Abstract
Efforts to eliminate the HIV epidemic will require increased HIV testing rates among high-risk populations. To inform the design of HIV testing interventions, a discrete choice experiment (DCE) with six policy-relevant attributes of HIV testing options elicited the testing preferences of 300 female barworkers and 440 male Kilimanjaro mountain porters in northern Tanzania. Surveys were administered between September 2017 and July 2018. Participants were asked to complete 12 choice tasks, each involving first- and second-best choices from 3 testing options. DCE responses were analyzed using a random effects latent class logit (RELCL) model, in which the latent classes summarize common participant preference profiles, and the random effects capture additional individual-level preference heterogeneity with respect to three attribute domains: (a) privacy and confidentiality (testing venue, pre-test counseling, partner notification); (b) invasiveness and perceived accuracy (method for obtaining the sample for the HIV test); and (c) accessibility and value (testing availability, additional services provided). The Bayesian Information Criterion indicated the best model fit for a model with 8 preference classes, with class sizes ranging from 6% to 19% of participants. Substantial preference heterogeneity was observed, both between and within latent classes, with 12 of 16 attribute levels having positive and negative coefficients across classes, and all three random effects contributing significantly to participants’ choices. The findings may help identify combinations of testing options that match the distribution of HIV testing preferences among high-risk populations; the methods may be used to systematically design heterogeneity-focused interventions using stated preference methods.
Keywords: Discrete choice experiment, Preference heterogeneity, Random effects latent class logit (RELCL), HIV counseling and testing, Tanzania
1. Introduction
Ambitious targets have been set by the Joint United Nations Programme on HIV/AIDS (UNAIDS), the President’s Emergency Plan for AIDS Relief (PEPFAR), and by Ministries of Health across the globe to eliminate the HIV epidemic. For the year 2030, these targets include what is known as 95–95-95 - diagnosing 95% of all persons living with HIV (PLWH), initiating treatment for 95% of those diagnosed, and achieving viral suppression for 95% of those treated (UNAIDS, 2014). Progress towards diagnosing 95% of PLWH by 2030 is contingent on accelerating the uptake of HIV testing, both among higher-risk populations and across the population at large. The number of undiagnosed HIV infections is considered a major hindrance to achieving the UNAIDS targets and ending the epidemic (The Lancet, 2017).
Discrete choice experiments (DCEs) are commonly used to elicit information about individuals’ preferences for varying characteristics of multi-attribute products. DCE results can be used to develop targeted, preference-informed interventions; optimal interventions may vary across and within population subgroups. DCEs have been used in various contexts related to HIV, including testing (Indravudh et al., 2017; Johnson et al., 2010; Ostermann et al., 2014, 2015; Phillips et al., 2002; Strauss et al., 2018a, 2018b), prevention (Cameron et al., 2013; Newman et al., 2016; Quaife et al., 2018a; Terris-Prestholt et al., 2013), service delivery (d’Elbee et al., 2018; Kruk et al., 2016; Zanolini et al., 2018), and treatment (Beusterien et al., 2007; Bregigeon-Ronot et al., 2017; Hauber et al., 2009; Ostermann et al., 2020a; Mühlbacher et al., 2013). We previously characterized the HIV testing preferences in a community sample in Tanzania and identified substantial preference heterogeneity (Ostermann et al., 2014). To our knowledge, DCEs have not been used to systematically characterize the distribution of HIV testing preferences among populations at high risk of HIV infection.
To inform the design of HIV testing interventions for high-risk populations, this study used a DCE to characterize patterns of testing preferences among female barworkers and male Kilimanjaro mountain porters, two high-risk populations in northern Tanzania. The DCE focused on policy-relevant characteristics of HIV testing programs that may be adapted to match the preferences of these populations. To identify patterns of preferences, we modeled DCE responses using a random effects latent class logit (RELCL) model. RELCL models allow the simultaneous estimation of common preference profiles via latent classes, as well as class-independent individual variation via random effects (Zhou and Bridges, 2019; Greene and Hensher, 2013; Hess et al., 2014). The findings from this study may help identify combinations of testing options that match the distribution of HIV testing preferences among the two high risk populations included in this study. More generally, the analytic approach described here may inform the systematic design of interventions in the context of preference heterogeneity.
2. Methods
2.1. Ethical approval
The study protocol was approved by the Institutional Review Boards at Duke University and the University of South Carolina in the United States, as well as the Ethics Review Committee at Kilimanjaro Christian Medical University College and the National Institute for Medical Research in Tanzania. The protocol was registered in ClinicalTrials.gov (Protocol NCT02714140) on March 21, 2016 (Ostermann et al., 2020). Informed consent was obtained from all study participants.
2.2. Study setting
The study was conducted in Moshi, Tanzania. Moshi is the commercial center and administrative capital of the Kilimanjaro Region in Northern Tanzania and has an estimated population of about 200,000 (United Republic of Tanzania, 2012). Voluntary HIV counseling and testing (VCT) is available at 25 health facilities, including 2 free-standing VCT centers.
2.3. Study sample
Study participants were enrolled between September 2017 and July 2018. Participants comprised 300 women employed in bars, restaurants and guesthouses serving alcohol to patrons (henceforth referred to as “bars” and “female barworkers”, respectively) and 440 male mountain porters supporting climbers of nearby Mount Kilimanjaro (“male porters”). We previously showed that female barworkers and male porters engage in higher rates of HIV risk behaviors than randomly selected male and female community members in the same setting (Ostermann et al., 2015). A census of bars and barworkers, conducted by the study team between February and June of 2016, identified 612 bars within Moshi, with 2059 age-eligible female barworkers. There are an estimated 10, 000 porters in the Kilimanjaro Region (Mitchell et al., 2009; Peaty, 2012).
Eligible study participants were residents of Moshi, able to read, and ages 18 to 49. Female barworkers were recruited from randomly selected bars; male porters were sequentially approached as they exited Mount Kilimanjaro National Park. Eligible individuals were invited to the study’s research office for consent and enrollment; study compensation ranged from Tanzania Shilling (TSH) 5000 (~$2.15) to TSH 10,000 (~$4.30), including transport reimbursement.
2.4. Discrete choice experiment
HIV testing preferences were assessed using a DCE. The objective of the DCE was to present survey respondents with hypothetical HIV testing options that could feasibly be implemented in the study area. As such, the DCE was built on the characteristics of testing options that were available in the study area at the time of the survey, as well as characteristics that could feasibly be implemented. The design, administration, and analysis of the DCE followed the guidelines for DCE applications in healthcare (Bridges et al., 2011) and in low-income settings (Mangham et al., 2009). As with a previous DCE on HIV testing preferences in the same area, the selection of attributes was guided by focus group discussions with members of the target populations (Ostermann et al., 2014, 2015; Njau et al., 2014). The attributes and levels employed in our study were the following:
Attribute 1 – Testing venue.
In the study area, HIV testing is available at health facilities and at free-standing VCT centers. Several of these facilities also conduct outreach activities, including home-based testing, which involves a counselor coming to a client’s home for VCT. Testing venue was thus implemented as a three-level attribute: testing at a health facility, testing at a free-standing VCT center, and testing at home.
Attribute 2 – Testing availability.
The majority of testing venues in Moshi offer HIV testing only on weekdays. However, selected facilities started making testing available on weekends. In the DCE, testing availability was implemented as a two-level, ordered, attribute describing testing availability on either weekdays only or every day of the week.
Attribute 3 – Pre-test counseling.
National testing guidelines (National AIDS Control Programme, 2019) require that, before testing for HIV, a counselor provides the client with information about HIV, risk of infection, and the HIV test. This service is referred to as pre-test counseling. Pre-test counseling can be done one-on-one, in a group, or with a partner in the context of couples counseling and testing. Accordingly, this attribute was implemented as a three-level attribute.
Attribute 4 – Type of sample.
Three different methods may be used to collect the sample for the HIV test. Samples can be collected using blood from the arm (venipuncture) or the finger (finger prick), or saliva can be taken from the mouth using an oral swab. Accordingly, the attribute was implemented as a three-level attribute. It was emphasized that all three options give the same result; however, oral testing was not yet approved for general use in Tanzania at the time of the survey.
Attribute 5 – Additional services.
To decrease stigma and increase value, there have been efforts to integrate HIV testing with other health services (National AIDS Control Programme, 2017). This attribute was implemented as a three-level attribute describing the provision of additional services in conjunction with the HIV test, namely a complimentary screen for other sexually transmitted infections (STIs), a complimentary general health check (e.g., blood pressure, diabetes), versus no additional services.
Attribute 6 - Partner notification.
For persons testing positive for HIV, the notification and testing of sexual partners is critical for identifying or preventing additional HIV infections (Brown et al., 2011; Cherutich et al., 2017; Chiou et al., 2015; Garcia de Olalla et al., 2015; Henley et al., 2013; Kahabuka et al., 2017; Landis et al., 1992; Myers et al., 2016; Rosenberg et al., 2015; Udeagu et al., 2012, 2014). Partner notification was implemented as a three-level attribute. Self-disclosure involves clients testing positive being encouraged to advise their partners to test for HIV. Confidential provider notification involves clients being asked to give the name and contact information for their partners, and a counselor later contacting these partners to test for HIV without revealing the client’s name. Automatic disclosure involves the joint receipt of HIV test results by clients and their partners in the context of couples counseling.
2.4.1. Experimental design
We measured preferences over the full range of feasible combinations of attribute levels by presenting respondents with a range of HIV testing options, based on an experimental design, and observing their stated preferences. The experimental design of a DCE is the combination of choice tasks that allows for the independent estimation of the influence of each testing characteristic on preferences. Ngene software (ChoiceMetrics, 2017) version 1.12b was used to select an experimental design that minimized the D-error for a mixed logit model (Johnson et al., 2007). Two constraints were imposed in the selection of choice tasks for the experimental design:
To exclude non-feasible combinations of attribute levels for the pre-test counseling and partner notification attributes (e.g., couples counseling with self-disclosure), these two attributes were combined into a 5-level compound attribute. Four levels of the compound attribute described combinations of either one-on-one or group counseling with either self-disclosure or confidential provider notification; the fifth level described couples counseling with automatic partner notification.
Statistical priors were obtained from a pilot study with 236 female barworkers and male porters. Data were analyzed using a mixed logit model; the estimated means and standard deviations were used as priors in the search for a D-efficient design optimized for a mixed logit model.
The final design consisted of 120 tasks. Participants were randomized across 10 blocks with 12 tasks each. The order of choice tasks in a block was randomized across participants. Each choice task included three unlabeled testing alternatives; the order of alternatives was randomized within each choice task.
2.4.2. DCE administration
In-person DCE surveys were fielded by trained research staff, in Kiswahili (a language commonly used in the study area), on iPad devices, using Comet survey software (Selway Labs, 2017). Participants initially ranked the levels of each attribute. These data were used to populate a respondent-specific comprehension task with clearly dominant (preferred levels for all attributes) and dominated (worse levels for all attributes) alternatives, followed by 12 DCE choice tasks. In each choice task, participants were first asked to select their most preferred option from three testing options presented; in a follow-up task they were asked to select their most preferred from the two remaining options. A sample choice task is shown in Fig. 1.
Fig. 1.
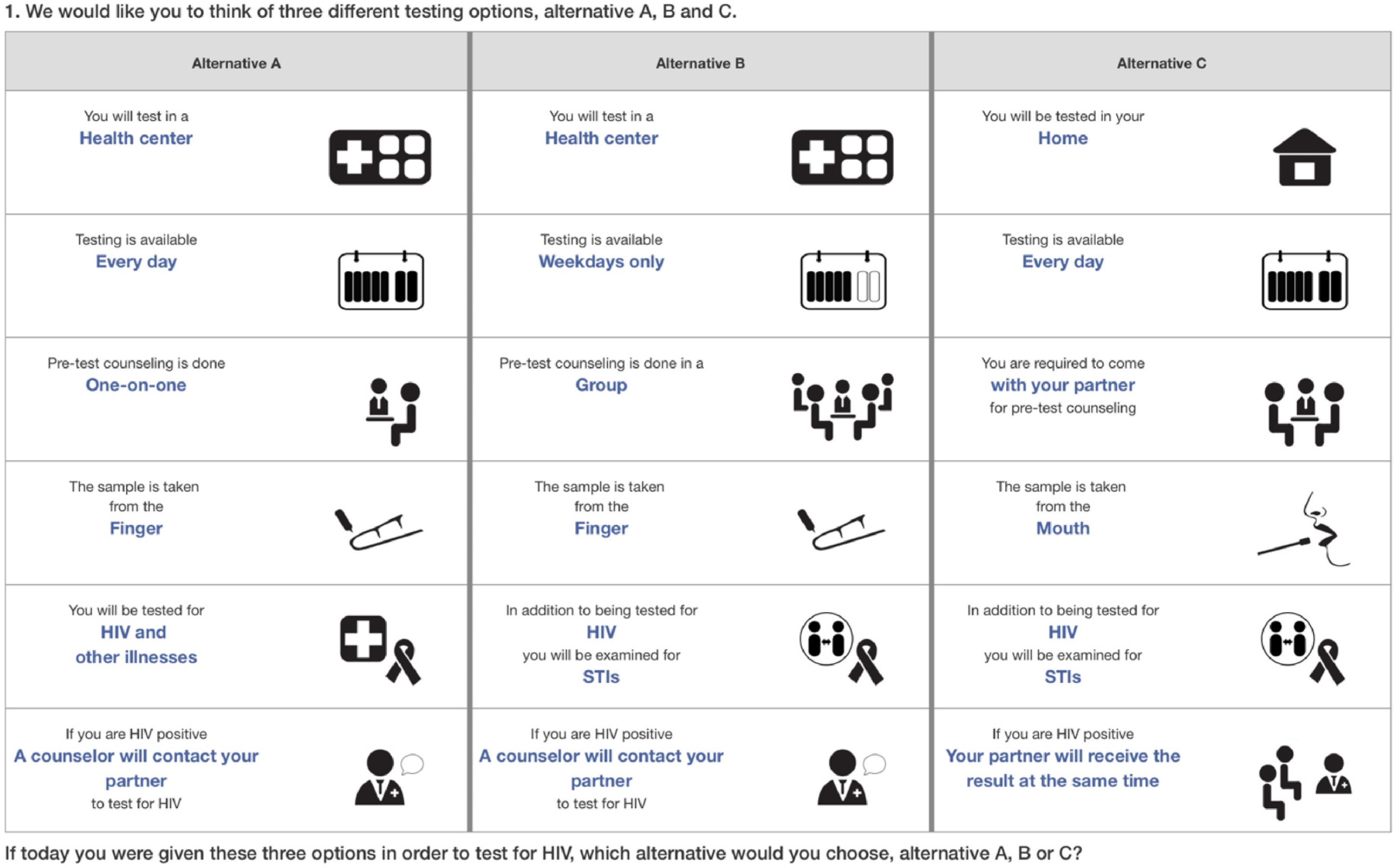
Sample DCE choice task.
2.5. Supplemental survey
A supplemental survey assessed sociodemographic characteristics and the HIV testing history of study participants.
2.6. Econometric model
Respondents’ rankings of the HIV testing options presented in the DCE choice tasks were modeled with a latent class conditional logit model with three individual-level, class independent, random effects. Our model did not include alternative-specific constants because choices were unlabeled and presented in random order. Let i index respondents and t index choice tasks (1 ≤ t ≤ T = 12). Further, let yit = m denote respondent i’s choice of option m in task t, and let M = 3 denote the number of alternatives in each ranking task. The probability of response m based on the basic latent class conditional logit model, without random effects, is
where x denotes latent class membership, zitm denotes the vector of attribute levels associated with alternative m for respondent i in task t; and ηm|x,zitm denotes the utility associated with alternative m conditional on membership in latent class x and attribute levels zitm. The linear model for the utility of alternative m characterized by attributes zitm for a member of latent class x is
where p indexes attribute levels of alternative m (p = 1, …, P); βxp is the regression coefficient for attribute level p for latent class x; and zitmp is the effects coding for attribute level p in alternative m for individual i in task t.
Let Fi denote individual i’s vector of d (d = 1, …, D) scores on D independent and standard normal random effects distributions. Adding Fi to the model, the probability of respondent i making choice m is
and the utility function is
| (1) |
where Fid is the random effect d for individual i, and λdmp is a coefficient (i.e., loading) relating random effect Fid and attribute level zitmp to the utility of alternative m.
The specific model estimated here includes D = 3 subject level random effects that capture individual-level preference heterogeneity with respect to the following attribute domains: (a) privacy and confidentiality (F.1, covering the testing venue, pre-test counseling, and partner notification attributes), (b) invasiveness and perceived accuracy (F.2, covering the type of sample attribute), and (c) accessibility and value (F.3, covering the testing availability and additional services attributes). These domains correspond to the three pillars of a conceptual model of preference-relevant HIV testing characteristics that we previously developed using qualitative work in the study area (Njau et al., 2014). The influence of the random effects (i.e., F.1, F.2, F.3) on the probability of choice m is determined by the corresponding λ weights. The product term (i.e., the third term in Equation (1)) modifies the latent class-specific preference term (i. e., the second term in Equation (1)) for the individual. Thus, this model accounts for preference heterogeneity in two ways: latent classes, which capture heterogeneity in preference profiles that are shared among groups of individuals (i.e., combinations of preferences that are likely to co-occur), and additional individual-level heterogeneity in the form of domain specific random effects that capture respondents’ unique attribute preferences, independent of class. Class membership is modeled as a function of only one covariate, participant type (barworkers vs. porters).
2.7. Statistical analysis
Differences in sociodemographic characteristics and HIV testing experiences between the two study cohorts were analyzed using Student’s t-tests and chi-squared statistics. The DCE data, composed of rankings of the three testing options presented to participants in each choice task, were analyzed as sequential choices: following an initial choice of the most preferred of the three options presented, a second choice involved the selection of the more preferred of the two remaining options. Random effects latent class logit (RELCL) models with 1–10 preference classes and 0 to 3 continuous, normally distributed random effects were estimated in Latent Gold Choice version 5.0 (Statistical Innovations Inc. 2018). Models were estimated using expectation-maximization (EM) and Newton–Raphson (NR) algorithms, with 250 EM and 50 NR iterations, and 16 different sets of random starting values. The Bayesian Information Criterion (BIC) was used to compare model fit. The best-fitting model was re-estimated with 150 different sets of random starting values to check that a global optimum was obtained. For the final model, correlates of class membership were evaluated using the bias-adjusted three-step approach described by Bakk et al. (2014). Additionally, separate models with the same specification were estimated for each risk group, and class membership predictions, based on modal estimated class membership probabilities, were compared between the aggregate and cohort-specific models.
3. Results
Table 1 details key demographic characteristics and the HIV testing history of study participants. Approximately half of the participants had at least some secondary school education, and most participants had tested for HIV at least once. Female barworkers were less likely to be married, had higher education, and were more likely to have tested for HIV than male Kilimanjaro mountain porters. Compared to a national sample of adults ages 18–49 residing in mainland urban Tanzania who participated in the 2016–17 Tanzania HIV IMPACT Survey (THIS) (Tanzania Commission for AIDS, 2018), female barworkers were less likely to be married, had more education, and both groups were somewhat more likely to have ever tested for HIV.
Table 1.
Characteristics of study participants.
| Study sampleb | National samplec | |||||
|---|---|---|---|---|---|---|
|
|
|
|
|
|
||
| (Ages 18–49, urban) | (Ages 18–49, urban) | |||||
|
|
|
|
|
|
||
| Female barworkers | Male porters | Females | Males | |||
|
| ||||||
| Number of participants | 300 | 440 | p-value | 5067 | 4077 | |
| Age | Mean (sd) | 29.7 (7.61) | 31.4 (6.71) | 0.001 | 29.6 (8.4) | 29.8 (8.4) |
| Marital status | Married | 31.8% | 65.6% | <0.001 | 58.5% | 65.9% |
| Not married | 68.2% | 34.4% | 41.5% | 34.1% | ||
| Education | Primary school or less | 42.1% | 55.4% | <0.001 | 62.5% | 51.8% |
| Secondary school | 57.9% | 44.6% | 37.5% | 48.2% | ||
| # of HIV tests | None | 5.3% | 20.0% | <0.001 | 12.3% | 26.6% |
| 1 | 13.7% | 20.0% | . | . | ||
| 2 | 19.3% | 23.4% | . | . | ||
| 3 | 26.3% | 18.4% | . | . | ||
| 4 | 15.3% | 6.8% | . | . | ||
| 5 or more | 20.0% | 11.4% | . | . | ||
| Most recent HIV test a | In the past year | 46.1% | 49.4% | 0.407 | 50.8% | 51.5% |
| More than 1 year ago | 53.9% | 50.6% | 49.2% | 48.5% | ||
Notes.
Sample restricted to adults ages 18–49, living in mainland urban Tanzania; https://phia-data.icap.columbia.edu/files.
Information not available.
Among those who tested at least once.
Demographic data (age, marital status, education) are missing for 1 porter and 1 barworker
Source: Survey-weighted means and percentages from the 2016–2017 Tanzania HIV Impact Survey (THIS).
Fig. 2 shows the performance of latent class logit (LCL) models with and without random effects specifications, as measured by the BIC. The RELCL models consistently outperformed LCL models without random effects, with model performance improving with additional random effects. Comparisons of the BIC across models indicated that, among models with 3 random effects, model fit continuously improved up to 8 classes, with only marginal improvements gained form additional classes.
Fig. 2.
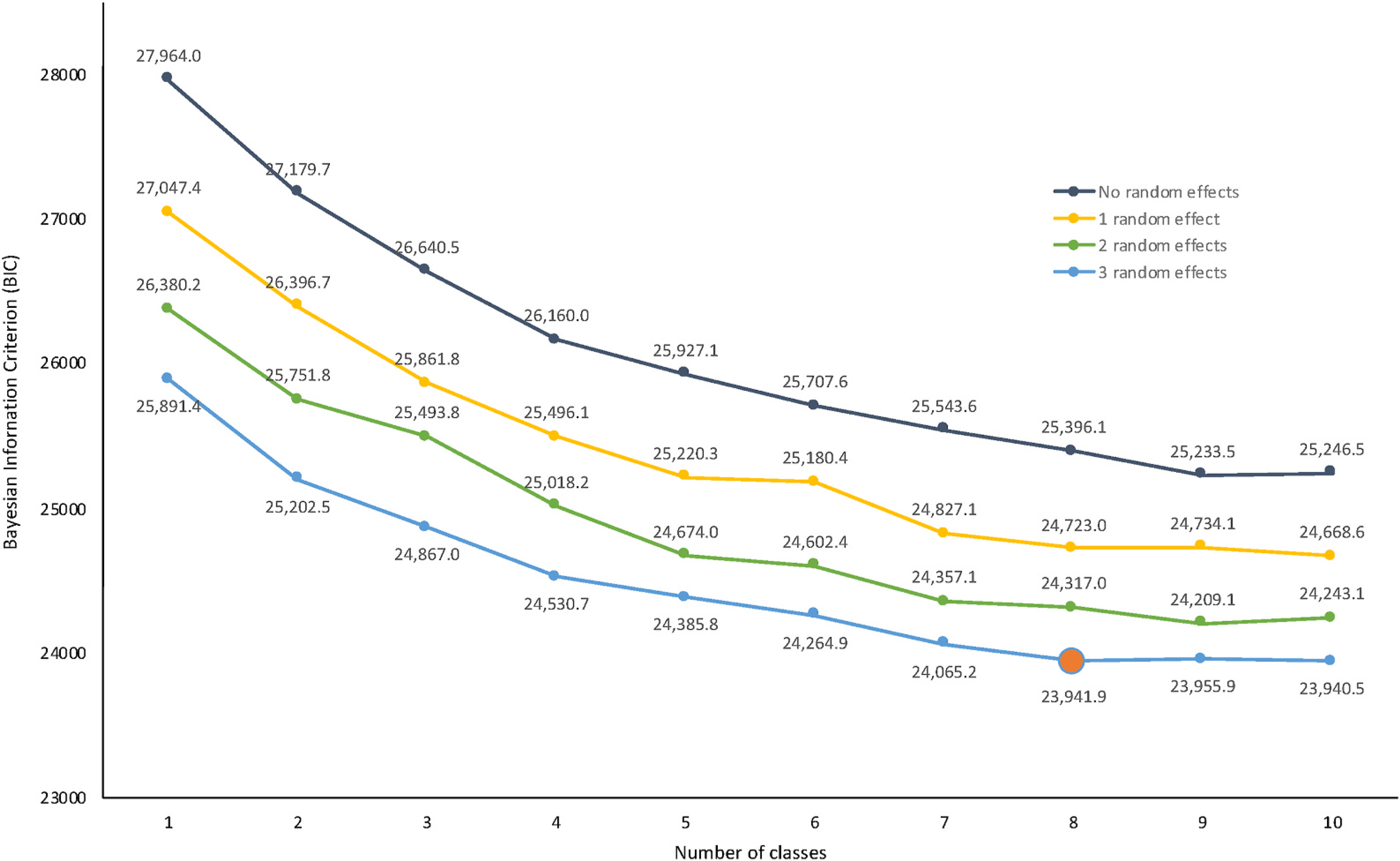
Relative performance of alternative latent class specifications with 0–3 random effects.
Note: The orange marker indicates the model presented below, selected based on the model’s relative performance on the Bayesian Information Criterion (BIC).
Table 2 shows the results of the RELCL model with 8 preference classes and 3 random effects. As with a standard latent class model, the 8 preference classes represent statistical groupings of individuals with similar sets of preferences. Unlike a standard latent class model, with a RELCL model, the three random effects capture additional class-independent individual preference heterogeneity with respect to the five attributes grouped broadly into three domains: privacy and confidentiality (testing venue; pre-test counseling; partner notification); invasiveness and perceived accuracy (type of sample); and accessibility and value (testing availability; additional services).
Table 2.
Heterogeneous HIV testing preferences: Results from a random effects latent class logit model (RELCL; N = 740).
| Class1 | Class2 | Class3 | Class4 | Class5 | Class6 | Class7 | Class8 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| Class sizes | |||||||||||||||||||
| All participants | 18.9% | 18.7% | 13.5% | 12.8% | 12.5% | 10.1% | 7.5% | 6.1% | |||||||||||
| Female barworkers | 20.0% | 18.0% | 15.4% | 13.8% | 12.6% | 9.7% | 4.6% | 6.1% | |||||||||||
| Male mountain porters | 18.3% | 19.1% | 12.2% | 12.1% | 12.5% | 10.3% | 9.5% | 6.1% | |||||||||||
| Class-specific coefficient estimates, β | Loadings, λ a | ||||||||||||||||||
| Testing venue | |||||||||||||||||||
| Home | −1.34 | (0.09) | −1.06 | (0.08) | −1.18 | (0.09) | −1.19 | (0.11) | −0.07 | (0.08) | −0.84 | (0.09) | 1.26 | (0.12) | −0.87 | (0.13) | −0.168 | 1 | |
| Health facility | 1.65 | (0.13) | 1.45 | (0.10) | 1.87 | (0.12) | 0.16 | (0.14) | 0.17 | (0.09) | 1.30 | (0.11) | −0.48 | (0.10) | −0.78 | (0.15) | 0.103 | 1 | |
| Voluntary counseling and testing center | −0.31 | (0.10) | −0.39 | (0.08) | −0.69 | (0.10) | 1.03 | (0.17) | −0.10 | (0.08) | −0.46 | (0.10) | −0.78 | (0.11) | 1.65 | (0.18) | 0.065 | 1 | |
| Testing availability | |||||||||||||||||||
| Weekdays only | −0.15 | (0.07) | −0.72 | (0.06) | −1.09 | (0.08) | −0.35 | (0.09) | 0.00 | (0.07) | −0.33 | (0.09) | −0.61 | (0.08) | −1.13 | (0.13) | 0.408 | 3 | |
| Every day | 0.15 | (0.07) | 0.72 | (0.06) | 1.09 | (0.08) | 0.35 | (0.09) | 0.00 | (0.07) | 0.33 | (0.09) | 0.61 | (0.08) | 1.13 | (0.13) | −0.408 | 3 | |
| Type of sample | |||||||||||||||||||
| Arm (venipuncture) | 0.50 | (0.11) | −0.66 | (0.12) | 1.45 | (0.16) | 0.64 | (0.19) | −0.55 | (0.13) | 0.59 | (0.14) | 0.26 | (0.17) | 1.29 | (0.21) | 0.911 | 2 | |
| Finger (finger prick) | −0.17 | (0.08) | 1.55 | (0.10) | −0.81 | (0.10) | −0.14 | (0.11) | 0.87 | (0.08) | −0.15 | (0.09) | −0.15 | (0.09) | −0.42 | (0.16) | 0.114 | 2 | |
| Mouth (oral swab) | −0.33 | (0.12) | −0.89 | (0.13) | −0.64 | (0.14) | −0.50 | (0.22) | −0.32 | (0.13) | −0.44 | (0.15) | −0.11 | (0.18) | −0.87 | (0.19) | −1.025 | 2 | |
| Additional services | |||||||||||||||||||
| HIV test only (no additional services) | −0.66 | (0.06) | −1.09 | (0.08) | −1.57 | (0.11) | −0.71 | (0.10) | −0.45 | (0.06) | −1.18 | (0.09) | −1.04 | (0.09) | −2.01 | (0.16) | 0.049 | 3 | |
| Health check | −0.02 | (0.08) | 0.87 | (0.09) | 2.07 | (0.15) | 0.44 | (0.11) | −0.22 | (0.08) | 0.96 | (0.12) | 1.10 | (0.12) | 2.68 | (0.22) | 0.391 | 3 | |
| STI examination | 0.68 | (0.08) | 0.22 | (0.08) | −0.51 | (0.09) | 0.26 | (0.10) | 0.67 | (0.09) | 0.22 | (0.10) | −0.06 | (0.10) | −0.67 | (0.14) | −0.440 | 3 | |
| Pre-test counseling and partner notification | |||||||||||||||||||
| One-on-one counseling; self-disclosure | 0.32 | (0.10) | −0.08 | (0.12) | 0.07 | (0.14) | 0.37 | (0.21) | 0.38 | (0.13) | −0.72 | (0.15) | −0.30 | (0.16) | −0.58 | (0.22) | 0.735 | 1 | |
| Group counseling; self-disclosure | −0.14 | (0.10) | −0.86 | (0.10) | −0.63 | (0.12) | −0.44 | (0.11) | −0.43 | (0.10) | 0.76 | (0.14) | −0.30 | (0.13) | −1.09 | (0.22) | 0.175 | 1 | |
| One-on-one counseling; provider notification | −0.18 | (0.12) | −0.22 | (0.15) | −0.62 | (0.17) | −0.22 | (0.24) | 0.63 | (0.15) | −0.72 | (0.18) | −0.25 | (0.17) | 0.35 | (0.26) | 0.786 | 1 | |
| Group counseling; provider notification | −0.68 | (0.09) | −0.63 | (0.09) | −1.18 | (0.11) | −0.87 | (0.13) | −0.29 | (0.10) | 0.92 | (0.14) | −0.34 | (0.11) | −0.79 | (0.16) | 0.059 | 1 | |
| Couples counseling (automatic disclosure) | 0.69 | (0.20) | 1.80 | (0.28) | 2.36 | (0.30) | 1.17 | (0.45) | −0.28 | (0.26) | −0.24 | (0.29) | 1.19 | (0.29) | 2.11 | (0.38) | −1.755 | 1 | |
Notes: Estimates from a RELCL model with 8 preference classes and 3 class-independent random effects.
1, 2, and 3 indicate the corresponding attribute domain: λ1 – privacy and confidentiality; λ2 – invasiveness and perceived accuracy; λ3 – accessibility and value.
The data included 17760 choices of 740 participants, totaling 8880 rankings (12 best and second-best choices per participant yield 12 rankings * 740 participants = 8880 rankings). The latent preference classes range in size from 6% to 19% of participants; distributions are similar between female barworkers and male porters.
Class-specific preferences for each attribute level included in the DCE are described by the estimated (effects coded) coefficients. Results indicate substantial heterogeneity; the variation in parameters within and across classes, combined with a comparatively large number of classes, preclude the labeling of classes based on patterns of coefficients. All attribute levels except testing availability (which was constrained to be ordered), oral testing, and HIV testing only (without additional services) have positive and negative coefficients across classes, indicating that they are preferred by some groups of participants (classes) and disliked by others. The largest coefficient ranges across preference classes were observed for a health check alongside the HIV test, couples counseling with automatic disclosure of a positive HIV test result, the different testing venues, and preferences for venipuncture or finger prick.
The final column in Table 2 shows the effects of the random effects on utility. The estimated parameters (“loadings”) describe the extent to which the random effects amplify (same sign for the class-specific utility weight and the loading) or offset (different signs for the class-specific utility weight and the loading) the class-specific effect of each attribute level on utility. The effects differ greatly across attribute levels. The largest loadings were observed for (a) couples counseling and testing with automatic disclosure of a positive HIV test result, (b) oral swabs, and (c) venipuncture. The estimates indicate that for 3 out of 6 classes in which couples counseling was positively associated with utility, a difference of one standard deviation in random effect 1 more than offsets these utility gains. Similarly, a difference of one standard deviation in random effect 2 more than offsets the (average) aversion to oral swabs for all 8 classes and more than doubles the positive effect of venipuncture on utility among 4 out of 6 preference classes.
Given that the random effects have standard normal distributions, the absolute values of the loadings also characterize the variability of (class-specific) preference estimates across individuals. This individual-level heterogeneity, alongside the heterogeneity in preference profiles described by the latent classes, is visualized in Fig. 3. The distributions describe individual level relative preferences for (positive values) or aversion against (negative values) the respective characteristics evaluated in the DCE, conditional on predicted class membership (which is represented by different colors). The largest absolute loading in Table 2 (the λ value for couples counseling and testing with automatic disclosure) corresponds to the widest distributions of individual level preference estimates around class-specific means.
Fig. 3.
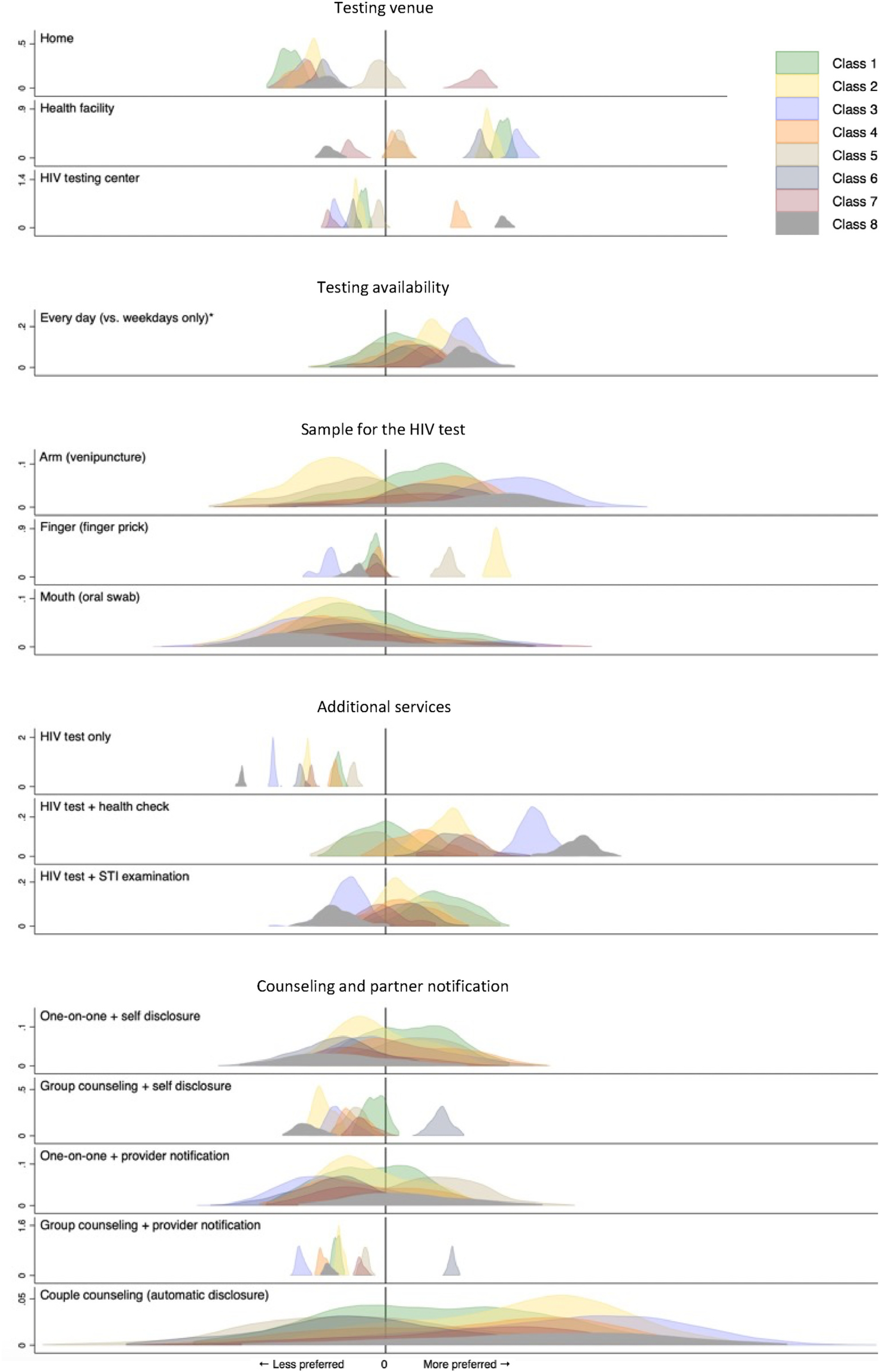
Visualization of between- and within-class preference heterogeneity; estimates from a random effects latent class logit model (N = 740) Notes: Distributions represent kernel densities of individual-level preference estimates conditional on modal class membership probability and individuals’ posterior scores on three domain-specific random effects. Each color represents one preference class. Within attribute levels, kernel densities were scaled in proportion to class size; y-axis scales vary across attribute levels. * Estimates for the weekdays only attribute level are symmetric around x = 0. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 3 documents systematic variation in estimated class membership probabilities with demographic characteristics and HIV testing history. Older, less educated, never testers were more likely to be members of Class 1; frequent testers were most likely to be members of classes 7 and 8. Whilst gender, and thus risk group, was associated with class membership (e.g., male porters were less likely to be members of classes 1, 3, and 4), the corresponding parameter estimates were smaller than those of variables describing testing history. Age, education, marital status, and testing history were significantly associated with the random effects for the confidentiality and privacy domain (λ1), while education and a prior HIV test were associated with the random effect for the invasiveness and perceived accuracy domain (λ2). Strong concordance was observed between study participants’ groupings into preference classes based on gender-specific vs. aggregate RELCL models (Appendix 1).
Table 3.
Correlates of latent class membership and individual-specific random effects (N = 740).
| Age | Some secondary school education | Married | Tested once | Tested more than once | Male porters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Class 2 | −0.08 | *** | (0.00) | −0.03 | (0.05) | 0.12 | * | (0.06) | 1.01 | *** | (0.09) | 0.67 | *** | (0.08) | 0.35 | *** | (0.06) | |
| Class 3 | −0.07 | *** | (0.00) | −0.49 | *** | (0.06) | 0.02 | (0.06) | 0.88 | *** | (0.10) | 0.62 | *** | (0.08) | −0.05 | (0.06) | ||
| Class 4 | −0.06 | *** | (0.00) | −0.29 | *** | (0.06) | 0.10 | (0.07) | 0.16 | (0.10) | −0.21 | ** | (0.08) | −0.13 | (0.07) | |||
| Class 5 | −0.02 | *** | (0.00) | −0.09 | (0.06) | −0.48 | *** | (0.07) | 0.41 | *** | (0.09) | −0.03 | (0.07) | 0.24 | *** | (0.06) | ||
| Class 6 | −0.09 | *** | (0.00) | 0.04 | (0.07) | −0.13 | * | (0.07) | 0.78 | *** | (0.11) | 0.64 | *** | (0.08) | 0.45 | *** | (0.06) | |
| Class 7 | −0.05 | *** | (0.01) | −0.23 | *** | (0.06) | 0.09 | (0.07) | 1.46 | *** | (0.12) | 1.11 | *** | (0.11) | 1.09 | *** | (0.08) | |
| Class 8 | −0.06 | *** | (0.01) | −0.52 | *** | (0.08) | −0.42 | *** | (0.08) | 1.51 | *** | (0.13) | 1.16 | *** | (0.11) | 0.39 | *** | (0.07) |
| λ1 | 0.01 | * | (0.01) | 0.14 | * | (0.07) | −0.29 | *** | (0.07) | −0.13 | (0.11) | −0.32 | ** | (0.09) | −0.02 | (0.07) | ||
| λ2 | −0.01 | (0.01) | −0.16 | * | (0.07) | 0.05 | (0.07) | 0.22 | * | (0.11) | 0.12 | (0.10) | −0.09 | (0.07) | ||||
| λ3 | 0.00 | (0.01) | −0.03 | (0.07) | −0.05 | (0.07) | 0.19 | (0.11) | −0.01 | (0.09) | 0.03 | (0.07) | ||||||
Notes: Coefficients and standard errors for correlates of class membership estimated using a bias-adjusted multinomial logit model; class 1 is the reference class; constants not shown. Coefficients and standard errors for correlates of individual-specific random effects estimated using linear regression models.
*, **, and *** indicate statistical significance at the 0.05, 0.01, and 0.001 levels, respectively.
4. Discussion
In this study of the HIV testing preferences of 300 female barworkers and 440 male Kilimanjaro mountain porters, two high-risk populations in Northern Tanzania, we identified substantial preference heterogeneity across individuals. Our findings provide strong support for the provision of an array of diverse HIV testing options in the study area that target the heterogeneous testing preferences of high-risk populations.
To our knowledge this study is the largest DCE of preferences for HIV counseling and testing focused on high-risk populations and on policy-relevant testing attributes. In addition, this study is the first to specifically focus on preference heterogeneity. While prior studies, including our own (Ostermann et al., 2014, 2015), have documented preference heterogeneity, the analysis of sources of heterogeneity was limited to systematic variation in mean preference parameters between population subgroups identified on the basis of covariates. This study uses a RELCL model to jointly characterize heterogeneity in preference profiles that are shared among groups of respondents (i.e., combinations of preferences that are likely to co-occur among the two high-risk populations) and additional individual-level heterogeneity that captures respondents’ unique attribute preferences, independent of class. Specifically, the latent classes capture some of the correlations among all the estimated part-worth utilities. Additionally, the three random effects, each linked to an attribute domain, and the corresponding loadings, further describe individual-level heterogeneity in the magnitude of the part-worth utilities and correlations among the part-worth utilities across attribute levels within attribute domains. Fig. 3 illustrates substantial variation in the distributions of the estimated preferences across attribute levels, classes, and individuals, thereby highlighting the distributional flexibility of the RELCL model employed in this study.
We acknowledge several limitations of the study. First, study participants were recruited from two high-risk populations in Northern Tanzania, and HIV testing options were described with only six characteristics. The number of attributes and levels presented could not cover all testing characteristics that might be important to a given participant. To ensure policy relevance, our selection of attribute levels was guided by actual and feasible characteristics across the 25 HIV counseling and testing providers in the study area. Other characteristics of HIV testing options may influence testing preferences and uptake in other settings and populations.
Second, while our study identified substantial preference heterogeneity, it was unable to discern the sources or consequences of this variation. Class membership probabilities and the distribution of random effects varied systematically with age, education, marital status, and HIV testing history (Table 3), however, the estimated distributions of the two distinct sub-populations across preference classes were nearly identical, there were no systematic differences in the distributions of random effects between barworkers and porters, and gender-specific models resulted in similar groupings of individuals as the aggregate model. While additional studies are needed to characterize the extent to which specific individual-level characteristics (e.g., knowledge and information, prior experiences with HIV testing, perceptions of HIV risk, anticipated consequences of a positive HIV test), correlate with preferences, our results suggest that a substantial share of preference heterogeneity may not be explainable by general demographic and risk characteristics. From a policy perspective, it may thus be more important to evaluate the extent to which heterogeneous population preferences align with the characteristics of existing testing options, and to explore associations with testing uptake among high-risk populations.
Third, we acknowledge several methodological limitations. These include the lack of experimental design software that would have allowed us to identify an experimental design optimized for a latent class model; the omission of interactions; and general limitations of DCEs, such as the potential for hypothetical bias (Quaife et al., 2018b).
5. Conclusion
This study describes substantial heterogeneity in preferences for HIV testing among two high-risk populations in Tanzania, including distinct preference profiles that are shared among groups of individuals, and additional, random variation across individuals. From a practical perspective, the study results provide strong support for the provision of an array of HIV testing options to target preference heterogeneity and maximize uptake of HIV testing among high-risk populations. The methods we describe may be applicable to other populations, settings, and choice contexts in which similar preference heterogeneity is suspected and can serve as a starting point for the systematic design of heterogeneity-focused interventions using stated preference methods.
Acknowledgements
The authors are grateful to the study participants and to the study research assistants, Martha Masaki, Beatrice Mandao, Elizabeth Mbuya, Honoratha Israel, Yombya Madukwa, Mohamed Mcharo, Upendo Nnko, Stephen Sikumbili, Edward Singo, Blandina Zenze, Leonia Rugalabamu, Suzan Kitomari, Stanny Komu, and Beldad Mmari, for input on study procedures and study implementation.
The authors thank the staff of the Kilimanjaro Clinical Research Institute, especially Professor Blandina Mmbaga, Dr. Aisa Shayo, and Zuhura Lintu, the University of South Carolina’s Arnold School of Public Health, especially the Department of Health Services Policy & Management and the Center for Health Care Quality, the Duke Global Health Institute and Duke University’s Center for Health Policy and Inequalities Research, for administrative support; and members of the Duke Center for AIDS Research and the study’s Scientific Advisory Board for feedback on study feasibility, design, analytic methods, and implementation.
Finally, the authors acknowledge Dr. Credianus Mgimba (Regional Medical Officer, Kilimanjaro Region), Dr. Best Magoma (former Regional Medical Officer, Kilimanjaro Region), Dr. Eligy Mosille (Regional AIDS Control Coordinator, Kilimanjaro Region), Ms. Dafrosa Itemba (Director, Tanzania Women Research Foundation), and members of the Moshi District Council administration, for their support of the study’s development and implementation.
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health in the United States under Award Numbers R01MH106388 and R21MH96631 and by the Duke University Center for AIDS Research (CFAR), an NIH funded program (P30AI064518). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix 1. Estimates from gender-specific models and strong concordance between class membership predictions from gender-specific vs. aggregate models
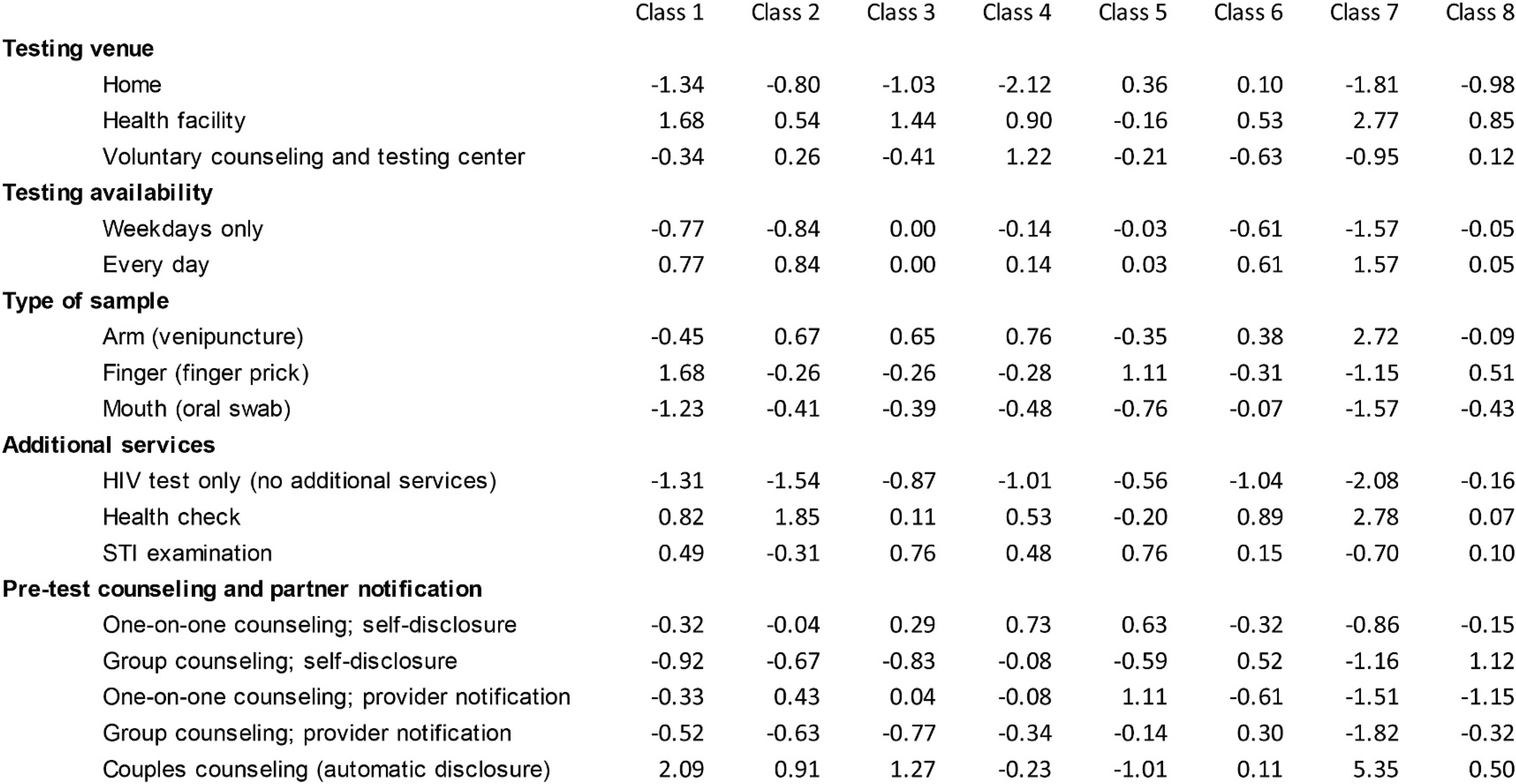
Panel A. Parameter estimates for female barworkers (N = 300).
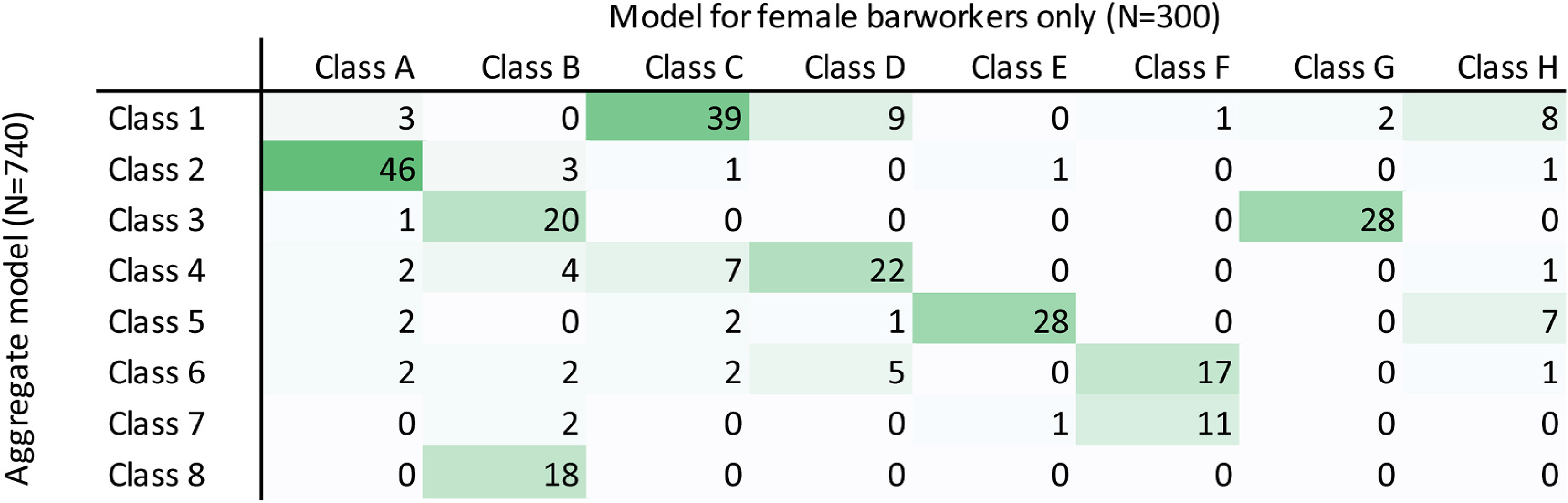
Panel B. Concordance between class membership predictions from gender-specific (N = 300 female barworkers only) vs. aggregate (N = 740) latent class models.
Panel C. Parameter estimates for male porters (N = 440).
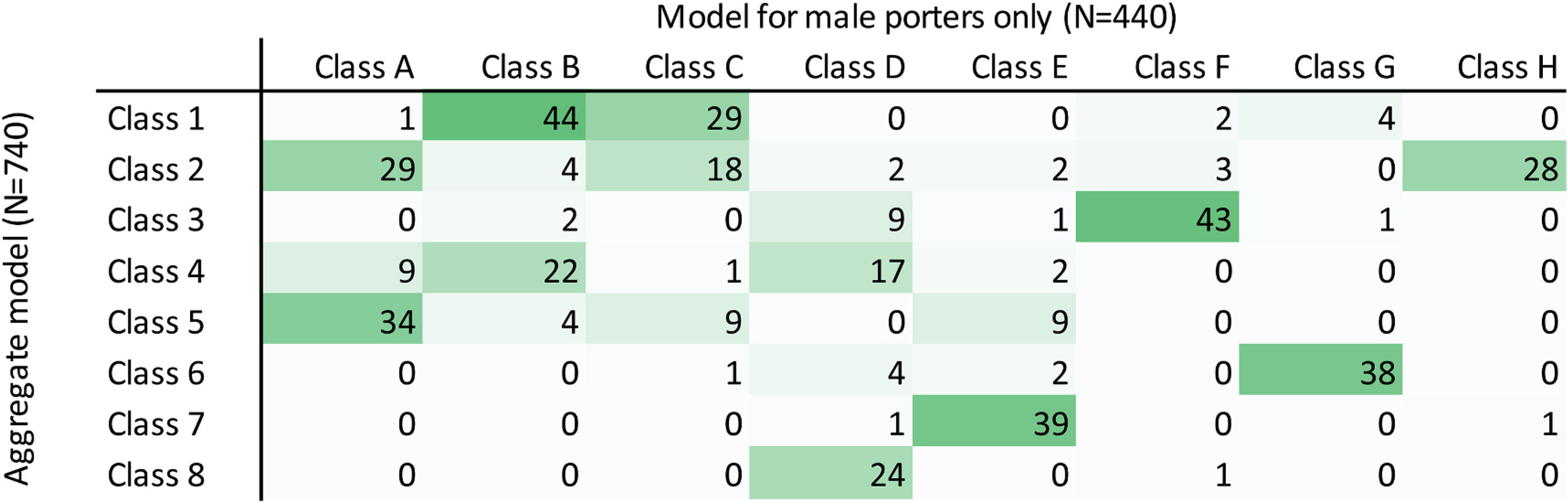
Panel D. Concordance between class membership predictions from gender-specific (N = 440 male porters only) vs. aggregate (N = 740) latent class models
Notes: Estimates are based on gender-specific RELCL models with the same specification as the aggregate model presented in Table 2 and Fig. 3. Class membership predictions are based on modal class membership probability.
Footnotes
Declaration of competing interest
The authors declare that they have no conflict of interests.
Ethics approvals
The protocol was registered in ClinicalTrials.gov (Protocol NCT02714140) on March 21, 2016. The protocol was approved by the Institutional Review Boards at Duke University (Duke University Health System IRB, Protocol Pro00075996) and the University of South Carolina (Health Sciences South Carolina IRB, facilitated review, Pro00060760) in the United States; as well as the Ethics Review Committee at Kilimanjaro Christian Medical University College (Protocols #273 and #901) and the National Institute for Medical Research (NIMR/HQ/R.8a/Vol. IX/1 363 and NIMR/HQ/R.8a/Vol. IX/2 603) in Tanzania.
References
- Bakk Z, Oberski D, Vermunt J, 2014. Relating latent class Assignments to external variables: standard errors for correct inference. Polit. Anal 22 (4), 520–540. [Google Scholar]
- Beusterien KM, Dziekan K, Schrader S, et al. , 2007. Patient preferences among third agent HIV medications: a US and German perspective. AIDS Care 19 (8), 982–988. [DOI] [PubMed] [Google Scholar]
- Bregigeon-Ronot S, Cheret A, Cabie A, et al. , 2017. Evaluating patient preference and satisfaction for human immunodeficiency virus therapy in France. Patient Prefer. Adherence 11, 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JF, Hauber AB, Marshall D, et al. , 2011. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health 14 (4), 403–413. [DOI] [PubMed] [Google Scholar]
- Brown LB, Miller WC, Kamanga G, et al. , 2011. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J. Acquir. Immune Defic. Syndr 56 (5), 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MP, Newman PA, Roungprakhon S, Scarpa R, 2013. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine 31 (36), 3712–3717. [DOI] [PubMed] [Google Scholar]
- Cherutich P, Golden MR, Wamuti B, et al. , 2017. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV 4 (2), e74–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou PY, Lin LC, Chen YM, et al. , 2015. The effects of early multiple-time PN counseling on newly HIV-diagnosed men who have sex with men in Taiwan. AIDS Behav. 19 (10), 1773–1781. [DOI] [PubMed] [Google Scholar]
- d’Elbee M, Indravudh PP, Mwenge L, et al. , 2018. Preferences for linkage to HIV care services following a reactive self-test: discrete choice experiments in Malawi and Zambia. AIDS 32 (14), 2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Olalla P, Molas E, Barbera MJ, et al. , 2015. Effectiveness of a pilot partner notification program for new HIV cases in Barcelona, Spain. PloS One 10 (4), e0121536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WH, Hensher DA, 2013. Revealing additional dimensions of preference heterogeneity in a latent class mixed multinomial logit model. Appl. Econ 45 (15), 1897–1902. [Google Scholar]
- Hauber AB, Mohamed AF, Watson ME, Johnson FR, Hernandez JE, 2009. Benefits, risk, and uncertainty: preferences of antiretroviral-naive African Americans for HIV treatments. AIDS Patient Care STDS 23 (1), 29–34. [DOI] [PubMed] [Google Scholar]
- Henley C, Forgwei G, Welty T, et al. , 2013. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex. Transm. Dis 40 (12), 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S, 2014. Chapter 14: latent class structures: taste heterogeneity and beyond. In: Hess S, Daly A (Eds.), Handbook of Choice Modelling. Edward Elgar Publishing. [Google Scholar]
- Indravudh PP, Sibanda EL, d’Elbee M, et al. , 2017. ‘I will choose when to test, where I want to test’: investigating young people’s preferences for HIV self-testing in Malawi and Zimbabwe. AIDS 31 (Suppl. 3), S203–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Kanninen B, Bingham M, Özdemir S, Bj K, 2007. Experimental design for stated choice studies. In: Valuing Environmental Amenities Using Stated Choice Studies. Springer, Dordrecht. [Google Scholar]
- Johnson FR, Ozdemir S, Phillips KA, 2010. Effects of simplifying choice tasks on estimates of taste heterogeneity in stated-choice surveys. Soc. Sci. Med 70 (2), 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahabuka C, Plotkin M, Christensen A, et al. , 2017. Addressing the first 90: a highly effective partner notification approach reaches previously undiagnosed sexual partners in Tanzania. AIDS Behav. 21 (8), 2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk ME, Riley PL, Palma AM, et al. , 2016. How can the health System retain women in HIV treatment for a lifetime? A discrete choice experiment in Ethiopia and Mozambique. PloS One 11 (8), e0160764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SE, Schoenbach VJ, Weber DJ, et al. , 1992. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N. Engl. J. Med 326 (2), 101–106. [DOI] [PubMed] [Google Scholar]
- Mangham LJ, Hanson K, McPake B, 2009. How to do (or not to do) ... Designing a discrete choice experiment for application in a low-income country. Health Pol. Plann 24 (2), 151–158. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Keane J, Laidlaw J, 2009. Making Success Work for the Poor: Package Tourism in Northern Tanzania. Arusha, Tanzania. Overseas Development Institute, SNV Connecting People’s Capacities. [Google Scholar]
- Mühlbacher AC, Stoll M, Mahlich J, Nübling M, 2013. Patient preferences for HIV/AIDS therapy - a discrete choice experiment. Health Econ Rev 3 (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RS, Feldacker C, Cesar F, et al. , 2016. Acceptability and effectiveness of assisted human immunodeficiency virus partner services in Mozambique: results from a pilot program in a public, urban clinic. Sex. Transm. Dis 43 (11), 690–695. [DOI] [PubMed] [Google Scholar]
- National AIDS Control Programme, 2017. Health Sector HIV and AIDS Strategic Plan (HSHSP IV) 2017–2022. Minstry of Health, Community Development, Gender, Elderly and Children. [Google Scholar]
- National AIDS Control Programme, 2019. In: National guidelines for HIV testing services in Tanzania. Minstry of Health, Community Development, Gender, Elderly and Children. [Google Scholar]
- Newman PA, Cameron MP, Roungprakhon S, Tepjan S, Scarpa R, 2016. Acceptability and preferences for hypothetical rectal microbicides among a community sample of young men who have sex with men and transgender women in Thailand: a discrete choice experiment. AIDS Behav. 20 (11), 2588–2601. [DOI] [PubMed] [Google Scholar]
- Njau B, Ostermann J, Brown D, Muhlbacher A, Reddy E, Thielman N, 2014. HIV testing preferences in Tanzania: a qualitative exploration of the importance of confidentiality, accessibility, and quality of service. BMC Publ. Health 14, 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann Jan, Mühlbacher Axel, Brown Derek, et al. , 2020a. Heterogeneous patient preferences for modern antiretroviral therapy: results of a discrete choice experiment. Value in Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Njau B, Brown DS, Muhlbacher A, Thielman N, 2014. Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PloS One 9 (3), e92100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Njau B, Mtuy T, Brown DS, Muhlbacher A, Thielman N, 2015. One size does not fit all: HIV testing preferences differ among high-risk groups in Northern Tanzania. AIDS Care 27 (5), 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Njau B, Hobbie A, et al. , 2020. Using discrete choice experiments to design interventions for heterogeneous preferences: protocol for a pragmatic randomised controlled trial of a preference-informed, heterogeneity-focused, HIV testing offer for high-risk populations. BMJ Open 10 (11), e039313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaty D, 2012. Kilimanjaro tourism and what it means for local porters and for the local environment. Journal of Ritsumeikan Social Sciences and Humanities 4, 1–12. [Google Scholar]
- Phillips KA, Maddala T, Johnson FR, 2002. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv. Res 37 (6), 1681–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife M, Eakle R, Cabrera Escobar MA, et al. , 2018a. Divergent preferences for HIV prevention: a discrete choice experiment for multipurpose HIV prevention products in South Africa. Med. Decis. Making 38 (1), 120–133. [DOI] [PubMed] [Google Scholar]
- Quaife M, Terris-Prestholt F, Di Tanna GL, Vickerman P, 2018b. How well do discrete choice experiments predict health choices? A systematic review and meta-analysis of external validity. Eur. J. Health Econ 19 (8), 1053–1066. [DOI] [PubMed] [Google Scholar]
- Rosenberg NE, Mtande TK, Saidi F, et al. , 2015. Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial. Lancet HIV 2 (11), e483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M, George GL, Rhodes BD, 2018a. Determining preferences related to HIV counselling and testing services among high school learners in KwaZulu-natal: a discrete choice experiment. AIDS Behav. 22 (1), 64–76. [DOI] [PubMed] [Google Scholar]
- Strauss M, George G, Mantell JE, et al. , 2018b. Stated and revealed preferences for HIV testing: can oral self-testing help to increase uptake amongst truck drivers in Kenya? BMC Publ. Health 18 (1), 1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzania Commission for AIDS, 2018. Tanzania HIV Impact Survey (THIS) 2016–2017: Final Report. Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC). https://phia.icap.columbia.edu/countries/tanzania/. Accessed 15-Apr-2021. [Google Scholar]
- Terris-Prestholt F, Hanson K, MacPhail C, Vickerman P, Rees H, Watts C, 2013. How much demand for new HIV prevention technologies can we really expect? Results from a discrete choice experiment in South Africa. PloS One 8 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divergent paths to the end of AIDS. In: The Lancet (Ed.), Lancet HIV 4 (9), e375. [DOI] [PubMed] [Google Scholar]
- Udeagu CC, Shah D, Shepard CW, Bocour A, Guiterrez R, Begier EM, 2012. Impact of a New York City Health Department initiative to expand HIV partner services outside STD clinics. Publ. Health Rep 127 (1), 107–114. [PMC free article] [PubMed] [Google Scholar]
- Udeagu CC, Bocour A, Shah S, Ramos Y, Gutierrez R, Shepard CW, 2014. Bringing HIV partner services into the age of social media and mobile connectivity. Sex. Transm. Dis 41 (10), 631–636. [DOI] [PubMed] [Google Scholar]
- UNAIDS, 2014. 90–90-90. An Ambitious Treatment Target to Help End the AIDS Epidemic. UNAIDS. Accessed August 7, 2018. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. [Google Scholar]
- United Republic of Tanzania, 2012. Tanzania Population and Housing Census. National Bureau of Statistics. https://www.nbs.go.tz/nbs/takwimu/census2012/Tanzania_Total_Population_by_District-Regions-2016_2017r.pdf. Published 2012. Accessed December 1, 2019. [Google Scholar]
- Zanolini A, Sikombe K, Sikazwe I, et al. , 2018. Understanding preferences for HIV care and treatment in Zambia: evidence from a discrete choice experiment among patients who have been lost to follow-up. PLoS Med. 15 (8), e1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Bridges JF, 2019. Explore preference heterogeneity for treatment among people with Type 2 diabetes: a comparison of random-parameters and latent-class estimation techniques. Journal of Choice Modelling 30, 38–49. [Google Scholar]


