cytometry staining depicting PD-1 and Thy-1.1 expression is shown. (B and C) Splenocytes from Pdcd1+/+ or Pdcd1−/− littermates were incubated with RMA-Thy1.1 or RMA-Pdcd1−/−Thy1.1. After 3 days, cells were stained with Thy-1.1 and PD-1 antibodies. NK cells were gated as singlets/live-NK1.1+NKp46+DX5+ events. CD8+ T cells were gated as singlets/live-CD3+CD8+ events and B cells as singlets/live-CD19+. (D) NK cells isolated from Pdcd1+/+ or Pdcd1−/− littermates were incubated with RMA-Thy1.1 or RMA-Pdcd1−/−Thy1.1. After 3 days, cells were stained with Thy-1.1 and PD-1 antibodies. The experiment depicted is representative of 15 performed with similar results.
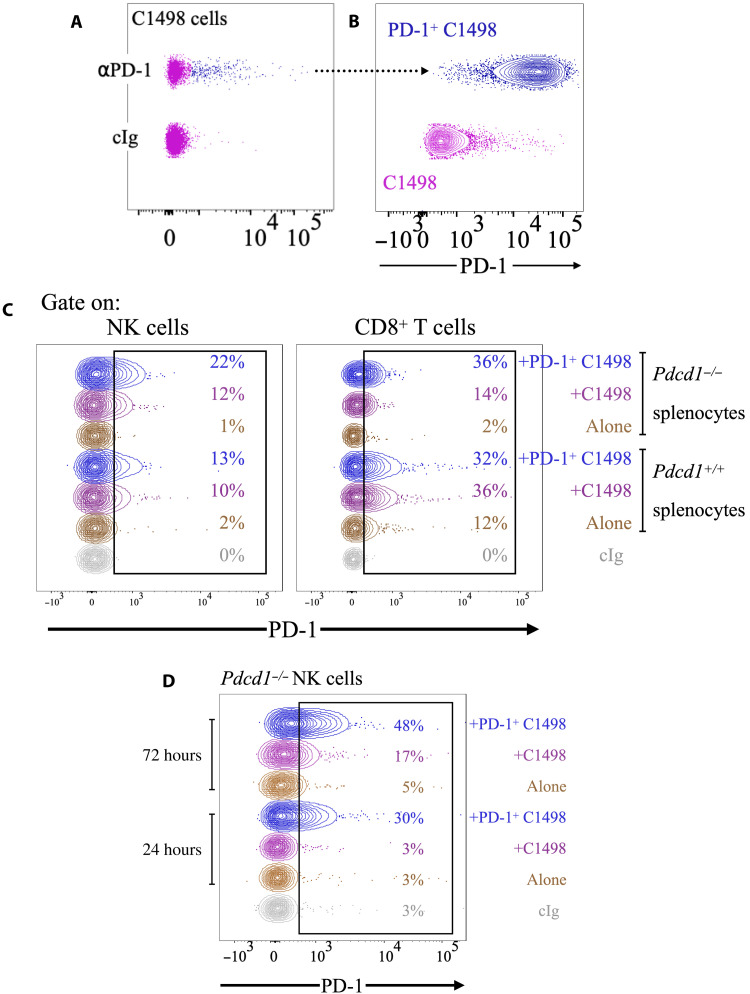
To expand on these results, we next used C1498 cells, an often used model of leukemia (47–49). A fraction of C1498 cells (~5%) endogenously expressed PD-1 in culture (Fig. 2A). We sorted PD-1+ C1498 cells, confirmed that they stably expressed PD-1 upon 2 weeks in culture (Fig. 2B), and then incubated them with splenocytes from Pdcd1+/+ or Pdcd1−/− littermates. In accordance with the results obtained with RMA cells, both NK cells and CD8+ T cells from Pdcd1−/− mice acquired PD-1 when incubated with C1498 cells, and more so if tumor cells had higher PD-1 expression (Fig. 2C). PD-1 staining observed in Pdcd1−/− mice was very similar to what was observed in the Pdcd1+/+ littermate controls, suggesting that even in the C1498 model, the most PD-1 was not endogenously expressed by immune cells, but rather came from the tumor cells. Similar experiments were repeated using purified NK cells from Pdcd1−/− NK cells. After 24 hours, NK cells incubated with PD-1+ C1498 cells stained positively for PD-1 (Fig. 2D). PD-1 staining was further increased at 72 hours, when we also observed a shift in NK cells incubated with C1498 parental cells (Fig. 2D). Corroboration that higher PD-1 expression on tumor cells resulted in higher levels of PD-1 acquisition on NK cells was further obtained in the RMA model, where two polyclonal sublines with different PD-1 expression donated PD-1 to NK cells at different levels (fig. S8). Together, these data show that NK cells and CD8 T cells acquire PD-1 from leukemia tumor cell lines in vitro.
Fig. 2. NK cells and T cells acquire PD-1 from C1498 cancer cells.
(A) C1498 cells were stained with PD-1 antibody or isotype control. (B) PD-1+ cells (in blue) were flow-sorted and, after 2 weeks in culture, stained for PD-1, alongside parental C1498 cells. (C) Splenocytes from Pdcd1+/+ or Pdcd1−/− littermates were cultured with C1498 or PD-1+ C1498 cells for 3 days and then stained with PD-1 antibodies. The experiment depicted is representative of three performed with similar results. The numbers in the graphs refer to the percentage of PD-1+ cells. (D) Splenic NK cells isolated from Pdcd1−/− mice were cocultured with C1498 or PD-1+ C1498 cells, or without tumor cells as a control, for 24 or 72 hours and stained for PD-1. The experiment depicted is representative of six performed with similar results. The numbers in the graphs refer to the percentage of PD-1+ cells.
Trogocytosis is responsible for intercellular transfer of PD-1 from tumor to NK cells
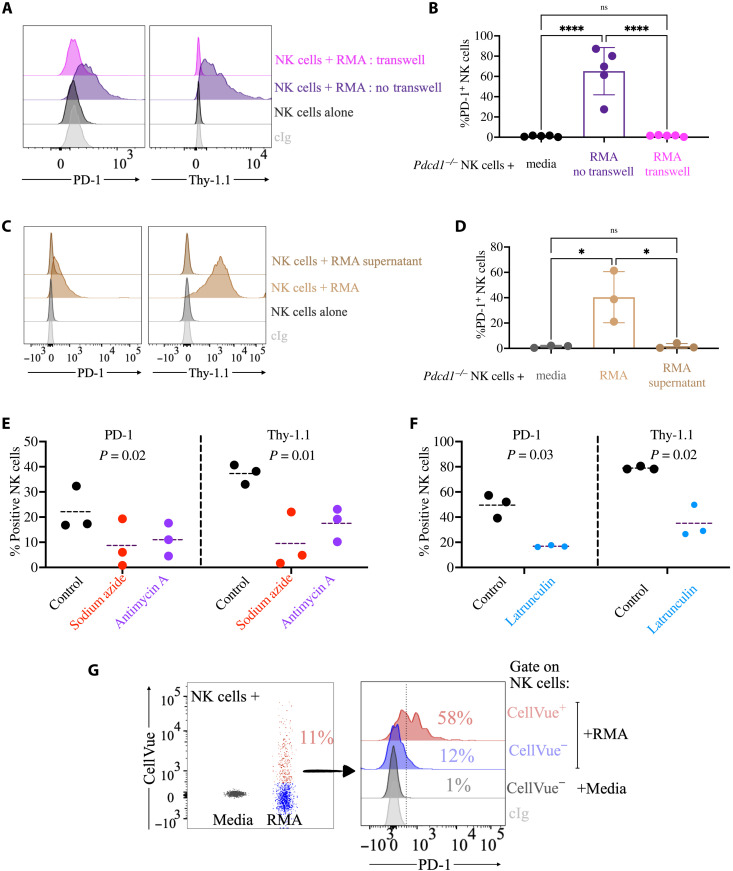
Once we established that NK cells acquired PD-1 from tumor cells, we next investigated whether trogocytosis was responsible for PD-1 transfer. Cell-cell contact is required for trogocytosis. Consistent with our hypothesis that PD-1 is acquired by trogocytosis, Pdcd1−/− NK cells cultured in transwell with tumor cells (where physical interaction between the two cell types is precluded) failed to stain for PD-1 and Thy-1.1 (Fig. 3, A and B). Further, NK cells incubated with supernatant conditioned by RMA cells failed to stain for PD-1 (Fig. 3, C and D). These experiments reveal that cell-cell contact is required for PD-1 acquisition by NK cells and suggest that soluble or exosomal PD-1 is not responsible for PD-1 transfer.
Fig. 3. Trogocytosis is responsible for intercellular transfer of PD-1 from tumor to NK cells.
(A and B) Splenic NK cells isolated from a Pdcd1−/− mouse were cocultured for 24 hours with RMA cells separated or not by a transwell semipermeable membrane before staining for PD-1 and Thy-1.1. The experiment depicted is representative of four performed with similar results. Cumulative analysis is shown in (B) (n = 3), where the statistical analysis was performed with a one-way ANOVA with multiple comparisons, ****P < 0.0001. (C and D) Splenic NK cells isolated from a Pdcd1−/− mouse were cocultured for 24 hours with RMA cells or with medium conditioned for 3 days by RMA cells and then stained for PD-1 and Thy-1.1. The experiment depicted is representative of three performed with similar results. Cumulative analysis is shown in (D) (n = 3), where the statistical analysis was performed with a one-way ANOVA with multiple comparisons, *P < 0.05. (E) Splenic NK cells isolated from Pdcd1−/− mice were pretreated with sodium azide or antimycin A and then cocultured for 1 hour with RMA cells and stained for PD-1 and Thy-1.1. Three independent experiments are plotted. Statistical analysis with one-way ANOVA with repeated measurements. (F) Splenic NK cells were isolated from Pdcd1−/− mice and treated for 3 days with rhIL-2. NK cells were then pretreated with latrunculin and cocultured with RMA-Thy1.1 cells. Three independent experiments are plotted. Statistical analysis with two-tailed paired Student’s t test. (G) NK cells were incubated with RMA cells prelabeled with CellVue for 24 hours. CellVue and PD-1 staining on NK cells is depicted on the left and right, respectively. Numbers in the histogram flow plot refer to the frequency of PD-1+ cells.
While a specific pharmacological inhibitor of trogocytosis is not available, blocking of adenosine triphosphate (ATP) synthesis (10) or of actin polymerization (50) is known to interfere with trogocytosis. Consistent with the idea that PD-1 is acquired via trogocytosis by NK cells, pretreatment of NK cells with sodium azide or antimycin A, which both prevent ATP synthesis, or with latrunculin A, which stops actin polymerization, resulted in a strong reduction of PD-1 and Thy-1.1 acquisition (Fig. 3, E and F). Last, pretreatment of NK cells with actinomycin D, which blocks transcription, did not prevent Pdcd1+/+ NK cells from staining for PD-1, confirming that PD-1 protein on the surface of NK cells did not derive from endogenous Pdcd1 transcripts (fig. S9).
Transfer of proteins via trogocytosis is accompanied by transfer of membrane lipids. PD-1 transfer was coupled with acquisition of lipids from tumor cells, as revealed by experiments wherein NK cells were cocultured with RMA cells previously labeled with CellVue, a dye that intercalates in the lipid regions of the cellular membrane (Fig. 3G). Not only did NK cells become robustly positive for the dye, but also PD-1 staining was more abundantly detected on NK cells that also acquired lipids from tumor cells (Fig. 3G).
To obtain direct evidence that PD-1 was trogocytosed by NK cells, we complemented PD-1–deficient RMA cells with a PD-1–green fluorescent protein (GFP) fusion protein. In live imaging experiments, NK cells acquired GFP signal after a few minutes of coincubation with tumor cells (fig. S10 and movie S1). Together, these experiments indicate that PD-1, together with other proteins, is acquired via trogocytosis by NK cells.
SLAM receptors drive NK cell trogocytosis of PD-1 from tumor cells
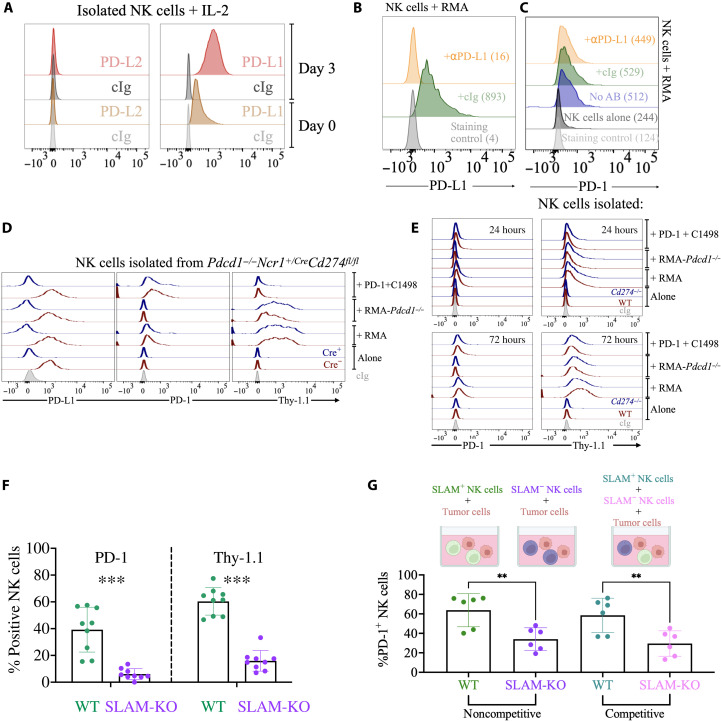
Acquisition of proteins from donor cells can be facilitated by receptor-ligand engagement, a process known as trans-endocytosis, which NK cells are known to mediate (51). In culture, NK cells fail to express PD-L2 but express PD-L1 [Fig. 4A and (52)], which could therefore serve as a ligand for trans-endocytosis–driven PD-1 acquisition. However, a saturating dose of PD-L1 blocking antibody did not reduce PD-1 acquisition (Fig. 4, B and C), as we would expect if trans-endocytosis was involved. Similar results were obtained blocking PD-1 on RMA cells with an antibody that prevents binding of PD-L1. Despite the antibodies saturated PD-1 on the membrane of RMA cells (fig. S11A), PD-1 and Thy-1.1 were still effectively transferred to NK cells (fig. S11B). These experiments not only suggest that PD-1/PD-L1 binding is not required for PD-1 transfer but also imply that Fc receptor engagement by PD-1 antibodies does not facilitate trogocytosis (53). Last, we sought genetic corroboration using PD-L1–deficient NK cells from two different mouse strains: a full-body PD-L1 knockout (Cd274−/−) (54) and an NK cell–specific PD-L1 knockout (Ncr1+/Cre × Cd274fl/fl) (55) that we crossed with PD-1–deficient mice (Pdcd1−/− Ncr1+/Cre Cd274fl/fl). PD-L1–deficient NK cells acquired PD-1 and Thy-1.1 at levels similar to NK cells isolated from PD-L1–expressing controls (Fig. 4, D and E, and figs. S12 and S13). PD-L1 was also dispensable for PD-1 and Thy-1.1 acquisition in CD8+ T cells and B cells (fig. S12).
Fig. 4. SLAM receptors are essential for trogocytosis.
(A) Splenic NK cells isolated from a Pdcd1−/− mouse were cultured for 3 days, and then PD-L2 and PD-L1 expression was analyzed by flow cytometry. Representative of three experiments performed with similar results. (B and C) NK cells were incubated with RMA cells in the presence of a PD-L1 blocking antibody or an isotype control for 24 hours, before being stained for PD-1 and PD-L1. As additional controls, NK cells were (i) cocultured with RMA without adding any antibody or (ii) cultured alone without adding tumor cells. The experiment depicted is representative of three performed. The numbers in the flow histograms represent the geometric mean fluorescence intensity (MFI) of PD-L1 or PD-1. (D and E) NK cells were isolated from the spleen of Pdcd1−/−Ncr1+/CreCd274fl/fl (D) or Cd274−/− (E) mice (or control littermates, in red) and cocultured for 1 or 3 days with RMA or C1498 tumor cells, when PD-1 and Thy-1.1 staining was assessed by flow cytometry. (F) Splenocytes from SLAM-deficient mice or control littermates were cocultured for 3 days with RMA or C1498 cells. PD-1 and Thy-1.1 staining on NK cells was then assessed by flow cytometry. Three independent experiments are depicted, n = 9. Statistical analysis with two-tailed unpaired Student’s t test. ***P < 0.001. (G) SLAM-sufficient (CD45.1) or SLAM-deficient (CD45.2) NK cells were negatively isolated and cultured with rhIL-2 for 3 days. NK cells were then mixed in a 1:1 ratio (competitive condition) or not (noncompetitive condition) and cocultured with RMA-Thy1.1 tumor cells. PD-1 staining on NK cells is shown from two different experiments, n = 6. Statistical analysis with two-tailed Student’s t test, unpaired for the noncompetitive condition and paired for the competitive condition. **P < 0.01.
SLAM receptors are important mediators of cell-cell interactions between hematopoietic cells and are abundantly expressed not only by NK cells but also by T and B cells (56). Given the broad expression of SLAM family members, their importance in regulating the activation of different immune cells and considering that PD-1 was trogocytosed by both innate and adaptive lymphocytes, we hypothesized that SLAM receptors promoted PD-1 trogocytosis. To test this hypothesis, we cultured splenocytes from mice where the whole SLAM locus was deleted (57) with tumor cells and then assessed PD-1 and Thy-1.1 staining on NK, T, and B cells. Consistent with our hypothesis, NK cells from SLAM-deficient mice failed to acquire PD-1 from RMA cells (Fig. 4F and fig. S14). Not only was PD-1 acquisition abolished, but also, more broadly, SLAM-deficient NK cells failed to perform trogocytosis with tumor cells, as revealed by the lack of Thy-1.1 transfer (Fig. 4F and fig. S14). In addition to NK cells, SLAM-deficient T and B cells also displayed reduced trogocytosis (fig. S15), confirming that SLAM receptors are key mediators for trogocytosis between immune cells and leukemia cells. As SLAM receptors are broadly expressed by many leukocytes, we investigated whether cell-intrinsic expression by NK cells was required for PD-1 trogocytosis. To this end, we isolated splenic NK cells from CD45.1 (SLAM-sufficient) or CD45.2 (SLAM-deficient) mice and cocultured them, in a competitive or noncompetitive fashion, with RMA cells. As expected, and consistent with experiments depicted in Fig. 4F, isolated NK cells lacking SLAM receptors were less efficient in acquiring PD-1 from tumor cells. This was true not only when SLAM-sufficient and SLAM-deficient cells were compared vis a vis upon coculture with tumor cells but also in competitive settings, where the two genotypes were cultured together in the presence of tumor cells (Fig. 4G).
Given the importance of SLAM receptors in mediating cell-cell interactions, we analyzed whether deficiency in other adhesion molecules also interfered with trogocytosis. LFA-1 is a key adhesion molecule, but NK cells lacking expression of CD11a (58), a subunit of LFA-1, did not present a deficit in PD-1 or Thy-1.1 acquisition (fig. S16). Similar results were also observed analyzing CD8+ T and B cells (fig. S17). NKG2D, an activating receptor ubiquitously expressed by NK cells (59), was also not involved in mediating trogocytosis between NK and RMA cells (fig. S18). Together, these results indicate that SLAM receptors mediate PD-1 trogocytosis from tumor to immune cells.
Activated NK cells acquire PD-1 from tumor cells in vivo
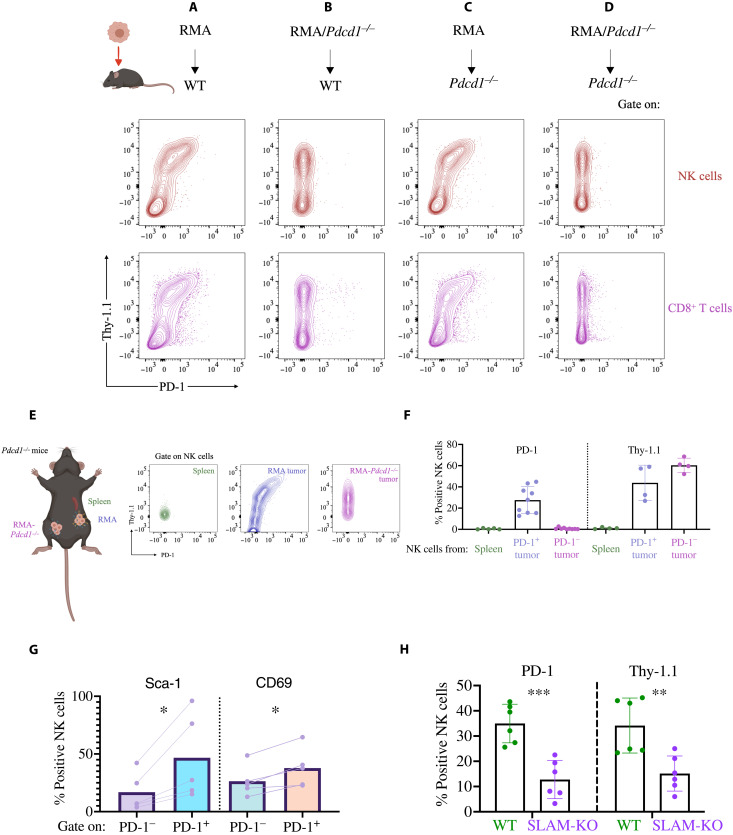
Next, we performed in vivo studies to determine whether intratumoral NK cells trogocytosed PD-1. We injected Pdcd1+/+ or Pdcd1−/− littermates with RMA or RMA-Pdcd1−/− cells, both expressing Thy-1.1, and when tumors reached ~300 mm3, we analyzed PD-1 staining on intratumoral lymphocytes (fig. S19). In all cohorts of mice, NK cells infiltrating the tumors stained intensely for Thy-1.1 (Fig. 5, A to D, y axis), showing that trogocytosis occurred in vivo. Strikingly, high levels of PD-1 were detected on the surface of NK cells only when tumor cells expressed PD-1 not only in Pdcd1+/+ mice but also in Pdcd1−/− mice (Fig. 5, A and C versus B and D, and figs. S20 and S21). These data show not only that PD-1 is acquired by tumor-infiltrating NK cells but also that trogocytosis is the major mechanism leading to PD-1 presence on the surface of NK cells in the RMA model. Consistent observations were made for CD8+ T cells (Fig. 5, A to D). Corroboration that PD-1 was trogocytosed in vivo came from experiments where NK cells infiltrating tumors expressing a PD-1–GFP fusion protein costained for PD-1 and GFP (fig. S22, A to D). In addition to PD-1 and Thy-1.1, NK cells also trogocytosed TCRvβ12 from tumor cells (fig. S22, E to J). Last, in experiments where Pdcd1−/− mice were injected with RMA or RMA-Pdcd1−/− cells in either flank, only NK cells infiltrating RMA tumors, but not PD-1–deficient tumors or splenic NK cells, stained for PD-1 (Fig. 5, E and F). Together, these data support the idea that NK cells acquire PD-1 from cancer cells in the tumor microenvironment.
Fig. 5. Intratumoral lymphocytes acquire PD-1 from tumor cells.
(A to D) Pdcd1+/+ or Pdcd1−/− mice were injected with RMA or RMA-Pdcd1−/− tumors. PD-1 and Thy-1.1 staining was assessed by flow cytometry on tumor-infiltrating NK and T cells (gated as in Fig. 1, B and C, respectively). The experiment shown is representative of five performed with similar results. (E) RMA or RMA-Thy1.1 cells were injected in either flank of a Pdcd1−/− mouse. PD-1 and Thy-1.1 staining was analyzed in intratumoral or splenic NK cells. The experiment depicted is representative of three performed, n = 9 for PD-1 staining and n = 4 for Thy-1.1 staining. The three experiments are pooled in (F). (G) Expression of Sca-1 and CD69 was analyzed on Pdcd1−/− NK cells infiltrating RMA-Thy1.1 tumors, stratifying NK cells for PD-1 staining. Representative of two experiments performed, n = 5. Statistical analysis with two-tailed paired Student’s t test; *P < 0.05. (H) SLAM-deficient mice or control littermates were injected with RMA-Thy1.1 cells. PD-1 staining was determined on tumor-infiltrating NK cells. Data depict two experiments performed, n = 6. Statistical analysis with two-tailed paired Student’s t test; **P < 0.01; ***P < 0.001.
In our previous study, we reported that PD-1 staining was higher on activated NK cells (38). Analysis of NK and T cells from Pdcd1−/− mice infiltrating RMA tumors confirmed that PD-1+ NK and T cells also stained more brightly for activation markers such as Sca-1 and CD69 (Fig. 5G). When analyzing PD-1+ versus PD-1− NK cells in the tumor microenvironment, we found that PD-1–acquiring NK cells were more activated (fig. S23, A to C), expressed higher levels of activating receptors (fig. S23, D and E), and were distributed in the three CD11b/CD27-defined maturation states, with a slight preference toward CD11b+CD27+ (R2) cells, which are mature and functional (fig. S23F). On the other hand, we failed to observe a correlation with KLRG1 expression and only noticed a weak correlation with NKG2A (fig. S23, G and H), whereas other checkpoint receptors, including CTLA-4, TIM3, and TIGIT, were only poorly expressed. From the functional standpoint, in agreement with our previously published data (38), NK cells acquiring PD-1 were better interferon-γ (IFN-γ) producers and degranulated more robustly (fig. S23, I and J). These data support the idea that the NK cells that are activated by the encounter with tumor cells are also the ones more susceptible to acquiring PD-1 and therefore being inhibited by it.
Last, we tested the requirement of SLAM receptors for PD-1 trogocytosis in vivo. Consistent with ex vivo experiments (Fig. 4, F and G), SLAM-deficient NK cells infiltrating RMA tumors acquired lower levels of PD-1 and Thy-1.1 (Fig. 5H), highlighting the importance of SLAM receptors as drivers of trogocytosis.
PD-1 acquired via trogocytosis inhibits NK cell responses against cancer
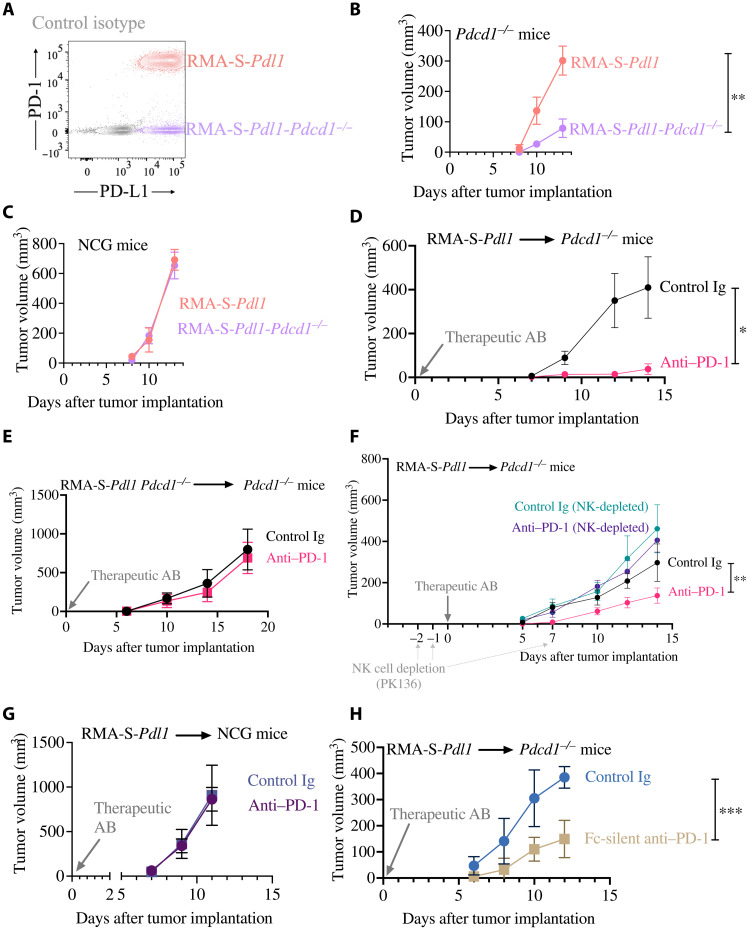
Once we established that NK cells trogocytose PD-1 in vivo, we sought to determine whether trogocytosed PD-1 suppressed antitumor immunity. For these studies, rather than using RMA cells [which are resistant to both T and NK cell responses (38, 46, 47, 60)], we took advantage of RMA-S-Pdl1 cells we previously generated (38). Like RMA, these cells express high levels of PD-1, but differently than RMA, they lack MHC class I expression and are therefore susceptible to NK-mediated control (38). Using CRISPR-Cas9, we generated an RMA-S-Pdl1 variant lacking PD-1 expression (Fig. 6A). When injected in Pdcd1−/− mice, where the only source of PD-1 on NK cells are tumor cells, we observed a marked deceleration in outgrowth of tumor cells lacking PD-1 expression (Fig. 6B). However, lack of PD-1 did not delay cell growth in vitro, nor it prevented RMA-S-Pdl1-Pdcd1−/− cells from growing tumors in immunodeficient mice (Fig. 6C). These data indicate that, rather than having cell-intrinsic growth defects, PD-1–deficient tumor cells have reduced capacity of forming ectopic tumors as they fail to inhibit NK cells via PD-1 transfer.
Fig. 6. PD-1 blockade is effective in Pdcd1−/− mice when NK cells are present and tumor cells express PD-1.
(A) PD-1 and PD-L1 expression in RMA-S-Pdl1 or RMA-S-Pdl1-Pdcd1−/− cells was analyzed by flow cytometry. (B to H) In all experiments, the indicated cell lines were resuspended in Matrigel and injected, alone or mixed with different PD-1 blocking or control antibody. Tumor growth was assessed over time, and data were analyzed with two-way ANOVA. (B) n = 6 per group; the experiment depicted is representative of two performed with similar results. (C) n = 6 per group; the experiment depicted is representative of two performed with similar results. (D) n = 4 per group; the experiment depicted is representative of two performed with similar results. (E) n = 6 per group; the experiment depicted is representative of three performed with similar results. (F) n = at least 5 per group; the experiment depicted is representative of two performed with similar results. (G) n = 5 per group; the experiment depicted is representative of two performed with similar results. (H) n = 5 per group; the experiment depicted is representative of two performed with similar results. *P < 0.05; **P < 0.01; ***P < 0.001.
Using the RMA-S-Pdl1 model, we previously showed that PD-1 blockade rescued the ability of NK cells to control tumor growth in vivo (38). Considering that PD-1 expression in tumor cells promoted in vivo growth in a cell-extrinsic fashion, we reasoned that the therapeutic effect of PD-1 blockade should also be observed in Pdcd1−/− mice. In accordance with our hypothesis, when we treated PD-1–deficient mice injected with RMA-S-Pdl1 cells with a PD-1 blocking antibody (RMP1-14), we observed a marked reduction in tumor outgrowth (Fig. 6D). On the other hand, when we injected PD-1–deficient mice with PD-1–deficient RMA-S-Pdl1 cells, PD-1 blockade had no therapeutic effect (Fig. 6E).
To confirm that NK cells, and no other components of the immune response, were inhibited by tumor-derived PD-1, we injected RMA-S-Pdl1 cells in mice where NK cells were depleted using a monoclonal antibody (PK136) and treated the mice with PD-1 blockade. NK cell depletion was sufficient to abolish the therapeutic effect of the blocking antibody, whereas PD-1 blockade delayed tumor outgrowth in the control group (Fig. 6F). Corroboration of these results came from experiments where PD-1 antibodies failed in immunocompromised mice (Fig. 6G and fig. S24A). Confirmatory results were obtained by depleting NK cells with a different antibody (anti–asialo-GM-1; fig. S24B). The similar in vivo growth of tumor cells in immunocompromised mice (Fig. 6G) excluded a tumor cell–intrinsic effect of PD-1 blocking antibodies.
Last, to rule out that the therapeutic effect of PD-1 antibodies was due to antibody-dependent cellular cytotoxicity (ADCC) potentially mediated by NK cells against cancer cells coated with PD-1 antibodies, we used an engineered version of anti–PD-1 that lacks the ability to bind to Fc receptors (Fc-silent RMP1-14) (61). Treatment with Fc-silent PD-1 antibodies delayed the outgrowth of PD-1–expressing tumors (Fig. 6H), indicating that the therapeutic effect of PD-1 antibodies in Pdcd1−/− mice was not due to ADCC. Together, these results indicate that trogocytosed PD-1 inhibits the antitumor activity of NK cells, which can be rescued by PD-1 blocking antibodies.
Identification of an NK cell population in multiple myeloma patients staining for tumor cell markers and PD-1
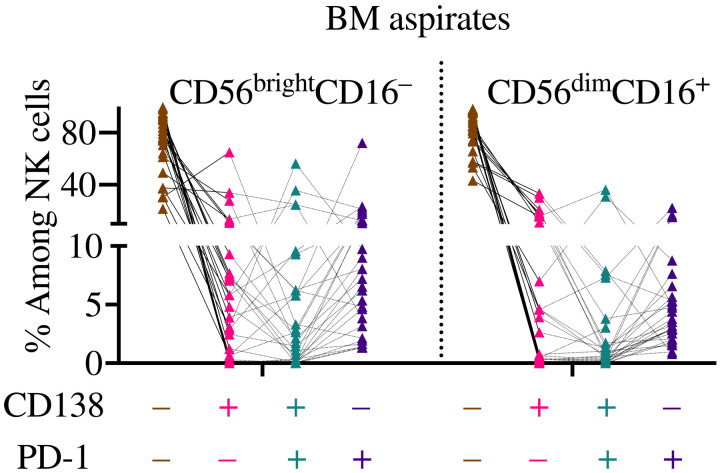
Last, to determine whether NK cells acquire PD-1 from tumor cells in cancer patients, we analyzed PD-1 staining in NK cells from the bone marrow (BM) of patients with clonal plasma cell disorders (Table 1 details patients’ information). Pathological analysis showed that of the 28 patients analyzed, 21 were diagnosed with multiple myeloma (MM), 3 with monoclonal gammopathy of undetermined significance (MGUS), 3 with smoldering myeloma, and 1 with solitary plasmacytoma. For these studies, we relied on CD138, a protein frequently expressed by clonal plasma cells but not by NK cells (62), as a surrogate trogocytosis marker. Flow cytometry analysis confirmed the presence in several patients of a high scattering CD138+ population (fig. S25), which we identified as clonal plasma cells. In support of our gating strategy, patients diagnosed with MM presented a higher CD138+ frequency than MGUS patients. NK cells were instead gated as low scattering events, live, CD3−CD56+CD16+/−, and in most samples, CD7 was also used (fig. S25). In most samples analyzed, high scattering CD138+ cells expressed PD-1 (fig. S25). Indication that NK cells performed trogocytosis came from analysis of BM aspirates where a CD138+ NK cell population, in both CD56brightCD16− and CD56dimCD16+ NK cell subsets, was identified (Fig. 7, in pink and green), corroborating the results obtained in murine models. Notably, we found a sizeable and consistent (albeit often small) population of NK cells that stained for both CD138 and PD-1 (Fig. 7, in green), supporting the idea that NK cells in patients with clonal plasma cell disorders acquire PD-1 and cancer cell markers from tumor cells.
Table 1. Information on the analyzed patients.
| Diagnosis | |||||
| MM | MGUS | Smoldering myeloma | Solitary plasmacytoma | ||
| n | 21 | 3 | 3 | 1 | |
| Sex | Male | 12 | 3 | 2 | 1 |
| Female | 9 | 0 | 1 | 0 | |
| Age | Range | 50–82 | 60–72 | 70–89 | 77 |
| Median | 68 | 71 | 79 | 77 | |
| % Plasma cell blasts | Range | 0–90 | 0–9 | 12–50 | 0 |
| Medium | 21 | 8 | 13 | 0 | |
Fig. 7. NK cells costain for CD138 and PD-1 in the BM of patients with clonal plasma cell disorders.
The BM aspirates of 28 patients with clonal plasma cell disorders were analyzed by flow cytometry. The frequency of NK cells staining for either, neither, or both CD138 and PD-1 is depicted.
In conclusion, this study identifies trogocytosis as a new mechanism by which PD-1 is acquired from tumor cells by NK and T cells. PD-1 trogocytosis strongly relies on SLAM receptors and functionally suppresses the ability of NK cells to eliminate tumors in vivo.
DISCUSSION
The nature of PD-1 expression on NK cells remains elusive, with contrasting evidence indicating that PD-1 is either expressed on or not expressed in NK cells (44, 45). Considering the importance of PD-1 in suppressing the immune response to cancer and given the tremendous interest in the development of NK cell–based cancer immunotherapies (32, 63), understanding whether PD-1 directly inhibits NK cell function is of the utmost importance. We recently reported that PD-1 suppresses NK cells in several mouse models of cancer (38), but previously have not yet deciphered the mechanisms leading to PD-1 up-regulation in murine NK cells infiltrating lymphoma mouse models. The lack of PD-1 induction in NK cells following several ex vivo stimulations, combined with the analysis of the Pdcd1 locus in resting and cytokine-stimulated NK cells, prompted us to hypothesize that PD-1 was not endogenously expressed by NK cells but rather was derived from other sources. Several cellular processes have been shown to be responsible for protein transfer. Among these processes, trogocytosis, the intercellular exchange of whole membrane fragments, is highly performed by NK cells (10–12). Trogocytosis can have a substantial impact for immune responses, and it often serves an immunoregulatory purpose (1, 20). For example, proteins acquired via trogocytosis can trigger inhibitory cis-signaling in the receiving cell (64), whereas ligands for activating receptors can be removed from target cells reducing lymphocyte activation (65, 66). In cancer, trogocytosis has been associated with reduced immune responses and with the failure of immunotherapy. For example, a recent study highlighted how CAR (Chimeric Antigen Receptor) T cells trogocytose antigens from tumor cells and become susceptible to fratricide, greatly limiting the response to cellular therapy (50). Despite such evidence and the immense interest in elucidating the mechanisms underlying resistance to PD-1 blockade, whether PD-1 is trogocytosed by immune cells has been largely unexplored, even if a recent study showed that PD-L1 is trogocytosed by monocytes (67). Here, we show that trogocytosis is a major mechanism by which PD-1 becomes localized on the surface of immune cells. This was true not only for NK cells but also for adaptive lymphocytes. PD-1 acquisition happened in a cell-cell contact–dependent fashion, contextualized within the transfer of other proteins and whole-membrane fragments, and was strongly suppressed by ATP depletion or inhibition of actin polymerization, indicating that PD-1 was trogocytosed by immune cells. PD-1 antibodies did not elicit PD-1 trogocytosis by NK cells, suggesting that PD-1 could be acquired by NK cells even in the absence of Fc receptor engagement. Mechanistic studies using blocking antibodies and transgenic mice allowed us to exclude a role for PD-L1, abundantly expressed by NK cells, in PD-1 acquisition, ruling out trans-endocytosis as a mechanism of PD-1 transfer. On the other hand, receptors belonging to the SLAM family proved to be pivotal for intercellular transfer of PD-1 from tumor to immune cells. SLAM receptors are important regulators of immune function and ubiquitously expressed not only by NK cells (56) but also by tumors of hematopoietic origin, including MM (68). Our finding that SLAMs promote the transfer of PD-1 from tumor to immune cells requires consideration of trogocytosis as an important biological variable when designing monotherapy or combination therapy targeting these receptors.
Trogocytosed PD-1 was functional and suppressed the anticancer activity of NK cells. The in vivo studies performed here further expand on our previous findings that NK cells contribute to the therapeutic efficacy of PD-1 blockade (38), and explain why checkpoint blockade relies on NK cells despite their lack of PD-1 expression.
While more translational studies are required to follow up on these mechanistic data, we successfully identified a subset of NK cells that stained for CD138 in the BM of patients with clonal plasma cell disorders. As CD138 is not expressed by NK cells, we relied on CD138 staining to identify BM NK cells that performed trogocytosis. Consistent with our in vivo results, CD138+ NK cells also stained for PD-1, and flow cytometry and bioinformatic analysis of a published dataset indicated that MM cells can express PD-1 (69). On the basis of our in vivo results, we propose that PD-1 expression, in addition to benefiting cancer cells with intrinsic signaling (70), also promotes immune escape. Tumor cells expressing PD-1 can donate this powerful inhibitory receptor to activated immune cells when they are in direct contact. PD-1 acquisition can, however, be therapeutically abrogated by checkpoint blockade, potentially rescuing the ability of NK cells to promote anticancer immunity.
In our current analysis (and differing from our murine results), PD-1 was also found in a fraction of human NK cells that did not stain for CD138. These data are consistent with the idea that human NK cells endogenously express PD-1, as recently corroborated in healthy donors and patients undergoing hematopoietic stem cell transplantation (44). Endogenous expression of PD-1 does not exclude the possibility that immune cells also rely on trogocytosis to gain further PD-1 protein from neighbor cells. This notion is well supported by a recent study that identified trogocytosing NK cells in a broad spectrum of hematopoietic malignancies (71). In accordance with our data, NK cells labeled with tumor cell markers also stained for PD-1 (71). Whether endogenously expressed by NK cells or acquired from cancer or other immune cells, several reports, including the present one, have highlighted the importance of PD-1 in suppressing NK cells (38–44).
Last, considering these results, it will be important for future immune-profiling efforts based on transcriptomic analysis to consider that proteins are acquired, sometimes at unexpectedly high levels, by immune cells in the tumor microenvironment. Pursuant to our previous studies and given its known importance in suppressing anticancer responses, we focused on PD-1; however, it is conceivable that other proteins with immunomodulatory potential will be acquired by NK and T cells while interacting with tumor cells. Further characterization of the mechanisms underlying membrane transfer and identification of molecules transferred to immune cells is required to elucidate how immune cells are regulated by checkpoint receptors, and other proteins, in a transcription-independent fashion.
MATERIALS AND METHODS
Mice and in vivo procedures
Mice were maintained at the University of Ottawa. Pdcd1 knockout mice (B6.Cg-Pdcd1tm1.1Shr/J) (72) were purchased from The Jackson Laboratory and crossed with C57BL/6J mice purchased from The Jackson Laboratory to obtain Pdcd1 heterozygous mice. Heterozygous mice were bred to obtain Pdcd1+/+ and Pdcd1−/− littermates. Ncr1+/Cre mice (73) were gifted by E. Vivier (INSERM, Marseille, France) and crossed with Cd274fl/fl mice (55), gifted by P.G.F. (Trinity College, Dublin, Ireland). Mice were then crossed with Pdcd1−/− mice. Cd274−/− mice (54) were obtained from A.H.S. (Harvard Medical School, Boston, MA). SLAM-ko mice (57) were donated by A.V. (Institut de recherches cliniques de Montréal, Montréal, QC). Itgal1−/− mice (58) were purchased from The Jackson Laboratory. Klrk1−/− mice (74) and B6 Cd45.1 mice were gifted by D. H. Raulet (University of California, Berkeley, Berkeley, CA). NCG mice were purchased from Charles Rivers Laboratories. For all experiments, sex-matched (both males and females) and age-matched (7 to 18 weeks old) mice were used.
For subcutaneous injections, tumor cells were resuspended in 100 μl of phosphate-buffered saline (PBS) and injected in the left flank. Tumors were collected when tumor volume was approximately 300 mm3. In some experiments, 0.5 × 106 tumor cells were resuspended in 100 μl of growth factor–reduced Matrigel (BD Biosciences) and injected in both the left and right flank of the same mouse.
Tumor outgrowth of parental or PD-1–deficient RMA-S-Pdl1 cells was assessed in Pdcd1−/− or NCG mice injected with 0.1 × 106 tumor cells resuspended in 100 μl of Matrigel. For immunotherapy experiments, 0.5 × 106 tumor cells were resuspended in 100 μl of Matrigel mixed with 20 μg of anti–PD-1 (RMP1-14) or control antibody (1-1) (both by Leinco). In some experiments, Fc-silent RMP1-14 (61) was used. When indicated, mice were depleted of NK cells with intraperitoneal injection of 200 μg of NKR-P1C antibody (PK136, Leinco) or 10 μl (resuspended in 90 μl of PBS) of anti–asialo-GM-1 (BioLegend).
Cell lines
All cell lines were cultured at 37°C in a humidified atmosphere containing 5% CO2 and maintained in RPMI culture medium containing penicillin (100 U/ml), streptomycin (100 μg/ml), glutamine (0.2 mg/ml), gentamicin sulfate (10 μg/ml), 20 mM Hepes, and 5% fetal calf serum. Cell line identity was confirmed by flow cytometry when possible, and cells were regularly tested for mycoplasma.
Generation of cell line variants
RMA and C1498 cells were transduced with the retroviral expression vector MSCV-IRES-Thy1.1-DEST (Addgene, 17442), by spin infection (800g for 2 hours at 37°C) with polybrene (8 μg/ml), and Thy1.1+ cells were sorted.
Single-guide RNA (sgRNA) targeting the first exon of the Pdcd1 gene (sequence: TGTGGGTCCGGCAGGTACCC) was cloned into the LentiCRISPR lentiviral backbone vector (Addgene 52961), also containing the Cas9 gene. Lentiviral expression vectors were generated by transfecting 293T cells with 2 μg of vector with 2 μg of packaging plus polymerase-encoding plasmids using Lipofectamine 2000. Virus-containing supernatants were used to transduce RMA-Thy1.1 cells by spin infection, and PD-1–negative cells were sorted. PD-1+ C1498 cells were obtained by sorting the PD-1+ fraction of C1498 parental cells. Generation of RMA-S-Pdl1 cells was previously described (38).
To generate PD-1–GFP fusion protein, the full-length open reading frame of mouse Pdcd1 (Origene Technologies Inc., Rockland, MD, USA; catalog number NM_008798) was subcloned as an Sgf I/Mlu I fragment into the corresponding sites of a lentiviral vector (Origene, catalog number PS100071) such that cells transduced with the resulting lentivirus would express PD-1 fused in frame at the C terminus with monomeric GFP. Lentiviral expression vectors were generated by transfecting 293T cells with 2 μg of vector with 2 μg of packaging plus polymerase-encoding plasmids (pCMV-dR8.2 dvpr, Addgene #8455, and pCMV-VSV-G, Addgene #8454) using Lipofectamine 2000. Virus-containing supernatants were used to transduce RMA-Thy1.1-Pdcd1−/− cells by spin infection, and PD-1–GFP+ cells were sorted as bright or dim. All engineered cells were regularly assessed for phenotype maintenance by flow cytometry.
Ex vivo experiments
Murine splenic NK cells were isolated using the EasySep Mouse NK Cell Isolation Kit (StemCell Technologies). In all experiments with isolated NK cells, recombinant human IL-2 (rhIL-2) [1000 U/ml; National Institutes of Health (NIH) Biological Resources Branch Preclinical Repository] was added to the culture medium. In most coculture experiments, NK cells were labeled with CellTrace Violet proliferation dye (BD Biosciences) and tumor cells with carboxyfluorescein diacetate succinimidyl ester (CFSE) (BioLegend), or vice versa. A total of 100,000 NK cells were cocultured with tumor cells at a 1:1 ratio in 24-well plates in a final volume of 1 ml. When whole spleens were used, 200,000 splenocytes were cocultured with tumor cells at a 2:1 ratio in six-well plates, in a final volume of 3 ml. In other experiments, splenocytes or purified NK cells were treated with rhIL-2 (1000 U/ml) for 3 days and cocultured with tumor cells for 2 to 3 hours at 37°C in a 1:5 ratio.
In ex vivo cytokine stimulation experiments, isolated splenic NK cells were cultured with interleukin-15 (IL-15) (10 or 100 ng/ml; PeproTech), IL-2 (1000 U/ml), IL-5 (100 ng/ml; PeproTech), IL-6 (100 ng/ml; PeproTech), IL-12 (20 ng/ml; PeproTech) + IL-18 (100 ng/ml; Leinco), transforming growth factor–β1 (TGF-β1) (10 ng/ml; PeproTech), type I IFN (1000 U/ml; PBL Assay), or 25 nM of the glucocorticoid corticosterone (Sigma-Aldrich) for 3 days. For transwell experiments (0.4-μm filter, Millipore), coculture was set up in six-well plates with a final volume of 3 ml.
In sup transfer experiments, RMA cells were seeded at 200,000 cells/ml and cultured for 3 days. Conditioned medium was collected, centrifuged, filtered, diluted 1:1 with fresh medium, and added to NK cells for 24 hours.
For membrane dye transfer experiments, NK cells were labeled with CFSE and RMA cells with CellVue Claret Far Red (Sigma-Aldrich). A total of 10,000 NK cells were then cocultured with RMA cells at a 1:10 ratio in 96-well V-bottom plates with a final volume of 100 μl. In experiments where ATP production was pharmacologically blocked, NK cells were pretreated with 50 mM sodium azide (Sigma-Aldrich) for 2 hours or with 13 μM antimycin A (Sigma-Aldrich) for 1 hour, washed, and then incubated with RMA cells for 1 hour. In experiments where actin polymerization was inhibited, negatively isolated splenic NK cells were activated with rhIL-2 (1000 U/ml) for 3 days, then pretreated with latrunculin A for 30 min at 37°C, and cultured with RMA cells at a 1:5 ratio at 37°C for 2 hours. In experiments where transcription was inhibited, negatively isolated splenic NK cells were activated with rhIL-2 (1000 U/ml) for 3 days, then pretreated with actinomycin D for 1 hour at 37°C, and cultured with RMA cells at a 1:5 ratio at 37°C for 3 hours. In experiments where PD-L1 was blocked, 5 μg of PD-L1 blocking antibody clone 10F.9G2 (or isotype control) was added to the coculture.
In experiments where PD-1 was blocked in vitro, tumor cells were incubated with 5 μg of RMP1-14, Fc-silent RMP1-14, or control isotype for 20 min, and then NK cells were added to the culture. After 2 days, additional 5 μg of antibodies was added to the coculture and cells were harvested and analyzed after 24 hours. To check PD-1 saturation, an aliquot of coculture or tumor cell alone was stained with directly conjugated RMP1-14 or a noncompeting PD-1 antibody (29F.1A12).
Microscopy
For microscopy experiments, splenic NK cells were negatively isolated and cultured in rhIL-2 for 3 days. NK cells were then resuspended with tumor cells (1:3 ratio) in 1% methylcellulose (Sigma-Aldrich), placed in Delta T culture dishes (Bioptechs, Butler, PA, USA), and kept at 34°C in 5% CO2 and 21% O2 during imaging. Live cell microscopy was performed using the 100× oil immersion lens on a Zeiss Axiovert 200M, with differential interference contrast, and green fluorescent frames were captured using AxioVision software at 10-min intervals for periods extending to 5 hours. Merged frames were imported in Adobe Photoshop as layers and then converted into time-lapse animations.
Flow cytometry
When needed, tumors were excised from mice, cut in pieces, resuspended in serum-free medium, and dissociated using a gentle magnetic-activated cell sorting dissociator (Miltenyi). Following dissociation, the single-cell suspension was passed through a 40-μm filter, and cells were washed and resuspended in PBS for staining. Spleens were harvested, gently dissociated through a 40-μm filter, and washed, and red blood cells were lysed using ACK (Ammonium-Chloride-Potassium) buffer (Sigma-Aldrich), then washed, and resuspended in PBS for staining.
The cellular preparation was stained with the Zombie NIR Fixable Viability Dye (BioLegend) for 20 min in PBS to label dead cells. Cells were then washed with flow buffer (PBS + 0.5% bovine serum albumin) and incubated for 20 min with purified rat anti-mouse CD16/CD32 (clone 2.4G2) (BD Biosciences) to block FcγRII/III receptors, followed by washing in flow buffer, and then incubated for a further 20 min with primary specific antibodies. Cells were washed and resuspended in flow buffer for sample acquisition or fixed in BD Cytofix/Cytoperm and acquired within 7 days. Flow cytometry was performed using LSRFortessa (BD Biosciences) or Celesta (BD Biosciences), and data were analyzed with FlowJo software (Tree Star Inc.)
Antibodies
For experiments with murine cells, the following antibodies were used: (i) from BD Biosciences: anti-CD3ε (clone 145-2C11), anti-CD8a (clone 53-6.7), anti-CD11b (clone M1/70), anti-CD11c (clone HL3), anti-CD45.2 (clone 104), anti-CD49b (clone DX5), anti-CD69 (clone H1.2F3), anti-Ly6G (clone 1A8), anti–NKR-P1C (clone PK136), and anti–Sca-1 (clone D7); (ii) from BioLegend: anti-CD4 (clone RM4-5), anti-CD19 (clone 6D5), anti-TCRvβ12 (clone MR11-1), anti–Thy-1.1 (clone OX-7), anti-F4/80 (clone BM8), anti-Ly6c (clone HK1.4), anti-NKp46 (clone 29A1.4), anti–PD-1 (clone 29F.1A12), anti–PD-L1 (clone 10F.9G2), rat IgG2a isotype control, and mouse IgG1 isotype control; (iii) from Abcam: anti-CD45.1 (clone A20).
For experiments with MM patients, the following antibodies were used: anti-CD3 (clone SK7), anti-CD7 (clone M-T701), anti-CD16 (clone 3G8), anti-CD38 (clone HIT2), anti CD45 (clone HI30), anti-CD56 (clone NCAM16.2), anti-CD138 (clone MI15), and anti–PD-1 (clone EH12.1), all from BD Biosciences.
ATAC-seq
Genomic snapshots were generated using IGV software (Broad Institute) using data available on Gene Expression Omnibus (GEO): GSE77695 (75) and GEO: GSE145299 (76).
Analysis of patients
BM aspirates were obtained from patients with clonal plasma cell disorder enrolled at the Ottawa Hospital Research Institute and at the Division of Hematology (“Sapienza” University of Rome). BM samples were lysed using a buffer composed of 1.5 M NH4Cl, 100 mM NaHCO3, and 10 mM EDTA and then stained as described above.
Statistical analysis
Differences in tumor growth curves were analyzed with a two-way analysis of variance (ANOVA). Comparison between two groups was performed with Student’s t test (two-tailed, paired, or unpaired). Comparison between three groups was performed with ANOVA. P < 0.05 was considered a statistically significant difference.
Study approvals
Mouse studies were reviewed and approved by Animal Care Veterinary Services at the University of Ottawa in accordance with the guidelines of Canadian Institutes of Health Research. For human studies, informed and written consent in accordance with the Declaration of Helsinki was obtained from all patients, and approval was obtained from the Ethics Committee of the Sapienza University of Rome (RIF.CE: 5191) or of The Ottawa Hospital (REB 20180221-02H).
Acknowledgments
We thank members of the Ardolino laboratory and L. Horan, B. Vanderhyden, F. Scott, M. C. Bourgeois-Daigneault, D. Kissov, and S. Piconese for critically reading the manuscript; F. Ortiz, V. Tang, W. Stanford, C. Ito, and the CHEO flow-core for support with flow cytometry; and the ACVS facility at the University of Ottawa for support with animal studies. We are thankful to J. V. Ravetch (Rockefeller University) for providing us with Fc-silent PD-1 antibodies and to D. H. Raulet for providing us with mouse lines and continuous support. This manuscript is dedicated to the memory of Lucas Horan, who loved science and the Bay.
Funding: M.A. is supported by Ride for Dad, CIHR, and Myeloma Canada; H.-Y.S. by the NIH intramural research programs and the National Eye Institute (1ZIAEY000569-01); G.S. and A.S. by AIRC: 5×1000-21147 (A.S.) and MFAG-21311 (G.S.); A.Z. by Sapienza University; P.G.F. by Science Foundation Ireland; and A.H.S. by NIH (PO1 grant 56299). E.V. is supported by an AIRC fellowship. M.M. is the recipient of a CAAIF postdoctoral fellowship. J.J.H. and D.P.C. are recipients of a Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award from CIHR. D.P.C. is also the recipient of a Banting post-doctoral fellowship. S.A. is a recipient of an OGS scholarship.
Author contributions: Conceptualization: M.S.H., H.-Y.S., G.S., A.Z., D.A.G., A.M., and M.A. Formal analysis: M.M., H.-Y.S., D.P.C., G.S., D.A.G., and M.A. Funding acquisition: H.-Y.S., A.S., G.S., A.Z., A.M., and M.A. Investigation: M.S.H., M.M., J.J.H., E.V., O.J.M., S.A., A.K.S., O.M., F.G.A., D.A.G., and M.A. Methodology: M.S.H. and M.A. Resources: K.P.B., R.L., M.T.P., A.S., P.G.F., A.H.S., A.V., A.Z., and A.M. Supervision: A.S., G.S., A.Z., and M.A. Visualization: H.-Y.S., D.A.G., and M.A. Writing—original draft: E.V., D.A.G., A.M., and M.A. Writing—review and editing: all authors.
Competing interests: M.A. received monetary compensation from Alloy Therapeutics for consulting. M.A. is under a contract agreement to perform sponsored research with Actym Therapeutics and Dragonfly Therapeutics. Neither consulting nor sponsored research is related to the present article. A.H.S. has patents/pending royalties from Roche and Novartis on intellectual property on the PD-1 pathway (patent 7,432,059 with royalties paid from Roche, Merck, Bristol Myers Squibb, EMD Serono, Boehringer Ingelheim, AstraZeneca, Leica, Mayo Clinic, Dako, and Novartis; patent 7,722,868 with royalties paid from Roche, Merck, Bristol Myers Squibb, EMD Serono, Boehringer Ingelheim, AstraZeneca, Leica, Mayo Clinic, Dako, and Novartis; patents 8,652,465 and 9,457,080 licensed to Roche; patents 9,683,048, 9,815,898, 9,845,356, 10,202,454, and 10,457,733 licensed to Novartis; and patents 9,580,684, 9,988,452, and 10,370,446 issued to none). The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S25
Other Supplementary Material for this manuscript includes the following:
Movie S1
REFERENCES AND NOTES
- 1.Hudrisier D., Joly E., What is trogocytosis and what is its purpose? Nat. Immunol. 4, 815 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Bettadapur A., Miller H. W., Ralston K. S., Biting off what can be chewed: Trogocytosis in health, infection and disease. Infect. Immun. 88, e00930-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J. F., Yang Y., Sepulveda H., Shi W., Hwang I., Peterson P. A., Jackson M. R., Sprent J., Cai Z., TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science 286, 952–954 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Hwang I., Huang J. F., Kishimoto H., Brunmark A., Peterson P. A., Jackson M. R., Surh C. D., Cai Z., Sprent J., T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J. Exp. Med. 191, 1137–1148 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudrisier D., Riond J., Mazarguil H., Gairin J. E., Joly E., Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J. Immunol. 166, 3645–3649 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Hudrisier D., Riond J., Garidou L., Duthoit C., Joly E., T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur. J. Immunol. 35, 2284–2294 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Hudrisier D., Aucher A., Puaux A. L., Bordier C., Joly E., Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J. Immunol. 178, 3637–3647 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Hudrisier D., Clemenceau B., Balor S., Daubeuf S., Magdeleine E., Daëron M., Bruhns P., Vié H., Ligand binding but undetected functional response of FcR after their capture by T cells via trogocytosis. J. Immunol. 183, 6102–6113 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Batista F. D., Iber D., Neuberger M. S., B cells acquire antigen from target cells after synapse formation. Nature 411, 489–494 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Carlin L. M., Eleme K., McCann F. E., Davis D. M., Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J. Exp. Med. 194, 1507–1517 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjöström A., Eriksson M., Cerboni C., Johansson M. H., Sentman C. L., Kärre K., Höglund P., Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J. Exp. Med. 194, 1519–1530 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabiasco J., Espinosa E., Hudrisier D., Joly E., Fournié J. J., Vercellone A., Active trans-synaptic capture of membrane fragments by natural killer cells. Eur. J. Immunol. 32, 1502–1508 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Herrera O. B., Golshayan D., Tibbott R., Ochoa F. S., James M. J., Marelli-Berg F. M., Lechler R. I., A novel pathway of alloantigen presentation by dendritic cells. J. Immunol. 173, 4828–4837 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Aucher A., Magdeleine E., Joly E., Hudrisier D., Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood 111, 5621–5628 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daubeuf S., Lindorfer M. A., Taylor R. P., Joly E., Hudrisier D., The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. J. Immunol. 184, 1897–1908 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Miyake K., Shiozawa N., Nagao T., Yoshikawa S., Yamanishi Y., Karasuyama H., Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc. Natl. Acad. Sci. U.S.A. 114, 1111–1116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabiasco J., Vercellone A., Meggetto F., Hudrisier D., Brousset P., Fournié J. J., Acquisition of viral receptor by NK cells through immunological synapse. J. Immunol. 170, 5993–5998 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Miner C. A., Giri T. K., Meyer C. E., Shabsovich M., Tripathy S. K., Acquisition of activation receptor ligand by trogocytosis renders NK cells hyporesponsive. J. Immunol. 194, 1945–1953 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern-Ginossar N., Nedvetzki S., Markel G., Gazit R., Betser-Cohen G., Achdout H., Aker M., Blumberg R. S., Davis D. M., Appelmelk B., Mandelboim O., Intercellular transfer of carcinoembryonic antigen from tumor cells to NK cells. J. Immunol. 179, 4424–4434 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Miyake K., Karasuyama H., The role of trogocytosis in the modulation of immune cell functions. Cell 10, 1255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel D. M., Arnold P. Y., White G. A., Nardella J. P., Mannie M. D., Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J. Immunol. 163, 5201–5210 (1999). [PubMed] [Google Scholar]
- 22.Zimmer J., Ioannidis V., Held W., H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: Implications for NK cell function. J. Exp. Med. 194, 1531–1539 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeMaoult J., Caumartin J., Daouya M., Favier B., Rond S. L., Gonzalez A., Carosella E. D., Immune regulation by pretenders: Cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 109, 2040–2048 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Brown R., Kabani K., Favaloro J., Yang S., Ho P. J., Gibson J., Fromm P., Suen H., Woodland N., Nassif N., Hart D., Joshua D., CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis. Blood 120, 2055–2063 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Reed J., Wetzel S. A., Trogocytosis-mediated intracellular signaling in CD4(+) T cells drives TH2-associated effector cytokine production and differentiation. J. Immunol. 202, 2873–2887 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomaru U., Yamano Y., Nagai M., Maric D., Kaumaya P. T. P., Biddison W., Jacobson S., Detection of virus-specific T cells and CD8+ T-cell epitopes by acquisition of peptide-HLA-GFP complexes: Analysis of T-cell phenotype and function in chronic viral infections. Nat. Med. 9, 469–475 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Rosenits K., Keppler S. J., Vucikuja S., Aichele P., T cells acquire cell surface determinants of APC via in vivo trogocytosis during viral infections. Eur. J. Immunol. 40, 3450–3457 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Haastert B., Mellanby R. J., Anderton S. M., O’Connor R. A., T cells at the site of autoimmune inflammation show increased potential for trogocytosis. PLOS ONE 8, e81404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown R., Suen H., Favaloro J., Yang S., Ho P. J., Gibson J., Joshua D., Trogocytosis generates acquired regulatory T cells adding further complexity to the dysfunctional immune response in multiple myeloma. Onco. Targets Ther. 1, 1658–1660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg G., Uzana R., Pato A., Frankenburg S., Merims S., Yefenof E., Ferrone S., Peretz T., Machlenkin A., Lotem M., Imprinting of lymphocytes with melanoma antigens acquired by trogocytosis facilitates identification of tumor-reactive T cells. J. Immunol. 190, 5856–5865 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iannello A., Thompson T. W., Ardolino M., Marcus A., Raulet D. H., Immunosurveillance and immunotherapy of tumors by innate immune cells. Curr. Opin. Immunol. 38, 52–58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodgins J. J., Khan S. T., Park M. M., Auer R. C., Ardolino M., Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Invest. 129, 3499–3510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roda-Navarro P., Vales-Gomez M., Chisholm S. E., Reyburn H. T., Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc. Natl. Acad. Sci. U.S.A. 103, 11258–11263 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCann F. E., Eissmann P., Onfelt B., Leung R., Davis D. M., The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J. Immunol. 178, 3418–3426 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K., Nakayama M., Kawano M., Amagai R., Ishii T., Harigae H., Ogasawara K., Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc. Natl. Acad. Sci. U.S.A. 110, 9421–9426 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez V. D., Huang Y. W., Delgado-Gonzalez A., Chen S. Y., Donoso K., Sachs K., Gentles A. J., Allard G. M., Kolahi K. S., Howitt B. E., Porpiglia E., Fantl W. J., High-grade serous ovarian tumor cells modulate NK cell function to create an immune-tolerant microenvironment. Cell Rep. 36, 109632 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caumartin J., Favier B., Daouya M., Guillard C., Moreau P., Carosella E. D., LeMaoult J., Trogocytosis-based generation of suppressive NK cells. EMBO J. 26, 1423–1433 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu J., Hodgins J. J., Marathe M., Nicolai C. J., Bourgeois-Daigneault M. C., Trevino T. N., Azimi C. S., Scheer A. K., Randolph H. E., Thompson T. W., Zhang L., Iannello A., Mathur N., Jardine K. E., Kirn G. A., Bell J. C., McBurney M. W., Raulet D. H., Ardolino M., Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 128, 4654–4668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benson D. M. Jr., Bakan C. E., Mishra A., Hofmeister C. C., Efebera Y., Becknell B., Baiocchi R. A., Zhang J., Yu J., Smith M. K., Greenfield C. N., Porcu P., Devine S. M., Rotem-Yehudar R., Lozanski G., Byrd J. C., Caligiuri M. A., The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beldi-Ferchiou A., Lambert M., Dogniaux S., Vély F., Vivier E., Olive D., Dupuy S., Levasseur F., Zucman D., Lebbé C., Sène D., Hivroz C., Caillat-Zucman S., PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 7, 72961–72977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pesce S., Greppi M., Tabellini G., Rampinelli F., Parolini S., Olive D., Moretta L., Moretta A., Marcenaro E., Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 139, 335–346.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Cheng Y., Xu Y., Wang Z., du X., Li C., Peng J., Gao L., Liang X., Ma C., Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 36, 6143–6153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vari F., Arpon D., Keane C., Hertzberg M. S., Talaulikar D., Jain S., Cui Q., Han E., Tobin J., Bird R., Cross D., Hernandez A., Gould C., Birch S., Gandhi M. K., Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 131, 1809–1819 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis Z., Felices M., Lenvik T., Badal S., Walker J. T., Hinderlie P., Riley J. L., Vallera D. A., Blazar B. R., Miller J. S., Low-density PD-1 expression on resting human natural killer cells is functional and upregulated after transplantation. Blood Adv. 5, 1069–1080 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Judge S. J., Dunai C., Aguilar E. G., Vick S. C., Sturgill I. R., Khuat L. T., Stoffel K. M., van Dyke J., Longo D. L., Darrow M. A., Anderson S. K., Blazar B. R., Monjazeb A. M., Serody J. S., Canter R. J., Murphy W. J., Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J. Clin. Invest. 130, 3051–3068 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kärre K., Ljunggren H. G., Piontek G., Kiessling R., Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986). [DOI] [PubMed] [Google Scholar]
- 47.Ardolino M., Azimi C. S., Iannello A., Trevino T. N., Horan L., Zhang L., Deng W., Ring A. M., Fischer S., Garcia K. C., Raulet D. H., Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J. Clin. Invest. 124, 4781–4794 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curran E., Chen X., Corrales L., Kline D. E., Dubensky T. W. Jr., Duttagupta P., Kortylewski M., Kline J., STING pathway activation stimulates potent immunity against acute myeloid leukemia. Cell Rep. 15, 2357–2366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruzicka M., Koenig L. M., Formisano S., Boehmer D. F. R., Vick B., Heuer E. M., Meinl H., Kocheise L., Zeitlhöfler M., Ahlfeld J., Kobold S., Endres S., Subklewe M., Duewell P., Schnurr M., Jeremias I., Lichtenegger F. S., Rothenfusser S., RIG-I-based immunotherapy enhances survival in preclinical AML models and sensitizes AML cells to checkpoint blockade. Leukemia 34, 1017–1026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamieh M., Dobrin A., Cabriolu A., van der Stegen S. J. C., Giavridis T., Mansilla-Soto J., Eyquem J., Zhao Z., Whitlock B. M., Miele M. M., Li Z., Cunanan K. M., Huse M., Hendrickson R. C., Wang X., Rivière I., Sadelain M., CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anton O. M., Peterson M. E., Hollander M. J., Dorward D. W., Arora G., Traba J., Rajagopalan S., Snapp E. L., Garcia K. C., Waldmann T. A., Long E. O., Trans-endocytosis of intact IL-15Rα–IL-15 complex from presenting cells into NK cells favors signaling for proliferation. Proc. Natl. Acad. Sci. U.S.A. 117, 522–531 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong W., Wu X., Ma S., Wang Y., Nalin A. P., Zhu Z., Zhang J., Benson D. M., He K., Caligiuri M. A., Yu J., The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 9, 1422–1437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor R. P., Lindorfer M. A., Fcγ-receptor–mediated trogocytosis impacts mAb-based therapies: Historical precedence and recent developments. Blood 125, 762–766 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Latchman Y. E., Liang S. C., Wu Y., Chernova T., Sobel R. A., Klemm M., Kuchroo V. K., Freeman G. J., Sharpe A. H., PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. U.S.A. 101, 10691–10696 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz C., Khan A. R., Floudas A., Saunders S. P., Hams E., Rodewald H. R., McKenzie A. N. J., Fallon P. G., ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control. J. Exp. Med. 214, 2507–2521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu N., Veillette A., SLAM family receptors in normal immunity and immune pathologies. Curr. Opin. Immunol. 38, 45–51 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Guo H., Cranert S. A., Lu Y., Zhong M. C., Zhang S., Chen J., Li R., Mahl S. E., Wu N., Davidson D., Waggoner S. N., Veillette A., Deletion of Slam locus in mice reveals inhibitory role of SLAM family in NK cell responses regulated by cytokines and LFA-1. J. Exp. Med. 213, 2187–2207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding Z. M., Babensee J. E., Simon S. I., Lu H., Perrard J. L., Bullard D. C., Dai X. Y., Bromley S. K., Dustin M. L., Entman M. L., Smith C. W., Ballantyne C. M., Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 163, 5029–5038 (1999). [PubMed] [Google Scholar]
- 59.Long E. O., Kim H. S., Liu D., Peterson M. E., Rajagopalan S., Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 31, 227–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ljunggren H. G., Kärre K., Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J. Exp. Med. 162, 1745–1759 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahan R., Sega E., Engelhardt J., Selby M., Korman A. J., Ravetch J. V., FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 28, 285–295 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Stadtmauer E. A., Faitg T. H., Lowther D. E., Badros A. Z., Chagin K., Dengel K., Iyengar M., Melchiori L., Navenot J. M., Norry E., Trivedi T., Wang R., Binder G. K., Amado R., Rapoport A. P., Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv. 3, 2022–2034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller J. S., Lanier L. L., Natural killer cells in cancer immunotherapy. Annu. Rev. Cancer Biol. 3, 77–103 (2019). [Google Scholar]
- 64.Nakashima M., Watanabe M., Uchimaru K., Horie R., Trogocytosis of ligand-receptor complex and its intracellular transport in CD30 signalling. Biol. Cell. 110, 109–124 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Ho W. T., Pang W. L., Chong S. M., Castella A., al-Salam S., Tan T. E., Moh M. C., Koh L. K., Gan S. U., Cheng C. K., Schwarz H., Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Cancer Res. 73, 652–661 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Lee K. Y., Wong H. Y., Zeng Q., le Lin J., Cheng M. S., Kuick C. H., Chang K. T. E., Loh A. H. P., Schwarz H., Ectopic CD137 expression by rhabdomyosarcoma provides selection advantages but allows immunotherapeutic targeting. Onco. Targets Ther. 10, 1877459 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawashima M., Carreras J., Higuchi H., Kotaki R., Hoshina T., Okuyama K., Suzuki N., Kakizaki M., Miyatake Y., Ando K., Nakayama M., Umezu S., Horie R., Higuchi Y., Katagiri K., Goyama S., Kitamura T., Chamoto K., Yano S., Nakamura N., Kotani A., PD-L1/L2 protein levels rapidly increase on monocytes via trogocytosis from tumor cells in classical Hodgkin lymphoma. Leukemia 34, 2405–2417 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Radhakrishnan S. V., Bhardwaj N., Luetkens T., Atanackovic D., Novel anti-myeloma immunotherapies targeting the SLAM family of receptors. Onco. Targets Ther. 6, e1308618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen Y. C., Zada M., Wang S. Y., Bornstein C., David E., Moshe A., Li B., Shlomi-Loubaton S., Gatt M. E., Gur C., Lavi N., Ganzel C., Luttwak E., Chubar E., Rouvio O., Vaxman I., Pasvolsky O., Ballan M., Tadmor T., Nemets A., Jarchowcky-Dolberg O., Shvetz O., Laiba M., Shpilberg O., Dally N., Avivi I., Weiner A., Amit I., Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing. Nat. Med. 27, 491–503 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zha H., Jiang Y., Wang X., Shang J., Wang N., Yu L., Zhao W., Li Z., An J., Zhang X., Chen H., Zhu B., Li Z., Non-canonical PD-1 signaling in cancer and its potential implications in clinic. J. Immunother. Cancer 9, e001230 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vo D. N., Constantinides M., Allende-Vega N., Alexia C., Cartron G., Villalba M., Dissecting the NK cell population in hematological cancers confirms the presence of tumor cells and their impact on NK population function. Vaccines 8, 727 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keir M. E., Freeman G. J., Sharpe A. H., PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179, 5064–5070 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Narni-Mancinelli E., Chaix J., Fenis A., Kerdiles Y. M., Yessaad N., Reynders A., Gregoire C., Luche H., Ugolini S., Tomasello E., Walzer T., Vivier E., Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl. Acad. Sci. U.S.A. 108, 18324–18329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guerra N., Tan Y. X., Joncker N. T., Choy A., Gallardo F., Xiong N., Knoblaugh S., Cado D., Greenberg N. R., Raulet D. H., NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28, 571–580 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shih H. Y., Sciumè G., Mikami Y., Guo L., Sun H. W., Brooks S. R., Urban J. F. Jr., Davis F. P., Kanno Y., O’Shea J. J., Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell 165, 1120–1133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sciumè G., Mikami Y., Jankovic D., Nagashima H., Villarino A. V., Morrison T., Yao C., Signorella S., Sun H.-W., Brooks S. R., Fang D., Sartorelli V., Nakayamada S., Hirahara K., Zitti B., Davis F. P., Kanno Y., O’Shea J. J., Shih H.-Y., Rapid enhancer remodeling and transcription factor repurposing enable high magnitude gene induction upon acute activation of NK cells. Immunity 53, 745–758.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S25
Movie S1