Abstract
Alzheimer’s disease (AD) and vascular dementia are two of the most prevalent dementias that afflict the aging population in the United States (US). Studies have made great strides in understanding the neuropathology of these diseases; however, many studies are conducted in the context of non-Hispanic whites (NHWs), and few include the rapidly growing underrepresented populations that reside in the US. We sought to characterize current knowledge of the neuropathologic landscape of AD and vascular dementia of the largest growing US minority groups, namely Latinos/Hispanics, Black Americans, and Asian Americans, compared with NHWs being the majority group. It is vital to note these historic categories are social constructs and cultural and social associations may underlie differences. We conducted a literature search utilizing specific criteria to yield neuropathology papers that addressed the demographics and neuropathologies of relevance, then collated the findings into this review. We reveal that while there has been much progress in neuropathological research involving Latinos/Hispanics and Black Americans in the past decade, no cohesive conclusions could be extrapolated from the existing data due to the dearth of minority participants and even smaller amount of information related to the heterogeneity within each minority group, especially Latinos/Hispanics. Furthermore, we reveal an even greater scarcity in neuropathological studies involving Asian Americans, also a very heterogeneous group. We hope the presented findings will illuminate the paucity of minority representation in not just neuropathological research but the field of clinical research overall and serve to inspire clinicians and researchers to help reduce the health disparities underrepresented groups in the US face.
Keywords: Disparities, Dementia, Neuropathology, Hispanic, Latino, Asian, African American, Brain
Introduction
Clinically, Alzheimer’s disease (AD) is defined as a type of dementia distinguished by neurodegeneration that results in memory loss and deterioration of cognitive functions24, 38. Neuropathologically, AD is defined by aggregations of the amyloid-β (Aβ) protein, in the form of Aβ plaques, and tau protein, in the form of neurofibrillary tangles (NFTs)13, 25, 97. In addition to Aβ plaques and NFTs, neuropathologies associated with vascular dementia can also be concomitant57, 78, 101. Vascular dementia is the second most common cause of dementia following AD and neuropathologically can manifest as infarcts and hemorrhages as well as other vascular pathologies such as cerebral amyloid angiopathy (CAA) and arteriolosclerosis9, 17, 23, 63, 90, 101. While there has been progress with understanding disease phenotype (for review see 89) and progress with therapies having evidence of targeting and reducing Aβ plaques in the brain, there have yet to be treatments that fully cure or stop the progression of the disease20, 22. Although great strides have been made to combat this disease, most studies have focused on select populations or cohorts, specifically composed of persons identifying as non-Hispanic whites (NHWs)28, 43, 73, 97.
The population of persons age 65 and over in the United States (US) has significantly increased in the past decade—as much as a 36% increase2. US ethnoracial minorities made up 20% of this age demographic at the beginning of the decade, and increased to 24% by the end of it2. The NHW population of age 65 and over is projected to increase 29% by 2040 in comparison to the 115% increase of the ethnoracial minority population2. The largest US minority population is Latinos/Hispanics, followed by Black Americans, then Asian Americans, which are the fastest growing demographic18, 53. Other notable underrepresented groups are American Indians (Native Americans), Native Hawaiians, and Pacific Islanders4. It is important to note in this paper we will utilize historic terms (such as those within the US census) and these categories are social constructs and cultural and social associations may underlie differences. Diversity in studies maximizes variability in risk and protective factors. This can aid in studying clinically relevant transitions across the spectrum of a disease with the goal of identifying modifiable pathways to support maintenance of normal functions. As racial/ethnic groups in the US continue to grow in addition to longer life expectancies, more diverse elderly individuals will be seen for diagnosis and treatment of neurodegenerative diseases; thus, more research reflecting the population diversity is needed so that prevention, treatment, and prognosis strategies encompass all who are affected by the devastating impact of dementia.
Heterogeneity of race and ethnic categories
Race and ethnicity are two terms fundamentally distinct from one another. Race historically has been used to describe the physical traits of an individual such as their eyes, hair, and skin, whereas ethnicity has been used to describe an individual’s cultural identity74. It is essential to remember these terms are socially constructed and hold no bearing on an individual’s biology. The categorizations for race and ethnicity in the US have evolved over time as self-identification shifts, immigration, and mixed racial heritage became more prevalent1.The term “race” has regretfully implied a sole focus on an individual’s morphology, and historically has not accounted for other background variables such as geographic origin, environmental factors, and sociocultural characteristics that influence these differences43, 98. While the historic terms employed in this review are not optimal given how restrictive and tentative they are, the U.S. Census uses them, which many neuropathology studies have utilized as well, and thus are presented as such in this review. Thankfully, there is a growing awareness that these historic terms alone do not aptly stratify these individuals in a scientific context and new approaches are being encouraged1, 6, 43, 49.
Furthermore, it is important to recognize there is heterogeneity within racial/ethnic categories. The terms Latinos and Hispanics are sometimes used interchangeably65. The term Hispanics historically has been used to describe those who are from Spain or other Spanish-speaking country, while Latino signifies those who originate from Latin America, regardless of their spoken language62. Moreover, it is noted the term Caribbean Hispanics is used to describe the Latino population that resides in Puerto Rico, Dominican Republic, and Cuba96. To further expand on distinctions within the Latino/Hispanic population of the US, some studies have partitioned this ethnicity based on area of decedents’ self-reported origin: Mexican, South American, Central American, and Caribbean99, 100. These categories are standardized by the US Census; hence studies have followed the same format64. The Mexican descendent population is the largest Latino/Hispanic group in the US and is spread out through the country, with more density in the Southwest27. Individuals from South and Central America also follow this distribution pattern, whereas Caribbean Latinos/Hispanics are more concentrated in eastern states3. These geographical distributions when examining AD cohorts can be immensely important as there have been reported socioeconomic and cultural differences within these groups that are associated with risk factors for AD77.
There is also heterogeneity within the Black American population. The largest subgroup within the US Black population is single-race non-Hispanic, comprising of 87% of the total US Black population95. The following largest is the multiracial non-Hispanic population, constituting 8% of the overall US Black population, with Black Hispanics making up the remainder 5%95. The majority of Black Americans are of West/Central African and European heritage, and some also have Native American roots34. An estimated 90% of the total Black population was born in the US in which most are descendants of enslaved people84, 94. Despite the Great Migration where the Black population had dispersed to areas in the US away from the South, the distribution in the South has begun to grow in the past few decades92. Another observed migration pattern in the last few decades has been a substantial increase among the foreign-born US Black population, making up 10% of the current US Black population in which the majority of foreign-born US Black persons, 88%, were born in African or Caribbean nations94.
Similarly, there is immense diversity within the Asian American population. There are 21 distinct Asian subgroups living in the US according to gathered data from the Census American Community Survey, with the largest being Chinese (including Taiwanese), followed by Asian Indian, Filipino, Vietnamese, Korean, and Japanese15, 16. Almost half (45%) of Asian Americans live in the western US, with nearly one third of the nation’s population living in California alone (30%), while 24% reside in southern states, 19% in the northeast, and 12% in the midwest15. Data have shown a substantial proportion of Chinese, Filipino, Vietnamese, Korean, and Japanese inhabit the western US, amounting to at least 43% distribution across all groups46. On the other hand, Asian Indians occupied the northeastern and southern regions of the US more frequently, accounting for over 29% distribution for each region46. By addressing the geographic, ethnic, and cultural variations that exist among race and ethnic subgroups, AD research can be refined to yield more precise methodology and analyses.
Differences in clinical AD based on race and ethnicity
Differences in the prevalence and incidence of clinically defined AD and related dementias (ADRD) have been observed when comparing across race/ethnicity. AD prevalence is highest in Black Americans and Latinos/Hispanics, followed by NHWs, and then Asian Americans21, 33, 40, 41, 61, 66-68, 70, 72, 79, 86, 96. Notably, one study showed US-born NHWs, Hispanics, and other uncategorized races had lower frequency of dementia compared to their immigrant counterparts, except for non-Hispanic Blacks (NHBs), where it was an inverse correlation72. Studies have also shown differences in dementia prevalence between Latino subgroups. One study revealed a 4.8% prevalence of dementia among Mexican Americans ≥65 years residing in Sacramento County, California, with up to 31% prevalence in those aged 85 years or older42. In contrast, a study of Caribbean Hispanics residing in North Manhattan estimated a dementia prevalence for individuals aged 65-74 of 7.5%, 27.9% for those 75-84, and 62.9% for those 85 and older41. Latinos/Hispanics have been shown to have an earlier age of onset of AD when compared to NHWs and Black Americans, though the difference was marginal for the latter in some cohorts21, 33, 42, 61, 72. Both Latinos/Hispanics and Black Americans have a high prevalence of cardiovascular risk factors associated with ADRD, while prevalence of these risk factors is lower among NHWs followed by Asian Americans40, 42, 61, 67, 96, 102. In a California study that examined dementia incidence in Asian American subgroups, Filipino Americans had the highest incidence rate at 17.3 per 1000 person-years, and South Asian Americans (i.e., Asian Indian, Pakistani, Bangladeshi, Sri Lankan, or Nepalese) had the lowest rate at 12.1 per 1000 person-years67.
While many observable trends have been correlated with AD from a clinical perspective across different race/ethnicities, they do not confirm the presence of the hallmark protein aggregates that are currently are the gold standard for diagnoses of ADRD, in other words, the neuropathology. Do the neuropathological profiles of individuals with ADRD of the three largest minority groups in the US differ, as there have been noted cultural and geographic differences in these groups? Neuropathology studies have been conducted on predominantly NHW cohorts, so little is known about ADRD in these other racial/ethnic groups6, 28, 43, 73. For this review, we will discuss the current landscape of neuropathological findings in AD and vascular dementia in Latinos/Hispanics, Black Americans, and Asian Americans. We seek a more comprehensive understanding of the disease profile in these groups of individuals to provide improved diagnoses and develop effective countermeasure therapies or methods to allay the risk of AD and vascular dementia.
Methods
Inclusion and exclusion criteria
All literature in this review focused on signature pathologies associated with AD and vascular dementia in Latino/Hispanic, Black American, and Asian American cohorts. Studies meeting the inclusion criteria were published 1995 and onwards, peer-reviewed, specifically presented AD or vascular dementia neuropathological findings, and were conducted in the US. Neuropathologic evaluations of interest included but were not limited to Braak neurofibrillary tangle (NFT) stage, Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuritic plaque score; Thal amyloid phase; and National Institute of Aging and Alzheimer’s Association (NIA-AA) criteria of overall AD diagnosis based on neuropathologic changes13, 48, 71, 97. For vascular dementia, pathologies of interest included, but were not limited to, infarcts, hemorrhages, arteriolosclerosis, atherosclerosis, and CAA. As for staging schemes and creating a consensus for diagnosing vascular dementia, there have been multiple attempts23, 55, 83, 90, 92; however, there is no universally used system in place. Exclusion criteria included papers that contained only living cohorts (i.e., cohorts not having neuropathologic evaluation of persons after death) presented data with no mention of AD neuropathologies specifically (e.g., genotyping, neuropsychological tests, etc.), studies that were not conducted on human subjects, and articles that were not sourced from peer-reviewed journals.
Search strategy
The literature review was conducted by searching specific MeSH terms in PubMed, Scopus, and Web of Science to yield the peer-reviewed articles investigating AD and vascular neuropathology among Latinos/Hispanics, African Americans/Black Americans, and Asian Americans on November 19, 2021. The MeSH search term refinement process for these databases was guided by a UC Davis Health medical librarian. The full list of search terms can be found in the supplemental methods at the end of this review.
Results
Neuropathologic findings on US minority groups were often compared to NHWs. For the purposes of this review, we will use Latinos/Hispanics as the collective term to describe this demographic unless a mentioned study further partitions out subgroups, in which the terms that are consistent with the study will be used. This same principle applies to the term Black Americans being used for mentions of this group unless the study denotes otherwise. Details of selected main papers are within Table 1 relating to neuropathological data of Latinos/Hispanics and/or Black Americans as many of these studies compared these groups. Additional studies on Asian Americans are in Table 2.
Table 1.
Literature with neuropathological data of Latinos/Hispanics and/or Black Americans. For the ethnicity/race column, the mentioned terms used in each respective paper stated are followed with the universal synonym in parentheses to provide consistency.
|
Citation (PMID) |
Cohort and location* | Numbers of Ethnicity/Race(s) examined | Total cohort size |
Pathologies examined |
Inclusion/Exclusion Criteria | Main findings |
|---|---|---|---|---|---|---|
|
Sandberg G, …, Troncoso JC. Neurobiol Aging, 2001. (11182466) |
Maryland ME office; study carried out at University of Maryland |
☐ 58 African Americans (Black Americans) ☐ 80 Whites (NHWs) |
138 |
☐ AD |
Inclusion
|
|
| Wilkins CH, …, Morris JC. Arch Neurol, 2006. (16401740). | Washington University ADRC; greater metropolitan St. Louis, MO |
☐ 10 African Americans (Black Americans) ☐ 10 Whites (NHWs) |
20 |
☐ AD ☐ CVD ☐ LBD |
Inclusion
|
|
|
Riudavets MA, …, Troncoso JC. J Neuropathol Exp Neurol 2006. (17146288) |
Maryland ME office; study carried out at University of Maryland |
☐ 100 Blacks (Black Americans) ☐ 100 Whites (NHWs) |
200 |
☐ AD ☐ CVD |
Inclusion
|
|
| Mehta KM, ..., Miller BL. Neurology, 2008. (18003939)# | >30 ADCs; NACC database |
☐ 1,301 Latinos (Hispanics) ☐ 3,563 African Americans (Black Americans) ☐ 451 Asian ☐ 162 American Indians ☐ 25,160 Whites (NHWs) |
30,916 (3,017 with npath) |
☐ AD ☐ CVD |
Inclusion:
Exclusion:
|
|
| Ringman JM, ..., Vinters HV. JAMA Neurology, 2014. (24797962)* | >30 ADCs; NACC database |
☐ Hispanic (Latinos) ☐ African Americans (Black Americans) ☐ Whites (NHWs) |
425 |
☐ AD ☐ CVD |
Inclusion
Exclusion
|
|
| Barnes LL, …, Schneider JA. Neurology, 2015. (26180136). | Rush University, ADRC Chicago, Illinois |
☐ 41 Blacks (Black Americans) ☐ 81 Whites (NHWs) |
122 |
☐ AD ☐ CVD ☐ LBD |
Inclusion
|
|
| Graff-Radford NR, …, Dickson DW. Alz Dement, 2016. (27094726) | 32 past/present ADCs; NACC database |
☐ 110 African Americans (Black Americans) ☐ 2,500 White (NHWs) |
2,610 |
☐ AD ☐ CVD ☐ LBD ☐ TDP/FTD |
Inclusion
Exclusion
|
|
| Kamara DM, …, Walker LC. J Alzheimers Dis, 2018. (29614657). | Emory ADRC Atlanta, GA |
☐ 18 African Americans (Black Americans) ☐ 19 Caucasians (NHWs) |
37 |
☐ AD ☐ CVD |
Inclusion
|
|
| Soria JA, …, Rissman RA. J Alzheimers Dis, 2018 (30412501) | UCSD ADRC San Diego, California |
☐ 53 Latinos (Hispanics) |
53 |
☐ AD ☐ CVD ☐ LBD |
Inclusion
Exclusion
|
|
| Filshtein TJ, …, DeCarli C. J Alzheimers Dis, 2019. (30775996) | University of California, Davis (UCD) ADC; Sacramento, CA |
☐ 28 Hispanic (Latinos) ☐ 35 Black (Black Americans) ☐ 360 NHWs |
423 |
☐ AD ☐ CVD |
Inclusion:
|
|
| Santos OA, ..., Murray ME. Alz Dement, 2019. (30792090) | Florida Autopsies Multi-Ethnic cohort (FLAME) State of Florida brain bank |
☐ 67 Hispanic (Latinos) ☐ 19 African Americans (Black Americans) ☐ 1,539 Caucasian (NHWs) |
2,809 |
☐ AD ☐ LBD |
Inclusion:
Exclusion
|
|
| Weissburger GH, ..., Salmon DP. J. Alzheimers Dis., 2019. (30636736) | UCSD ADRC San Diego, California |
☐ 14 Hispanic (Latinos) ☐ 20 NHWs |
34 |
☐ AD ☐ CVD |
Inclusion
Exclusion
**Insulin-dependent diabetes, major stroke or neurological illness, or self-reported alcohol or drug abuse |
|
# for Mehta et al. numbers of Ethnicity/Race(s) examined represent deceased persons, only a subset of cases (n= 3,017) had neuropathology data--npath details of ethnoracial group were not listed. *for Ringman et al. data within paper not sufficient to derive numbers of Ethnicity/Race(s) examined with neuropathologic evaluations. ** previous UCSD ADRC cohort studies state this exclusion, although not stated directly within paper. Abbreviations: AD=Alzheimer’s disease, ADC/ADRC= Alzheimer’s Disease (research) Center, CAA=cerebral amyloid angiopathy, CVD=Cerebrovascular disease related pathologies, DLB=Dementia with Lewy Bodies, DRS=Dementia rating scale, dx=diagnosis, NACC=National Alzheimer’s Coordinating Center, ME= medical examiner, npath=neuropathology, LBD= Lewy body dementia, FTD=Frontotemporal dementia, NFT=neurofibrillary tangles, NHW=Non-Hispanic Whites, UCSD=University of California San Diego. *Cohort name was listed if given and location includes medical center/institution/data source.
Table 2.
Literature of neuropathological data of Asian Americans. For the ethnicity/race column, the mentioned terms used in each respective paper stated are followed with the universal synonym in parentheses to provide consistency.
|
Citation (PMID) |
Cohort and location* | Numbers of Ethnicity/Race(s) examined | Cohort size | Pathologies examined | Inclusion Criteria | Main findings |
|---|---|---|---|---|---|---|
| White L, …, Markesbery W. Ann N Y Acad Sci, 2002. (12480729) | HAAS, Oahu, HI |
|
285 |
☐ AD ☐ CVD ☐ LBD |
Came to autopsy between 1991 to 1999 |
|
| Petrovitch H, …, White LR. Ann Neurol 2005. (15562458) | HAAS, Oahu, HI |
|
333 |
☐ AD ☐ CVD |
|
|
| White L. J Alzheimers Dis. 2009. (19661625) | HAAS, Oahu, HI |
|
443 |
☐ AD ☐ CVD ☐ LBD |
Came to autopsy between 1992 to 2004 |
|
|
Launer LJ, …White LR Ann Neurol 2011. (22162060) |
HAAS, Oahu, HI |
|
436 |
☐ AD ☐ CVD |
Came to autopsy between 1992 and 2001 |
|
| White LR, …, Montine TJ; Neurology, 2016. (26888993) | HAAS, Oahu, HI and the Nun Study (NS) from Montreal, Canada |
|
1,108 |
☐ AD ☐ CVD ☐ LBD |
|
|
| Latimer CS, … Montine TJ. J Neuropathol Exp Neurol, 2017. (28499012) | HAAS, Oahu, HI and the Nun Study (NS) Montreal, Canada |
|
1,262 |
☐ AD ☐ CVD ☐ LBD |
Completed NIA-AA ABC scores |
|
All published studies within table included individuals from the Honolulu Asian Aging Study (HAAS) which is a continuation of the Honolulu Heart Program54. Persons within HAAS consisted of Japanese American men born between 1900 and 1920 living in Oahu during baseline examination in 1965. Participants were evaluated for dementia starting in 1991 (age 73 to 94 years), the autopsy program began in 1992. Abbreviations: AD=Alzheimer’s Disease, CVD=Cerebrovascular disease, Hpscl=Hippocampal Sclerosis, LBD=Lewy body disease, NP=neuritic plaques, NFT=Neurofibrillary Tangle
AD and vascular dementia neuropathology in Latino/Hispanics
One observed neuropathological trend in Latinos/Hispanics was cerebrovascular pathologies, such as infarcts, CAA, arteriolosclerosis, and atherosclerosis, that were typically associated with dementia and/or AD diagnoses (See Table 1). A 2010 study using the National Alzheimer’s Coordinating Center (NACC) database showed Latinos/Hispanics were more likely to have neurovascular pathology compared to NHWs70. In persons with dementia during life, studies conducted at the Alzheimer’s Disease Research Centers (ADRCs) at both University of California, Davis (UCD) and the University of California, San Diego (UCSD) reported a higher frequency of concomitant neurovascular pathologies compared to NHWs29, 102. One comparison of interest between these studies with respect to cerebrovascular disease (CVD) pathologies—specifically microinfarcts and macroinfarcts—is Filshtein et al. found occurrence was higher in Latinos/Hispanics compared to NHWs in the UCD cohort29, while Weissburger et al. discovered there were no significant differences between the two groups in their UCSD cohort102. This contradiction may be because persons with evidence of in-vivo hemorrhages, strokes, and other major agonal infarcts were excluded from the UCSD study102. A study also using the National Alzheimer’s Coordinating Center database illustrated this theme in the specific context of CAA, in which Latino individuals with neuropathologically confirmed AD were more probable to have severe CAA than NHWs81. The study done by Weissburger et al. also supported this trend102. Another study by UCSD further validated this pattern by finding higher CAA burden in the AD group compared to the no pathology group, which was defined as not having significant brain pathologies, and the non-AD pathology group was defined as only have tauopathies, frontotemporal dementia (FTD), progressive supranuclear palsy (PSP), dementia with Lewy bodies (DLB), or Parkinson’s Disease (PD) with neocortical Lewy bodies, in an all-Latino cohort91.
As for hallmark AD pathologies, Aβ plaques and NFTs, there were more inconsistent patterns between Latinos/Hispanics and NHWs. The study done by Filshtein et al. utilizing demented cases from the Alzheimer’s Disease Center at UCD, a California based cohort, revealed Latinos/Hispanics had the lowest occurrence of AD clinicopathological diagnosis without the involvement of CVD, including lower frequencies of persons at higher Braak NFT stage compared to NHWs and Black Americans29. This is consistent with literature where Latino cohorts tended to exhibit concomitant neurovascular pathologies with their AD diagnoses29, 102. Conversely, Santos et al., in a Florida based cohort, demonstrated Latinos/Hispanics were twice as likely to have a higher Braak NFT stage than NHWs86, while a study conducted by Mehta et al., including cases with a clinical possible/probable diagnoses of AD, revealed Braak NFT stage did not differ significantly between Latinos/Hispanics and NHWs70. The study by Weissburger et al. also showed both groups (NHWs and Latinos/Hispanics) had similar Braak NFT stage102. Regarding plaques, which can include neuritic plaques (amyloid plaques containing dystrophic neurites) in some literature, Mehta et al. found neuritic plaques were more frequent in Latinos/Hispanics compared to NHWs70. However, the results by Filshtein et al. revealed Latinos/Hispanics had the lowest proportion of CERAD frequent neuritic plaque score, implying that neuritic plaques may not be as much of a major contributing pathology to their dementia29. Santos et al., excluding persons that did not have autopsy confirmed AD and cases with known mutations, opted to use Thal amyloid phase to categorize plaque presence, in which the phases did not differ between Latinos/Hispanics and NHWs86. Notably, Latino/Hispanic participants in these studies may represent diverse ethnic groups in terms of geography and nation of origin leading to seemingly contradictory findings. For instance, Santos et al. had utilized the Florida Autopsies Multi-Ethnic (FLAME) cohort located at the Mayo Clinic of Florida for their study, which consisted of individuals primarily from the Caribbean origin for their Latino group, whereas Weissburger et al. and Soria et al. had utilized cohorts from the UCSD ADRC, which comprised of individuals primarily of Mexican descent for their Latino/Hispanic group86, 91, 102. Furthermore, these studies also had slightly different inclusion and exclusion criteria, as outlined in Table 1, that may also contribute to discrepancies.
AD and vascular dementia neuropathology in Black Americans
Like Latinos/Hispanics, cerebrovascular pathologies are also commonly observed in Black Americans, but the pattern is not completely consistent which may be due to cohort inclusion/exclusion criteria, demographic locations, and recruitment strategies. A study at the Rush Alzheimer’s Disease Clinical Core, based in the Chicago Illinois area, revealed Black decedents had significantly greater severity in both atherosclerosis and arteriolosclerosis when compared to NHWs7. The study by Filshtein et al. corroborates this finding, in which their results of persons with dementia demonstrated Black participants had a higher proportion of CVD compared to NHWs29. Another study utilizing patient data from over 30 Alzheimer’s disease centers across the country also found Black Americans were more likely to have had a contributing diagnosis of vascular dementia than NHWs, although this study was based on small group numbers and did not account for center biases40. Interestingly, Mehta et al. revealed Black Americans had similar neurovascular pathology rates as NHWs on autopsy, in contrast to the consensus of the other studies70. Likewise, the results from a study on a cohort based in Washington University’s Alzheimer’s Disease Research Center (ADRC) also showed no differences in cerebrovascular infarcts between the NHW and Black American participants107. Multiple studies found CAA burden did not differ significantly between Black Americans and NHWs56, 81, 82. In contrast, Graff-Radford et al. observed Black Americans had significantly greater frequencies of CAA in addition to the other vascular neuropathologies (i.e., infarcts, hemorrhages, arteriolosclerosis, atherosclerosis) in comparison to NHWs40; however this study utilized data from multiple cohorts and did not control for center biases.
For AD pathologies, there were also contradictions in the literature. Findings from Barnes et al. revealed Black decedents were less likely to have AD-only pathology, defined by neuritic plaques and NFTs as the single contributing pathology to their dementia diagnosis compared to NHW decedents7. Along similar conclusions, Filshtein et al. demonstrated mixed pathologies were more common in Black decedents than in NHW decedents29. With respect to hallmark AD proteinopathy comparisons, such as neuritic and diffuse plaque counts, Thal amyloid phase, and likelihood of higher Braak NFT stage, multiple studies showed no significant neuropathological differences in both categories for either patient demographic82, 85, 86, 107. In contrast, more than one study demonstrated Black American decedents were more likely to exhibit higher Braak NFT stage29, 40, 70. Graff-Radford et al. also indicated Black American participants had greater CERAD-frequent scores for neuritic and diffuse plaques40, whereas Mehta et al. revealed Black Americans had similar neuritic and diffuse plaque counts as NHWs70. Both studies had utilized data from the NACC database, but this conflict in findings may be due to the fact the Mehta et al. study had a larger sample size from the longitudinal window from 1984 through 200570, compared to the Graff-Radford et al. study which recruited data from a smaller sample size from 2005 to 201540. As with the previous section, discrepancies may lie within cohort selection criteria as highlighted in Table 1.
AD and vascular dementia neuropathology in Asian Americans
AD and vascular dementia pathological trends for Asian Americans compared to NHWs are largely unexplored for all pathological categories as literature for this minority group in this specific context is still sparse. Of the studies conducted, most have focused on Japanese Americans, specifically men, through the Honolulu Asian Aging Study (HAAS) which includes very few, if any other subgroups. The HAAS was established in 1991 and comprised surviving participants of the Honolulu Heart Program, a prospective, community-based cohort study of heart disease and stroke established in 196536, 54, 93, 105. For neurovascular pathologies, a study revealed microinfarcts were significantly more common in Japanese American men in HAAS compared to Caucasian women in the Nun Study (NS)106. Another finding from the HAAS showed that the frequency of microvascular lesions as the contributing dementia pathology was nearly the same as AD pathologies104; however, a later paper relative to this one showed that microvascular infarcts as the dominant or exclusive contributing lesion to dementia were the most frequent among decedents, then followed by AD lesions103. There were additional findings on the HAAS revealing dementia frequency increased with neuritic plaques in decedents with NFTs and even further with CVD lesions76. Interestingly, one analysis showed microinfarcts were strongly associated with poor cognitive function score in non-demented individuals, whereas NFTs were strongly associated with poor cognitive function score in demented individuals59. For neuropathologic change involving AD proteinopathies, a more recent study showed that the HAAS was more resistant to Aβ accumulation, but the NS was more resistant to neurofibrillary degeneration for individuals without Aβ accumulation58.
Native American, Alaska Native, Native Hawaiian, other groups, and points of further research
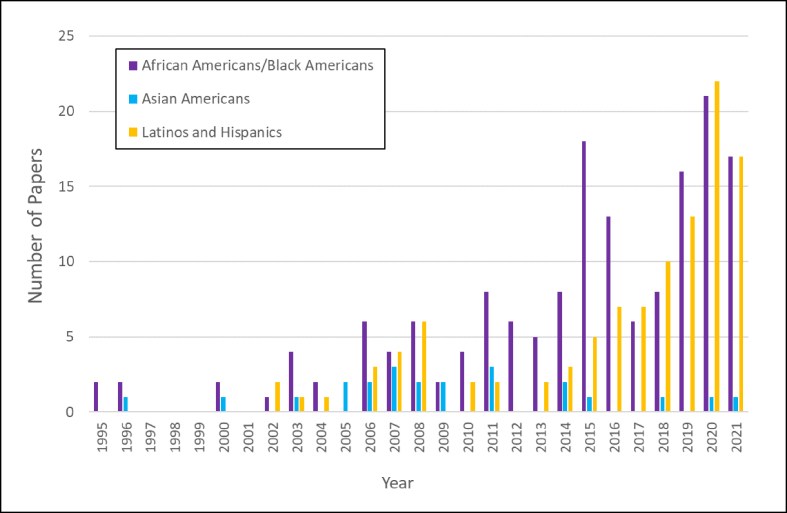
The scope of this paper had focused on the neuropathology of the three largest minority groups of the US, as those were the demographics that offered adequate findings to collate into a cohesive and purposeful review. The current presented literature offers a foundation for AD and vascular dementia research in underrepresented US groups and seems to only expand each year (see Figure 1), with more research being conducted on a greater variety of cohorts and sites. The existing findings are concentrated and substantial enough to serve as preliminary data for comparison of future findings, depending on the objective demographic. Nonetheless, despite the upward trends of more AD and vascular dementia research being conducted in diverse cohorts, there are still many gaps that need to be filled and other demographics that need to be considered. For example, Alaskan Natives and American Indians (Native Americans) constitute the fourth largest distinct (i.e. one race) population of the US4, yet there is a paucity of medical studies on persons of these backgrounds. This may be due to cultural aspects, where those who valued tradition (including religious beliefs) strongly advocated for the body to buried whole52. This dearth of information also applies to Native Hawaiians and other Pacific Islanders, despite being the fifth largest single race population and second fastest growing race in the country behind Asian Americans4, 45. The lack of neuropathology literature that captures the diversity of Asian Americans also highlights imbalances in research, as it is most probable the neuropathological trends of Japanese Americans would not accurately encompass the depth and breadth of diversity of persons across the Asian continent. An overall paucity of literature presently exists in comprehensive studies centering on these mentioned groups and is not limited to specifically neuropathology studies.
Figure 1.
Number of papers found on PubMed as of November 19, 2021 by year using the search terms “Alzheimer’s brain pathology” along with the demographic term (categories in legend, see supplemental section for further information).
While there has been advances in neuropathology literature focused on Latinos/Hispanics and Black Americans in recent years compared to other minority groups, there are still limited participants in these cohorts. The existing literature is limited in sample size, which varies widely between studies and typically with the minority groups representing a small fraction of the cohort (see Tables 1 and 2). Furthermore, studies can have certain inclusion and exclusion criteria that may hinder participation in select groups; for example, exclusion of CVD for AD studies may decrease frequencies of certain minority groups with higher frequencies of CVD50.
These constraints from low minority group recruitment may be due to numerous factors, including lack of access to healthcare, historical abuses of minority groups for medical research, mistrust of the healthcare system making participants less likely to agree to participate in clinical trials or autopsy programs, and language barriers5, 8, 10, 11, 30, 31, 37, 49, 75, 100. With respect to retention, a systematic review highlights a lack of literature that examines retention exclusively from recruitment39. Socioeconomic circumstance was shown to be the most powerful contributor to the absence of participants for longitudinal studies involving ethnoracial minorities26. Low socioeconomic status largely impacts access to health care resources such as regular visits to a health professional as a result of being uninsured10, 87, 88, in which patients may not only lack the direct care they need but also the general awareness of clinical study enrollment opportunities. Flexible scheduling played a substantial role in participation as many individuals were restrained by work or childcare obligations for their appointments as well as transportation26, 32, 69. Financial compensation was a major influencer in recruitment amongst ethnoracial minorities51, 69; it has been reported that members of the Latino/Hispanic community were motivated by monetary compensation for their time because they experienced economic hardships69.
Patients may also feel discrimination in the process of seeking care, especially among non-Whites for their race, color, and/or ethnicity35. Half of Black Americans report they have faced healthcare discrimination, and one third of Asian Americans and Latino Americans similarly report having experienced healthcare discrimination as well35. A vast majority of non-White Americans believe that in the importance of having AD and dementia care providers to understand their ethnic/racial backgrounds, such as Native Americans, Black Americans, Latinos/Hispanics, and Asian Americans35. However, less than half of Black and Native Americans are confident there are culturally competent providers, and only roughly 3 in 5 Asian Americans and Latinos/Hispanics are confident35. A few studies have attempted to understand barriers and willingness for brain donation across major US racial ethnic groups: NHWs, Latinos/Hispanics, Black Americans, and Asian Americans11, 12. While conducting focus groups, the first study revealed concerns, attitudes and beliefs around brain donation that fell into three categories: 1) religious beliefs 2) concerns and misconceptions about brain research and 3) the role of the family7. A follow up study surveying NHWs as well as 169 African Americans, 50 Asians, and 61 Hispanics revealed older age, Latino ethnicity and understanding of brain use by researchers and what participants need to do to ensure brain is donated were positive predictive factors, while the belief that the body should remain whole at burial, African/African American race, and concern researchers might not be respectful of the body during autopsy were negative predictive factors11, 12. The belief that the body should remain whole was shared amongst Latinos/Hispanics, African Americans, and Asian Americans, which was a similar sentiment of Alaskan Natives and American Indians mentioned earlier52. This further illustrates the substantial role cultural barriers may play in cohort participation from US minority groups.
Knowledge, stigma, and apprehension of ADRD also differ across ethnic/racial groups. For example, one study revealed that NHWs tended to have greater knowledge about AD compared to Black Americans, and Black Americans had same or greater levels of concern about getting AD as NHWs depending on their geographic location47. Another study discovered concern about developing ADRD in Native Americans, Black Americans, and Latinos/Hispanics is noticeably lower compared to NHWs35, which contradicts the finding about Black Americans in the aforementioned study, possibly due to region differences where the data was taken. It has also been shown that Asian Americans do not exhibit a strong concern of ADRD as many believed it was a natural occurrence for aging people14. Multiple papers have denoted that Asian Americans had beliefs of stigma of persons with AD, which played a significant role in seeking care from primary care providers for AD14, 19, 60. Limited knowledge on not only ADRD but also the brain removal process poses some hesitance on minority subject participation8, 11. As stated above, some themes that subjects or family members of subjects shared skepticism on were understanding the purpose of studying a decedent’s brain, misconceptions on how the brain is used or collected for research, and overall knowledge about the brain donation procedure11.
It is important to recognize the existing inadequacies and confines of the study recruitment process for US minority groups to further advance the representation of these populations in biomedical research. Fortunately, there has been progress to minimize these barriers. The UC Davis ADC utilized many avenues to increase diversity in enrollment in research cohorts, such as satellite clinic sites, increasing face to face screening at community events, options of in-home visits, compensation for transportation to clinic visits, dedicated drivers to transport participants to visits, and employing bicultural and bilingual individuals with proficiency of the involved populations44. These methods facilitated a substantial increase in the number of ethnic minority participants, as much as a four-fold increase44; this approach also led to more diversity in other variables as well, such as educational background44. A later study showed that mailing recruitment letters was the most successful method in a multi-modal recruitment approach in enrolling more ethnoracial minorities for ADRD cohorts80. As these issues get addressed on a more widespread scale, significant advancements can be made not only in the field of neuropathology, but all fields of clinical research.
Acknowledgements
This work was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers AG062517, AG052132, AG050782 and AG056519, and supported by the California Department of Public Health Alzheimer’s Disease Program (Grant # 19-10611) with partial funding from the 2019 California Budget Act. The views and opinions expressed in this manuscript are those of the author and do not necessarily reflect the official policy or position of any public health agency of California or of the United States government.
Supplementary Material
References
- In: Anderson NB, Bulatao RA, Cohen B, eds. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington (DC)2004. [PubMed]
- 2020 Profile of Older Americans: Administration for Community Living, May 2021.
- Hispanic Population and Origin in Select U.S. Metropolitan Areas, 2014 [online]. Available at: https://www.pewresearch.org/hispanic/interactives/hispanic-population-in-select-u-s-metropolitan-areas/. Accessed 19 November 2021.
- Race and Ethnicity in the United States: 2010 Census and 2020 Census [online]. Available at: https://www.census.gov/library/visualizations/interactive/race-and-ethnicity-in-the-united-state-2010-and-2020-census.html. Accessed 19 November 2021.
- Amorrortu RP, Arevalo M, Vernon SW, et al. Recruitment of racial and ethnic minorities to clinical trials conducted within specialty clinics: an intervention mapping approach. Trials 2018;19:115. [DOI] [PMC free article] [PubMed]
- Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: Update and areas of immediate need. Alzheimers Dement 2019;15:292-312. [DOI] [PMC free article] [PubMed]
- Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 2015;85:528-534. [DOI] [PMC free article] [PubMed]
- Bilbrey AC, Humber MB, Plowey ED, et al. The Impact of Latino Values and Cultural Beliefs on Brain Donation: Results of a Pilot Study to Develop Culturally Appropriate Materials and Methods to Increase Rates of Brain Donation in this Under-Studied Patient Group. Clin Gerontol 2018;41:237-248. [DOI] [PMC free article] [PubMed]
- Blevins BL, Vinters HV, Love S, et al. Brain arteriolosclerosis. Acta Neuropathol 2021;141:1-24. [DOI] [PMC free article] [PubMed]
- Blustein J, Weiss LJ. Visits to specialists under Medicare: socioeconomic advantage and access to care. J Health Care Poor Underserved 1998;9:153-169. [DOI] [PubMed]
- Boise L, Hinton L, Rosen HJ, Ruhl M. Will My Soul Go to Heaven If They Take My Brain? Beliefs and Worries About Brain Donation Among Four Ethnic Groups. Gerontologist 2017;57:719-734. [DOI] [PMC free article] [PubMed]
- Boise L, Hinton L, Rosen HJ, et al. Willingness to Be a Brain Donor: A Survey of Research Volunteers From 4 Racial/Ethnic Groups. Alzheimer Dis Assoc Disord 2017;31:135-140. [DOI] [PMC free article] [PubMed]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239-259. [DOI] [PubMed]
- Braun KL, Browne CV. Perceptions of dementia, caregiving, and help seeking among Asian and Pacific Islander Americans. Health Soc Work 1998;23:262-274. [DOI] [PubMed]
- Budiman A, Ruiz NG. Key facts about Asian Americans, a diverse and growing population [online]. Available at: https://www.pewresearch.org/fact-tank/2021/04/29/key-facts-about-asian-americans/. Accessed 30 November 2021.
- Budiman A, Ruiz NG. Key facts about Asian origin groups in the U.S. [online]. Available at: https://www.pewresearch.org/fact-tank/2021/04/29/key-facts-about-asian-origin-groups-in-the-u-s/. Accessed 25 January 2022.
- Burns A, Iliffe S. Dementia. BMJ 2009;338:b75. [DOI] [PubMed]
- Bustamante LN, Hugo-Lopez M, Krogstad JM. U.S. Hispanic Population Surpassed 60 Million in 2019, but Growth Has Slowed [online]. Available at: https://www.pewresearch.org/fact-tank/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/. Accessed October 17 2020.
- Casado BL, Hong M, Lee SE. Attitudes Toward Alzheimer's Care-Seeking Among Korean Americans: Effects of Knowledge, Stigma, and Subjective Norm. Gerontologist 2018;58:e25-e34. [DOI] [PubMed]
- Cavazzoni P. FDA's Decision to Approve New Treatment for Alzheimer's Disease [online]. Available at: https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease. Accessed 7 June 2021.
- Clark CM, DeCarli C, Mungas D, et al. Earlier onset of Alzheimer disease symptoms in latino individuals compared with anglo individuals. Arch Neurol 2005;62:774-778. [DOI] [PubMed]
- Cummings J, Ritter A, Zhong K. Clinical Trials for Disease-Modifying Therapies in Alzheimer's Disease: A Primer, Lessons Learned, and a Blueprint for the Future. J Alzheimers Dis 2018;64:S3-S22. [DOI] [PMC free article] [PubMed]
- Deramecourt V, Slade JY, Oakley AE, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology 2012;78:1043-1050. [DOI] [PMC free article] [PubMed]
- Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 2010;9:1118-1127. [DOI] [PubMed]
- Dugger BN, Dickson DW. Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol 2017;9. [DOI] [PMC free article] [PubMed]
- Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist 2011;51 Suppl 1:S33-45. [DOI] [PMC free article] [PubMed]
- Ennis SR, Rios-Vargas M, Albert NG. The Hispanic Population: 2010: U.S. Census Bureau, 2011 May.
- Filshtein TJ, Brenowitz WD, Mayeda ER, et al. Reserve and Alzheimer's disease genetic risk: Effects on hospitalization and mortality. Alzheimers Dement 2019;15:907-916. [DOI] [PMC free article] [PubMed]
- Filshtein TJ, Dugger BN, Jin LW, et al. Neuropathological Diagnoses of Demented Hispanic, Black, and Non-Hispanic White Decedents Seen at an Alzheimer's Disease Center. J Alzheimers Dis 2019;68:145-158. [DOI] [PMC free article] [PubMed]
- Frates J, Garcia Bohrer G. Hispanic perceptions of organ donation. Prog Transplant 2002;12:169-175. [DOI] [PubMed]
- Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health 1997;87:1773-1778. [DOI] [PMC free article] [PubMed]
- Gamboa CJ, Julion WA. Proactive Recruitment of Older African-Americans for Alzheimer's Research with Brain Donation: a Cohort Case Study of Success. J Racial Ethn Health Disparities 2021;8:463-474. [DOI] [PubMed]
- Garcia MA, Saenz J, Downer B, Wong R. The role of education in the association between race/ethnicity/nativity, cognitive impairment, and dementia among older adults in the United States. Demogr Res 2018;38:155-168. [DOI] [PMC free article] [PubMed]
- Gates HLJ. In Search of Our Roots: How 19 Extraordinary African Americans Reclaimed Their Past. New York: Crown Publishing, 2009.
- Gaugler J JB, Johnson T, Reimer J, and Weuve J. . 2021 Alzheimer's disease facts and figures. Alzheimers Dement 2021;17:327-406. [DOI] [PubMed]
- Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res 2012;9:664-672. [DOI] [PMC free article] [PubMed]
- George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health 2014;104:e16-31. [DOI] [PMC free article] [PubMed]
- Giaccone G, Arzberger T, Alafuzoff I, et al. New lexicon and criteria for the diagnosis of Alzheimer's disease. Lancet Neurol 2011;10:298-299; author reply 300-291. [DOI] [PubMed]
- Gilmore-Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: A systematic review. Alzheimers Dement (N Y) 2019;5:751-770. [DOI] [PMC free article] [PubMed]
- Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer's Coordinating Center. Alzheimers Dement 2016;12:669-677. [DOI] [PMC free article] [PubMed]
- Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 1999;14:481-493. [PubMed]
- Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 2003;51:169-177. [DOI] [PubMed]
- Hill CV, Perez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethn Dis 2015;25:245-254. [DOI] [PMC free article] [PubMed]
- Hinton L, Carter K, Reed BR, et al. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord 2010;24:234-241. [DOI] [PMC free article] [PubMed]
- Hixson L, Hepler B, Kim MO. Native Hawaiian and Other Pacific Islander Population: U.S. Census Bureau, 2012 May.
- Hoeffel EM, Rastogi S, Kim MO, Shahid H. The Asian Population 2010: U.S. Census Bureau, 2012 March.
- Howell JC, Soyinka O, Parker M, et al. Knowledge and Attitudes in Alzheimer's Disease in a Cohort of Older African Americans and Caucasians. Am J Alzheimers Dis Other Demen 2016;31:361-367. [DOI] [PMC free article] [PubMed]
- Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1-13. [DOI] [PMC free article] [PubMed]
- Ighodaro ET, Nelson PT, Kukull WA, et al. Challenges and Considerations Related to Studying Dementia in Blacks/African Americans. J Alzheimers Dis 2017;60:1-10. [DOI] [PMC free article] [PubMed]
- Indorewalla KK, O'Connor MK, Budson AE, Guess DiTerlizzi C, Jackson J. Modifiable Barriers for Recruitment and Retention of Older Adults Participants from Underrepresented Minorities in Alzheimer's Disease Research. J Alzheimers Dis 2021;80:927-940. [DOI] [PMC free article] [PubMed]
- Jefferson AL, Lambe S, Chaisson C, Palmisano J, Horvath KJ, Karlawish J. Clinical research participation among aging adults enrolled in an Alzheimer's Disease Center research registry. J Alzheimers Dis 2011;23:443-452. [DOI] [PMC free article] [PubMed]
- Jernigan M, Fahrenwald N, Harris R, Tsosie U, Baker LO, Buchwald D. Knowledge, beliefs, and behaviors regarding organ and tissue donation in selected tribal college communities. J Community Health 2013;38:734-740. [DOI] [PMC free article] [PubMed]
- Jones N, Marks R, Ramirez R, Rios-Vargas M. 2020 Census Illuminates Racial and Ethnic Composition of the Country [online]. Available at: https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html. Accessed 9 November 2021.
- Kagan A. The Honolulu heart program : an epidemiological study of coronary heart disease and stroke. Amsterdam: Harwood Academic, 1996.
- Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 2004;226:75-80. [DOI] [PubMed]
- Kamara DM, Gangishetti U, Gearing M, et al. Cerebral Amyloid Angiopathy: Similarity in African-Americans and Caucasians with Alzheimer's Disease. J Alzheimers Dis 2018;62:1815-1826. [DOI] [PMC free article] [PubMed]
- Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017;134:171-186. [DOI] [PMC free article] [PubMed]
- Latimer CS, Keene CD, Flanagan ME, et al. Resistance to Alzheimer Disease Neuropathologic Changes and Apparent Cognitive Resilience in the Nun and Honolulu-Asia Aging Studies. J Neuropathol Exp Neurol 2017;76:458-466. [DOI] [PMC free article] [PubMed]
- Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol 2011;70:774-780. [DOI] [PMC free article] [PubMed]
- Liu D, Hinton L, Tran C, Hinton D, Barker JC. Reexamining the relationships among dementia, stigma, and aging in immigrant Chinese and Vietnamese family caregivers. J Cross Cult Gerontol 2008;23:283-299. [DOI] [PMC free article] [PubMed]
- Livney MG, Clark CM, Karlawish JH, et al. Ethnoracial differences in the clinical characteristics of Alzheimer's disease at initial presentation at an urban Alzheimer's disease center. Am J Geriatr Psychiatry 2011;19:430-439. [DOI] [PMC free article] [PubMed]
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? [online]. Available at: https://www.pewresearch.org/fact-tank/2021/09/23/who-is-hispanic/. Accessed 17 October 2021.
- Love S, Chalmers K, Ince P, et al. Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. Am J Neurodegener Dis 2014;3:19-32. [PMC free article] [PubMed]
- Marks R, Jones N. Collecting and Tabulating Ethnicity and Race Responses in 2020 Census: U.S. Census Bureau, 2020.
- Martinez DE, Gonzalez KE. “Latino” or “Hispanic”? The Sociodemographic Correlates of Panethnic Label Preferences among U.S. Latinos/Hispanics. Sociological Perspectives 2021;64:365-386.
- Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015-2060) in adults aged >/=65 years. Alzheimers Dement 2019;15:17-24. [DOI] [PMC free article] [PubMed]
- Mayeda ER, Glymour MM, Quesenberry CP, Jr., Whitmer RA. Heterogeneity in 14-year Dementia Incidence Between Asian American Subgroups. Alzheimer Dis Assoc Disord 2017;31:181-186. [DOI] [PMC free article] [PubMed]
- Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216-224. [DOI] [PMC free article] [PubMed]
- McDougall GJ, Jr., Simpson G, Friend ML. Strategies for research recruitment and retention of older adults of racial and ethnic minorities. J Gerontol Nurs 2015;41:14-23; quiz 24-15. [DOI] [PMC free article] [PubMed]
- Mehta KM, Yaffe K, Perez-Stable EJ, et al. Race/ethnic differences in AD survival in US Alzheimer's Disease Centers. Neurology 2008;70:1163-1170. [DOI] [PMC free article] [PubMed]
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479-486. [DOI] [PubMed]
- Moon H, Badana ANS, Hwang SY, Sears JS, Haley WE. Dementia Prevalence in Older Adults: Variation by Race/Ethnicity and Immigrant Status. Am J Geriatr Psychiatry 2019;27:241-250. [DOI] [PubMed]
- Napoles AM, Chadiha LA, Resource Centers for Minority Aging R. Advancing the science of recruitment and retention of ethnically diverse populations. Gerontologist 2011;51 Suppl 1:S142-146. [DOI] [PMC free article] [PubMed]
- Pariona A. What Is The Difference Between Race And Ethnicity? [online]. Available at: https://www.worldatlas.com/articles/what-is-the-difference-between-race-and-ethnicity.html. Accessed 14 June 2021.
- Peters TG, Kittur DS, McGaw LJ, Roy MRs, Nelson EW. Organ donors and nondonors. An American dilemma. Arch Intern Med 1996;156:2419-2424. [PubMed]
- Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol 2005;57:98-103. [DOI] [PubMed]
- Proctor BD, Semega JL, Kollar MA. Income and Poverty in the United States: 2015. US Census Bureau: U.S. Census Bureau, 2016 September.
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329-344. [DOI] [PubMed]
- Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA. Prevalence and incidence of clinically diagnosed Alzheimer's disease dementia from 1994 to 2012 in a population study. Alzheimers Dement 2019;15:1-7. [DOI] [PMC free article] [PubMed]
- Reuland M, Sloan D, Antonsdottir IM, Spliedt M, Johnston MCD, Samus Q. Recruitment of a diverse research cohort in a large metropolitan area for dementia intervention studies. Contemp Clin Trials 2022;112:106622. [DOI] [PMC free article] [PubMed]
- Ringman JM, Sachs MC, Zhou Y, Monsell SE, Saver JL, Vinters HV. Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol 2014;71:878-883. [DOI] [PMC free article] [PubMed]
- Riudavets MA, Rubio A, Cox C, Rudow G, Fowler D, Troncoso JC. The prevalence of Alzheimer neuropathologic lesions is similar in blacks and whites. J Neuropathol Exp Neurol 2006;65:1143-1148. [DOI] [PubMed]
- Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250-260. [DOI] [PubMed]
- Rucker WC. The River Flows On: Black resistance, culture, and identity formation in early America: LSU Press., 2006.
- Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuropathological lesions of Alzheimer's disease is independent of race and gender. Neurobiol Aging 2001;22:169-175. [DOI] [PubMed]
- Santos OA, Pedraza O, Lucas JA, et al. Ethnoracial differences in Alzheimer's disease from the FLorida Autopsied Multi-Ethnic (FLAME) cohort. Alzheimers Dement 2019;15:635-643. [DOI] [PMC free article] [PubMed]
- Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat 10 2012:1-207. [PubMed]
- Schneider LS. Drug development, clinical trials, cultural heterogeneity in Alzheimer disease: the need for pro-active recruitment. Alzheimer Dis Assoc Disord 2005;19:279-283. [DOI] [PubMed]
- Shakir MN, Dugger BN. Advances in Deep Neuropathological Phenotyping of Alzheimer Disease: Past, Present, and Future. J Neuropathol Exp Neurol 2022;81:2-15. [DOI] [PMC free article] [PubMed]
- Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement 2018;14:280-292. [DOI] [PubMed]
- Soria JA, Huisa BN, Edland SD, et al. Clinical-Neuropathological Correlations of Alzheimer's Disease and Related Dementias in Latino Volunteers. J Alzheimers Dis 2018;66:1539-1548. [DOI] [PubMed]
- Strozyk D, Dickson DW, Lipton RB, et al. Contribution of vascular pathology to the clinical expression of dementia. Neurobiol Aging 2010;31:1710-1720. [DOI] [PMC free article] [PubMed]
- Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: introduction. Am J Epidemiol 1975;102:477-480. [DOI] [PubMed]
- Tamir C. Key findings about Black America [online]. Available at: https://www.pewresearch.org/fact-tank/2021/03/25/key-findings-about-black-america/. Accessed 14 June 2021.
- Tamir C, Budiman A, Noe-Bustamante L, Mora L. Facts About the U.S. Black Population [online]. Available at: https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/. Accessed 14 June 2021.
- Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49-56. [DOI] [PubMed]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791-1800. [DOI] [PubMed]
- Tishkoff SA, Kidd KK. Implications of biogeography of human populations for 'race' and medicine. Nat Genet 2004;36:S21-27. [DOI] [PubMed]
- Vargas Bustamante A, Chen J, Rodriguez HP, Rizzo JA, Ortega AN. Use of preventive care services among Latino subgroups. Am J Prev Med 2010;38:610-619. [DOI] [PubMed]
- Vega IE, Cabrera LY, Wygant CM, Velez-Ortiz D, Counts SE. Alzheimer's Disease in the Latino Community: Intersection of Genetics and Social Determinants of Health. J Alzheimers Dis 2017;58:979-992. [DOI] [PMC free article] [PubMed]
- Vinters HV, Zarow C, Borys E, et al. Review: Vascular dementia: clinicopathologic and genetic considerations. Neuropathol Appl Neurobiol 2018;44:247-266. [DOI] [PubMed]
- Weissberger GH, Gollan TH, Bondi MW, et al. Neuropsychological Deficit Profiles, Vascular Risk Factors, and Neuropathological Findings in Hispanic Older Adults with Autopsy-Confirmed Alzheimer's Disease. J Alzheimers Dis 2019;67:291-302. [DOI] [PMC free article] [PubMed]
- White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis 2009;18:713-725. [DOI] [PubMed]
- White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci 2002;977:9-23. [DOI] [PubMed]
- White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA 1996;276:955-960. [PubMed]
- White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology 2016;86:1000-1008. [DOI] [PMC free article] [PubMed]
- Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol 2006;63:87-90. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.